Neuroscience
The easiest way to divide the cortex is into the frontal and posterior cortex. The frontal cortex (frontal lobe) specializes in action, ranging from cognition, emotion, and autonomic control to movements and speech. The posterior cortex (parietal, temporal, and occipital lobes) is concerned with perception and memory. The frontal and posterior cortex, subcortical structures, and the peripheral nervous system provide the hierarchically arranged feedback loops that allow us to interact with our environment to achieve goals successfully.
The prefrontal cortex (PFC) (cortex rostral to the motor association cortex) directs the cognitive and emotional processes, called perception-action cycles, that adapt (and preadapt) us to our environment. The PFC predicts and creates the future. Working with networked brain structures, the PFC marshals its executive functions of planning, attention, working memory, and decision-making to develop innovative and sophisticated actions to pursue future goals (Fuster, 2015).
The nervous system maintains homeostasis by using bottom-up (feedforward) and top-down (feedback) processing. The relationship between the thalamus and cortex best illustrates the interconnectedness of neural networks. Ascending thalamocortical neurons distribute sensory information to appropriate cortical (and subcortical) regions, and descending corticothalamic neurons convey instructions to the thalamus. The nervous system generates EEG activity, ranging from DC potentials to beta-gamma rhythms, using multiple generators that operate as neuroscientist William Calvin's "cerebral symphony." Graphic © adike/Shutterstock.com.

IQCB Blueprint Coverage
This unit addresses II. Neuroscience (8 hours). These areas will be covered in the formal IQCB examination, and we recommend that you review readings over their topics. Please take a ClassMarker exam after you complete each of five sections to assess your mastery.

This unit covers:
A. Cortical and Subcortical Structures Macro and Microanatomy
B. Sensory Pathways
C. Autonomic Nervous System
D. Major Networks
E. Behavioral Correlates to Brain Regions and Networks
Please click on the podcast icon below to hear a full-length lecture for Section A Part 1.

A. CORTICAL AND SUBCORTICAL STRUCTURES MACRO AND MICROANATOMY
We will review Navigating the Brain, The Unfixed Brain, Dissecting Brains, Meninges, Cerebral Ventricles, Glymphatic System, The Brain's Vascular System, General Cortical and Subcortical Divisions, Subcortical and Cortical Generators, and Microanatomy.
Navigating the Brain
Orientations
Three customary planes for viewing the body and brain are sagittal, coronal, and horizontal. The sagittal plane divides the body into right and left halves. The coronal plane separates the body into front and back parts. Finally, the horizontal (transverse) plane divides the brain into upper and lower parts (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.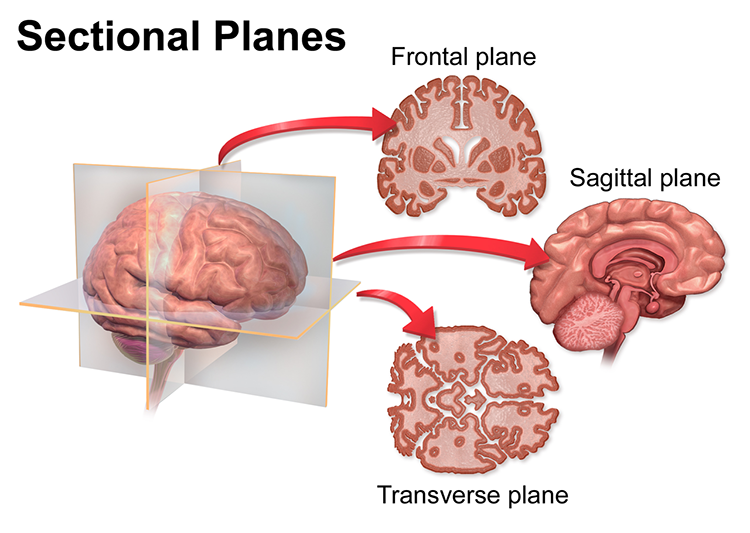
Directional Terms
Important directional terms include medial (toward the middle) and lateral (toward the side), ipsilateral (same side) and contralateral (opposite side), superior (above) and inferior (below), anterior/rostral (toward the head), and caudal (toward the tail), proximal (near the center) and distal (toward the periphery, and dorsal (toward or at the back) and ventral (toward the belly) (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.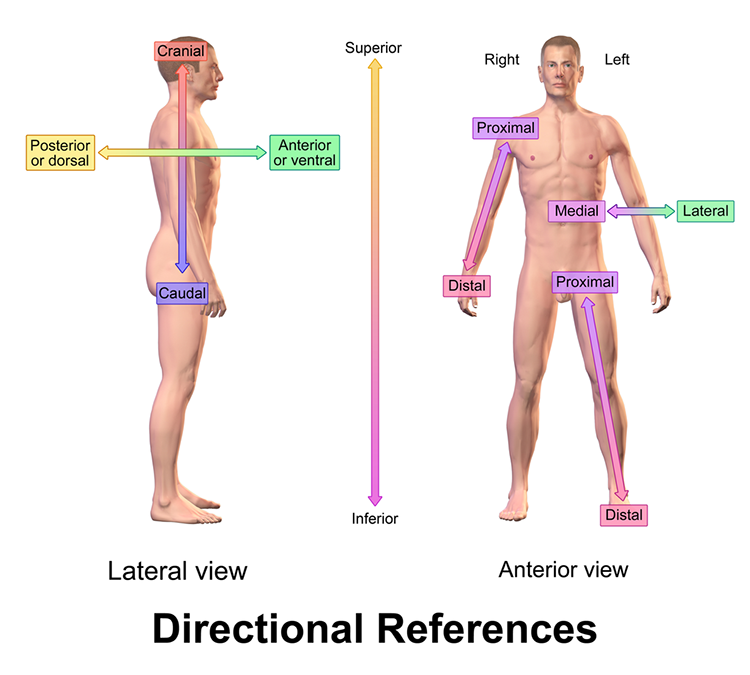
Cortical Features
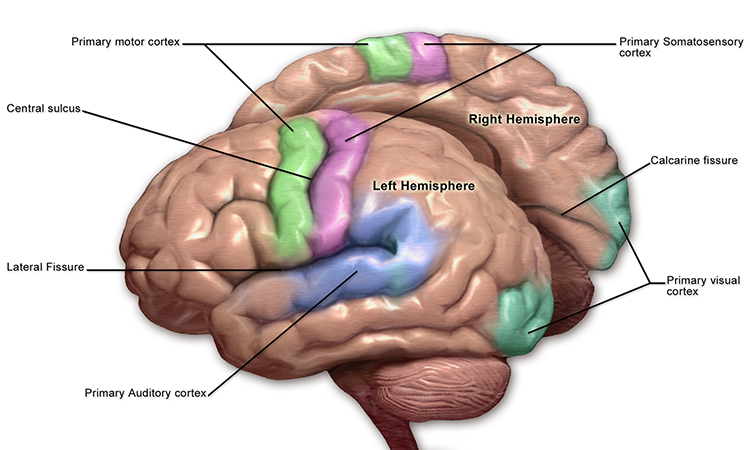
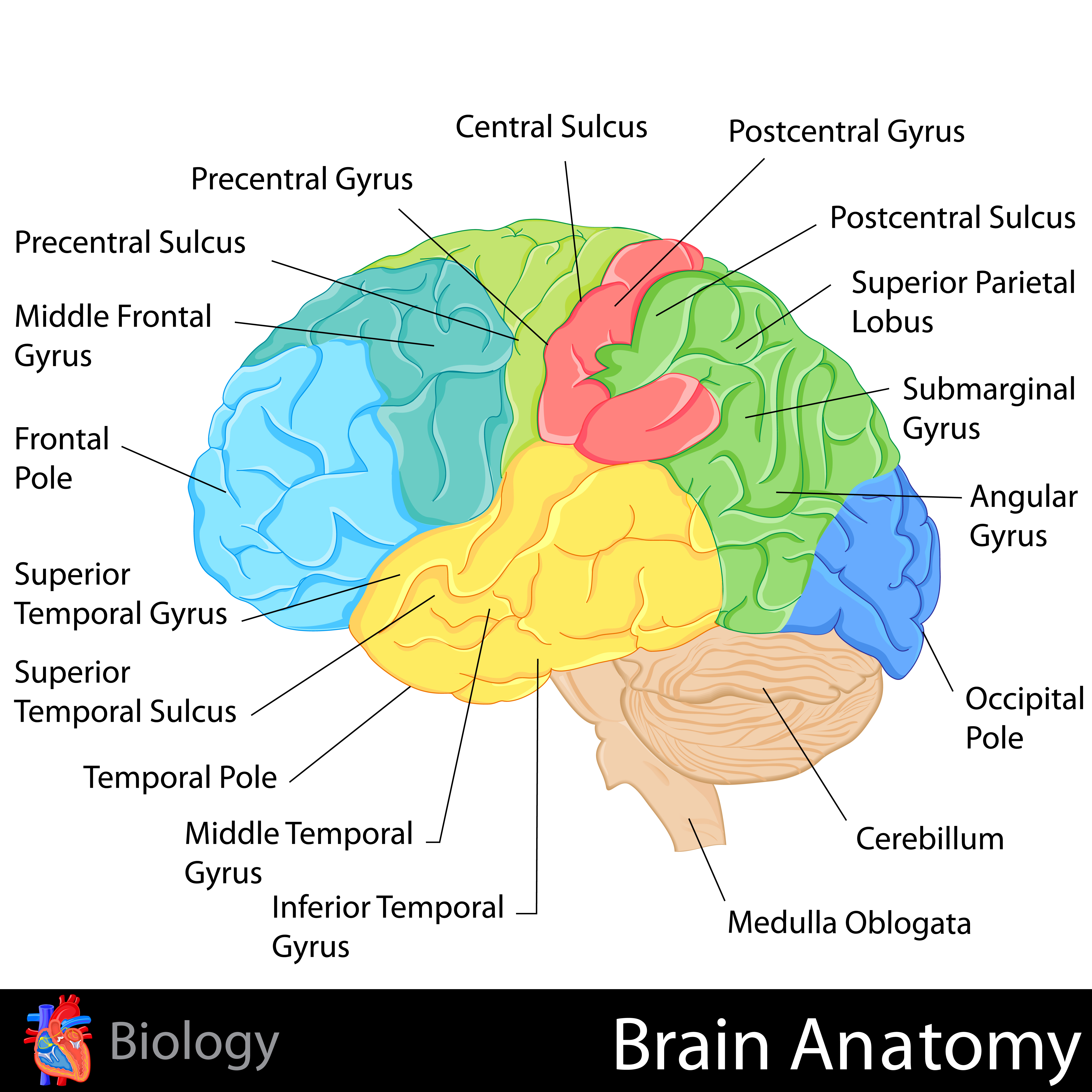
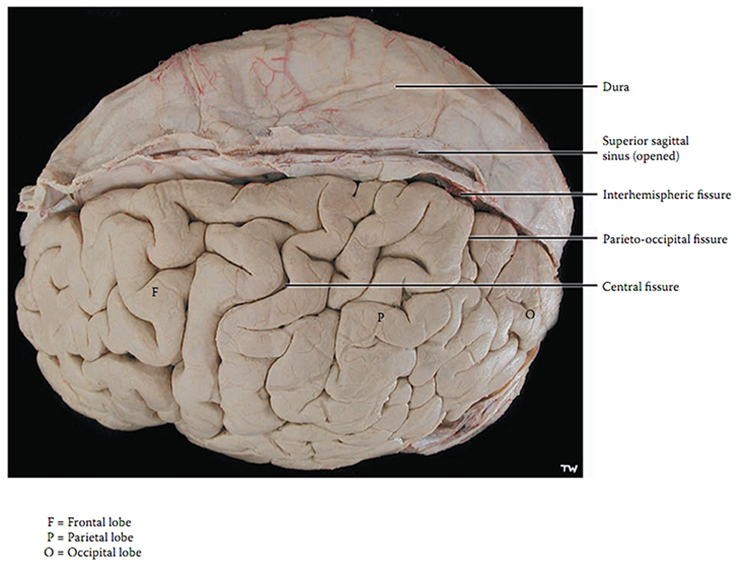
The adult human brain has a volume of about 1100 cm2 and requires convolutions to fit within the skull (Bear, Connors, & Paradiso, 2020). Two-thirds of the cortical surface lies within these folds (Breedlove & Watson, 2020). Anatomists distinguish three topographical features of the cerebral cortex: gyrus, sulcus, and fissure.A gyrus is a ridged area of the brain. The precentral gyrus, anterior to the central sulcus, is the primary motor cortex (controls muscles and movements). The postcentral gyrus, posterior to the central sulcus, is the primary somatosensory cortex (receives somatosensory information).
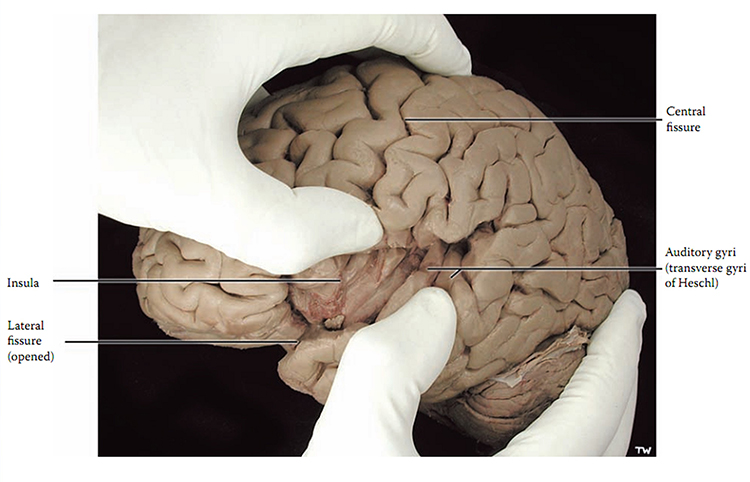
A sulcus is a groove in the cortical surface. As we observed, the central sulcus separates the primary motor cortex from the primary somatosensory cortex. A fissure is a deep groove. The Sylvian fissure (also called the lateral fissure or lateral sulcus) is the upper boundary of the temporal lobe (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.
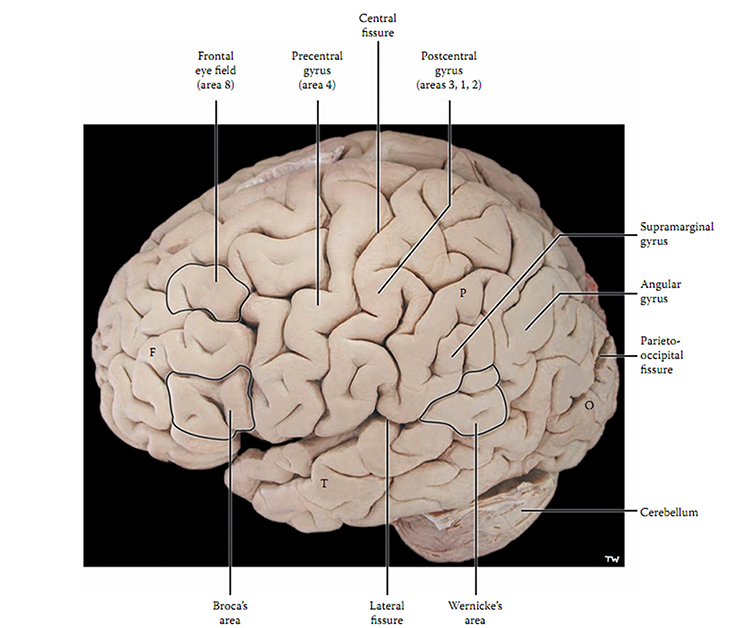
The Unfixed Brain
This video was produced by Suzanne Stensaas, PhD, Department of Neurobiology and Anatomy, and the Spencer S. Eccles Health Sciences Library, University of Utah.
Dissecting Brains
This video is courtesy of the Wellcome Collection.
Brain Subdivisions
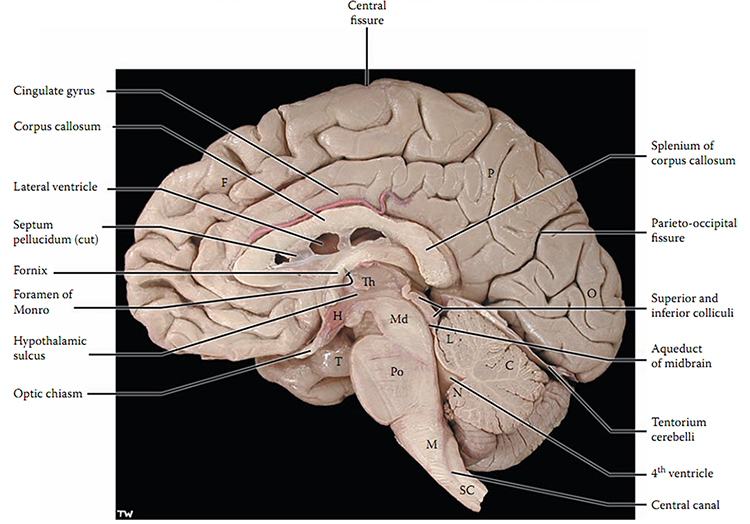
The brain is divided into three major subdivisions: forebrain, midbrain, and hindbrain. Brain landmark graphic © snapgalleria/Shutterstock.com.
Median brain section graphic © NatthapongSachan/Shutterstock.com.
.jpg)
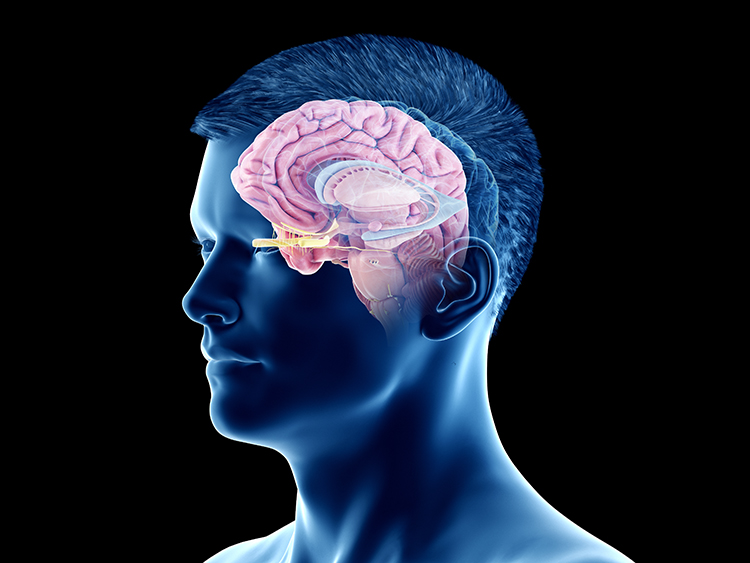
The forebrain consists of the telencephalon (cerebral hemispheres) and the diencephalon. The telencephalon encompasses the cerebral cortex and the deeper structures of the basal ganglia and limbic system. Limbic system graphic © SciePro/Shutterstock.com.
The diencephalon in the posterior forebrain contains the thalamus and hypothalamus. Thalamus graphic © SciePro/Shutterstock.com.
The midbrain consists of the mesencephalon, which includes the inferior colliculi, superior colliculi, and substantia nigra. The degeneration of the substantia nigra is a key step in developing Parkinson's disease. Substantia nigra graphic © Kateryna Kon/Shutterstock.com.
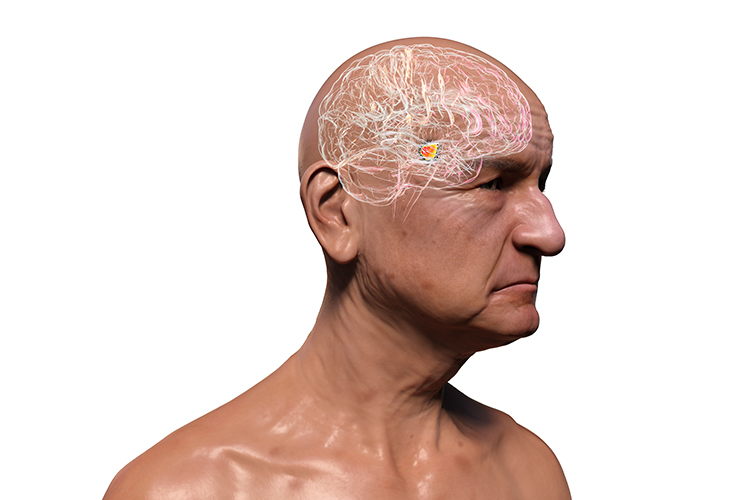
The hindbrain contains the metencephalon and myelencephalon. The metencephalon is comprised of the cerebellum and pons. The cerebellum plays a role in higher-level functions like emotional and cognitive regulation, speed, capacity, consistency, and appropriateness of cognitive and emotional processes. Damage to the cerebellum can reduce general intelligence. Cerebellum graphic with highlighted Purkinje neuron © Kateryna Kon/Shutterstock.com.
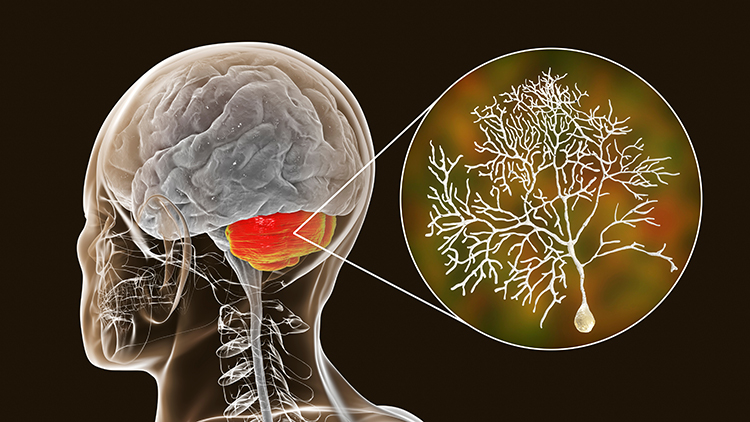
The myelencephalon consists of the medulla. The medulla plays a critical role in the speeding and slowing of the heart across each breathing cycle, a phenomenon called respiratory sinus arrhythmia or RSA. Alcohol, opioids, and sedative-hypnotics can fatally depress brainstem respiratory centers, slowing and halting breathing. Medulla graphic © mkfilm/Shutterstock.com.
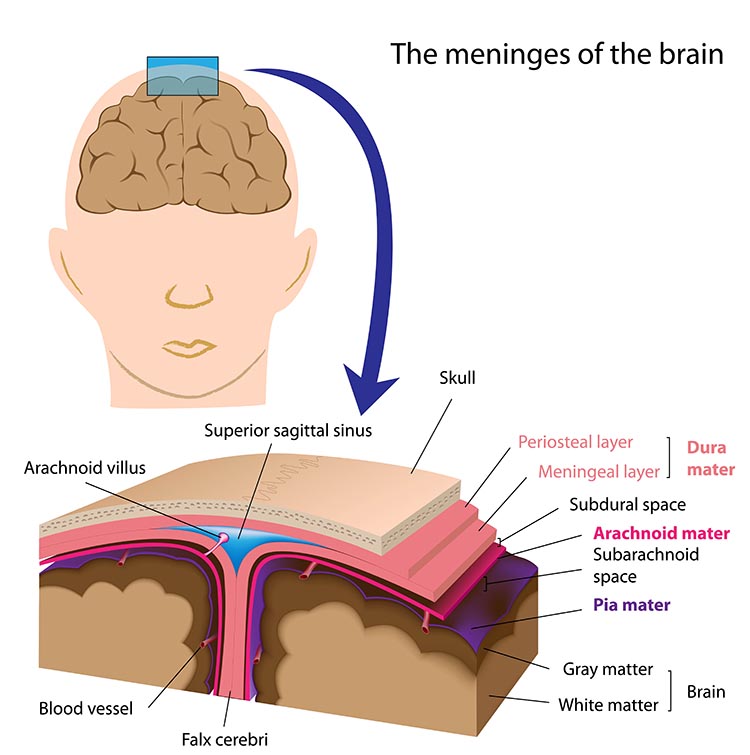
Meninges
Three meninges protect the brain and spinal cord, which are housed within the skull and vertebrae. These membranes include the dura mater, pia mater, and arachnoid (Breedlove & Watson, 2023). Graphic © Alilia Medical Media/Shutterstock.com.
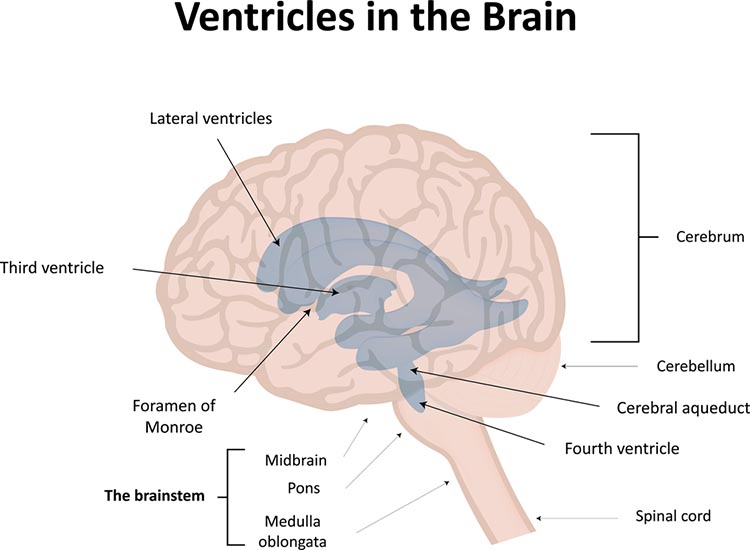
Cerebral Ventricles
The cerebral ventricles are a network of fluid-filled chambers that protect the brain from trauma due to abrupt head movements and that facilitate the exchange of nutrients and wastes between blood vessels and the brain. These cavities, found within all four lobes of each hemisphere, include the lateral, third, and fourth ventricles. The ventricular system circulates cerebrospinal fluid (CSF) produced by the choroid plexus membrane of the lateral ventricles (Breedlove & Watson, 2023). Graphic © joshya/ Shutterstock.com.
Glymphatic System
The belief of an absence of conventional lymphatic vessels in the CNS contributed to the concept that the brain, in spite of its high metabolic rate, represents an immune privileged region. This idea left questioned how cerebral interstitial fluid is cleared from waste products. It was generally thought that clearance depended on cerebrospinal fluid (CSF), acting as a pseudo-lymphatic system (Natale et al., 2021).
The human brain contains a recently-discovered glymphatic system. This astrocyte-controlled lymphatic system removes cellular debris, proteins, and wastes (Xie et al., 2013). The flushing of toxic substances may protect us from neurological disorders like Alzheimer’s (Breedlove & Watson, 2023).
The glymphatic system is a newly discovered lymphatic system in the brain. This recently discovered system provides a flow of CSF through the brain's interior that helps clear cellular debris, proteins, and other wastes. Glymphatic system graphic © Claus Lunau/Science Photo Library.
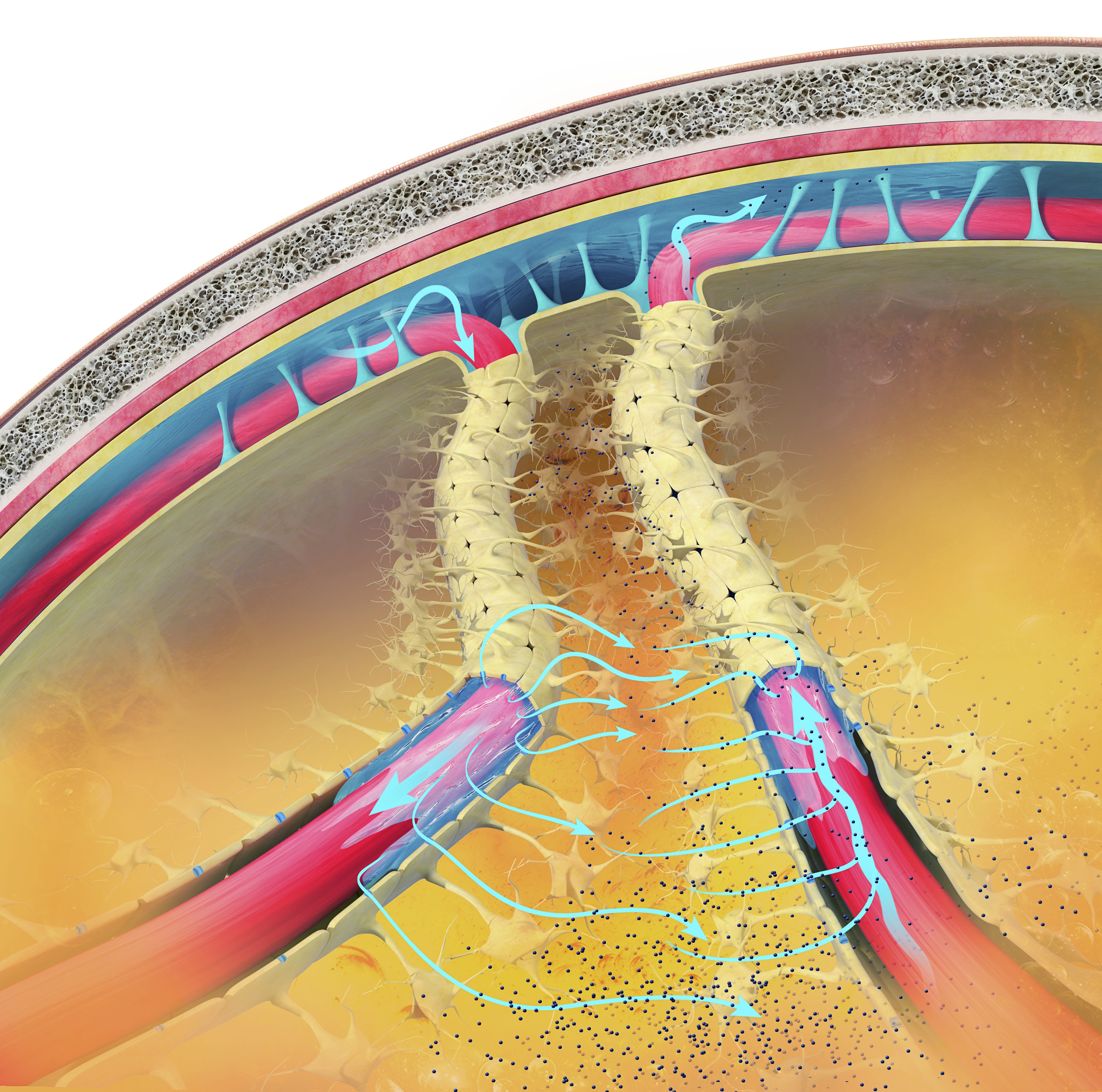
Caption: The glymphatic clearance pathway or the paravascular system clears waste and fluid from the vertebrate central nervous system (CNS). Interstitial fluid is removed via the cerebrospinal fluid (CSF). It is similar to the lymphatic system but removes waste products from the brain and spinal cord. This view shows the subarachnoid space (across the top) between the brain and its membranes. The blue arrows show the movement of interstitial fluid and solutes.
By removing harmful substances such as the amyloid and tau proteins implicated in Alzheimer’s and Parkinson's disease, the glymphatic flow may protect us from various neurological disorders (Breedlove & Watson, 2023). The glymphatic system removes most of its waste during stage 3 sleep, called slow-wave sleep.
The Brain's Vascular System
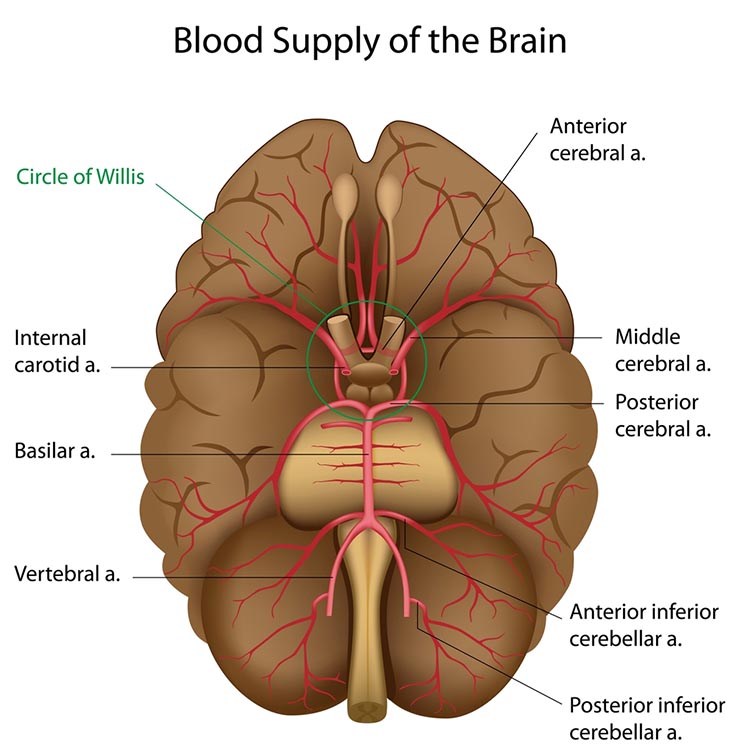
The resting brain consumes over 20% of the body's energy. The internal carotid artery's anterior and middle cerebral arterial branches deliver blood to about two-thirds of the cerebral hemispheres. The posterior cerebral arteries' left and right posterior vertebral arterial branches supply blood to the posterior cerebral hemispheres, cerebellum, and brainstem (Breedlove & Watson, 2023). Graphic © Alilia Medical Media/Shutterstock.com.
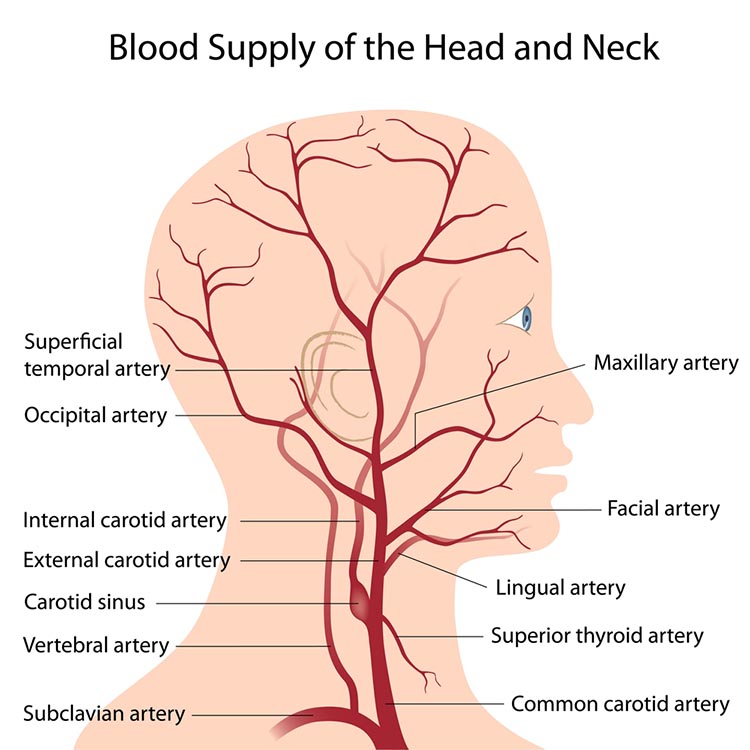
The effects of a stroke due to blood vessel blockage or rupture are limited because paired arteries supply each brain hemisphere. The circle of Willis, located at the base of the brain, is a vascular network comprised of the carotid and basilar arteries. This structure may provide another route for delivering blood when a major artery is compromised by disease or traumatic injury. Graphic © Alilia Medical Media/Shutterstock.com.
General Cortical and Subcortical Divisions
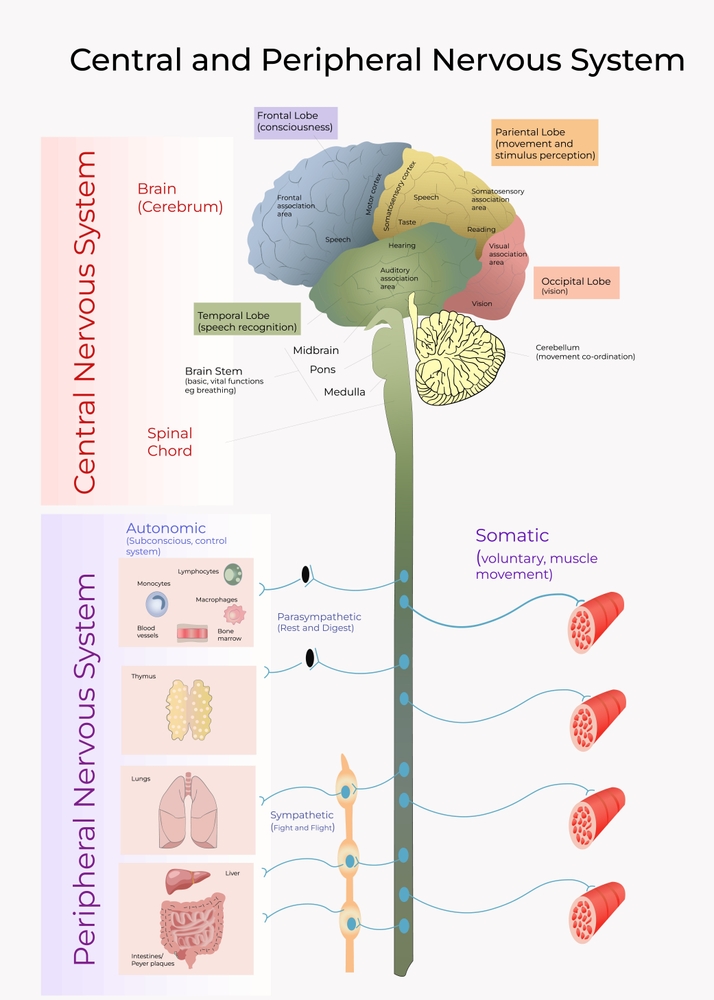
The human nervous consists of the central nervous system and peripheral nervous system. The central nervous system (CNS) consists of the brain, spinal cord, and retina.
The 3-pound brain has approximately 86 billion neurons (Chan et al., 2009; Voytek, 2013). Graphic © Jasada Sabai/Shutterstock.com.
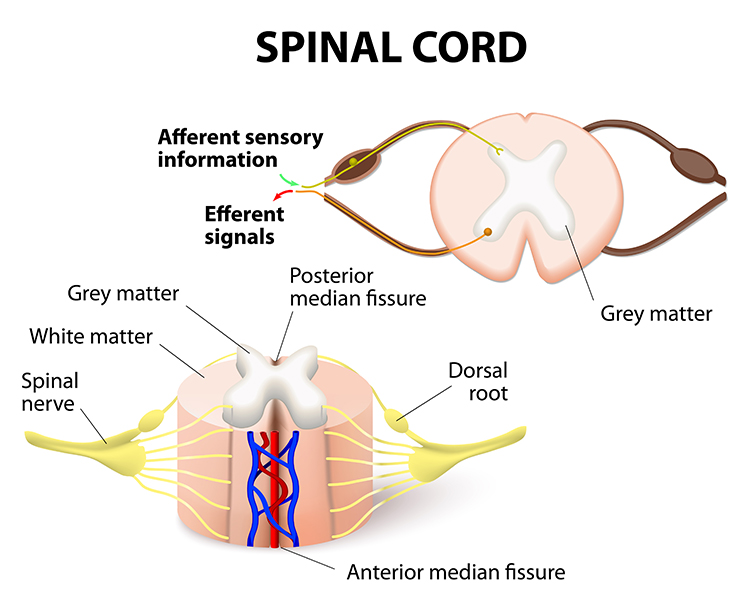
The cylindrical spinal cord consists of nervous tissue extending from the medulla (brainstem) to the vertebral column's lumbar (lower back) segment. The spinal cord distributes sensory information from the body to the brain, and CNS commands are sent from the brain to the body. The spinal cord also contains networks that control reflexes and central pattern generators.
The peripheral nervous system (PNS) consists of neurons and nerves outside the brain and spinal cord. The peripheral nervous system graphic Illustration 276621112 © Karen Harding | Dreamstime.com.
The peripheral nervous system is comprised of the autonomic nervous system and somatic nervous system. © Elena Ladanovskayai/Shutterstock.com.
Nerves
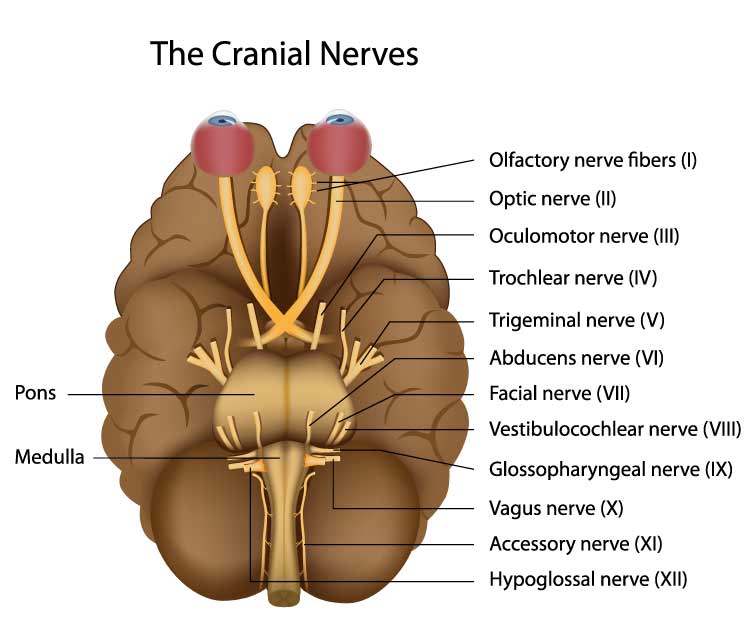
Nerves are bundles of axons that lie outside of the central nervous system. Motor nerves distribute instructions from the CNS to the rest of the body. Sensory nerves transmit information from sensory receptors to the CNS.There are three major systems of nerves: cranial nerves, spinal nerves, and the autonomic nervous system. The 12 pairs of cranial nerves distribute sensory and motor information. There are three exclusively sensory pathways to the brain: olfactory (I), optic (II), and vestibulocochlear (VIII). There are five exclusively motor pathways from the brain: oculomotor (III), trochlear (IV), abducens (VI), spinal accessory (XI), and hypoglossal (XII). Finally, four cranial nerves carry sensory and motor information: trigeminal (V), facial (VII), glossopharyngeal (IX), and vagus (X). Graphic © Alila Medical Media/Shutterstock.com.
Thirty-one pairs of spinal nerves, each member serving one side of the body, leave the spinal cord through openings in the backbone. Graphic © Sebastian Kaulitzki/Shutterstock.com.
.jpg)
Each spinal nerve carries sensory projections from the body (dorsal root) and motor commands from the spinal cord to skeletal muscles (ventral root). Graphic © Designua/Shutterstock.com.
Cortical Generators
The cerebral cortex (gray matter) consists of neuronal cell bodies, glial cells, and blood vessels. White matter lies beneath the neocortex, which consists of myelinated nerves, nonmyelinated fibers, and glial cells. The EEG mainly originates from pyramidal neurons in layers 3, 5, and 6 of gray matter approximately 5 mm thick. Pyramidal neuron graphic © Juan Gaertner/Shutterstock.com.
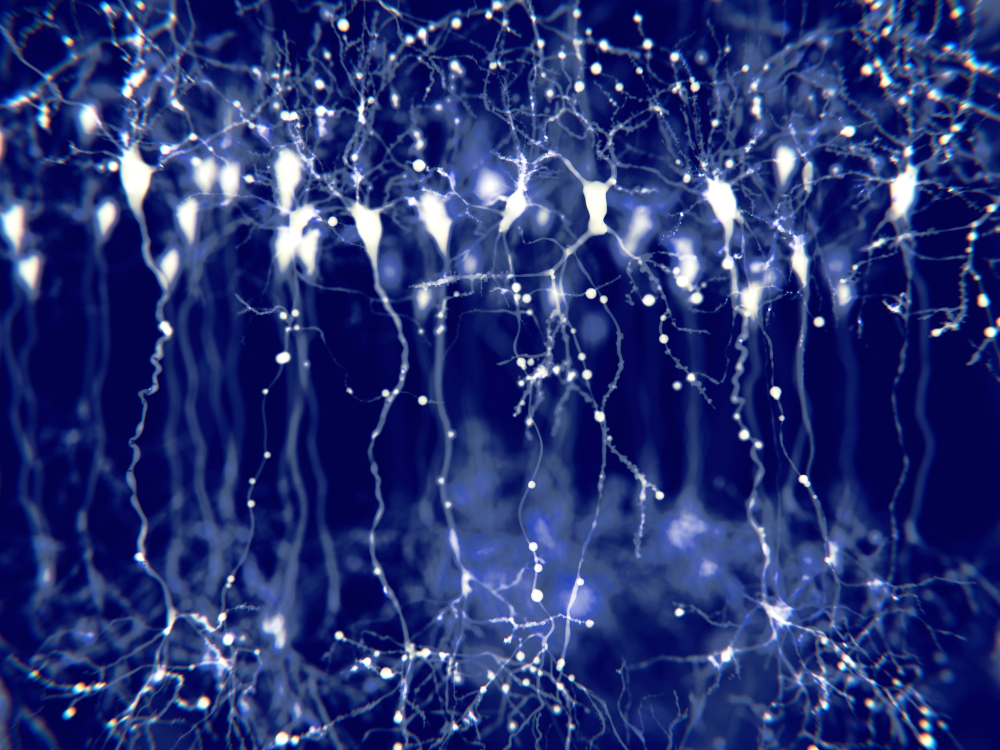
Vertical cortical macrocolumns contain hundreds of pyramidal neurons and supporting stellate and basket cells (Thompson & Thompson, 2016). Each pyramidal neuron may receive more than 100,000 synapses. These macrocolumns are positioned side by side and perpendicular to the cortical surface. Since neighboring macrocolumns often receive the same afferent messages, this increases the probability that they will fire together and generate a potential we can detect from the scalp. A reliable scalp EEG requires a minimum of 6 cm² of synchronized cortex (Dyro, 1989).
Although thalamic pacemakers generate EEG rhythms, resonant loops between cortical macrocolumns may be another source (Traub et al., 1989). Over 97 percent of the conversations within the brain are cortical-to-cortical (Thompson & Thompson, 2016). This communication is primarily confined within the same hemisphere. A resonant loop develops when macrocolumns that share afferent input fire synchronously to generate an electrical potential. The distance between the cortical macrocolumns that participate in a resonant loop is one determinant of EEG frequency. The closer the macrocolumns in a resonant loop, the higher the frequency they can generate (Lubar, 1997).
There are three types of resonant loops driven by afferent input or thalamic pacemakers: local, regional, and global. Local loops couple neighboring macrocolumns and may generate frequencies above 30 Hz in the high-beta and gamma ranges. Regional loops couple macrocolumns separated by several centimeters and may produce alpha and beta rhythms. Finally, global loops couple macrocolumns as distant as 7 cm (for example, between the frontal and parietal lobes) and may create delta and theta rhythms.
While only 3 percent of these linkages are thalamocortical, they greatly influence the EEG by subcortically connecting distant cortical regions and producing most synchronous activity (Steriade, 1990). Lubar (1997) proposed a violin analogy where the thalamic pacemakers that fire at varying frequencies are the strings, and the resonant loops that introduce different time delays are the instrument's resonant cavity.
Spindling is a synaptically generated oscillation in a circuit that includes the reticular nuclei (Steriade, 2005). The video of alpha spindling © John S. Anderson.
Different spindle frequencies are due to corresponding durations of thalamocortical neuron hyperpolarization. For example, longer hyperpolarizations associated with EEG synchronized states produce 7-Hz or lower-frequency spindles. In contrast, relatively short hyperpolarizations result in 14 Hz spindles (Steriade, 2005).
The electrical potentials generated by the thalamus can volume conduct near the speed of light through cerebrospinal fluid (CSF), brain tissue, the skull, and the scalp so that nearly identical waveforms can simultaneously appear at distant sites (Fisch, 1999; Thompson & Thompson, 2016).
Thalamic Generators
Anderson and Anderson (1968) advanced the facultative pacemaker theory that thalamic neurons activate cortical neurons and thalamic inhibitory interneurons via recurrent collaterals. While these thalamocortical neurons only excite a limited number of cortical neurons, thalamic interneurons inhibit a large pool of thalamocortical relay neurons. When the inhibition ends after one-tenth of a second, the thalamocortical neurons experience rebound excitation. This synchronized depolarization excites both cortical neurons and thalamic inhibitory interneurons, inhibiting a more extensive pool of thalamocortical relay neurons and initiating another excitation and inhibition cycle that produces EEG rhythms (Fisch, 1999). Graphic courtesy of Zachary Barry and featured in Wikipedia's article Recurrent ThalamoCortical Resonance.
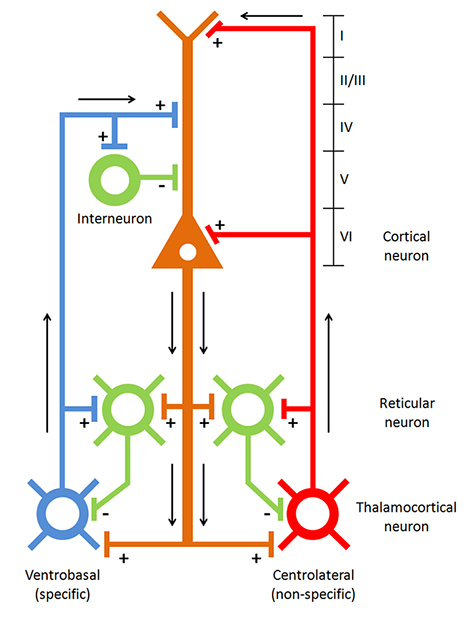
Caption: Thalamocortical circuit diagram depicting specific/sensory and non-specific intralaminar thalamocortical systems.
The networking of excitatory and inhibitory thalamic neurons imposes a group rhythm on its members that is transmitted to cortical macrocolumns by thalamocortical neurons (Bear, Connors, & Paradiso, 2016).
The nucleus reticularis of the thalamus may function as a pacemaker by releasing the inhibitory transmitter GABA at synapses with thalamocortical neurons. These neurons depolarize cortical neurons and thalamic inhibitory interneurons via burst discharges when their inhibition ends.
Oscillatory activity may involve an interaction between thalamocortical relay neurons (TCR), nucleus reticularis neurons (RE), and interneurons. Diverse neurotransmitters, including acetylcholine and GABA, mediate these interactions.
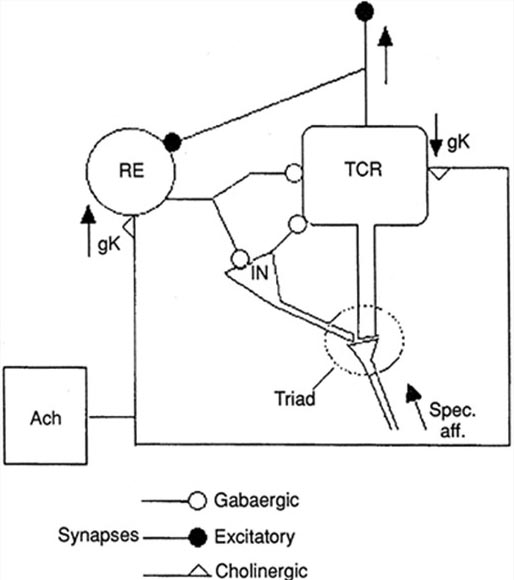
The thalamus is the dominant pacemaker for rhythmic EEG activity, including theta (3-8 Hz), alpha (8-12 Hz), and SMR (13-15 Hz) (Amzica & Lopes da Silva, 2018). Thalamocortical pathway graphic by Yeh et al. (2018). Creative Commons Attribution-Share Alike 4.0.
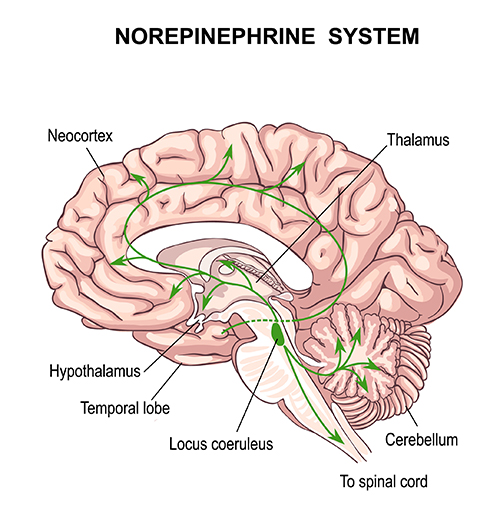
The Locus Coeruleus Inhibits Thalamic Alpha Generators
When we are inattentive, thalamic pacemakers generate the alpha rhythm. When we need to focus attention, we activate the brainstem noradrenergic locus coeruleus. The increased release of norepinephrine by this 15-millimeter network focuses attention and abolishes alpha oscillations. The locus coeruleus enhances the brain's sensory information processing by suppressing thalamic alpha generators. This may be an underlying mechanism of the phenomenon of alpha blocking.Although researchers cannot noninvasively monitor locus coeruleus activity in human participants, it is correlated with pupil dilation. In human studies, the greater the alpha blocking response and pupil dilation, the better the performance on demanding attention tasks (Dahl et al., 2020; Dahl et al., 2022). Graphic © Vasilisa Tsoy/Shutterstock.
The alpha rhythm is not a cause but a sign that incoming stimulation is too weak to overcome inhibition by the reticular nucleus. EEG activity is not causal and reflects network activity that has already occurred.
Additional Subcortical Generators
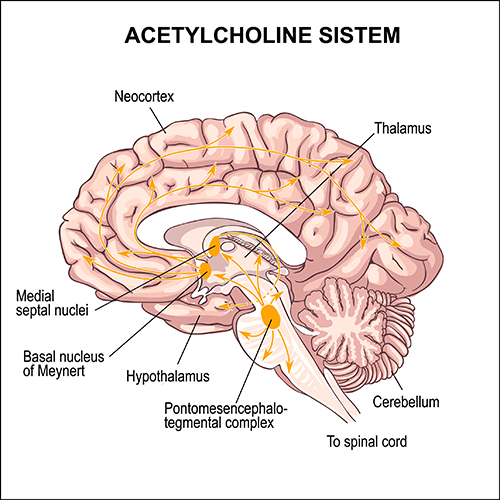
Ascending projections from the basal forebrain, reticular formation, locus coeruleus, and raphe systems disrupt brain rhythms. These neurons receive information from most sensory systems and cortical regions and directly desynchronize the EEG through synapses on cortical neurons and indirectly through innervation of thalamic pacemakers. Desynchronization shifts pyramidal neurons from burst firing to more continuous firing or the generation of single spikes (Fisch, 1999).The cholinergic basal forebrain, located in the ventral frontal lobe and anterior hypothalamus, influences cerebral blood flow and cognitive activity. Graphic © Vasilisa Tsoy/Shutterstock.
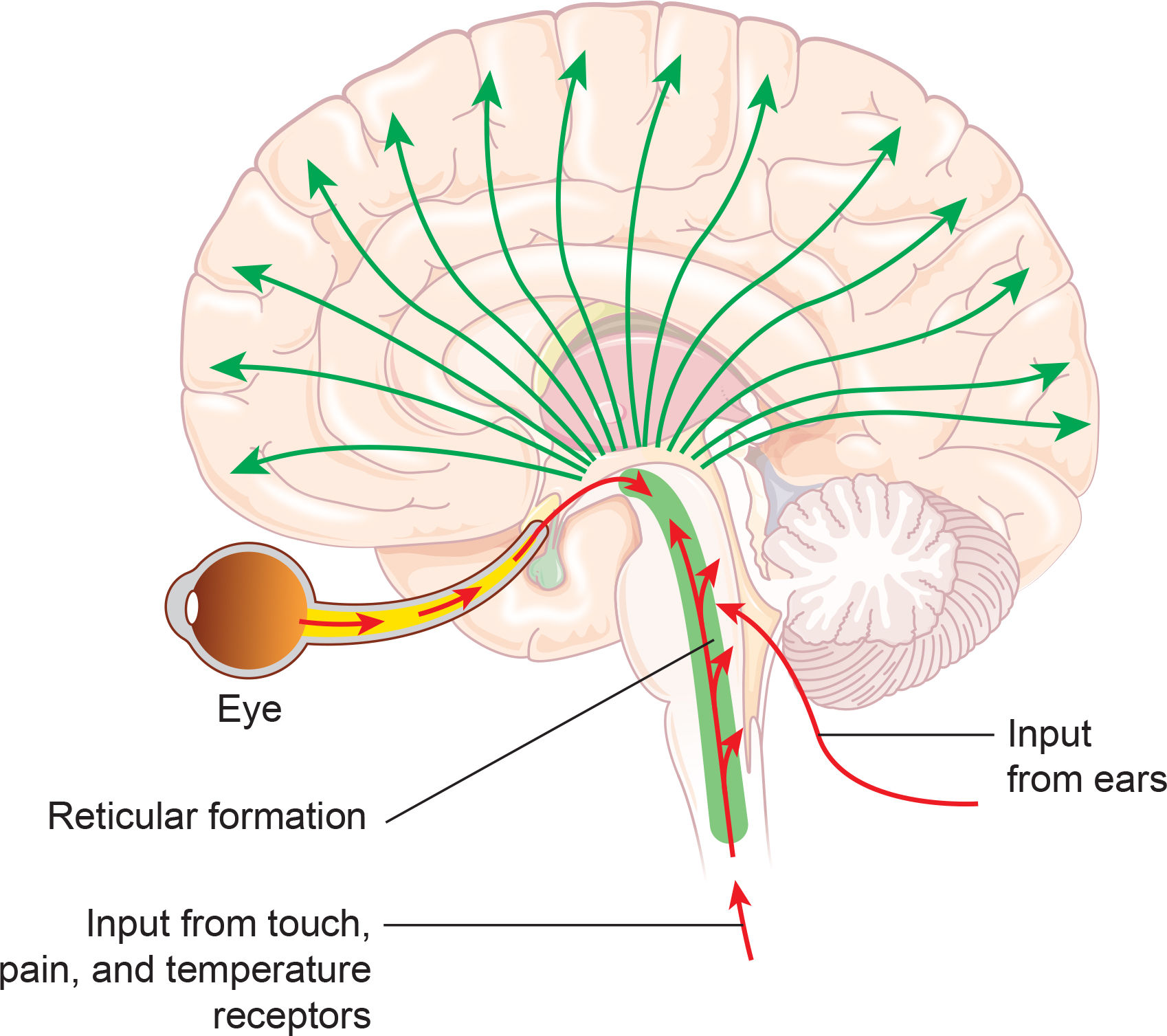
The reticular activating system (RAS) includes a network of 90 nuclei within the central brainstem from the lower medulla through the thalamus that activates the brain to promote attention, consciousness, and wakefulness. This network receives input from ascending sensory tracts (auditory, olfactory, somatosensory, and visual systems). The RAS projects to the thalamus and diffusely to the cortex. The RAS also has diffuse cortical projections that bypass the thalamus. Reticular formation graphic redrawn by minaanandag on Fiverr.com.
The noradrenergic brainstem locus coeruleus system, which projects to the thalamus, limbic system, and cerebral cortex, also contributes to wakefulness and vigilance for salient stimuli. Graphic © Vasilisa Tsoy/Shutterstock.

