Current Trends in Neurofeedback
Overview
Intake and initial EEG assessment provide a baseline against which subsequent progress, or lack of progress, can be gauged. This is important because, despite the robust research evidence for the efficacy and effectiveness of neurofeedback as shown in groups that receive training (Schwartz & Andrasik, 2016; Tan, Shaffer, Lyle, & Teo, 2016), benefits are not guaranteed for individual clients. One purpose of ongoing assessment is to verify that expected outcomes occur and, if a good result does not happen, use continuous assessment data to adjust training protocols.
Ethical principles involved in the ongoing assessment are related to questions of benevolence (i.e., is training helping?) and nonmalevolence (i.e., is training causing any harm?) (Beauchamp, 2003). Ongoing assessment is necessary to assure and avoid the latter, even if the harm is the ongoing cost of time and finances for a treatment that does not help a particular individual. The ethical principle of autonomy involves whether the client wants to continue training. Ongoing assessment helps the client remain well-informed about the training outcome and can continue to provide consent to continue training. The ethical principle of justice may apply to neurofeedback training insofar as training should be efficient (e.g., am I providing training efficiently enough to the current client that I do not unreasonably deny access to training to others?).
Additionally, ongoing assessment supports maintaining a positive working relationship by allowing the client and practitioner to examine outcomes and make evidence-based decisions collaboratively. Seeing progress through continuous assessment also motivates the client to persevere with training and apply skills that build on neurofeedback effects outside of the training session.
The process of reviewing data from ongoing assessment also may help the client to see relationships between their subjective states and behavior, or even environmental events. For example, the client may develop a self-awareness that when the subjective state they experience during neurofeedback occurs in a given situation outside the training environment, they can behave more effectively. Such self-awareness can motivate the client to reproduce the subjective state they have intentionally learned with neurofeedback in advance of or during relevant situations.
Neurofeedback trains the EEG activity of the brain. The ongoing assessment of EEG activity provides an index of whether neurofeedback has the intended effect on brain activity. However, the client and practitioner are most likely interested in whether changes in EEG activity generalize to other domains such as emotional experience, cognition, physiological condition, and real-life behavior in situations that matter to the client. Therefore, in addition to ongoing assessment of EEG activity, it is usually the case that the client and practitioner will at least periodically use non-EEG measures during the continuous assessment. Therefore, ongoing assessment typically includes using the same EEG variables as those used during intake and repeating non-EEG measures used during intake to assess the generalization of possible EEG changes to changes in those problems that originally motivated the client to request neurofeedback.
BCIA Blueprint Coverage
This unit covers X. Current Trends in Neurofeedback.

We have divided this unit into A. Current trends (e.g., z-score training, LORETA z-score training) and B. Combining neurofeedback with other modalities, including heart rate variability (HRV), respiration, HEG, and neuromodulation systems.
NEUROFEEDBACK TRENDS
We have divided this section into qEEG-Based Neurofeedback Training, The Development of Z-Score Training, Infra-Low Training, Infra-Slow Training, and Connectivity Training.
qEEG-Based Neurofeedback Training
Following the development and proliferation of high-quality EEG recording devices capable of recording 19 scalp electrode locations simultaneously and software capable of creating images derived from the recorded data, the use of a quantitative approach to the EEG began to be more broadly utilized.
Caption: qEEG treatment of ADHD
The quantitative assessment of scalp electrical recordings was made possible by the development of the Fast Fourier Transformation (FFT) algorithm in 1965 (Cooley & Tukey, 1965; Dumermuth & Fluhler, 1967).
This method of computation allowed for the deconstruction of the complex EEG information, consisting of multiple frequency components with different amplitudes and characteristics, into frequency and power spectral displays, initially as tables and later as representative values mapped onto topographic head maps (brain maps).
As inexpensive and powerful personal computers became more available in the 1990s, clinicians and researchers could utilize these advanced computational methods and begin to identify the components associated with various conditions and disorders. This was facilitated by developing EEG normative databases by E. Roy John at New York University and Robert Thatcher and the University of Maryland, Frank Duffy of Harvard University, and others.
 |
 |
Caption: E. Roy John and Frank Duffy
The EEG has been shown to have strong stability and specificity across multiple ethnic and cultural groups (John, Ahn, & Prichep, 1980) and to be highly consistent in evaluations of test-retest reliability (Fein et al., 1983; Oken & Chiappa, 1988).
Because of this consistency and reliability, the quantitative assessment of the EEG (qEEG) and the availability of qEEG databases became useful clinical tools for individualized training protocol development. Each client could be assessed using multiple techniques, including a qEEG with normative database comparison (see the Assessment unit).
As described by Thatcher (1998), the value of the normative EEG database comparison included the ability to assess the neuropsychological status of the client to help determine the potential basis for the client’s complaints. Using a normative EEG database also helped identify strengths and weaknesses of the client’s neurophysiology to design the optimal training regime and aid in the evaluation of results following a given intervention.

Caption: Robert Thatcher
Many clinicians began to utilize these tools to determine the best training approach for their clients. If an area of the brain showed excess or deficient activity in one or more frequencies, that area would be targeted with uptraining or down-training methods to address those specific issues. This often led to the resolution of the client’s presenting complaints associated with these underlying patterns of dysregulation.
The benefits of these approaches for training protocol development were obvious. There was no need to guess at the location of training based upon an understanding of neuroanatomy or neurophysiology. There was no need to guess at the frequency to target in training and no need to guess at the direction of that training – whether to reward increases or decreases in a given frequency or set of frequencies. Finally, the training results were identifiable with follow-up qEEG assessment upon subsequent analysis.
As database-guided training progressed, a few difficulties emerged. One of the first and most often called out by neurologists (Nuwer & Coutin-Churchman, 2014) is the artifact problem in the EEG. Inexperienced practitioners who had little training in electroencephalography often made errors in data selection and rejection when evaluating an EEG for subsequent processing and database comparison. The presence of eye artifacts, including blink, lateral eye movement, eye flutter, and others, leads to an overabundance of delta frequency activity in frontal and lateral frontal EEG sensor locations compared to a normative database. Graphic © eegatlas-online.com.
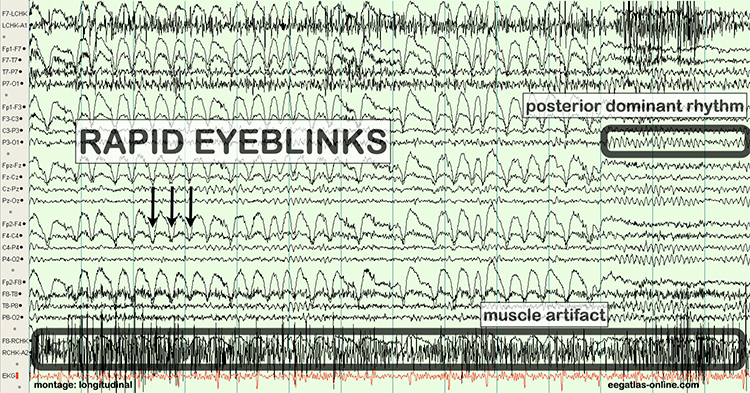
This is due to the frequency of eye artifact (slow, in the 1-4 Hz range) and amplitude (generally quite high, in the 50 to 100 uV range or higher) that is thus in the same frequency as the delta frequency band but with much higher voltages than typical resting delta activity.
Several other artifacts produced additional false-positive findings in the database report. These include EMG (muscle activation) artifact that artificially elevates beta and fast or high beta frequencies, cable sway artifact (in the delta range), electrical “mains” artifact or electromagnetic frequency (EMF) artifact that increases fast beta findings as well as a wide variety of other artifacts. Please see the section on EEG artifacts.
The second major error was to train any finding that showed deviation from normal or typical values compared to a database of well screened, typical subjects.
As inexpensive and powerful personal computers became more available in the 1990s, clinicians and researchers utilized these advanced computational methods and began to identify the components associated with various conditions and disorders. This was facilitated by the development of EEG normative databases developed by E. Roy John (NxLink, later known as BrainDX) at New York University, Robert Thatcher (NeuroGuide) at the University of Maryland, Frank Duffy of Harvard University (BEAM), and others. More recent additions have included the Human Brain Institute (HBI) database developed by Juri Kropotov and the iSyncBrain database developed by a group of Korean researchers.
In some cases, training an abnormal finding resulted in the client reporting worsening symptoms, the return of previously resolved symptoms and in some cases, the emergence of serious negative symptoms that were new to the client.
This led to the understanding that not all abnormal or out-of-range findings on a qEEG assessment were associated with pathology. Findings on qEEG assessments needed to be correlated with other assessment findings and client-reported symptoms. It became clear that some changes in the EEG were compensatory and reflected the system’s attempts to find balance or homeostasis. Removing these compensatory behaviors often resulted in the negative effects noted previously.
From this developing understanding grew an idea that training could address only the specific symptom or network that was most problematic for the client and that would have built-in mechanisms to correct for movement of EEG variables in undesirable directions. This has led to the creation of direct database training or z-score training.
The Development of Z-Score Training
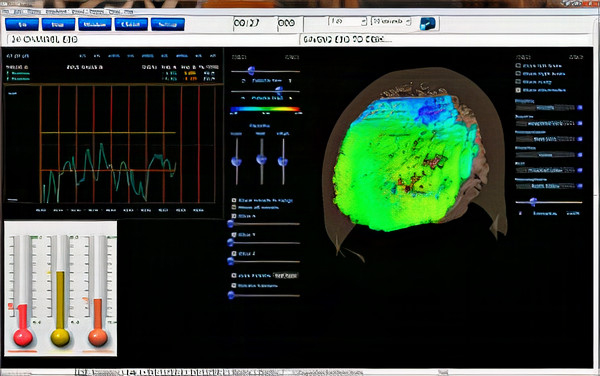
To meet the need for z-score training, Robert Thatcher, developer of the NeuroGuide database, and Thomas Collura of BrainMaster Technologies developed Live Z-Score Training (LZT). In 1996 (Thatcher et al., 2019), they started with a simple 1- or 2- channel approach that quickly developed into a 4-channel system for training clients by providing them with real-time information about how closely their EEG matched a database of age-matched typical controls. There were approximately 72 variables available within the Thatcher database, including power, relative power, phase and coherence values, and more for the standard EEG frequencies.
Caption: Z-PLUS LZT Live Z-Score Training with Z-Bars and Z-Maps Display
The client’s EEG values were compared to these values, and differences were displayed in standard deviations. The goal was to train toward z-zero standard deviations.

