qEEG
IQCB Blueprint Coverage
This unit addresses III. qEEG Technical (9 Hours).

This unit covers:
A. Understanding the Uniqueness of QEEG Analysis from Other Neuroimaging Techniques and Conventional Metrics
B. Use of QEEG Norms and Methods Used to Derive QEEG Norms
C. The Functional Correlates of Abnormal EEG Changes
D. The Role of QEEG Metrics Toward Understanding and Treating Specific Clinical Presentations and the Relationship of the QEEG to Other Clinical Examinations
E. Demonstrate Basic Knowledge of Brodmann Areas in Terms of How Areas Were Defined and Most Common Functional Attributes of These Regions
F. Demonstrate Knowledge of Networks and Connectivity and Definition of Terms
G. Demonstrate Knowledge of Current Source Density Maps, Metrics, and Graphic Methods of Such
H. Reports Based on QEEG Metrics Should Relate These to Clinical History, Symptoms, and Other Clinical Assessments
A. Understanding the Uniqueness of QEEG Analysis from our Neuroimaging Techniques and Conventional Metrics
Neuroimaging methods can be thought of as either structural or functional. Structural methods include CT and MRI and present images of brain structures. Functional methods include EEG, MEG, fMRI, PET, and SPECT, each of which constructs images showing the location of differing levels of brain activity.
Please click on the podcast icon below to hear a lecture over Section A.

The different functional neuroimaging methods use different biologic signals as their index of function. EEG and MEG use brain electrical activity, fMRI uses blood oxygen level, PET uses positron-emitting radioisotopes bound to glucose, and SPECT uses gamma-emitting radioisotopes. Therefore, PET and SPECT are more invasive and pose more significant risks to patients and research participants (Breedlove & Watson, 2023).
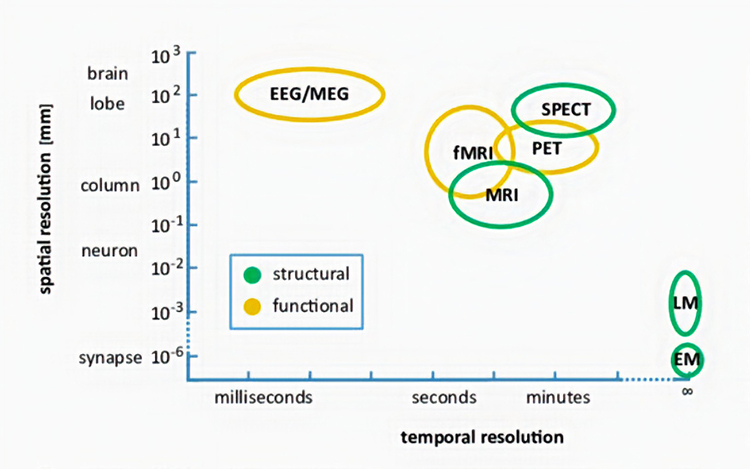
Each functional neuroimaging method can be rated with respect to how quickly it can detect changes in function (temporal resolution) and over how small an area it detects changes in function (spatial resolution). Whereas EEG and MEG methods detect changes in function most quickly, they are less able to detect the precise area where functional changes occur compared to fMRI. Graphic from Pfister et al. (2012) © Mathematics and Visualization. EM = electron microscope and LM = light microscope.
STRUCTURAL TECHNIQUES
The main structural imaging techniques are computerized axial tomography and magnetic resonance imaging.Computerized Axial Tomography
Computerized Axial Tomography (CAT or CT) provides medium-resolution images of brain structure by moving an x-ray source along an arc surrounding the head (Breedlove & Watson, 2023). Watch the Blausen CAT Scans animation. Graphic © Tyler Olson/Shutterstock.com.
CT scans allow physicians to visualize structural abnormalities like stroke damage and tumors. Graphic © Triff/Shutterstock.com.
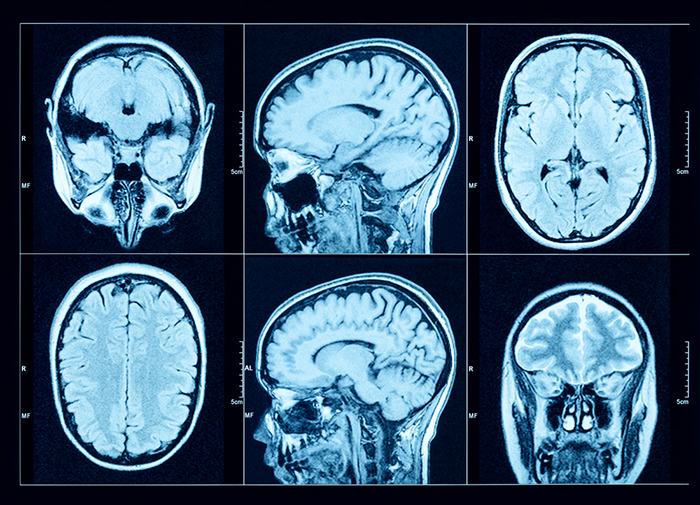
Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) constructs higher-resolution images than CT scans. Since MRI scans use powerful magnetic fields and radio wave pulses to construct images of living brains, they are safer than CT scans because there is no radiation exposure. MRI scans allow a detailed examination of brain topography, including the location and volume of specific brain regions. Graphic © Peastock/Shutterstock.com.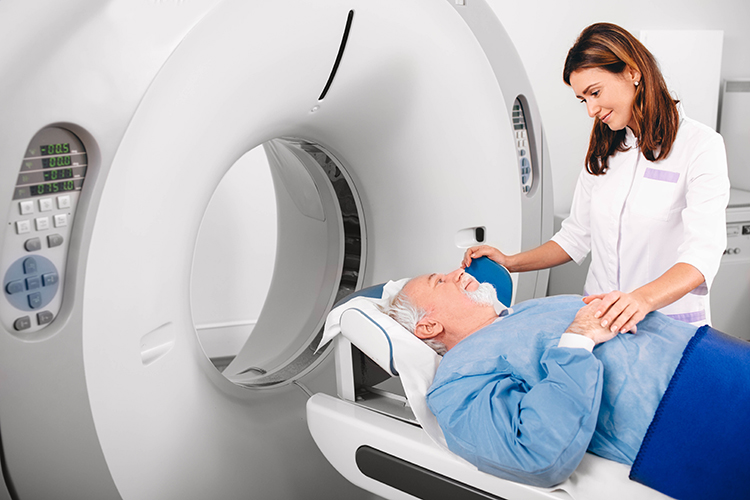
MRI scans' superior spatial resolution can detect demyelination in disorders like multiple sclerosis that CT scans would miss (Breedlove & Watson, 2023). Graphic © MriMan/Shutterstock.com.
FUNCTIONAL TECHNIQUES
Functional techniques reviewed in this section include the EEG and qEEG, magnetoencephalography (MEG), functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and single-photon computerized emission tomography (SPECT). See Lebby (2013) for an excellent overview of these techniques. Also, consult the McGill brain imaging tool module.EEG
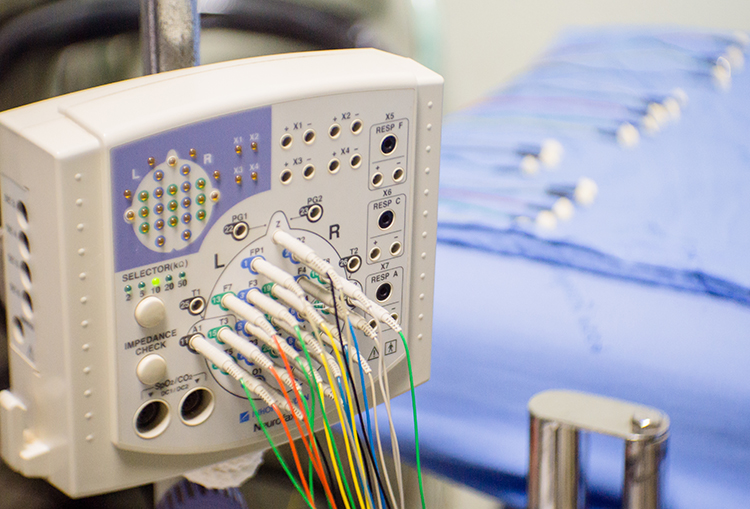
The EEG and qEEG can be conceptualized as functional imaging techniques. A single-channel EEG performs “neuroimaging” by displaying an image of microvolts in adjacent 1-Hz bins or adjacent bands (e.g., a 2D spectrogram (shown below) or with a 3D spectrogram. Graphic © John S. Anderson.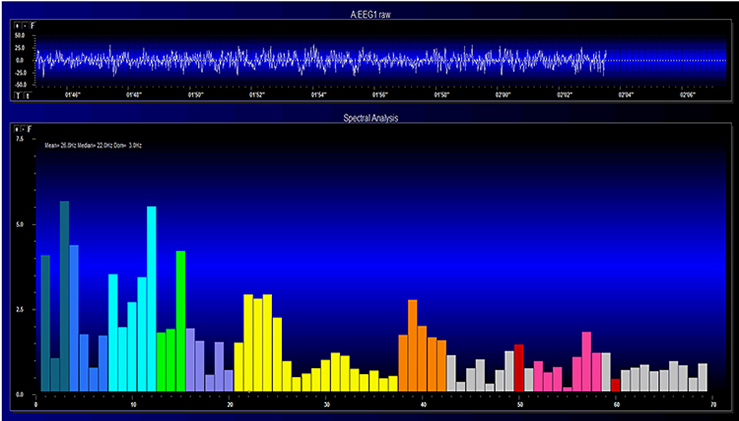
Further, 19-channel qEEG methods show images of activity as it is distributed across the brain’s convexity (i.e., over a 2D 10-20 map) or in 3 dimensions using more advanced qEEG methods (e.g., LORETA). Graphic courtesy of BrainMaster Technologies.
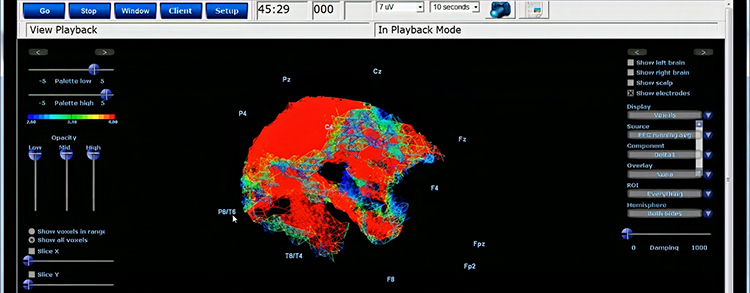
This movie is a 19-channel BioTrace+ /NeXus-32 display of EEG recording © John S. Anderson.
Magnetoencephalography
Magnetoencephalography (MEG) is a noninvasive functional imaging technique that uses SQUIDs (superconducting quantum interference devices) to detect the weak magnetic fields generated by neuronal activity. As with the EEG/qEEG, spatial resolution is inferior (cm compared to mm) to the functional MRI (fMRI) (Breedlove & Watson, 2023). Graphic © Steve Shoup/Shutterstock.com.
MEG's millisecond temporal resolution allows it to measure rapidly shifting patterns of cortical circuit activation (Breedlove & Watson, 2023). Researchers may combine MEG with MRI to better delineate the cortical structures generating the magnetic fields (Lin et al., 2004). The MEG graphic below is courtesy of the University of Montreal MEG Laboratory website.
Functional Magnetic Resonance Imaging (fMRI)
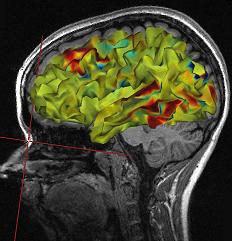
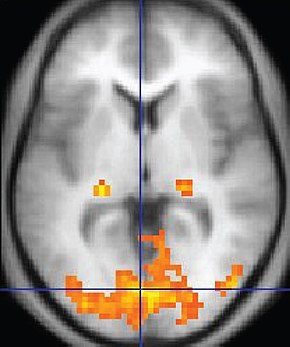
Functional Magnetic Resonance Imaging (fMRI) generates intense magnetic fields to indirectly detect brain regions' oxygen use during specific tasks.
fMRI images represent the blood flow changes that result from electrical and chemical activity in neuronal networks. Although the fMRI is limited by significant (100-ms to several-s) delays, it can reveal network contribution to cognitive performance. The fMRI trades spatial resolution for PET's superior temporal resolution (Breedlove & Watson, 2023). A fMRI image © Wikipedia is shown below.
Watch the Blausen MRI animation.
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that injects radioactive chemicals into the brain's circulation to measure brain activity (Breedlove & Watson, 2023).
PET scans achieve low temporal resolution (seconds to minutes) with moderate spatial resolution. PET images © Yok_onepiece/Shutterstock.com are shown below.
Single-Photon Emission Computerized Tomography
Single-photon emission computerized tomography (SPECT) is a functional imaging technique that uses gamma rays to create three-dimensional images and slice images of cerebral blood flow averaged over several minutes. Graphic © rumruay/Shutterstock.com.
SPECT achieves limited temporal (minutes) and spatial resolution (centimeters).
Comparison with Other Neuroimaging Techniques
Temporal Resolution
The qEEG offers superior temporal resolution compared to other neuroimaging techniques. While functional Magnetic Resonance Imaging (fMRI) and Positron Emission Tomography (PET) provide excellent spatial resolution, they lack the millisecond-level temporal resolution of the qEEG (Michel & Murray, 2012). This high temporal resolution allowsthe qEEG to capture rapid neural dynamics, making it particularly useful for studying cognitive processes and brain connectivity (Michel et al., 2004).Spatial Resolution
In contrast, techniques like fMRI and PET excel in spatial resolution, providing detailed images of brain structures and their functions. fMRI measures blood oxygenation level-dependent (BOLD) signals, reflecting neural activity indirectly, while PET uses radioactive tracers to visualize metabolic processes (Logothetis, 2008). The qEEG, however, has limited spatial resolution, which is a significant drawback when precise localization of brain activity is required (Sanei & Chambers, 2013).Cost and Accessibility
The qEEG is relatively cost-effective and accessible compared to fMRI and PET. The latter require expensive equipment and facilities, making them less accessible for routine clinical use. qEEG systems are portable, less expensive, and easier to use, which allows for broader application in diverse settings, including outpatient clinics and remote areas (Coburn et al., 2006).Non-Invasiveness and Safety
The qEEG is non-invasive and poses no risk of radiation exposure, unlike PET, which involves the administration of radioactive substances. This makes the qEEG suitable for repeated measurements and use in vulnerable populations such as children and pregnant women (Hughes & John, 1999).Comparison with Conventional Metrics
Objective MeasurementConventional clinical metrics, such as behavioral assessments and self-report questionnaires, rely heavily on subjective data, which can be influenced by various biases. The qEEG provides objective, quantifiable data on brain function, reducing the reliance on subjective reports and enhancing the accuracy of diagnoses and treatment evaluations (Boutros et al., 2011).
Early Detection and Prognosis
The qEEG can detect subtle abnormalities in brain function that may not be evident through conventional metrics. This capability allows for earlier detection of neurological and psychiatric conditions, potentially leading to more timely and effective interventions (John et al., 1988). Moreover, qEEG metrics have been shown to correlate with treatment outcomes, offering valuable prognostic information (Prichep et al., 1993).
Clinical and Research Applications
The qEEG has been extensively used in the diagnosis and monitoring of various neurological and psychiatric disorders, including epilepsy, ADHD, and depression (Hughes & John, 1999; Clarke et al., 2001). Its ability to provide real-time feedback on brain activity has also facilitated the development of neurofeedback therapies, which have shown promise in treating conditions such as anxiety and PTSD (Hammond, 2005).
Conclusion
The qEEG stands out among neuroimaging techniques and conventional clinical metrics due to its high temporal resolution, cost-effectiveness, non-invasiveness, and objective measurement capabilities. While it may lack the spatial resolution of techniques like fMRI and PET, its unique attributes make it an invaluable tool in both clinical and research settings. Future advancements in qEEG technology and analysis methods are likely to further enhance its utility, paving the way for new applications and insights into brain function.Glossary
computerized axial tomography (CAT or CT): the creation of medium-resolution images of brain structure by moving an x-ray source along an arc surrounding the head.
functional magnetic resonance imaging (fMRI): an imaging technique to detect brain regions' oxygen use during specific tasks indirectly.
magnetoencephalography (MEG): a noninvasive functional imaging technique that uses SQUIDs (superconducting quantum interference devices) to detect the weak magnetic fields generated by neuronal activity.
magnetic resonance imaging (MRI): a noninvasive imaging technique that uses strong magnetic fields and bursts of RF energy to construct highly detailed images of the living brain.
single photon emission computerized tomography (SPECT): a functional imaging technique that uses gamma rays to create three-dimensional and slice images of cerebral blood flow averaged over several minutes.
spatial resolution: the ability to distinguish between two separate points or structures in the brain. It defines the level of detail that an imaging technique can provide about the spatial arrangement of brain structures. Higher spatial resolution means more detailed images, allowing for finer distinctions between adjacent brain regions.
temporal resolution: the ability to capture rapid changes in brain activity over time. It defines how frequently an imaging technique can sample brain activity. Higher temporal resolution allows for more precise tracking of the timing of neural events, capturing fast-paced dynamics of brain function.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET PLUS
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Assignment
Now that you have completed this module, describe what fMRI scans can add to assessment.
References
Boutros, N. N., Arfken, C., Galderisi, S., Warrick, J., Pratt, G., & Iacono, W. (2011). QEEG in psychiatric disorders: A review of diagnostic utility and specificity. Clinical EEG and Neuroscience, 42(1), 45-51. https://doi.org/10.1177/155005941104200110
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Clarke, A. R., Barry, R. J., McCarthy, R., & Selikowitz, M. (2001). EEG analysis in attention-deficit/hyperactivity disorder: A comparative study of two subtypes. Psychiatry Research, 103(1), 63-73. https://doi.org/10.1016/S0165-1781(01)00261-3
Coburn, K. L., Lauterbach, E. C., Boutros, N. N., Black, K. J., Arciniegas, D. B., & Coffey, C. E. (2006). The value of quantitative EEG in clinical psychiatry: A report by the committee on research of the American Neuropsychiatric Association. Journal of Neuropsychiatry and Clinical Neurosciences, 18(4), 460-500.https://doi.org/10.1176/jnp.2006.18.4.460
Hammond, D. C. (2005). Neurofeedback treatment of depression and anxiety. Journal of Adult Development, 12(2-3), 131-137. https://doi.org/10.1007/s10804-005-7029-5
Hughes, J. R., & John, E. R. (1999). Conventional and quantitative electroencephalography in psychiatry. Journal of Neuropsychiatry and Clinical Neurosciences, 11(2), 190-208. https://doi.org/10.1176/jnp.11.2.190
John, E. R., Prichep, L. S., & Easton, P. (1988). Normative data banks and neurometrics: Basic concepts, methods and results of norm constructions. In Computer-Aided Electrophysiology (pp. 21-38). Springer.https://doi.org/10.1007/978-1-4684-5517-5_2
Lebby, P. C. (2013). Brain imaging: A guide for clinicians. Oxford University Press.
Lin, F., Witzel, T., Hamalainen, M. S., Dale, A. M., Belliveau, J. W., & Stufflebeam, S. M. (2004). Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage, 2(3), 582-595. https://dx.doi.org/10.1016%2Fj.neuroimage.2004.04.027
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869-878.https://doi.org/10.1038/nature06976
Michel, C. M., & Murray, M. M. (2012). Towards the utilization of EEG as a brain imaging tool. NeuroImage, 61(2), 371-385. https://doi.org/10.1016/j.neuroimage.2011.12.039
Michel, C. M., Murray, M. M., Lantz, G., Gonzalez, S., Spinelli, L., & de Peralta, R. G. (2004). EEG source imaging. Clinical Neurophysiology, 115(10), 2195-2222. https://doi.org/10.1016/j.clinph.2004.06.001
Pfister, H., Kaynig, V., Botha, C. P., Bruckner, S., Dercksen, V., & Hege, H.-C. (2012). Visualization in connectomics. Mathematics and Visualization, 37. https://doi.org/10.1007/978-1-4471-6497-5_21
Prichep, L. S., Sutton, S., Hauer, J. F., & Kuperman, S. (1993). Quantitative EEG in the evaluation of stimulant effects in attention deficit disorder. Clinical Electroencephalography, 24(1), 8-18. https://doi.org/10.1177/155005949302400107
Sanei, S., & Chambers, J. A. (2013). EEG signal processing. John Wiley & Sons. https://doi.org/10.1002/9780470688031
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
B. Use of QEEG Norms and Methods Used to Derive QEEG Norms
Quantitative electroencephalography (qEEG) has proven to be a valuable tool in clinical neuropsychology and psychiatry, offering objective, quantifiable data on brain function. One of the significant advantages of the qEEG is the use of normative databases, which allow clinicians and researchers to compare an individual's brain activity patterns to those of a healthy population. This section examines the use of qEEG norms and the methodologies employed to derive these norms.
Please click on the podcast icon below to hear a lecture over Section B.

Use of qEEG Norms
“Normative data [are data] that characterize what is usual in a defined population at a specific point or period of time” (O’Connor, 1990, p. 307).The word norm shares the same etymology (Online Etymology Dictionary, 2023) as normal, deriving from the Latin word “norma,” which means a carpenter’s square, standard, or pattern. Norms are descriptions for something that is “usual, typical, standard, or expected” or the usual amount (Cambridge Dictionary, 2023). They may be rules or standards of behavior in a group, for example, shaking hands when greeting a stranger at a business meeting in North America or describing what is typical or most characteristic for a group of things or people. That is, the norm may be qualitative or categorical.
However, many norms can be quantified, giving the amount expected of something in numerical form. An example of a quantitative norm is the value (63.5 inches) of the variable (height), which gives what is expected, on average, among adult women living in North America. A qEEG norm for the variable of alpha band amplitude, measured at a posterior 10-20 site in a healthy adult with eyes closed, is in the range of 20-60 uV (Lewine & Orrison, 1995). qEEG normative databases, however, quantify the expected range of values such variables can take on and, more precisely, quantify variables with specific average or mean values and measure of variability or standard deviation for the variable’s range. qEEG norms, then, quantify what is normal for the participants selected to meet expectations for normal health and whose EEG data were collected, as will be described further below.
qEEG norms can also be thought of as electronic tables that provide means and standard deviations (described below) for numerous EEG variables that are calculated, for instance, for each 10-20 site and pairs of sites where the EEG has been collected from normally healthy individuals in adjacent age groups, from various states and tasks. The quantified EEG data (values, averages/means, variability shown by standard deviations) for healthy normal participants are organized in the database by qualitative variables (i.e., 10-20 site, site pairs, age group, state or type of task) because the normal EEG is affected by those variables, allowing deviations from normalcy to be seen which may suggest a hypothesis of pathology or impairment to be followed by correlation with other sources of information (e.g., neurological examination or neuropsychological testing), and possibly by treatment (e.g., neurofeedback training).
qEEG norms serve as a reference for identifying deviations in brain function that may be associated with neurological or psychiatric conditions. By comparing a patient's EEG data to a normative database, clinicians can identify abnormal patterns that may not be evident through standard EEG analysis. This comparison can aid in diagnosing various disorders, including attention-deficit/hyperactivity disorder (ADHD), depression, anxiety, and epilepsy.
Normative databases are essential for the utility of QEEG in clinical practice. They enable the quantification of EEG data by converting raw EEG recordings into z-scores, representing the number of standard deviations a particular data point is from the mean of the normative group. These z-scores can highlight deviations in power spectral densities, coherence, phase lag, and other EEG metrics indicative of underlying neuropathology (John et al., 1988).
One of the key applications of qEEG norms is in the identification of neurodevelopmental disorders such as ADHD. Studies have shown that children with ADHD exhibit distinct qEEG patterns compared to their neurotypical peers, such as increased theta activity and decreased beta activity. These patterns can be quantitatively assessed using normative databases, aiding diagnosis and treatment planning (Clarke et al., 2001).
Purposes of qEEG Normative Databases
Developers of databases begin by formulating a purpose for the database. These include description, comparison, diagnosis, exploration of biomarkers, training protocol development, and scientific discovery.Description
Generally, qEEG norms characterize or describe the range of what is expected for particular EEG variables seen in healthy participants. In this, they are similar to norms used in health care for height and weight, for example. As Anastasi writes regarding psychological test scores, “Norms represent the test performance of the standardization sample. The norms are thus empirically established by determining what a representative group of persons do on a test. Any individual’s raw score is then referred to the distribution of scores obtained by the standardization sample, to discover where he falls in that distribution (Anastasi, 1976, p. 67).”The norms of a qEEG database have a descriptive purpose that presents what is normal for the sample of healthy individuals selected to represent the entire population of healthy people.
Comparison
The normative description, say, of the mean and variability of alpha power seen in a group of healthy participants in a resting eyes closed condition, can then also serve the purpose of a benchmark used for comparison of an individual, such as a new client, to what is normal so that one can determine whether the client’s EEG is consistent with normal expectations or deviates from them. The database user may ask, “Does this client’s alpha power conform with the prediction I would make based on knowing the normal range of alpha power?”This comparative purpose resembles the practice in medicine of evaluating a patient’s blood pressure in relation to the normal expectation for the range of blood pressure values for healthy people of similar age to the patient. In the context of a typical assessment before conducting neurofeedback, a qEEG normative database compares the participant’s EEG to that found in the database, describing how and where findings differ significantly from what is expected.
The comparative function of norms addresses what Anastasi (1976) writes in the context of psychological test theory about test scores, which applies equally to the value of an EEG variable of any individual participant: “In the absence of additional interpretive data, a raw score on any psychological test is meaningless (p. 67).” This suggests, essentially, that to give meaning to a participant’s EEG finding, one must place it in context, that is, compare it to the range of what is normal, and identify the degree to which the finding is either above or below normal expectation based on the sample of healthy participants who were assessed for the normative database, their average value, and how variable the values were among the database’s sample of participants.
Basic statistical methods described below are used to quantify the degree of difference from expectation and its statistical significance that this comparison of the individual findings to normative values provides.
Diagnosis
In clinical settings, findings that a client’s EEG differs from the norm may be integrated with other assessment findings to understand better and diagnose problems presented by the client. However, deviations from the norms in a qEEG database are not diagnostic in themselves. A statistically significant difference between client EEG findings and norms in a qEEG database does not necessarily have clinically significant importance or predict how the client functions in their daily activities. However, statistical significance can suggest hypotheses to explore and, together with information from other sources, may contribute to making a valid diagnosis by a qualified health provider.qEEG findings for a participant of statistically significant difference from database values must be considered in the context of factors such as clinical presentation, medical history, findings of other relevant tests, and understanding of the anatomy, physiology, and function of the brain together with the knowledge of base rates in the setting where the clinical clinician works (i.e., how often diagnoses of various types occur). The discussion above is instructive because it notes the limitations of qEEG findings.
Exploration of Biomarkers
Biomarkers are biological markers, being “objective indications of medical state observed from outside the patient – which can be measured accurately and reproducibly” (Strimbu & Tavel, 2010, p. 463). Related to biomarkers are endophenotypes (i.e., “within phenotypes”), quantifiable phenomena that link underlying biological processes to symptoms and are more stable than the symptoms themselves. For example, McVoy and colleagues (2019) present a recent review of possible biomarkers in child psychiatric disorders.Several databases have been used to explore EEG biomarkers and endophenotypes, examples of which are for ADHD (Arns et al., 2008; Ji et al., 2022), Alzheimer’s disease (Jeong, Park, & Kang, 2022), Parkinson’s disease (Caviness et al., 2016 and traumatic brain injury (Thatcheret al., 1989).
Training Protocol Development
Differences between the client’s EEG and normative values, seen both before and after neurofeedback or some other intervention, can also be compared to determine whether the client’s EEG has achieved a degree of normalization as a result of neurofeedback or other intervention, fulfilling the purpose of outcome evaluation.Scientific Discovery
In a research setting, a special group (e.g., individuals with major depression or mild traumatic brain injury) may be compared to the normative group in the qEEG database to identify what best differentiates the two groups, as in studies that use discriminant function analyses (DFAs) to differentiate groups, for instance, with a traumatic brain injury (Thatcher et al., 1989). DFAs may be used clinically (see below).Databases may also be used in research settings to investigate the association between EEG variables and measures of psychological functions such as IQ (Thatcher, North, & Biver, 2008). For example, Thatcher et al. (2008) found that the qEEG variables of phase difference, phase reset per second, phase reset locking interval, and coherence were most strongly related to IQ. Comparisons may also be made between measures of brain function by qEEG and other neuroimaging methods such as MRI (Thatcher et al., 1998a; Thatcher et al., 1998b) to understand brain structure and function better. These investigators found good correspondence between qEEG and MRI results.
In summary, qEEG normative databases are used in clinical and research contexts. They can be used for description, comparison, diagnosis, biomarker identification, treatment planning and evaluation, and scientific exploration.
The Contents of qEEG Normative Databases
The sections above indicate that the contents of a qEEG normative database are the values of multiple EEG variables measured among normally healthy individuals of various age ranges. The database uses these values to calculate the variables' means and standard deviations, ensuring the values are normally distributed. The database organizes its contents by age group, recording condition, and variable type. Variables included in databases are relevant to understanding the brain’s structure and function and their integrity in clinical settings.Variables
Database variables usually include power, relative power, and power ratios for typical EEG frequency bands for each 10-20 sites and pair of sites. Methods of qEEG analysis are also progressing so that calculations can be made for variables from subcortical (e.g., cerebellar) sites (Thatcher, Biver, Soler, Lubar, & Koberda, 2020). Amplitude may also be calculated for single hertz bins.Some databases with inverse solutions provide amplitude for Brodmann Areas and anatomical structures. Some databases can produce reports showing results for sites that comprise various brain networks (e.g., salience network, default mode network) and for sites involved in various conditions (e.g., depression, anxiety). Connectivity measures such as coherence (or comodulation, Kaiser, 2008) and various aspects of phase may be database features. Asymmetry of amplitude between homologous sites and peak frequency may also be calculated.
Variables can also be calculated for various montages (e.g., linked ears, average reference, Laplacian). Both raw and z-score values for these variables are included in a database; z-score calculations make it possible to compare an individual to the healthy normative population and delivery of z-score neurofeedback..
EEG variables not in qEEG databases are wave patterns such as epileptiform events that are better appreciated with different quantitative methods than those used in qEEG databases reviewed in this section of qEEG Tutor. Sometimes, waveforms of significance, such as beta spindling and changes in mental state, are also identified more clearly by visual inspection.
Values
Each variable contains values of that variable, one value for each participant in the database. The values for a variable are aggregated or summarized with statistics such as the mean and standard deviation, described below, that are the basis for calculating z-scores and the statistical significance of the difference from normal that a clinical participant might show.Montages
Many variables in a database can be calculated in different versions based on the montage the user selects. For example, linked ears, average reference, and Laplacian montages provide different views of the EEG and calculate EEG variables differently to do so.Probability and Statistics or Database Use
The mathematical analysis of data in a qEEG normative database serves important purposes, as suggested above. At a basic level, databases compute statistical quantities for variables such as means (averages) and standard deviations (a measure of variability). Databases also ensure that their data are normally distributed in the manner of a bell-shaped curve referred to as Gaussian so that values can be interpreted with reference to the statistical significance of their difference from the mean of the sample collected for the database.Descriptive statistics aim to describe and estimate the true value of a variable in the entire population of interest so that results can be generalized or used with new participants who were not assessed for the database. For example, the population of interest may be 30-40-year-old adults. This means we want to use the information in the database for some purpose with people anywhere on the planet. If it were possible to measure alpha power at P4 for all people in that age range and calculate its average, then the result would be represented by the variable μ, the Greek letter mu. The variability of the alpha power at P4 for all 30-40-year-olds in the world would be represented by the variable δ, the Greek letter sigma.
However, because it is not possible to measure every 30-40-year-old, it is necessary to collect a sample of 30-40-year-olds who can represent or stand in for the entire population and measure alpha power at P4 to estimate what the true values of μ and δ would be if we could measure the height of the entire population. When the alpha power at P4 for the 30-40-year-olds in the sample is measured, then the average of those measures, the mean (often represented as X) estimates μ, and the variability of those measures, the standard deviation (often presented as S) estimates δ. To the degree that the sample of participants for the database is similar to or representative enough of the 30-40-year-olds across the globe, the database results may be said to generalize and be validly used with 30-40-year-olds worldwide.
Mean
By way of quick review, the mean or average of a variable is calculated by adding all values for that variable in the database’s sample of normal individuals and dividing the sum by the number of those individuals in the sample. It is often shown by X (or by M or x).Standard Deviation
Databases show how much the range of values for a variable differ from each other or vary. This is shown quantitatively by the standard deviation (S). (sometimes represented by SD). The standard deviation for a variable is calculated by finding the difference between each value and the mean, squaring each difference, and dividing the sum of squared differences by the number of individuals in the database’s sample. The result is called the variance. The square root of the variance is called the standard deviation. It is more commonly used than the variance to show how individual values deviate or differ from the average.z-scores
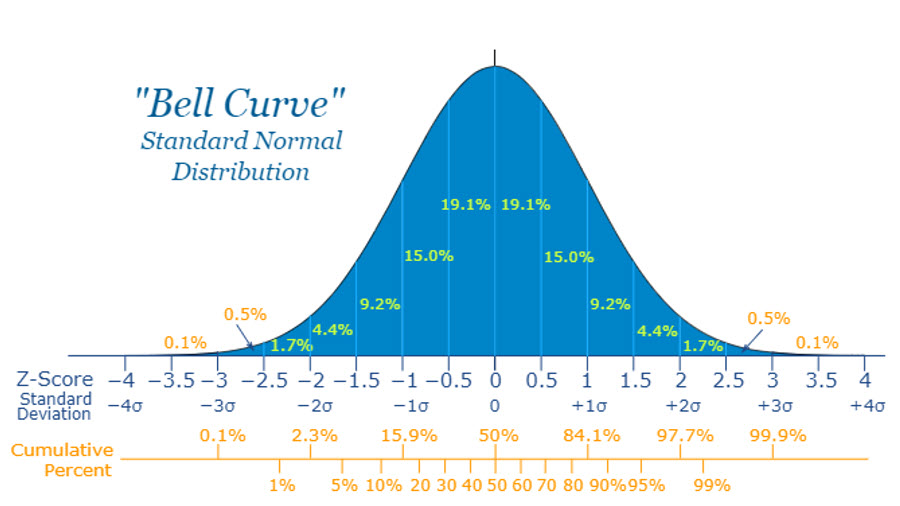
Z-scores, also called standard scores, are very useful for describing and comparing qEEG findings and for delivering neurofeedback.We calculate a z-score using the mean and standard deviation for a variable. We subtract a value from the mean and divide the difference by the standard deviation. When done for all variable values, the resulting z-scores have a mean of 0 and a standard deviation of + 1. This quickly shows for any value how far from the mean is, and, if the values are normally distributed in a bell-shape Gaussian manner, how likely they are to occur if randomly selected from the sample in the database.
As the discussion below of Gaussian normal distributions explains, about 68 percent of database participants will have a value between +1 and -1 for any variable. Slightly more than 95 percent will have a value between +2 and -2. A value that exceeds + 2 is likely to occur among normally healthy participants in the database with a probability or expectation of somewhat less than 5 percent of the time. As described below, the criterion or threshold for what is “normal” can be quantitatively defined, albeit somewhat arbitrarily, in z-score terms. For instance, a user of a qEEG normative database could decide to set “normal” to mean a z-score in a range probable to be seen among 95 percent of healthy people. That z-score range would be -2.00 to +2.00. Z-scores found outside that range would be called statistically significant from the mean.
When a client’s data are compared to the database, the client’s data can also be converted to z-scores. This has great utility. It provides a common unit of measure, the z-score, so that the database user can quickly see the statistically significant difference between the client’s value and the mean value of normal participants in the database.