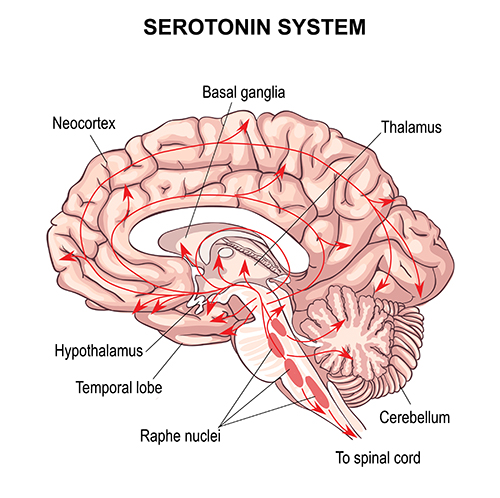
Finally, the serotonergic raphe system is a midline network of cell bodies within the brainstem and midbrain that may influence alertness and vigilance through reciprocal connections with the suprachiasmatic nucleus of the hypothalamus (Monti & Jantos, 2008). Graphic © Vasilisa Tsoy/Shutterstock.
Cortical and Subcortical Generators of Specific EEG Rhythms
Please click on the podcast icon below to hear a full-length lecture for Section A Part 2.

Slow Cortical Potentials (0-1 Hz)
Slow cortical potentials (SCPs) have been identified in cortical neurons, the thalamus, and glial cells. Cortical neurons in layers II to VI generate slow oscillations when the thalamus is removed or when cortical tissue is studied in vitro (in an artificial environment) or in vivo (within a living organism). Thalamic reticular neurons exhibit similar slow spontaneous oscillations when studied in vitro, and synchronized intracortical oscillations may depend on a corticothalamic network that targets these thalamic neurons.Glial cells generate slow SCPs when they burn sugar, producing negatively charged bicarbonate ions. Unlike EEG rhythms like delta, SCPs do not summate dendritic potentials. SCPs are associated with glial cells and gap junctions. Glial cells chemically communicate among themselves and with neurons. The slow oscillations of glial cells may influence the timing of neuronal firing through their control of potassium ion outflow (Steriade, 2005). These slow oscillations appear to organize the generation of other brain rhythms.
"The concept of a unified corticothalamic network that generates diverse types of brain rhythms grouped by the cortical slow oscillation (Steriade, 2001a,b) is supported by EEG studies in humans" (Molle et al., 2002).
Caton (1875) observed that the cortex's direct current baseline becomes negative whenever it is more active. The voltage gradients range from 150-200 μV. Underlying "tone" or valence factors determine the firing characteristics of neurons within a network. When SCPs are more positive, there is reduced firing of cortical neurons due to hypopolarization. When SCPs are more negative, there is increased firing due to depolarization.
The following 19-channel BioTrace+/NeXus-32 display of 0.1-1 Hz SCP activity © John S. Anderson.
Perspective on Fast Cortical Potentials
EEG "bands" are somewhat arbitrary ranges of frequencies that have evolved from observation and usage. The following BioTrace+ /NeXus-32 video of raw and spectral EEG displays © John S. Anderson. Frequency is plotted along the horizontal axis, and amplitude is shown on the vertical axis.While frequency band labels are helpful descriptors, they can also be misleading. Classification of an EEG rhythm is based on context (measurement conditions and EEG activity during the specific epoch), frequency, and waveform morphology.
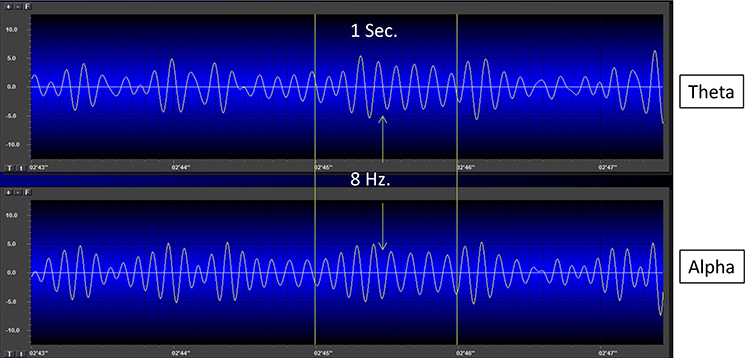
The process of up-training or down-training signal amplitude in one or more of the EEG bands using an EEG is called EEG biofeedback or neurofeedback. Minimum EEG voltages of 20-30 μV are seen in children and adults (Kraus et al., 2011).
Brainwaves Reflect Behavior
The ratio of slow (theta) to faster (beta) brainwaves shows how alert you are. This is the theta/beta ratio.In the next section, we will examine delta, theta, rhythmic slow-wave, alpha, mu, synchronous "alpha," SMR, beta, high or fast beta, and gamma activity.
Local Versus Global Decision-making
The short time windows of fast oscillators facilitate local integration and decision-making, primarily because of the limitations of the axon conduction delays. The long time windows of slow oscillators can involve many neurons in large or distant brain areas and favor complex, global decisions.Delta (0.5-3 Hz)
There are two delta rhythms, a slow oscillation under 1 Hz and a traditional 1-4 Hz oscillation. The slow 0.3-0.4 Hz oscillation originates in the neocortex and persists when the thalamus is removed. Thalamocortical neurons generate the 1-4 Hz oscillations observed during human stage-3 sleep. Slow neocortical oscillations may synchronize the thalamic delta rhythm (Steriade, 2005).Delta activity is generated by cortical neurons when other connections do not activate them and is found predominantly in frontal areas. Delta is associated with sleep and infancy. Delta is associated with sleep and infancy. During stage-3 sleep, delta allows astrocytes to rebuild their stores of glycogen. Clinicians observe delta in clients diagnosed with ADHD, brain tumors, learning disorders, and traumatic brain injury (TBI). Rhythmic high-amplitude delta is associated with TBI, mainly if localized. Diffuse delta may be found in ADHD and learning disorders.
Normal Amplitudes
Delta should not be present in significant amounts in the awake adult EEG. "Apparent" delta is usually an eye movement artifact. Some delta activity probably occurs in the waking adult EEG.Delta bands are inhibited or down-trained but rarely rewarded. Delta desynchronization can be rewarded. The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 1-4 Hz activity from a 10-year-old male © John S. Anderson.
Theta (3-8 Hz)
The mechanisms that generate the theta rhythm are poorly understood. Theta differs depending on location and source. Amzica and Lopes da Silva (2011) consider the classic septal/diagonal band pacemaker model incomplete. Hippocampal interneurons, which innervate the hypothetical medial septum pacemaker, exercise top-down control. The hypothalamic supramammillary nucleus, with extensive connections to the brainstem, diencephalon, and medial septum, may also pace and modulate hippocampal theta. Further, a non-cholinergic theta source has been found within the entorhinal cortex of the hippocampus.Theta is associated with creativity, global synchronization, memory formation, and recall. Increased theta amplitudes correspond with hypo-perfusion and decreased glucose metabolism. Excessive frontal theta is linked with depression, daydreaming, distractibility, and inattention. A theta/beta (T/B) ratio of 3.0 may indicate ADHD depending on age, as T/B ratios are developmentally mediated (Monastra et al., 1999).
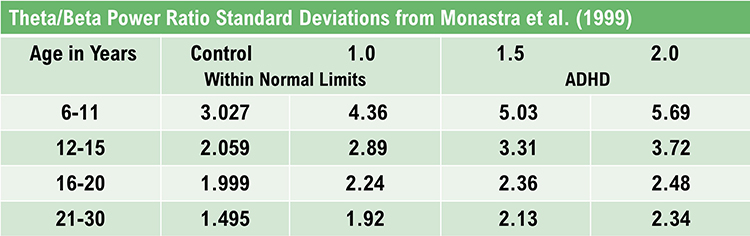
Normal Amplitudes
Theta voltage is age-related in the awake EEG. Voltage diminishes from age 8-30 with minimal amounts over age 30. A typical 6-7 Hz rhythm in the frontal midline (FCz) is associated with mental activity such as problem-solving and other functions. This rhythm appears to be limbic in origin. It is higher in amplitude and more synchronous when processing the feedback that an error has occurred. The 4-Hz rhythm is associated with childhood pleasurable experiences and adult memory searches.Rhythmic Slow Wave (RSW or Theta)
Inhibit theta to remediate symptoms. Reward posterior RSW in alpha/theta training for addictions, global synchronization, optimal performance, and PTSD. RSW is generally not increased frontally. Clinicians may train for increases or decreases in phase synchrony. When awake with eyes open, RSW is mainly seen in the frontal-midline (FCz). The limbic system and thalamus generate RSW. Depending on location, RSW may be slowed-alpha as thalamic output slows.The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 4-8 Hz activity from a 10-year-old boy © John S. Anderson.
Alpha (8-13 Hz)
The 8-13-Hz alpha rhythm differs from spindle waves in both its source and the activity during which it is observed. Alpha 1 (low alpha) ranges from 8-10 Hz, and alpha 2 (high alpha) from 10-13 Hz (Thompson & Thompson, 2016). Alpha rhythms depend on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons (Hughes & Crunelli, 2005). Cortical networks maintain and propagate the alpha rhythm (Amzica & Lopes da Silva, 2018). Connectome graphic © Image Source Trading Ltd/Shutterstock.
Researchers have correlated the alpha rhythm with relaxed wakefulness. There are age- and function-related differences. Spindle waves, in contrast, originate in the thalamus and occur during unconsciousness and stage 2 sleep (Steriade, 2005).
Alpha is the dominant rhythm in adults and is located posteriorly. The 8-10 Hz range is associated with ADHD, daydreaming, fogginess, OCD, and TBI. Frontal asymmetry is associated with depression. The 10-12 Hz range is seen with inner calm (calm and alert) and meditation. Clinicians train alpha amplitude and phase synchrony up or down for remediation of symptoms, depending on location.
Posterior Dominant Rhythm (PDR)
The posterior alpha rhythm is visible at about 4 months with a frequency of around 4 Hz. Between 3-5 years, this rhythm is approximately 8 Hz with amplitudes as high as 100 μV. From 6-15 years, this rhythm is 9 Hz by age 7 and 10 Hz by ages 10-15 with a mean amplitude of 50-60 μV. Girls show a statistically faster acceleration of posterior alpha frequency than boys. From 13-21 years, the mean alpha frequency is 10 Hz, and amplitudes decline throughout this period. Faster alpha frequencies are associated with higher IQ and better memory performance.The following 19-channel BioTrace+ /NeXus-32 display of the response of the posterior dominant rhythm to eyes opening and closing © John S. Anderson.
Normal Amplitudes
The typical adult alpha frequency ranges from 9.5-10.5 Hz. Alpha below 8 Hz is considered abnormal. There are age-related differences. Alpha frequency declines after age 70. Adult amplitudes are 50 μV or less:60% have ~ 20-60 μV
28% have < 25 μV
6% have > 60 μV
Higher alpha amplitudes are observed over the non-dominant (right) hemisphere (alpha asymmetry). Most studies show no effect of handedness. Asymmetry is generally no more than 20 μV or 20% of the greater of the two amplitudes (Amzica & Lopes da Silva, 2018).
Causes of Excessive Alpha Amplitudes
Sleep deprivation or metabolic exhaustion can result in high amplitude and slowing of the peak frequency and persistent alpha during an eyes-open condition. Meditation practices can cause increased amplitudes and slowing, a faster alpha response to an eyes-closed condition, and persistent alpha in an eyes-open condition. Marijuana use and abuse can cause increased amplitudes and slowing, persistent alpha in an eyes-open condition, depending on the type of marijuana. These effects can persist for many years following abstinence.The following 19-channel BioTrace+ /NeXus-32 display of eyes-closed 8-12 Hz activity from a 13-year-old girl © John S. Anderson.
Mu Rhythm (7-11 Hz)
While the 7-11-Hz mu rhythm usually overlaps with the alpha range, its morphology deviates from the alpha waveform as one end is pointed. The mu rhythm can be recorded at C3 and C4 in a minority of subjects and may represent suppression of hand movement or imagining hand movement (Thompson & Thompson, 2016).Mu rhythms appear to regulate motor cortex activities via prefrontal cortical mirror neurons. These circuits may play a critical role in imitation learning and our ability to understand the actions of others. Mu rhythms facilitate the conversion of visual and auditory input into integrated skill-building functions. Attenuating the mu rhythm appears to be associated with activating this function (Pineda, n.d.). The mu rhythm is highlighted below.
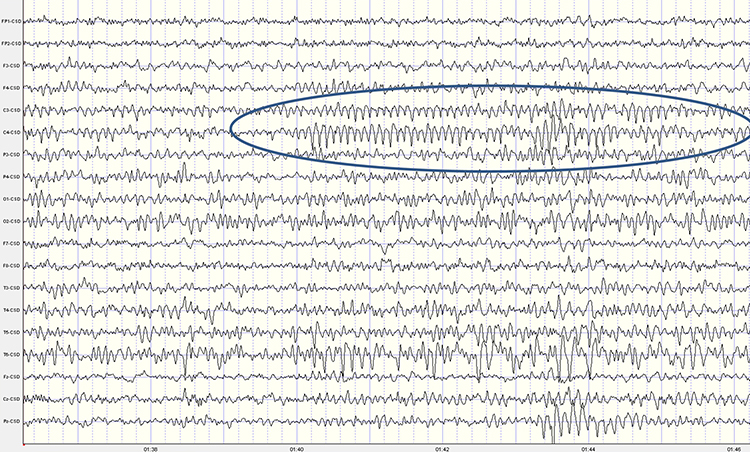
This second example of the mu rhythm shows a classic 10-11 Hz and 19-20 Hz "Owl Eye" presentation.
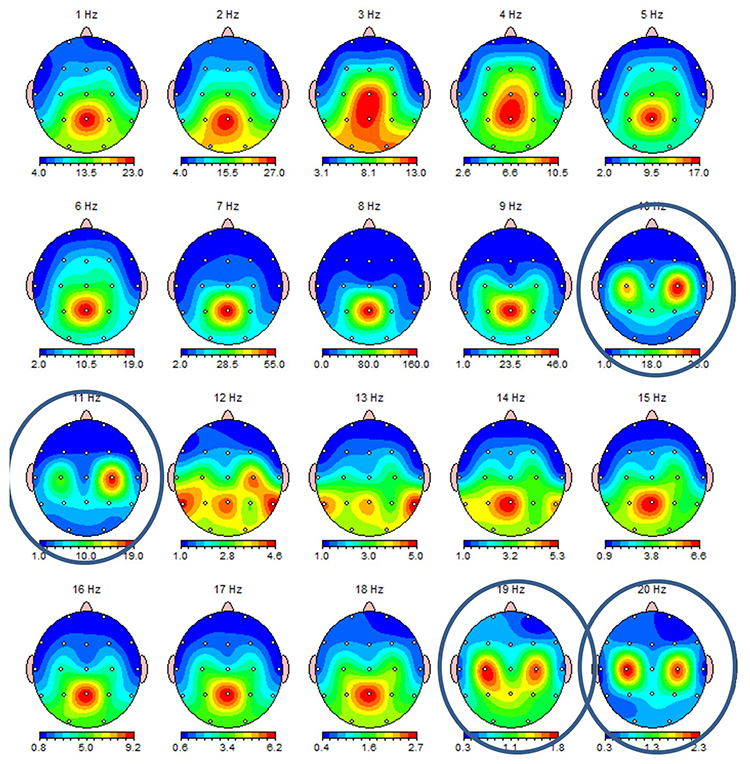
Synchronous "Alpha"
Various sensory systems, such as our auditory, somatosensory, and visual systems, produce localized and semi-independent "alpha" activity. Synchronous, distributed alpha integrates perception and facilitates action. Synchronous "alpha" appears to block the localized alpha-like patterns such as mu and the posterior rhythm in favor of more broadly distributed network integration.Sensorimotor Rhythm (13-15 Hz)
The sensorimotor rhythm (SMR) is beta 1 located on the sensorimotor strip (C3, Cz, C4). SMR amplitude increases when the motor circuitry is idle. SMR increases with stillness and decreases with movement. Deficient SMR may be observed in movement spectrum complaints like hyperactivity and tics. SMR appears as sleep spindles during stage-2 sleep. SMR is associated with neutral blood perfusion of the brain and resting levels of glucose metabolism. Clinicians typically reward increased SMR amplitude to calm hyperactivity and during theta/beta ratio training.The following 19-channel BioTrace+ /NeXus-32 display of 12-15 Hz activity © John S. Anderson.
Beta (over 12Hz)
Beta consists of rhythmic activity between 13-38 Hz. There are four beta ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz). Beta is located mainly in the frontal lobes. Beta is associated with focus, analysis, and relaxed thinking (Thompson & Thompson, 2015).Excessive beta is observed in anxiety, depression (asymmetry), insomnia, OCD, and sleep disorders. Deficient beta is seen in ADHD, cognitive decline, and learning disorders.
Since beta overlaps with the EMG range, clinicians must be careful when up-training this rhythm and use an EMG inhibit. Beta is generated by the brainstem and cortex and is associated with hyper-perfusion and increased glucose metabolism.
Normal 16-20+ Hz Beta Amplitudes
Beta amplitudes are minimal in children up to 12 years. There is a significant increase in beta amplitude and organization between 12-30 years. Beta is commonly seen in nearly all adults with amplitudes of 20 μV or less. Interhemispheric amplitude asymmetries exceeding 35% are abnormal. The following 19-channel BioTrace+ /NeXus-32 display of 13-21 Hz activity © John S. Anderson.Fast or High Beta Rhythms (20-35 Hz)
Fast 20-35-Hz oscillations are generated by activation of the mesencephalic reticular formation. Thalamocortical, rostral thalamic intralaminar, and cortical neurons spontaneously oscillate in this range. This activity is primarily seen in the frontal lobes and is associated with hyper-perfusion and increased glucose metabolism. Persistent excessive activity can lead to metabolic exhaustion.This activity may be associated with peak performance and cognitive processing and related to specificity and precision in information processing. Excessive high beta is associated with alcoholism, anxiety, OCD, rumination, and worry. Clinicians often inhibit high beta activity but rarely reward it.
The following 19-channel BioTrace+ /NeXus-32 display of eyes-closed ~ 25 Hz fast beta activity © John S. Anderson.
Gamma Rhythms (28-80 Hz)
Amzica and Lopes da Silva (2011) concluded that gamma oscillations might speed information distribution and processing. Gamma bursts occur during problem-solving, and the absence of gamma is associated with cognitive deficits and learning disorders. Gamma synchrony is related to cognitive processing and is essential in coding by contributing specificity and precision to information processing. Gamma is theorized to serve as a "binding rhythm" that integrates sensory inputs into perception and consciousness.The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 36-44 Hz activity in a 10-year-old boy © John S. Anderson.
Gamma rhythms are linked with SCPs. The following BioTrace+ /NeXus-32 display of SCP and gamma activity © John S. Anderson.
GLOSSARY
acetylcholine: an amine neurotransmitter that binds to nicotinic and muscarinic ACh receptors.
acetylcholine esterase (AChE): the enzyme that deactivates ACh.
AChE-R: an abnormal form of acetylcholine esterase (AChE), which may render dendrites with acetylcholine receptors more excitable when stressed.
action potential: a propagated electrical signal that usually starts at a neuron’s axon hillock and travels to presynaptic axon terminals.
adenylate cyclase: at a metabotropic receptor, an enzyme that transforms ATP into the second messenger cyclic AMP.
afferent: a neuron that transmits sensory information towards the central nervous system, or from one region to another.
all-or-none law: once an action potential is triggered in an axon, it is propagated, without decrement, to the end of the axon. The amplitude of the action potential is unrelated to the intensity of the stimulus that triggers it.
alpha blocking: arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it entirely while increasing EEG power in the beta range.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: regular bursts of alpha activity.
alpha-subunit: a subunit of a G protein associated with the neuron membrane that breaks away to activate enzymes within the neuron when a ligand binds to a metabotropic receptor.
amino acid neurotransmitters: the oldest family of transmitters. These molecules bind to ionotropic and metabotropic receptors, transmitting information and modulating neuronal activity. Most synaptic communication is accomplished in the brain by glutamate (generally excitatory) and GABA (generally inhibitory).
AMPA (glutamate) receptors: ionotropic receptors which open sodium channels, depolarizing the neuron's membrane (producing an EPSP), and dislodging a Mg+ ion that blocks an adjacent NMDA (glutamate) receptor's calcium channel. AMPA receptors are responsible for most activity at glutamatergic synapses.
amplitude: the energy or power contained within the EEG signal measured in microvolts or picowatts.
amygdala: a limbic system structure plays a crucial role in learning about the consequences of our actions and creating declarative memories for events with emotional significance.
angular gyrus: the region located near the superior temporal lobe (BA 39) and involved in reading, math, and copying writing.
anion: a negative ion, for example, chloride (Cl-).
anterior: near or toward the front of the head, for example, the anterior cingulate.
anterior cingulate cortex (ACC): a division of the prefrontal cortex (Fpz, Fz, Cz, Pz) that plays a vital role in attention and is activated during working memory. The ACC mediates emotional and physical pain and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components.
anterior commissure: a bundle of nerve fibers that crosses the midline and connects the left and right temporal lobes, hippocampus, and amygdala.
apical dendrite: a dendrite that arises from the top of the pyramid and extends vertically to layer 1 of the neocortex.
arousal: a process that combines alertness and wakefulness, produced by at least five neurotransmitters, including acetylcholine, histamine, hypocretin, norepinephrine, and serotonin.
astrocytes: star-shaped glial cells that communicate with and support neurons and help determine whether synapses will form.
asynchronous waves: voltages produced when neurons depolarize and hyperpolarize independently.
ATP: the energy source for a neuron’s sodium-potassium transporters.
auditory cortex: temporal cortex that processes auditory information within the dorsal and ventral streams.
autonomic nervous system: a subdivision of the peripheral nervous system that innervates glands and internal organ smooth muscles and includes enteric, parasympathetic, and sympathetic divisions.
autoreceptors: metabotropic receptors that can be located on the membrane of any part of a neuron. They detect neurotransmitters released the neuron releases, generate IPSPs that inhibit the neuron from reaching the excitation threshold, and regulate internal processes like transmitter synthesis and release through the second messenger system.
axoaxonic synapses: junctions between two axons that do not affect the generation of an action potential, only the amount of neurotransmitter distributed.
axodendritic synapses: junctions between axons and dendrites determine whether the axon hillock will initiate an action potential.
axon: long, cylindrical structures that convey information from the soma to the terminal buttons. An axon also transports molecules in both directions along the outer surface of protein bundles called microtubules.
axon hillock: a swelling in the cell body where a neuron integrates the messages it has received from other neurons and decides whether to fire an action potential.
axonal varicosity: a swelling in an axon wall allowing neurotransmitter release through the wall via volume transmission.
axoplasmic transport: the movement of molecules in both directions along the outer surface of protein bundles called microtubules.
basal dendrite: dendrite that horizontally branches out from the 30-μm base of the pyramid through the layer where the neuron resides.
basal forebrain: a cholinergic network located in the ventral frontal lobe and anterior hypothalamus that influences cerebral blood flow and cognitive activity.
basal ganglia: these forebrain structures consist of an egg-shaped nucleus that contains the putamen and globus pallidus and a tail-shaped structure called the caudate, which together are responsible for the production of movement. The basal ganglia have also been implicated in obsessive-compulsive disorder, Parkinson’s disease, and Huntington’s chorea.
benzodiazepine receptor agonist (BZRA) hypnotics: nonbenzodiazepines like zolpidem (Ambien).
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
bilateral synchronous slow waves: a pathological sign observed in drowsy children. When detected in alert adults, intermittent bursts of high amplitude slow waves may signify gray matter lesions in deep midline structures.
Broca's area: area located inferior frontal gyrus (BA 44 and 45) of the dominant hemisphere (F7-T3 in the left hemisphere) concerned with speech production, grammar, language comprehension, and sequencing.
Brodmann areas: 47 numbered cytoarchitectural zones of the cerebral cortex based on Nissl staining.
cation: a positive ion, for example, sodium (Na+).
caudal: away from the front of the head.
cell body or soma: contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
central nervous system (CNS): the division of the nervous system that includes the brain, spinal cord, and retina.
central nucleus of the amygdala: the nucleus that orchestrates the nervous system's response to essential stimuli by activating circuits in the brainstem (autonomic arousal) and the basal ganglia and periaqueductal gray (defensive behavior).
central sulcus: the fissure that separates the frontal and parietal lobes.
cerebral cortex: the layer of gray matter that covers the cerebral hemispheres. The cerebral cortex consists of gray matter and white matter.
cerebral ventricles: a network of fluid-filled chambers that protects the brain from trauma due to abrupt head movements and facilitates the exchange of nutrients and wastes between blood vessels and the brain.
cerebrospinal fluid (CSF): fluid produced by the choroid plexus membrane of the lateral ventricles that fills the ventricular system.
chemical synapses: junctions between neurons that transmit molecules across gaps of less than 300 angstroms. Neurons use chemical synapses to produce short-duration (milliseconds) and long-duration (seconds to hours) changes in the nervous system. Chemical synapses are capable of more extensive communication and initiate more diverse and long-lasting changes than electrical synapses.
circle of Willis: vascular network located at the base of the brain comprised of the carotid and basilar arteries. This structure may provide another route for delivering blood when a major artery is compromised by disease or traumatic injury.
classical routes for EEG activation: specific sensory pathways like the visual (retina to the visual cortex), auditory (cochlea to the auditory cortex), and somatosensory (chemoreceptors and mechanoreceptors to the somatosensory cortex) systems. Increased transmission of information through these pathways desynchronizes EEG activity in the cortical regions to which these afferent neurons project, as specialized circuits of neurons independently process this information.
coherence: the degree of coupling between separate cortical regions and reflects neural network connectivity and dynamics. Coherence evaluates the linear association or correlation between the EEG waveforms recording from two different scalp locations (two referential montages).
commissures: axon tracts. The left and right hemispheres communicate using the corpus callosum, anterior commissure, and posterior commissure.
co-modulation: the degree of association in the magnitude of signals detected from two different sources (sites). Co-modulation, which can be measured using the Pearson Product-Moment Correlation Coefficient, shows the degree to which signals strengthen and weak in a correlated manner.
complex: a sequence of waves.
COMT: a degrading enzyme that only targets the catecholamines dopamine and norepinephrine.
connectivity: the degree of synchrony between the oscillations of specialized brain regions (nodes) within a network.
contingent negative variation (CNV): a steady, negative shift in potential (15 μV in young adults) detected at the vertex. This slow cortical potential may reflect expectancy, motivation, intention to act, or attention. The CNV appears 200-400 ms after a warning signal (S1), peaks within 400-900 ms, and sharply declines after a second stimulus that requires the performance of a response (S2).
continuous irregular delta: slow waves produced by white matter lesions seen in disorders like multiple sclerosis.
contralateral: structures that are located on opposite sides of the body. For example, neurons in the left primary motor cortex control muscles on the right side of the body.
coronal plane: the plane that separates the body into front and back parts.
corpus callosum: the largest commissure that connects the left and right frontal, parietal, and occipital lobes.
corticothalamic network: a unified network that generates diverse types of brain rhythms grouped by slow cortical oscillations.
cranial nerves: 12 pairs of nerves connected to the brain and are part of the sensory and motor systems of the head and neck.
cyclic AMP: a second messenger that moves about the neuron, activating other enzymes. Protein kinase A, which controls the excitability of ion channels, is a crucial enzyme target of cyclic AMP. Cyclic AMP also travels to the nucleus to regulate gene expression.
Dale's principle: the incorrect view that a neuron can only release one neurotransmitter. They often release two to four.
delta rhythm: 0.05-3-Hz oscillations generated by thalamocortical neurons during stage-3 sleep.
dendrite: a branched structure designed to receive messages from other neurons via axodendritic synapses (junctions between axons and dendrites), determining whether the axon hillock will initiate an action potential.
dendritic spines: protrusions on the dendrite shaft where axons typically form axodendritic synapses.
dendrodendritic synapses: junctions between dendrites that communicate chemically across synapses and electrically across gap junctions.
depolarize: to make the membrane potential more positive by making the inside of the neuron more positive with respect to its outside.
desynchronization: the absence or loss of coordinated neuronal firing and synchronization of brain waves.
diencephalon: the posterior forebrain subdivision that contains the thalamus and hypothalamus.
diffusion: the distribution of molecules from areas of high concentration to low concentration.
diphasic wave: a wave that contains both a negative and positive deflection from the baseline.
dipole: the electrical field generated between the sink (where current enters the neuron) and the source (place at the other end of the neuron where current leaves) may be located anywhere along the dendrite.
distal: toward the periphery.
dominant frequency: EEG frequency with the greatest amplitude.
dopamine: a monoamine neurotransmitter that exerts its postsynaptic effects on at least six receptors linked to G proteins. This means that dopamine functions as a neuromodulator. The two major families include D1 (D1 and D5) and D2 (D2A, D2B, D3, and D4).
dorsal: toward the upper back or head.
dorsal stream (auditory): the pathway from the temporal to the parietal lobes that helps spatially localize sounds.
dorsal stream (visual): the pathway from the primary visual cortex (V1) to the parietal lobe that helps to localize objects and guide movements towards them.
dorsolateral prefrontal cortex (DLPFC): the region of the middle frontal gyrus (BA 9 and 4) that shares responsibility with cortical and subcortical networks for executive functions like abstract reasoning, cognitive flexibility, decision-making, inhibition, planning, and working memory (Miller & Cummings, 2007) and exercises the highest cortical level of motor control.
D-serine: a neurotransmitter that binds to the glycine site on the NMDA receptor to trigger calcium entry into a dendritic spine when glutamate binds to its site, resulting in a large, prolonged increase in intracellular calcium.
dual-action antidepressants: medications that activate 5-HT1 receptors to produce antidepressant and anxiolytic effects, while they blockade 5-HT2 (agitation, restlessness, and sexual dysfunction) and 5-HT3 (nausea, headache, and vomiting) receptors to minimize their side effects.
EEG activity: a single wave or successive waves.
EEG power: the signal energy in the EEG spectrum. Most EEG power falls within the 0-20 Hz frequency range. EEG power is measured in microvolts or picowatts.
efferent: a motoneuron that transmits information towards the periphery.
electrical synapse: a symmetrical synapse where neurons communicate information bidirectionally across gap junctions between adjacent membranes using ions. Transmission across electrical synapses is instantaneous, compared with the 10 ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of distant neurons to synchronize their activity and simultaneously fire.
electroencephalogram (EEG): the voltage difference between at least two electrodes, where at least one electrode is located on the scalp or inside the brain. The EEG is a recording of both EPSPs and IPSPs that occur primarily in dendrites in pyramidal cells located in macrocolumns, several mm in diameter, in the upper cortical layers.
electrostatic pressure: the attractive or repulsive force between ions that moves them from one region to another.
entorhinal cortex: a structure located in the caudal region of the temporal lobe and that receives pre-processed sensory information from all modalities and reports on cognitive operations. The entorhinal cortex provides the main input to the hippocampus, is involved in memory consolidation and spatial localization, and provides input into the septohippocampal system that may generate the 4-7 Hz theta rhythm.
enzymatic deactivation: the process in which an enzyme breaks a neurotransmitter apart into inactive fragments. For example, acetylcholine transmission is ended by the enzyme acetylcholine esterase (AChE). Deactivating enzymes located in the synaptic cleft degrade a neurotransmitter molecule when it detaches from its binding site.
evoked potential: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). An evoked potential has a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
excitatory postsynaptic potential (EPSP): a brief positive shift in a postsynaptic neuron's potential produced when neurotransmitters bind to receptors and cause positive sodium ions to enter the cell. An EPSP pushes the neuron towards the excitation threshold when it can initiate an action potential.
exocytosis: the process of neurotransmitter release. When an action potential arrives and depolarizes the terminal button, calcium ions enter the terminal button from the extracellular fluid. Calcium binds with clusters of protein molecules that join the vesicles with the presynaptic membrane. The clusters move apart, forming a hole through both membranes called a fusion pore, and the neurotransmitter leaves the terminal button for the synaptic cleft or extracellular fluid.
exogenous ERP: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual).
explicit learning: behavioral changes that occur with our conscious awareness that require processing by the hippocampus.
extracellular dipole layers: macrocolumns of pyramidal cells, which lie parallel to the surface of the cortex, send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons.
extracellular fluid: the fluid surrounding a neuron.
facultative pacemaker theory: Anderson and Anderson's (1968) theory that thalamic neurons activate cortical neurons and thalamic inhibitory interneurons via recurrent collaterals.
fast cortical potentials: EEG rhythms that range from 0.5 Hz-100 Hz. The main frequency ranges include delta, theta, alpha, sensorimotor rhythm, and beta.
feature binding: the process of linking information to perceptual objects (linking an apple's color to its shape) that may involve the 40-Hz rhythm.
fissures: deep grooves, for example, the lateral fissure.
focal waves: EEG waves detected within a limited area of the scalp, cerebral cortex, or brain.
forebrain: the anterior brain subdivision that consists of the cerebral hemispheres (telencephalon) and the thalamus and hypothalamus (diencephalon), also called the prosencephalon.
frequency: the number of cycles completed each second expressed in hertz (Hz).
frequency synchrony: when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
frontal lobes: the most anterior cortical lobes of the brain (F7, F3, Fz, F8, F4) that are divided into the primary motor cortex, motor association cortex, Broca's area, and prefrontal cortex.
fusion pore: a hole through a vesicle and presynaptic membrane that allows neurotransmitters to leave the terminal button for the synaptic cleft or extracellular fluid.
G protein: a protein located inside a neuron’s membrane next to a metabotropic receptor, activated when the receptor binds a ligand. An alpha-subunit of the G protein then breaks away to perform actions within the cell.
GABA: an amino acid that is often inhibitory. GABA may be the most important inhibitory neurotransmitter in the brain. There are several types of GABA receptors, each producing inhibition differently.
gamma rhythms: a 28-80 Hz rhythm that includes the 38-42 Hz Sheer rhythm and is associated with learning and problem-solving, meditation, mental acuity, and peak brain function in children and adults.
gap junction: an electrical synapse, which is a symmetrical synapse where neurons communicate information bidirectionally across gap junctions between adjacent membranes using ions. Transmission across electrical synapses is instantaneous, compared with the 10-ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of distant neurons to synchronize their activity and simultaneously fire.
generalized asynchronous slow waves: waves seen in sleepy children and those with elevated temperatures. This may indicate degenerative disease, dementia, encephalopathy, head injury, high fever, migraine, and Parkinson's disease in adults.
glial cells: nonneural cells that guide, insulate, and repair neurons and provide structural, nutritional, and information-processing support. Glial cells generate slow cortical potentials (SCPs). Glial cells include astrocytes, microglia, oligodendrocytes, radial glial cells, and Schwann cells.
global loops: cortical macrocolumns separated by as much as 7 cm and receive shared input fire synchronously to generate delta and theta rhythms.
glutamate: an amino acid that is often excitatory and that may be the primary excitatory neurotransmitter in the brain. Its receptors are found on the surface of almost all neurons. There are at least 13 different receptors for glutamate, 5 ionotropic and 8 metabotropic. Most presynaptic neurons in the brain excite postsynaptic neurons via ionotropic glutamate receptors in the postsynaptic membrane. Metabotropic glutamate receptors may play a regulatory function, either augmenting or suppressing the activation of ionotropic glutamate receptors.
glycine: an amino acid that is often inhibitory and has a binding site on the NMDA receptor.
gray matter: brain tissue that looks grayish brown and comprises cell bodies, dendrites, unmyelinated axons, glial cells, and capillaries.
gyrus: ridge of cortex demarcated by sulci or fissures, for example, the precentral gyrus.
hertz (Hz): unit of frequency, an abbreviation for cycles per second.
high alpha (alpha 2): 10-12-Hz alpha associated with open awareness.
high beta (beta 4): 25-38-Hz activity mostly seen in the frontal lobes and is associated with hyper-perfusion and increased glucose metabolism. High or fast beta activity may be related to peak performance and cognitive processing and related to specificity and precision in information processing. Excessive high beta is associated with alcoholism, anxiety, OCD, rumination, and worry.
hindbrain: posterior brain division that consists of the cerebellum, pons, and medulla.
hippocampus: a seahorse-shaped limbic structure. The hippocampus is required to form declarative memories and plays a vital role in emotion, navigation, spatial memory, and dampening the endocrine stress response. The hippocampus also contains leukocyte receptors, making it part of the feedback loop for immune system regulation. Hippocampal neurons and networks that include it are sources of the theta rhythm.
horizontal (transverse) plane: the plane that divides the brain into upper and lower parts.
hubs: highly centralized nodes through which other node pairs communicate; hubs allow efficient communication.
hyper-coherence: excessive coupling due to a failure to selectively activate cortical regions. Hyper-coherence may interfere with multitasking and rapid decision-making.
hypo-coherence: deficient coupling due to a breakdown in communication between regions that should generally communicate with each other. Hypo-coherence often results from traumatic brain injuries.
hyperpolarize: a negative shift in membrane potential (the inside becomes more negative with respect to the outside) due to the loss of positive ions or gain of negative ions.
inferior colliculi: midbrain structures that integrate information about spatial localization and multiple sensory modalities, including somatosensory information.
inhibitory postsynaptic potential (IPSP): a brief negative shift in a postsynaptic neuron's potential produced when cations like potassium leave a neuron or anions (negative ions) like chloride enter a neuron, which hyperpolarizes the cell. An IPSP pushes the neuron away from the threshold of excitation.
insular cortex: cortex that lies deep within the lateral sulcus that divides the temporal and parietal lobes (BA 13). The insula is involved in emotional and autonomic responses to external stimuli and is part of the salience network.
integration: the addition of EPSPs and IPSPs at the axon hillock. Neurons sum EPSPs and IPSPs over their surface in spatial integration and over ms of time in temporal integration to raise the membrane from its resting potential to the excitation threshold. EPSPs and IPSPs last from 15-200 ms, while action potentials occur in 1-2 ms.
internal carotid artery: a major paired artery that supplies blood to nearly two-thirds of the cerebral hemispheres.
interneurons: neurons that receive input from and distribute output to other neurons. They have short processes and are confined to the central nervous system. They provide the integration required for decisions, learning and memory, perception, planning, and movement.
intracellular fluid: the watery cytoplasm contained within a neuron.
ion: a charged atom or molecule with a positive or negative charge. Positive ions are called cations, and negative ions are called anions.
ionotropic receptor: receptor protein that contains a binding site for a ligand and an ion channel that opens when the neurotransmitter attaches to this site.
ipsilateral: structures that are located on the same side of the body. For example, the left olfactory bulb distributes axons to the left hemisphere.
irregular waves: successive waves that constantly alter their shape and duration.
kappa rhythm: bursts of alpha or theta and is detected over the temporal lobes of subjects during cognitive activity.
lambda waves: saw-toothed transient waves from 20-50 mV in amplitude and 100-250 ms in duration detected over the occipital cortex during wakefulness. These positive deflections are time-locked to saccadic movements and observed during visual scanning, as during reading.
lateral: to the side, away from the center, as in the lateral geniculate nucleus.
lateral geniculate nucleus (LGN): thalamic nucleus that relays visual information to the cortex.
lateral nucleus of the amygdala: a nucleus that processes sensory information and distributes it throughout the amygdala.
lateralized waves: waves that are primarily detected on one side of the scalp and may indicate pathology.
Layers I-III: cortical layers that receive corticocortical afferent fibers that connect the left and right hemispheres.
Layer III: the cortical layer that is the primary source of efferent corticocortical fibers.
Layer IV: the cortical layer that is the primary destination of thalamocortical afferents and intra-hemispheric corticocortical afferents.
Layer V: the cortical layer that is the primary origin of efferent fibers that target subcortical structures that have motor functions.
Layer VI: the cortical layer that projects cortico-thalamic efferent fibers to the thalamus, which, together with the thalamocortical afferents, creates a dynamic and reciprocal relationship between these two structures.
left dorsolateral prefrontal cortex: the division of the prefrontal cortex concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals.
limbic system: a poorly-defined widespread network of nuclei involved in emotion, motivation, learning, memory, and navigation. Three important limbic structures are the hippocampus, amygdala, and septal nuclei.
local loops: neighboring cortical macrocolumns that share input generate frequencies above 30 Hz in the high-beta and gamma ranges.
local synchrony: synchrony that occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals.
localized slow waves: waves that may indicate a transient ischemic attack (TIA) or stroke, migraine, mild head injury, or tumors above the tentorium. Deep lesions result in bilateral or unilateral delta.
locus coeruleus system: the noradrenergic branch of the ascending reticular activating system that projects to the thalamus, limbic system, and cerebral cortex, and contributes to wakefulness and vigilance for salient stimuli. Subnormal norepinephrine transmission may contribute to ADHD.
long-latency potentials: potentials that have extended latencies following stimulus onset, for example, P300 and N400 ERPs.
long-term depression (LTD): a persistent decrease in synaptic strength following low-frequency stimulation.
long-term potentiation (LTP): a persistent increase in synaptic strength following high-frequency stimulation.
low alpha (alpha 1): 8-10-Hz alpha below a client's peak alpha frequency when eyes are closed.
macrocolumns: circuits of cortical pyramidal neurons several millimeters in diameter that create extracellular dipole layers parallel to the surface of the cortex, that send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons. Since the pyramidal neurons are all aligned with the cortical surface, the postsynaptic potentials at cells within the same macrocolumn add together because they have the same positive or negative charge. The macrocolumns fire synchronously.
medial: toward the center of the body, away from the side. For example, the medial geniculate nucleus.
medial geniculate nucleus (MGN): thalamic nucleus that projects to several cortical auditory areas using two separate pathways. The MGN mainly relays frequency, amplitude, and binaural information to the auditory cortex in the temporal lobe.
medial prefrontal cortex: the division of the prefrontal cortex that integrates cognitive-affective information and helps control the hypothalamic–pituitary–adrenal (HPA) axis during emotional stress.
membrane potential: a neuron’s electrical charge created by a difference in ion distribution within and outside the neuron. A typical resting potential is about -70 mV (thousandths of a volt) since the inside of a resting axon is more negatively charged than the outside.
meninges: three protective layers (dura mater, pia mater, and arachnoid) that enclose the brain and spinal cord.
mesocortical neurons: dopaminergic neurons that project from the ventral tegmental area of the midbrain to the prefrontal cortex and excite prefrontal cortical neurons that control working memory, planning, and strategy preparation for problem solving. Underactivity in this pathway is associated with the negative symptoms of schizophrenia-like attentional deficits.
metabotropic receptors: include all G protein-linked receptors located on neurons, including autoreceptors. Neurotransmitters that bind to G protein-linked receptors are often called neuromodulators. Metabotropic receptors, which indirectly control the cell's operations, expend energy, and produce slower, longer-lasting, and more diverse changes than ionotropic receptors. Their effects can last several seconds, instead of milliseconds, because of the long-lived activity of G proteins and cyclic AMP.
metencephalon: the hindbrain subdivision that consists of the cerebellum and pons.
microtubules: hollow cylindrical protein bundles that are involved in axoplasmic transport.
midbrain: the middle division called the mesencephalon, which includes the inferior colliculi, superior colliculi, and substantia nigra.
mirror neurons: neurons activated when we perform a movement or observe others perform the same activity. Mirror neurons may facilitate observational learning, understanding others' actions and intentions, and empathy.
modulating effects: neuromodulators like the monoamines alter the performance of diffuse networks of target neurons by indirectly controlling cellular operations when they bind to metabotropic receptors.
module: a set of interconnected nodes in a neural network.
monoamine neurotransmitters: amine neurotransmitters that include dopamine, norepinephrine, epinephrine (catecholamines), and serotonin (indoleamine). These neurotransmitters are released using volume transmission and generally have modulating effects, altering the performance of diffuse networks of target neurons.
monoamine oxidase (MAO): an enzyme that degrades and inactivates the monoamine neurotransmitters dopamine, norepinephrine, and serotonin.
monoamine oxidase inhibitors (MAOIs): antidepressant drugs that interfere with MAO's breakdown of monoamines and increase monoamine availability to treat clinical depression.
monophasic wave: either a single negative (upward) or positive (downward) deflection from baseline.
motor cortex: a subdivision of the frontal lobe located in the precentral gyrus and guides fine motor coordination (like writing).
motor ERPs: event-related potentials detected over the primary motor cortex (precentral gyrus) during movement. Their amplitude is proportional to the force and rate of skeletal muscle contraction.
motor nerves: efferent neurons that convey commands to glands, muscles, and other neurons.
movement-related potentials (MRPs): slow cortical potentials that occur at 1 second as subjects prepare for unilateral voluntary movements. MRPs are distributed bilaterally with maximum amplitude at Cz. The supplementary motor area and primary motor and somatosensory cortices primarily generate these potentials.
mu rhythm: 7-11-Hz waves resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
multiple spike-and-slow-wave complex: multiple spikes associated with at least one slow wave.
muscarinic receptors: metabotropic ACh receptors that are stimulated by muscarine and blocked by atropine. Muscarinic receptors control smooth muscle and predominate in the CNS. In the CNS, muscarinic receptors help mediate learning, memory, attention, arousal, EEG, and postural control.
myelencephalon: the hindbrain subdivision that consists of the medulla.
myelinated axons: axons insulated by myelin by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system.
N1-P2: a sensory event-related potential in the auditory cortex of the temporal cortex that reveals whether an uncommunicative person can hear a stimulus.
N400 potential: an event-related potential (ERP) elicited when we encounter semantic violations like ending a sentence with a semantically incongruent word ("The handsome prince married the beautiful fish"), or when the second word of a pair is unrelated to the first (BATTLE/GIRL).
negative SCPs: slow cortical potentials produced by glial cells that increase the probability of neuron firing.
nerve: bundled axons outside of the central nervous system.
neural network: a system of interconnected ensembles of neurons that collaborate to achieve a goal. These networks communicate and perform functions via hub- or node-based communication systems.
neuroaxis: an imaginary line that runs centrally through the central nervous system (CNS) from the front of the prefrontal cortex to the base of the spinal cord.
neuromodulator: neurochemical that modifies the effect of neurotransmitters through mechanisms like binding to metabotropic receptors.
neuron: a nerve cell that is the fundamental anatomical unit of the nervous system.
nicotinic ACh receptor: an ionotropic receptor that is stimulated by nicotine and blocked by curare. They are mainly found in the PNS on skeletal muscles. At CNS axoaxonic synapses, they produce presynaptic facilitation (increase neurotransmitter release). In the CNS, nicotinic receptors help regulate cortical blood flow, anxiety reduction, and decision-making.
nigrostriatal pathway: a dopaminergic pathway from the substantia nigra to the basal ganglia (caudate nucleus and putamen) that controls movement. The nigrostriatal pathway is progressively destroyed in Parkinson’s disease.
nitric oxide: a gaseous retrograde transmitter that is involved in long-term potentiation (LTP).
NMDA (glutamate) receptors: ligand-gated and voltage-gated glutamate receptors that bind the glutamate agonist NMDA. NMDA receptors play an important role in long-term potentiation (LTP).
node: a vertex within a neural network.
nodes of Ranvier: gaps between myelinated axon segments where the axon membrane is exposed to extracellular fluid and action potentials are regenerated by sodium ion entry.
norepinephrine: a monoamine neurotransmitter that exerts postsynaptic effects at alpha and beta receptors, each with two subtypes. All norepinephrine receptors are G protein-linked. The cell bodies of the most critical noradrenergic system are located in the locus coeruleus, a nucleus found in the dorsal pons.
nucleus accumbens: a limbic structure that receives dopamine released by the mesolimbic pathway. The nucleus accumbens plays a critical role in reinforcing diverse activities, including ingestion of drugs like central nervous system stimulants.
nucleus reticularis: a thalamic nucleus that may function as a pacemaker by releasing the inhibitory transmitter GABA at synapses with thalamocortical neurons.
occipital lobes: cortical lobes (Oz, O1, O2) posterior to the parietal lobes. The primary visual cortex (VI) is located within the calcarine sulcus (BA 17). They process visual information from the eyes in collaboration with the frontal, parietal, and temporal lobes.
odd-ball stimulus: a meaningful stimulus that is different from others in a series used to elicit the P300 potential. For example, a colored playing card is presented in a series of monochrome cards.
open awareness: the ability to adaptively respond to various environmental changes.
orbitofrontal cortex (OFC): the frontal lobe subdivision (BA 10, 11, and 47) that may aid planning by evaluating the consequences (rewards and punishments) of our actions and helping to generate the motivation to ingest drugs. The OFC appears to adjust decision-making based on the stakes involved and enables us to switch between substantial (investments) and trivial (snacks) choices.
orienting response: Pavlov’s "What is it?" reaction to stimuli like the sound of a vase crashing that includes (1) increased sensory sensitivity, (2) head (and ear) turning toward the stimulus, (3) increased muscle tone (reduced movement), (4) EEG desynchrony, (5) peripheral constriction and cephalic vasodilation, (6) a rise in skin conductance, (7) heart rate slowing, and (8) slower, deeper breathing.
P300 potential: an event-related potential (ERP) with a 300-900 ms latency and greatest positive peaks located over parietal lobe sites. The P300 potential may reflect an event’s subjective probability, meaning, and transmission of information.
parahippocampal gyri: structures located within the medial temporal lobe that form spatial and nonspatial contextual associations, which serve as building blocks for contextual processing, episodic memory, navigation, and scene processing. They may also play a role in emotional responsiveness.
parietal lobes: cortical lobes (Pz, P3, P4) posterior to the frontal lobes divided into the primary somatosensory cortex (postcentral gyrus) and secondary somatosensory cortex. Their primary function is to process somatosensory information like pain and touch.
perception-action cycles: cognitive and emotional processes that adapt (and preadapt) us to our environment.
peripheral nervous system (PNS): autonomic and somatic nervous system neurons and nerves outside the skull and spinal cord.
phase: the degree to which the peaks and valleys of EEG waveforms coincide. Phase measures the time shift between EEG activity in two brain regions.
phase reset: a sudden change in phase difference (phase shift duration or SD) followed by a period of phase locking (lock duration or LD). PR = SD + LDs.
phase synchrony: synchrony when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
polyphasic (multiphasic) wave: a wave that contains two or more deflections of opposite polarity from baseline.
positive SCPs: slow cortical potentials produced by glial cells that decrease the probability of neuron firing.
postcentral gyrus: primary somatosensory cortex, posterior to the central sulcus.
posterior: near or toward the back of the head.
posterior basic rhythm: posterior alpha rhythm associated with IQ and memory performance.
posterior cerebral arteries: the left and right posterior arterial branches of the basilar artery that supply blood to the posterior cerebral hemispheres, cerebellum, and brainstem.
posterior commissure: axon tracts located below the corpus callosum connect the right and left diencephalon and mesencephalon.
posterior cortex: parietal, temporal, and occipital cortical areas concerned with perception and memory.
precentral gyrus: primary motor cortex, anterior to the central sulcus.
prefrontal cortex (PFC): the most anterior frontal lobe division (BA 9, 10, 11, 12, 46, 47) that is subdivided into dorsolateral, medial, orbitofrontal, and anterior cingulate regions and is responsible for executive functions like attention, working memory, prediction of the outcomes of current and hypothetical actions, the ability to work toward goals, problem-solving, planning, and the ability to suppress actions that could lead to unwanted outcomes.
premotor cortex (motor association cortex): the frontal lobe subdivision (BA 6) that is anterior to the motor cortex and helps to program and execute head, trunk, and limb movements.
presynaptic facilitation: a modulatory process in which a neuron increases the presynaptic neuron's neurotransmitter release by delivering a neurotransmitter that increases calcium ion entry into its terminal button.
presynaptic inhibition: a modulatory process in which a neuron decreases neurotransmitter release by reducing calcium ion entry.
primary motor cortex: the frontal lobe region located along the precentral gyrus (BA 4) that organizes the opposite side of the body's muscles and movements required for fine motor coordination in tasks like writing. Lesions can result in loss of motor control, including rigid paralysis.
primary somatosensory cortex (S1): parietal lobe subdivision located in the parietal lobe's postcentral gyrus posterior to the central sulcus (BA 3, 1, and 2). S1 maps touch and pain information from the opposite side of the body.
primary visual cortex (V1): the occipital lobe region (also called striate cortex) which receives most visual information from the lateral geniculate nucleus of the thalamus.
protein kinase A: an intracellular enzyme that controls the excitability of ion channels and is a vital enzyme target of cyclic AMP.
raphe system: the midline network of cell bodies within the brainstem and midbrain that may influence alertness and vigilance through reciprocal connections with the suprachiasmatic nucleus of the hypothalamus.
rate law: the principle that neurons represent the intensity of a stimulus by variation in the rate of axon firing.
readiness potential: slow-rising, negative potential (10-15 μV) detected at the vertex before voluntary and spontaneous movement. This slow cortical potential precedes voluntary action by 0.5 to 1 s and peaks when the subject responds.
regional loops: cortical macrocolumns that share input and are separated by several centimeters generate alpha and beta rhythms.
regular or monomorphic waves: successive waves with identical shapes. Regular waves may resemble sine waves (sinusoidal) or maybe arched (resembling wickets), or saw-toothed (asymmetrical and triangular).
resonant loop: the synchronous firing by macrocolumns that share afferent input to generate an electrical potential.
resting potential: the membrane potential of a neuron when it is not influenced by messages from other neurons.
reticular activating system (RAS): a network of 90 nuclei within the central brainstem from the lower medulla to the upper midbrain. The reticular formation sends axons to the spinal cord, thalamus, and cortex, contributing to diverse functions like neurological reflexes, muscle tone and movement, attention, arousal, and sleep.
reuptake: the primary method that neurons terminate the action of neurotransmitters. Reuptake transporters located in terminal buttons and astrocytes remove neurotransmitters from the synaptic cleft.
reward deficiency syndrome: Blum’s hypothesis that an abnormal form of the A1 allele is present in most severe alcoholics and results in defective D2 receptors. Reduced D2 receptor activity may reduce the activation of the nucleus accumbens and hypothalamus and result in dysphoria, drug craving, and compulsive drug-seeking and abuse.
rhythmic slow wave activity: a posterior waveform generated by the limbic system and thalamus that is mostly seen in the frontal-midline (FCz) when awake with eyes open.
right dorsolateral prefrontal cortex: division of the prefrontal cortex that organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
rostral: toward the front of the head.
sagittal plane: the plane that divides the body into right and left halves.
saltatory conduction: action potential conduction in myelinated axons in which action potentials jump from node to node for 200 times greater speed.
secondary somatosensory cortex (S2): the region of the parietal lobe adjacent to S1 (BA 40 and 43), receives projections from it and maps touch and pain from both sides of the body.
sensorimotor rhythm (SMR): 13-15 Hz (beta 1) rhythm that is located over the sensorimotor strip (C3, Cz, C4). The waves are synchronous. SMR increases with stillness and decreases with movement. Deficient SMR may be observed in movement spectrum complaints like hyperactivity and tics.
sensorimotor system: in Sterman’s model, ascending pathways that convey information about touch and proprioception to the thalamus, the thalamus and its thalamic projections to the sensorimotor cortex, and the sensorimotor cortex, and its efferent fibers.
sensory event-related potentials (ERPs): event-related potentials evoked by external sensory stimuli (auditory, olfactory, somatosensory, and visual). These evoked potentials or exogenous ERPs have a negative peak around 80-90 ms and a positive peak about 170 ms following stimulus onset. These changes in brain activity in response to specific stimuli. ERPs can be detected throughout the cortex. Investigators monitor ERPs by placing electrodes at locations like the midline (Fz, Cz, and Pz). A computer analyzes a subject's EEG responses to the same stimulus or task over many trials to subtract random EEG activity. ERPs always have the same waveform morphology. Their negative and positive peaks occur at regular intervals following the stimulus.
sensory nerves: neurons specialized for sensory intake. They are called afferent because they transmit sensory information towards the central nervous system.
septal nuclei: a limbic structure that contains several nuclei involved in emotion, control of aggressive behavior, reward, and addiction. The septohippocampal system contributes to the theta rhythm.
septohippocampal system: a subcortical circuit from the septum to the hippocampus that contributes to 4-7 Hz theta activity.
septum: a limbic structure that contains several nuclei involved in emotion and addiction and control of aggressive behavior.
sharp transients: a sequence that contains several sharp waves.
sharp waves: waves that resemble spikes with a pointed peak with a longer 70-200-ms duration.
sink: a site where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, represented by -ve.
slow cortical potentials (SCPs): gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials. SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms.
sodium (Na+) ions: positive ions that enter a neuron during EPSPs and action potentials.
sodium-potassium transporters: pumps that are powered by ATP and that exchange three sodium for two potassium ions.
soma or cell body: the part of a neuron that contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
somatic nervous system: spinal nerves that innervate somatosensory receptors in the skin, joints, and skeletal muscles.
source: the place at the end of the neuron opposite of the sink where current leaves. The source is symbolized by +ve. The extracellular area surrounding the source becomes electrically positive.
spatial summation: the addition of EPSPs and IPSPs over a neuron’s surface.
spike: a negative transient with a pointed peak at conventional paper speeds, 20-70 ms duration, and 40-100 μV amplitude.
spike-and-slow-wave complex: a spike followed by a higher amplitude slow wave at 3 Hz. In an absence seizure, the amplitudes are very high (e.g., 160 μV).
spinal cord: the column of neurons and glial cells within the vertebral canal that extend from the brainstem to the lumbar vertebrae. The spinal cord distributes sensory information from the body to the brain, and CNS commands from the brain to the body. The spinal cord also contains networks that control reflexes and central pattern generators.
spinal nerve: 31 pairs of nerves that exit the spinal cord.
spindle waves: waves that originate in the thalamus and occur during unconsciousness and stage-2 sleep.
striatal: basal ganglia (caudate nucleus and putamen).
stroke: cerebrovascular accident (CVA) involves the destruction of brain tissue (infarction) due to cerebral hemorrhage and cerebral ischemia affecting blood vessels that supply the brain. CVAs show abrupt onset and involve temporary or permanent neurological symptoms like aphasia, paralysis, or loss of sensation.
Stroop test: cognitive monitoring task where color and names conflict.
substantia nigra: the midbrain structure that projects to the basal ganglia (caudate nucleus and putamen) to control movement and is progressively destroyed in Parkinson’s disease.
sulcus: a shallow groove in the surface of the cerebral hemisphere, for example, the central sulcus.
superior colliculus: the dorsal midbrain structure that receives visual information and directs visual gaze and attention to selected stimuli.
superior olivary nuclei: midbrain structures that process binaural information to localize sound.
Sylvian fissure: deep fissure that serves as the upper boundary of the temporal lobe.
synapse-associated polyribosome complexes (SPRCs): organelles with dendrites that can produce proteins that allow rapid remodeling of synapses. A polyribosome complex consists of several ribosomes bound to messenger RNA (mRNA). SPRCs represent one mechanism underlying synaptic plasticity.
synaptic cleft: 20-40 nm fluid-filled gap between presynaptic and postsynaptic structures.
synchronous: adverb meaning that groups of neurons depolarize and hyperpolarize simultaneously.
synchronous "alpha": network-wide "alpha" that integrates perception and facilitates action. This distributed activity appears to block localized alpha-like patterns such as mu and the posterior rhythm in favor of more broadly distributed network integration.
synchrony: the coordinated firing of pools of neurons. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
telencephalon: the frontal forebrain subdivision that consists of the cerebral cortex, basal ganglia, and limbic system.
temporal summation: the addition of EPSPs and IPSPs over time. Summation is more effective when postsynaptic potentials are generated more closely in time.
temporal lobes: lobes separated from the rest of the cortical lobes by the Sylvian fissure (BA 15, 20, 21, 22, 37, 38, 39, 40, 52). The temporal lobes process hearing, smell, and taste information and help us understand spoken language and recognize visual objects and faces.
terminal buttons: buds located on the ends of axon branches that form synapses and release neurochemicals to other neurons. They contain vesicles that store neurotransmitters for release when an action potential arrives. A terminal button’s presynaptic membrane may have reuptake transporters that return neurotransmitters from the synapse or extracellular space for repackaging.
thalamus: the forebrain structure above the hypothalamus that consists of specialized nuclei that process and relay data to and from the telencephalon (cerebral cortex, basal ganglia, and limbic system). The thalamus analyzes all sensory data except olfaction before distributing this information to the cortex via thalamocortical afferent fibers. The thalamus contributes to SCPs, delta, theta, alpha, SMR activity, and beta-gamma activity.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
threshold of excitation: the membrane potential at which an axon initiates an action potential, nominally -40 mV.
transient: a single wave or sequence of regular waves, called a complex, distinguishable from background EEG activity.
triphasic wave: a wave that contains three deflections from baseline.
unmyelinated axons: smaller-diameter axons without fatty insulation that conduct more slowly than myelinated axons.
+ve: The source is the place at the other end of the neuron where the current leaves. The source is symbolized by +ve.
-ve: A sink is where the current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, symbolized by -ve.
ventral: toward the base of the skull or front of the body.
ventral posterior nucleus (VPN): a thalamic nucleus that receives somatosensory information following crossover at the medulla and projects to the primary somatosensory cortex (S1).
ventral stream (auditory): the subcortical auditory pathway to the auditory cortex that appears to analyze sound components, including speech sounds.
ventral stream (visual): the pathway from the primary visual cortex (V1) to the inferior temporal and frontal areas that allows us to identify objects and faces.
ventral striatum: the olfactory tubercle and nucleus accumbens.
ventral tegmental area: the midbrain structure that distributes dopaminergic axons to the nucleus accumbens. Serotonin receptors on endorphin-releasing neurons in the hypothalamus may increase the activity of dopaminergic reward pathways by inhibiting the release of GABA at receptors on cell bodies of the ventral tegmental area neurons.
ventromedial prefrontal cortex (VMPFC): the ventromedial reward network (BA 10, 14, 25, 32, and parts of 11, 12, and 13) implicated in making decisions where the outcomes are uncertain and where moral values must be applied to actual situations.
vigilance system: in Sterman’s model, a system that consists of both specific brainstem nuclei (e.g., locus coeruleus and raphe nuclei) and their diffuse connections with the thalamus and other subcortical structures, and the cortex. Several neurotransmitter systems mediate vigilance, including cholinergic/glutamatergic (reticular formation), noradrenergic (locus coeruleus), and serotonergic (raphe) neurons.
volume conduction: the movement of the EEG through body tissues and interstitial fluid to the scalp.
volume transmission: extrasynaptic neurotransmitter release from axonal varicosities, dendrites, and terminal button into the extracellular space. Monoamines like norepinephrine and serotonin are released outside the synaptic cleft.
waveform: the shape and form of an EEG signal.
Wernicke's area: area of the temporoparietal cortex (BA 22) of the dominant hemisphere specialized for speech perception and production. Damage can result in an inability to understand the meaning of speech and construct intelligible sentences.
white matter: the layer beneath the cortex that mainly consists of myelinated axons.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.