Caption: Tom Collura
Subsequent advances resulted in 19-channel, real-time surface z-score training, and then 19-channel LORETA z-score training, using the LORETA source localization method to train in 3 dimensions. Ultimately, Collura and his colleagues shifted to the E. Roy John database, currently known as BrainDX. Then they more recently shifted again to a database known as qEEGPro, which was developed by using client EEG recordings that were cleaned of clinical EEG patterns identified by a client questionnaire (qEEG.pro/database/). This approach is based upon questionable assumptions, and many qEEG researchers consulted about this expressed skepticism regarding this database approach (personal communication, John Anderson, 2019-2021).
Caption: qEEGPro 19-Channel sLORETA Z-Score Training
Robert Thatcher and his team continued to work with the Neuroguide database and have continued to develop the features of 19-channel z-score training, most recently with the development of swLORETA, a more precise and accurate iteration of the LORETA source localization method.
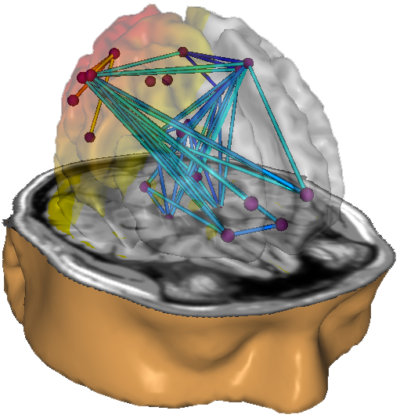
Caption: NeuroNavigator swLORETA
This has resulted in a large base of clinicians using this approach. More than 50 publications have presented evidence of the efficacy of z-score neurofeedback training, suggesting widespread scientific acceptance (https://www.appliedneuroscience.com/PDFs/Z_Score_NFB_Publications.pdf).
Infra-Low Training
Two prominent individuals who have contributed substantially to the field and have a large following are Susan and Siegfried Othmer. They initially studied with Margaret Ayers when she worked with their son, Brian, who experienced temporal lobe epilepsy. After several transitions, they developed a training institute and proceeded to work with clients and train new practitioners.
Caption: Sigfried and Susan Othmer
The evolution of the Othmer method led to training in a frequency below 0.1 Hz, known as infra-low frequencies. This caused even more controversy in the field because these portions of the EEG frequency spectrum are poorly understood. The mechanisms of control of this area of electrophysiology are even less clear. This is also within the frequency spectrum occupied by such artifacts as eye blink, eye movement, cable sway, electrodermal responses (GSR), electrode drift, and other factors. Siegfried Othmer has a doctorate in physics and has explained that these issues have been addressed and that the training proceeds effectively despite these factors due to proprietary signal processing methods (personal communication, 2021).There have been multiple published reports regarding infra-low frequency training (Legarda et al., 2011; Othmer & Legarda, 2011; Sasu & Othmer, 2020). Other clinicians have also developed their own applications associated with training in these areas of brain electrophysiology below 1 Hz.
Following the move to ILF frequencies training, the Othmers continued to incorporate A-T training, using a two-channel sum training approach. They have recently included a two-channel synchrony training approach using the alpha and gamma frequency bands.
The Othmers have a large following, and many clinics and individual practitioners employ these methods and report positive results with their clients. An explanation of what is being trained in the ILF protocol is difficult to come by and must wait for future revelations.
Infra-Slow-Training
Another prominent clinician in the neurofeedback field, Mark Smith, has also pursued a similar low-frequency training that he terms infra-slow frequency training, utilizing different amplifiers and software. He initially trained with the Othmers but then began developing his approach in providing a feedback signal for activity below 0.1 Hz. As time went on, he began to incorporate database training, also known as z-score training, using the Neuroguide database developed by Robert Thatcher. He incorporated quantitative EEG, learning from Jonathan Walker, MD, and others.
Caption: Mark Smith
Smith's explanation of his approach to infra-slow-frequency (ISF) training in collaboration with Thomas Collura (Smith et al., 2014) is that the training involves providing the client with information regarding the phase of the ISF signal rather than the typical amplitude fluctuations of an alternating current EEG frequency derived from a peak to peak digital processing function. As noted in the above-mentioned article, the time constant is quite long, requiring significant time (3 minutes is noted in the article example) for the filters to return to baseline following a significant shift in the signal being measured.
Unfortunately, as noted in the Othmers' ILF training discussion, significant voltage shifts occur with typical eye movement, eye blink, electrodermal, and cable sway artifacts. There is no discussion in the article regarding these issues, and therefore, the validity of the training signal feedback is in question. There may be more to the signal processing than is explained in the article to mitigate these effects.
Again, the training results with this approach, using a combination of ISF, z-score, synchrony, and referential enhancement training, have been positive and clinical examples and book chapters (Smith, 2014, 2017, 2018) support continued study.
Functional Connectivity Training
Studying how different brain regions are interconnected has become a major focus of interest in the EEG and quantitative EEG communities and among researchers using functional magnetic resonance imaging (fMRI) and other methods to identify and track the blood oxygen level-dependent (BOLD) signal. Recently, fMRI has even been used for neurofeedback, including for connectivity training (Yamashita et al., 2017).One of the benefits of EEG analysis is the faster time scale of the EEG signal compared to the BOLD signal. Blood flow changes occur in a seconds-to-minutes time scale, while EEG changes happen within milliseconds. Evoked potential (EP) and event-related potential (ERP) studies using EEG show brain responses to stimuli in as little as 50 milliseconds (ms) and observable responses in as little as 100 ms. Additionally, the costs of training using fMRI are orders of magnitude greater than those using EEG neurofeedback.
While recent connectivity studies have often focused on fMRI, EEG connectivity studies of connectivity have a long and well-documented history.
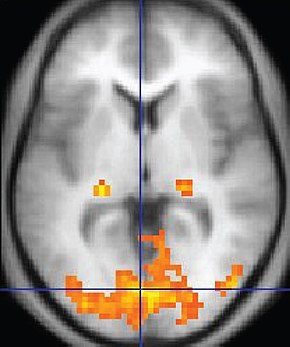
Connectivity changes following even a single neurofeedback session have been demonstrated (Kluetsch et al., 2014) using pre- and post-fMRI measurements to identify changes in network communication. Kluetsch and colleagues demonstrated a rebound effect on the EEG following alpha desynchronization training and increased connectivity within the salience (SN) and default mode networks (DMN). Graphic courtesy of BrainMaster Technologies.
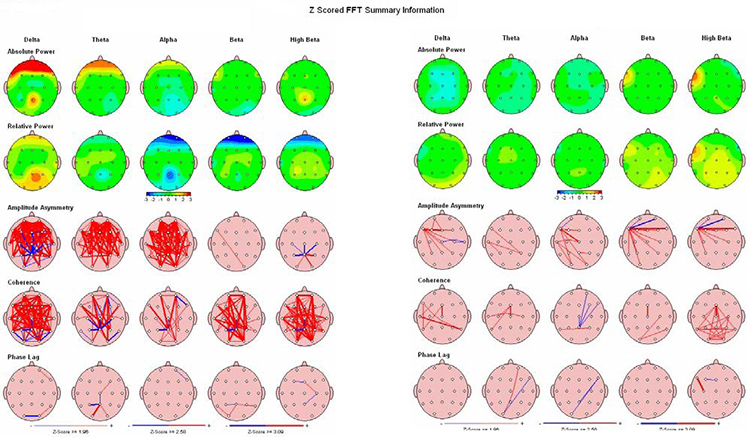
Caption: Pre- and Post-treatment NeuroGuide QEEG maps (eyes open) showing effects of 23 sessions of 4-channel Multivariate Proportional live Z-Score training
Coben and colleagues (2018) showed significant changes in EEG power values in 174 participants selected from 11 different clinical sites in the US, who were given an average of 21.49 sessions of coherence feedback training. They also showed 50% greater change in participants randomly assigned to four-channel coherence coherence training vs. those receiving two-channel training (113 vs. 61 participants). Coben’s group experimented with various methods for measuring coherence, particularly in relation to the 4 channel coherence training. This more sophisticated approach may have accounted for the improved results within this group.
One of the early proponents of coherence training, Joseph Horvath, studied whether coherence training or power training was more efficacious in outcome results (Walker & Horvath, 2010). The Walker and Horvath study showed no clear preference for power or coherence training but found that power training often resulted in more coherence abnormalities that would need to be addressed by direct coherence training.
One issue associated with coherence training using EEG is the effect of reference contamination from the linked ears or linked mastoid reference. This is a common issue and is usually mitigated by using multiple montage views (see the section in Neurofeedback Tutor on quantitative EEG assessment). However, current approaches to coherence measures in such databases as NeuroGuide and NxLink and others utilize the linked ears montage. They don’t have the option of using other montages for coherence assessment or training.
Nunez and Srinivasan (2006) showed that using either a single mastoid or the averaged potential of the linked mastoids increases coherence in all electrode combinations due to the contribution of the reference signal to all electrodes. They reported that using the common average reference is superior: “averaged reference coherence estimates obtained from dense electrode arrays accurately mimic theoretical reference-free coherence estimates but remain elevated by volume conduction for electrode pairs closer than about 8-10 cm.” (p. )
Therefore, future approaches to coherence training using EEG should consider these findings to improve accuracy in such training outcomes. Additionally, the work of Coben and colleagues suggests that further work on the mathematical analysis and display of coherence measures should also be undertaken to identify the optimum method.
It appears that many neurofeedback training approaches improve functional connectivity, whether designed to address connectivity directly or not. This is consistent with other findings suggesting that training brain activity necessarily involves brain network functions. Nothing occurs in the central nervous system (CNS) without activated and exercised network structures. Behavioral improvements appear to correlate with changes in functional connectivity. Therefore, it may be helpful for the long-term efficacy of neurofeedback training to identify those interventions that maximally improve effective network communication.
fMRI Neurofeedback
Real-time neurofeedback based on functional magnetic resonance imaging (rt-fMRI) has been applied to a range of conditions such as pain, anxiety, depression, schizophrenia, cognitive function, and post-stroke motor behavior (Linhartova et al., 2019; Watanabe et al., 2017).
fMRI provides more precise spatial resolution than the EEG and can image cerebral structures that are not amenable to EEG investigation. However, because it relies on blood oxygen level responses, the temporal resolution of fMRI is less than that of EEG. Recent work has begun to study the use of rt-fMRI neurofeedback with EEG neurofeedback (Bezmaternykh et al., 2021).
COMBINING NEUROFEEDBACK WITH OTHER MODALITIES
Clinicians integrate modalities like heart rate variability (HRV), respiration, hemoencephalography (HEG), and neuromodulation with neurofeedback training to accelerate the acquisition of self-regulation skills and improve training outcomes.
HEART RATE VARIABILITY
"HRV is the organized fluctuation of time intervals between successive heartbeats defined as interbeat intervals" (Shaffer, Meehan, & Zerr, 2020).
The oscillations of a healthy heart are complex. HRV indexes how efficiently we mobilize and utilize limited self-regulatory resources to maintain homeostasis. HRV plays a vital role in regulatory capacity, executive functions, health, and performance. A healthy heart can rapidly adjust to sudden challenges due to the cooperation of interlocking and better-calibrated control systems. HRV is crucial to health, performance, and resilience. Behavioral interventions like aerobic exercise, healthy breathing, compassion, and mindfulness meditation are powerful strategies for increasing HRV. Graphic © Maridav/Shutterstock.com.
We have divided this section into The Meaning of HRV, A Healthy Heart is Not a Metronome, How HRV Biofeedback Can Support Neurofeedback, and Heart-Brain Interactions.
The Meaning of HRV
Heart rate is the number of heartbeats per minute. Graphic © Crdjan/Shutterstock.com.
Elevated HR is associated with dementia and cognitive decline
Imahori et al. (2021) conducted a cohort study of 2147 adults ≥ 60 who were free of dementia when they entered the study. Resting heart rates (RHR) ≥80 (compared with 60-69 bpm) were associated with a greater risk of dementia and more rapid cognitive decline, independent of cardiovascular disease (CV).Elevated HR limits HRV
HR is important because a high rate can reduce heart rate variability (HRV), the changes in the time intervals between consecutive heartbeats (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Graphic © arka38/Shutterstock.com.
Listen to a mini-lecture on a Heart Rate Variability Overview © BioSource Software LLC.
We measure the time intervals between successive heartbeats in milliseconds. Graphic courtesy of Dick Gevirtz.
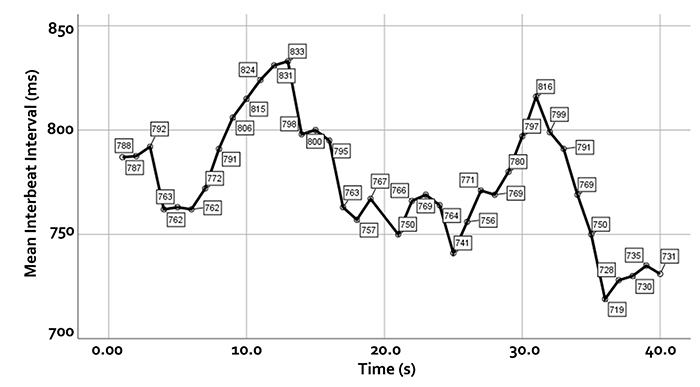
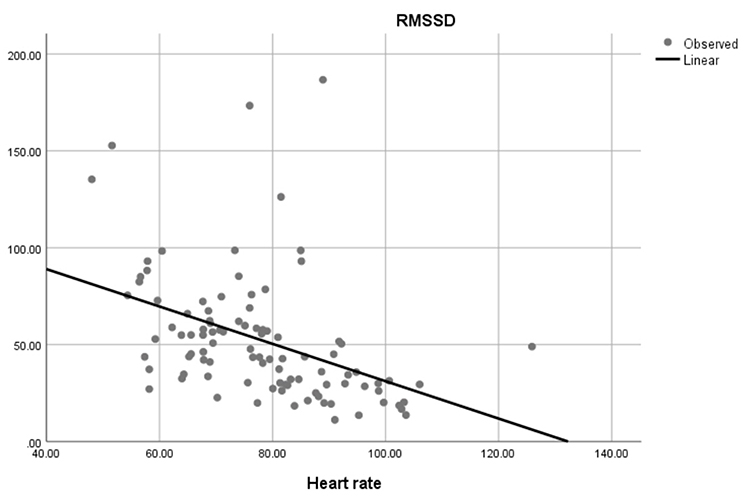
Faster HRs reduce the time between successive beats and the opportunity for interbeat intervals (IBIs) to vary. Faster HRs lower HRV. Resting HRs that exceed 90 bpm are associated with an elevated risk of mortality (Zhang, Shen, & Qi, 2016). The next three scatterplots show an inverse relationship between HR and three widely used HRV metrics: RMSSD, SDNN, and low-frequency power.
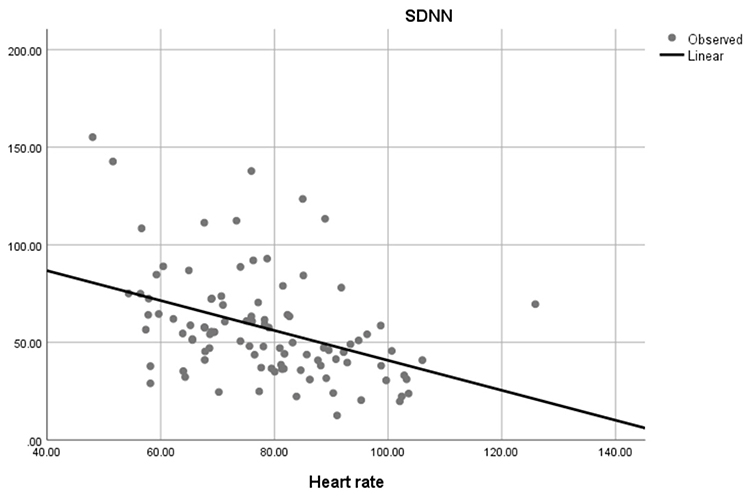
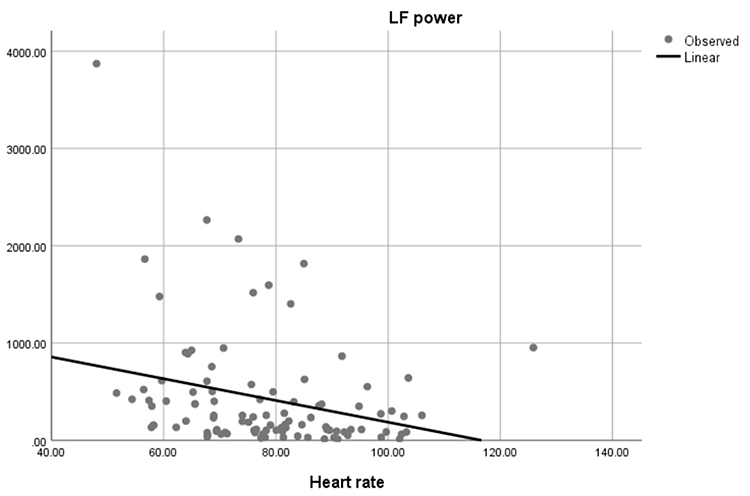
Conversely, the slower HRs seen in endurance sports like trail running increase the time between adjacent heartbeats and the chance for IBIs to vary. This raises HRV. This phenomenon is called cycle length dependence (McCraty & Shaffer, 2015). Graphic © Maridav/Shutterstock.com.

Typical non-athlete HRs are 60-80 bpm. Athletes may have HRs between 40-60 bpm (Khazan, 2019). Graphic © ESB Professional/Shutterstock.com.

Dr. Gevirtz explains HRV © Association for Applied Psychophysiology and Biofeedback. You can enlarge the video by clicking on the bracket icon at the bottom right of the screen. When finished, click on the ESC key.
A Healthy Heart Is Not a Metronome