Z-scores also provide a common unit of measure for different variables. For example, alpha power is measured in uV, but coherence is not. However, the values for both variables can be transformed to z-scores so that their relative significance, in terms of difference from normal, can be seen for a client. When assessing a client, converting findings to z-scores provides a common metric for examining which of many variables has the greatest statistical significance. The z-score has a mean of zero and a standard deviation of 1 in all cases.
Neurofeedback practitioners use qEEG databases to develop z-score training protocols for their clients. Neurofeedback software that performs z-score training allows the practitioner to identify variables found during the assessment with statistically significant or unusual values that are otherwise important. The practitioner then sets neurofeedback thresholds in z-score terms to specify “now how normal” the client’s EEG for that variable must be to produce feedback. The software calculates the value for the EEG variable and converts it to a z-score based on the database’s statistics for that variable.
Based on the client’s z-score and how far it deviates from the mean for normal participants, the software delivers feedback or not relative to how the practitioner has defined normal with z-score thresholds that may be + 2 or + 3 standard deviations from the mean for normal participants in the database. In the former case (i.e., a threshold of + 2 from the mean), the client’s EEG value must be consistent with 95 percent of the normal standardization group in the database before feedback is provided. Client values with z-scores greater than or less than a z-score of 2 are statistically significant because they are unlikely to occur among healthy normal people, with a probability of occurring only 5 percent of the time.
Normal Gaussian Distribution
qEEG normative databases contain means and standard deviations of EEG variables collected from healthy participants in adjacent age groups in defined assessment conditions (e.g., resting eyes open, resting eyes closed, various tasks). Using z-scores is advantageous in comparing an individual’s EEG assessment data with the healthy normal participants in the database. Using z-scores requires the distribution of values for a variable to be Gaussian or normally distributed. Normal distribution graphic © Andrii Ablohin/Shutterstock.com.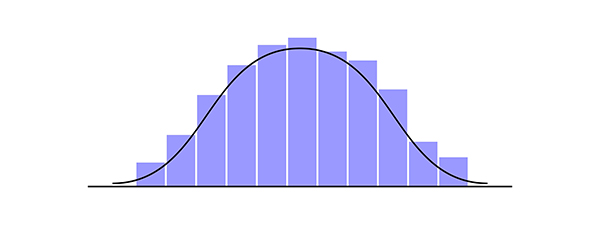
What is a Gaussian distribution? This type of distribution is named after the mathematician Carl Friedrich Gauss, who lived between 1777 and 1855. A Gaussian distribution is also called a normal distribution with a familiar bell-shaped curve. It represents the relationship between a variable, such as alpha power, and the probability or frequency of a specific value for alpha power in a sample of participants. A Gaussian distribution has a peak in the middle (neither too pointed nor too flat), called the mean or average, and is symmetric, having the same shape and symmetry on both sides. Gaussian distributions normal curve graphic © Elena Pimukova/Shutterstock.com.
When measured values are transformed into z-scores, the scores remain distributed in a Gaussian manner, as seen in the figure below. This figure shows the percentage of scores that fall between two symmetric z-score values. The figure also shows the cumulative percentage for any z-score, the percentage of z-scores less than the selected z-score. On the horizontal abscissa, the figure shows z-scores where μ represents the hypothetical mean of the entire population from which the database participants were sampled, and σ represents the similar hypothetical standard deviation.
By comparison, participants who comprise a database and have been selected to represent an entire population have their mean values represented by X and their standard deviations represented by S. In the figure below, the M equals a value of 0, and S equals 1 for z-scores calculated for database participants. What can be seen from these graphs is that the largest number of scores occur around the middle or the mean, with scores that are either very high or very low occurring with less frequency. Standard normal curve graphic © Math is Fun.
To make valid statistical comparisons between an individual’s EEG value and that of the comparison group in a database and to use z-scores, the distribution of values in the database must be normal, that is, to have a Gaussian bell-shaped distribution (Thatcher & Lubar, 2009). The Gaussianity of a distribution can be tested statistically.
Sometimes, however, the data are not normally distributed in Gaussian. In those cases, mathematical transformations are applied to the data so that the data will be Gaussian. Although many variables, such as height or weight, have a normal Gaussian distribution, some variables, such as some EEG variables, must first be transformed (e.g., with a log10 transformation) to normalize them so that z-scores may then be validly calculated (cf. Thatcher, North, & Biver, 2005a).
Gaussian Sensitivity
As described by Thatcher et al. (2003, p. 101), Gaussian sensitivity is important because it describes the degree of uncertainty (or accuracy and “certainty”) a data set has. In the forensic context, gaussian sensitivity is important because of its relationship to an instrument’s measurement error, which must be demonstrated as one of the Daubert standards (Daubert v Merrell Dow, 1993). Thatcher et al. (2005) define sensitivity as the difference between two data distributions, the ideal Gaussian distribution, and the distribution of observed data values for the sample whose EEG has been measured. The difference between the two distributions measures the sensitivity of the data measured in the sample to the ideal Gaussian distribution. Compare the definition of Gaussian sensitivity with the definition used to evaluate a diagnostic test where sensitivity is the test’s ability to identify individuals with a disease, and its related concept, specificity is defined as that test’s ability to identify individuals without a disease (McNamara & Martin, 2018).Normal Distributions
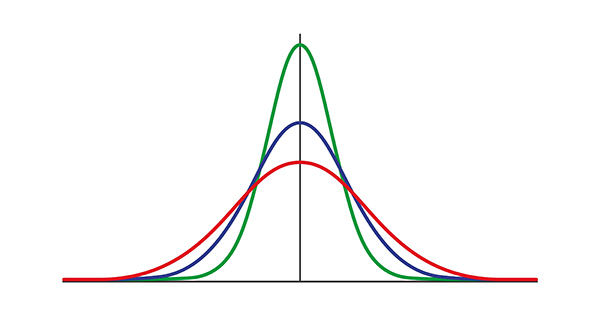
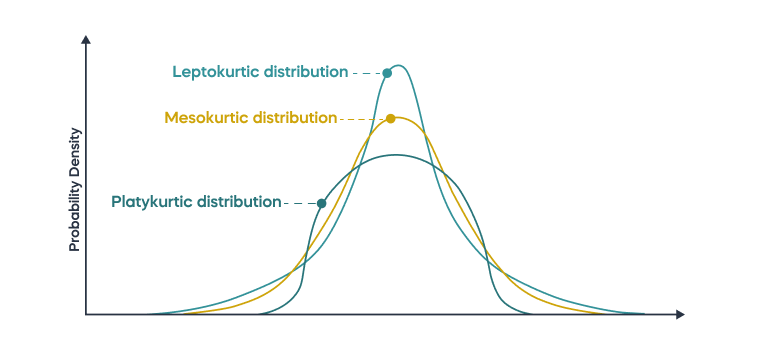
As described by Thatcher and colleagues (Thatcher, Walker, Biver, North, & Curtin, 2003), having normal distribution is important for minimizing the error with which those data estimate true scores. Data distributions can deviate from a normal Gaussianity in kurtosis and skewness.Kurtosis refers to the degree to which the tails of a distribution extend away from the mean, with “kurtosis” derived from the Greek word for bulging. Leptokurtic distributions have “lepto” or narrow bulging, and platykurtic distributions have a “platy” or flat bulging. The Gaussian normal distribution is a mesokurtic distribution with “meso” or medium bulging.
A leptokurtic distribution has data distributed with a peak that is more pointed than, and tails that extend farther from the mean than a normal distribution. This means that a leptokurtic distribution has more outliers, or data points that lie far outside the expected range of the data.
A platykurtic distribution has data distributed with a flatter peak, and the tails extend less far from the mean than a normal distribution. A leptokurtic distribution has data distributed with a more pointed peak and tails that extend farther from the mean than a normal distribution. This means that a leptokurtic distribution has more outliers or data points that lie far outside the expected range of the data.
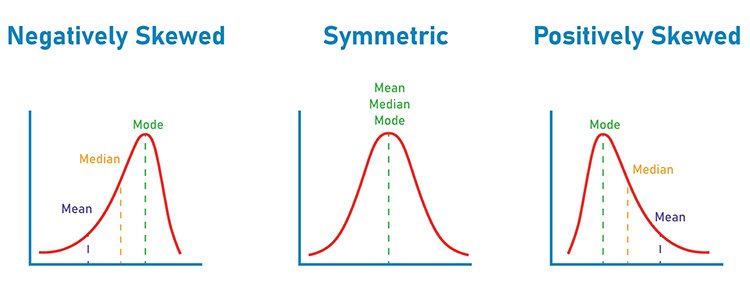
Skewness refers to the degree to which the symmetry of the distribution is reduced because the peak is located to one side or the other of the mean. Negatively skewed distributions have their mean less than their mode. Positively skewed distributions have their mean greater than their mode (i.e., the peak of the distribution or the value taken on by most observations). Skew graphic © Dream01/Shutterstock.com.
Both kurtosis and skewness for a distribution can be calculated to see how deviant the distribution is from the Gaussian normal distribution. If a distribution deviates significantly from normal, then the values in the distribution can be mathematically transformed, for example, with a log10 transformation, to produce normally distributed data that are more validly examined with statistics and have reduced errors of estimate.
Percentiles
Percentiles range in value from 0 to 100. Any given percentile indicates the percent of scores or participants that occur below that percentile. For example, a score on a test equal to the 70th percentile means that 70% of scores for that test were lower. Percentile graphic retrieved from XELPLUS.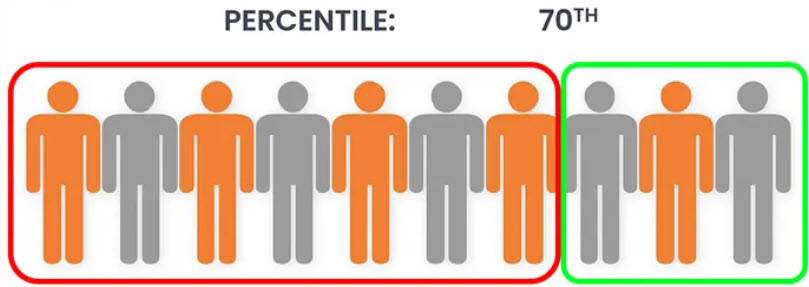
Definition of Normal
With reference to the figures above, it is possible to define “normal” statistically. For example, a database user could set the normal range as any z-score between -2.0 and +2.0 based on the knowledge that about 95 percent of z-scores for participants in the qEEG normative database will have values in that range. Such a definition, however, is somewhat arbitrary, with another user perhaps choosing to define normal as any z-score between -1.5 and +1.5 to narrow the range of what is considered normal and expand the range of what is considered abnormal (i.e., z-scores less than -1.5 or greater than +1.5). Z-scores outside the selected range are then defined as being of statistical significance, unlikely to have occurred by chance among normally healthy individuals, unexpected, or “abnormal.”Alpha Levels
Experimenters set what is known as an alpha level (e.g., α = .05) to specify the degree of certainty they want the results of statistical tests to achieve before concluding the significance of experimental results. Choosing α = .05 means that if statistical test results are less than .05, there is less than a 5% chance that the results occurred merely by chance.P-Values
P-values are the probability that a result from a statistical test is due to chance. For example, suppose the results of a statistical comparison of participants' test scores in two groups are significantly different to the degree that it would occur less than 5% by chance alone. In that case, the statistical test's significance is p< .05.Alpha levels and p-values are used by qEEG and neurofeedback providers to define the levels of significance necessary for the value of an EEG variable to be achieved before it is considered significant or abnormal.
Correlation Coefficients
A correlation coefficient is a measure of linear association between two variables. It shows how confidently one can expect one variable to change when a second variable changes. The values of correlation coefficients ranges between +1.00 and -1.00, with positive values meaning that the values of each variable tend to increase together and negative values meaning that the values of one variable tend to decrease as the values of the other variable increase (i.e., an inverse relationship). Strong correlation coefficients (i.e., close to either +1 or -1) can be tested for statistical significance. If significant, then each variable is a good predictor of the other. A correlation coefficient of 0.00 would mean no predictive relationship between the two variables.Correlation coefficients are measures of association and are used in the calculation of EEG measures such as coherence. The significance of correlation coefficients can also be evaluated with statistical tests
T-tests
A t-test is a statistical test used to compare the average scores for two groups measured at a single point in time to see if the difference is significant. Alternatively, a t-test may be used to compare findings for a single group measured at two points to see if a change has occurred, for example, due to neurofeedback training.Standard Error of Measurement
The standard error of measurement (SEM) is applied to calculate confidence intervals around obtained scores. Its calculation is based on a test’s standard deviation and reliability. The higher the reliability and the lower the standard deviation, the smaller the SEM and the more confidence we can place in the accuracy or the consistency of the value of an EEG variable we are measuring. The standard error of measurement (SEM) can be expressed in the same units as z-scores, standard deviation units.Confidence Intervals
A confidence interval is a range of values around a measured value. The range shows how confident one can be that the measured value is true or accurate. For example, a confidence interval of 95 percent can be calculated to show the probability that the true value of a measurement is within a specified range. Confidence intervals are calculated based on a sample’s mean and standard deviation, with the added assumption or requirement that the measures' distribution is normally distributed (i.e., in a Gaussian manner). Confidence interval graphic retrieved from Northwestern | Statistics.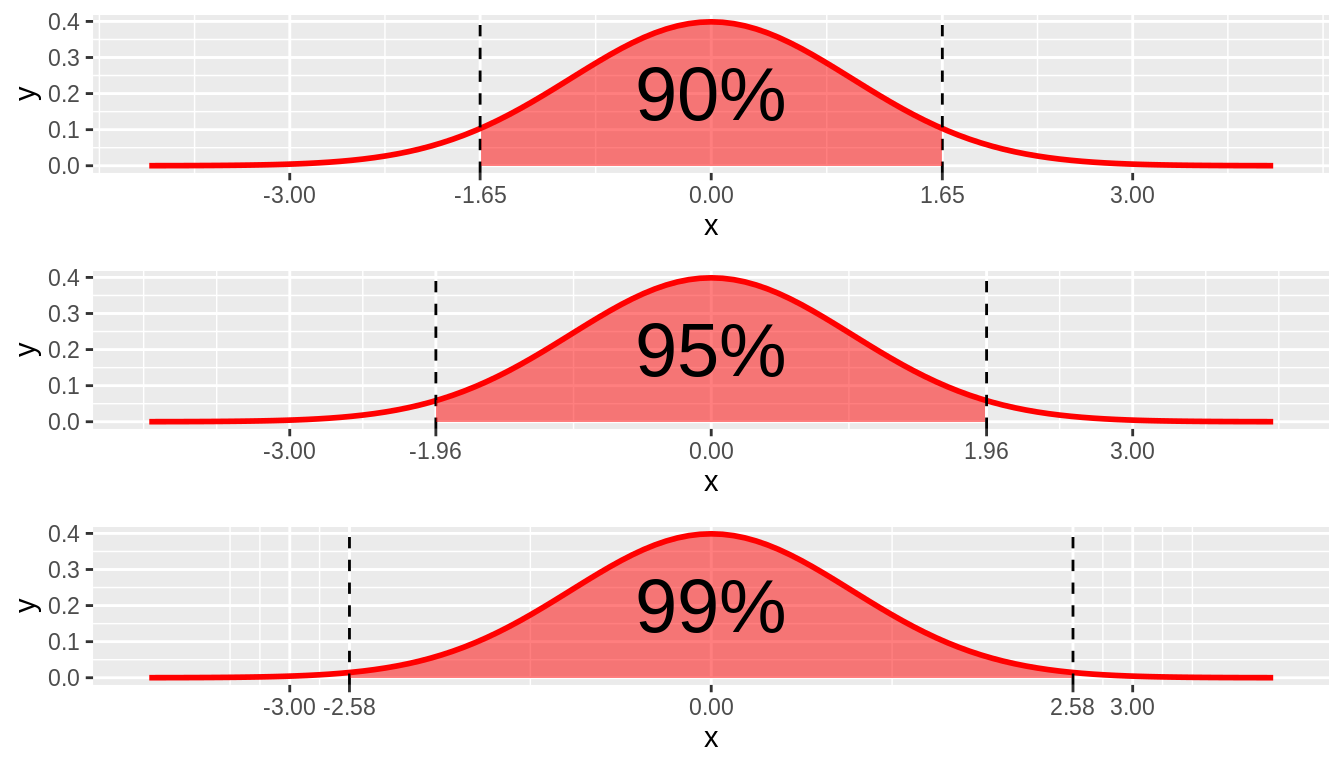
Caption: 90%, 95%, and 99% confidence intervals.
Summary
This section has reviewed the concept of a variable and the fact that a variable can take on any number of values. Further discussed was how the measurement of a variable in a sample of participants produces a range of values that have an average value, with other values being distributed on either side with less and less frequency in the sample and with less expected probability in the population from which the sample was drawn. The distribution of values then can be quantified by a measure of variability, the standard deviation, and the degree to which the distribution matches a normal Gaussian distribution. This information can then be used to set criteria for what will be considered normal, calculate z-scores, and compare the findings for EEG variables, using z-scores as a common metric, which is then used to define what is normal. The use of statistical tests of correlation and differences between two groups or between one group assessed at two times, is valuable when using qEEG normative databases.Methods Used to Derive qEEG Norms
qEEG normative database developers must seek approval from an approved institutional ethics review board to recruit participants and record their EEG. An element of participant recruitment may then be the use of an approved form by which the participant who has been recruited provides their consent for data collection after being informed about risks, benefits, their later knowledge of database progress, storage and use of data, and possible financial compensation.
Sample Selection and Data Collection
The first step in creating a normative database is selecting a representative sample from the general population. This sample should be sufficiently large and diverse to account for variations in age, sex, and other demographic factors. EEG data is then collected from these individuals under standardized conditions to ensure consistency in the recordings (John et al., 1988).
Data for qEEG normative databases come from individuals in a series of age ranges considered healthy within normal expectations, for whom EEG is collected in different conditions to calculate different EEG variables. The participants whose data comprise the database are selected to represent or stand in for the entire population of healthy individuals from whom it would be impossible to collect data exhaustively. The participants in the database and any future participants whose data will be compared to the database are assumed to come from the same or similar population. Database participants are selected to be representative of the entire population so that the findings in the database can be generalized, that is, applied to other participants in the future who are drawn from the same population as the participants in the database.
As stated above, a database developer first formulates a clear idea of the database’s purpose. The developer typically considers what the database will be used for, what population needs to be represented and described, and who will eventually be compared to the database. In the case of qEEG normative databases, the relevant population that the developer wishes to use is normally healthy individuals. Some databases will, however, limit their participants' range to children or adults, and some will span the entire developmental range. This will allow database users to validly compare their participants to those in the database to conclude whether the participant falls within the range of normal expectations for their age range.
Participant Recruitment
The database developer may use various recruitment strategies to achieve their objectives. Some examples of methods to recruit participants include posting flyers with contact information, making presentations at meetings that parents attend, selecting from a research participant pool at a university, making announcements in print publications, requesting family physicians and health clinics to forward contact information for patients who wish to be included, and making announcements on social media, television and radio announcements (Kubicek & Robies, 2016; UCLA Research Administration, 2021).Recruitment bias, however, may affect the external validity of research findings, such as those for a database, if some factor or variable systematically occurs in the sample but not in the population, the sample is supposed to represent. For example, if a sample for a database of blood pressure values includes White participants, the resulting data may not validly represent Black, Hispanic, Asian, or Native American participants (cf. Zakai, Minnier, Safford, et al., 2022). However, recruitment bias does not appear to affect qEEG databases, at least for culture strongly. For example, the findings of Matousek and Petersen (1973a, 1973b) in Scandinavia correspond well with a sample of children with different cultural and ethnic backgrounds (e.g., John, 1981).
Stratification
Because age is known to affect the EEG systematically (John et al. et al., 1977; Gasser et al., 1988; Gasser et al., 1988; Matousek & Peterson,1973a,b; Thatcher, 1991; Thatcher, 1992; Thacher, 1994; Thatcher et al., 2003), database participants are stratified by age range, with age ranges being more narrowly defined for children because normal development brings about relatively rapid changes in the EEG. For example, Matousek and Peterson used one-year age groupings to study children. Thatcher, Walker, and Guidice (1987) used a sliding age range where ranges overlap, with some participants being included who were chronologically somewhat younger or older than the nominal age of the range. This was done to improve the statistical properties of the sample and make it more amenable to analysis.In the NeuroGuide database (Thatcher & Lubar, 2009), spans of 2 years form the levels of age stratification from birth to age 16, after which age ranges widen to a maximum age range of 35 to 82 years for the oldest level of stratification. The database user then compares their participant to the database’s participants of similar age so that any differences found are not the result of age differences.
Because sex, race, socioeconomic status, and geographic location do not affect EEG, they are not needed for stratification. For example, a study that compared urban participants in the US with mixed urban and rural Scandinavian participants showed no major differences in results (John, 1981).
Representativeness
Database developers attempt to ensure that the sample they have recruited is representative of the population of interest. For qEEG normative databases, this is the population of people who are healthy within normal expectations. For example, suppose the developer intends for the database to be used with individuals of different races, cultures, geographic locations, socioeconomic levels, and intellectual abilities. In that case, the developer will attempt to include participants in the database who represent the typical range of values for those variables, even though those variables are not used for stratification.Inclusion and Exclusion Criteria for Participant Selection
The developers of EEG normative databases use inclusion and exclusion criteria to select participants whose data are included to ensure that the participants are healthy within normal expectations. Inclusion criteria are those that the participant must have to be included in the database. For example, screening instruments may be used to ensure that the candidate participant has met normal developmental milestones and is performing at grade level in school. Exclusion criteria disqualify a prospective participant from being in the database. These are often findings of neurological disease, psychiatric illness, developmental disorder, learning disability, or addictive behavior that could affect the EEG. The resulting pool of participants is therefore considered healthy and normal in that their physical and mental condition is within a range that makes them able to perform at least a minimum of reasonable age-appropriate day-to-day functions.The variables used for inclusion and exclusion criteria must be identified, and their measurement method must be specified. Methods for ascertaining the status of recruited participants concerning inclusion and exclusion criteria are varied. Participant or parent interviews, questionnaires, file reviews, and test results are often used.
Sample Size
Using the defined age strata and the criteria for inclusion and exclusion, the database developer recruits enough participants to produce normally distributed data (i.e., Gaussian) for the variables in the database. The so-called normalcy of the distribution is necessary for the valid calculation and use of the z-scores described above. In addition to adding participants or transforming values until a distribution is normal, statistical regression methods have also been used for constructing norms and calculating the number of participants needed (John, 1977; Timmerman et al., 2021).Thatcher and Lubar (2009) state, however, that sample size is less important than eliminating artifacts, amplifier calibration, use of good data collection methods, and the degree to which the measured values in the sample conform to a normal Gaussian distribution.
Data for qEEG Databases
Collection Methods, Conditions, and Sample Duration
Data for qEEG databases are collected from standard EEG electrode sites, with appropriately minimized impedance or offset, using standardized recording methods in well-controlled conditions. Participants must be rested and alert, taking their normal medication without having had poor sleep or excess coffee or intoxicant intake.Data are collected in defined conditions, for instance, with the participant resting and alert with their eyes open or closed. Sometimes, EEG data are collected while the participant performs a cognitive task, such as attending to or recalling verbal material, or ERP data are collected in response to various stimuli. For example, Kropotov and colleagues developed the Human Brain Institute database with several task and ERP conditions in addition to resting conditions with eyes open and with eyes closed (Kropotov, 2009; Kropotov et al., 2005). As Thatcher and Lubar (2009) write, however, many databases are based on recording the EEG during conditions when the participant is awake and resting with eyes closed and with eyes open because these conditions can easily be replicated with new participants whose results are to be compared with those in the database. Nevertheless, Thatcher and Lubar (2009) write that using active conditions can help show brain structures involved in specific tasks.
Although authors such as Salinsky, Oken, and Morehead (1991) suggest that as little as 60 seconds of data produce results that are 92% reliable for some variables, Hughes and John (1999), among others, recommend that between 2 and 5 minutes of artifact-free data be used to calculate EEG variables reliably.
A factor related to data collection and database use is amplifier matching. Different qEEG amplifiers have different electrical frequency response curves that can affect the EEG findings, especially in frequencies less than 2 Hz, which can calculate some z-score erroneous when based on data collection with an amplifier different from the one used to construct the database. This means, for example, that the absolute power finding for one amplifier may differ from that of a different amplifier when used under the same conditions with the same participant.
Before 1990, the study of absolute power was sometimes avoided if the researcher used an amplifier different from the one used during the construction of the database that the researcher was using. Different amplitudes were sometimes found with different amplifiers, particularly for low frequencies. Instead, power ratios were often used, according to Thatcher and Lubar (2009). Amplifier matching is a procedure that first compares the findings collected with the amplifier originally used to construct the database with a different amplifier with the same participants. Differences between the two are then applied in calculations that compensate for such differences so that the measures taken with the new amplifier will be consistent with those of the original amplifier. This allows a practitioner to confidently use an amplifier matched to the original amplifier and know that calculations of z-scores from data it collects will not be biased.
As Thatcher and Lubar (2009) described, the first use of amplifier matching was during the construction of the University of Maryland database (Thatcher et al., 2003). Sine wave signals of different frequencies were used as input to the amplifier used for the database and into a second amplifier so that the frequency response curves of the two amplifiers could be measured and differences, if any, determined. The resulting response ratios to the sine wave at each frequency were then used to scale the power spectral analysis coefficients derived from the second amplifier.
Calculations based on data collected with the second amplifier were adjusted based on these ratios. In this way, measures of EEG collected by the second amplifier would match those if the first (i.e., database) amplifier had been used. This means that a researcher or clinician who uses the second amplifier can have confidence that the results can be validly compared to the findings in the database.
Artifact Removal
Raw EEG data are susceptible to various artifacts, such as eye movements, muscle activity, and electrical interference. These artifacts can distort the EEG signal and must be removed through preprocessing techniques. Many artifacts that can invalidate the collected EEG activity must be removed before calculating the values of variables in a qEEG database. According to Thatcher and Lubar (2009), it is important to use visual inspection to remove artifacts, at least to provide an initial template that subsequent computer-based methods apply to exclude artifactual data from the final sample of EEG for a participant. Using such methods results in satisfactory reliability or consistency of measures necessary for accurate and valid quantification.
Common methods include visual inspection, automated algorithms, and independent component analysis (ICA) to separate and eliminate artifact components from the EEG data (Sanei & Chambers, 2013). Thatcher and Lubar (2009) comment that artifact removal methods that rely on independent or principal components analysis should be avoided because they produce inaccurate EEG coherence and phase measures.
Calculation of EEG Variables
EEG bandwidths are defined, and the preprocessed EEG data are subjected to frequency analysis, typically using Fast Fourier Transforms (FFT) or wavelet transforms. This analysis decomposes the EEG signal into its constituent frequencies, allowing for calculating power spectral densities across different frequency bands (delta, theta, alpha, beta, and gamma). These power spectra are then used to derive normative values for each frequency band (Hughes & John, 1999). More recently, individual 1 Hz frequency bins have become the standard for such analysis to refine further the assessment's specificity (Nunez & Srinivasan, 2006).
The data, then, for multiple participants in a given age group under specified conditions (e.g., 30-40-year-old individuals resting with eyes open) are then aggregated to produce a mean and standard deviation for each EEG variable of interest at each 10-20 site or combination of sites (e.g., the mean and standard deviation for the alpha band of 8-10 Hz at O2 for 30-40 year-olds resting with eyes open; alpha-band coherence between F7 and T3)..
Reliability and Validity
Understanding qEEG normative databases benefits from knowing some basics, not only about probability and statistics but also from information related to psychological test theory and construction (Thatcher, 2010). The objective is to have tools that produce stable and true measures of the variable of interest. Discussion of the truth or certainty of a particular measure rests on its reliability and validity.Reliability
Reliability, in the sense used for psychological tests, means consistency (Anastasi, 1976). Measures of reliability have a hypothetical range of 0.00 (two or more measurements are completely inconsistent) to +1.00 (complete consistency). A common standard for acceptable reliability is 0.90 or above. Because the quantification of a variable is done with data collected in one recording session by standardized mathematical calculations, the reliability of the data in qEEG databases depends primarily on how well artifacts are removed from analog data from a participant in a consistently conscious state during data collection, and on standardization of the methods used for data collection.Two types of reliability measures are sometimes used (Nunnally, 1967). Split-half reliability calculates the similarity of values between alternating short epochs of artifact-free EEG data from a single recording session, then calculates the correlation between odd and even epochs in the sequence.
Test-retest reliability correlates artifact-free epochs taken from the first half of the qEEG recording with those taken from the second half. This type of reliability is sensitive to changes in consistency of the data that may occur from the beginning to the end of the recording session. Reliability figures greater than 0.9 are often found with these methods (Duffy et al., 1994; John, 1977; John, Princhep, & Easton, 1987; Thatcher, 1998; Thatcher et al., 2003).
Test-retest reliability has also been assessed for qEEG variables over longer than within-session time frames. Gudmundsson, Runarsson, Sigurdssson, Eiriksdottir, and Johnsen (2007) found that the highest reliability values over 2 months were for spectral variables, with reliability increasing up to an epoch length of 40 seconds, and with coherence being least reliably measured. Cannon and colleagues (Cannon et al., 2012) also used methods available with the NeuroGuide database and low-resolution electromagnetic tomography analysis (LORETA et al., 1994) to find good test-retest stability of EEG measures.
Validity
In psychological test theory, “validity concerns what the test measures and how well it does so” (Anastasi, 1976, p. 134). The several types of validity include content validity, concurrent validity, predictive validity, construct validity, face validity, and ecological validity. The two types of validity that are most applicable to qEEG databases are content validity and concurrent validity.Content validity refers to how well the test or the qEEG database covers the domain of interest. In the case of qEEG databases, content validity has to do with whether the EEG variables and the data recording conditions in the database include those of most interest to clinicians and researchers. A review of the variables and recording conditions included in databases is presented above, and the outline of selected databases is given below. The variables included in qEEG normative databases can be seen to have good content validity, given that they cover the range of important EEG variables.
Concurrent validity is criterion-related validity that refers to how well a measure is simultaneously associated with a gold standard or other measure of the same variable.
Concurrent validity is relevant to how well the values of two databases agree simultaneously. The degree of association, like measures of reliability described above, is described by a correlation coefficient whose value ranges between -1.00 and +1.00. Concurrent validity coefficients above +0.75 are considered excellent and show good agreement between measures. Similarly, when comparing two databases, they show good concurrent validity if the correlation between their values is high.
Hughes and John (1999) reviewed information that supports the view that EEG normative databases have good concurrent validity, as shown by acceptable correlations between the values of their variables. Thatcher and Lubar (2009) reported that CNS Response compared z-scores calculated by the NYU and University of Maryland databases for 19-channel qEEG findings for psychiatric patients ages 6 to 84. Correlations between z-scores ranged from 0.857 to 0.979, where a correlation of +1.00 or -1.00 is perfect agreement. These findings, therefore, show good agreement between z-score calculations of the NYU (i.e., NxLink) and the University of Maryland (i.e., NeuroGuide) databases.
The measured and quantified values of EEG variables might be compared to values based on visual inspection, as an exercise in concurrent validity. However, as reviewed by Thatcher (2010), visual inspection results in less reliable measures than qEEG methods, which necessarily limits the validity of visual inspection. Further, some EEG activity (e.g., coherence, phase) is not well appreciated visually.
Another form of criterion-related validity, predictive validity, concerns how accurately one measure predicts a different measure. The relevance of predictive validity for qEEG databases has to do with how well equations using the normative information in the database can be constructed to predict something or some state in the future. Concerning qEEG normative databases, studies have examined, for example, how well their data can be used to predict IQ (Thatcher et al., 1983; Thatcher et al., 2008) and membership in a mild traumatic brain injury group (mTBI) when used in a discriminant function analysis with EEG data from mTBI participants (Thatcher et al., 1989).
In another sense, the question of validity that is related to the database is, “How well do a participant’s significant differences from the normative values in a qEEG database correlate with other measures and observations such as findings of CT or MR imaging, impairment seen through neuropsychological test results, and neurologist examination?” Results showing a significant relationship between qEEG and MRI variables among participants with traumatic brain injuries (Thatcher et al., 1998; Thatcher et al., 1998) encourage the consideration that normative qEEG databases may have validity for use in the assessment of individuals with TBI as shown in their possibly significant deviations from the normal range of EEG variables.
Cross-validation is the statistical analysis of how closely the data of a new sample match the characteristics of the data of the original sample. Suppose the findings of the second sample replicate or match those of the first sample to a satisfactory degree. In that case, one can conclude that the findings of the first sample represent those of the entire population from which that sample was drawn. Successful cross-validation gives confidence that the sample’s results are valid. For example, the average power of the alpha frequency at O1 during eyes closed collection, together with its standard deviation and distribution shape for a sample of 10-year-old children resting with their eyes closed, is compared to findings for the same variable among a different sample of 10-year-old children resting with their eyes closed.
The usual method for conducting cross-validation is a “leave-one-out” procedure. This first takes a sample, for instance, of 100 10-year-old normally developing healthy children, measures alpha amplitude at O1 with eyes closed, and calculates the mean and standard deviation. Results for even-numbered participants are compared to those for odd-numbered participants. To the degree that the findings are identical, the cross-validation indicates that the findings are a valid estimate of the true values that would have been found if the entire population from which the samples were drawn was measured. This type of analysis has been conducted successfully by the developers of several databases (Gasser et al., 1988; Gasser et al., 1988; John et al., 1987; Thatcher et al., 1983).
Thatcher et al. (2003) have also presented results for a related type of study using what is referred to as a Gaussian cross-validation. These investigators validated the NeuroGuide database by comparing participants who were separated into two groups based on a leave-one-out method, finding the validity results satisfactory. Thatcher and colleagues (2003) also discuss what is referred to as the NeuroGuide database’s sensitivity and specificity in terms of its Gaussian analysis. This analysis showed the NeuroGuide database to be statistically accurate and sensitive.
A question about validity discussed above is, “How well, or validly, do the measures in a database represent normal EEG activity?” Such validity rests on the combined effects of how the participants for the qEEG normative database were sampled, exclusion and inclusion criteria, data collection methods, the effectiveness of artifact removal, variables selected for inclusion in the database, and mathematical methods used to quantify EEG variables.
Whether the use of qEEG normative databases and the comparison of test groups to database values have validity in predicting various conditions or cognitive characteristics remains a valuable field for study. Similarly, the validity of comparisons of client and database findings for planning and conducting EEG neurofeedback remains a valuable field for study.
qEEG Database Development History
Thatcher and Lubar (2009) report that the earliest qEEG database was made in the 1950s at UCLA and comprised several hundred candidates for the NASA space program, university faculty, and students (Adey et al. 1961, 1964a, 1964b). Inclusion and exclusion criteria were not employed. These authors analyzed their data by calculating means, standard deviations, and measures of distribution shape for selected variables.
Matousek and Petersen (1973a, 1973b) published the first peer-reviewed normative database. Their work described inclusion and exclusion criteria and standards for statistical analyses. Over 400 participants were selected, of whom just over half were females. Participants aged from two months to 22 years were all from an urban Scandinavian area. Individuals with a history of significant illness that may have affected brain function were excluded from the sample, and school-aged participants were required to perform at grade level. Eighteen to 49 participants were included in each one-year age group. Matousek and Peterson (1973a, 1973b) were also the first to employ t-tests and z-scores.
Subsequent normative databases constructed by John and colleagues (John, 1977; John et al., 1977, 1987), Thatcher and colleagues (Thatcher, 1988; Thatcher et al., 1983; 1987; 2003; 2005a; 2005b; Thatcher & Krause, 1986; Thatcher, Krause, & Hrybyk, 1986), and by Gordon and colleagues (Gordon et al., 2005) used similar subject selection criteria to ensure a representative sample.