References
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Chan, C. Y., Ke, D. S., & Chen, J. Y. (2009). Essential fatty acids and human brain. Acta Neurol Taiwan, 18(4), 231-241. PMID: 20329590
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer: Basic principles of digital and analog EEG (3rd ed.). Elsevier.
Fuster, J. (2015). The prefrontal cortex (5th ed.). Academic Press.
Hughes, S. W., & Crunelli, V. (2005). Thalamic mechanisms of EEG alpha rhythms and their pathological implications. The Neuroscientist, 11(4), 357-372. https://doi.org/10.1177/1073858405277450
Lubar, J. F. (1997). Neocortical dynamics: Implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Applied Psychophysiology and Biofeedback, 22(2), 111-126. https://doi.org/10.1023/a:1026276228832.
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct, 214(5-6), 655-667. https://dx.doi.org/10.1007%2Fs00429-010-0262-0
Miller, B. L., & Cummings, J. L. (Eds.) (2007). The human frontal lobes: Functions and disorders (2nd ed.). Guilford Press.
Monastra, V. J., Lubar, J. F., & Linden, M, VanDeusen, P., Green, G., Wing, W., . . Fenger, T.N. (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validiation study. (2001). Neuropsychology, 13(3), 424-433. https://doi.org/10.1037/0894-4105.13.3.424
Monti, J. M., & Jantos, H. (2008). The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res, 172, 625-646. https://doi.org/10.1016/S0079-6123(08)00929-1
Neumann, N., Strehl, U., & Birbaumer, N. (2003). A primer of electroencephalographic instrumentation. In M. Schwartz & F. Andrasik (Eds.). Biofeedback: A practitioner's guide (3rd ed.). Guilford Press.
Steriade, M. (2001). The intact and sliced brain. MIT.
Steriade, M. (2005). Cellular substrates of brain rhythms. In E. Niedermeyer, & F. Lopes da Silva (Eds.). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Steriade, M., & Llinás (1988). The functional states of the thalamus and the associated neuronal interplay. Physiol Rev, 68(3), 649-742. https://doi.org/10.1152/physrev.1988.68.3.649
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Traub, R. D., Miles, R., & Wong, R. K. S. (1989). Model of the origin of rhythmic population oscillations in the hippocampal slice. Science, 243, 1319-1325. https://doi.org/10.1126/science.2646715
Voytek, B. (2013). Brain metrics: How measuring brain biology can explain the phenomena of mind. Scitable by Nature Education.
Microanatomy: Neurons and Glia
The brain uses a sophisticated communication and command-and-control system that monitors and manages interactions between roughly 100 billion neurons, each with 5,000-10,000 synaptic connections, for as many as 500 trillion synapses in adults. Neuroscientists have learned a great deal more about neuronal function since graduate school. The most important findings are that the adult human brain creates new neurons, silent synapses may mediate neuroplasticity in adulthood, the lymphatic system extends to the brain, networks of neurons exhibit mirroring properties, neurons can release more than one neurotransmitter (NT), release NTs outside of a synapse, conduct two-way conversations, modulate NT release and action, talk to the astrocytes that enclose the synapse, and electrically communicate almost instantaneously. Research has expanded our understanding of the roles of astrocytes in regulating neuron growth, function, signaling, information processing, synapse formation and elimination, and brain waves.
We will review Types of Neurons, Neuron Structure, Excitatory and Inhibitory Postsynaptic Potentials, Integrating Postsynaptic Potentials, Action Potentials, Synaptic Transmission, Extra-Synaptic Transmission, Modulation, Types of Neurotransmitters, Neurotransmitter Pathways, Termination of Neurotransmitter Action, Electrical Synapses, Discoveries Since Graduate School, Cortical Architecture, and Neurons in the Cortex.
Please click on the podcast icon below to hear a full-length lecture for Section A Part 3.

Types of Neurons
Neuron graphic © SciePro/Shutterstock.com.
We can divide neurons into sensory, motor, and interneurons. Glial cells like astrocytes and microglia work in partnership with neurons.
Sensory neurons are specialized for sensory intake. They are called afferent because they transmit sensory information towards the central nervous system (brain and spinal cord). Sensory neuron graphic © TimeLineArtist/Shutterstock.com.
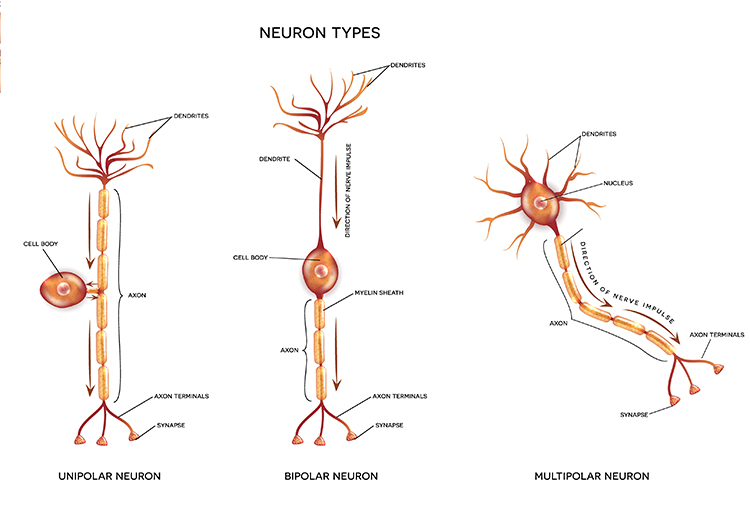
Motor neurons convey commands to glands, muscles, and other neurons. They are called efferent because they convey information towards the periphery. Motor and sensory neuron graphic © iso-form llc/Shutterstock.com.
.jpg)
Interneurons provide the integration required for decisions, learning and memory, perception, planning, and movement. They have short processes, analyze incoming information, and distribute their analysis with other neurons in their network. Interneurons are entirely confined to the central nervous system, account for many of its neurons, and comprise most of the brain (Breedlove & Watson, 2023).
Local interneurons analyze small amounts of information provided by neighboring neurons. Relay interneurons connect networks of local interneurons from separate regions to enable diverse functions like perception, learning, and memory, and executive functions like planning (Carlson & Birkett, 2021). Neuron graphic © Aldona Griskeviciene/Shutterstock.com.
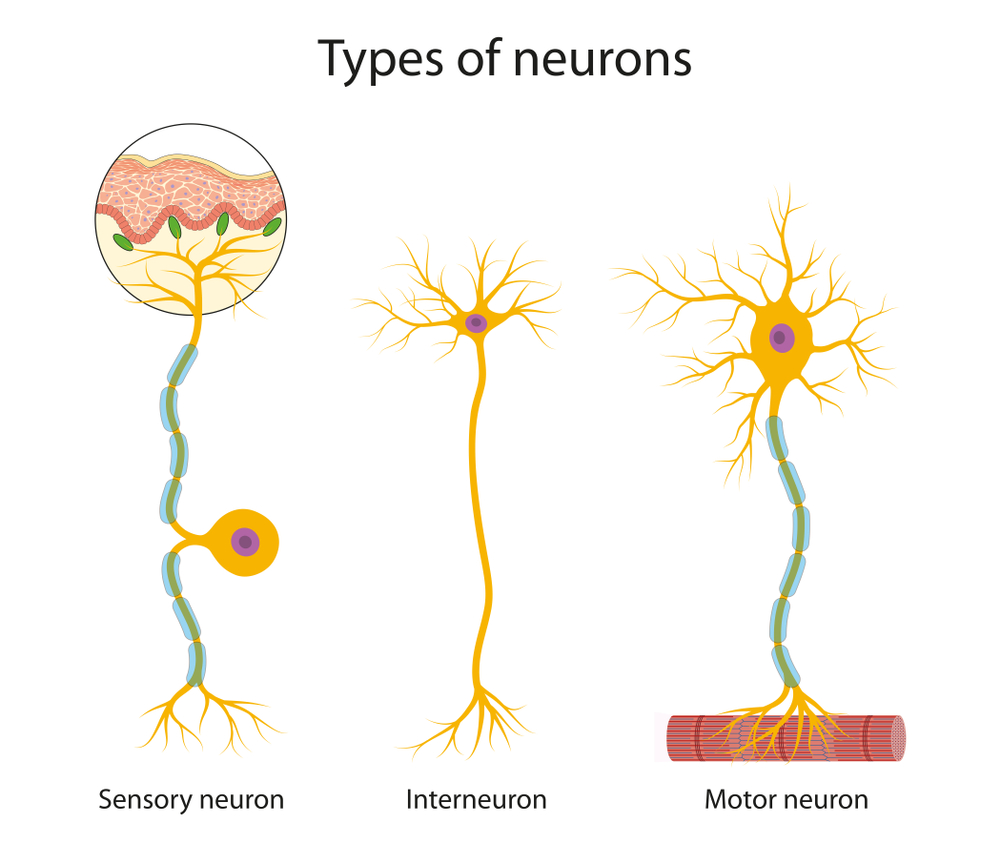
Reflex arc graphic © SANDIP NEOGI/Shutterstock.com.
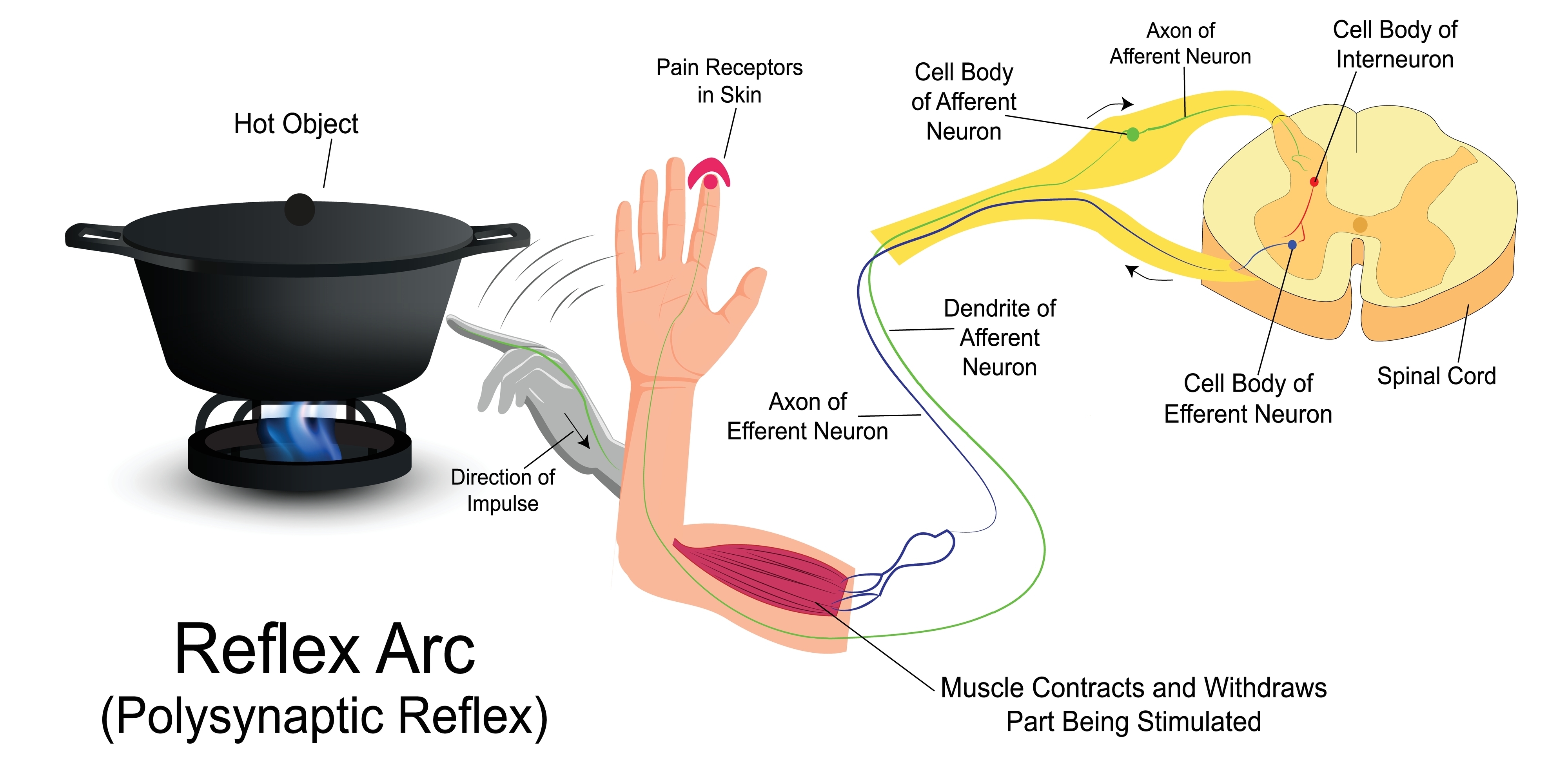
Neuron Structure
While neurons have over 200 different designs to perform specialized jobs in the nervous system, they generally have five structures: a cell body or soma, dendrites, an axon and axon hillock, and terminal buttons. Graphic © Designua/Shutterstock.com.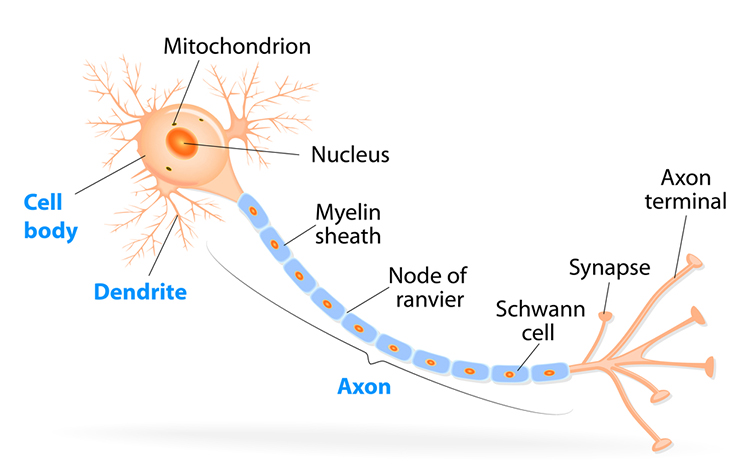
The cell body or soma contains the machinery for the neuron’s life processes. It receives and integrates EPSPs and IPSPs, small graded positive and negative changes in membrane potential generated by axons. The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across. The cell body is the primarily location where neurons manufacture proteins (like enzymes, receptors, and ion channels) and peptides (neurotransmitters like oxytocin). There is increasing evidence of distributed protein manufacturing via local mRNA translation (Nagano & Araki, 2021). Check out the Khan Academy YouTube video, Anatomy of a Neuron. Cell body graphic © MattL_Images/Shutterstock.com.
Dendrites are branched structures designed to receive messages from other neurons via axodendritic synapses (junctions between axons and dendrites shown below) and send messages to other neurons dendrodendritic synapses (junctions between the dendrites of two neurons). Dendrites receive thousands of synaptic contacts and have specialized proteins called receptors for neurotransmitters released into the synaptic cleft (Bear, Connors, & Paradiso, 2016).
A neuron's dendrites are called a dendritic tree, and each branch of the tree is called a dendritic branch. Graphic by BruceBlaus from Wikipedia article Neuron.
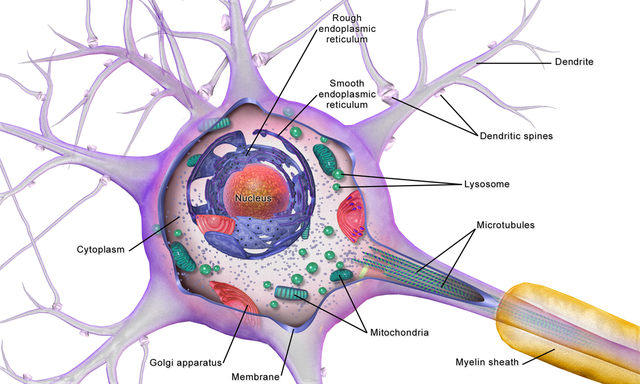
Biological psychologists classify neurons based on whether their dendrites feature spines. Dendritic spines are protrusions on the dendrite shaft where axons typically form axodendritic synapses. Graphic © Jose Luis Calvo/Shutterstock.com.
Spiny neurons have dendritic spines, while aspinous neurons do not (Bear, Connors, & Paradiso, 2016).
During learning, spines' number, size, and shape may change to adjust the space for receptors (neuroplasticity).
An axon is a cylindrical structure only found in neurons specialized for distributing information within the central and peripheral nervous systems. Axons range from 1 to 25 µm in diameter and 0.1 mm to more than a meter in length. Over 90% of neurons are interneurons whose axons and dendrites are very short and do not extend beyond their cell cluster. Axons usually branch repeatedly. Each branch is called an axon collateral.
Axons transmit action potentials toward a neuron's terminal buttons. Using microtubules, an axon also bidirectionally transports molecules between the cell body and terminal buttons.
An axon hillock is a swelling of the cell body where the axon begins. The middle of an axon is the axon proper, and the end is the axon terminal (Bear, Connors, & Paradiso, 2016). Graphic by M.alijar3i from the Wikipedia article Axon Hillock.
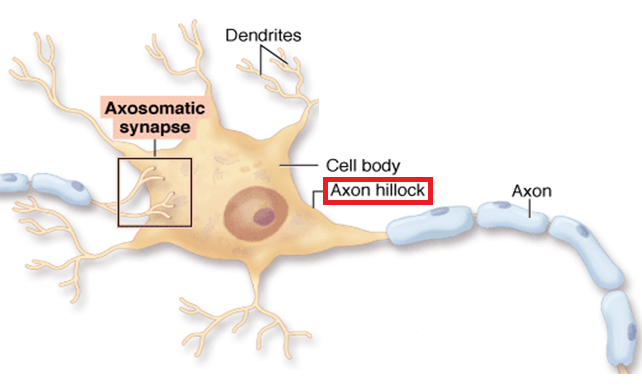
The axon hillock sums EPSPs and IPSPs over milliseconds to generate an action potential.
Axon terminals are buds on the ends of axon branches that form synapses and release neurochemicals to other neurons. Axon terminals contain vesicles that store neurotransmitters for release when an action potential arrives. Their presynaptic membrane may have reuptake transporters that return neurotransmitters (NTs) from the synapse or extracellular space for repackaging. The graphic of serotonin reuptake transporters below is courtesy of NIDA.
Types of Glial Cells
While there are hundreds of types of neurons, there are only four main categories of glial cells (astrocytes, microglia, oligodendrocytes, and Schwann cells).Old school view: glial cells mainly provide structural support (glia is derived from the Greek for glue).
New school view: glial cells help neurons process information, including modulating neuron excitability.
Check out the YouTube video, Neurology - Glial Cells, White Matter and Gray Matter.
Astrocytes are star-shaped glial cells in the central nervous system (i.e., brain and spinal cord) that perform vital functions. Astrocyte endfeet form junctions with capillaries comprising part of the protective blood-brain barrier. They regulate blood flow to the neuron, delivering stored glucose during peak metabolic demand (Schummers et al., 2008). Astrocyte graphic © Kateryna Kon/Shutterstock.com.
 (1).jpg)
Astrocytes enclose synapses, determine where synapses can form by releasing specialized molecules, regulate synapse maturation, bidirectionally communicate with synapses, prune surplus synapses, help neurons regulate brain microcirculation, and eavesdrop on nearby synapse activity (Breedlove & Watson, 2023; Parri & Crunelli, 2003: Shan et al., 2021).
Astrocytes transport amino acid NTs (e.g., GABA and glutamate) from the synaptic cleft.
Astrocytes are theorized to participate in gliotransmission between neurons and each other (Eroglu & Barres, 2010; Perea et al., 2009). However, gliotransmission remains controversial.
. . . the physiological role of gliotransmission is highly debatable . . . as gliotransmitter release has been reliably demonstrated only in vitro in cultures and brain slice experiments that are often accompanied by manipulations (e.g., high frequency stimulation) which can affect astrocytic channels or receptors leading to impaired signaling cascades. This experimental design imposes questions about the existence of gliotransmission . . . and whether it plays a physiological role in the brain . . . (Buskila et al., 2019).
The presynaptic and postsynaptic neurons and astrocytes comprise a tripartite synapse.
An essential role of astrocytes is regulating the chemical content of this extracellular space. For example, astrocytes envelop synaptic junctions in the brain, thereby restricting the spread of neurotransmitter molecules that have been released. Astrocytes also have special proteins in their membranes that actively remove many neurotransmitters from the synaptic cleft. A recent and unexpected discovery is that astrocytic membranes also possess neurotransmitter receptors that, like the receptors on neurons, can trigger electrical and biochemical events inside the glial cell (Bear et al., 2020, p. 49).
Astrocyte glutamate release may be essential for hippocampal long-term depression (LTD), a long-lasting reduction in transmission strength, and long-term memory modulation (Navarrete et al., 2019). Astrocyte calcium and brain-derived neurotrophic factor (BDNF) release appear critical for late-phase hippocampal long-term potentiation (LTP), a long-lasting increase in transmission strength, and long-term memory regulation (Liu et al., 2021).
Astrocytes communicate with each other through gap junctions (Bennett et al., 2003).
Finally, astrocytes may contribute to brainwaves by regulating synapses via gap junctions and calcium signaling.
These capabilities allow astrocytes to regulate neuronal excitability via glutamate uptake, gliotransmission and tight control of the extracellular K+ levels via a process termed K+ clearance. Spatio-temporal synchrony of activity across neuronal and astrocytic networks, both locally and distributed across cortical regions, underpins brain states and thereby behavioral states, and it is becoming apparent that astrocytes play an important role in the development and maintenance of neural activity underlying these complex behavioral states (Buskila et al., 2019).
Microglia
Microscopic microglial cells participate in the immune response. Microglial cells scavenge and engulf diverse materials (phagocytosis), release cytotoxins to control infection, present antigens to T-cells, remove branches from neurons near damaged tissue to aid regrowth (synaptic stripping), promote tissue repair, and promote chronic neuroinflammation in the CNS that amplifies neurodegeneration. They assist synaptic remodeling by removing unnecessary synapses. Finally, microglia cross the blood-brain barrier to promote homeostasis (Bear, Connors, & Paradiso, 2016). Graphic © Juan Gaertner/Shutterstock.com.
Description: yellow = neurons, orange = astrocytes, grey = oligodendrocytes, white = microglia.
Oligodendrocytes, which are smaller than astrocytes, form up to 50 segments of myelin that only insulate adjacent axons within the brain and spinal cord of the central nervous system. Graphic © Designua/Shutterstock.com.
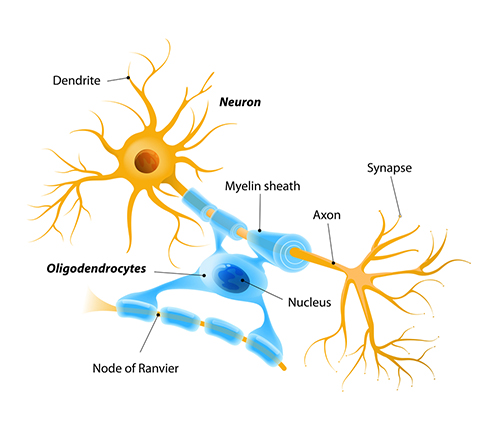
Oligodendrocytes block axonal regeneration by releasing growth inhibitory proteins. These molecules are part of the reason for minimal functional recovery in the CNS following spinal cord damage. Multiple sclerosis, a demyelinating disease, destroys oligodendrocytes.
Schwann cells provide myelin for single PNS axons and facilitate axonal regeneration following damage (Breedlove & Watson, 2020). Graphic © Tefi/Shutterstock.com.
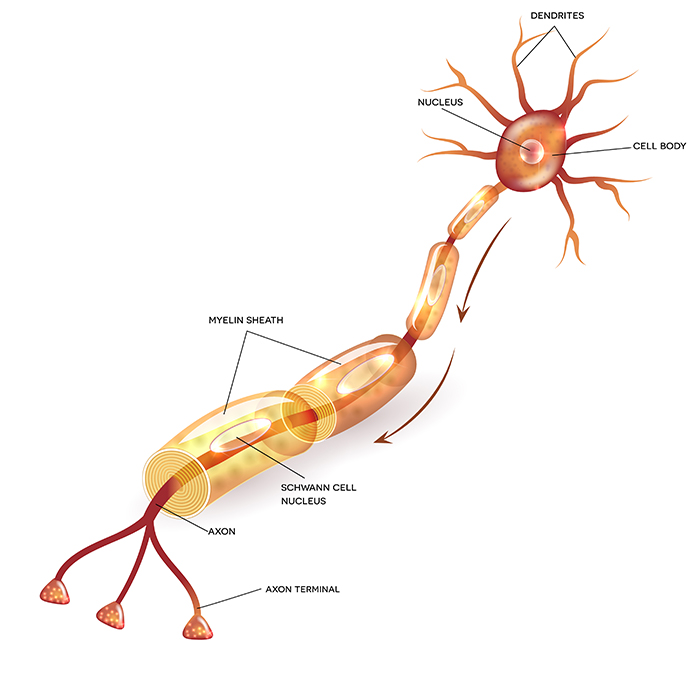
Excitatory and Inhibitory Postsynaptic Potentials
Graded positive and negative changes in membrane potential, called excitatory postsynaptic potentials and inhibitory postsynaptic potentials, are essential to the EEG and neuronal communication.An excitatory postsynaptic potential (EPSP) is a subthreshold depolarization that makes the membrane potential more positive and pushes the neuron toward its excitation threshold. EPSPs are produced when neurotransmitters bind to receptors and cause positive sodium ions to enter the cell. A postsynaptic membrane may have tens to thousands of transmitter-gated ion channels at a single synapse. The amount of transmitter released determines how many of these channels will be activated. The size of an EPSP will be a multiple of the number of vesicles, each containing several thousand transmitter molecules.
An inhibitory postsynaptic potential (IPSP) is a hyperpolarization that makes the membrane potential more negative and pushes the neuron away from its excitation threshold. At most inhibitory synapses, IPSPs are produced when neurotransmitters like GABA or glycine bind to receptors and cause negative chloride ions to enter the cell. When an inhibitory synapse is closer to the soma than an excitatory synapse, it can counteract positive current flow and decrease the size of the EPSP. This mechanism is called shunting inhibition (Bear, Connors, & Paradiso, 2016).
Integrating Postsynaptic Potentials
Integration is the summation of EPSPs and IPSPs at the unmyelinated axon hillock. Interneuron graphic © Dee-sign/Shutterstock.com.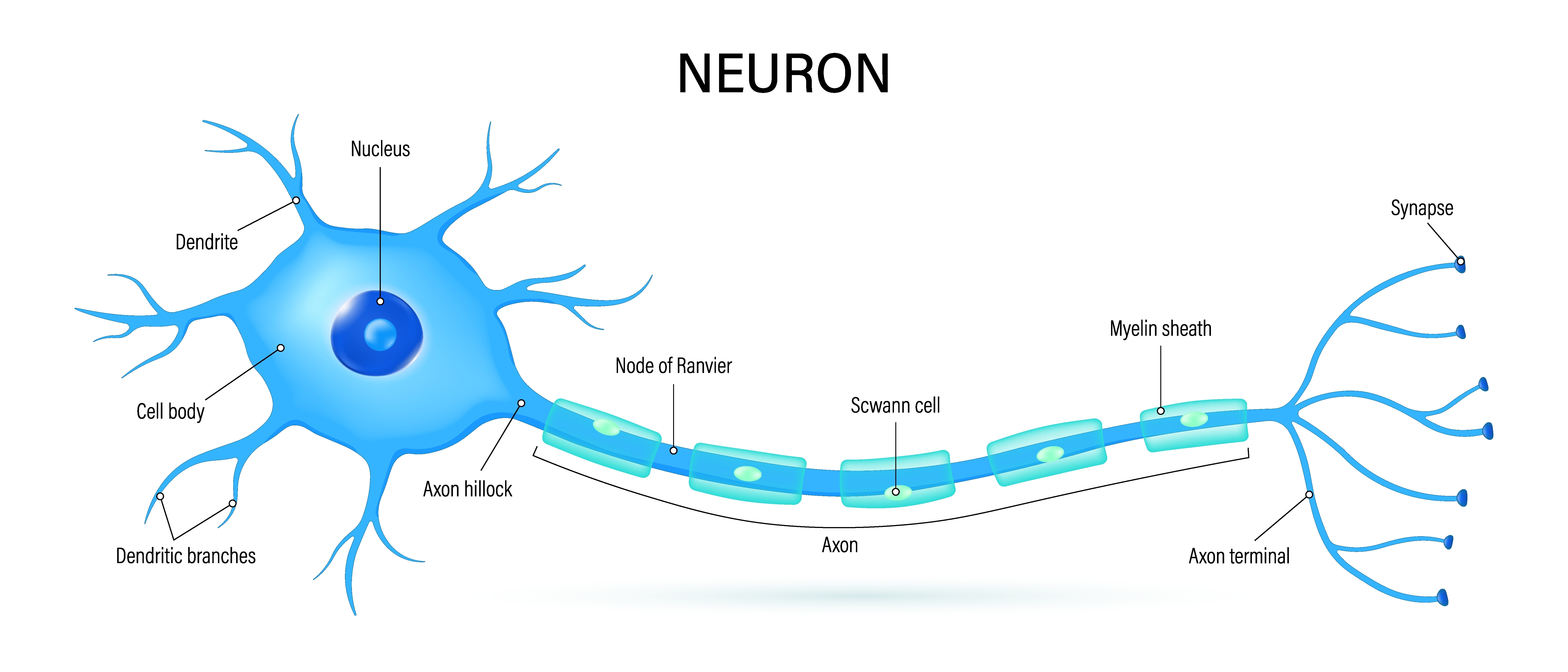
The axon hillock of a postsynaptic neuron uses two methods to sum EPSPs and IPSPs: spatial and temporal summation.
In spatial summation, the axon hillock sums the simultaneous postsynaptic potentials (PSPs) from thousands of synapses on dendrites. In temporal summation, the axon hillock adds the PSPs from presynaptic neurons that repeatedly fire within a 1-15-ms time window.
Each EPSP depolarizes the axon hillock by about 0.5 mV. Without competing IPSPs, it would take about 30 EPSPs to trigger an action potential. Each IPSP hyperpolarizes the axon hillock by about 0.5 mV. If the summated EPSPs and IPSPs move the axon hillock from a resting potential of -70 mV to a threshold of excitation of -55 mV, sodium channels in the axon hillock membrane open, and an action potential propagates down the axon. Graphic © 2003 Josephine Wilson.
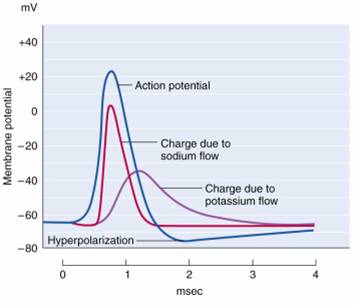
Check out the YouTube video, Best Action Potential Explanation.
Action Potentials
An action potential is a brief electrical impulse that transmits information from the axon hillock to the terminal button. This wave of positive charge only travels in one direction because the preceding segment is refractory due to the closing of its sodium channels. An action potential takes 1-2 ms from the point the axon hillock reaches its threshold to its repolarization to a negative resting potential.Action potentials travel down axons, which branch multiple times and terminate at synapses. The all-or-none law and rate laws describe action potential transmission. The all-or-none law states that once an action potential is triggered in an axon, it is propagated, without decrement, to the end of the axon. The rate law states that neurons represent the intensity of a stimulus by variation in the rate of axon firing. More intense stimuli shorten the interval before a neuron can fire again, allowing a neuron to fire more rapidly. An intense stimulus can cause a neuron to fire every 2 or 3 ms, while a weak stimulus might lengthen the time lag to every 4 or 5 ms. Action potential graphic © extender_01/Shutterstock.com.
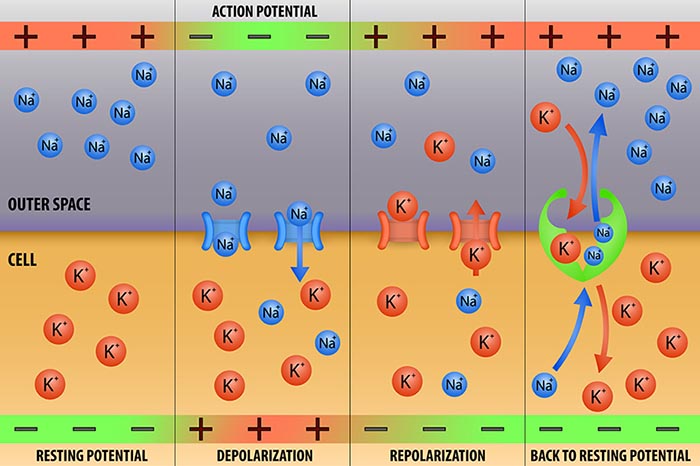
We can compare action potential conduction to water movement through a leaky garden hose.
Garden hose: water can take two paths, inside the hose or through holes in its wall, and most of the water will flow where movement is easiest. Most of the water will travel through the leaks for a small-diameter hose with many large holes. Conversely, the bulk of the water will remain inside a large-diameter hose with only a few small holes.
Axon: positive charge can take two paths, inside the axon or through pores in its membrane. Like water, a positive charge will take the path of least resistance. For a small-diameter axon with many open sodium ion channels, the majority of the current will exit the axonal membrane to the extracellular fluid. Small diameter, unmyelinated axons transmit action potentials without weakening since sodium ion channels constantly regenerate this signal. This method is slow because the signal travels step-by-step, small segment by small segment, and waits for sodium channels to admit enough positive ions to reach the excitation threshold. Graphic © 2003 Josephine Wilson.
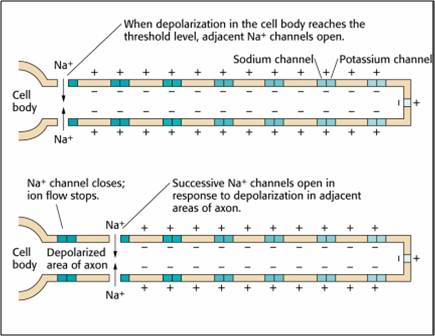
This method also consumes considerable energy since sodium-potassium transporters, powered by ATP, are located across the axon membrane to exchange three sodium for two potassium ions.
Conversely, the bulk of the current will remain inside the axon's interior for a large-diameter axon with few open ion channels. Wider spacing between adjacent ion channels means that the action potential can depolarize a longer axon segment, which increases conduction velocity (Bear, Connors, & Paradiso, 2016).
Medium-to-large diameter myelinated axons transmit action potentials using a method called saltatory conduction. Each segment of insulating myelin is almost 1 mm long. The gaps between segments, called nodes of Ranvier, are 1 to 2 thousandths of a millimeter. An action potential weakens under each myelinated segment (cable properties) and is then regenerated at each Ranvier node. The destruction of this insulation by demyelinating diseases like multiple sclerosis (MS) can be devastating because it disrupts neuron-to-neuron communication.
Saltatory conduction can be 200 times faster because the action potential jumps from node to node in 1-mm steps,” instead of steps that are a thousand times smaller. This method is also more energy-efficient because sodium-potassium transporters are only needed at the nodes of Ranvier, where ion exchange is possible. These transporters account for about 40% of a neuron’s energy expenditure (Breedlove & Watson, 2023; Garrett, 2003). Graphic © 2003 Josephine Wilson.
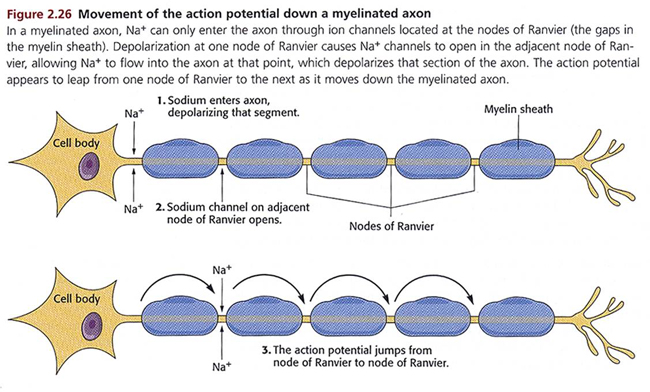
Synaptic Transmission
Neurons communicate through the release of over 200 neurochemicals and ions. Axon terminal buttons release neurochemicals across a 20-50-nm fluid-filled gap between presynaptic and postsynaptic structures called a synaptic cleft and into the extracellular fluid surrounding the neuron (Bear et al., 2020). Chemical synapses produce short-duration (millisecond) and long-duration (seconds to days) changes in the nervous system. Synapse animation without sound © 3Dme Creative Studio/Shutterstock.com.
They are functionally asymmetrical because the presynaptic neuron sends a chemical message, and the postsynaptic neuron receives it. They are structurally asymmetrical because the presynaptic element (axon) contains vesicles containing NTs, and the postsynaptic element (dendrite) doesn’t. NT release from a terminal button is called exocytosis (Breedlove & Watson, 2023). Synapse graphic © SciePro/Shutterstock.com.
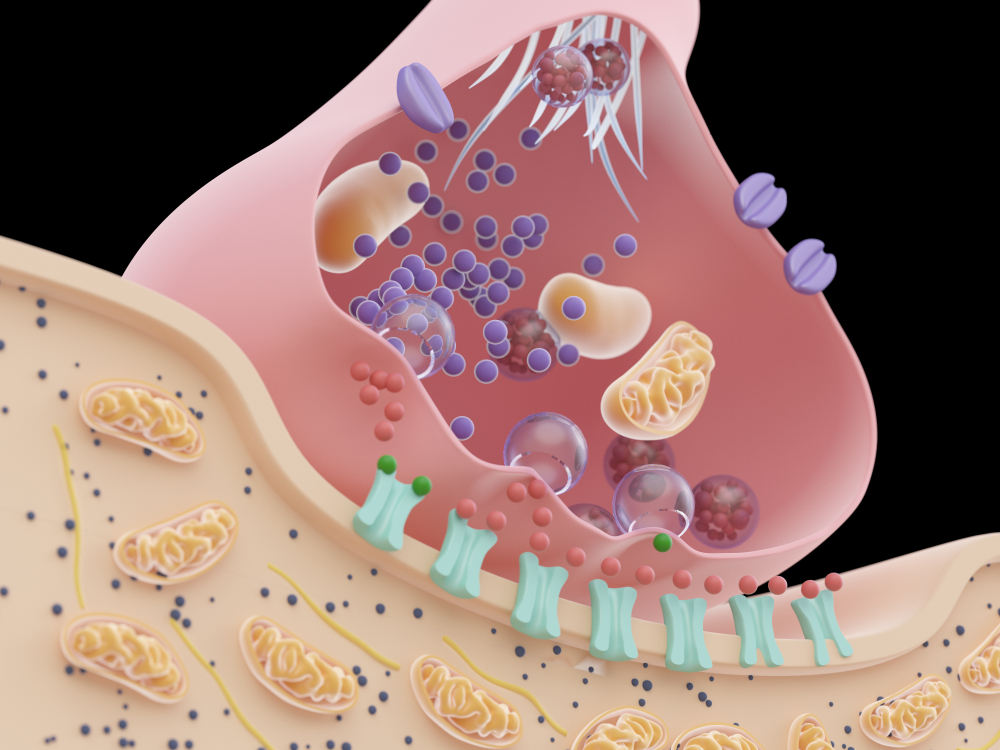
In the graphic below, an axon terminal button releases NTs into the synaptic cleft. NTs briefly form covalent bonds with receptors on a dendritic spine and then disengage after they initiate small graded potential changes (e.g., EPSPs or IPSPs) or more diverse, gradual, and long-lived actions (e.g., creating second messengers inside the target neuron). Chemical synapse graphic © nobeastsofierce/Shutterstock.com.
.jpg)
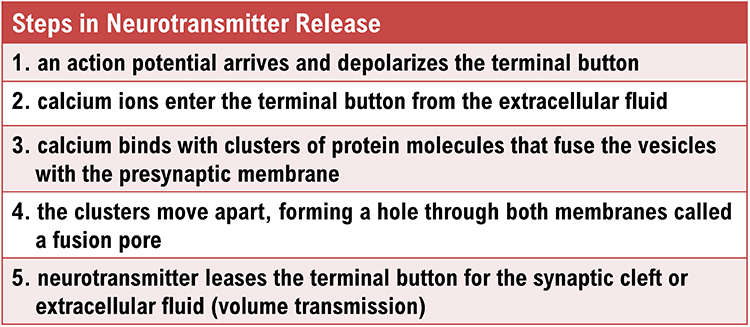
Neurotransmitter Co-Release
Old-school view: according to Dale’s law, a neuron can only release one NT at a synapse.New-school view: neurons can release a classical NT and a peptide.
Dale's law proposed that a neuron releases only one NT. However, researchers have found increasing evidence of NT co-release (Svensson et al., 2019). A neuron can store different NTs in separate types of vesicles (Hökfelt et al., 2003). Neurons can also store multiple NTs in the same vesicles (e.g., ATP and glutamate), although they may not release them simultaneously (Merighi et al., 2011; Xia et al., 2009).
Extra-Synaptic Transmission: Think Outside the Cleft
Neurons release NTs outside of classical synapses. These mechanisms include release from terminal buttons into the extracellular space, axonal varicosities, and retrograde transmission.
Old-school view: axon terminals only release NTs into the synaptic cleft.
New-school view: NT release also occurs outside of the synaptic cleft. Axonal varicosities (swellings in axon walls), dendrites, and the terminal button can release NTs into the extracellular space. Graphic © 3Dme Creative Studio/Shutterstock.com.
Volume Transmission
Volume transmission involves NT release and eventual binding to a receptor outside the synaptic cleft (Coggan et al., 2005). Graphic adapted from the American Scientist..jpg)
Axonal Varicosities
Axons can release NTs into the extracellular space through varicosities (swellings) along their length, analogous to drip irrigation (Breedlove & Watson, 2023).
Most neurons that release norepinephrine do not do so through terminal buttons on the ends of axonal branches. Instead, they usually release them through axonal varicosities, beadlike swellings of the axonal branches (Carlson & Birkett, 2019, pp. 82-83).
Retrograde Transmission
In retrograde transmission, a presynaptic neuron sends a chemical message to the postsynaptic neuron. In response, the postsynaptic neuron synthesizes and distributes an endocannabinoid (e.g., anandamide) or gas (e.g., nitrous oxide) to the presynaptic neuron and its immediate active neighbors. Neurons synthesize these NTs on demand since vesicles cannot contain them.. . . this gaseous signal has a range of influence that extends well beyond the cell of origin, diffusing a few tens of micrometers from its site of production before it is degraded. This property makes NO a potentially useful agent for coordinating the activities of multiple cells in a localized region and may mediate certain forms of synaptic plasticity that spread within small networks of neurons (Purves, 2017, pp. 142-143).
Retrograde NTs can bind to membrane-bound receptors or diffuse into the target cell, initiating second messenger production to adjust synaptic efficiency in learning and memory (Breedlove & Watson, 2023).
Modulation
Modulation is analogous to a volume control knob instead of an on/off switch on a stereo preamplifier, analog instead of digital.
.jpg)
We will consider two of countless modulation mechanisms: modulation of NT release and modulation of NT action at its receptor.
Neurotransmitter Release Modulation
Axons can influence the amount of NT released when an action potential arrives at an axon terminal through axoaxonic synapses (junctions between two axons). Axoaxonic synapse graphic (see examples 2 and 6) by Blausen.com staff (2014) under Creative Commons Attribution 3.0.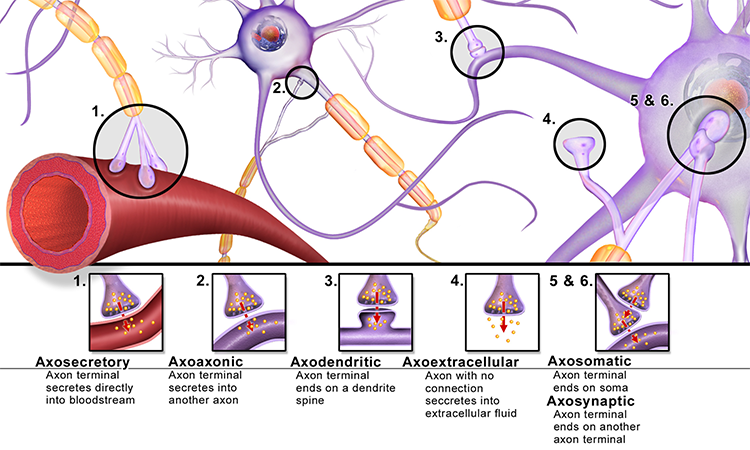 Axoaxonic synapses do not affect the generation of an action potential, only the amount of NT distributed. In presynaptic facilitation, a neuron increases the
presynaptic neuron's NT release by delivering a NT that increases calcium ion
entry into its terminal button. In presynaptic inhibition, a neuron decreases
NT release by reducing calcium ion entry. These modulatory effects are confined to a single
synapse (Breedlove & Watson, 2023).
Axoaxonic synapses do not affect the generation of an action potential, only the amount of NT distributed. In presynaptic facilitation, a neuron increases the
presynaptic neuron's NT release by delivering a NT that increases calcium ion
entry into its terminal button. In presynaptic inhibition, a neuron decreases
NT release by reducing calcium ion entry. These modulatory effects are confined to a single
synapse (Breedlove & Watson, 2023).
Autoreceptors Modulate Neurotransmitter Release
Autoreceptors are metabotropic receptors on the presynaptic membrane. When NTs released into the synaptic cleft bind to autoreceptors, this hyperpolarizes the axon terminal button so it will release less NT when the next action potential arrives. Autoreceptor graphic © gritsalak karalak/Shutterstock.com.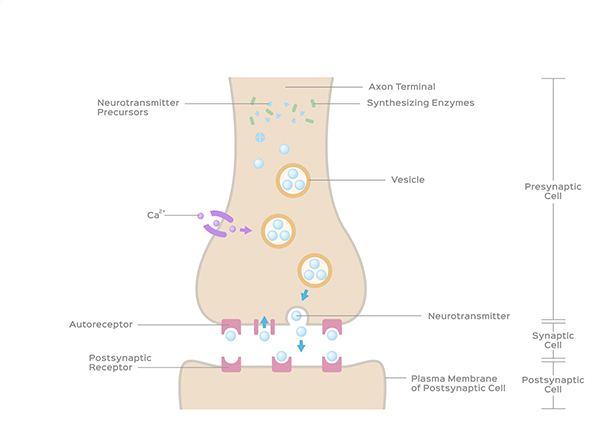
Neuromodulators Adjust Neurotransmitter Action
Receptors contain binding sites for a NT like GABA and drugs like alcohol. When alcohol binds to its allosteric site, it strengthens GABA's covalent bond with its orthosteric site, causing greater chloride entry into the neuron and increasing its hyperpolarization. Ingesting multiple CNS depressants (e.g., alcohol and barbiturates) can yield dangerous additive effects, amplifying GABA's action to a level that can depress or stop breathing.Types of Neurotransmitters
Please click on the podcast icon below to hear a full-length lecture for Section A Part 4.