When the time intervals between heartbeats significantly change across successive breathing cycles, this shows that the cardiovascular center can effectively modulate vagal tone.
Listen to a mini-lecture on Why Is Heart Rate Variability Important? © BioSource Software LLC.
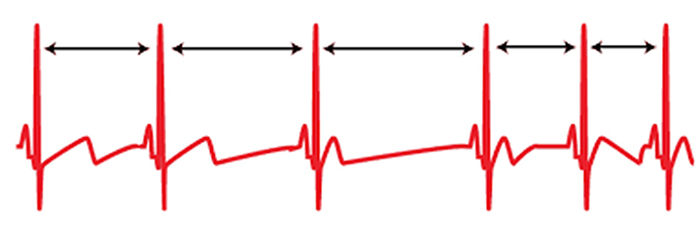
The record below shows healthy variability. The time intervals between successive heartbeats differ.
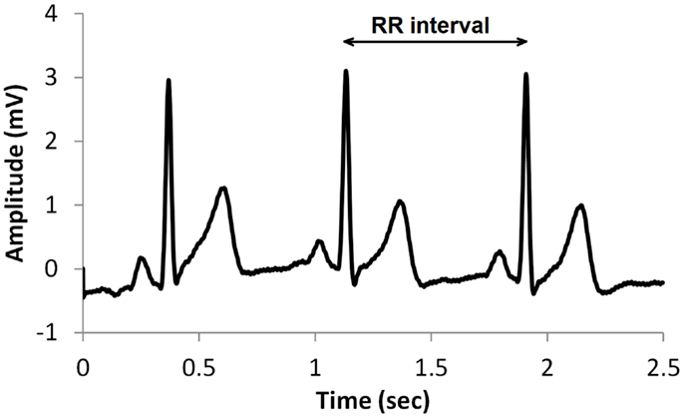
In contrast, this record shows no variability since the IBIs are identical. This display could represent a heart driven by a pacemaker or a heart that needs one.
"The complexity of a healthy heart rhythm is critical to the maintenance of homeostasis because it provides the flexibility to cope with an uncertain and changing environment...HRV metrics are important because they are associated with regulatory capacity, health, and performance and can predict morbidity and mortality" (Shaffer, Meehan, & Zerr, 2020).
Check out the YouTube video HRV Training and its Importance. Graphic © Axynia/Shutterstock.com."... HRV is associated with executive function, regulatory capacity, and health... Cardiac vagal control indexes how efficiently we mobilize and utilize limited self-regulatory resources during resting, reactivity, and recovery conditions" (Shaffer, Meehan, & Zerr, 2020).
Vagal tone modulation helps maintain the dynamic autonomic balance critical to cardiovascular health. Autonomic imbalance due to deficient vagal inhibition is implicated in increased morbidity and all-cause mortality (Thayer, Yamamoto, & Brosschot, 2010).HRV appears to index autonomic functioning, BP, neurocardiac functioning, digestion, oxygen and carbon dioxide exchange, vascular tone (diameter of resistance vessels), and possibly facial muscle regulation (Gevirtz et al., 2016). HRV reflects the vagal contribution to executive functions, affective control, and social self-regulation (Byrd et al., 2015; Laborde et al., 2017; Mather & Thayer, 2018).
Vagal tank theory (Laborde et al., 2018) argues that vagal traffic to the heart indicates how efficiently we mobilize and use scarce self-regulatory resources.
How HRV Biofeedback Can Support Neurofeedback
HRV biofeedback (HRVB) is the real-time display of HRV back to the individual. When HRVB training uses a paced-breathing protocol, clinicians assess and correct dysfunctional breathing behaviors that can interfere with HRVB and neurofeedback. Increased HRV is associated with improved executive function and may strengthen descending medial prefrontal cortex regulation of emotion (Mather & Thayer, 2018; McCraty & Shaffer, 2015), assisting NF. Reciprocal heart-brain interactions make these benefits possible.Dr. Gevirtz discusses Evgeny Vaschillo's findings regarding HRVB effects on the BOLD signal peak in multiple brain areas © Association for Applied Psychophysiology and Biofeedback. You can enlarge this video by clicking on the bracket icon at the bottom right of the screen. When finished, click on the ESC key.
Heart-Brain Interactions
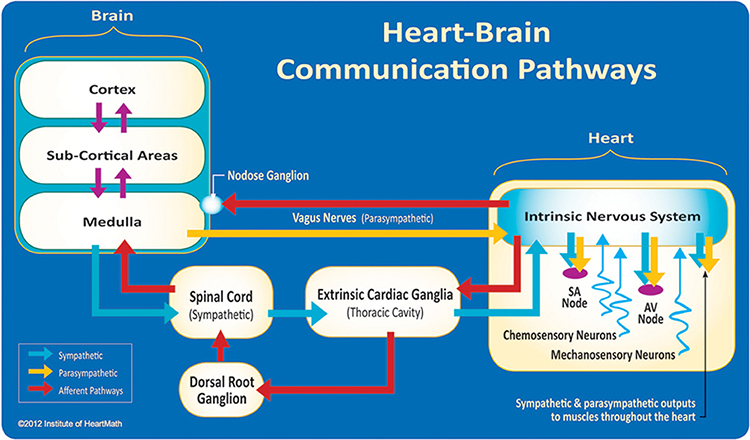
Thayer and Lane (2000) outline a neurovisceral integration model that describes how a central autonomic network (CAN) links the brainstem NST with forebrain structures (including the anterior cingulate, insula, ventromedial prefrontal cortex, and the amygdala and hypothalamus) through feedback and feed-forward loops. They speculate that a breakdown in negative feedback may produce the increased SNS arousal that characterizes anxiety disorders. Thayer et al. (2012, p. 754) contend that regions that include the amygdala and medial prefrontal cortex, which evaluate "threat and safety," help regulate HRV through their connections with the NST.Shaffer, McCraty, and Zerr (2014) propose that interconnected cardiac ganglia create an intrinsic nervous system within the heart that influences the S-A and A-V node pacemakers and forms reciprocal connections with the extrinsic cardiac ganglia found in the chest cavity and the medulla. The sensory, interconnecting, afferent, and motor neurons within the heart can function independently and constitute a "little brain" on the mammalian heart.
Listen to a mini-lecture on Heart-Brain Interactions © BioSource Software LLC. Graphic © Naeblys/ Shutterstock.com.
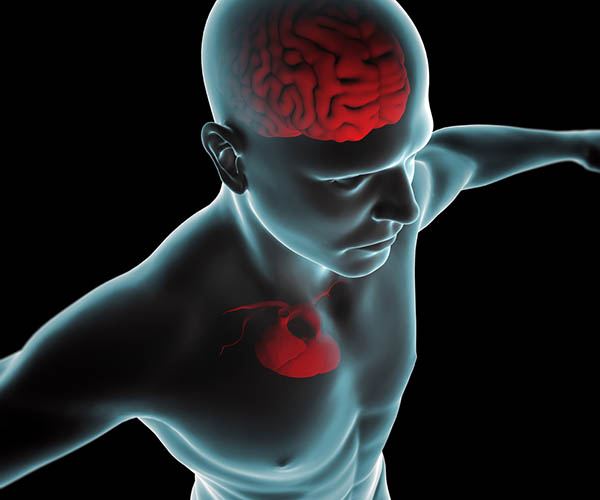
The ascending afferent nerves help to regulate the heart and its rhythms physiologically and influence efferent SNS and PNS activity. From 85-90% of vagus nerve fibers are afferents, and more afferents from the heart target the brain than any other major organ.
Afferent signals from the intrinsic cardiac nervous system appear to affect attention, motivation, perceptual sensitivity, and emotional processing (Shaffer, McCraty, & Zerr, 2014). Graphic © 2012 Institute of HeartMath.
MacKinnon, Gevirtz, McCraty, and Brown (2013) reported that HRV influences the amplitude of heartbeat event-related potentials (HERPs). The amplitude of these negative EEG potentials that appear about 200-300 ms after each R-spike indexes cardiac afferent communication with the brain. Both negative and positive emotion conditions reduced HRV and HERP amplitude. In contrast, RF breathing increased HRV above baseline and increased HERP amplitude.
The authors speculated that RF breathing reduces interference with vagal afferent signal transmission from the heart to the cerebral cortex.
The following intrinsic ganglia img © 2012 Dr. Andrew Armour and the Institute of HeartMath.
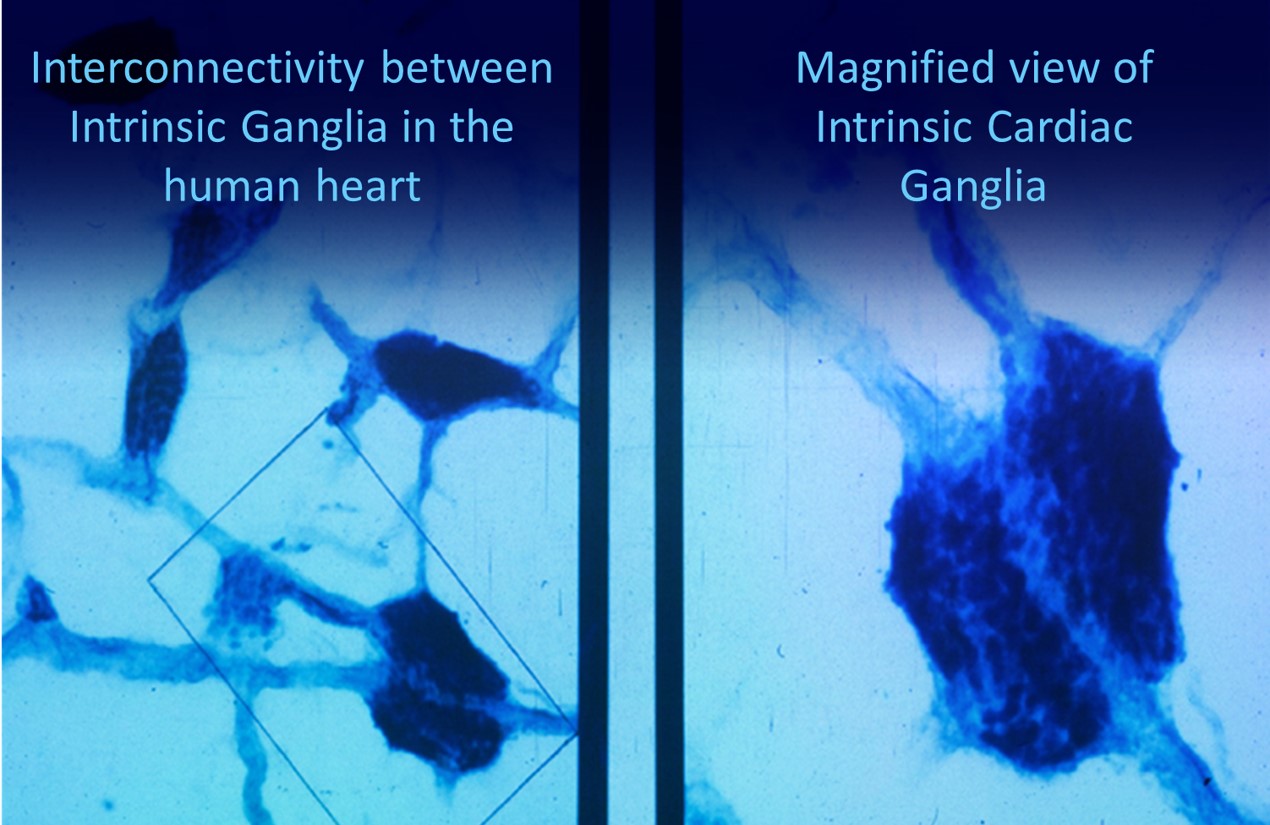
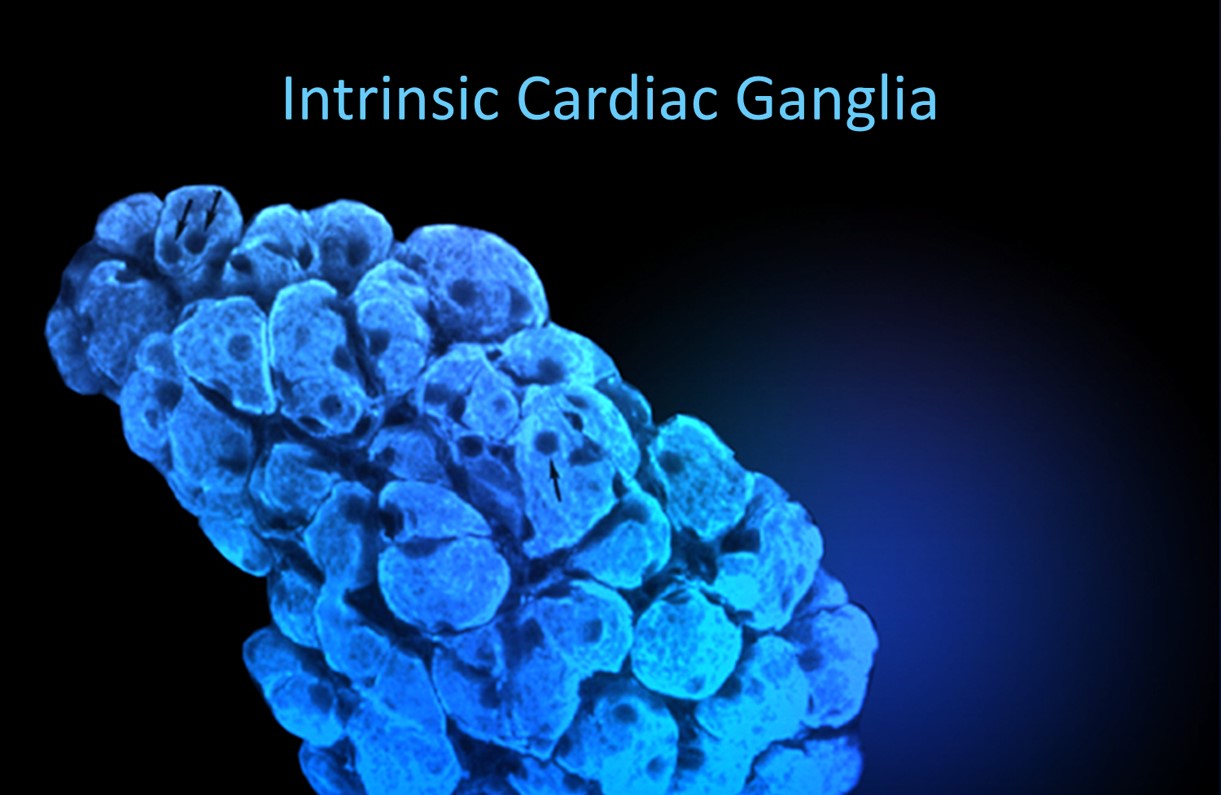
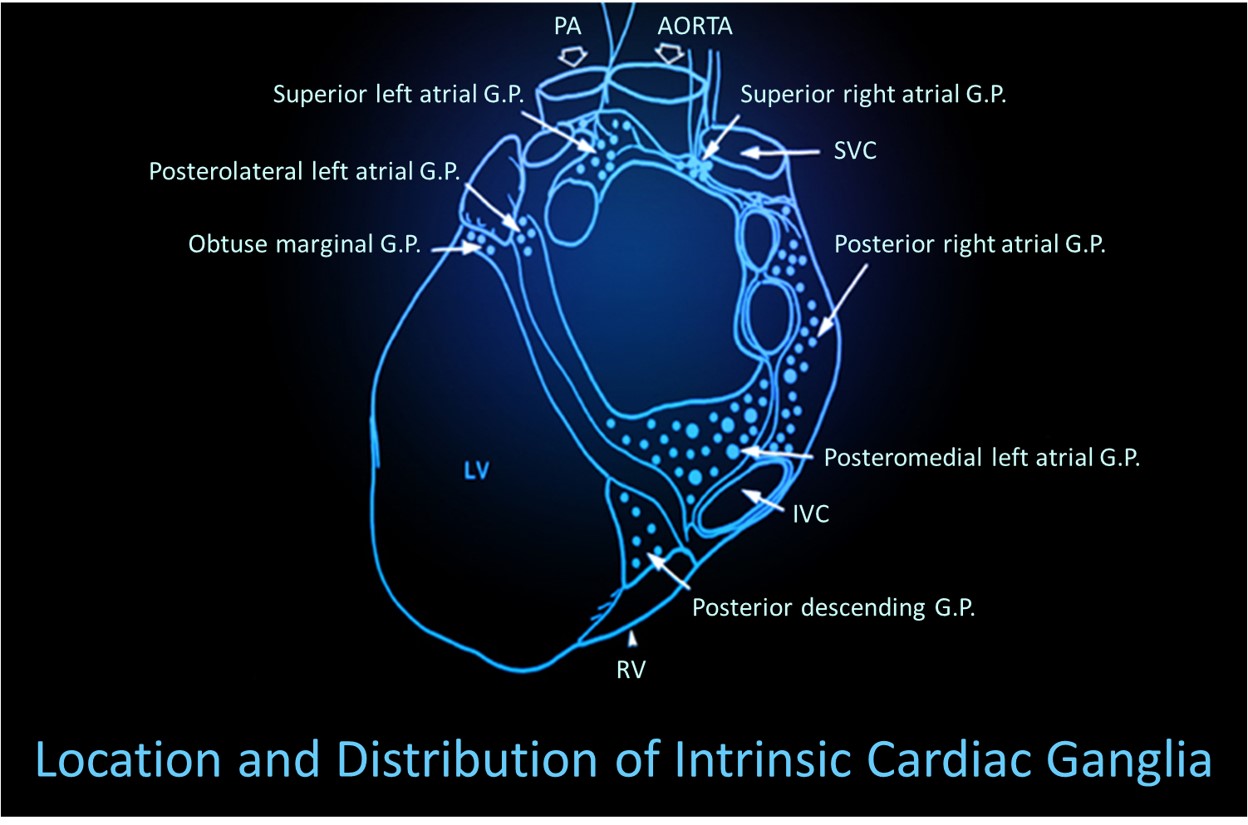
RESPIRATION
Healthy breathing is critical to HRV biofeedback and neurofeedback training success. These interventions may be less effective or fail altogether unless clinicians identify and correct dysfunctional breathing patterns like overbreathing. We have divided this section into Respiratory Anatomy and Physiology, Physiological Mechanisms That Regulate Blood Gasses, Disordered Breathing, Healthy Breathing, and The Effects of Overbreathing on the EEG.Respiratory Anatomy and Physiology
The respiratory cycle
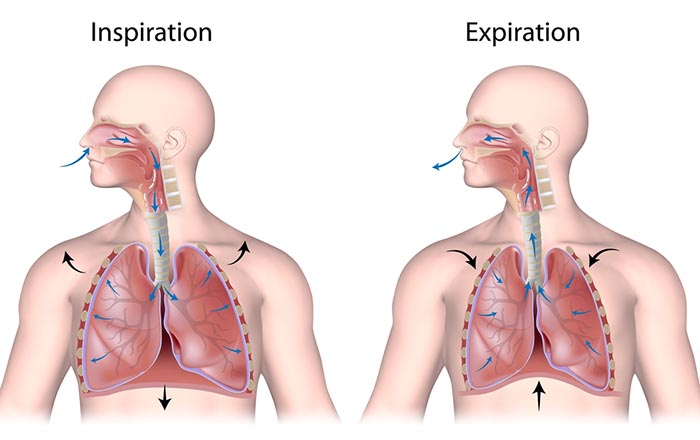
We breathe about 20,000 times a day. Typical adult resting breathing rates are 12-14 breaths per minute (bpm; Khazan, 2019a). Disorders that affect respiration may raise rates to 18-28 bpm (Fried, 1987; Fried & Grimaldi, 1993).The respiratory cycle consists of inhalation (breathing in) and exhalation (breathing out), controlled by separate mechanisms. Animation © weicheltfilm/iStockphoto.com.
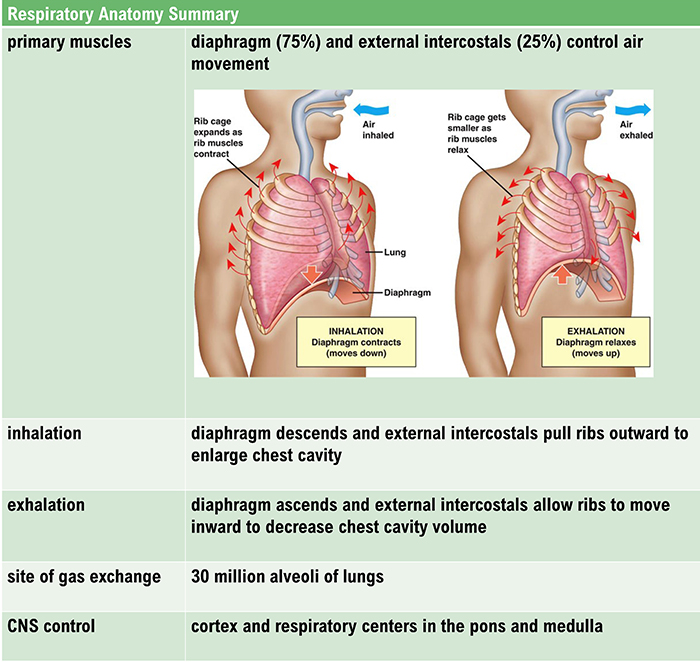
The lungs cannot inflate themselves since they lack skeletal muscles. Instead, they passively inflate by creating a partial vacuum by the diaphragm and external intercostal muscles (Gevirtz, Schwartz, & Lehrer, 2016).
During inhalation, contraction by the diaphragm and external intercostal muscles ventilate the lungs. Check out the Blausen Intercostals animation.
The dome-shaped diaphragm muscle plays the lead role during inhalation. The diaphragm comprises the floor of the thoracic cavity. When the diaphragm contracts, it flattens, and its dome drops, increasing the thoracic cavity volume. Contraction of the diaphragm pushes the rectus abdominis muscle of the stomach down and out. Check out the Blausen Diaphragm animation.
In the animation below, watch the lungs inflate as the diaphragm descends. Animation © look_around/iStockphoto.com.
In relaxed breathing, a 1-cm descent creates a 1-3 mmHg pressure difference and moves 500 milliliters of air. In labored breathing, a 10-cm descent produces a 100-mmHg pressure difference and transports 2-3 liters of air. The diaphragm accounts for about 75% of air movement into the lungs during relaxed breathing.
The external intercostals play a supporting role during inhalation. External intercostal muscle contraction pulls the ribs upward and enlarges the thoracic cavity. The external intercostals account for about 25% of air movement into the lungs during relaxed breathing.
The contraction of the diaphragm and the external intercostals expands the thoracic cavity, increases lung volume, and decreases the pressure within the lungs below atmospheric pressure. This pressure difference causes air to inflate the lungs until the alveolar pressure returns to atmospheric pressure.
During forceful inhalation, accessory muscles of inhalation (sternocleidomastoid, scalene, pectoralis major and minor, serratus anterior, and latissimus dorsi) also contract (Khazan, 2021). Graphic © Designua/Shutterstock.com.
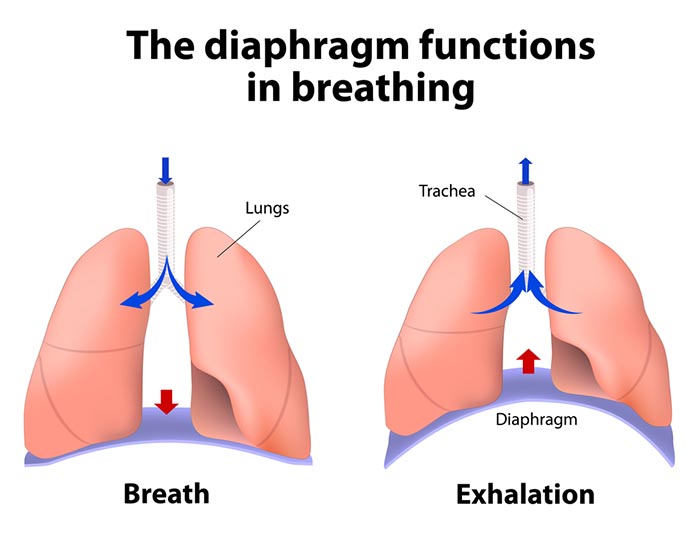
The dome-shaped diaphragm muscle ascends during normal expiration.
Exhalation during relaxed breathing is produced by the relaxation of the diaphragm and external intercostal muscles, contraction of the internal intercostals, the elastic recoil of the chest wall and lungs, and surface tension. When the diaphragm relaxes, its dome moves upward. When the external intercostals relax, the ribs move downward. These changes reduce the volume of the thoracic cavity and the lungs and increase the pressure within the lungs above atmospheric pressure. This pressure difference causes air to deflate the lungs until the alveolar pressure returns to atmospheric pressure.Forceful exhalation during exercise recruits the rectus abdominis, external and internal obliques, and transversus abdominis abdominal muscles (Khazan, 2021; Lorig, 2007; Tortora & Derrickson, 2021). Graphic © Alila Medical Media/ Shutterstock.com.
The BioGraph ® Infiniti display below shows healthy inhalation and exhalation in which the abdomen gradually expands and then contracts.
Types of respiration
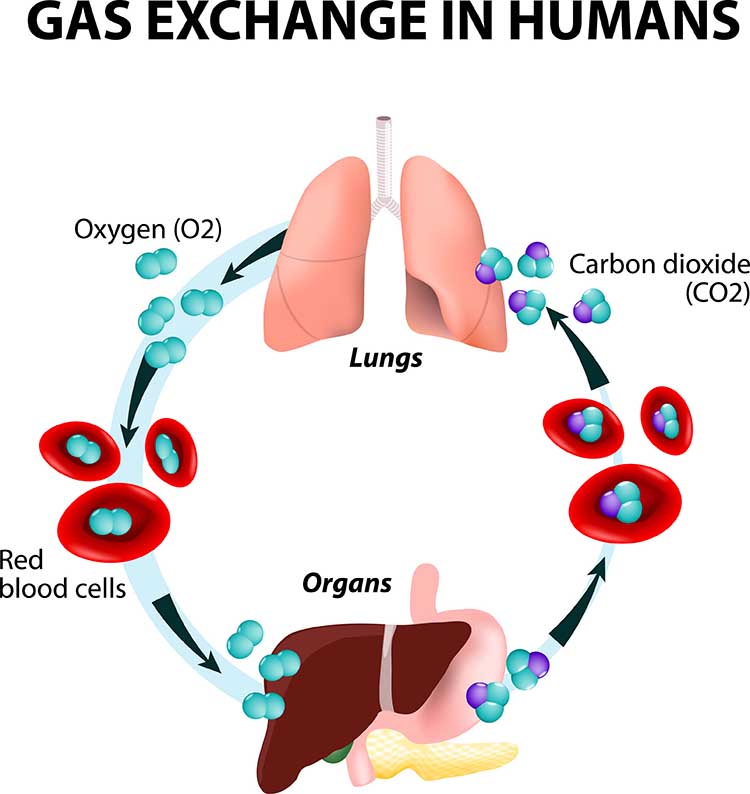
The term respiration refers to external, internal, and cellular processes. External respiration transports gases in and out of our lungs. Internal respiration transports oxygen from the air we inhale, delivers it to our cells, and returns metabolic CO2 to the lungs for 12-15% to be exhaled and 85-88% retained to regulate pH (Khazan, 2021).Respiratory gases are exchanged (between the lungs and blood) across the respiratory membrane, comprised of the alveolar and capillary walls. Check out the Blausen Overview of the Respiratory Tract animation. Graphic © Designua/Shutterstock.com.
The lungs contain an estimated 30 million alveoli (air sacs) that create an incredible 753 ft2 surface for gas exchange (Tortora & Derrickson, 2021). Check out the Blausen Bronchi animation. Graphic © Designua/Shutterstock.com.
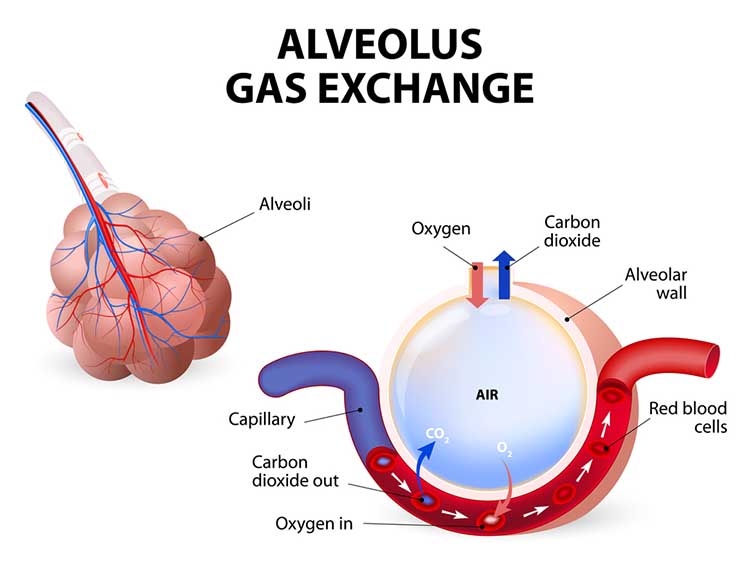
The alveoli collapse like “wet balloons” during normal breathing. Since deflated alveoli cannot absorb normal oxygen levels, the brain triggers sighs to reopen these air sacs. Humans initiate sighs every 5 minutes to increase oxygen delivery and activate the brain through double inhalation (Long, 2016).
The respiratory cycle consists of an inspiratory phase, inspiratory pause, expiratory phase, and expiratory pause. Abdominal respirometer excursion, which indexes respiratory amplitude (the peak-to-trough difference), is often greatest during the inspiratory pause. The diagram below was adapted from Stern, Ray, and Quigley (1991).
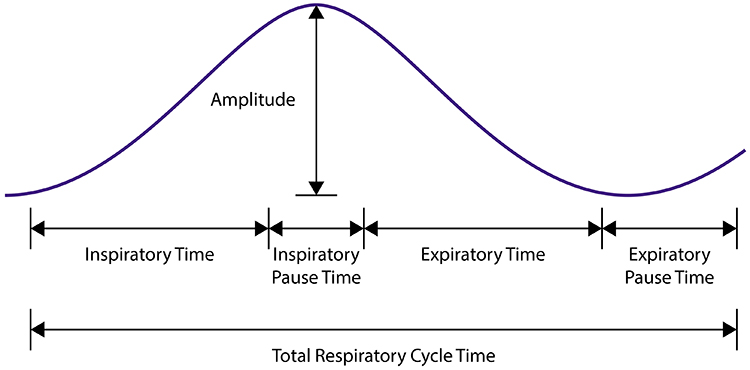
Clinicians should examine all components of the respiratory cycle—not just respiration rate—to understand their clients' respiratory mechanics. Everyday activities like speaking and writing checks may affect individual components differently. Apnea, breath suspension, lowers respiration rate. Clinicians teaching effortless breathing training may instruct their clients to lengthen the expiratory pause with respect to the inspiratory pause. Simple inspection of their respiration rates will not show whether they have successfully changed the relative durations of these two pauses. Finally, in heart rate variability (HRV) biofeedback, clinicians encourage slow (5-7 bpm) and rhythmic breathing.
Neural control of respiration
Respiration is controlled by a respiratory center in the medulla and pontine respiratory group. The dorsal respiratory group (DRG) and ventral respiratory group (VRG) are neuron clusters in two medulla regions. Pacemaker cells located in the VRG (analogous to the heart's sinoatrial node) organize the basic breathing rhythm. Check out the Khan Academy YouTube video The Respiratory Center.Medulla: DRG and VRG
The DRG's Role in Breathing
The DRG collects information from peripheral stretch and chemoreceptors and distributes it to the VRG to modify its breathing rhythms. The DRG is responsible for normal quiet breathing. The majority of VRG neurons are inactive at this time. During forceful breathing, DRG neurons activate the VRG, which stimulates the diaphragm, sternocleidomastoid, pectoralis minor, scalene, and trapezius muscles to contract (Tortora & Derrickson, 2021).The VRG's Role in Breathing
The phrenic and intercostal nerves transmit VRG inspiratory neuron action potentials to the diaphragm and external intercostal muscles. The contraction of these muscles expands the thoracic cavity and inflates the lungs.VRG pacemaker cells influence the rate of DRG action potentials. VRG expiratory neurons inhibit DRG inspiratory neuron firing. Exhalation passively results from diaphragm and external intercostal muscle relaxation and recoil by the chest wall and lungs. The DRG and VRG neurons' continuous reciprocal activity results in a 12-15 bpm respiratory rate, with 2-second inspiratory and 3-second expiratory phases.
Why Drug Overdoses Can be Lethal
An overdose of a CNS depressant like alcohol or morphine can completely inhibit VRG neurons in the medulla and stop breathing. The graphic below depicting the medulla is courtesy of Wikimedia Commons.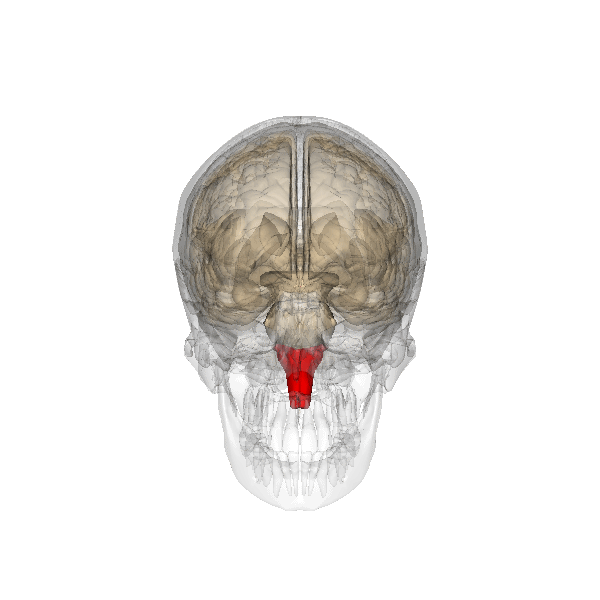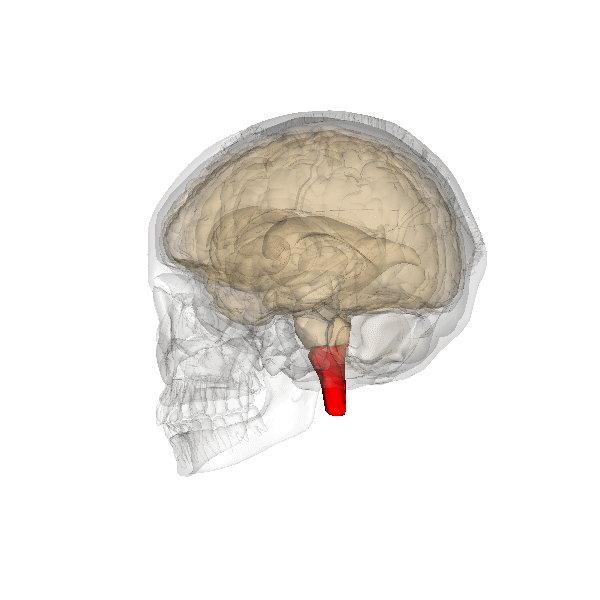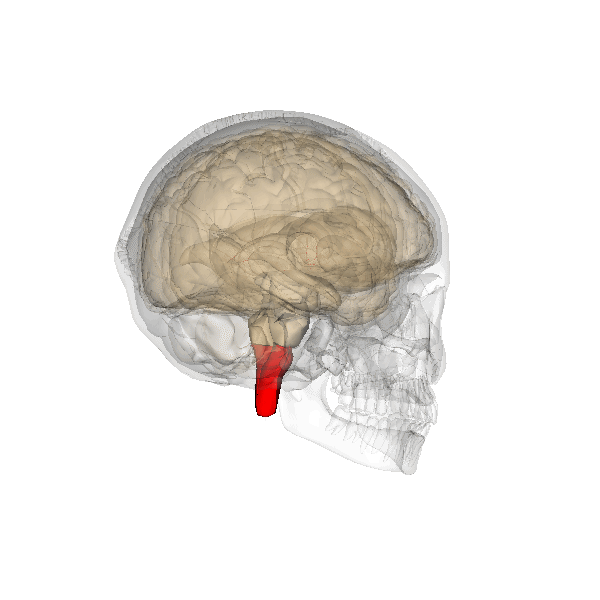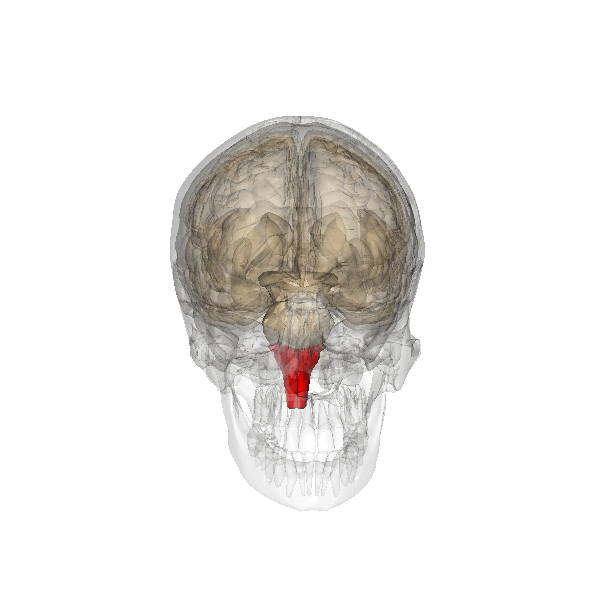
The pons: The respiratory group's role
The pontine respiratory group adjusts VRG breathing rhythms based on descending input from brain structures and peripheral sensory input. The graphic below depicting the pons is courtesy of Wikimedia Commons.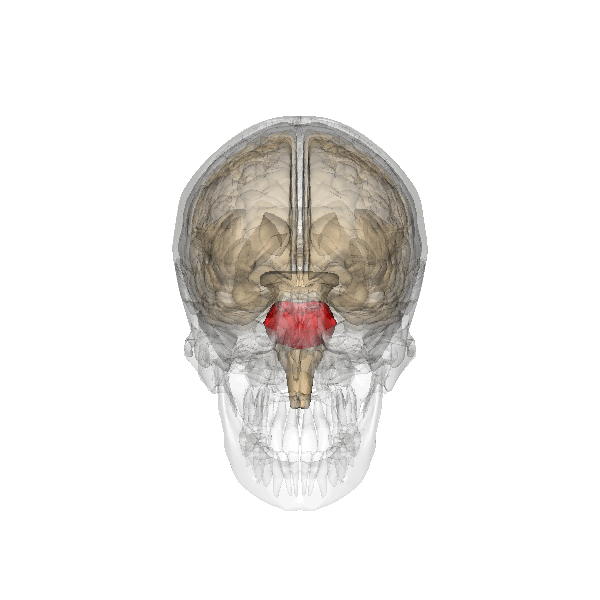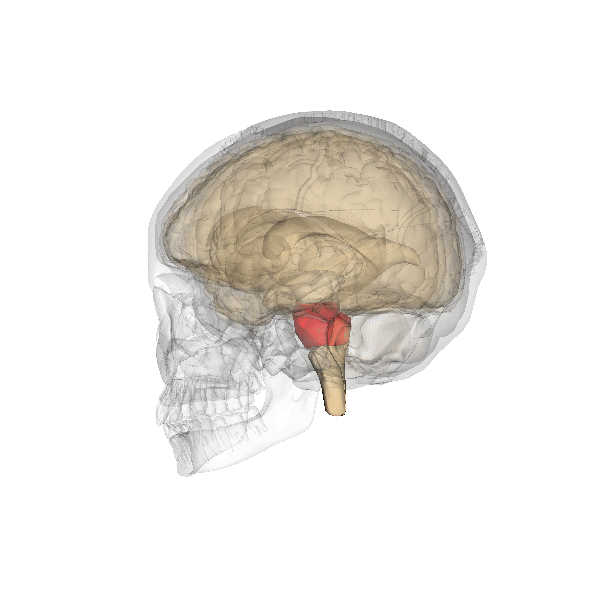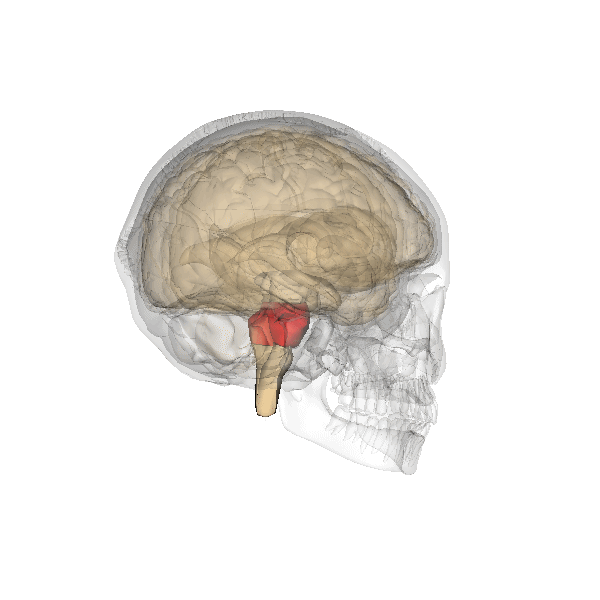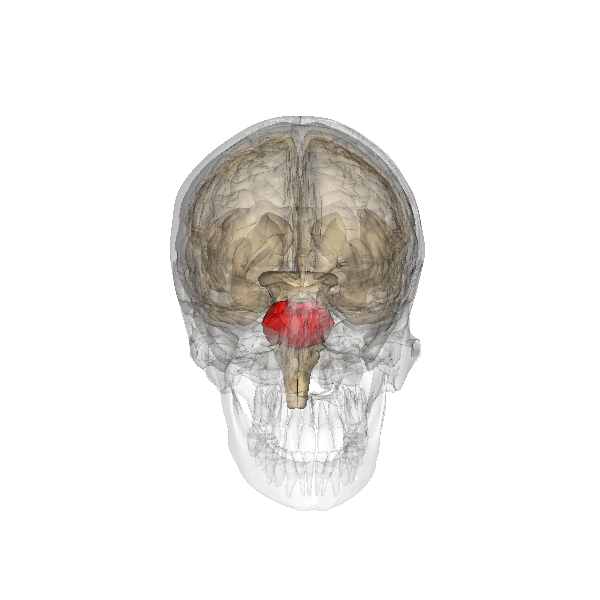
The pontine respiratory group modifies breathing during varied activities like exercise, sleep, and speech (Marieb & Hoehn, 2019). The body's cells require about 200 ml of oxygen at rest. This demand increases 15-20 times during strenuous exercise; 30 times for elite athletes. Brainstem diagram below © 2003 Josephine Wilson.
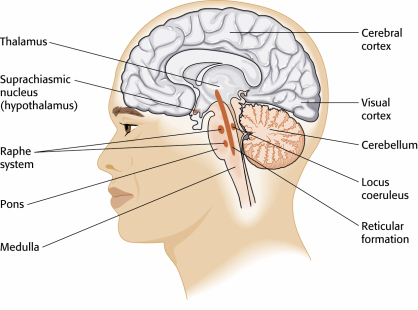
The cerebral cortex's role in breathing
Cortical control of respiratory centers in the medulla and pons allows us to stop or change our breathing patterns voluntarily. This voluntary control protects against lung damage from water or toxic gases.Why You Can't Hold Your Breath Indefinitely
The rise of CO2 and H+ in the blood limits our ability to suspend breathing by stimulating the inspiratory area when a critical level is reached. When chemoreceptors monitoring cerebrospinal fluid pH detect decreased pH (greater acidity), the DRG region of the medulla initiates the next breath. This homeostatic mechanism prevents us from harming ourselves by holding our breath (Tortora & Derrickson, 2021). Graphic © 2003 by Josephine F. Wilson.
Nasal breathing effects on the limbic system
Inhalation through the nose, but not the mouth, activates the amygdala, hippocampus, and olfactory cortex neurons. Graphic © joshya/shutterstock.com.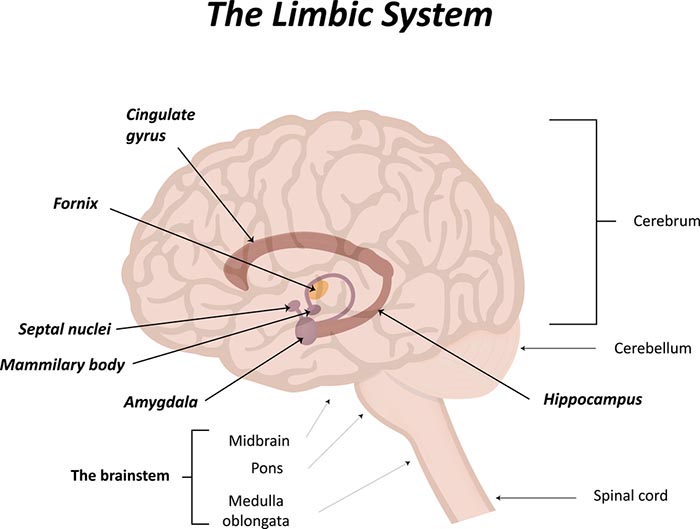
Nasal inhalation may accelerate our response to physical threats and impact fear and memory. During panic, breathing is faster, and we spend more time inhaling (Zelano et al., 2016). Check out the YouTube video How you breathe affects memory and fear.
Startled zebra graphic © WOLF AVNI/Shutterstock.com.

