EEG
An electroencephalograph (EEG) monitors brainwave activity at various frequencies, from DC shifts (slow cortical potentials) to fast potentials exceeding 50 Hz. The EEG records the excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) propagated by the apical dendrites of large pyramidal cells arranged in thousands of cortical columns.
Local field potentials, the aggregate effect of interconnected neuron firing and modulation by glial cells, regulate neuron excitability and firing. Action potential animation © NIMEDIA/Shutterstock.com.
International QEEG Certification Board Blueprint Coverage
This unit addresses IV. EEG (8 hours).

This unit covers:
A. Basic Knowledge of Neurophysiology of the EEG
B. Editing and Identifying Artifacts
C. Normal Waveform Patterns
D. Standards of EEG Acquisition Procedures Including Activation
E. Abnormal EEG Waveforms and Rhythms
F. The Use of Different EEG Montages for Waveform Analysis
A. Basic Knowledge of Neurophysiology of the EEG
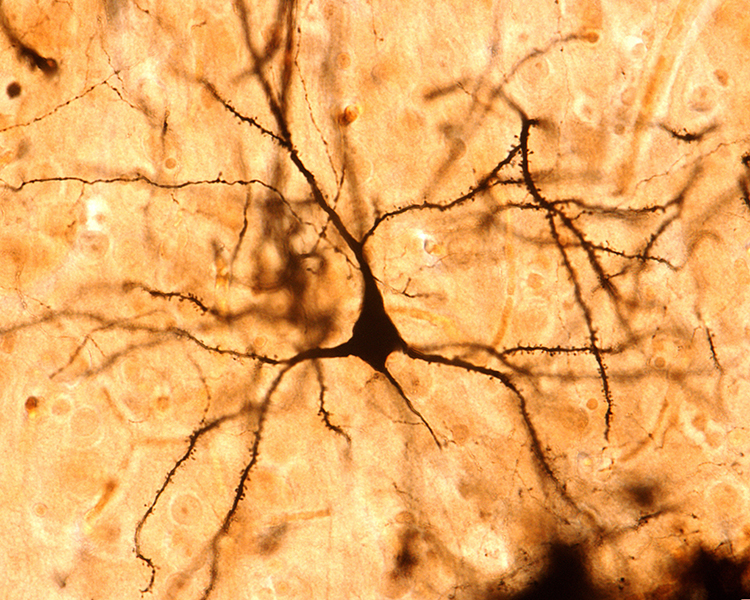
The scalp EEG is the voltage difference between two recording sites recorded over time. The EEG is primarily generated by large pyramidal neurons in layers 3 and 5 of the 2-4.5-mm-thick cortical gray matter. Please click on the podcast icon below to hear a full-length lecture over Section A.

Image of a pyramidal neuron revealed using Golgi silver chrome © Jose Luis Calvo/Shutterstock.com. Note that the apical dendrite arising from the cell body and basilar dendrites feature an extensive network of spines.
Local activity is a composite of local and network influences. Network communication systems and local cortical functions show different characteristics across the cortex and produce unique and specific EEG patterns in other regions.
The movie below is a BioTrace+/NeXus-32 display of the raw EEG with voltage shown as μV peak to peak © John S. Anderson.
What Can the EEG Tell Us?
With the EEG, we can follow the progression from stimulus to behavior response. This allows us to determine the correct function at each step and identify causal factors in dysfunctional outcomes or responses.Source of the Scalp EEG
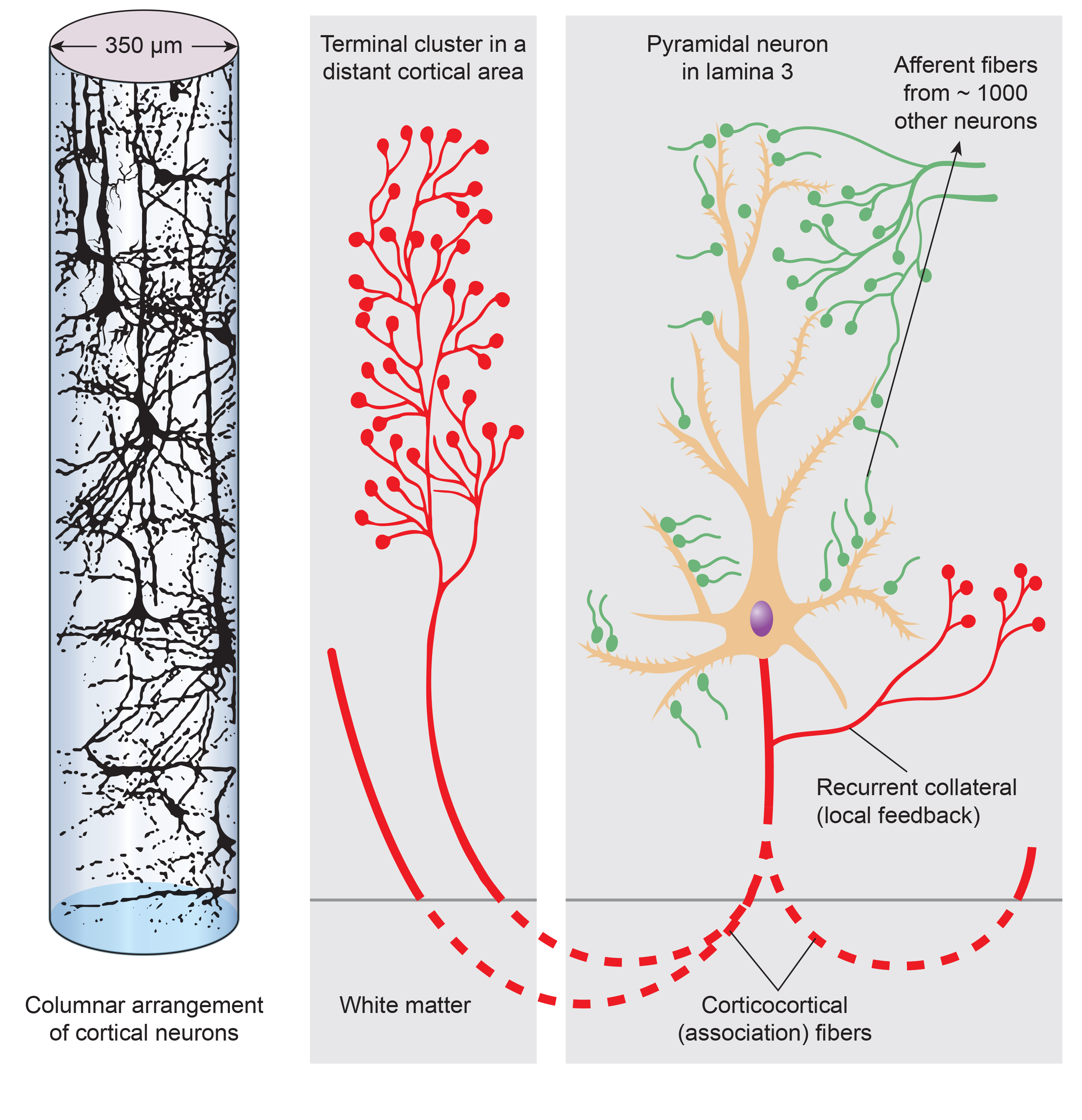
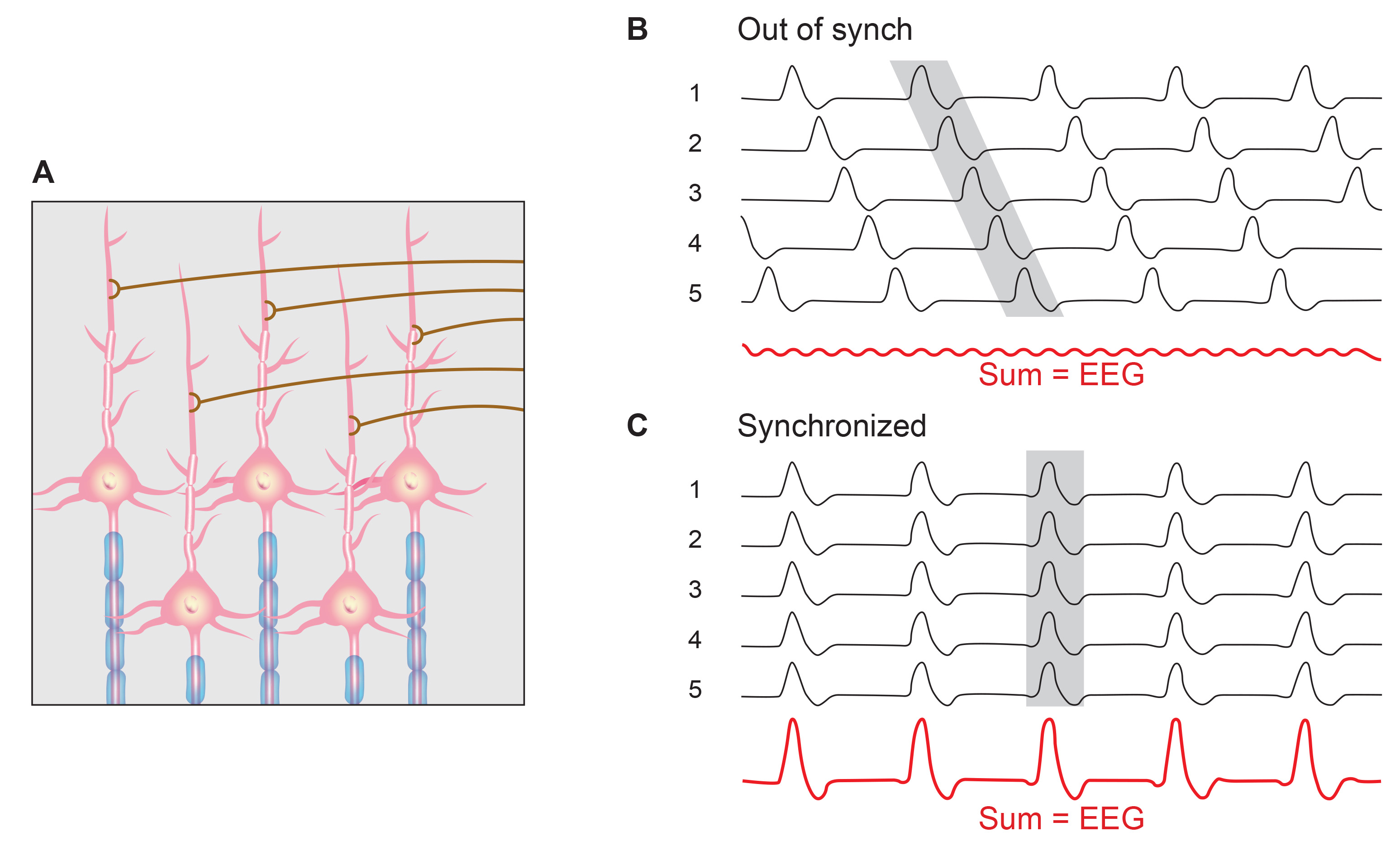
The scalp EEG results from summating large areas of gray matter activity. Areas are polarized synchronously due to oscillatory or transient evoked activity input. These areas comprise thousands of cortical columns containing large pyramidal cells aligned perpendicularly to the cortical surface. Cortical layer graphic © Anna Him/Shutterstock.com.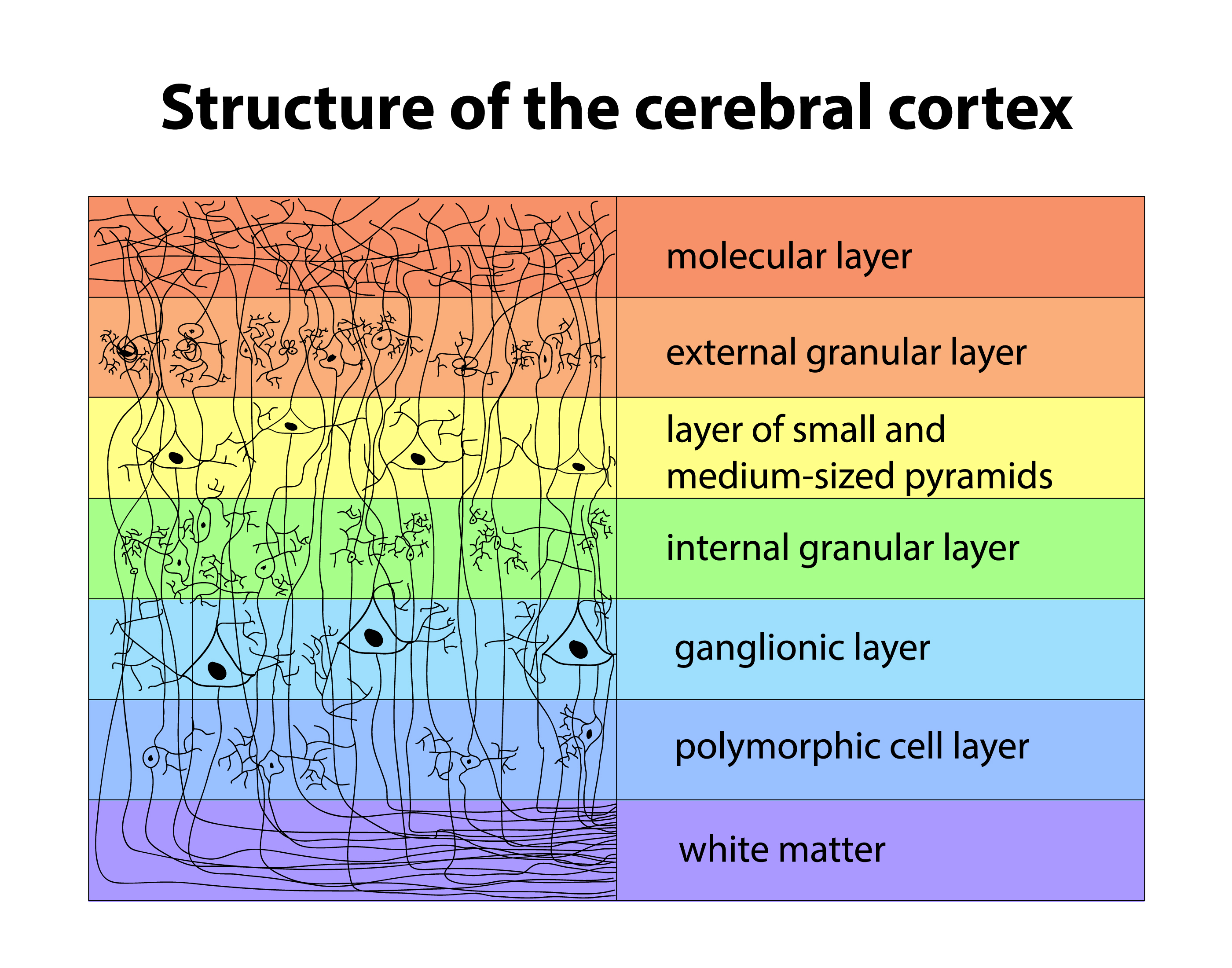
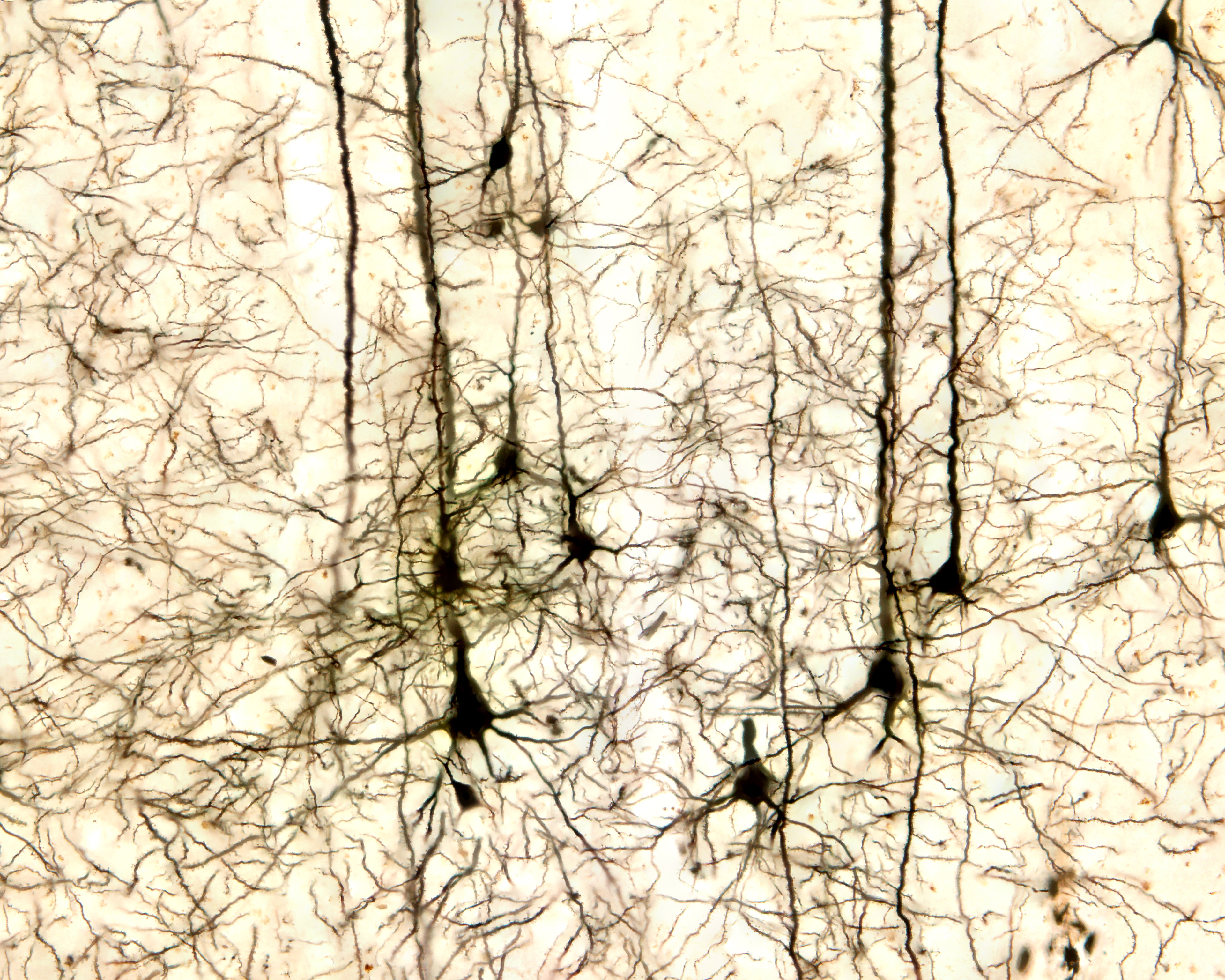
Pyramidal neurons of the cerebral cortex stained with the Golgi silver chromate © Jose Louis Calvo/Shutterstock.com.
Pyramidal neurons are found in all cortical layers except layer 1 and represent the primary type of output neuron in the cerebral cortex. Pyramidal neuron graphic © Kateryna Kon/Shutterstock.com.
The scalp EEG results from the summation of EPSPs and IPSPs in thousands of cortical columns containing large pyramidal cells perpendicular to the cortical surface. The columns are synchronously polarized (made more negative) and depolarized (made less negative) due to the input of oscillatory or transient evoked activity. Graphic redrawn by minaanandag on Fiverr.com.
Artist: Dani S@unclebelang. This WEBTOON is part of our Real Genius series.