While the actual number of NTs is unknown, more than 200 molecules have been identified. Each neurotransmitter may have multiple receptors. A NT's effect, excitatory or inhibitory, depends on its interaction with specific receptors. The same NT can produce opposite results at different receptor subtypes (Breedlove & Watson, 2023).
The principal NT families include amino acid neurotransmitters (GABA, glutamate), amine neurotransmitters (acetylcholine, dopamine serotonin), peptide neurotransmitters, also called neuropeptides (oxytocin, vasopressin), gas neurotransmitters (nitric oxide, carbon dioxide), and lipid neurotransmitters (anandamide and AG-2). The table below is adapted from Breedlove and Watson (2023).
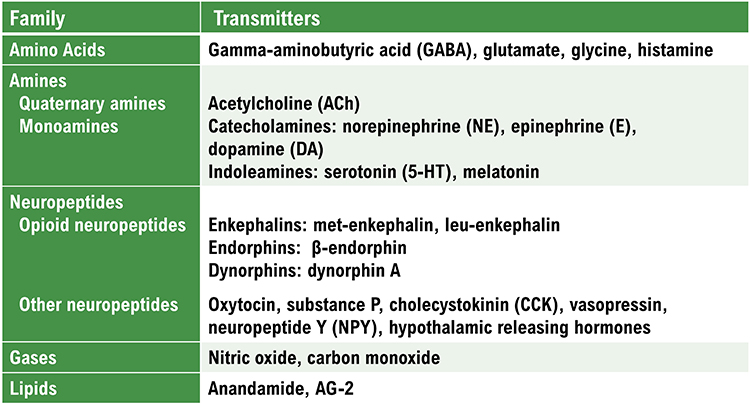
Neurotransmitter Pathways
Researchers have identified pathways for acetylcholine, dopamine, norepinephrine, and serotonin. The reproduced diagrams are © Vasilisa Tsoy/Shutterstock.com.Cholinergic pathways
.jpg)
Cholinergic cell bodies and their projections originate in the basal forebrain and brainstem. Cholinergic pathways are involved in arousal, attention, memory, motivation, muscle contraction, and sleep.
Dopaminergic pathways
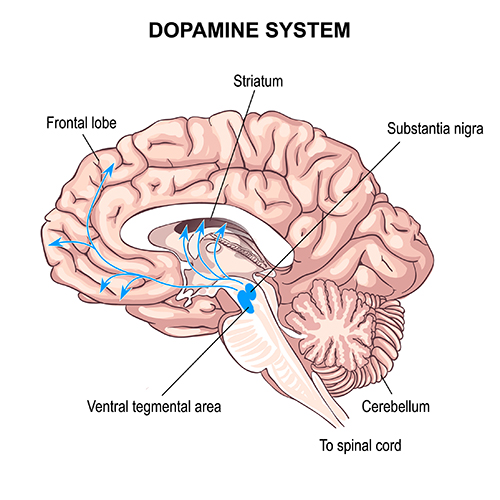
Two major dopaminergic pathways originate in the midbrain: the mesostriatal and mesolimbocortical pathways. Dopaminergic pathways are involved in addiction, motor control, and salience (reward- and threat-based motivation).
Noradrenergic pathways

The noradrenergic pathways originate in the midbrain locus coeruleus and lateral tegmental area. Noradrenergic pathways are involved in arousal, attention, memory, vigilance, sleep, and the fight-or-flight response and mobilizing the brain and body for action.
Serotonergic pathways

The serotonergic pathways originate in the brainstem and midbrain raphe nuclei. Serotonergic pathways are involved in appetite, mood, and sleep.
Termination of Neurotransmitter Action
Following exocytosis, NT action is terminated by reuptake and enzymatic degradation. In reuptake, reuptake transporters located in the presynaptic terminal and astrocytes that enclose the synapse return NT molecules to the presynaptic neuron. Astrocytes remove glutamate from the synapse. Graphic © Blamb/Shutterstock.com.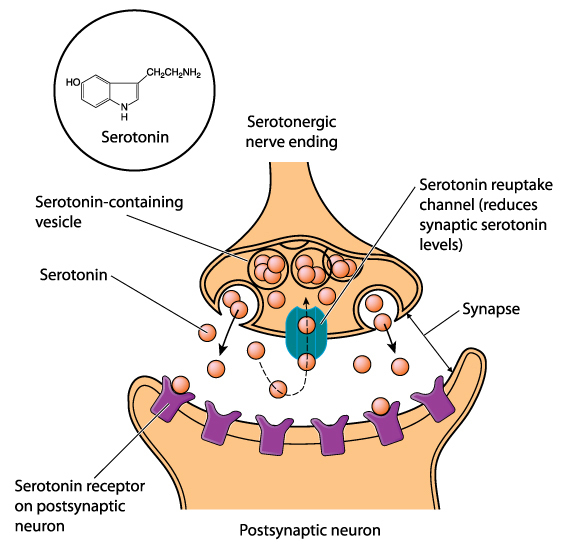
In enzymatic degradation, enzymes located in the synaptic cleft and the cytoplasm of the presynaptic neuron's terminal button split neurotransmitter molecules apart (e.g., acetylcholine). Graphic © Designua/Shutterstock.com.
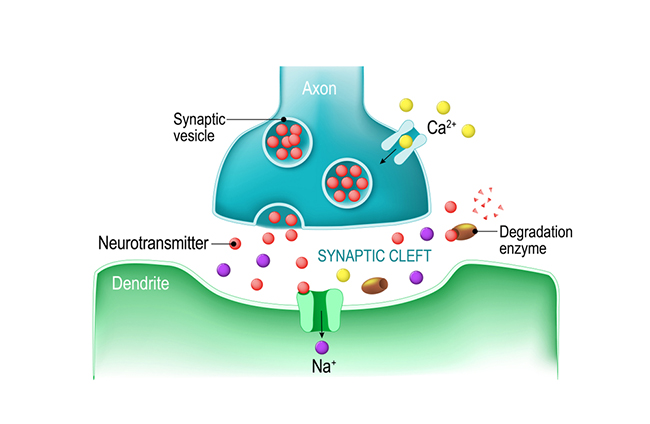
Electrical Synapses
Electrical synapses communicate information across gap junctions between adjacent membranes using ions. Gap junctions are narrow spaces between two cells bridged by connexons (protein channels) that allow ions near-instantaneous travel. Gap junction illustration © VectorMine/Shutterstock.com..jpg)
Electrical synapses are generally symmetrical. As long as the gap junction remains open, ions flow across a 3-nm gap junction into the more negatively charged neuron. Whether neurons are presynaptic or postsynaptic depends on their respective charges. When two neurons are electrically coupled, an action potential in one induces a postsynaptic potential (PSP) in the paired neuron.
Transmission across electrical synapses is nearly instantaneous, compared with the 10-ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of neurons to synchronize their activity and simultaneously fire.
Studies in recent years have revealed that electrical synapses are common in every part of the mammalian CNS. When two neurons are electrically coupled, an action potential in the presynaptic neuron causes a small amount of ionic current to flow across the gap junction channels into the other neuron. This current causes an electrically mediated postsynaptic potential (PSP) in the second neuron. Note that, because most electrical synapses are bidirectional, when that second neuron generates an action potential, it will in turn induce a PSP in the first neuron (Bear et al., 2020, p. 113).
Neurons that secrete hormones use electrical synapses to release their chemical messengers simultaneously. Neonatal brains may use gap junctions to activate many neurons at once. Image of long, fibrous astrocyte processes using Golgi's silver chromate technique © Jose Luis Calvo/Shutterstock.com.
Gap junctions may be a preliminary step toward developing chemical synapses between these neurons, eventually replacing their electrical synapses. Prenatally and postnatally, gap junctions enable nearby neurons to coordinate their development by sharing electrical and chemical communications (Bear, Connors, & Paradiso, 2016; Breedlove & Watson, 2023).
Old school view: synapses are either electrical or chemical.
New school view: synapses can be both electrical and chemical
Discoveries Since Graduate School
Neuroscientists have learned a great deal more about neuron-to-neuron communication since graduate school. The most important findings are that the adult brain creates new neurons, silent synapses may mediate neuroplasticity in adulthood, the lymphatic system extends to the brain, neuronal networks exhibit mirroring properties, and neurons can release more than one NT, release NTs outside of a synapse, conduct two-way conversations, modulate NT release and action, talk to astrocytes that enclose synapses, and electrically communicate almost instantaneously.
Neurogenesis
Neuroscience has challenged the doctrine that the adult human brain does not create new neurons. There is a consensus that neurogenesis, creating new neurons in adults, occurs in the hippocampus (Eriksson et al., 1998) and olfactory bulb (Lim & Alvarez-Buylla, 2016). However, neurogenesis outside the hippocampus remains controversial. Animal research has yielded evidence of functionally significant neurogenesis in the amygdala, caudate nucleus and putamen (striatum), cortex, hypothalamus, and substantial nigra (Jurkowski et al., 2020). The neurogenesis graphic by Rebeca Cuesta is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.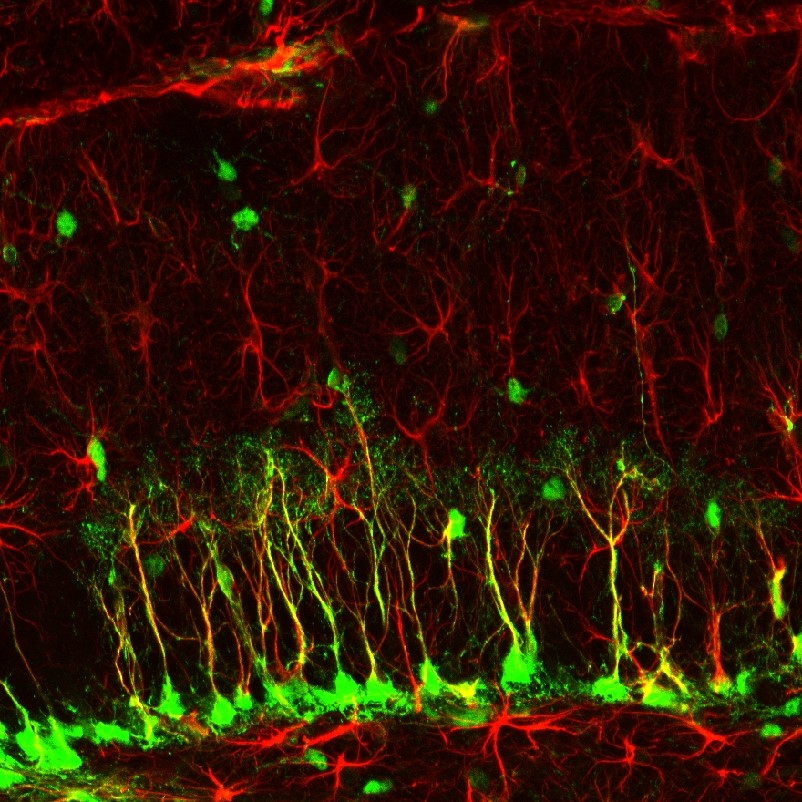
Silent Synapses
Silent synapses are inactive due to the absence of glutamate AMPA receptors. Researchers studying adult mice discovered these synapses on the ends of threadlike filopodia. The simultaneous firing of two neurons connected by a silent synapse causes missing AMPA receptors to appear on the filopodia cell membrane and remodel it to resemble a dendritic spine (Vardalaki et al., 2022). The next step is determining whether the adult human brain also contains silent synapses. If it does, they are a potential target for increasing cognitive flexibility in the elderly. Filopodia photomicrograph by Aurea D. Sousa and Richard E. Cheney under the Creative Commons Attribution-Share Alike 4.0 International license.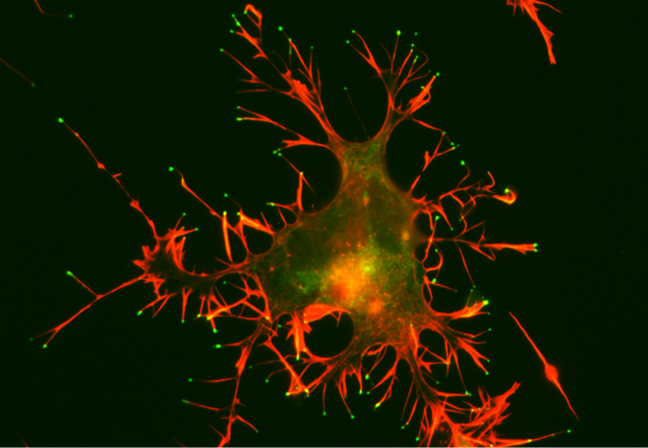
Caption: A CAD cell (a neuronal cell line) expressing GFP-Myo10 (green) was stained for actin filaments (red) to visualize the slender cellular protrusions known as filopodia. Overexpressing Myo10 induces large numbers of filopodia and is responsible for this cell's unusually large number of filopodia.
Holly Barker (2022) writing for The Scientist, explained:
The study may explain how the brain is able to learn new things without having to sacrifice existing connections, the researchers say. The ability of the brain to use different synapses 'solves the plasticity versus flexibility dilemma,' says Harnett. If all the brain’s synapses are flexible, then you can’t preserve old information. But if they’re all stable, then it is difficult to learn new things, he says. Instead, the brain employs both: spiny synapses for stability and filopodia for flexibility.
But instead of distinct categories, Harnett’s group are beginning to think about dendritic projections as existing on a continuum, from filopodia on one end to mature spines at the other. 'It is a spectrum of maturity, strength, and plasticity,' says study author Dimitra Vardalaki, a PhD candidate in Harnett’s lab.
Mirror Neuron System
Researchers discovered primate neurons with motor and visual properties in the premotor cortex. The mirror properties are due to a neuron's connections, not its construction. The cortex graphic © Vasilisa Tsoy/Shutterstock.com..jpg)
These mirror neurons fired when primates grasped and manipulated objects and another primate or human performed the same action (Di Pelligrino et al., 1992; Rizzolatti & Craighero, 2004). Mirroring extends across species, including facial expressions.
Molenberghs et al. (2011) unexpectedly found neurons with mirroring properties in the cerebellum, limbic system, and primary visual cortex. Graphic courtesy of Wikimedia Commons.
The authors proposed that a core network is responsible for observing and executing movements. The nervous system recruits additional areas for non-motor affective, auditory, and somatosensory functions.

Mirror neurons look like other neurons when examined using a microscope. Their mirror properties emerge from their connections between sensory, motor, and emotional systems. Perhaps most mirror neurons may be tuned by experience (Catmur, Walsh, & Heyes, 2007).
The mirror neuron system (MNS) appears to encode the goal of a motor act and its component movements, whether a model manipulates an object or mimes the action. The MNS encodes the actions of others and stores them to predict their future actions (Rajmohan & Mohandas, 2007).
Soon after birth, an immature mirror neuron system may allow babies to imitate their parents' mouth movements, like thrusting out the tongue. Graphic courtesy of Wikimedia Commons.

Note. Image from Gross L. Evolution of neonatal imitation. PLoS Biol.
Ramachandran (2011) has called the mirror neurons activated when they observe others' movements "monkey see—monkey do neurons." He calls mirror neurons activated by others' emotional displays "Gandhi neurons." Check out Ramachandran's TED Talk, The Neurons that Shaped Civilization.

Investigators have speculated that the human MNS may mediate empathy, imitation learning, language, social cognition, and theory of mind (Buccino et al., 2006; Rajmohan & Mohandas, 2007; Schmidt et al., 2021).
Rizzolatti and Sinigaglia (2008) hypothesized that the primary role of the MNS is to help us understand others’ intentions, which allows us to achieve empathy. When we observe others’ facial expressions of emotion, visual information may be directly transmitted to mirror neurons in the insula, producing the visceral changes that color our emotions.
In autism, mirror neurons may not fire when observing other individuals performing actions. This may help explain deficits in empathy, social skills, language, and the development of a theory of mind (Enticott et al., 2011).
Heyes et al. (2021) summarized the state of our knowledge about the MNS.
For action understanding, multivoxel pattern analysis, patient studies, and brain stimulation suggest that mirror-neuron brain areas contribute to low-level processing of observed actions (e.g., distinguishing types of grip) but not to high-level action interpretation (e.g., inferring actors’ intentions). In the area of speech perception, although it remains unclear whether mirror neurons play a specific, causal role in speech perception, there is compelling evidence for the involvement of the motor system in the discrimination of speech in perceptually noisy conditions. For imitation, there is strong evidence from patient, brain-stimulation, and brain-imaging studies that mirror-neuron brain areas play a causal role in copying of body movement topography. In the area of autism, studies using behavioral and neurological measures have tried and failed to find evidence supporting the 'broken-mirror theory' of autism.
Cortical Architecture
While no one has counted the neurons in the human nervous system, a recent estimate is that an adult brain contains about 86 billion neurons (Voytek, 2013). Each neuron connects with an average of 40,000 synapses. There are 10 times more glial cells than neurons, comprising 50% of the brain’s volume (Breedlove & Watson, 2023). The 2 trillion glial cells are considerably smaller than neurons, with somas between 6 to 10 μm in diameter (Hammond, 1996). Animation © nmlfd/iStockphoto.com.The cerebral cortex comprises neuronal cell bodies, glial cells, and blood vessels. Beneath the neocortex lies myelinated nerves (white matter), unmyelinated fibers, and glial cells.
The cerebral cortex covers the cerebral hemispheres and consists of gray and white matter. Gray (or grey) matter, which looks grayish brown, comprises cell bodies. White matter gains its opaque white color from myelinated axons. The cerebral cortex of a macaque brain is shown below, courtesy of Wikipedia. Note that staining imparts a darker shade to gray matter.
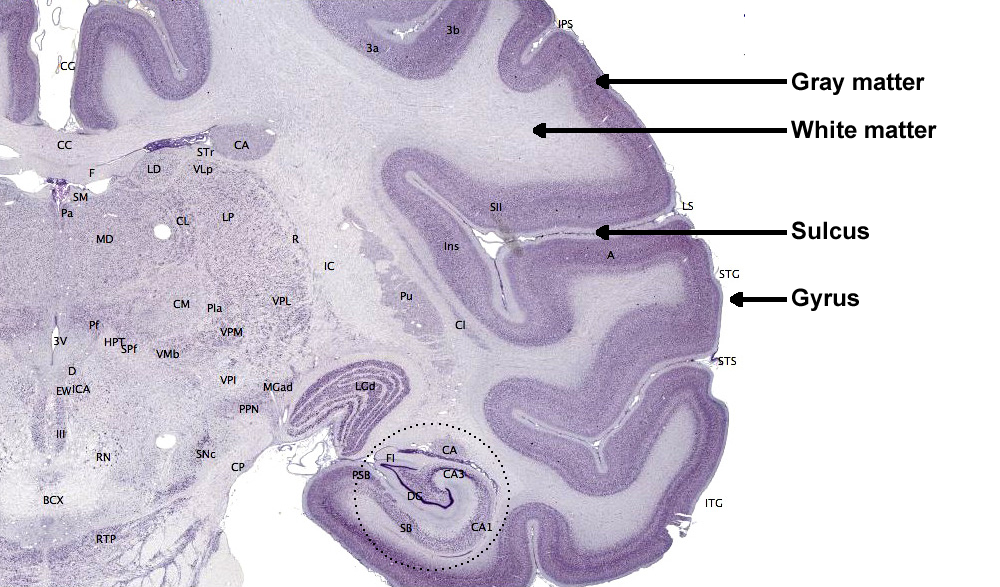
The convolutions of the cerebral cortex contain two-thirds of its surface area and maximize the volume of cortical tissue housed within the skull. Cerebral cortical convolutions include sulci, which are shallow grooves in the surface of the cerebral hemisphere (central sulcus); fissures, which are deep grooves (lateral fissure); and gyri, which are ridges of cortex demarcated by sulci or fissures (precentral gyrus) (Carlson & Birkett, 2021).
There are two main types of cortex: neocortex and allocortex.
The neocortex or isocortex consists of six layers that are 3 mm thick with a surface area of about 2360 cm2 and white matter underneath. Layers I-III receive corticocortical afferent fibers that connect the left and right hemispheres. Layer III is the main source of corticocortical efferent fibers. Layer IV is the primary destination of thalamocortical afferents and intra-hemispheric corticocortical afferents. Layer V is the primary origin of efferent fibers that target subcortical structures with motor functions. Layer VI projects corticothalamic efferent fibers to the thalamus, which, together with the thalamocortical afferents, creates a dynamic and reciprocal relationship between these two structures (Creutzfeldt, 1995). Diagram courtesy of Wikimedia Commons.
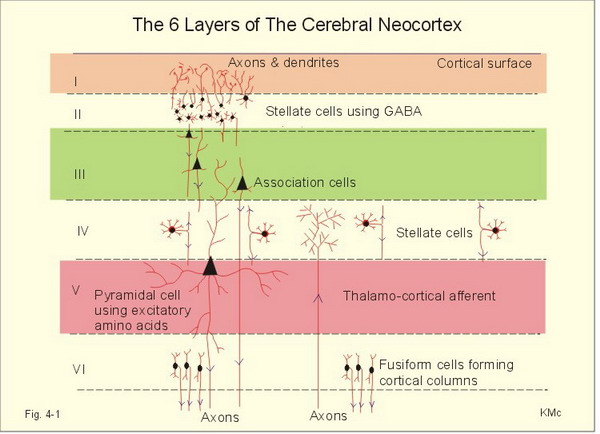
Allocortex, which means other cortex, usually has three or four layers, compared with the neocortex's six layers. The allocortex has less volume than the neocortex and comprises the olfactory system and hippocampus.
A transitional region between the neocortex and allocortex is called the paralimbic cortex.
For a basic overview of the cortex, watch the Khan Academy video Cerebral Cortex.
Neurons in the Cortex
We can classify cerebral cortical neurons as whether their dendrites display spines. Spiny neurons, which have either pyramidal or stellate (star-like)-shaped cell bodies, are usually excitatory. While all pyramidal cells are spiny neurons, stellate cells can be spiny or aspinous (Bear, Connors, & Paradiso, 2016).The graphic below depicts dendritic spines.neurons, which have either pyramidal or stellate (star-like)-shaped cell bodies, are usually excitatory. While all pyramidal cells are spiny neurons, stellate cells can be spiny or aspinous (Bear, Connors, & Paradiso, 2020). Spiny and aspinous dendrite graphic redrawn by minaanandag on Fiverr.com.
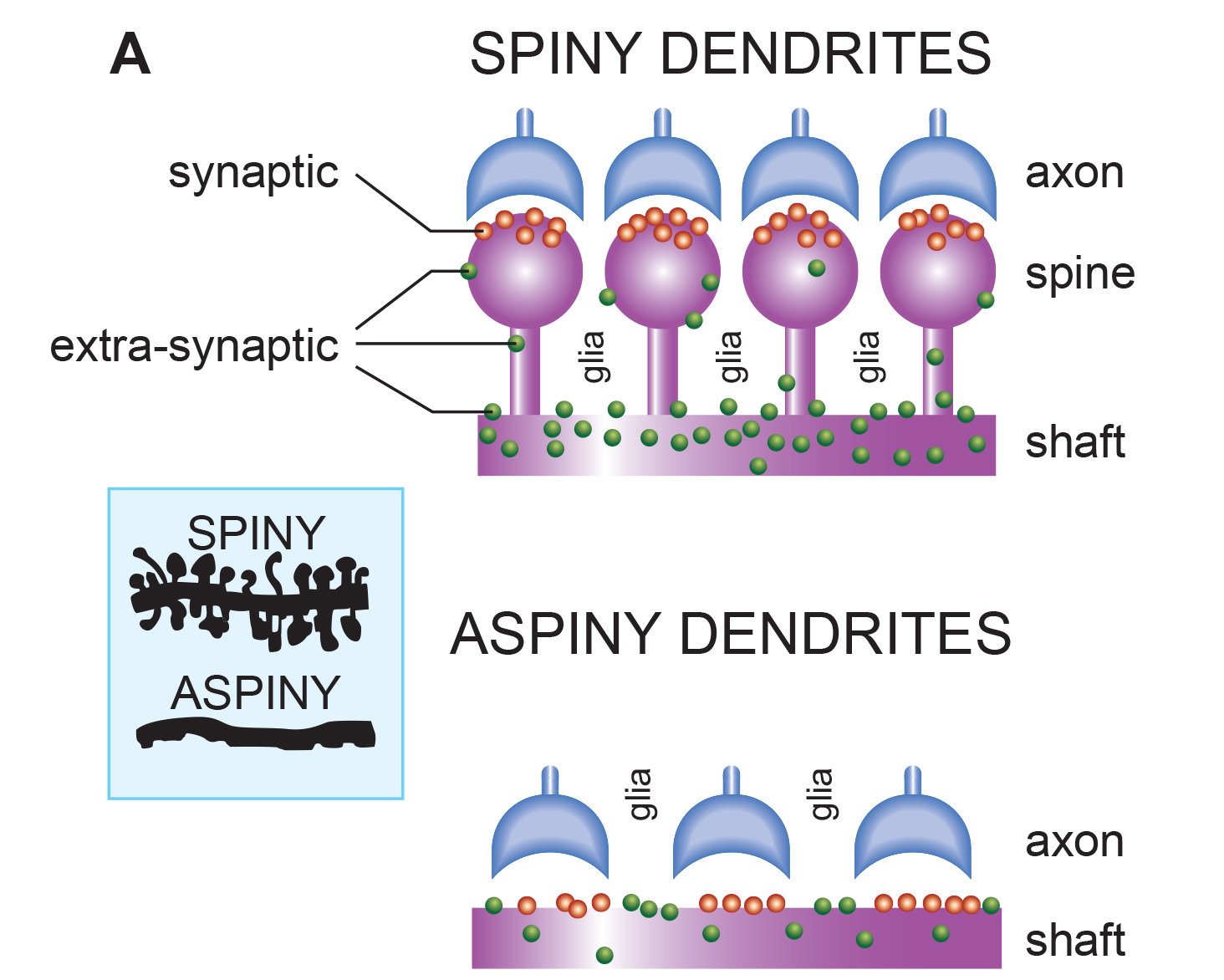
Dr. John C. Fiala, and Dr. Kristen M. Harris created this reconstruction of a dendritic spine. Creative Commons Attribution-Share Alike 3.0.
_and_massive_aggregation_of_polyribosomes_(black)..jpg)
There are many types of aspinous (smooth) neurons which are believed to be inhibitory.
Glossary
40-Hz rhythm: gamma rhythm hypothesized to be associated with feature binding (linking an apple's color to its shape) and attributed to the neocortex and thalamocortical neurons.
absolute refractory period: the time when a neuron cannot initiate a second action potential regardless of stimulus intensity.
acetylcholine: an amine NT that binds to nicotinic and muscarinic ACh receptors.
acetylcholine esterase (AChE): the enzyme that deactivates ACh.
AChE-R: an abnormal form of acetylcholine esterase (AChE) may render dendrites with acetylcholine receptors more excitable when stressed.
action potential: a propagated electrical signal that usually starts at a neuron’s axon hillock and travels to presynaptic axon terminals.
active zone: presynaptic terminal button region, including the presynaptic membrane, specialized for neurotransmitter release.
adenylate cyclase: at a metabotropic receptor, an enzyme that transforms ATP into the second messenger cyclic AMP.
afferent: a neuron that transmits sensory information towards the central nervous system or from one region to another.
all-or-none law: once an action potential is triggered in an axon, it is propagated, without decrement, to the end of the axon. The amplitude of the action potential is unrelated to the intensity of the stimulus that triggers it.
allocortex: cortex that contains three or four layers and is comprised of the olfactory system and hippocampus.
allosteric site: modulatory binding site on a NT's receptor complex.
alpha blocking: arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it entirely while increasing EEG power in the beta range.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons that are linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: regular bursts of alpha activity.
alpha-subunit: a subunit of a G protein associated with the neuron membrane that breaks away to activate enzymes within the neuron when a ligand binds to a metabotropic receptor.
amino acid: protein building block containing both a carboxyl (—COOH) and an amino (—NH2) group.
AMPA (glutamate) receptors: ionotropic receptors which open sodium channels, depolarize the neuron's membrane (producing an EPSP), and dislodge a Mg+ ion that blocks an adjacent NMDA (glutamate) receptor's calcium channel. AMPA receptors are responsible for most activity at glutamatergic synapses.
amplitude: the energy or power contained within the EEG signal measured in microvolts or picowatts.
amygdala: the limbic system structure that participates in evaluating whether stimuli are salient (rewarding or threatening), establishing unconscious emotional memories, learning conditioned emotional responses, and producing anxiety and fear responses.
anandamide: a fatty acid NT derived from arachidonic acid.
anion: a negative ion, for example, chloride (Cl-).
anterior: near or toward the front of the head, for example, the anterior cingulate.
anterior cingulate: a division of the prefrontal cortex that plays a vital role in attention and is activated during working memory. It mediates emotional and physical pain, and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components.
anterior commissure: a bundle of nerve fibers that crosses the midline and connects the left and right temporal lobes and the hippocampus and amygdala.
apical dendrite: a dendrite that arises from the top of the pyramid and extends vertically to layer 1 of the neocortex.
arousal: a process that combines alertness and wakefulness, produced by at least five NTs, including acetylcholine, histamine, hypocretin, norepinephrine, and serotonin.
aspinous (smooth) neurons: neurons without dendritic spines that are believed to be inhibitory.
astrocyte endfeet: specialized astrocyte processes that comprise part of the blood-brain barrier.
astrocytes: star-shaped glial cells that communicate with and support neurons and help determine whether synapses will form.
asynchronous waves: neurons depolarize and hyperpolarize independently.
ATP: energy source for a neuron’s sodium-potassium transporters.
autoreceptors: metabotropic receptors that can be located on the membrane of any part of a neuron. They detect neurotransmitters the neuron releases, generate IPSPs that inhibit the neuron from reaching the excitation threshold, and regulate internal processes like NT synthesis and release through the second messenger system.
axoaxonic synapses: junctions between two axons that do not affect the generation of an action potential, only the amount of nNT distributed.
axodendritic synapses: junctions between axons and dendrites that determine whether the axon hillock will initiate an action potential.
axon: long, cylindrical structures that convey information from the soma to the terminal buttons. An axon also transports molecules in both directions along the outer surface of protein bundles called microtubules.
axon hillock: a swelling in the cell body where a neuron integrates the messages it has received from other neurons and decides whether to fire an action potential.
axon terminal button: an axon's bulblike termination specialized for NT release.
axonal varicosity: swellings along an axon's length through which neurotransmitters are released.
axoplasmic transport: the movement of molecules in both directions along the outer surface of protein bundles called microtubules.
basal dendrite: a dendrite that horizontally branches out from the 30 μm base of the pyramid through the layer where the neuron resides.
basal ganglia: forebrain structures consisting of an egg-shaped nucleus that contains the putamen and globus pallidus and a tail-shaped structure called the caudate, which together are responsible for the production of movement. The basal ganglia have also been implicated in obsessive-compulsive disorder, Parkinson’s disease, and Huntington’s chorea.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
bilateral synchronous slow waves: a pathological sign observed in drowsy children. When detected in alert adults, intermittent bursts of high-amplitude slow waves may signify gray matter lesions in deep midline structures.
blood-brain barrier: a semipermeable barrier of capillary endothelial cells and astrocyte endfeet that regulates large and small molecule entry into the brain, and protects against circulating pathogens and toxins.
brain-derived neurotrophic factor (BDNF): a protein belonging to the neurotrophin growth factor family that is involved in neuron growth and repair. BDNF is a neuromodulator that mediates neuronal plasticity essential to learning and memory.
cation: positive ion, for example, sodium (Na+).
caudal: away from the front of the head.
cell body or soma: part of a neuron that contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
central nucleus of the amygdala: nucleus that orchestrates the nervous system's response to important stimuli by activating circuits in the brainstem (autonomic arousal) and the basal ganglia and periaqueductal gray (defensive behavior).
cerebral cortex: the layer of gray matter that covers the cerebral hemispheres. The cerebral cortex consists of gray matter and white matter.
chemical synapses: junctions between neurons that transmit molecules across gaps of less than 300 angstroms. Neurons use chemical synapses to produce short-duration (millisecond) and long-duration (seconds to hours) changes in the nervous system. Chemical synapses are capable of more extensive communication and initiating more diverse and long-lasting changes than electrical synapses.
classical routes for EEG activation: specific sensory pathways like the visual (retina to the visual cortex), auditory (cochlea to the auditory cortex), and somatosensory (chemoreceptors and mechanoreceptors to the somatosensory cortex) systems. Increased transmission of information through these pathways desynchronizes EEG activity in the cortical regions to which these afferent neurons project, as specialized circuits of neurons independently process this information.
co-release: the release of multiple neurotransmitters by the same neuron (e.g., GABA and glutamate).
co-storage: storing multiple neurotransmitters in the same vesicles (e.g., GABA and glutamate).
commissures: axon tracts. The left and right hemispheres communicate using the corpus callosum, anterior commissure, and posterior commissure.
complex: a sequence of waves.
COMT: a degrading enzyme that only targets the catecholamines dopamine and norepinephrine.
connexon: a protein comprised of six connexins that forms a gap junction between adjacent cell cytoplasm.
contingent negative variation (CNV): a steady, negative shift in potential (15 microvolts in young adults) detected at the vertex. This slow cortical potential may reflect expectancy, motivation, intention to act, or attention. The CNV appears 200-400 ms after a warning signal (S1), peaks within 400-900 ms, and sharply declines after a second stimulus that requires the performance of a response (S2).
continuous irregular delta: slow waves produced by white matter lesions seen in disorders like multiple sclerosis.
contralateral: structures that are located on opposite sides of the body. For example, neurons in the left primary motor cortex control muscles on the right side of the body.
corpus callosum: the largest commissure that connects the left and right frontal, parietal, and occipital lobes.
corticothalamic network: a unified network that generates diverse types of brain rhythms grouped by slow cortical oscillations.
cyclic AMP: a second messenger that moves about the neuron, activating other enzymes. Protein kinase A, which controls the excitability of ion channels, is a crucial enzyme target of cyclic AMP. Cyclic AMP also travels to the nucleus, where it can regulate gene expression.
Dale's principle: incorrect view that a neuron can only release one neurotransmitter. They often release two to four.
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
dendrite: a branched structure designed to receive messages from other neurons via axodendritic synapses (junctions between axons and dendrites), determining whether the axon hillock will initiate an action potential.
dendritic spine: a small protrusion from a dendritic shaft that increases the surface area for expressing receptors.
dendrodendritic synapses: junctions between dendrites that communicate chemically across synapses and electrically across gap junctions.
depolarization: a membrane potential shift to a less negative value.
diffusion: the distribution of molecules from areas of high concentration to low concentration.
diphasic wave: a wave that contains both a negative and positive deflection from the baseline.
dipole: the electrical field generated between the sink (where current enters the neuron ) and the source (place at the other end of the neuron where current leaves), which may be located anywhere along the dendrite.
dominant frequency: the EEG frequency with the most significant amplitude.
dopamine: a monoamine neurotransmitter exerts its postsynaptic effects on at least six receptors linked to G proteins. This means that dopamine functions as a neuromodulator. The two families include D1 (D1 and D5) and D2 (D2A, D2B, D3, and D4).
dorsal: toward the upper back or head.
dorsolateral prefrontal cortex: the left dorsolateral prefrontal cortex is concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals. The right dorsolateral prefrontal cortex organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
D-serine: a neurotransmitter that binds to the glycine site on the NMDA receptor to trigger calcium entry into a dendritic spine when glutamate binds to its site, resulting in a large, prolonged increase in intracellular calcium.
dual-action antidepressants: medications that activate 5-HT1 receptors to produce antidepressant and anxiolytic effects, while they blockade 5-HT2 (agitation, restlessness, and sexual dysfunction) and 5-HT3 (nausea, headache, and vomiting) receptors to minimize their side effects.
EEG activity: single wave or successive waves.
EEG power: the signal energy in the EEG spectrum. Most EEG power falls within the 0-20 Hz frequency range. EEG power is measured in microvolts or picowatts.
efferent: motoneuron that transmits information towards the periphery.
electrical synapse: a gap junction between neurons for the rapid and often bidirectional movement of ions and small molecules.
electroencephalogram (EEG): the voltage difference between at least two electrodes, where at least one electrode is located on the scalp or inside the brain. The EEG is a recording of EPSPs and IPSPs that occur primarily in dendrites in pyramidal cells located in macrocolumns, several millimeters in diameter, in the upper cortical layers.
electrostatic pressure: the attractive or repulsive force between ions that moves them from one region to another.
entorhinal cortex: a structure located in the caudal region of the temporal lobe and receives pre-processed sensory information from all modalities and reports on cognitive operations. The entorhinal cortex provides the main input to the hippocampus, and is involved in memory consolidation, spatial localization, and provides input into the septohippocampal system that may generate the 4-7 Hz theta rhythm.
enzymatic deactivation: the process in which an enzyme breaks a neurotransmitter apart into inactive fragments. For example, acetylcholine transmission is ended by the enzyme acetylcholine esterase (AChE). Deactivating enzymes located in the synaptic cleft degrade a neurotransmitter molecule when it detaches from its binding site.
evoked potential: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). An evoked potential has a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
excitatory amino acid transporter (EAAT): transporters in CNS glial cells and neurons that remove glutamate from the synaptic cleft.
excitatory postsynaptic potential (EPSP): a brief positive shift in a postsynaptic neuron's potential produced when neurotransmitters bind to receptors and cause positive sodium ions to enter the cell. An EPSP pushes the neuron towards the threshold of excitation when it can initiate an action potential.
exocytosis: the process of neurotransmitter release. When an action potential arrives and depolarizes the terminal button, calcium ions enter the terminal button from the extracellular fluid. Calcium binds with clusters of protein molecules that connect the vesicles to the presynaptic membrane. The clusters move apart, forming a hole through both membranes called a fusion pore, and the neurotransmitter leaves the terminal button for the synaptic cleft or extracellular fluid.
exogenous ERP: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual).
explicit learning: behavioral changes that occur with our conscious awareness that require processing by the hippocampus.
extracellular dipole layers: macrocolumns of pyramidal cells, which lie parallel to the surface of the cortex, send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons.
extracellular fluid: the fluid surrounding a neuron.
fast cortical potentials: EEG rhythms that range from 0.5 Hz-100 Hz. The main frequency ranges include delta, theta, alpha, sensorimotor rhythm, and beta.
feature binding: the process of linking information to perceptual objects (linking an apple's color to its shape) that may involve the 40-Hz rhythm.
fissures: deep grooves, for example, the lateral fissure.
focal waves: EEG waves that are detected within a limited area of the scalp, cerebral cortex, or brain.
frequency: the number of cycles completed each second expressed in hertz (Hz).
frequency synchrony: when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
frontal lobes: the most anterior cortical lobes of the brain that are divided into the motor cortex, premotor cortex, and prefrontal cortex.
fusion pore: a hole through a vesicle and presynaptic membrane that allows neurotransmitter to leave the terminal button for the synaptic cleft or extracellular fluid.
G protein: a protein located inside a neuron’s membrane next to a metabotropic receptor that is activated when the receptor binds a ligand. An alpha-subunit of the G protein then breaks away to perform actions within the cell.
GABA: an amino acid that is often inhibitory and that may be the most important inhibitory neurotransmitter in the brain. There are several types of GABA receptors, each of which produces inhibition differently.
gamma rhythm: EEG activity frequencies above 30 or 35 Hz. Frequencies from 25-70 Hz are called low gamma, while those above 70 Hz represent high gamma.
gap junction: narrow space between two cells bridged by connexons (protein channels) that allow ions to travel between them rapidly.
generalized asynchronous slow waves: waves that are seen in sleepy children and those with elevated temperatures. These waves may indicate degenerative disease, dementia, encephalopathy, head injury, high fever, migraine, and Parkinson's disease in adults.
glial cells: nonneural cells that guide, insulate, and repair neurons and provide structural, nutritional, and information-processing support. Glial cells generate slow cortical potentials (SCPs). Glial cells include astrocytes, microglia, oligodendrocytes, radial glial cells, and Schwann cells.
gliotransmission: calcium-dependent astrocyte neurotransmitter release.
glutamate: an amino acid that is often excitatory and that may be the primary excitatory neurotransmitter in the brain. Its receptors are found on the surface of almost all neurons. There are at least 13 different receptors for glutamate, 5 ionotropic and 8 metabotropic. Most presynaptic neurons in the brain excite postsynaptic neurons via ionotropic glutamate receptors in the postsynaptic membrane. Metabotropic glutamate receptors may play a regulatory function, either augmenting or suppressing the activation of ionotropic glutamate receptors.
glycine: an amino acid that is often inhibitory and has a binding site on the NMDA receptor.
glymphatic system: an astrocyte-controlled lymphatic system that removes cellular debris, proteins, and wastes from the brain.
gray matter: brain tissue that looks grayish brown and comprises cell bodies, dendrites, unmyelinated axons, glial cells, and capillaries.
gyrus: ridge of cortex demarcated by sulci or fissures, for example, the precentral gyrus.
hertz (Hz): the unit of frequency, an abbreviation for cycles per second.
hippocampus: a limbic structure located in the medial temporal lobe involved in 4-7 Hz theta activity, control of the endocrine system’s response to stressors, formation of explicit memories, and navigation. Cortisol binding to this structure disrupts these functions, interferes with creating new neurons, and harms and kills hippocampal neurons.
hubs: highly centralized nodes through which other node pairs communicate; hubs allow efficient communication.
hyperpolarization: a negative membrane potential shift.
inhibitory postsynaptic potential (IPSP): a brief negative shift in a postsynaptic neuron's potential produced when cations like potassium leave a neuron or anions (negative ions) like chloride enter a neuron, which hyperpolarize the cell. An IPSP pushes the neuron away from its excitation threshold.
integration: the addition of EPSPs and IPSPs at the axon hillock. Neurons sum EPSPs and IPSPs over their surface in spatial integration and over milliseconds in temporal integration to raise the membrane from its resting potential to the excitation threshold. EPSPs and IPSPs last from 15-200 ms, while action potentials occur in 1-2 ms.
interneurons: neurons that receive input from and distribute output to other neurons. They have short processes and are confined to the central nervous system. They provide the integration required for decisions, learning and memory, perception, planning, and movement.
intracellular fluid: the watery cytoplasm contained within a neuron.
ion: a charged atom or molecule with a positive or negative charge. Positive ions are called cations, and negative ions are called anions.
ionotropic receptor: receptor protein that contains a binding site for a ligand and an ion channel that opens when the neurotransmitter attaches to this site.
ipsilateral: structures that are located on the same side of the body. For example, the left olfactory bulb distributes axons to the left hemisphere.
irregular waves: successive waves that constantly alter their shape and duration.
kappa rhythm: bursts of alpha or theta and is detected over the temporal lobes of subjects during cognitive activity.
lambda waves: saw-toothed transient waves from 20-50 μV in amplitude and 100-250 ms in duration detected over the occipital cortex during wakefulness. These positive deflections are time-locked to saccadic movements and observed during visual scanning, as during reading.
lateral: to the side, away from the center, as in the lateral geniculate nucleus.
lateral nucleus of the amygdala: a nucleus that processes sensory information and distributes it throughout the amygdala.
lateralized waves: waves that are primarily detected on one side of the scalp and that may indicate pathology.
Layers I-III: cortical layers that receive corticocortical afferent fibers that connect the left and right hemispheres.
Layer III: the cortical layer that is the primary source of corticocortical efferent fibers.
Layer IV: the cortical layer that is the primary destination of thalamocortical afferents and intra-hemispheric corticocortical afferents.
Layer V: the cortical layer that is the primary origin of efferent fibers that target subcortical structures that have motor functions.
Layer VI: the cortical layer that projects corticothalamic efferent fibers to the thalamus, which, together with the thalamocortical afferents, creates a dynamic and reciprocal relationship between these two structures.
left dorsolateral prefrontal cortex: the division of the prefrontal cortex concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals.
local field potential: the aggregate effect of the firing of the interconnected pyramidal neurons within the cortical columns plus additional mechanisms like glial cell modulation of the cortical electrical gradient.
local synchrony: synchrony that occurs when high-amplitude EEG signals are produced by the coordinated firing of cortical neurons.
localized slow waves: waves that may indicate a transient ischemic attack (TIA) or stroke, migraine, mild head injury, or tumors above the tentorium. Deep lesions result in bilateral or unilateral delta.
locus coeruleus: the noradrenergic branch of the ascending reticular activating system, which is responsible for vigilance. Subnormal norepinephrine transmission may contribute to ADHD.
long-latency potentials: potentials that have extended latencies following stimulus onset, for example, P300 and N400 ERPs.
long-term depression (LTD): a weakening of synaptic connections for hours or longer caused by low-frequency stimulation.
long-term potentiation (LTP): a strengthening of synaptic connections for hours or longer caused by high-frequency stimulation.
macrocolumns: circuits of cortical pyramidal neurons several millimeters in diameter that create extracellular dipole layers parallel to the surface of the cortex that send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons. Since the pyramidal neurons are all aligned with the cortical surface, the postsynaptic potentials at cells within the same macrocolumn add together. This summation occurs because they share the same charge and the macrocolumns fire synchronously.
medial: toward the center of the body, away from the side. For example, the medial geniculate nucleus.
medial prefrontal cortex: the division of the prefrontal cortex that integrates cognitive-affective information and helps control the hypothalamic–pituitary–adrenal (HPA) axis during emotional stress.
membrane potential: a neuron’s electrical charge created by a difference in ion distribution within and outside the neuron. A typical resting potential is about -70 mV (thousandths of a volt), since the inside of a resting axon is more negatively charged than the outside.
mesocortical neurons: dopaminergic neurons that project from the ventral tegmental area of the midbrain to the prefrontal cortex and excite prefrontal cortical neurons that control working memory, planning, and strategy preparation for problem-solving. Underactivity in this pathway is associated with the negative symptoms of schizophrenia-like attentional deficits.
metabotropic receptors: include all G protein-linked receptors located on neurons, including autoreceptors. Neurotransmitters that bind to G protein-linked receptors are often called neuromodulators. Metabotropic receptors, which indirectly control the cell's operations, expend energy, and produce slower, longer lasting, and more diverse changes than ionotropic receptors. Their effects can last several seconds, instead of milliseconds, because of the long-lived activity of G proteins and cyclic AMP.
microglia: microscopic glial cells that participate in the immune response.
microtubules: hollow cylindrical protein bundles that are involved in axoplasmic transport.
mirror neuron: a neuron activated when performing a movement and when observing another person making the exact movement.
modulating effects: neuromodulators like the monoamines alter the performance of diffuse networks of target neurons by indirectly controlling cellular operations when they bind to metabotropic receptors.
module: a set of interconnected nodes in a neural network.
monoamine: a biogenic amine containing a single amino group, including dopamine, epinephrine, norepinephrine, and serotonin.
monoamine oxidase (MAO): an enzyme that degrades and inactivates the monoamine neurotransmitters dopamine, norepinephrine, and serotonin.
monoamine oxidase inhibitors (MAOIs): antidepressant drugs that interfere with MAO's breakdown of monoamines and increase monoamine availability to treat clinical depression.
monophasic wave: either a single negative (upward) or positive (downward) deflection from baseline.
motor cortex: the subdivision of the frontal lobe located in the precentral gyrus and guides fine motor coordination (like writing).
motor ERPs: event-related potentials detected over the primary motor cortex (precentral gyrus) during movement. Their amplitude is proportional to the force and rate of skeletal muscle contraction.
motor neurons: efferent neurons that convey commands to glands, muscles, and other neurons.
movement-related potentials (MRPs): slow cortical potentials that occur at 1 second as subjects prepare for unilateral voluntary movements. MRPs are distributed bilaterally with maximum amplitude at Cz. The supplementary motor area and primary motor and somatosensory cortices primarily generate these potentials.
mu rhythm: 7-11-Hz waves resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
multiple spike-and-slow-wave complex: multiple spikes associated with at least one slow wave.
muscarinic receptors: metabotropic ACh receptors that are stimulated by muscarine and blocked by atropine. Muscarinic receptors control smooth muscle and predominate in the CNS. In the CNS, muscarinic receptors help mediate learning, memory, attention, arousal, EEG, and postural control.
myelinated axons: axons that are insulated by myelin by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system.
N1-P2: a sensory event-related potential in the auditory cortex of the temporal cortex that reveals whether an uncommunicative person can hear a stimulus.
N400 potential: an event-related potential (ERP) elicited when we encounter semantic violations like ending a sentence with a semantically incongruent word ("The handsome prince married the beautiful fish"), or when the second word of a pair is unrelated to the first (BATTLE/GIRL).
negative SCPs: slow cortical potentials produced by glial cells that increase the probability of neuron firing.
neuroaxis: an imaginary line that runs centrally through the central nervous system (CNS) from the front of the prefrontal cortex to the base of the spinal cord.
neuromodulator: a neurochemical that modifies the effect of neurotransmitters through mechanisms like binding to metabotropic receptors.
neuron: a nerve cell that is the fundamental anatomical unit of the nervous system.
nicotinic ACh receptor: an ionotropic receptor that is stimulated by nicotine and blocked by curare. They are mainly found in the PNS on skeletal muscles. At CNS axoaxonic synapses, they produce presynaptic facilitation (increase neurotransmitter release). In the CNS, nicotinic receptors help regulate cortical blood flow, anxiety reduction, and decision-making.
nigrostriatal pathway: dopaminergic pathway from the substantia nigra to the basal ganglia (caudate nucleus and putamen) that controls movement. The nigrostriatal pathway is progressively destroyed in Parkinson’s disease.
nitric oxide: a gaseous retrograde transmitter that is involved in long-term potentiation (LTP).
NMDA (glutamate) receptors: ligand-gated and voltage-gated glutamate receptors that bind the glutamate agonist NMDA. NMDA receptors play an essential role in long-term potentiation (LTP).
node: vertex within a neural network.
nodes of Ranvier: gaps between myelinated axon segments where the axon membrane is exposed to extracellular fluid and action potentials are regenerated by sodium ion entry.
norepinephrine: a monoamine neurotransmitter that exerts postsynaptic effects at alpha and beta receptors, each with two subtypes. All norepinephrine receptors are G protein-linked. The cell bodies of the core noradrenergic system are located in the locus coeruleus, a nucleus found in the dorsal pons.
nucleus accumbens: a limbic structure that is a target of dopamine released by the mesolimbic pathway. The nucleus accumbens plays a critical role in the reinforcement of diverse activities, including ingestion of drugs like central nervous system stimulants.
occipital lobes: cortical lobes that are posterior to the parietal lobes. They process visual information from the eyes in collaboration with the frontal, parietal, and temporal lobes.
odd-ball stimulus: a meaningful stimulus that is different from others in a series used to elicit the P300 potential. For example, a colored playing card in a series of monochrome cards.
oligodendrocytes: glial cells that insulate adjacent axons within the brain and spinal cord of the central nervous system.
orbitofrontal cortex: the frontal lobe subdivision that is concerned with affective evaluation. It decodes the punishment and reward value of stimuli and helps inhibit inappropriate behavior. Phineas Gage's profound personality changes were produced by damage to this region.
orienting response: Pavlov’s "What is it?" reaction to stimuli like the sound of a vase crashing that includes (1) increased sensory sensitivity, (2) head (and ear) turning toward the stimulus, (3) increased muscle tone (reduced movement), (4) EEG desynchrony, (5) peripheral constriction and cephalic vasodilation, (6) a rise in skin conductance, (7) heart rate slowing, and (8) slower, deeper breathing.
orthosteric site: a NT's binding site.
P300 potential: an event-related potential (ERP) with a 300-900-ms latency. The largest amplitude positive peaks are located over the parietal lobe. The P300 potential may reflect an event’s subjective probability, meaning, and transmission of information.
paralimbic cortex: a transitional region between neocortex and allocortex.
parietal lobes: cortical lobes posterior to the frontal lobes that are divided into the primary somatosensory cortex (postcentral gyrus) and secondary somatosensory cortex. Their primary function is to process somatosensory information like pain and touch. The right posterior parietal lobe helps guide movements, locate objects in three-dimensional space, and create body boundaries.
peptide: a short amino acid chain linked by peptide bonds (e.g., opioid peptides, oxytocin, substance P).
phase: the degree to which the peaks and valleys of EEG waveforms coincide.
phase synchrony: synchrony when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
polarization: to make the membrane potential more negative by making the inside of the neuron more negative with respect to its outside.
polyphasic (multiphasic) wave: a wave that contains two or more deflections of opposite polarity from baseline.
positive SCPs: slow cortical potentials that are produced by glial cells that decrease the probability of neuron firing.
posterior: near or toward the back of the head.
posterior commissure: axon tracts located below the corpus callosum that connect the right and left diencephalon and mesencephalon.
precision: the number of voltage gradations or steps.
prefrontal cortex: the most anterior frontal lobe division and is subdivided into dorsolateral, medial, orbitofrontal, and anterior cingulate regions responsible for executive functions like attention and planning.
premotor cortex: nonprimary motor cortex anterior to the primary motor cortex.
presynaptic facilitation: a neuron increases the presynaptic neuron's NT release at an axoaxonic synapse by delivering a NT that increases calcium ion entry into its terminal button, depolarizing it.
presynaptic inhibition: a neuron decreases the presynaptic neuron's NT release at an axoaxonic synapse by delivering a NT that decreases calcium ion entry into its terminal button, hyperpolarizing it.
primary somatosensory cortex (S1): the parietal lobe subdivision located at the postcentral gyrus that processes information about touch and pain.
protein kinase A: an intracellular enzyme that controls the excitability of ion channels and is a critical enzyme target of cyclic AMP.
raphe nuclei: serotonergic cell bodies in the midbrain, pons, and medulla give rise to most of the brain’s serotonergic neurons.
rate law: the principle that neurons represent the intensity of a stimulus by variation in the rate of axon firing.
readiness potential: slow-rising, negative potential (10-15 µV) detected at the vertex before voluntary and spontaneous movement. This slow cortical potential precedes voluntary movement by 0.5 to 1 second and peaks when the subject responds.
regular or monomorphic waves: successive waves with identical shapes. Regular waves may resemble sine waves (sinusoidal) or maybe arched (resembling wickets) or saw-toothed (asymmetrical and triangular).
resting potential: the membrane potential of a neuron when it is not influenced by messages from other neurons.
reticular formation: a network of 90 nuclei within the central brainstem from the lower medulla to the upper midbrain. The reticular formation sends axons to the spinal cord, thalamus, and cortex, contributing to diverse functions like neurological reflexes, muscle tone and movement, attention, arousal, and sleep.
retrograde transmission: a signaling process in which the postsynaptic neuron dendrite or cell body synthesizes and distributes an endocannabinoid (e.g., anandamide) or gas (e.g., nitrous oxide) to the presynaptic neuron and its immediate active neighbors.
reuptake: the primary method that neurons terminate the action of neurotransmitters. Reuptake transporters located in terminal buttons and astrocytes remove neurotransmitters from the synaptic cleft.
reward deficiency syndrome: Blum’s hypothesis that an abnormal form of the A1 allele is present in most severe alcoholics and results in defective D2 receptors. Reduced D2 receptor activity may reduce the activation of the nucleus accumbens and hypothalamus and result in dysphoria, drug craving, and compulsive drug-seeking and abuse.
right dorsolateral prefrontal cortex: the division of the prefrontal cortex that organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
rostral: toward the front of the head.
saltatory conduction: action potential conduction in myelinated axons in which action potentials jump from node to node for 200 times greater speed.
sampling rate: the number of measurements per second (Hz).
Schwann cells: glial cells that provide myelin for single PNS axons and facilitate axonal regeneration following damage.
secondary somatosensory cortex (S2): a region of the parietal lobe that receives somatosensory information from the primary somatosensory cortex (S1).
sensorimotor rhythm (SMR): EEG rhythm that ranges from 12-15 Hz and is located over the sensorimotor cortex (central sulcus). The waves are synchronous. The sensorimotor rhythm is associated with the inhibition of movement and reduced muscle tone. The SMR is generated by "ventrobasal relay cells in the thalamus and thalamocortical feedback loops."
sensorimotor system: in Sterman’s model, ascending pathways that convey information about touch and proprioception to the thalamus, the thalamus and its thalamic projections to the sensorimotor cortex, and the sensorimotor cortex, and its efferent fibers.
sensory event-related potentials (ERPs): event-related potentials evoked by external sensory stimuli (auditory, olfactory, somatosensory, and visual). These evoked potentials or exogenous ERPs have a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. These changes in brain activity in response to specific stimuli. ERPs can be detected throughout the cortex. Investigators monitor ERPs by placing electrodes at locations like the midline (Fz, Cz, and Pz). A computer analyzes a subject's EEG responses to the same stimulus or task over many trials to subtract random EEG activity. ERPs always have the same waveform morphology. Their negative and positive peaks occur at regular intervals following the stimulus.
sensory neurons: neurons specialized for sensory intake. They are called afferent because they transmit sensory information towards the central nervous system (brain and spinal cord).
septal nuclei: in Sieb’s model, when the prefrontal cortex receives information about high-priority environmental events, it signals cell bodies in the septum to induce a beta rhythm in the hippocampus to remove its inhibition of vigilance centers.
septohippocampal system: a subcortical circuit from the septum to hippocampus that contributes to 4-7 Hz theta activity.
septum: a limbic structure that contains several nuclei involved in emotion and addiction and control of aggressive behavior.
sharp transients: a sequence that contains several sharp waves.
sharp waves: waves that resemble spikes with a pointed peak with a longer 70-200-ms duration.
sink: a site where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, represented by -ve.
slow cortical potentials (SCPs): gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials. SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms.
sodium (Na+) ions: positive ions that enter a neuron during EPSPs and action potentials.
sodium-potassium transporters: pumps that are powered by ATP and that exchange three sodium for two potassium ions.
soma or cell body: the region of a neuron that contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
source: the place at the end of the neuron opposite of the sink where current leaves, represented by +ve. The extracellular area surrounding the source becomes electrically positive.
spatial summation: the addition of EPSPs and IPSPs over a neuron’s surface.
spike: a negative transient with a pointed peak at conventional paper speeds, 20-70-ms duration, and 40-100 μV amplitude; rare dendritic action potentials.
spike-and-slow-wave complex: a spike followed by a higher amplitude slow wave at 3 Hz. In an absence seizure, the amplitudes are very high (e.g., 160 μV).
spindle waves: waves that originate in the thalamus and occur during unconsciousness and stage II sleep.
spiny neurons: neurons with dendritic spines that are usually excitatory.
striatal: basal ganglia (caudate nucleus and putamen).
Stroop test: cognitive monitoring task where color and names conflict.
substantia nigra: midbrain structure that projects to the basal ganglia (caudate nucleus and putamen) to control movement and that is progressively destroyed in Parkinson’s disease.
sulcus: a shallow groove in the surface of the cerebral hemisphere, for example, the central sulcus.
synapse-associated polyribosome complexes (SPRCs): organelles with dendrites that can produce proteins that allow rapid remodeling of synapses. A polyribosome complex consists of several ribosomes bound to messenger RNA (mRNA). SPRCs represent one mechanism underlying synaptic plasticity.
synaptic cleft: a 20-50-nm fluid-filled gap between presynaptic and postsynaptic structures.
synchronous: adverb meaning that groups of neurons depolarize and hyperpolarize simultaneously.
synchrony: the coordinated firing of pools of neurons. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
telencephalon: the frontal subdivision of the forebrain, including the cerebral cortex, basal ganglia, and limbic system.
temporal summation: the addition of EPSPs and IPSPs over time. Summation is more effective when postsynaptic potentials are generated more closely in time.
temporal lobes: lobes separated from the rest of the cortical lobes by the Sylvian fissure. The temporal lobes process hearing, smell, and taste information and help us understand spoken language and recognize visual objects and faces. The amygdala and hippocampus, which lie beneath the temporal cortex, play crucial roles in emotion, declarative, emotional, and working memory, and navigation.
terminal buttons: buds located on the ends of axon branches that form synapses and release neurochemicals to other neurons. They contain vesicles that store neurotransmitters for release when an action potential arrives. A terminal button’s presynaptic membrane may possess reuptake transporters that return neurotransmitters from the synapse or extracellular space for repackaging.
thalamus: forebrain structure above the hypothalamus that receives, filters, and distributes most sensory information. The thalamus contains neurons that can block or relay ascending sensory information. When these thalamic neurons rhythmically fire, this blocks the transmission of information to the cortex. When they depolarize in response to sensory information, this integrates and transmits this information to the cortex. Inputs to the thalamus determine whether these neurons block or relay sensory information.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
threshold of excitation: the membrane potential at which an axon initiates an action potential, nominally -40 mV.
traveling waves: EEG oscillations that move across the cortex that may mediate large-scale coordination of brain networks and support connectivity.
transient: a single wave or sequence of regular waves, called a complex, distinguishable from background EEG activity.
triphasic wave: a wave that contains three deflections from baseline.
unmyelinated axons: smaller-diameter axons without fatty insulation that conduct more slowly than myelinated axons.
+ve: the source is the place at the other end of the neuron where current leaves, and is represented by +ve.
-ve: a sink is where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, represented by -ve.
ventral: toward the base of the skull or front of the body.
ventral striatum: the olfactory tubercle and nucleus accumbens.
ventral tegmental area: the midbrain structure that distributes dopaminergic axons to the nucleus accumbens. Serotonin receptors on endorphin-releasing neurons in the hypothalamus may increase the activity of dopaminergic reward pathways by inhibiting the release of GABA at receptors on cell bodies of the ventral tegmental area neurons.
vigilance system: in Sterman’s model, a system that consists of both specific brainstem nuclei (e.g., locus coeruleus and raphe nuclei) and their diffuse connections with the thalamus and other subcortical structures, and the cortex. Several neurotransmitter systems mediate vigilance, including cholinergic/glutamatergic (reticular formation), noradrenergic (locus coeruleus), and serotonergic (raphe) neurons.
voltage-gated sodium channel: a membrane-bound sodium ion channel required for action potentials that opens and closes in response to the membrane potential.
volume transmission: widespread neurotransmitter distribution via the extracellular fluid and cerebrospinal fluid.
waveform: the shape and form of an EEG signal.
white matter: the layer beneath the cortex that mainly consists of myelinated axons.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.