The findings of Matousek and Petersen were subsequently replicated with a sample of children from a different culture than John and colleagues (John, 1977; John et al., 1977, 1987), who used age regression methods to produce appropriate means and standard deviations for different ages. These investigators and Duffy (et al., 1994) emphasized the importance of variables having Gaussian distributions and standards of reliability, replication, and cross-validation.
Thatcher and Lubar (2009) reviewed the history of current source normative databases that can render images of the cortex in three dimensions. Variable Resolution Electromagnetic Tomographic Analysis (VARETA) is a z-score EEG database Bosch-Bayard et al. (2001) developed and showed high sensitivity and specificity. VARETA differs from LORETA in that it uses a probabilistic mask, allowing the smoothness of the inverse solution to vary and estimates distributed and discrete sources with equal accuracy (Thatcher & Lubar, 2009). Machado et al. (2004) successfully used VARETA in cases of stroke.
Pascual-Marqui and colleagues (Pascual-Marquiet et al., 2001) developed an inverse solution for localizing EEG sources in three dimensions termed Low-Resolution Electromagnetic Tomographic Analysis (LORETA). Thatcher and colleagues (Thatcher et al., 2005a; 2005b) developed a normative database for LORETA, showing good cross-validation results and sensitivity relative to surface EEG measures. Hoffman (2006) also found high accuracy in using this LORETA database in assessing patients with various neurological diagnoses. Thatcher, Biver, and North (2007) extended the development of a LORETA database with z-scores to measure EEG connectivity. Thatcher and Lubar (2009) described that EEG z-score databases have been used with real-time EEG biofeedback. That process involves using complex demodulation as a joint-time frequency analysis (JTFA) to calculate a virtually instantaneous NFB z-score. The JTFA calculation, however, results in a smaller z-score than the usual FFT calculation, producing a more conservative estimate of deviation from the values in the database.
Thatcher (1998) and colleagues developed a z-score normative EEG database (NeuroGuide) in the 1980s and 1990s, and its functionality has increased considerably since that time (Thatcher, 2021). He notes that at the time of his writing in the late 1990s, there were several other EEG databases, such as the NxLink z-score normative database developed by John and colleagues (John, Princep, & Easton, 1987). Several qEEG normative databases, in addition to NeuroGuide, are currently commercially available. The NxLink database has been updated and is now called BrainDX (BrainDX, 2023). Other qEEG databases that have been commonly used include Human Brain Institute (Kropotov, 2009), Sterman-Kaiser Imaging Lab (Kaiser, 2008), EureKa! (Congedo, 2005), NeuroRep (Coben & Hudspeth, 2008), iMediSync (Jeong, Park, & Kang, 2022), qEEG-Pro (Keizer, 2021), and TDBRAIN (van Dijk, van Wingen, Denys, Olbrich, van Ruth, Arns, 2022).
Features of Selected Current Databases
We will discuss NeuroGuide, Human Brain Institute, Jewel, NewMind, TDBRAIN, iMediSync, and the QEEG Pro databases.
NeuroGuide
Thatcher and his collaborators (e.g., Thatcher, 1998, 2021; Thatcher et al., 2020) have developed the NeuroGuide database over decades, beginning in the 1980s (e.g., Thatcher et al., 1983) and adding additional participants and features as they have made innovations in analytic methods.
Caption: Robert Thatcher
Developed initially at the University of Maryland, NeuroGuide completed an ethics review and required informed consent from participants. As Thatcher and colleagues (2007) reported, the database has 727 participants aged 2 to 82. Most participants are younger than 14 to address the changes in EEG that occur with normal aging. Fifty-nine percent are male. Seventy-one percent are white, 24 percent black, and 3 percent Asian. Participants are drawn from urban and rural environments and have a range of socioeconomic statuses (Thatcher, 1991, 1992, 1994, 1998; Thatcher & Krause, 1986; Thatcher, Walker, & Guidice, 1987). Applied Neuroscience is the company that develops and sells NeuroGuide.
NeuroGuide uses 20 age groups with sliding windows to increase its age resolution. The youngest 15 age groups span a 2-year range and overlap by 6 months for participants up to age 15 (N = 470). The remaining 5 age groups total 155 participants, each overlapping by five years (Thatcher et al., 2003). According to Thatcher and Lubar (2008), the purpose of using so-called sliding age ranges is to have a more stable database solution with higher age resolution. The oldest age group spans 35-82. The smallest sample size (N = 16) is for the 2-3 year age range, and the largest (N = 45) is for the 3-4 year age range. Participant inclusion and exclusion were based on questionnaire responses and results from IQ, academic achievement tests, and neuropsychological tests. History regarding school grades and teacher reports, work, childhood development, environmental toxin exposure, loss of consciousness, neurological and psychiatric disorders, head injury with CNS symptoms, convulsions, skull size, and handedness were considered as well.
EEG eyes-open and closed data were collected with an Applied Neurosciences Laboratory amplifier with an impedance of less than 10Kohms (Thatcher et al., 2003). The frequency response was 0.5-30 Hz, and recording durations ranging from 59 to 142 seconds were reported by Thatcher and colleagues (2003).
The resulting data were tested for Gaussianity and log transformed when necessary. NeuroGuide calculates numerous variables: amplitude, power, relative power, power ratios, asymmetry, coherence, phase metrics, peak frequencies, and burst metrics. These are transformed into z-scores, both for surface and sLORETA analysis. Further, data are analyzed in terms of several brain networks. New developments in NeuroGuide include analysis of subcortical activity (i.e., amygdala, cerebellum) and effective connectivity (Thatcher et al., 2020). Analyses are used to generate reports, and a symptom checklist can be used to select neurofeedback protocols. The NeuroGuide database is the basis for z-score surface and sLORETA neurofeedback.
Thatcher and colleagues (Thatcher, 2018; Thatcher, Biver, & North, 2007a; Thatcher, Biver, & North, 2007b; Thatcher, Lubar, & Koberda, 2019) have used Joint Time-Frequency Analysis (JTFA) with the NeuroGuide database to calculate z-scores in real time for neurofeedback. Thatcher and Lubar (2009) outline the mathematical methods for this analysis. These methods, however, result in a conservative estimate of the instantaneous value’s deviation from normal (Thatcher & Lubar, 2009). Graphs showing session-by-session change in data can also be produced. Care has been taken to match many available amplifiers to those used for data collection in NeuroGuide. NeuroGuide has developed a standardized weighted LORETA method (swLORETA) for its NeuroNavigator imaging feature with 12,400 voxels. NeuroNavigator also includes an index of phase slope for analyzing information flow. Unique to NeuroGuide is imaging of the cerebellum, red nucleus, nucleus accumbens, and the habenula.
Among the other features of NeuroGuide (Thatcher, 2021) are EEG automatic artifact rejection that does not use ICA/PCA methods, display of conventional EEG with amplitude time and event markers, and multiple montages. Other features include conventional EEG display with time domain LORETA for viewing epilepsy and focal disorders, EEG JTFA and FFT analyses, EEG auto- and cross-spectral analyses, bi-spectral analyses, Gabor adaptive spectrogram, brain function and concussion indices, automatic qEEG clinical report writer, color topographic maps of ANOVA and t-tests, and other research tools for statistical analyses and digital signal processing of the EEG such as NeuroStat that can, for example, produce pre-post comparisons for EEG variables.
Gaussian cross-validation was established (Thatcher et al., 2003). A discriminant function for mild traumatic brain injury has also been cross-validated (Thatcher et al., 1989). Multiple peer-reviewed publications have used NeuroGuide. NeuroGuide is registered with the FDA and can be obtained through Applied Neuroscience at https://appliedneuroscience.com, among other vendors.
Human Brain Institute
The qEEG normative database developed by the Human Brain Institute is described in Juri Korpotov’s (2009) text on qEEG and event-related potentials (ERPs) and in Tereshchenko et al. (2010).
Caption: Juri Kropotov
Not surprisingly, it contains normative data not only for EEG variables but also for ERPs. Participants include 1000 healthy individuals ranging in age from 7 to 89 years. The sample for children and adolescents between ages 7 and 17 is 300 participants, with 500 adult participants aged 18-60 and 200 participants over 60. Inclusion criteria are an uneventful perinatal period and average or better academic achievement. Exclusion criteria are neurological and psychiatric disease, convulsions, or head injury with brain-related symptoms.
Additionally, data are included for participants with clinical conditions such as ADHD, epilepsy, OCD, addiction, depression, and whiplash. However, these data have not yet been included in the commercially available software (Juri Kropotov, personal communication, 2023). Data collection conditions used by the HBI normative database are eyes closed and eyes open, each with a minimum of 3 minutes of data. Recordings are also made for several task conditions: a go/no-go task, mathematical calculation, reading, auditory recognition, and an auditory oddball task. The database uses WinEEG software for Mitsar amplifiers, which uses automated artifact marking and correction and spike detection. However, manual artifact deletion is also available. Artifacts are defined as amplitudes above 100uV, 0-1 Hz waves with amplitude above 50uV, and 20-35 Hz frequencies above 35uV. Also, eye movement artifacts are automatically corrected using a template and ICA.
Data include a frequency range of 0-45 Hz with 0.25-Hz resolution. EEG features available are absolute magnitude, absolute power, relative power, coherence, wavelet transformation, and ERPs. Montages available are linked ears, global average, and local average. Z-scores are calculated, and the database has sLORETA capability. qEEG data are normalized to calculate valid means and standard deviations. In addition to typical EEG variables such as power and coherence, wavelet transformations and dipole approximations are available. ICA extracts separate components of ERPs related to particular cognitive functions. Time dynamics and topography are provided for each component so that the user can determine the amplitude and latency of ERP components, which are important for understanding stages of information processing.
A Mitsar amplifier is required for use. The software generates topograms showing deviations from normal and produces training recommendations. Data from new participants are compared to an age-matched subset of participants in the database having a sample of at least 30.
Sites providing further information about the HBI database are: https://www.hbimed.com/en/hbi-database/ https://www.hbimed.com/en/hbi-database/#tab-id-6 https://bio-medical.com/hbi-human-brain-indices-database.html The HBI database is registered with the FDA.
The North American vendor for the HBI normative database is bio-medical.com.
Jewel
Jewel is a qEEG z-score database created by John Demos (Demos, 2023) and installed on the user’s computer.
Caption: John Demos
Participants are ages 7 to adult, were recruited from John Demos’ clinical practice, and provided informed consent. Data for the first 75 participants were collected with a Lexicor Neurosearch 24 amplifier, and a BrainMaster Discovery amplifier was used for the remaining 1000 participants.
Data without artifacts were manually selected for analysis. Amplitude, power, and coherence measures are calculated for several bands: Delta (1.5-4 Hz), Theta (4-8 Hz), Alpha (8-12 Hz), Beta (12-25 Hz), Gamma (35-45 Hz), Theta1 (4-6 Hz), Theta2 (6-8 Hz), Alpha1 (8-10 Hz), Alpha2(10-12 Hz), Beta1 (12-14 Hz), Beta2 (14-16 Hz), Beta3 (16-20 Hz), Beta4 (20-24 Hz), Beta5 (24-28Hz), and Beta6 (28-32 Hz). Jewel offers unique eyes open monopolar and bipolar z-scores and z-scores based on amplitude rather than power.
Jewel has an automatic report generation feature based on qEEG findings and symptoms, as reported with a free online questionnaire that can also be sent to the client’s therapist and used to generate a client report with homework. The report includes neurofeedback training suggestions for amplitude training, surface and sLORETA z-score protocols, low-frequency training, HEG training, and photic stimulation. Information from a knowledge base is also included in the report. Protocol generation uses both qEEG findings and questionnaire results. The Jewel brain map shows 10-20 sites corresponding to symptoms, and a table produced by Jewel correlates symptoms and bandwidths for training.
Power and coherence findings are linked to corresponding symptoms, and Jewel produces a table showing how symptoms and bandwidth findings relate to selecting protocols. Protocols can be developed using several methods, for example, based on symptoms or cortical sites. Jewel and qEEG-Pro databases can be processed through Jewel to prepare BrainAvatar z-score neurofeedback training protocols. Jewel has a feature allowing a side-by-side comparison of findings from its database and those of qEEG-PRO. Z-score training options for 1-, 2-, 4-, and 6-channel protocols are supported. Jewel also easily generates non-z-score neurofeedback suggestions based on symptoms. Jewel also generates weekly tracking sheets and pre-post qEEG comparisons.
Users must use BrainMaster’s Discovery amplifier and BrainAvatar software with an analysis software upgrade. A Jewel subscription can be purchased from either John Demos at www.eegshopping.com or from BrainMaster at www.brainmaster.com.
NewMind
The NewMind normative qEEG database is designed to simplify qEEG interpretation compared to other databases and to guide neurofeedback training. Its developer, Richard Soutar, began collecting data for this database in 2006 and has periodically added participants, bringing its current sample size to 797 healthy normal individuals aged 5 to 90.
Caption: Richard Soutar
The sample reportedly represents a wide variety of socioeconomic, racial, and ethnic backgrounds. Participants originate from over 600 primarily US clinics where they sought neurofeedback training for peak performance or amelioration of different disorders. However, the participants were carefully screened with cognitive, physiological, and emotional function measures so that only healthy individuals were included in the database.
Data were recorded with BrainMaster and NewMind amplifiers that had been frequency-matched (Soutar, 2020). A sampling rate of 256 Hz, bandwidth of DC-40 Hz, input impedance of 500 MOhm, DC offset of +/- 300 LSB, and common mode rejection of 100 dB are reported. Eyes closed and eyes open data were collected with a linked ears montage. One minute of clean data after automatic artifacting was used for both conditions. Time domain components analyzed by the database are for the following bands: Delta (1-3 Hz), Theta (4-7 Hz), Alpha (8-12 Hz), LoBeta (13-15 Hz), Beta (15-20 Hz), and High Beta (20-30 Hz). Results are provided for magnitude, dominant frequency, interhemispheric coherence and phase, and asymmetry. Results are shown in 1-standard deviation increments for each 10-20 site.
Development of the NewMind database involved cross-validation and testing of statistical sensitivity and Gausianity. Bootstrapping and log transformations were used when necessary to normalize the distribution of variables.
The NewMind software integrates qEEG findings with comprehensive client self-report questionnaire results and tracks training progress.
The web page https://www.newmindmaps.com/demo/demo.htm states that: The NewMind automated EEG analysis also identifies primary and secondary z-score locations. The z-score location suggestions assist the practitioner in identifying the locations to use when z-score protocol Neurofeedback training is being used. Finding z-score locations and their order can be a long and painful process; the NewMind z-score analysis helps simplify and speed up this analysis process. The database has a dashboard that facilitates easy use in generating provider and client reports, including head maps. Features include control sliders, an interpretive dashboard, midline analysis, a symptom tracker, a normed physiological checklist, a cross-validated cognitive checklist, a normed socio-emotional assessment, a normed cognitive computerized performance tests, and a pre-post maps comparison tool that reports the percentage change in standard deviation units. The software automatically generates suggested neurofeedback training protocols, for which BrainMaster designs can be downloaded.
The NewMind database is not registered with the FDA and requires a NewMind, Discovery, or Mitsar amplifier. The NewMind cloud-based database can be found at www.newmindmaps.com and www.brainmaster.com.
TDBRAIN and the Brainclinics Brainmarker Platform
The TDBRAIN (Two Decades – Brainclinics Research Archive for Insights in Neurophysiology) database originates in Nijmegen, The Netherlands, where associates of the Research Institute Brainclinics of the Brainclinics Foundation have conducted considerable EEG and related research (e.g., van Dijk et al., 2022). Not surprisingly, given the name of the database, data were collected between 2001 and 2021. The TDBRAIN database is available as a free open-access database at https://brainclinics.com/resources/. Besides EEG data, it includes rich phenotype data such as baseline severity (BDI, ADHD-RS), personality (NEO-FFI), sleep, neuropsychological, and especially treatment response data to rTMS and neurofeedback.Participants for the database number 1274 (620 female) ranging in age from 5 to 88 years, 47 of whom are identified as “healthy,” with another 255 having “unknown” diagnoses (if any), and the remainder presenting a heterogeneous collection of diagnoses (e.g., major depressive disorder, ADHD, subjective memory complaints, obsessive-compulsive disorder, etc.). An extended version of the database, TDBRAIN+, comprising about 4500 EEG records, is used internally at Brainclinics for the development of the Brainclinics Brainmarker platform for which several validation studies have been published (e.g., EEG-based stratification between methylphenidate and neurofeedback for ADHD: Voetterl et al., 2022) or will soon be published e.g., EEG based stratification between antidepressants and brain stimulation treatments (Voetterl et al., 2023).
Participants provided informed consent and satisfied several requirements for the use of medications, caffeine, alcohol, and tobacco before data collection, which was done with a Neuroscan Nuamps amplifier. EEG data, EOG, EMG, and ECG data were collected during two-minute samples for eyes open and closed. In addition to resting eyes closed and eyes open recording conditions, data were collected during an auditory oddball task and a visual 1-backtest. Available data is raw unprocessed EEG, but free Python code for artifact removal is available from the same source where artifacts can be automatically removed, including those related to EMG, sharp channel jumps, kurtosis, extreme voltage swing, residual eyeblinks, electrode bridging and extreme correlations (van Dijk et al., 2022).
Studies have demonstrated high reliability (Williams et al.,2005) and cross-cultural validation (Paul et al., 2007). Data were also validated using alpha power changes from eyes open to eyes closed conditions and alpha peak frequency maturational changes (van Dijk et al., 2022).
A major clinical use of this database is to identify biomarkers, or so-called Brainmarkers, as prognostic stratification markers for the selection of the treatment that is best-matched to the individual, for example, neurofeedback, psychostimulant medication, antidepressants, or rTMS (for a review on stratified psychiatry including examples see: Arns et al., 2022).
A video of TDBRAIN+ and validation of the Brainmarker platform can be found at https://www.youtube.com/watch?v=-UgYXFnkbfg.
The TDBRAIN database and related code can be freely downloaded at https://brainclinics.com/resources/, and more information about the Brainmarker platform can be obtained via https://brainclinics.com/the-brainmarker-assessment-suite/.
iSyncBrain
The iMediSync corporation has developed a normative database, iSyncBrain, that uses artificial intelligence-guided analytics and is cloud-based (Jeong, Park, & Kang, 2022; Ji et al., 2022; Ko et al., 2021). The developers completed the ethics review, and participants provided informed consent. Participants were recruited from the Korean EEG Center at the Seoul National University, totaling 1289 participants, with 553 male and 736 female participants, whose ages ranged from 4 to 82. Participants were stratified by age and sex. Nonlinear regression methods were used to stratify based on sex, and a de-noising process based on qEEG variability was used to stratify between female and male participants at various ages. The sample size for age ranges was over 250 for the youngest group, with less than 50 for the oldest age range. The database has 15 age groups for ages 4-19 and four age groups for ages above 19.The inclusion and exclusion criteria included personal history (medical and academic) and cognitive, emotional, and behavioral factors. Participants were excluded if their history was positive for any psychiatric or neurological disease, problematic academic or social activity, head trauma, epilepsy, behavioral or conduct disorders, or medical treatment that might have affected brain function. Participants were also excluded if they met quantified criteria on a measure of cognition (i.e., K-WPPSI, CNS Vital Signs, MMSE), emotional function (State-Trait Anxiety Inventory – Korean, Child Depression Inventory, Beck Depression Inventory), or behavior (Korean Child Behavior Checklist).
Four minutes of data were collected in eyes-closed and eyes-open conditions with a frequency range of 1-45 Hz. Artifacts were addressed with a notch filter, re-referencing to Common Average Reference, Artifact Subspace Reconstruction, advanced mixture ICA, automated EEG denoising, and EMG and EOG artifacts removal. A cloud-based AI-driven auto-analyzing platform was used. Amplitude was calculated for the Delta, Theta, Alpha, Beta, and Gamma bands. In addition, absolute and relative power spectra were calculated with occipital peak alpha, power ratios, coherence, and sLORETA. Topographic maps and 3D viewing were also provided. Log transformations were used when significant skewness was found, with subsequent curve-fitting, a continuous method, to avoid disconnection or large jumps in z-scores between successive age bands. Non-linear regression using a spline method was used for curve fitting. Z-scores were finally calculated. Average reference and common reference montages can be used.
The iSyncBrain database has been validated concurrently with the qEEG-Pro normative database, and the results show good agreement (Ko et al., 2021). ADHD and Alzheimer’s disease participants have also been evaluated using the database (Jeong, Park, & Kang, 2022; Ji et al., 2022). The database can generate reports, including that of a biomarker for Mild Cognitive Impairment. The database does not indicate FDA registration. It can be obtained through https://www.imedisync.com/en/products/isyncbrain/.
qEEG-Pro
qEEG-Pro was developed by Keizer, with participants recruited from the Neurofeedback Institute Netherlands (Keizer, 2019; Keizer, 2023; Kerson, 2023). Participants ranged in age from 6 to 83 years. There were 1482 participants in the eyes-open condition (female = 527, male = 955) and 1232 in the eyes-closed condition (female = 432, male = 799). The database provides different norms based on sex and recording conditions, with age regression methods using a sliding window to compare a new participant’s assessment to similar-aged database participants. Most database participants were younger than 20 years, with the largest age groups totaling between 70 and 80 participants. qEEG-Pro uses a moving average of 150 age subgroups, ranging from 1 to 75.5 years, with a 6-month age resolution (Keizer, 2018; Kerson, 2023). The manual (Keizer, 2018, p. 31) indicates that these subgroups were created by “selecting 200 participants who had a minimum age difference with age bin of interest.”Participants were screened with the CNC-1020 questionnaire (Brownback, 2023). Regression analysis was conducted on all metrics, including those that had been log-transformed, using the CNC-1020’s 47 categories of psychopathology, which data were also log-transformed. The resulting residuals were then used to calculate the means and standard deviations for all metrics in the qEEG database (Keizer, 2018, p. 31). Participants were excluded if EEG data showed epileptiform activity or recordings were less than 1 minute.
Data were collected in eyes-open and eyes-closed conditions using a Demed TruScan32 amplifier. Fully automated artifact rejection was used but did not employ ICA or PCA methods. The artifact rejection eliminated data below the high pass filter and within the notch filter range. Noisy channels were also eliminated. Data showing other artifacts (i.e., eye blink, horizontal eye movement, low- and high-frequency artifacts) were also removed.
The resulting data were analyzed into bands ranging from 1 to 45 Hz (i.e., Delta 1-3 Hz, Theta 4-7 Hz, Alpha 8-12 Hz, Beta 15-20 Hz, and Gamma 35-45 Hz) with a 1-Hz resolution. Z-scores are calculated for power, relative power, power ratios, asymmetry, phase coherence, phase lag, burst metrics (average peak power, bursts per second), and comodulation (cross-frequency power correlation). The database also has sLORETA functionality. Linked ears and Laplacian montages can be used.
qEEG-Pro generates a report that includes maps for absolute power, relative power, and z-scores for single hertz bins from 1 to 40 Hz. Additional maps are generated for extreme z-score development (relevant for identifying possible maturational lag), fluctuation time (for examination of power variability), and percentage deviant activity (showing the percentage of time power values exceed + 2.3 SD for site by frequency map). The report includes tables for absolute and relative power (raw and z-score), amplitude asymmetry, phase coherence, FFT power distribution, peak alpha frequency at each 10-20 site, and z-score alpha peak distribution maps.
Concurrent validity studies have been conducted with qEEG-Pro and iBrainSync (Ko, Park, Kim, & Kang, 2021) and NeuroGuide (Thatcher et al., 2020) showing good correlation. The database development and its use are presented in peer-reviewed publications (Keizer, 2021; Ko et al., 2020).
A list of suitable amplifiers for data collection can be found at: https://qeeg.pro/order-qeeg/. A BrainMaster Discovery amplifier must be used for neurofeedback. The qEEG-Pro database is FDA-approved and available from the preceding qEEG-Pro website and from https://brainmaster.com/. https://www.brownbackmason.com/shop (accessed February 27, 2023).
Multiple Tests and Comparisons Risk
Thatcher and Lubar (2009) commented on the frequent practice of subjecting EEG data to analysis with multiple normative databases. They suggested that doing so may produce results that are invalid from those databases that have not met the minimal standards they outline above. This may result in spurious, inconsistent findings that could mislead a clinician. Thatcher and Lubar (2009) stated that database users must know how well the databases they use follow scientific standards and disclose to their clients the corresponding certainty and validity of their shared results. Additionally, the risk of spuriously and misleadingly significant findings by chance increases with the number of tests one uses, so using a database, or more than one database, must employ these tools selectively (Schretlen & Campbell, 2013).
Summary
This section has reviewed basic concepts relevant for understanding and using qEEG normative databases. Thatcher and Lubar (2009) described “gold standards” for evaluating qEEG normative databases (p. 54).
The clinician must evaluate the claims made by product developers to determine if the claimed methods and product features are valid. This may present some difficulty for clinicians who are not experts in database development, statistical analysis, EEG theory, and practice. Some of the databases we discussed were developed using client EEGs rather than carefully screened participants. The claim that such individuals' EEG recordings can be “cleaned” of abnormal patterns to allow their data to be included in a normative database is questionable at best. Additionally, the clinical database approach using recordings from clinical participants depends upon the developer’s knowledge and understanding of neurophysiology, neuropsychology, electroencephalography, and more. Therefore, some of these may be effective, valid, and useful in clinical settings, while others may not. Ultimately, the clinician is responsible for using a reliable, validated instrument with known and well-documented parameters that have withstood the scrutiny of multiple peer-reviewed publications. One or two publications the developer(s) authored do not meet these criteria.
The somewhat revised list of criteria below is based on Thatcher and Lubar (2009) and summarizes the several steps reviewed in this section that meet good scientific standards for developing qEEG normative databases:
Judgment of qEEG normative databases that follow the plan above can also be made based on the results of these steps published in peer-reviewed journals.
Glossary
biomarkers: biological indicators used to measure and evaluate physiological states or conditions.
concurrent validity: the extent to which test results align with results from other established measures of the same construct, administered at the same time.
confidence interval: a range of values derived from data, within which a population parameter is expected to lie with a specified probability.
content validity: the degree to which a test or measurement reflects the entire range of material it is supposed to assess.
correlation: a statistical measure that indicates the extent to which two or more variables fluctuate together.
cross-validation: a method for assessing how a predictive model performs with an independent dataset, different from the one used for model development.
criterion-related validity: the extent to which a measure is related to an outcome, assessed by comparing it with a criterion known to be associated with the construct.
descriptive statistics: statistical methods that summarize and describe the main features of a dataset.
exclusion criteria: specific conditions or attributes that disqualify individuals from participating in normative database construction.
Fast-Fourier Transformation: a mathematical algorithm that converts time-domain EEG signals into their frequency-domain components. This process allows the identification and analysis of different brain wave frequencies (delta, theta, alpha, beta, gamma), facilitating the study of brain activity patterns.
Gaussian curve: the normal distribution or bell curve. A Gaussian curve in the qEEG represents the distribution of a set of values (such as EEG amplitudes or frequencies) where most of the data points cluster around the mean, with fewer points appearing as you move away from the mean.
Gaussian sensitivity: the degree to which a system or method is responsive to data following a normal distribution.
inclusion criteria: specific conditions or attributes required for subjects to be eligible to participate in normative database construction.
independent components analysis (ICA): a computational method used in the qEEG to separate a multichannel EEG signal into independent sources. It helps isolate artifacts (such as eye blinks or muscle movements) from the brain signals, improving the accuracy of the analysis.
joint-time frequency analysis (JTFA): techniques used to analyze signals with time-varying frequency content, providing information in both time and frequency domains.
kurtosis: a statistical measure that describes the shape of a distribution's tails in relation to its overall shape.
leptokurtic: distributions with positive kurtosis, having heavy tails and a sharp peak.
low-resolution electromagnetic tomography (LORETA): a method for estimating the location of brain activity from EEG/MEG data with low spatial resolution.
mean (M): the average value of a set of numbers.
mesokurtic: distributions with kurtosis similar to that of a normal distribution.
normal curve: a unimodal and symmetrical curve used to describe the distribution of EEG data across a population. It helps in comparing an individual's EEG data to the normative population data, identifying deviations that may indicate abnormalities.
normative databases: EEG data collected from a large, healthy population. These databases provide reference standards that allow clinicians to compare an individual’s qEEG results against typical patterns, aiding in the identification of abnormal brain activity.
p-value: the probability that observed data would occur by chance under a null hypothesis.
percentiles: values below which a given percentage of observations in a group fall.
predictive validity: the extent to which a score on a scale or test predicts future performance on a related criterion.
reliability: the consistency and stability of a measurement over time.
skew: a measure of asymmetry in a distribution.
split-half reliability: a measure of consistency where a test is split into two parts, and the scores from both halves are compared.
standard deviation (S): a statistical measure of the amount of variation or dispersion in a set of values. In the qEEG, it is used to quantify the variability in brain wave activity within a population, helping to determine how far an individual's EEG data deviate from the norm.
standard error of measurement (SEM): the standard deviation of an individual's observed scores from their true score.
stratification: the process of dividing the population data into distinct subgroups or strata based on specific characteristics or variables. These strata are typically chosen to ensure that the normative database accurately reflects the diversity within the population and allows for more precise comparisons.
t-test: a statistical test used to compare the means of two groups or the mean of a single sample to a known value or population mean.
test-retest reliability): the consistency of test scores over repeated administrations of the same test.
validity: the degree to which a test accurately measures what it intends to measure.
wavelet transforms: a method used to analyze EEG signals at different scales or resolutions. Wavelet transforms allow for the examination of both frequency and time information simultaneously, providing a more detailed analysis of brain wave patterns.
z-scores: the number of standard deviations a data point (such as a specific EEG frequency) is from the mean of a normative database. Positive or negative z-scores indicate how much an individual's brain activity deviates from the average, assisting in the diagnosis and treatment of neurological conditions.
Test Yourself on ClassMarker
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, explain how researchers construct normative databases.
References
Adey, W. R., (1964a). Data acquisition and analysis techniques in a brain research institute. Annals of the New York Academy of Science, 31, 884-866. PMID: 14214053
Adey, W. R. (1964b). Biological instrumentation, electrophysiological recording and analytic techniques. Physiologist, 72, 65-68. PMID: 14154035
Adey, W. R., Walter, D. O., & Hendrix, C. E. (1961). Computer techniques in correlation and spectral analyses of cerebral slow waves during discriminative behavior. Experimental neurology, 3, 501–524. https://doi.org/10.1016/s0014-4886(61)80002-2
Anastasi, A. (1976). Psychological testing (4th ed.). Macmillan.
Applied Neuroscience (accessed January 9, 2023). https://appliedneuroscience.com/
Arns, M., Gunkelman, J., Breteler, M., & Spronk, D. (2008). EEG phenotypes predict treatment outcome to stimulants in children with ADHD. Journal of Integrative Neuroscience, 7(3), 421–438. https://doi.org/10.1142/s0219635208001897
Australian Bureau of Statistics (accessed January 17, 2023). ttps://www.abs.gov.au/websitedbs/D3310114.nsf/home/statistical+language+-+what+are+variables<
Bosch-Bayard, J., Valdés-Sosa, P., Virues-Alba, T., Aubert-Vázquez, E., John, E. R., Harmony, T., Riera-Díaz, J., & Trujillo-Barreto, N. (2001). 3D statistical parametric mapping of EEG source spectra by means of variable resolution electromagnetic tomography (VARETA), Clinical EEG (electroencephalography), 32(2), 47–61. https://doi.org/10.1177/155005940103200203
Boutros, N. N., Arfken, C., Galderisi, S., Warrick, J., Pratt, G., & Iacono, W. (2011). QEEG in psychiatric disorders: A review of diagnostic utility and specificity. Clinical EEG and Neuroscience, 42(1), 45-51. https://doi.org/10.1177/155005941104200110
BrainDX. (accessed 7 January 2023). https://braindx.net/research.php#content
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Cambridge Dictionary. (accessed 17 January 2023). https://dictionary.cambridge.org/dictionary/english/norm
Cannon, R. L., Baldwin, D. R., Shaw, T. L., Diloreto, D. J., Phillips, S. M., Scruggs, A. M., & Riehl, T. C. (2012). Reliability of quantitative EEG (qEEG) measures and LORETA current source density at 30 days. Neuroscience letters, 518(1), 27–31. https://doi.org/10.1016/j.neulet.2012.04.035
Caviness, J. N., Utianski, R. L., Hentz, J. G., Beach, T. G., Dugger, B. N., Shill, H. A., Driver-Dunckley, E. D., Sabbagh, M. N., Mehta, S., & Adler, C. H. (2016). Differential spectral quantitative electroencephalography patterns between control and Parkinson's disease cohorts. European Journal of Neurology, 23(2), 387–392. https://doi.org/10.1111/ene.12878
Clarke, A. R., Barry, R. J., McCarthy, R., & Selikowitz, M. (2001). EEG analysis in attention-deficit/hyperactivity disorder: a comparative study of two subtypes. Psychiatry Research, 103(1), 63-73. https://doi.org/10.1016/S0165-1781(01)00261-3
Coben, R., & Hudspeth, W. J. (2008). Introduction to advances in EEG connectivity. Journal of Neurotherapy, 12, 93-98. https://doi.org/10.1080/10874200802429843
Coburn, K. L., Lauterbach, E. C., Boutros, N. N., Black, K. J., Arciniegas, D. B., & Coffey, C. E. (2006). The value of quantitative EEG in clinical psychiatry: A report by the committee on research of the American Neuropsychiatric Association. Journal of Neuropsychiatry and Clinical Neurosciences, 18(4), 460-500. https://doi.org/10.1176/jnp.2006.18.4.460
Congedo, M. (2005). EureKa! (Version 3.0). Nova Tech EEG, Inc.
Daubert v Merrell Dow Pharmaceuticals (Daubert), 61 U.S.L. W 4805 (U.S. June 29, 1993).
Duffy, F. H., Hughes, J. R., Miranda, F., Bernad, P., & Cook, P. (1994). Status of quantitative EEG (QEEG) in clinical practice, 1994. Clinical EEG (Electroencephalography), 25(4), VI–XXII. https://doi.org/10.1177/155005949402500403
Gasser, T., Verleger, R., Bächer, P., & Sroka, L. (1988). Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalography and Clinical Neurophysiology, 69(2), 91–99. https://doi.org/10.1016/0013-4694(88)90204-0
Gasser, T., Jennen-Steinmetz, C., Sroka, L., Verleger, R., & Möcks, J. (1988). Development of the EEG of school-age children and adolescents. II. Topography. Electroencephalography and Clinical Neurophysiology, 69(2), 100–109. https://doi.org/10.1016/0013-4694(88)90205-2
Gordon, E., Cooper, N., Rennie, C., Hermens, D., & Williams, L. M. (2005). Integrative neuroscience: the role of a standardized database. Clinical EEG and Neuroscience, 36(2), 64–75. https://doi.org/10.1177/155005940503600205
Gudmundsson, S., Runarsson, T. P., Sigurdsson, S., Eiriksdottir, G., & Johnsen, K. (2007). Reliability of quantitative EEG features. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(10), 2162–2171. https://doi.org/10.1016/j.clinph.2007.06.018
Hoechstetter, K., Bornfleth, H., Weckesser, D., Ille, N., Berg, P., & Scherg, M. (2004). BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography, 16(4), 233–238. https://doi.org/10.1023/b:brat.0000032857.55223.5d
Hoffman, D. (2006). LORETA: An attempt at a simple answer to a complex controversy. Journal of Neurotherapy, 10, 57-72. https://doi.org/10.1300/J184v10n01_05
Hughes, J. R., & John, E. R. (1999). Conventional and quantitative electroencephalography in psychiatry. Journal of Neuropsychiatry and Clinical Neurosciences, 11(2), 190-208. https://doi.org/10.1176/jnp.11.2.190
Jeong, T., Park, U., & Kang, S. W. (2022). Novel quantitative electroencephalogram feature image adapted for deep learning: Verification through classification of Alzheimer's disease dementia. Frontiers in Neuroscience, 16, 1033379. https://doi.org/10.3389/fnins.2022.1033379
Characteristics of attention-deficit/hyperactivity disorder subtypes in children classified as using quantitative electroencephalography.