Local Field Potentials
The local field potential (LFP) is the aggregate effect of the firing of the interconnected pyramidal neurons within the cortical columns, plus additional mechanisms like glial cell modulation of the cortical electrical gradient.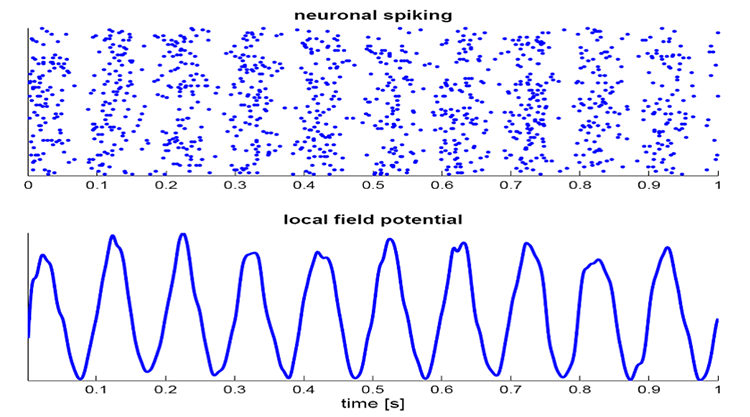
Caption from Wikipedia's article on Neural Oscillation. Simulation of neural oscillations at 10 Hz. The upper panel shows the spiking of individual neurons (with each dot representing an individual action potential within the population of neurons). On the lower panel, the local field potential reflects their summed activity. This figure illustrates how synchronized patterns of action potentials may result in macroscopic oscillations that can be measured outside the scalp.
Do not confuse the "spiking" of individual neurons with epileptogenic spikes in the scalp EEG.
Scalp Electrical Potentials
Scalp electrical potentials represent the sum of all available electrical fields. Fields of opposite polarity (+/-) cancel each other out so that scalp potentials are greater when large aggregates of neurons polarize and depolarize synchronously. The scalp EEG represents a weighted sum of all active currents within the brain that generate open fields, including non-cortical sources.Action potentials reflect neuronal output. They are seen in extracellular recordings as fast (~300 Hz) activity that exceeds 90 mV, lasting less than 2 ms. Action potentials play a minor role in scalp surface EEG. They fall below 60 V outside of a 50-μm (0.050-mm) radius. Scalp electrodes are several centimeters from cortical neurons and are generally aligned away from the scalp. Therefore, action potentials are unlikely to contribute significant voltages to the scalp EEG.
Local Field Potentials Regulate Neuron Excitability and Firing
Neurons are most likely to fire during the depolarizing phase of the local field potential. Neurons are more excitable when they are "in phase" with respect to the local field potential (LFP) and are inhibited when they are out of phase with the LFP. Thus, at any instant in time, the amplitude and frequency of the EEG are regulated by the LFP, which, in turn, is influenced by oscillatory mechanisms such as slow cortical potentials.The movie is a 19-channel BioTrace+ /NeXus-32 display of SCPs © John S. Anderson. Negative SCPs drift up, and positive SCPs drift down, depending on the software settings. However, the convention in electroencephalography is to show negative up. SCPs represent a global shift in DC voltage across the cortex and represent a generally higher (negative SCPs) or lower (positive SCPs) state of cortical excitability regulating neural networks.
The EEG is a moment-to-moment measure of the excitability of action potential firing, like gates opening and closing on the half cycle. The synchronous activity of large pyramidal neurons networked in cortical columns creates the EEG.
The Composition of the EEG
The EEG is composed of electrical potentials, varying in two dimensions: frequency and amplitude.Sources of IPSP and EPSP Inputs
Many sources contribute input that results in IPSP and EPSP activity within cortical neurons. These sources contribute to influences such as oscillatory generator input or ascending event-related evoked input.EEG Sources
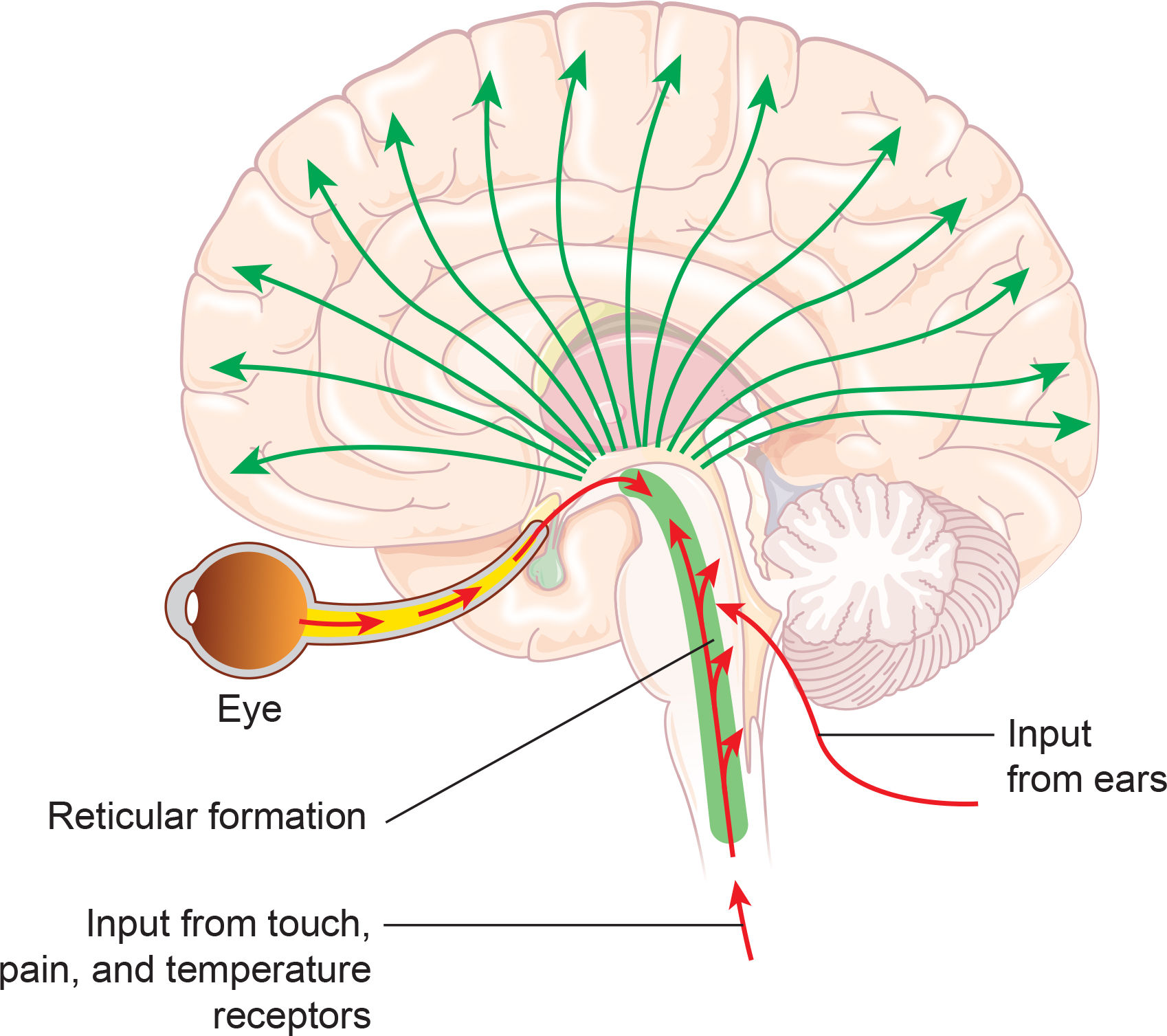
Generators like the thalamus produce oscillatory activity among many interconnected neurons, including EEG patterns like the alpha rhythm.
Movie © John S. Anderson. The recording begins with eyes open. The eyes-closed condition starts at 14’01” and clearly shows increased 8-12 Hz voltage (posterior dominant rhythm or PDR) in occipital and parietal locations in the line tracing and topographic maps to the right of the tracing.
The eyes open again at 14’31”, and alpha attenuates (alpha blocking). This shows the posterior dominant rhythm (generally known as "alpha") appearing in the eyes-closed condition when visual sensory input is stopped. The attenuation or blocking of this rhythm as sensory input returns in the eyes-open condition.
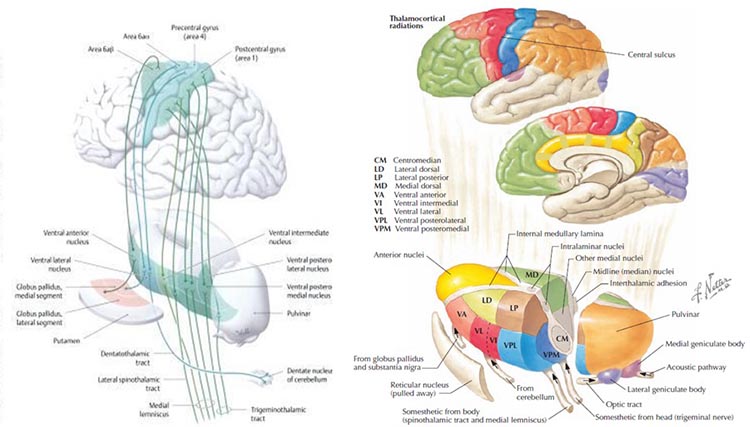
The thalamus contributes to slow cortical potentials, 1-4 Hz delta, 8-12 Hz alpha, and 20-38 Hz beta (including 40-Hz activity). The diagram shows the connections between the pulvinar (bottom right) and the thalamus's reticular nuclei (bottom left) and cortex © Elsevier Inc. - Netterimages.com.
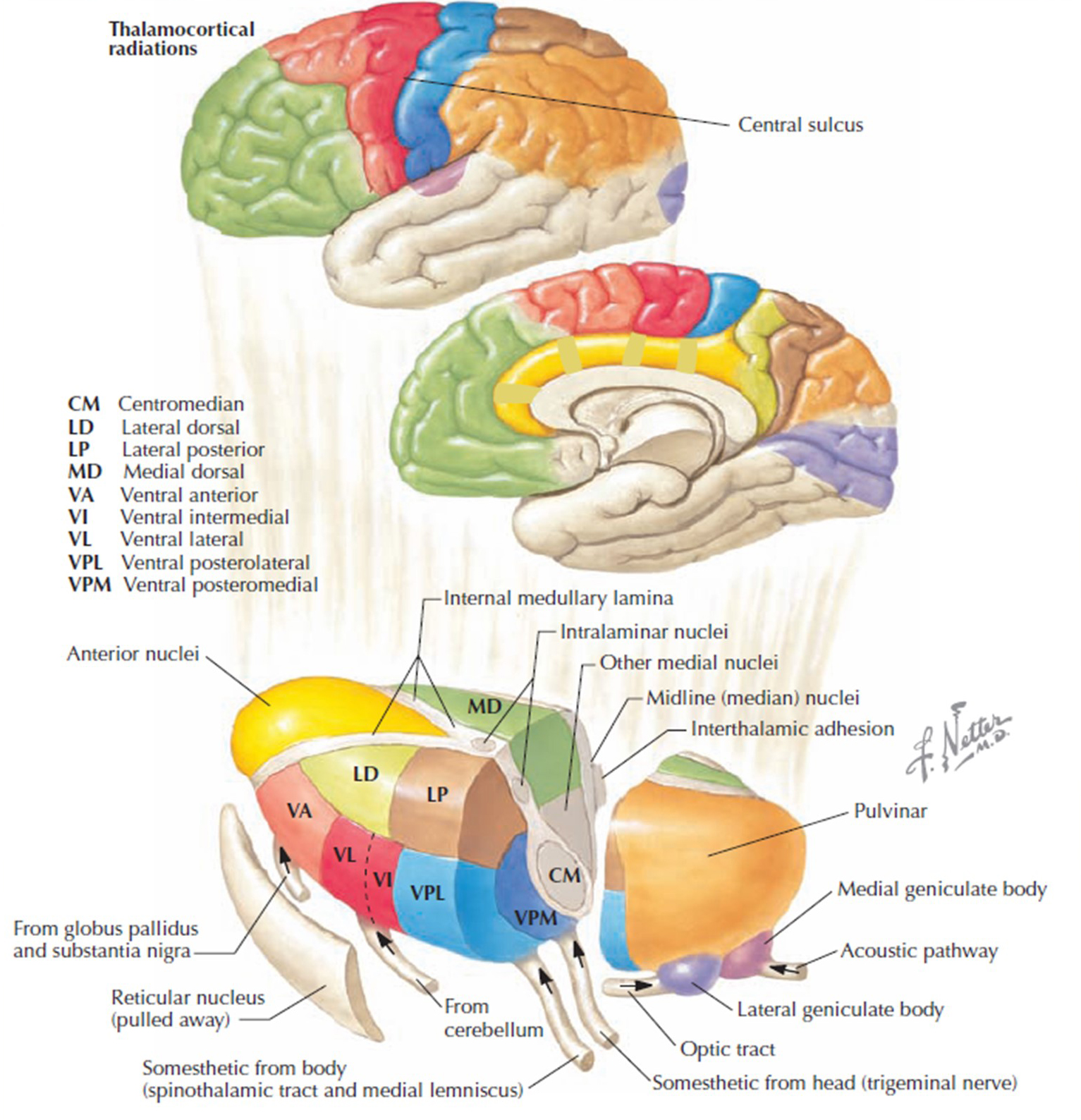
Thalamocortical cells are subject to excitatory drive from their system afferents, monosynaptic corticothalamic fibers, and brainstem reticular formation (ascending reticular activating system, ARAS). They receive inhibitory drive from local interneurons and neurons in the reticular nucleus of the thalamus (RNT). The RNT neurons are excited by activity in thalamocortical and corticothalamic cells. The connections are precisely organized. For example, each column in a primary cortical area sends corticothalamic fibers back to the same part of its specific thalamic nucleus that sends its thalamocortical fibers to that cortical column.
The corticothalamic fibers also synapse on the RNT cells, which receive input from that part of the thalamic nucleus. Each cortical receiving area is said to be "reciprocally connected" with its specific thalamic nucleus. Like the thalamocortical cells, RNT cells and cortical neurons also receive excitatory drive from the ARA (Jackson & Stoney, 2006).
The EEG is generated by thalamocortical (alpha) and cortical-cortical (beta) sources.
Neurons in the ascending reticular activating system produce event-related potentials in response to diverse stimuli like a flashing light or sound. Event-related potentials (ERPs) are the brain's response to externally applied stimuli, events, or cognitive/motor tasks. They are time-locked measures of brain electrical activity. Graphic redrawn by minaanandag on Fiverr.com.
Dipole Generators
Large cortical pyramidal neurons organized in macrocolumns are oriented with an apical dendrite projecting toward the scalp and an axon descending in the opposite direction. An "Equivalent Dipole Generator" usually represents the sum of all multipolar current sources. Summed generators are modeled as dipoles to aid the conceptual understanding of the electrical fields involved. Graphic adapted from Lipping (2017).
EEG Signals (Brainwaves)
The EEG represents changes in a brain area's electrical activity (potential) compared to a "neutral" site or another brain area. The EEG is displayed as oscillations or voltage fluctuations, which show a "wave" pattern when plotted on a graph.
"These oscillations are generated spontaneously in several areas of the cerebral cortex as neuronal networks transiently form assemblies of synchronously firing cells." Klaus Linkenkaer-Hansen.
Sink, Source, and Dipole
We can describe pyramidal cells in terms of their sink, source, and dipole. A sink (-ve), located at the bottom, middle, or top of the apical dendrite, is where positive ions enter the dendrite. Cation (positive ion) entry gives the extracellular space a negative charge. The source (+ve) is where the current exits the cell. Finally, the dipole is the field created between the sink and the source (Thompson & Thompson, 2016).The postsynaptic potentials (EPSPs and IPSPs) propagated by the apical dendrites in layers 2 and 3 create an extracellular dipole layer parallel to the cortical surface. The dipole layer's electrical polarity is the opposite of the deeper cortical layers 4 and 5 (Fisch, 1999).
A cortical dipole is created when pyramidal neurons depolarize simultaneously. This phenomenon is called local synchrony. Fewer than 5% of pyramidal neurons can generate more than 90% of the power in the EEG signal because most pyramidal neurons usually fire asynchronously so that their potentials counteract each other. A small fraction of these neurons firing in step can produce visible changes in EEG feedback. This creates the potential for operant conditioning to help clients learn to modify EEG activity through neurofeedback.
Cortical dipoles have three properties: site (depends on source), size (oscillation frequency and voltage), and relative position with respect to sulci and gyri (Collura, 2014).
The EEG is Mainly Sensitive to Radially Oriented Dipoles
Evolution has convoluted the human brain to increase its computing power without enlarging the skull. This enfolding has created two easily visible anatomical features: gyri and sulci. Cortex photograph © IdeaGeneration/Shutterstock.com.
Recall that a gyrus is a ridge of the convoluted cerebral cortex, while a sulcus is a valley. The graphic below is adapted from Wikipedia.com from the article Sulcus (neuroanatomy) by minaanandag.
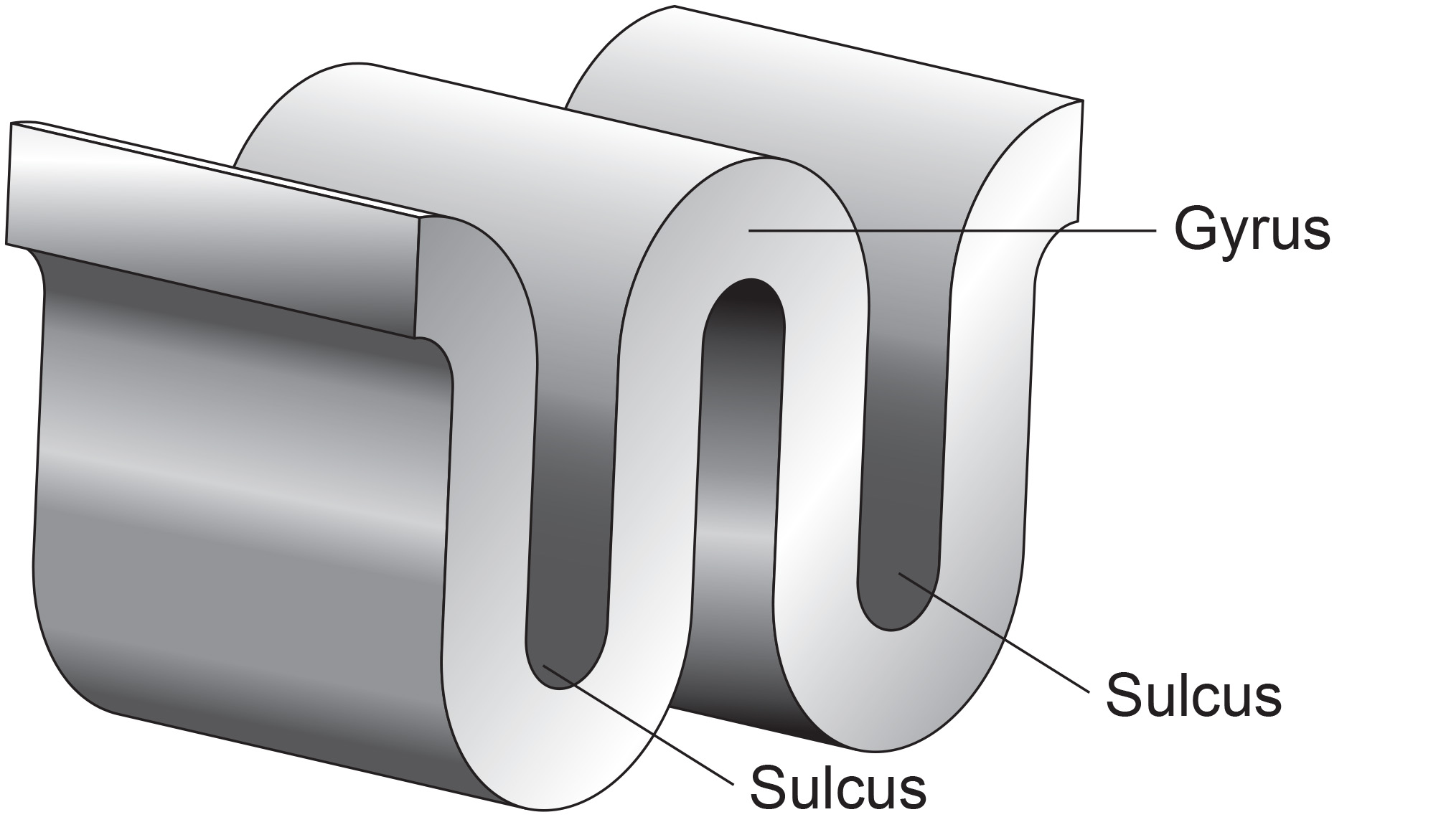
The EEG is most sensitive to a correlated dipole layer in gyri. The EEG is less sensitive to a correlated dipole layer in sulci, valleys within the cortex. Finally, the EEG is insensitive to an opposing dipole layer in sulci.
The EEG is composed of electrical potentials that vary along the dimensions of amplitude and frequency.
EEG Amplitude
The "amount" or amplitude and the "pattern" or morphology of any EEG frequency band reflect the number of neurons discharging simultaneously at that frequency. Lower neuron firing rates correspond to lower signal amplitude. Amplitude graphic © petrroudny43/Shutterstock.com.
Amplitude measures the energy in the signal, which is usually expressed in microvolts.
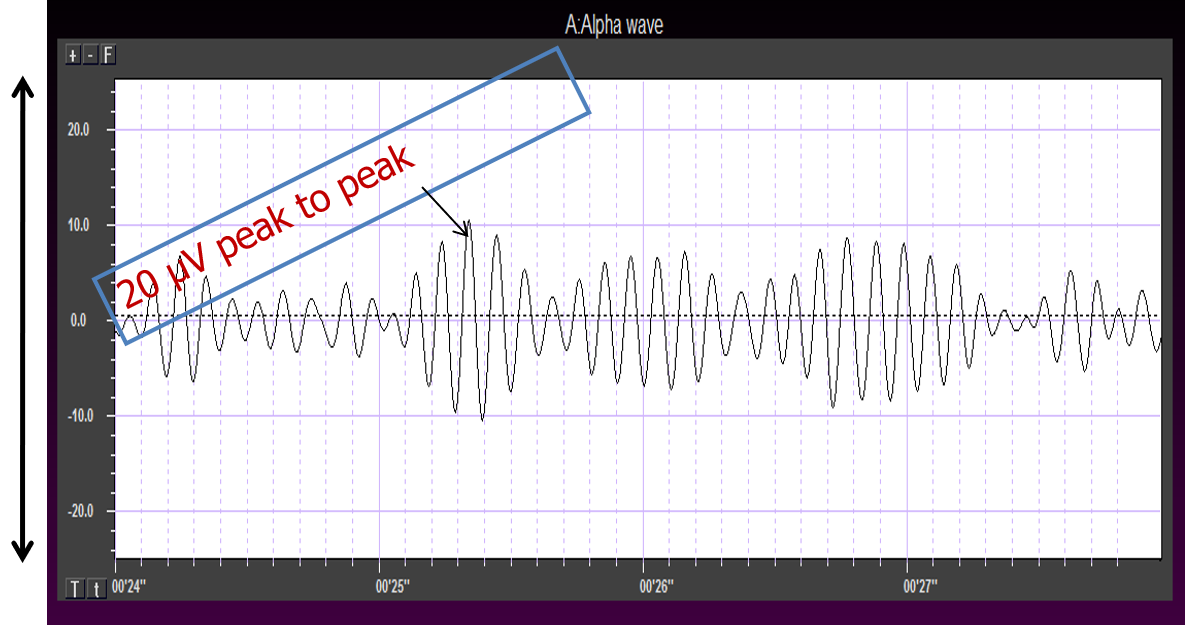
Greater synchrony in firing among neurons results in higher amplitude, as shown with alpha in the graphic below.
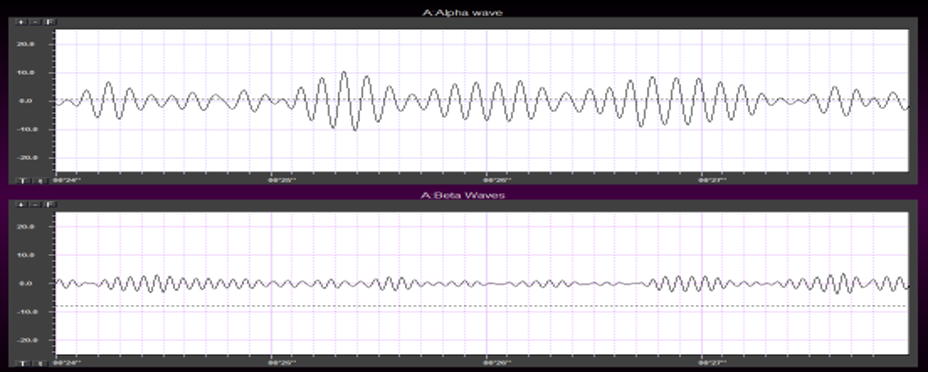
Greater firing synchrony produces larger EEG potentials that can be measured from the scalp surface. Graphic redrawn by minaanandag on Fiverr.com.
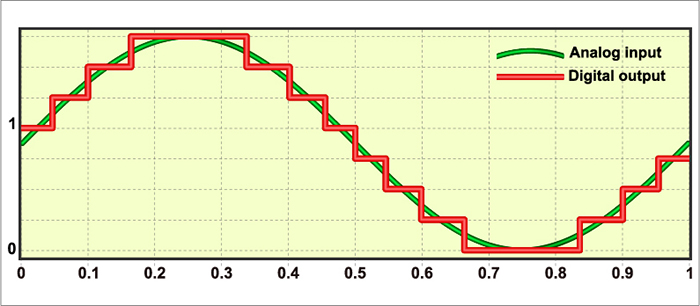
The EEG plots voltage changes over time, which can be displayed on a graph. The sampling rate is the number of measurements per second (Hz). Precision is the number of voltage gradations or steps.
The analog-to-digital (A/D) converters that transform voltages into numerical values vary in precision: more bits correspond to greater accuracy. Graphic © Fouad A. Saad/Shutterstock.com.
EEG Frequencies
The raw EEG contains all EEG frequencies, just as white light contains all light frequencies. Digital filters separate the EEG frequencies just as a prism separates individual colors. Graphic © kmls/ Shutterstock.com.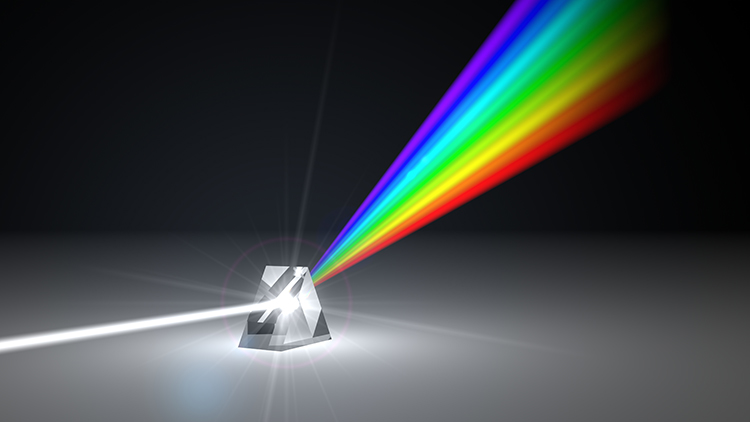
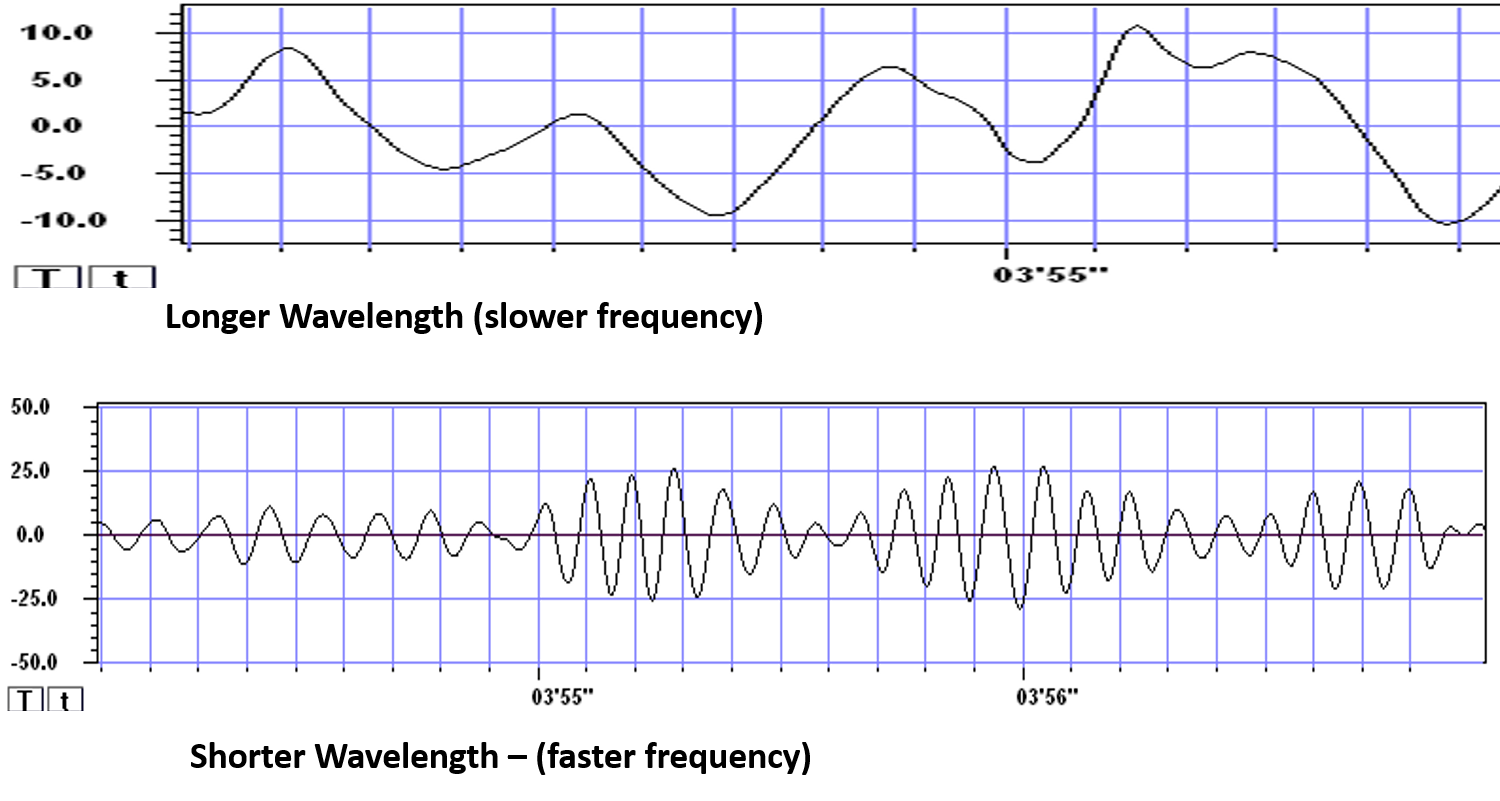
EEG frequency is measured in cycles per second or Hz. Count the number of peaks or count the number of zero (0.0) crossings divided by 2.
The slower the waves, the lower the EEG frequency. Frequency graphic © Bany's beautiful art/Shutterstock.com.
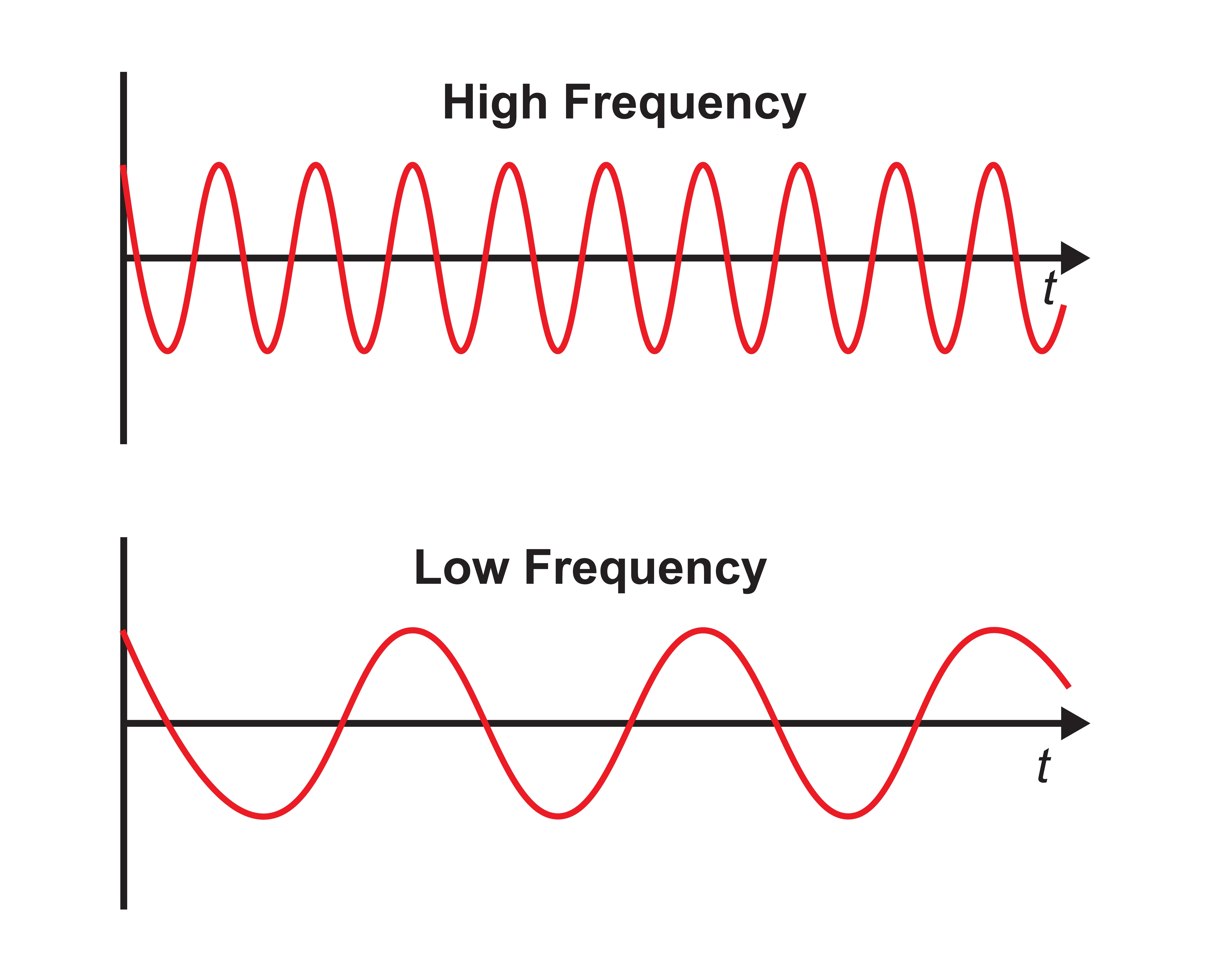
The longer the wavelength, the slower the frequency.
The movie is a 19-channel BioTrace+ /NeXus-32 display of EEG activity from 1-64 Hz activity broken into its component delta, theta, alpha, and beta frequency bands by digital filters © John S. Anderson.
The movie is a 19-channel BioTrace+ /NeXus-32 display of alpha activity © John S. Anderson. Brighter colors represent higher alpha amplitudes. Frequency histograms are displayed for each channel. Notice the runs of high-amplitude alpha waves.
EEG Oscillations
The generation of oscillatory activity, sometimes called spindle behavior, is likely due to the interaction between thalamocortical relay neurons (TCR), reticular nucleus neurons (RE), and interneurons. Diverse neurotransmitters, including acetylcholine and GABA, mediate these interactions.Circuits Contributing to the EEG
Feedforward, thalamocortical, and intra-cortical networks help generate the EEG.Spindling or Bursting Activity
Spindling is a synaptically-generated oscillation in a circuit that necessarily includes reticular nucleus neurons (RE).The movie below is a BioTrace+ /NeXus-32 display of EEG spindling activity © John S. Anderson.
The various spindle frequencies, which have often been interpreted as reflecting different types of oscillations, merely depend on various durations of the hyperpolarizations (negative shifts) in thalamic-cortical relay neurons. Long duration hyperpolarizations, as during ... deeply EEG-synchronized states, are associated with 7 Hz or even lower-frequency spindles, while relatively short hyperpolarizations result in ... higher frequencies (14 Hz) (Steriade, 2005).
The Purpose of Oscillatory Activity
A single neuron can influence multiple postsynaptic targets located between 0.5 and 5 mm away with conduction periods between 1 and 10 ms. This time difference becomes progressively more pronounced when more complex events involve progressively larger assemblies of neurons. It may take hundreds of thousands of neurons, stimulating multiple postsynaptic neurons, for the desired outcome to occur. When these many neurons are involved, it becomes increasingly clear that there is a need for organization and structure to manage this diverse activity.Timing is everything since action potentials arrive from a large number of sources. The nervous system must correctly register arrival times to recognize a face, recall a name, or remember personal history and context.
Hierarchical Processing
Complex events require the systems to operate within a spatial and temporal hierarchy. Each oscillatory cycle is a window of time within which processing can occur. Each cycle has a beginning and an end within which encoded or transferred messages must complete their tasks. Groups of neurons, close or distant, interact most effectively when firing windows are synchronous. The brain does not operate continuously but in discontinuous packets.Multiple Oscillators
"Oscillatory classes in the cerebral cortex show a linear progression of the frequency classes on the log scale. In each class, the frequency ranges ('bandwidth') overlap with those of the neighboring classes, so that frequency coverage is more than four orders of magnitude" (Buzaki, 2006). Graphic adapted from Buzaki by minaanandag at Fiverr.com..jpg)
Frequency Determines Complexity
The wavelength or frequency of the EEG band determines how long the processing window will remain open and, therefore, the size of the neuronal pool involved. Because of the distances involved, longer wavelengths (slower frequencies) allow larger groups of more distant neurons to be assembled and coordinated. Different frequencies organize different types of connections and different levels of computational complexity.Local Versus Global Decision-Making
Short time windows of fast oscillators facilitate local integration, primarily because of the limitations of axon conduction delays. Fast oscillations favor local decisions. Slow oscillators can involve many neurons in large and/or distant brain areas. Slow oscillations favor complex, global decisions.Complexity Versus Frequency
Complex sensory integration and decision-making tasks were associated with 4-7 Hz synchronization. Intermediate tasks such as identifying spoken and written words and pictures increased 13-18 Hz beta activity. Simpler, more localized tasks, such as the visual processing of grid displays, were associated with faster-frequency activity (24-32 Hz) (Sarnthein et al., 1998; Von Stein et al., 1999).Traveling Waves Help Coordinate Widespread Brain Networks
Zhang et al. (2018) proposed that traveling waves between 2 to 15 Hz, moving at 0.25-0.75 meters per second across the cortex, mediate large-scale coordination of brain networks and support connectivity.Summary of EEG Oscillations
When the CNS processes incoming content, separate areas detect features of salient content, including visual, auditory, tactile, kinesthetic, and olfactory information. The CNS shares, integrates, compares immediate with previous content, analyzes, and makes decisions regarding memory and responses. Interacting networks linked by electrical and chemical signals perform this work. We record the electrical potentials generated by this complex and dynamic network activity as the EEG.The movie below of bursting alpha shows the sequential synchronization/desynchronization of groups of neurons. Higher voltage bursts are followed by voltage decreasing toward zero. These voltage fluctuations reflect rhythmic changes in the local field potential. This BioTrace+ /NeXus-32 video © John S. Anderson.
DEFINITION OF ERPs AND SCPs
Sensory evoked potentials are a subset of event-related potentials (ERPs)
Event-related potentials (ERPs) represent the brain's responses to external stimuli, events, or cognitive/motor tasks. ERPs can be detected throughout the cortex. Investigators monitor ERPs by placing electrodes at areas like the midline (Fz, Cz, and Pz). A computer analyzes a subject's EEG responses to the same stimulus or task over many trials to subtract random EEG activity. ERPs always have the same waveform morphology. Their negative and positive peaks occur at regular intervals following the stimulus.Sensory evoked potentials are a subset of ERPs elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). They have a negative peak at 80-90 ms and a positive peak at about 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
Motor ERPs are detected over the primary motor cortex (precentral gyrus) during movement, and their amplitude is proportional to the force and rate of skeletal muscle contraction (Thompson & Thompson, 2016).
Slow cortical potentials modulate the excitability of associated neurons
Slow cortical potentials (SCPs) are gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials, and exclude event-related potentials (ERPS) (Andreassi, 2007).SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms (Steriade, 2005).
The movie is a 19-channel BioTrace+ /NeXus-32 display of SCPs © John S. Anderson. Brighter colors represent higher SCP amplitudes. Negative SCPs drift down, and positive SCPs drift up. Negative SCPs are produced by the depolarization of apical dendrites and increase the probability of neuron firing. Positive SCPs are produced by the hyperpolarization of these dendrites and decrease the likelihood of neuron firing.
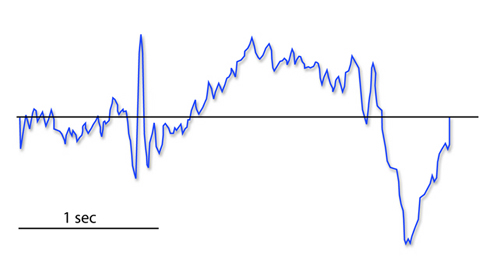
The contingent negative variation (CNV) is a steady, negative shift in
potential (15 µV in young adults) detected at the vertex. This
slow cortical potential may reflect expectancy, motivation, intention to
act, or attention. The CNV appears 200-400 ms after a warning signal
(S1), peaks within 400-900 ms, and sharply declines after a second
stimulus that requires the performance of a response (S2). John Balven adapted the graphic below from Stern, Ray,
and Quigley (2001).
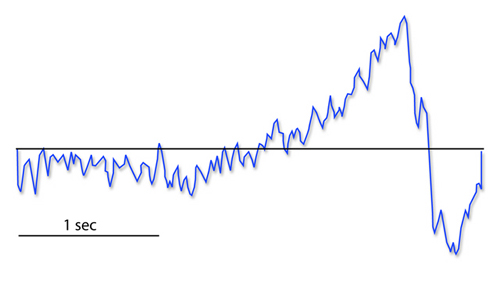
The readiness potential is a slow-rising, negative potential (10-15
µV) detected at the vertex before voluntary and spontaneous
movement. This slow cortical potential precedes voluntary movement by 0.5
to 1 second and peaks when the subject responds. This potential is
separate from the CNV. John Balven adapted the graphic below from Stern, Ray, and Quigley (2001).
Movement-related potentials (MRPs) occur at 1 second as subjects prepare for
unilateral voluntary movements.
MRPs are distributed bilaterally with maximum amplitude at Cz. The
supplementary motor area and primary motor and somatosensory cortices
generate these potentials (Babiloni et al., 2002).
P300 and N400 ERPs are classified as long-latency potentials due to their
extended latencies
following stimulus onset.
The P300 potential is an event-related potential (ERP) with a 300-900-ms
latency.
The largest amplitude positive peaks are located over the parietal lobe. Researchers
elicit the P300 potential by exposing subjects to an odd-ball stimulus, a
meaningful
stimulus different from others in a series (a colored playing card
presented in a series of monochrome cards). The P300
potential may reflect an event’s subjective probability, meaning, and
transmission of information. Research shows this is separate from
the contingent negative variation (CNV) (Stern, Ray, & Quigley, 2001).
Shorter P300 latencies may reflect better allocation of attention, and
researchers have measured longer P300 latencies in ADD than non-ADD
samples. Experimental subjects show longer latencies when lying than when
telling the truth (Farwell & Donchin, 1991; Thompson & Thompson, 2016).
The N400 potential is an
event-related potential (ERP) elicited when we encounter semantic
violations like ending a sentence with a semantically incongruent word
("The handsome prince married the beautiful fish"),
or when the second word of a pair is unrelated to the first (BATTLE/GIRL). Warren and
McIlvane (1998) speculate that the N400 potential is evoked
whenever a general conceptual system that produces category judgments
encounters a mismatch that violates equivalence relations. Halgren and
colleagues (2002) consider it an index of the difficulty of semantic
processing.
A Deep Dive Into SCPs
In 1875, Richard Caton identified what may have been the first evidence of SCPs in an article in the British Medical Journal titled "The Electric Currents of the Brain."He stated, "The cortex's Direct Current baseline waxes negative whenever it is more active. Gradients of 150-200 μV/mm are noted." He later noted, "when any part of the gray matter is in a state of functional activity, its electric current usually exhibits negative variation." Some later researchers suggested that this signaled the discovery of the "steady potential" or the DC potential of the brain. However, others have noted the possibility of equipment-based artifacts in his recordings (Niedermeyer, 1999).
From the late 1800s through the early 1900s, research into brain electrical activity turned toward observations of electrical stimulation and spontaneous electrical activity in animal studies. As technology improved, the ability of researchers to identify EEG rhythms also improved. Hans Berger is famous for his description of alpha-blocking with cognitive activity, made possible partly because of his use of more sensitive equipment (Niedermeyer, 1999).
Slow Cortical Potential Generators
Several neural mechanisms and structures within the brain generate SCPs. The generation of SCPs is primarily cortical, as evidenced by their persistence even after extensive thalamic destruction and corpus callosum transection, indicating that the thalamus is not essential for their genesis (Steriade, Nuñez, & Amzica, 1993).SCPs have been identified in cortical neurons, the thalamus, and glial cells. Cortical neurons in layers II to VI generate slow oscillations when the thalamus is removed or when cortical tissue is studied in vitro (in an artificial environment) or in vivo (within a living organism). Thalamic reticular neurons exhibit similar slow spontaneous oscillations when studied in vitro, and synchronized intracortical oscillations may depend on a corticothalamic network that targets these thalamic neurons.
The source and nature of slow cortical potentials are in dispute. The prevailing theory holds that negative SCPs result from synchronous postsynaptic potentials in the apical dendrites of cortical pyramidal cells. Others hold that SCPs are supported and produced by glial cells within the cortex. It appears that pyramidal neurons may be the source of these potentials and that the glial system is the "sink" in electrical terms (Strehl, 2005, personal communication).
Increased neuronal activity is associated with an increased outflow of potassium ions leading to increased extracellular potassium concentrations. Glial cells depolarize when extracellular potassium concentrations increase, resulting in intracellular and extracellular current flows similar to typical neuronal synaptic transmissions (Speckmann & Elger, 1999). Since glial cells are widely interconnected and have extensive processes, it appears likely that the glial system is responsible for the potential changes that produce SCP values recorded from the scalp in response to neuronal activity.
Despite some discussion regarding the source of SCP activity, it is clear that scalp SCPs represent the cortex's excitability potential. SCP negativity is associated with increased cortical excitability. High cortical negativity has been correlated with a greater likelihood of seizures (Speckmann et al., 1984) and migraines (Siniatchkin et al., 2000) in susceptible individuals.
SCP positivity is associated with increased cortical inhibition. Higher-than-expected positive SCPs have been noted in children with elevated blood lead levels (PbB; Otto & Reiter, 1984). Children diagnosed with ADHD show deficient SCP self-regulation skills compared with controls (Heinrich et al., 2004). SCPs have been used to monitor the depth of anesthesia during surgical procedures (Sebel et al., 1997) because they appear to be excellent indicators of the arousal level.
Cortical Neurons
SCPs are primarily generated by the synchronized activity of large populations of cortical neurons. The slow shifts in membrane potential are thought to reflect changes in the overall excitability of cortical networks (Birbaumer et al., 1990). Neuron graphic © SciePro/Shutterstock.com.
Thalamocortical Interactions
Interactions between the thalamus and cortex also play a significant role in generating SCPs. Through its relay and integrative functions, the thalamus modulates cortical excitability and contributes to the slow potential changes observed in SCPs (Lopes da Silva, 1991). Thalamocortical graphic © Netter.
Glial Cells
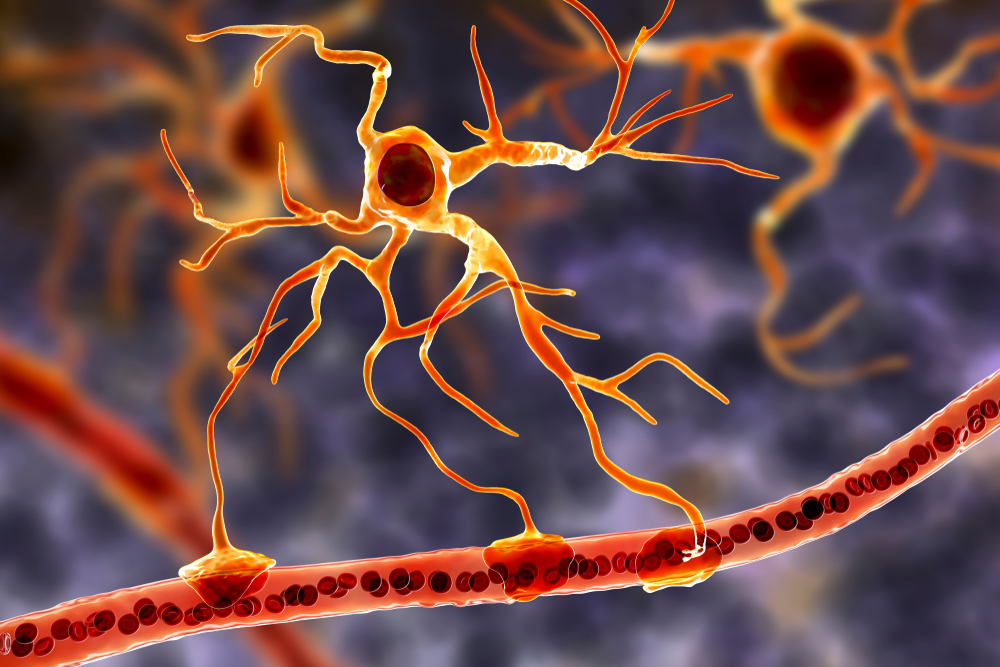
Emerging evidence suggests that glial cells, particularly astrocytes, may influence SCPs by modulating the extracellular environment and supporting neuronal function (Amzica & Steriade, 2002).Glial cells generate slow SCPs when they burn sugar, producing negatively charged bicarbonate ions. Unlike EEG rhythms like delta, SCPs do not summate dendritic potentials. SCPs are associated with glial cells and gap junctions. Glial cells chemically communicate among themselves and with neurons. The slow oscillations of glial cells may influence the timing of neuronal firing through their control of potassium ion outflow (Steriade, 2005). Astrocyte graphic © Kateryna Kon/Shutterstock.com.
"The concept of a unified corticothalamic network that generates diverse types of brain rhythms grouped by the cortical slow oscillation is supported by EEG studies in humans" (Mölle et al., 2002).
The Meaning of SCP EEG Activity
SCPs indicate shifts in cortical excitability and are associated with various functional states of the brain. Surface-negative SCPs reflect synchronized depolarization of neuronal assemblies, indicating increased cortical activity. Surface-positive SCPs correspond to decreased cortical excitation, often involving inhibitory processes (Hinterberger et al., 2003).The negative SCPs detected at the scalp during neuronal depolarization may seem counterintuitive at first.
When neurons are activated, their cell bodies become more positive internally due to the influx of positive ions. This leaves the immediate extracellular space around the neuron more negative. The negative charge in the extracellular space is conducted through brain tissue, cerebrospinal fluid, skull, and scalp. EEG electrodes on the scalp detect this conducted negative potential, resulting in a negative deflection on the EEG trace.
This phenomenon is often referred to as paradoxical negativity in EEG literature (Birbaumer et al., 1990). It's important to note that what we record on the scalp is not a direct measure of neuronal membrane potential, but rather the result of complex electrical field propagation through various tissues (Elbert et al., 1980). Paradoxical positivity occurs when neurons are hyperpolarized.
This relationship is crucial in understanding the neurophysiological mechanisms underlying SCPs (Birbaumer et al., 1990). This paradoxical negativity graphic (Brienza & Mecarelli, 2019) is available under the license CC BY 3.0.
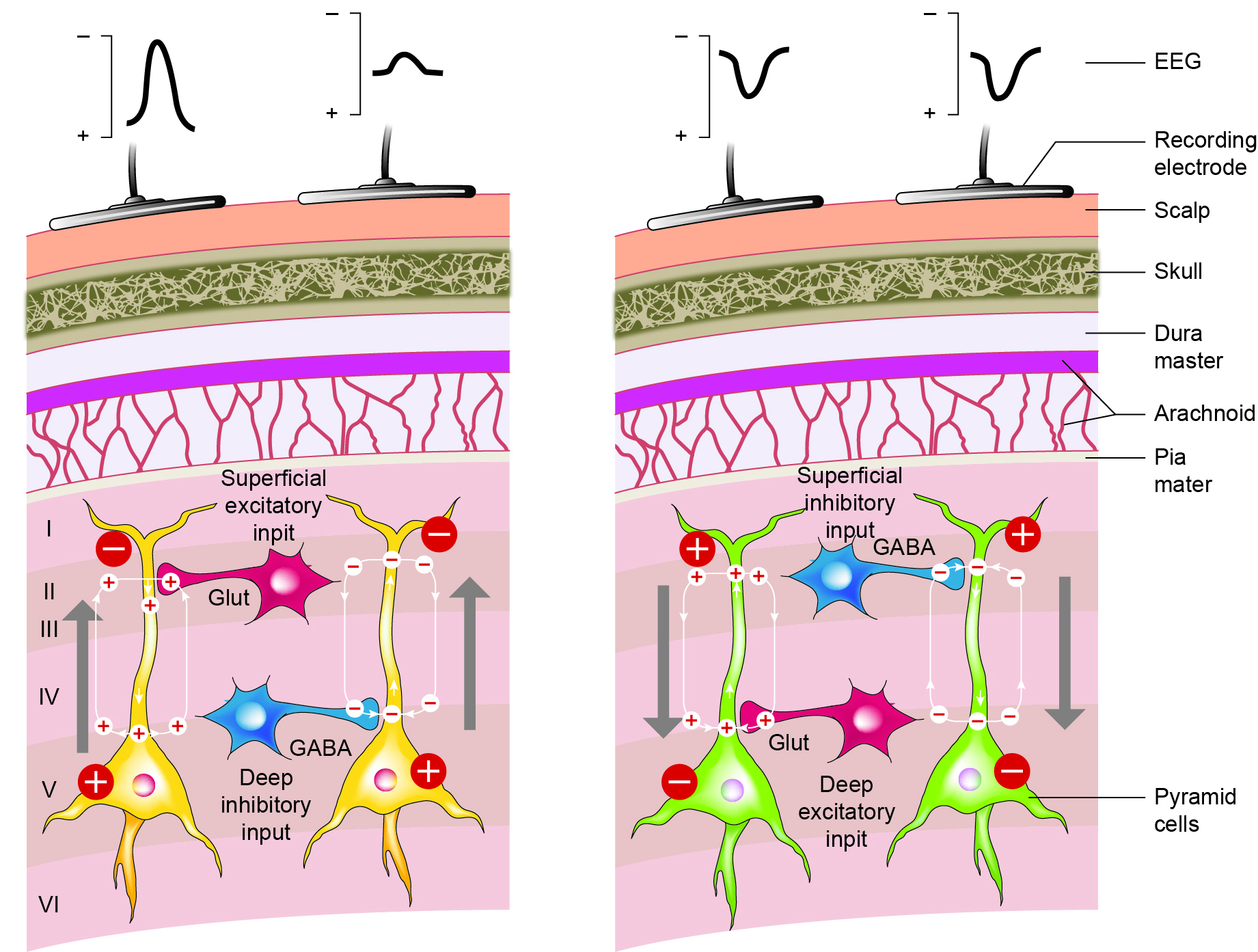
Caption: Schematic drawing of the scalp EEG registering negative (A) and positive (B) deflections elicited from summated EPSPs and IPSPs derived from pooled pyramidal cells. Cells releasing glutamate and GABA provide excitatory and inhibitory superficial and deep synaptic connections, resulting in an electrophysiological sink or source. EEG = electroencephalography; EPSPs = excitatory postsynaptic potentials; GABA = gamma-aminobutyric acid; IPSPs = inhibitory postsynaptic potentials. Figure courtesy of Anteneh Feyissa M.D. and Mayo Clinic.
Caton (1875) observed that the cortex's direct current baseline becomes negative whenever it is more active. The voltage gradients range from 150-200 μV. Underlying "tone" or valence factors determine the firing characteristics of neurons within a network. When SCPs are more positive, cortical neurons fire less due to hyperpolarization. When SCPs are more negative, firing increases due to depolarization.
SCPs participate in cognitive processes such as attention, preparation, and intention. Negative SCP shifts are often linked to increased cortical excitability and readiness to respond, while positive shifts are associated with decreased excitability and relaxation (Birbaumer et al., 1990).
Some types of SCPs are event-related. These include the Bereitschaftspotenial (BP or so-called readiness potential), contingent negative variation (CNV), and stimulus-preceding negativity (SPN). These represent slow negative waves related to anticipating a stimulus or preparing for a movement (Brunia et al., 2012). The BP occurs before the execution of a self-paced movement. CNV occurs when a preparatory stimulus foretells the imminent presentation of a stimulus that requires a response. SPN occurs after a movement when waiting for a stimulus that will provide feedback about the accuracy of the movement.
The slow rhythm of SCPs is often combined with delta oscillations, and these rhythms are phase-locked, suggesting a close interaction between different frequency bands in the brain's electrical activity (Steriade, Nuñez, & Amzica, 1993).
SCPs play a crucial role in motor preparation and execution. The readiness potential (Bereitschaftspotential), a type of SCP, precedes voluntary movements and reflects the planning and initiation of motor actions.
SCPs are also associated with emotional and motivational states. Negative SCPs can indicate increased arousal and emotional engagement, whereas positive SCPs can reflect relaxation and disengagement (Hinterberger et al., 2004).
Psychological and Medical Disorders
SCPs have been extensively studied in various psychological and medical conditions.Attention-Deficit/Hyperactivity Disorder (ADHD)
Individuals with ADHD often exhibit abnormal SCP patterns, with a reduced ability to generate negative SCP shifts. Neurofeedback training targeting SCPs has shown promise in improving attention and reducing hyperactivity in these individuals (Heinrich et al., 2004). Epilepsy SCP neurofeedback has been explored as a treatment for epilepsy. Training individuals to increase positive SCP shifts can reduce cortical excitability and decrease the frequency of seizures (Rockstroh et al., 1993). Parkinson's Disease Studies have shown that patients with Parkinson's disease (PD) exhibit abnormal SCP patterns, particularly during motor tasks (Brittain & Brown, 2014). These abnormalities include altered amplitude and timing of SCPs, associated with the impaired initiation and execution of voluntary movements seen in PD.Research has shown that during non-rapid eye movement (NREM) sleep, cortico-basal slow wave delta activity increases, while beta activity decreases. Deep brain stimulation (DBS) further modulates this altered activity, enhancing cortical delta activity and reducing alpha and low beta power. These findings suggest that SCPs and their interaction with other brain rhythms are significantly altered in PD, contributing to sleep dysfunction and spontaneous awakenings (Anjum et al., 2023).
SCPs are used to monitor the effects of therapeutic interventions, such as deep brain stimulation (DBS), on cortical function in PD patients.
Slow cortical potential (SCP) neurofeedback has shown potential efficacy as a complementary treatment for Parkinson's disease (PD). SCP neurofeedback aims to train individuals to self-regulate their brain activity, particularly focusing on slow cortical potentials associated with motor control.
Research suggests that SCP neurofeedback can improve motor function in PD patients (Kober & Wood, 2014). Some studies have reported that participants undergoing SCP neurofeedback training demonstrated better control over motor symptoms, such as tremors and rigidity, and experienced enhanced overall motor performance. Additionally, SCP neurofeedback has been associated with improvements in non-motor symptoms, including mood and cognitive function, which are often affected in PD.
While promising, the current evidence is based on a limited number of studies with small sample sizes. More extensive and rigorous clinical trials are needed to establish SCP neurofeedback's long-term efficacy and generalizability for PD.
Depression
SCP abnormalities are observed in depression, with patients often showing reduced amplitude of SCP shifts. Neurofeedback interventions aiming to normalize SCP patterns have shown potential in alleviating depressive symptoms (Strehl et al., 2017).Sleep
SCPs play a role in sleep regulation and quality.Sleep Onset and Maintenance
SCPs are involved in the transition from wakefulness to sleep. Positive SCP shifts are associated with sleep initiation and maintaining sleep stability (Sterman, 1996).Sleep Disorders
SCPs are closely linked to sleep rhythms, particularly during NREM sleep (Anjum et al., 2023). The slow oscillations of SCPs facilitate the synchronization of neuronal activity, which is essential for the restorative functions of sleep. In PD, the suppression of slow waves and the increase in subcortical beta activity before spontaneous awakenings highlight the critical role of SCPs in maintaining sleep stability and quality.Abnormal SCP patterns have been linked to sleep disorders such as insomnia. Neurofeedback training targeting SCPs can improve sleep onset latency and enhance overall sleep quality (Hoedlmoser et al., 2008).
Performance
Enhancing SCP activity through neurofeedback training has improved performance in various cognitive and motor tasks.Cognitive Performance
SCP training can enhance cognitive performance, including attention, memory, and executive function. This is likely due to the improved cortical excitability and better regulation of cognitive states (Vernon et al., 2003).Motor Performance
SCP neurofeedback has been shown to improve motor performance, particularly in tasks requiring precise timing and coordination. This enhancement is attributed to the role of SCPs in motor preparation and execution (Gruzelier et al., 2014).SCP Research
Research into the electrical characteristics of the human brain became primarily focused on phasic phenomena from AC-coupled recordings. This trend continues today with the current practice of EEG biofeedback or neurofeedback, focusing primarily on training AC frequencies, generally in the range of 1 to 60 Hz.The study of SCPs continued in physiology and animal research. Only recently has there been increased interest in observing SCPl values in the human EEG and correlating them with cognitive activity, sensory processing, and motor activity. SCPs are distinguished from short-latency, event-related potentials (ERP) up to 500 ms. SCPs reflect cortical processes that require more than one second to complete and are associated with more global, task-related activities. Such changes occur in task-specific areas of the cortex and can be displayed using topographic maps. Areas of activation show surface negative potential changes (Altenmuller & Gerloff, 1999).
Operant conditioning of SCP changes is an even more recent study area. One reason for increased interest in SCP training is the excellent work done by Birbaumer and colleagues (1999) at the University of Tubingen in Germany, demonstrating that SCPs can be operantly conditioned with positive outcomes for a variety of disorders. The recent availability of DC-coupled amplifiers for EEG recording has also contributed to this interest (Altenmuller & Gerloff, 1999).
According to Niedermeyer (1999), "DC" can mean several things. DC means direct current, which is a current without oscillations. From an electrophysiological perspective, "DC shifts" are ultra-slow potentials below the typical EEG in the oscillation frequency and are generally around 0.1-0.2 cycles per second. However, they may extend up to 1 cycle per second. So SCPs are not true direct current, though their oscillations are so slow that they are "DC-like" phenomena.
DC also refers to "direct coupling" (Niedermeyer, 1999) and describes a type of amplifier that does not use capacitors between the amplification stages and uses an infinite time constant to provide for optimal DC recording. Until recently, this has been quite difficult to achieve for EEG recording. Most conventional EEG amplifiers use capacitors in the input stage, which reject DC voltages and create a finite time constant that interferes with access to DC phenomena.
An approximation of DC information can be obtained from an alternating current amplifier by using a rectifier or extending the time constant to approximately 10 seconds (Kotchoubey et al., 1999). A thorough discussion of amplifier characteristics is beyond the scope of this article. Several excellent chapters relating to this subject can be found in Niedermeyer and Lopes da Silva (1999).
Recent studies have used SCPs to evaluate various task-oriented responses. Birbaumer and colleagues have trained SCPs to reduce seizures (Daum et al., 1993; Kotchoubey et al., 1997, 1999, 2001, 2002), and other groups have applied SCP feedback training to improve ADHD (Heinrich et al., 2004; Strehl, 2004, personal communication) and schizophrenia (Schneider et al., 1992).
SCP feedback training appears to be an approach that targets general characteristics of arousal using a single measure, compared to other types of EEG training that often reward increases and/or decreases in certain combinations of frequencies to accomplish changes in arousal. SCP feedback may provide a less complex approach to training neuronal activity in the clinical setting, providing greater accessibility via clinician-supervised home training devices.
Most research to date has been conducted using the Cz electrode site. However, at least one investigation involved training left hemisphere language sites. This approach demonstrated improved word processing results following the negativity training condition and diminished performance following the positivity condition (Pulvermuller, 2000). Studying the effects of SCP training at other electrode sites would be interesting.
Some efforts have been to identify SCP values using multiple electrodes in a quantitative EEG assessment paradigm. Basile and colleagues (2004) used four 32-channel DC-coupled amplifiers to identify differences in SCP responses in schizophrenic patients compared with normal controls. They found significant variations in response patterns, with normal controls showing simply-organized positivity and negativity patterns, while schizophrenic patients showed much more complex, fragmented patterns of activation and inhibition.
There are only a few clinically available DC-coupled amplifiers capable of accurately monitoring SCP activity. An Internet search yielded several devices aimed at the research institution market with correspondingly high prices and a couple of other devices with prices within a clinical practice’s reach. A new 32-channel DC-coupled data acquisition device for quantitative EEG assessments has also recently been released.
One potential attraction of using a DC amplifier is the capability of monitoring and/or training both SCPs and typical EEG frequencies. This is because DC amplifiers are optimized for SCP and have the capacity to record faster frequencies. This is particularly true for amplifiers with better analog-to-digital (AD) conversion characteristics (bit size, not sampling rate) because this allows them to record AC potentials without exceeding amplifier capabilities, which can be a problem in an amplifier without capacitors at the input stages.
Higher analog-to-digital (A/D) conversion values (more bits of data per sample) allow newer DC amplifiers to process EEG at a much lower voltage while retaining a high degree of resolution for signals that are often in the millivolt range (compared to microvolt values for most EEG signals).
The training of SCP shifts is a fairly new endeavor. Much remains to be learned about the effects of training both the positivity and negativity conditions at various electrode sites for individuals with various presenting concerns and specific neurophysiological characteristics.
This author's recent, brief clinical experiences suggest that training SCP using new, more accurate amplifiers may result in more pronounced changes occurring more quickly. This occurred on several occasions, even when using previously well-tested protocols alternating 8- to 10-second trials of both the positivity and negativity conditions. Thus, it will be important to develop protocols with more specificity and flexibility to meet the needs of non-homogeneous client populations that also consider changes in equipment and software characteristics that may affect the rate of skill acquisition and subsequent outcomes.
Training SCPs
Various approaches to training slow cortical shifts have been applied. Early research (Birbaumer, 1990; Birbaumer, 1999) showed a correlation between cortical negativity and reaction time, signal detection, and short-term memory. This was identified primarily through evoked and event-related potential (EP, ERP) research, which led to a focus on the event-related potential in developing training paradigms for research and clinical interventions. Protocols involved presenting clients/participants with sequences of 8-second trials, training both positive and negative shifts in the cortical gradient by providing visual and/or auditory feedback showing such shifts in real-time. When the goal was greater cortical positivity, more positive shift trials were provided, and the opposite was true when cortical negativity was desired. Additional transfer trials were included that asked the individual to produce a shift but did not provide visual or auditory feedback to test the level of skill acquisition. This approach was the primary paradigm during the early years of SCP research (Strehl, 2009).
Other clinicians and researchers, including Susan and Siegfried Othmer and Mark Smith, addressed these slow gradient shifts with training called variously Infra-Low Frequency Neurofeedback (Othmer, 2020) and Infraslow Neurofeedback (Smith, 2013).
Post-traumatic stress, anxiety, and other characteristics of excessive cortical activation and excitability have been addressed by training to increase overall cortical positivity, using a 4-channel/location approach that rewards a gradual shift in the cortical gradient by providing proportional audio feedback reflecting directional shifts in the gradient. This has resulted in several client self-reports of an altered state experience characterized by a marked decrease in cognitive activity while retaining awareness. The positive shift in cortical gradient appears to correspond with a reduction in conscious thought while allowing a sense of self-awareness to remain (multiple clinical observations shared with John Anderson).
Conclusion
Slow cortical potentials (SCPs) are characterized by low-frequency oscillations in the EEG, typically below 1 Hz, with significant depolarizing-hyperpolarizing components. These potentials, occurring at approximately 0.3 Hz, are crucial indicators of cortical excitability and are associated with various cognitive and motor processes. Generated by cortical neurons, thalamocortical interactions, and glial cells, SCPs reflect shifts in cortical excitability linked to attention, motor preparation, and emotional states. Negative SCP shifts indicate increased excitability and readiness to respond, while positive shifts are associated with relaxation and decreased excitability.
SCPs play roles in psychological and medical disorders like ADHD, epilepsy, and depression, and are vital in sleep regulation and performance enhancement. SCP neurofeedback shows promise in improving symptoms and cognitive functions in various conditions.
The authors would like to thank Ute Strehl of the University of Tubingen in Germany, David Seiver of Mind Alive, LTD in Canada, and Erwin Hartsuiker of Mind Media BV in the Netherlands for technical assistance in preparing this article.
NEUROPLASTICITY (LTD AND LTP)
Neuroplasticity, the remodeling of neurons and neural networks with experience, is responsible for learning and memory. Memory storage involves remodeling neurons in terms of synaptic transmission, interneuron modulation, formation of new synapses, and rewiring of neural pathways (Bear, Connors, & Paradiso, 2020). Animal studies have shown that operant conditioning can induce astrogliogenesis (creation of new astrocytes) and neurogenesis (creation of new neurons) in structures like the medial prefrontal cortex and hippocampus (Rapanelli, Frick, & Zanutto, 2011).
The graphic by Rebeca Cuesta is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
Neuroplasticity appears to involve a simple rule: when some synapses strengthen, adjacent synapses weaken to prevent overload due to increased input. A protein called Arc is crucial to this process (El-Boustani et al., 2018).
Neurofeedback, which involves the operant conditioning of CNS electrical activity, would be impossible without neuroplasticity. In neurofeedback, clients may learn to change the activity of local, regional, and global cortical resonant loops and the connectivity between brain regions (Collura, 2014; Thompson & Thompson, 2016).
To learn more about neuroplasticity, view the Khan Academy video Neuroplasticity.
Long-Term Depression and Long-term Potentiation
Two of the diverse processes involved in neuroplasticity are long-term depression and long-term potentiation.In long-term depression (LTD), synaptic transmission that coincides with slight depolarization of the postsynaptic neuron weakens synapses due to pre- and postsynaptic changes. Relatively low-frequency stimulation of afferent neurons reduces the magnitude of their response to future stimulation.
In long-term potentiation (LTP), synaptic transmission that coincides with strong depolarization of the postsynaptic neuron strengthens synapses due to pre- and postsynaptic changes. Strong stimulation of afferent neurons results in a stable and persistent (weeks or more) increase in synaptic effectiveness. LTP involves diverse changes, including creating new synapses, enhancing previous synapses, and building new dendritic branches and spines (Breedlove & Watson, 2023).
To learn more, watch the Khan Academy video Long Term Potentiation and Synaptic Plasticity.
Glossary
amplitude: the height of a wave, indicating the strength or intensity of a signal.
astrocytes: a type of glial cell in the brain supporting neuronal function and modulating the extracellular environment.
basal ganglia: a group of nuclei in the brain that controls voluntary motor movements, procedural learning, and routine behaviors.
cognitive performance: the efficiency of cognitive functions such as attention, memory, and executive function.
contingent negative variation (CNV): a slow cortical potential shift between a warning stimulus and an imperative stimulus requiring a motor response. It reflects the anticipation and preparation for a motor act and involves brain regions such as the prefrontal cortex and supplementary motor area. CNV is used to study cognitive processes like attention, expectation, and motor preparation.
cortical negativity: a state where the cortical surface exhibits a negative electrical potential.
cortical neurons: nerve cells in the cortex responsible for generating and transmitting electrical impulses.
cortical positivity: a state where the cortical surface exhibits a positive electrical potential.
deep brain stimulation (DBS): a neurosurgical procedure involving the implantation of electrodes in specific brain areas to modulate neuronal activity.
depolarization: a reduction in membrane potential, making the inside of a cell less negative relative to the outside.
dipole: the electrical field generated between the sink (where current enters the neuron ) and the source (place at the other end of the neuron where current leaves), which may be located anywhere along the dendrite.
event-related potential (ERP):a measured brain response that is the direct result of a specific sensory, cognitive, or motor event. ERPs are measured using electroencephalography (EEG), which records the electrical activity of the brain via electrodes placed on the scalp. ERPs are typically extracted by averaging the EEG activity time-locked to the onset of a stimulus or event, thus isolating the brain's response to that particular event from background noise.
evoked potential: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). An evoked potential has a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
excitability: the ability of neurons to respond to stimuli and generate action potentials.
excitatory postsynaptic potential (EPSP): a brief positive shift in a postsynaptic neuron's potential.
glial cells: non-neuronal cells in the central nervous system that process information and support and protect neurons.
hyperpolarization: an increase in membrane potential, making the inside of a cell more negative relative to the outside.
local field potential (LFP): the aggregate effect of the firing of the interconnected pyramidal neurons within the cortical columns plus additional mechanisms like glial cell modulation of the cortical electrical gradient.
local synchrony: synchrony that occurs when high-amplitude EEG signals are produced by the coordinated firing of cortical neurons.
localized slow waves: waves that may indicate a transient ischemic attack (TIA) or stroke, migraine, mild head injury, or tumors above the tentorium. Deep lesions result in bilateral or unilateral delta.
locus coeruleus: the noradrenergic branch of the ascending reticular activating system, which is responsible for vigilance. Subnormal norepinephrine transmission may contribute to ADHD.
long-latency potentials: potentials that have extended latencies following stimulus onset, for example, P300 and N400 ERPs.
long-term depression (LTD): a persistent decrease in synaptic strength following low-frequency stimulation.
long-term potentiation (LTP): a persistent increase in synaptic strength following high-frequency stimulation.
motor control: the process by which humans and animals use their brains and muscles to perform movements.
movement-related potentials (MRPs): slow cortical potentials that occur at 1 second as subjects prepare for unilateral voluntary movements. MRPs are distributed bilaterally with maximum amplitude at Cz. The supplementary motor area and primary motor and somatosensory cortices primarily generate these potentials.
N1-P2: a sensory event-related potential in the auditory cortex of the temporal cortex that reveals whether an uncommunicative person can hear a stimulus.
N400 potential: an event-related potential (ERP) elicited when we encounter semantic violations like ending a sentence with a semantically incongruent word ("The handsome prince married the beautiful fish"), or when the second word of a pair is unrelated to the first (BATTLE/GIRL).
neuroplasticity: the brain's ability to adapt and reorganize its structure and function in response to internal and external experiences. It encompasses various processes, including synaptic plasticity (changes in the strength or efficacy of connections between neurons), neurogenesis (the generation of new neurons), and changes in neural circuits and networks.
odd-ball stimulus: a meaningful stimulus that is different from others in a series used to elicit the P300 potential. For example, a colored playing card in a series of monochrome cards.
orienting response: Pavlov’s "What is it?" reaction to stimuli like the sound of a vase crashing that includes (1) increased sensory sensitivity, (2) head (and ear) turning toward the stimulus, (3) increased muscle tone (reduced movement), (4) EEG desynchrony, (5) peripheral constriction and cephalic vasodilation, (6) a rise in skin conductance, (7) heart rate slowing, and (8) slower, deeper breathing.
P300 potential: an event-related potential (ERP) with a 300-900-ms latency. The largest amplitude positive peaks are located over the parietal lobe. The P300 potential may reflect an event’s subjective probability, meaning, and transmission of information.
paradoxical negativity: in the context of SCPs, it refers to surface-negative EEG shifts when neurons are depolarized due to volume conduction of negative potentials from the extracellular space to the scalp.
paradoxical positivity: in the context of SCPs, it refers to surface-positive EEG shifts when neurons are hyperpolarized due to volume conduction of positive potentials from the extracellular space to the scalp.
Parkinson's disease (PD): a progressive neurodegenerative disorder characterized primarily by motor symptoms such as tremor, rigidity, bradykinesia (slowness of movement), and postural instability.
precision: the number of voltage gradations or steps.
readiness potential: a slow cortical potential that precedes voluntary movements, reflecting the planning and initiation of motor actions.
sampling rate: the number of measurements per second (Hz).
scalp EEG: the non-invasive recording of the electrical activity of the brain using electrodes placed on the scalp. It measures the collective electrical activity generated by large groups of neurons firing synchronously in the brain. Scalp EEG provides valuable information about brain function and can be used to study various neurological conditions, cognitive processes, and states of consciousness.
sensory event-related potentials (ERPs): event-related potentials evoked by external sensory stimuli (auditory, olfactory, somatosensory, and visual). These evoked potentials or exogenous ERPs have a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. These changes in brain activity in response to specific stimuli. ERPs can be detected throughout the cortex. Investigators monitor ERPs by placing electrodes at locations like the midline (Fz, Cz, and Pz). A computer analyzes a subject's EEG responses to the same stimulus or task over many trials to subtract random EEG activity. ERPs always have the same waveform morphology. Their negative and positive peaks occur at regular intervals following the stimulus.
sink: a site where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, represented by -ve.
slow cortical potentials (SCPs): gradual voltage shifts in the EEG characterized by low-frequency oscillations, typically below 1 Hz.
source: the place at the end of the neuron opposite of the sink where current leaves, represented by +ve. The extracellular area surrounding the source becomes electrically positive.
stimulus-preceding negativity (SPN): a slow negative potential shift observed before a stimulus that signals important or relevant information, such as feedback or a reward. SPN reflects anticipatory attention and affective processes involving regions like the insula and orbitofrontal cortex. SPN is associated with emotional and cognitive anticipation.
surface-negative: a negative SCP shift typically associated with increased cortical excitability and response readiness.
surface-positive: a positive SCP shift typically associated with decreased cortical excitability and relaxation.
synchronization: the coordination of neuronal activity across different regions of the brain. thalamocortical interactions: interactions between the thalamus and cortex that play a significant role in generating SCPs.
+ve: the source is the place at the other end of the neuron where current leaves.
-ve: a sink is where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink.
voltage: the electrical potential difference between two points.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which teaches the neuroscience underlying neurofeedback, and qEEG100, which provides extensive multiple-choice testing over the International QEEG Board's Blueprint and Reading List.