References
Aloisi, F. (2001). Immune function of microglia. Glia, 36, 165–179. https://doi.org/10.1002/glia.1106
Altenmuller, E. O., & Gerloff, C. (1999). Psychophysiology and the EEG. In E. Niedermeyer & F. Lopes da Silva (Eds.), Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
E. Niedermeyer & F. Lopes da Silva (Eds.) (1999). Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
Andreassi, J. L. (2007). Psychophysiology: Human behavior and physiological response (5th ed.). Lawrence Erlbaum and Associates, Inc.
Arnsten, A. F. (2006). Fundamentals of Attention-Deficit/Hyperactivity Disorder: Circuits and pathways. Journal of Clinical Psychiatry, 67 (Suppl. 8), 7-12.
Babiloni, C., Babiloni, F., Carducci, F., Cincotti, F., Del Percio, C., Hallett, M., Moretti, D. V., Romani, G. L., & Rossini, P. M. High resolution EEG of sensorimotor brain functions: Mapping ERPs or mu ERD? In R. C. Reisin, M. R. Nuwer, M. Hallett, & C. Medina (Eds.). Advances in Clinical Neurophysiology (Supplements to Clinical Neurophysiology Vol. 54). Elsevier Science B. V.
Barker, H. (2022). Silent synapses may provide plasticity in adulthood. The Scientist.com.
Basile, L. F. H., Yacubian, J., Ferreira, B. L. C., Valim, A. C., & Gattaz, W. F. (2004). Topographic abnormality of slow cortical potentials in schizophrenia. Brazilian Journal of Medical and Biological Research, 37(1), 97-109. https://doi.org/10.1590/s0100-879x2004000100014
Bellocchio, E. E., Reimer, R. J., Fremeau, R. T., Jr, & Edwards, R. H. (2000). Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science, 289(5481), 957–960. https://doi.org/10.1126/science.289.5481.957
Bennett, M. V., Contreras, J. E., Bukauskas, F. F., & Sáez, J. C. (2003). New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends in Neurosciences, 26(11), 610–617. https://doi.org/10.1016/j.tins.2003.09.008
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Buccino, G., Solodkin, A., & Small, S. L. (2006). Functions of the mirror neuron system: implications for neurorehabilitation. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 19(1), 55–63. https://doi.org/10.1097/00146965-200603000-00007
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature, 10, 186-198. https://doi.org/10.1038/nrn2575
Buskila, Y., Bellot-Saez, A., & Morley, J. W. (2019). Generating brain waves, the power of astrocytes. Frontiers in Neuroscience, 13, 1125. https://doi.org/10.3389/fnins.2019.01125
Cameron, H. A., & Dayer, A. G. (2008). New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry, 63(7), 650-655. https://dx.doi.org/10.1016%2Fj.biopsych.2007.09.023
Carlson, N. R., & Birkett, M. A. (2021). Physiology of behavior (13th ed.). Pearson.
Chan, C. Y., Ke, D. S., & Chen, J. Y. (2009). Essential fatty acids and human brain. Acta Neurol Taiwan, 18(4), 231-241. PMID: 20329590
Coggan, J. S., Bartol, T. M., Esquenazi, E., Stiles, J. R., Lamont, S., Martone, M. E., Berg, D. K., Ellisman, M. H., & Sejnowski, T. J. (2005). Evidence for ectopic neurotransmission at a neuronal synapse. Science, 309(5733), 446–451. https://doi.org/10.1126/science.1108239
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Costanzo, R. M. (1991). Regeneration of olfactory receptor cells. CIBA Found Symp, 160, 233-242. https://doi.org/10.1002/9780470514122.ch12
Creuzfeldt, O. D. (1995). Cortex cerebri. Oxford University Press.
Damasio, A. (2010). Self comes to mind. Pantheon Books.
Daum, I., Rockstroh, B., Birbaumer, N., Elbert. T., Canavan, A., & Lutzenberger W. (1993). Behavioural treatment of slow cortical potentials in intractable epilepsy: Neuropsychological predictors of outcome. J Neurol Neurosurg Psychiatry, 56(1) 94-97. https://doi.org/10.1136/jnnp.56.1.94
deCharms, R. C., Fumiko, M., Glover, G. H., Ludlow, D., Pauly, J. M., Soneji, D., Gabrieli, J. D. E., & Mackey, S. C. (2005). Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences, 102(51), 18626-18631. https://doi.org/10.1073/pnas.0505210102
DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci, 13(7), 281-285. https://doi.org/10.1016/0166-2236(90)90110-v
Demos, J. N. (2019). Getting started with neurofeedback. (2nd ed.). W. W. Norton & Company.
di Pellegrino, G., Fadiga, L., Fogassi, L., Gallese, V., & Rizzolatti, G. (1992). Understanding motor events: a neurophysiological study. Experimental Brain Research, 91(1), 176–180. https://doi.org/10.1007/BF00230027
Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302, 290-292. https://doi.org/10.1126/science.1089134
El-Boustani, S., Ip, J., Breton-Provencher, V., Knott, G., Okuno, H., Bito, H., & Sur, M. (2018). Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science, 360(6395), 1349-1354. https://doi.org/10.1126/science.aao0862
Enticott, P. G., Kennedy, H. A., Rinehart, N. J., Tonge, B. J., Bradshaw, J. L., Taffe, J. R., Daskalakis, Z. J., & Fitzgerald, P. B. (2012). Mirror neuron activity associated with social Impairments but not age in Autism Spectrum Disorder. Biol Psychiatry, 71(5), 427-433. https://doi.org/10.1016/j.biopsych.2011.09.001
Evans, J. R., & Abarbanel, A. (1999). Introduction to quantitative EEG and neurofeedback. San Diego: Academic Press.
Farwell, L. A., & Donchin, E. (1991). The truth will out: Interrogative polygraphy (“lie detection”) with event-related brain potentials. Psychophysiology, 28, 531–547. https://doi.org/10.1111/j.1469-8986.1991.tb01990.x
Fox, S. I., & Rompolski, K. (2022). Human physiology (16th ed.). McGraw-Hill.
Garrett, B. (2003). Brain and behavior. Thompson/Wadsworth.
Giachello, C. N., Montarolo, P. G., & Ghirardi, M. (2012). Synaptic functions of invertebrate varicosities: What molecular mechanisms lie beneath. Neural Plasticity, 2012, 670821. https://doi.org/10.1155/2012/670821
Gordleeva, S. Y., Ermolaeva, A. V., Kastalskiy, I. A., & Kazantsev, V. B. (2019). Astrocyte as spatiotemporal integrating detector of neuronal activity. Frontiers in Physiology, 10, 294. https://doi.org/10.3389/fphys.2019.00294
Gross, L. (2006). Evolution of neonatal imitation. PLoS Biology, 4(9), e311. https://doi.org/10.1371/journal.pbio.0040311
Hansson, E., & Ronnback, L. (2003.) Glial neuronal signaling in the central nervous system. FASEB J, 17, 341-348. https://doi.org/10.1096/fj.02-0429rev
Heinrich, H., Gevensleben, H., Freisleder, F. J., Moll, G. H., & Rothenberger, A. (2004). Training of slow cortical potentials in attention-deficit/hyperactivity disorder: Evidence for positive behavioral and neurophysiologic effects. Biological Psychiatry, 55(7), 772–775. https://doi.org/10.1016/j.biopsych.2003.11.013
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Jurkowski, M. P., Bettio, L., K Woo, E., Patten, A., Yau, S. Y., & Gil-Mohapel, J. (2020). Beyond the hippocampus and the SVZ: Adult neurogenesis throughout the brain. Frontiers in Cellular Neuroscience, 14, 576444. https://doi.org/10.3389/fncel.2020.576444 Kalat, J. W. (2019). Biological psychology (13th ed.). Cengage Learning.
Kennerley, S. W., Behrens, T. E., & Wallis, J. D. (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci, 14(12), 1581-1589. doi:10.1038/nn.2961
Kitamura, T., Saitoh, Y., Takashima, N., Murayama, A., Niibori, A., Ageta, H., . . . Inokuchi, K. (2009). Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell, 139(4), 814-827. https://doi.org/10.1016/j.cell.2009.10.020
Klein, S. B., & Thorne, B. M. (2007). Biological psychology. New York: Worth Publishers.
Kotchoubey, B., Blankenhorn, V., Fröscher, W., Strehl, U. & Birbaumer, N. (1997). Stability of cortical self-regulation in epilepsy patients. Neuroreport, 27(8)1867-1870. https://doi.org/10.1097/00001756-199705260-00015
Kotchoubey, B., Busch, S., Strehl, U. & Birbaumer, N. (1999). Changes in EEG power spectra during biofeedback of slow cortical potentials in epilepsy. Applied Psychophysiology and Biofeedback, 24(4) 213-233. https://doi.org/10.1023/a:1022226412991
Kotchoubey, B., Kubler. A., Strehl. U., Flor, H., & Birbaumer, N. (2002). Can humans perceive their brain states? Consciousness and Cognition, 11(1), 98-113. https://doi.org/10.1006/ccog.2001.0535
Kotchoubey, B., Schneider, D., Schleichert, H., Strehl, U., Uhlmann, C., Blankenhorn, V., Fröscher, W., & Birbaumer, N. (1996). Self-regulation of slow cortical potentials in epilepsy: A retrial with analysis of influencing factors. Epilepsy Research, 25(3), 269-276. https://doi.org/10.1016/s0920-1211(96)00082-4
Kotchoubey, B., Schneider, D., Uhlmann, C., Schleichert, H., & Birbaumer, N. (1997). Beyond habituation: Long-term repetition effects on visual event-related potentials in epileptic patients. Electroencephalographer, 103(4), 450-456. https://doi.org/10.1016/s0013-4694(97)00026-6
Kotchoubey, B., Strehl, U., Holzapfel, S., Blankenhorn, V., Fröscher. W., & Birbaumer, N. (1999). Negative potential shifts and the prediction of the outcome of neurofeedback therapy in epilepsy. Clinical Neurophysiology, 110(4), 683-686. https://doi.org/10.1016/s1388-2457(99)00005-x
Kotchoubey. B., Strehl, U., Holzapfel, S., Schneider. D., Blankenhorn, V., & Birbaumer, N. (1999). Control of cortical excitability in epilepsy. Adv Neurol, 81, 281-290.
Kotchoubey, B., Strehl, U., Uhlmann, C., Holzapfel, S., Konig, M., Fröscher. W., Blankenhorn, V., & Birbaumer, N. (2001). Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia, 42(3), 406-416.
Kropotov, J. D. (2009). Quantitative EEG, event-related potentials and neurotherapy. Academic Press.
Landisman, C. E., & Connors, B. W. (2005). Long-term modulation of electrical synapses in the mamillimeteralian thalamus. Science, 310(5755), 1809-1813. https://doi.org/10.1126/science.1114655
Lim, D. A., & Alvarez-Buylla, A. (2016). The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harbor Perspectives in Biology, 8(5), a018820. https://doi.org/10.1101/cshperspect.a018820
Liu, J. H., Zhang, M., Wang, Q., Wu, D. Y., Jie, W., Hu, N. Y., Lan, J. Z., Zeng, K., Li, S. J., Li, X. W., Yang, J. M., & Gao, T. M. (2022). Distinct roles of astroglia and neurons in synaptic plasticity and memory. Mol Psychiatry, 27, 873–885. https://doi.org/10.1038/s41380-021-01332-6
Merighi A. (2018). Costorage of high molecular weight neurotransmitters in large dense core vesicles of mammalian neurons. Frontiers in Cellular Neuroscience, 12, 272. https://doi.org/10.3389/fncel.2018.00272
Meshorer et al. (2002). Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science, 295(5554), 508-512. https://doi.org/10.1126/science.1066752
Molenberghs, P., Cunnington, R., & Mattingley, J. B. (2011). Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev, 36(1), 341-349. https://doi.org/10.1016/j.neubiorev.2011.07.004
Munro, C. A., McCaul, M. E., Wong, D. F., Oswald, L. M., Zhou, Y., Brasic, J., Kuwabara, H., Kumar, A., Alexander, M., Ye, W., & Wand, G. S. (2006). Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry, 59(10), 966-974. https://doi.org/10.1016/j.biopsych.2006.01.008
Nagano, S., & Araki, T. (2021). Axonal transport and local translation of mRNA in neurodegenerative diseases. Frontiers in Molecular Neuroscience, 14, 697973. https://doi.org/10.3389/fnmol.2021.697973
Nash, J. M. (2011). The gift of mimicry. Your brain: A user's guide. Time.
Natale, G., Limanaqi, F., Busceti, C. L., Mastroiacovo, F., Nicoletti, F., Puglisi-Allegra, S., & Fornai, F. (2021). Glymphatic system as a gateway to connect neurodegeneration from periphery to CNS. Frontiers in Neuroscience, 15, 639140. https://doi.org/10.3389/fnins.2021.639140
Niedermeyer, E. (1999). Historical aspects. In E. Niedermeyer & F. Lopes da Silva (Eds.), Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
E. Niedermeyer & F. Lopes da Silva (Eds.) (1999). Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
Otto, D., & Reiter, L. (1984) Developmental changes in slow cortical potentials of young children with elevated body lead burden: Neurophysiological considerations. Annals of the New York Academy of Sciences, 425(1), 377-383. https://doi.org/10.1111/j.1749-6632.1984.tb23559.x
Parri, R., & Crunelli, V. (2003). An astrocyte bridge from synapse to blood flow. Nature Neuroscience, 6(1), 5–6. https://doi.org/10.1038/nn0103-5
Purves, D. (2017). Neuroscience (6th ed.). Oxford University Press Academic.
Pulvermüller, F., Mohr, B., Schleichert, H., & Veit, R., (2000). Operant conditioning of left-hemispheric slow cortical potentials and its effect on word processing. Biological Psychology, 53(2-3), 177-215. https://doi.org/10.1016/S0301-0511(00)00046-6
Radley, J. J., Arias, C. M., & Sawchenko, P. E. (2006). Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. The Journal of Neuroscience, 26(50), 12967-12976. https://doi.org/10.1523/JNEUROSCI.4297-06.2006
Raichle, M. E., & Gusnard, D. A. (2002). Appraising the brain's energy budget. PNAS, 99(16), 10237-10299. https://doi.org/www.pnas.orgcgidoi10.1073pnas.172399499
Rajmohan, V., & Mohandas, E. (2007). Mirror neuron system. Indian Journal of Psychiatry, 49(1), 66–69. https://doi.org/10.4103/0019-5545.31522
Rapanelli, M., Frick, L. R., & Zanutto, B. S. (2011). Learning an operant conditioning task differentially induces gliogenesis in the medial prefrontal cortex and neurogenesis in the hippocampus. PLoS ONE, 6(2), e14713. https://doi.org/10.1371/journal.pone.0014713
Rizzolatti, G., & Craighero, L. (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. https://doi.org/10.1146/annurev.neuro.27.070203.144230
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L., & von Stein, A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci, 95(12), 7092-7096. https://doi.org/10.1073/pnas.95.12.7092
Schacter, D. L. (1977). EEG theta waves and psychological phenomena: A review and analysis. Biological Psychology, 5, 47-82. https://doi.org/10.1016/0301-0511(77)90028-x
Schmidt, S. N. L., Hass, J., Kirsch, P., & Mier, D. (2021). The human mirror neuron system-A common neural basis for social cognition? Psychophysiology, 58(5), e13781. https://doi.org/10.1111/psyp.13781
Schneider, F., Rockstroh, B., Heimann, H., Lutzenberger, W., Mattes, R., Elbert, T., Birbaumer, N, & Bartels, M. (1992). Self-regulation of slow cortical potentials in psychiatric patients: Schizophrenia. Biofeedback and Self-Regulation, 17(4), 277-292. https://doi.org/10.1007/bf01000051
M. S. Schwartz, & F. Andrasik (Eds.). (2003). Biofeedback: A practitioner's guide (3rd ed.). The Guilford Press.
Sebel, P. S., Lang, E., Rampil, I. J., White, P. F., Cork, R., Jopling, M., Smith, N. T., Glass, P. S., & Manberg, P. (1997). A multicenter study of bispectral electroencephalogram analysis monitoring anesthetic effect. Anesthesia and Analgesia, 84(4), 891-899. https://doi.org/10.1097/00000539-199704000-00035
Shan, L., Zhang, T., Fan, K., Cai, W., & Liu, H. (2021). Astrocyte-neuron signaling in synaptogenesis. Frontiers in Cell and Developmental Biology, 9, 680301. https://doi.org/10.3389/fcell.2021.680301
Siniatchkin, M., Hierundar, A., Kropp, P., Kuhnert, R., Gerber, W-D., & Stephani, U. (2000). Self-regulation of slow cortical potentials in children with migraine: An exploratory study. Applied Psychophysiology & Biofeedback, 25(1), 13-32. https://doi.org/10.1023/a:1009581321624
Speckmann, E.-J., & Elger, C. E. (1984). The neurophysiological basis of epileptic activity: A condensed overview. R. Degen & E. Niedermeyer (Eds.), Epilepsy, sleep, and sleep deprivation (pp.23-34). Elsevier. PMID: 1760082
Speckmann, E.-J., & Elger, C. E. (1999). Introduction to the neurophysiological basis of the EEG and DC potentials. In E. Niedermeyer & F. Lopes da Silva (Eds.), Electroencephalography: Basic principles, clinical applications, and related fields (4th ed.). Williams and Wilkins.
Stahl, S. M. (2008). Stahl’s essential psychopharmacology: Neuroscientific basis and practical applications (3rd ed.). Cambridge University Press.
Steriade, M. (2005). Cellular substrates of brain rhythms. In E. Niedermeyer, & F. Lopes da Silva (Eds.). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Sterman, M. B. (2000). EEG markers for attention deficit disorder: Pharmacological and neurofeedback applications. Child Study Journal, 30(1), 1-24.
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Streit, W. J. (2006). Microglial senescence: Does the brain's immune system have an expiration date? Trends in Neurosciences, 29(9), 506–510. https://doi.org/10.1016/j.tins.2006.07.001
Svensson, E., Apergis-Schoute, J., Burnstock, G., Nusbaum, M. P., Parker, D., & Schiöth, H. B. (2019). General principles of neuronal co-transmission: Insights from multiple model systems. Frontiers in Neural Circuits, 12, 117. https://doi.org/10.3389/fncir.2018.00117
Thompson, M., & Thompson, L. (2016). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Thompson, M., & Thompson, L. (2009). Asperger’s syndrome intervention: Combining neurofeedback, biofeedback, and metacognition. In T. H. Budzynski, H. K. Budzynski, J. R. Evans, & A. Abarbanel (Eds.). Introduction to quantitative EEG and neurofeedback (2nd ed.). Academic Press.
Vardalaki, D., Chung, K., & Harnett, M. T. (2022). Filopodia are a structural substrate for silent synapses in adult neocortex. Nature, 612(7939), 323–327. https://doi.org/10.1038/s41586-022-05483-6
Voytek, B. (2013). Brain metrics: How measuring brain biology can explain the phenomena of mind. Scitable by Nature Education.
Warren, A. M., & McIlvane, W. J. (1998). Stimulus equivalence and the N400 effect. Poster presented at the 1998 Annual Meeting of the Cognitive Neuroscience Society in San Francisco, CA.
Wilson, J. (2003). Biological foundations of human behavior. Wadsworth/Thompson Learning.
Wilson, V. E., Thompson, M., Thompson, L., Thompson, J., Fallahpour, K., & Linden, M. K. (2011). Introduction to biofeedback (Neurofeedback). In B. W. Strack, M. K. Linden, & V. S. Wilson (Eds.). Biofeedback & neurofeedback applications in sport psychology. Association for Applied Psychophysiology and Biofeedback.
Winn, P. (2001). (Ed.), Dictionary of biological psychology. Routledge.
Wostyn, P., & Goddaer, P. (2022). Can meditation-based approaches improve the cleansing power of the glymphatic system? Explor Neuroprot Ther, 2,110–117. https://doi.org/10.37349/ent.2022.00022
Xu, T., Yu, X., Perlik, A., Tobin, W., Zweig, J., Tennant, K., Jones, T., & Zuo, Y. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature, 462(7275), 915-919. https://doi.org/10.1038/nature08389
Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., Poo, M., & Duan, S. (2003). Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proceedings of the National Academy of Sciences of the United States of America, 100(25), 15194-15199. https://doi.org10.1073/pnas.2431073100
Zhang, H., Watrous, A., Patel, A., & Jacobs, J. (2018). Theta and alpha oscillations are traveling waves in the human neocortex. Neuron. https://doi.org/10.1016/j.neuron.2018.05.019
B. SENSORY PATHWAYS
We will review the Visual System, Auditory System, Somatosensory System, and Somatosensory Nervous System.
Please click on the podcast icon below to hear a full-length lecture for Section B.

Sensory input produced by activities like reading a novel and listening to music can desynchronize cortical activity, resulting in lower amplitude, higher-frequency EEG waveforms (Neumann, Strehl, & Birbaumer, 2003). Arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it, a phenomenon called alpha blocking, while increasing EEG power in the beta range (Andreassi, 2007).
The classical routes for EEG activation consist of ascending sensory pathways that distribute information to specialized thalamic nuclei and then project the results of thalamic processing to the appropriate cortical regions. The exception to this rule is olfaction (smell), which goes directly to the primary olfactory cortex.
The old-school view is that these ascending sensory pathways exercise bottom-up perception control as feedforward circuits. This overlooks the fact that ten times more cortical efferent neurons target the sensory thalamus as thalamic afferent neurons project to the cortex.
The new-school view is that extensive interconnections between the thalamus and cortex permit a degree of top-down cortical control over perception (Kandel et al., 2021).
We will examine the visual, auditory, and somatosensory systems to better understand the ascending sensory pathways to the cortex.
Visual System
Retinal ganglion cells, which comprise the optic nerves, ascend to the midline optic chiasm where the two optic nerves meet. Here, temporal axons (toward the side of the head) continue as part of their own side's optic tract, and the nasal halves cross over to join the opposite side's optic tract. Most optic tract axons project information to the lateral geniculate nucleus of the thalamus.
Meanwhile, the lateral geniculate nucleus (LGN) relays visual information to the cortex, brainstem, and cortical neurons, which modulate its activity. Brainstem neurons that mediate alertness and attention can adjust the LGN's response to visual input. Moreover, the cortex can exert a top-down selection of visual input to increase attention to a salient region of the visual field at the expense of others (Bear, Connors, & Paradiso, 2016).
LGN neurons form the optic radiations and project to the primary visual cortex (V1) in the occipital lobe in cortical layer IV. A minority of retinal ganglion cell axons target the dorsal midbrain superior colliculus, which is concerned with visual gaze direction and attention to selected visual objects (Breedlove & Watson, 2023).
The cortex contains many specialist regions to process visual properties like color, shape, location, motion, and orientation. Evolution has organized these visual areas into dorsal and ventral streams that begin in the primary visual cortex. The dorsal stream, which projects from V1 to the parietal lobe, helps us localize objects and guide movements. There are neurons with visual and motor properties in the adjacent motor association cortex called mirror neurons. Networks of mirror neurons may play a role in learning how to perform actions by observing others' movements, understanding others' actions and intentions, and empathy.
The lower ventral stream, which projects to inferior temporal and frontal areas, allows us to identify objects and faces. Graphic by Andrey Vyshedskiy, Shreyas Mahapatra, and Rita Dunn. Creative Commons Attribution 4.0.
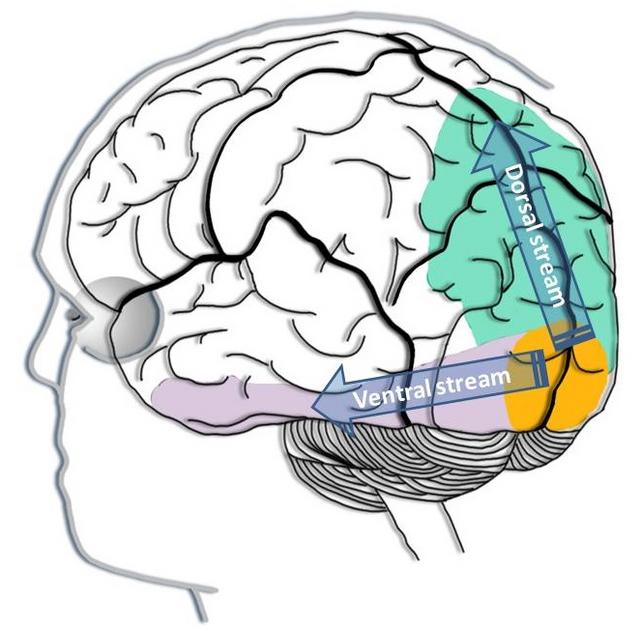
Auditory System
The cochlea's inner hair cells within the organ of Corti send 30,000 to 50,000 auditory fibers to several destinations: the midbrain superior olivary nuclei and inferior colliculi and the medial geniculate nucleus of the thalamus. They process binaural (two-ear) information to localize sound. All ascending auditory neurons innervate the inferior colliculi, some via intermediate relays.
The inferior colliculi integrate information about spatial localization and multiple sensory modalities, including somatosensory information. They project to the thalamus' medial geniculate nucleus (MGN), which projects to several cortical auditory areas using two separate pathways. The MGN mainly relays frequency, amplitude, and binaural information to the auditory cortex in the temporal lobe.
The auditory cortex processes auditory information within dorsal and ventral streams. The dorsal stream extends to the parietal lobe and helps us spatially localize sounds. The lower ventral stream, which projects to the temporal lobe, appears to analyze sound components, perhaps including speech sounds (Breedlove & Watson, 2023).
As with the visual pathways, the auditory system involves extensive feedback. Neurons in the brainstem innervate the outer hair cells that adjust the sensitivity of the basilar membrane within the organ of Corti to specific frequencies. Also, auditory cortex axons innervate the inferior colliculi and MGN to exercise top-down control (Bear, Connors, & Paradiso, 2016). Auditory pathway graphic by Jonathan E. Peelle. Creative Commons Attribution 4.0.
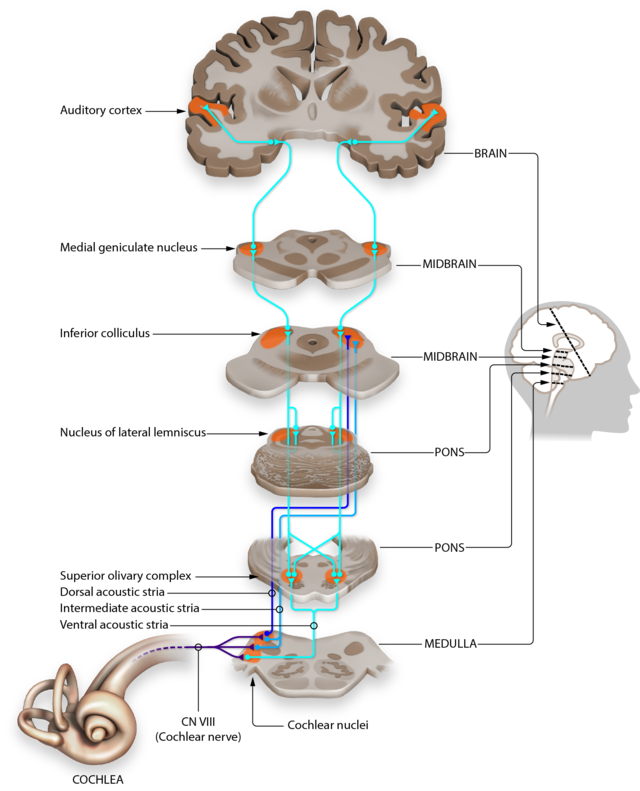
Somatosensory System
The somatosensory system employs specialized receptors to perceive itch, pain, temperature, and touch. For touch, a unipolar neuron's axon enters the spinal cord's dorsal horn and synapses with a dorsal column neuron in the medulla. Axons from this neuron decussate (cross the midline) and innervate the thalamus' ventral posterior nucleus (VPN). The VPN, in turn, distributes this information to the primary somatosensory cortex (S1). While each hemisphere's S1 maps touch information from the opposite side of the body, the secondary somatosensory cortex (S2) maps both sides. The maps are overlaid so that the left and right arms are represented in the same region of the body surface map (Breedlove & Watson, 2023). Polygon data were generated by Database Center for Life Science(DBCLS). Creative Commons Attribution-Share Alike 2.1 jp.
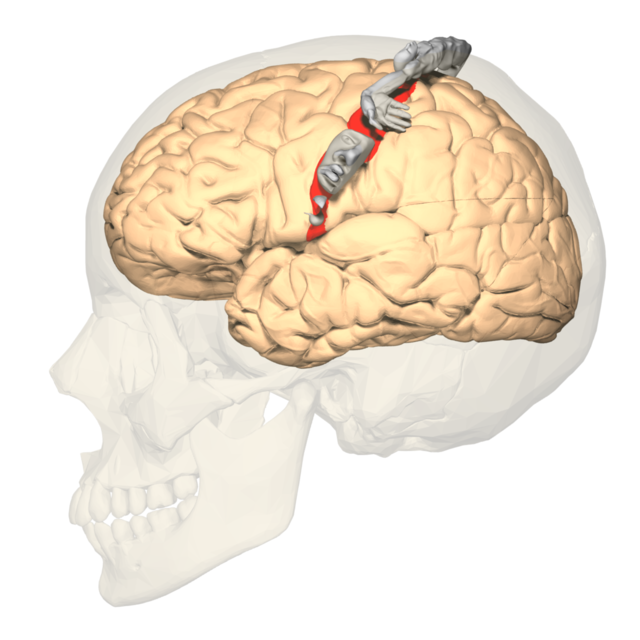
As with the visual and auditory systems, the ascending pathways do far more than relay information. These networks process and alter sensory information at each successive synapse. The cortex exercises top-down control over neurons in the dorsal column and VPN to dynamically adjust cortical inputs (Bear, Connors, & Paradiso, 2016).
Somatic Nervous System
The somatic nervous system comprises spinal nerves that innervate somatosensory receptors in the skin, joints, and skeletal muscles. While somatic motoneuron cell bodies lie in the CNS, most of their axons are in the PNS. The cell bodies of somatic sensory neurons are in the PNS dorsal root ganglia. The somatosensory association area is located at 2 © Sakurra/Shutterstock.com.
Dorsal root graphic © stihii/Shutterstock.com.
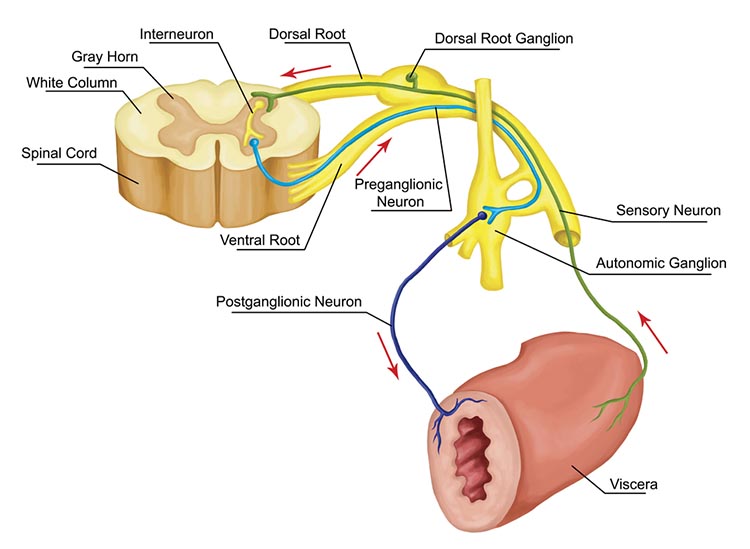
Glossary
alpha blocking: the attenuation of alpha band (8-13 Hz) electroencephalographic (EEG) activity, typically observed during states of heightened attention or cognitive engagement.
auditory cortex: the region of the temporal lobe responsible for processing auditory information, including sound localization, pitch discrimination, and speech comprehension.
classical routes for EEG activation: the ascending pathways mediating sensory input to the cortex, primarily through the thalamus, which generate characteristic EEG patterns corresponding to different sensory modalities.
dorsal stream (auditory): the auditory pathway projecting from the auditory cortex to the parietal lobe, involved in sound localization and spatial processing ("where").
dorsal stream (visual): the visual pathway extending from the primary visual cortex to the parietal lobe, responsible for spatial perception, motion analysis, and visuomotor guidance ("where").
lateral geniculate nucleus (LGN): the thalamic relay nucleus for visual information, receiving input from the retina and projecting to the primary visual cortex.
medial geniculate nucleus (MGN): the thalamic relay nucleus for auditory information, receiving input from the inferior colliculus and projecting to the primary auditory cortex.
mirror neurons: neurons that fire both during the execution of an action and the observation of another individual performing the same action, implicated in motor learning, empathy, and social cognition.
primary somatosensory cortex (S1): the region of the parietal lobe responsible for processing tactile information from the body, including touch, pressure, temperature, and pain.
primary visual cortex (V1): the region of the occipital lobe responsible for initial processing of visual information, including edge detection, orientation selectivity, and binocular integration.
secondary somatosensory cortex (S2): the region adjacent to S1 involved in higher-order processing of somatosensory information, integrating tactile input with other sensory modalities and contributing to object recognition.
somatic nervous system: the division of the peripheral nervous system responsible for voluntary motor control and sensory input from the skin, muscles, and joints.
superior colliculus: a midbrain structure involved in visual reflexes, saccadic eye movements, and multisensory integration.
superior olivary nuclei: a group of nuclei in the brainstem involved in sound localization, binaural hearing, and acoustic reflex modulation.
ventral posterior nucleus (VPN): a thalamic nucleus relaying somatosensory information from the body to the primary somatosensory cortex.
ventral stream (auditory): the auditory pathway projecting from the auditory cortex to the temporal lobe, involved in sound identification and object recognition ("what").
ventral stream (visual): the visual pathway extending from the primary visual cortex to the temporal lobe, responsible for object recognition, face perception, and semantic processing ("what").
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

References
Bear, M. F., Connors, B. W., & Paradiso, M. A. (2020). Neuroscience: Exploring the brain (4th ed.). Jones & Bartlett Learning.
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Buccino, G., Solodkin, A., & Small, S. L. (2006). Functions of the mirror neuron system: implications for neurorehabilitation. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 19(1), 55–63. https://doi.org/10.1097/00146965-200603000-00007
Carlson, N. R., & Birkett, M. A. (2021). Physiology of behavior (13th ed.). Pearson.
Damasio, A. (2010). Self comes to mind. Pantheon Books.
C. AUTONOMIC NERVOUS SYSTEM
The autonomic nervous system regulates cardiac and smooth muscle and glands, transmits sensory information to the CNS, and innervates muscle spindles.
We will review the Autonomic Nervous System (ANS), Sympathetic Division, Parasympathetic Division, The Relationship Between the Sympathetic and Parasympathetic Branches, Porges' Polyvagal Theory, and the Enteric Division.
Please click on the podcast icon below to hear a full-length lecture for Section C.

The autonomic nervous system is divided into three main systems: sympathetic, parasympathetic, and enteric. Check out the YouTube video The Autonomic Nervous System.
The parasympathetic branch generates the variability between adjacent heartbeats. The sympathetic branch regulates slower heart rate changes across several beats. The hormones angiotensin, epinephrine, and vasopressin modulate heart rate over seconds to hours (Karemaker, 2020).
The three autonomic nervous system branches work cooperatively and competitively to maintain homeostasis. Contrary to popular books on stress, the sympathetic branch is not our enemy, just as the parasympathetic branch is not always our friend. We can't rise from a couch without fainting or sprint across a field without increased sympathetic activation. Graphic © masisyan/Shutterstock.com.

Porges' (2011) polyvagal theory challenged the traditional vagus nerve (10th cranial nerve) model. Porges proposes that the unmyelinated and myelinated vagus are responsible for competing adaptive responses to threats to our survival. Graphic © Chu KyungMin/Shutterstock.com.
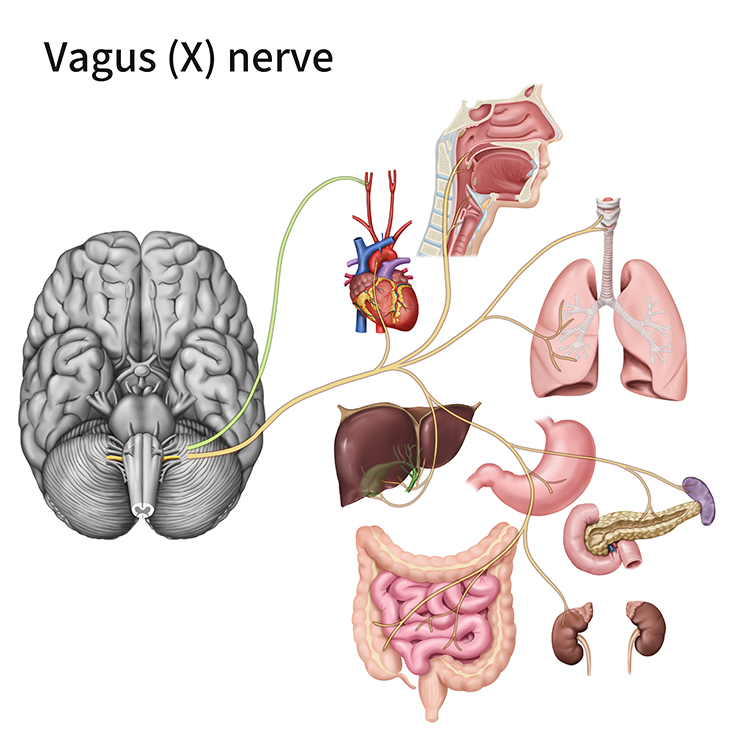
This section will challenge the simplistic belief that the sympathetic division fight-or-flight response is our only means of responding to stressors. The parasympathetic branch expands our response options through immobilization, feigning death, passive avoidance, shutdown, or social engagement supported by the release of the hormone oxytocin.

Sympathetic Division
The sympathetic nervous system (SNS) readies us for action and regulates activities that expend stored energy.
Listen to a mini-lecture on the Sympathetic Nervous System Function © BioSource Software LLC.

In concert with the endocrine system, the SNS responds to threats to our safety through mobilization, fight-or-flight, and active avoidance. The SNS responds more slowly (> 5 seconds) and for a more extended period than the more rapid (< 1 second) parasympathetic vagus system (Nunan et al., 2010). Porges (2011) theorized that the SNS inhibits the unmyelinated vagus (dorsal vagal complex) to mobilize us for action.
Dr. Gevirtz provides an overview of the sympathetic branch © Association for Applied Psychophysiology and Biofeedback. You can enlarge the video by clicking on the bracket icon at the bottom right of the screen. When finished, click on the ESC key.
SNS cell bodies are found in the gray matter of the thoracic (from T1) and lumbar (to L2) spinal cord segments. The sympathetic division is thoracolumbar. SNS preganglionic neurons, which originate in the CNS, exit the spinal cord via the ventral root. Most of these axons synapse with the sympathetic chain's autonomic ganglia (collection of neurons), paralleling the spinal cord on each side.
Listen to a mini-lecture on the Sympathetic Nervous System Organization © BioSource Software LLC.
Preganglionic neurons branch extensively and can communicate with sympathetic ganglia in complex ways:
1. Divergence – one preganglionic neuron synapses with multiple ganglia.
2. Convergence – many preganglionic neurons from different spinal levels synapse with one ganglion cell.
Both divergence and convergence produce mass activation, allowing integrated sympathetic action (e.g., increased blood pressure, heart rate, and respiration rate) during emergencies but not at rest. The sympathetic branch does not exhibit this degree of integration during resting conditions (Lehrer & Gevirtz, 2021).
SNS preganglionic axons also directly innervate the adrenal medulla (the central portion of the adrenal gland). When stimulated, the adrenal medulla releases epinephrine and norepinephrine, reinforcing sympathetic activation of visceral organs. The release of epinephrine and norepinephrine increases muscle blood flow and converts stored nutrients into glucose to power skeletal muscle contraction. Postganglionic neurons exit the sympathetic chain and project axons to target organs like the heart, lungs, and sweat glands.
Adrenergic receptors produce changes through G-proteins. The following table is adapted from Fox and Rompolski (2022).
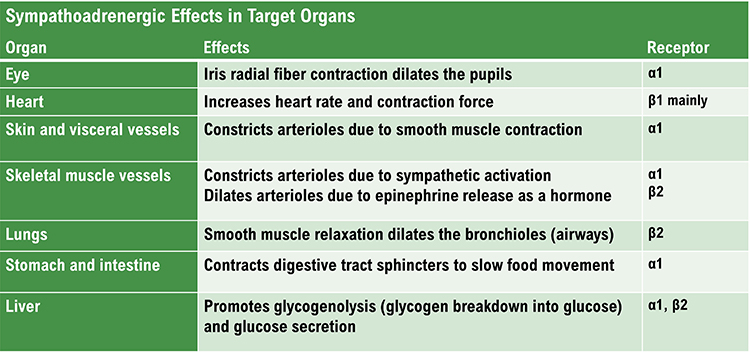
Organs With Sympathetic Innervation Only
The adrenal medulla, arrector pili muscles of the skin, cutaneous sweat glands, and most blood vessels receive sympathetic innervation exclusively (Fox & Rompolski, 2022).Preganglionic and Postganglionic Neurotransmitters Are Different
SNS preganglionic and postganglionic axons release different neurotransmitters. SNS preganglionic axons secrete acetylcholine, while the postganglionic axons secrete norepinephrine. Sweat glands and skeletal muscle blood vessels are the exceptions to this rule. The postganglionic axons that innervate them release acetylcholine (Fox & Rompolski, 2022). The autonomic nervous system diagrams below © 2003 Josephine Wilson.
The Sympathetic Branch and Heart Rate Variability
Heart rate variability (HRV) consists of the beat-to-beat changes in HR, including changes in the time intervals between consecutive heartbeats. The SNS does not appear to contribute significantly to the low-frequency (LF; 0.04-0.15 Hz) component of HRV under resting conditions, as was previously believed.Stephen Porges has emphasized that most stressors do not require the sympathetic branch's intensive energy expenditure during fight-or-flight.
When we perceive daily stressors as threatening, this causes vagal withdrawal instead of sympathetic activation. In vagal withdrawal, we disengage parasympathetic control of our viscera (e.g., large internal organs) and reduce HRV.
We shift from a more calm, socially engaged state. We are now ready for mobilization, either fight or flight (mediated by the sympathetic nervous system) or immobilization.
Vagal withdrawal increases power in the very-low-frequency (VLF) band (≤ 0.04 Hz) and lowers it in the high-frequency (HF) band (0.15 – 0.40 Hz).
Artist: Dani S@unclebelang. This WEBTOON is part of our Real Genius series.