Ji, Y., Choi, T. Y., Lee, J., Yoon, S., Won, G. H., Jeong, H., Kang, S. W., & Kim, J. W. (2022). Characteristics of Attention-Deficit/Hyperactivity Disorder subtypes in children classified using quantitative Electroencephalography. Neuropsychiatric Disease and Treatment, 18, 2725–2736. https://doi.org/10.2147/NDT.S386774
John, E. R. (1977). Functional neuroscience. In E. R. John and R. W. Thatcher (Eds.). Neurometrics Vol. II: Quantitative electrophysiological analyses. Lawrence Erlbaum Associates. Lawrence Erlbaum Associates.
John E. R. (1981). Neurometric evaluation of brain dysfunction related to learning disorders. Acta Neurologica Scandinavica, 64 (Supplement 89), 87–100. https://doi.org/10.1111/j.1600-0404.1981.tb02367.x
John, E. R., Karmel, B. Z., Corning, W. C., Easton, P., Brown, D., Ahn, H., John, M., Harmony, T., Prichep, L., Toro, A., Gerson, I., Bartlett, F., Thatcher, R., Kaye, H., Valdes, P., & Schwartz, E. (1977). Neurometrics: Numerical taxonomy identifies different profiles of brain functions within groups of behaviorally similar people. Science, 196, 1393-1410. Science. https://doi.org/867036
John, E. R., Princhep, L. S., Easton, P. (1987). Normative data banks and neurometrics: Basic concepts, methods and results from norm construction. In A. Redmond (Ed.), Handbook of electroencephalography and clinical neurophysiology III: Computer analysis of the EEG and other neurophysiological signals (pp. 449-495). Elsevier.
John, E. R., Prichep, L. S., & Easton, P. (1988). Normative data banks and neurometrics: Basic concepts, methods and results of norm constructions. In Computer-aided electrophysiology (pp. 21-38). Springer. https://doi.org/10.1007/978-1-4684-5517-5_2
Kaiser, D. A. (2008). Ultradian and circadian effects in electroencephalography activity. Biofeedback, 36, 148-151.
Keizer, A. W. (2018). qEEG-Pro manual (v1.5). Neurofeedback Institute Netherlands.
Keizer, A. W. (2021). Standardization and personalized medicine using quantitative EEG in clinical settings. Clinical EEG and Neuroscience, 52 82-89. https://doi.org/10.1177/155005941987494
Keizer, A. W. (February 2, 2023). Personal communication on normative databases.
Kerson, C. (February 20, 2023). Personal communication
Ko, J., Park, U., Kim, D., & Kang, S. W. (2021). Quantitative electroencephalogram standardization: A sex- and age-differentiated normative database. Frontiers in Neuroscience, 15, 766781. https://doi.org/10.3389/fnins.2021.766781
Kropotov, J. (2009). Quantitative EEG, event-related potentials and neurotherapy. Academic Press.
Kropotov, J. D., Grin-Yatsenko, V. A., Ponomarev, V. A., Chutko, L. S., Yakovenko, E. A., & Nikishena, I. S. (2005). ERPs correlates of EEG relative beta training in ADHD children. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 55(1), 23–34. https://doi.org/10.1016/j.ijpsycho.2004.05.011
Kubicek, K. & Robles, M. (2016, November 11). Resource for integrating community voices into a rresearch Study: Community advisory board toolkit. Southern California Clinical and Translational Science Institute grant UL1TR001855.
Lebby, P. C. (2013). Brain imaging: A guide for clinicians. Oxford University Press.
Lewine, J. D., & Orrison, W. W. (1995). Clinical electroencephalography and event-telated potentials. In W. W. Orrison, J. D. Lewine, J. A. Sanders, & M. F. Hartshorne (Eds.), Functional brain imaging (pp. 327-368). Mosby.
Lin, F., Witzel, T., Hamalainen, M. S., Dale, A. M., Belliveau, J. W., & Stufflebeam, S. M. (2004). Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage, 2(3), 582-595. https://dx.doi.org/10.1016%2Fj.neuroimage.2004.04.027
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869-878.https://doi.org/10.1038/nature06976
Machado, C., Cuspineda, E., Valdés, P., Virues, T., Llopis, F., Bosch, J., Aubert, E., Hernández, E., Pando, A., Alvarez, M. A., Barroso, E., Galán, L., & Avila, Y. (2004). Assessing acute middle cerebral artery ischemic stroke by quantitative electric tomography. Clinical EEG and neuroscience, 35(3), 116–124. https://doi.org/10.1177/155005940403500303
Matousek, M., & Petersén, I. (1973). Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalography and Clinical Neurophysiology, 35(6), 603–612. https://doi.org/10.1016/0013-4694(73)90213-7
Matousek, M., & Peterson, I. (1973b). Frequency analysis of EEG background activity by means of age-dependent EEG quotients. In P. Kellaway, I. Peterson, R. K., Otnes, and L. Enochson (Eds.), Automation of clinical electroencephalography (pp. 75-102). Raven Press.
McNamara, L. A., & Martin, S. W. (2018). Sensitivity, specificity, and predictive value. In S. S. Long, C. G. Prober, & M. Fischer (eds.), Principles and practice of pediatric infections diseases (5th ed.). Elsevier.
McVoy, M., Lytle, S., Fulchiero, E., Aebi, M. E., Adeleye, O., & Sajatovic, M. (2019). A systematic review of quantitative EEG as a possible biomarker in child psychiatric disorders. Psychiatry Research, 279, 331–344. https://doi.org/10.1016/j.psychres.2019.07.004
Michel, C. M., & Murray, M. M. (2012). Towards the utilization of EEG as a brain imaging tool. NeuroImage, 61(2), 371-385. https://doi.org/10.1016/j.neuroimage.2011.12.039
Michel, C. M., Murray, M. M., Lantz, G., Gonzalez, S., Spinelli, L., & de Peralta, R. G. (2004). EEG source imaging. Clinical Neurophysiology, 115(10), 2195-2222. https://doi.org/10.1016/j.clinph.2004.06.001
Nunnally, J. C. (1967). Psychometric theory. McGraw-Hill.
O'Connor P. J. (1990). Normative data: Their definition, interpretation, and importance for primary care physicians. Family Medicine, 22(4), 307–311. PMID: 2200734
Online Etymology Dictionary. (accessed January 17, 2023). https://www.etymonline.com/word/norm
Pascual-Marqui, R. D., Michel, C. M., & Lehmann, D. (1994). Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 18(1), 49–65. https://doi.org/10.1016/0167-8760(84)90014-x
Pascual-Marqui, R. D., Koukkou, M., Lehmann, D., & Kochi, K. (2001). Functional localization and functional connectivity with LORETA comparison to normal controls and first episode drug naïve schizophrenics. Journal of Neurotherapy, 4, 35-37. https://doi.org/10.1300/J184v04n04_06
Pfister, H., Kaynig, V., Botha, C. P., Bruckner, S., Dercksen, V., & Hege, H.-C. (2012). Visualization in connectomics. Mathematics and Visualization, 37. https://doi.org/10.1007/978-1-4471-6497-5_21
Prichep, L. S., Sutton, S., Hauer, J. F., & Kuperman, S. (1993). Quantitative EEG in the evaluation of stimulant effects in attention deficit disorder. Clinical Electroencephalography, 24(1), 8-18. https://doi.org/10.1177/155005949302400107
Salinsky, M. C., Oken, B. S., & Morehead, L. (1991). Test-retest reliability in EEG frequency analysis. Electroencephalography and Clinical neurophysiology, 79(5), 382–392. https://doi.org/10.1016/0013-4694(91)90203-g
Sanei, S., & Chambers, J. A. (2013). EEG signal processing. John Wiley & Sons. https://doi.org/10.1002/9780470688031
Schretlen, D. J., & Sullivan, C. (2013). Intraindividual variability in cognitive test performance. In S. Koffler, J. Morgan, I. S. Baron, & M. F. Greiffenstein (Eds.), Neuropsychology: Science and practice: Vol I (pp. 39-60). Oxford University Press.
Strimbu, K., & Tavel, J. A. (2010). What are biomarkers? Current Opinion in HIV and AIDS, 5(6), 463–466. https://doi.org/10.1097/COH.0b013e32833ed177
Tereshchenko, E. P., Ponomarev, V. A., Muller, A., & Kropotov, I.uD. (2010). Fiziologiia Cheloveka, 36(1), 5–17. PMID: 20196443
Thatcher, R. W. (1991). Maturation of the human frontal lobes: Physiological evidence for staging. Developmental Neuropsychology, 7(3), 397–419. https://doi.org/10.1080/87565649109540500
Thatcher R. W. (1992). Cyclic cortical reorganization during early childhood. Brain and Cognition, 20(1), 24–50. https://doi.org/10.1016/0278-2626(92)90060-y
Thatcher, R. W. (1994). Psychopathology of early frontal lobe damage: Dependence on cycles of development. Development and Psychopathology, 6(4), 565–596. https://doi.org/10.1017/S0954579400004697
Thatcher, R. W. (1998). EEG normative databases and EEG biofeedback. Journal of Neurotherapy, 2, 8-39. https://doi.org/10.1300/J184v02n04_02
Thatcher, R. W. (2010). Validity and reliability of quantitative electroencephalography. Journal of Neurotherapy, 14, 122-152. https://doi.org/10.1080/10874201003773500
Thatcher, R. W. (2018). NeuroGuide help manual. Applied Neuroscience.
Thatcher, R. W. (2021). Handbook of quantitative EEG and EEG biofeedback (3rd ed.). ANI Publishing.
Thatcher, R. W., Biver, C., McAlaster, R., Camacho, M., & Salazar, A. (1998). Biophysical linkage between MRI and EEG amplitude in closed head injury. NeuroImage, 7(4 Pt 1), 352–367. https://doi.org/10.1006/nimg.1998.0330
Thatcher, R. W., Biver, C. J., & North, D. N. (2007a). Accessed January 9, 2023 from https://www.appliedneuroscience.com/PDFs/Z_Score_Biofeedback.pdf Z-score biofeedback: Technical Foundations. Applied Neuroscience.
Thatcher, R. W., Biver, C. J., & North, D. (2007). Spatial-temporal current source correlations and cortical connectivity. Clinical EEG and Neuroscience, 38(1), 35–48. https://doi.org/10.1177/155005940703800109
Thatcher, R. W., Biver, C. J., Soler, E. P., Lubar, J., & Koberda, J. L. (2020). New advances in electrical neuroimaging, brain networks and neurofeedback protocols. Journal of Neurology and Neurobiology, 6, 1-14. dx.doi.org/10.16966/2379-7150.168
Thatcher, R. W., Krause, P. J., & Hrybyk, M. (1986). Cortico-cortical associations and EEG coherence: a two-compartmental model. Electroencephalography and Clinical Neurophysiology, 64(2), 123–143. https://doi.org/10.1016/0013-4694(86)90107-0
Thatcher, R. W., Krause, P. J., & Hrybyk, M. (1986). Cortico-cortical associations and EEG coherence: A two-compartmental model. Electroencephalography and Clinical Neurophysiology, 64(2), 123–143. https://doi.org/10.1016/0013-4694(86)90107-0
Thatcher, R. W., & Lubar, J. F. (2009). History of the scientific standards of QEEG normative databases. In T. H. Budzinski, H. K. Budzinski, J. R. Evans, (Eds.), Introduction to quantitative EEG and neurofeedback: Advanced theory and applications (2nd ed., pp. 29-59). Academic Press.
Thatcher, R. W., Lubar, J. R., & Koberda, J. L. (2019). Z-score EEG biofeedback: Past, present, and future. Applied Psychophysiology and Biofeedback, 47, 89-103. https://doi.org/10.5298/1081-5937-47.4.04
Thatcher, R. W., McAlaster, R., Lester, M. L., Horst, R. L., & Cantor, D. S. (1983). Hemispheric EEG asymmetries related to cognitive functioning in children. In A. Perecuman (Ed.), Cognitive processing in the right hemisphere (pp. 125-145). Academic Press.
Thatcher, R. W., North, D. N., & Biver, C. J. (2005a). EEG inverse solutions and parametric vs. non-parametric statistics of Low Resolution Electromagnetic Tomography (LORETA). Clinical EEG and Neuroscience, 36, 1-9.
Thatcher, R. W., North, D., & Biver, C. J. (2005b). Evaluation and validity of a LORETA normative EEG database. Clinical EEG and Neuroscience, 36(2), 116–122. https://doi.org/10.1177/155005940503600211
Thatcher, R. W., North, D. M., & Biver, C. J. (2008). Intelligence and EEG phase reset: A two compartmental model of phase shift and lock. NeuroImage, 42(4), 1639–1653. https://doi.org/10.1016/j.neuroimage.2008.06.009
Thatcher, R. W., Walker, R. A., Biver, C. J., North, D. N., & Curtin, R. (2003). Quantitative EEG normative databases: Validation and clinical correlation. Journal of Neurotherapy, 7, 87-121. https://doi.org/10.1300/J184v07n03_05
Thatcher, R. W., Walker, R. A., Gerson, I., & Geisler, F. H. (1989). EEG discriminant analyses of mild head trauma. Electroencephalography and Clinical Neurophysiology, 73(2), 94–106. https://doi.org/10.1016/0013-4694(89)90188-0
Thatcher, R. W., Walker, R. A., & Giudice, S. (1987). Human cerebral hemispheres develop at different rates and ages. Science, 236(4805), 1110–1113. https://doi.org/10.1126/science.3576224
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Timmerman, M. E., Voncken, L., & Albers, C. J. (2021). A tutorial on regression-based norming of psychological tests with GAMLSS. Psychological Methods, 26(3), 357–373. https://doi.org/10.1037/met0000348
UCLA Research Administration: Human Research Protection Program. (2021). Guidance and procedure: Recruitment and screening methods and materials. Author.
University of New South Wales. (2023, January 17). https://studyonline.unsw.edu.au/blog/types-of-data#:~:text=Psychologist%20Stanley%20Stevens%20developed%20the,to%20properly%20analyse%20the%20data
van Dijk, H., van Wingen, G., Denys, D., Olbrich, S., van Ruth, R., & Arns, M. (2022). The two decades brainclinics research archive for insights in neurophysiology (TDBRAIN) database. Scientific Data, 9(1), 333. https://doi.org/10.1038/s41597-022-01409-z
Zakai, N. A., Minnier, J., Safford, M. M., Koh, I., Irvin, M. R., Fazio, S., Cushman, M., Howard, V. J., & Pamir, N. (2022). Race-dependent association of high-density lipoprotein cholesterol levels with incident coronary artery disease. Journal of the American College of Cardiology, 80(22), 2104–2115. https://doi.org/10.1016/j.jacc.2022.09.027
C. The Functional Correlates of Abnormal EEG Changes
Abnormal EEG changes can indicate a variety of neurological and psychiatric conditions, with distinct functional correlates depending on the nature and location of these changes. This review explores the functional correlates of abnormal EEG changes, focusing on their clinical significance and the underlying neural mechanisms.
Please click on the podcast icon below to hear a lecture over Section C.

Introduction
EEG abnormalities can manifest as changes in wave patterns, frequency, amplitude, and coherence. These alterations are often associated with specific neurological and psychiatric conditions, reflecting underlying pathophysiological processes. Understanding the functional correlates of these abnormalities helps in diagnosing and managing disorders such as epilepsy, dementia, and mood disorders.Abnormal EEG Changes in Epilepsy
Epilepsy is one of the most well-studied conditions associated with abnormal EEG activity. The hallmark of epilepsy on EEG is the presence of epileptiform discharges, including spikes, sharp waves, and spike-and-wave complexes. These abnormalities are highly correlated with seizure activity and can provide critical information about seizure focus and type.Epileptiform discharges indicate hyperexcitable neuronal networks, which can lead to the synchronous firing characteristic of seizures. The location of these discharges can pinpoint the seizure onset zone, guiding surgical interventions and treatment plans (Niedermeyer & da Silva, 2004). For instance, temporal lobe epilepsy often shows interictal spikes in the temporal regions, correlating with impaired memory and emotional regulation due to the involvement of the hippocampus and amygdala (Engel, 2001).
Abnormal EEG Changes in Dementia
Dementia, particularly Alzheimer's disease, is associated with distinct EEG changes, such as a slowing of the dominant frequency and decreased coherence. These changes reflect widespread cortical dysfunction and neural network disintegration. In Alzheimer's disease, EEG typically shows an increase in delta and theta activity and a decrease in alpha and beta activity, particularly in the posterior regions of the brain (Jeong, 2004).These EEG changes correlate with cognitive decline, particularly in memory and executive function. The slowing of EEG rhythms is thought to result from the loss of cholinergic neurons in the basal forebrain, which play a crucial role in cortical arousal and cognitive processes (Babiloni et al., 2006). EEG slowing can also indicate the progression of the disease, providing a non-invasive biomarker for monitoring dementia (Jelic et al., 2000).
Abnormal EEG Changes in Mood Disorders
Mood disorders, including depression and bipolar disorder, are associated with specific EEG abnormalities. In major depressive disorder (MDD), EEG often reveals increased alpha activity in the frontal regions, which is thought to reflect altered cortical arousal and affective dysregulation (Olbrich & Arns, 2013). These changes are functionally correlated with symptoms such as anhedonia, impaired concentration, and changes in sleep patterns.In bipolar disorder, EEG may show increased beta activity during manic phases and increased theta activity during depressive phases. These oscillatory changes are thought to reflect the underlying neurophysiological alterations associated with the bipolar spectrum, including dysregulated neurotransmitter systems and neural connectivity (Bruder et al., 2005).
Abnormal EEG Changes in Anxiety Disorders
Anxiety disorders are also associated with characteristic EEG changes. Patients with generalized anxiety disorder (GAD) often exhibit increased beta activity, particularly in the frontal and central regions. This heightened beta activity correlates with excessive worry, hypervigilance, and autonomic arousal (Hammond, 2005).Similarly, in post-traumatic stress disorder (PTSD), increased beta and decreased alpha activity have been reported, reflecting heightened arousal and impaired relaxation. These EEG patterns are thought to result from dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and altered neural circuitry involved in fear and stress responses (Huang et al., 2014).
Summary
Abnormal EEG changes provide critical insights into the functional correlates of various neurological and psychiatric disorders. By identifying specific EEG patterns associated with conditions such as epilepsy, dementia, mood disorders, and anxiety disorders, clinicians can better understand the underlying neural mechanisms and develop targeted interventions. Continued research into the functional correlates of abnormal EEG changes will enhance our ability to diagnose, monitor, and treat these disorders effectively.Naming the EEG Components
Historically, the naming of the EEG frequencies followed a somewhat chaotic path (Schomer & Lopes da Silva, 2017), beginning with Berger’s (1929) designations of alpha, as the “first” rhythm and beta as everything faster than alpha.
Jasper and Andrews (1938) used the term gamma rhythm for frequencies above 30 or 35 Hz. The term gamma has become somewhat fluid in its definition, depending on the person defining it. Crick (1994) defined gamma as a frequency of 40 Hz (generally defined as 38-42 Hz or 36-44 Hz with a center frequency of 40 Hz), as did Sheer and colleagues (1992). Others, such as Lutz and colleagues (2004), used the frequency band 25-42 Hz to represent gamma, and Swingle also used the term to denote faster beta activity above 25 Hz. Amzica and Lopes da Silva (2017) also used the term gamma to define a frequency of 30-50 Hz. St. Louis and Frey (2016) stated that frequencies from 25-70 Hz are called low gamma, while those above 70 Hz represent high gamma. These sometimes conflicting definitions can lead to confusion as to the precise meaning of this term
Walter (1936) chose the term delta to represent all the frequencies below the alpha band and later added the term theta to identify the portion in the 4-7.5 Hz range. Currently, these terms remain fairly well defined, with delta representing 1-4 Hz and theta representing 4-7 or 4-8 Hz.
Following the naming of these five frequency bands, many other electroencephalographers have sought to name EEG patterns, sometimes choosing names for patterns that already have descriptive designations, such as the pattern known as positive occipital sharp transients of sleep with the acronym POSTS.
These are also being called rho waves and posterior slow rhythms of 3-4 Hz being called the pi rhythm. These two terms and several others have fallen out of favor. Schomer and Lopes da Silva (2017) suggest that the use of Greek words to identify EEG activity should be confined to the classic bands of alpha, beta, gamma, delta, and theta. As we will see in our alpha and beta frequencies discussions, the mu rhythm is one exception to this restriction.
A lack of precision in terminology makes numerical designations a better choice for defining EEG observations. This ensures that one’s meaning is clear when speaking of an EEG frequency band. Adding the location and the client’s state (eyes open, eyes closed, asleep, etc.) provides the necessary detail for communicating one’s findings to others. Therefore, describing eyes-closed 8-12 Hz activity, with a dominant frequency of 10 Hz and amplitude between 20-30 uV, in specific occipital and parietal sensor locations, is quite useful for assessment purposes instead of a general finding of “posterior alpha.”
Before proceeding further with the discussions of individual frequencies, it is important to note that brain activity in general and more specifically the scalp EEG patterns that reflect that brain activity are organized and regulated by multiple cortical, sub-cortical, and general network mechanisms. One of the most important mechanisms is cross-frequency synchronization (CFS), which describes waves of one type of frequency, such as beta or gamma, occurring synchronously with the wave patterns of slower frequencies such as delta, theta or alpha. These are often described as nested rhythms.
Siebenhuhner and colleagues (2021) propose that cross-frequency synchronization “integrates processing among synchronized neuronal networks from theta to gamma frequencies to link sensory and attentional functions.”
When considering the activity of individual EEG frequencies, it is important to recognize how they interact, how they are interdependent, and how they reflect the activity of the broader neuronal networks in the brain, central nervous system, and the organism as a whole. The EEG does not cause behavior. Instead, it reflects behaviors that have already happened or are currently occurring and are the result of a highly complex dance of interactions.
8-12 Hz EEG – the Alpha Rhythm
Due to alpha being the first EEG pattern to be identified historically, we will begin by defining the alpha rhythm. The International Federation of Societies for Electro-encephalography and Clinical Neurophysiology (IFSECN) (1974) offered the following definition:
Rhythm at 8 to 13 Hz occurring during wakefulness over the posterior regions of the head, generally with higher voltage over the occipital areas. Amplitude is variable but is mostly below 50 μV in adults. Best seen with eyes closed and under conditions of physical relaxation and relative mental inactivity. Blocked or attenuated by attention, especially visual and mental effort.
Such a rhythm must meet all the above criteria to qualify for the designation, eliminating patterns such as mu rhythm in the Rolandic areas, which often has the same frequency but different morphology (characteristic wave shape and pattern) and behavioral correlations.
Source of 8-12 Hz Alpha Activity
Activity in the 8-12 Hz frequency range appears associated with reduced sensory and cognitive activity. Why is this so? What mechanism is responsible for this rhythmic activity?
One of the main communication pathways between the external world, the senses that perceive and transmit this information, and the cortical neurons that receive it, is the thalamic-cortical relay system, often designated the TCR system.
The thalamus receives incoming sensory input (a paired structure in the brain's center – see the image below). The individual nuclei of the thalamus transmit that sensory information to appropriate areas of the cortex. The occipital and parietal areas of the cortex are the primary visual processing areas, just as the temporal areas process most of the auditory information. In contrast, central Rolandic areas process tactile and other signals from the skin and muscles. Of course, current findings indicate that brain activity is associated with coordination within and between cortical networks and influences from subcortical structures and local and TCR influences. Still, the TCR system is a primary pathway for determining which areas of the cortex receive each type of sensory input. Thalamic-cortical relay system graphic © Elsevier Inc. - Netterimages.com.
When the eyes close, the neurons responsible for processing incoming visual information no longer have “ work” to do, and so they respond to another signal coming through the TCR system. This is a rhythmic signal mediated by a membrane of inhibitory GABAergic neurons that surround most of the thalamus and provide inhibitory regulation of the signals traveling to the cortex. This is called the reticular nucleus of the thalamus (TRN) or nucleus reticularis of the thalamus (NRT). The function of this system is much too complex for this section, but a good treatment is available in Crabtree (2018), and an examination of the role of the TCR and TRN systems in consciousness is found in Min (2010).
The rhythmic signal from the TCR and NRT interaction produces a 10-Hz (8-12 Hz range) input to the visual processing neurons when visual sensory input is withdrawn (eyes closing), resulting in those neurons firing synchronously in that frequency in response to this input. The voltage of a specific EEG frequency, measured at the scalp surface, is directly proportional to the number of cortical neurons firing synchronously in that frequency (Nunez & Srinivasan, 2006). Therefore, when the eyes are closed, and the signal from the TCR system changes from sensory input to a rhythmic 10-Hz input, visual neurons respond to that input and the voltage of alpha, and most specifically in adults, 10-Hz activity increases in voltage. The image below shows a spectral display of an eyes-closed EEG. Graphic redrawn by minaanandag on Fiverr.com.
Note: eyes-closed EEG in longitudinal bipolar montage represented as a spectral display. The x-axis shows frequency from 0-30 Hz, and the y-axis shows absolute power (uV Sq). Note the peak at about 10 Hz. Voltage is higher in the P3-O1 derivation than in the P4-O2 derivation, revealing a small asymmetry. The highest power is in the T4-T6 derivation.
Identifying alpha activity in parietal and/or occipital areas of the scalp is usually quite easy, particularly in the eyes-closed condition. The image below shows an eyes-closed alpha pattern from a 15 -year-old male.
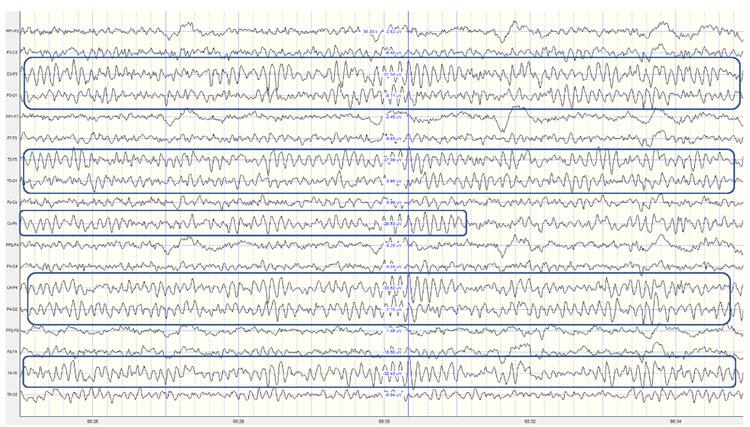
Note: eyes-closed EEG filtered to 1-45 Hz in the longitudinal bipolar montage with a 50-uV scale. Boxes indicate the most prominent 8-12 Hz activity. This montage represents a series of adjacent electrode comparisons or derivations, as each signal tracing is derived from each pair of electrode comparisons.
This longitudinal bipolar montage is displayed below.
Note: longitudinal bipolar montage (commonly known as the “double banana” montage).
Observe that the rhythmic activity is well-defined and has the typical bursting or spindling pattern of the alpha rhythm, resulting from the input of the TCR and NRT systems. Spindling consists of a series of distinct oscillations of a particular frequency that begin with relatively low amplitude, increase in amplitude, and then decrease in amplitude, giving the appearance of a spindle such as one used in spinning, with fiber wound around it. Graphic © New Africa/Shutterstock.com.

The waves are quite sinusoidal (waving up and down in a smooth rhythm similar to a sine curve) and continue throughout the recording with minimal disruption. The voltage indicator shows that the maximum voltage at the moment of the line placement was from about 20 to 30 uV at the peak of the waveform in this montage.
One can determine the wave's frequency by either counting the number of peaks in a one-second segment or counting the number of times the wave crossed the zero line and dividing by 2 (zero crossings/2). Either method gives a 8-9 Hz value when multiple one-second epochs are counted. This is somewhat slow for a 15-year-old, although the voltage appears to be within normal limits. When compared to a normative database, we can see that indeed, it is a slow peak alpha when compared to other 15-year-old males as indicated by the chart below from the NeuroGuide database.
Note: the alpha peak frequency z-scores are at least 1 SD below expected values (> -1.0 SD) in all locations and many areas show deviations exceeding the significance cutoff of -1.96 SD (blue highlight). The database also plots the theta peak frequency as fast (red highlight) due to slow alpha in the 8-Hz frequency bin or segment. There are likely other slow components of the dominant rhythm that contribute to this incorrect plot of the frequency information. Ideally, the peak frequency of the EEG should be calculated within a broad range from approximately 6 Hz to about 14-16 Hz to avoid this type of error. This is another problem with the somewhat arbitrary designation of frequency bands using set values.
The same data processed by the iSynchBrain database show similar findings for O1 and O2 below:
Note: peak frequency power and frequency comparisons at O1 and O2 showing slow peak frequency at 9.2 Hz bilaterally, resulting in z-scores of -1.15 and -1.23, respectively, with voltage (power) on the left at 0.63 SD and on the right at 1.01 SD, showing slightly elevated values. This database provides single-Hz calculations for both frequency and amplitude rather than using an arbitrary band to define alpha.
The alpha peak frequency is age-dependent, though not called ” alpha” until the frequency reaches 8 Hz. In early infancy, it is designated as the posterior basic rhythm or posterior dominant rhythm (PDR). It appears around the age of 4 months with a frequency of about 4 cycles per second (c/s) or Hz (Schomer & Lopes da Silva, 2017). The PDR increases (speeds up) during maturation and is approximately 6 c/s at 1 year and up to 8 c/s at around 3 years of age. This is when it can be called the alpha rhythm.
The frequency reaches 10 Hz by approximately 10 years of age, and that (10.2 ± 0.9/sec) is the peak frequency of adulthood (Petersén & Eeg-Olofsson, 1971). From the previous example, we see why a peak frequency between 8-9 Hz is slow for a 15-year-old.
Meaning and Importance of the Alpha Peak Frequency
The speed or frequency of the alpha peak frequency is often mentioned, even in a neurologist’s report. For the neurofeedback practitioner, it is helpful to understand the factors associated with different alpha frequencies. The alpha peak frequency is a measure of the frequency of the rhythmic pattern of the posterior rhythm (generally called alpha). This has traditionally been an important measure. Although it has recently been somewhat de-emphasized in some EEG circles, it remains an interesting measure. It has a great deal of research supporting it as a useful metric for assessment.
A slow peak alpha frequency has been associated with some forms of cognitive decline and memory impairment (Stam, 2017) as well as mild traumatic brain injury (mTBI; Jabbari et al., 1985; Williams, 1941). A fast peak alpha frequency has been associated with improved scores on timed IQ tests. It has also been associated with enhanced memory and cognitive performance in various age groups (Grandy et al., 2013). A faster peak alpha frequency is associated with advanced reading skills in precocious children (Suldo et al., 2001).
The peak alpha frequency changes through the lifespan. Therefore, age-normed values for the peak alpha frequency are important for assessment purposes. The normal adult peak alpha frequency is 9.5-10.5 Hz. Jabbari et al., 1985 Scholarly literature states that the peak alpha frequency is not abnormal until it is below 8 Hz (Schomer & Lopes da Silva, 2017). However, it is commonly thought to be potentially meaningful when the frequency is below 9 Hz for an adult.
Stam (2017) stated that slowing the alpha peak frequency by more than 1 Hz (9 Hz for an adult) is generally a sign of pathology. A slow alpha frequency can be associated with fatigue, cognitive decline, and memory impairment. Slowing of the background alpha rhythm is also a sign of generalized cerebral dysfunction (Nayak & Anilkumar, 2021). Rathee and colleagues (2020) related the speed of the peak alpha frequency to reading comprehension. They found that a slower peak alpha frequency is associated with poor comprehension.
Negative correlations of a peak alpha frequency faster than 10.5 Hz, possibly associated with an overly activated central nervous system, may include sleep initiation problems, anxiety, intrusive thoughts, and difficulty with self-soothing and self-calming skills.
Meaning and Importance of the Eyes-Closed Alpha Response Voltage
In addition to the peak frequency within the 8-12 or 8-13 Hz alpha band, the amplitude of the activity can also be meaningful. The alpha voltage will be partially affected by the montage that is used. For example, keeping in mind the discussion of differential amplifiers in the Instrumentation and Electronics section, the closer two electrodes are to each other, the more the rhythmic, synchronous patterns will be attenuated. Common-mode rejection (CMR) is most sensitive to frequency synchronization, so waves that are the same frequency and also synchronous (e.g., 10 Hz waves, waving up and down at the same time at the two sensor locations + and – [also commonly called “active” and “ reference”]) will be rejected. Comparing the O1 and O2 occipital electrodes to each other will result in a lower apparent voltage of alpha if the two waveforms are synchronous, which is quite likely.
Conversely, comparing either O1 or O2 to an ear reference or possibly to a forehead reference would result in almost no rejection of alpha activity. Therefore, the rhythmic patterns are unlikely to be similar at these distant locations and will be retained. When viewing standard voltage information in an atlas or a research paper or textbook, try to identify the montage used when those standards were developed.
Simonova et al. (1967) found amplitudes between 20 and 60 μV in 66% of their subjects, while values below 20 μV were found in 28% and above 60 μV in only 6%. Schomer and Lopes da Silva (2017) suggest that values between 10 and 60 μV are typical. However, other sources such as the John Hopkins Atlas of EEG (2011) and Libenson’s Practical Approach to Electroencephalography (2009) cite 20 μV as the minimum voltage for adults. These differences may seem insignificant but can represent the difference between a low voltage fast EEG finding and a typical assessment.
Rhythmic alpha activity represents the synchronization of the EEG. It represents part of the excitation/inhibition cycle. When either large or small groups of neurons perform tasks, this results in the desynchronization of the EEG during work, as each group of neurons performs its function somewhat locally and somewhat independently. This is followed by a resting or inhibitory phase that results in the synchronization of the EEG and hence an increase in alpha amplitude as many neurons fire synchronously. This is clearly seen in the shift from active visual processing when the eyes are open to a synchronous pattern of oscillatory activity when neurons do not have incoming visual input to process and can rest. This measure of alpha voltage change from eyes open to eyes closed, known as the alpha response, and the decrease of alpha with eyes opening, called alpha blocking, helps identify if the work/rest cycle is occurring correctly.
Someone with an eyes-closed posterior dominant rhythm voltage below 20 μV suggests to the neurofeedback assessor that the person does not easily shift to a state of decreased arousal/alertness necessary for alpha amplitude to increase. The disconnection from the outside world upon eyes closing should result in decreased sensory processing of vision and other senses and decreased cognitive activity, leading to an increase in 8-12 Hz amplitude or power.
Typically, alpha activity voltage should increase as more neurons fire synchronously in this frequency. When this increase is less than 50% above the resting baseline eyes-open alpha voltage, it usually indicates some difficulty turning off the mind, meaning that neurons remain activated and working and thus prevented from entering a resting state. The lack of a typical alpha increase may be associated with heightened states of alertness and vigilance, meaning that these clients maintain their external perceptive focus and/or cognitive activity, even when the eyes are closed, likely meaning that the ability to achieve global synchronous activity is being inhibited. Resulting behavioral consequences can include fatigue, as the neurons are constantly engaged and are not allowed to rest. This pattern may be associated with a history of trauma and/or a history of hypervigilance for various reasons.