Assignment
Now that you have completed this unit, how would you explain the relationship between local field potentials and the EEG? How does anatomy explain why the EEG is comprised of EPSPs and IPSPs instead of action potentials?
References
Advokat, C. D., & Comaty, J. E., & Julien, R. M. (2019). Julien's primer of drug action (15th ed.). Worth Publishers.
Aloisi, F. (2001). Immune function of microglia. Glia, 36, 165–179. https://doi.org/10.1002/glia.1106
Altenmuller, E. O., & Gerloff, C. (1999). Psychophysiology and the EEG. In E. Niedermeyer & F. Lopes da Silva (Eds.), Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
Amzica, F., & Steriade, M. (2002). The functional significance of K-complexes. Sleep Medicine Reviews, 6(2), 139–149. https://doi.org/10.1053/smrv.2001.0181
Andreassi, J. L. (2007). Psychophysiology: Human behavior and physiological response (5th ed.). Lawrence Erlbaum and Associates, Inc.
Anjum, M., Smyth, C., Dijk, D., Starr, P., Denison, T., & Little, S. (2023). Multi-night cortico-basal recordings reveal mechanisms of NREM slow wave suppression and spontaneous awakenings at high-temporal resolution in Parkinson’s disease. Research Square. https://doi.org/10.21203/rs.3.rs-3484527/v1
Arnsten, A. F. (2006). Fundamentals of Attention-Deficit/Hyperactivity Disorder: Circuits and pathways. Journal of Clinical Psychiatry, 67 (Suppl. 8), 7-12.
Babiloni, C., Babiloni, F., Carducci, F., Cincotti, F., Del Percio, C., Hallett, M., Moretti, D. V., Romani, G. L., & Rossini, P. M. High resolution EEG of sensorimotor brain functions: Mapping ERPs or mu ERD? In R. C. Reisin, M. R. Nuwer, M. Hallett, & C. Medina (Eds.). Advances in Clinical Neurophysiology (Supplements to Clinical Neurophysiology Vol. 54). Elsevier Science B. V.
Basile, L. F. H., Yacubian, J., Ferreira, B. L. C., Valim, A. C., & Gattaz, W. F. (2004). Topographic abnormality of slow cortical potentials in schizophrenia. Brazilian Journal of Medical and Biological Research, 37(1), 97-109. https://doi.org/10.1590/s0100-879x2004000100014
Bear, M. F., Connors, B. W., & Paradiso, M. A. (2020). Neuroscience: Exploring the brain (4th ed.). Jones & Bartlett Learning.
Birbaumer, N. (1999). Slow cortical potentials: Plasticity, operant control, and behavioral effects. The Neuroscientist, 5, 74–78. https://doi.org/10.1177/1073858499005002
Birbaumer, N., Elbert, T., Canavan, A. G., & Rockstroh, B. (1990). Slow potentials of the cerebral cortex and behavior. Physiological Reviews, 70(1), 1–41. https://doi.org/10.1152/physrev.1990.70.1.1
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Brienza, M., & Mecarelli, O. (2019). Neurophysiological basis of EEG. In O. Mecarelli (Ed.), Clinical electroencephalography (pp. 25-45). Springer. https://doi.org/10.1007/978-3-030-04573-9_2
Brunia, C. H. M., van Boxtel, G. J. M., & Böcker, B. E. (2012). Negative slow waves as indices of anticipation: The Bereitschaftspotential, the Contingent Negative Variation, and the Stimulus-Preceding Negativity. In E. S. Kappenman & S. J. Luck (eds.), The Oxford handbook of Event-Related Potential components (pp. 190-208). Oxford Academic. https://doi.org/10.1093/oxfordhb/9780195374148.013.0108
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature, 10, 186-198. https://doi.org/10.1038/nrn2575
Cameron, H. A., & Dayer, A. G. (2008). New interneurons in the adult neocortex: Small, sparse, but significant? Biol Psychiatry, 63(7), 650-655. https://dx.doi.org/10.1016%2Fj.biopsych.2007.09.023
Carlson, N. R., & Birkett, M. A. (2021). Physiology of behavior (13th ed.). Pearson.
Caton, R. (1875). The electric currents of the brain. British Medical Journal, 2, 278.
Chan, C. Y., Ke, D. S., & Chen, J. Y. (2009). Essential fatty acids and human brain. Acta Neurol Taiwan, 18(4), 231-241. PMID: 20329590
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Costanzo, R. M. (1991). Regeneration of olfactory receptor cells. CIBA Found Symp, 160, 233-242. https://doi.org/10.1002/9780470514122.ch12
Creuzfeldt, O. D. (1995). Cortex cerebri. Oxford University Press.
Damasio, A. (2010). Self comes to mind. Pantheon Books.
Daum, I., Rockstroh, B., Birbaumer, N., Elbert. T., Canavan, A., & Lutzenberger W. (1993). Behavioural treatment of slow cortical potentials in intractable epilepsy: Neuropsychological predictors of outcome. J Neurol Neurosurg Psychiatry, 56(1) 94-97. https://doi.org/10.1136/jnnp.56.1.94
deCharms, R. C., Fumiko, M., Glover, G. H., Ludlow, D., Pauly, J. M., Soneji, D., Gabrieli, J. D. E., & Mackey, S. C. (2005). Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences, 102(51), 18626-18631. https://doi.org/10.1073/pnas.0505210102
DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci, 13(7), 281-285. https://doi.org/10.1016/0166-2236(90)90110-v
Demos, J. N. (2019). Getting started with neurofeedback. (2nd ed.). W. W. Norton & Company.
Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302, 290-292. https://doi.org/10.1126/science.1089134
Elbert, T., Rockstroh, B., Lutzenberger, W., & Birbaumer, N. (1980). Biofeedback of slow cortical potentials. I. Electroencephalography and Clinical Neurophysiology, 48(3), 293-301. https://doi.org/10.1016/0013-4694(80)90265-5
El-Boustani, S., Ip, J., Breton-Provencher, V., Knott, G., Okuno, H., Bito, H., & Sur, M. (2018). Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science, 360(6395), 1349-1354. https://doi.org/10.1126/science.aao0862
Enticott, P. G., Kennedy, H. A., Rinehart, N. J., Tonge, B. J., Bradshaw, J. L., Taffe, J. R., Daskalakis, Z. J., & Fitzgerald, P. B. (2012). Mirror neuron activity associated with social Impairments but not age in Autism Spectrum Disorder. Biol Psychiatry, 71(5), 427-433. https://doi.org/10.1016/j.biopsych.2011.09.001
Evans, J. R., & Abarbanel, A. (1999). Introduction to quantitative EEG and neurofeedback. San Diego: Academic Press.
Farwell, L. A., & Donchin, E. (1991). The truth will out: Interrogative polygraphy (“lie detection”) with event-related brain potentials. Psychophysiology, 28, 531–547. https://doi.org/10.1111/j.1469-8986.1991.tb01990.x
Fox, S. I., & Rompolski, K. (2022). Human physiology (16th ed.). McGraw-Hill.
Garrett, B. (2003). Brain and behavior. Thompson/Wadsworth.
Hansson, E., & Ronnback, L. (2003.) Glial neuronal signaling in the central nervous system. FASEB J, 17, 341-348. https://doi.org/10.1096/fj.02-0429rev
Heinrich, H., Gevensleben, H., Freisleder, F. J., Moll, G. H., & Rothenberger, A. (2004). Training of slow cortical potentials in attention-deficit/hyperactivity disorder: Evidence for positive behavioral and neurophysiologic effects. Biological Psychiatry, 55(7), 772–775. https://doi.org/10.1016/j.biopsych.2003.11.013
Hinterberger, T., Veit, R., Wilhelm, B., Weiskopf, N., Vatine, J. J., & Birbaumer, N. (2005). Neuronal mechanisms underlying control of a brain-computer interface. The European Journal of Neuroscience, 21(11), 3169–3181. https://doi.org/10.1111/j.1460-9568.2005.04092.x
Hoedlmoser, K., Pecherstorfer, T., Gruber, G., Anderer, P., Doppelmayr, M., Klimesch, W., & Schabus, M. (2008). Instrumental conditioning of human sensorimotor rhythm (12-15 Hz) and its impact on sleep as well as declarative learning. Sleep, 31(10), 1401–1408.
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Kalat, J. W. (2019). Biological psychology (13th ed.). Cengage Learning.
Kalia, L. V., & Lang, A. E. (2015). Parkinson's disease. Lancet (London, England), 386(9996), 896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Kennerley, S. W., Behrens, T. E., & Wallis, J. D. (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci, 14(12), 1581-1589. doi:10.1038/nn.2961
Kitamura, T., Saitoh, Y., Takashima, N., Murayama, A., Niibori, A., Ageta, H., . . . Inokuchi, K. (2009). Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell, 139(4), 814-827. https://doi.org/10.1016/j.cell.2009.10.020
Klein, S. B., & Thorne, B. M. (2007). Biological psychology. New York: Worth Publishers.
Kotchoubey, B., Blankenhorn, V., Fröscher, W., Strehl, U. & Birbaumer, N. (1997). Stability of cortical self-regulation in epilepsy patients. Neuroreport, 27(8)1867-1870. https://doi.org/10.1097/00001756-199705260-00015
Kotchoubey, B., Busch, S., Strehl, U. & Birbaumer, N. (1999). Changes in EEG power spectra during biofeedback of slow cortical potentials in epilepsy. Applied Psychophysiology and Biofeedback, 24(4) 213-233. https://doi.org/10.1023/a:1022226412991
Kotchoubey, B., Kubler. A., Strehl. U., Flor, H., & Birbaumer, N. (2002). Can humans perceive their brain states? Consciousness and Cognition, 11(1), 98-113. https://doi.org/10.1006/ccog.2001.0535
Kotchoubey, B., Schneider, D., Schleichert, H., Strehl, U., Uhlmann, C., Blankenhorn, V., Fröscher, W., & Birbaumer, N. (1996). Self-regulation of slow cortical potentials in epilepsy: A retrial with analysis of influencing factors. Epilepsy Research, 25(3), 269-276. https://doi.org/10.1016/s0920-1211(96)00082-4
Kotchoubey, B., Schneider, D., Uhlmann, C., Schleichert, H., & Birbaumer, N. (1997). Beyond habituation: Long-term repetition effects on visual event-related potentials in epileptic patients. Electroencephalographer, 103(4), 450-456. https://doi.org/10.1016/s0013-4694(97)00026-6
Kotchoubey, B., Strehl, U., Holzapfel, S., Blankenhorn, V., Fröscher. W., & Birbaumer, N. (1999). Negative potential shifts and the prediction of the outcome of neurofeedback therapy in epilepsy. Clinical Neurophysiology, 110(4), 683-686. https://doi.org/10.1016/s1388-2457(99)00005-x
Kotchoubey. B., Strehl, U., Holzapfel, S., Schneider. D., Blankenhorn, V., & Birbaumer, N. (1999). Control of cortical excitability in epilepsy. Adv Neurol, 81, 281-290.
Kotchoubey, B., Strehl, U., Uhlmann, C., Holzapfel, S., Konig, M., Fröscher. W., Blankenhorn, V., & Birbaumer, N. (2001). Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia, 42(3), 406-416.
Kropotov, J. D. (2009). Quantitative EEG, event-related potentials and neurotherapy. Academic Press.
Landisman, C. E., & Connors, B. W. (2005). Long-term modulation of electrical synapses in the mamillimeteralian thalamus. Science, 310(5755), 1809-1813. https://doi.org/10.1126/science.1114655
Lopes da Silva, F. (1991). Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalography and Clinical Neurophysiology, 79(2), 81–93. https://doi.org/10.1016/0013-4694(91)90044-5
Meshorer et al. (2002). Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science, 295(5554), 508-512. https://doi.org/10.1126/science.1066752
Molenberghs, P., Cunnington, R., & Mattingley, J. B. (2011). Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev, 36(1), 341-349. https://doi.org/10.1016/j.neubiorev.2011.07.004
Mölle, M., Marshall, L., Gais, S., & Born, J. (2002). Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(24), 10941–10947. https://doi.org/10.1523/JNEUROSCI.22-24-10941.2002
Munro, C. A., McCaul, M. E., Wong, D. F., Oswald, L. M., Zhou, Y., Brasic, J., Kuwabara, H., Kumar, A., Alexander, M., Ye, W., & Wand, G. S. (2006). Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry, 59(10), 966-974. https://doi.org/10.1016/j.biopsych.2006.01.008
Nash, J. M. (2011). The gift of mimicry. Your brain: A user's guide. Time.
Niedermeyer, E. (1999). Historical aspects. In E. Niedermeyer & F. Lopes da Silva (Eds.), Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
E. Niedermeyer & F. Lopes da Silva (Eds.) (1999). Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
Othmer, S., & Othmer, S. (2020). Toward a theory of infra-low frequency neurofeedback. In H. W. Kirk (Ed.), Restoring the brain. Routledge, eBook ISBN 9780429275760
Otto, D., & Reiter, L. (1984) Developmental changes in slow cortical potentials of young children with elevated body lead burden: Neurophysiological considerations. Annals of the New York Academy of Sciences, 425(1), 377-383. https://doi.org/10.1111/j.1749-6632.1984.tb23559.x
Pulvermüller, F., Mohr, B., Schleichert, H., & Veit, R., (2000). Operant conditioning of left-hemispheric slow cortical potentials and its effect on word processing. Biological Psychology, 53(2-3), 177-215. https://doi.org/10.1016/S0301-0511(00)00046-6
Radley, J. J., Arias, C. M., & Sawchenko, P. E. (2006). Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. The Journal of Neuroscience, 26(50), 12967-12976. https://doi.org/10.1523/JNEUROSCI.4297-06.2006
Raichle, M. E., & Gusnard, D. A. (2002). Appraising the brain's energy budget. PNAS, 99(16), 10237-10299. https://doi.org/www.pnas.orgcgidoi10.1073pnas.172399499
Rapanelli, M., Frick, L. R., & Zanutto, B. S. (2011). Learning an operant conditioning task differentially induces gliogenesis in the medial prefrontal cortex and neurogenesis in the hippocampus. PLoS ONE, 6(2), e14713. https://doi.org/10.1371/journal.pone.0014713
Rockstroh, B., Elbert, T., Birbaumer, N., Wolf, P., Düchting-Röth, A., Reker, M., Daum, I., Lutzenberger, W., & Dichgans, J. (1993). Cortical self-regulation in patients with epilepsies. Epilepsy Research, 14(1), 63–72. https://doi.org/10.1016/0920-1211(93)90075-i
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L., & von Stein, A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci, 95(12), 7092-7096. https://doi.org/10.1073/pnas.95.12.7092
Schacter, D. L. (1977). EEG theta waves and psychological phenomena: A review and analysis. Biological Psychology, 5, 47-82. https://doi.org/10.1016/0301-0511(77)90028-x
Schneider, F., Rockstroh, B., Heimann, H., Lutzenberger, W., Mattes, R., Elbert, T., Birbaumer, N, & Bartels, M. (1992). Self-regulation of slow cortical potentials in psychiatric patients: Schizophrenia. Biofeedback and Self-Regulation, 17(4), 277-292. https://doi.org/10.1007/bf01000051
M. S. Schwartz, & F. Andrasik (Eds.). (2003). Biofeedback: A practitioner's guide (3rd ed.). The Guilford Press.
Sebel, P. S., Lang, E., Rampil, I. J., White, P. F., Cork, R., Jopling, M., Smith, N. T., Glass, P. S., & Manberg, P. (1997). A multicenter study of bispectral electroencephalogram analysis monitoring anesthetic effect. Anesthesia and Analgesia, 84(4), 891-899. https://doi.org/10.1097/00000539-199704000-00035
Shibasaki, H., & Hallett, M. (2006). What is the Bereitschaftspotential? Clinical Neurophysiology, 117(11), 2341-2356. https://doi.org/10.1016/j.clinph.2006.04.025
Siniatchkin, M., Hierundar, A., Kropp, P., Kuhnert, R., Gerber, W-D., & Stephani, U. (2000). Self-regulation of slow cortical potentials in children with migraine: An exploratory study. Applied Psychophysiology & Biofeedback, 25(1), 13-32. https://doi.org/10.1023/a:1009581321624
Smith, M. L. (2013). Infra-slow fluctuation training; On the down-low in neuromodulation. NeuroConnections.
Speckmann, E.-J., & Elger, C. E. (1984). The neurophysiological basis of epileptic activity: A condensed overview. R. Degen & E. Niedermeyer (Eds.), Epilepsy, sleep, and sleep deprivation (pp.23-34). Elsevier. PMID: 1760082
Speckmann, E.-J., & Elger, C. E. (1999). Introduction to the neurophysiological basis of the EEG and DC potentials. In E. Niedermeyer & F. Lopes da Silva (Eds.), Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
E. Niedermeyer & F. Lopes da Silva (Eds.) (1999). Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
Stahl, S. M. (2008). Stahl’s essential psychopharmacology: Neuroscientific basis and practical applications (3rd ed.). Cambridge University Press.
Steriade, M. (2005). Cellular substrates of brain rhythms. In E. Niedermeyer, & F. Lopes da Silva (Eds.). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Steriade, M., Nuñez, A., & Amzica, F. (1993). Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 13(8), 3266–3283. https://doi.org/10.1523/JNEUROSCI.13-08-03266.199
Sterman M. B. (1996). Physiological origins and functional correlates of EEG rhythmic activities: Iimplications for self-regulation. Biofeedback and Self-regulation, 21(1), 3–33. https://doi.org/10.1007/BF02214147
Sterman, M. B. (2000). EEG markers for attention deficit disorder: Pharmacological and neurofeedback applications. Child Study Journal, 30(1), 1-24.
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Strehl, U. (2009). Slow cortical potentials neurofeedback. Journal of Neurotherapy, 13(2), 117–126. https://doi.org/10.1080/10874200902885936
Strehl, U., Aggensteiner, P., Wachtlin, D., Brandeis, D., Albrecht, B., Arana, M., Bach, C., Banaschewski, T., Bogen, T., Flaig-Röhr, A., Freitag, C. M., Fuchsenberger, Y., Gest, S., Gevensleben, H., Herde, L., Hohmann, S., Legenbauer, T., Marx, A. M., Millenet, S., Pniewski, B., … Holtmann, M. (2017). Neurofeedback of slow cortical potentials in children with Attention-Deficit/Hyperactivity Disorder: A multicenter randomized trial controlling for unspecific effects. Frontiers in Human Neuroscience, 11, 135. https://doi.org/10.3389/fnhum.2017.00135
Streit, W. J. (2006). Microglial senescence: Does the brain's immune system have an expiration date? Trends in Neurosciences, 29(9), 506–510. https://doi.org/10.1016/j.tins.2006.07.001
Thompson, M., & Thompson, L. (2016). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Thompson, M., & Thompson, L. (2009). Asperger’s syndrome intervention: Combining neurofeedback, biofeedback, and metacognition. In T. H. Budzynski, H. K. Budzynski, J. R. Evans, & A. Abarbanel (Eds.). Introduction to quantitative EEG and neurofeedback (2nd ed.). Academic Press.
Vernon, D., Egner, T., Cooper, N., Compton, T., Neilands, C., Sheri, A., & Gruzelier, J. (2003). The effect of training distinct neurofeedback protocols on aspects of cognitive performance. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 47(1), 75–85. https://doi.org/10.1016/s0167-8760(02)00091-0
Voytek, B. (2013). Brain metrics: How measuring brain biology can explain the phenomena of mind. Scitable by Nature Education.
Warren, A. M., & McIlvane, W. J. (1998). Stimulus equivalence and the N400 effect. Poster presented at the 1998 Annual Meeting of the Cognitive Neuroscience Society in San Francisco, CA.
Wilson, J. (2003). Biological foundations of human behavior. Wadsworth/Thompson Learning.
Wilson, V. E., Thompson, M., Thompson, L., Thompson, J., Fallahpour, K., & Linden, M. K. (2011). Introduction to biofeedback (Neurofeedback). In B. W. Strack, M. K. Linden, & V. S. Wilson (Eds.). Biofeedback & neurofeedback applications in sport psychology. Association for Applied Psychophysiology and Biofeedback.
Winn, P. (2001). (Ed.), Dictionary of biological psychology. Routledge.
Xu, T., Yu, X., Perlik, A., Tobin, W., Zweig, J., Tennant, K., Jones, T., & Zuo, Y. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature, 462(7275), 915-919. https://doi.org/10.1038/nature08389
Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., Poo, M., & Duan, S. (2003). Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proceedings of the National Academy of Sciences of the United States of America, 100(25), 15194-15199. https://doi.org10.1073/pnas.2431073100
Zhang, H., Watrous, A., Patel, A., & Jacobs, J. (2018). Theta and alpha oscillations are traveling waves in the human neocortex. Neuron. https://doi.org/10.1016/j.neuron.2018.05.019
B. Editing and Identifying Artifacts
Contamination of the EEG by physiological and exogeneous artifacts requires that clinicians take extensive precautions, examine the raw EEG record, and remove contaminated epochs through artifacting. Impedance tests and behavioral tests help ensure the fidelity of EEG recording.
Please click on the podcast icon below to hear a full-length lecture over Section B.

BCIA's Neurofeedback Essential Skills List requires applicants to identify and remove artifact sources in EEG recordings. Review the Technical unit to recognize normal EEG patterns and identify and correct noncerebral origin signals like bridging artifacts. Once you understand the mechanics and appearance of common artifacts, Peper et al. (2008) recommend that you intentionally reproduce them to recognize and prevent them.
Brain map validity depends on the integrity of the raw EEG. You will need 45 seconds of reasonably clean data to construct a valid brain map from raw EEG recordings of 3-10 minutes. However, the ideal amount of clean data is approximately 2 minutes for adequate database comparison. Realize that longer recordings risk increased drowsiness artifacts and sleep. Therefore, speaking to the client occasionally to help them maintain alertness is helpful. Simply saying how much time is left every 1-2 minutes is usually enough.
Stage 1 sleep is a subtle, drowsy state that clients often do not recognize. This state change is seen as a decrease in alpha and an increase in theta amplitudes. Slow eye-rolling movements and decreased EMG and beta will be observed. Stage 1 sleep graphic © John S. Anderson. Note the increased theta amplitudes in the spectral displays for channels 1 and 2.
The less frequent the artifact, the shorter the required recording period. Disable low-pass and high-pass filters before editing to visualize electro-ocular and SEMG artifacts better.
Worst case, as with a hyperactive child, none of the EEG channels may contain usable data, and you will need to repeat the assessment. Where artifact only contaminates a few channels, you may base the assessment on the clean channels (Demos, 2019).
Strategies to Reduce Artifacts
Demos (2019) recommends several precautions to reduce artifacts in raw EEG recordings:- Demonstrate how to create artifacts for your clients using screen displays while they clench their teeth, move their eyes, blink, swallow, and fidget
- Confirm the cap fits properly
- Use reclining chairs with negligible neck cushioning that can force the head downward to minimize SEMG artifacts
- Limit eyelid movement with cotton balls gently touching the closed eyelids, secured by a loose sleep mask, flexible band, or tape in the eyes-closed recording. There should be no pressure against the eyes
- Ensure that impedance values or DC offset values are appropriate for your amplifier (values under 5 Kohms are expected for publishable research, but values of less than 20 Kohms are acceptable for general clinical sessions and do not require excessive skin abrasion)
- Only record qEEG data when the raw waveforms appear clean
The movie features a 19-channel BioTrace+ /NeXus-32 display of EEG artifacts © Mary Tracy.
EEG artifacts, consisting of noncerebral electrical activity, can be divided into physiological and exogenous artifacts. Physiological artifacts include electromyographic, electro-ocular (eye blink and eye movement), cardiac (pulse), sweat (skin impedance), drowsiness, and evoked potential. Exogenous artifacts include movement, 60 Hz and field effect, and electrode (impedance, bridging, and electrode pop) artifacts.
Electromyographic (EMG) Artifacts
EMG artifacts interfere with EEG recording by volume-conducting signals from skeletal muscles. This artifact contains high-frequency activity that resembles a "buzz" of fast activity during a contraction. EMG is seen as fast beta activity in the qEEG. While some frequencies are between 10-70 Hz, most are 70 Hz or higher.The graphic shows how high-frequency filter (HFF) selection can affect contamination by this artifact. A high-frequency filter (low-pass filter) attenuates frequencies above a cutoff frequency. In the examples below, the cutoffs are 35 Hz and 15 Hz.
All the channels on the left side of the tracing show SEMG artifacts admitted by a 35-Hz high-frequency filter. The right tracing is free from SEMG artifacts since its 15-Hz high-frequency filter attenuates the higher frequencies that contain these artifacts.
The next graphic shows how gum chewing can generate SEMG artifacts by contracting the muscles of mastication. Graphic © eegatlas-online.com.
The frequency spectrum for SEMG artifacts ranges from 2-1,000 Hz. While strong muscular contraction can contaminate all frequency bands, including 10 Hz, the beta rhythm (at 70 Hz or higher) is most affected by this artifact. EMG artifact may create the appearance of greater beta activity than is present. Graphic © eegatlas-online.com.
Below is a BioGraph ® Infiniti EMG artifact display. Note how the amplitude of the EEG spectrum increases with each contraction.
Thompson and Thompson (2016) observed that EMG artifacts are readily detected because they affect one or two channels, particularly at T3 and T4 at the periphery and less often at O1, O2, Fp1, and Fp2.
You can identify EMG artifacts by visually inspecting the raw signal. The next graphic shows SEMG artifacts using a 70-Hz high-frequency filter.
Electro-Ocular Artifacts
Electro-ocular artifacts contaminate EEG recordings with potentials generated by eye blinks, eye flutter, and other eye movements. For example, an anxious patient's eyelid flutter may cause deflections at Fp1 and Fp2 (Klass, 2008).Thiese artifacts are due to the eye’s electrical field movement when the eye rotates and the contraction of the extraocular muscles. The eye creates an electropositive dipole at the front and electronegative at the back. Bell’s Phenomenon refers to the upward rotation of the eye when it closes and causes an artifact seen as an apparent increase in EEG.
The next two graphics show eye movement artifacts due to rapid blinking. Graphics © eegatlas-online.com.
The next graphic shows eye blinks, sharp lateral eye movement, and slow lateral eye movement.
Below is a BioGraph ® Infiniti EEG display of eye movement artifact.
Below is a NeXus display of eye blink and EMG © John S. Anderson.
An upward eye movement will create a positive deflection at Fp1, while a downward eye movement may create a negative deflection. In a longitudinal sequential montage, the artifact is typically seen at frontal sites (Fp1- F3 and Fp2-F4). A left movement may produce a positive deflection at F7 and a negative deflection at F8 (Thompson & Thompson, 2015).
Rapid eye flutter may resemble seizure activity. Graphic redrawn by minaanandag on fiverr.com.
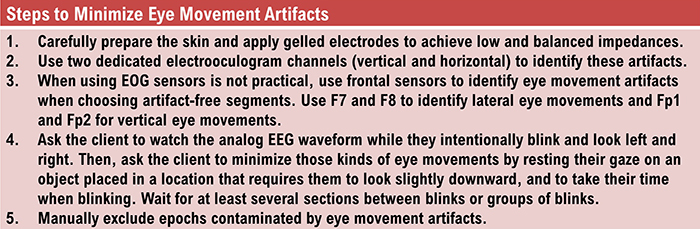
Cardiac and Pulse Artifacts
Cardiac artifacts occur when the ECG signal appears in the EEG (Jiang et al., 2019). This artifact may be produced when electrode impedance is imbalanced, too high, or when an ear electrode contacts the neck. The frequency range for ECG artifact is 0.05-80 Hz, contaminating the delta through beta bands. Since multiple electrodes detect this artifact, it can create the appearance of greater coherence than is present. Graphic © eegatlas-online.com.
You can detect cardiac artifacts by inspecting the raw EEG waveform's chart recorder, data acquisition, or oscilloscope displays. Cardiac artifacts appear as a wave that repeats about once per second (Thompson & Thompson, 2016). Below is a BioGraph ® Infiniti ECG artifact display.
ECG artifacts are easily recognized, especially with a separate ECG tracing, allowing a direct comparison between recorded ECG and artifact in the EEG. Sharp, regular, consistent artifacts at about 1 per second in the EEG are likely the result of the ECG. These artifacts are observed best in referential montages using earlobe electrodes A1 and A2 or mastoid electrodes M1 and M2. Any patterns in the EEG that are synchronous with the ECG tracing can be identified as such an artifact. ECG artifact graphic was produced by Garces et al. (2007).
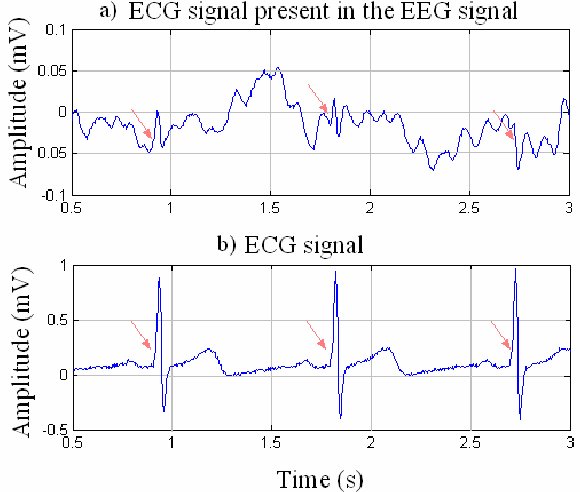
Another cardiac-related artifact are the pulse artifacts that occur when an EEG electrode is placed directly over a blood vessel. The mechanical movement of the electrode as a vessel expands and contracts will produce a slow-wave pattern in the EEG that can be mistaken for delta activity and will affect topographic maps created from data containing such an artifact. This is sometimes called the cardioballistic artifact, though another similar-looking artifact is thought to be related to the subtle movement of the body as the heart beats. Graphic © eegatlas-online.com.
Sweat (Impedance) Artifacts
Sweat artifacts result from sweat on the skin changing the conductive properties under and near the electrode sites (i.e., bridging artifact). Sweating reduces electrode contact with the scalp and generates large-scale up-and-down EEG line movements in several frontal channels. Abrupt, unexpected stimuli often elicit this artifact and usually appear as isolated 1-2 Hz slow waves of 1-2-s duration at frontal and temporal sites (Thompson & Thompson, 2015). Graphic © eegatlas-online.com.

Bridging Artifacts
A short circuit produces bridging artifacts between adjacent electrodes due to excessive application of electrode paste or a client sweating excessively or arriving with a wet scalp. Bridging artifacts can cause adjacent electrodes to create a short circuit that produces identical referential EEG recordings or a flat line with a bipolar montage. The Fp1-F3 channel's reduced amplitude and frequency illustrate a bridging artifact.
Drowsiness Artifacts
Drowsiness artifacts involve the appearance of stage 1 or stage 2 sleep in the EEG. Stage 1 and stage 2 of sleep are most likely during eyes-closed recording. Sleep may occur during eye-closed awake recording. Graphic © eegatlas-online.com.
Two additional drowsiness artifact examples appear below. The first shows a brief episode of drowsiness lasting about 5 seconds with a dropout of the alpha rhythm (posterior dominant rhythm - PDR) and then a return of the PDR. The second example shows the end of a longer period of light sleep with a K-complex indicated in the F3-C3 derivation, followed by a return to a typical alpha rhythm.
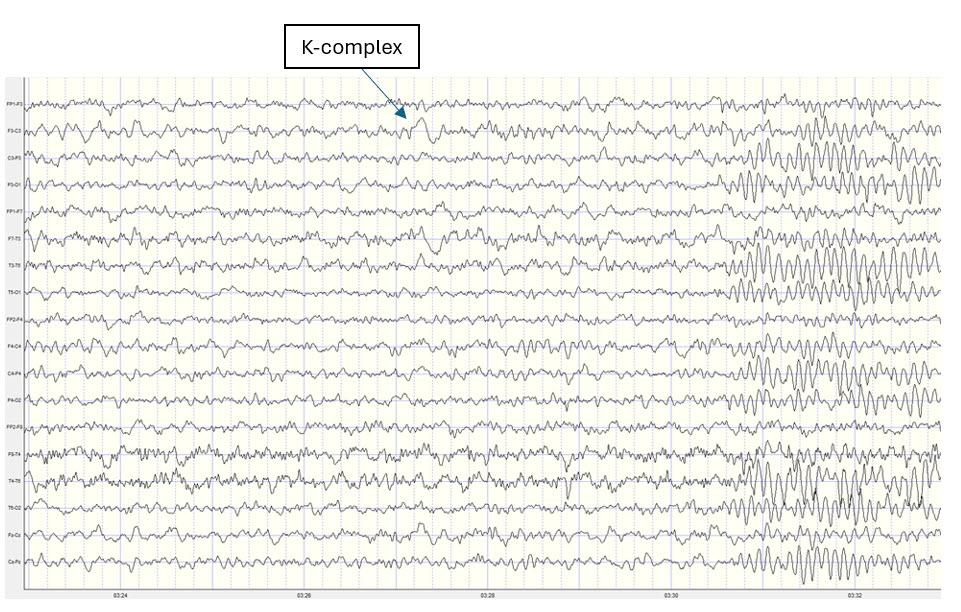
Stage 1 sleep is a subtle, drowsy state that clients often do not recognize. Alpha amplitude (especially occipital) may decrease, and theta (especially frontal) may increase. Reductions in EMG and beta amplitude will accompany slow eye-rolling movements. Sleepiness may be accompanied by spike-like transients (vertex or V-waves). The graphic below © John S. Anderson shows increased theta during stage 1 sleep.
When you detect drowsiness artifact during a training session, suspend recording and instruct your clients to move their hands and legs to increase wakefulness. To avoid this artifact, ask them to retire early and sleep for 9 hours if possible (Thompson & Thompson, 2003).
Evoked Potential Artifacts
Evoked potential artifacts (also called event-related potential artifacts) consist of somatosensory, auditory, and visual signal processing-related transients that may contaminate multiple channels of an EEG record. While evoked potentials increase recording variability and reduce reliability, they minimally affect averaged data (Thompson & Thompson, 2015).Watch BPM Biosignals' YouTube video EEG: Visually evoked potentials (VEP).
Movement Artifacts
Movement artifacts are caused by client movement or the movement of electrode wires by other individuals. Most of these artifacts are produced by brief changes in the electrode-skin surface connection. Cable movement is called cable sway.Movement artifacts can produce high-frequency and high-amplitude voltages identical to EEG and EMG signals. While the delta rhythm is most affected by this artifact, it may also contaminate the theta band (Thompson & Thompson, 2016). Graphic © eegatlas-online.com.
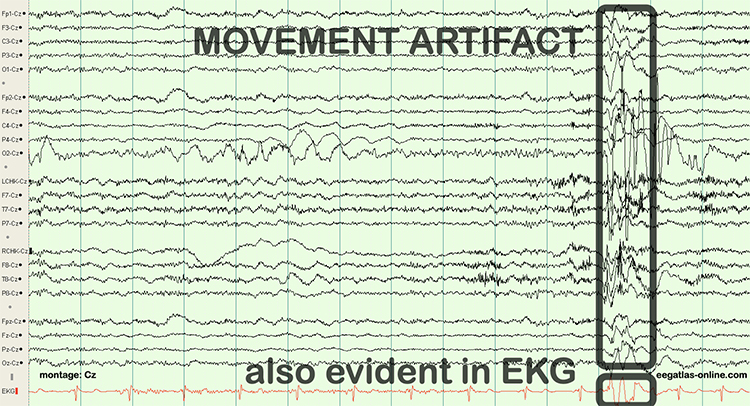
The graphic below shows movement artifacts due to head movement (left), respiration (center), and tongue movement (right).
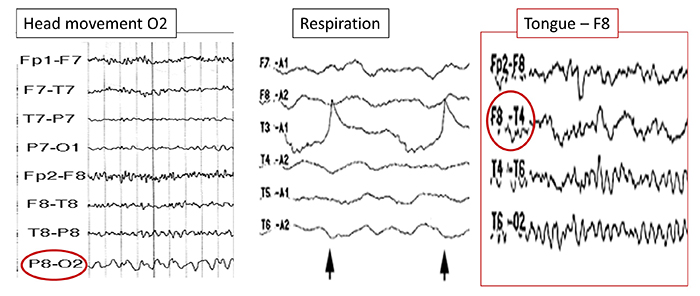
Below is a BioGraph ® Infiniti cable movement artifact display. Note the two voltage spikes at the beginning of the recording.
50/60 Hz and Field Artifacts
Both 50/60 Hz and field artifacts are external artifacts transmitted by nearby electrical sources. While 60-Hz artifact is a risk in North America, where AC voltage is transmitted at 60 Hz, 50-Hz artifact is a problem in other locations that generate power at 50 Hz. Their fundamental frequency is 50 or 60 Hz, with harmonics at 100/120 Hz, 150/180 Hz, and 200/240 Hz. Imbalanced electrode impedances increase an EEG amplifier's vulnerability to these artifacts. The 60-Hz artifact graphic below © John S. Anderson.
A BioGraph ® Infiniti display of 60-Hz artifact is shown below in red. Note the cyclical voltage fluctuations and 60-Hz peak in the power spectral display.
Radiofrequency Artifacts
Radiofrequency (RF) artifacts radiate outward like a cone from the front of televisions and computer monitors. Graphic © eegatlas-online.com.
Electrode Artifacts
There are several sources of electrode artifacts. Even with proper care, electrode surfaces can become corroded and the leads and connectors damaged. Using sensors with mismatched electrode metals can cause polarization of amplifier input stages.Impedance Artifacts
Unless skin-electrode impedance is low (under 5 KΩ for research and 20 KΩ for training) and balanced (under 1-3 KΩ ), diverse artifacts like 50/60 Hz and movement can contaminate the EEG signal, as seen in the P3 and Pz electrodes. Graphic © eegatlas-online.com.
Electrode Pop Artifacts
Mechanical disturbance can produce a unique artifact even when the impedance is low and balanced. Electrode pop artifact has a sudden large deflection in at least one channel when an electrode abruptly detaches from the scalp. This may also happen when there is a bubble or other defect in the gel or paste, and a charge builds up and subsequently "jumps" across the gap, resulting in a large electrical discharge. Graphic © John S. Anderson.
Glossary
50/60 Hz: external artifacts transmitted by nearby electrical sources.
bridging artifacts: a short circuit between adjacent electrodes due to excessive application of electrode paste or a client who is sweating excessively or who arrives with a wet scalp.
cable sway: the movement or displacement of electrode cables during the recording session. This movement can introduce artifacts or noise into the EEG signal, potentially interfering with the accurate interpretation of brain activity.
cardiac artifact: the contamination of the EEG by the ECG signal.
drowsiness artifact: in adults, 1-Hz (or slower) waveforms can be detected with greatest amplitude and reverse polarity at F7 and F8 may progress to 1-2 Hz slowing of the alpha rhythm.
EEG artifacts: noncerebral electrical activity in an EEG recording can be divided into physiological and exogenous artifacts.
electro-ocular artifacts: contamination of EEG recordings by potentials generated by eye blinks, eye flutter, and eye movements.
electrode pop artifacts: sudden large deflections in at least one channel when an electrode abruptly detaches from the scalp.
EMG artifact: interference in EEG recording by volume-conducted signals from skeletal muscles.
evoked potential artifact (event-related potential artifact): somatosensory, auditory, and visual signal processing-related transients that may contaminate multiple channels of an EEG record.
exogenous artifacts: noncerebral electrical activity generated by movement, 50/60 Hz and field effect, bridging, and electrode (electrode “pop" and impedance) artifacts.
field artifacts: external artifacts transmitted by nearby electrical sources.
high-frequency filter (HFF): a filter that attenuates frequencies above a cutoff frequency.
impedance artifacts: high and/or imbalanced impedance between the scalp electrodes and the skin can distort or disrupt the EEG signal, affecting the accuracy of the recorded brain activity.
movement artifacts: voltages caused by client movement or the movement of electrode wires by other individuals.
physiological artifacts: noncerebral electrical activity that includes electromyographic, electro-ocular (eye blink and eye movement), cardiac (pulse), sweat (skin impedance), drowsiness, and evoked potential.
pulse artifacts: noncerebral voltages due to the mechanical movement of an electrode in relation to the skin surface due to the pressure wave of each heartbeat
radiofrequency (RF) artifacts: interference caused by external electromagnetic signals, typically from nearby electronic devices or radio frequency sources. These signals can contaminate the EEG recording, leading to distortions or noise in the recorded brain activity.
sweat artifacts: changes in the EEG signal when sweat on the skin changes the conductive properties under and near the electrode sites (i.e., bridging artifact).
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Assignment
Now that you have completed this module, explain why low-and-balanced skin-electrode impedances are important in neurofeedback training. Describe the precautions you take to achieve acceptable impedance values. How do you measure impedance with your neurofeedback system?
References
Andreassi, J. L. (2000). Psychophysiology: Human behavior and physiological response. Lawrence Erlbaum and Associates, Inc.
Basmajian, J. V. (Ed.). (1989). Biofeedback: Principles and practice for clinicians. Williams & Wilkins.
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Cacioppo, J. T., & Tassinary, L. G. (Eds.). (1990). Principles of psychophysiology. Cambridge University Press.
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
Floyd, T. L. (1987). Electronics fundamentals: Circuits, devices, and applications. Columbus: Merrill Publishing Company.
Garces, A., Laciar, E,, Patiño, H., & Valentinuzzi, M. (2007). Artifact removal from EEG signals using adaptive filters in cascade. Journal of Physics: Conference Series, 90(1), 012081. https://10.1088/1742-6596/90/1/012081.
Grant, A. (2015). Four elements earn permanent seats on the periodic table. Science News.
Halford, J. J., Sabau, D., Drislane, F. W., Tsuchida, T. N., & Sinha, S. R. (2016). American Clinical Society Guideline 4: Recording clinical EEG on digital media. Journal of Clinical Neurophysiology, 33(4), 317-319. https://doi.org/10.1080/21646821.2016.1245563
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Hughes, J. R. (1994). EEG in clinical practice (2nd ed.). Butterworth-Heinemann.
Jiang, X., Bian, G. B., & Tian, Z. (2019). Removal of artifacts from EEG signals: A review. Sensors (Basel, Switzerland), 19(5), 987. https://doi.org/10.3390/s19050987
Khazan, I. Z. (2019). Biofeedback and mindfulness in everyday life: Practical solutions for improving your health and performance. W. W. Norton & Company.
Klass, D. W. (2008). The continuing challenge of artifacts in the EEG. EEG artifacts. American Society of Electroneurodiagnostic Technologists, Inc. https://doi.org/10.1080/00029238.1995.11080524
Kubala, T. (2009). Electricity 1: Devices, circuits, and materials (9th ed.). Cengage Learning.
Lau, T. M., Gwin, J. T., & Ferris, D. P. (2012). How many electrodes are really needed for EEG-based mobile brain imaging? Journal of Behavioral and Brain Science, 2(3), 387-393. https://doi.org/10.4236/jbbs.2012.23044
Lebby, P. C. (2013). Brain imaging: A guide for clinicians. Oxford University Press.
Libenson, M. H. (2010). Practical approach to electroencephalography. Saunders Elsevier.
Lin, F., Witzel, T., Hamalainen, M. S., Dale, A. M., Belliveau, J. W., & Stufflebeam, S. M. (2004). Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage, 2(3), 582-595. https://dx.doi.org/10.1016%2Fj.neuroimage.2004.04.027
Min, B.-K. (2007). The top-down function of prestimulus EEG alpha activity. Dissertation.
Montgomery, D. (2004). Introduction to biofeedback. Module 3: Psychophysiological recording. Association for Applied Psychophysiology and Biofeedback.
Nilsson, J. W., & Riedel, S. A. (2008). Electric circuits (8th ed.). Pearson Prentice-Hall.
Peek, C. J. (2016). A primer of traditional biofeedback instrumentation. In M. S. Schwartz, & F. Andrasik (Eds.). (2016). Biofeedback: A practitioner's guide (4th ed.). The Guilford Press.
Picton, T. W., & Hillyard, S. A. (1972). Cephalic skin potentials in electroencephalography. Encephalogr Clin Neurophysiol, 33, 419-424. https://doi.org/10.1016/0013-4694(72)90122-8
Pfister, H., Kaynig, V., Botha, C. P., Bruckner, S., Dercksen, V., & Hege, H.-C. (2012). Visualization in connectomics. Mathematics and Visualization, 37. https://doi.org/10.1007/978-1-4471-6497-5_21
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Thomas, C. (2007). What is a montage? EEG instrumentation. American Society of Electroneurodiagnostic Technologists, Inc.
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
C. Normal Waveform Patterns
This section covers normal EEG patterns, including the posterior dominant rhythm, differences between eyes open and eyes-closed resting conditions, developmental aspects of the EEG, diurnal influences on the EEG, subjective characteristics of EEG frequency bands, and waveform morphology.
Please click on the podcast icon below to hear a lecture over the first half of Section C.