PHYSIOLOGICAL MECHANISMS THAT REGULATE BLOOD GASSES
Breathing ensures healthy CO2 levels
The main functions of breathing are gas exchange and acid-base (pH) regulation. The respiratory system exchanges oxygen for carbon dioxide (CO2) released by cells during metabolism. CO2 regulates our physiology by increasing oxygen delivery when tissues are more active. Our body uses 85-88% of CO2 to ensure a healthy acid-base balance, making gas exchange possible through the Bohr effect (Khazan, 2021).Dr. Khazan explains internal respiration © Association for Applied Psychophysiology and Biofeedback. You can enlarge the video by clicking on the bracket icon at the bottom right of the screen. When finished, click on the ESC key.
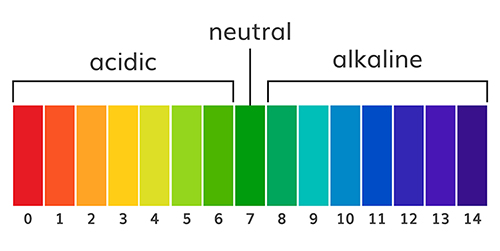
The abbreviation pH refers to the power of hydrogen, which is the concentration of hydrogen ions. Acidic solutions have a low pH (< 7) due to a high concentration of hydrogen ions. A neutral solution of distilled water has a pH of 7. Alkaline or basic solutions have a high pH (>7) due to a low concentration of hydrogen ions. The pH level regulates oxygen and nitric oxide release. Graphic © AlexVector/ Shutterstock.com.
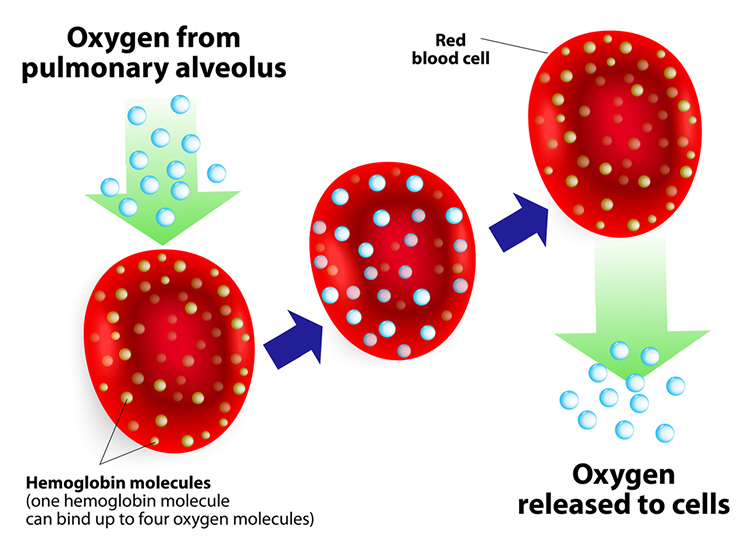
Hemoglobin molecules on red blood cells transport oxygen and nitric oxide through the bloodstream. One hemoglobin molecule can carry four oxygen molecules. Red blood cell graphic © royaltystockphoto.com/ Shutterstock.com.
.jpg)
Relaxed breathing increases the carbon dioxide concentration of arterial blood compared to thoracic breathing. At rest, we only excrete 12-15% of blood CO2. Conserving CO2 lowers blood pH, weakens the bond between hemoglobin and oxygen, and increases oxygen delivery to body tissues. This phenomenon is called the Bohr effect. Check out MEDCRAMvideos YouTube lecture Oxygen Hemoglobin Dissociation Curve Explained Clearly!
Conversely, low CO2 levels due to overbreathing or hyperventilation raise blood pH and reduce oxygen delivery to body tissues since oxygen remains tightly bound to the hemoglobin molecules (Fox & Rompolski, 2022). Graphic © Designua/Shutterstock.com.
Conserve CO2
We do not need more oxygen! (Khazan, 2021). Near sea level, the air healthy clients inhale contains 21% oxygen, while the air they exhale has 15%. We only use ¼ of inhaled oxygen and don’t need more. We need to conserve CO2 by retaining 85-88% of it.Breathing serves more than gas exchange
The respiratory system also delivers odorants to the olfactory epithelium, produces the airway pressure required for speech, anticipates cognitive and skeletal muscle metabolic demands, and helps to modulate systems regulated by the autonomic nervous system (ANS), especially the cardiovascular system. Respiration is an important regulator of heart rate variability, consisting of beat-to-beat changes in the heart rhythm (Lorig, 2007). Check out the YouTube video The Respiratory System.Disordered Breathing
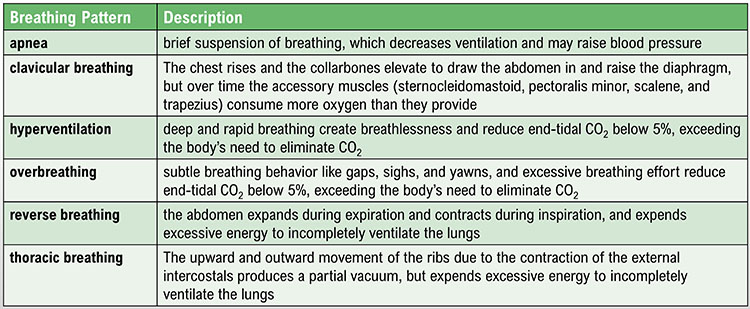
Clinicians encounter six abnormal breathing patterns which reduce oxygen delivery to the lungs: thoracic breathing, clavicular breathing, reverse breathing, overbreathing, hyperventilation, and apnea.Thoracic breathing
In thoracic breathing, the chest muscles are mainly responsible for breathing. The external intercostals lift the rib cage up and out. The diaphragm is pushed upward as the abdomen is drawn in. Upward and outward movement of the ribs enlarges the thoracic cavity producing a partial vacuum. Negative pressure expands the lungs but is too weak to ventilate their lower lobes. Thoracic breathing reduces ventilation since the lower lobes receive a disproportionate share of the blood supply due to gravity.Thoracic breathing expends excessive energy, incompletely ventilates the lungs, and strains our accessory muscles.
In the BioGraph ® Infiniti screen below, the abdominal (blue trace) strain gauge exhibits minimal excursion, and the respiration rate exceeds the desired 5-7 breaths-per-minute range.Are you a thoracic breather? Place your left hand on your chest and your right hand on your navel. If both hands shallowly rise and fall at about the same time, you are breathing thoracically.
Clavicular breathing
In clavicular breathing, the chest rises, and the collarbones are elevated to draw the abdomen in and raise the diaphragm (Khazan, 2021). Clavicular breathing may accompany thoracic breathing. Patients may breathe through their mouths to increase air intake. This pattern provides minimal pulmonary ventilation. Over time, the accessory muscles (sternocleidomastoid, pectoralis minor, scalene, and trapezius) use more oxygen than clavicular breathing provides.Clavicular breathing may be accompanied by thoracic and mouth breathing, produce an oxygen deficit, reduce CO2, and cause overbreathing.
In the BioGraph ® Infiniti screen below, the purple trace represents the chest strain gauge, and the red trace represents accessory SEMG activity. Note the rapid shallow chest movement and fluctuating accessory SEMG values that increase with the shoulder elevation that accompanies each inhalation.Are you a clavicular breather? Have an observer lightly place one hand on your shoulder (the observer's shoulder must be relaxed). If this hand rises as you inhale, you are showing clavicular breathing.
Reverse breathing
Reverse breathing, where the abdomen expands during exhalation and contracts during inhalation, often accompanies thoracic breathing and results in incomplete ventilation of the lungs.In the BioGraph ® Infiniti screen below, the client starts at the left with inhalation, followed by exhalation. Note how the stomach contracts during inhalation (falling blue trace) and expands during exhalation (rising blue trace). This pattern is the opposite of healthy breathing.
Are you a reverse breather? If the hand on your stomach falls and the hand on your chest rises when you inhale, you are reverse breathing. Reverse breathing expends excessive energy and incompletely ventilates the lungs.
Overbreathing
Overbreathing is a mismatch between breathing rate and depth (Khazan, 2021). This disparity may involve rapid breathing and or increased tidal volume (the amount of air exhaled during a breath) as well as more subtle behaviors like gasps and sighs.
Gasps and sighs involve the quick intake of a large air volume, accompanied by breath-holding. They may comprise part of a defensive reaction. When clients expel excessive CO2, this lowers end-tidal CO2 (the percentage of CO2 at the end of exhalation) and causes hypocapnia, which is deficient CO2. Acute overbreathing produces various symptoms.
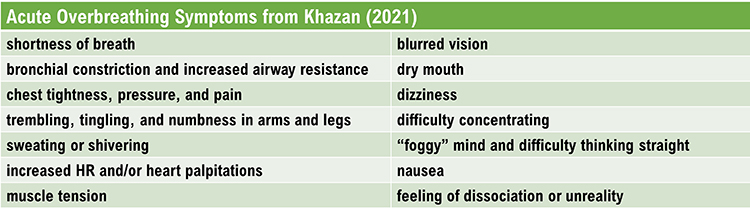
Hypocapnia disrupts homeostasis
Hypocapnia disrupts homeostasis by disturbing the body's acid-base (pH) and electrolyte balance, blood flow, and oxygen delivery. Hypocapnia may force the kidneys to expel bicarbonates to restore pH balance (Khazan, 2021).Electrolytes are substances like acids or salts that can dissociate into free ions when dissolved (e.g., NaCl → Na+ + Cl-). Hypocapnia deprives cells (e.g., neurons, cardiac muscle, and skeletal muscle) of the ions (Ca+2 and Na+) required for typical membrane potentials and communication with other cells.
The Effects of Ca+2 Movement
Hypocapnia can move Ca+2 from the interstitial fluid into muscle cells with disastrous results. In skeletal muscle, calcium entry can cause spasms, fatigue, and weakness. In blood vessel smooth muscle, it can produce vasoconstriction. In the bronchioles of the lungs, it can trigger bronchonstriction. Finally, in GI tract smooth muscle, it can result in nausea and change motility.The Effects of Na+ Movement
Na+ ion movement into neurons from extracellular fluid increases excitability, metabolism, and demand for oxygen while reducing oxygen availability for other organs. The brain can experience ischemia and excitotoxicity. Vasoconstriction due to Na+ ion entry and less nitric oxide release greatly diminishes glucose delivery to the tissues, especially to the outermost layers of the cortex (Gevirtz, Schwartz, & Lehrer, 2016). Graphic © Magic mine/Shutterstock.com.
Healthy end-tidal CO2 values range from 35-45 mmHg. Moderate overbreathing can reduce oxygen delivery to the brain by 30%-40%, and severe overbreathing can reduce it by 60%.
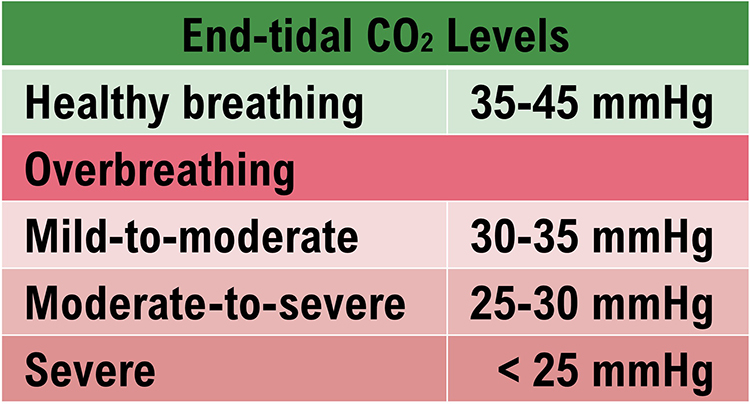
Conversely, low CO2 levels due to overbreathing or hyperventilation raise blood pH and reduce oxygen delivery to body tissues since oxygen remains tightly bound to the hemoglobin molecules (Fox & Rompolski, 2022). Overbreathing can produce acute and chronic vasoconstriction effects and reduced delivery of oxygen and glucose to body tissues, especially the brain (Khazan, 2013). Graphic adapted from Inna Khazan.
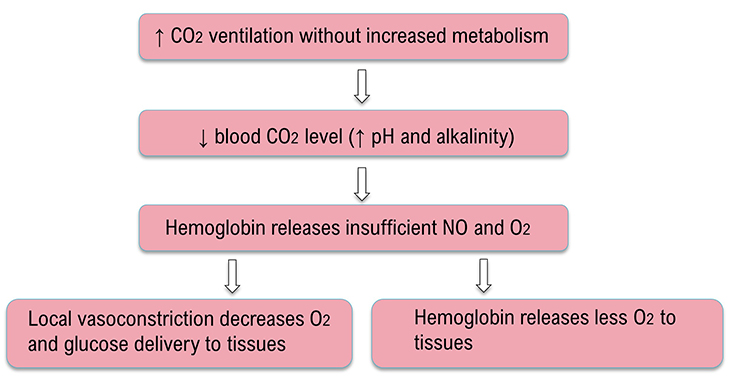
The Effects of Overbreathing on the EEG
Overbreathing causes cerebral vasoconstriction and reduced glucose, nitric oxide, and oxygen delivery to the nervous system. Hyperventilation can increase frontal delta and theta (Thompson & Thompson, 2015). Hyperventilation graphic © EEGpedia.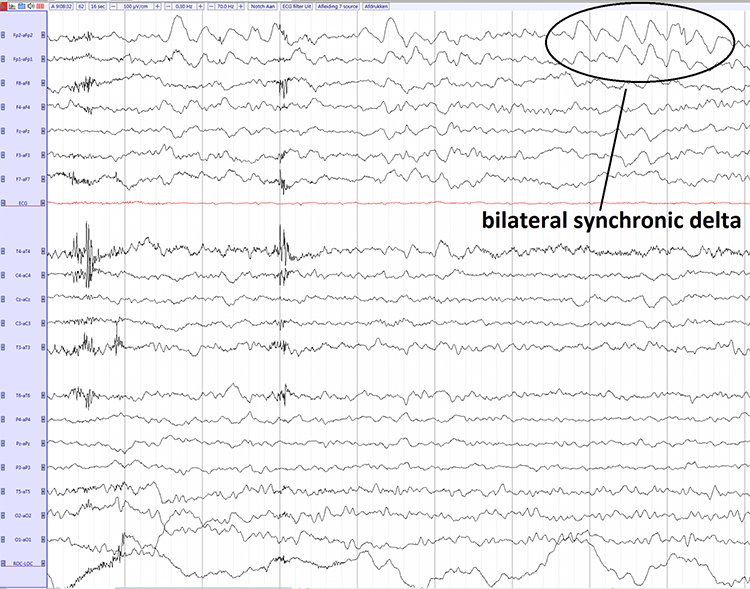
The effects of chronic overbreathing
Clients who overbreathe may experience chronic hypocapnia. Since the body cannot function with sustained high pH, the kidneys excrete bicarbonates to return pH to near-normal levels. Bicarbonates are salts of carbonic acid that contain HC03. Acid buffering can only restore homeostasis in the short run because increased metabolism raises acidity until needed bicarbonates are depleted. Clients experience fatigue, muscle pain, reduced physical endurance, and a sodium deficit when this happens. Acidosis may increase overbreathing in a failed attempt to reduce acidity (Khazan, 2021).Why do clients overbreathe?
Clients overbreathe as part of the fight-or-flight response in response to stressors, when they experience difficult emotions, and when they suffer chronic pain. They can learn this dysfunctional breathing pattern through classical and operant conditioning and social learning.If clients practice overbreathing long enough, it can become a habit when this lowers the body's setpoint for CO2. Now, when breathing slows, the respiratory centers attempt to restore low CO2 levels by removing it through behaviors like breath-holding, sighing, and yawning (Gevirtz, Schwartz, & Lehrer, 2016). Reduced blood CO2 levels may contribute to asthma, panic, phobia, and pain disorders like chronic low back pain.
Overbreathing and hyperventilation have different clinical presentations
Whereas overbreathing and hyperventilation produce the same physiological changes, hyperventilation involves distinctive behaviors and subjective sensations. Graphic © Pixel-Shot/Shutterstock.com.
Hyperventilation syndrome (HVS) involves abnormal CO2 loss from the blood due to excessive breathing rate and depth. HV accounts for about 60% of major city ambulance calls due to frightening symptoms like chest pain, breathlessness, dizziness, and panic. Common panic symptoms © DruZhi Art/Shutterstock.com.
Clients who present with HVS breathe thoracically, deeply, and rapidly (over 20 bpm) using accessory muscles (the sternum moves forward and upward) and restricting diaphragm movement. Their rapid breathing can lower end-tidal CO2 from 5% to 2.5%, although many patients have normal values during attacks (Kern, 2021).
Like overbreathing, this pattern exceeds the body's need to eliminate CO2, reduces oxygen delivery to body tissues and NO release, and curtails their supply of glucose (Khazan, 2013). Check out the YouTube video Breathing Pattern Disorders Such as Hyperventilation.
The BioGraph ® Infiniti display below shows the shallow, rapid breathing that characterizes hyperventilation.
In contrast to HVS, overbreathing is usually so subtle that the patients are unaware that their sighs and yawns produce hypocapnia.