Dr. Gevirtz explains vagal withdrawal © Association for Applied Psychophysiology and Biofeedback.
Parasympathetic Division
The parasympathetic division regulates activities that increase the body’s energy reserves, including salivation, gastric (stomach) and intestinal motility, gastric juice secretion, and increased blood flow to the gastrointestinal system (rest and digest). When we discuss Porges' polyvagal theory, we will learn that this system is also involved in self-regulation, social engagement, and passive responses to threats.
Listen to a mini-lecture on the Parasympathetic Nervous System Function © BioSource Software LLC.
Heart rate variability biofeedback uses slow-paced breathing and rhythmic skeletal muscle contraction to restore healthy parasympathetic activity.
PNS cell bodies are found in the nuclei of four cranial nerves (especially the vagus) and the spinal cord's sacral region (S2-S4). The parasympathetic division is craniosacral.Listen to a mini-lecture on the Parasympathetic Nervous System Organization © BioSource Software LLC.
Unlike the sympathetic division, parasympathetic ganglia are located near their target organs. This arrangement means that preganglionic axons are relatively long, postganglionic axons are relatively short, and PNS changes can be selective.
Preganglionic neurons travel with the oculomotor, facial, glossopharyngeal, and vagus cranial nerves. Preganglionic neurons that exit the vagus (X) nerve at the medulla synapse with terminal ganglia within the heart, lungs, esophagus, stomach, pancreas, liver, and intestines. Both PNS preganglionic and postganglionic axons release acetylcholine (Fox & Rompolski, 2022).
Dr. Gevirtz provides an overview of the parasympathetic branch © Association for Applied Psychophysiology and Biofeedback.
Dampening Inflammation
The vagus nerve's sensory branch detects inflammation/infection via tissue necrosis factor (TNF) and interleukin-1 (IL-1).The motor branch of the vagus signals descending neurons to release norepinephrine, which prompts spleen immune cells to release acetylcholine to macrophages to dampen inflammation (Schwartz, 2015). Resonance frequency breathing may influence the vagal cholinergic cytokine control system (Gevirtz, 2013; Tracey, 2007).
Read the Science News article Viva vagus: Wandering nerve could lead to range of therapies.
Chronic inflammation is implicated in various disorders, including Alzheimer's, cancer, cardiovascular diseases, depression, and diabetes (Dhar, Lambert, & Barton, 2016; Poole, Dickens, & Steptoe, 2011). Graphic © arka38/Shutterstock.com.
The Relationship Between the Sympathetic and Parasympathetic Branches
Berntson, Cacioppo, and Quigley (1993) challenge the concept of a continuum ranging from SNS to PNS dominance. They argue that the two autonomic branches do not only act antagonistically (reciprocally). They also exert complementary, cooperative, and independent actions.
Antagonistic Actions
The SNS and PNS branches compete for control of target organs such as the heart. For example, the PNS can slow the heart by 20 to 30 beats per minute or briefly stop it (Tortora & Derrickson, 2021). Since these divisions generally produce contradictory actions, like speeding and slowing the heart, their effect on an organ depends on their current balance of activity. This competitive relationship is called accentuated antagonism (Olshansky et al., 2011). This graphic © Macrovector/Shutterstock.com summarizes the main competing responses.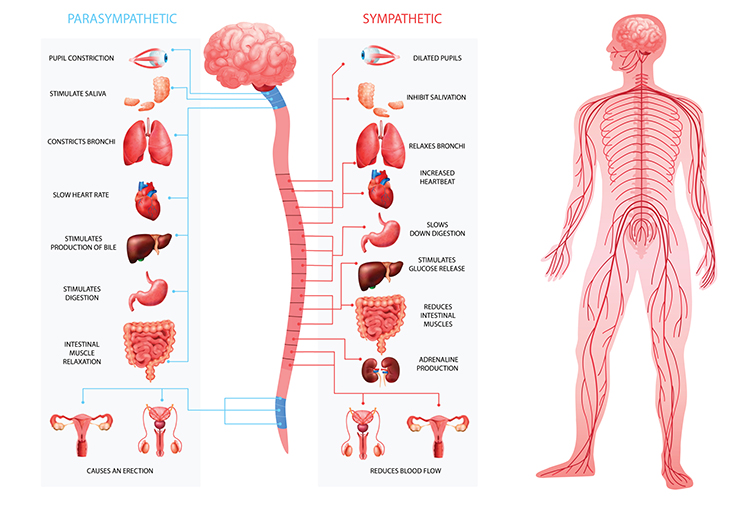
This adrenergic and cholinergic effects table was adapted from Fox and Rompolski (2022). Adrenergic receptors are alpha (α) or beta (β), and cholinergic receptors are muscarinic (M).
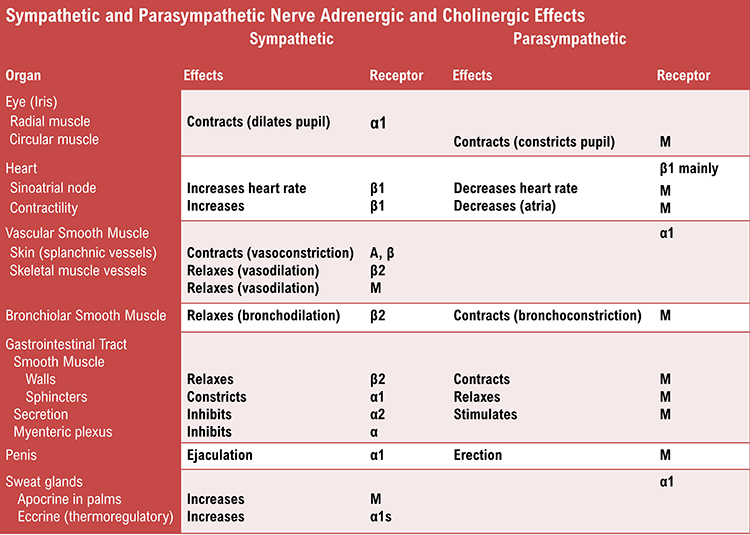
Dr. Gevirtz discusses accentuated antagonism © Association for Applied Psychophysiology and Biofeedback.
Complementary Actions
SNS and PNS actions are complementary when they produce similar changes in the target organ. Saliva production serves as an example. PNS activation produces watery saliva, and SNS activation constricts salivary gland blood vessels, producing thick saliva.Cooperative Actions
SNS and PNS actions are cooperative when their different effects result in a single action. Sexual function provides an example. PNS activation produces erection and vaginal secretions, while SNS activation produces ejaculation and orgasm.Exclusive Sympathetic Control
The SNS exclusively innervates several organs. They are controlled by increasing or decreasing the firing of SNS postganglionic fibers: adrenal medulla, arrector pili muscle, sweat glands, and most blood vessels.The Relationship Between the SNS and PNS is Dynamic
There is a dynamic relationship between sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) influences in a healthy heart. The synergistic relationship between these autonomic branches is complex: sometimes reciprocal, additive, or subtractive (Gevirtz, Schwartz, & Lehrer, 2016).PNS control predominates at rest, resulting in an average heart rate of 75 beats per minute (bpm) that is significantly slower than the SA node's intrinsic rate, which decreases with age, from an average of 107 bpm at 20 years to 90 bpm at 50 years (Opthof, 2000).
Parasympathetic nerves exert their effects more rapidly (< 1 second) than sympathetic nerves (> 5 seconds) (Ecksberg & Eckberg, 1982; Nunan et al., 2010; Shaffer, McCraty, & Zerr, 2014; Tortora & Derrickson, 2017).
While the SNS can suppress PNS activity, it can also increase PNS reactivity (Gellhorn, 1957). Parasympathetic rebound may occur following high stress levels, resulting in increased nighttime gastric activity (Nada et al., 2001) and asthma symptoms (Ballard, 1999).
The relationship between the PNS and SNS branches is complex (both linear and nonlinear) and should not be described as a “zero-sum” system (Ginsberg, 2017). Increased PNS activity may be associated with decreased, increased, or no change in SNS activity.
For example, immediately following aerobic exercise, heart rate recovery involves PNS reactivation while SNS activity remains elevated (Billman, 2017). Likewise, teaching clients to breathe slowly when they experience high levels of SNS activity can engage both branches and increase respiratory sinus arrhythmia (RSA) (Ginsberg, 2017).
This is analogous to a Formula 1 ® driver speeding through a turn while gently applying the left foot to the brake, a maneuver called “left-foot braking” (Ginsberg, 2017). Graphic © Digital Storm/Shutterstock.com.

The complex relationship between SNS and PNS nerve activity means that the LF and HF power ratio may not index autonomic balance (Billman, 2013). The graphic below © Frontiers in Physiology shows how varying levels of SNS and PNS nerve activity can co-determine the LF/HF ratio.
We must reject the simplistic view that increased sympathetic activity is unhealthy. This is false when you swim laps in the pool or are startled by an unexpected sound. Adaptive SNS activation in response to an increased physical workload or sudden threat is desirable.
A depressed SNS response could signal physical depletion and compromised ability to cope. In fact, 24-hour HRV recordings of patients diagnosed with medical and psychological disorders show low SNS and normal PNS activity.
Reduced very-low-frequency (VLF) power is strongly associated with future health crises like sudden cardiac death (Arai et al., 2009; McCraty, 2013).
The degree and duration of SNS activation should be appropriate to the current challenge, and recovery should be rapid.
Porges' Polyvagal Theory
The vagus, the 10th cranial nerve, inhibits the heart and increases bronchial tone in the lungs. The vagus, the 10th cranial nerve, inhibits the heart and increases bronchial tone in the lungs. Graphic © VectorMine/Shutterstock.com.
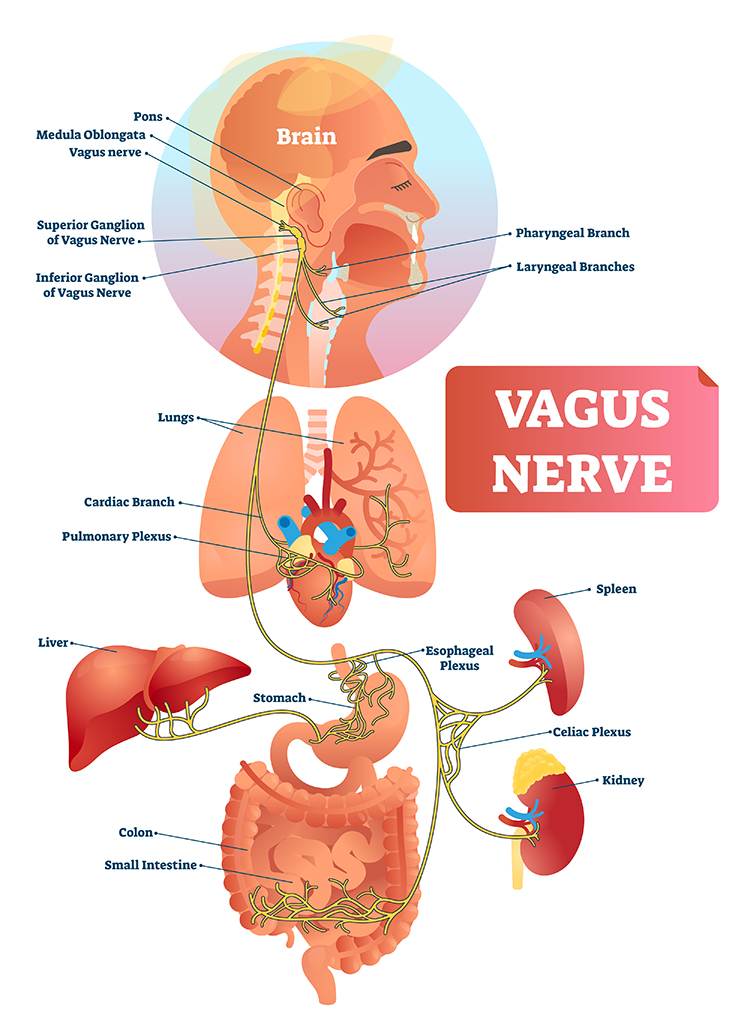
The vagus contains specialized subsystems that control competing adaptive responses. Graphic © Alila Medical Media/Shutterstock.com.
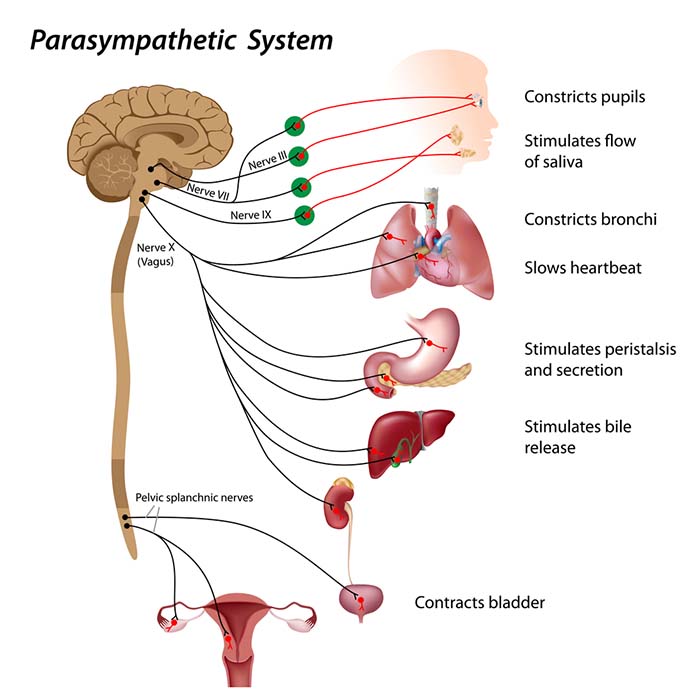
According to Porges' (2011) polyvagal theory, the autonomic nervous system must be considered a “system,” with the vagal nerve containing specialized subsystems that regulate competing adaptive responses.

Caption: Stephen Porges
His theory proposes competing roles for the unmyelinated fibers in the vagus, which originate in the dorsal motor complex, and newer myelinated nerves, which originate in the nucleus ambiguus. Although the polyvagal theory is controversial (Grossman & Taylor, 2007), it highlights diverse PNS adaptive functions.
Listen to a mini-lecture on the Polyvagal Perspective © BioSource Software LLC.
Click on the Read More button to learn about myelinated vagus, sympathetic branch, and unmyelinated vagus roles in polyvagal theory.
The Myelinated Vagus
Porges theorizes that the evolution of the autonomic nervous system was central to developing emotional experience and affective processes involved in social behavior. We are not limited to fight, flight, or freezing behavioral responses as human beings. When we encounter stressors, we can self-regulate and initiate pro-social behaviors (e.g., the tend-and-befriend response). Graphic © greenland/Shutterstock.com.

Porges calls this the social engagement system, and the theory suggests that this system depends upon the healthy functioning of the myelinated vagus, a vagal brake. From Porges' perspective, we only activate the myelinated vagus when our nervous system perceives we are safe. Social engagement is a mutual process and may be integral to playing with others. Graphic © vvvita/Shutterstock.com.

Social engagement often involves eye contact. Graphic © XiXinXing/Shutterstock.com.

Social engagement also includes voluntary close physical proximity. Graphic © Lucky Business/ Shutterstock.com.

The myelinated vagus enables us to self-regulate, calm ourselves, and inhibit sympathetic outflow to the heart. Graphic © Boirkina Marina/Shutterstock.com.

The myelinated vagus allows us to engage prefrontal cortex executive functions (e.g., attention and mindfulness). Graphic © wavebreakmedia/Shutterstock.com.

Professionals increasingly provide HRV biofeedback training before starting neurofeedback to activate the prefrontal cortex.

Daily stressors inhibit the myelinated vagus, producing vagal withdrawal, which interferes with attention and social engagement.

In contrast, positive events may produce happiness that facilitates the myelinated vagus while increasing sympathetic activation. Graphic © kareinoppe/Shutterstock.com.

The Sympathetic Nervous System
In concert with the endocrine system, the SNS responds to threats to our safety through mobilization, including fight-or-flight and active avoidance. The SNS responds more slowly and for a more extended period (i.e., more than a few seconds) than the parasympathetic vagus system. The SNS responds more slowly and for a more extended period (i.e., more than a few seconds) than the parasympathetic vagus system.The SNS inhibits the unmyelinated vagus to mobilize us for action instead of fainting or freezing. In contrast, the parasympathetic myelinated vagus rapidly adjusts cardiac output, promotes social engagement (the tend-and-befriend response), and enables self-regulation. These three changes promote biofeedback training.
According to this theory, quality communication and pro-social behaviors can only be effectively engaged when defensive circuits are inhibited (Shaffer, McCraty, & Zerr, 2014). Graphic © pixelheadphoto/Shutterstock.com.

The Unmyelinated Vagus
Porges hypothesizes that the unmyelinated fibers regulate the freeze response and respond to threats through immobilization, feigning death, passive avoidance, and shutdown. Check out the YouTube video The Polyvagal Theory and PTSD with Stephen Porges, PhD. Graphic © DenisNata/Shutterstock.com.
Summary
When our nervous system perceives safety, we activate the myelinated vagus to conserve and rebuild energy stores (rest and digest), socially bond with others (tend and befriend), and engage in executive functions like self-regulation and planning.When our nervous system perceives danger, we activate the sympathetic and the endocrine systems' SAM pathway and HPA axis, inhibiting the unmyelinated vagus for fight or flight or active avoidance.
When our nervous system perceives that our life is threatened and that fight, flight, or active avoidance will not succeed, like a mouse in the jaws of a cat, we activate the unmyelinated vagus. This results in passive avoidance through dissociation, fainting, feigning death, immobilization, and shutdown. Dani S@unclebelang on fiverr.com created the Polyvagal Theory graphic from the author's design.
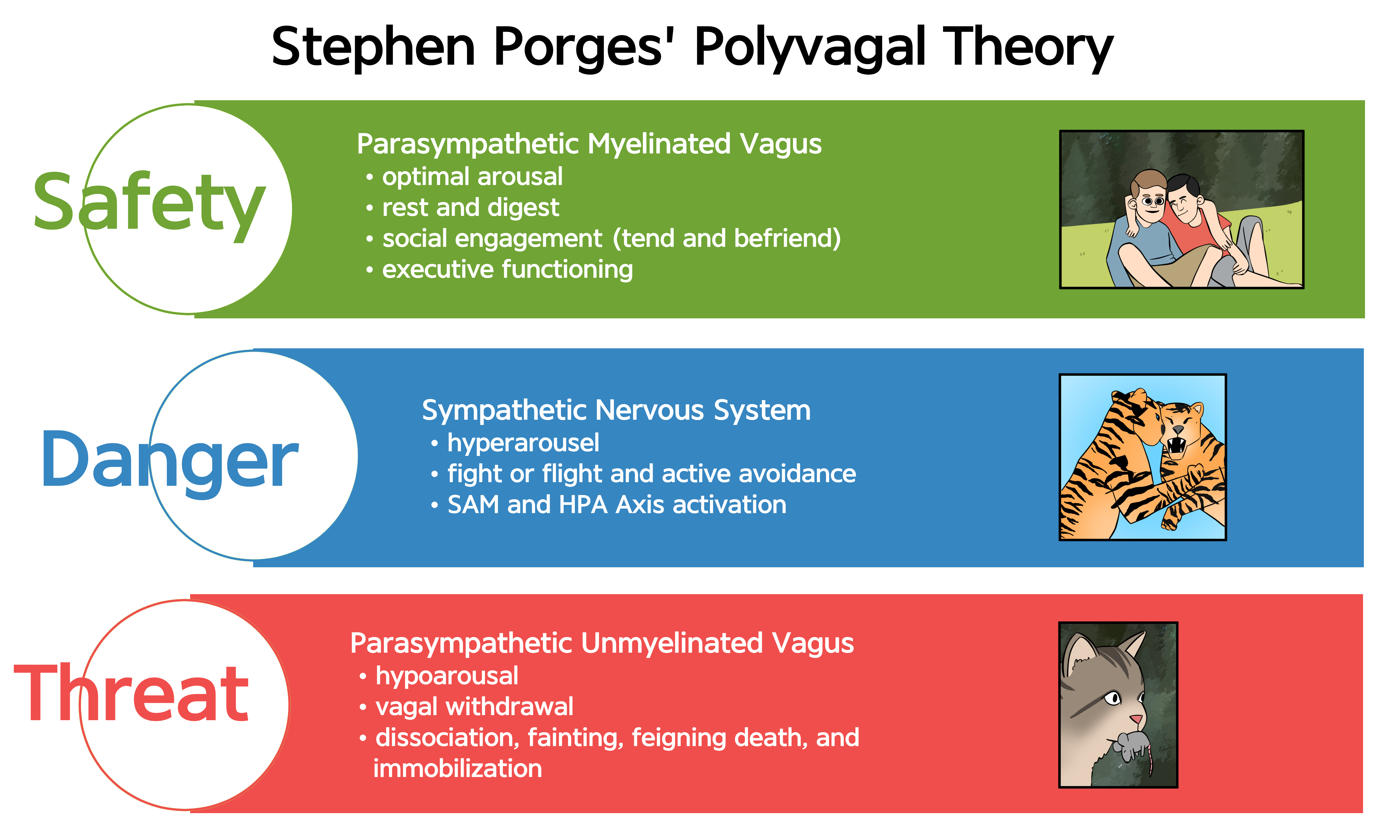
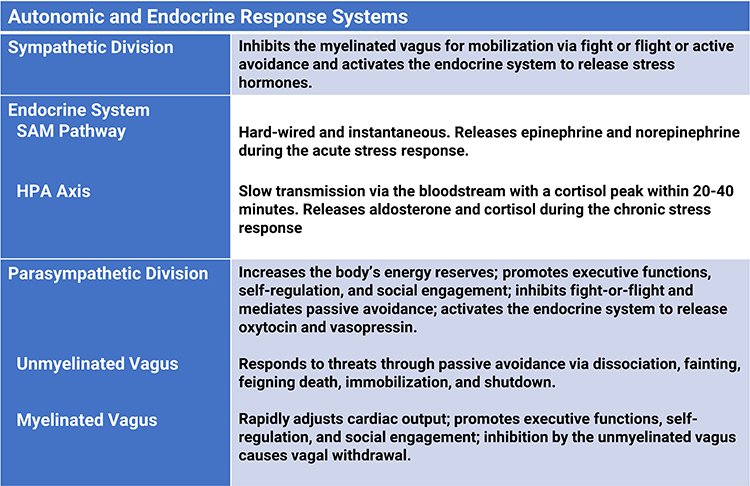
Enteric Division
The enteric division is the largest part of the autonomic nervous system (Rao & Gershon, 2016). The ENS controls peristalsis and enzyme secretion in the GI tract to maintain the balance of fluid and nutrients (Breedlove & Watson, 2023).
Listen to a mini-lecture on the Enteric Nervous System © BioSource Software LLC.
The enteric division contains approximately 100 million neurons (like the spinal cord; Boron & Boulpaep, 2017), which release a comparable range of neurotransmitters to the CNS (Gershon, 1999). The ENS contains interneurons, sensory and autonomic motor neurons, and neuroglial cells. A subset of intestinal sensory neurons travels in the vagus nerve to influence ANS activity (Fox & Rompolski, 2022). Graphic of abdominal anatomy © Sebastian Kaulitzki/ Shutterstock.com.
The gut contains more than 90% of the body’s serotonin (Khazan, 2013) and about 50% of its dopamine. Check out the Khan Academy YouTube video Control of the GI Tract.
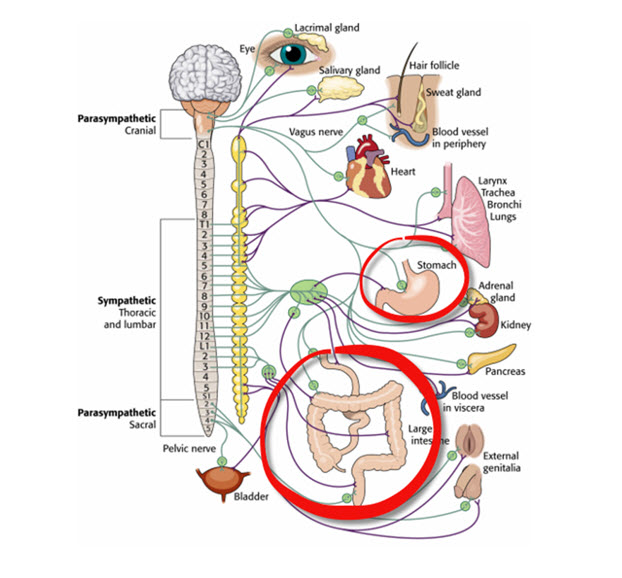
Enteric neurons are concentrated in the myenteric and submucosal ganglia. While the ENS locally regulates intestinal functions, sympathetic and parasympathetic efferents can override its activity during intense emotions like anger and fear (Fox & Rompolski, 2022). During physical activity and stress, sympathetic firing inhibits intestinal motility. During rest, parasympathetic firing promotes it by exciting enteric preganglionic neurons (Khazan, 2013). Graphic © Lightspring/Shutterstock.com.
Glossary
accentuated antagonism: the parasympathetic nervous system's ability to directly oppose sympathetic action, such as slowing the heart by 20 or 30 beats.
adrenal medulla: the inner region of the adrenal gland that produces the hormones epinephrine and norepinephrine.
autonomic nervous system: the subdivision of the peripheral nervous system that includes enteric, parasympathetic, and sympathetic divisions.
central nervous system: the division of the nervous system that includes the brain, spinal cord, and retina.
ganglia: a collection of neuronal cell bodies outside of the CNS.
hypothalamus: the forebrain structure located below the thalamus that dynamically maintains homeostasis through its control of the autonomic nervous system, endocrine system, survival behaviors, and interconnections with the immune system.
homeostat: a device that maintains homeostasis. For example, the hypothalamus.
mass activation: the simultaneous stimulation of adjacent ganglia (cell bodies) in the sympathetic chain allows the sympathetic nervous system to produce many coordinated changes simultaneously. For example, increased heart rate, respiration rate, and sweat gland activity.
medulla: the brainstem structure that regulates blood pressure, defecation, heart rate, respiration, and vomiting. The medulla influences the autonomic nervous system and distributes signals between the brain and spinal cord.
myelinated vagus: the phylogenetically newer ventral vagal complex that rapidly adjusts cardiac output and promotes social engagement.
parasympathetic division: the autonomic nervous system subdivision that regulates activities that increase the body’s energy reserves, including salivation, gastric (stomach) and intestinal motility, gastric juice secretion, and increased blood flow to the gastrointestinal system.
parasympathetic withdrawal: daily stressors can inhibit the myelinated vagus suppressing parasympathetic activity.
peripheral nervous system: nervous system subdivision that includes autonomic and somatic branches.
polyvagal theory: theory that the unmyelinated vagus (dorsal vagus complex) and newer myelinated vagus (ventral vagal complex) mediate competing adaptive responses.
somatic nervous system: peripheral nervous system subdivision that receives external sensory and somatosensory information and controls skeletal muscle contraction.
sympathetic division: autonomic nervous system branch that regulates activities that expend stored energy, such as when we are excited.
sympathetic preganglionic neurons: the neurons that originate in the CNS, leave the spinal cord via the ventral root, and mainly synapse with sympathetic chain ganglia.
unmyelinated vagus:
the phylogenetically older dorsal vagus complex that responds to threats through immobilization, feigning death, passive avoidance, and shutdown.
vagal withdrawal: the inhibition of the myelinated vagus, often by daily stressors.
vagus nerve: the tenth cranial nerve, which supplies parasympathetic nervous system innervation for the heart.
very low frequency (VLF): the ECG frequency range of 0.003-.04 Hz that may represent
temperature regulation, plasma renin fluctuations, endothelial, and physical activity influences, and possible intrinsic cardiac nervous system, and PNS contributions.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

References
Arai, Y. C., et al. (2009). Increased heart rate variability correlation between mother and child immediately pre-operation. Acta Anaesthesiol Scand, 53(5), 607-610. https://doi.org/10.1111/j.1399-6576.2009.01912.x
Ballard, R. D. (1999). Sleep, respiratory physiology, and nocturnal asthma. Chronobiology International, 16(5), 565-580. https://doi.org/10.3109/07420529908998729
Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin, 114, 296-322. https://doi.org/10.1037/0033-2909.114.2.296
Billman, G. E. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. https://doi.org/10.3389/fphys.2013.00026
Billman, G. E. (2017). Personal communication to J. P. Ginsberg regarding the LF/HF ratio.
Boron, W. F., & Boulpaep, E. L. (2017). Medical physiology (3rd ed.). Saunders.
Breedlove, S., M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Eckberg, D. L., & Eckberg, M. J. (1982). Human sinus node responses to repetitive, ramped carotid baroreceptor stimuli. American Journal of Physiology, 242 (Heart and Circulatory Physiology, 11), H638–H644. https://doi.org/10.1152/ajpheart.1982.242.4.h638
Fox, S. I., & Rompolski, K. (2022). Human physiology (16th ed.). McGraw-Hill.
Gershon, M. D. (1999). The enteric nervous system: A second brain. Hosp Pract, 34(7), 31–32, 35–38, 41–2 passim. https://doi.org/10.3810/hp.1999.07.153
Gevirtz, R. (2013). The nerve of that disease: The vagus nerve and cardiac rehabilitation. Biofeedback, 41(1), 32-38. http://dx.doi.org/10.5298/1081-5937-41.1.01
Gevirtz, R. N., Lehrer, P. M., & Schwartz, M. S. (2016). Cardiorespiratory biofeedback. In M. S. Schwartz & F. Andrasik (Eds.). Biofeedback: A practitioner’s guide (4th ed.). The Guilford Press.
Ginsberg, J. P. (2017). Personal communication regarding autonomic balance.
Grossman, P., & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 74, 263–285. https://doi.org/10.1016/j.biopsycho.2005.11.014
Karemaker, J. M. (2020). Interpretation of heart rate variability: The art of looking through a keyhole. Front. Neurosci. https://doi.org/10.3389/fnins.2020.609570
Khazan, I. Z. (2013). The clinical handbook of biofeedback: A step-by-step guide for training and practice with mindfulness. John Wiley & Sons, Ltd.
Lehrer, P. M., & Gevirtz, R. (2021). BCIA HRV Biofeedback didactic workshop. Association for Applied Psychophysiology and Biofeedback.
McCraty, R. (2013). Personal communication concerning autonomic balance.
Nada, T., Nomura, M., Iga, A., Kawaguchi, R., Ochi, Y., Saito, K., Nakaya, Y., & Ito, S. (2001). Autonomic nervous function in patients with peptic ulcer studied by spectral analysis of heart rate variability. Journal of Medicine, 32(5-6), 333-347. PMID: 11958279
Nunan, D., Sandercock, G. R. H., & Brodie, D. A. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology, 33(11), 1407-1417. https://doi.org/10.1111/j.1540-8159.2010.02841.x
Olshansky, B., Sabbah, H. N., Hauptman, P. J., & Colucci, W. S. (2008). Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation, 118, 863-871. https://doi.org/10.1161/circulationaha.107.760405
Porges, S. W. (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. W. W. W. Norton & Company.
Schwartz, S. (2015). Viva vagus: Wandering nerve could lead to range of therapies. Science News, 188(11), 18.
Tortora, G. J., & Derrickson, B. H. (2021). Principles of anatomy and physiology (16th ed.). John Wiley & Sons, Inc.
Tracey, K. J. (2007). Physiology and immunology of the cholinergic anti-inflammatory pathway. Journal of Clinical Investigation, 117(2), 289-296. https://doi.org/10.1172/jci30555
D. MAJOR NETWORKS
Neurofeedback has evolved from training activity at single discrete scalp sites corresponding to discrete Brodmann areas to modifying network communication. Quantitative EEG (qEEG) normative databases can reveal the key parts of a network that need training and the required direction.
We will review A Neurofeedback Paradigm Shift, Commonsense About Functional Brain Areas, Key Anatomical Terms, Brodmann Areas, Brain Organization and Dynamics, Network Overview, Attentional Processes, Two Cortical Networks Regulate Attention, and The Pulvinar Mediates Attentional Shifts.
Please click on the podcast icon below to hear a full-length lecture for Section D.

A Neurofeedback Paradigm Shift
With neurofeedback, we want to know where to place active electrodes and what brain activity to train. It's normal to think that the sensor should go over the part of the brain corresponding to a Brodmann area that needs to be up-trained or down-trained. That may work for some conditions that respond to single-channel training. But, one site is connected to another site, and so on, in networks.
Networks, which can be functional or structural, mediate connectivity. In functional networks, there is correlated activity between regions over time. Structural networks consist of axonal projections and pathways.
Although functional and structural networks can overlap, functional connectivity does not automatically imply an underlying anatomical connection. Fornito et al. (2016) explained the importance of time scale in their convergence: ". . . as functional connectivity is averaged over longer time periods, it may converge onto structural connectivity, although it is important to remember that structural and functional connectivity are different measures and may thus yield connectomes with different values of some topological parameters (Zalesky et al., 2012b)" (p. 25).
Interventions as different as immobilizing a limb and neurofeedback can change functional connectivity.
Newbold and Dosenbach (2021) put a cast on participants' (non-broken) dominant hand for a few weeks. They performed multiple fMRI scans to see how this impacted the functional connectivity of their motor cortices. After just a day or two, functional connectivity between the left and right motor cortex was nearly non-existent, a faster timescale than many predicted. Recovery was also quite rapid in 2 of the 3 people.
The premise of connectivity training is that neurofeedback (EEG and functional MRI) can increase or decrease functional connectivity to improve performance.
Commonsense About Functional Brain Areas
Peterson and Fiez (1993) observed: "A functional area of the brain is not a task area; there is no 'tennis forehand area' to be discovered. Likewise, no area of the brain is devoted to a very complex function; 'attention' or 'language' is not localized in a particular Brodmann area or lobe. Any task or 'function' utilizes a complex and distributed set of brain areas.
The areas involved in performing a particular task are distributed in different locations in the brain, but the processing involved in task performance is not diffusely distributed among them. Each area makes a specific contribution to the performance of the task, and the contribution is determined by where the area resides within its richly connected parallel, distributed hierarchy."
Key Anatomical Terms
Long brain region names can be intimidating. These six descriptions can serve as your decoder ring. Anterior or rostral refers to toward the head end, whereas posterior or caudal means toward the tail. Dorsal means toward the top of the brain, while ventral means toward the bottom. A gyrus is a ridge of convoluted brain tissue, whereas a sulcus is a furrow (Breedlove & Watson, 2023).
Networks have functional and anatomical names. For example, the dorsal attention network (DAN) corresponds to the dorsal frontoparietal network (D-FPN).
Brodmann Areas
The original Brodmann areas consisted of 47 numbered cytoarchitectural zones of the cerebral cortex based on Nissl staining. They require subdividing certain areas and vary in size and shape across individuals. Brodmann graphic © sciencepics/Shutterstock.com.
Gordon et al. (2016) compared the original Brodmann areas and several other brain atlases using fMRI. They concluded: "The Brodmann parcellation (Brodmann 1909) [. . .] does successfully represent structure in the data, but is too underparcellated to represent true cortical areas. This perspective agrees with modern attempts to anatomically parcellate human cortex, which frequently observe more fine-grained architectonic divisions than those reported by Brodmann (e.g., Morris et al. 2000, retrosplenial cortex; Öngür et al. 2003, orbitofrontal cortex; Morosan et al. 2005, superior temporal gyrus; Caspers et al. 2006, inferior parietal cortex; Scheperjans et al. 2008, superior parietal cortex; Kujovic et al. 2013, extrastriate visual cortex)."
Human Brodmann Areas
Areas 3, 1, 2: Primary somatosensory cortexArea 4: Primary motor cortex
Area 5: Somatosensory association cortex
Area 6: supplementary motor cortex and premotor cortex
Area 7: Somatosensory association cortex
Area 8: Frontal eye fields
Area 9 and 46: Dorsolateral prefrontal cortex (DLPFC)
Area 10: Anterior prefrontal cortex
Areas 11 and 12: Orbitofrontal cortex
Areas 13 and 16: Insular cortex
Area 17: Primary visual cortex (V1)
Area 18: Secondary visual cortex (V2)
Area 19: Associative visual cortex (V3, V4, V5)
Area 20: Inferior temporal gyrus
Area 21: Middle temporal gyrus
Area 22: Superior temporal gyrus, including Wernicke's area
Area 23: Ventral posterior cingulate cortex
Area 24: Ventral anterior cingulate cortex
Area 25: Subgenual region of the ventromedial prefrontal cortex
Area 26: Ectosplenial region of the retrosplenial cerebral cortex
Area 27: Pyriform cortex
Area 28: Ventral entorhinal cortex
Area 29: Retrosplenial cingulate cortex
Area 30: Cingulate cortex
Area 31: Dorsal posterior cingulate cortex
Area 32: Dorsal anterior cingulate cortex
Area 33: Anterior cingulate cortex
Area 34: Dorsal entorhinal cortex
Areas 35 and 36: Perirhinal cortex
Area 37: Fusiform gyrus
Area 38: Temporopolar area
Area 39: Angular gyrus
Area 40: Supramarginal gyrus
Areas 41 and 42: Auditory cortex
Area 43: Primary gustatory cortex
Area 44: Pars opercularis (inferior temporal gyrus and part of Broca's area)
Area 45: Pars triangularis (inferior temporal gyrus and part of Broca's area)
Area 46: Dorsolateral prefrontal cortex (DLPFC)
Area 47: Pars orbitalis (part of inferior frontal gyrus)
Area 48: Retrosubicular area (small area of the medial temporal lobe)
Area 52: Parainsular area (junction of the temporal lobe and insula)
Source: Brodmann Area article in Wikipedia.
The revised Brodmann maps shown above reveal 180 regions, 100 of which were not previously identified (Glasser et al., 2016). The revised maps were contributed by Mark Dow, Research Assistant at the Brain Development Lab at the University of Oregon, to Wikimedia Commons.
Cognitive neuroscience tends to use modern brain atlases of defined regions (e.g., Yeo et al., 2011) and exact coordinates like Montreal Neurological Institute (MNI) space and Talairach space (Chau & McIntosh, 2005). Researchers sometimes "convert" these coordinates to corresponding Brodmann areas since they are well-known.
Brodmann areas participate in networks. They play an important role in neurofeedback because they help us target functional and structural networks instead of discrete, disconnected scalp sites. Neuroscience reveals the connections between Brodmann areas and how different networks can become active or quiet together during diverse conditions.
For example, attention to an object's location activates sites in four main areas that comprise the dorsal attention network (dorsal frontoparietal network). The five areas comprising the ventral attention network (ventral frontoparietal network) process automatically unanticipated environmental events. Flexible attention control involves the dynamic interaction of these top-down and bottom-up systems (Vossel et al., 2014).
Psychological disorders can be associated with shifts from normal activity in particular brain areas and their connections.
In the aftermath of traumatic brain injury (TBI), neuronal connections across the entire brain change. For example, surviving long axonal projections no longer target inhibitory neurons (Frankowski et al., 2022).
.jpg)
Current neurofeedback protocols allow us to train networks involved in cognitive functions like attention or psychological disorders like depression using multiple electrodes simultaneously. Connectivity training enables us to increase or decrease communication between brain locations to treat symptoms and improve performance. Activations and deactivations are both important.
Quantitative EEG (qEEG) normative databases can reveal the key parts of a network that need training and the required direction. Graphic courtesy of NeuroNavigator’s swLORETA Effective Connectivity Normative Database.
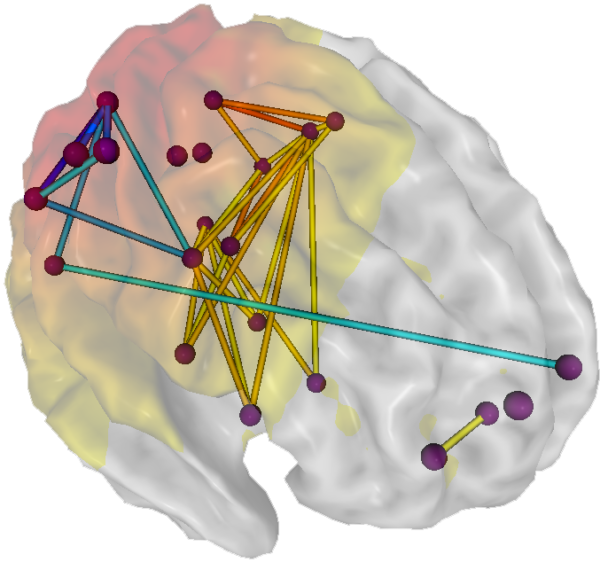
The more than 100-year-old Brodmann areas lack some of the specificity needed for in vivo and functional studies.
Brain Organization and Dynamics
The brain is organized into interactive functional distributed networks with spatial, temporal, and content-based relationships. These networks interact through feedback loops and transiently organized aggregates of neurons, all mediated by rhythmic, oscillatory electrical discharges that ultimately produce the EEG. This process is further controlled/informed by selective attention to specific interest categories. The graphic below shows the default, salience, and central executive networks.
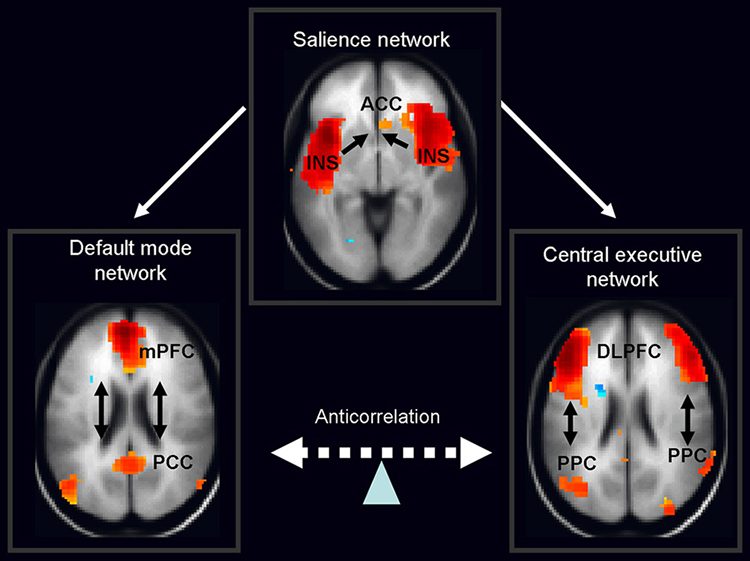
Nekovarova, Fajnerova, Horacek, Spaniel, CC BY 3.0, via Wikimedia Commons
Each type of local cognitive, sensory processing, or emotional network produces oscillatory activity and contains internal stabilizing characteristics. These local networks exist within a global dynamic network system that links and provides an interactive capacity to the smaller networks, also operating within an oscillatory framework.
A densely-connected lateral prefrontal and posterior parietal cortical network orchestrates responses to novel cognitive tasks using flexible hubs. The frontoparietal network assigns tasks to the most appropriate brain regions and shares information among these regions to master new skills (Cole et al., 2013).
The central nervous system processes incoming content. Separate regions process specialized content (e.g., auditory, kinesthetic, tactile, visual). Content is shared, integrated, compared to previous content, and analyzed. Decisions are made regarding memory and responses. This central nervous system activity occurs within interacting networks linked by electrical/chemical signals. Electrical discharges from network activity are recorded from the scalp surface as the EEG.
Network Overview
The networks most relevant to attention include the oculomotor, motor, affective, central autonomic, social, and executive circuits.
Oculomotor Network
The frontal eye field (FEF), in concert with the dorsolateral prefrontal cortex, posterior parietal cortex, basal ganglia, and thalamus, programs and initiates voluntary eye movements, inhibits eye movements toward distracting stimuli, and allows us to return our focus to locations we've experienced in the past (Thompson & Thompson, 2016). Simone Vossel, Joy J. Geng, Gereon R. Fink, CC BY 3.0, via Wikimedia Commons.
Motor Network
The supplementary motor area (SMA), in concert with the premotor cortex, primary motor cortex, sensorimotor cortex, and cerebellum, plans, initiates, and inhibits voluntary movements and muscle contractions (Breedlove & Watson, 2023; Thompson & Thompson, 2016). Chouinard PA and Paus T, CC BY 3.0, via Wikimedia Commons.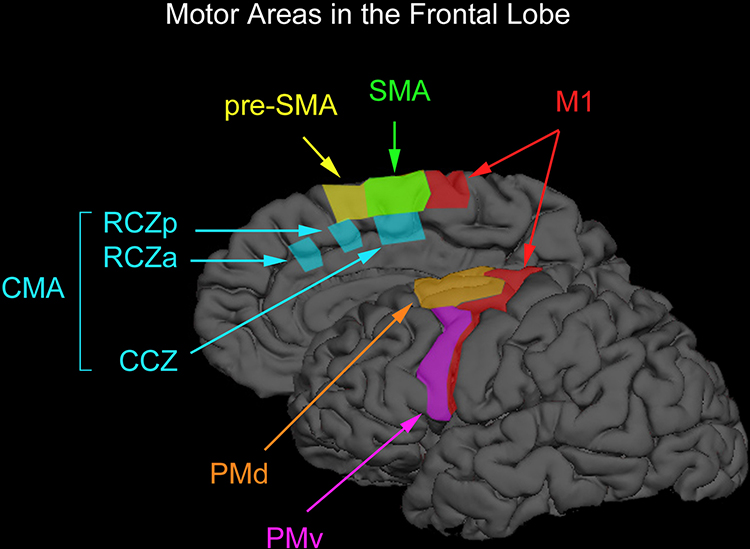
Caption: Motor areas in the frontal lobe. The premotor cortex on the lateral surface of the brain can be divided into the dorsal and ventral premotor areas (PMd and PMv), and the supplementary motor cortex on the medial wall of the brain can be divided into the supplementary motor and pre-supplementary motor areas (SMA and pre-SMA). Premotor cortex below the superior frontal sulcus is typically considered PMv, whereas premotor cortex above this anatomical landmark is typically considered PMd. The vertical anterior-commissural line is often used to denote the boundary between SMA and pre-SMA. One can further divide PMd according to a rostral subdivision located along the superior frontal gyrus and a caudal subdivision located along the precentral gyrus. However, one cannot dissociate these two subdivisions from the TMS easily, and we therefore do not discuss them separately. There also exist two cingulate motor areas (RCZa and RZp) anterior to the vertical anterior-commissural line and one cingulate motor area (CCZ) posterior to the vertical anterior-commissural line.
Somato-Cognitive Action Network (SCAN)
Gordon and colleagues (2023), using precision fMRI from seven participants and fMRI datasets from the Adolescent Brain Cognitive Development Study, Human Connectome Project, and UK Biobank from 50,000 individuals, found three interconnected primary motor cortex (M1) regions that participate in the integrated movement of multiple body parts.The somato-cognitive action network (SCAN) consists of M1, the SMA, Centromedian nucleus (CM) and Ventral Intermediate nucleus (VIM) of the thalamus, posterior putamen, and vermis and the flocculonodular lobe of the cerebellum, which mediate posture and balance. Connectivity analysis revealed that the SCAN communicates with the cingulo-opercular network (CON) or salience network, which mediates cognitive control and maintains task focus over extended periods. SCAN graphic by Johnson et al. (2024).
Caption: Somato-cognitive action network (SCAN). (A) Resting state functional connectivity (RSFC) seeded from the middle inter-effector node in the primary motor cortex (bilaterally) in a representative individual (P1; 356 min resting-state fMRI). In cortex the SCAN includes three inter-effector nodes (superior, middle, inferior) that alternate with effector-specific foot, hand, and mouth primary motor regions, as well as two nodes on the dorsal midline in the SMA (supplementary motor area) and dACC (dorsal anterior cingulate cortex) that are interleaved with the effector-specific regions of the SMA/pre-SMA
M1's two interlacing systems establish a pattern of integration and isolation: regions specific to effectors, such as the foot, hand, and mouth, are responsible for isolating fine motor control, while the SCAN integrates goals, body movement, and physiology.
The apparent relative expansion of SCAN regions in humans could suggest a role in complex actions specific to humans, such as coordinating breathing for speech, and integrating hand, body and eye movement for tool use. A common factor across this wide range of processes is that they must be integrated if an organism is to achieve its goals through movement while avoiding injury and maintaining physiological allostasis. The SCAN provides a substrate for this integration, enabling pre-action anticipatory postural, breathing, cardiovascular and arousal changes (such as shoulder tension, increased heart rate or ‘butterflies in the stomach’). The finding that action and body control are melded in a common circuit could help explain why mind and body states so often interact.
Mesa (2023) placed these findings in context:
The dominant paradigm states that the motor cortex is simplistic. Planning, cognition, and conscious initiation of movements happen elsewhere in the brain; the motor cortex just receives these signals, relaying them directly to muscles.
In concert with the salience network, the SCAN is responsible for complex adaptations (e.g., allostasis). These findings are consistent with primate studies showing that more M1 neurons are responsible for movements independent of the muscles used than for the contraction of specific muscles (Griffin et al., 2015; Kaufman et al., 2014). Together, these findings challenge the 1870 cortical homunculus model, a distorted human figure with body parts' size reflecting the amount of cortical area dedicated to them. Graphic by Mpj29. Mpj29, CC BY-SA 4.0, via Wikimedia Commons.
.gif)
Affective Network
The affective network is essential for processing and regulating emotions, linking emotions with memories, facilitating social interactions, and maintaining emotional well-being. Its proper functioning is critical for adaptive emotional responses and social behavior.The pre- and subgenual areas of the anterior cingulate cortex (ACC) participate in affective circuits triggered when we make mistakes (Arnsten, 2009). The dorsal rostral cingulate zone monitors cognitive activity to predict when errors are likely and greater executive control may be needed (Thompson & Thompson, 2016). The ventromedial prefrontal cortex projects to the amygdala, basal ganglia, hypothalamus, and brainstem arousal and reward pathways. Graphic by Gonzalez-Madruga et al. (2022). The affective network is designated by AN.
Caption: Illustration of six resting-state networks: The default mode (DMN), affective (AN), frontoparietal control (FPCN), dorsal attention (DAN), salience (SN), and the somatomotor/sensory networks (SMN). Arrows reflect findings of within and between network hypo-and hyperconnectivity in individuals with ADHD as identified by Gao et al. (2019).
Central Autonomic Network
The central autonomic network (CAN) comprises forebrain, limbic, and brainstem regions (Bennaroch, 1993). Neuroimaging studies reveal a cortico-limbic network responsible for autonomic control, consisting of the ventromedial prefrontal cortex (VMPFC), cingulate cortex, insula, mediodorsal thalamus, hypothalamus, and amygdala (Beissner et al., 2013; Shoemaker et al., 2015; Schuman et al., 2021; Thayer et al., 2012).The central autonomic network is essential for integrating sensory, motor, and cognitive information to regulate autonomic functions, maintain homeostasis, coordinate stress responses, and link physiological states with emotional and behavioral processes.
Thayer and Lane (2000) proposed a neurovisceral integration model. Their model places the prefrontal cortex at the top of a hierarchical structure, with direct functional links with the insula and cingulate (Thayer et al., 2012). The limbic system extends these connections through the amygdala to downstream subcortical regions, such as the hypothalamus and brainstem nuclei, which are integral for the parasympathetic and sympathetic heart rate modulation at the lowest level of this model. The neurovisceral integration model highlights the crucial role of the prefrontal cortex and its superior control over subcortical structures in heart rate regulation and in linking sympathovagal balance with cognitive and emotional processes (Thayer et al., 2009). Neurovisceral model graphic redrawn from Nikolin et al. (2017).
Caption: Neurovisceral integration model. (A) Simplified depiction of the neurovisceral integration model described by Thayer and Sternberg [1]. (B) Brain regions relevant to the neurovisceral integration model. PC, prefrontal cortex; CC, cingulate cortex; Hyp, hypothalamus; Ins, insula; Amy, amygdala; BS, brainstem.
Magnetic resonance imaging (MRI) and resting-state functional connectivity (RSFC) studies have reinforced the critical role of interplay between the medial prefrontal cortex and limbic regions in controlling heart rate (Kumral et al., 2019; Sakaki et al., 2016). Individuals with slower heart rates exhibited significantly heightened RSFC within a functional network comprising various central autonomic and sensorimotor system regions compared to those with faster heart rates (de la Cruz et al., 2019). Slower heart rates were associated with an elevated RSFC between the prefrontal cortex (VMPFC) and the anterior insula.
Social Network
The orbitofrontal cortex (OFC), along with the basal ganglia and thalamus, orchestrates the highest level of emotional processing in the nervous system. The social network is responsible for socially responsible behavior, empathy, behavioral inhibition, emotional regulation, and sound judgment (Thompson & Thompson, 2016). Graphic courtesy of Han and colleagues (2021).
The blue nodes represent the mentalizing network, including the vmPFC (ventromedial prefrontal cortex), OFC (orbitofrontal cortex), dlPFC (dorsolateral prefrontal cortex), and dmPFC (dorsomedial prefrontal cortex). This network enables us to think about our own and others' mental states (Hoskinson et al., 2019). The orange dots are the mirror network (superior temporal sulcus, STS), active during our performance and observations of others' actions. The mirror network supports observational learning and social cognition (Sadeghi et al., 2022). The green dot is the amygdala, which detects salient stimuli. The yellow dot is the entorhinal cortex. Finally, the red dot is the anterior insular cortex, AIC.
Executive Network
The dorsolateral prefrontal cortex plays a critical role in executive functions, which Kropotov (2009) described as "the coordination and control of motor and cognitive actions to attain specific goals." Executive functions include allocation of attention, cognitive inhibition, behavioral inhibition, working memory, and cognitive flexibility. The executive network focuses and maintains continuous attention (Faraone et al., 2015). Seven-network graphic by Feirrera et al. (2022). The Executive Network is shown in blue.
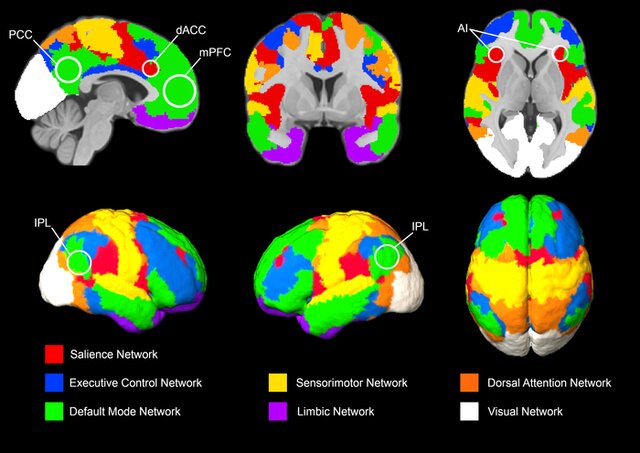
Caption: Brain networks: One atlas with seven networks. Seven brain networks derived from resting‐state fMRI data were adapted from Schaefer et al. (2018).
Attentional Processes
Attention is the selection of sensory information or cognition for enhanced processing. We can overtly or covertly attend to stimuli. In overt attention, our attentional focus and sensory orientation coincide. For example, you parse this sentence as you focus your gaze on it. In covert attention, we shift our attentional focus from our sensory orientation. For example, you attend to a reminder on the corner of your screen while you gaze at this sentence. While the midbrain superior colliculus is mainly implicated in overt attention, it may also regulate covert attention (Breedlove & Watson, 2023).
Cortical Regions That Guide Attention
The dorsal attention network (dorsal frontoparietal system), which comprises the intraparietal sulcus and frontal eye field, is responsible for the top-down direction of attention (Breedlove & Watson, 2023). This system is responsible for endogenous (voluntary) attention, directing the attentional spotlight to support cognitive system priorities.The intraparietal sulcus (IPS) in the parietal lobe provides voluntary top-down attention steering (Corbetta & Shulman, 1998). The frontal eye field (FEF), found in the premotor region of the frontal lobes, directs our gaze toward targets selected by the IPS (Paus et al., 1991). Target selection is guided by cognitive goals (top-down processing) rather than stimulus characteristics (bottom-up processing). Graphic by Aboitiz et al. (2014). The dorsal attention network is shown below in gold.
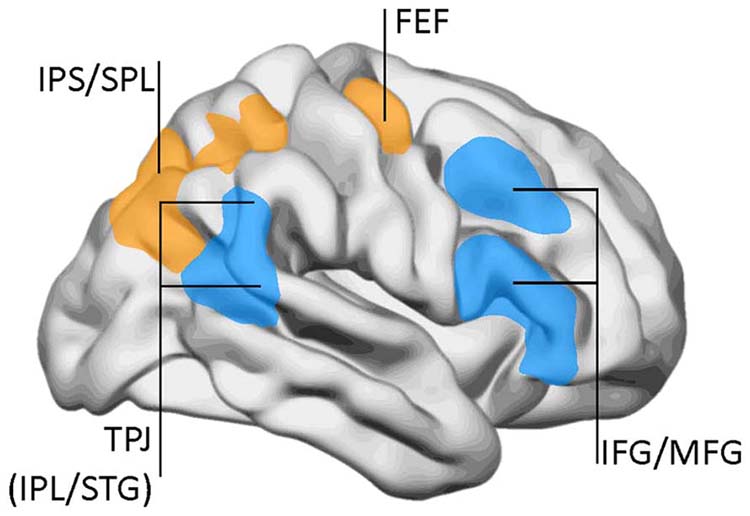
Caption: The dorsal attentional network (DAN) and the ventral attention network (VAN). DAN (yellow/orange): FEF, frontal eye fields; IPS, inferior parietal sulcus; SPL, superior parietal lobe. VAN (blue): IFG, inferior frontal gyrus; IPL, inferior parietal lobe (posterior aspect); MFG, middle frontal gyrus; TPJ, temporo-parietal junction; STG, superior temporal gyrus. Data from Corbetta and Shulman (2002), with permission.
In contrast, the ventral attention network (ventral frontoparietal network), where the superior temporal gyrus and inferior parietal lobe intersect, mediates bottom-up shifts in attention in response to stimulus attributes (Corbetta & Shulman, 2002). It controls involuntary reflexive attention, redirecting attention based on the novelty or importance of incoming stimuli. The TPJ functions like a circuit breaker by overruling immediate attentional priorities and reallocating attentional resources to a new target (Breedlove & Watson, 2023).
Attention system graphic by deBoer et al. (2020). The dorsal attention network (blue) is responsible for top-down attention, and the ventral attention network (gold) mediates bottom-up attention.
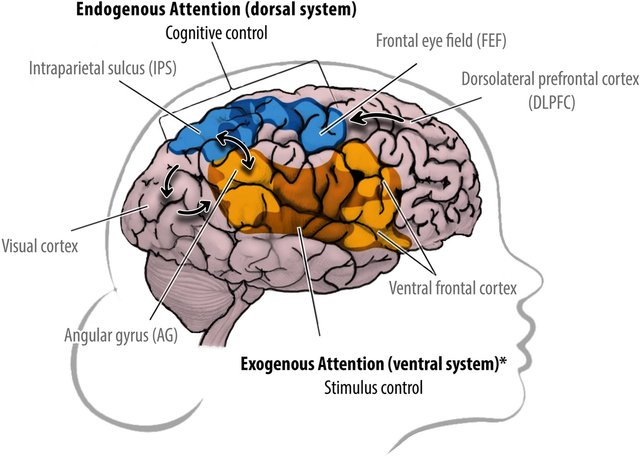
Caption: Humans can perceive the world from different perspectives: an endogenous (Self) versus exogenous (Other) focus of attention. Self-produced signals are predicted in the brain (e.g., efference copies), whereas external signals are not. These predictions allow the brain to early discriminate self-produced signals from external stimulation and keep track of its own spatiotemporal location (perspective). Endogenous attention: the dorsal system is goal-directed and processes self-produced signals. ‘Cognitive control’ enables the top-down voluntary selection of stimuli and responses, resp.: dorsolateral prefrontal cortex (dlPFC), frontal eye field (FEF), intraparietal sulcus (IPS), and visual cortex. Exogenous attention * lateralized to the right hemisphere: the ventral system is stimulus-driven and processes external signals. ‘Stimulus control’ enables the automatic orientation and bottom-up detection of behaviorally relevant stimuli, resp.: visual cortex, angular gyrus (AG; part of the temporoparietal junction), and the ventral frontal cortex. These attention systems keep external and self-produced signal processing separated and interconnect at the right AG (double arrow).
Extensive interconnections between the dorsal and ventral attention systems allow us to fluidly redirect attention from stimuli that are forebrain priorities (IPS) to those that are unexpected.
Two Cortical Networks Regulate Attention
Salience Network
The salience network (midcingulo-insular network; M-CIN) comprises structures that monitor our external and internal environments to determine which inputs are essential and require further processing and attention. The insula, primarily the anterior insula, is a crucial network component because it facilitates bottom-up access to the brain’s attentional and working memory resources (Menon & Uddin, 2010). The cingulate gyrus is another crucial component, particularly the right dorsal anterior cingulate cortex (Thompson & Thompson, 2016). Graphic by van Ettinger-Veenstra et al. (2019).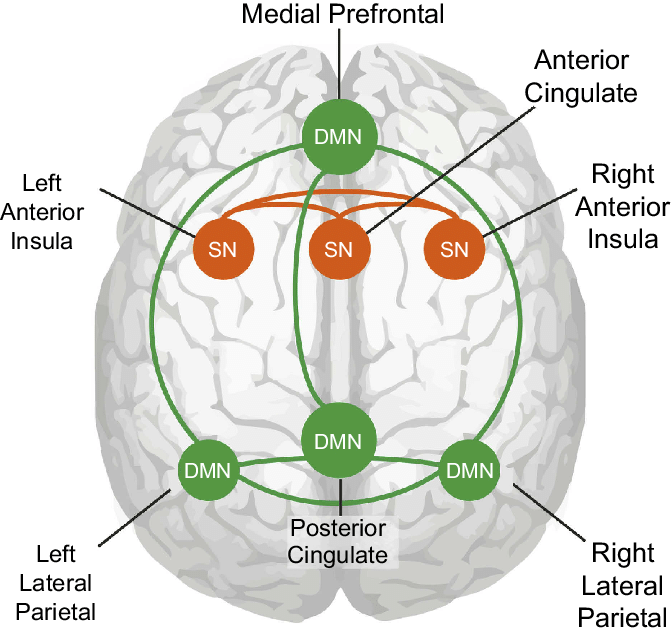
Caption: Node connections of the default mode network and the salience network. Cartoon of node connections used in this study as seen on a see-through brain. Green shows the connections between the nodes of the DMN, and orange shows the connection between the nodes of the SN.
The clinical literature on ADHD, depression, and schizophrenia has explored the role of the anterior cingulate gyrus (ACC) in these disorders. The ACC's deep brain stimulation has successfully improved treatment-resistant depression (Mayberg et al., 2005).
The insula, a cortical region located within the lateral sulcus, functions as an integrative and organization hub for the salience network. The insula integrates interoceptive awareness, emotional experience, and external perception to facilitate an individual's global perception of the world and its relationship. The insula directs specific networks in processing salient stimuli and generating appropriate responses to stimuli (Wiebking & Northoff, 2014).
Yasmine Y. Fathy, Susanne E. HoogersHenk W. BerendseYsbrand D. van der WerfPieter J. VisserFrank J. de JongWilma D.J. van de Berg, CC BY 4.0, via Wikimedia Commons.
.png)
Caption: Insular Cortex Sub-regions in MRI. The central sulcus generally divides the insular cortex into anterior and posterior subdivisions. The anterior insular cortex is further divided into ventral (red) and dorsal sub-regions (green), and the dorsal insula can be divided into an anterior and mid-region. The left figure shows the sub-regions of the insular cortex based on the Hammers_mith probabilistic atlas (Faillenot et al., 2017). Surface rendering of the insular sub-regions in 3D is shown on the right. A: anterior, dAI: dorsal anterior insula, I: inferior, P: posterior, PI: posterior insula, S: superior, vAI: ventral anterior insula.
The insula interfaces with the human brain's cognitive, homeostatic, and affective systems. It links the areas of the brain that monitor internal signals and those engaged in watching incoming external sensory streams. The insula detects salient events via afferent pathways and switches between other large-scale networks when these events are recognized to guide attention and working memory.
The anterior and posterior insula interact to regulate autonomic responses to salient stimuli. Interactive communication between the insula and anterior cingulate cortex facilitates access to the motor system (Menon & Uddin, 2010).
This network appears to help us switch between task-oriented (executive) and default mode (attention) networks (Seeley et al., 2007; Shirer et al., 2012).
Default Mode Network (DMN)
Brain regions are selectively active when we are conscious (Breedlove & Watson, 2020). The default mode network (DMN) consists of frontal, temporal, and parietal lobe circuits active during spontaneous cognition like introspection, daydreaming, and streams of consciousness. The DMN appears to contribute to flexible memory retrieval, idea generation, and critical creativity elements. The DMN is relatively inactive when pursuing external goals (Andrews-Hanna et al., 2010). Shim G, Oh JS, Jung WH, et al., CC BY 3.0, via Wikimedia Commons..jpg)
Caption: Default mode and task-related maps for healthy subjects. On a green background, the default mode network is highlighted in warm colors (red and yellow), and the task-related network is highlighted in cold colors (blue and light blue) depending on the p-value of a one-sample t-test.
ADHD has been linked to irregular connectivity among brain regions, including within the DMN (Cao et al., 2014). Notably, the strength of specific brain connections can accurately forecast variations in one's capacity to maintain attention (Rosenberg et al., 2016, 2017). This is true even in a resting state when the individual is not actively engaged in any specific task.
There has been increasing discussion about whether some of the DMN regions are part of a distinctively separate Parietal Memory Network (PMN) that includes the precuneus (PCU), the mid-cingulate cortex (MCC), and the posterior inferior parietal lobule/dorsal angular gyrus (pIPL/dAG; Gilmore, Nelson, & McDermott, 2015; Hu et al., 2016).
Deactivations can be as important as activations. For example, the degree of DMN deactivation seems critically important for aiding attentional control – people who can suppress it more can learn new material more easily (e.g., Nelson et al., 2016; Zerr et al., 2018). The DMN also helps to synthesize details into single coherent events and is important for envisioning the future (e.g., Gilmore, Nelson, Chen, & McDermott, 2018).
The DMN may contribute to creative fluency, generating innovative ideas like alternative uses for everyday objects. A study of neurosurgical patients showed that left DMN stimulation reduced the number of uses but not their originality (Shofty et al., 2022).
Understanding Ourselves
The posterior cingulate cortex (PCC) and precuneus combine bottom-up attention with information from memory and perception. The ventral (lower) part of the PCC activates in all tasks that involve the DMN, including those related to the self or others, remembering the past, thinking about the future, processing concepts, and spatial navigation. The dorsal (upper) part of PCC mediates involuntary awareness and arousal. The precuneus is concerned with visual, sensorimotor, and attentional information.The medial prefrontal cortex (mPFC) participates in decisions about the self, such as personal information, autobiographical memories, future goals and events, and decision-making regarding those close to us, like family members. The ventral (lower) part involves positive emotional information and reward.
The angular gyrus connects perception, attention, spatial cognition, and action and helps us recall episodic memories.
Understanding Others
The major functional hubs include the PCC, mPFC, and angular gyrus. The dorsal medial prefrontal cortex (dmPFC) analyzes others' objectives. The temporoparietal junction (TPJ) constructs theories of mind, which are models of others' cognitive processes, emotions, knowledge, and motivation. The lateral temporal cortex concerns short-term verbal memory, naming, and reading. Finally, the anterior temporal pole is part of a bilateral semantic system representing object concepts and a left hemisphere-dominant network concerned with naming and understanding object names.Autobiography and Future Simulations
The major functional hubs include the PCC, mPFC, and angular gyrus. The hippocampus forms new declarative memories. The parahippocampal cortex (PHC) mediates spatial memory, navigation, and high-level visual processing like facial recognition. The retrosplenial cortex (RSC) participates in episodic memory, navigation, predicting future events, and analyzing visual scenes. Finally, the posterior inferior parietal lobe (pIPL) integrates sensory information and participates in top-down attentional orienting. Graphic courtesy of the New Scientist (Fox, 2008).
The Pulvinar Mediates Attentional Shifts
The pulvinar nucleus, which comprises the posterior quarter of the human thalamus, processes visual information and directs attention. The pulvinar plays a pivotal role in processing visual information and shares widespread connections with the cingulate, parietal cortex, and superior colliculus. The pulvinar is crucial for orienting, shifting attention, and filtering out irrelevant stimuli. Tasks that present subjects with more distracting stimuli increase pulvinar activation, as the functional MRI (fMRI) shows. Thalamus graphic © Songkram Chotik-anuchit/Shutterstock.com.
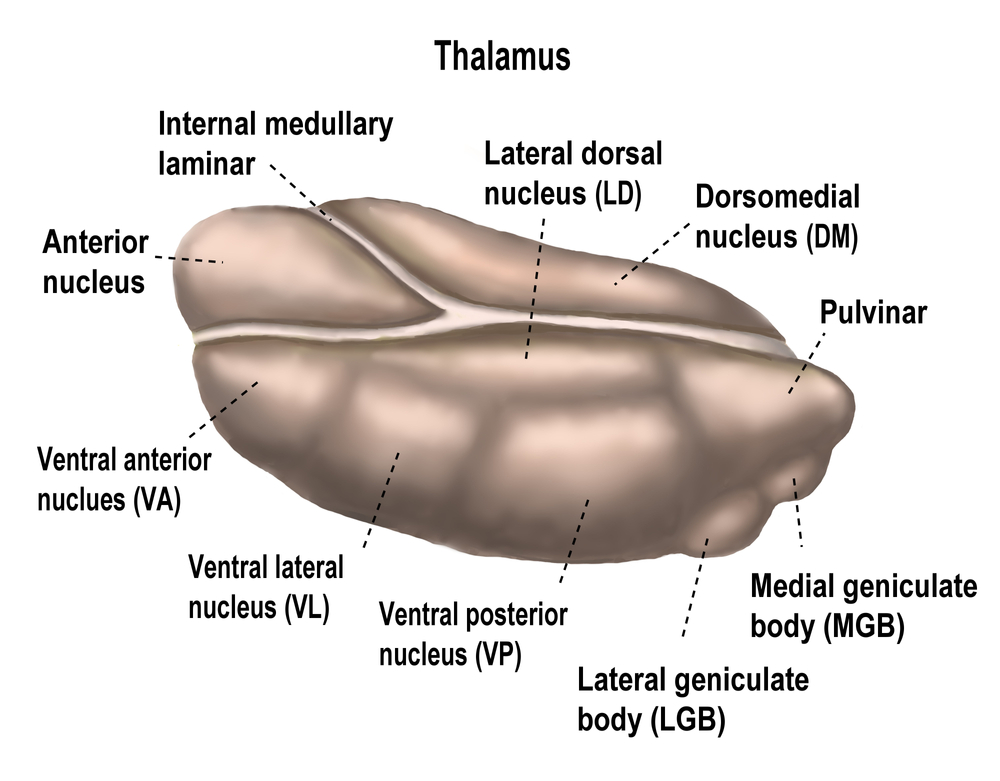
Overall, the pulvinar guides the processing of relevant information in wide-ranging cortical networks based on dynamically changing attentional priorities (Breedlove & Watson, 2023; Saalmann et al., 2012).
Glossary
affective network: a network that is triggered when we make mistakes and that monitors cognitive activity to predict when errors are likely, and greater executive control may be needed. The affective network includes the anterior cingulate cortex, hippocampal cortex, entorhinal cortex, superior temporal gyrus, inferior temporal gyrus, posterior parietal cortex, globus pallidus internal segment, substantia nigra, pars reticulata, and medial dorsal nucleus of the thalamus.
angular gyrus: located in the parietal lobe near the junction of the temporal and occipital lobes, the angular gyrus corresponds to Brodmann area 39. It plays a role in language processing, attention, spatial cognition, and integration of sensory information.
anterior: toward the front of the brain.
anterior cingulate gyrus (ACC): located in the parietal lobe near the junction of the temporal and occipital lobes, the angular gyrus corresponds to Brodmann area 39. It plays a role in language processing, attention, spatial cognition, and integration of sensory information.
anterior temporal pole: brain region involved in social cognition, semantic memory, and emotional processing.
Brodmann areas: a system of dividing the cerebral cortex into numbered regions based on cytoarchitecture.
caudal: toward the back of the brain.
central autonomic network (CAN): a complex system of brain regions that is involved in the regulation of the autonomic nervous system. This network includes several brain structures like the prefrontal cortex, anterior cingulate cortex, insula, amygdala, hypothalamus, periaqueductal gray, parabrachial complex, the nucleus of the solitary tract, and the medulla oblongata. These structures work together to regulate the body's physiological states, such as heart rate, blood pressure, respiration, digestion, and thermoregulation.
cingulo-opercular network (CON) network: a brain network involved in cognitive control and attention.
connectivity: the strength of connections between brain regions.
creative fluency: the ability to generate a large number of creative ideas.
default mode network (DMN): frontal, temporal, and parietal lobe circuits that are active during introspection and daydreaming and relatively inactive when we pursue external goals.
dorsal: toward the top of the brain.
dorsal attention network (dorsal frontoparietal network): a brain network involved in attentional control.
dorsal medial prefrontal cortex (dmPFC): brain region involved in self-referential processing, social cognition, and theory of mind.
dorsolateral prefrontal cortex: the left dorsolateral prefrontal cortex is concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals. The right dorsolateral prefrontal cortex organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
flexible hubs: brain regions that play a role in multiple brain networks.
frontal eye field (FEF): a region of the premotor cortex that directs gaze towards targets selected by the IPS.
frontoparietal network: a brain network involved in cognitive control and attention.
functional networks: correlated activity between regions over time.
gyrus: a ridge or fold on the surface of the cerebral cortex.
hippocampus: brain region involved in memory formation and spatial navigation.
insula: the cortical region located within the lateral sulcus of the frontal, parietal, and temporal lobes that functions as an integrative and organization hub for the salience network.
lateral temporal cortex: brain region involved in language processing, semantic memory, and auditory perception.
magnetic resonance imaging (MRI): a non-invasive imaging technology that uses a strong magnetic field and radio waves to produce detailed images of the inside of the body. It's especially useful for imaging soft tissues and organs like the brain, spinal cord, muscles, and heart. It provides high-resolution, 3D images that can be viewed from different angles, making it a valuable tool in medical diagnosis and research.
mentalizing network: a brain network involved in understanding the mental states of others.
mirror network (superior temporal sulcus, STS): a brain network involved in understanding the actions and intentions of others.
networks: groups of interconnected brain regions that work together to perform a specific function.
neurovisceral integration model: a theoretical framework that suggests the heart, brain, and other bodily systems are interconnected and communicate with each other to maintain overall health and well-being. It posits that autonomic, attentional, and affective systems are integrated within the central autonomic network and that imbalances within this network may underlie the associations between stress, disease, and cognitive function.
parahippocampal cortex (PHC): brain region involved in memory encoding and retrieval.
parietal memory network (PMN): a brain network involved in episodic memory retrieval.
posterior: toward the back of the brain.
posterior cingulate cortex (PCC): brain region involved in memory consolidation, self-referential processing, and default mode network activity.
posterior inferior parietal lobe (pIPL): brain region involved in attention, perception, and numerical cognition.
pre- and subgenual areas of the anterior cingulate cortex (ACC): brain regions involved in emotional processing and regulation.
precuneus: brain region involved in self-referential processing, episodic memory, and visuospatial imagery.
pulvinar nucleus: the posterior region of the thalamus that processes visual information and directs attention.
resting-state functional connectivity (RSFC): a method for measuring the functional connectivity between brain regions while the participant is at rest.
retrosplenial cortex (RSC): found in the posterior part of the cingulate cortex, the retrosplenial cingulate cortex covers Brodmann areas 29 and 30. It is involved in spatial memory, navigation, and contextual processing.
rostral: toward the front of the brain.
salience network: structures including the insula and anterior cingulate cortex that seek to monitor our external and internal environments to determine which of these inputs are salient and require further processing and attention.
social network: the network that mediates socially responsible behavior, empathy, behavioral inhibition, emotional regulation, and sound judgment. The social network includes the orbitofrontal cortex, superior temporal gyrus, inferior temporal gyrus, anterior cingulate cortex, caudate, globus pallidus internal segment, substantia nigra, pars reticulata, ventral anterior nucleus of the thalamus, and medial dorsal nucleus of the thalamus.
somato-cognitive action network (SCAN): a brain network involved in integrating sensory and motor information to guide behavior.
structural networks: axonal projections and pathways.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