Example of a typical alpha response upon eyes closing:
Note: this is a 19-channel recording of the transition from eye-open to eyes-closed conditions. This is a longitudinal bipolar montage, and the scale is 50 μV. Note the eyes closing at minute 2:40 (indicated by the eye movement) and the immediate response of the alpha rhythm appearing.
Meaning and Importance of the Alpha-Blocking Response
Conversely, the continued presence of alpha once the eyes are open suggests a lack of appropriate alpha blocking. This appears to result from a lack of inhibition of synchronous generator mechanisms. Hartoyo and colleagues (2020) showed a simple mechanism: excitatory input to inhibitory cortical neurons. This differs from the excitatory cortical neurons that typically reduce synchronous cortical firing in favor of local responses to incoming stimuli when the eyes are opened.
Note that activation of inhibitory mechanisms results in increased inhibition, even though the function is initially excitatory. Conversely, activation of excitatory mechanisms results in greater activation. This can seem confusing, and it may help to focus on the result, whether excitatory or inhibitory, rather than the initial behavior.
There should be a dynamic balance between excitation and inhibition in the human neocortex (Dehghani et al., 2016). When this balance is disrupted, we see the behavioral effects noted here. Alpha blocking represents the re-activation of visual processing neurons when visual input returns. Typically, as these neurons are no longer in a common or general resting state but are involved in task-oriented behaviors that are more localized, synchronous activity should decrease (be inhibited). Therefore, the overall voltage will decrease because of less synchronization. This does not imply that more neurons are firing when alpha amplitude is higher. Greater synchronization results in higher amplitude while desynchronization results in lower amplitude.
Imagine an auditorium full of people initially clapping synchronously (eyes-closed alpha rhythm). The noise is loud because everyone is clapping at the same moment (higher amplitude) and completely quiet in between claps, and the frequency of the claps reflects that synchrony. Then, imagine everyone clapping independently, possibly in synchrony with immediate neighbors but not with the audience as a whole. This is like the eyes-open condition and, though there will always be some noise, because someone is always clapping, resulting in a faster frequency of clapping. At no one moment will it be as loud as when the entire audience clapped synchronously. So, the overall voltage is lower even though the frequency of clapping is faster. Thus, alpha amplitude decreases when the eyes are opened, and this should occur quickly, in 1-2 seconds and certainly in less than 10-15 seconds. Any delay in alpha blocking suggests difficulty returning to the task. Desynchronization of the EEG occurs in posterior areas as visual processing begins when the eyes are opened.
The most common reasons for the lack of appropriate alpha blocking (meaning alpha activity persists after eyes are opened) are:
1. Fatigue, including sleep deprivation
2. Long-term meditation practice, particularly mantra meditation
3. Marijuana use and abuse, generally long-term, chronic
4. Cerebral dysfunction due to disease, injury, or possibly chemical exposure
Below is an example of correct alpha blocking following the eyes opening:
Note: this is a 19-channel recording of the transition from eyes-closed to eyes-open conditions. This is a longitudinal bipolar montage, and the scale is 50 μV. Note the eyes opening at minute 1:12 (indicated by the eye movement) and the immediate blocking of the alpha rhythm.
Clearly, the response of 8-12 Hz EEG activity can be quite revealing and provides the clinician with helpful information about the client. However, it is important to note that other factors can affect the EEG recording. We have already noted the effects of artifacts on the EEG generally. Additionally, the client’s state of mind, level of anxiety, comfort with the application of sensors to the scalp, level of trust of the practitioner conducting the recording, amount of sleep, the use of caffeine and other stimulants and common medications can all affect the results of the recording.
Once an assessment is made of excess or deficient alpha activity, lack of an alpha response or persistent alpha following the eyes opening, or a slow or fast peak alpha frequency, the clinician can proceed with training to address these findings. There are multiple approaches to training the 8-12 Hz frequency band, including training specific segments of that band to achieve training goals. For example, if a lack of an alpha response to eyes closing is associated with anxiety and possibly insomnia, training for an increase in the 8-10 Hz portion of the posterior alpha rhythm in the eyes-closed condition may be an effective intervention. If the peak alpha frequency is slow, training for increases in the 10-12 Hz portion may help speed up this frequency. If there is persistent alpha in the eyes-open condition, inhibiting or downtraining 8-12 Hz generally may be helpful.
Of course, with any intervention, other causal factors must be addressed as well. Persistent alpha and/or frontal alpha can signify fatigue secondary to a sleep disorder such as sleep apnea. Therefore, a referral to a physician for a sleep study may be helpful. Over-arousal patterns that correspond to a lack of alpha response can be associated with a history of emotional, psychological, physical, or sexual trauma. These issues may need to be addressed by the neurofeedback clinician or by referring the client to an appropriate therapist.
13 or 14 to 25 or 30 Hz EEG – the Beta Rhythm
The following description is from Kane et al. (2017):
Beta band: Frequency band of 14–30 Hz inclusive. Greek letter: β.
Beta rhythm or activity: Any EEG rhythm between 14 and 30 Hz (wave duration 33–72 ms). Most characteristically recorded over the fronto-central regions of the head during wakefulness. The amplitude of the fronto-central beta rhythm varies but is mostly below 30 µV. Blocking or attenuation of the beta rhythm by contralateral movement or tactile stimulation is especially obvious in electrocorticograms. Other beta rhythms are most prominent in other locations or are diffuse and may be drug-induced (for example, alcohol, barbiturates, benzodiazepines, and intravenous anesthetic agents).
Beta activity appears to result from multiple factors associated with active patterns organized by slower rhythms. Bursts of beta occur in frontal-central, frontal-temporal, frontal-parietal, and central-parietal areas and are associated with different tasks based upon the structures underlying these areas. Because all functions are distributed to multiple sites, it is difficult to precisely define which part of the CNS is responsible for each function. The observations in this section are based on both animal and human studies, the latter primarily in lesion studies and during neurosurgery.
Beta is also associated with the negative shift of the DC gradient (Speckmann et al., 2017). The DC gradient, only measurable by a DC-coupled EEG amplifier, measures the overall electrical gradient of cortical areas under the recording sensors. This gradient shows a slow oscillation of less than 1 second and is usually in the 0.1-0.2 Hz range. A shift of the gradient from its current state, becoming more electrically positive or negative, occurs every 5-10 seconds and sometimes quite a bit less often.
Buzsaki (2011) states that there is a progression of frequencies whose bandwidths overlap and interact with each other, from frequencies that take 15-40 seconds to complete each cycle up to those that oscillate at 200-600 cycles per second. Gunkelman (2005) called beta and gamma activity “emergent properties of bound networks.” This means that, as slower frequencies of EEG synchronize across networks, beta and gamma emerge in bursts of activity in coordination with that synchrony.
Beta activity is associated with "work" and reflects the ongoing excitatory/inhibitory cycles occurring on multiple time scales. While the work/rest cycle mentioned in relation to the alpha response and alpha blocking is one type of excitatory/inhibitory cycle on a broader scale, beta activity reflects actions that occur millisecond by millisecond and appear to be more locally generated.
Beta frequencies have a relatively low voltage compared to slower frequencies. As noted in the above definition, amplitudes are generally less than 30 μV and frequently less than 20 μV. Remember our example of the audience clapping. In the example below, “clapping” occurs more often but with less power due to less synchronous activity.
Below is an example of eyes-open beta activity:
Note: this is a 19-channel EEG recording in the eyes-open condition. This is a longitudinal bipolar montage, and the scale is 50 μV. Observe the alpha activity continuing at a much lower voltage in parietal and occipital derivations, compared to the previous eyes-closed examples, resulting from the attenuation of alpha with the opening of the eyes. There is beta activity in the 20-30 Hz range at less than 10 μV, seen mostly in frontal, central, parietal, and temporal derivations (comparisons between adjacent sensors) and somewhat slower 15-20 Hz patterns.
Kropotov (2016) describes the existence of several beta rhythms with different frequencies, various locations, and distinct functions. From this information, he states that there is likely no single neuronal mechanism for generating localized beta activity. This fits with the understanding that beta activity is associated with local tasks and therefore is mediated more by local mechanisms, all with overall coordination from network systems and other rhythmic activity.
When beta activity is typical for the client based on age, state (eyes-open, eyes-closed, under task, etc.), and location, it suggests that those areas are functioning as expected. If beta activity is deficient, that may mean that the site is under-functioning or under-activated for some reason. Reasons may include damage of some sort, metabolic deficits, fatigue, or other factors. An area that is consistently over-functioning may, in time because of overuse and fatigue, end up with a lower level of functioning and hence less beta activity.
Higher-than-typical beta amplitude at a given location may mean that the area is over-functioning or overly activated. Whether the beta amplitude is higher or lower than average, the clinician will want to understand the underlying functional neuroanatomy of the area to aid in the assessment process. For example, suppose the right posterior temporal/parietal junction that roughly underlies the area between T4 and T6 (TP8 in the 10-10 system) shows excess beta amplitude. In that case, it may be associated with heightened sensitivity to or attention to non-verbal communication such as tone of voice, facial expression, and body language associated with angry outbursts or that may signal danger. If this area is under-activated, it may represent a self-protective “disconnection” from these same signals (Gunkelman, 2021).
Individuals who typically show excess beta activity at sleep onset and during sleep stages have a higher incidence of insomnia (Perlis et al., 2001). Meier and colleagues (2014) found a correlation between excess beta power in frontal, central, and temporal areas with delinquent behavior in adult men with concurrent ADHD symptomatology. In Rowan’s Primer of EEG (2nd ed.), Marcuse and colleagues (2016) identify excess interhemispheric beta asymmetry as an important diagnostic tool. The side with reduced relative beta power points to the pathological hemisphere. They identify brain abscesses, stroke, tumor, vascular malformations, and cortical dysplasia as associated with a focal decrease or enhancement of beta activity.
A reference to a normative database can be useful when assessing beta activity, particularly if a traumatic brain injury is suspected. Comparison with other EEG frequencies is also important, as an excess or lack of beta activity is often accompanied by differences from expected values for other frequencies.
Rhythms with Similar Frequencies But Different Characteristics – Not Beta
There are a few special cases where activity in the supposed beta frequency range may represent other EEG patterns. One important example is a rhythmic pattern seen over the sensory-motor cortex, generally at C3 and C4, known as the mu rhythm. This rhythm has a frequency of between 10-15 Hz and, therefore, may be mistaken for alpha or beta activity. Kane and colleagues (2017) describe the mu rhythm as follows:
Mu rhythm: Rhythm at 7–11 Hz, composed of arch-shaped waves occurring over the central or centro-parietal regions of the scalp during wakefulness. Amplitude varies but is mostly below 50 mV. Blocked or attenuated most clearly by contralateral movement, the thought of movement, readiness to move or tactile stimulation.
Greek letter: μ. Synonyms: rhythm rolandique en arceau, comb rhythm (use of terms discouraged).
The mu rhythm can occur with a frequency over 13 Hz and can have spectral peaks in alpha (8-13 Hz) and beta (14-25 Hz) frequency bands (Jenson, 2020) and hence appear in either the alpha or beta frequency bands in quantitative EEG analysis. Mu can also show an additional characteristic, known as a harmonic effect in the EEG recording (Cheyne, 2013; Jones et al., 2009). It usually manifests as a peak in a spectrograph at twice the frequency of the mu rhythm. So, a 10-Hz mu rhythm would often have a 20-Hz pattern associated with it, whereas a 13-Hz mu rhythm may show a 26-Hz harmonic.
Magnetoencephalography (MEG) studies by Jones and colleagues (2009) show that what they call mu-alpha (mu rhythm in the alpha frequency range) and mu-beta (mu rhythm in the beta frequency range) co-occur at a rate greater than chance, suggesting similar mechanisms associated with their presence in the recording. They also show that this activity is not an artifact of the recording process but that it is more difficult to differentiate the faster beta component when using EEG instead of MEG. MEG studies have demonstrated that beta frequency activity originates in precentral locations and alpha frequency activity in post-central locations (Hari et al., 1997).
Below are two examples of mu rhythm activity as a tracing with a 19 channel recording and as a topographic representation of the same data.
Note: this example shows a mu rhythm with the typical negative-going wave (up in this example) appearing somewhat rounded and the sharp positive-going wave (down) at C3 and C4. This is a Laplacian montage with a scale of 300 uA (microamperes). Counting the frequency yields a mu rhythm of 10-11 Hz.
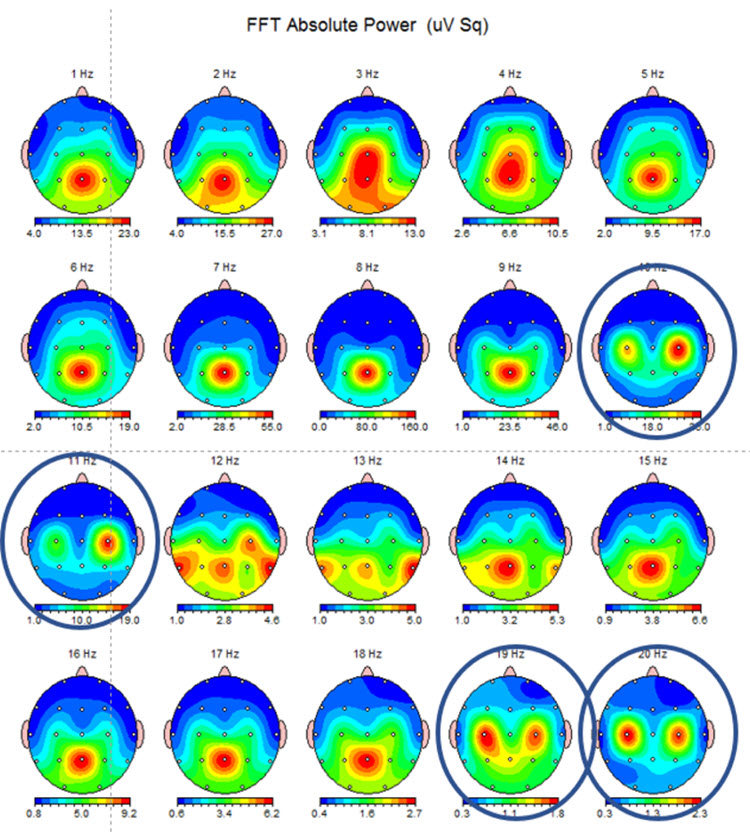
Note: this example shows the same EEG data in a topographic display showing the 10-11 Hz mu activity at C3 and C4 and the double harmonic at 19-20 Hz in the same locations. This is often referred to as the “owl eye” display because the presence of mu rhythm at C3 and C4 produces a display that looks like the face of an owl.
The appearance of the mu rhythm over the sensory-motor cortex has been associated with pathology (Gastaut & Bert, 1954; Gastaut et al., 1959), such as psychosomatic symptoms in individuals identified as neurotic. Chatrian and colleagues (1959) demonstrated that suppression of mu activity is associated with one’s movements or when observing someone else moving. In the early 1950s, mu was thought to be quite rare, and various studies identified the prevalence of between 2.9-14% (Schnell & Klass, 1966). In an early version of the textbook Electroencephalography by Niedermayer and Lopes da Silva, the incidence of mu was estimated at approximately 18%. More recent studies using computerized spectral analysis and coherence calculations (Kuhlman, 1978; Schoppenhorst et al., 1980) show the presence of mu in 60% of their study participants.
Mu activity has been correlated with motor cortex functions, as noted above. It appears to be similar to the alpha activity described in posterior areas in response to eyes-open and eyes-closed conditions. Similarly, mu activity seems to be present when the motor cortex is idle and blocked or attenuated when the motor cortex becomes active. Interestingly, mu is blocked with the physical movement of the person being recorded, when that person observes movement, and when the person even visualizes movement. However, unlike alpha, mu activity does not block with eye-opening.
Mu activity has been associated with what has been called the mirror neuron system (MNS), which is a network of locations that are associated with what might be termed learning by observation, mimicry, or imitation. Bernier and colleagues (2007) identify mu as reflecting an underlying execution/observation matching system and studied the relationship of abnormalities in response patterns of the mu rhythm in individuals with autism spectrum disorder (ASD). They show decreased attenuation of mu rhythm when adult individuals with ASD observed physical movement in others compared to age- and IQ-matched typical adults. Montirosso and colleagues (2019) show different patterns of mu desynchronization between pre-term and full-term infants at 14 months of age during an action observation/execution task, with full-term infants showing more broadly distributed areas of mu rhythm desynchronization (blocking of mu due to cortical excitation) compared to the pre-term infants.
Okada and colleagues (1992) identified patients with mu rhythm activity that increased with drowsiness, photic stimulation, and hyperventilation as being more likely to experience intractable epilepsy or to suffer from organic brain disorders compared to another group with more well-controlled epilepsy and psychiatric disorders where the mu rhythm showed more typical behavior, i.e., not blocking with eye-opening but blocking appropriately with spontaneous movement or with sensorimotor stimulation.
Finally, Siyang and colleagues (2016) have correlated mu with blood oxygen level-dependent (BOLD) signals. They have shown that mu activity's higher power (amplitude) is negatively correlated with the BOLD signal over the sensorimotor network, the attention control network, and the mirror neuron system. This means there is a decrease in blood oxygen utilization when the mu rhythm is higher in amplitude, confirming its nature as a resting state indicator for these systems and networks. Additionally, higher mu amplitude was positively correlated with the BOLD signal in areas of the salience network such as the anterior cingulate and anterior insula. So, it appears that some systems are at rest when mu is active, and some are more active when this is true. This, again, speaks to the complexity of EEG activity and the global, system-wide relationships associated with all EEG frequencies.
Thus, mu rhythm has become an area of increased study and interest, and training mu activity over the sensory-motor cortex has become a fairly common neurofeedback intervention. For example, a psychiatric clinic in Nashville, TN has seen improvement in multiple symptoms when Rolandic mu is trained using z-score neurofeedback (Tim Caldwell, 2020).
The visual identification of mu rhythm activity can be helpful, and task-oriented assessments that show the response to movement or observations of movement should be included in the EEG assessment of potential clients.
There is a similar pattern of activity in the sensory-motor cortex in the 12-15 Hz range that some identify as mu rhythm but which is also labeled as the sensory-motor rhythm (SMR). This is a pattern noted by Sterman (1967) and reported in multiple publications regarding his work with cats (Sterman & Enger, 2006) and subsequently in his work with human participants (Sterman, 1996). Sterman initially trained cats to increase this EEG frequency activity and found that it appeared to prevent seizure activity in the presence of toxic substances. He then taught human participants with intractable epilepsy to produce increased amplitude of SMR with excellent results, and this work was replicated in multiple publications. An excellent review of the history and development of this area of EEG research and training is available in Enger and Sterman (2006).
Neurofeedback training of SMR eventually progressed to working with ADHD clients (Lubar & Shouse, 1976) and others. This pattern is associated with decreased motor excitability and is similar to the sleep spindles seen in stage 2 sleep. Clients learning to increase the voltage of this 12-15 Hz pattern show a marked decrease in hyperactive behaviors. Training in this frequency band is commonly used for clients with ADHD hyperactive or combined subtypes. It is included within the broad, generic beta frequency band. Still, it shows specific characteristics that suggest it doesn’t have the same behavioral or local generation characteristics that more typical beta activity seems to have.
Below is an example of SMR activity in an eyes-open recording.
Note: this is an image of a filtered EEG recording – isolating only the 12-15 Hz frequency band. The montage is longitudinal bipolar, and the scale is 10 uV due to the low voltage of this signal. Observe that activity in this band is present in all electrode locations but does show more of a persistent bursting or spindling pattern in derivations involving central electrodes.
The 12-15 Hz (SMR as defined by Sterman) EEG over the sensory-motor cortex was one of the first EEG patterns to become the focus of neurofeedback training. As noted, many published studies demonstrated the efficacy of training this frequency band in central electrodes for conditions as diverse as seizure disorders and attention deficit disorder. Training this frequency continues to be a commonly used intervention in the neurofeedback field. The controversy regarding whether SMR activity is the same as Rolandic mu or a distinct pattern with unique characteristics is difficult to resolve. When viewing SMR activity in individuals with prominent mu rhythm, the bursting patterns do not appear synchronous, suggesting a separate mechanism of action. Still, these are simply the author's observations and have not been verified by rigorous studies.
The presence of more precisely identified frequency patterns in the EEG with more clearly defined behavioral correlates (e.g., the beta frequency band) is a good reason to be wary of broadly defined EEG frequency bands with a lack of specificity by location or behavior. We will also see this occur in the other frequencies that are covered in this discussion.
The assessment of beta activity includes location specificity and differences between locations, so training choices also target these findings. When training is focused on correcting atypical results, attention must be paid to compensatory behaviors reflected in the EEG. This will be discussed in more detail toward the end of this section.
0.5-3.5 Hz or 1-4 Hz or 0.1-4 Hz – The Delta Frequency Band
Continuing with our somewhat historically-defined EEG frequency discussion, we move on to the delta frequency band of 1-4 Hz or sometimes defined as 0.5-3.5 Hz and even as 0.1-4 Hz. Kane (2017) describes delta as follows:
A frequency band of 0.1–<4 Hz. Greek letter: δ. Comment: for practical purposes, the lower frequency limit is 0.5 Hz, as DC potential differences are not monitored in conventional EEGs (due to the use of AC amplifiers rather than DC-coupled amplifiers for most EEG recordings – author’s note).
This somewhat limited description is partially due to the difficulty of defining cellular activities associated with the EEG from 0.1-4 Hz. Amzica and Lopes da Silva (2017) discuss the delta frequency band in some depth and conclude that the activity in this frequency range represents more than one phenomenon and that frequency-band definitions do not reflect the underlying mechanisms of the various sub-components of this activity.
Amzica and Lopes da Silva state that activities associated with delta frequencies reflect two different EEG phenomena, waves and oscillations. They describe oscillation as a repeated variation of a parameter such as current or voltage between two values with the possible additional characteristic of a regular pattern to that variation. They define a wave as a single variation of a parameter between two extreme values and state that oscillations appear to be made up of waves. Current thinking identifies at least two cellular sources of delta, the thalamus and the cortex.
Thalamic delta oscillations result from two inward currents of thalamocortical cells (Soltesz et al., 1991). These currents appear to be associated with low-frequency membrane potential oscillations in thalamocortical cells that continue even in vitro after removal from the organism. This was identified in studies in rat and cat subjects (Leresche et al., 1991) and verified in human studies during in vivo monitoring (Crunelli et al., 2018). There are intrinsic mechanisms within these cells that result in regular electrical discharges. Crunelli and colleagues show that these oscillations have a rhythm-regulation function as expected and a plasticity function that can shape ongoing oscillations during inattention and NREM sleep, reconfiguring thalamic-cortical networks to facilitate information processing during attentive wakefulness.
Cortical delta oscillations continue even when those neurons are disconnected from thalamic input. Again, this suggests an intrinsic mechanism within cortical neurons that allow these very slow rhythms to occur even without communication from other sources. Grey Walter (1936) identified delta activity in the scalp EEG overlying areas of cerebral tumors. More recently, localized delta activity has been associated with areas of traumatic brain injury (Buchanan et al., 2021). This is one area where quantitative analysis of the EEG can be extremely helpful when evaluating individuals post-stroke or post-mTBI. Areas disconnected from local and/or global networks generally show increased delta rhythm activity and do not function as expected. Neurofeedback training can often help resolve these disconnections or facilitate a reorientation of function to a different set of neurons.
Activity in the delta frequency range appears to represent multiple brain functions. As noted earlier in the discussion of cross-frequency synchronization, delta appears to play a significant role as an underlying pacemaker for organizing and coordinating everything from local activation that results in beta frequencies to global network functions that integrate local information processing results from multiple sensory areas, to interpretive, problem-solving, and decision-making activities, to command-and-control outcomes.
These broadly distributed functions that must be coordinated in both time and space require an underlying physiological mechanism to facilitate such coordination. Crick (1994) suggested the 40 Hz (gamma) rhythm as the binding mechanism, and others have also recommended this. Gunkelman (2005) suggested the glial system as the physiological mechanism and the slow cortical gradient and delta frequencies as the EEG components that reflect the activity of this system, with gamma as an emergent property that only appears when the slow cortical gradient/delta system is bound or synchronized.
This latter concept is supported by more recent findings regarding glial cells. Recent neurophysiological understanding of brain function has been focused on chemical synapses, through which neurons communicate, and other systems and subcortical mechanisms influence cortical activity. This sub-cortical influence is seen in the ascending pathways from brainstem areas that project to the thalamus and cortex. Two examples, shown below, are serotonergic and dopaminergic pathways.
Serotonergic pathways
Dopaminergic pathways
The glia are linked in a gap junction-based network, with glial cells connecting through direct electrical coupling via proteins known as connexons that form gap junctions. Alvarez-Maubecin and colleagues (2000) have demonstrated the same type of connection between glia and neurons. Gap junctions are capable of nearly instantaneous communication instead of the relatively slow communication in the neurochemical synaptic transmission system. Thus, large and broadly-distributed networks of gap junction-linked glia can communicate and organize neuronal activity by generating slow oscillations mediated by calcium and potassium concentrations and by glia-to-neuron gap junction communication. This is in marked contrast to the notion that the slow oscillation results from neuronal activation and reflects the effect of postsynaptic potentials on the local field potential. Amzica and Lopes da Silva (2017) clearly show the neuronal activation that follows glial influences. The synchronization between the two reflects this global coordination mechanism.
Delta activity appears to be a component of this slow oscillation, particularly at the slower delta frequencies below 2 Hz. The intrinsic cellular oscillations noted earlier occur at these less-than-2-Hz frequencies and likely should be viewed independently from the faster components of the delta band. These oscillations likely result from the glial involvement discussed above, which appears to continue even when neurons are not receiving sensory input. Again, we see that more precise and specific discrimination of EEG frequency activity and the physiological and behavioral correlations associated with those patterns can be helpful when analyzing EEG recordings. This is also true of subsequent training approaches.
The slow cortical gradient or slow cortical potential (SCP) has been studied concerning the incidence of cortical activation. There is a clear correlation between greater cortical negativity (an electrically negative shift) and increased neuronal firing. When the cortex shifts to a more electro-negative state, this lowers the firing threshold for cortical neurons and increases cortical excitation. The opposite is true, and cortical positivity is associated with reduced neuronal firing.
Kotchoubey and colleagues (2002), in collaboration with Neils Birbaumer of the University of Tubingen in Germany, showed reduced seizure frequency in participants with refractory epilepsy when trained to create an electro-positive SCP shift, with the goal of reducing cortical excitability and hence reducing the frequency and intensity of seizure activity. In a 10-year follow-up of study participants (Strehl et al., 2014), experimental group participants continued to show reduced seizure frequency compared to the initial pre-study baseline. Those participants were also able to demonstrate the same control of the SCP gradient in three follow-up training sessions. This sustained improvement occurred without any intervening “booster” training sessions. Other studies of the usefulness of SCP gradient training for migraines and ADHD have also been conducted.
Training the cortical gradient has also been a component of the various ultra-slow and infra-slow neurofeedback training approaches discussed in the Selecting Training Protocols section. Though they don’t generally reference the cortical gradient directly in discussions of their approaches, the mechanism of action is likely associated with the glial system communication and organization properties.
Clients identified with excess localized delta activity and traumatic brain injury, stroke, or other focal abnormalities have improved from neurofeedback training as noted earlier. Local training to inhibit or downtrain the delta activity at the site or sites of abnormally high voltage is one approach. Still, more recently, a global approach to neurofeedback training via sLORETA and swLORETA neurofeedback seeks to correct network-wide dysregulation, including errors of connectivity which, as mentioned earlier, may underly the local finding of excess delta activity. This highlights the need for a comprehensive assessment, including an EEG assessment that shows connectivity metrics that can help recognize global disruptions that may need to be addressed.
Regarding the delta frequency band assessment, some artifacts mimic both delta and slow cortical gradient patterns. These include lateral eye movements that may represent normal nystagmus (slow drift or slow phasic shift of the eyes laterally when the eyes are open) or similar shifts when the eyes are closed. Saccades or quick side-to-side eye movements are faster and less likely to be mistaken for delta waves during visual inspection or artifact removal.
Another artifact that influences the slower component of the delta band, as well as the slow cortical gradient, is skin electrodermal activity, variously called the skin conductance response (SCR), electrodermal response (EDR) or galvanic skin response (GSR). This skin response activity shows a fairly slow oscillation at rest, reflecting tonic sympathetic nervous system (SNS) activity, and also shows more phasic responses to stress or challenge. The slow, tonic change in the skin's conductive properties changes the electrical conductivity under the EEG recording electrode on the scalp. It can cause an apparent (although false) slow oscillation in the cortical gradient measure and the delta frequency band recording under certain conditions.
Another issue, particularly when recording the SCP, ultra-low or infra-slow frequency ranges, is the presence of electrode drift, which is a function of metals used in EEG electrodes that can lead to unstable signals due to erratic ion exchange. This is most commonly seen in tin electrodes, which are unfortunately quite frequently used, particularly in EEG electrode caps. They are solid electrodes rather than coated with a conductive substance that can wear off. Other materials such as solid silver and gold-plated silver have varying degrees of drift as the electrode/conductive gel/skin surface interfaces come into equilibrium. The best electrode material to reduce electrode artifact is the sintered silver/ silver chloride (Ag/AgCl) electrode, a mixture of silver and chloride compressed into a solid pellet. The second best is an Ag/AgCl coated electrode. However, the coating can wear over time. For a full analysis of electrode materials and drift, see the excellent study by Tallgren and colleagues (2004).
Finally, older EEG amplifiers, particularly those using long EEG leads from the scalp to the amplifier, show a phenomenon called cable sway when EEG leads move through the electromagnetic field of the recording environment. Leads can sway due to the movement of the client, movement by the EEG technician, or even from air currents. These cable sway movements can result in fairly high amplitude and slow oscillations that can be mistaken for SCP and delta activity.
4-8 Hz - Theta Frequency Band
The last of our basic frequency bands is known as theta. The very brief description from Kane (2017) is below:
Theta band: Frequency band from 4-8 Hz. Greek letter: Ɵ
Theta rhythm: Rhythm with a frequency of 4-8 Hz.
Theta wave: Wave with a duration of 1/4 to over 1/8 s (125–250 ms)
EEG activity in the theta frequency band is quite specific to the location where it is recorded. 4-8 Hz activity in temporal areas has different functional and behavioral correlates from the same frequency activity in frontal midline or posterior areas. This, again, reaffirms that location and behavior are essential components when analyzing scalp EEG.
Below is an example of filtered (4-8 Hz) theta activity:
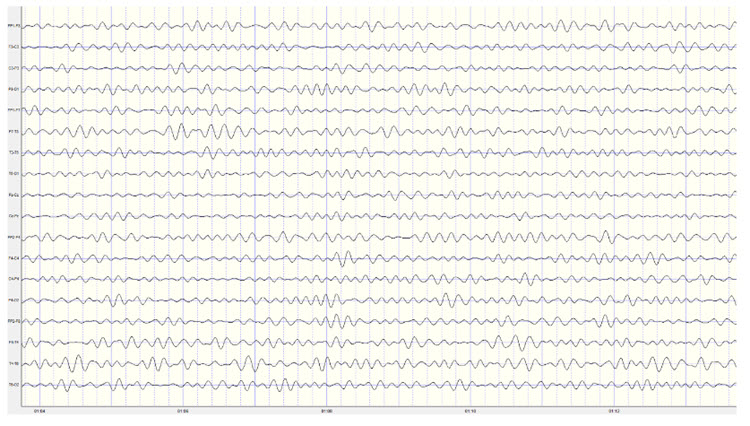
Note: this is an eyes-closed recording in the longitudinal bipolar montage with a 20-uV scale. The EEG is displayed using a 4-8 Hz filter to isolate that frequency band from the full band EEG. Observe the slightly greater amplitude and rhythmicity in temporal derivations.
Below is 1-45 Hz display of the same EEG recording at the same time location:
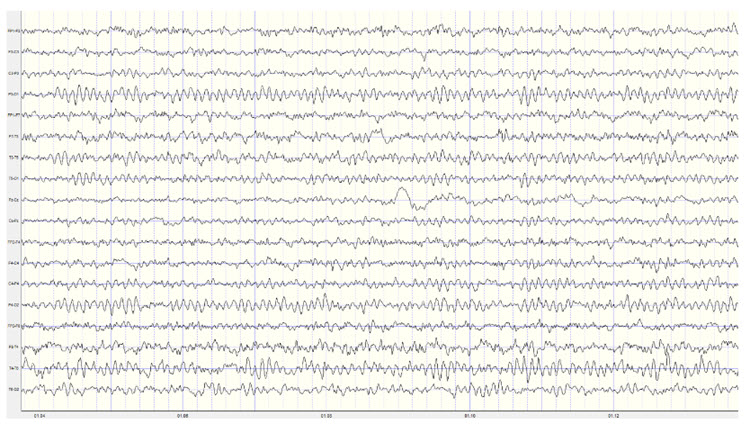
Note: this is an eyes-closed recording in the longitudinal bipolar montage with a 50-uV scale. The EEG is displayed using a 1-45 Hz filter to show the relatively full EEG band.
Amzica and Lopes da Silva (2017) cite various studies regarding the theta rhythm they identify as 4-7 Hz. As discussed earlier, they note that normal theta activity should not be confused with pathologic theta, which represents a slowing of the alpha frequency band into the theta range. They suggest that this slowing of alpha may result from reducing cerebral blood flow or metabolic encephalopathies. Metabolic encephalopathies can result from chemical imbalance due to various causal factors from kidney or liver dysfunction, diabetes, or a variety of other health issues (MyHealthAlberta.com).
Arnolds et al. (1980) found significant differences between behavioral conditions when viewing hippocampal theta recorded with depth electrodes. Writing resulted in faster frequency and greater rhythmicity but lower amplitude than sitting or walking. In contrast, a word association task resulted in faster frequency, greater rhythmicity, and increased amplitude in the period of silence immediately following the question but before the answer was given.
Ekstrom and colleagues (2005) studied hippocampal and neocortical theta activity during a virtual driving task that involved location finding. They found that both areas increased theta during all tasks associated with the driving simulation. A significant correlation between all areas showed increased coordination between multiple areas while accomplishing the tasks. They concluded that both cortical and hippocampal theta oscillations and coordination between these areas are associated with attention and sensorimotor integration.
Historically, the study of attention disorders has focused on excess frontal theta activity in individuals with inattentive ADHD. The ratio of theta (4-8 Hz) activity to beta (13-21 Hz) activity, or the theta/beta ratio (T/B ratio), was developed to make the analysis of this metric easier. It was initially calculated using a single channel, vertex location at Cz (Monastra et al., 1999). Other studies compared multiple locations and found that the Cz location was accurate and represented the location of the largest deviation of the ratio between previously diagnosed ADHD clients and typical controls (Lubar, 1991). Identifying an elevated T/B ratio (meaning more than typical theta compared to the amount of beta) compared to typical controls appeared to be an accurate way to assess attention disorders.
The sensitivity of an assessment measure determines how accurate it is at identifying individuals known to have a particular condition. Specificity is a measure of how accurately the measure correctly eliminates individuals without the condition from identification as having the condition.
In a large, blinded, multi-center validation of the theta/beta ratio in comparison with rating scales for the assessment of ADHD, the researchers calculated the sensitivity and specificity of measures. They found that the T/B ratio achieved superior results than commonly used rating scales (Snyder et al., 2008). With a sample size of 159 individuals, the EEG assessment showed a sensitivity of 87% and a specificity of 94%, for an overall accuracy of 89% compared to the next closest measure, the Connors’ Rating Scale – Teacher version (CRS – Teacher), with a sensitivity of 67% and specificity of 41%, for an overall accuracy of 58%.
The sensitivity of an assessment measure determines how accurate it is at identifying individuals known to have a particular condition. Specificity is a measure of how accurately the measure correctly eliminates individuals without the condition from identification as having the condition.