Normal EEG Patterns
The healthy adult EEG is a cerebral symphony comprised of theta, alpha, sensorimotor rhythm, beta, and gamma activity. We will survey the generators, distributions, and behavioral correlates of these rhythms. EEG rhythms correlate with patterns of behavior (level of attentiveness, sleeping, waking, seizures, and coma), occur in distinct frequency ranges, and are characterized by synchrony and desynchrony.Synchrony means that pools of neurons coordinate their firing due to pacemakers (left) and mutual coordination (right). Synchrony graphic redrawn from Bear, Connors, and Paradiso (2002) by minaanandag at Fiverr.com.
The synchronized EEG graphic below © John S. Anderson.
Desynchrony means that pools of neurons fire independently due to stimulation of specific sensory pathways up to the midbrain and high-frequency stimulation of the reticular formation and nonspecific thalamic projection nuclei.
The desynchronized EEG graphic below © John S. Anderson.
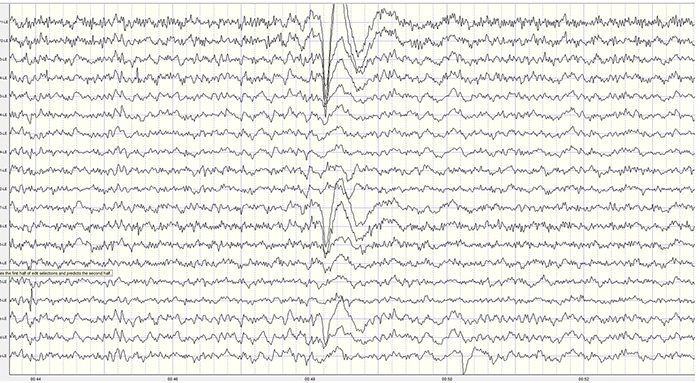

Graphic © John S. Anderson.
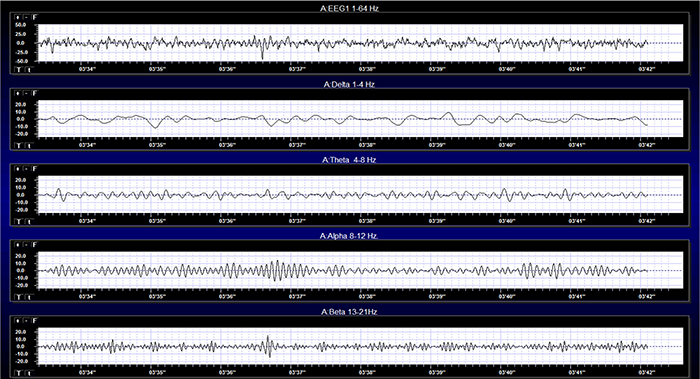
Effect of Eyes Open and Closed Conditions on the EEG
The alpha rhythm is strongly modulated by visual input. Opening the eyes blocks or reduces the occipital alpha rhythm. Hans Berger (1929) originally described this phenomenon. In contrast, eyes-closed alpha is associated with alert wakefulness and reduced visual input (Thompson & Thompson, 2016).The movie below is a 19-channel BioTrace+ /NeXus-32 display of eyes open and closed EEG © John S. Anderson. Note the appearance of alpha activity with eyes closed at about 14 seconds and alpha-blocking with eyes open at about 45 seconds.
The graphic below illustrates alpha-blocking and was uploaded to ResearchGate by the author, Byoung-Kyong Min. The two trials show alpha-blocking during eyes-open conditions.
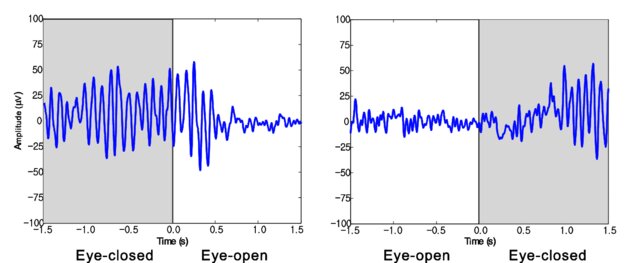
Posterior Dominant Rhythm
The posterior dominant rhythm (PDR) is the highest-amplitude frequency detected at the posterior scalp when eyes are closed. A healthy adult PDR is 10 Hz. Values below 9 Hz and above 11 Hz are abnormal, may result from psychoactive drugs, and may be associated with clinical symptoms like anxiety (Demos, 2019). Graphic © eegatlas-online.com. See the PDR at P3-O1.
Developmental Aspects of EEG
Delta is dominant below age 3, theta from 3 to 5, and low alpha from 6 to 8. Alpha frequency increases to a peak around 10 Hz after age 10. Peak frequencies slow during adulthood with aging (Thompson & Thompson, 2016).The PDR rises with age: 1 year (6 Hz), 8 years (8 Hz), 10-12 years (9 Hz), and 13-14 years (10 Hz) (Demos, 2019).
Diurnal Influences on the EEG
Alpha and theta rhythm amplitudes vary across the day, with the highest values at 11 am, 1 pm, and 3 pm. Fatigue and individual differences (but not eating) influence the magnitude of change and precise peak times. Serial assessments should be conducted at the same time of day to control for diurnal fluctuations (Thompson & Thompson, 2015).Evaluation of Subject Variables During Acquisition
Subject variables are crucial to the interpretation of EEG measurements. In this section, we will examine the importance of alertness-drowsiness, physical relaxation, and anxiety. We covered the effects of eyes closed/eyes open in the previous section and medication effects in the Psychopharmacology unit.Alertness-Drowsiness
A client's age determines how drowsiness is expressed in the EEG. Between 6 months and 2 years (and rarely after 12 years), most children present with hypnogogic hypersynchrony (abrupt appearance of low-amplitude spikes that resemble epileptiform activity otherwise normal record). Beta activity between 20-25 Hz also maximally appears centrally and posteriorly. When older children and adults are drowsy or enter stage 1 and 2 sleep, frontocentral beta may be activated (Fisch, 1999).Adult EEG drowsiness patterns increasingly appear by age 10. Slow lateral eye movements are associated with 1-Hz (or slower) waveforms that can be detected with the greatest amplitude and reverse polarity at F7 and F8. Continued drowsiness may be related to 1-2 Hz slowing of the alpha rhythm. For this reason, it is crucial to confirm client wakefulness when assessing alpha rhythm frequency (Fisch, 1999. Graphic © fizkes/Shutterstock.com.

From ages 10 to 20, drowsiness is often accompanied by rhythmic frontal theta (Fisch, 1999).
Physical Relaxation
The human stress response is multidimensional and involves diverse systems, ranging from the central nervous system to the immune system. Each person uniquely responds to stressors. This is called response stereotypy.Individuals differ in which systems are involved, the degree of their activation or suppression, and the impact of these changes on their health.
Since clients show widely different response stereotypies, assessment should monitor multiple physiological channels, including blood volume pulse (BVP) for heart rate and heart rate variability, electromyography (EMG), a respirometer for respiration rate and pattern, skin conductance (SC), and skin temperature.
A capnometer, which measures end-tidal CO2, can complement the information provided by a respirometer by detecting CO2 reductions due to overbreathing, which is more subtle than hyperventilation (Khazan, 2019).
Professionals can find the normative values for these measures in Moss and Shaffer's (2019) Physiological Recording Technology in Biofeedback and Neurofeedback.
Stress responses that affect physiologic responses outside the CNS can cause artifacts in EEG recordings. Therefore, when client distress produces excessive artifact, a professional may have to coach the client to relax guided by one or more biofeedback modalities.
Clients should be monitored in a comfortable but upright position.
Anxiety
Anxious clients often present with decreased alpha and increased 19-21 Hz or 20-23 Hz beta activity. Conversely, anxious adults diagnosed with ADHD may show increased alpha activity. Even without perceptible sweating, anxious patients may present with intermittent biphasic slow-wave activity (Picton & Hillyard, 1972). Graphic © Peshkova/Shutterstock.com.

Clients who experience panic may exhibit paroxysmal EEG activity (Thompson & Thompson, 2015).
Subjective Characteristics of Frequency Bands
Most EEG power or signal energy falls within the 0-20 Hz frequency range. You may recall that hertz (Hz) is an abbreviation for cycles per second. The dominant frequency is the frequency with the greatest amplitude. It is at least 13 Hz in awake adults. EEG power is measured in microvolts or picowatts.
Higher frequencies reflect cognitive activity and active processing of sensory input. They involve relatively desynchronized activity like alert wakefulness and REM sleep. Lower frequencies reflect strongly synchronized activity like interactive neuronal communication, control of network activity, nondreaming sleep, and coma.
The table below is adapted from Wilson et al. (2011) and based on Thompson and Thompson (2015). Different authors define frequency bandpasses differently. For example, delta 0.5-3 Hz or 1-4 Hz.
DELTA (0.5-3 or 4 HZ)
Delta EEG activity, characterized by low-frequency oscillations (0.5 to 3 or 4 Hz), plays a crucial role in various brain functions, particularly during sleep and in pathological conditions.
We will explore the generators of delta activity, its significance in brain function, and its association with disease. Public Domain, https://commons.wikimedia.org/w/index.php?curid=453193
.jpg)
Synchronous means that groups of neurons depolarize and hyperpolarize at the same time. Delta comprises less than 5% of a healthy adult's percent of amplitude compared with 70% for occipital alpha (Thatcher, 1999). The greatest amplitude or signal strength is found in the central region of the scalp. The delta rhythm is the dominant frequency from ages 1-2 and is associated in adults with deep sleep and brain pathologies like trauma and tumors, and learning disability (Hugdahl, 1995; Thompson & Thompson, 2015).
Sleep deprivation can increase delta amplitude. Adult high-amplitude rhythmic delta indicates pathology like traumatic brain injury (TBI). Undergraduates performing problem-solving tasks exhibit arrhythmic delta (Lubar et al., 2001). Children diagnosed with ADHD or learning disabilities may present with diffuse delta and theta. When this occurs, clinicians may inhibit 2-7 Hz instead of 4-7 Hz. Amplitude training is appropriate for inhibiting but not rewarding delta (Demos, 2019).
Low-amplitude delta may be associated with ADHD, anxiety, insomnia, and TBI. Z-score training is safest for uptraining delta (Demos, 2019).
The movie below is a 19-channel BioTrace+ /NeXus-32 display of delta activity © John S. Anderson. Brighter colors represent higher delta amplitudes. Higher peaks represent higher delta amplitudes in the graphs at the end of each line. Frequency histograms are displayed for each channel.
Delta Rhythm Generators
Delta waves are primarily associated with deep stages of sleep, especially slow-wave sleep (SWS), and are generated by the thalamocortical network. This network's synchronization of neuronal activity is critical for generating delta rhythms. Several mechanisms contribute to the generation of delta waves.Thalamic Pacemaker Neurons
Thalamic neurons, particularly those in the thalamic reticular nucleus (TRN), act as pacemakers for delta activity. These neurons exhibit rhythmic burst firing patterns propagated to the cortex, resulting in synchronized delta oscillations (Steriade et al., 1993).Cortical Neurons
Cortical neurons, especially those in the neocortex, also play a significant role in generating delta waves. The interplay between excitatory pyramidal neurons and inhibitory interneurons within cortical columns contributes to the rhythmicity observed in delta activity (Destexhe et al., 1999). Pyramidal neuron graphic © Juan Gaertner/Shutterstock.com.
Thalamocortical Interactions
The reciprocal connections between the thalamus and cortex are essential for generating and maintaining delta rhythms. These interactions facilitate the synchronization of neuronal firing across large cortical areas, leading to the widespread presence of delta waves during sleep (Amzica & Steriade, 1998).The Meaning of Delta EEG Activity
Delta activity is most prominently observed during the deep stages of non-REM sleep, which are believed to play several critical roles.
Sleep and Restoration
Delta waves are a hallmark of slow-wave sleep, a phase critical for physical and cognitive restoration. During this phase, the brain undergoes synaptic pruning, and memory consolidation.During SWS, the body undergoes several restorative processes, including tissue repair, muscle growth, and the release of growth hormones. Delta waves facilitate these processes by ensuring deep, uninterrupted sleep, which is necessary for effective physical recovery (Tononi & Cirelli, 2014). Healthy adult hypnogram from Spieshoeffer et al. (2019).
.jpg)
Caption: representative polysomnogram showing healthy sleep architecture with characteristic and repetitive passage through sleep cycles. N3 represents slow-wave sleep, which occurs more during the first half of the night, whereas REM sleep is more common during the second half. Pathological sleep is characterized by reduced slow-wave sleep and/or REM sleep and/or sleep fragmentation. W: awake; N1: non-REM I sleep; N2: non-REM II sleep; N3: non-REM III sleep; R: REM sleep.
Delta activity helps in clearing metabolic waste products from the brain, such as beta-amyloid, which, if accumulated, can contribute to neurodegenerative diseases like Alzheimer's. This glymphatic clearance system is more active during SWS, supported by delta oscillations (Xie et al., 2013).
The glymphatic system is a newly discovered lymphatic system in the brain. It provides a flow of CSF through the brain's interior that helps clear cellular debris, proteins, and other wastes. Glymphatic system graphic © Claus Lunau/Science Photo Library.
Delta EEG activity during sleep, particularly in the prefrontal cortex, is associated with better performance on neuropsychological tasks specific to the left prefrontal cortex in healthy older adults (Anderson & Horne, 2003). Sleep deprivation can increase delta waking amplitude.
Cognitive Performance
Delta EEG activity increases during mental tasks requiring attention to internal processing, such as difficult mental calculations and short-term memory tasks. This suggests that delta activity is related to the cognitive effort involved in internal processing (Harmony et al., 1996).Memory Consolidation
Delta oscillations are associated with the consolidation of declarative memory. The synchronous activity of delta waves helps to transfer information from the hippocampus to the neocortex, facilitating long-term memory storage (Marshall & Born, 2007).Homeostatic Regulation
Delta activity reflects the homeostatic regulation of sleep. Higher amounts of delta activity indicate a higher sleep pressure, which is the body's way of balancing sleep and wakefulness to maintain overall health (Achermann & Borbély, 2003).Neuroprotection
Delta activity supports the maintenance and health of neurons, potentially protecting against the accumulation of neurotoxic substances. Regular deep sleep with sufficient delta activity is linked to a lower risk of developing neurodegenerative diseases (Varga et al., 2016).Emotional Regulation
Adequate delta activity during deep sleep contributes to emotional stability and resilience.Deep sleep, characterized by delta waves, helps regulate mood and emotional responses. Disruptions in delta sleep are associated with mood disorders such as depression and anxiety (Goldstein & Walker, 2014).
The Delta Rhythm in Disease
Neurodegenerative Disorders
Alterations in delta activity have been observed in neurodegenerative diseases such as Alzheimer's and Parkinson's disease. Patients with Alzheimer's disease, for example, exhibit disrupted delta oscillations, which correlate with cognitive decline and memory impairment (Varga et al., 2016).In nondemented, amyloid-positive subjects, higher delta power is associated with clinical progression from subjective cognitive decline to mild cognitive impairment or dementia. This indicates that delta activity may be a prognostic marker for cognitive decline (Gouw et al., 2017).
Sleep Disorders
Changes in delta activity are also linked to sleep disorders like insomnia and sleep apnea. Reduced delta power during sleep is often associated with poor sleep quality and increased daytime fatigue (Chokroverty, 2017).Psychological Disorders
Delta activity is implicated in various psychiatric disorders, including depression and schizophrenia. Abnormal delta oscillations are often observed in these conditions, suggesting a link between disrupted delta activity and the pathophysiology of these disorders (Gardner et al., 2014).Increased delta activity, along with decreased alpha activity, differentiates psychotic disorders such as schizophrenia, bipolar disorder with psychotic features, and methamphetamine-induced psychosis. This pattern indicates dysfunctional thalamocortical connectivity and may serve as a neurophysiological biomarker for these conditions (Howells et al., 2018).
Children diagnosed with ADHD or learning disabilities may present with diffuse delta and theta. When this occurs, clinicians may inhibit 2-7 Hz instead of 4-7 Hz. Amplitude training is appropriate for inhibiting but not rewarding delta (Demos, 2019).
Low-amplitude delta may be associated with ADHD, anxiety, insomnia, and TBI. Z-score training is the safest way to uptrain delta (Demos, 2019).
Encephalopathy
Specific delta EEG patterns, such as continuous slowing and frontal intermittent delta activity (FIRDA), are associated with different pathological conditions and outcomes in encephalopathic patients. For example, delta activity is linked to alcohol/drug abuse and HIV infection, while FIRDA is associated with past cerebrovascular accidents (Sirin et al., 2019; Sutter, Stevens, & Kaplin, 2012).Brain Lesions
Focal delta activity on EEG is significantly associated with structural brain lesions, such as those caused by strokes, tumors, and trauma. This correlation highlights the importance of delta activity in identifying underlying brain abnormalities (Gilmore & Brenner, 1981; Nazish, 2020).Amnesic Mild Cognitive Impairment
In patients with amnesic mild cognitive impairment not due to Alzheimer's disease, those with epileptiform EEG activity show higher temporal delta source activities. This suggests the role of neural hypersynchronization in their brain dysfunctions (Babiloni et al., 2020).Conclusion
Delta EEG activity, generated primarily by the thalamocortical network and cortical neurons, is essential for several critical brain functions, especially during slow-wave sleep (SWS). It facilitates physical and cognitive restoration, including synaptic pruning, memory consolidation, and the clearance of metabolic waste. Delta activity is also linked to cognitive performance, homeostatic sleep regulation, neuroprotection, and emotional stability.
Recent research highlights the significance of delta activity in various health and disease contexts. In neurodegenerative disorders like Alzheimer's and Parkinson's disease, disrupted delta oscillations correlate with cognitive decline. Similarly, sleep disorders such as insomnia and sleep apnea are associated with reduced delta power, leading to poor sleep quality and increased fatigue. Psychiatric conditions, including depression and schizophrenia, often exhibit abnormal delta activity, suggesting a role in their pathophysiology. Specific delta patterns are also indicative of encephalopathy, brain lesions, and amnesic mild cognitive impairment, serving as potential neurophysiological biomarkers.
THETA (3-8 HZ)
The theta rhythm ranges from 3-7 Hz, 4-7 Hz, or 4-8 Hz with 20-100 microvolts (Thompson & Thompson, 2015). Theta may be arrhythmic or rhythmic (Demos, 2019). Theta is seen during drowsiness or starting to sleep, hypnagogic imagery (intense imagery experienced before sleep onset), and hypnosis (Libenson, 2024).
The greatest amplitude is found in the frontal and temporal regions of the scalp. Since there may be several theta generators, the theta rhythm is associated with different behavioral processes. The theta rhythm is associated with creativity, but also with anxiety, daydreaming, depression, inattention, and minor TBI. Excessive left hemisphere (LH) theta may be associated with depression, and right hemisphere (RH) theta may be linked to anxiety (Demos, 2019).
EEG activity in the theta frequency band is quite specific to the location where it is recorded. 4-8 Hz activity in temporal areas has different functional and behavioral correlates from the same frequency activity in frontal midline or posterior areas. This, again, reaffirms that location and behavior are essential components when analyzing scalp EEG.
Below is an example of filtered (4-8 Hz) theta activity.
Caption: This is an eyes-closed recording in the longitudinal bipolar montage with a 20-µV scale with dark vertical lines showing the beginning of each new 1-second segment. The EEG is displayed using a 4-8 Hz filter to isolate that frequency band from the full band EEG. Observe the slightly greater amplitude and rhythmicity in temporal derivations.
Below is a 1-45 Hz display of the same EEG recording at the same time location.
Caption: This is an eyes-closed recording in the longitudinal bipolar montage with a 50-µV scale. The EEG is displayed using a 1-45 Hz filter to show the relatively full EEG band.
Theta activity in an awake adult is considered abnormal (Libenson, 2024).
Amzica and Lopes da Silva (2018) cite various studies regarding the theta rhythm, which they identify as 4-7 Hz. As discussed earlier, they note that normal theta activity should not be confused with pathologic theta, which represents a slowing of the alpha frequency band into the theta range. They suggest that this slowing of alpha may result from reducing cerebral blood flow or metabolic encephalopathies. Metabolic encephalopathies can result from chemical imbalances due to various causal factors, from kidney or liver dysfunction, diabetes, or a variety of other health issues.
Arnolds et al. (1980) found significant differences between behavioral conditions when viewing hippocampal theta recorded with depth electrodes. Writing resulted in faster frequency and greater rhythmicity but lower amplitude than sitting or walking. In contrast, a word association task resulted in faster frequency, greater rhythmicity, and increased amplitude in the period of silence immediately following the question but before the answer was given.
Ekstrom and colleagues (2005) studied hippocampal and neocortical theta activity during a virtual driving task that involved location finding. They found that both areas increased theta during all tasks associated with the driving simulation. A significant correlation between all areas showed increased coordination between multiple areas while accomplishing the tasks. They concluded that cortical and hippocampal theta oscillations and coordination between these areas are associated with attention and sensorimotor integration.
Childhood Disorders
The theta rhythm is the dominant frequency in healthy young children (Thompson & Thompson, 2015). Theta amplitudes and normative theta-to-beta ratios are higher in children than in older adults. Children diagnosed with ADHD often have higher ratios than children without ADHD. Theta-to-beta ratios greater than 3:1 may indicate a slow-wave disorder, and children with a slow-wave disorder may have ratios as high as 6:1 (Demos, 2019). Excessive theta graphic retrieved from ADDYSSEY.
Historically, the study of attention disorders has focused on excess frontal theta activity in individuals with inattentive ADHD. The ratio of theta (4-8 Hz) activity to beta (13-21 Hz) activity, or the theta/beta ratio (T/B ratio), was developed to make the analysis of this metric easier. It was initially calculated using a single channel vertex location at Cz (Monastra et al., 1999). Other studies compared multiple locations and found that the Cz location was accurate and represented the location of the largest deviation of the ratio between previously diagnosed ADHD clients and typical controls (Lubar, 1991). Identifying an elevated T/B ratio (meaning more than typical theta compared to the amount of beta) compared to typical controls appeared to be an accurate way to assess attention disorders.
In a large, blinded, multi-center validation of the theta/beta ratio compared to rating scales for assessing ADHD, the researchers calculated the sensitivity and specificity of measures. They found that the T/B ratio achieved superior results than commonly used rating scales (Snyder et al., 2008). With a sample size of 159 individuals, the EEG assessment showed a sensitivity of 87% and a specificity of 94%, for an overall accuracy of 89% compared to the next closest measure, the Connors’ Rating Scale – Teacher version (CRS – Teacher), with a sensitivity of 67% and specificity of 41%, for an overall accuracy of 58%.
The sensitivity of an assessment measure determines how accurately it identifies individuals known to have a particular condition. Specificity measures how accurately the measure correctly eliminates individuals without the condition from being identified as having the condition.
For example, the Conners Parent Rating Scale-Revised (CPRS-R) shows a sensitivity of 77%, correctly identifying 77 percent of clients with ADHD. It has a specificity of 73%, correctly identifying 73 percent of clients known not to have ADHD. The theta/beta ratio biomarker described above outperforms the CPRS-R by correctly identifying more true positive ADHD and negative non-ADHD clients (Chang et al., 2016).
However, Chang and colleagues note that the Child Behavior Checklist (CBCL), also included in their study, provides a more comprehensive analysis of the client and is more effective at identifying possible comorbidities often mistaken for the different subtypes of ADHD. They also note that using such a checklist approach provides the clinician with information they may not otherwise be able to identify in the clinical setting. It appears that a combined approach using a well-validated checklist in combination with objective measures such as the theta/beta ratio or other quantitative EEG assessment tools and a continuous performance test such as the Test of Variables of Attention (TOVA) would provide a comprehensive assessment for childhood disorders of attention.
In the research of Snyder and colleagues (2008), only 6% of typical children were incorrectly identified using the T/B ratio. This suggests that, at a minimum, a simple, single-channel EEG assessment should be included as a component of a comprehensive approach to identifying ADHD children before prescribing medication.
Interestingly, Van Son and colleagues (2019) expanded the associations that could be identified with the T/B ratio. They determined that a higher T/B ratio was negatively correlated with prefrontal executive control, including response inhibition and negative affect control. This suggests that excess theta in relation to beta activity could lead to greater impulsivity and to a lack of control of negative behaviors. They also found an association between higher T/B ratios and reward-motivated decision-making, possibly selecting immediate gratification at the expense of long-term benefit. Finally, they correlated the T/B ratio with increased mind wandering, decreased executive network functions, and increased default mode network (DMN) activity.
As with all the previous EEG frequencies and assessment measures, the correct amount of a particular frequency activity is important. For example, someone who lacks appropriate default mode functioning may experience a lack of the type of resting-state activity that appears to have a therapeutic effect. The DMN has also been called the resting state network (RSN) due to its functions that differ from task-oriented behaviors. It is thought to be important for a variety of reasons. Therefore, a person with a lower-than-typical T/B ratio may benefit from increased theta voltage and some training in activating the DMN. In contrast, an individual diagnosed with ADHD may benefit from training to reduce or inhibit excess theta voltage.
Temporal lobe theta likely reflects the hippocampal theta identified using depth electrodes. It appears to be associated with route finding and navigation, both hippocampal functions. Differential, interhemispheric (bipolar montage) training of temporal lobe areas in the theta frequency range has been a component of certain approaches to neurofeedback for some time (Othmer, 2007). This approach is used for various conditions, including migraine, tinnitus, PMS, and many others. Frequencies are adjusted to facilitate the optimal response and may range from the alpha frequencies through the theta frequencies down to the infra-low frequencies below 1 Hz.
Again, the analysis of theta activity is often aided by using a normative database. Otherwise, determining whether an amount is too high or too low in amplitude is difficult. Fortunately, for the T/B ratio assessment, Monastra (2001) has provided a table of values with three age ranges: 6-11 yrs, 12-15 yrs, and 16-20 yrs. The table of mean T/B power ratios is below.
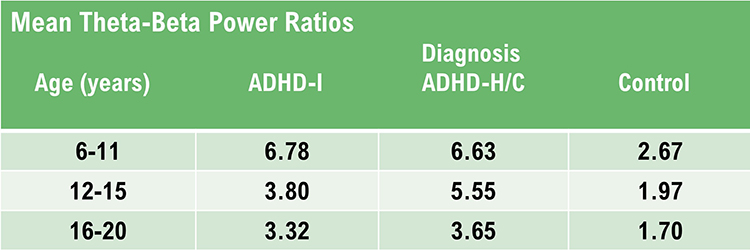
Caption: ADHD-I = attention deficit-hyperactivity disorder, inattentive type; ADHD-H/C = attention deficit-hyperactivity disorder, hyperactive-combined type.
Assessment of theta activity, more generally, beyond the T/B ratio in the central midline, is more challenging. As van Son (2019) noted, assessment under task may be essential to determine these results more accurately.
Two strategies to reduce high theta-to-beta ratios are amplitude training (down-training theta) and ratio training (rewarding decreases in the theta-to-beta ratio; Demos, 2019).
Cognitive Decline
Brief memory lapses (senior moments) are associated with LH bursts of rhythmic temporal theta (BORTTs) due to sleepiness or reduced hippocampal perfusion. Assessment and training should incorporate memory tasks. The protocol should inhibit LH temporal lobe theta, particularly at T3.
Where BORTTs are produced by drowsiness, training should include behavioral interventions to reduce insomnia (Demos, 2019).
While alpha/theta protocols to treat substance use disorders up-train theta, this should be proscribed in epilepsy in the frontal lobes, where it could impair attention or decisions, or in PSTD due to the risk of provoking flashbacks (Demos, 2019).
The movie below is a 19-channel BioTrace+ /NeXus-32 display of theta activity © John S. Anderson. Brighter colors represent higher theta amplitudes. Frequency histograms are displayed for each channel.
Substance Use Disorders
While alpha/theta protocols to treat substance use disorders up-train theta, this should be proscribed in epilepsy in the frontal lobes, where it could impair attention or decisions, or in PSTD due to the risk of provoking flashbacks (Demos, 2019). Using alpha-theta training protocols has generated caution from some practitioners and instructors, as indicated above in Demos (2019) and in conference presentations (personal experience). These cautions may result from a misunderstanding of the mechanism of alpha-theta training. In an upcoming post, John S. Anderson will address some of these issues and questions.
Final Notes
Recognition of standard EEG frequencies, analysis of such activity, and use of this information for training and re-assessment are important parts of neurofeedback practice. This section has attempted to provide an overview of this area to aid new practitioners and experienced clinicians in furthering their understanding of this complex study area.
One of the greatest benefits of neuroscience in general and electroencephalography and neurofeedback specifically is that they encourage lifelong learning. Suppose this section appeared overwhelming, with few hard and fast rules or concrete facts to hold on to. In that case, it is important to remember that one can do useful and effective neurofeedback without an in-depth understanding of this area. Understanding the EEG comes gradually through regular exposure to educational materials, lectures, and workshops, working with an experienced mentor, and regular interaction with clients and their EEG recordings. Pursuing and maintaining certification with the Biofeedback Certification International Alliance (BCIA) is a good way to engage in continuing professional education and lifelong learning.
There is no substitute for viewing large numbers of EEG recordings. As mentioned in the beginning, this can start with an EEG atlas and then progress to examining your own recordings, whether a single channel, a couple of channels, or multiple channels.
Patience with one’s process is an integral part of any learning experience. Think of the time it takes to learn any worthwhile skill, from swimming to learning a musical instrument to learning a new computer or phone operating system. Managing one’s expectations is crucial.
ALPHA (8-12 or 13 HZ)
Does the Alpha Rhythm Range From 8-12 or 8-13 Hz?
In electroencephalography (EEG), the alpha rhythm is generally defined as having a frequency range of 8-12 Hz or 8-13 Hz. This discrepancy arises from variations in historical definitions, regional practices, and updates in scientific standards.
Many sources traditionally define the alpha rhythm within the 8-12 Hz range. This is common in older literature and is used in some clinical contexts to describe the typical frequency band associated with a relaxed, awake state. For example, Jadeja (2021) describes clinically relevant EEG frequency bands, including the alpha rhythm, as 8-12 Hz.
More recent or alternative standards may extend this range to 8-13 Hz to accommodate a broader spectrum of alpha activity observed in various populations and conditions. For example, Kane and colleagues (2017) offered the following revised definition:
Rhythm at 8–13 Hz inclusive occurring during wakefulness over the posterior regions of the head, generally with maximum amplitudes over the occipital areas. Amplitude varies but is mostly below 50 µV in the adult, but often much higher in children. Best seen with the eyes closed, during physical relaxation and relative mental inactivity. Blocked or attenuated by attention, especially visual, and mental effort. Comment: use of term rhythm must be restricted to those rhythms that fulfill these criteria. Activities in the alpha band which differ from the alpha rhythm as regards their topography and/or reactivity, should either have specific appellations (for instance: the mu rhythm and alpha coma) or should be referred to as rhythms of alpha frequency or alpha activity.
Summary
The slight variation in definitions typically does not impact the practical applications significantly but reflects different methodological approaches or updates in EEG research standards. The choice of definition can depend on the specific requirements of a study or the preference of a clinical guideline being followed. Both ranges are correct.The Source of the Alpha Rhythm
Activity in the 8-12 Hz frequency range appears associated with reduced sensory and cognitive activity. Why is this so? What mechanism is responsible for this rhythmic activity?
One of the main communication pathways between the external world, the senses that perceive and transmit this information, and the cortical neurons that receive it is the thalamic-cortical relay system, often designated the TCR system.
The thalamus receives incoming sensory input (a paired structure in the brain's center. The individual nuclei of the thalamus transmit that sensory information to appropriate areas of the cortex. The occipital and parietal areas of the cortex are the primary visual processing areas, just as the temporal areas process most of the auditory information. In contrast, central Rolandic areas process tactile and other signals from the skin and muscles. Of course, current findings indicate that brain activity is associated with coordination within and between cortical networks and influences from subcortical structures and local and TCR influences. Still, the TCR system is a primary pathway for determining which cortex areas receive each type of sensory input.
When the eyes close, the neurons responsible for processing incoming visual information no longer have “ work” to do, and so they respond to another signal coming through the TCR system. This is a rhythmic signal mediated by a membrane of inhibitory GABAergic neurons that surround most of the thalamus and provide inhibitory regulation of the signals traveling to the cortex. This is called the reticular nucleus of the thalamus (TRN) or nucleus reticularis of the thalamus (NRT). The function of this system is much too complex for this section, but a good treatment is available in Crabtree (2018), and an examination of the role of the TCR and TRN systems in consciousness is found in Min (2010).
The rhythmic signal from the TCR and NRT interaction produces a 10-Hz (8-12 Hz range) input to the visual processing neurons when visual sensory input is withdrawn (eyes closing), resulting in those neurons firing synchronously in that frequency in response to this input. The voltage of a specific EEG frequency, measured at the scalp surface, is directly proportional to the number of cortical neurons firing synchronously in that frequency (Nunez & Srinivasan, 2006). Therefore, when the eyes are closed, and the signal from the TCR system changes from sensory input to a rhythmic 10-Hz input, visual neurons respond to that input, and the voltage of alpha, and most specifically in adults, 10-Hz activity increases in voltage. The image below shows a spectral display of an eyes-closed EEG. Graphic redrawn by minaanandag on Fiverr.com.
.jpg)
Caption: An eyes-closed EEG in a longitudinal bipolar montage is represented as a spectral display. The x-axis shows frequency from 0-30 Hz, and the y-axis shows absolute power (uV Sq). Note the peak at about 10 Hz. Voltage is higher in the P3-O1 derivation than in the P4-O2 derivation, revealing a small asymmetry. The highest power is in the T4-T6 derivation.
Identifying alpha activity in the scalp's parietal and/or occipital areas is usually quite easy, particularly in the eyes-closed condition. The image below shows an eyes-closed alpha pattern from a 15 -year-old male.
.jpg)
Caption: eyes-closed EEG filtered to 1-45 Hz in the longitudinal bipolar montage with a 50-uV scale. Boxes indicate the most prominent 8-12 Hz activity. This montage represents a series of adjacent electrode comparisons or derivations, as each signal tracing is derived from each pair of electrode comparisons.
A longitudinal bipolar montage is displayed below.
.jpg)
Caption: Longitudinal bipolar montage (commonly known as the “double banana” montage).
Observe that the rhythmic activity is well-defined and has the typical bursting or spindling pattern of the alpha rhythm, resulting from the input of the TCR and NRT systems. Spindling consists of a series of distinct oscillations of a particular frequency that begin with relatively low amplitude, increase in amplitude, and then decrease in amplitude, giving the appearance of a spindle such as one used in spinning, with fiber wound around it. Graphic © New Africa/Shutterstock.com.

The waves are quite sinusoidal (waving up and down in a smooth rhythm similar to a sine curve) and continue throughout the recording with minimal disruption. The voltage indicator shows that the maximum voltage at the moment of the line placement was from about 20 to 30 uV at the peak of the waveform in this montage.
One can determine the wave's frequency by counting the number of peaks in a one-second segment or counting the number of times the wave crossed the zero line and dividing by 2 (zero crossings/2). Either method gives an 8-9 Hz value when multiple one-second epochs are counted. This is somewhat slow for a 15-year-old, although the voltage appears to be within normal limits. When compared to a normative database, we can see that, indeed, it is a slow peak alpha when compared to other 15-year-old males, as indicated by the chart below from the NeuroGuide™ database.
Caption: the alpha peak frequency z-scores are at least 1 SD below expected values (> -1.0 SD) in all locations, and many areas show deviations exceeding the significance cutoff of -1.96 SD (blue highlight). The database also plots the theta peak frequency as fast (red highlight) due to slow alpha in the 8-Hz frequency bin or segment. There are likely other slow components of the dominant rhythm that contribute to this incorrect plot of the frequency information. Ideally, the peak frequency of the EEG should be calculated within a broad range from approximately 6 Hz to about 14-16 Hz to avoid this type of error. This is another problem with the somewhat arbitrary designation of frequency bands using set values.
The same data processed by the iSynchBrain database show similar findings for O1 and O2 below.
Caption: Peak frequency power and frequency comparisons at O1 and O2 show a slow peak frequency at 9.2 Hz bilaterally, resulting in z-scores of -1.15 and -1.23, respectively. Voltage (power) on the left is 0.63 SD, and on the right is 1.01 SD, showing slightly elevated values. This database provides single-Hz calculations for frequency and amplitude rather than using an arbitrary band to define alpha.
The alpha peak frequency is age-dependent, though not called "alpha" until the frequency reaches 8 Hz. It is designated as the posterior basic rhythm or posterior dominant rhythm (PDR) in early infancy. It appears around 4 months with a frequency of about 4 cycles per second (c/s) or Hz (Schomer & Lopes da Silva, 2017). The PDR increases (speeds up) during maturation and is approximately 6 c/s at 1 year and up to 8 c/s at around 3 years of age. This is when it can be called the alpha rhythm.
The frequency reaches 10 Hz by approximately 10 years of age, and that (10.2 ± 0.9/sec) is the peak frequency of adulthood (Petersén & Eeg-Olofsson, 1971). The previous example shows why a peak frequency between 8-9 Hz is slow for a 15-year-old.
The Alpha Frequency's Meaning and Importance
The speed or frequency of the alpha peak frequency is often mentioned, even in a neurologist’s report. For the neurofeedback practitioner, it is helpful to understand the factors associated with different alpha frequencies. The alpha peak frequency measures the frequency of the rhythmic pattern of the posterior rhythm (generally called alpha). This has traditionally been an important measure. Although it has recently been somewhat de-emphasized in some EEG circles, it remains an interesting measure. A great deal of research supports it as a useful metric for assessment.Researchers such as Klimesch and others (Haegens et al., 2014; Hanslmayr et al., 2005; Mierau et al., 2017) have found it important to first assess the individual peak alpha frequency for normal subjects before investigating the cognitive effects of alpha neurofeedback.
A slow peak alpha frequency has been associated with some forms of cognitive decline and memory impairment (López-Sanz et al., 2016), as well as mild traumatic brain injury (mTBI; Jabbari et al., 1985; Williams, 1941). A fast peak alpha frequency has been associated with improved scores on timed IQ tests. It has also been associated with enhanced memory and cognitive performance in various age groups (Grandy et al., 2013). A faster peak alpha frequency is associated with advanced reading skills in precocious children (Suldo et al., 2001).
Negative correlations of a peak alpha frequency faster than 10.5 Hz, possibly associated with an overly activated central nervous system, may include sleep initiation problems, anxiety, intrusive thoughts, and difficulty with self-soothing and self-calming skills.
The peak alpha frequency changes throughout the lifespan. Therefore, age-normed values for the peak alpha frequency are important for assessment purposes. The normal adult peak alpha frequency is 9.5-10.5 Hz (Jabbari et al., 1985). Scholarly literature states that the peak alpha frequency is not abnormal until it is below 8 Hz (Schomer & Lopes da Silva, 2017). However, it is commonly thought to be potentially meaningful when the frequency is below 9 Hz for an adult.
Angelakis et al. (2004) stated that slowing the alpha peak frequency by more than 1 Hz (9 Hz for an adult) is generally a sign of pathology. A slow alpha frequency can be associated with fatigue, cognitive decline, and memory impairment. Slowing of the background alpha rhythm is also a sign of generalized cerebral dysfunction (Nayak & Anilkumar, 2021). Rathee and colleagues (2020) related the speed of the peak alpha frequency to reading comprehension. They found that a slower peak alpha frequency is associated with poor comprehension.
Asymmetries in the posterior rhythm are common, and some are expected. Typically, the posterior rhythm has a higher voltage over the right, nondominant hemisphere. Surprisingly, a complete absence of the posterior rhythm can occur in a small percentage of otherwise normal individuals. While the absence of the posterior rhythm can also be seen in individuals with brain injuries or other abnormalities, such cases usually exhibit additional EEG abnormalities. Therefore, an EEG that only shows the absence of the posterior rhythm without other abnormalities should be considered normal (Libenson, 2024).
However, other sources correlate the absence of the posterior alpha rhythm with a variety of other indicators, even when additional abnormal EEG patterns are not present.
Niedermeyer (1997) discusses the absence of the posterior alpha rhythm and points out correlations between chronic alcoholism and vertebrobasilar artery insufficiency. He suggests that a lack of posterior alpha may be associated with dysfunctional synchronization mechanisms and states:
The crucial factor in these considerations might be the question: is absence of the alpha rhythms in the scalp EEG (and even in recordings from deeper structures) synonymous with alpha absence in the microstructure? In other words: is alpha rhythm a truly universal phenomenon in healthy persons regardless of EEG-alpha-absence caused by lack of synchronizing mechanisms? Accordingly, persons with an inherited low voltage fast pattern do have a posterior alpha but are unable to show it in their EEG records.Other conditions that show an absence of the posterior alpha rhythm include seizure disorders. Aich (2014) and colleagues found a significant correlation (<0.01) between the presence of seizure activity and the absence of the alpha rhythm. This study identified 48.3% of their identified seizure disorder participants as having no visible alpha rhythm.
Eyes-Closed Alpha Response Voltage Meaning and Importance
The amplitude of the activity can also be meaningful in addition to the peak frequency within the 8-12 or 8-13 Hz alpha band. Anxiety and TBI can reduce alpha activity during baselines. Since concentration and thinking can suppress alpha, instruct clients to refrain from these activities during recording (Demos, 2019).The alpha voltage will be partially affected by the montage that is used. For example, considering the discussion of differential amplifiers in the Instrumentation and Electronics section, the closer two electrodes are to each other, the more the rhythmic, synchronous patterns will be attenuated. Common-mode rejection (CMR) is most sensitive to frequency synchronization, so waves that are the same frequency and also synchronous (e.g., 10 Hz waves, waving up and down at the same time at the two sensor locations + and – [also commonly called “active” and “ reference”]) will be rejected. Comparing the O1 and O2 occipital electrodes to each other will result in a lower apparent alpha voltage if the two waveforms are synchronous, which is quite likely.
Conversely, comparing either O1 or O2 to an ear reference or possibly to a forehead reference would result in almost no rejection of alpha activity. Therefore, the rhythmic patterns are unlikely to be similar at these distant locations and will be retained. When viewing standard voltage information in an atlas, a research paper, or a textbook, try to identify the montage used when those standards were developed.
Simonova et al. (1967) found amplitudes between 20 and 60 μV in 66% of their subjects, while values below 20 μV were found in 28% and above 60 μV in only 6%. Schomer and Lopes da Silva (2017) suggest that values between 10 and 60 μV are typical. However, other sources such as the John Hopkins Atlas of EEG (2011) and Libenson’s (2024) Practical Approach to Electroencephalography (2nd ed.) cite 20 μV as the minimum voltage for adults. These differences may seem insignificant but can represent the difference between a low voltage fast EEG finding and a typical assessment.
Rhythmic alpha activity represents the synchronization of the EEG. It represents part of the excitation/inhibition cycle. When either large or small groups of neurons perform tasks, this results in the desynchronization of the EEG during work, as each group of neurons performs its function locally and independently. This is followed by a resting or inhibitory phase that results in the synchronization of the EEG and, hence, an increase in alpha amplitude as many neurons fire synchronously. This is seen in the shift from active visual processing when the eyes are open to a synchronous pattern of oscillatory activity when neurons do not have incoming visual input to process and can rest. This measure of alpha voltage change from eyes open to eyes closed, known as the alpha response, and the decrease of alpha with eyes opening, called alpha blocking, helps identify if the work/rest cycle is occurring correctly.
Someone with an eyes-closed posterior dominant rhythm voltage below 20 μV suggests to the neurofeedback assessor that the person does not easily shift to a state of decreased arousal/alertness necessary for the alpha amplitude to increase. The disconnection from the outside world upon eyes closing should result in decreased sensory processing of vision and other senses and decreased cognitive activity, leading to an increase in 8-12 Hz amplitude or power.
Typically, alpha activity voltage should increase as more neurons fire synchronously in this frequency. When this increase is less than 50% above the resting baseline eyes-open alpha voltage, it usually indicates some difficulty turning off the mind, meaning that neurons remain activated and working and thus prevent them from entering a resting state. The lack of a typical alpha increase may be associated with heightened states of alertness and vigilance, meaning that these clients maintain their external perceptive focus and/or cognitive activity, even when the eyes are closed, likely inhibiting the ability to achieve global synchronous activity. Resulting behavioral consequences can include fatigue, as the neurons are constantly engaged and are not allowed to rest. This pattern may be associated with a history of trauma and/or a history of hypervigilance for various reasons.
Example of a typical alpha response upon eyes closing.
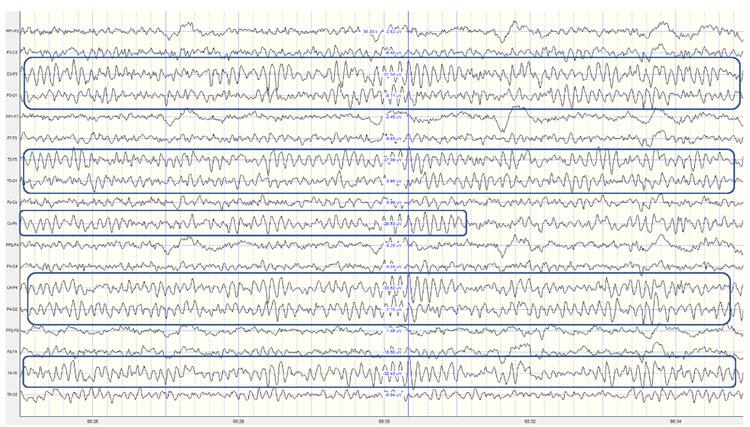
Caption: This is a 19-channel recording of the transition from eye-open to eye-closed conditions. It is a longitudinal bipolar montage, and the scale is 50 μV. Note that the eyes close at minute 2:40 (indicated by the eye movement), and the immediate response of the alpha rhythm appears.
The movie below is a 19-channel BioTrace+ /NeXus-32 display of alpha activity under eyes-closed and eyes-open conditions © John S. Anderson.
Alpha-Blocking Response Meaning and Importance
Conversely, the continued presence of alpha once the eyes are open suggests a lack of appropriate alpha blocking. This appears to result from a lack of inhibition of synchronous generator mechanisms. Hartoyo and colleagues (2020) showed a simple mechanism: excitatory input to inhibitory cortical neurons. This differs from the excitatory cortical neurons that typically reduce synchronous cortical firing in favor of local responses to incoming stimuli when the eyes are opened.
Note that activation of inhibitory mechanisms results in increased inhibition, even though the function is initially excitatory. Conversely, activation of excitatory mechanisms results in greater activation. This can seem confusing, and it may help to focus on the result, whether excitatory or inhibitory, rather than the initial behavior.
There should be a dynamic balance between excitation and inhibition in the human neocortex (Dehghani et al., 2016). When this balance is disrupted, we see the behavioral effects noted here. Alpha blocking represents the re-activation of visual processing neurons when visual input returns. Typically, as these neurons are no longer in a common or general resting state but are involved in task-oriented behaviors that are more localized, synchronous activity should decrease (be inhibited). Therefore, the overall voltage will decrease because of less synchronization. This does not imply that more neurons are firing when the alpha amplitude is higher; it means less synchronization and, hence, lower voltage.
Imagine an auditorium where everyone claps in unison (eyes-closed alpha rhythm). The noise is loud because the claps are synchronized (higher amplitude), with silence in between. Now, picture everyone clapping independently, perhaps in sync with immediate neighbors but not the whole audience. This resembles the eyes-open condition when recording the alpha rhythm: there is continuous noise because someone is always clapping, resulting in a faster frequency of claps. However, it’s never as loud as synchronized clapping, leading to lower overall voltage despite the higher frequency. Thus, alpha amplitude decreases quickly when eyes open, within 1-2 seconds, and certainly within 10-15 seconds. Delays in alpha blocking suggest difficulty returning to the task. Desynchronization occurs in posterior areas as visual processing begins when the eyes are opened.
The most common reasons for the lack of appropriate alpha blocking (meaning alpha activity persists after eyes are opened) are:
1. Fatigue, including sleep deprivation
2. Long-term meditation practice, particularly mantra meditation
3. Marijuana use and abuse, generally long-term, chronic
4. Cerebral dysfunction due to disease, injury, or possibly chemical exposure
Below is an example of correct alpha-blocking following the eyes opening.
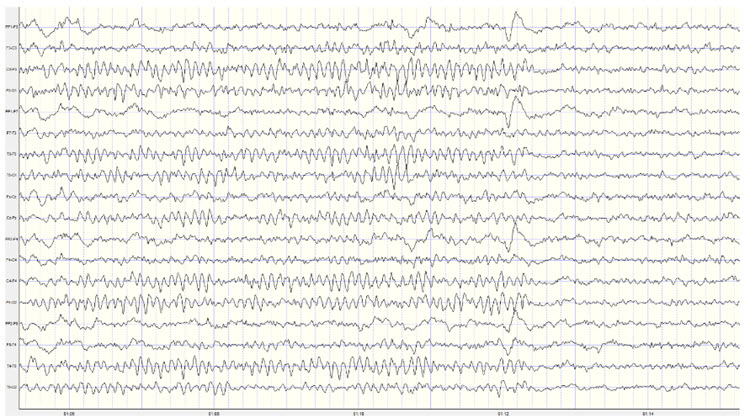
Caption: This is a 19-channel recording of the transition from eyes-closed to eyes-open conditions. This is a longitudinal bipolar montage, and the scale is 50 μV. Note the eyes opening at minute 1:12 (indicated by the eye movement) and the immediate blocking of the alpha rhythm.
Clearly, the response of 8-12 Hz EEG activity can be quite revealing and provides the clinician with helpful information about the client. However, it is important to note that other factors can affect the EEG recording. We have already noted the effects of artifacts on the EEG in general. Additionally, the client’s state of mind, level of anxiety, comfort with the application of sensors to the scalp, level of trust of the practitioner conducting the recording, amount of sleep, the use of caffeine and other stimulants and common medications can all affect the results of the recording.
Once an assessment is made of excess or deficient alpha activity, lack of an alpha response or persistent alpha following the eyes opening, or a slow or fast peak alpha frequency, the clinician can proceed with training to address these findings. There are multiple approaches to training the 8-12 Hz frequency band, including training specific segments of that band to achieve training goals. For example, if a lack of an alpha response to eyes closing is associated with anxiety and possibly insomnia, training for an increase in the 8-10 Hz portion of the posterior alpha rhythm in the eyes-closed condition may be an effective intervention. If the peak alpha frequency is slow, training for increases in the 10-12 Hz portion may help speed up this frequency. If there is persistent alpha in the eyes-open condition, inhibiting or downtraining 8-12 Hz generally may be helpful.
Of course, with any intervention, other causal factors must be addressed as well. Persistent alpha and/or frontal alpha can signify fatigue secondary to a sleep disorder such as sleep apnea. Therefore, a referral to a physician for a sleep study may be helpful. Over-arousal patterns that correspond to a lack of alpha response can be associated with a history of emotional, psychological, physical, or sexual trauma. The neurofeedback clinician may need to address these issues or refer the client to an appropriate therapist.
Summary
The alpha rhythm is characterized by a frequency range of 8-12 Hz or 8-13 Hz, with the discrepancy arising from variations in historical definitions, regional practices, and scientific standards. Traditionally, the 8-12 Hz range is used in older literature and clinical contexts. However, more recent standards extend this to 8-13 Hz to include a broader spectrum of alpha activity.
The alpha rhythm is primarily observed when a person is awake, relaxed, and with their eyes closed. This activity is mediated by the thalamic-cortical relay (TCR) system, involving the thalamus and cortical neurons. When visual input ceases (e.g., eyes closed), the reticular nucleus of the thalamus (TRN) sends rhythmic signals to the visual processing neurons, producing the alpha waves detected in EEG recordings.
Clinically, the alpha rhythm is crucial for diagnosing sleep disorders, cognitive function, and various neurological conditions. A slow peak alpha frequency is linked to cognitive decline and memory impairment, while a faster peak frequency correlates with better cognitive performance and higher IQ scores. Neurofeedback protocols often use individual peak alpha frequencies to enhance cognitive functions. Deviations from the normal alpha frequency range can indicate neurological or psychiatric conditions, such as generalized cerebral dysfunction or persistent alpha activity with eyes open.
The analysis of alpha rhythms in EEG is essential for clinical and research purposes, aiding in diagnosing and understanding various conditions and developing targeted treatments. The amplitude and frequency of alpha activity provide insights into a patient's cognitive and emotional state, supporting personalized therapeutic interventions. The importance of alpha rhythms underscores their role in cognitive neuroscience and clinical neurophysiology.
SENSORIMOTOR RHYTHM (12 or 13-15 HZ)
Sensorimtor rhythm (SMR) activity falls within the frequency range of 12 to 15 Hz, overlapping with the beta band's lower and alpha bands' higher ends.Please click on the podcast icon below to hear a lecture over the second half of Section C.