Apnea
Apnea involves the suspension of breathing. While commonly associated with sleep, breath-holding while awake may occur during stressful situations as part of a defensive response. A client may also hold breaths during ordinary activities like opening a jar, speaking, or writing a check. Episodes of apnea decrease ventilation and may increase blood pressure.Don't confuse apnea with post-expiratory pauses. Graphic © Khazan (2021).
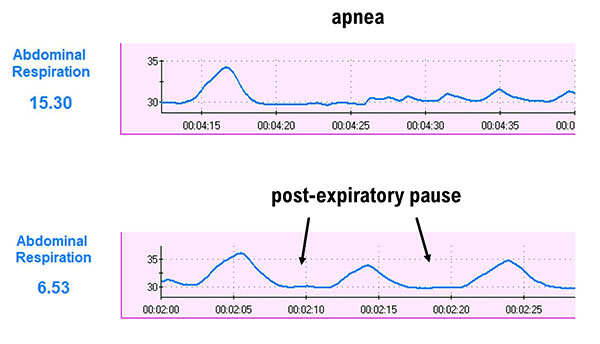
In the BioGraph ® Infiniti display below, the blue abdominal strain gauge trace briefly flattens when the patient suspends breathing after the second breath.
HEALTHY BREATHING
We teach clients to use sound breathing mechanics throughout the day. Slow-paced breathing is appropriate during practice but not during the increased workloads of aerobic exercise. Graphic © goodluz/Shutterstock.com.
Medical Cautions
Clients who overbreathe may experience chronic hypocapnia. Since the body cannot function with sustained high pH, the kidneys excrete bicarbonates to return pH to near-normal levels. Graphic © Alhovic/Shutterstock.com.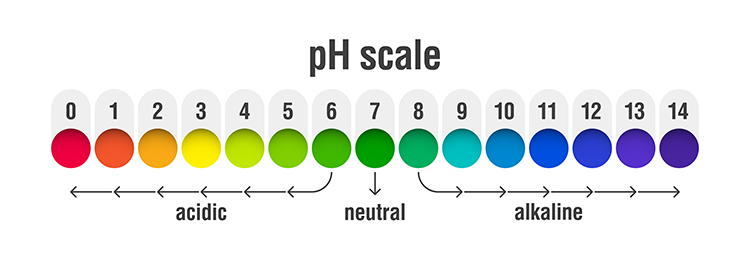
Bicarbonates are salts of carbonic acid that contain HC03. Acid buffering can only restore homeostasis in the short run because increased metabolism raises acidity until needed bicarbonates are depleted. Clients experience fatigue, muscle pain, reduced physical endurance, and a sodium deficit when this happens. Acidosis may increase overbreathing in a failed attempt to reduce acidity (Khazan, 2021).
Effortless breathing could be hazardous if your client suffers from diseases that produce metabolic acidoses like diabetes and kidney disease. In these cases, overbreathing is an attempt to compensate for abnormal acid-base balance, and slow-paced breathing could endanger health.
Clients diagnosed with low blood pressure should be careful since slow-paced breathing might further lower blood pressure.
Finally, slow-paced breathing might produce a functional overdose if your client takes anti-hypertensive medication, insulin, or a thyroid supplement. If medication adjustment appears necessary, your client should consult the supervising physician before reducing dosage (Fried & Grimaldi, 1993).