References
Aggleton, J. P., & Brown, M. W. (1999). Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. The Behavioral and Brain Sciences, 22(3), 425–489.
Ahuja, A., & Yusif Rodriguez, N. (2022). Is the dorsolateral prefrontal cortex actually several different brain areas? The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 42(33), 6310–6312. https://doi.org/10.1523/JNEUROSCI.0848-22.2022
Amaral, D. G., & Witter, M. P. (1995). Hippocampal formation. In G. Paxinos (Ed.), The rat nervous system (pp. 443-493). Academic Press.
Amunts, K., Schleicher, A., Bürgel, U., Mohlberg, H., Uylings, H. B., & Zilles, K. (1999). Broca's region revisited: Cytoarchitecture and intersubject variability. The Journal of Comparative Neurology, 412(2), 319–341. https://doi.org/10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R., & Buckner, R. L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. https://doi.org/10.1016/j.neuron.2010.02.005
Arnsten A. F. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. https://doi.org/10.1038/nrn2648
Augustine J. R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research. Brain Research Reviews, 22(3), 229–244. https://doi.org/10.1016/s0165-0173(96)00011-2
Avidan, G., & Behrmann, M. (2009). Functional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Current Biology: CB, 19(13), 1146–1150. https://doi.org/10.1016/j.cub.2009.04.060
Baliki, M. N., Geha, P. Y., & Apkarian, A. V. (2009). Parsing pain perception between nociceptive representation and magnitude estimation. Journal of Neurophysiology, 101(2), 875–887. https://doi.org/10.1152/jn.91100.2008
Beissner, F., Meissner, K., Bär, K. J., & Napadow, V. (2013). The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(25), 10503–10511. https://doi.org/10.1523/JNEUROSCI.1103-13.2013
Benarroch E. E. (1993). The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings, 68(10), 988–1001. https://doi.org/10.1016/s0025-6196(12)62272-1
Bizley, J. K., & Cohen, Y. E. (2013). The what, where and how of auditory-object perception. Nature Reviews. Neuroscience, 14(10), 693–707. https://doi.org/10.1038/nrn3565
Booth, J. R., Wood, L., Lu, D., Houk, J. C., & Bitan, T. (2007). The role of the basal ganglia and cerebellum in language processing. Brain Research, 1133(1), 136–144. https://doi.org/10.1016/j.brainres.2006.11.074
Braak, H., & Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica, 82(4), 239–259. https://doi.org/10.1007/BF00308809
Breedlove, S., M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Bressler, S. L., & Menon, V. (2010). Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. https://doi.org/10.1016/j.tics.2010.04.004
Brodmann, K. (1909). Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth.
Broyd, S. J., Demanuele, C., Debener, S., Helps, S. K., James, C. J., & Sonuga-Barke, E. J. (2009). Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 33(3), 279–296. https://doi.org/10.1016/j.neubiorev.2008.09.002
Buchsbaum, M. S., Buchsbaum, B. R., Chokron, S., Tang, C., Wei, T. C., & Byne, W. (2006). Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers. Neuroscience Letters, 404(3), 282-287. https://doi.org/10.1016/j.neulet.2006.05.063
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1-38. https://doi.org/10.1196/annals.1440.011
Buckner, R. L., Snyder, A. Z., Shannon, B. J., LaRossa, G., Sachs, R., Fotenos, A. F., Sheline, Y. I., Klunk, W. E., Mathis, C. A., Morris, J. C., & Mintun, M. A. (2005). Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(34), 7709–7717. https://doi.org/10.1523/JNEUROSCI.2177-05.2005
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. https://doi.org/10.1016/s1364-6613(00)01483-2
Cao, Z., Ottino-Gonzalez, J., Cupertino, R. B., Schwab, N., Hoke, C., Catherine, O., Cousijn, J., Dagher, A., Foxe, J. J., Goudriaan, A. E., Hester, R., Hutchison, K., Li, C. R., London, E. D., Lorenzetti, V., Luijten, M., Martin-Santos, R., Momenan, R., Paulus, M. P., Schmaal, L., … Garavan, H. (2021). Mapping cortical and subcortical asymmetries in substance dependence: Findings from the ENIGMA Addiction Working Group. Addiction Biology, 26(5), e13010. https://doi.org/10.1111/adb.13010
Carey, L. M., Abbott, D. F., Egan, G. F., O'Keefe, G. J., Jackson, G. D., Bernhardt, J., & Donnan, G. A. (2006). Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabilitation and Neural Repair, 20(1), 24–41. https://doi.org/10.1177/1545968305283053
Carlén M. (2017). What constitutes the prefrontal cortex? Science, 358(6362), 478–482. https://doi.org/10.1126/science.aan8868
Caspers, S., Geyer, S., Schleicher, A., Mohlberg, H., Amunts, K., & Zilles, K. (2006). The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. NeuroImage, 33(2), 430–448. https://doi.org/10.1016/j.neuroimage.2006.06.054
Castellanos, F. X., Margulies, D. S., Kelly, C., Uddin, L. Q., Ghaffari, M., Kirsch, A., Shaw, D., Shehzad, Z., Di Martino, A., Biswal, B., Sonuga-Barke, E. J., Rotrosen, J., Adler, L. A., & Milham, M. P. (2008). Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry, 63(3), 332–337. https://doi.org/10.1016/j.biopsych.2007.06.025
Catani, M., Jones, D. K., Donato, R., & Ffytche, D. H. (2003). Occipito-temporal connections in the human brain. Brain: A Journal of Neurology, 126(Pt 9), 2093–2107. https://doi.org/10.1093/brain/awg203
Chau, W., & McIntosh, A. R. (2005). The Talairach coordinate of a point in the MNI space: How to interpret it. NeuroImage, 25(2), 408–416. https://doi.org/10.1016/j.neuroimage.2004.12.007
Cole, M. W., Reynolds, J. R., Power, J. D., Repovs, G., Anticevic, A., & Braver, T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16, 1348–1355. https://doi.org/10.1038/nn.3470
Corbetta, M., & Shulman, G. I. (1998). Human cortical mechanisms of visual attention during orienting and search. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 353(1373), 1353-1362. https://doi.org/10.1098/rstb.1998.0289
Craig A. D. (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10(1), 59–70. https://doi.org/10.1038/nrn2555
Critchley H. D. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology, 493(1), 154–166. https://doi.org/10.1002/cne.20749
Critchley H. D. (2009). Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 73(2), 88–94. https://doi.org/10.1016/j.ijpsycho.2009.01.012
Davidson, R. J. (2000). The functional neuroanatomy of affective style. XCognitive neuroscience of emotion V. Oxford University Press, 371–388.
de la Cruz, F., Schumann, A., Köhler, S., Reichenbach, J. R., Wagner, G., & Bär, K. J. (2019). The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. NeuroImage, 196, 318–328. https://doi.org/10.1016/j.neuroimage.2019.04.014
Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain: A Journal of Neurology, 118 ( Pt 1), 279–306. https://doi.org/10.1093/brain/118.1.279
Dosenbach, N. U., Fair, D. A., Miezin, F. M., Cohen, A. L., Wenger, K. K., Dosenbach, R. A., Fox, M. D., Snyder, A. Z., Vincent, J. L., Raichle, M. E., Schlaggar, B. L., & Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. https://doi.org/10.1073/pnas.0704320104
Doty R. L. (2008). The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Annals of Neurology, 63(1), 7–15. https://doi.org/10.1002/ana.21327
Drevets, W. C., Price, J. L., & Furey, M. L. (2008). Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure & Function, 213(1-2), 93–118. https://doi.org/10.1007/s00429-008-0189-x
Du, F., Whetsell, W. O., Jr, Abou-Khalil, B., Blumenkopf, B., Lothman, E. W., & Schwarcz, R. (1993). Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Research, 16(3), 223–233. https://doi.org/10.1016/0920-1211(93)90083-j
Eichenbaum, H., Yonelinas, A. P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152. https://doi.org/10.1146/annurev.neuro.30.051606.094328
Epstein R. A. (2008). Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences, 12(10), 388–396. https://doi.org/10.1016/j.tics.2008.07.004
Etkin, A., Egner, T., & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. https://doi.org/10.1016/j.tics.2010.11.004
Faraone, S. V., Biederman, J., & Mick, E. (2006). The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychological Medicine, 36(2), 159–165. https://doi.org/10.1017/S003329170500471X
A. Fornito, A. Zalesky, & E. T. Bullmore (Eds.) (2016). Fundamentals of brain network analysis. Elsevier. https://doi.org/10.1016/C2012-0-06036-X
Fox, D. (2008). The secret life of the brain. New Scientist, 2681.
Frank, G. K., Shott, M. E., Riederer, J., & Pryor, T. L. (2016). Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Translational Psychiatry, 6(11), e932. https://doi.org/10.1038/tp.2016.199
Frankowski, J. C., Tierno, A., Pavani, S., Cao, Q., Lyon, D. C., & Hunt, R. F. (2022). Brain-wide reconstruction of inhibitory circuits after traumatic brain injury. Nat Commun, 13, 3417. https://doi.org/10.1038/s41467-022-31072-2
Friederici A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. https://doi.org/10.1152/physrev.00006.2011
Fuster J. M. (2001). The prefrontal cortex--An update: Time is of the essence. Neuron, 30(2), 319–333. https://doi.org/10.1016/s0896-6273(01)00285-9
Gevensleben, H., Moll, G. H., Rothenberger, A., & Heinrich, H. (2014). Neurofeedback in attention-deficit/hyperactivity disorder - Different models, different ways of application. Frontiers in Human Neuroscience, 8, 846. https://doi.org/10.3389/fnhum.2014.00846
Gilmore, A. W., Nelson, S. M., Chen, H. Y., & McDermott, K. B. (2018). Task-related and resting-state fMRI identify distinct networks that preferentially support remembering the past and imagining the future. Neuropsychologia, 110, 180–189. https://doi.org/10.1016/j.neuropsychologia.2017.06.016
Gilmore, A. W., Nelson, S. M., & McDermott, K. B. (2015). A parietal memory network revealed by multiple MRI methods. Trends in Cognitive Sciences, 19(9), 534–543. https://doi.org/10.1016/j.tics.2015.07.004
Glasser, M. F., Coalson, T. S., Robinson, E. C., Hacker, C. D., Harwell, J., Yacoub, E., Ugurbil, K., Andersson, J., Beckmann, C. F., Jenkinson, M., Smith, S. M., & Van Essen, D. C. (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536(7615), 171–178. https://doi.org/10.1038/nature18933
Gordon, E. M., Chauvin, R. J., Van, A. N., Rajesh, A., Nielsen, A., Newbold, D. J., Lynch, C. J., Seider, N. A., Krimmel, S. R., Scheidter, K. M., Monk, J., Miller, R. L., Metoki, A., Montez, D. F., Zheng, A., Elbau, I., Madison, T., Nishino, T., Myers, M. J., Kaplan, S., … Dosenbach, N. U. F. (2023). A somato-cognitive action network alternates with effector regions in motor cortex. Nature, 617(7960), 351–359. https://doi.org/10.1038/s41586-023-05964-2
Gorges, M., Müller, H. P., & Kassubek, J. (2018). Structural and functional brain mapping correlates of impaired eye movement control in Parkinsonian Syndromes: A systems-based concept. Frontiers in Neurology, 9, 319. https://doi.org/10.3389/fneur.2018.00319
Gottfried J. A. (2010). Central mechanisms of odour object perception. Nature Reviews. Neuroscience, 11(9), 628–641. https://doi.org/10.1038/nrn2883
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., Reiss, A. L., & Schatzberg, A. F. (2007). Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62(5), 429–437. https://doi.org/10.1016/j.biopsych.2006.09.020
Griffin, D. M., Hoffman, D. S., & Strick, P. L. (2015). Corticomotoneuronal cells are "functionally tuned." Science, 350(6261), 667–670. https://doi.org/10.1126/science.aaa8035
Griffiths, T. D., & Warren, J. D. (2002). The planum temporale as a computational hub. Trends in Neurosciences, 25(7), 348–353. https://doi.org/10.1016/s0166-2236(02)02191-4
Grill-Spector, K., Knouf, N., & Kanwisher, N. (2004). The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience, 7(5), 555–562. https://doi.org/10.1038/nn1224
Gross J. J. (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39(3), 281–291. https://doi.org/10.1017/s0048577201393198
Hafting, T., Fyhn, M., Molden, S., Moser, M. B., & Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436(7052), 801–806. https://doi.org/10.1038/nature03721
Hamilton, J. P., Glover, G. H., Hsu, J. J., Johnson, R. F., & Gotlib, I. H. (2011). Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Human Brain Mapping, 32(1), 22–31. https://doi.org/10.1002/hbm.20997
Han, M., Jiang, G., Luo, H., & Shao, Y. (2021). Neurobiological bases of social networks. Frontiers in Psychology, 12, 626337. https://doi.org/10.3389/fpsyg.2021.626337
Heckers, S., Rauch, S. L., Goff, D., Savage, C. R., Schacter, D. L., Fischman, A. J., & Alpert, N. M. (1998). Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience, 1(4), 318–323. https://doi.org/10.1038/1137
Hoeft, F., Meyler, A., Hernandez, A., Juel, C., Taylor-Hill, H., Martindale, J. L., McMillon, G., Kolchugina, G., Black, J. M., Faizi, A., Deutsch, G. K., Siok, W. T., Reiss, A. L., Whitfield-Gabrieli, S., & Gabrieli, J. D. (2007). Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the United States of America, 104(10), 4234–4239. https://doi.org/10.1073/pnas.0609399104
Holmes, A. J., MacDonald, A., 3rd, Carter, C. S., Barch, D. M., Andrew Stenger, V., & Cohen, J. D. (2005). Prefrontal functioning during context processing in schizophrenia and major depression: An event-related fMRI study. Schizophrenia Research, 76(2-3), 199–206. https://doi.org/10.1016/j.schres.2005.01.021
Hoskinson, K. R., Bigler, E. D., Abildskov, T. J., Dennis, M., Taylor, H. G., Rubin, K., Gerhardt, C. A., Vannatta, K., Stancin, T., & Yeates, K. O. The mentalizing network and theory of mind mediate adjustment after childhood traumatic brain injury. Soc Cogn Affect Neurosci., 14(12), 1285-1295. https://doi.org/10.1093/scan/nsaa006. PMID: 31993655; PMCID: PMC7137721.
Hu, Y., Wang, J., Li, C., Wang, Y. S., Yang, Z., & Zuo, X. N. (2016). Segregation between the parietal memory network and the default mode network: Effects of spatial smoothing and model order in ICA. Science Bulletin, 61(24), 1844–1854. https://doi.org/10.1007/s11434-016-1202-z
Hyman, B. T., Van Hoesen, G. W., Damasio, A. R., & Barnes, C. L. (1984). Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science, 225(4667), 1168–1170. https://doi.org/10.1126/science.6474172
Jackson, D. C., Malmstadt, J. R., Larson, C. L., & Davidson, R. J. (2000). Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology, 37(4), 515–522.
Jasper, H. H. (1958). The Ten-Twenty Electrode System of the International Federation. Electroencephalography and Clinical Neurophysiology, 10, 371-375.
Kaufman, M. T., Churchland, M. M., Ryu, S. I., & Shenoy, K. V. (2014). Cortical activity in the null space: Permitting preparation without movement. Nature Neuroscience, 17(3), 440–448. https://doi.org/10.1038/nn.3643
Khan, U. A., Liu, L., Provenzano, F. A., Berman, D. E., Profaci, C. P., Sloan, R., Mayeux, R., Duff, K. E., & Small, S. A. (2014). Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nature Neuroscience, 17(2), 304–311. https://doi.org/10.1038/nn.3606
Kluetsch, R. C., Ros, T., Théberge, J., Frewen, P. A., Calhoun, V. D., Schmahl, C., Jetly, R., & Lanius, R. A. (2014). Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatrica Scandinavica, 130(2), 123–136. https://doi.org/10.1111/acps.12229
Kringelbach M. L. (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews. Neuroscience, 6(9), 691–702. https://doi.org/10.1038/nrn1747
Kroptov, J. D. (2009). Quantitative EEG, event-related potentials and neurotherapy. Academic Press, Elsevier.
Kumral, D., Schaare, H. L., Beyer, F., Reinelt, J., Uhlig, M., Liem, F., Lampe, L., Babayan, A., Reiter, A., Erbey, M., Roebbig, J., Loeffler, M., Schroeter, M. L., Husser, D., Witte, A. V., Villringer, A., & Gaebler, M. (2019). The age-dependent relationship between resting heart rate variability and functional brain connectivity. NeuroImage, 185, 521–533. https://doi.org/10.1016/j.neuroimage.2018.10.027
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure & Function, 214(5-6), 519–534. https://doi.org/10.1007/s00429-010-0255-z
Leech, R., & Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain: A Journal of Neurology, 137(Pt 1), 12–32. https://doi.org/10.1093/brain/awt162
Linden D. E. (2006). How psychotherapy changes the brain--The contribution of functional neuroimaging. Molecular Psychiatry, 11(6), 528–538. https://doi.org/10.1038/sj.mp.4001816
Liston, C., Malter Cohen, M., Teslovich, T., Levenson, D., & Casey, B. J. (2011). Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: Pathway to disease or pathological end point? Biological Psychiatry, 69(12), 1168–1177. https://doi.org/10.1016/j.biopsych.2011.03.022
Makovac, E., Meeten, F., Watson, D. R., Herman, A., Garfinkel, S. N., D Critchley, H., & Ottaviani, C. (2016). Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in Generalized Anxiety Disorder. Biological Psychiatry, 80(10), 786–795. https://doi.org/10.1016/j.biopsych.2015.10.013
Margulies, D. S., Kelly, A. M., Uddin, L. Q., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. NeuroImage, 37(2), 579–588. https://doi.org/10.1016/j.neuroimage.2007.05.019
Margulies, D. S., Vincent, J. L., Kelly, C., Lohmann, G., Uddin, L. Q., Biswal, B. B., Villringer, A., Castellanos, F. X., Milham, M. P., & Petrides, M. (2009). Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America, 106(47), 20069–20074. https://doi.org/10.1073/pnas.0905314106
Mayberg H. S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin, 65, 193–207. https://doi.org/10.1093/bmb/65.1.193
Mayberg, H. S., Lozano, A. M., Voon, V., McNeely, H. E., Seminowicz, D., Hamani, C., Schwalb, J. M., & Kennedy, S. H. (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45(5), 651–660. https://doi.org/10.1016/j.neuron.2005.02.014
Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483-506. https://doi.org/10.1016/j.tics.2011.08.003
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5-6), 655–667. https://doi.org/10.1007/s00429-010-0262-0
Mesa, N. (2023). New brain network connecting mind and body discovered. Retrieved from The Scientist.
Morosan, P., Rademacher, J., Schleicher, A., Amunts, K., Schormann, T., & Zilles, K. (2001). Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage, 13(4), 684–701. https://doi.org/10.1006/nimg.2000.0715
Neef, N. E., Anwander, A., Bütfering, C., Schmidt-Samoa, C., Friederici, A. D., Paulus, W., & Sommer, M. (2018). Structural connectivity of right frontal hyperactive areas scales with stuttering severity. Brain: A Journal of Neurology, 141(1), 191–204. https://doi.org/10.1093/brain/awx316
Nelson, S. M., Savalia, N. K., Fishell, A. K., Gilmore, A. W., Zou, F., Balota, D. A., & McDermott, K. B. (2016). Default mode network activity predicts early memory decline in healthy young adults aged 18-31. Cerebral Cortex, 26(8), 3379–3389. https://doi.org/10.1093/cercor/bhv165
Neville, K. R., & Haberly, L. B. (2004). Olfactory cortex. The synaptic organization of the brain. In G. M. Shepherd (Ed.). Oxford University Press, 415–454. https://doi.org/10.1093/acprof:oso/9780195159561.003.0010
Newbold, D. J., & Dosenbach, N. U. F. (2021). Tracking plasticity of individual human brains. Current Opinion in Behavioral Sciences, 40, 161-168. https://doi.org/10.1016/j.cobeha.2021.04.018
Niendam, T. A., Laird, A. R., Ray, K. L., Dean, Y. M., Glahn, D. C., & Carter, C. S. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective & Behavioral Neuroscience, 12(2), 241–268. https://doi.org/10.3758/s13415-011-0083-5
Nikolin, S., Boonstra, T. W., Loo, C. K., & Martin, D. (2017). Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PloS one, 12(8), e0181833. https://doi.org/10.1371/journal.pone.0181833
Ogawa H. (1994). Gustatory cortex of primates: anatomy and physiology. Neuroscience Research, 20(1), 1–13. https://doi.org/10.1016/0168-0102(94)90017-5
Olson, I. R., Plotzker, A., & Ezzyat, Y. (2007). The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain: A Journal of Neurology, 130(Pt 7), 1718–1731. https://doi.org/10.1093/brain/awm052
Ongür, D., Ferry, A. T., & Price, J. L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology, 460(3), 425–449. https://doi.org/10.1002/cne.10609
Ottaviani, C., Watson, D. R., Meeten, F., Makovac, E., Garfinkel, S. N., & Critchley, H. D. (2016). Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biological Psychology, 119, 31–41. https://doi.org/10.1016/j.biopsycho.2016.06.009
Padmanabhan, A., Lynch, C. J., Schaer, M., & Menon, V. (2017). The Default Mode Network in autism. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 2(6), 476–486. https://doi.org/10.1016/j.bpsc.2017.04.004
Paus, T., Kalina, M., Patocková, L., Angerová, Y., Cerný, R., Mecir, P., Bauer, J., & Krabec, P. (1991). Medial vs lateral frontal lobe lesions and differential impairment of central-gaze fixation maintenance in man. Brain, 114, 2051-2067. https://doi.org/10.1093/brain/114.5.2051
Peng, K., Steele, S. C., Becerra, L., & Borsook, D. (2018). Brodmann area 10: Collating, integrating and high level processing of nociception and pain. Progress in Neurobiology, 161, 1–22. https://doi.org/10.1016/j.pneurobio.2017.11.004
Petersen, S. E., & Fiez, J. A. (1993). The processing of single words studied with positron emission tomography. Annual Review of Neuroscience, 16, 509-530. https://doi.org/10.1146/annurev.ne.16.030193.002453
Price, J. L., & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 192–216. https://doi.org/10.1038/npp.2009.104
Purves, D. (2018). Neuroscience (6th ed.). Oxford University Press.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. https://doi.org/10.1073/pnas.98.2.676
Rajkowska, G., & Goldman-Rakic, P. S. (1995). Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cerebral Cortex, 5(4), 323–337. https://doi.org/10.1093/cercor/5.4.323
Ramnani, N., & Owen, A. M. (2004). Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews. Neuroscience, 5(3), 184–194. https://doi.org/10.1038/nrn1343
Rogers, M. A., Kasai, K., Koji, M., Fukuda, R., Iwanami, A., Nakagome, K., Fukuda, M., & Kato, N. (2004). Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Rresearch, 50(1), 1–11. https://doi.org/10.1016/j.neures.2004.05.003
Rolls E. T. (2006). Brain mechanisms underlying flavour and appetite. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361(1471), 1123–1136. https://doi.org/10.1098/rstb.2006.1852
Rolls, E. T., Cheng, W., & Feng, J. (2020). The orbitofrontal cortex: Reward, emotion and depression. Brain Communications, 2(2), fcaa196. https://doi.org/10.1093/braincomms/fcaa196
Ros, T., J Baars, B., Lanius, R. A., & Vuilleumier, P. (2014). Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Frontiers in Human Neuroscience, 8, 1008. https://doi.org/10.3389/fnhum.2014.01008
Rosenberg, M. D., Finn, E. S., Scheinost, D., Constable, R. T., & Chun, M. M. (2017). Characterizing attention with predictive network models. Trends in Cognitive Sciences, 21(4), 290–302. https://doi.org/10.1016/j.tics.2017.01.011
Rosenberg, M. D., Finn, E. S., Scheinost, D., Papademetris, X., Shen, X., Constable, R. T., & Chun, M. M. (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19(1), 165–171. https://doi.org/10.1038/nn.4179
Roy, A. K., Shehzad, Z., Margulies, D. S., Kelly, A. M., Uddin, L. Q., Gotimer, K., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. https://doi.org/10.1016/j.neuroimage.2008.11.030
Roy, M., Shohamy, D., & Wager, T. D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. https://doi.org/10.1016/j.tics.2012.01.005
Rudebeck, P. H., Putnam, P. T., Daniels, T. E., Yang, T., Mitz, A. R., Rhodes, S. E., & Murray, E. A. (2014). A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proceedings of the National Academy of Sciences of the United States of America, 111(14), 5391–5396. https://doi.org/10.1073/pnas.1317695111
Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X., & Kastner, S. (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science, 337(6095), 753-756. https://doi.org/10.1126/science.1223082
Sadeghi, S., Schmidt, S., Mier, D., & Hass, J. (2022). Effective connectivity of the human mirror neuron system during social cognition. Social Cognitive and Affective Neuroscience, 17(8), 732–743. https://doi.org/10.1093/scan/nsab138
Sakaki, M., Yoo, H. J., Nga, L., Lee, T. H., Thayer, J. F., & Mather, M. (2016). Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage, 139, 44–52. https://doi.org/10.1016/j.neuroimage.2016.05.076
Sanders, R. H., & Levitin, D. J. (2020). Towards an understanding of control of complex rhythmical "wavelike" coordination in humans. Brain Sciences, 10(4), 215. https://doi.org/10.3390/brainsci10040215
Shoemaker, J. K., Norton, K. N., Baker, J., & Luchyshyn, T. (2015). Forebrain organization for autonomic cardiovascular control. Autonomic Neuroscience: Basic & Clinical, 188, 5–9. https://doi.org/10.1016/j.autneu.2014.10.022
Schumann, A., de la Cruz, F., Köhler, S., Brotte, L., & Bär, K. J. (2021). The influence of heart rate variability biofeedback on cardiac regulation and functional brain connectivity. Frontiers in Neuroscience, 15, 691988. https://doi.org/10.3389/fnins.2021.691988
Sedley, W., Parikh, J., Edden, R. A., Tait, V., Blamire, A., & Griffiths, T. D. (2015). Human auditory cortex neurochemistry reflects the presence and severity of tinnitus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(44), 14822–14828. https://doi.org/10.1523/JNEUROSCI.2695-15.2015
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., Reiss, A. L., & Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Seghier M. L. (2013). The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist: A Review Journal Bridging Neurobiology, Neurology and Psychiatry, 19(1), 43–61. https://doi.org/10.1177/1073858412440596
Sheline, Y. I., Price, J. L., Yan, Z., & Mintun, M. A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11020–11025. https://doi.org/10.1073/pnas.1000446107
Shepherd G. M. (2007). Perspectives on olfactory processing, conscious perception, and orbitofrontal cortex. Annals of the New York Academy of Sciences, 1121, 87–101. https://doi.org/10.1196/annals.1401.032
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex, 22. https://doi.org/158–165.10.1093/cercor/bhr099
Shofty, B., Gonent, T., Bergmann, E., Mayseless, N., Korn, A., Shamay-Tsoory, S., Grossman, R., Jalon, I., & Ram, Z. (2022). The default network is causally linked to creative thinking. Molecular Psychiatry. https://doi.org/10.1038/s41380-021-01403-8
Small, D. M., Zald, D. H., Jones-Gotman, M., Zatorre, R. J., Pardo, J. V., Frey, S., & Petrides, M. (1999). Human cortical gustatory areas: A review of functional neuroimaging data. Neuroreport, 10(1), 7–14. https://doi.org/10.1097/00001756-199901180-00002
Suzuki, W. A., & Amaral, D. G. (1994). Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. The Journal of Comparative Neurology, 350(4), 497–533. https://doi.org/10.1002/cne.903500402
Thayer, J. F., Hansen, A. L., Saus-Rose, E., & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 37(2), 141–153. https://doi.org/10.1007/s12160-009-9101-z
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. https://doi.org/10.1016/s0165-0327(00)00338-4
Thompson, M., & Thompson, L. (2015). The neurofeedback book (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Utevsky, A. V., Smith, D. V., & Huettel, S. A. (2014). Precuneus is a functional core of the default-mode network. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(3), 932–940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
van Ettinger-Veenstra, H., Lundberg, P., Alföldi, P., Södermark, M., Graven-Nielsen, T., Sjörs, A., Engström, M., & Gerdle, B. (2019). Chronic widespread pain patients show disrupted cortical connectivity in default mode and salience networks, modulated by pain sensitivity. Journal of pain research, 12, 1743–1755. https://doi.org/10.2147/JPR.S189443
Van Hoesen, G., & Pandya, D. N. (1975). Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Research, 95(1), 1–24. https://doi.org/10.1016/0006-8993(75)90204-8
van Strien, N. M., Cappaert, N. L., & Witter, M. P. (2009). The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nature Reviews. Neuroscience, 10(4), 272–282. https://doi.org/10.1038/nrn2614
Vann, S. D., Aggleton, J. P., & Maguire, E. A. (2009). What does the retrosplenial cortex do? Nature Reviews. Neuroscience, 10(11), 792–802. https://doi.org/10.1038/nrn2733
Vogt B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews. Neuroscience, 6(7), 533–544. https://doi.org/10.1038/nrn1704
Vogt, B. A., Finch, D. M., & Olson, C. R. (1992). Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex, 2(6), 435–443. https://doi.org/10.1093/cercor/2.6.435-a
Vossel, S., Geng, J. J., & Fink, G. R. (2014). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(2), 150–159. https://doi.org/10.1177/1073858413494269
Watkins, K. E., Vargha-Khadem, F., Ashburner, J., Passingham, R. E., Connelly, A., Friston, K. J., Frackowiak, R. S., Mishkin, M., & Gadian, D. G. (2002). MRI analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain: A Journal of Neurology, 125(Pt 3), 465–478. https://doi.org/10.1093/brain/awf057
Wesson, D. W., & Wilson, D. A. (2011). Sniffing out the contributions of the olfactory tubercle to the sense of smell: Hedonics, sensory integration, and more? Neuroscience and Biobehavioral Reviews, 35(3), 655–668. https://doi.org/10.1016/j.neubiorev.2010.08.004
Wiebking, C., & Northoff, G. (2014). Interoceptive awareness and the insula - Application of neuroimaging techniques in psychotherapy. GSTF International Journal of Psychology, 1(1), 53-60. https://doi.org/10.5176/0000-0002_1.1.8
Witter, M. P., Wouterlood, F. G., Naber, P. A., & Van Haeften, T. (2000). Anatomical organization of the parahippocampal-hippocampal network. Annals of the New York Academy of Sciences, 911, 1–24. https://doi.org/10.1111/j.1749-6632.2000.tb06716.x
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., Roffman, J. L., Smoller, J. W., Zöllei, L., Polimeni, J. R., Fischl, B., Liu, H., & Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. https://doi.org/10.1152/jn.00338.2011
Zerr, C. L., Berg, J. J., Nelson, S. M., Fishell A. K., Savalia, N. K., & McDermott, K. B. (2018). Learning efficiency: Identifying individual differences in learning rate and retention in healthy adults. Psychological Science, 956797618772540. PMID 29953332 https://doi.org/10.1177/0956797618772540
Zotev, V., Krueger, F., Phillips, R., Alvarez, R. P., Simmons, W. K., Bellgowan, P., Drevets, W. C., & Bodurka, J. (2011). Self-regulation of amygdala activation using real-time FMRI neurofeedback. PloS one, 6(9), e24522. https://doi.org/10.1371/journal.pone.0024522
E. BEHAVIORAL CORRELATES TO BRAIN REGIONS AND NETWORKS
We will review Cortical Lobes, Subcortical Structures, and A Deep Dive Into Brodmann Areas.
Please click on the podcast icon below to hear a full-length lecture for Section D.