It is typically recorded over the sensorimotor cortex and characterized by rhythmic oscillations distinct from the higher-frequency beta and lower-frequency alpha waves (Sterman, 1996).
The SMR rhythm is generated by thalamic ventrobasal relay cells and reentrant thalamocortical loops (Thompson & Thompson, 2015). These circuits involve the thalamus sending sensory information to the cortex, where it is processed and sent back to the thalamus, creating rhythmic oscillations (Lopes da Silva, 1991). The sensorimotor cortex, particularly the primary motor cortex (M1) and the primary somatosensory cortex (S1), contains neurons that contribute to the generation of SMR. The synchronized activity of these neurons results in the rhythmic patterns observed in SMR (Pfurtscheller & Lopes da Silva, 1999).
The movie below is a 19-channel BioTrace+ /NeXus-32 display of SMR activity © John S. Anderson. Brighter colors represent higher SMR amplitudes. Frequency histograms are displayed for each channel. Notice the runs of high-amplitude SMR activity.
The Meaning of the Sensorimotor Rhythm
SMR activity is associated with a state of relaxed wakefulness and motor inhibition. The sensorimotor rhythm may signal an internal focus (Demos, 2019). It is believed to reflect the brain's ability to maintain a calm, focused state without engaging in motor activity. SMR is often considered a marker of optimal sensorimotor integration linked to several cognitive and motor functions.SMR is related to the suppression of motor activity, indicating a state where the brain is inhibiting unnecessary movements. This is important for stillness and precision tasks (Sterman, 1996). Increased SMR activity is associated with improved motor performance and reduced motor activity (Corsi-Cabrera et al., 2001; Sazgar & Young, 2019).
Increased SMR activity is associated with improved attention and focus, as it reflects a state where the brain is not distracted by extraneous motor activity and instead concentrates on sensory input and cognitive tasks (Egner & Gruzelier, 2001).
Psychological and Medical Disorders
Attention-Deficit Hyperactivity Disorder (ADHD)
Individuals with ADHD often exhibit reduced SMR activity. Neurofeedback training to increase SMR can improve attention and behavioral control (Lubar, 1991). Based on six RCTs, Stefanie Enriquez-Geppert and colleagues (2023) rated neurofeedback for ADHD as efficacious and specific in Evidence-Based Practice in Biofeedback and Neurofeedback(4th ed.).Generalized Seizures
Abnormal SMR activity can be indicative of dysfunctional thalamocortical circuits, which are often implicated in the generation of epileptic seizures. Modulating SMR activity through neurofeedback or other interventions has been explored as a potential therapeutic approach for reducing seizure frequency and severity (Moffet et al., 2017).Frey (2023) rated SMR-based and slow cortical potential (SCP)-based neurofeedback as efficacious for seizures, SMR-based NFB as probably efficacious for non-seizure manifestations of epilepsy, and connectivity-based NFB as not empirically supported for seizures.
In two meta-analyses (Soroush et al., 2023; Tan et al., 2009), SMR- and SCP-based treatments were associated with fewer seizures. In a sham-controlled RCT, neurofeedback for pediatric focal epilepsy was associated with improved quality of life, but only SMR neurofeedback improved cognition (Morales-Quezada et al., 2019).
Anxiety and Depression
SMR neurofeedback has also been explored as a treatment for anxiety and depression. Increasing SMR activity can help alleviate symptoms by promoting a state of calm and reducing hyperarousal (Hammond, 2005).Sleep
During sleep, particularly in non-rapid eye movement (NREM) sleep, SMR activity is less prominent than during wakefulness. However, the broader beta band, which includes SMR frequencies, shows distinct patterns across different sleep stages. For instance, beta activity is generally reduced during NREM sleep but can show transient increases during sleep spindles, which are bursts of oscillatory brain activity during stage 2 NREM sleep (Krystal et al., 2002; Merica & Fortune, 2005).SMR activity plays a role in sleep regulation and quality. Higher levels of SMR activity are associated with better sleep onset and maintenance. Individuals with higher SMR activity tend to fall asleep more easily and have more stable sleep patterns (Hoedlmoser et al., 2008). SMR neurofeedback training has been shown to improve sleep quality by increasing SMR activity, leading to deeper and more restorative sleep (Cortoos et al., 2010).
Performance
Enhancing SMR activity through neurofeedback training has been shown to improve cognitive performance, including memory, attention, and executive function. This is likely due to the increased focus and reduced distractibility associated with higher SMR levels (Vernon et al., 2003).Increased SMR activity is associated with improved motor performance, particularly in tasks requiring fine motor control and precision. This is because SMR reflects a state of motor inhibition, allowing for more controlled and deliberate movements (Gruzelier et al., 2014).
The Sensorimotor Rhythm and Sleep Spindles
Sleep spindles and sensory-motor rhythm (SMR) share several characteristics and underlying mechanisms, highlighting their interconnected roles in brain function, particularly in relation to sleep. The sleep spindle graphic © eegatlas-online.com..png)
Similarities and Overlapping Features
Both SMR and sleep spindles operate within overlapping frequency ranges. SMR typically ranges from 12 to 15 Hz, while sleep spindles are generally observed in the 12 to 16 Hz range. This overlap suggests a possible functional and mechanistic connection between the two types of brain activity (De Gennaro & Ferrara, 2003; Sterman, 1996).Thalamocortical circuits generate both SMR and sleep spindles. The thalamus, particularly the thalamic reticular nucleus, is crucial in generating sleep spindles by interacting with the cortex to produce rhythmic bursts of activity. Similarly, the thalamocortical loops involved in SMR generation facilitate synchronized oscillations in the sensorimotor cortex (Steriade et al., 1993; Lopes da Silva, 1991).
SMR is associated with relaxed wakefulness and motor inhibition, which can facilitate the transition to sleep. Sleep spindles occur during stage 2 of non-REM sleep and are associated with maintaining sleep, particularly by protecting the sleeper from external stimuli and aiding sleep consolidation (De Gennaro & Ferrara, 2003; Hammond, 2005).
Functional Roles
Sleep spindles play a critical role in maintaining sleep stability by reducing the brain’s responsiveness to external stimuli. SMR, by promoting a calm and relaxed state, may facilitate the onset of sleep and enhance the stability of the sleep cycle, thereby supporting the generation of sleep spindles (Hoedlmoser et al., 2008).SMR and sleep spindles are implicated in memory consolidation processes. Sleep spindles are known to be involved in consolidating declarative and procedural memories during sleep. SMR, by supporting focused attention and cognitive control during wakefulness, may indirectly enhance memory processes by optimizing brain function before sleep (Marshall & Born, 2007; Vernon et al., 2003).
Neurofeedback training targeting SMR has been shown to improve sleep quality and cognitive performance. Similarly, interventions aimed at enhancing sleep spindle activity have been explored for their potential to improve memory and cognitive function. The shared thalamocortical mechanisms suggest that training in one of these rhythms could potentially influence the other, offering combined benefits for sleep and cognitive health (Cortoos et al., 2010; Hoedlmoser et al., 2008).
Summary
SMR EEG activity, occurring in the frequency range of 12 to 15 Hz, is primarily generated by neurons in the sensorimotor cortex, particularly those involved in motor control and sensory processing. Thalamic ventrobasal relay cells and reentrant thalamocortical loops are crucial in generating SMR. The thalamus sends sensory information to the cortex, which processes it and sends it back to the thalamus, creating rhythmic oscillations. The synchronized activity of neurons in the primary motor cortex (M1) and primary somatosensory cortex (S1) results in the rhythmic patterns observed in SMR.SMR activity is associated with relaxed wakefulness and motor inhibition, reflecting the brain's ability to maintain a calm, focused state without engaging in motor activity. It is considered a marker of optimal sensorimotor integration linked to several cognitive and motor functions, such as improved attention, focus, and motor performance.
In psychological and medical disorders, SMR activity is often reduced in individuals with ADHD, but neurofeedback training can improve attention and behavioral control. Abnormal SMR activity is also implicated in generalized seizures, anxiety, and depression, with neurofeedback training showing potential therapeutic benefits.
SMR plays a role in sleep regulation and quality. Higher SMR levels are associated with better sleep onset and maintenance, and SMR neurofeedback training has been shown to improve sleep quality, leading to deeper and more restorative sleep.
Enhancing SMR activity through neurofeedback training improves cognitive performance, including memory, attention, and executive function, as well as motor performance, particularly in tasks requiring fine motor control and precision.
SMR and sleep spindles share several characteristics and underlying mechanisms, particularly their generation by thalamocortical circuits. Both play critical roles in maintaining sleep stability and are involved in memory consolidation processes. Neurofeedback training targeting SMR can improve sleep quality and cognitive performance, and interventions to enhance sleep spindle activity show similar benefits.
BETA (over 12 HZ)
The beta rhythm exceeds 12 Hz with 2-20 microvolts asynchronous waves. Asynchronous means the neurons depolarize and hyperpolarize independently.
The beta rhythm in EEG is typically defined within the frequency range of 13-30 Hz. However, some sources may use slightly different ranges. The variation in definitions can be attributed to differences in historical research, regional practices, and specific research contexts (Jadeja, 2021).
The beta rhythm is distributed across the scalp, with the highest amplitude in the frontal and precentral areas.
Beta Rhythm Generators
Whereas the sensorimotor cortex generates the sensorimotor rhythm, beta is generated in the brainstem and cortex. Ascending sensory traffic from the brainstem can override thalamic pacemakers (Andreassi, 2000), and specific cortical regions can produce localized beta activity beneath active electrodes (Thompson & Thompson, 2015). Reticular activating system graphic redrawn by minaanandag.

Kane and colleagues (2017) offered the following revised definition:
Any EEG rhythm between 14 and 30 Hz (wave duration 33–72 ms). Most characteristically recorded over the fronto-central regions of the head during wakefulness. Amplitude of fronto-central beta rhythm varies but is mostly below 30 µV. Blocking or attenuation of the beta rhythm by contralateral movement or tactile stimulation is especially obvious in electrocorticograms. Other beta rhythms are most prominent in other locations or are diffuse, and may be drug-induced (for example alcohol, barbiturates, benzodiazepines and intravenous anaesthetic agents).
Beta is also associated with the negative shift of the DC gradient (Speckmann et al., 2017). The DC gradient, only measurable by a DC-coupled EEG amplifier, measures the overall electrical gradient of cortical areas under the recording sensors. This gradient shows a slow oscillation of less than 1 second and is usually in the 0.1-0.2 Hz range. A shift of the gradient from its current state, becoming more electrically positive or negative, occurs every 5-10 seconds and sometimes quite a bit less often.
Buzsaki (2011) states that there is a progression of frequencies whose bandwidths overlap and interact with each other, from frequencies that take 15-40 seconds to complete each cycle up to those that oscillate at 200-600 cycles per second. Gunkelman (2005) called beta and gamma activity “emergent properties of bound networks.” This means that, as slower frequencies of EEG synchronize across networks, beta and gamma emerge in bursts of activity in coordination with that synchrony.
Beta activity is associated with "work" and reflects the ongoing excitatory/inhibitory cycles occurring on multiple time scales. While the work/rest cycle mentioned in relation to the alpha response and alpha blocking is one type of excitatory/inhibitory cycle on a broader scale, beta activity reflects actions that occur millisecond by millisecond and appear to be more locally generated.
Below is an example of eyes-open beta activity.
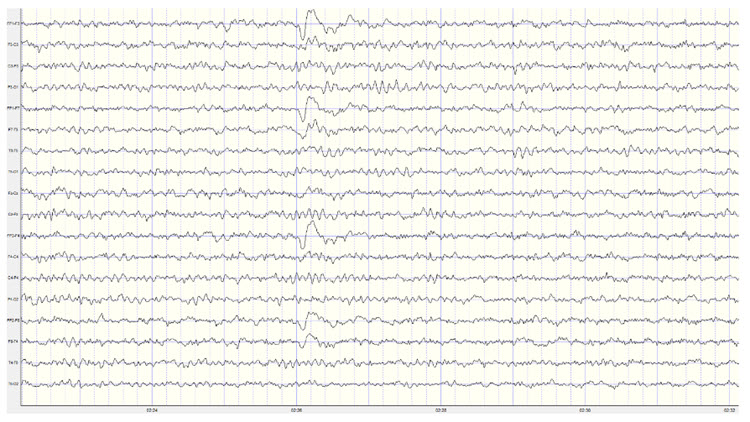
Caption: This is a 19-channel EEG recording in the eyes-open condition. This is a longitudinal bipolar montage, and the scale is 50 μV. Observe the alpha activity continuing at a much lower voltage in parietal and occipital derivations, compared to the previous eyes-closed examples, resulting from the attenuation of alpha with the opening of the eyes. There is beta activity in the 20-30 Hz range at less than 10 μV, seen mostly in frontal, central, parietal, and temporal derivations (comparisons between adjacent sensors) and somewhat slower 15-20 Hz patterns.
Kropotov (2016) describes the existence of several beta rhythms with different frequencies, various locations, and distinct functions. From this information, he states there is likely no single neuronal mechanism for generating localized beta activity. This fits the understanding that beta activity is associated with local tasks and, therefore, is mediated more by local mechanisms, with overall coordination from network systems and other rhythmic activity.
Beta is divided into narrower bands associated with cortical performance and subjective experience. These bands include low beta, high beta, and gamma. Demos (2019) advises that you should specify the actual range of interest.
Low Beta (16-20+ Hz)
The cortex produces low beta when we solve problems like multiplication. When a child correctly answers a math problem, the 17-Hz amplitude may increase while theta and 8-10 Hz alpha amplitude simultaneously decrease. While detected between 12-15 Hz, it is less often seen above 20 Hz (Thompson & Thompson, 2015).High Beta (20-35 Hz)
High beta is correlated with multi-tasking and optimal performance and anxiety, migraine, obsessive-compulsive disorder (OCD), rumination, and worrying. While elevations may indicate a range of disorders, they may represent the brain's compensation for elevated theta. Clinicians rarely reinforce high beta. Instead, they may inhibit high beta and theta (Demos, 2019).Beta Spindles
Beta spindles are trains of spindle-like waveforms with frequencies lower than 20 Hz but more often fall between 22 and 25 Hz. Beta spindles may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia (Arns et al., 2015; Demos, 2019; Thompson & Thompson, 2015).
19-21 Hz or 20-23 Hz
Clients diagnosed with anxiety disorder frequently show increased power in the 19-21 Hz or 20-23 Hz range compared with 16-18 Hz. Elevations in these ranges may be associated with emotional intensity. The Thompsons (2015) advise clinicians to use open-ended questions to question clients about their mental activity and emotional state when these bands are elevated without telegraphing their expectations.24-36 Hz
Clients who are distressed, hypervigilant, and who overthink, worry, and ruminate may show marked elevations in this range. A peak in this range may be associated with family or personal substance use disorder and may indicate the instrumental use of drugs to control anxiety. High-amplitude beta in this range is not always a negative indicator since it is also observed when highly intelligent individuals multi-task (Thompson & Thompson, 2015).The movie below is a 19-channel BioTrace+ /NeXus-32 display of low beta (13-21 Hz) and high beta (22-34 Hz) activity © John S. Anderson. Brighter colors represent higher beta amplitudes. Frequency histograms are displayed for each channel.
Beta Rhythm Abnormalities
High voltage or abundant beta activity is the most common fast activity abnormality in EEGs due to pharmacologic effects. Benzodiazepines (e.g., diazepam, lorazepam) and barbiturates (e.g., phenobarbital) often cause this increase, as they are used for seizure management. This drug-induced beta activity is usually diffuse or frontally predominant and can also appear transiently during drowsiness, subsiding with deeper sleep (Libenson, 2024).
When beta activity is typical for the client based on age, state (eyes-open, eyes-closed, under task, etc.), and location, it suggests those areas are functioning as expected. If beta activity is deficient, that may mean that the site is under-functioning or under-activated for some reason. Reasons may include damage of some sort, metabolic deficits, fatigue, or other factors. An area that is consistently over-functioning may, in time, because of overuse and fatigue, end up with a lower level of functioning and, hence, less beta activity.
Higher-than-typical beta amplitude at a given location may mean the area is over-functioning or overly activated. Whether the beta amplitude is higher or lower than average, the clinician will want to understand the underlying functional neuroanatomy of the area to aid in the assessment process. For example, suppose the right posterior temporal/parietal junction that roughly underlies the area between T4 and T6 (TP8 in the 10-10 system) shows excess beta amplitude. In that case, it may be associated with heightened sensitivity to or attention to non-verbal communication, such as tone of voice, facial expression, and body language associated with angry outbursts or that may signal danger. If this area is under-activated, it may represent a self-protective “disconnection” from these same signals (Gunkelman, 2021).
Individuals who typically show excess beta activity at sleep onset and during sleep stages have a higher incidence of insomnia (Perlis et al., 2001). Meier and colleagues (2014) found a correlation between excess beta power in frontal, central, and temporal areas with delinquent behavior in adult men with concurrent ADHD symptomatology. In Rowan’s Primer of EEG (2nd ed.), Marcuse and colleagues (2016) identify excess interhemispheric beta asymmetry as an important diagnostic tool. The side with reduced relative beta power points to the pathological hemisphere. They identify brain abscesses, stroke, tumors, vascular malformations, and cortical dysplasia as associated with a focal decrease or enhancement of beta activity.
A reference to a normative database can be useful when assessing beta activity, particularly if a traumatic brain injury is suspected. Comparison with other EEG frequencies is also important, as an excess or lack of beta activity often accompanies differences from expected values for other frequencies.
Significant cerebral dysgenesis, like lissencephaly, can cause increased beta activity, typically accompanied by other EEG abnormalities and intellectual disability. In such cases, the beta activity is often slower. Increased beta activity can also appear in normal EEGs without explanation, potentially due to incomplete medication lists or residual drug effects.
Reductions in beta activity frequency (18-30 Hz) often indicate diffuse cortical injury, such as post-anoxic episodes, or the effects of sedative medications. Severe cortical injuries may show an absence of beta activity, though some patients naturally exhibit less beta activity as a normal variant. Low amplifier gains can obscure normal fast activity in EEG traces with high-voltage slow activity.
A true asymmetry in fast activity between brain regions is a significant abnormality, suggesting potential cortical damage. Fast waves are generated at the cortical level, reflecting the activity of circuits near the scalp surface rather than deeper or more medial regions of the brain. Asymmetry in fast activity often indicates cortical damage, with the area of lower voltage marking the abnormality, such as in the case of a cortical stroke where beta activity is reduced. Rarely, an abnormal cortical area may show increased fast activity.
Spindling Excessive Beta (SEB)
Spindling excessive beta (SEB) refers to a specific pattern observed in electroencephalogram (EEG) recordings characterized by high-frequency beta waves (13-30 Hz) that display a spindle-like appearance. These beta spindles often exceed 20 μV in amplitude and can occur in both frontal and central regions of the brain. SEB is distinguished from typical beta activity by its spindling morphology and higher amplitude (Arns et al., 2015; Krepel et al., 2021). Beta spindle graphic retrieved from ADDYSSEY.
SEB appears to be a transdiagnostic EEG feature, meaning it is observed across various psychiatric disorders and is not limited to a single diagnosis (Arns et al., 2015; Krepel et al., 2021). SEB has been associated with a range of psychiatric and neurological conditions, making it a critical biomarker in clinical neurophysiology. Its clinical importance is primarily seen in sleep disorders, Attention-Deficit Hyperactivity Disorder, medication resistance, and epilepsy.
Sleep Disorders
SEB is often linked with sleep disturbances, particularly insomnia. Insomnia complaints are more prevalent in individuals with frontal SEB patterns (Arns et al., 2015). Studies indicate that individuals with SEB may experience difficulties in maintaining sleep, leading to chronic insomnia (Swatzyna et al., 2022). SEB may be a marker of hypoarousal or sub-vigil states.Attention-Deficit/Hyperactivity Disorder (ADHD)
There is a notable association between SEB and ADHD. Patients exhibiting SEB often report symptoms of inattention, hyperactivity, and impulsivity. Children with excess beta activity in their EEGs are a small subset of ADHD children, similar to other ADHD children but more prone to temper tantrums and moodiness (Clarke et al., 2001). This association suggests that SEB can be used as an objective marker for ADHD diagnosis and management (Swatzyna et al., 2022).Medication Resistance
SEB is frequently observed in patients who have failed multiple medication trials for various psychiatric conditions. This resistance to medication highlights the need for alternative therapeutic approaches for individuals exhibiting SEB (Swatzyna et al., 2022).Epilepsy
Beta spindles can co-occur with seizure disorders, although patients with a history of seizures were excluded from some SEB studies to isolate its effects in other conditions. The presence of SEB in epilepsy patients can complicate the clinical picture, necessitating careful EEG interpretation (Swatzyna et al., 2022).Mechanisms and Hypotheses
SEB might reflect a sub-vigil state, which is an intermediate state between wakefulness and sleep. This state can lead to cognitive impairments and behavioral disturbances observed in conditions like ADHD and insomnia (Swatzyna et al., 2022). Benzodiazepines and other substances affecting GABAergic transmission can induce SEB, suggesting a neurochemical basis for its occurrence. This insight provides avenues for pharmacological interventions targeting the GABA system (Swatzyna et al., 2022).Treatment Implications
Recognizing SEB in patients can influence treatment strategies. For instance, behavioral interventions targeting sleep hygiene may benefit those with SEB-related insomnia. Similarly, neurofeedback and other non-pharmacological treatments might offer alternative approaches for ADHD patients exhibiting SEB, especially those resistant to conventional medications (Swatzyna et al., 2022).Conclusion
SEB is a distinctive EEG pattern with significant clinical implications across various psychiatric and neurological disorders. Its presence can aid in the diagnosis, understanding, and treatment of conditions such as insomnia, ADHD, and medication-resistant psychiatric disorders. Continued research into SEB will further elucidate its mechanisms and optimize therapeutic strategies.Summary
The beta rhythm, typically defined within the 13-30 Hz range, is characterized by asynchronous waves with amplitudes between 2-20 microvolts. Asynchronous in this context means that neurons depolarize and hyperpolarize independently. The brainstem and cortex generate the beta rhythm, with the highest amplitude in the frontal and precentral areas. Beta activity reflects ongoing excitatory and inhibitory cycles, correlates with tasks and states such as attention, problem-solving, and anxiety, and is sensitive to pharmacological influences. Beta abnormalities can indicate various clinical conditions, including insomnia, ADHD, and epilepsy.
Spindling Excessive Beta (SEB) is a distinct EEG pattern characterized by high-frequency beta waves (13-30 Hz) with a spindle-like appearance, often exceeding 20 μV in amplitude. SEB is a transdiagnostic feature associated with psychiatric and neurological conditions such as insomnia, ADHD, medication resistance, and epilepsy. SEB is thought to reflect sub-vigil states, which are intermediate between wakefulness and sleep, potentially leading to cognitive impairments and behavioral disturbances. Recognizing SEB can influence treatment strategies, including behavioral interventions for sleep and neurofeedback for ADHD. Continued research is essential to further elucidate the mechanisms and therapeutic approaches for SEB.
GAMMA (28-80 Hz)
Gamma ranges from 30-100 Hz and includes the Sheer rhythm, which extends from 38-42 Hz (Buzsáki & Wang, 2012; Fries, 2015; Jensen et al., 2007). The difference in reported gamma frequency bands may reflect methodological differences and biological variability.
Methodological Differences
Different EEG systems and setups can have varying sensitivities and resolutions, affecting the frequency range they can accurately capture. High-density EEG systems may detect higher frequencies more reliably than standard setups (Hari & Puce, 2017; Nunez & Srinivasan, 2006).Data processing variations, such as filters, window lengths, and analytical algorithms, can influence the detection and categorization of gamma frequencies. High-frequency oscillations can be more susceptible to noise and artifacts, such as muscle activity (EMG) or electrical interference. Different studies may apply varying degrees of filtering and artifact rejection, affecting the upper limit of detectable gamma frequencies (Niedermeyer & da Silva, 2004).
For these reasons, different studies might use different criteria for identifying gamma oscillations.
Biological Variability
Due to genetic, developmental, and health-related factors, the EEG patterns of different individuals can be significantly variable. This can lead to different observed upper limits for gamma oscillations (Başar, 2012; Ulhaas & Singer, 2010).The cognitive or sensory task during EEG recording can affect the frequency and power of gamma oscillations. Different tasks may induce gamma activity in different frequency ranges.
Gamma oscillations can vary across different regions of the brain. For instance, the gamma frequency range observed in the visual cortex during visual processing tasks may differ from that observed in the hippocampus during memory tasks.
The overlap between gamma (30-100 Hz) and surface EMG (SEMG) (13-200 Hz) requires that clinicians examine the raw EEG to ensure that training does not reward muscle contraction. The movie below is a 19-channel BioTrace+ /NeXus-32 display of gamma activity © John S. Anderson. . Brighter colors represent higher gamma amplitudes. Frequency histograms are displayed for each channel. Notice that the mean frequency falls between 38 and 39 Hz.
This section delves into the generators of gamma activity, its meaning in the context of brain function, and recent findings in the field. Research has linked gamma activity to cortical activation, cognitive processes, attention, emotion, and motor functions, with implications for conditions like ADHD, GAD, and epilepsy.
Gamma Rhythm Generators
Gamma oscillations are primarily generated by the synchronized activity of excitatory and inhibitory neurons in the brain, particularly within the cortical regions. The interplay between pyramidal neurons and interneurons, especially those expressing parvalbumin, is crucial for generating these high-frequency oscillations. These interneurons provide rhythmic inhibitory input to pyramidal cells, creating a network capable of producing gamma waves (Buzsáki & Wang, 2012).Thalamocortical interactions also play a role in gamma generation. The thalamus, acting as a relay station for sensory information, can influence cortical gamma activity through its connections with the cortex. This interaction is particularly evident in sensory processing areas, where gamma oscillations are modulated by sensory input (Fries, 2015).
Furthermore, gamma activity is not uniformly distributed across the brain. Still, it is particularly prominent in regions involved in higher-order cognitive functions, such as the prefrontal cortex, hippocampus, and visual cortex. These areas utilize gamma oscillations for attention, memory encoding, and sensory perception (Bosman et al., 2014).
The Meaning of Gamma EEG Activity
Gamma oscillations have been implicated in various cognitive, perceptual, and motor functions, emotions, epilepsy, schizophrenia, Alzheimer's disease, and ADHD.Binding Sensory Information
One of the primary roles of gamma activity is in binding sensory information. This concept, known as the binding problem, refers to how the brain integrates information from different sensory modalities to create a coherent perceptual experience. Gamma synchrony is thought to facilitate this process by providing a temporal framework that binds disparate neural signals (Singer, 2013).Attention
In the context of attention, gamma oscillations have been shown to enhance the processing of relevant stimuli while suppressing irrelevant information. This selective attention mechanism is believed to be mediated by synchronizing gamma activity between different brain regions, thereby enhancing communication and processing efficiency (Jensen et al., 2007). Enhanced gamma activity is associated with better performance on cognitive tasks (Hasib et al., 2023).Working and Long-Term Memory
Gamma activity is crucial for working memory and long-term memory encoding. Studies have demonstrated that gamma oscillations in the hippocampus and prefrontal cortex are involved in maintaining and manipulating information in working memory and encoding and retrieving long-term memories (Colgin, 2016).Motor Performance
Gamma event-related synchronization (ERS) is linked to motor performance, with distinct low (35-50 Hz) and high (75-100 Hz) gamma bands showing different temporal and spatial characteristics during motor tasks (Amo et al., 2016, 2017; Crone et al., 1998). Enhanced gamma activity is observed during voluntary movements (Amo et al., 2016).The Sheer rhythm is also associated with peak performance (Spydell, Ford, & Sheer, 1979). For example, 40-Hz activity is generated when athletes correct their balance after leaning forward on a balance board. Athletes who have sustained a concussion and suffer impaired balance do not increase 40-Hz power (Demos, 2019; Thompson & Thompson, 2015).
Emotions
Gamma fluctuations are associated with emotional experiences, with higher gamma activity linked to difficult emotions during worry inductions in GAD patients (Oathes, 2008).Epilepsy
Increased gamma activity is noted in patients with primary generalized epilepsy, potentially serving as a marker for underlying dysfunctions and a prerequisite for seizures (Willoughby et al., 2003).Schizophrenia
In schizophrenia, for example, deficits in gamma oscillations are thought to contribute to the cognitive impairments and sensory processing anomalies observed in patients (Uhlhaas & Singer, 2010).Alzheimer's Disease
Recent studies have suggested that gamma oscillations might play a role in clearing amyloid-beta plaques, a hallmark of the disease. Optogenetic stimulation of gamma oscillations in mouse models has reduced amyloid-beta levels, pointing to potential therapeutic avenues (Iaccarino et al., 2016).ADHD
Reduced gamma activity is observed in adults with ADHD, particularly in the right centroparietal region, correlating with symptom severity and suggesting altered network function (Tombor et al., 2019).Recent Findings
Advancements in neuroimaging and electrophysiological techniques have allowed for more precise mapping of gamma activity and its sources. Magnetoencephalography (MEG) and high-density EEG have provided more detailed spatial and temporal resolution, enhancing our understanding of gamma oscillations in health and disease (Hari & Puce, 2017).Conclusion
Gamma oscillations in EEG, ranging from 30-100 Hz, are critical for numerous brain functions, including cognitive processes, attention, memory, motor functions, and emotional experiences. This range includes the Sheer rhythm (38-42 Hz). Variations in reported gamma frequency bands stem from differences in EEG methodologies, such as system sensitivity and data processing techniques, and biological variability among individuals. High-density EEG systems can capture higher frequencies more reliably than standard setups. Gamma oscillations are generated by the synchronized activity of excitatory and inhibitory neurons, particularly in cortical regions, and their interplay is essential for producing these high-frequency waves.Gamma oscillations play a vital role in binding sensory information, a process known as the binding problem, which integrates information from different sensory modalities to create a coherent perceptual experience. In the context of attention, gamma oscillations enhance the processing of relevant stimuli while suppressing irrelevant information, thereby improving cognitive task performance. Gamma activity is crucial for both working memory and long-term memory encoding, particularly in the hippocampus and prefrontal cortex.
Motor performance is also linked to gamma oscillations, with distinct low (35-50 Hz) and high (75-100 Hz) gamma bands showing different characteristics during motor tasks. The Sheer rhythm is associated with peak performance, such as athletes correcting their balance, where increased 40-Hz activity is observed. Emotional experiences are also linked to gamma fluctuations, with higher gamma activity correlating with difficult emotions in conditions like generalized anxiety disorder (GAD).
Gamma oscillations are implicated in several neurological and psychiatric conditions. In epilepsy, increased gamma activity is observed and may serve as a marker for underlying dysfunctions. In schizophrenia, deficits in gamma oscillations contribute to cognitive impairments and sensory processing anomalies. Alzheimer's disease research suggests that gamma oscillations might help clear amyloid-beta plaques, pointing to potential therapeutic avenues. In ADHD, reduced gamma activity is noted, particularly in the right centroparietal region, correlating with symptom severity.
Recent advancements in neuroimaging, such as magnetoencephalography (MEG) and high-density EEG, have improved the spatial and temporal resolution of gamma activity mapping, enhancing the understanding of gamma oscillations in both healthy and diseased states. These technologies allow for more precise identification of gamma rhythm sources and their roles in brain function.
Gamma oscillations' importance in various brain functions and their implications in several conditions highlight the need for continued research to fully elucidate their mechanisms and therapeutic potential.
Waveform Morphology
A wave is a change in the potential difference between two EEG electrodes. Waveform and morphology refer to the shape of the signal generated by oscillating potential differences. Neurofeedback professionals examine the raw waveform's morphology before considering the filtered and quantified EEG (Demos, 2019). EEG activity means a single wave or series of waves (Fisch,1999).
We can distinguish between regular and irregular activity. A regular or monomorphic series of waves are rhythmic with the same frequency and morphology. Rhythmic waves that resemble sine waves are called sinusoidal.
Regular waves may be arch-shaped and resemble wickets. Graphic © eegatlas-online.com.
Saw-toothed waves resemble asymmetrical triangles. Graphic © eegatlas-online.com.
Irregular waves continuously change shape and duration. The graphic below shows runs of irregular high-amplitude delta waves.
EEG waves may be monophasic or polyphasic. Monophasic waves possess a single upward or downward deflection. Two polyphasic waveforms containing waveforms with two or more elements are diphasic and triphasic waves. Diphasic waves have two elements--one positive and one negative. Triphasic waveforms contain three elements with alternating directions.
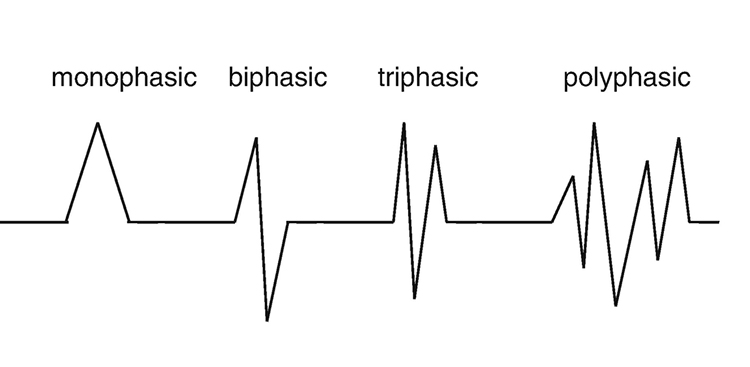
A transient is a single wave or series of waves distinct from background EEG activity.
Sharp transients are waves with steep peaks that are not produced by epilepsy.