For example, the Connors’ Rating Scale – Parent (CRS – Parent) shows a sensitivity of 78%, meaning that it identifies 78% of clients known to have ADHD. However, it does this at the expense of identifying 86% of typical controls as also having ADHD (specificity of 14%). This could mean that many typical children could be diagnosed with ADHD and possibly treated with medication if the CRS-Parent was the only tool used for such an assessment – something that is quite often the case. Even if the teacher version of the rating scale were used, it would still result in 59% of typical children being misdiagnosed (specificity of 41%).
When using the T/B ratio, only 6% of typical children were incorrectly identified. This suggests that, at a minimum, the use of a simple, single-channel EEG assessment should be included as a component of a comprehensive approach to identify ADHD children before prescribing medication.
Interestingly, Van Son and colleagues (2019) expanded the associations that could be identified with the T/B ratio. They determined that a higher T/B ratio was negatively correlated with prefrontal executive control, including response inhibition and negative affect control. This suggests that excess theta in relation to beta activity could lead to greater impulsivity and to a lack of control of negative behaviors. They also found an association with higher T/B ratios and reward-motivated decision making, possibly selecting immediate gratification at the expense of long-term benefit. Finally, they correlated the T/B ratio with increased mind wandering, decreased executive network functions, and increased default mode network (DMN) activity.
As with all the previous EEG frequencies and assessment measures, the correct amount of a particular frequency activity is important. For example, someone who lacks appropriate default mode functioning may experience a lack of the type of resting-state activity that appears to have a therapeutic effect. The DMN has also been called the resting state network (RSN) due to its functions that differ from task-oriented behaviors. It is thought to be important for a variety of reasons. Therefore, a person with a lower-than-typical T/B ratio may benefit from increased theta voltage and some training in activating the DMN. In contrast, an individual diagnosed with ADHD may benefit from training to reduce or inhibit excess theta voltage.
Temporal lobe theta likely reflects the hippocampal theta identified using depth electrodes. It appears to be associated with route finding and navigation, both hippocampal functions. Differential, interhemispheric (bipolar montage) training of temporal lobe areas in the theta frequency range has been a component of certain approaches to neurofeedback for some time (Othmer & Othmer, 2007). This approach is used for various conditions, including migraine, tinnitus, PMS, and many others. Frequencies are adjusted to facilitate the optimal response and may range from the alpha frequencies, through the theta frequencies, down to the infra-low frequencies below 1 Hz.
Again, the analysis of theta activity is often aided by using a normative database. Otherwise, determining of whether an amount is too high or too low in amplitude is difficult to discern. Fortunately for T/B ratio assessment, Monastra (2001) has provided a table of values with three age ranges, 6-11 yrs, 12-15 yrs, 16-20 yrs. The table of mean T/B power ratios is below.
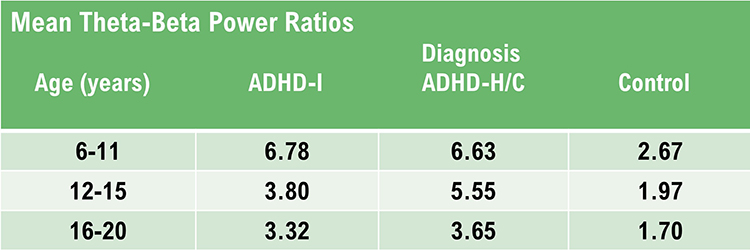
Note: ADHD-I = attention deficit-hyperactivity disorder, inattentive type; ADHD-H/C = attention deficit-hyperactivity disorder, hyperactive-combined type.
Assessment of theta activity more generally, beyond the T/B ratio in the central midline is more challenging. As noted in Van Son (2019), assessment under task may be essential to determine these results more accurately.
Final Notes
The recognition of standard EEG frequencies, the analysis of such activity, and using this information for training and re-assessment purposes is an important part of neurofeedback practice. This section has attempted to provide an overview of this area to aid the new practitioner and the experienced clinician alike in furthering their understanding of this complex area of study.
One of the greatest benefits of neuroscience in general and electroencephalography and neurofeedback specifically is that they encourage lifelong learning. If this section appeared overwhelming, with few hard and fast rules or concrete facts to hold on to, it is important to keep in mind that one can do useful and effective neurofeedback even without an in-depth understanding of this area. Understanding of EEG comes gradually through regular exposure to educational materials, lectures and workshops, work with an experienced mentor, and regular interaction with clients and their EEG recordings.
There is no substitute for viewing large numbers of EEG recordings. As mentioned in the beginning, this can start with an EEG atlas and then progress to examining your own recordings, whether a single channel, a couple of channels, or multiple channels.
Patience with one’s process is an integral part of any learning experience. Think of the time it takes to learn any worthwhile skill, from swimming to learning a musical instrument to learning a new computer or phone operating system. Managing one’s expectations is crucial.
A Few Final Notes on the Evaluation of the EEG – Compensatory Behaviors
As mentioned earlier, when evaluating the EEG, keep in mind that when an area of the brain is not functioning optimally, other areas may increase their activity to compensate. This is also true of emotional and psychological factors. The client may have developed coping strategies that result in unexpected EEG findings. These include excess right hemisphere activation in beta frequencies, excess alpha in left frontal areas or lack of alpha response in posterior areas. When training to resolve these differences in EEG activity, keep in mind that differences from typical do not necessarily mean pathological. Taking away a compensatory skill that serves the client well and not causing additional disorder is not a desirable approach. It is one of the reasons for being careful when training to a quantitative EEG with database comparison. It is important to match symptoms with findings so that truly troublesome issues can be addressed.One example of the effective application of this principle is Thatcher’s surface and LORETA neurofeedback program within the NeuroGuide™ program. This system utilizes a symptom checklist matching function that correlates qEEG findings with the client's presenting symptoms and then only targets those areas with the greatest correlation to both symptoms and findings. Other systems will likely be developed to accomplish this same goal, and an experienced mentor can also provide similar guidance.
Glossary
alpha blocking response: reduction in alpha wave activity (8-12 Hz) when a person opens their eyes or engages in mental activity, indicating a transition from a relaxed, awake state to an alert, attentive state.
alpha peak frequency: the specific frequency within the alpha band (8-12 Hz) where the power spectrum of the EEG signal is highest, often associated with individual differences in cognitive processing and brain health.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges, often defined as: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
cable sway: voltage fluctuations produced when EEG leads move through the electromagnetic field of the recording environment. Older EEG amplifiers are particularly vulnerable to this artifact.
common-mode rejection (CMR): using a differential amplifier, eliminating simultaneous, in-phase signals that occur at the two electrode sites.
cross-frequency synchronization (CFS): a mechanism in which waves of one type of frequency, such as beta or gamma, occur synchronously with the wave patterns of slower frequencies such as delta, theta, or alpha. These are often described as nested rhythms.
DC gradient: a measure of slow shifts in the baseline of the EEG signal, often related to very low-frequency brain activity or physiological processes.
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
EEG slowing: a decrease in the dominant frequency of brain waves, often seen in conditions like dementia, brain injury, or metabolic disturbances.
frequency: the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
gamma rhythm: high-frequency brain waves in the range of 30-100 Hz, associated with higher-order cognitive functions, such as attention, memory, and consciousness.
gap junction: electrical synapses that send bidirectional signals between adjacent neurons. Transmission across electrical synapses is instantaneous, compared with the 10-ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of distant neurons to synchronize their activity and simultaneously fire.
generalized anxiety disorder (GAD): A psychiatric condition characterized by chronic, excessive worry, and physiological hyperarousal, often associated with abnormal beta activity in the qEEG.
high gamma: gamma waves in the upper range of the gamma band (60-100 Hz), linked to complex cognitive processing and neural integration.
hypothalamic pituitary (HPA) axis: a system of endocrine glands involved in stress responses, regulating cortisol release, and impacting brain function and mental health.
low gamma: gamma waves in the lower range of the gamma band (30-60 Hz), associated with sensory processing and cognitive functions. mu rhythm: 7-11-Hz waves that resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
mu rhythm: brainwaves in the 8-13 Hz range, similar to alpha waves but found over the sensorimotor cortex, associated with motor functions and mirror neuron activity.
nystagmus: an involuntary rhythmic side-to-side, up and down, or circular motion of the eyes that occurs with a variety of conditions.
pi rhythm: a lesser-known, variable frequency brain wave pattern, not commonly used or defined in standard EEG terminology.
posterior dominant rhythm (PDR): highest-amplitude frequency detected at the posterior scalp when eyes are closed. Also called posterior basic rhythm.
posterior slow rhythms: slower brain waves observed in the posterior regions, often indicative of pathological conditions such as encephalopathy or dementia.
reticular nucleus of the thalamus (TRN): GABAergic thalamic neurons that modulate signals from other thalamic nuclei and do not project to the cortex. Also called the nucleus reticularis of the thalamus (NRT).
sensitivity: the ability of a diagnostic test or metric to correctly identify individuals with a specific condition (true positive rate).
sensorimotor rhythm (SMR): 13-15 Hz spindle-shaped sensorimotor rhythm (SMR) detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity.
skin electrodermal activity: electrical potentials produced by eccrine sweat glands.
slow cortical potential (SCP): slow event-related direct-current shifts of the electroencephalogram. Cortical potential shifts precede the depolarization of large cortical assemblies. The negative shift reflects the actions of gap junction cells. That shift reduces the excitation threshold of pyramidal neurons, leading to increased firing or depolarization of large assemblies of pyramidal neurons.
specificity: The ability of a diagnostic test or metric to correctly identify individuals without a specific condition (true negative rate).
thalamic-cortical relay (TCR) system: the primary pathway for determining which areas of the cortex receive each type of sensory input.
theta/beta ratio: the ratio between 4-7 Hz theta and 13-21 Hz beta, measured as amplitude squared most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated by a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
voltage: the electrical potential difference measured by EEG, reflecting the summed activity of neurons and used to assess brain function.
Test Yourself on ClassMarker
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, explain why posterior slowing may be a cause for concern.
References
Babiloni, C., Frisoni, G. B., Pievani, M., Vecchio, F., Geroldi, C., De Carli, C., ... & Rossini, P. M. (2006). Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. NeuroImage, 31(1), 180-189. https://doi.org/10.1016/j.neuroimage.2005.11.046
Bernier, R., Dawson, G., Webb, S., & Murias, M. (2007). EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition, 64(3), 228–237. https://doi.org/10.1016/j.bandc.2007.03.004
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Buchanan, D. M., Ros, T., & Nahas, R. (2021). Elevated and slowed EEG oscillations in patients with Post-Concussive Syndrome and chronic pain following a motor vehicle collision. Brain Sciences, 11(5), 537. https://doi.org/10.3390/brainsci11050537
Buzsaki, G. (2011). Rhythms of the brain. Oxford University Press.
Chatrian, G. E., Petersen, M. C., & Lazarte, J. A. (August 1959). The blocking of the Rolandic wicket rhythm and some central changes related to movement. Electroencephalography and Clinical Neurophysiology, 11(3), 497–510. https://doi.org/10.1016/0013-4694(59)90048-3
Cheyne D. O. (2013). MEG studies of sensorimotor rhythms: A review. Experimental Neurology, 245, 27–39. https://doi.org/10.1016/j.expneurol.2012.08.030
Clarke, A. R., Barry, R. J., McCarthy, R., & Selikowitz, M. (2001). EEG analysis in attention-deficit/hyperactivity disorder: A comparative study of two subtypes. Psychiatry Research, 103(1), 63-73. https://doi.org/10.1016/S0165-1781(01)00261-3
Crabtree J. W. (2018). Functional diversity of thalamic reticular subnetworks. Frontiers in Systems Neuroscience, 12, 41. https://doi.org/10.3389/fnsys.2018.00041
Crick, F. (1994). The astonishing hypothesis: The scientific search for the soul. Charles Scribner’s Sons.
Crunelli, V., Lőrincz, M. L., Connelly, W. M., David, F., Hughes, S. W., Lambert, R. C., Leresche, N., & Errington, A. C. (2018). Dual function of thalamic low-vigilance state oscillations: Rhythm-regulation and plasticity. Nature Reviews. Neuroscience, 19(2), 107–118. https://doi.org/10.1038/nrn.2017.151
Ekstrom, A. D., Caplan, J. B., Ho, E., Shattuck, K., Fried, I., & Kahana, M. J. (2005). Human hippocampal theta activity during virtual navigation. Hippocampus, 15(7), 881–889. https://doi.org/10.1002/hipo.20109
Engel, J. Jr. (2001). Mesial temporal lobe epilepsy: What have we learned? Neuroscientist, 7(4), 340-352. https://doi.org/10.1177/107385840100700410
Gastaut, H. J., & Bert, J. (1954). EEG changes during cinematographic presentation; Moving picture activation of the EEG. Electroencephalography and Clinical Neurophysiology, 6(3), 433–444. https://doi.org/10.1016/0013-4694(54)90058-9
Hammond, D. C. (2005). Neurofeedback treatment of depression and anxiety. Journal of Adult Development, 12(2-3), 131-137. https://doi.org/10.1007/s10804-005-7029-5
Huang, M. X., Yurgil, K. A., Risbrough, V. B., Nichols, S. L., Chen, Y. H., McClelland, J. M., ... & Baker, D. G. (2014). Resting-state magnetoencephalography reveals different patterns of aberrant functional connectivity in combat-related mild traumatic brain injury. Journal of Neurotrauma, 31(2), 131-144. https://doi.org/10.1089/neu.2013.3059
Hughes, J. R., & John, E. R. (1999). Conventional and quantitative electroencephalography in psychiatry. Journal of Neuropsychiatry and Clinical Neurosciences, 11(2), 190-208. https://doi.org/10.1176/jnp.11.2.190
Jelic, V., Julin, P., Shigeta, M., Nordberg, A., Lannfelt, L., Winblad, B., & Wahlund, L. O. (2000). Apolipoprotein E epsilon4 allele decreases functional connectivity in Alzheimer’s disease as measured by EEG coherence. Journal of Neurology, Neurosurgery & Psychiatry, 69(2), 223-229. https://doi.org/10.1136/jnnp.69.2.223
Lutz, A., Greischar, L. L., Rawlings, N. B., Ricard, M., & Davidson, R. J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America, 101(46), 16369–16373. https://doi.org/10.1073/pnas.0407401101
Niedermeyer, E., & da Silva, F. L. (2004). Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins.
Olbrich, S., & Arns, M. (2013). EEG biomarkers in major depressive disorder: discriminative power and prediction of treatment response. International Review of Psychiatry, 25(5), 604-618. https://doi.org/10.3109/09540261.2013.816269
D. The Role of QEEG Metrics in Understanding and Treating Specific Clinical Presentations and the Relationship of the QEEG to Other Clinical Examinations
Quantitative electroencephalography (qEEG) has emerged as a critical tool in both understanding and treating various clinical presentations. By providing detailed analyses of brain wave patterns, The qEEG offers insights that complement traditional clinical examinations.
Please click on the podcast icon below to hear a lecture over Section D.

This section explores the role of qEEG metrics in the diagnosis and treatment of specific clinical conditions and examines how the qEEG integrates with other clinical assessments. The following lecture discusses the quantitative analysis of the EEG © John S. Anderson.
Introduction
The qEEG involves the statistical analysis of the electrical activity of the brain, providing objective and quantifiable data on brain function. This technology is particularly valuable in identifying abnormal brain patterns associated with various neurological and psychiatric conditions. The use of qEEG metrics has expanded our understanding of these conditions and informed treatment strategies. Moreover, the qEEG often works synergistically with other clinical examinations, enhancing diagnostic accuracy and treatment efficacy.
QEEG Metrics in Specific Clinical Presentations
Attention-Deficit/Hyperactivity Disorder (ADHD)
Attention-Deficit/Hyperactivity Disorder (ADHD) is characterized by symptoms of inattention, hyperactivity, and impulsivity. qEEG metrics have been instrumental in identifying specific brain wave patterns associated with ADHD. Studies have consistently shown increased theta activity and decreased beta activity in children with ADHD, resulting in a high theta/beta ratio. This pattern is particularly evident in the frontal cortex, which is involved in executive functions and attention (Arns et al., 2013). These findings have not only improved the diagnostic process but also guided neurofeedback therapies that aim to normalize these brain wave patterns.Major Depressive Disorder (MDD)
Major Depressive Disorder (MDD) is another condition where the qEEG has proven useful. Depressed patients often exhibit increased alpha and theta activity, particularly in the frontal lobes. These abnormalities correlate with the severity of depressive symptoms and cognitive dysfunction. qEEG metrics can help differentiate MDD from other psychiatric disorders and predict treatment response. For instance, patients with higher frontal alpha asymmetry are more likely to respond to selective serotonin reuptake inhibitors (SSRIs) (Bruder et al., 2008). This predictive capability can inform personalized treatment plans and improve outcomes.Anxiety Disorders
In anxiety disorders, the qEEG typically reveals increased beta activity, reflecting heightened arousal and vigilance. This is often seen in generalized anxiety disorder (GAD) and panic disorder. The increased beta activity is associated with symptoms such as excessive worry, restlessness, and hyperarousal (Hammond, 2005). qEEG-guided neurofeedback has been effective in reducing these symptoms by training patients to reduce excessive beta activity and increase alpha activity, promoting relaxation and reducing anxiety.Epilepsy
The qEEG is invaluable in the diagnosis and management of epilepsy. It helps in identifying epileptiform discharges and localizing seizure foci, which is crucial for surgical planning and treatment. qEEG metrics can also monitor the efficacy of antiepileptic drugs and adjust treatment plans accordingly. By providing a continuous assessment of brain activity, the qEEG aids in managing epilepsy more effectively than traditional EEG alone (Niedermeyer & da Silva, 2004).Relationship of the qEEG to Other Clinical Examinations
The qEEG does not function in isolation but rather complements other clinical assessments to provide a comprehensive understanding of a patient's condition. Here are a few examples of how the qEEG integrates with other clinical tools.
Neuroimaging Techniques
While the qEEG provides excellent temporal resolution, neuroimaging techniques such as MRI and fMRI offer superior spatial resolution. Combining the qEEG with these imaging techniques can provide a more detailed picture of brain function. For example, the qEEG can identify abnormal brain wave patterns, while fMRI can pinpoint the anatomical location of these abnormalities, aiding in more accurate diagnoses and targeted treatments (Michel et al., 2004).Neuropsychological Testing
Neuropsychological tests assess cognitive functions such as memory, attention, and executive function. When used alongside the qEEG, these tests can correlate specific cognitive deficits with abnormal brain wave patterns. For instance, a patient with memory impairment may show abnormal theta activity in the hippocampus region on a qEEG, correlating with poor performance on memory tests. This integration can enhance the understanding of cognitive dysfunctions and guide rehabilitation strategies (Babiloni et al., 2006).Clinical Interviews and Questionnaires
Clinical interviews and self-report questionnaires remain essential tools for diagnosing psychiatric disorders. The qEEG can validate and quantify the subjective symptoms reported in these assessments. For instance, a patient reporting anxiety symptoms may show increased beta activity on a qEEG, confirming the clinical diagnosis and providing objective data to track treatment progress (Olbrich & Arns, 2013).Conclusion
qEEG metrics play a crucial role in understanding and treating specific clinical presentations, providing objective data that complement traditional clinical examinations. By identifying abnormal brain wave patterns associated with conditions such as ADHD, depression, anxiety disorders, and epilepsy, the qEEG enhances diagnostic accuracy and informs treatment strategies. The integration of the qEEG with other clinical tools, including neuroimaging techniques, neuropsychological testing, and clinical interviews, provides a comprehensive approach to patient care, improving outcomes and advancing our understanding of brain function and dysfunction.
Glossary
anxiety disorders: a group of mental health conditions characterized by excessive fear, worry, and related behavioral disturbances, impacting daily functioning and quality of life.
Attention-Deficit/Hyperactivity Disorder (ADHD): a neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity that interfere with functioning or development.
clinical interviews: structured or semi-structured conversations between a clinician and a patient used to gather detailed information about the patient’s symptoms, history, and overall mental health.
epilepsy: a neurological disorder marked by recurrent, unprovoked seizures due to abnormal electrical activity in the brain.
Major Depressive Disorder (MDD): a mental health disorder characterized by persistent feelings of sadness, loss of interest or pleasure, and various physical and cognitive symptoms that impair daily functioning.
neuroimaging techniques: methods used to visualize the structure and function of the brain, including MRI, fMRI, PET, and CT scans, aiding in the diagnosis and understanding of neurological and psychiatric conditions.
neuropsychological tests: standardized assessments designed to measure cognitive functions such as memory, attention, language, and executive functioning, often used to diagnose and monitor brain disorders.
self-report questionnaires: tools in which patients provide information about their symptoms, behaviors, and feelings, commonly used in clinical assessments to gather subjective data.
spatial resolution: The ability of a neuroimaging technique to distinguish between two separate points or structures in the brain, determining the level of detail in the images produced.
temporal resolution: the ability of a neuroimaging technique to capture rapid changes in brain activity over time, reflecting the precision with which the timing of neural events can be measured.
Test Yourself on ClassMarker
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, explain how clinicians integrate qEEG findings with psychological assessment tools.
References
Ancoli, S., & Kamiya, J. (1978). Methodological issues in alpha biofeedback training. Biofeedback and Self-Regulation, 3(2). 159-183. https://doi.org/0.1007/BF00998900
Arns, M., Conners, C. K., & Kraemer, H. C. (2013). A decade of EEG Theta/Beta Ratio research in ADHD: A meta-analysis. Journal of Attention Disorders, 17(5), 374-383. https://doi.org/10.1177/1087054712460087
Babiloni, C., Frisoni, G. B., Pievani, M., Vecchio, F., Geroldi, C., De Carli, C., ... & Rossini, P. M. (2006). Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. NeuroImage, 31(1), 180-189. https://doi.org/10.1016/j.neuroimage.2005.11.046
Berger, H. (1929). Über das Elektrenkephalogramm des Menschen. Archiv f. Psychiatrie, 87, 527–570.
Boyce, R., Glasgow, S. D., Williams, S., & Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science, 352(6287), 812-816. https://doi.org/10.1126/science.aad5252
Brown, B. (1974). New mind, New body: Biofeedback: New directions for the mind. Harper & Row.
Bruder, G. E., Sedoruk, J. P., Stewart, J. W., McGrath, P. J., Quitkin, F. M., & Tenke, C. E. (2008). Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: Pre-and post-treatment findings. Biological Psychiatry, 63(12), 1171-1177. https://doi.org/10.1016/j.biopsych.2007.12.029
Budzynski, T. H. (1976). Biofeedback and the twilight states of consciousness. In G. E. Schwartz & D. Shapiro (Eds.). Consciousness and self-regulation. Springer.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. (2005). An open clinical trial utilizing real-time EEG operant conditioning as an adjunctive therapy in the treatment of crack cocaine dependence. Journal of Neurotherapy, 9(2). https://doi.org/10.1300/J184v09n02_03
Buzsáki, G. (2006). Rhythms of the brain. Oxford University Press.
Cox, C. L., Huguenard, J. R., & Prince, D. A. (1997). Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proceedings of the National Academy of Sciences, 94(16), 8854-8859. https://doi.org/10.1073/pnas.94.16.8854
Davey, C. G., & Harrison, B. J. (2018). The brain's center of gravity: How the default mode network helps us to understand the self. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 17(3), 278-279. https://doi.org/10.1002/wps.20553
Hammond, D. C. (2005). Neurofeedback treatment of depression and anxiety. Journal of Adult Development, 12(2-3), 131-137. https://doi.org/10.1007/s10804-005-7029-5
Hardt, J., & Kamiya, J. (1976). Some comments on Plotkin’s self-regulation of the electroencephalographic alpha. J. Exp. Psychol., 105(1), 100-108.
Michel, C. M., Murray, M. M., Lantz, G., Gonzalez, S., Spinelli, L., & de Peralta, R. G. (2004). EEG source imaging. Clinical Neurophysiology, 115(10), 2195-2222. https://doi.org/10.1016/j.clinph.2004.06.001
Nicholson, A. A., Densmore, M., MicKinnon, M. C., Neufeld, R. W. J., Frewen, P. A. Théberge, J., Jetly, R., Richardson, J. D., & Lanius, R. A. (2019). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol Med, 49(12), 2049-2059. https://doi.org/10.1017/S003329171800286
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., & Lanius, R. A. (2020). A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. Neuroimage Clin., 28, 102490. https://doi.org/10.1016/j.nicl.2020.102490
Nicholson, A. A., Ros, T., Jetly, R., & Lanius, R. (2020). Regulating posttraumatic stress disorder symptoms with neurofeedback: Regaining control of the mind. Journal of Military Veteran and Family Health 6(S1), 3-15. https://doi.org/10.3138/jmvfh.2019-0032
Niedermeyer, E., & da Silva, F. L. (2004). Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins.
Olbrich, S., & Arns, M. (2013). EEG biomarkers in major depressive disorder: discriminative power and prediction of treatment response. International Review of Psychiatry, 25(5), 604-618. https://doi.org/10.3109/09540261.2013.816269
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy, 3, 37–55.
Scott, W., & Kaiser, D. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455-469. https://doi.org/10.1081/ada-200056807
Weise, R., von Mengden, I., Glos, M., Garcia, C., & Penzel, T. (2013). P 153. Influence of transcranial slow oscillation current stimulation (tSOS) on EEG, sleepiness and alertness. Clinical Neurophysiology, 124(10), e137. https://doi.org/10.1016/j.clinph.2013.04.230
E. Demonstrate Basic Knowledge of Brodmann Areas in Terms of How These Areas Were Defined and the Most Common Functional Attributes of These Regions
We recommend our Neuroscience unit for an indepth coverage of Brodmann areas.
Please click on the podcast icon below to hear a lecture over Section E.

Brodmann areas are a system of classification for the cerebral cortex based on the organization of neurons in different regions. Defined by the German anatomist Korbinian Brodmann in the early 20th century, these areas have become foundational in understanding brain function and neuroanatomy.
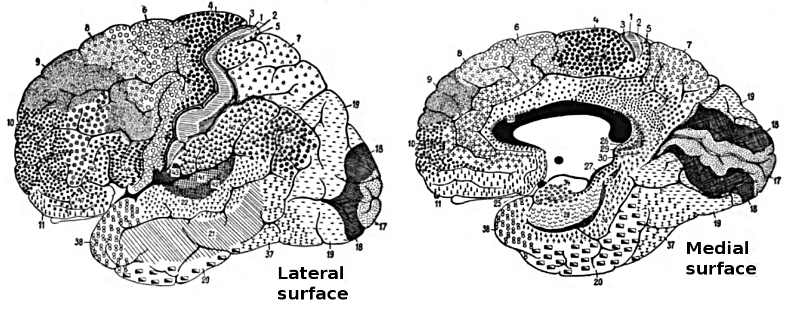
Definition of Brodmann Areas
Korbinian Brodmann defined the areas of the cerebral cortex based on their cytoarchitectonic characteristics, which involve the microscopic appearance and organization of cells. Using Nissl staining to highlight cell bodies, Brodmann examined the cortical layers and identified variations in cellular structure across different regions. In 1909, he published a map dividing the human cortex into 52 distinct areas, each with unique structural features (Brodmann, 1909).
Brodmann's classification was primarily anatomical, focusing on the differences in cell types, densities, and arrangements within the cortical layers. Despite its anatomical basis, the Brodmann map has shown significant correlations with functional areas of the brain, making it a valuable tool in both neuroanatomy and neurophysiology (Amunts & Zilles, 2015).
Functional Attributes of Brodmann Areas
The functional attributes of Brodmann areas are diverse, corresponding to various sensory, motor, and cognitive processes. Below are descriptions of some of the most well-studied Brodmann areas and their associated functions. The graphic redrawn by minaanandag on fiverr.com shows the correspondence between 10-20 sites and Brodmann areas.
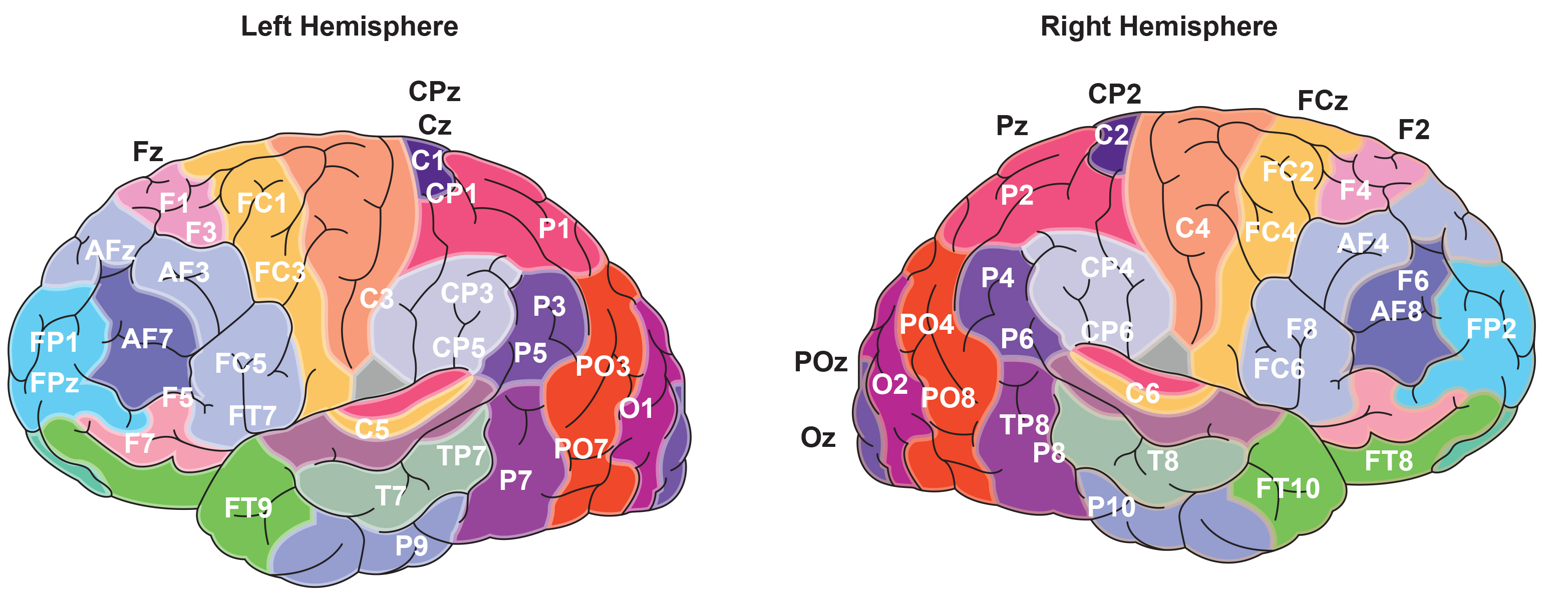
Brodmann Areas 1, 2, 3 (Primary Somatosensory Cortex)
These areas, located in the postcentral gyrus of the parietal lobe, are collectively known as the primary somatosensory cortex. They are responsible for processing tactile information, including touch, proprioception, and nociception. Area 3 receives the bulk of thalamocortical input and is involved in the initial processing of sensory information, while areas 1 and 2 integrate this information and contribute to the perception of complex tactile stimuli (Kaas, 1993). Graphic © Big8/Shutterstock.com..jpg)
Brodmann Area 4 (Primary Motor Cortex)
Located in the precentral gyrus of the frontal lobe, Brodmann area 4 is known as the primary motor cortex (M1). It is essential for voluntary motor control, with neurons in this area sending signals to the spinal cord to initiate muscle contractions. The primary motor cortex is organized somatotopically, meaning different regions correspond to different parts of the body, a concept illustrated by the motor homunculus (Penfield & Boldrey, 1937).Graphic © Big8/Shutterstock.com..jpg)
Brodmann Area 17 (Primary Visual Cortex)
Brodmann area 17, located in the occipital lobe, corresponds to the primary visual cortex (V1). This area is crucial for processing visual information received from the retina. It is characterized by a high degree of retinotopic organization, where spatial information from the visual field is mapped onto the cortex. Neurons in V1 are involved in detecting basic visual features such as edges, orientation, and motion (Hubel & Wiesel, 1968). Graphic © Big8/Shutterstock.com..jpg)
Brodmann Area 22 (Superior Temporal Gyrus, Part of Wernicke's Area)
Part of Brodmann area 22, located in the superior temporal gyrus, is involved in language comprehension. This region, known as Wernicke's area, is crucial for the processing of spoken and written language. Damage to this area can result in Wernicke's aphasia, characterized by fluent but nonsensical speech and impaired understanding of language (Geschwind, 1970). Graphic © Big8/Shutterstock.com..jpg)
Brodmann Area 40 (Supramarginal Gyrus)
Brodmann area 40, located in the parietal lobe, is part of the supramarginal gyrus. This area plays a role in phonological processing, language perception, and spatial attention. It is also involved in integrating sensory information to facilitate motor planning and coordination (Rizzolatti et al., 2002). Graphic © Big8/Shutterstock.com..jpg)
Brodmann Area 46 (Dorsolateral Prefrontal Cortex)
Brodmann area 46, part of the dorsolateral prefrontal cortex (DLPFC), is associated with executive functions, including working memory, decision-making, and cognitive flexibility. The DLPFC is critical for goal-directed behavior, problem-solving, and the regulation of emotional responses (Miller & Cohen, 2001). Graphic © Big8/Shutterstock.com..jpg)
Conclusion
Brodmann's classification of cortical areas based on cytoarchitectonic features has provided a foundational framework for understanding the structure and function of the human brain. The functional attributes of these areas, from sensory and motor processing to complex cognitive functions, underscore the diverse roles of different cortical regions. By correlating anatomical distinctions with functional capabilities, Brodmann areas continue to guide research and clinical practice in neuroscience.
Glossary
Brodmann areas: cerebral cortex regions defined by their distinct cytoarchitectonic characteristics, based on the cellular composition and organization, mapped by Korbinian Brodmann.
dorsolateral prefrontal cortex (DLPFC): a frontal lobe region associated with executive functions such as working memory, decision-making, and cognitive control.
primary motor cortex (M1): a region located in the precentral gyrus of the frontal lobe, responsible for voluntary motor control and execution of movement.
primary somatosensory cortex: a region situated in the postcentral gyrus of the parietal lobe, responsible for processing tactile and proprioceptive information from the body.
primary visual cortex (V1): a region located in the occipital lobe, responsible for the initial processing of visual information received from the retinas.
supramarginal gyrus: a part of the parietal lobe involved in phonological processing, language perception, and integration of sensory information.
superior temporal gyrus: a region in the temporal lobe involved in auditory processing and language comprehension, including part of Wernicke's area.
Wernicke's area: a region located in the posterior part of the superior temporal gyrus, critical for language comprehension; damage to this area can result in Wernicke's aphasia.
Test Yourself on ClassMarker
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, describe important dorsolateral prefrontal cortex functions.
References
Amunts, K., & Zilles, K. (2015). Architectonic mapping of the human brain beyond Brodmann. Neuron, 88(6), 1086-1107. https://doi.org/10.1016/j.neuron.2015.12.001
Ancoli, S., & Kamiya, J. (1978). Methodological issues in alpha biofeedback training. Biofeedback and Self-Regulation, 3(2). 159-183. https://doi.org/0.1007/BF00998900
Berger, H. (1929). Über das Elektrenkephalogramm des Menschen. Archiv f. Psychiatrie, 87, 527–570.
Boyce, R., Glasgow, S. D., Williams, S., & Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science, 352(6287), 812-816. https://doi.org/10.1126/science.aad5252
Brodmann, K. (1909). Vergleichende lokalisationslehre der großhirnrinde in ihren prinzipien dargestellt auf grund des zellenbaues. Barth.