Breathing Basics
Healthy breathing achieves a match between metabolic needs, production of CO2, and breath depth and rate. We should maintain optimal breathing chemistry for each activity level and breathing rate. Although rapid breathing does not always signal overbreathing and slow breathing does not always indicate health, there are correlations (Khazan, 2021). Breathing should be mindful with focus on the abdomen, effortless, between 5-7 breaths per minute, supported by loose clothing, posture, and ergonomics that promote healthy breathing.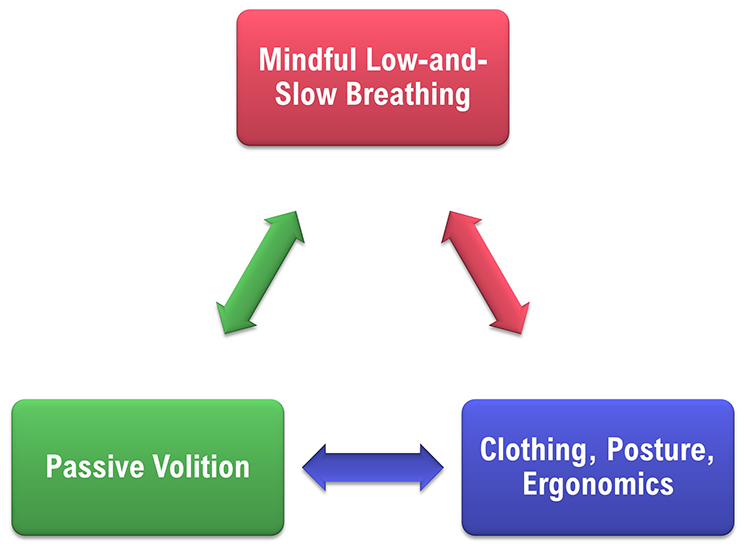
Breathe Effortlessly
Encourage your clients to breathe effortlessly (Peper & Tibbets, 1994). Your client should experience their body "breathing itself." Breathing is 5-7 bpm for adults. The pauses following expiration are longer than those following inspiration. Tidal volume is between 1,000 and 3,000 ml. Airflow is smooth and continuous. Their attention settles on the abdomen. The stomach expands, and the lower ribs and back widen during inhalation). Graphic © mimagephotography/Shutterstock.com.
Discourage Deep Breaths
They should breathe at a comfortable depth (like smelling a flower), exhaling longer than inhaling. Breathing will calm your client when its depth and rate satisfy their resting body’s metabolic needs (Khazan, 2021). Elena Sherengovskaya/Shutterstock.com.
Discourage typical deep breathing, where a client inhales a massive breath and inevitably exhales too quickly because this promotes overbreathing and expels too much CO2 (Khazan, 2021).

Breathing will calm your client when its depth and rate satisfy the resting body’s metabolic needs. Don’t encourage deeper or larger breaths. Graphic fizkes © Shutterstock.com.

Encourage Your Clients to Dress for Success
Caution clients to avoid the designer jean syndrome (Peper & Tibbets, 1994) in which tight clothing prevents abdominal expansion and promotes thoracic breathing. Graphic © kbrowne41/Shutterstock.com.
Inhale Through the Nostrils
Encourage your clients to inhale through the nostrils to filter, moisten, and warm the air. Depending on training goals and health, they should exhale through the mouth or nose. Graphic © Yuliya Evstratenko/ Shutterstock.com
Great Posture Promotes Healthy Breathing
“Make sure you choose a comfortable chair that allows you to sit with a straight back, the vertebrae of your spine stacked neatly on top of one another. Your feet should be flat on the floor, legs uncrossed, knees at a 90-degree angle” (Lagos, 2020, p. 58). Clients may also enjoy practicing in natural settings. Graphic © BalanceFormCreative/Shutterstock.com.
Enhance Your Clients' Respiratory Feedback
Teach your clients to exhale using pursed lips as if blowing out a candle (Khazan, 2021). Graphic © JPRFPhotos/Shutterstock.com.
Invite clients to breathe with a weight on the abdomen while lying on the floor with knees bent.

Alternatively, they can place their hands on their abdomens. Graphic stockfour/Shutterstock.com.

Physiological Effects of Healthy Breathing
Breathing at typical rates during physical activity increases the carbon dioxide concentration of arterial blood compared to thoracic breathing. At rest, we only excrete 12-15% of blood CO2. Conserving CO2 lowers blood pH, weakens the bond between hemoglobin and oxygen, and increases oxygen delivery to body tissues. This phenomenon is called the Bohr effect. Check out MEDCRAMvideos YouTube lecture Oxygen Hemoglobin Dissociation Curve Explained Clearly! The breathing chemistry graphics were adapted from Inna Khazan.
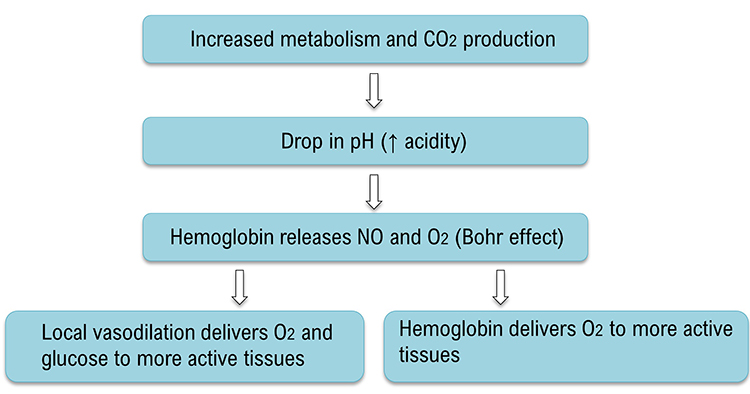
Slow-paced breathing in the resonance frequency (RF) range (e.g., 4.5 to 7.5 bpm for adults) conserves CO2, reduces pH, releases NO and O2, and better distributes glucose and oxygen to more active tissues.
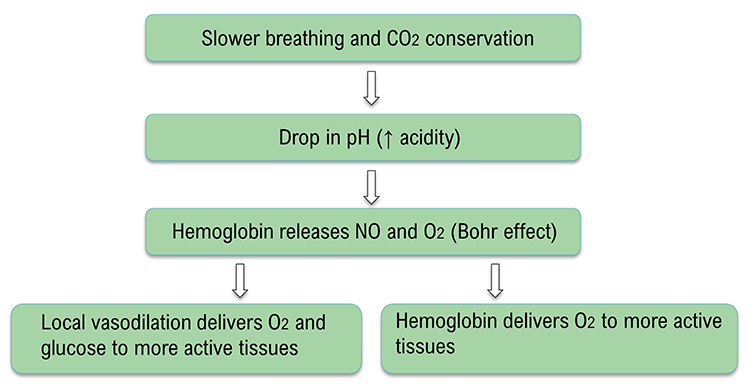
Healthy breathing can increase peripheral blood flow when the RR slows to the RF range, and end-tidal CO2 normalizes to 5% or 36 mmHg. Peripheral vasodilation can increase perfusion and delivery of oxygen and glucose to the brain, reduce peripheral resistance, and promote hand-warming. These changes are crucial for executive functioning and treating hypertension, vascular headache, and Raynaud's disease. Breathing in the RF range can slow HR and increase vagal tone and HRV.
Sinha et al. (2020) reported increased theta amplitude at F3, F4, P3, P4, O1, and O2 sites during deep breathing at 30 bpm for 3 minutes.
HEMOENCEPHALOGRAPHY
Hemoencephalography (HEG) utilizes passive infrared (pIR) and near-infrared (NIR) to estimate cerebral blood flow (CBF) and metabolism. HEG indirectly measures frontal lobe activity based on the mechanism of neurovascular coupling. A brief rise in neural activity is associated with a proportional CBF increase (Huneau et al., 2015). The large-scale CBF increase provides nutrients and removes wastes to support the operation of ion channels (Attwell & Laughlin, 2001) and metabolic regulation (Iadecola & Nedergaard, 2007) by neurons and associated astrocytes. The complex mechanism responsible for this functional hyperemia may involve several vasoactive substances.
HEG Instrumentation
pIR-HEG detects frontal lobe infrared light to estimate the heat generated by cortical activity and the metabolic activity that supports it. Greater frontal lobe activity is correlated with increased metabolism and cortical perfusion, resulting in more infrared radiation emitted by the skin surface. A Thought Technology Ltd. pIR-HEG sensor is pictured below.
In NIR-HEG, diodes emit alternating infrared light at 660 nm (red light) and 850 nm (infrared light) into the skull, and photodetectors measure the refracted light at these wavelengths. Although the skull largely absorbs red and infrared wavelengths, oxygenated blood reflects red light. As hemoglobin oxygen saturation increases to support rising metabolism, blood backscatters more red light. The ratio of oxygenated (HbO2) to deoxygenated hemoglobin (Hb) increases. Since the skull's absorption of infrared light does not notably change with perfusion, NIR-HEG uses the fraction of this wavelength reflected as a fairly steady comparative baseline (Toomim, 2000).
As cortical firing, metabolic demand, and blood oxygenation increase, the sensors detect more red than infrared light. The increased HEG ratio (refracted red vs. infrared light) is associated with the heightened frontal lobe blood oxygenation required by more rapid neuronal firing and supporting metabolic activity (Pecyna & Pokorski, 2013). Graphic © Biofeedback Institute of Los Angeles.