In contrast, epileptiform activity consists of spikes and sharp waves. An epileptiform spike has a sharp appearance and lasts 20-70 ms. Watch the Blausen Epilepsy and Seizure animations. Graphic © eegatlas-online.com.
Less steeply shaped sharp waves last 70-200 ms. Complex denotes a series of waves that share a similar shape. See the K-complex in the EEG record below. Graphic © eegatlas-online.com.
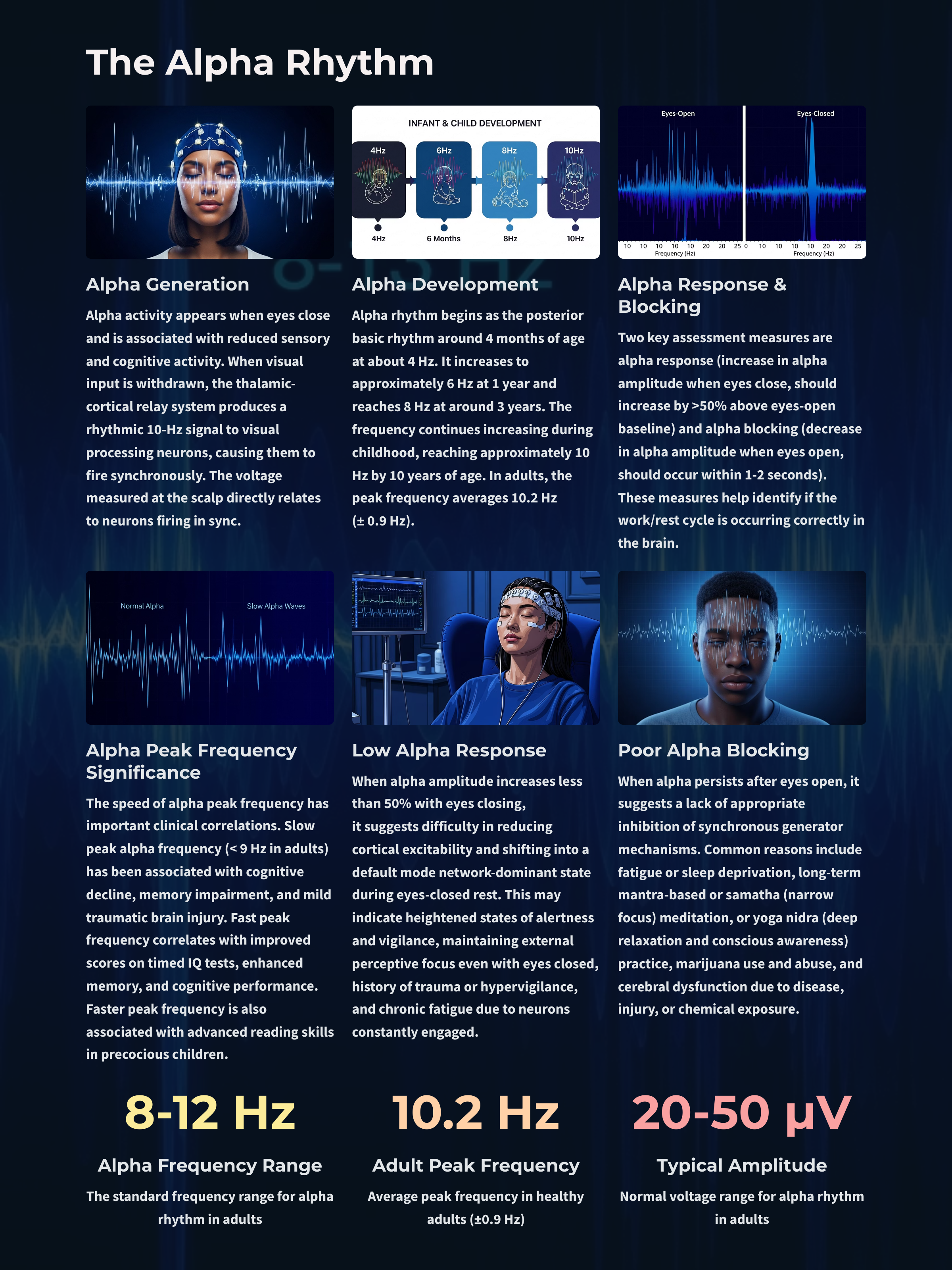
Glossary
alpha blocking: the replacement of the alpha rhythm by low-amplitude desynchronized beta activity during movement, attention, mental effort like complex problem-solving, and visual processing. alpha blocking normally occurs when eyes have just been opened. Arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it while increasing EEG power in the beta range.
alpha response: increased alpha amplitude.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: trains of alpha waves that are visible in the raw EEG and are observed during drowsiness, fatigue, and meditative practice
amplitude: the height of a wave, indicating the strength or intensity of a signal.
amyloid-beta: a protein fragment that accumulates in the brains of individuals with Alzheimer's disease, forming plaques.
analog: the representation of a signal by a continuously variable physical property like voltage.
analog filter: analog circuits designed using components like capacitors, resistors, and operational amplifiers designed to remove or enhance signal components.
analog-to-digital (A/D) converter: an electronic device that converts continuous signals to discrete digital values.
asynchronous waves: EEG activity where neurons depolarize and hyperpolarize independently.
attention: the cognitive process of selectively concentrating on one aspect of the environment while ignoring others.
attention-deficit hyperactivity disorder (ADHD): a neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
beta spindles: trains of spindle-like waveforms with frequencies that can be lower than 20 Hz but more often fall between 22 and 25 Hz. They may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia.
binding problem: the question of how the brain integrates information from different sensory modalities to create a coherent perceptual experience.
bit: binary digit: the smallest measurement unit for quantifying information that assumes a value of 0 or 1.
bit number: the number of voltage levels that an A/D converter can discern. A resolution of 16 bits means that the converter can discriminate among 65,536 voltage levels.
brain lesions: abnormal tissue in the brain resulting from injury or disease, such as strokes, tumors, or trauma.
bursts of rhythmic temporal theta (BORTTs): transient periods of rhythmic theta activity observed in the temporal regions of the brain during EEG recording. These bursts typically manifest as short-lived increases in theta oscillations and are often associated with cognitive processes such as memory encoding and retrieval.
cognitive performance: the ability to use mental processes to perform tasks, including memory, attention, and executive function.
cognitive processes: mental activities involved in acquiring, storing, and using knowledge. electrophysiological techniques: methods used to study the electrical properties of biological cells and tissues.
cognitive restoration: the process during sleep where the brain undergoes various activities that help improve mental functions, including memory consolidation and the clearance of metabolic waste.
complex: a series of waves that share a similar shape.
cortical neurons: neurons located in the brain's cortex that play a key role in various brain functions, including the generation of delta waves.
declarative memory: a type of long-term memory involving facts and information that can be consciously recalled.
delta waves: low-frequency brain oscillations ranging from 0.5 to 4 Hz, typically observed during deep stages of non-REM sleep.
desynchrony: pools of neurons fire independently due to stimulation of specific sensory pathways up to the midbrain and high-frequency stimulation of the reticular formation and nonspecific thalamic projection nuclei.
digital: representation of a signal property like voltage using a series of the digits 0 and 1.
digital filter: a circuit that uses digital processors, like a digital signal processing (DSP) chip, to remove or enhance signal components.
digitization: encoding analog information like continuously changing voltage into a series of the digits 0 and 1.
diphasic waves: waves that possess two elements--one positive and one negative.
dominant frequency: the frequency with the greatest amplitude; at least 13 Hz in awake adults.
dynamic range: the ability to sample a wide range of signal voltages.
EEG activity: a single wave or series of waves.
encephalopathy: a broad term for any diffuse disease of the brain that alters brain function or structure, often characterized by altered mental states and various neurological symptoms.
epileptiform activity: abnormal, paroxysmal EEG patterns that resemble those seen in epilepsy, often indicating a predisposition to seizures.
epoch: signal sampling period; commonly, a 1-s sample of EEG activity.
exact low resolution brain electromagnetic tomography (eLORETA): a version of LORETA that claims no localization error.
excitatory neurons: neurons that increase the likelihood of a firing action potential in the recipient neuron.
Fast Fourier Transform (FFT): a mathematical transformation that converts a complex signal into component sine waves whose amplitude can be calculated.
FFT filter: a filter that uses Fast Fourier transforms to calculate the average voltage of an EEG signal's component frequencies for a specified period.
filter: an electronic circuit that removes or enhances signal components.
fine motor control: the ability to make small, precise movements, often involving the coordination of muscles and nerves.
FIR filter: a filter that continuously updates its averaging of EEG voltage with new data points.
frequency: the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
gamma: 28-80 Hz rhythm that includes the 38-42 Hz Sheer rhythm and is associated with learning and problem-solving, meditation, mental acuity, and peak brain function in children and adults.
gamma event-related synchronization (ERS): a phenomenon where gamma oscillations (30-100 Hz) in the brain become more synchronized in response to a specific event or stimulus, often associated with increased neural processing and cognitive functions such as attention and memory encoding.
gamma oscillations: brain waves in the frequency range of approximately 30 to 100 Hz, associated with various cognitive functions.
glymphatic clearance system: a system in the brain responsible for removing waste products, which is more active during sleep.
hertz (Hz): unit of frequency measured in cycles per second.
high beta: 20-35 Hz rhythm correlated with multi-tasking and optimal performance and anxiety, migraine, obsessive-compulsive disorder (OCD), rumination, and worry.
high-frequency filter (HFF; low-pass filter): a filter that attenuates frequencies above a cutoff frequency.
hippocampus: a brain region involved in the formation and retrieval of memories.
hippocampal-neocortical transfer: the process during sleep where information is transferred from the hippocampus to the neocortex for long-term storage.
homeostatic sleep regulation: the process by which the body balances sleep and wakefulness to maintain overall health, often indicated by the amount of delta activity.
hyperarousal: a state of increased psychological and physiological tension, often associated with anxiety and stress.
IIR filter: a recursive filter that uses part of its output as input. IIR filters attenuate frequencies outside the bandpass more sharply than FIR filters with the same order, have greater time delay (that depends on frequency) due to greater filter sharpness, achieve faster computation due to their lower order, and are less stable than FIR filters.
inhibitory neurons: neurons that decrease the likelihood of a firing action potential in the recipient neuron.
irregular waves: EEG waves that continuously change shape and duration.
Joint time-frequency analysis (JTFA): an algorithm that computes values on each data point at rates up to 256 times per second without using a fixed epoch length. Where FFT simultaneously calculates amplitudes for all frequency bands, JTFA analyzes preselected bands.
K-complex: sharp negative waveforms that reach amplitudes over microvolts succeeded by a longer moderate-to-high-amplitude positive wave observed in Stage 2 sleep.
kappa rhythm: very low amplitude activity in the alpha or theta range detected over temporal sites during mental activity.
long-term memory: the storage of information over an extended period.
low beta: 16-20+ Hz rhythm associated with successful problem-solving.
low resolution electromagnetic tomography (LORETA): Pascual-Marqui's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp.
low-frequency filter (high-pass filter): a circuit that filters out low-frequency activity and passes only the frequencies above a set value (e.g., 1.6 Hz).
magnetoencephalography (MEG): a neuroimaging technique for mapping brain activity by recording magnetic fields produced by electrical currents occurring naturally in the brain.
magnitude: the average amplitude over a unit of time using quantification methods like peak-to-peak (P-P) and root mean square (RMS).
memory consolidation: the process by which short-term memories are transformed into long-term memories during sleep.
microvolt: one-millionth of a volt.
mild cognitive impairment (MCI): a condition involving noticeable cognitive decline, greater than expected for a person's age, but not severe enough to interfere significantly with daily life or independent function.
monomorphic waves: series of waves that are rhythmic with the same frequency and morphology.
morphology: the shape of the signal generated by oscillating potential differences.
motor inhibition: suppressing motor activity, allowing for stillness and precision in movements.
mu rhythm: 7-11-Hz waves resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
neocortex: the part of the brain involved in higher-order brain functions, including sensory perception, cognition, and generation of delta waves.
neural oscillations: rhythmic or repetitive neural activity in the central nervous system.
neurodegenerative diseases: disorders characterized by the progressive degeneration of neurons, often associated with disrupted delta activity.
non-rapid eye movement (NREM) sleep: a phase of sleep that includes stages 1-3, with delta waves being most prominent in stage 3, also known as slow-wave sleep.
notch filter: a circuit that suppresses a narrow band of frequencies, such as those produced by line current at 50/60Hz.
optogenetic stimulation: a technique that uses light to control neurons genetically modified to express light-sensitive ion channels. This method allows precise control over the activity of specific neurons in the brain, enabling researchers to study the functions of neural circuits and their roles in behavior and neurological conditions.
order: the maximum delay in samples used in creating each output sample.
parvalbumin: a protein expressed in certain inhibitory interneurons.
peak-to-peak (p-p): a signal quantification method that measures waveform "height" from peak to trough.
percent power: the expression of power within a frequency band as a percentage of total EEG power.
physical restoration: the process during sleep where the body repairs tissues, grows muscles, and releases growth hormones.
polyphasic waves: waveforms with two or more elements; diphasic and triphasic waves.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
power: amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
prefrontal cortex: a part of the brain involved in complex cognitive behavior, decision-making, and moderating social behavior.
primary motor cortex (M1): a brain region involved in planning, controlling, and executing voluntary movements.
primary somatosensory cortex (S1): a brain region responsible for processing sensory information from the body.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
raw EEG signal: oscillating electrical potential differences detected from the scalp.
reference electrode: an electrode that is placed on the scalp, earlobe, or mastoid.
regular waves: rhythmic waves with the same frequency and morphology.
relaxed wavefulness: a state of being awake but calm and free from active motor activity.
reticular nucleus of the thalamus (TRN): GABAergic thalamic neurons that modulate signals from other thalamic nuclei and do not project to the cortex. Also called the nucleus reticularis of the thalamus (NRT).
root mean square (RMS): a signal quantification method that calculates the area under the EEG waveform and is analogous to the weight of an object.
sampling rate: the number of times per second that an ADC samples the EEG signal.
sampling resolution: a chronic brain disorder characterized by symptoms such as hallucinations, delusions, and cognitive impairments.
saw-toothed waves: waves that resemble asymmetrical triangles.
schizophrenia: a chronic brain disorder characterized by symptoms such as hallucinations, delusions, and cognitive impairments.
sensorimotor cortex: a region of the brain that processes and integrates sensory inputs and motor outputs.
sensorimotor rhythm (SMR): 12 to 15 Hz oscillations associated with motor inhibition and focused attention.
sensory perception: the process of recognizing and interpreting sensory stimuli.
sharp transients: waves with steep peaks that are not produced by epilepsy.
sharp waves: steeply-shaped waves with 70-200 ms duration.
Sheer rhythm: a brain oscillation characterized by high-frequency activity (38-42 Hz) that occurs in short, transient bursts. These bursts are typically synchronized across large neural populations and are thought to play a role in the timing and coordination of neuronal activity, particularly in relation to cognitive processes and sensory perception.
sinusoidal: rhythmic waves that resemble sine waves.
sleep pressure: the body's need for sleep, which increases with prolonged wakefulness and is reflected by the amount of delta activity during sleep.
sleep spindle: waves that range from 12-15 Hz and last from 0.5 to several seconds widely distributed over the scalp and are observed during Stage 2 and 3 sleep.
slow cortical potentials (SCPs): gradual voltage changes in the EEG that reflect changes in cortical excitability and are associated with various cognitive and motor processes.
slow-wave sleep (SWS): the deepest phase of non-REM sleep characterized by high amplitude, low-frequency delta waves.
spike: a waveform with a sharp appearance that lasts 20-70 ms.
spike-and-wave complexes: spikes that are succeeded by slow waves.
spinding excessive beta (SEB): an EEG pattern characterized by high-frequency beta waves (13-30 Hz) with a spindle-like appearance, often exceeding 20 μV in amplitude. It is associated with various psychiatric and neurological conditions, including ADHD, insomnia, and medication-resistant psychiatric disorders.
standardized LORETA (sLORETA): a refinement of LORETA that estimates each voxel's electrical potentials without regard to their frequency, expresses normalized F-values, and achieves a 1-cubic-cm resolution.
sub-vigil states: intermediate states between wakefulness and sleep, characterized by reduced sensory and cognitive activity. These states can lead to cognitive impairments and behavioral disturbances, often observed in conditions like ADHD and insomnia.
surface EMG (SEMG): a non-invasive technique used to measure and record the electrical activity produced by skeletal muscles. Sensors placed on the skin's surface detect the electrical potentials muscle fibers generate during contraction, providing information about muscle function, activation patterns, and fatigue.
surface Laplacian (SL) analysis: a family of mathematical algorithms that provide two-dimensional images of radial current flow from cortical dipoles to the scalp.
synaptic pruning: the process of eliminating weaker synaptic connections in the brain during sleep, which is believed to be facilitated by delta waves.
synchrony: the simultaneous occurrence of events or processes, in this context, the coordinated activity of neurons.
Talairach coordinate: coordinate assignment based on vertical distance from a horizontal line from the anterior commissure (origin) to the posterior commissure; referenced to the Talairach or Montreal atlases.
thalamic pacemaker neurons: neurons in the thalamus that generate rhythmic burst firing patterns, contributing to the synchronization of delta waves.
thalamic pacemaker neurons: neurons in the thalamus that generate rhythmic burst firing patterns, contributing to the synchronization of delta waves.
temporal framework: the timing structure that allows for the coordination of neural activity. thalamocortical interactions: the connections and communications between the thalamus and the cerebral cortex.
thalamic-cortical relay (TCR) system: the primary pathway for determining which cortical areas receive each type of sensory input.
thalamocortical circuits: neural pathways that connect the thalamus with the cortex, which generate rhythmic brain activity.
theta/beta ratio: the ratio between 4-7 Hz theta and 13-21 Hz beta, measured as amplitude squared, most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
transient: a single wave or series of waves distinct from background EEG activity.
triphasic wave: a wave that consists of three elements with alternating directions.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
vertex sharp transient (V wave): a negative-polarity waveform detected at the vertex in sleep records but are not usually observed during wakefulness.
voxel: a volumetric unit.
wave: a plot of voltage using a bipolar (positive/negative) scale with zero in the middle; the analog form of the signal in which voltage continuously varies.
waveform: the shape of the signal that is generated by oscillating potential differences between two electrodes.
working memory: a cognitive system responsible for temporarily holding information available for processing.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Assignment
Now that you have completed this module, write down the frequency bands you uptrain and downtrain in clinical or peak performance practice and explain the rationale for these choices.
References
Achermann, P., & Borbély, A. A. (2003). Mathematical models of sleep regulation. Frontiers in Bioscience, 8, s683-s693. https://doi.org/10.2741/1074
Aich T. K. (2014). Absent posterior alpha rhythm: An indirect indicator of seizure disorder? Indian Journal of Psychiatry, 56(1), 61–66. https://doi.org/10.4103/0019-5545.124715
Amo, C., Castillo, M., Barea, R., Santiago, L., Martínez-Arribas, A., Amo-López, P., & Boquete, L. (2016). Induced gamma-band activity during voluntary movement: EEG analysis for clinical purposes. Motor Control, 20(4), 409-28. https://doi.org/10.1123/mc.2015-0010
Amo, C., Santiago, L., Barea, R., López-Dorado, A., & Boquete, L. (2017). Analysis of Ggamma-band activity from human EEG using empirical mode decomposition. Sensors (Basel, Switzerland), 17. https://doi.org/10.3390/s17050989
Amzica, F., & Lopes da Silva, F. H. (2018). Cellular substrates of brain rhythms. In D. L. Schomer & F. H. Lopes da Silva (Eds.), Niedermeyer’s electroencephalography: Basic principles, clinical applications and related fields (7th ed., pp. 20–62). Oxford University Press.
Angelakis, E., Lubar, J. F., Stathopoulou, S., & Kounios, J. (2004). Peak alpha frequency: an electroencephalographic measure of cognitive preparedness. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(4), 887–897. https://doi.org/10.1016/j.clinph.2003.11.034
Anderson, C., & Horne, J. (2003). Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology, 40(3), 349-57. https://doi.org/10.1111/1469-8986.00038
Arns, M., Swatzyna, R., Gunkelman, J., & Olbrich, S. (2015). Sleep maintenance, spindling excessive beta and impulse control: an RDoC arousal and regulatory systems approach? Neuropsychiatric Electrophysiology, 1, 1-11. https://doi.org/10.1186/S40810-015-0005-9
Arnolds, D. E., Lopes da Silva, F. H., Aitink, J. W., Kamp, A., & Boeijinga, P. (1980). The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalography and Clinical Neurophysiology, 50(3-4), 324–328. https://doi.org/10.1016/0013-4694(80)90160-1
Babiloni, C., Noce, G., Bonaventura, C., Lizio, R., Pascarelli, M., Tucci, F., Soricelli, A., Ferri, R., Nobili, F., Famà, F., Palma, E., Cifelli, P., Marizzoni, M., Stocchi, F., Frisoni, G., & Percio, C. (2020). Abnormalities of cortical sources of resting state delta electroencephalographic rhythms are related to epileptiform activity in patients with amnesic mild cognitive impairment not due to Alzheimer's disease. Frontiers in Neurology, 11. https://doi.org/10.3389/fneur.2020.514136
Bosman, C. A., Lansink, C. S., & Pennartz, C. M. (2014). Functions of gamma-band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. European Journal of Neuroscience, 39(11), 1982-1999. https://doi.org/10.1111/ejn.12606
Buzsáki, G., & Wang, X. J. (2012). Mechanisms of gamma oscillations. Annual Review of Neuroscience, 35, 203–225. https://doi.org/10.1146/annurev-neuro-062111-150444
Chang, L. Y., Wang, M. Y., & Tsai, P. S. (2016). Diagnostic accuracy of rating scales for Attention-Deficit/Hyperactivity Disorder: A meta-analysis. Pediatrics, 137(3), e20152749. https://doi.org/10.1542/peds.2015-2749
Clarke, A., Barry, R., McCarthy, R., & Selikowitz, M. (2001). Excess beta activity in children with attention-deficit/hyperactivity disorder: an atypical electrophysiological group. Psychiatry Research, 103, 205-218. https://doi.org/10.1016/S0165-1781(01)00277-3
Colgin, L. L. (2016). Rhythms of the hippocampal network. Nature Reviews Neuroscience, 17(4), 239-249.
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Corsi-Cabrera, M., Pérez-Garci, E., Río-Portilla, Y., Ugalde, E., & Guevara, M. (2001). EEG bands during wakefulness, slow-wave, and paradoxical sleep as a result of principal component analysis in the rat. Sleep, 24(4), 374-80. https://doi.org/10.1093/sleep/23.6.1a.
Cortoos, A., De Valck, E., Arns, M., Breteler, M. H., & Cluydts, R. (2010). An exploratory study on the effects of tele-neurofeedback and tele-biofeedback on objective and subjective sleep in patients with primary insomnia. Applied Psychophysiology and Biofeedback, 35(2), 125-134. https://doi.org/10.1007/s10484-009-9116-z
Crabtree J. W. (2018). Functional diversity of thalamic reticular subnetworks. Frontiers in Systems Neuroscience, 12, 41. https://doi.org/10.3389/fnsys.2018.00041
Crone, N., Miglioretti, D., Gordon, B., & Lesser, R. (1998). Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain: A Journal of Neurology, 121( Pt 12), 2301-15. https://doi.org/10.1093/BRAIN/121.12.2301
De Gennaro, L., & Ferrara, M. (2003). Sleep spindles: An overview. Sleep Medicine Reviews, 7(5), 423-440. https://doi.org/10.1053/smrv.2002.0252
Dehghani, N., Peyrache, A., Telenczuk, B., Le Van Quyen, M., Halgren, E., Cash, S. S., Hatsopoulos, N. G., & Destexhe, A. (2016). Dynamic balance of excitation and inhibition in human and monkey neocortex. Scientific Reports, 6, 23176. https://doi.org/10.1038/srep23176
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Egner, T., & Gruzelier, J. H. (2001). Learned self-regulation of EEG frequency components affects attention and event-related brain potentials in humans. NeuroReport, 12(18), 4155-4159. https://doi.org/10.1097/00001756-200112210-00058
Ekstrom, A. D., Caplan, J. B., Ho, E., Shattuck, K., Fried, I., & Kahana, M. J. (2005). Human hippocampal theta activity during virtual navigation. Hippocampus, 15(7), 881–889. https://doi.org/10.1002/hipo.20109
Enriquez-Geppert, S., Brown, T., Henrich, H., Arns, M., & Pimenta, M. G. (2023). Attention Deficit Hyperactivity Disorder. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
Fries P. (2015). Rhythms for Cognition: Communication through coherence. Neuron, 88(1), 220–235. https://doi.org/10.1016/j.neuron.2015.09.034
Frey, L. (2023). Epilepsy. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Gilmore, P., & Brenner, R. (1981). Correlation of EEG, computerized tomography, and clinical findings. Study of 100 patients with focal delta activity. Archives of Neurology, 38(6), 371-372. https://doi.org/10.1001/ARCHNEUR.1981.00510060073013.
Goldstein, A. N., & Walker, M. P. (2014). The role of sleep in emotional brain function. Annual Review of Clinical Psychology, 10, 679-708. https://doi.org/10.1146/annurev-clinpsy-032813-153716
Gouw, A., Alsema, A., Tijms, B., Borta, A., Scheltens, P., Stam, C., & Flier, W. (2017). EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiology of Aging, 57, 133-142. https://doi.org/10.1016/j.neurobiolaging.2017.05.017
Grandy, T. H., Werkle-Bergner, M., Chicherio, C., Schmiedek, F., Lövdén, M., & Lindenberger, U. (2013). Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology, 50(6), 570–582. https://doi.org/10.1111/psyp.12043
Gruzelier, J. H., Inoue, A., Smart, R. G., Steed, A., & Steffert, T. (2014). Acting performance and flow state enhanced with sensory-motor rhythm neurofeedback comparing ecologically valid immersive VR and training screen scenarios. Neuroscience Letters, 480(2), 112-116. https://doi.org/10.1016/j.neulet.2014.04.027
Haegens, S., Cousijnc, H., Wallis, G., Harrison, P. J., & Nobre, A. C. (2014). Inter- and intra-individual variability in alpha peak frequency. NeuroImage, 92, 46-55. doi.org/10.1016/j.neuroimage.2014.01.049
Hammond, D. C. (2005). Neurofeedback with anxiety and affective disorders. Child and Adolescent Psychiatric Clinics of North America, 14(1), 105-123. https://doi.org/10.1016/j.chc.2004.07.008
Hanslmayr, S., Sauseng, P., Doppelmayr, M., Schabus, M., & Klimesch, W. (2005). Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Applied Psychophysiology and Biofeedback, 30(1), 1–10. https://doi.org/10.1007/s10484-005-2169-8
Hari, R., & Puce, A. (2017). MEG-EEG primer. Oxford University Press.
Harmony, T., Fernández, T., Silva, J., Bernal, J., Díaz-Comas, L., Reyes, A., Marosi, E., Rodriguez, M., & Rodríguez, M. (1996). EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 24(1-2), 161-71. https://doi.org/10.1016/S0167-8760(96)00053-0
Hartoyo, A., Cadusch, P. J., Liley, D. T. J., & Hicks, D. G. (2020). Inferring a simple mechanism for alpha-blocking by fitting a neural population model to EEG spectra. PLoS Computational Biology, 16(4), e1007662. https://doi.org/10.1371/journal.pcbi.1007662
Hasib, T., & Vengadasalam, V. (2023). Analysing gamma frequency components in EEG signals: A comprehensive extraction approach. Journal of Informatics and Web Engineering. https://doi.org/10.33093/jiwe.2023.2.2.11
Hoedlmoser, K., Pecherstorfer, T., Gruber, G., Anderer, P., Doppelmayr, M., Klimesch, W., & Schabus, M. (2008). Instrumental conditioning of human sensorimotor rhythm (12-15 Hz) and its impact on sleep as well as declarative learning. Sleep, 31(10), 1401–1408.
Howells, F., Temmingh, H., Hsieh, J., Dijen, A., Baldwin, D., & Stein, D. (2018). Electroencephalographic delta/alpha frequency activity differentiates psychotic disorders: A study of schizophrenia, bipolar disorder and methamphetamine-induced psychotic disorder. Translational Psychiatry, 8. https://doi.org/10.1038/s41398-018-0105-y
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Iaccarino, H. F., Singer, A. C., Martorell, A. J., Rudenko, A., Gao, F., Gillingham, T. Z., Mathys, H., Seo, J., Kritskiy, O., Abdurrob, F., Adaikkan, C., Canter, R. G., Rueda, R., Brown, E. N., Boyden, E. S., & Tsai, L. H. (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature, 540(7632), 230–235. https://doi.org/10.1038/nature20587
Jadeja, N. M. (2021). Frequencies and rhythms. In How to read an EEG. Cambridge University Press. https://doi.org/10.1017/9781108918923.008
Jensen, O., Kaiser, J., & Lachaux, J. P. (2007). Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences, 30(7), 317–324. https://doi.org/10.1016/j.tins.2007.05.001
Kane, N., Acharya, J., Benickzy, S., Caboclo, L., Finnigan, S., Kaplan, P. W., Shibasaki, H., Pressler, R., & van Putten, M. J. A. M. (2017). A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clinical Neurophysiology Practice, 2, 170–185. https://doi.org/10.1016/j.cnp.2017.07.002
Kayser, J., & Tenke, C. E. (2015). On the benefits of using surface Laplacian (current source density) methodology in electrophysiology. Int J Psychophysiol, 97(3), 171-173. https://dx.doi.org/10.1016%2Fj.ijpsycho.2015.06.001
Krepel, N., van Dijk, H., Sack, A. T., Swatzyna, R. J., & Arns, M. (2021). To spindle or not to spindle: A replication study into spindling excessive beta as a transdiagnostic EEG feature associated with impulse control. Biological Psychology, 165, 108188. https://doi.org/10.1016/j.biopsycho.2021.108188
Libenson, M. H. (2024). Practical approach to electroencephalography (2nd ed.). Saunders Elsevier.
Lopes da Silva, F. H. (1991). Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalography and Clinical Neurophysiology, 79(2), 81-93. https://doi.org/10.1016/0013-4694(91)90044-5
López-Sanz, D., Bruña, R., Garcés, P., Cámara, C., Serrano, N., Rodríguez-Rojo, I., Delgado, M., Montenegro, M., López-Higes, R., Yus, M., & Maestú, F. (2016). Alpha band disruption in the AD-continuum starts in the Subjective Cognitive Decline stage: A MEG study.Scientific Reports, 6. https://doi.org/10.1038/srep37685.
Lubar, J. F. (1991). Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Biofeedback and Self-regulation, 16(3), 201–225. https://doi.org/10.1007/BF01000016
Lubar, J. F. (2001). Rationale for choosing bipolar versus referential training. Journal of Neurotherapy, 4(3), 94–97.
Marshall, L., & Born, J. (2007). The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences, 11(10), 442-450. https://doi.org/10.1016/j.tics.2007.09.001
Merica, H., & Fortune, R. (2005). Spectral power time-courses of human sleep EEG reveal a striking discontinuity at approximately 18 Hz marking the division between NREM-specific and wake/REM-specific fast frequency activity. Cerebral Cortex, 15(7), 877-884.
Mierau, M., Klimesch, W., & Lefebvre, J. (2017). State-dependent alpha peak frequency shifts: Experimental evidence, potential mechanisms and functional implications. Neuroscience, 360, 146-154. doi.org/10.1016/j.neuroscience.2017.07.037
Min, B. K., & Park, H. J. (2010). Task-related modulation of anterior theta and posterior alpha EEG reflects top-down preparation. BMC Neuroscience, 11, 79. https://doi.org/10.1186/1471-2202-11-79
Moffett, S., O’Malley, S., Man, S., Hong, D., & Martin, J. (2017). Dynamics of high frequency brain activity. Scientific Reports, 7. https://doi.org/10.1038/s41598-017-15966-6.
Monastra, V. J., Lubar, J. F., & Linden, M. (2001). The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: Reliability and validity studies. Neuropsychology, 15(1), 136–144. https://doi.org/10.1037//0894-4105.15.1.136
Monastra, V. J., Lubar, J. F., Linden, M., VanDeusen, P., Green, G., Wing, W., Phillips, A., & Fenger, T. N. (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: an initial validation study. Neuropsychology, 13(3), 424–433. https://doi.org/10.1037/0894-4105.13.3.424
Morales-Quezada, L., Martinez, D., El-Hagrassy, M. M., Kaptchuk, T. J., Sterman, M. B., & Yeh, G. Y. (2019). Neurofeedback impacts cognition and quality of life in pediatric focal epilepsy: An exploratory randomized double-blinded sham-controlled trial. Epilepsy & Behavior: E&B, 101(Pt A), 106570. https://doi.org/10.1016/j.yebeh.2019.106570
Nazish, S. (2020). Clinical and radiological correlates of different electroencephalographic patterns in hospitalized patients. Clinical EEG and Neuroscience, 52(4), 280-286. https://doi.org/10.1177/1550059420910
Neurophysiological parameters influencing sleep–wake discrepancy in insomnia disorder: A preliminary analysis on alpha rhythm during sleep onset. (2023). Brain Sciences. MDPI. https://www.mdpi.com/2076-3425/13/2/260
Niedermeyer E. (1997). Alpha rhythms as physiological and abnormal phenomena. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 26(1-3), 31–49. https://doi.org/10.1016/s0167-8760(97)00754-x
Niedermeyer, E., & da Silva, F. L. (2004). Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins.
Nunez, P. L., & Srinivasan, R. (2019). Electric fields of the brain: The neurophysics of EEG (2nd ed.). Oxford Academic. https://doi.org/10.1093/acprof:oso/9780195050387.001.0001
Oathes, D., Ray, W., Yamasaki, A., Borkovec, T., Castonguay, L., Newman, M., & Nitschke, J. (2008). Worry, generalized anxiety disorder, and emotion: Evidence from the EEG gamma band. Biological Psychology, 79, 165-170. https://doi.org/10.1016/j.biopsycho.2008.04.005
Othmer S. ( 2007). Progress in neurofeedback for the autism spectrum. Paper presented at the 38th Annual Meeting of the Association for Applied Psychophysiology & Biofeedback Monterey, Canada, 15–18 February 2007.
Petersén, I., & Eeg-Olofsson, O. (1971). The development of the electroencephalogram in normal children from the age of 1 through 15 years. Non-paroxysmal activity. Neuropadiatrie, 2(3), 247–304. https://doi.org/10.1055/s-0028-1091786
Pfurtscheller, G., & Lopes da Silva, F. H. (1999). Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110(11), 1842-1857. https://doi.org/10.1016/S1388-2457(99)00141-8
Rathee, S., Bhatia, D., Punia, V., & Singh, R. (2020). Peak alpha frequency in relation to cognitive performance. Journal of Neurosciences in Rural Practice, 11(3), 416–419. https://doi.org/10.1055/s-0040-1712585
Rowan, A. J., & Tolunsky, E. (2003). Primer of EEG with a mini-atlas. Butterworth-Heinemann.
Sazgar, M., & Young, M. (2019). Normal EEG awake and sleep. Absolute Epilepsy and EEG Rotation Review. https://doi.org/10.1007/978-3-030-03511-2_6
Schomer, D. L., & Lopes da Silva, F. H. (Eds.). (2017). Niedermeyer's electroencephalography: Basic principles, clinical applications, and related fields (7th ed.). Oxford Academic. https://doi.org/10.1093/med/9780190228484.001.0001
Simon, M., Schmidt, E. A., Kincses, W. E., Fritzsche, M., Bruns, A., Aufmuth, C., Bogdan, M., Rosenstiel, W., & Schrauf, M. (2011). EEG alpha spindle measures as indicators of driver fatigue under real traffic conditions. Clinical Neurophysiology, 122(6), 1168–1178. https://doi.org/10.1016/j.clinph.2010.10.044
Simonová, O., Roth, B., & Stein, J. (1967). EEG studies of healthy population--Normal rhythms of resting recording. Acta Universitatis Carolinae. Medica, 13(7), 543–551. PMID: 5620888
Singer W. (2013). Cortical dynamics revisited. Trends in Cognitive Sciences, 17(12), 616–626. https://doi.org/10.1016/j.tics.2013.09.006
Sirin, T., Şirinocak, P., Arkalı, B., Akıncı, T., & Yeni, S. (2019). Electroencephalographic features associated with intermittent rhythmic delta activity. Neurophysiologie Clinique, 49, 227-234. https://doi.org/10.1016/j.neucli.2019.01.036
Snyder, S. M., Quintana, H., Sexson, S. B., Knott, P., Haque, A. F., & Reynolds, D. A. (2008). Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatry Research, 159(3), 346–358. https://doi.org/10.1016/j.psychres.2007.05.00
Soroush-Vala, A., Rahmanian, M., Jadid, M., & Hassanvandi, S. (2023). Application of neurofeedback in treating epilepsy: A systematic review and meta-analysis. International Journal of Body, Mind and Culture, 10, 143-157. https://doi.org/10.22122/ijbmc.v10i2.506
Spiesshoefer, J., Linz, D., Skobel, E., Arzt, M., Stadler, S., Schoebel, C., Fietze, I., Penzel, T., Sinha, A. M., Fox, H., Oldenburg, O., & German Cardiac Society Working Group on Sleep Disordered Breathing (AG 35-Deutsche Gesellschaft für Kardiologie Herz und Kreislaufforschung e.V.) (2021). Sleep - the yet underappreciated player in cardiovascular diseases: A clinical review from the German Cardiac Society Working Group on Sleep Disordered Breathing. European Journal of Preventive Cardiology, 28(2), 189–200. https://doi.org/10.1177/2047487319879526
Spydell, J. D., Ford, M. R., & Sheer, D. E. (1979). Task dependent cerebral lateralization of the 40 Hertz EEG rhythm. Psychophysiology, 16(4), 347–350. https://doi.org/10.1111/j.1469-8986.1979.tb01474.x
Steriade, M., McCormick, D. A., & Sejnowski, T. J. (1993). Thalamocortical oscillations in the sleeping and aroused brain. Science, 262(5134), 679-685. https://doi.org/10.1126/science.8235588
Sterman, M. B. (1996). Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedback and Self-regulation, 21(1), 3-33. https://doi.org/10.1007/BF02214146
Sterman, M. B. (2000). Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clinical Electroencephalography, 31(1), 45-55. https://doi.org/10.1177/155005940003100111
Suldo, S. M., Olson, L. A., & Evans, J. R. (2002). Quantitative EEG Evidence of Increased Alpha Peak Frequency in Children with Precocious Reading Ability. Journal of Neurotherapy, 5(3), 39–50. https://doi.org/10.1300/J184v05n03_0
Sutter, R., Stevens, R., & Kaplan, P. (2012). Clinical and imaging correlates of EEG patterns in hospitalized patients with encephalopathy. Journal of Neurology, 260, 1087-1098. https://doi.org/10.1007/s00415-012-6766-1
Swatzyna, R. J., Arns, M., Tarnow, J. D., Turner, R. P., Barr, E., MacInerney, E. K., Hoffman, A. M., & Boutros, N. N. (2022). Isolated epileptiform activity in children and adolescents: Prevalence, relevance, and implications for treatment. European Child & Adolescent Psychiatry, 31(4), 545–552. https://doi.org/10.1007/s00787-020-01597-2
Tan, G., Thornby, J., Hammond, D. C., Strehl, U., Canady, B., Arnemann, K., & Kaiser, D. A. (2009). Meta-analysis of EEG biofeedback in treating epilepsy. Clinical EEG and Neuroscience, 40(3), 173–179. https://doi.org/10.1177/155005940904000310
Thatcher, R. W., Palmero-Soler, E., & Otte, G. (2020). Independent components analysis “artifact correction” distorts EEG phase in artifact free segments. Journal of Neurology and Neurobiology, 6, 1-14. https://doi.org//10.16966/2379-7150.172
The Johns Hopkins atlas of digital EEG: An interactive training guide. (2011). Johns Hopkins University Press.
Thompson, M., & Thompson, L. (2015). The neurofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Thornton, K. E., & Carmody, D. P. (2009). Eyes-closed and activation QEEG databases in predicting cognitive effectiveness in and the inefficiency hypothesis. Journal of Neurotherapy, 13, 1–21. https://doi.org/10.1080/10874200802429850
Tombor, L., Kakuszi, B., Papp, S., Réthelyi, J., Bitter, I., & Czobor, P. (2019). Decreased resting gamma activity in adult attention deficit/hyperactivity disorder. The World Journal of Biological Psychiatry, 20, 691 - 702. https://doi.org/10.1080/15622975.2018.1441547
Tononi, G., & Cirelli, C. (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron, 81(1), 12-34. https://doi.org/10.1016/j.neuron.2013.12.025
Uhlhaas, P. J., & Singer, W. (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews. Neuroscience, 11(2), 100–113. https://doi.org/10.1038/nrn2774
van Son, D., de Rover, M., De Blasio, F. M., van der Does, W., Barry, R. J., & Putman, P. (2019). Electroencephalography theta/beta ratio covaries with mind wandering and functional connectivity in the executive control network. Annals of the New York Academy of Sciences, 1452(1), 52–64. https://doi.org/10.1111/nyas.14180
Varga, A. W., Kishi, A., Mantua, J., Lim, J., Koushyk, V., Leiberg, S., Heintz, C., Naik, S., Rapoport, D. M., & Ayappa, I. (2016). Abnormal sleep spindles and slow waves in sleep apnea and Alzheimer disease. Sleep, 39(4), 775-787. https://doi.org/10.5665/sleep.5634
Vernon, D., Egner, T., Cooper, N., Compton, T., Neilands, C., Sheri, A., & Gruzelier, J. (2003). The effect of training distinct neurofeedback protocols on aspects of cognitive performance. International Journal of Psychophysiology, 47(1), 75-85. https://doi.org/10.1016/S0167-8760(02)00091-0
Williams D. (1941). The electro-encephalogram in acute injuries. Journal of Neurology and Psychiatry, 4(2), 107–130. https://doi.org/10.1136/jnnp.4.2.107
Willoughby, J., Fitzgibbon, S., Pope, K., Mackenzie, L., Medvedev, A., Clark, C., Davey, M., & Wilcox, R. (2003). Persistent abnormality detected in the non-ictal electroencephalogram in primary generalised epilepsy. Journal of Neurology, Neurosurgery & Psychiatry, 74, 51 - 55. https://doi.org/10.1136/jnnp.74.1.51
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., O'Donnell, J., Christensen, D. J., Nicholson, C., Iliff, J. J., Takano, T., Deane, R., & Nedergaard, M. (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373-377. https://doi.org/10.1126/science.1241224
D. Standards of EEG Acquisition Procedures Including Activation
Introduction
Quantitative electroencephalography (qEEG) is a clinically validated methodology that uses digitized EEG signals to quantify brain activity. Its effectiveness depends on the integrity of data acquisition, inspection, and processing, as outlined in the 2025 IQCB minimum technical requirements (Collura et al., 2025). These guidelines extend foundational standards originally established by the American Clinical Neurophysiology Society (Sinha et al., 2016), offering a comprehensive framework for acquiring and processing EEG data suitable for quantitative analysis.This unit details each stage of QEEG workflow—from acquisition to artifact rejection and advanced source localization. Topics include the role of spectral analysis, inverse solutions such as LORETA, and the appropriate use of discriminant algorithms. The essay also addresses procedural steps recommended by IQCB for quality assurance and board certification, and closes with an overview of process flows and critical clinical applications. These stages are fundamental for establishing the clinical reliability of QEEG in contexts ranging from neurodiagnostic screening to forensic evaluation, and the following sections demonstrate how each component contributes to generating accurate and clinically meaningful interpretations of brain electrical activity.
Please click on the podcast icon below to hear a full-length lecture over Section D.

Acquisition
Electrodes must be applied using the International 10-20 system, ensuring 19 standard recording sites (Sinha et al., 2016). This standardization supports anatomical consistency across sessions and subjects, facilitating accurate data interpretation and database comparisons. At least 10 minutes of recording in eyes-open (EO) and eyes-closed (EC) conditions is required, providing enough data to select 2–5 minutes of artifact-free EEG for the qEEG (Collura et al., 2025).Extended recordings are sometimes necessary when the subject exhibits high levels of movement or arousal, as in cases of hyperactivity, anxiety, or sensory sensitivity. The technician must continuously monitor electrode impedance to keep it below 5 kΩ, ensure skin preparation minimizes drift, and regularly check signal quality. During EO recording, participants are encouraged to focus on a fixed point and limit blinking; EC recording emphasizes relaxed alertness without drowsiness.
Coaching before starting a recording can help the subject understand what may cause artifacts and how to avoid them. While continuous recording without breaks is recommended for each condition, it is also sometimes necessary to pause the recording to provide the subject with further coaching.
Activation procedures, such as photic stimulation and hyperventilation, are generally excluded to preserve a resting baseline unless specific clinical indications require their inclusion (Collura et al., 2025). Some qEEG databases have norms for activation conditions (e.g., Thornton & Carmody, 2009) that can be used in addition to the usual resting eyes-open and eyes-closed conditions. Real-time monitoring from acquisition software helps identify problematic channels, allowing immediate corrections. The acquisition phase thus requires a blend of technical expertise, patient coaching, and adherence to standardized protocols to ensure the resulting data are both valid and diagnostically useful.
Visual Inspection
Following acquisition, a certified reviewer must inspect the EEG using multiple montages (e.g., linked ears, bipolar, average reference, Laplacian) to identify usable segments and anomalies (Collura et al., 2025). This review involves not only technical quality checks but also clinical interpretation of observed rhythms, waveforms, and transient events. The inspection should assess background rhythms, such as alpha during EC and desynchronization during EO, as well as abnormal discharges like spikes, sharp waves, or slow-wave activity.The IQCB encourages reviewers to classify findings as "rule out" (potentially abnormal), "throw out" (artifact), or "refer out" (requires medical evaluation) based on clinical significance and artifact profile. A well-structured report should document the overall signal quality, the quantity and type of artifact observed, the length of clean segments available, and any clinical abnormalities such as asymmetries, paroxysmal bursts, or unusual frequency components. Additionally, the reviewer should evaluate subject-related state changes such as drowsiness or cognitive fluctuation, often indicated by vertex events, alpha dropout, or temporal slowing.
IQCB certification ensures that only qualified professionals perform this crucial step, ensuring that important clinical information is not overlooked. The visual inspection stage acts as a bridge between raw acquisition and analytical processing, and it plays a crucial role in ensuring that the qEEG reflects true neurophysiological function rather than artifact contamination or misinterpretation.
Selection and Artifact Rejection
The artifact rejection phase uses manual editing or algorithmic tools like principal component analysis (Buzzell et al., 2022) and independent component analysis (Delorme & Makeig, 2004; Kang et al., 2018). These tools help isolate and remove common artifacts such as eye movements, blinks, muscle tension, and electrical interference. ICA is often preferred because it can preserve good data while isolating artifactual components across channels, allowing for their targeted removal. However, the reliability of ICA depends on proper training, dataset quality, and the number of independent components relative to the number of electrodes. Some (Thatcher et al., 2020) argue, however, that use of ICA distorts connectivity metrics. The total recording duration selected for analysis must be 1–5 minutes in length, ideally contiguous and representative of baseline brain activity (Collura et al., 2025).It is also critical to avoid stitching together too many short segments, as this can distort spectral characteristics and reduce coherence between sites. Clinically relevant transients like FIRDA, OIRDA, and posterior slow waves of youth must be preserved, as they contribute meaningful information for diagnostic interpretation. Automated methods must be validated by visual confirmation to prevent false rejection of valid EEG features or retention of noise. Proper rejection techniques ensure that the qEEG reflects cortical activity rather than artifactual input, supporting accurate computation of metrics and interpretation. The integration of manual and automated approaches, along with careful clinical judgment, is essential to achieving high-quality artifact-free data that meet IQCB standards for clinical and research application.
We encourage you to review qEEG Tutor's coverage of normal and abnormal waveforms, and of artifacts for more detail about how to recognize artifactual recording segments to reject.
Computation of Metrics
Standard QEEG analysis uses Fast-Fourier transform (FFT) to convert time-domain signals into frequency-domain metrics, enabling calculation of symmetry, phase, and coherence (Collura et al., 2025). These metrics quantify the amount and distribution of neural oscillations in various frequency bands—delta, theta, alpha, beta, and gamma—across cortical regions.Metrics are mapped onto topographical representations that display activity across scalp regions and allow visual interpretation of spectral abnormalities. Frequency bands should align with published standards (Sinha et al., 2016), although some variation may be justified depending on the normative database used. Absolute power measures the raw amplitude of activity in each band, while relative power expresses the proportion of activity in one band relative to the total power. Symmetry metrics assess interhemispheric balance, and coherence measures the degree of functional connectivity between electrode sites. Phase measures the degree of wave peaks of a given frequency at two different sitesCross-device comparisons require amplifier amplitude normalization to preserve metric integrity.
Normative databases used to assess statistical deviation from the normal healthy average for an age group must be constructed with attention to demographic diversity and clinical validity, with many seeking FDA 510(k) clearance. Proper metric computation transforms EEG from a descriptive modality into a quantitative framework capable of identifying biomarkers of dysfunction and guiding treatment interventions.
Low-Resolution Electromagnetic Tomography
LORETA and its variants (sLORETA, eLORETA) estimate the cortical sources of scalp-recorded EEG by solving the inverse problem under smoothness constraints (Thatcher et al., 2005). These algorithms compute three-dimensional current source density (CSD) maps that show the probable origins of EEG activity within the brain. They assume that neighboring cortical regions activate together, enabling smooth interpolation across space.These maps align well with neuroimaging modalities like PET and fMRI, validating their utility in identifying functional abnormalities in disorders such as ADHD, depression, and epilepsy (Lantz et al., 1997). LORETA enables clinicians to visualize not just which frequencies are elevated or reduced, but where in the cortex these alterations are occurring. This adds an anatomical dimension to spectral data and supports precision treatment planning, such as neurofeedback or targeted pharmacotherapy. The accuracy of these estimates depends heavily on the quality of input data, the spatial fidelity of the head model, and the electrode montage. When applied rigorously, LORETA enhances the interpretability of the qEEG, deepens clinical insight, and supports integration with other diagnostic modalities.
ICA and PCA
Independent Component Analysis (ICA) and Principal Component Analysis (PCA) are essential tools in modern EEG preprocessing, particularly for artifact removal and signal decomposition. PCA works by reducing the dimensionality of data, identifying principal components that account for the most variance, and allowing for the elimination of those that correspond to noise. This approach is helpful when broad systemic artifacts are present but comes at the cost of losing signal from all channels in the affected epochs. ICA, on the other hand, assumes statistical independence among signal sources and separates mixed signals into constituent components. This allows specific artifact sources, such as eye blinks, cardiac signals, or muscle noise, to be isolated and removed without discarding entire channels.The success of ICA depends on the amount and quality of data, and the experience of the user in recognizing artifact components from cortical sources. Moreover, ICA-processed data must be consistent with preprocessing used in the normative database for valid comparison. Machine learning and template-based artifact recognition are sometimes integrated with ICA to increase specificity. However, manual inspection remains critical to validate the decomposition results. Improper use of ICA can result in discarding clinically relevant signals or retaining contamination, which can skew qEEG metrics. As such, both ICA and PCA must be implemented judiciously, ideally under the supervision of certified experts, to ensure their contribution to data quality and analytical fidelity aligns with IQCB standards.
Discriminants and Classification Algorithms
Discriminant analysis refers to statistical methods that distinguish between clinical groups based on multivariate EEG features. These algorithms generate composite scores that indicate the likelihood that a subject belongs to a diagnostic category such as mild cognitive impairment, depression, or ADHD. They are often developed using large datasets in which group membership is known, and features such as spectral power, asymmetry, or coherence are used to build predictive models.IQCB guidelines emphasize that such models must only be used for confirmatory purposes—never as primary diagnostic tools—due to their sensitivity to false positives, particularly in diverse populations (Collura et al., 2025). These tools are best employed when there is an existing diagnostic hypothesis and sufficient clinical justification. Misapplication of these models in unscreened populations could lead to diagnostic error or unnecessary intervention. Moreover, classification algorithms based on machine learning or artificial intelligence require rigorous training, cross-validation, and external testing before clinical application.
The integrity of input data, consistency with training conditions, and adherence to ethical review are essential for defensible use. When employed properly, discriminants can streamline assessment, support diagnosis, and predict treatment response. However, they must always be accompanied by human oversight and contextual clinical knowledge to maintain their validity and reliability.
Recommended Procedure for EEG Recording for QEEG Evaluation
The IQCB has laid out a detailed procedural protocol for EEG recording to ensure consistency and suitability for qEEG analysis (Collura et al., 2025). The procedure begins with obtaining informed consent and clinical history, establishing a quiet recording environment, and ensuring that all electronic devices are removed to minimize interference. Electrode application follows, with impedance checks ensuring all sensors meet the resistance threshold. Participants are coached on behavior during EO and EC recordings, including maintaining a fixed gaze and minimizing movement. EO recording is typically conducted first to avoid drowsiness in EC data, which are collected afterward. Continuous observation during both sessions allows the technician to note EMG activity, artifacts, and behaviors of interest. Upon completion, visual inspection should be carried out across at least three montages to verify data quality and identify segments suitable for analysis.The goal is to collect 2–5 minutes of artifact-free EO and EC data for further processing. This rigorous procedure supports the collection of high-quality, interpretable EEG data, facilitating accurate and standardized QEEG assessment in clinical and research settings.
Process and Data Flow for QEEG Evaluation
The QEEG evaluation process is systematic, beginning with raw EEG acquisition under standardized conditions and progressing through data cleaning, generation of metrics, and analysis (Collura et al., 2025). Once EEG data are collected, the technician or clinician performs artifact rejection using visual inspection and computational methods. Clean data, ideally 2–5 minutes in length, are then processed using FFT to derive spectral metrics. These metrics are compared against age-regressed normative databases to identify deviations, often visualized in tables and color-coded topographic maps. Additional analyses may include asymmetry, phase coherence, and source localization using LORETA. The results are compiled into a report that includes raw data review, metric analysis, and clinical interpretation. This report can guide treatment planning, monitor progress, or support diagnostic decisions.The process flow ensures that every phase—acquisition, inspection, selection, computation, and interpretation—is executed with precision and consistency. Flow diagrams reinforce this structure, making procedures transparent and replicable across settings. Adhering to this standardized workflow enhances QEEG's scientific reliability and practical utility in clinical practice.
Conclusion
The 2025 IQCB guidelines mark a major advancement in standardizing QEEG practice, ensuring data quality and clinical reliability (Collura et al., 2025). The integration of ACNS standards (Sinha et al., 2016), spectral methods, and advanced source localization expands QEEG’s utility across neuropsychiatric contexts. Tools like ICA, PCA, and LORETA enhance analysis when used properly, while procedural protocols ensure data integrity. Emphasis on artifact management, certification, and validated metrics supports accurate interpretation and reinforces QEEG’s role as a complement to traditional clinical EEG.Standardized data flow models and process recommendations increase reproducibility and support training and certification. Collectively, these advances strengthen the scientific and clinical foundation of QEEG and improve its capacity to aid diagnosis, inform treatment, and contribute meaningfully to neurobehavioral healthcare.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Neurofeedback Blueprint.

Assignment
Now that you have completed this section, create a flowchart of the process by which you acquire the qEEG.
Glossary
acquisition: the process of recording the EEG using standardized electrode placement and controlled conditions to ensure consistent and reliable data suitable for quantitative analysis.
artifact: any non-neural signal contaminating eeg data, including movements, eye blinks, muscle activity, and electrical interference.
coherence: a metric that quantifies the degree of synchronization between EEG signals from two electrode sites, often used to assess functional connectivity.
discriminant analysis: a statistical method that uses multiple EEG features to predict group membership, such as diagnostic categories.
eyes-closed (EC): a condition in eeg acquisition where subjects close their eyes, enhancing alpha rhythm visibility and reducing visual input.
eyes-open (EO): a condition in eeg acquisition where subjects keep their eyes open, typically used to contrast with ec states and monitor attentional modulation.
Fast-Fourier Transform (FFT): an algorithm that converts time-domain EEG signals into frequency-domain data, allowing computation of spectral power within predefined bands.
frequency band: ranges of EEG signal frequency associated with different brain states, typically including delta, theta, alpha, beta, and gamma bands.
impedance: the resistance to electrical current at the electrode-scalp interface, which must be minimized for accurate EEG recording.
independent component analysis (ICA): a computational technique that separates mixed signals in eeg data into statistically independent components, facilitating artifact removal.
International 10–20 System: a standardized method for electrode placement on the scalp used to ensure consistency in eeg recording across individuals and studies.
IQCB: the International QEEG Certification Board, which establishes standards and certification procedures for clinical QEEG practice.
LORETA: low-resolution electromagnetic tomography; a technique that estimates the cortical sources of scalp-recorded EEG activity by solving the inverse problem with spatial smoothing constraints.
metric: a quantitative measure derived from EEG data, such as absolute power, relative power, symmetry, or coherence.
montage: a configuration of EEG electrode pairings used for data display and analysis, such as linked ears, average reference, or bipolar setups.
normative database: a reference dataset of EEG metrics collected from healthy individuals used for statistical comparison in QEEG analysis.
PCA: principal component analysis; a method for dimensionality reduction in EEG data that identifies uncorrelated variables (components) explaining the most variance.
photic stimulation: a procedure using rhythmic light flashes during EEG recording to provoke specific brain responses, often excluded from a resting-state qEEG. qEEG: quantitative electroencephalography; the mathematical and statistical analysis of digitized EEG signals to derive metrics indicative of brain function or dysfunction.
relative power: the proportion of spectral power in a given frequency band relative to the total power across all bands.
rule out/throw out/refer out: categories used in visual inspection of eeg data to classify findings as potentially significant, artifactual, or requiring clinical referral.
source localization: the process of estimating the origin of eeg activity within the brain, often using algorithms like LORETA.
symmetry: a qEEG metric assessing the balance of power or activity between homologous regions of the two hemispheres.
transients: brief EEG events that deviate from background activity, which may be physiological or pathological, such as vertex waves or epileptiform discharges.
References
Buzzell, G. A., Niu, Y., Aviyente, S., & Bernat, E. (2022). A practical introduction to EEG time-frequency principal components analysis (TF-PCA). Developmental Cognitive Neuroscience, 55, 101114. https://doi.org/10.1016/j.dcn.2022.101114
Collura, T., Cantor, D., Chartier, D., Crago, R., Hartzoge, A., Hurd, M., Kerson, C., Lubar, J., Nash, J., Prichep, L. S., Surmeli, T., Thompson, T., Tracy, M., & Turner, R. (2025). Guideline minimum technical requirements for performing clinical quantitative electroencephalography. Clinical EEG and Neuroscience, 0(0), 1–9. https://doi.org/10.1177/15500594241308654
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Kang, G., Jin, S.-H., Kim, D. K., & Kang, S. W. (2018). EEG artifacts removal using machine learning algorithms and independent component analysis. Clinical Neurophysiology, 129, e24. https://doi.org/10.1016/j.clinph.2018.04.060
Lantz, G., Michel, C. M., Pascual-Marqui, R. D., & Spinelli, L. (1997). Extracranial localization of intracranial interictal epileptiform activity using LORETA. Electroencephalography and Clinical Neurophysiology, 102(5), 414–422. https://doi.org/10.1016/S0921-884X(96)96551-0
Sinha, S. R., Sullivan, L., Sabau, D., Dombrowski, K. E., Halford, J. J., Gloss, D., Herman, S. T., Kanner, A. M., LaFrance, W. C., & Lambrakis, C. F. (2016). American Clinical Neurophysiology Society Guideline 1: Minimum technical requirements for performing clinical electroencephalography. Journal of Clinical Neurophysiology, 33(4), 303–307. https://doi.org/10.1097/WNP.0000000000000308
Thatcher, R. W., North, D., & Biver, C. (2005). Evaluation and validity of a LORETA normative EEG database. Clinical EEG and Neuroscience, 36(2), 116–1.
E. Abnormal EEG Waveforms and Rhythms
Abnormal EEG patterns include abnormal slow activity, paroxysmal epileptogenic abnormalities, and abnormal periodic paroxysmal patterns.
Please click on the podcast icon below to hear a lecture over Section E.