Brown, B. (1974). New mind, New body: Biofeedback: New directions for the mind. Harper & Row.
Budzynski, T. H. (1976). Biofeedback and the twilight states of consciousness. In G. E. Schwartz & D. Shapiro (Eds.). Consciousness and self-regulation. Springer.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. (2005). An open clinical trial utilizing real-time EEG operant conditioning as an adjunctive therapy in the treatment of crack cocaine dependence. Journal of Neurotherapy, 9(2). https://doi.org/10.1300/J184v09n02_03
Buzsáki, G. (2006). Rhythms of the brain. Oxford University Press.
Cox, C. L., Huguenard, J. R., & Prince, D. A. (1997). Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proceedings of the National Academy of Sciences, 94(16), 8854-8859. https://doi.org/10.1073/pnas.94.16.8854
Davey, C. G., & Harrison, B. J. (2018). The brain's center of gravity: How the default mode network helps us to understand the self. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 17(3), 278-279. https://doi.org/10.1002/wps.20553
Geschwind, N. (1970). The organization of language and the brain. Science, 170(3961), 940-944. https://doi.org/10.1126/science.170.3961.940
Green, E., & Green, A. (1977). Beyond biofeedback. Knoll Publishing Company.
Gruzelier, J. H., Holmes, P., Hirst, L., Bulpin, K., Rahman, S., van Run, C., & Leach, J. (2014). Replication of elite music performance enhancement following alpha/theta neurofeedback and application to novice performance and improvisation with SMR benefits. Biological Psychology, 95, 86-107. https://doi.org/10.1016/j.biopsycho.2013.11.001
Hardt, J., & Kamiya, J. (1976). Some comments on Plotkin’s self-regulation of the electroencephalographic alpha. J. Exp. Psychol., 105(1), 100-108.
Hubel, D. H., & Wiesel, T. N. (1968). Receptive fields and functional architecture of monkey striate cortex. Journal of Physiology, 195(1), 215-243. https://doi.org/10.1113/jphysiol.1968.sp008455
Kaas, J. H. (1993). The organization of somatosensory cortex in primates. In A. D. Keller & J. H. Asanuma (Eds.), Cerebral Cortex (pp. 195-245). Springer.
Kamiya, J. (1961). Behavioral, subjective, and physiological aspects of drowsiness and sleep. In D. W. Fiske, & S. R. Maddi (Eds.). Functions of varied experience. Dorsey Press.
Kamiya, J. (1962). Conditioned discrimination of the EEG alpha rhythm in Humans. Abstract presented at the Western Psychological Association meeting.
Kamiya, J. (1968). Conscious control of brain waves. Psychology Today, 1, 56-63.
Kamiya, J. (1969). Operant control of the EEG alpha rhythm and some of its reported effects on consciousness. In C. Tart (Ed.), Altered states of consciousness. John Wiley and Sons.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167-202. https://doi.org/10.1146/annurev.neuro.24.1.167
Nicholson, A. A., Densmore, M., MicKinnon, M. C., Neufeld, R. W. J., Frewen, P. A. Théberge, J., Jetly, R., Richardson, J. D., & Lanius, R. A. (2019). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol Med, 49(12), 2049-2059. https://doi.org/10.1017/S003329171800286
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., & Lanius, R. A. (2020). A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. Neuroimage Clin., 28, 102490. https://doi.org/10.1016/j.nicl.2020.102490
Nicholson, A. A., Ros, T., Jetly, R., & Lanius, R. (2020). Regulating posttraumatic stress disorder symptoms with neurofeedback: Regaining control of the mind. Journal of Military Veteran and Family Health 6(S1), 3-15. https://doi.org/10.3138/jmvfh.2019-0032
Penfield, W., & Boldrey, E. (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain, 60(4), 389-443. https://doi.org/10.1093/brain/60.4.389
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy, 3, 37–55.
Rizzolatti, G., Fogassi, L., & Gallese, V. (2002). Motor and cognitive functions of the ventral premotor cortex. Current Opinion in Neurobiology, 12(2), 149-154. https://doi.org/10.1016/S0959-4388(02)00308-2
Scott, W., & Kaiser, D. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455-469. https://doi.org/10.1081/ada-200056807
Weise, R., von Mengden, I., Glos, M., Garcia, C., & Penzel, T. (2013). P 153. Influence of transcranial slow oscillation current stimulation (tSOS) on EEG, sleepiness and alertness. Clinical Neurophysiology, 124(10), e137. https://doi.org/10.1016/j.clinph.2013.04.230
F. Demonstrate Knowledge of Networks and Connectivity and Definition of Terms
The study of brain networks and connectivity has become a central focus in neuroscience, offering profound insights into how different regions of the brain communicate and function collectively. This report explores the fundamental concepts of brain networks and connectivity, defining key terms and highlighting their significance in understanding brain function and dysfunction.
Please click on the podcast icon below to hear a lecture over Section F.

Introduction
Brain networks and connectivity refer to the complex interactions between different brain regions that enable cognitive, sensory, and motor functions. These interactions can be studied at various levels, from local circuits to large-scale networks, using a variety of neuroimaging and electrophysiological techniques. Understanding these networks is crucial for deciphering the neural basis of behavior and the pathophysiology of neurological and psychiatric disorders.
Definition of Key Terms
Functional Connectivity
Functional connectivity refers to the temporal correlation between spatially remote neurophysiological events, indicating that different regions of the brain are functionally linked. This concept is typically assessed using methods such as functional Magnetic Resonance Imaging (fMRI), which measures the BOLD (Blood Oxygen Level Dependent) signal, and Electroencephalography (EEG), which captures electrical activity. Functional connectivity does not necessarily imply direct anatomical connections but reflects the coherence of activity patterns over time (Friston, 2011).Structural Connectivity
Structural connectivity describes the physical connections between different brain regions, primarily formed by white matter tracts. This type of connectivity is often investigated using Diffusion Tensor Imaging (DTI), a form of MRI that maps the diffusion of water molecules along white matter fibers. Structural connectivity provides the anatomical basis for functional interactions, revealing the pathways through which information flows in the brain (Behrens & Sporns, 2012).Effective Connectivity
Effective connectivity refers to the causal influence that one brain region exerts over another. Unlike functional connectivity, which is symmetrical and undirected, effective connectivity involves directionality and causality. Techniques such as Dynamic Causal Modeling (DCM) and Granger causality analysis are used to infer effective connectivity, providing insights into the directional flow of information and the hierarchical organization of brain networks (Friston et al., 2013).Small-World Network
A small-world network is a type of network characterized by high clustering of connections and short path lengths between nodes. In the context of brain networks, this means that while neurons or regions form tightly connected clusters, there are also long-range connections that enable efficient communication across the brain. Small-world properties are believed to optimize both local specialization and global integration of information (Bassett & Bullmore, 2006).Hub
Hubs are highly connected and central nodes within a network, playing a critical role in facilitating communication and integration across different regions. In the brain, hubs are typically found in regions that are crucial for various cognitive functions, such as the precuneus, posterior cingulate cortex, and anterior cingulate cortex. Hubs are integral to the brain's small-world architecture and are thought to be vulnerable in many neurodegenerative diseases (Crossley et al., 2014).Connectivity Metrics
qEEG connectivity metrics quantify the relationships between different brain regions, helping to understand the functional and structural networks underlying various cognitive and behavioral functions. This report explores the key QEEG connectivity metrics, their significance, and their applications in clinical and research settings.
Introduction
qEEG connectivity metrics are used to assess the interaction between different regions of the brain. These metrics can be divided into functional and effective connectivity. Functional connectivity measures the statistical dependencies between neural signals, while effective connectivity assesses the causal influence one neural system exerts over another. Both types of connectivity provide important information about how different parts of the brain communicate and coordinate.
Key qEEG Connectivity Metrics
Coherence, comodulation, phase lag index, imaginary coherence, amplitude envelope correlation, and Granger casuality are important connectivity metrics.
Coherence
Coherence measures the degree of synchronization between two EEG signals across different frequency bands. It is calculated as the normalized cross-spectral density between two signals, providing a value between 0 (no synchronization) and 1 (perfect synchronization). Coherence is used to assess functional connectivity, reflecting how different brain regions work together during various tasks or at rest (Thatcher et al., 1986).Clinical Significance: Increased coherence in specific frequency bands may indicate enhanced communication between brain regions, while decreased coherence may suggest disconnection or dysfunction. For example, reduced coherence in the alpha band has been associated with cognitive decline in Alzheimer's disease (Babiloni et al., 2006).
Comodulation refers to the synchronization or co-occurrence of amplitude fluctuations in different frequency bands across different brain regions. In the context of qEEG connectivity, comodulation analysis helps in understanding how different parts of the brain communicate with each other by examining the coupling between the amplitudes of brain wave oscillations in different frequency bands.
Comodulation is crucial for understanding the complex dynamics of brain activity and connectivity. Unlike coherence, which measures phase synchronization, comodulation focuses on the amplitude correlation between different frequencies, offering a complementary perspective on how brain regions interact. This can be particularly useful in identifying patterns of neural coordination that are not evident from phase-based measures alone. Comodulation can help in identifying and characterizing neural networks involved in various cognitive and behavioral functions. For example, the coupling between theta and gamma oscillations has been linked to working memory and cognitive control processes (Canolty et al., 2006).
Clinical significance: Abnormal comodulation patterns can be indicative of neurological and psychiatric disorders. For instance, disrupted comodulation between different frequency bands has been observed in conditions such as epilepsy and schizophrenia, providing potential biomarkers for diagnosis and treatment monitoring (Lopes da Silva, 2013). Amplitude Envelope Extraction: The amplitude envelopes of the decomposed signals are computed, often using the Hilbert transform. Comodulation Calculation: The correlation or coherence between the amplitude envelopes of different frequency bands is calculated to assess the degree of comodulation.
Phase Lag Index
The phase lag index (PLI) measures the consistency of phase differences between EEG signals, providing information about the directionality of communication between brain regions. Unlike coherence, PLI is less sensitive to volume conduction effects, making it a more reliable measure of true brain connectivity (Stam et al., 2007).Clinical Significance: Abnormal PLI values can indicate disrupted neural synchronization. For instance, altered PLI patterns have been observed in patients with epilepsy, reflecting abnormal connectivity associated with seizure activity (van Diessen et al., 2013).
Imaginary Coherence
Imaginary coherence assesses the synchronization between EEG signals, focusing on the imaginary part of the cross-spectrum. This metric helps to eliminate the effects of volume conduction, providing a clearer picture of genuine brain connectivity (Nolte et al., 2004).Clinical Significance: Imaginary coherence has been used to study connectivity in various psychiatric and neurological disorders. For example, decreased imaginary coherence in the beta band has been linked to schizophrenia, suggesting disrupted long-range connectivity (Hinkley et al., 2011).
Amplitude Envelope Correlation
The amplitude envelope correlation (AEC) measures the correlation between the amplitude envelopes of EEG signals. This metric reflects the strength of functional connectivity by quantifying how the amplitude of oscillatory activity in one brain region correlates with that in another region over time (Engel et al., 2013).Clinical Significance: AEC can reveal changes in connectivity associated with different brain states. For example, reduced AEC in the alpha band has been associated with impaired consciousness in disorders such as coma and vegetative states (Boly et al., 2008).
Granger Causality
Granger causality is a measure of effective connectivity that assesses the directional influence one time series exerts over another. It uses predictive modeling to determine whether past values of one signal can predict future values of another signal, indicating causal relationships (Granger, 1969).Clinical Significance: Granger causality has been applied to study the directional interactions in various brain disorders. For instance, abnormal Granger causality patterns have been found in patients with autism, reflecting atypical information flow in neural networks (Billeci et al., 2013).
Applications of QEEG Connectivity Metrics
Neurological Disorders
QEEG connectivity metrics are widely used to investigate the neural basis of neurological disorders. For instance, in epilepsy, these metrics can identify abnormal connectivity patterns that contribute to seizure activity, guiding treatment strategies such as surgery or neurostimulation (Gotman, 2013).Psychiatric Disorders
In psychiatric disorders, QEEG connectivity metrics help to uncover the underlying neural mechanisms. For example, altered connectivity patterns in the default mode network (DMN) have been associated with depression and anxiety disorders, providing insights into their pathophysiology and potential biomarkers for treatment response (Greicius et al., 2007).Cognitive and Developmental Disorders
QEEG connectivity metrics are also valuable in studying cognitive and developmental disorders. In ADHD, for example, disrupted connectivity patterns have been linked to attentional deficits and hyperactivity, informing the development of targeted interventions such as neurofeedback (Arns et al., 2013).Conclusion
qEEG connectivity metrics offer powerful tools for analyzing brain function and dysfunction. By quantifying the interactions between different brain regions, these metrics provide critical insights into the neural basis of various cognitive, behavioral, and clinical phenomena. The integration of QEEG connectivity metrics with other neuroimaging and clinical data can enhance our understanding of brain disorders and guide the development of more effective diagnostic and therapeutic strategies.Brain Networks
Default Mode Network (DMN)
The Default Mode Network (DMN) is a prominent brain network that is active during rest and involved in self-referential and introspective activities. The DMN includes regions such as the medial prefrontal cortex, posterior cingulate cortex, and inferior parietal lobule. Abnormalities in DMN connectivity have been associated with various psychiatric conditions, including depression and schizophrenia (Raichle, 2015). Shim G, Oh JS, Jung WH, et al., CC BY 3.0, via Wikimedia Commons..jpg)
Caption: Default mode and task-related maps for healthy subjects. On a green background, the default mode network is highlighted in warm colors (red and yellow) and the task-related network is highlighted in cold colors (blue and light blue) depending on the p-value of one-sample t-test.
Salience Network
The Salience Network is responsible for detecting and filtering salient stimuli, integrating sensory, emotional, and cognitive information. It includes regions such as the anterior insula and anterior cingulate cortex. Dysfunction in the Salience Network has been implicated in disorders such as autism and frontotemporal dementia (Menon, 2015). Graphic by van Ettinger-Veenstra et al. (2019).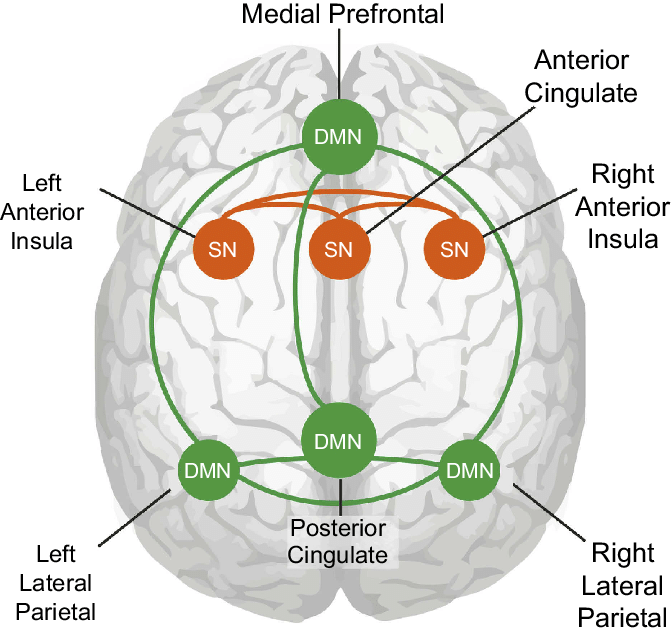
Caption: Node connections of the default mode network and the salience network. Cartoon of node connections used in this study as seen on a see-through brain. Green shows the connections between the nodes of the DMN and orange the connection between the nodes of the SN.
Executive Network
The Executive Network, also known as the Executive Control Network, is involved in high-level cognitive functions such as working memory, problem-solving, and attention. Key regions include the dorsolateral prefrontal cortex and posterior parietal cortex. Impaired connectivity within the executive network is often observed in conditions like ADHD and Alzheimer's disease (Seeley et al., 2007).Seven-network graphic by Feirrera et al. (2022). The Executive Network is shown in blue.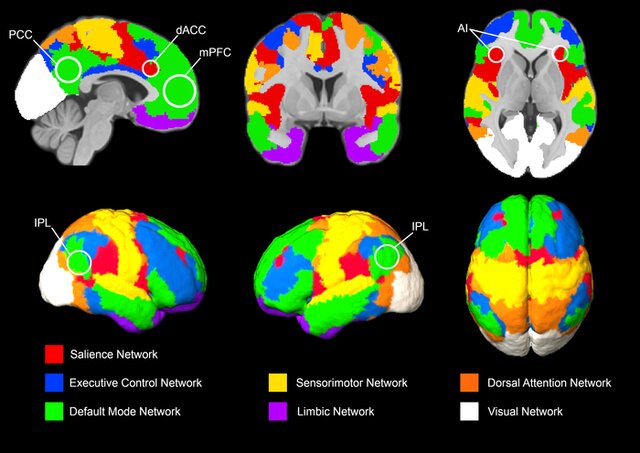
Caption: Brain networks: One atlas with seven networks. Seven brain networks derived from resting‐state fMRI data were adapted from Schaefer et al. (2018).
Integration with Clinical Examinations
Understanding brain networks and connectivity enhances the diagnostic and therapeutic approaches for neurological and psychiatric disorders. Functional and structural connectivity analyses can identify network dysfunctions that correlate with clinical symptoms, guiding more targeted interventions. For example, network-based approaches can improve the accuracy of early diagnosis in Alzheimer's disease by identifying disrupted connectivity patterns even before significant cognitive decline (Greicius et al., 2004).
Conclusion
The study of brain networks and connectivity provides a comprehensive framework for understanding the complex interactions that underlie brain function. By defining key terms such as functional, structural, and effective connectivity, and exploring the characteristics of major brain networks, we can appreciate the intricate architecture of the brain. This knowledge not only advances basic neuroscience but also has profound implications for clinical practice, offering new avenues for diagnosing and treating brain disorders.
Glossary
amplitude envelope correlation (AEC): the correlation between the amplitude envelopes of EEG signals, indicating the strength of functional connectivity by quantifying how the amplitude of oscillatory activity in one brain region correlates with that in another over time.
coherence: the degree of synchronization between two EEG signals across different frequency bands, reflecting functional connectivity between brain regions.
Default Mode Network (DMN): a brain network that is active during rest and involved in self-referential and introspective activities, including regions such as the medial prefrontal cortex, posterior cingulate cortex, and inferior parietal lobule.
Diffusion Tensor Imaging (DTI): a type of MRI technique used to visualize and measure the integrity of white matter tracts in the brain, aiding in the assessment of structural connectivity.
Dynamic Causal Modeling (DCM): a computational method used to infer and quantify effective connectivity by modeling the causal relationships between different brain regions based on observed neural data.
effective connectivity: the causal influence that one brain region exerts over another, indicating the directionality and strength of interactions between neural systems.
executive network: a brain network involved in high-level cognitive functions such as working memory, problem-solving, and attention, primarily including regions like the dorsolateral prefrontal cortex and the posterior parietal cortex.
functional connectivity: the statistical dependencies between spatially remote neurophysiological events, indicating that different brain regions are functionally linked.
Granger causality analysis: a method for assessing effective connectivity by determining whether past values of one time series can predict future values of another, indicating causal relationships between brain signals.
hubs: highly connected and central nodes within a brain network that play a critical role in facilitating communication and integration across different brain regions.
imaginary coherence: a measure of the synchronization between EEG signals by focusing on the imaginary part of the cross-spectrum, reducing the influence of volume conduction and highlighting true brain connectivity.
phase lag index (PLI)): a measure of the consistency of phase differences between EEG signals, providing information about the directionality and strength of functional connectivity while minimizing volume conduction effects.
salience network: a brain network involved in detecting and filtering salient stimuli, integrating sensory, emotional, and cognitive information, including regions like the anterior insula and anterior cingulate cortex.
small-world network: a type of network characterized by high clustering of connections and short path lengths between nodes, optimizing both local specialization and global integration of information.
structural connectivity: the physical connections between different brain regions, primarily formed by white matter tracts, providing the anatomical basis for functional and effective connectivity.
Test Yourself on ClassMarker
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, explain how activating the Default Mode Network could impair athletic performance.
References
Ancoli, S., & Kamiya, J. (1978). Methodological issues in alpha biofeedback training. Biofeedback and Self-Regulation, 3(2). 159-183. https://doi.org/0.1007/BF00998900
Bassett, D. S., & Bullmore, E. (2006). Small-world brain networks. The Neuroscientist, 12(6), 512-523. https://doi.org/10.1177/1073858406293182
Behrens, T. E., & Sporns, O. (2012). Human connectomics. Current Opinion in Neurobiology, 22(1), 144-153. https://doi.org/10.1016/j.conb.2011.08.014
Berger, H. (1929). Über das Elektrenkephalogramm des Menschen. Archiv f. Psychiatrie, 87, 527–570.
Boyce, R., Glasgow, S. D., Williams, S., & Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science, 352(6287), 812-816. https://doi.org/10.1126/science.aad5252
Brown, B. (1974). New mind, New body: Biofeedback: New directions for the mind. Harper & Row.
Budzynski, T. H. (1976). Biofeedback and the twilight states of consciousness. In G. E. Schwartz & D. Shapiro (Eds.). Consciousness and self-regulation. Springer.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. (2005). An open clinical trial utilizing real-time EEG operant conditioning as an adjunctive therapy in the treatment of crack cocaine dependence. Journal of Neurotherapy, 9(2). https://doi.org/10.1300/J184v09n02_03
Buzsáki, G. (2006). Rhythms of the brain. Oxford University Press.
Cox, C. L., Huguenard, J. R., & Prince, D. A. (1997). Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proceedings of the National Academy of Sciences, 94(16), 8854-8859. https://doi.org/10.1073/pnas.94.16.8854
Crossley, N. A., Mechelli, A., Vértes, P. E., Winton-Brown, T. T., Patel, A. X., Ginestet, C. E., ... & Bullmore, E. T. (2014). Cognitive relevance of the community structure of the human brain functional coactivation network. Proceedings of the National Academy of Sciences, 110(28), 11583-11588. https://doi.org/10.1073/pnas.1220826110
Davey, C. G., & Harrison, B. J. (2018). The brain's center of gravity: How the default mode network helps us to understand the self. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 17(3), 278-279. https://doi.org/10.1002/wps.20553
Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1(1), 13-36. https://doi.org/10.1089/brain.2011.0008
Friston, K. J., Harrison, L., & Penny, W. (2013). Dynamic causal modelling. NeuroImage, 19(4), 1273-1302. https://doi.org/10.1016/S1053-8119(03)00202-7
Green, E., & Green, A. (1977). Beyond biofeedback. Knoll Publishing Company.
Greicius, M. D., Srivastava, G., Reiss, A. L., & Menon, V. (2004). Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences, 101(13), 4637-4642. https://doi.org/10.1073/pnas.0308627101
Gruzelier, J. H., Holmes, P., Hirst, L., Bulpin, K., Rahman, S., van Run, C., & Leach, J. (2014). Replication of elite music performance enhancement following alpha/theta neurofeedback and application to novice performance and improvisation with SMR benefits. Biological Psychology, 95, 86-107. https://doi.org/10.1016/j.biopsycho.2013.11.001
Hardt, J., & Kamiya, J. (1976). Some comments on Plotkin’s self-regulation of the electroencephalographic alpha. J. Exp. Psychol., 105(1), 100-108.
Kamiya, J. (1961). Behavioral, subjective, and physiological aspects of drowsiness and sleep. In D. W. Fiske, & S. R. Maddi (Eds.). Functions of varied experience. Dorsey Press.
Kamiya, J. (1962). Conditioned discrimination of the EEG alpha rhythm in Humans. Abstract presented at the Western Psychological Association meeting.
Kamiya, J. (1968). Conscious control of brain waves. Psychology Today, 1, 56-63.
Kamiya, J. (1969). Operant control of the EEG alpha rhythm and some of its reported effects on consciousness. In C. Tart (Ed.), Altered states of consciousness. John Wiley and Sons.
Menon, V. (2015). Salience Network. In A. W. Toga (Ed.), Brain mapping: An encyclopedic reference (Vol. 2, pp. 597-611). Academic Press. https://doi.org/10.1016/B978-0-12-397025-1.00112-4
Nicholson, A. A., Densmore, M., MicKinnon, M. C., Neufeld, R. W. J., Frewen, P. A. Théberge, J., Jetly, R., Richardson, J. D., & Lanius, R. A. (2019). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol Med, 49(12), 2049-2059. https://doi.org/10.1017/S003329171800286
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., & Lanius, R. A. (2020). A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. Neuroimage Clin., 28, 102490. https://doi.org/10.1016/j.nicl.2020.102490
Nicholson, A. A., Ros, T., Jetly, R., & Lanius, R. (2020). Regulating posttraumatic stress disorder symptoms with neurofeedback: Regaining control of the mind. Journal of Military Veteran and Family Health 6(S1), 3-15. https://doi.org/10.3138/jmvfh.2019-0032
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy, 3, 37–55.
Raichle, M. E. (2015). The brain's default mode network. Annual Review of Neuroscience, 38, 433-447. https://doi.org/10.1146/annurev-neuro-071013-014030
Scott, W., & Kaiser, D. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455-469. https://doi.org/10.1081/ada-200056807
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., ... & Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349-2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Weise, R., von Mengden, I., Glos, M., Garcia, C., & Penzel, T. (2013). P 153. Influence of transcranial slow oscillation current stimulation (tSOS) on EEG, sleepiness and alertness. Clinical Neurophysiology, 124(10), e137. https://doi.org/10.1016/j.clinph.2013.04.230
G. Demonstrate Knowledge of Networks and Connectivity and Definition of Terms
Current Source Density (CSD) analysis is a powerful technique used in neuroscience to localize and quantify the sources of electrical activity in the brain. We provide an overview of CSD maps, the metrics used in their analysis, and the graphic methods employed to represent these data. Understanding these aspects is crucial for interpreting electrophysiological data accurately and enhancing our comprehension of brain function.
Please click on the podcast icon below to hear a lecture over Section G.

Introduction
Current Source Density (CSD) mapping is a method used to determine the locations and magnitudes of current sources and sinks within the brain. It involves analyzing the spatial distribution of voltage gradients recorded by electrodes, typically in the form of electroencephalography (EEG) or local field potentials (LFPs). By applying mathematical transformations to these data, researchers can create detailed maps that reveal the underlying neural activity with high spatial resolution (Nicholson & Freeman, 1975).
Current Source Density Maps
CSD maps provide a visualization of where electrical currents enter (sinks) and leave (sources) the extracellular space. These maps are created by differentiating the voltage signals recorded at multiple spatially arranged electrodes. The resulting data can show the spatial distribution of neuronal activity more clearly than raw voltage recordings, as it eliminates the influence of volume conduction, which can obscure the true locations of neuronal sources (Freeman & Nicholson, 1975).
Generation of CSD Maps
To generate CSD maps, the recorded EEG or LFP data are first preprocessed to remove noise and artifacts. The voltage differences between adjacent electrodes are then calculated to obtain the first spatial derivative. A second derivative is subsequently computed to produce the CSD values, which represent the current density at each point in the electrode array. These values are then visualized as maps, where color gradients indicate the intensity and direction of current flow (Poghosyan & Ioannides, 2007).
Metrics Used in CSD Analysis
CSD analysis involves several key metrics that provide quantitative insights into the characteristics of neuronal sources and sinks.
Source Strength
Source strength quantifies the magnitude of current sources and sinks. It is typically expressed in units of current density (A/m²) and provides a measure of the intensity of neuronal activity at different locations within the brain (Tenke et al., 2015).Source Distribution
Source distribution describes the spatial extent and distribution of current sources and sinks. It can be used to identify patterns of neural activation and to determine the localization of functional areas within the cortex (Kayser & Tenke, 2006).Source Dynamics
Source dynamics refer to the temporal changes in current density over time. This metric is crucial for understanding the timing and sequence of neural events, which is particularly important in studies of sensory processing and cognitive function (Mitzdorf, 1985).Graphic Methods for CSD Representation
Graphical representation of CSD data is essential for interpreting the spatial and temporal patterns of neuronal activity. Several methods are commonly used to visualize CSD maps.
Color Maps
Color maps are one of the most common methods for displaying CSD data. In these maps, different colors represent different magnitudes of current density, with gradients indicating the intensity and direction of currents. Color maps provide an intuitive and easily interpretable visualization of CSD data, making it easier to identify areas of high activity (Tenke et al., 2015). Mu rhythm color map © John S. Anderson.
Note: this example shows the same EEG data in a topographic display showing the 10-11 Hz mu activity at C3 and C4 and the double harmonic at 19-20 Hz in the same locations. This is often referred to as the “owl eye” display because the presence of mu rhythm at C3 and C4 produces a display that looks like the face of an owl.
Contour Plots
Contour plots use lines to represent areas of equal current density, similar to topographic maps used in geography. These plots can be useful for identifying the precise locations of sources and sinks and for visualizing the spatial structure of neural activity (Freeman & Nicholson, 1975). Contour plot by Li et al. (2022), available via license: CC BY 4.0.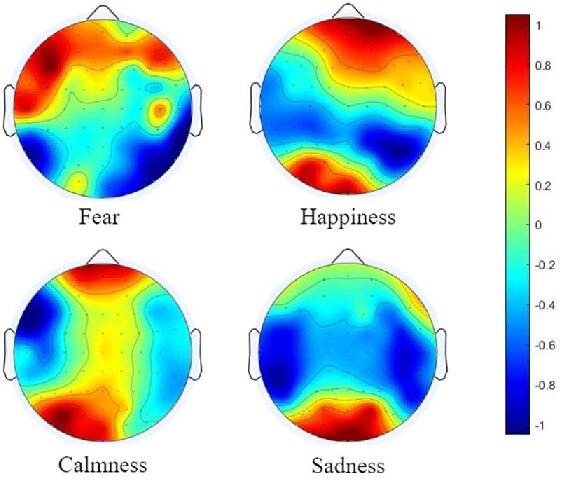
3D Surface Plots
3D surface plots provide a three-dimensional representation of CSD data, where the height of the surface corresponds to the magnitude of current density. These plots can offer a more detailed view of the spatial distribution of neural activity, making it easier to identify complex patterns and interactions between different brain regions (Nicholson & Freeman, 1975). 3D surface plot © NeuroGuide™.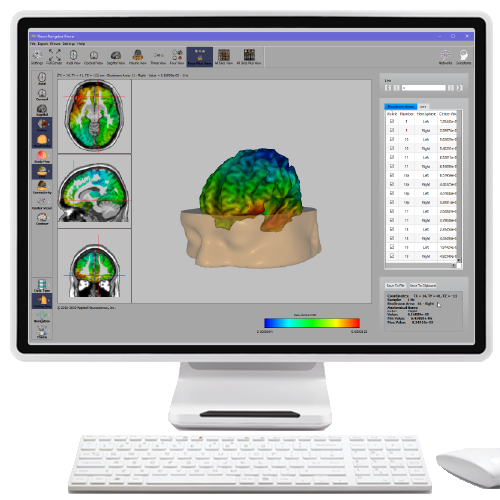
Time-Series Plots
Time-series plots display the temporal evolution of CSD values at specific locations or across the entire electrode array. These plots are crucial for understanding the dynamics of neural activity and for correlating CSD data with behavioral or sensory events (Mitzdorf, 1985). EEG time-series plot by Samuelgthorpe|plotly.
LORETA, sLORETA, and eLORETA
Low resolution electromagnetic tomography (LORETA) is Pascual-Marqui, Michel, and Lehman's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp. In this context, tomography refers to two-dimensional coronal, horizontal, and sagittal brain slices. LORETA does not identify subcortical sources like the amygdala or thalamus located below the cortical hemispheres (Thompson & Thompson, 2015).LORETA represents cortical sites using three-dimensional voxels, which are volumetric units. While its original voxels had a 7-mm spatial resolution (7 mm x 7 mm x 7 mm), the spatial resolution has increased to 5 mm (5 mm x 5 mm x 5 mm). LORETA values are expressed in amperes per cubic centimeter.
LORETA assigns each voxel x, y, and z Talairach coordinates referencing the original Talairach atlas and subsequent atlases like the Montreal Neurological Institute (MNI) atlas. Talairach coordinate assignment is based on vertical distance from a horizontal line from the anterior commissure (origin) to the posterior commissure. For example, x46, y33, z40 corresponds to Brodmann area 8.
These stereotaxic coordinates are primarily independent of brain shape and volume, which has permitted their use in other imaging methods (e.g., positron emission tomography (PET) and magnetic resonance imaging (MRI). Graphic courtesy of BrainMaster Technologies.
Standardized LORETA (sLORETA) achieves a resolution of 1 cubic centimeter. Smaller voxels more precisely localize cortical EEG sources of surface potentials. sLORETA estimates individual voxel's electrical potentials without regard to their frequency. sLORETA values are expressed in normalized F values. This refinement of LORETA trades absolute units of current density for reduced noise and more precise source localization. This is important because LORETA's "three-sphere model," which assumes different cortex, skull, and skin conductivity, suffers from artifacts ("ghost images") and limited source localization (Thompson & Thompson, 2015). Graphic courtesy of BrainMaster Technologies.

A third version of LORETA, eLORETA, exact low resolution brain electromagnetic tomography, claims no localization error (Thompson & Thompson, 2015).
Laplacian Analysis
Surface Laplacian (SL) analysis, which is also called current source density (CSD) and scalp current density (SCD), is a family of mathematical algorithms that provide two-dimensional images of radial current flow from cortical dipoles to the scalp. Positive values represent the current flow from the cortex to the scalp (sources). Negative values represent the current flow from the scalp to the brain (sinks).Unlike the LORETA family of inverse solutions, SL analysis is independent of reference recording procedures--all reference schemes will yield the same current flow estimates and polarity. SL analysis better localizes the EEG signal than surface potentials because it minimizes scalp EEG blurring produced by volume conduction. Finally, unlike inverse solutions, SL makes no assumptions about different tissue conductivity, functional neuroanatomy, cortical geometry and shape, and EEG sources (Kayser & Tenke, 2015). This figure was uploaded by Zoltan Juhasz to ResearchGate.
Applications of CSD Analysis
CSD analysis is widely used in both basic and clinical neuroscience research. In basic research, it helps to elucidate the mechanisms of sensory processing, cognitive functions, and neural oscillations. In clinical settings, CSD analysis can aid in the diagnosis and treatment of neurological disorders by providing detailed maps of brain activity that are not accessible through traditional EEG or LFP recordings (Kayser & Tenke, 2006).
Conclusion
Current Source Density (CSD) analysis is a valuable tool in neuroscience, offering detailed insights into the spatial and temporal patterns of neural activity. By using metrics such as source strength, distribution, and dynamics, and employing various graphic methods for representation, researchers can gain a deeper understanding of brain function and dysfunction. The continued development and application of CSD analysis techniques promise to advance our knowledge of the brain and improve clinical outcomes.
Glossary
3D surface plots: three-dimensional representations of qEEG data where the height of the surface corresponds to the magnitude of current density, providing a detailed view of the spatial distribution of neural activity.
color maps: visual representations of qEEG data where different colors indicate varying magnitudes of brain activity or connectivity, allowing for intuitive interpretation of complex data.
comodulation: a measure of the relationship between the amplitude envelopes of oscillations in different frequency bands, either within the same brain region or across different regions. It reflects how the modulation of oscillatory power in one frequency band is coupled with the modulation of power in another band, providing insights into the functional interactions and connectivity within the brain.
contour plots: graphs that use lines to represent areas of equal current density or activity in qEEG data, similar to topographic maps, to visualize the spatial structure of neural activity.
Current Source Density (CSD) mapping: a technique that calculates the local current sources and sinks in the brain by analyzing the spatial distribution of voltage gradients from EEG data, providing high spatial resolution of neural activity.
Exact Low-Resolution Brain Electromagnetic Tomography (eLORETA): an advanced source localization method that improves upon LORETA by incorporating both electric and magnetic field data to enhance the accuracy of identifying the sources of brain activity.