HEG Applications
John Demos (2019) trains forehead sites corresponding to the orbital gyrus, ventral lateral, and ventral medial prefrontal cortex. After removing oil from the skin with alcohol, the sensors can be quickly positioned at sites like Fpz by adjusting their position on an elastic band wrapped around the scalp. He recommends that clinicians treat aggression, autism, depression, inattention, and impulsivity with NIR-HEG.Michael and Lynda Thompson (2015) recommended combining pIR-HEG with neurofeedback and peripheral biofeedback modalities. In their ADD Centre experience, clients learn to increase forehead temperature fairly rapidly. This can teach them fundamental self-regulation skills like sustained attention, increase their perceived self-efficacy, and create positive expectancies as they progress to the more gradual learning process of neurofeedback. As with all adjunctive procedures, the Thompsons could not quantify the percentage of client improvement due to pIR-HEG training itself.
The Biofeedback Institute of Toronto screen displays the left and right average temperature (pIR in degrees) as a green waveform at the top © Association for Applied Psychophysiology and Biofeedback. The client's goal is to increase forehead temperature through a "calm, relaxed yet intense focus." (p. 612)
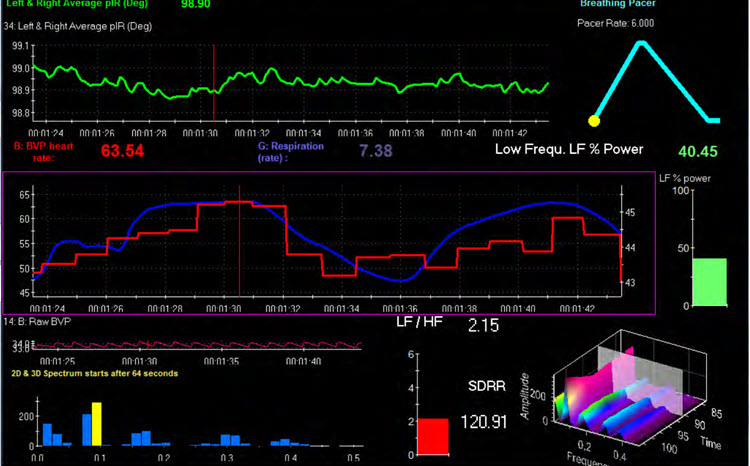
NEUROMODULATION
Neuromodulation, stimulating the nervous system to produce physiological change, is not neurofeedback because it acts on the nervous system instead of providing it with information about its performance. This section will briefly describe audio and visual entrainment, transcranial alternating current stimulation, electromagnetic stimulation, and near-infrared light stimulation.
Audio and Visual Entrainment
The delivery of visual and auditory stimuli pulsed at low frequencies (e.g., below 30 Hz) can lead to amplitude increases in corresponding EEG frequencies and promote decreased arousal at lower frequencies or increased arousal at higher frequencies (Collura & Siever, 2009; Siever, 2007). These changes in EEG activity are associated with changes in mood, cognition, and behavior and may be beneficial despite their usually short-term effects (Basu & Banerjee, 2020; Hanslmayret al., 2019). Subliminal auditory stimulation has also been used to “drive” EEG toward states that can be reinforced by simultaneous EEG neurofeedback (Swingle, 2015). The MindAlive Inc. David Delight is shown below.
Transcranial Alternating Current Stimulation
Transcranial alternating current stimulation (tACS) uses an alternating electrical current to entrain brain oscillations (Tavakoli & Yun, 2017). It is beginning to be used for various psychiatric conditions such as ADHD, depression, OCD, schizophrenia, and dementia (Elyamany et al., 2020). Graphic © The Scientist Magazine.
Electromagnetic Stimulation
Cranial microcurrent electrical stimulation of the brain has been used to treat anxiety, PTSD, depression, insomnia, and pain. Low-intensity current (50 μA to 4 mA) is typically applied to the earlobes. Although shortcomings in the designs of research investigations are documented (Brunye et al., 2021), a consistent body of evidence suggests safe and beneficial effects for many clients (Shekelle et al., 2018).Repetitive transcranial electromagnetic stimulation (rTMS) is used clinically to treat depression, anxiety, autism spectrum disorder, and substance abuse (Marques et al., 2019). Using electromagnetic coils held above the scalp, it delivers an electromagnetic stimulus to the brain to either stimulate or inhibit local brain tissue depending on the rTMS frequency. In transcranial direct current stimulation (tDCS), a low-intensity direct current is applied to the scalp via saline-saturated electrodes, for example, to treat depression (Jog et al., 2019).
Pulsed electromagnetic field therapy (PEMF therapy) uses lower power than rTMS to deliver stimulation by electromagnetic coils often arrayed in a helmet, though also delivered through a mat for some conditions. Clinicians use PEMF to treat depression and pain (Larsen et al., 2020; Martiny & Lunde, 2010).
Near-Infrared Light Stimulation
Photobiomodulation (PBM) uses near-infrared light emitted from lasers or light-emitting diodes in the range of 600-1100 nm either transcranially or intranasally to increase mitochondrial activity in the brain. It also affects EEG activity (Jahanet al., 2019), stimulates blood flow and oxygenation, reduces inflammation, promotes antioxidative activity and neurogenesis, and promotes the healing of injured tissue (Cardoso et al., 2021; Hamblin, 2017). Some findings show that PBM activates intrinsic brain networks. Among normal subjects, these effects translate into a better mood and cognitive function and improved executive function and sleep among those who have sustained a traumatic brain injury. In addition to brain injury and stroke, benefits from PBM have been seen for neurodegenerative conditions (e.g., Alzheimer’s Disease) and psychiatric disorders (e.g., depression, anxiety, PTSD) (Berman & Nichols, 2019; Guitierrez-Menendez et al., 2020; Hamblin, 2016, 2017).
Glossary
accessory muscles: the sternocleidomastoid, pectoralis minor, scalene, and trapezius muscles, which are used during forceful breathing, as well as during clavicular and thoracic breathing.
apnea: a dysfunctional respiratory pattern in which an individual suspends breathing.
audio and visual entrainment (AVE): the delivery of visual and auditory stimuli pulsed at low frequencies (e.g., below 30 Hz) to increase the amplitude of corresponding EEG frequencies.
bicarbonates: salts of carbonic acid that contain HCO3.
Bohr effect: the influence of carbon dioxide on hemoglobin release of nitric oxide and oxygen.
cerebral cortex: the 2-4 millimeter-thick outer layers that cover the cerebral hemispheres that contain circuitry essential to complex brain functions like cognition and consciousness.
clavicular breathing: a dysfunctional breathing pattern that primarily relies on the external intercostals and the accessory muscles to inflate the lungs, resulting in a more rapid respiration rate, excessive energy consumption, and incomplete ventilation of the lungs.
diaphragm: a dome-shaped muscle whose contraction enlarges the vertical diameter of the chest cavity and accounts for about 75% of air movement into the lungs during relaxed breathing.
dorsal respiratory group (DRG): neuron clusters in the medulla of the brainstem that collect information from peripheral stretch and chemoreceptors and distribute this information to the VRG to modify its breathing rhythms.
electromagnetic stimulation: cranial microcurrent electrical stimulation of the brain.
end-tidal CO2: the percentage of CO2 in exhaled air at the end of exhalation.
external intercostals: the muscles of inhalation that pull the ribs upward and enlarge the thoracic cavity. The external intercostals account for about 25% of air movement into the lungs during relaxed breathing.
heart rate variability (HRV): beat-to-beat changes in heart rate, including changes in the R-R intervals between consecutive heartbeats.
HEG ratio: the ratio of refracted red vs. infrared light.
hemoencephalography (HEG): using passive infrared (pIR) and near infrared (NIR) to estimate cerebral blood flow (CBF) and metabolism.
hemoglobin: red blood cell protein that carries oxygen throughout the circulatory system.
hyperventilation (HV): a dysfunctional breathing pattern in which deep and rapid breathing results in breathlessness and reduces end-tidal CO2 below 5%, exceeding the body's need to eliminate CO2.
hypocapnia: decreased CO2 in arterial blood.
near-infrared hemoencephalography (NIR-HEG): projecting alternating infrared light at 660 nm (red light) and 850 nm (infrared light) into the skull and measuring the refracted light at these wavelengths to estimate the metabolic activity that supports cortical activity.
neuromodulation: stimulating the nervous system to produce physiological change.
neurovascular coupling: the mechanism in which vascular blood flow increases to provide nutrients and remove wastes to satisfy the greater metabolic demands of accelerated neuronal activity.
near-infrared hemoencephalography (NIR-HEG): projecting alternating infrared light at 660 nm (red light) and 850 nm (infrared light) into the skull and measuring light refracted from cortical tissue at these wavelengths to estimate the metabolic activity that supports cortical activity.
nitric oxide (NO): a gaseous neurotransmitter that promotes vasodilation and long-term potentiation.
overbreathing: mismatch between breathing rate and depth due to excessive breathing effort and subtle breathing behaviors, like sighs and yawns. Overbreathing can reduce arterial CO2.
pH: the power of hydrogen; the acidity or basicity of an aqueous solution determined by the concentration of hydrogen ions.
passive infrared hemoencephalograpy (pIR-HEG): a pIR sensor measures skin surface temperature that indexes cortical blood flow and related cerebral processing.
photobiomodulation (PBM): the delivery of near-infrared light from lasers or light-emitting diodes in the range of 600-1100 nm either transcranially or intranasally to increase brain mitochondrial activity.
pons: the brainstem structure above the medulla that contains breathing centers that adjust VRG breathing rhythms based on descending input from brain structures and peripheral sensory input.
pontine respiratory group (PRG): neurons located in the pons that communicate with the dorsal respiratory group (DRG) in the medulla to modify the basic breathing rhythm.
pulsed electromagnetic field therapy (PEMF therapy): stimulation at lower power than rTMS by electromagnetic coils arrayed in a helmet or a mat.
rectus abdominis: the muscle of forceful expiration that depresses the inferior ribs and compresses the abdominal viscera to push the diaphragm upward.
repetitive transcranial electromagnetic stimulation (rTMS): a technique that delivers a repetitive electromagnetic stimulus to the brain that either activates or deactivates the tissue depending on the speed of repetitive stimulation.
respiratory amplitude: the excursion of an abdominal strain gauge.
respiratory cycle: consists of an inspiratory phase, inspiratory pause, expiratory phase, and expiratory pause.
respiratory membrane: the site of respiratory gas exchange that is comprised by alveolar and capillary walls.
reverse breathing: a dysfunctional breathing pattern in which the abdomen expands during exhalation and contracts during inhalation, often resulting in incomplete ventilation of the lungs.
thoracic breathing: a breathing pattern that primarily relies on the external intercostals to inflate the lungs, resulting in a more rapid respiration rate, excessive energy consumption, and insufficient lung ventilation.
transcranial alternating current stimulation (tACS): using an alternating electrical current to entrain brain oscillations.
ventral respiratory group (VRG): neurons located in the medulla that initiate inhalation and exhalation.
Test Yourself
Click on the ClassMarker logo to take 10-question tests over this unit without an exam password.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Human Physiology and Physiological Psychology, which each satisfy BCIA's Anatomy and Physiology requirement for HRV Biofeedback Certification, and HRV Biofeedback100, which provides extensive multiple-choice testing over the BCIA HRV Biofeedback Blueprint.