Abnormal Slow Activity
Abnormal slow activity includes generalized intermittent slow activity, focal and lateralized intermittent slow activity, and persistent slow activity.Generalized intermittent slow activity is asynchronous, under 8 Hz, and involves the majority or all of both hemispheres. These bursts typically consist of polymorphic delta (Benbadis & Rielo, 2018). Alerting and opening the eyes reduce, whereas hyperventilation challenge and relaxation increase these slow waves (Fisch, 1999). Graphic © Medscape.
Focal and lateralized intermittent slow activity is under 8 Hz and is usually confined to a single or a couple of adjacent electrodes. These bursts have an irregular appearance, are composed of several frequencies, and rarely involve an entire hemisphere (Fisch, 1999). Graphic © eegatlas-online.com.
Persistent slow activity consists of theta and delta waveforms. Distribution may be anterior or widespread in encephalopathies (Richardson & Benbadis, 2019). Graphic © Medscape.
Paroxysmal Epileptogenic Abnormalities
Paroxysmal epileptogenic abnormalities include interictal epileptiform discharges (focal, generalized), ictal, secondary bilateral synchrony, and epileptiform patterns of doubtful significance.Interictal epileptiform discharges typically consist of individual spikes and sharp waves and complexes that contain both waveforms that last less than 2 seconds (Fisch, 1999). Graphic courtesy of Teppei Matsubara.
Ictal epileptiform discharges may consist of prolonged relatively normal-appearing interictal periods. These may include 3-Hz spike-and-wave discharges, slow spike-and-wave discharges, sharp-and-slow-wave discharges, amplitude and frequency fluctuations in rhythms of 10 Hz or higher, and irregular multiple spike-and-wave or spike-and-wave discharges (Fisch, 1999). Graphic © eegatlas-online.com.
Secondary bilateral synchrony (SBS) involves spikes with a single phase reversal around the midline (Jin, 2007). Graphic © Neurology Asia.
Epileptogenic patterns of doubtful significance are brief and not associated with seizures or neurological disorders. Examples are 6-Hz spike-and-slow-wave, 14- and 16-Hz positive bursts, benign epileptiform transients of sleep (BETS), rhythmical mid-temporal discharge (RMTD), small sharp spikes (SSS), and wicket spikes (Fisch, 1999). Six-Hz spike-and-wave graphic © Mayo Foundation for Medical Education.
Fourteen and 16 Hz positive bursts from EEGpedia.org.
.png.crdownload)
Caption: 6-Hz positive burst of 0.5 second, bilateral (average). This pattern consists of brief bursts lasting 0.5 to 1 second, occurring at either 14 Hz (ranging from 13 to 17 Hz) or 6 Hz (ranging from 5 to 7 Hz). The activity may be unilateral—more often on the right side—or bilateral, either asynchronous or synchronous. The waveforms have an arch-like shape with prominent positive sharp peaks and are most prominent in the posterior temporal region. They typically appear during drowsiness and light sleep in young adults and have no known association with epilepsy.
Benign Epileptiform Transients of Sleep (BETS) AKA Small Sharp Spikes from eegatlas-online.com.
Caption: This waveform typically appears during stage 1 or 2 of non-rapid eye movement (NREM) sleep. It is usually monophasic in shape, though it can occasionally present as diphasic. It originates in the temporal region but is widely distributed across a broad field. The background activity is generally undisturbed, although a single slow wave may occasionally follow. The waveform amplitude is typically under 50 microvolts, and its duration is less than 50 milliseconds.
Rhythmical mid-temporal discharge (RMTD) from eegatlas-online.com.
Caption: Rhythmic theta waves right temporal (average)
Wicket spikes from EEGpedia.org.
.png)
Caption: Run of wicket spikes. This pattern consists of short runs of spikes in the 6 to 11 Hz range, sometimes appearing as isolated spikes. The activity generally falls within the alpha frequency range and takes the form of sharp, arciform monophasic waveforms with amplitudes between 60 and 200 microvolts. These waveforms are typically observed in the anterior or mid-temporal regions and usually appear unilaterally, with the dominant side shifting. There is no accompanying slow wave. This activity is seen during relaxed wakefulness, drowsiness, and light sleep, and is most commonly observed in middle to late adulthood. Clinically, this pattern is considered an analogue of the auditory alpha rhythm, may diminish in response to auditory stimulation, and is not associated with epilepsy.
Abnormal Periodic Paroxysmal Patterns
Abnormal periodic paroxysmal patterns include generalized periodic paroxysmal patterns and lateralized periodic paroxysmal patterns. Generalized periodic paroxysmal patterns involve the same areas of both hemispheres. The waveforms exhibit similar composition, amplitude, and phase in each hemisphere but may slightly vary within a hemisphere (Fisch, 1999). Graphic © Epilepsy & Behavior.
Lateralized periodic paroxysmal patterns differ from generalized periodic paroxysmal patterns in their unilateral distribution. Both patterns share the same waveform morphology (Fisch, 1999). Graphic © Internal Medicine.
EEG Biomarkers and Clinical Utility
The electroencephalogram (EEG) is a critical tool for identifying electrophysiological biomarkers that reflect diverse patterns of cortical and subcortical dysfunction. While diffuse slowing is a hallmark of encephalopathy—a reversible condition caused by metabolic, hypoxic, or toxic disturbances—other EEG biomarkers such as intermittent epileptiform discharges (IEDs) and spindling excessive beta (SEB) indicate cortical hyperexcitability or dysregulation that frequently present in psychiatric and neurodevelopmental populations (Boutros et al., 2016; Johnstone et al., 2005; Swatzyna et al., 2020, 2024).This section delineates these three biomarkers individually, tracing their defining electrophysiological signatures, associated behavioral presentations, underlying neurophysiology, and implications for differential diagnosis and targeted treatment. By distinguishing between these biomarkers and avoiding the overgeneralization of their significance, clinicians can adopt a more nuanced, physiologically grounded approach to complex psychiatric and neurological presentations.
Diffuse Slowing as a Marker of Encephalopathy
Diffuse slowing is the canonical EEG indicator of encephalopathy, defined by the replacement of the normal alpha rhythm (8–12 Hertz) with widespread delta (0.5–4 Hertz) or theta (4–7 Hertz) activity.
Caption: Encephalopathy: 13 y/o male lead toxicity: Generalized mild slow pattern consistent with metabolic, toxic or anoxic encephalopathy.
This pattern reflects disruption of thalamocortical circuits and diminished cortical responsiveness due to systemic metabolic, toxic, or hypoxic insults (Brenner, 2021; Swatzyna et al., 2024). Unlike focal slowing, which is restricted to a single cortical region and may indicate localized structural pathology, diffuse slowing signifies widespread cortical-subcortical disconnection or dysfunction (Swatzyna et al., 2024; Yamada & Meng, 2018). This pattern often emerges in systemic medical conditions such as metabolic encephalopathy, hypoxic injury, or toxic exposure.
From a physiological perspective, disruptions to thalamocortical relay loops and diminished cholinergic or mitochondrial function drive the shift from alpha resonance to lower-frequency oscillations. In the clinical EEG, diffuse slowing presents with a posterior dominant rhythm less than eight Hertz, reduced reactivity to eye opening, and blunted engagement with mental tasks.
Clinically, diffuse slowing is highly significant and predictive of poor outcomes. Large-scale cohort studies have shown that its presence on an EEG is associated with a twofold increase in 30-day mortality among hospitalized patients, independent of age or comorbidity burden. In outpatient psychiatric populations, particularly those with refractory symptoms or abrupt onset of behavioral changes, diffuse slowing appears in approximately eleven percent of adults and up to 14 percent of children. The morphological presentation varies but often includes waxing-and-waning frontal delta seen in hepatic or renal encephalopathies, a theta–delta fusion pattern following diffuse axonal injury, or mixed theta activity masked by sedative-induced beta. Inter-rater agreement for detecting diffuse slowing is modest (kappa = 0.46), but improves significantly when quantitative spectral overlays and low-frequency montages are used, highlighting the importance of comprehensive review.
Routine EEG captures mild encephalopathy in only 40–60% of cases, with sensitivity increasing substantially through extended monitoring or activation techniques such as hyperventilation or sleep deprivation (Grant et al., 2020).
Quantitative EEG (qEEG) improves detection through spectral analysis, enabling topographic mapping of slow-wave distribution and amplitude asymmetries. For example, anterior-predominant slowing correlates with executive dysfunction, while posterior slowing is linked to visuospatial deficits and emotional detachment (Rios & Sousa, 2020).
Topographic analysis of diffuse slowing reveals amplitude gradients that correspond with distinct symptom profiles. Frontal-predominant slowing often correlates with executive dysfunction, impulsivity, and apathy—symptoms linked to frontostriatal and default-mode network hypoactivation. Posterior-dominant slowing, by contrast, is associated with visuospatial deficits and emotional blunting. sLORETA and magnetoencephalography studies support these associations, showing maximal delta current density in the midline thalamus and precuneus. These findings emphasize the importance of noting spatial distribution in EEG reports, allowing for tailored psychiatric and neurocognitive interventions.
Misinterpretation of diffuse slowing can lead to significant clinical error. Dismissing it as nonspecific may result in the unnecessary escalation of dopamine-blocking agents, worsening catatonia or confusion, while mislabeling age-related slowing as encephalopathy can prompt unnecessary diagnostics. Educational gaps among neurologists and psychiatrists further complicate interpretation. Most neurology residencies devote fewer than 15 hours to non-epileptic EEG interpretation, contributing to underreporting. Structured narrative EEG reports that describe background frequency, symmetry, and reactivity, along with clinical correlations, can mitigate misinterpretation and promote interdisciplinary communication. At institutions like the Houston Neuroscience Brain Center, co-review by neuropsychiatrists and EEG-certified neurologists enhances diagnostic precision.
Functionally, diffuse slowing impacts processing speed, working memory, and sustained attention. Patients may present with vague complaints of “brain fog,” cognitive inefficiency, or emotional dysregulation. In children, this may mimic attention-deficit/hyperactivity disorder but without stimulant response. In adults, symptoms are often misattributed to personality disorders or treatment-resistant depression. Identifying and treating reversible causes such as sleep apnea, hypothyroidism, or substance exposure can reverse EEG slowing and lead to symptomatic improvement. Diffuse slowing therefore serves as a biomarker for cortical inefficiency and a signal to reevaluate pharmacologic and systemic treatment strategies. Structured EEG reports that frame findings within a behavioral context improve communication and reduce unnecessary psychotropic escalation, making diffuse slowing a key target in precision psychiatric practice.
Intermittent Epileptiform Discharges as a Marker of Cortical Hyperexcitability
Intermittent epileptiform discharges (IEDs) are paroxysmal waveforms—typically spikes, sharp waves, or spike-and-wave complexes—lasting under 200 milliseconds and reflecting transient cortical hyperexcitability. Pediatric IED graphic courtesy of Papadelis et al. (2016).
Caption: 1 s of highlighted section that contains one sharp wave is presented on the right panels in an extended time scale display. Red dots indicate the peak of the IEDs. The convention for displaying EEG activity, such as in this figure, is for up to represent negative while down represents positive (cf. Greenfield et al., 2010, pp. 8-9).
Though classically linked to epilepsy, IEDs are also found in neurodevelopmental and psychiatric populations without seizures, where they signal neuronal instability and may contribute to cognitive and emotional dysregulation (Boutros et al., 2016; Swatzyna et al., 2020). IEDs may be focal, multifocal, or generalized and are especially prevalent during drowsiness or early non-REM sleep, which is why routine EEG often fails to detect them. Sleep-deprived or overnight EEG increases sensitivity, particularly in children with attention-deficit/hyperactivity disorder (ADHD) or autism spectrum disorder (ASD), where prevalence can exceed 25% (Hara, 2007; Swatzyna et al., 2017).
Swatzyna et al. (2022) further explored the prevalence of isolated epileptiform discharges across psychiatric conditions. Their systematic review and cross-sectional analysis found that IEDs occurred in 25.2% of ADHD cases and 63.3% of ASD cases. Rates for mood and anxiety disorders were lower but still significant, with cross-sectional data suggesting rates of approximately 39%. These findings underscore the value of EEG as a tool for guiding medication selection, especially in refractory psychiatric cases where the trial-and-error approach can be harmful. Identifying IEDs may prevent inappropriate use of psychotropics that exacerbate cortical excitability. Moreover, isolated discharges have also been observed in Alzheimer's disease and other cognitive impairments, raising important implications for broader diagnostic strategies. For instance, EEG may help determine driving safety in patients with subclinical discharges.
The clinical relevance of IEDs depends on their anatomical location. Frontal IEDs are associated with disinhibition and irritability, temporal discharges with memory impairment and mood lability, and parietal discharges with sensory integration deficits. When misattributed to primary psychiatric conditions, such as mood or personality disorders, IEDs may lead to pharmacologic mismanagement. For example, selective serotonin reuptake inhibitors (SSRIs), stimulants, and antipsychotics can lower seizure threshold and exacerbate cortical instability in susceptible individuals (Swatzyna et al., 2020). Conversely, EEG-guided interventions—including low-dose anticonvulsants or targeted neurofeedback—can reduce discharge frequency and improve behavioral outcomes.
In patients with treatment resistance, IEDs should be considered a red flag prompting extended EEG evaluation and reconsideration of diagnosis. Their identification reflects the potential for subclinical electrophysiological contributors to cognitive and affective dysfunction, separate from encephalopathy.
This pattern reflects disruption of thalamocortical circuits and diminished cortical responsiveness due to systemic metabolic, toxic, or hypoxic insults (Brenner, 2021; Swatzyna et al., 2024). EEGs showing diffuse slowing often lack reactivity to eye opening or auditory commands, particularly in the posterior dominant rhythm. In hepatic encephalopathy, this slowing is typically frontally maximal and may present as waxing-and-waning frontal intermittent rhythmic delta activity (FIRDA), while a theta–delta fusion pattern is more common in diffuse axonal injury. Additionally, sedative-induced beta activity may obscure slow-wave patterns unless appropriate low-frequency filters (e.g., 0.3 Hertz) are employed (Swatzyna et al., 2024).
Routine EEG captures mild encephalopathy in only 40–60% of cases, with sensitivity increasing substantially through extended monitoring or activation techniques such as hyperventilation or sleep deprivation (Grant et al., 2020). Quantitative EEG (qEEG) improves detection through spectral analysis, enabling topographic mapping of slow-wave distribution and amplitude asymmetries. For example, anterior-predominant slowing correlates with executive dysfunction, while posterior slowing is linked to visuospatial deficits and emotional detachment (Rios & Sousa, 2020). Inter-rater reliability in identifying diffuse slowing is modest (κ ≈ 0.46), but improves significantly when quantitative background metrics are included (Grant et al., 2020).
Clinical misinterpretation—especially dismissing diffuse slowing as “non-specific” or as age-related—can result in missed diagnoses of metabolic or toxic encephalopathy, inappropriate medication escalation, and failure to investigate reversible etiologies. As a result, diffuse slowing remains a clinically actionable EEG biomarker that guides systemic workup and alerts clinicians to underlying pathophysiology.
Spindling Excessive Beta as a Marker of Cortical Hyperarousal
Spindling excessive beta (SEB) is defined as rhythmic beta activity (13–30 Hertz) with a spindle-shaped morphology that occurs diffusely in awake EEG recordings. The SEB graphic is courtesy of Dr. Swatzyna.
Unlike physiologic beta, SEB has a waxing-and-waning pattern resembling sleep spindles but appears during wakefulness in individuals with cortical hyperarousal (Gibbs & Gibbs, 1950; Johnstone et al., 2005). SEB has been described as a non-specific transdiagnostic marker of cortical dysregulation and is frequently observed in patients with ADHD, PTSD, generalized anxiety disorder, and ASD—particularly in refractory presentations (Swatzyna et al., 2015, 2024). In a study of over 1,200 medication-refractory patients, SEB was present in more than 25% of cases and was often associated with treatment intolerance and paradoxical medication reactions (Swatzyna et al., 2024).
SEB is thought to arise from dysfunctional GABAergic modulation and disrupted thalamocortical feedback, sometimes precipitated or exacerbated by chronic benzodiazepine use, stimulant exposure, or environmental toxins such as lead and mercury (Krepel et al., 2021; Swatzyna et al., 2024). Behavioral correlates include insomnia, anxiety, irritability, and heightened startle reflex, while functional EEG analysis often reveals elevated beta power frontocentrally. SEB does not indicate encephalopathy but represents a distinct electrophysiological phenotype of cortical overactivation. Clinical misinterpretation of SEB as a sign of vigilance or medication responsiveness may lead to inappropriate stimulant or sedative use, worsening symptoms. When SEB is identified, clinicians should assess for hyperarousal syndromes and consider down-training protocols via neurofeedback, medication tapering, or detoxification strategies. As with IEDs, SEB should prompt broader evaluation for underlying contributors to neuropsychiatric instability and guide individualized, physiology-informed treatment.
Conclusion: Distinct Biomarkers, Divergent Mechanisms
Diffuse slowing, intermittent epileptiform discharges, and spindling excessive beta are three well-established EEG biomarkers with distinct physiological origins and clinical implications. Diffuse slowing is a marker of encephalopathy and global cortical underactivation caused by systemic dysfunction (Brenner, 2021; Swatzyna et al., 2024). In contrast, IEDs signify localized cortical hyperexcitability, often observed in seizure-prone or neurodevelopmentally vulnerable individuals (Swatzyna et al., 2020), while SEB represents excessive beta synchrony linked to cortical hyperarousal and dysregulated thalamocortical gating (Johnstone et al., 2005; Krepel et al., 2021). Though these markers may coexist in complex cases, they require different diagnostic and therapeutic approaches. Recognizing their unique signatures and pathophysiological underpinnings enables clinicians to reframe treatment-resistant psychiatric presentations through a neurophysiological lens. EEG biomarkers thus provide a bridge between subjective symptoms and objective brain function, guiding precision medicine across neurology, psychiatry, and integrative care.Glossary
A (auricular): International 10-20 system earlobe reference placement.
abnormal periodic paroxysmal patterns: generalized periodic paroxysmal patterns and lateralized periodic paroxysmal patterns.
abnormal slow activity: generalized intermittent slow activity, focal and lateralized intermittent slow activity, and persistent slow activity.
amplitude: the strength of the EEG signal measured in microvolt or picowatts.
artifact: false signals like 50/60Hz noise produced by line current.
benign epileptiform transients of sleep (BETS): sharp waves are seen alone or as a low-amplitude spike and a smaller after-going slow wave. BETS can be monophasic or diphasic and occur during light sleep. There is no disturbance of background activity, and it does not progress.
benign small sharp spikes (BSSS): sharp waves are seen alone or as a low-amplitude spike and a smaller after-going slow wave. BETS can be monophasic or diphasic and occur during light sleep. There is no disturbance of background activity, and it does not progress.
bilateral independent periodic discharges (BIPDs): asynchronous discharges that occur independently in the left and right hemispheres, appear as sharp waves or spikes, 100-300 µV, and recur at rates up to 3 Hz.
bilateral independent periodic lateralized epileptiform discharges (BIPLEDs): asynchronous discharges that occur independently in both hemispheres, appear as sharp waves or spikes, 40-100 µV in bipolar montages, and recur at rates from 0.5-1.5 Hz.
bridging artifact: a short circuit between adjacent electrodes due to excessive application of electrode paste or a client who is sweating excessively or arrives with a wet scalp.
brief duration: 10-60 s.
bursts of rhythmic temporal theta (BORTTs): transient periods of rhythmic theta activity observed in the temporal regions of the brain during EEG recording. These bursts typically manifest as short-lived increases in theta oscillations and are often associated with cognitive processes such as memory encoding and retrieval.
C (central): sites in the International 10-20 system that detect frontal, parietal-occipital activity and temporal EEG activity.
channel: an EEG amplifier input from three leads (active, reference, and ground electrodes) placed on the head.
drowsiness artifact: in adults, 1-Hz (or slower) waveforms can be detected with the greatest amplitude, and reverse polarity at F7 and F8 may progress to 1-2 Hz slowing of the alpha rhythm.
encephalopathy: a broad term for any diffuse disease of the brain that alters brain function or structure, often characterized by altered mental states and various neurological symptoms.
EEG artifacts: noncerebral electrical activity in an EEG recording can be divided into physiological and exogenous artifacts.
electro-ocular artifact: contamination of EEG recordings by potentials generated by eye blinks, eye flutter, and eye movements.
electrode pop artifact: sudden large deflections in at least one channel when an electrode abruptly detaches from the scalp.
EMG artifact: interference in EEG recording by volume-conducted signals from skeletal muscles.
epileptogenic patterns of doubtful significance: brief EEG patterns not associated with seizures or neurological disorders. Examples are 6-Hz spike-and-slow-wave, 14- and 16-Hz positive bursts, benign epileptiform transients of sleep (BETS), rhythmical mid-temporal discharge (RMTD), small sharp spikes (SSS), and wicket spikes.
exogenous artifacts: noncerebral electrical activity generated by movement, 50/60 Hz and field effect, bridging, and electrode (electrode “pop" and impedance) artifacts.
F (frontal): sites in the International 10-20 system that detect frontal lobe EEG activity.
focal and lateralized intermittent slow activity: EEG activity under 8 Hz and usually confined to a single or a couple of adjacent electrodes. These bursts have an irregular appearance, are composed of several frequencies, and rarely involve an entire hemisphere.
Fp (frontopolar or prefrontal): sites in the International 10-20 system that detect prefrontal cortical EEG activity.
frontal intermittent rhythmic delta activity (FIRDA): bilateral and synchronous discharges that exceed 100 µV and recur at rates up to 3 Hz.
generalized intermittent slow activity: asynchronous EEG activity under 8 Hz that involves the majority or all of both hemispheres. These bursts typically consist of polymorphic delta.
generalized periodic discharges (GPDs): asynchronous EEG activity under 8 Hz that involves the majority or all of both hemispheres. These bursts typically consist of polymorphic delta.
generalized periodic epileptiform discharges (GPEDs or GPDs): bilateral and synchronous discharges that exceed 100 µV and recur at rates up to 3 Hz.
generalized periodic paroxysmal patterns: epileptiform discharges in the same areas of both hemispheres. The waveforms exhibit similar composition, amplitude, and phase in each hemisphere but may slightly vary within a hemisphere.
generalized rhythmic delta activity (GRDA): generalized rhythmic delta activity. The term frontal intermittent rhythmic delta activity (FIRDA) was used before ACNS standardization in 2012. These bilateral and synchronous discharges exceed 100 µV and recur at rates up to 3 Hz.
hertz (Hz): the unit of frequency measured in cycles per second.
high amplitude: 60-200+ µV.
ictal epileptiform discharges: individual spikes and sharp waves, and complexes that contain both waveforms that last less than 2 s.
impedance (Z): the complex opposition to an AC signal measured in Kohms.
impedance meter: a device that uses an AC signal to measure impedance in an electric circuit, such as between active and reference electrodes.
impedance test: automated or manual measurement of skin-electrode impedance.
inion: bony prominence on the back of the skull.
interictal epileptiform discharges: high-amplitude spike-and-wave or polyspike-and-wave complexes that repeat at a frequency of 3 Hz.
intermediate duration: 1-1.5 minutes.
intermittent rhythmic delta activity (IRDA): bilateral synchronous discharges recur between 2-2.5 Hz and appear in brief bursts.
International 10-20 system: a standardized procedure for 21 recording and one ground electrode on adults.
lateralized periodic discharges (LPDs): lateralized periodic discharges. These unilateral discharges appear as sharp waves or spikes, 100-300 µV, and recur at rates up to 3 Hz.
lateralized periodic paroxysmal patterns: epileptiform discharges that differ from generalized periodic paroxysmal patterns in their unilateral distribution. Both patterns share the same waveform morphology.
lateralized rhythmic delta activity (LRDA): unilateral discharges recur at rates up to 3 Hz. LRDA runs typically last less than 1 minute and is shorter than LPDs.
low amplitude: less than 20 µV.
mastoid bone: the bony prominence behind the ear.
microvolt (μV): the unit of amplitude (signal strength) that is one-millionth of a volt.
movement artifact: voltages caused by client movement or the movement of electrode wires by other individuals.
multifocal (Mf): an EEG abnormality detected at several scalp locations.
nasion: the depression at the bridge of the nose.
neuroplasticity: the brain's creation and remodeling of synapses in response to experience, injury, and learning.
notch filter: a filter that suppresses a narrow band of frequencies, such as those produced by line current at 50/60Hz.
O (occipital): sites in the International 10-20 system that detect occipital lobe EEG activity.
ohm (Ω): unit of impedance or resistance.
P (parietal): sites in the International 10-20 system that detect parietal lobe EEG activity.
paroxysmal epileptogenic abnormalities: interictal epileptiform discharges (focal, generalized), ictal, secondary bilateral synchrony, and epileptiform patterns of doubtful significance.
periodic lateralized epileptiform discharges (PLEDs): lateralized or focal and exhibit regular periodic, negative spike-and-sharp wave patterns with a 20-1000 ms duration and 50-300 µV amplitude.
physiological artifacts: noncerebral electrical activity that includes electromyographic, electro-ocular (eye blink and eye movement), cardiac (pulse), sweat (skin impedance), drowsiness, and evoked potential.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
positive occipital sharp transients of sleep (POSTS): sharply contoured with a frequency of 4-5 Hz and seen alone or in groups in occipital areas. POSTS are usually bilaterally synchronous but opposite in phase.
preauricular point: the slight depression located in front of the ear and above the earlobe.
prolonged duration: 5-60 minutes.
protracted duration: greater than 60 minutes.
pulse artifacts: noncerebral voltages due to mechanical movement of an electrode in relation to the skin surface due to the pressure wave of each heartbeat.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
Research Domain Criteria (RDoC): The Research Domain Criteria (RDoC) is a research framework developed by the National Institute of Mental Health (NIMH) that aims to integrate various domains of information (including genetics, neuroscience, and behavioral science) to understand mental disorders. It moves beyond traditional diagnostic categories to focus on fundamental biological and psychological processes that cut across different disorders.
rhythmic temporal theta of drowsiness (RMTD): bitemporal left is greater than right in this longitudinal bipolar montage. Noted are notched rhythmic waveforms localized to the temporal regions, some of which are sharply contoured.
secondary bilateral synchrony (SBS): spikes with a single phase reversal around the midline.
sharp-wave (SW): transient with pointed peak and 70 to 200 ms duration.
spike-wave (SW): transient with pointed peak and 20 to under 70 ms duration.
spinding excessive beta (SEB): an EEG pattern characterized by high-frequency beta waves (13-30 Hz) with a spindle-like appearance, often exceeding 20 μV in amplitude. It is associated with various psychiatric and neurological conditions, including ADHD, insomnia, and medication-resistant psychiatric disorders.
SREDA (or SCREDA): sub-clinical rhythmic electrographic discharges in adults.
stimulus induced rhythmic, periodic, or ictal discharges (SIRPIDs): EEG discharges reliably produced by alerting stimuli.
theta–delta fusion pattern:an electroencephalographic background in which a slowed posterior dominant rhythm in the theta range (≈ 4–7 Hz) co-exists and continuously blends with lower-frequency delta activity (≤ 4 Hz), producing indistinct, mixed‐frequency waves across widespread scalp regions. This pattern typically appears in diffuse axonal or hypoxic–ischemic injury and signals moderate cerebral dysfunction rather than focal pathology.
tragus: the flap at the opening of the ear.
transient: isolated waveforms or complexes that can be distinguished from background activity.
typical amplitude: 20-50 µV, depending on age.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
very brief duration: less than 10 s.
wicket waves: EEG activity with arciform appearance, no after-going slow-wave, and no background disruption or disturbance.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Assignment
Now that you have completed this module, write down the frequency bands you uptrain and downtrain in clinical or peak performance practice and explain the rationale for these choices.
References
Benbadis, S. R., & Rielo, D. A. (2018). Encephalopathic EEG patterns. Medscape. Retrieved from https://emedicine.medscape.com/article/1140530-overview.
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
Ferree, T. C., Luu, P., Russell, G. S., & Tucker, D. M. (2001). Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology, 112(3), 536–544. https://doi.org/10.1016/s1388-2457(00)00533-2
Greenfield, L. J., Carney, P. R., & Geyer, J. D. (2010). Reading EEGs: A practical approach (2nd ed.). Wolters Kluwer.
ISNR Board of Directors (2010). What is neurofeedback? Retrieved from https://isnr.org/what-is-neurofeedback.
Jin, L. (2007). A reappraisal of secondary bilateral synchrony. Neurology Asia, 12, 29-35.
Johnson, E. L., & Kaplan, P. W. (2017). Population of the ictal-interictal zone: The significance of periodic and rhythmic activity. Clin Neurophysiol Pract, 2, 107-118. https://doi.org/10.1016/j.cnp.2017.05.001
Kappenman, E. S., & Luck, S. J. (2010). The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology, 47(5), 888-904. https://doi.org/10.1111/j.1469-8986.2010.01009.x.
Khazan, I. Z. (2019). Biofeedback and mindfulness in everyday life: Practical solutions for improving your health and performance. W. W. Norton & Company.
Neurofeedback essential skills list. (2020). Biofeedback Certification International Alliance.
Peper, E., Gibney, K. H., Tylova, H., Harvey, R., & Combatalade, D. (2008). Biofeedback mastery: An experiential teaching and self-training manual. Association for Applied Psychophysiology and Biofeedback.
Richardson, C. A., & Benbadis, S. R. (2019). Generalized EEG waveform abnormalities. Medscape. Retrieved from https://emedicine.medscape.com/article/1140075-overview.
Swatzyna, R. J., Arns, M., Tarnow, J. D., Turner, R. P., Barr, E., MacInerney, E. K., Hoffman, A. M., & Boutros, N. N. (2022). Isolated epileptiform activity in children and adolescents: Prevalence, relevance, and implications for treatment. European Child & Adolescent Psychiatry, 31(4), 545–552. https://doi.org/10.1007/s00787-020-01597-2
Swatzyna, R. J., Brown, T., Henrich, H., & Arns, M. (2024). Evidentiary significance of routine EEG in refractory psychiatric cases. Clinical EEG and Neuroscience. Advance online publication. https://doi.org/10.1177/15500594241234567
Swatzyna, R. J., Kozlowski, G. P., & Tarnow, J. D. (2015). Pharmaco-EEG: Individualized medicine in clinical practice. Clinical EEG and Neuroscience, 46(3), 192-199. https://doi.org/10.1177/1550059414555932
G. Tan, F. Shaffer, R. R. Lyle, & I. Teo (Eds.). Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Yamada, T., & Meng, E. (2018). Practical guide for clinical neurophysiology (4th ed.). Oxford University Press.
F. The Use of Different EEG Montages for Waveform Analysis
Peer-reviewed evidence suggests that more EEG channels provide a more accurate assessment and achieve superior clinical or performance outcomes than fewer channels (Lau et al., 2012).
Please click on the podcast icon below to hear a lecture over Section F.

Although some practitioners conduct assessment and training with a single channel, assessments and training methods that use more than one channel have become more available. For example, the cost of a full 19-channel EEG assessment has decreased substantially. Such an assessment can provide not only EEG amplitude data from all sites in the 10-20 system but additionally makes calculations of metrics such as coherence and phase that give information on how well the 10-20 sites communicate with each other. These data from multi-channel methods are beneficial for complex symptom profiles like those associated with Autism Spectrum Disorders, epilepsy, and traumatic brain injury (Thompson & Thompson, 2016). Graphic © Chaikom/Shutterstock.com.

Montage Options and Their Consequences
Electroencephalography (EEG) is a powerful method for recording the brain's electrical activity, but its interpretive value depends heavily on how the electrode data are referenced and compared. These reference strategies are called montages. The choice of montage affects signal quality, spatial localization, artifact suppression, and clinical utility (Niedermeyer & da Silva, 2005). EEG montages group derivations, or electrode pairs, into configurations that guide the visualization and interpretation of cerebral activity. The differential amplifier at the heart of EEG systems subtracts shared signals from each electrode pair to isolate differences, a process known as common mode rejection (Sanei & Chambers, 2007). Understanding how this process interacts with various montage types is essential for clinical accuracy and effective neurofeedback.
In this essay, we will examine the principal montage configurations used in clinical and research EEG: referential (monopolar), sequential (bipolar), average reference, linked ears reference, Laplacian (local average), common average, source derivation, and Cz reference. Each of these montages structures the EEG data differently and offers unique strengths and weaknesses depending on the clinical context. We will explore how these montages are constructed, when they are used, how they manage artifacts, and their limitations. We will begin by examining the fundamental role of differential amplifiers and then proceed to evaluate each montage in turn. We will conclude with an integrative summary that provides practical guidance for selecting montages in clinical neurofeedback and EEG analysis.
Differential Amplifiers and Common Mode Rejection
A channel is an EEG amplifier output resulting from scalp electrical activity from three electrode/sensor connections to the scalp. These sensors are commonly known as active, reference, and ground electrodes, though they are more appropriately called positive +, negative - and reference. They are placed on the head in the following manner: an active or positive electrode is placed over a known EEG generator like Cz. A reference or negative electrode may be located on the scalp, earlobe, or mastoid. A ground/reference electrode may also be placed on an earlobe or mastoid (Thompson & Thompson, 2015).Active and reference sensors are identical balanced inputs and interchangeable. However, some neurofeedback data acquisition systems require the designation of a specific sensor as a "reference," as in a linked-ears reference.
The graphic below was redrawn from John Demos' BCIA-recommended Getting Started with EEG Neurofeedback (2nd ed.). The ear references are connected as a common reference for the four active electrodes (F7, T3, T4, and T5).
A derivation is the assignment of two electrodes to an amplifier's inputs 1 and 2. For example, Fp1 to O2 means that Fp1 is placed in input 1 and O2 in input 2.
A montage groups electrodes together (combines derivations) to record EEG activity (Thomas, 2007).
All montages compare EEG activity between one or more pairs of electrode sites.
Modern amplifiers record all input sensors in reference to a common sensor - often Cz - and all montage (sensor comparison) changes are performed in the software. Amplifiers no longer require manual switching of electrodes between inputs.The narrated video below © John S. Anderson displays the same 21-channel recording viewed using different montages with a 60-Hz notch filter on and off.
The differential amplifier is the core element of EEG acquisition. It takes input from two electrodes—designated as positive and negative—and amplifies the voltage difference between them while suppressing any voltage common to both. This suppression of shared signal, known as common mode rejection, is vital in reducing electrical artifacts such as 50 or 60 Hertz interference from power lines (Huigen et al., 2002). The effectiveness of this process is quantified as the common mode rejection ratio (CMRR), which ideally exceeds 100,000:1 or 100 decibels (MettingVanRijn et al., 1990). Such performance ensures that genuine cerebral signals are preserved while artifacts are minimized.
Each EEG channel represents this differential comparison between two inputs. Modern amplifiers typically include a third electrode, the ground or system reference, which serves as the return path for currents and stabilizes the electrical circuit. This tripartite configuration—positive, negative, and ground—is the basis for clean signal acquisition. The voltages at the active and reference electrodes are each calculated relative to the ground, and the differential amplifier then subtracts one from the other, multiplying the result by a predefined gain to produce the final output.
The location of the reference electrode has a profound influence on what is seen in the EEG tracing (Nunez & Srinivasan, 2006). If the reference site shares common electrical activity with the active electrode, that activity is subtracted out, potentially obscuring important signals. Conversely, if the reference contains unique artifacts such as cardiac or muscle activity, these signals can be erroneously attributed to the active electrode. This phenomenon, called reference contamination, is particularly problematic when the reference is placed near sources of high-amplitude physiological noise, such as the jaw, neck, or posterior scalp.
Differential amplifiers do not distinguish between real brain activity and artifact; they merely process differences. This means that highly rhythmic EEG signals like posterior alpha activity can be attenuated if they are in phase and similar in amplitude at both electrodes. On the other hand, any signal that is present at only one of the electrodes or that differs significantly in phase or amplitude will be retained and potentially amplified. Therefore, understanding the geometry of signal sources and the spatial orientation of electrodes is essential for correct interpretation.
Artifact suppression using differential amplifiers is not limited to electrical noise. Common biological artifacts—such as electrocardiographic (ECG) signals, electromyographic (EMG) activity, and eye movements—can also be suppressed to varying degrees. For instance, ECG artifacts, which span from 0.05 to 80 Hertz, can contaminate a broad range of EEG frequency bands. If these artifacts are present at both electrodes in a derivation, the amplifier will subtract them. However, if they are present only at the reference site, they may appear in the signal of interest, misleading the clinician (Gasser et al., 1985).
In summary, the differential amplifier and its common mode rejection capabilities form the technological foundation of EEG recording. All montage configurations—whether monopolar, bipolar, or complex derivations—rely on this principle. Understanding how signal subtraction and artifact suppression occur enables clinicians and researchers to make informed choices about electrode placement and montage selection, which ultimately improves the reliability and interpretability of EEG data.
Referential (Monopolar) Montage
The referential or monopolar montage is one of the most commonly used EEG configurations in clinical and neurofeedback settings. In this setup, a single active electrode is placed at a site of interest on the scalp and referenced to a site assumed to be electrically neutral, such as the earlobe or mastoid process (Thompson & Thompson, 2015). The goal is to isolate activity at the active electrode by comparing it to a stable reference point. This configuration is often favored for its simplicity and ease of interpretation, especially in routine EEGs and neurofeedback applications.A referential (monopolar) montage places one active electrode (A) on the scalp and a "neutral" reference (R) and ground (G) on the ear or mastoid. Graphic adapted by minaanandag on fiverr.com.
A referential montage assumes that the EEG activity seen on the computer screen represents the active (+) site because the reference (-) site is assumed to be neutral (i.e., producing no EEG activity) and because of the subtraction of signals produced by noise and artifacts that are common to both active and reference sites (common-mode rejection).
The graphics below were redrawn from from John Demos' BCIA-recommended Getting Started with EEG Neurofeedback (2nd ed.). In the right diagram, the active electrode "sees" 7 microvolts while the reference "sees" 0 microvolts, for a total voltage of 7 microvolts.
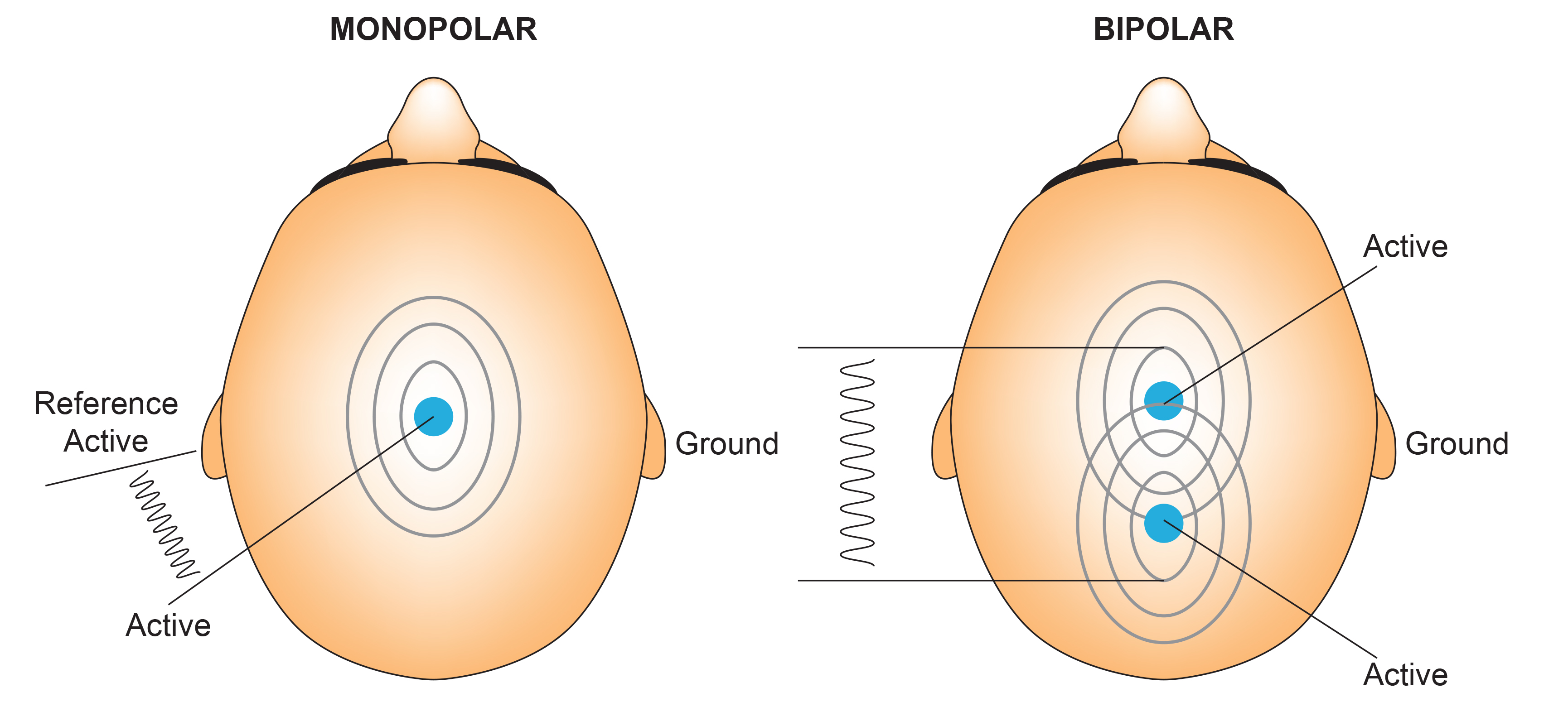
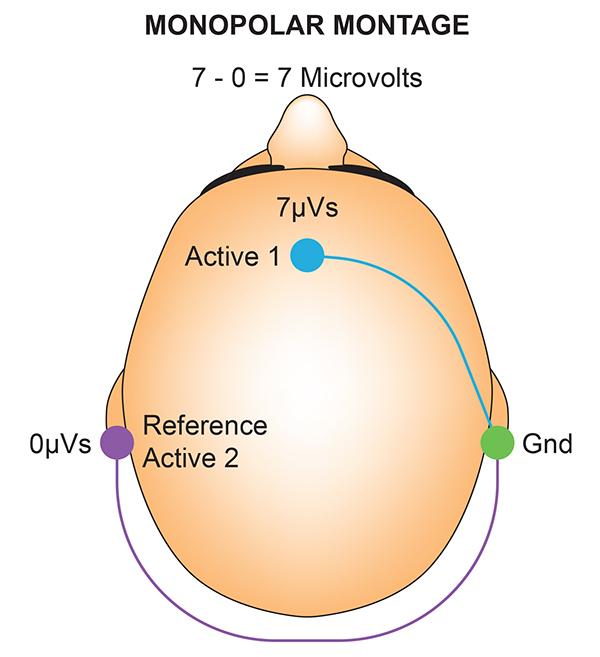
In the photograph below, the blue cable would be used for the active electrode, the yellow cable with an ear clip for reference, and the black cable with an ear clip for the ground.

However, this montage is vulnerable to artifacts from the contraction of facial muscles (Demos, 2019). The ear reference is also known to produce reference contamination, where EEG signals from this electrode are contributed or added to other electrodes via the mechanism of the differential amplifier, where anything different between the "active" and "reference" sensors is retained. This commonly results in alpha activity produced by posterior alpha sources close to the ear.
The graphic below was redrawn from John Demos' BCIA-recommended Getting Started with EEG Neurofeedback (2nd ed.). A differential amplifier rejects the common voltage (e.g., 3 feet) and outputs the voltage difference (e.g., 4 feet). A single-ended amplifier outputs the entire voltage (e.g., 7 feet, EEG artifact and signal value).
One of the primary advantages of the referential montage is its capacity to highlight localized activity at the active electrode site. This can be particularly useful for detecting abnormalities such as focal slowing, spikes, or epileptiform discharges. By choosing a reference site that contributes minimal electrical activity, the differential amplifier can emphasize the true signal from the active site. When used correctly, this montage is especially effective in identifying asymmetries and focal disturbances.
However, the assumption that the reference site is electrically neutral is frequently violated (Demos, 2019). Reference contamination occurs when the reference site generates its own neural or artifactual signals, which then appear in the recorded EEG as if they originated at the active electrode. This contamination is particularly problematic in posterior regions with strong alpha activity. For example, reference electrodes near T5 or T6 can introduce rhythmic alpha activity into anterior traces due to the effects of volume conduction and common mode rejection (Johnstone et al., 2005).
In addition to contamination from neural signals, the referential montage is sensitive to physiological artifacts. Muscle activity from the temporalis or sternocleidomastoid muscles, as well as eye movements and cardiac signals, can all distort the EEG when they appear at or near the reference site. These artifacts may be mistaken for cortical activity unless clinicians are trained to recognize their characteristic signatures (Nunez & Srinivasan, 2006).
Another limitation of the referential montage lies in its susceptibility to misinterpretation in the presence of symmetrical brain activity. When both the active and reference electrodes detect similar signals, the resulting subtraction may cancel out real EEG features. Conversely, when only the reference site contains activity, such as alpha or ECG artifact, the differential amplifier may erroneously attribute this to the active site, creating a false impression of localized activity.
Despite these limitations, the referential montage remains a foundational configuration in EEG. With careful attention to electrode placement, impedance matching, and artifact rejection, it can provide valuable insights into cerebral function (Nuwer, 1997). Understanding the sources of contamination and their effects on signal interpretation is essential for maximizing the utility of this montage in both clinical and research contexts.
Sequential (Bipolar) Montage
The sequential or bipolar montage consists of electrode pairs placed on the scalp in which both electrodes serve as active recording sites.
The sequential (bipolar) montage detects the difference in EEG between the positive and the negative electrodes (active and reference), as the referential montage does. The signal for the channel represents the difference between two sources of EEG activity.
The graphics below were redrawn from from John Demos' BCIA-recommended Getting Started with EEG Neurofeedback (2nd ed.). In the diagram on the right, the active "sees" 7 microvolts while the reference "sees" 3 microvolts. A differential amplifier subtracts these voltages, leaving 4 microvolts.
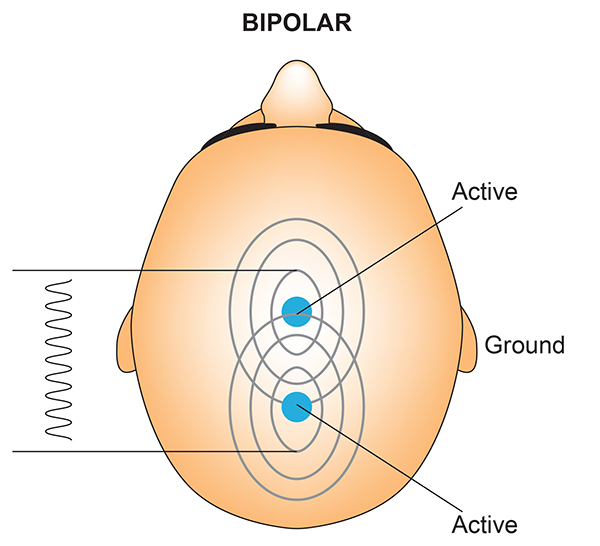
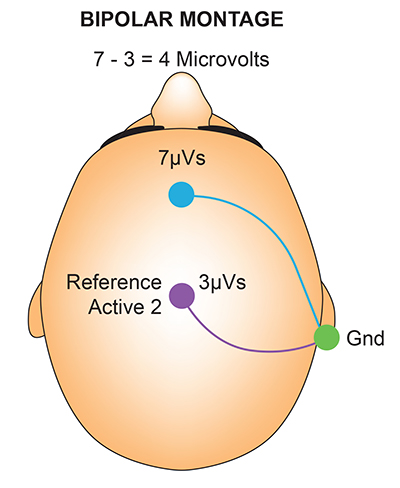
In cases when 19-channels are used, this montage is usually presented with electrode pairs shown in sequence. Note that only the black cable for the ground has an ear clip in the photograph below.