Laplacian analysis: a mathematical method used in QEEG to enhance spatial resolution by calculating the second spatial derivative of the EEG signal, effectively highlighting local sources of brain activity.
local field potentials (LFPs): electrical potentials recorded from the brain that reflect the summed electrical activity of a group of neurons in a localized area, often used in conjunction with QEEG to study brain function.
Low Resolution Electromagnetic Tomography (LORETA): a technique used in qEEG to localize the sources of brain activity with low spatial resolution, providing a 3D representation of the electrical activity within the brain.
source distribution: the spatial extent and pattern of current sources and sinks in the brain, providing information about the localization and spread of neural activity.
source dynamics: the temporal changes in current density over time, reflecting the timing and sequence of neural events, crucial for understanding the dynamics of brain activity.
source strength: a measure of the magnitude of current sources and sinks in the brain, expressed in units of current density (A/m²), indicating the intensity of neuronal activity.
Standardized Low-Resolution Brain Electromagnetic Tomography (sLORETA): a variation of LORETA that standardizes the current density values, improving the statistical reliability and localization accuracy of brain activity sources.
Surface Laplacian (SL) analysis: a method that enhances the accuracy of LORETA by standardizing current density values, improving the localization of brain activity and statistical reliability of the results.
Talairach coordinate: a system of anatomical coordinates used to identify locations within the brain based on a standardized brain atlas, facilitating the comparison of neuroimaging data across different studies.
time-series plots: graphs that display the temporal evolution of EEG or QEEG data at specific locations or across the entire electrode array, used to analyze the dynamics of brain activity over time.
voxel: a three-dimensional pixel representing a volumetric element in neuroimaging data, such as QEEG or MRI, used to localize and quantify brain activity within a specific region.
Test Yourself on ClassMarker
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, explain how eLORETA can enhance source localization.
References
Ancoli, S., & Kamiya, J. (1978). Methodological issues in alpha biofeedback training. Biofeedback and Self-Regulation, 3(2). 159-183. https://doi.org/0.1007/BF00998900
Berger, H. (1929). Über das Elektrenkephalogramm des Menschen. Archiv f. Psychiatrie, 87, 527–570.
Boyce, R., Glasgow, S. D., Williams, S., & Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science, 352(6287), 812-816. https://doi.org/10.1126/science.aad5252
Brown, B. (1974). New mind, New body: Biofeedback: New directions for the mind. Harper & Row.
Budzynski, T. H. (1976). Biofeedback and the twilight states of consciousness. In G. E. Schwartz & D. Shapiro (Eds.). Consciousness and self-regulation. Springer.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. (2005). An open clinical trial utilizing real-time EEG operant conditioning as an adjunctive therapy in the treatment of crack cocaine dependence. Journal of Neurotherapy, 9(2). https://doi.org/10.1300/J184v09n02_03
Buzsáki, G. (2006). Rhythms of the brain. Oxford University Press.
Canolty, R. T., Edwards, E., Dalal, S. S., Soltani, M., Nagarajan, S. S., Kirsch, H. E., ... & Knight, R. T. (2006). High gamma power is phase-locked to theta oscillations in human neocortex. Science, 313(5793), 1626-1628. https://doi.org/10.1126/science.1128115
Cox, C. L., Huguenard, J. R., & Prince, D. A. (1997). Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proceedings of the National Academy of Sciences, 94(16), 8854-8859. https://doi.org/10.1073/pnas.94.16.8854
Davey, C. G., & Harrison, B. J. (2018). The brain's center of gravity: How the default mode network helps us to understand the self. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 17(3), 278-279. https://doi.org/10.1002/wps.20553
Freeman, J. A., & Nicholson, C. (1975). Experimental optimization of current source-density technique for anuran cerebellum. Journal of Neurophysiology, 38(2), 369-382. https://doi.org/10.1152/jn.1975.38.2.369
Green, E., & Green, A. (1977). Beyond biofeedback. Knoll Publishing Company.
Gruzelier, J. H., Holmes, P., Hirst, L., Bulpin, K., Rahman, S., van Run, C., & Leach, J. (2014). Replication of elite music performance enhancement following alpha/theta neurofeedback and application to novice performance and improvisation with SMR benefits. Biological Psychology, 95, 86-107. https://doi.org/10.1016/j.biopsycho.2013.11.001
Hardt, J., & Kamiya, J. (1976). Some comments on Plotkin’s self-regulation of the electroencephalographic alpha. J. Exp. Psychol., 105(1), 100-108.
Kamiya, J. (1961). Behavioral, subjective, and physiological aspects of drowsiness and sleep. In D. W. Fiske, & S. R. Maddi (Eds.). Functions of varied experience. Dorsey Press.
Kamiya, J. (1962). Conditioned discrimination of the EEG alpha rhythm in Humans. Abstract presented at the Western Psychological Association meeting.
Kamiya, J. (1968). Conscious control of brain waves. Psychology Today, 1, 56-63.
Kamiya, J. (1969). Operant control of the EEG alpha rhythm and some of its reported effects on consciousness. In C. Tart (Ed.), Altered states of consciousness. John Wiley and Sons.
Kayser, J., & Tenke, C. E. (2006). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology, 117(2), 348-368. https://doi.org/10.1016/j.clinph.2005.08.034
Lopes da Silva, F. (2013). EEG and MEG: Relevance to neuroscience. >Neuron, 80(5), 1112-1128. https://doi.org/10.1016/j.neuron.2013.10.017
Mitzdorf, U. (1985). Current source-density method and application in cat cerebral cortex: Investigation of evoked potentials and EEG phenomena. Physiological Reviews, 65/(1), 37-100. https://doi.org/10.1152/physrev.1985.65.1.37
Nicholson, A. A., Densmore, M., MicKinnon, M. C., Neufeld, R. W. J., Frewen, P. A. Théberge, J., Jetly, R., Richardson, J. D., & Lanius, R. A. (2019). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol Med, 49(12), 2049-2059. https://doi.org/10.1017/S003329171800286
Nicholson, C., & Freeman, J. A. (1975). Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. Journal of Neurophysiology, 38(2), 356-368. https://doi.org/10.1152/jn.1975.38.2.356
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., & Lanius, R. A. (2020). A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. Neuroimage Clin., 28, 102490. https://doi.org/10.1016/j.nicl.2020.102490
Nicholson, A. A., Ros, T., Jetly, R., & Lanius, R. (2020). Regulating posttraumatic stress disorder symptoms with neurofeedback: Regaining control of the mind. Journal of Military Veteran and Family Health 6(S1), 3-15. https://doi.org/10.3138/jmvfh.2019-0032
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy, 3, 37–55.
Poghosyan, V., & Ioannides, A. A. (2007). Precise mapping of early visual responses in space and time. NeuroImage, 35(2), 759-770. https://doi.org/10.1016/j.neuroimage.2006.11.052
Ros, T., Enriquez-Geppert, S., Zotev, V., Young, K. D., Wood, G., Whitfield-Gabrieli, S., & Kober, S. E. (2014). Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain, 137(6), e278. https://doi.org/10.1093/brain/awu203
Scott, W., & Kaiser, D. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455-469. https://doi.org/10.1081/ada-200056807
Tenke, C. E., Kayser, J., & Murray, M. M. (2015). Reference-free quantification of EEG spectra: Combining current source density (CSD) and frequency principal components analysis (fPCA). Clinical Neurophysiology, 126(12), 2324-2337. https://doi.org/10.1016/j.clinph.2015.03.012
Weise, R., von Mengden, I., Glos, M., Garcia, C., & Penzel, T. (2013). P 153. Influence of transcranial slow oscillation current stimulation (tSOS) on EEG, sleepiness and alertness. Clinical Neurophysiology, 124(10), e137. https://doi.org/10.1016/j.clinph.2013.04.230
H. Reports Based on QEEG Metrics Should Relate These to Clinical History, Symptoms, and Other Clinical Assessments
Quantitative electroencephalography (qEEG) has gained prominence in clinical neuroscience for its ability to provide detailed, objective data on brain function. To maximize its clinical utility, qEEG metrics should be interpreted in the context of a patient’s clinical history, symptoms, and other clinical assessments.
Please click on the podcast icon below to hear a lecture over Section H.

This comprehensive approach enhances diagnostic accuracy and informs personalized treatment strategies. This report discusses the importance of integrating qEEG metrics with clinical data and explores how this integration can be effectively achieved. The following lecture discusses qEEG assessment © John S. Anderson.
Introduction
The qEEG involves the quantitative analysis of EEG data to identify abnormalities in brain activity. While the qEEG provides valuable insights into neural function, its interpretation can be significantly enriched by relating the findings to clinical history, presenting symptoms, and results from other clinical assessments. This holistic approach ensures that qEEG data are used not in isolation but as part of a broader diagnostic and therapeutic framework.
The following lecture discusses the clinical utility of qEEG assessment © John S. Anderson.
An Integrative Assessment Approach
Measures and Methods
EEG Variables
A natural measure to use for ongoing assessment is the EEG variable (e.g., SMR power) that has been chosen for training, based on the hypothesis that neurofeedback should produce a change in that variable (e.g., rewarding SMR should lead to SMR increase). Recent research has shown that neurofeedback at a single site may also have effects that generalize to other sites and brain networks (e.g., Nicholson, Ros, Densmore, Frewen, et al., 2020). Therefore, practitioners who have access to 19-channel qEEG hardware and software may additionally be interested in assessing how single-channel changes may have generalized to changes in network function (Thatcher, 2020).The movie below is a 19-channel BioTrace+ /NeXus-32 display of SMR activity © John S. Anderson. Brighter colors represent higher SMR amplitudes. Frequency histograms are displayed for each channel. Notice the runs of high-amplitude SMR activity.
The EEG variable targeted for training is usually measured during a pre-training baseline for each session. The saved data can be artifacted. The value of the EEG variable can then be graphed from session to session, with the value from the intake assessment being used as a baseline for these within-training session baselines (see Figure 1).
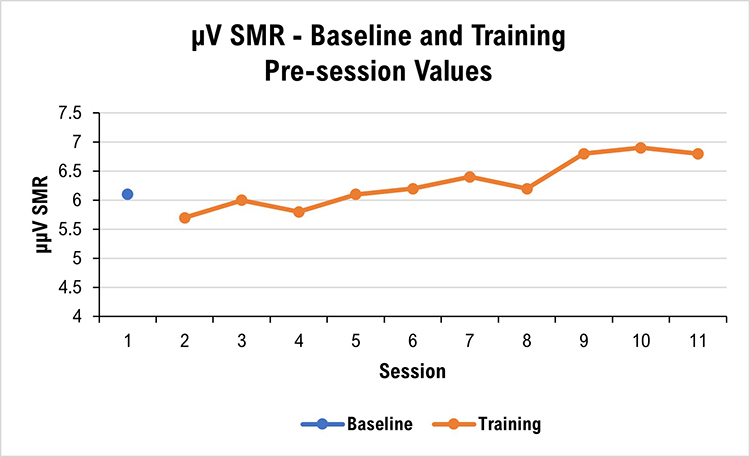
Many neurofeedback platforms make it easy to collect and graph EEG variables of interest, whether derived from single or multiple channels. Some platforms include a graphing capability, while the results of other platforms need to be copied and then pasted into a spreadsheet for graphing.
The EEG variable can also be measured again during a training session’s post-training baseline. This allows comparison with that session’s pre-training baseline and may show that the session’s training has produced a change in the intended direction. The pre-training and post-training baseline data from a series of sessions can be graphed separately or together, or a series of within-session change scores can be graphed (see Figure 2).
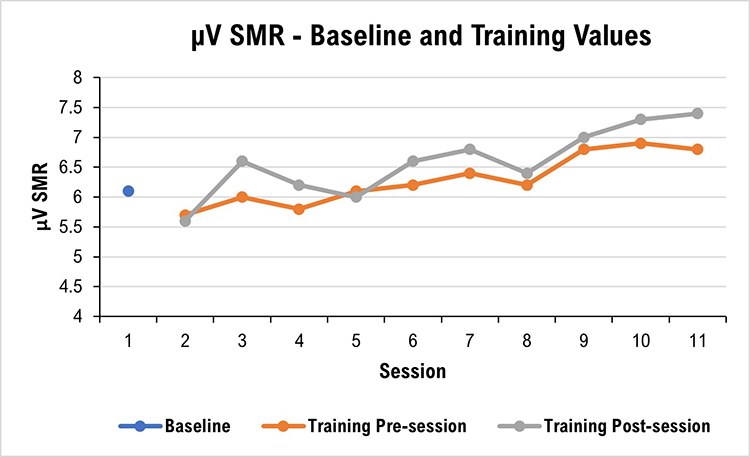
It can sometimes be interesting to graph EEG data from training blocks during a session. For example, if the client receives 10, 3-minute trials of neurofeedback in a session, the clinician can graph the series to show whether changes occur (see Figure 3).
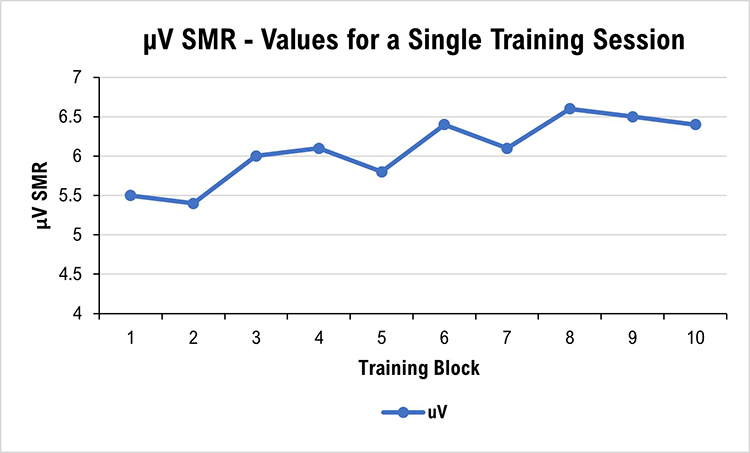
Qualitative Anecdotal Report
One of the products of intake assessment is the choice of a goal that has practical consequences for the client (e.g., feel less depressed, feel more relaxed, experience fewer headaches, concentrate better, study longer, avoid people less). Goals may be specified in positive or negative terms (e.g., more of A, or less of B) and refer to either internal states (e.g., thinking, emotion, somatic experience) or to behavior (e.g., number of pages written) (Hurn, Kneebone, & Cropley, 2006).Ongoing assessment of a qualitative nature can provide a detailed and informative description of how the client’s presenting problem changes during training. Anecdotal reporting invites the client to describe a personal situation or anecdote, together with the various dimensions of internal experience and behavioral performance, to represent the current state of their presenting problem or goal achievement.
At the beginning of each session, the neurofeedback provider can ask the client or a collateral informant to describe changes they are observing in the problem or goal for which they initially sought neurofeedback training. For example, both the child receiving neurofeedback and their parent can be asked how distractible the child has been during the past few days and their responses compared. Or, they could be asked to describe the situation in which they were most distractible to provide an anecdotal report linked to a specific problem at a particular time and place. Practitioner notes can be compared from session to session to give an impressionistic view of change.
Ongoing qualitative assessment, at least for the first few training sessions, is also helpful concerning monitoring for the possibility of unwanted side-effects of training (e.g., fatigue, excessive arousal, headache). Graphic courtesy of Migraine Buddy.

Quantitative Self-Report
Although such problems and goals may be expressed somewhat imprecisely and colloquially by clients, it can be helpful to define them more formally or quantitatively. Quantification makes it possible to graph progress over time and more easily see change (Greenhalgh & Meadows, 1999).Internal subjective states can be quantified with visual analog or Likert rating scales that label points on the scale. It is helpful to structure items with either Likert or visual analog scales (VAS). The rating can then be quantified and graphed for later review by the client and practitioner (Joshi et al., 2015; Wewers & Lowe, 1990). Definition of the scale item and labeling points on the scale help make the scale more reliable and valid as a measurement tool. At the beginning of a session, the client can mark their typical state during the previous few days or their state at the time of the session. VAS pain scale graphic © Overearth/Shutterstock.com.
Behavioral problems or goals can be operationally defined (i.e., in terms of the operations used to construct the definition; Martin & Pear, 2019). For example, suppose increased concentration (an internal state) is the goal. In that case, duration of study time may be a useful behavioral goal to define in terms of minutes elapsed the previous day. At the beginning of a session, the neurofeedback provider can then ask the client to retrospectively estimate their study time during the day before the session.
One way of defining action-oriented or behavioral goals more formally is to use the SMART goals framework. SMART stands for specific, measurable, achievable, relevant, and time-bound (Bovend’Eerdt et al., 2009). Behavior analytic tactics (Cooper et al., 2007) can be used to address the specific, measurable, and time-bound components of SMART goals, for example, by defining goals in terms of frequency, intensity, and duration, and in terms of the context in which they should be met (e.g., during a typical week with ordinary stressors, a headache will occur not more than twice with an average duration of not more than 1 hour, and average severity of not more than 2 on a scale of 0 to 5). SMART goals graphic © Appleing/Shutterstock.com.
After intake assessment and definition of a SMART goal, the practitioner can quickly ask the client at the beginning of training sessions about the measurable feature of the SMART goal (e.g., for a socially anxious client, how many times did they speak at all to anyone besides the cashier during their most recent three trips for coffee?). Because the SMART goals are concrete and specific, the measurable rating that the client gives will tend to be reliable and, therefore, more valid than a response to a more open-ended question related to a general goal. Because a SMART goal can be easily quantified, their baseline and session-by-session values can be graphed for visual inspection and interpretation by the client and practitioner.
Self-Monitoring
Like the within-session reports described in the previous two sections, clients can be asked to make ratings at assigned times in assigned situations outside the neurofeedback session (e.g., at home after dinner, rate your level of calmness for the day taken as a whole). This type of self-monitoring of symptoms (e.g., stress, distractibility) or abilities (e.g., sustained attention, duration of time at a task, accuracy of task completion) relevant to the client’s presenting concerns can be started after intake assessment.Self-monitoring benefits the client by increasing their self-awareness in day-to-day situations and potentially strengthening their ability to self-regulate “internal” subjective experiences such as emotion, cognition, and tonic or reactive physiological states in service of effective behavior. Graphic courtesy of the Optimism family of mental health applications.
It can be useful to structure self-monitoring by identifying specific situations or times to use self-monitoring scales between training sessions. For example, the client can be asked to rate their concentration level during one or more particular classroom activities (e.g., completing algebra problems). Selecting a specific situation for rating helps to make the rating more reliable and valid by reducing variability due to differences among many situations.
It may be more relevant for other clients to use a time-based method for self-monitoring. The client can be asked, for example, to rate their level of calmness at the beginning of the day after their first hour of work or the end of the day based on their overall impression of the day as a whole. When self-monitoring between training sessions is used, it is essential to plan with the client to remember when to implement the measure. Specifically defined situational or temporal cues are effective in this regard. If self-monitoring in a particular situation is required, then verbal or visual reminders are helpful. For example, a post-it note in a notebook used for algebra can cue clients to rate their concentration level after class. Electronic alarms can also serve to prompt completion of the rating, and smartphone apps can be used in place of paper-and-pencil forms (Bakker & Rickard, 2018; Melbye et al., 2020).
Behavioral Observation
Direct observation of behavior (Cooper et al., 2007; Kamphaus & Dever, 2016) is often used in research and clinical settings to assess the baseline level with which an observable behavior of interest occurs. For example, a parent may count the number of interruptions a child makes during a period to calculate a rate per hour. A teacher may time record whether or not a student is studying at defined times during a class. These observations are readily quantifiable and can be graphed to track progress during an ongoing assessment. Graphic © Photographee.eu/Shutterstock.com.
Rating Scales
In contrast to self-report scales, rating scales have been evaluated for their psychometric properties (Baer & Blais, 2010; Tate, 2010). They provide a quick and valid method to assess progress during neurofeedback training, especially if they have already been employed during intake assessment. The domains that rating scales sample are numerous, including emotional, cognitive, and physiological experience and functional abilities. Completion of rating scales relevant to the client’s presenting concerns can be done periodically before training sessions or at home between training sessions. Examples of rating scales are the Beck Depression Inventory-II (Beck, 1996) and the Depression, Anxiety and Stress Scale (Lovibond & Lovibond, 1995). Graphic © PsychCorp.
Practitioners are increasingly teaching clients mindfulness meditation in conjunction with neurofeedback and biofeedback (Khazan, 2013). Mindfulness meditation produces physiological, cognitive, and emotional benefits. There is reason to think that adding mindfulness to neurofeedback training may improve training effects. The client taught to apply mindfulness during neurofeedback sessions is likely to be more conscious of the mind-body state that occurs when feedback is either present or absent. The resulting self-awareness can then be the basis for voluntary self-control, that is, the intentional recreation, either during a training session or during a relevant non-session situation, of the mind-body state reinforced during neurofeedback. If the practitioner does teach mindfulness meditation to their client, several psychometrically sound instruments can be used to measure the client’s increasing skill and application (Lau et al., 2006; MacKillop & Anderson, 2007).
Cognitive Tests
Tests of thinking and cognition can also be readministered during neurofeedback training to assess progress. However, challenges to doing so are the practitioner's time to administer the tests and the potential for spuriously inflated results due to practice effects. The former can be addressed in some cases with computerized tests that the client can complete after the practitioner provides instructions (e.g., CNS Vital Signs; Gaultieri & Johnson, 2006), while the latter can be addressed using alternate forms of tests that are vulnerable to practice effects (e.g., Repeatable Battery for the Assessment of Neuropsychological Status; Randolph et al., 1998). Graphic © Pearson.
Psychophysiological Assessment
Several platforms provide the ability to conduct concurrent neurofeedback and peripheral biofeedback. With such systems, psychophysiological measures can be easily recorded simultaneously with EEG measures. As described above, this may be done during each session before starting neurofeedback training or just following training. If peripheral biofeedback training is not provided, these measures assess the generalization of neurofeedback training effects to other relevant domains (e.g., skin conductance, skin temperature, respiration, heart rate variability, EMG).Decision-Making
Ongoing assessment during neurofeedback occurs within the framework of evidence-based practice. The main components of evidence-based practice are scientific evidence, client condition, values and preferences, and available resources integrated by practitioner expertise (McMaster University Health Sciences Library, 2021, May 5; Melnyk, Fineout-Overholt, Stillwell, & Williamson, 2010) (Figure 4). In particular, ongoing assessment collects data that informs decision-making by the client and practitioner about whether to continue the neurofeedback protocol with which they began training, change the protocol, or discontinue training. Graphic created by authors.
Assessment results that show improvement encourage the continuation of neurofeedback until goals or a point of diminishing returns are reached. At that point, neurofeedback may be discontinued or new goals addressed.
When results show insufficient change, then the client and practitioner can consider different evidence-based protocols to apply (Tan et al., 2016).
Alternatively, reassessment may be helpful. If 19-channel qEEG methods have not already been used during the initial assessment, a 19-channel assessment may identify sites and networks that neurofeedback up to that point has not addressed.
For many clients, it may be the case that, however helpful neurofeedback is, it may be insufficient to resolve a presenting problem or reach a goal by itself. Integrating neurofeedback with other interventions, whether by one or more practitioners, is becoming more common. For example, Thompson and Thompson (2015) describe complementary methods such as heart rate variability training and transcranial electrical stimulation. Weiner (2017) reviews how neurofeedback and psychotherapy can be conducted together.
Some clients experience significant overriding developments during neurofeedback training that preclude successful treatment. For example, a client may develop a new medical condition or experience considerable family disruption. In these cases, training may be postponed until the resolution of these new concerns.
Summary
This section has reviewed approaches to ongoing assessment and placed them within ethical and evidence-based health care frameworks. In addition, decision-making options have been suggested based on observed results of training.
Importance of Integrating QEEG with Clinical Data
Enhanced Diagnostic Accuracy
qEEG metrics can reveal subtle abnormalities in brain function that might not be apparent through clinical observation alone. By integrating qEEG data with a patient’s clinical history and symptoms, clinicians can achieve a more accurate diagnosis. For instance, The qEEG can help differentiate between types of attention-deficit/hyperactivity disorder (ADHD) by identifying specific patterns of brain activity associated with each subtype (Clarke et al., 2001).Personalized Treatment Planning
Understanding the relationship between qEEG metrics and clinical symptoms allows for more personalized treatment strategies. For example, in depression, the qEEG can identify patterns of frontal alpha asymmetry, which have been associated with treatment response to certain antidepressants (Bruder et al., 2008). This information can guide the selection of appropriate medications and therapeutic interventions, potentially improving treatment outcomes.Monitoring Treatment Efficacy
The qEEG provides a quantitative measure of brain activity that can be used to monitor changes over the course of treatment. By comparing pre- and post-treatment QEEG data and correlating these changes with clinical symptoms, clinicians can assess the effectiveness of interventions and adjust treatment plans as needed. This approach is particularly useful in conditions such as epilepsy, where the qEEG can track the impact of antiepileptic drugs on seizure activity (Niedermeyer & da Silva, 2004).Methods of Integrating the qEEG with Clinical Data
Clinical History
A detailed clinical history is essential for interpreting qEEG data. This includes information on the patient’s medical, psychiatric, and family history, as well as any previous treatments and their outcomes. For example, in patients with a history of traumatic brain injury (TBI), QEEG can identify patterns of slow-wave activity that correlate with cognitive deficits, helping to tailor rehabilitation programs (Thornton & Carmody, 2009).Symptomatology
Relating qEEG findings to specific symptoms can enhance the clinical relevance of the data. For instance, increased theta/beta ratio observed in the qEEG is often associated with inattention in ADHD (Arns et al., 2013). By linking these metrics to clinical symptoms, clinicians can better understand the underlying neural mechanisms and develop targeted interventions.Other Clinical Assessments
Combining the qEEG with other clinical assessments, such as neuroimaging, neuropsychological tests, and behavioral evaluations, provides a more comprehensive view of the patient’s condition. For example, combining a qEEG with MRI can correlate functional abnormalities with structural changes, offering deeper insights into disorders such as Alzheimer’s disease (Babiloni et al., 2006).Case Examples
Attention-Deficit/Hyperactivity Disorder (ADHD)
In ADHD, the qEEG has been used to identify increased theta/beta ratios, which are indicative of attentional deficits. When combined with clinical history and behavioral assessments, these findings can differentiate ADHD subtypes and guide treatment. For example, neurofeedback therapy targeting the normalization of theta/beta ratios has shown promise in reducing ADHD symptoms (Arns et al., 2013).Major Depressive Disorder (MDD)
Patients with MDD often exhibit increased alpha activity in the frontal cortex. qEEG metrics can help predict treatment response to SSRIs, as patients with greater frontal alpha asymmetry are more likely to benefit from these medications (Bruder et al., 2008). By integrating qEEG data with clinical assessments of depressive symptoms, clinicians can personalize treatment plans and improve outcomes.Epilepsy
In epilepsy, the qEEG can identify epileptiform discharges and localize seizure foci. When combined with clinical history and seizure diaries, the qEEG helps to tailor antiepileptic drug regimens and evaluate their efficacy. This comprehensive approach enhances the management of epilepsy and reduces the frequency of seizures (Niedermeyer & da Silva, 2004).Summary
Integrating qEEG metrics with clinical history, symptoms, and other clinical assessments provides a holistic approach to diagnosing and treating neurological and psychiatric conditions. This comprehensive strategy enhances diagnostic accuracy, informs personalized treatment plans, and allows for effective monitoring of treatment efficacy. By leveraging the strengths of qEEG and clinical data, clinicians can offer more targeted and effective care for their patients.
Glossary
Attention-Deficit/Hyperactivity Disorder (ADHD): a neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity that interfere with functioning or development.
behavioral observation: the systematic recording and analysis of a person's behaviors and actions in a naturalistic or clinical setting, used to gather objective data on their psychological functioning.
clinical history: a comprehensive account of a patient's medical, psychological, and social background, including past diagnoses, treatments, and significant life events, used to inform assessment and treatment planning.
cognitive tests: standardized assessments designed to measure various aspects of cognitive functioning, such as memory, attention, language, and problem-solving skills, often used to diagnose cognitive impairments or disorders.
collateral informant: an individual who can provide detailed background information or more accurate information regarding deviant behavior.
ongoing assessment: the continuous evaluation of client progress.
psychophysiological assessment: the evaluation of the physiological processes underlying psychological states, using measures such as heart rate, skin conductance, and brain activity to assess the interplay between mind and body.
qualitative anecdotal report: a narrative description of changes in the problem or goal by the client or collateral informant.
quantitative self-report: a client's numerical ratings of symptoms, subjective states, and behaviors.
rating scales: tools used to assess the severity or frequency of specific symptoms or behaviors, often completed by the patient or clinician to provide standardized measurements for comparison and evaluation.
self-monitoring: a client's ratings at assigned times in specified situations outside the neurofeedback session to increaseself-awareness.
SMART: a framework that prescribes goals that are specific, measurable, achievable, relevant, and time-bound.
symptomatology: the set of symptoms and signs associated with a particular psychological disorder or condition, used to guide diagnosis and treatment planning.
Test Yourself on ClassMarker
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, which sounds do you prefer when you have succeeded during neurofeedback training? Which visual displays are more motivating for you?
References
Arns, M., Conners, C. K., & Kraemer, H. C. (2013). A decade of EEG Theta/Beta Ratio research in ADHD: A meta-analysis. Journal of Attention Disorders, 17(5), 374-383. https://doi.org/10.1177/1087054712460087
Babiloni, C., Frisoni, G. B., Pievani, M., Vecchio, F., Geroldi, C., De Carli, C., ... & Rossini, P. M. (2006). Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. NeuroImage, 31(1), 180-189.
https://doi.org/10.1016/j.neuroimage.2005.11.046
Baer, L., & Blais, M. A. (2010). (Eds.). Handbook of clinical rating scales and assessment in psychiatry and mental health. Humana Press.
Bakker, D., & Rickard, N. (2018). Engagement in mobile phone app for self-monitoring of emotional wellbeing predicts changes in mental health: MoodPrism. Journal of Affective Disorders, 227, 432-442. https://doi.org/10.1016/j.jad.2017.11.016
Beauchamp, T. L. (2003). Methods and principles in biomedical ethics. Journal of Medical Ethics, 29, 269-274. https://doi.org/10.1136/jme.29.5.269
Beck, A. T. (1996). Beck Depression Inventory – II. San Antonio, TX: Psychological Corporation.
Bovend’Eerdt, T., J. H., Botell, R. E., & Wade, D. T. (2009). Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clinical Rehabilitation, 23, 352-361. https://doi.org/10.1177/0269215508101741
Bruder, G. E., Sedoruk, J. P., Stewart, J. W., McGrath, P. J., Quitkin, F. M., & Tenke, C. E. (2008). Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: Pre-and post-treatment findings. Biological Psychiatry, 63(12), 1171-1177. https://doi.org/10.1016/j.biopsych.2007.12.029
Clarke, A. R., Barry, R. J., McCarthy, R., & Selikowitz, M. (2001). EEG analysis in attention-deficit/hyperactivity disorder: A comparative study of two subtypes. Psychiatry Research, 103(1), 63-73. https://doi.org/10.1016/S0165-1781(01)00261-3
Cooper, J. O., Heron, T. E., & Heward, W. L. (2007). Applied behavior analysis (2nd ed.). Pearson Merrill Prentice Hall.
Gaultieri, T., & Johnson, L. G. (2006). Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Archives of Clinical Neuropsychology, 21, 623-643. https://doi.org/10.1016/j.acn.2006.05.007
Greenhalgh, J., & Meadows, K. (1999). Effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: A literature review. Journal of Evaluation in Clinical Practice, 5, 401-416. https://doi.org/0.1046/j.1365-2753.1999.00209.x
Hurn, J., Kneebone, I., & Cropley, M. (2006). Goal-setting as an outcome measure: A systematic review. Clinical Rehabilitation, 20, 756-72. https://doi.org/10.1177/0269215506070793
Joshi, A., Kale, S., Chandel, S., & Pal, D. K. (2015). Likert Scale: Explored and Explained. Current Journal of Applied Science and Technology, 7, 396-403.
Kamphaus, R., & Dever, B. V. (2016). Behavioral observations and assessment. In J. C. Norcross, G. R. VandenBos, D. K. Freedheim, & R. Krishnamurthy (Eds.), APA handbook of clinical psychology: Applications and methods (Vol. 3) (pp. 17-29). American Psychological Association.
Khazan, I. Z. (2013). Clinical handbook of biofeedback: A step-by-step guide for training and practice with mindfulness. Wiley-Blackwell.
Lau, M. A., Bishop, S. R., Segal, Z. V., Buis, T., Anderson, N. D., Carlson, L., Shapiro, S., & Carmody, J. (2006). Toronto Mindfulness Scale: Development and validation. Journal of Clinical Psychology, 62, 1445-1467. https://doi.org/10.1002/jclp.20326
Lovibond, S. H. & Lovibond, P. F. (1995). Manual for the Depression Anxiety & Stress Scales (2nd Ed.). Psychology Foundation.
MacKillop, J., & Anderson, E. J. (2007). Further psychometric validation of the Mindful Attention Awareness Scale (MAAS). Journal of Psychopathology and Behavioral Assessment, 29, 289-293. https://doi.org/10.1007/s10862-007-9045-1
Martin, G., & Pear, J. J. (2019). Behavior modification: What it is and how to do it (11th ed.). Routledge.
McMaster University Health Sciences Library. (2021, May 5). https://hslmcmaster.libguides.com/c.php?g=306765&p=2044668
Melbye, S., Kessing, L. V., Bardram, J. E., & Fourholt-Jepsen, M. (2020). Smartphone-base self-monitoring, treatment, and automatically generated data in children, adolescents, and young adults with psychiatric disorders: Systematic review. JMIR Mental Health, 7, e17453.
Melnyk, B. M., Fineout-Overholt, E., Stillwell, S. B., & Williamson, K. M. (2010). Evidence-based practice step by step. American Journal of Nursing, 110, 51-53. https://doi.org/10.1097/01.NAJ.0000366056.06605.d2
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Theberge, J., Jetly, R., & Lanius, R. A. (2020). Randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. NeuroImage: Clinical, 28, e102490. https://doi.org/10.1016/j.nicl.2020.102490
Niedermeyer, E., & da Silva, F. L. (2004). Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins.
Randolph, C., Tierney, M. C., Mohr, E., & Chase, T. N. (1998). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 20, 310–319. https://doi.org/10.1076/jcen.20.3.310.823
Schwartz, M. S., & Andrasik, F. (Eds.). (2016). Biofeedback: A practitioner’s guide (4th ed.). Guilford Press.
Tan, G., Shaffer, F., Lyle, R., & Teo, I. (2016). Evidence-based practice in biofeedback & neurofeedback (3rd Ed.). Association for Applied Psychophysiology and Biofeedback.
Tate, R. (2010). Compendium of tests, scales and questionnaires: Practitioner's guide to measuring outcomes after acquired brain impairment. Psychology Press.
Thatcher, R. W. (2020). Handbook of quantitative EEG and EEG biofeedback (2nd Ed.). ANI Publishing.
Thompson, M., & Thompson, L. (2015). Neurofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Advancement of Psychophysiology and Biofeedback.
Thornton, K. E., & Carmody, D. P. (2009). Traumatic brain injury rehabilitation: QEEG biofeedback treatment protocols.
Weiner, G. (2017). Evolving as a neurotherapist: Integrating psychotherapy and neurofeedback. In T. Collura and J. A. Frederick (Eds.), Handbook clinical QEEG and neurotherapy (pp. 45-54). Routledge.
Wewers, M. E., & Lowe, N. K. (1990). Critical review of visual analogue scales in the measurement of clinical phenomena. Research in Nursing and Health, 13, 227-236. https://doi.org/10.1002/nur.4770130405