Assignment
Now that you have completed this module, explain how heart rate variability biofeedback and healthy breathing training can complement neurofeedback. How might hemoencephalography prepare clients for neurofeedback training?
References
Attwell, D., & Laughlin, S. B. (2001). An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145. https://doi.org/10.1097/00004647-200110000-00001
Basu, S., & Banerjee, B. (2020). Prospect of brainwave entrainment to promote well-being in individuals: A brief review. Psychological Studies, 65, 296-306. https://doi.org/10.1007/s12646-020-00555
Berman, M. H., & Nichols, T. W. (2019). Treatment of neurodegeneration: Integrating photobiomodulation and neurofeedback in Alzheimer’s dementia and Parkinson’s: A review. Photobiomodulation, Photomedicine, and Laser Surgery, 10, 623-634. https://doi.org/10.1089/photob.2019.4685
Bezmaternykh, D. D., Kalgin, K. V., Maximova, P. E., Mel’nikov, M. Y., Petroskii, E. D., Predtechenskaya, E. V., Savelov, A. A., Semenikhina, A. A., Tsaplina, T. N., Shtark, M. B., & Shurunova, A. V. (2021). Application of fMRI and simultaneous fMRI-EEG neurofeedback in post-stroke motor rehabilitation. Bulletin of Experimental Biological Medicine, 171, 379-383. https://doi.org/10.1007/s10517-021-05232-1
Brunye, T. T., Patterson, J. E., Wooten, T., & Hussey, E. K. (2021). Critical review of cranial electrotherapy stimulation for neuromodulation in clinical and non-clinical samples. Frontiers in Human Neuroscience: Brain Imaging and Stimulation, 15, 625321. https://doi.org/10.3389/fnhum.2021.625321
Byrd, D. L., Reuther, E. T., McNamaram J. P. H., DeLucca, T. L., & Berg, W. K. (2015). Age differences in high frequency phasic heart rate variability and performance response to increased executive function load in three executive function tasks. Front Psychol, 5, 1470. https://doi.org/10.3389/fpsyg.2014.01470. eCollection 2014
Cardoso, F. D. S., Gonzalez-Lima, F., & da Silva, S. G. (2021). Photobiomodulation for the aging brain. Ageing Research Reviews, 70, 101415. https://doi.org/10.1016/j.arr.2021.101415
Collura, T. F., & Siever, D. (2009). Audio-visual entrainment in relation to mental health and EEG. In T. H. Budzynski, H. K. Budzynski, J. R. Evans, A. Abarbanel, A. (Eds.). Introduction to quantitative EEG and neurofeedback: Advanced theory and applications (pp. 195-224). Academic Press.
Coben, R., Middlebrooks, M., Lightstone, H., & Corbell, M. (2018). Four channel multivariate coherence training: Development and evidence in support of a new form of neurofeedback. Front. Neurosci., 12, 729. https://doi.org/10.3389/fnins.2018.00729
Demos, J. N. (2019). Getting started with EEG neurofeedback (2nd ed.). W. W. Norton & Company.
Dobrushina, O. R., Vlasova, R. M., Rumshiskaya, A. D., Litvinova, L. D., Mershina, E. A., Sinitsyn, V. E., & Pechenkova, E. (2020). Modulation of intrinsic brain connectivity by implicit electroencephalographic neurofeedback. Frontiers in Human Neuroscience, 14. https://doi.org/10.3389/fnhum.2020.00192
Elyamany, O., Leicht, G., Herrmann, C. S., & Mulert, C. (2020). Transcranial alternating current stimulation (tACS): From basic mechanisms towards first applications in psychiatry. European Archives of Psychiatry and Clinical Neuroscience, 271, 135-156. https://doi.org/10.1007/s00406-020-01209-9
Fox, S. I., & Rompolski, K. (2022). Human physiology (16th ed.). McGraw-Hill.
Fried, R. (1987). The hyperventilation syndrome: Research and clinical treatment. Johns Hopkins University Press.
Fried, R., & Grimaldi, J. (1993). The psychology and physiology of breathing. Springer.
Gevirtz, R. N., Lehrer, P. M., & Schwartz, M. S. (2016). Cardiorespiratory biofeedback. In M. S. Schwartz & F. Andrasik (Eds.). Biofeedback: A practitioner’s guide (4th ed.). The Guilford Press.
Gevirtz, R. N., Schwartz, M. S., & Lehrer, P. M. (2016). Cardiorespiratory measurement and assessment in applied psychophysiology. In M. S. Schwartz and F. Andrasik (Eds.). Biofeedback: A practitioner’s guide (4th ed.). The Guilford Press.
Gutierrez-Menendez, A., Marcos-Nistal, M., Mendez, M., & Arias, J. L. (2020). Photobiomodulation as a promising new tool in the management of psychological disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 119, 242-254. https://doi.org/10.1016/j.neubiorev.2020.10.002
Hamblin, M. R. (2016). Shining light on the head: Photobiomodulation for brain disorders. BBA Clinical, 6, 113-124. https://doi.org/0.1016/j.bbacli.2016.09.002
Hamblin, M. R. (2017). Photobiomodulation for traumatic brain injury and stroke. Journal of Neuroscience Research, 4, 731-743. https://doi.org/10.1002/jnr.24190
Hanslmayr, S., Axmacher, N., & Inman, C. S. (2019). Modulating human memory via entrainment in brain oscillations. Trends in Neurosciences, 42, 485-499. https://doi.org/10.1016/j.tins.2019.04.004
Huneau, C., Benali, H., & Chabriat, H. (2015). Investigating human neurovascular coupling using functional neuroimaging: A critical review of dynamic models. Frontiers in Neuroscience, 9. 10.3389/fnins.2015.00467
Iadecola, C., & Nedergaard, M. (2007). Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376. https://doi.org/10.1038/nn2003
Imahori, Y., Vetrano, D. L., Xia, X., Grande, G., Ljungman, P., Fratiglioni, L., & Qui, C. (2021). Association of resting heart rate with cognitive decline and dementia in older adults: A population-based cohort study. Alzheimer's & Dementia. https://doi.org/10.1002/alz.12495
Jahan, A., Nazari, M. A., Mahmoudi, J., Salehpour, F., & Salimi, M. M. (2019). Transcranial near-infrared photobiomodulatiocould modulate brain electrophysiological features and attentional performance in healthy young adults. Lasers in Medical Science, 6, 1193-1200. https://doi.org/10.1007/s10103-018-2710-3
Jog, M. V., Wang, D. J. J., & Narr, K. L. (2019). Review of transcranial direct current stimulation (tDCS) for the individualized treatment of depressive symptoms. Personalized Medicine in Psychiatry, 17-18, 17-22. https://doi.org/10.1016/j.pmip.2019.03.001
Khazan, I. (2021). Respiratory anatomy and physiology. BCIA HRV Biofeedback Certificate of Completion Didactice workshop.
Laborde, S., Mosley, E., & Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research - Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. https://doi.org/10.3389/fpsyg.2017.00213
Larsen, E. R., Licht, R. W., Nielsen, R. E., Lolk, A., Borck, B., Sørensen, C., Christensen, E. M., Bizik, G., Ravn, J., Martiny, K., Vinberg, M., Jankuviené, O., Jørgensen, P. B., Videbech, P., & Bech, P. (2020). Transcranial pulsed electromagnetic fields for treatment-resistant depression: A multicenter 8-week single-arm cohort study. European psychiatry: the Journal of the Association of European Psychiatrists, 63(1), e18. https://doi.org/10.1192/j.eurpsy.2020.3
Legarda, S. B., McMahon, D., Othmer, S., & Othmer S. (2011). Clinical neurofeedback: Case studies, proposed mechanism, and implications for pediatric neurology practice. J Child Neurol., 26(8),1045-1051. https://doi.org/10.1177/0883073811405052
Linhartova, P., Latalova, A., Kosa, B., Kasparek, T., Schmahl, C., & Paret, C. (2019). fMRI neurofeedback in emotional regulation: A literature review. NeuroImage, 193, 75-92. https://doi.org/10.1016/j.neuroimage.2019.03.011
Kern, B. (2021). Hyperventilation syndrome treatment & management. eMedicine. Retrieved from Medscape. https://emedicine.medscape.com/article/807277-treatment
Khazan, I. Z. (2013). The clinical handbook of biofeedback: A step-by-step guide for training and practice with mindfulness. John Wiley & Sons, Ltd.
Khazan, I. Z. (2019). A guide to normal values for biofeedback. In D. Moss & F. Shaffer (Eds.). Physiological recording technology and applications in biofeedback and neurofeedback (pp. 2-6). Association for Applied Psychophysiology and Biofeedback.
Kluetsch, R. C., Ros, T., Théberge, J., Frewen, P. A., Calhoun, V. D., Schmahl, C., Jetly, R., & Lanius, R. A. (2014). Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr. Scand., 130, 123–136. https://doi.org/10.1111/acps.12229
Laborde, S., Mosley, E., & Mertgen, A. (2018). Vagal tank theory: The three Rs of cardiac vagal control functioning – resting, reactivity, and recovery. Front. Neursci., 12, 458. https://doi.org/10.3389/fnins.2018.00458
Lagos, L. (2020). Heart breath mind: Train your heart to conquer stress and achieve success. HapperCollins Publishers.
Leong, S. L., Vanneste, S., Lim, J., Smith, M., Manning, P., & De Ridder, D. (2018). A randomised, double-blind, placebo-controlled parallel trial of closed-loop infraslow brain training in food addiction. Sci Rep 8(11659). https://doi.org/10.1038/s41598-018-30181-7
Long, K. (2016, February 9). The science behind the sigh. The Wall Street Journal, D3.
Lorig, T. S. (2007). The respiratory system. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson, (Eds.). Handbook of psychophysiology (3rd ed.). Cambridge University Press.
Lubar, J. F., & Bahler, W. W. (1976). Behavioral management of epileptic seizures following EEG biofeedback training of the sensorimotor rhythm. Biofeedback Self Regul, 1(1), 77-104. https://doi.org/10.1007/BF00998692.
Lubar, J. F., Shabsin, H. S, Natelson, S. E., Holdson, S. E., Holder, G. S., Whittsett, S. F., Pamplin, W. E., & Krulikowski, D. I. (1981). EEG operant conditioning in intractable epileptics. Archives of Neurology, 38, 700–704. https://doi.org/10.1001/archneur.1981.00510110060009
Lubar, J. F., & Shouse, M. N. (1976). EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR): A preliminary report. Biofeedback Self Regul,1(3), 293-306. https://doi.org/10.1007/BF01001170. PMID: 990355.
MacKinnon, S., Gevirtz, R., McCraty, R., & Brown, M. (2013). Utilizing heartbeat evoked potentials to identify cardiac regulation of vagal afferents during emotion and resonant breathing. Applied Psychophysiology and Biofeedback, 38, 241–255. https://doi.org/10.1007/s10484-013-9226-5
Marieb, E. N., & Hoehn, K. (2019). Human anatomy and physiology (11th ed.). Pearson Benjamin Cummings.
Marques, R. C., Vieira, L., Marques, D., & Cantilino, A. (2019). Transcranial magnetic stimulation of the medial prefrontal cortex for psychiatric disorders: A systematic review. Brazilian Journal of Psychiatry, 41, 447-457. https://doi.org/10.1590/1516-4446-2019-0344
Martiny, K., Lunde, M., & Bech, P. (2010). Transcranial low voltage pulsed electromagnetic fields in patients with treatment-resistant depression. Biological Psychiatry, 68, 163-169. https://doi.org/10.1016/j.biopsych.2010.02.017
Marzbani, H., Marateb, H. R., & Mansourian, M. (2016). Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic and clinical neuroscience, 7(2), 143–158.
Mather, M., & Thayer, J. (2018). How heart rate variability affects emotion regulation brain networks. Curr Opin Behav Sci., 19, 98-104. https://doi.org/10.1016/j.cobeha.2017.12.017
McCraty, R. & Shaffer, F. (2015). Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob Adv Health Med, 4(1). 46-61. https://doi.org/10.7453/gahmj.2014.073.
Monastra, V. J., Lubar, J. F., Linden, M., VanDeusen, P., Green, G., Wing, W., Phillips, A., & Fenger, T. N. (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validation study. Neuropsychology, 13(3), 424–433. https://doi.org/10.1037/0894-4105.13.3.424
Nuwer, M. R., & Coutin-Churchman, P. (2014). Brain mapping and quantitative electroencephalogram. Encyclopedia of the Neurological Sciences (2nd ed.). Elsevier.
Oken, B. S., & Chiappa, K. H. (1988). Short-term variability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol, 69, 191–198. https://doi.org/10.1016/0013-4694(88)90128-9
Pecyna, M. B., & Pokorski, M. (2013). Near-infrared hemoencephalography for monitoring blood oxygenation in prefrontal cortical areas in diagnosis and therapy of developmental dyslexia. Adv exp Med Biol., 788, 175-180. https://doi.org/10.1007/978-94-007-6627-3_26
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271 – 279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brain wave training. Medical Psychotherapy, 3, 37–55. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peper, E., & Tibbetts, V. (1992). Fifteen-month follow-up with asthmatics utilizing EMG/incentive inspirometer feedback. Biofeedback and Self-Regulation, 17(2), 143-151. https://doi.org/10.1007/BF01000104
Putman, J. A., Othmer, S. F., Othmer, S., & Pollock, V. E. (2005). TOVA results following inter-hemispheric bipolar EEG training. Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience, 9(1), 37-52. https://doi.org/10.1300/J184v09n01_04
Ribas, V. R., Ribas, R., & Martins, H. (2016). The learning curve in neurofeedback of Peter Van Deusen: A review article. Dementia & Neuropsychologia, 10(2), 98–103. https://doi.org/10.1590/S1980-5764-2016DN1002005
Rogala, J., Jurewicz, K., Paluch, K., Kublik, E., Cetnarski, R., & Wróbel, A. (2016). The do's and don'ts of neurofeedback training: A review of the controlled studies using healthy adults. Frontiers in Human Neuroscience, 10, 301. https://doi.org/10.3389/fnhum.2016.00301
Shaffer, F., Meehan, Z. M., & Zerr, C. L. (2020). A critical review of ultra-short-term heart rate variability norms research. Frontiers in Neuroscience, 14, 594880. https://doi.org/10.3389/fnins.2020.594880
Shekelle, P., Cook, I., Miake-Lye, I., Mak, S., Booth M. S., Shanman, R., & Beroes, J. M. (2018). Effectiveness and risks of cranial electrical stimulation for the treatment of pain, depression, PTSD, and insomnia: A systematic review. Department of Veterans Affairs (US); 2018 Feb. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493132/
Shouse, M. N., & Lubar, J. F. (1979). Operant conditioning of EEG rhythms and ritalin in the treatment of hyperkinesis. Biofeedback and Self-Regulation, 4, 299–312. https://doi.org/10.1007/BF00998960
Siever, D. (2007). Audio-visual entrainment: History, physiology, and clinical studies. In J. R. Evans (Ed.), Handbook of neurofeedback: Dynamics and clinical applications (pp. 155-183). Haworth Medical Press.
Sinha, M., Sinha, R., Ghate, J., & Sarnik, G. (2020). Impact of altered breathing patterns on interaction of EEG and heart rate variability. Annals of Neurosciences, 7(2). https://doi.org/10.1177/0972753120950075
Sittenfeld, P., Budzynski, T., & Stoyva, J. (1976). Differential shaping of EEG theta rhythms. Biofeedback and Self-Regulation, 1, 31–45. https://doi.org/10.1007/BF00998689
Smith, M. L., Collura, T. F., Ferrara, J., & de Vries, J. (2014). Infra-slow fluctuation training in clinical practice: A technical history. NeuroRegulation, 1(2), 187–207.
Smith, M. L., Leiderman, L., & de Vries, J. (2017). Infra-slow fluctuation (ISF) for autism spectrum disorders. In T. F. Collura & J. A. Frederick (Eds.), Handbook of clinical QEEG and neurotherapy. Routledge Taylor and Francis Group.
Sterman, M. B. (1976). Effects of brain surgery and EEG operant conditioning on seizure latency following monomethylhydrazine in the cat. Exp. Neurol., 50, 757-765.
Sterman, M. B., & Friar, L. (1972). Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol,33(1), 89-95. https://doi.org/10.1016/0013-4694(72)90028-4. PMID: 4113278.
Sterman, M. B., Lopresti, R. W., & Fairchild, M. D. (1969). Electroencephalographic and behavioral studies of monomethylhydrazine toxicity in the cat. AMRL-TR-69-3, Aerospace Medical Research Laboratory, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio.
Sterman, M. B., LoPresti, R. W., & Fairchild, M. D. (2010). Electroencephalographic and behavioral studies of monomethyl hydrazine toxicity in the cat. Journal of Neurotherapy, 14(4), 293-300. https://doi.org/10.1080/10874208.2010.523367
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Strehl, U. (2014). What learning theories can teach us in designing neurofeedback treatments. Frontiers in Human Neuroscience, 8(894). https://doi.org/10.3389/fnhum.2014.00894
Swingle, P. (2014). Clinical versus normative databases: Case studies of clinical Q assessments. NeuroConnections.
Swingle, P. G. (2015). Adding neurotherapy to your practice: Clinician’s guide to the ClinicalQ, neurofeedback, and braindriving. Springer.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043-1065. PMID: 8737210
Tavakoli, A. V., & Yun, K. (2017). Transcranial alternating current stimulation (tACS) mechanisms and protocols. Frontiers in Cellular Neurophysiology, 11, 00214.
Terrasa, J. L., Alba, G., Cifre, I., Rey, B., Montoya, P., & Muñoz, M. A. (2019). Power spectral density and functional connectivity changes due to a sensorimotor neurofeedback training: A preliminary study. Neural Plast., 2019, 7647204. https://doi.org/10.1155/2019/7647204
Thatcher, R. W. (1998). Normative EEG databases and EEG biofeedback. Journal of Neurotherapy, 2(4), 8-39. https://doi.org/10.1300/J184v02n04_02
Thatcher, R. W., Lubar, J. F., & Koberda, J. L. (2019). Z-Score EEG biofeedback: Past, present, and future. Biofeedback, 47(4), 89–103. https://doi.org/10.5298/1081-5937-47.4.04
Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J., & Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36, 747-756. https://doi.org/10.1016/j.neubiorev.2011.11.009
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201-216. https://doi.org/10.1016/s0165-0327(00)00338-4
Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol, 141(2), 122-131. https://doi.org10.1016/j.ijcard.2009.09.543
Thompson, M., & Thompson, L. (2015). The neurofeedback book (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Toomim, H. (2000). A report of preliminary data: QEEG, SPECT, and HEG; Targeted treatment positions for neurofeedback. Applied Psychophysiology and Biofeedback, 25(4), 253–254.
Tortora, G. J., & Derrickson, B. H. (2021). Principles of anatomy and physiology (16th ed.). John Wiley & Sons, Inc.
Walker, J. E., & Horvat, J. (2010). Is it better to train power first or coherence first? Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience, 14(2), 102-106. https://doi.org/10.1080/10874201003767239
Watanabe, T., Sasaki, Y., Shibata, K., & Kawato, M. (2017). Advances in fMRI real-time neurofeedback. Trends in Cognitive Sciences, 21, 997-1010. https://doi.org/10.1016/j.tics.2017.09.010
Yamashita, A., Hayasaka, S., Kawato, M., Imamizu, H. (2017). Connectivity neurofeedback training can differentially change functional connectivity and cognitive performance. Cerebral Cortex, 27(10), 4960–4970. https://doi.org/10.1093/cercor/bhx177
Zelano, C., Jiang, H., Zhou, G., Arora, N., Schuele, S., Rosenow, J., & Gottfried, J. A. (2016). Nasal respiration entrains human limbic oscillations and modulates cognitive function. Journal of Neuroscience, 36(49), 12448. https://doi.org/10.1523/JNEUROSCI.2586-16.2016
Zhang, D., Shen, X., & Qi, X. (2016). Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. CMAJ, 188(3), E53-63. https://doi.org/10.1503/cmaj.150535