Instead of referencing an active site to a neutral point, this montage measures the voltage difference between two cortical regions, making both electrodes functionally equivalent. This approach results in a differential signal that reflects the spatial contrast between neighboring sites, offering insights into the topography of electrical activity across the brain (Niedermeyer & da Silva, 2005).
The EEG record appears more similar when sensors are closer together and less similar when they are farther apart (Fp1-O2). When electrodes are spaced close together (Fp1-Fp2), this montage may reject actual EEG activity (Fp1-Fp1). Graphic © John S. Anderson.
Because a particular grouping of electrodes in a montage or array results in specific display characteristics, it is useful to understand them visually. We will examine the longitudinal, transverse, and circular bipolar montages.
Longitudinal Bipolar Montage
The longitudinal bipolar montage is also known as the “double banana” because the shapes resemble two bananas.
There are various approaches to this montage with different choices for which electrode combination to start with, but the image above shows a typical approach. The Fp1 electrode is compared to F7, then F7 is compared to T3, T3 to T5, and T5 to O1. Starting again from the front with Fp1 compared to F3 and so on for sides anterior to posterior and sometimes midline comparisons.
The benefit of this montage is that adjacent electrode sites can be compared in a sequence, and EEG features that appear in one combination can be compared to the next anterior or posterior combinations to see if the features appear there. Phase reversals, where the waves reverse polarity, and one shows a negative [up] direction while the other shows a positive [down] direction, point to the source as three derivations are compared in a sequence as shown below. That is, other bipolar montages can allow us to check the accuracy of the assumptions derived from the longitudinal bipolar montage. The transverse bipolar montage and the circular bipolar montage are two examples shown below.
Transverse Bipolar Montage
Circular (Circumferential) Bipolar Montage
The variants of bipolar montages are particularly advantageous in clinical contexts where spatial gradients are diagnostically informative. It is especially useful in epilepsy monitoring, where bipolar comparisons can reveal the propagation path of epileptiform discharges. Because it records from two active sites, this configuration increases sensitivity to focal abnormalities and enables the clinician to pinpoint the origin of abnormal waveforms by identifying phase reversals across adjacent pairs.
One strength of the sequential montage is its resistance to contamination from reference sites, which are eliminated altogether in this configuration (Tatum, 2014). Since both electrodes are located on the scalp and contribute meaningful cortical signals, the resulting output avoids the pitfalls of reference contamination seen in referential montages. This makes the bipolar configuration more robust for detecting authentic local neural activity.
However, sequential montages also have limitations. Since they display the difference between two active sites, generalized brain activity that affects both sites similarly may cancel out. As a result, this montage is less effective at capturing diffuse patterns such as generalized slowing or broad alpha rhythms. It is also more difficult to interpret absolute voltage levels since each channel reflects a subtraction of two signals rather than a single-site potential.
The effectiveness of the bipolar montage depends strongly on electrode spacing. If electrodes are placed too close together, similar signals may cancel out, leading to flat or diminished waveforms. Conversely, if electrodes are too far apart, the montage may integrate dissimilar signals, introducing artificial gradients or spurious activity. Optimal electrode spacing ensures a balance between sensitivity to local differences and reduction of volume-conducted noise (Lüders et al., 2000).
In summary, the sequential (bipolar) montage provides a powerful tool for enhancing spatial specificity and reducing reference-related artifacts. While it is less suitable for capturing generalized cortical states, it excels in applications requiring precise localization of cortical activity (Stern, 2005). By combining bipolar montages with other configurations such as average reference or Laplacian, clinicians and researchers can cross-validate findings and achieve a more comprehensive understanding of EEG dynamics (Nuwer, 1997).
Average Reference Montage
The average reference montage re-references each EEG channel to the mean voltage of all electrodes, effectively using a dynamically calculated common baseline. This method assumes that the average potential across the scalp approximates zero, especially when a high-density electrode array is used (Nunez & Srinivasan, 2006). This can help eliminate common sources and generalized EEG patterns in favor of activity that is more specific to each scalp electrode site. At the same time, it can minimize important EEG activity that exists at multiple sites, such as that in the example EEG. It can also contribute scalp EEG patterns to locations where they don’t actually occur.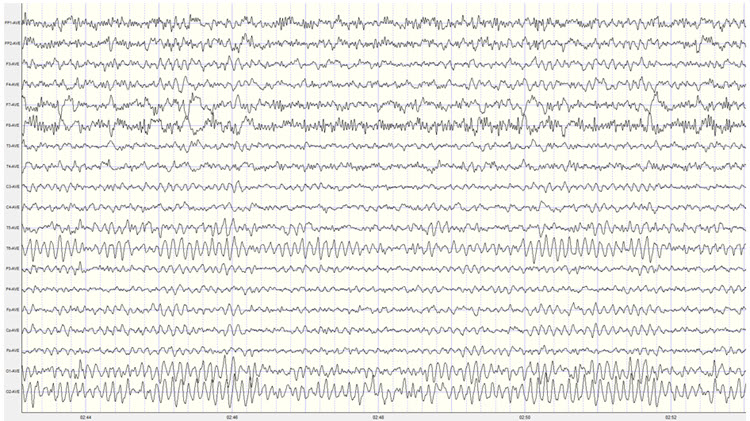
This montage is commonly used in research settings and clinical applications with full 10–20 or 10–10 coverage. It enhances the detection of distributed brain rhythms such as posterior alpha and frontal theta and facilitates comparisons across sessions or subjects (Essl & Rappelsberger, 1998).
It enhances the detection of spatially distributed brain rhythms such as the posterior alpha rhythm or frontal theta activity. Because every site is referenced to the same composite baseline, comparisons between channels are more consistent, facilitating topographic mapping and network analysis. The montage is particularly well suited for cognitive and affective neuroscience studies, where symmetrical referencing improves signal interpretability.
Despite its analytical advantages, the average reference montage depends on several assumptions that may not hold in all contexts. However, its effectiveness depends on uniform electrode distribution and good signal quality. Uneven or sparse coverage can introduce spatial bias into the calculated reference, skewing interpretation (Srinivasan et al., 1996). Additionally, artifacts in any single channel will influence all re-referenced channels unless properly rejected.
The average reference is not suitable for datasets with high noise or missing electrodes. When preprocessing includes automated rejection or interpolation, care must be taken to update the average accordingly (Kayser & Tenke, 2006). Otherwise, the residual artifact may distort spatial patterns. This montage is also susceptible to distortion from artifacts in a single channel.
Because each channel contributes to the average, high-amplitude noise at one site can propagate to all others through the reference calculation. Consequently, rigorous preprocessing is essential when using the average reference montage. Electrode impedance checks, bad channel interpolation, and artifact rejection must be performed with precision to preserve the accuracy of the derived signals.
Another drawback lies in the interpretability of the resulting waveforms. Since the signal at each site is calculated as a deviation from the mean, absolute voltage levels become ambiguous. Clinicians must be cautious when comparing recordings across sessions or individuals, as the average reference is a dynamic, data-dependent construct. For longitudinal studies or norm-referenced assessments, this instability can reduce diagnostic reliability.
Nevertheless, when applied under proper methodological conditions, the average reference montage is a powerful tool for multivariate EEG analysis. Its capacity to normalize comparisons and support global brain mapping makes it a valuable option in both clinical and experimental settings. When combined with complementary montages, it provides a more complete view of functional and dysfunctional brain activity.
Linked Ears Reference Montage
The linked ears reference montage uses the combined signal from both earlobes or mastoid processes as a shared reference for all active electrodes. This configuration is widely used in traditional neurofeedback and clinical EEG settings due to its simplicity and historical compatibility with early EEG systems. By averaging the signals from A1 and A2 (or M1 and M2), the montage attempts to create a neutral reference that is equidistant from the cortical recording sites (Thompson & Thompson, 2015).Linked ears are well known for adding cardiac activity, EMG activity from neck and jaw muscles, as well as EEG patterns such as alpha, theta, or transient activity to scalp electrodes. The example below shows this clearly in the circled epoch.
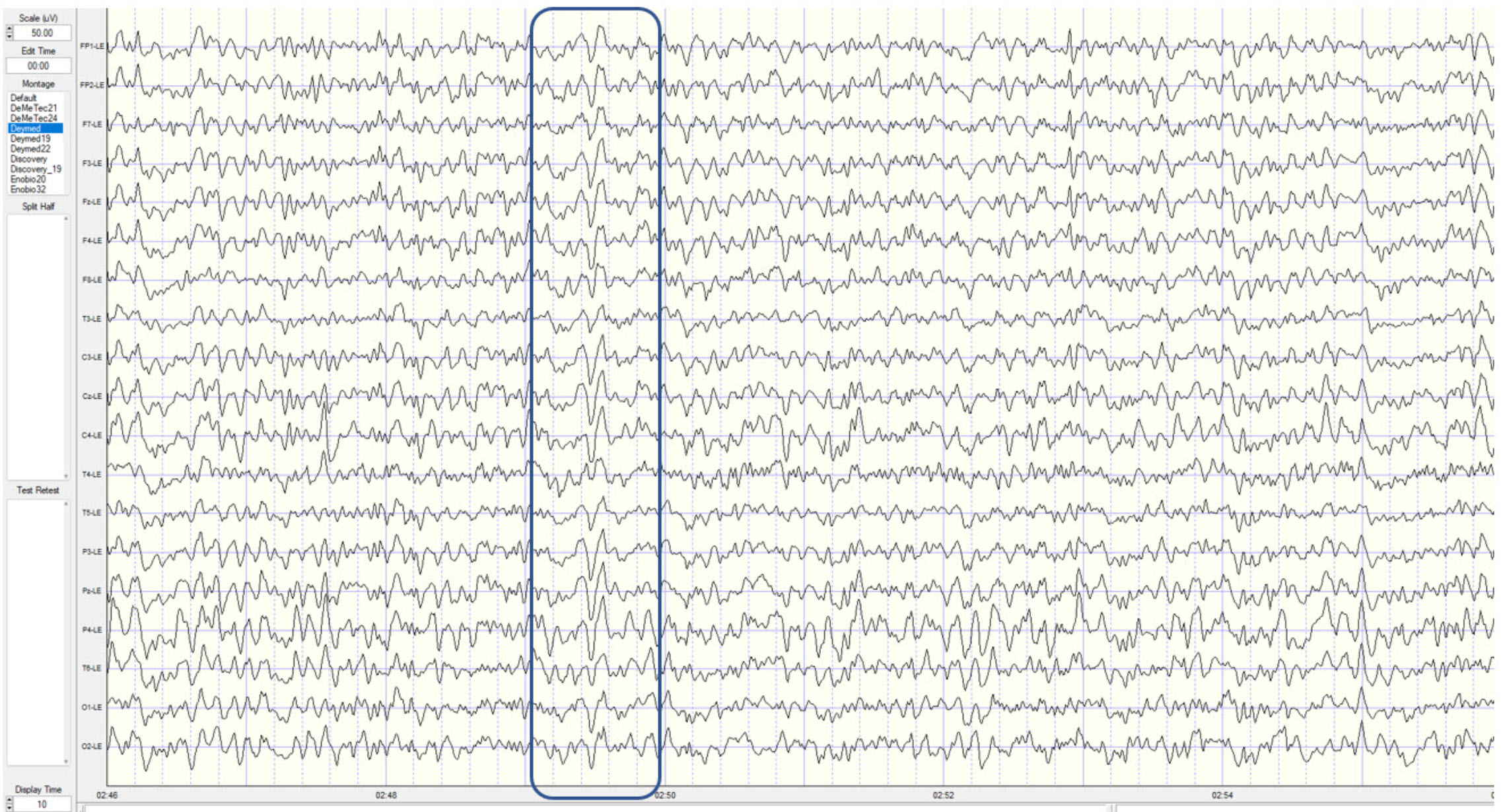
This montage allows for the visualization of broad cortical activity and supports symmetrical comparisons between hemispheres. It is particularly useful in applications focused on the vertex and central midline regions, which are physically distant from the ears and therefore less affected by reference contamination. In practice, the linked ears montage is often employed for protocols assessing posterior dominant rhythm, interhemispheric asymmetries, and training sensorimotor rhythms at Cz or C3/C4 (Johnstone et al., 2005).
However, one of the key drawbacks of the linked ears montage is reference contamination. Because the ears and mastoids are located close to the temporal lobes, they often capture alpha activity and muscle artifact, particularly from the jaw and neck (Scheller et al., 2016). When this signal is averaged and used as a reference, it may artificially inflate or distort the signals recorded at other electrodes. This is especially problematic for frontal and temporal electrodes, where contamination can obscure genuine cerebral activity.
Another concern is the asymmetry between the reference electrodes. Differences in impedance, signal quality, or artifact presence between the left and right ear can result in an unbalanced reference signal, further degrading the accuracy of the recording (Lantz et al., 2003). In patients with cranial asymmetries, conductive abnormalities, or hardware such as earrings or implants, obtaining consistent reference quality from both ears may be difficult.
Despite these limitations, the linked ears montage remains prevalent due to its operational ease and widespread adoption in legacy databases and training protocols. It is particularly well supported by many commercial neurofeedback systems and is often the default configuration in basic EEG recording setups. Its compatibility with normative databases, especially those developed using linked ear references, reinforces its continued use (Thatcher, 2010).
To maximize the effectiveness of the linked ears montage, clinicians should monitor reference quality carefully, check impedance regularly, and remain vigilant for signs of contamination. When used alongside other montage types such as bipolar or Laplacian configurations, the linked ears montage can provide a valuable reference point and contribute to a more comprehensive EEG analysis.
Laplacian Montage
The Laplacian or local average montage enhances spatial resolution by referencing each electrode to the average of its immediate neighbors. This configuration is distinct from global average reference strategies, as it emphasizes local activity while minimizing the influence of widespread or volume-conducted signals. By focusing on local field potentials, the Laplacian montage is particularly effective at isolating focal cortical sources.Electrode placement for the Laplacian montage requires relatively dense and evenly spaced coverage, ideally using 19 or more electrodes according to the 10–20 or 10–10 international systems. Each central electrode is compared mathematically to the average potential of the surrounding electrodes—typically those within a few centimeters in each direction. This yields a differential signal that highlights localized sources while suppressing broadly distributed background activity.
The Laplacian appears to differentiate the mu rhythm from the background most effectively.
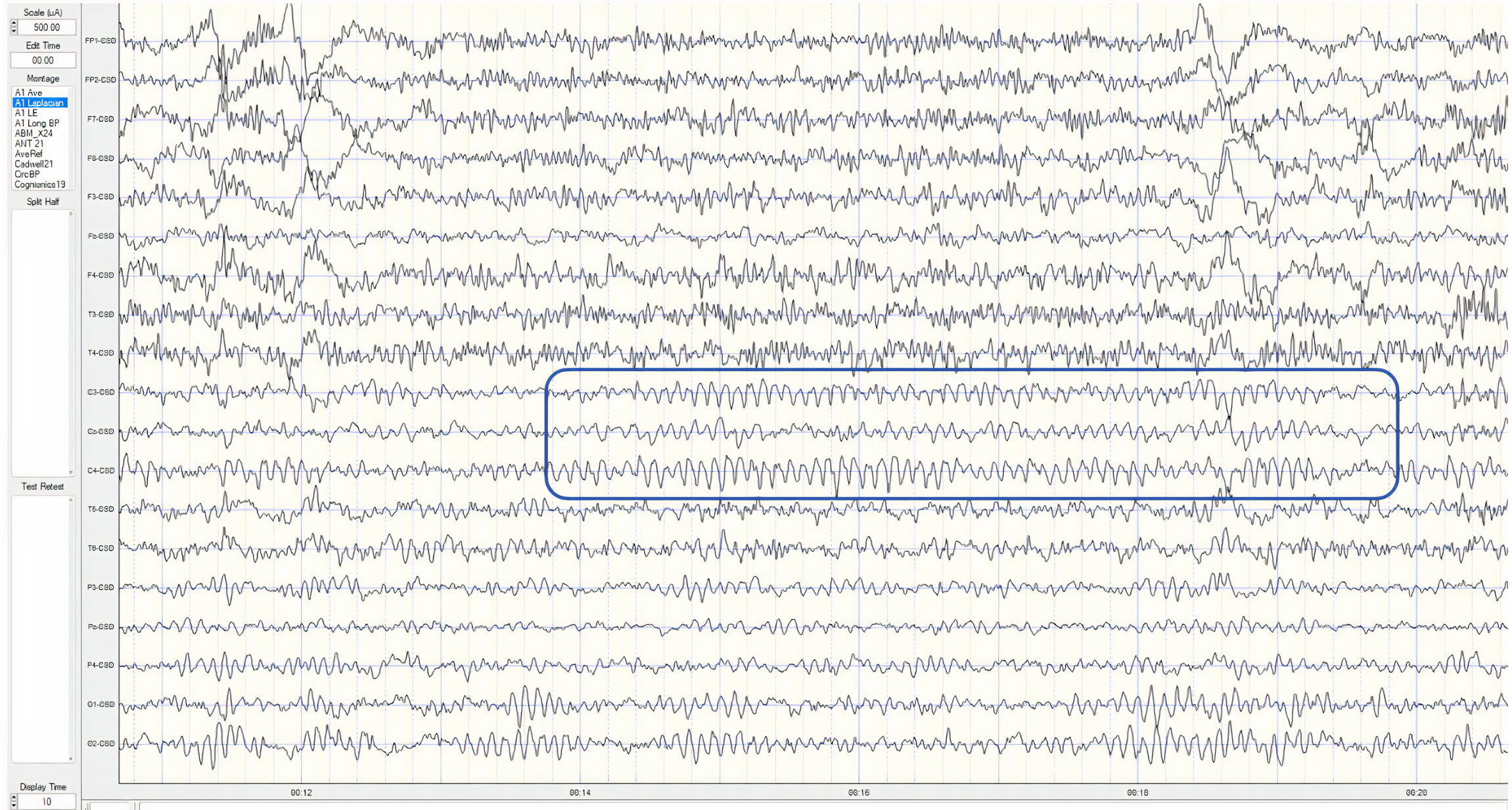
The primary strength of the Laplacian montage is its ability to improve source localization. It reduces the confounding effects of volume conduction and enhances the contrast between adjacent cortical areas (Kayser & Tenke, 2006). This montage is most effective with high-density electrode arrays and evenly spaced placements, as seen in the 10–10 or 10–5 systems. Each electrode is compared to a weighted average of its surrounding electrodes, producing a signal that reflects the degree of local activation. This setup is particularly useful in identifying sensorimotor rhythms and isolating activity in targeted cortical areas, such as during neurofeedback training or cortical mapping procedures (Carvalhaes & de Barros, 2015).
One of the major advantages of the Laplacian montage is its ability to minimize the influence of global noise and non-local sources. This is especially helpful in reducing contamination from muscle artifacts and from widespread electrical phenomena such as generalized slowing or medication effects (Gordon & Rzempoluck, 2004). It enhances the contrast between active and surrounding regions, improving the localization of pathological discharges, such as epileptiform spikes.
Nonetheless, the Laplacian montage presents technical and practical limitations. It suffers from the "edge effect," where electrodes located at the periphery of the cap, such as Fp1, O1, or T8, lack enough neighboring electrodes for accurate averaging, resulting in reduced reliability at the boundaries of the scalp array (Carvalhaes & de Barros, 2015). Additionally, spatial resolution is only as good as the density and quality of the electrode grid.
Another challenge is its susceptibility to localized artifacts. While global signals are suppressed, localized EMG or electrode noise can be exaggerated. Because the Laplacian filter enhances local differences, it requires meticulous electrode application and artifact rejection protocols to ensure meaningful output (Kayser & Tenke, 2015).
Despite its limitations, the Laplacian montage is widely regarded as a superior method for enhancing spatial detail in EEG. It offers a means to isolate localized cortical events and can serve as a complementary tool in clinical diagnostics and advanced neurofeedback. Its application is expanding with the adoption of high-density EEG systems and source modeling techniques that benefit from improved spatial specificity.
Common Average Montage
The common average montage expands on the principles of average referencing by allowing selective inclusion of electrode sites in the reference calculation. Unlike the pure average reference montage, which uses all electrode signals, the common average method excludes channels with high artifact or low signal quality, such as those at the scalp periphery or those contaminated by noise (Nunez & Srinivasan, 2006). This selective referencing enhances interpretability and reduces the influence of poor-quality signals.This montage is widely used in both clinical and research settings to improve topographic mapping and cross-electrode comparisons. It is particularly valuable in studies involving resting-state networks, cortical connectivity, and multichannel coherence metrics (Srinivasan et al., 1996). By mitigating the bias introduced by strongly active or noisy electrodes, the common average montage provides a more representative baseline.
In this montage, each channel is re-referenced to the mean of a selected subset of electrodes. This subset typically spans multiple scalp regions while omitting electrodes that exhibit high impedance, strong artifact contamination, or outlier amplitudes. This design makes the montage more robust in clinical environments, where signal quality may vary across electrode sites due to hair density, movement, or skin condition.
The strength of the common average montage lies in its adaptability and balance between global and regional specificity. It provides a relatively neutral reference that supports comparisons across hemispheres and cortical lobes. The montage is commonly used in studies of large-scale neural networks, including resting-state analysis and ERP paradigms, where stable and interpretable baselines are essential for cross-subject and cross-condition comparisons (Kayser & Tenke, 2006).
However, the method is sensitive to errors introduced by improperly excluded channels. A single artifact-ridden electrode mistakenly retained in the reference set can distort the entire dataset, propagating errors across all re-referenced channels (Huigen et al., 2002). Therefore, rigorous quality control procedures, including impedance checks and visual inspection, must accompany the use of this montage.
Another consideration is the interpretive challenge posed by relative voltage values. Because each electrode’s signal is measured against a composite baseline, absolute values become less meaningful. This can complicate clinical interpretation, especially when attempting to quantify focal abnormalities or assess responses to interventions.
The dynamic nature of the common average reference also complicates longitudinal comparisons. Because the reference voltage varies based on the subset of included electrodes, direct comparisons across time points or subjects require consistent referencing strategies and electrode sets (Nunez & Srinivasan, 2006). This limitation is particularly relevant when using normative databases or z-score maps.
Nevertheless, the common average montage remains a flexible and widely accepted configuration. It bridges the gap between local precision and whole-brain monitoring, offering clinicians and researchers a tool that balances noise suppression, artifact management, and analytical clarity. When paired with high-quality preprocessing, the common average montage can enhance the reliability of multi-channel EEG analysis in both experimental and applied contexts.
Source Derivation Montage
The source derivation montage enhances spatial resolution by estimating cortical source activity through mathematical transformations, such as the current source density (CSD) method. This approach calculates the second spatial derivative of the scalp potential field to identify regions where current flows into or out of the cortex (Perrin et al., 1989). It aims to minimize the effects of volume conduction and isolate localized brain activity more effectively than traditional voltage-based montages. The CSD method requires dense and evenly spaced electrode coverage, typically using at least 64 channels arranged according to the 10–10 or 10–5 system. The technique assumes radial symmetry and homogeneous conductivity across the scalp and cortex, which allows for the modeling of potential gradients (Kayser & Tenke, 2006). These assumptions, though idealized, have been shown to produce accurate estimates of neural activity, particularly for surface cortical generators.Source derivation is particularly effective in revealing focal cortical activity, such as epileptiform discharges or task-specific activations, and it supports functional brain mapping in research and clinical diagnostics (Carvalhaes & de Barros, 2015). It is also widely used in event-related potential (ERP) studies to highlight component-specific topographies by suppressing global background signals.
However, the accuracy of source derivation is compromised at peripheral electrode sites, where fewer neighboring electrodes are available to contribute to the spatial derivative calculation. This "edge effect" can reduce the fidelity of source estimates in regions such as Fp1, O1, or T8 (Kayser & Tenke, 2006). Additionally, the presence of noisy or artifact-laden electrodes in the neighborhood can distort the calculated source signals.
The interpretive framework for CSD data also differs from that of traditional EEG, as the values represent current densities rather than potentials. This difference requires specialized knowledge and prevents direct comparison with standard voltage-based normative databases (Tenke & Kayser, 2012). While CSD enhances the visibility of localized sources, it must be interpreted within its methodological constraints.
Despite these challenges, source derivation offers a valuable enhancement to EEG spatial resolution and is particularly well-suited for identifying cortical activity with high specificity. When integrated with normative modeling or functional imaging data, it can deepen the precision of brain mapping and expand the clinical utility of EEG in both diagnostic and therapeutic domains.
Cz Reference Montage
The Cz reference montage uses the centrally located vertex electrode (Cz) as a common reference point for all other channels. Its placement at the intersection of the midline and coronal planes provides an anatomically symmetrical site that is equidistant from many scalp electrodes, making it a convenient and standardized choice for various EEG paradigms (Niedermeyer & da Silva, 2005).Benefits from this montage include being able to visualize electrodes that are equidistant from the vertex, such as Fp1 and Fp2, F3 and F4, O1 and O2, and so on.
Also, it allows us to compare their characteristics to reveal differences between hemispheres and between frontal and posterior areas. This montage can be useful when identifying interhemispheric amplitude asymmetries and other metrics. In the example EEG below, right and left differences can be seen. However, activity in the general vicinity of Cz can be added to other electrodes in some cases.
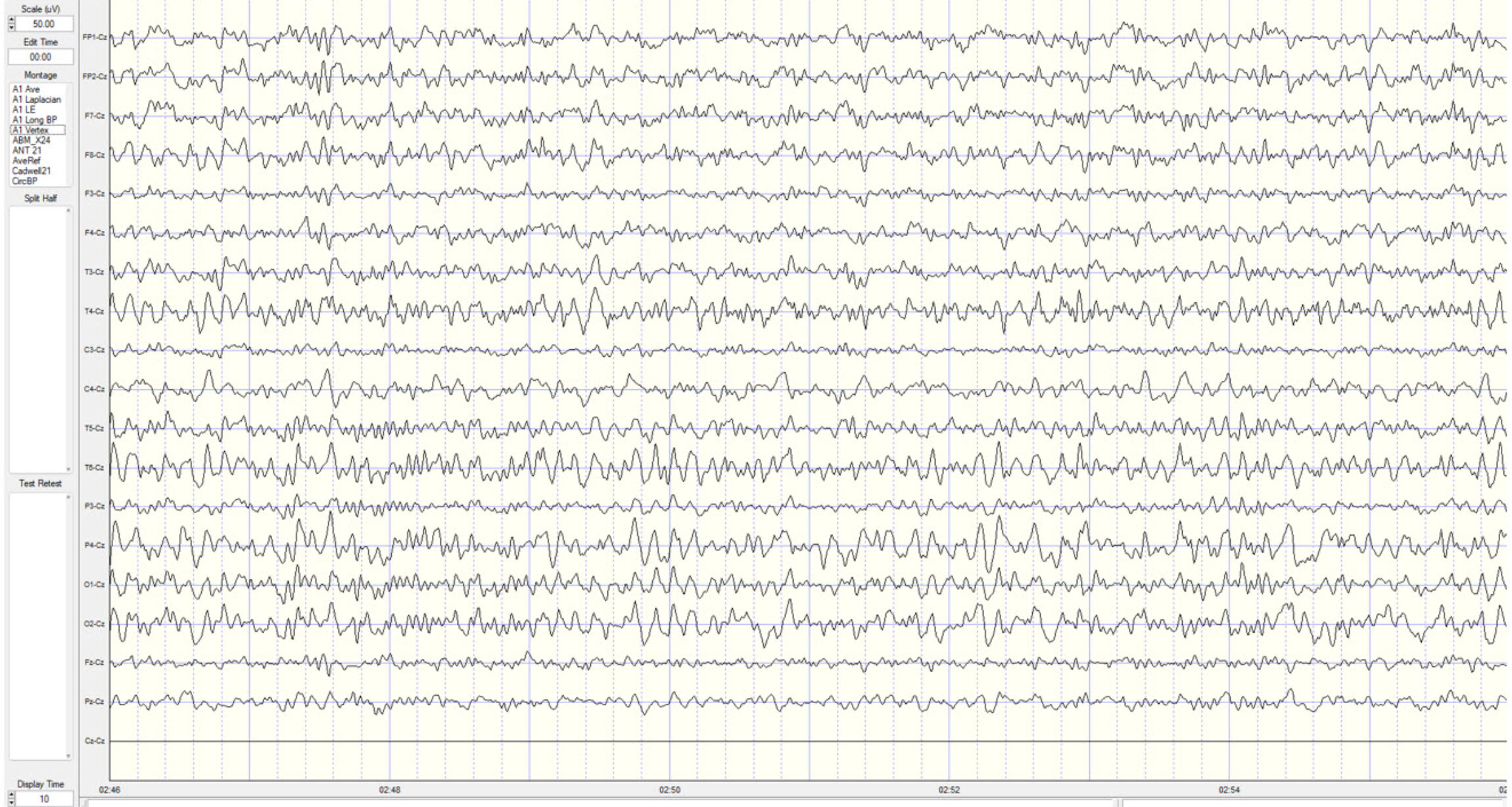
This montage is often favored in clinical and neurofeedback contexts due to its compatibility with sensorimotor tasks and studies targeting interhemispheric comparisons. Cz lies directly over the sensorimotor cortex, making it particularly useful for evaluating sensorimotor rhythm (SMR), mu rhythms, and motor-related cortical potentials (Thompson & Thompson, 2015).
A key advantage of the Cz montage is its consistency and ease of interpretation. By using a fixed and central reference, the montage supports symmetric comparisons between frontal and parietal lobes and across left and right hemispheres. It is commonly employed in event-related potential (ERP) research for analyzing P300 components and similar midline effects (Picton et al., 2000).
However, the Cz montage can obscure activity that originates at or near the Cz site itself. Because all other electrode signals are referenced to Cz, activity at Cz is subtracted out from each channel, potentially leading to underestimation or mislocalization of midline features such as vertex sharp waves or central spikes (Nunez & Srinivasan, 2006).
Additionally, any artifact present at Cz—such as from muscle tension or cranial electrical interference—can contaminate all other channels due to its role as the reference. As a result, it is important to monitor the Cz site for clean signal quality and ensure impedance levels are balanced to minimize reference noise propagation (Tatum, 2014).
Despite these drawbacks, the Cz reference montage remains a practical and widely adopted configuration. Its anatomical centrality, simplicity, and compatibility with standard cap systems make it especially useful for studies requiring standardized referencing. When interpreted cautiously and in conjunction with complementary montages, the Cz reference provides a reliable perspective on EEG topography and function.
Best Practices from the American Clinical Neurophysiology Society Guideline 3 (2016)
The Committee reaffirms the statements pertaining to montages set forth previously in the Guidelines of the American Clinical Neurophysiology Society (ACNS) and that are paraphrased as follows:(a) that no less than 16 channels of simultaneous recording be used, and that a larger number of channels be encouraged,
(b) that the full 21 electrode placements of the 10-20 system be used,
(c) that both bipolar and referential montages be used for clinical interpretation,
(d) that the electrode derivations of each channel be clearly identified at the beginning of each montage,
(e) that the pattern of electrode connections be made as simple as possible, and that montages should be easily comprehended,
(f) that the electrode pairs (bipolar) preferentially should run in straight (unbroken) lines and the interelectrode distances kept equal,
(g) that tracings from the more anterior electrodes be placed above those from the more posterior electrodes on the recording page, and
(h) that it is very desirable to have some of the montages comparable for all EEG laboratories.
2.2 The Committee recommends a “left above right” order of derivations, i.e., on the recording page, left-sided leads should be placed above right-sided leads for either alternating pairs of derivations or blocks of derivations. This recommendation coincides with the prevailing practice of most EEG laboratories, at least in North America and in many other areas.
Conclusion
The diversity of EEG montages provides a flexible framework for tailoring recordings to specific clinical or research goals. Each montage type—whether referential, bipolar, average-based, or source-derived—offers unique insights into cerebral activity and distinct methods of addressing the challenges of artifact suppression, spatial resolution, and reference bias. A foundational understanding of the differential amplifier and the principle of common mode rejection is critical to appreciating how each montage functions.Referential montages offer simplicity and are often suitable for identifying focal abnormalities, but they are vulnerable to reference contamination. Sequential montages emphasize spatial differences and are especially useful in epilepsy localization, though they may obscure global patterns. Average and common average montages promote uniform referencing and support whole-brain analysis, but they require clean, evenly distributed data. Linked ears references remain widely used but are limited by their susceptibility to temporal alpha and muscle artifact. Laplacian and source derivation montages enhance spatial resolution and suppress volume-conducted noise, though they are technically demanding and best suited for high-density systems. The Cz reference montage provides a central, symmetrical reference useful in many contexts but may obscure midline activity. Each configuration has strengths that must be weighed against its limitations, and no single montage is universally optimal. Instead, informed selection based on analytic objectives, subject characteristics, and electrode configuration is essential.
Best practices involve comparing multiple montages to validate findings and differentiate true neural activity from artifacts or montage-specific distortions. This multimodal approach can be especially beneficial in clinical settings where diagnostic precision is essential, or in research where reproducibility and spatial specificity are critical.
Continued developments in digital signal processing and source modeling are expanding the utility of advanced montages, particularly Laplacian and source derivation techniques. As EEG acquisition and analysis technology evolves, so too does the importance of understanding how different montage choices influence interpretation.
Ultimately, thoughtful montage selection enhances the diagnostic and analytic power of EEG. By integrating multiple referencing strategies, clinicians and researchers can achieve a more comprehensive and accurate depiction of brain function, guiding both assessment and intervention with greater precision.
Glossary
50/60 Hz: external artifacts transmitted by nearby electrical sources.
active electrode: an electrode placed over a site that is a known EEG generator like Cz.
alpha-blocking: the replacement of the alpha rhythm by low-amplitude desynchronized beta activity during movement, attention, mental effort like complex problem-solving, and visual processing.
amplitude: the strength of the EEG signal measured in microvolt or picowatts.
artifact: false signals like 50/60Hz noise produced by line current.
asynchronous waves: neurons depolarize and hyperpolarize independently.
average reference montage: an EEG montage in which each electrode is referenced to the mean voltage of all electrodes in the array.
bipolar montage: an EEG montage that measures the voltage difference between two adjacent active electrodes.
bridging artifact: a short circuit between adjacent electrodes due to excessive application of electrode paste or a client who is sweating excessively or who arrives with a wet scalp.
cardiac artifact: the contamination of the EEG by the ECG signal.
channel: an EEG amplifier output that is the result of scalp electrical activity from three electrode/sensor connections to the scalp.
common average montage: a modification of the average reference in which only selected electrodes are included in the average, typically excluding those with noise or edge placement.
common mode rejection: the suppression of shared signals between electrode pairs by a differential amplifier, enhancing the detection of localized brain activity.
computerized axial tomography (CAT or CT): the creation of medium-resolution images of brain structure by moving an x-ray source along an arc surrounding the head.
CSD (current source density): a technique used in source derivation montages that estimates the local net current flow into or out of the scalp at a given electrode site.
Cz: the central vertex electrode located at the intersection of the midline sagittal and coronal planes on the scalp.
derivation: the assignment of two electrodes to an amplifier's inputs 1 and 2.
desynchrony: pools of neurons fire independently due to stimulation of specific sensory pathways up to the midbrain and high-frequency stimulation of the reticular formation and nonspecific thalamic projection nuclei.
differential amplifier (balanced amplifier): a device that boosts the difference between two inputs: the active (input 1) and reference (input 2).
drowsiness artifact: in adults, 1-Hz (or slower) waveforms can be detected with greatest amplitude and reverse polarity at F7 and F8 may progress to 1-2 Hz slowing of the alpha rhythm.
EEG artifacts: noncerebral electrical activity in an EEG recording can be divided into physiological and exogenous artifacts.
electro-ocular artifact: contamination of EEG recordings by potentials generated by eye blinks, eye flutter, and eye movements.
electrode: a specialized conductor that converts biological signals like the EEG into currents of electrons.
electrode pop artifact: sudden large deflections in at least one channel when an electrode abruptly detaches from the scalp.
EMG artifact: interference in EEG recording by volume-conducted signals from skeletal muscles.
evoked potential artifact (event-related potential artifact): somatosensory, auditory, and visual signal processing-related transients that may contaminate multiple channels of an EEG record.
exogenous artifacts: noncerebral electrical activity generated by movement, 50/60 Hz and field effect, bridging, and electrode (electrode “pop" and impedance) artifacts.
field artifacts: external artifacts transmitted by nearby electrical sources.
frequency (Hz): the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
functional magnetic resonance imaging (fMRI): an imaging technique to detect brain regions' oxygen use during specific tasks indirectly.
ground electrode: a sensor placed on an earlobe, mastoid bone, or the scalp that is grounded to the amplifier.
hertz (Hz): unit of frequency measured in cycles per second.
high-frequency filter (HFF): a filter that attenuates frequencies above a cutoff frequency.
hypnogogic hypersynchrony: the abrupt appearance of low-amplitude spikes that resembles epileptiform activity in an otherwise normal record.
impedance (Z): the complex opposition to an AC signal measured in Kohms.
impedance meter: device that uses an AC signal to measure impedance in an electric circuit, such as between active and reference electrodes.
impedance test: automated or manual measurement of skin-electrode impedance.
inion: the bony prominence on the back of the skull.
International 10-10 system: a modified combinatorial system for electrode placement that expands the 10-20 system to 75 electrode sites to increase EEG spatial resolution and improve detection of localized evoked potentials.
International 10-20 system: a standardized procedure for placing 21 recording and one ground electrode on adults.
linked ears montage: an EEG montage that uses the average of both earlobe or mastoid electrodes as the reference for all other electrodes.
Laplacian montage: a local average montage where each electrode is referenced to the average of its immediate neighbors, enhancing spatial resolution.
longitudinal bipolar (LongBP) montage or double banana: EEG recording configuration involving the anterior-to-posterior chaining of adjacent electrodes in two lines on each side (Fp1 to O1 and Fp2 to O2) and connecting the midline electrodes (Fz to Pz).
magnetoencephalography (MEG): a noninvasive functional imaging technique that uses SQUIDs (superconducting quantum interference devices) to detect the weak magnetic fields generated by neuronal activity.
magnetic resonance imaging (MRI): a noninvasive imaging technique that uses strong magnetic fields and bursts of RF energy to construct highly detailed images of the living brain.
mastoid bone: the bony prominence behind the ear.
microvolt (μV): the unit of amplitude (signal strength) that is one-millionth of a volt.
monopolar recording: a recording method that uses one active and one reference electrode.
montage: a grouping of electrodes (combining derivations) to record EEG activity.
motor unit: an alpha motor neuron and the skeletal muscle fibers it innervates.
movement artifact: voltages caused by client movement or the movement of electrode wires by other individuals.
mu rhythm: an EEG rhythm typically recorded over the sensorimotor cortex, suppressed during movement or motor imagery.
nasion: the depression at the bridge of the nose.
notch filter: a filter that suppresses a narrow band of frequencies, such as those produce by line current at 50/60Hz.
ohm (Ω): the unit of impedance or resistance.
physiological artifacts: noncerebral electrical activity that includes electromyographic, electro-ocular (eye blink and eye movement), cardiac (pulse), sweat (skin impedance), drowsiness, and evoked potential.
polarization: chemical reactions produce separate regions of positive and negative charge where an electrode and electrolyte make contact, reducing ion exchange.
positron emission tomography (PET): a functional imaging technique that injects radioactive chemicals into the brain's circulation to measure brain activity.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
preauricular point: the slight depression located in front of the ear and above the earlobe.
pulse artifacts: noncerebral voltages due to mechanical movement of an electrode in relation to the skin surface due to the pressure wave of each heartbeat.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
reference contamination: the erroneous inclusion of signals from the reference electrode into the EEG trace of the active electrode.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
referential (monopolar) montage: the placement of one active electrode (A) on the scalp and a neutral reference (R) and ground (G) on the ear or mastoid.
response stereotypy: a person’s unique response pattern to stressors of identical intensity.
ssensorimotor rhythm (SMR): an EEG rhythm in the 12–15 Hz range, associated with relaxed wakefulness and sensorimotor inhibition.
sequential (bipolar) montage: placement of active (A) and reference (R) sensors on active scalp sites and the ground (G) to an earlobe or mastoid.
single photon emission computerized tomography (SPECT): a functional imaging technique that uses gamma rays to create three-dimensional and slice images of cerebral blood flow averaged over several minutes.
source derivation montage: a montage using mathematical algorithms, such as CSD, to estimate the underlying cortical generators of scalp-recorded EEG signals.
sweat artifact: changes in the EEG signal when sweat on the skin changes the conductive properties under and near the electrode sites (i.e., bridging artifact).
synchrony: the coordinated firing of pools of neurons due to pacemakers and mutual coordination.
tragus: the flap at the opening of the ear.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
vertex sharp wave: a midline EEG waveform typically seen during drowsiness, originating near the Cz electrode.
voxel: a three-dimensional pixel representing a specific volume of brain tissue, used to measure and analyze brain activity and structure.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Assignment
Now that you have completed this module, explain which montages you use in your practice and their strengths and limitations.
References
Andreassi, J. L. (2000). Psychophysiology: Human behavior and physiological response. Lawrence Erlbaum and Associates, Inc.
Acharya, J. N., Hani, A. J., Thirumala, P. D., & Tsuchida, T. N. (2016). American Clinical Neurophysiology Society Guideline 3: A Proposal for Standard Montages to Be Used in Clinical EEG. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 33(4), 312–316. https://doi.org/10.1097/WNP.0000000000000317
Basmajian, J. V. (Ed.). (1989). Biofeedback: Principles and practice for clinicians. Williams & Wilkins.
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Cacioppo, J. T., & Tassinary, L. G. (Eds.). (1990). Principles of psychophysiology. Cambridge University Press.
Carvalhaes, C., & de Barros, J. A. (2015). The surface Laplacian technique in EEG: Theory and methods. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 97(3), 174–188. https://doi.org/10.1016/j.ijpsycho.2015.04.023
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
Floyd, T. L. (1987). Electronics fundamentals: Circuits, devices, and applications. Columbus: Merrill Publishing Company.
Garces, A., Laciar, E,, Patiño, H., & Valentinuzzi, M. (2007). Artifact removal from EEG signals using adaptive filters in cascade. Journal of Physics: Conference Series, 90(1), 012081. https://10.1088/1742-6596/90/1/012081
Gasser, T., Bächer, P., & Steinberg, H. (1985). Test-retest reliability of spectral parameters of the EEG. Electroencephalography and Clinical Neurophysiology, 60(4), 312–319. https://doi.org/10.1016/0013-4694(85)91066-0
Gordon, E., & Rzempoluck, E. (2004). Laplacian methods and drug effects. Clinical EEG and Neuroscience, 35(2), 76–82. https://doi.org/10.1177/155005940403500207
Halford, J. J., Sabau, D., Drislane, F. W., Tsuchida, T. N., & Sinha, S. R. (2016). American Clinical Society Guideline 4: Recording clinical EEG on digital media. Journal of Clinical Neurophysiology, 33(4), 317-319. https://doi.org/10.1080/21646821.2016.1245563
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Hughes, J. R. (1994). EEG in clinical practice (2nd ed.). Butterworth-Heinemann.
Huigen, E., Peper, A., & Grimbergen, C. A. (2002). Investigation into the origin of the noise of surface electrodes. Medical and Biological Engineering and Computing, 40, 332–338. https://doi.org/10.1007/BF02345445
Johnstone, J., Gunkelman, J., & Lunt, J. (2005). Clinical database development: Characterization of EEG database. Journal of Neurotherapy, 9(3), 27–44. https://doi.org/10.1300/J184v09n03_04
Kayser, J., & Tenke, C. E. (2006). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns.Clinical Neurophysiology, 117 (2), 348–368. https://doi.org/10.1016/j.clinph.2005.08.03
Khazan, I. Z. (2019). Biofeedback and mindfulness in everyday life: Practical solutions for improving your health and performance. W. W. Norton & Company.
Klass, D. W. (2008). The continuing challenge of artifacts in the EEG. EEG artifacts. American Society of Electroneurodiagnostic Technologists, Inc. https://doi.org/10.1080/00029238.1995.11080524
Kubala, T. (2009). Electricity 1: Devices, circuits, and materials (9th ed.). Cengage Learning.
Lau, T. M., Gwin, J. T., & Ferris, D. P. (2012). How many electrodes are really needed for EEG-based mobile brain imaging? Journal of Behavioral and Brain Science, 2(3), 387-393. https://doi.org/10.4236/jbbs.2012.23044
Lebby, P. C. (2013). Brain imaging: A guide for clinicians. Oxford University Press.
Libenson, M. H. (2010). Practical approach to electroencephalography. Saunders Elsevier.
Lin, F., Witzel, T., Hamalainen, M. S., Dale, A. M., Belliveau, J. W., & Stufflebeam, S. M. (2004). Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage, 2(3), 582-595. https://dx.doi.org/10.1016%2Fj.neuroimage.2004.04.027
Lüders, H. O., Acharya, J. N., & Baumgartner, C. (2000). Semiology of epilepsy. Handbook of Clinical Neurology, 97, 21–51. https://doi.org/10.1016/S0072-9752(10)97003-2
MettingVanRijn, A. C., Peper, A., & Grimbergen, C. A. (1990). High-quality recording of bioelectric events: Part 1. Interference reduction, theory and practice. Medical and Biological Engineering and Computing, 28, 389–397. https://doi.org/10.1007/BF02441961
Min, B.-K. (2007). The top-down function of prestimulus EEG alpha activity. Dissertation.
Montgomery, D. (2004). Introduction to biofeedback. Module 3: Psychophysiological recording. Association for Applied Psychophysiology and Biofeedback.
Niedermeyer, E., & da Silva, F. L. (2005). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Nilsson, J. W., & Riedel, S. A. (2008). Electric circuits (8th ed.). Pearson Prentice-Hall.
Nunez, P. L., & Srinivasan, R. (2006). Electric fields of the brain: The neurophysics of EEG (2nd ed.). Oxford University Press.
Nuwer, M. R. (1997). Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology, 49(1), 277–292. https://doi.org/10.1212/WNL.49.1.277
Peek, C. J. (2016). A primer of traditional biofeedback instrumentation. In M. S. Schwartz, & F. Andrasik (Eds.). (2016). Biofeedback: A practitioner's guide (4th ed.). The Guilford Press.
Perrin, F., Pernier, J., Bertrand, O., & Echallier, J. F. (1989). Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology, 72(2), 184–187. https://doi.org/10.1016/0013-4694(89)90180-6
Picton, T. W., Bentin, S., Berg, P., Donchin, E., Hillyard, S. A., Johnson, R., ... & Taylor, M. J. (2000). Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37(2), 127–152. https://doi.org/10.1111/1469-8986.3720127
Picton, T. W., & Hillyard, S. A. (1972). Cephalic skin potentials in electroencephalography. Encephalogr Clin Neurophysiol, 33, 419-424. https://doi.org/10.1016/0013-4694(72)90122-8
Pfister, H., Kaynig, V., Botha, C. P., Bruckner, S., Dercksen, V., & Hege, H.-C. (2012). Visualization in connectomics. Mathematics and Visualization, 37. https://doi.org/10.1007/978-1-4471-6497-5_21
Sanei, S., & Chambers, J. A. (2007).EEG signal processing. Wiley-Interscience. https://doi.org/10.1002/0470035930
Srinivasan, R., Nunez, P. L., & Silberstein, R. B. (1996). Spatial filtering and neocortical dynamics: Estimates of EEG coherence. IEEE Transactions on Biomedical Engineering, 43(7), 712–720. https://doi.org/10.1109/10.502603
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Tatum, W. O. (2014). Handbook of EEG interpretation (2nd ed.). Demos Medical Publishing.
Tenke, C. E., & Kayser, J. (2012). Generator localization by current source density (CSD): Implications of volume conduction and field closure at intracranial and scalp resolutions. Clinical Neurophysiology, 123(12), 2328–2345. https://doi.org/10.1016/j.clinph.2012.06.005
Thatcher, R. W. (2010). Validity and reliability of quantitative EEG. Journal of Neurotherapy, 14(2), 122–152. https://doi.org/10.1080/10874201003773500
Thomas, C. (2007). What is a montage? EEG instrumentation. American Society of Electroneurodiagnostic Technologists, Inc.
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