Cortical Lobes
The cortical lobes are named for the overlying bones of the skull (Breedlove & Watson, 2023). Graphic © Sebastian Kaulitzki/Shutterstock.com.
The cortex is required for executive functions like attention, planning, and problem-solving.
Without a cerebral cortex, a person would be blind, deaf, dumb, and unable to initiate voluntary movement (Bear, Connors, & Paradiso, 2016, p. 205).
The five major cortical regions include the frontal, parietal, temporal, and occipital lobes, and the insula (not shown). Cortical lobes graphic © Madrock24/Shutterstock.com.
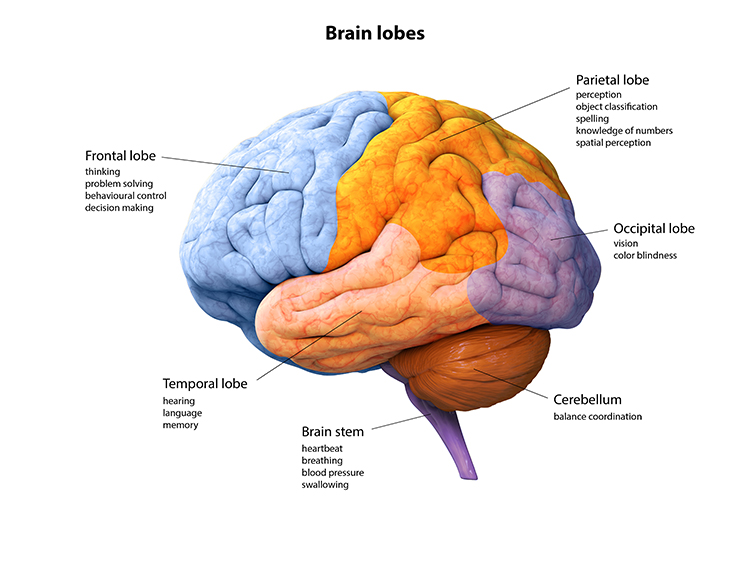
Frontal Lobes
The frontal lobes (F7, F3, Fz, F8, F4) consist of the cortex anterior to the central sulcus and consist of the primary motor cortex, motor association cortex, Broca's area, and prefrontal cortex.Essential left frontal lobe functions include working memory, concentration, planning, and positive emotion. The main clinical concern is Major Depressive Disorder (MDD).
Critical right frontal lobe functions include declarative memory, social awareness, and negative emotions. The main clinical concerns include Generalized Anxiety Disorder (GAD), fear, and impaired executive functioning.
Frontal lobe damage may result in impaired flexibility and problem-solving, increased risk-taking, changes in social behavior, an inability to use external cues, and deficits in emotional self-regulation. Graphic © ART-ur/Shutterstock.com.
The primary motor cortex is located in the precentral gyrus (Brodmann area 4, BA 4). It organizes the opposite side of the body's muscles and movements required for the fine motor coordination required by tasks like writing. Lesions can result in loss of motor control, including rigid paralysis. The graphic below, which shows the motor and sensory homunculi, was retrieved from the nccpbwikiproject.
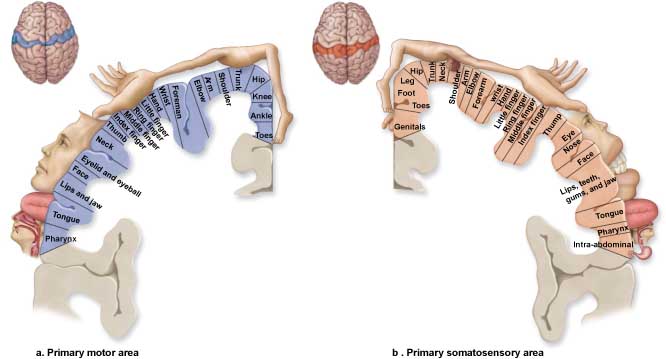
The motor association cortex (premotor cortex) is rostral to the primary motor cortex (BA 6) and helps program and execute movements. The motor association cortex is the piano player, and the primary motor cortex is the piano keyboard (Carlson & Birkett, 2016). The primary and motor association cortex collectively appear to map behaviors instead of specific muscles or movements (Breedlove & Watson, 2023).
Broca's area, which is located in the inferior frontal gyrus (BA 44 and 45) of the dominant hemisphere (F7-T3 in the left hemisphere), is concerned with speech production, grammar, language comprehension, and sequencing (Caplan, 2006). Lesions to Broca's area can produce dyslexia, deficits in grammar, spelling, and reading, and Broca's aphasia. Broca's area receives input from Wernicke's area via the arcuate fasciculus (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.
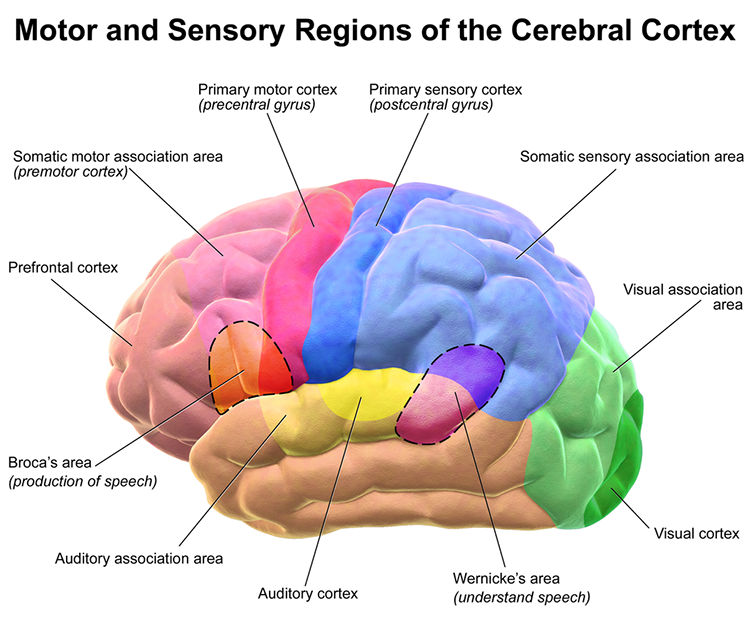
The prefrontal cortex (PFC) is rostral to the motor association area (BA 9, 10, 11, 12, 46, 47). It is responsible for executive functions, which involve attention, working memory, prediction of the outcomes of current and hypothetical actions, working toward goals, problem-solving, planning, and the ability to suppress actions that could lead to unwanted outcomes (Diamond, 2013). The PFC integrates emotion and reward in decision-making (Fuster, 2015).
Important subdivisions of the PFC include the orbitofrontal cortex, ventromedial, and dorsolateral PFC.
The orbitofrontal cortex (OFC) consists of Brodmann area (BA 10, 11, and 47) (Kringelbach, 2005). The OFC may aid planning by evaluating the consequences of our actions (rewards and punishments) and motivating us to ingest drugs. Phineas Gage's profound personality changes were produced by damage to this subdivision and the ventromedial PFC (VMPFC). The OFC appears to adjust decision-making based on the stakes involved, enabling us to switch between significant (investments) and trivial (snacks) choices. Finally, the OFC compares our current options with recent ones, while the anterior cingulate cortex registers our predictions and prediction errors (Kennerley et al., 2011).
Phineas Gage's profound personality changes were produced by damage to this subdivision and the ventromedial PFC (VMPFC). The OFC appears to adjust decision-making based on the stakes involved and enables us to switch between significant (investments) and trivial (snacks) choices. Finally, the OFC compares our current options with recent ones, while the anterior cingulate cortex registers our predictions and prediction errors (Kennerley et al., 2011).
The ventromedial prefrontal cortex (VMPFC) corresponds to the ventromedial reward network (Ongur & Price, 2000) and includes BA 10, 14, 25, 32, and parts of 11, 12, and 13. The VMPFC is implicated in making decisions where the outcomes are uncertain and moral values must be applied to actual situations. Patients with damage to the VMPFC choose outputs that lead to immediate reward, regardless of their future cost. They do not learn from their mistakes. Since they have difficulty understanding social cues, they may not recognize deception, irony, or sarcasm (Zaid & Andreotti, 2010). Likewise, they may not control their emotional reactions in social situations, particularly anger and violence (Carlson & Birkett, 2016).
The dorsolateral prefrontal cortex (DLPFC) is located in the middle frontal gyrus and includes BA 9 and 46. The DLPFC shares responsibility with cortical and subcortical networks for executive functions like abstract reasoning, cognitive flexibility, decision-making, inhibition, planning, and working memory (Miller & Cummings, 2007). It exercises the highest cortical level of motor control (Hale & Fiorello, 2004).
The left DLPFC is concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve them. The right DLPFC organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location. In unipolar depression and premenstrual dysphoric disorder, the right DLPFC may be more active than the left (alpha asymmetry). Prefrontal cortex subdivision graphic by minaanandag at fiverr.com.
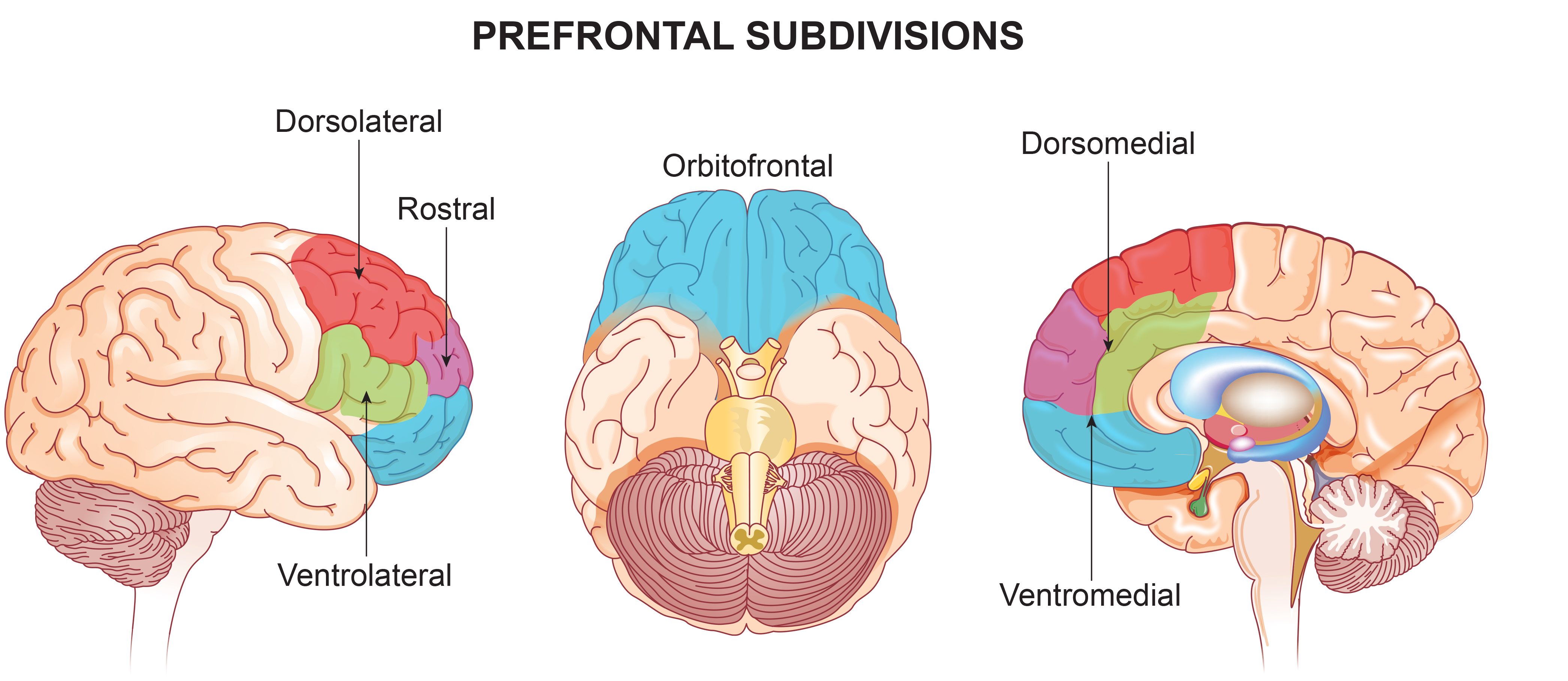
Anterior Cingulate Cortex (ACC)
The cingulate cortex has reciprocal connections with the parahippocampal gyri, integrates limbic functions, and is part of the salience network. Cingulate cortical functions include child nurturing, grooming, play, organization, and managing input/output functions.The anterior cingulate cortex (ACC) (Fpz, Fz, Cz, Pz) lies above the corpus callosum (BA 24, 32, 33). The dorsal ACC is connected to both the PFC and parietal cortex. The ACC plays a vital role in attention and is activated during working memory. The ACC mediates emotional and physical pain and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components. Graphic courtesy of Geoff B. Hall in Wikimedia Commons.
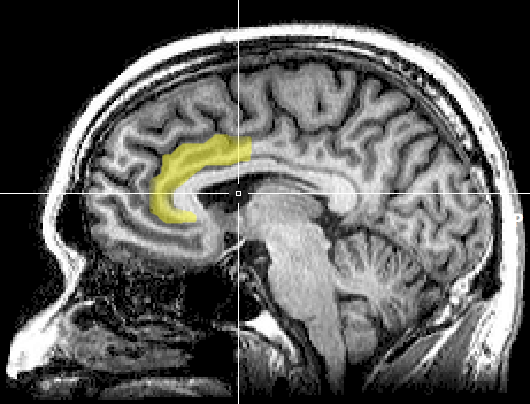
The Stroop test illustrates a cognitive monitoring task where color and names conflict. Discrepancies between facial and vocal cues show an affective conflict. The anterior cingulate recruits other brain areas to resolve these conflicts.
The anterior cingulate gyrus helps us allocate attention to focusing on a target and then disengaging, perceiving options, and making adaptive choices. The anterior cingulate gyrus, the prefrontal cortex, and the caudate function abnormally in children diagnosed with ADHD during selective attention tasks. fMRI evaluation showed that neurofeedback could teach children to normalize activity in these structures (Beauregard & Levesque, 2006).
The anterior cingulate gyrus is involved in motivation and perceiving emotional and physical pain. Eisenberger, Lieberman, and Williams (2003) used an fMRI to study the brains of subjects who believed that two companions playing an on-line baseball simulation dropped them suddenly from the game. Their emotional distress activated the anterior cingulate cortex, which evaluates the unpleasantness of physical pain. de Charms and colleagues (2005) provided real-time fMRI feedback from the anterior cingulate to subjects. They learned to reduce its metabolism and the intensity of experimental pain.
Lesions to the cingulate can produce akinetic mutism, in which a person cannot produce orienting responses. Cingulate malfunction can result in addictive behaviors (alcohol or drug abuse, eating disorders, chronic pain), obsessive-compulsive disorder and OCD spectrum disorders, and “road rage.”
Parahippocampal Gyri
The parahippocampal gyri are located within the medial temporal lobe. The parahippocampal gyri form spatial and nonspatial contextual associations, which are building blocks for contextual processing, episodic memory, navigation, and scene processing (Aminoff, Kveraga, & Bar, 2013). They may also play a role in emotional responsiveness. The parahippocampal gyrus graphic © mybox/Shutterstock.com.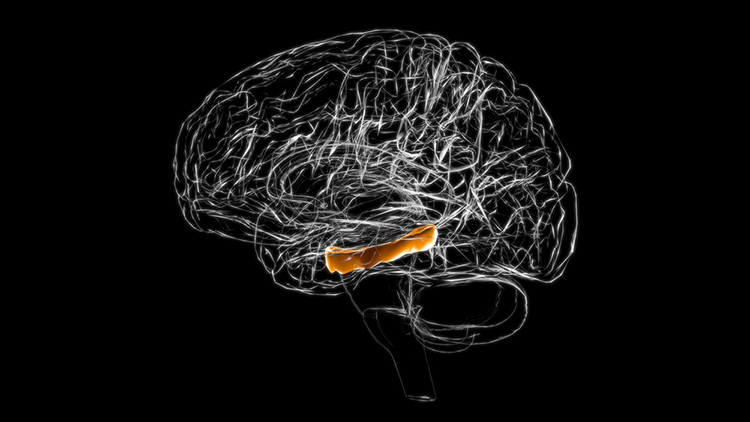
Parietal Lobes
The parietal lobes (Pz, P3, P4) are posterior to the frontal lobes (BA 1, 2, 3, 5, 7, 39, 40) and are divided into the primary somatosensory cortex and secondary somatosensory cortex. Their main function is to process somatosensory information like pain and touch.Major left parietal lobe functions include problem-solving, math, complex grammar, attention, and association. Essential right parietal lobe functions include spatial awareness and geometry. Graphic © ART-ur/Shutterstock.com.
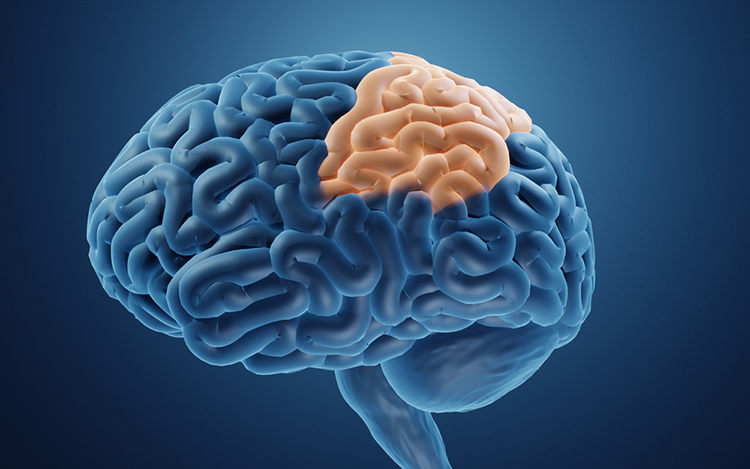
The primary somatosensory cortex (S1) is located in the parietal lobe's postcentral gyrus posterior to the central sulcus (BA 3, 1, and 2). S1 maps touch and pain information from the opposite side of the body. The secondary somatosensory cortex (S2) is adjacent to S1 (BA 40 and 43), receives projections from it, and maps touch and pain from both sides of the body (Breedlove & Watson, 2020). Graphic by Paskari from Wikimedia Commons.
The primary function of the parietal lobes is to process somatosensory information like pain and touch. The parietal cortex monitors our preparation for a movement and is responsible for our subjective feeling of intending to move (Sirigu et al., 2004).
The angular gyrus located near the superior temporal lobe (BA 39) is involved in reading, math, and copying writing. The graphic below highlights the angular gyrus in red © Kateryna Kon/Shutterstock.com.
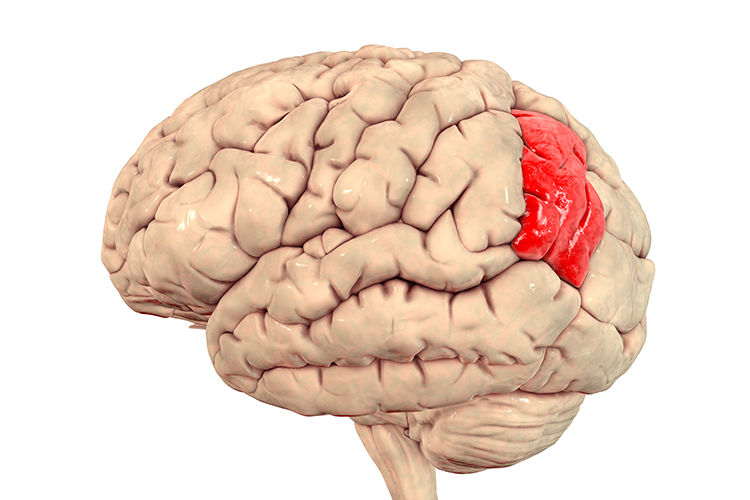
Major left hemisphere functions include attention, association, complex grammar, math, object names, and somatosensation.
Major right hemisphere functions include body boundary, geometry, guiding reaching with the hands, somatosensation, and spatial perception (Demos, 2019).
Temporal Lobes
The temporal lobes (T3, T4, T5, T6) are separated from the rest of the cortical lobes by the Sylvian fissure (BA 15, 20, 21, 22, 37, 38, 39, 40, 52). The temporal lobes process hearing, smell, and taste information and help us understand spoken language and recognize visual objects and faces (Breedlove & Watson, 2023).Wernicke's area, located in the temporoparietal cortex (BA 22) of the dominant hemisphere, is specialized for speech perception and production. Damage can result in an inability to understand speech's meaning and construct intelligible sentences. Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.

Major left hemisphere functions include affect, declarative memories, language comprehension, perception of movement, reading, and word recognition.
Important right hemisphere functions include face and object recognition, music, and social cues (Demos, 2019).
Occipital Lobes
The occipital lobes (Oz, O1, O2) are posterior to the parietal lobes. The primary visual cortex (VI) is located within the calcarine sulcus (BA 17). The occipital lobes process visual information from the eyes with the frontal, parietal, and temporal lobes. Graphic © ART-ur/Shutterstock.com.
Their primary functions are visual and include the analysis of orientation, color, spatial frequency, illusory contours, and complex patterns like concentric and radial stimuli (Breedlove & Watson, 2020).
Insular Cortex
The insular cortex lies deep within the lateral sulcus, which divides the temporal and parietal lobes (BA 13). The insula is involved in emotional and autonomic responses to external stimuli and is part of the salience network. The insula detects salient events via afferent pathways and switches between other large-scale networks when such events are identified, affecting attention and working memory. The anterior and poster insulae interact to regulate autonomic responses to salient stimuli. Interactive communication between the insula and anterior cingulate cortex facilitates motor control (Menon & Uddin, 2010). The right insula mediates awareness of our body, empathy, and understanding others’ points of view (Khazan, 2019).Increased heart rate variability strengthens the connectivity between the ACC and the insula for empathy and the ability to understand others’ emotions, feel gratitude, connect socially, understand our psychophysiological states, and restore nervous system balance. Mindfulness meditation increases insula gray matter and activation.
The insula functions as an integrative and organizational hub for the salience network. The insula integrates interoceptive awareness, emotional experience, and external perception to facilitate our global perception of and relationship with the world. The insula directs specific networks in processing salient stimuli and generating appropriate behavioral responses to these stimuli (Wiebking & Northoff, 2014).
The insula is the primary taste cortex and is activated when you see something that disgusts you (a fraternity bathroom) or see another person’s expression of disgust. Pictures of lovers also activate the anterior insula as opposed to friends. When the anterior insula was activated in neuroeconomic studies, subjects chose risk-avoidant financial strategies (choosing bonds instead of stocks). In the Prisoner’s Dilemma game, mutually cooperative decisions also resulted in the activation of this region. Insula graphic redrawn by minaanandag at fiverr.com.
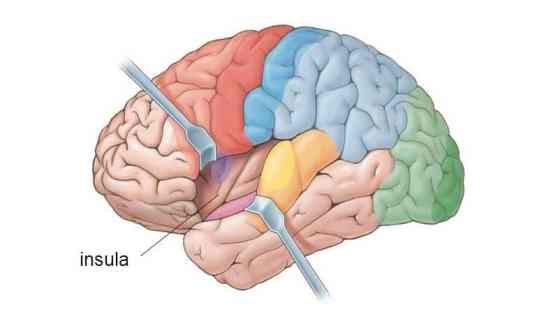
Antonio Damasio has proposed that this region helps map visceral states associated with emotional experience and generate conscious feelings. This could provide the basis of somatic markers like the discomfort produced by a risky decision.
The insular cortex has been implicated in the experience of pain and basic emotions, including anger, disgust, fear, happiness, and sadness. The insular cortex receives reports of internal states, like hunger and drug craving, and motivates individuals to engage in consummatory behavior. The insular cortex plays a crucial role in craving and impulse control. Drug-related cues stimulate this region and may activate memories of pleasurable drug-related experiences. Stroke damage to the insular cortex (see red area below) can eliminate nicotine addiction. Graphic from National Institute of Drug Abuse retrieved from Wikimedia Commons.
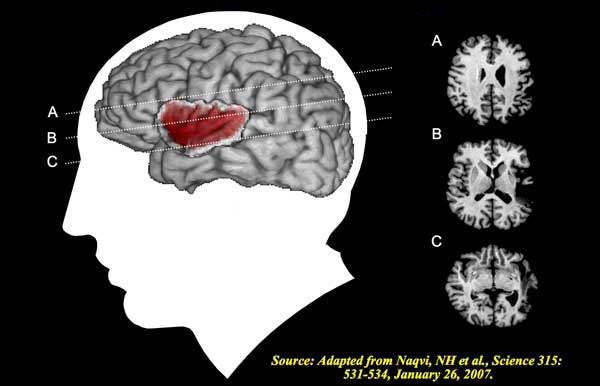
Cortical and Subcortical Connections
Please click on the podcast icon below to hear a full-length lecture for Section B.
Neocortical zones are connected with cortical and subcortical regions by specialized fiber tracts: association bundles, projection fibers, and commissural bundles. A meta-networking model proposes that the dynamic interaction of "distributed but relatively specialized networks" mediates brain functions like language (Herbet & Duffau, 2020, p. 1181).
Association Bundles
Association bundles link cortical regions in the same hemisphere using U-shaped white matter tracts. These include the arcuate fasciculus (AF), frontal aslant tract (FAT), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fascicle (ILF), middle longtitudinal fasciculus (MdLF), superior longitudinal fasciculus (SLF), and uncinate fasciculus (UF).Projection Fibers
Projection fibers connect the cortex with structures deep in the brain, the brainstem, and the spinal cord. The frontostriatal tract (FST) connects the premotor cortex with the caudate nucleus and putamen, thalamocortical, optic, and pyramidal tracts. Tractography animation Alfred Anwander, CC BY-SA 4.0, via Wikimedia Commons.
Commissural Bundles
The left and right hemispheres communicate using three commissures or axon tracts. The corpus callosum is the largest tract and connects the left and right frontal, parietal, and occipital lobes. Certain conditions, such as prenatal exposure to alcohol and other drugs, may result in agenesis of the corpus callosum, in which part or all of this fiber bundle is missing. Graphic © decade3d - anatomy online/Shutterstock.com.
The anterior commissure, shown above the third ventricle at the bottom of the diagram, is considerably smaller than the corpus callosum and connects the left and right temporal lobes and the hippocampus and amygdala. The posterior commissurebelow the corpus callosum connects the right and left diencephalon and mesencephalon (Breedlove & Watson, 2023). Anterior commissure graphic Winter, T. J. & Franz, E. A., CC BY 3.0, via Wikimedia Commons.
Subcortical Structures
Thalamus
The thalamus consists of specialized nuclei that process and relay data to and from the telencephalon (cerebral cortex, basal ganglia, and limbic system). The thalamus analyzes all sensory data except olfaction before distributing this information to the cortex via thalamocortical afferent fibers (Breedlove & Watson, 2023). The cortex also sends information to the thalamus to adjust its information processing via corticothalamic fibers. This two-way conversation creates feedback loops crucial for generating several EEG rhythms. The thalamus helps regulate arousal, sleep, and wakefulness (Steriade & Llinás, 1988). Its functional connection to the hippocampus plays a crucial role in episodic memory (Aggleton et al., 2010).The thalamus contributes to SCPs, delta, theta, alpha, SMR, and beta-gamma (Thompson & Thompson, 2016). Thalamus graphic © decade3d - anatomy online/Shutterstock.com.
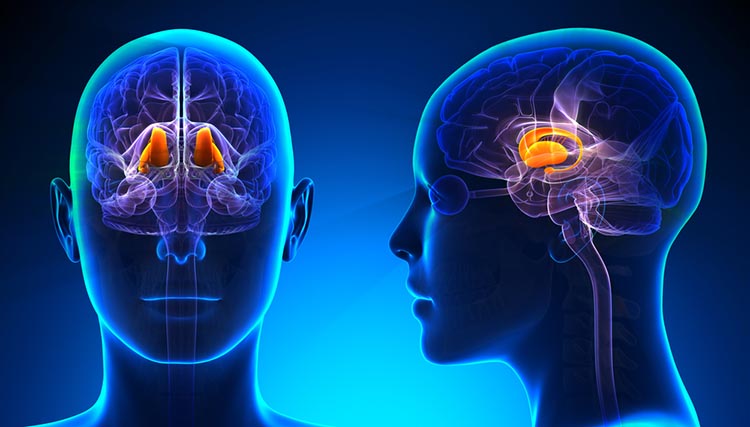
Basal Ganglia
The basal ganglia--the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra—-modulate movement. The basal ganglia, prefrontal cortex, cingulate cortex, and parietal cortex are involved in self-awareness, attention, and emotional regulation. Graphic © Kateryna Kon/Shutterstock.com.
Caption: The dorsal striatum consists of the caudate nucleus (green) and the putamen (orange). The amygdala is colored in blue
Limbic System
The limbic system is a poorly defined widespread network of nuclei involved in emotion, motivation, learning, memory, and navigation. Three important limbic structures are the hippocampus, amygdala, and septal nuclei (Breedlove & Watson, 2023). Limbic system graphic © decade3d - anatomy online/Shutterstock.com.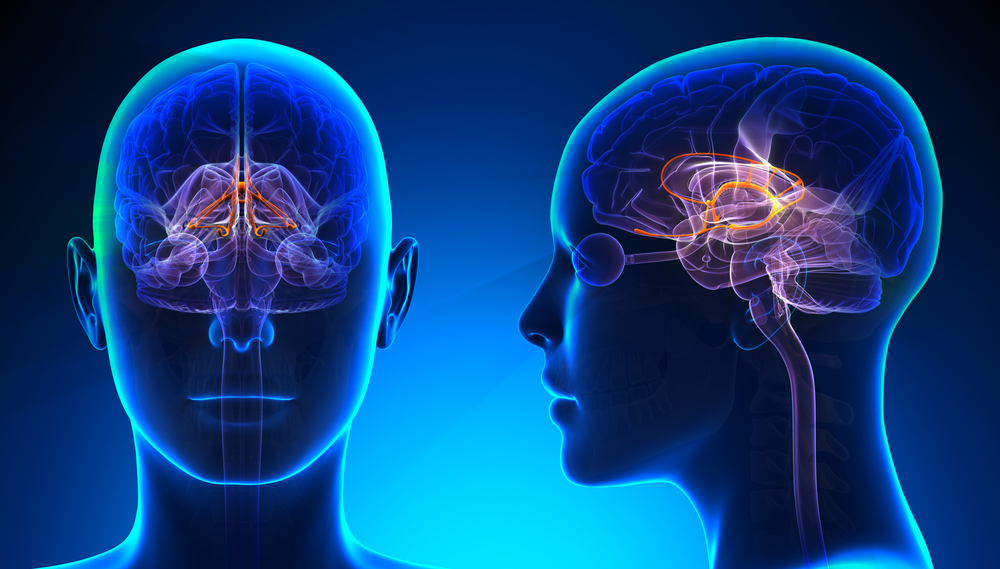
Hippocampus
The hippocampus is a seahorse-shaped limbic structure. The hippocampus is required to form declarative memories and plays a vital role in emotion, navigation, and spatial memory, and dampening the endocrine stress response. The hippocampus simultaneously integrates emotional, auditory, and visuospatial information to create episodic memories. The hippocampus also contains leukocyte receptors, making it part of the feedback loop for immune system regulation. Hippocampal neurons and networks that include it are sources of the theta rhythm (Amzica & Lopes da Silva, 2011).Hippocampal Brainwaves Travel in Two Directions
The old-school view was that hippocampal brainwaves travel in one direction. This model could not explain how the hippocampus integrates information from interconnected specialized systems. Based on recordings from human participants undergoing brain surgery, the new-school view is that brainwaves travel through the hippocampus in both directions: from the back to the front and from the front to the back (Kleen et al., 2021). Moreover, cognitive activity differentially influences the direction of movement for low (e.g., 1.9 Hz) and high (e.g., 13.8 Hz) frequency waveforms.Human hippocampal neuron graphic © Kateryna Kon/Shutterstock.com.
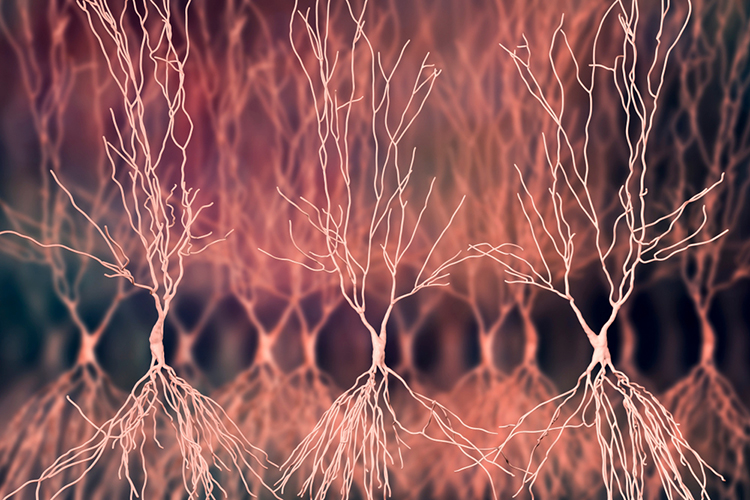
Watch Sam Kean's TED-Ed Talk, What Happens When You Remove the Hippocampus. Hippocampus graphic © decade3d - anatomy online/Shutterstock.com.
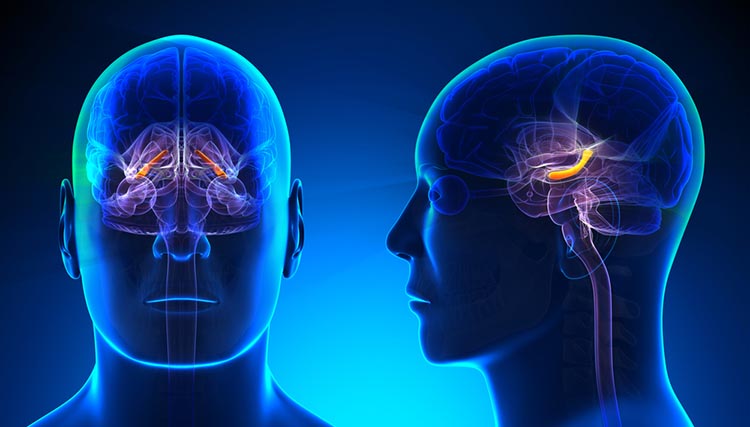
Amygdala
The amygdala is an essential limbic structure located deep within the medial temporal lobes at the end of the hippocampus. The amygdala comprises many nuclei, including the lateral and central nuclei. Rotating limbic system graphic lifesciencedb, CC BY-SA 2.1 JP, via Wikimedia Commons.
Caption: Human brain (hypothalamus = red, amygdala = green, hippocampus/fornix = blue, pons = gold, pituitary gland = pink).
The lateral nucleus processes sensory information and distributes it throughout the amygdala. The central nucleus orchestrates the nervous system's response to important stimuli by activating circuits in the brainstem (autonomic arousal), the basal ganglia, and periaqueductal gray (defensive behavior). The amygdala plays a crucial role in learning about the consequences of our actions and creating declarative memories of events with emotional significance (Breedlove & Watson, 2023). Amygdala graphic © decade3d - anatomy online/Shutterstock.com.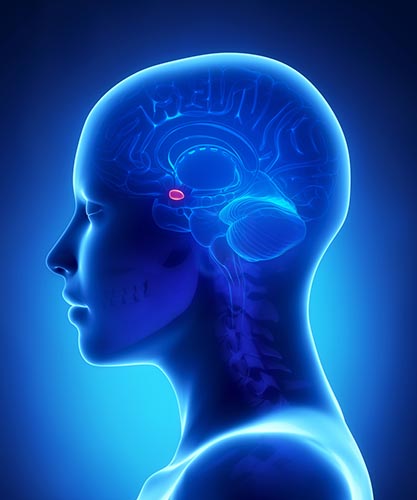
Septal Nuclei
The septal nuclei are a limbic structure that contains several nuclei involved in emotion, control of aggressive behavior, reward, and addiction (Breedlove & Watson, 2023). The septohippocampal system contributes to the theta rhythm (Amzica & Lopes da Silva, 2011). Septal nuclei graphic © MattL_Images/Shutterstock.com.
Glossary
affective network: a network that is triggered when we make mistakes and that monitors cognitive activity to predict when errors are likely, and greater executive control may be needed. The affective network includes the anterior cingulate cortex, hippocampal cortex, entorhinal cortex, superior temporal gyrus, inferior temporal gyrus, posterior parietal cortex, globus pallidus internal segment, substantia nigra, pars reticulata, and medial dorsal nucleus of the thalamus.
amygdala: a limbic system structure that participates in evaluating whether stimuli are salient (rewarding or threatening), establishing unconscious emotional memories, learning conditioned emotional responses, and producing anxiety and fear responses.
angular gyrus: located in the parietal lobe near the junction of the temporal and occipital lobes, the angular gyrus corresponds to Brodmann area 39. It plays a role in language processing, attention, spatial cognition, and integration of sensory information.
anterior cingulate cortex (ACC): located in the medial portion of the frontal lobes, the anterior cingulate cortex encompasses Brodmann areas 24, 25, 32, and 33. It plays a role in executive function, emotional regulation, attention, conflict monitoring, and error detection.
anterior prefrontal cortex (aPFC): found in the most anterior region of the prefrontal cortex, the anterior prefrontal cortex includes Brodmann areas 10 and 11. It involves complex cognitive processes such as planning, decision-making, working memory, and abstract reasoning.
attention: the selection of sensory information or cognition for enhanced processing.
attentional spotlight: a shift of selective attention to choose stimuli for enhanced processing.
auditory cortex: situated in the superior temporal gyrus, the auditory cortex encompasses Brodmann areas 41 and 42. It is responsible for processing and interpreting auditory information.
central autonomic network (CAN): a complex system of brain regions that is involved in the regulation of the autonomic nervous system. This network includes several brain structures like the prefrontal cortex, anterior cingulate cortex, insula, amygdala, hypothalamus, periaqueductal gray, parabrachial complex, nucleus of the solitary tract, and the medulla oblongata. These structures work together to regulate the body's physiological states, such as heart rate, blood pressure, respiration, digestion, and thermoregulation.
central executive network: structures including the dorsolateral prefrontal cortex, anterior cingulate cortex, and orbitofrontal cortex responsible for cognitive regulation of behavior.
cingulate cortex: a part of the limbic system, the cingulate cortex is situated in the medial aspects of the frontal and parietal lobes, covering Brodmann areas 23, 24, 30, 31, and 33. It involves emotion processing, memory, attention, and cognitive control.
covert attention: an attentional focus independent of sensory orientation.
creative fluency: generating creative ideas like alternative uses for everyday objects.
default mode network (DMN): frontal, temporal, and parietal lobe circuits that are active during introspection and daydreaming and relatively inactive when we pursue external goals.
dorsal anterior cingulate cortex (dACC): located in the dorsal region of the anterior cingulate cortex, the dorsal anterior cingulate cortex includes Brodmann areas 24 and 32. It plays a role in cognitive control, decision-making, and conflict monitoring.
dorsal entorhinal cortex (DEC): situated in the medial temporal lobe, the dorsal entorhinal cortex comprises parts of Brodmann area 28. It is involved in spatial memory and navigation.
dorsal frontoparietal system: the network comprised of the intraparietal sulcus and frontal eye field responsible for the top-down direction of attention.
dorsal posterior cingulate cortex (dPCC): located in the posterior part of the cingulate cortex, the dorsal posterior cingulate cortex covers Brodmann area 31. It is involved in self-referential thought, memory, and spatial awareness.
dorsolateral prefrontal cortex (DLPFC): the left dorsolateral prefrontal cortex is concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals. The right dorsolateral prefrontal cortex organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
early selection: filtering out lower-priority competing stimuli before preliminary perceptual and semantic analysis.
ectosplenial cerebral cortex: part of the retrosplenial cortex, the ectosplenial cerebral cortex is situated within the cingulate cortex and covers Brodmann area 29. It plays a role in spatial memory, navigation, and contextual processing.
endogenous attention: voluntary attention that directs the attentional spotlight to support cognitive system priorities.
executive network: a network responsible for allocating attention, cognitive inhibition, behavioral inhibition, working memory, and cognitive flexibility. The executive network includes the dorsolateral prefrontal cortex, posterior parietal cortex, arcuate premotor area, globus pallidus internal segment, substantia nigra, pars reticulata, ventral anterior nucleus of the thalamus, and medial dorsal nucleus of the thalamus.
exogenous attention): involuntary reflexive attention that redirects attention based on the novelty or importance of incoming stimuli.
frontal eye field (FEF): a region of the premotor cortex that directs gaze towards targets selected by the IPS.
functional networks: correlated activity between regions over time.
fusiform gyrus: situated in the ventral region of the temporal and occipital lobes, the fusiform gyrus includes Brodmann areas 37 and parts of 19 and 20. It is involved in face recognition, object recognition, and color and visual form processing.
inferior temporal gyrus (ITG): Located in the inferior temporal lobe region, the inferior temporal gyrus encompasses Brodmann areas 20 and 21. It plays a role in visual object recognition and semantic memory.
insular cortex (insula): the cortical region located within the lateral sulcus of the frontal, parietal, and temporal lobes that functions as an integrative and organization hub for the salience network.
intraparietal sulcus (IPS): the region of the parietal lobe that provides voluntary top-down steering of attention.
late selection: filtering out competing stimuli after performing extensive analysis.
magnetic resonance imaging (MRI): a non-invasive imaging technology that uses a strong magnetic field and radio waves to produce detailed images of the inside of the body. It's especially useful for imaging soft tissues and organs like the brain, spinal cord, muscles, and heart. It provides high-resolution, 3D images that can be viewed from different angles, making it a valuable tool in medical diagnosis and research.
middle temporal gyrus (MTG): located in the middle region of the temporal lobe, the middle temporal gyrus encompasses Brodmann areas 21 and 37. It plays a role in language processing, semantic memory, and visual motion processing.
motor network: the network that plans, initiates and inhibits voluntary movements and muscle contractions. The motor network includes the supplementary motor area, premotor cortex, primary motor cortex, primary somatosensory cortex, cerebellum, arcuate premotor area, globus pallidus internal segment, substantia nigra, pars reticulata, and ventral lateral nucleus of the thalamus.
neurvisceral integration model: a theoretical framework that suggests the heart, brain, and other bodily systems are interconnected and communicate with each other to maintain overall health and well-being. It posits that autonomic, attentional, and affective systems are integrated within the central autonomic network and that imbalances within this network may underlie the associations between stress, disease, and cognitive function.
oculomotor network: a network that programs and initiates voluntary eye movements, inhibits eye movements toward distracting stimuli, and allows us to return our focus to locations we've previously experienced. The oculomotor network includes the frontal eye field, dorsolateral prefrontal cortex, posterior parietal cortex, caudate, globus pallidus internal segment, substantia nigra, pars reticulata, ventral anterior nucleus of the thalamus, and medial dorsal nucleus of the thalamus.
orbitofrontal cortex (OFC): found in the ventral part of the frontal lobes, the orbitofrontal cortex includes Brodmann areas 10, 11, 12, 13, 14, and 47. It involves decision-making, reward processing, emotional regulation, and social cognition.
overt attention: the agreement between attentional focus and sensory orientation.
pars opercularis: located in the inferior frontal gyrus, the pars opercularis corresponds to Brodmann area 44. It plays a role in language production and is part of Broca's area.
pars orbitalis: situated in the ventral part of the inferior frontal gyrus, the pars orbitalis covers Brodmann area 47. It is involved in language processing, social cognition, and emotional regulation.
pars triangularis: located in the anterior part of the inferior frontal gyrus, the pars triangularis corresponds to Brodmann area 45. It is involved in language processing and is part of Broca's area.
parainsular area: found adjacent to the insular cortex, the parainsular area comprises parts of Brodmann areas 13 and 52. It involves auditory and somatosensory integration and processing pain and temperature sensations.
perirhinal cortex: situated in the medial temporal lobe, the perirhinal cortex encompasses Brodmann areas 35 and 36. It plays a role in object recognition, associative memory, and contextual processing.
prefrontal cortex: the most anterior region of the frontal lobes divided into orbitofrontal and ventromedial, dorsolateral prefrontal cortex, and anterior and ventral cingulate cortex subdivisions, and is responsible for the brain’s executive functions.
primary gustatory cortex: located within the insular cortex, the primary gustatory cortex corresponds to Brodmann area 43. It is responsible for processing taste information.
primary motor cortex (M1): situated in the precentral gyrus, the primary motor cortex corresponds to Brodmann area 4. It is responsible for voluntary movement control.
primary somatosensory cortex (S1): located in the postcentral gyrus, the primary somatosensory cortex covers Brodmann areas 1, 2, and 3. It is responsible for processing somatosensory information, including touch, pain, temperature, and proprioception.
primary visual cortex (V1): situated in the calcarine sulcus within the occipital lobe, the primary visual cortex corresponds to Brodmann area 17. It is responsible for processing basic visual information.
pulvinar nucleus: the posterior region of the thalamus that processes visual information and directs attention.
pyriform cortex: located in the ventral part of the temporal lobe, the pyriform cortex (also known as the primary olfactory cortex) includes parts of Brodmann areas 27, 28, and 34. It is responsible for processing olfactory information.
resting-state functional connectivity (RSFC): a neuroimaging method used to investigate brain networks that are active when a person is not focused on the outside world, often referred to as "at rest." These brain networks show synchronous activity when the person is not performing an explicit task. It's used in functional magnetic resonance imaging (fMRI) studies to assess connectivity and coordination between different parts of the brain. It helps in understanding brain organization and the baseline level of neural activity.
retrosplenial cingulate cortex: found in the posterior part of the cingulate cortex, the retrosplenial cingulate cortex covers Brodmann areas 29 and 30. It is involved in spatial memory, navigation, and contextual processing.
retrosubicular area: located in the medial temporal lobe, the retrosubicular area is part of the parahippocampal gyrus and corresponds to Brodmann area 27. It is involved in spatial navigation and memory.
salience network: structures including the insula and anterior cingulate cortex that seek to monitor our external and internal environments to determine which of these inputs are salient and require further processing and attention.
secondary visual cortex (V2): situated adjacent to the primary visual cortex in the occipital lobe, the secondary visual cortex corresponds to Brodmann area 18. It is involved in processing visual information, including recognition of shapes, colors, and spatial orientation.
social network: the network that mediates socially responsible behavior, empathy, behavioral inhibition, emotional regulation, and sound judgment. The social network includes the orbitofrontal cortex, superior temporal gyrus, inferior temporal gyrus, anterior cingulate cortex, caudate, globus pallidus internal segment, substantia nigra, pars reticulata, ventral anterior nucleus of the thalamus, and medial dorsal nucleus of the thalamus.
somatosensory association cortex (SAC): located in the parietal lobe, the somatosensory association cortex encompasses Brodmann areas 5 and 7. It is involved in the integration and interpretation of somatosensory information, such as touch, pain, temperature, and proprioception.
structural networks: axonal projections and pathways.
subgenual ventromedial prefrontal cortex (vmPFC): situated in the ventral part of the medial prefrontal cortex, the subgenual ventromedial prefrontal cortex includes parts of Brodmann areas 25, 32, and 14. It is involved in emotional regulation, decision-making, and social cognition.
supplementary motor cortex (SMA): located in the medial part of the superior frontal gyrus, the supplementary motor cortex corresponds to Brodmann area 6. It is involved in planning and coordinating complex movements and motor learning.
supramarginal gyrus: situated in the parietal lobe, the supramarginal gyrus is part of the inferior parietal lobule and corresponds to Brodmann area 40. It is involved in language processing, attention, and spatial cognition.
superior temporal gyrus (STG): located in the superior region of the temporal lobe, the superior temporal gyrus encompasses Brodmann areas 22, 41, and 42. It plays a role in auditory processing, language comprehension, and social cognition.
temporoparietal junction (TPJ): lthe intersection of the superior temporal gyrus and inferior parietal lobe that mediates bottom-up shifts in attention in response to stimulus attributes.
temporopolar area: situated in the most anterior part of the temporal lobe, the temporopolar area corresponds to Brodmann area 38. It is involved in olfactory processing, social cognition, and semantic memory.
ventral anterior cingulate cortex (vACC): located in the ventral region of the anterior cingulate cortex, the ventral anterior cingulate cortex includes parts of Brodmann areas 24, 25, and 33. It is involved in emotional regulation, attention, and pain processing.
ventral entorhinal cortex (VEC): situated in the medial temporal lobe, the ventral entorhinal cortex covers parts of Brodmann area 28. It is involved in object recognition, memory, and contextual processing.
ventral posterior cingulate cortex (vPCC): located in the ventral part of the posterior cingulate cortex, the ventral posterior cingulate cortex includes parts of Brodmann areas 23 and 31. It involves self-referential thought, episodic memory retrieval, and emotional processing.
ventromedial prefrontal cortex: a region of the prefrontal cortex that may play a role in calculating risk and the emotional responses of anxiety and fear. Cortisol binding to this structure increases anxiety and fear and disrupts and kills neurons.
visual association cortex: found in the occipital and parietal lobes, the visual association cortex includes Brodmann areas 18, 19, and parts of 7. It is responsible for higher-level visual processing, including object recognition, motion perception, and spatial awareness.
References
Aggleton, J. P., & Brown, M. W. (1999). Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. The Behavioral and Brain Sciences, 22(3), 425–489.
Ahuja, A., & Yusif Rodriguez, N. (2022). Is the dorsolateral prefrontal cortex actually several different brain areas? The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 42(33), 6310–6312. https://doi.org/10.1523/JNEUROSCI.0848-22.2022
Amaral, D. G., & Witter, M. P. (1995). Hippocampal formation. In G. Paxinos (Ed.), The rat nervous system (pp. 443-493). Academic Press.
Amunts, K., Schleicher, A., Bürgel, U., Mohlberg, H., Uylings, H. B., & Zilles, K. (1999). Broca's region revisited: Cytoarchitecture and intersubject variability. The Journal of Comparative Neurology, 412(2), 319–341. https://doi.org/10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R., & Buckner, R. L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. https://doi.org/10.1016/j.neuron.2010.02.005
Arnsten A. F. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. https://doi.org/10.1038/nrn2648
Augustine J. R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research. Brain Research Reviews, 22(3), 229–244. https://doi.org/10.1016/s0165-0173(96)00011-2
Avidan, G., & Behrmann, M. (2009). Functional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Current Biology: CB, 19(13), 1146–1150. https://doi.org/10.1016/j.cub.2009.04.060
Baliki, M. N., Geha, P. Y., & Apkarian, A. V. (2009). Parsing pain perception between nociceptive representation and magnitude estimation. Journal of Neurophysiology, 101(2), 875–887. https://doi.org/10.1152/jn.91100.2008
Beissner, F., Meissner, K., Bär, K. J., & Napadow, V. (2013). The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(25), 10503–10511. https://doi.org/10.1523/JNEUROSCI.1103-13.2013
Benarroch E. E. (1993). The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings, 68(10), 988–1001. https://doi.org/10.1016/s0025-6196(12)62272-1
Bizley, J. K., & Cohen, Y. E. (2013). The what, where and how of auditory-object perception. Nature Reviews. Neuroscience, 14(10), 693–707. https://doi.org/10.1038/nrn3565
Booth, J. R., Wood, L., Lu, D., Houk, J. C., & Bitan, T. (2007). The role of the basal ganglia and cerebellum in language processing. Brain Research, 1133(1), 136–144. https://doi.org/10.1016/j.brainres.2006.11.074
Braak, H., & Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica, 82(4), 239–259. https://doi.org/10.1007/BF00308809
Breedlove, S., M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Bressler, S. L., & Menon, V. (2010). Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. https://doi.org/10.1016/j.tics.2010.04.004
Brodmann, K. (1909). Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth.
Broyd, S. J., Demanuele, C., Debener, S., Helps, S. K., James, C. J., & Sonuga-Barke, E. J. (2009). Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 33(3), 279–296. https://doi.org/10.1016/j.neubiorev.2008.09.002
Buchsbaum, M. S., Buchsbaum, B. R., Chokron, S., Tang, C., Wei, T. C., & Byne, W. (2006). Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers. Neuroscience Letters, 404(3), 282-287. https://doi.org/10.1016/j.neulet.2006.05.063
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1-38. https://doi.org/10.1196/annals.1440.011
Buckner, R. L., Snyder, A. Z., Shannon, B. J., LaRossa, G., Sachs, R., Fotenos, A. F., Sheline, Y. I., Klunk, W. E., Mathis, C. A., Morris, J. C., & Mintun, M. A. (2005). Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(34), 7709–7717. https://doi.org/10.1523/JNEUROSCI.2177-05.2005
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. https://doi.org/10.1016/s1364-6613(00)01483-2
Cao, Z., Ottino-Gonzalez, J., Cupertino, R. B., Schwab, N., Hoke, C., Catherine, O., Cousijn, J., Dagher, A., Foxe, J. J., Goudriaan, A. E., Hester, R., Hutchison, K., Li, C. R., London, E. D., Lorenzetti, V., Luijten, M., Martin-Santos, R., Momenan, R., Paulus, M. P., Schmaal, L., … Garavan, H. (2021). Mapping cortical and subcortical asymmetries in substance dependence: Findings from the ENIGMA Addiction Working Group. Addiction Biology, 26(5), e13010. https://doi.org/10.1111/adb.13010
Carey, L. M., Abbott, D. F., Egan, G. F., O'Keefe, G. J., Jackson, G. D., Bernhardt, J., & Donnan, G. A. (2006). Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabilitation and Neural Repair, 20(1), 24–41. https://doi.org/10.1177/1545968305283053
Carlén M. (2017). What constitutes the prefrontal cortex? Science, 358(6362), 478–482. https://doi.org/10.1126/science.aan8868
Caspers, S., Geyer, S., Schleicher, A., Mohlberg, H., Amunts, K., & Zilles, K. (2006). The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. NeuroImage, 33(2), 430–448. https://doi.org/10.1016/j.neuroimage.2006.06.054
Castellanos, F. X., Margulies, D. S., Kelly, C., Uddin, L. Q., Ghaffari, M., Kirsch, A., Shaw, D., Shehzad, Z., Di Martino, A., Biswal, B., Sonuga-Barke, E. J., Rotrosen, J., Adler, L. A., & Milham, M. P. (2008). Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry, 63(3), 332–337. https://doi.org/10.1016/j.biopsych.2007.06.025
Catani, M., Jones, D. K., Donato, R., & Ffytche, D. H. (2003). Occipito-temporal connections in the human brain. Brain: A Journal of Neurology, 126(Pt 9), 2093–2107. https://doi.org/10.1093/brain/awg203
Chau, W., & McIntosh, A. R. (2005). The Talairach coordinate of a point in the MNI space: How to interpret it. NeuroImage, 25(2), 408–416. https://doi.org/10.1016/j.neuroimage.2004.12.007
Cole, M. W., Reynolds, J. R., Power, J. D., Repovs, G., Anticevic, A., & Braver, T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16, 1348–1355. https://doi.org/10.1038/nn.3470
Corbetta, M., & Shulman, G. I. (1998). Human cortical mechanisms of visual attention during orienting and search. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 353(1373), 1353-1362. https://doi.org/10.1098/rstb.1998.0289
Craig A. D. (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10(1), 59–70. https://doi.org/10.1038/nrn2555
Critchley H. D. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology, 493(1), 154–166. https://doi.org/10.1002/cne.20749
Critchley H. D. (2009). Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 73(2), 88–94. https://doi.org/10.1016/j.ijpsycho.2009.01.012
Davidson, R. J. (2000). The functional neuroanatomy of affective style. XCognitive neuroscience of emotion V. Oxford University Press, 371–388.
de la Cruz, F., Schumann, A., Köhler, S., Reichenbach, J. R., Wagner, G., & Bär, K. J. (2019). The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. NeuroImage, 196, 318–328. https://doi.org/10.1016/j.neuroimage.2019.04.014
Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain: A Journal of Neurology, 118 ( Pt 1), 279–306. https://doi.org/10.1093/brain/118.1.279
Dosenbach, N. U., Fair, D. A., Miezin, F. M., Cohen, A. L., Wenger, K. K., Dosenbach, R. A., Fox, M. D., Snyder, A. Z., Vincent, J. L., Raichle, M. E., Schlaggar, B. L., & Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. https://doi.org/10.1073/pnas.0704320104
Doty R. L. (2008). The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Annals of Neurology, 63(1), 7–15. https://doi.org/10.1002/ana.21327
Drevets, W. C., Price, J. L., & Furey, M. L. (2008). Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure & Function, 213(1-2), 93–118. https://doi.org/10.1007/s00429-008-0189-x
Du, F., Whetsell, W. O., Jr, Abou-Khalil, B., Blumenkopf, B., Lothman, E. W., & Schwarcz, R. (1993). Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Research, 16(3), 223–233. https://doi.org/10.1016/0920-1211(93)90083-j
Eichenbaum, H., Yonelinas, A. P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152. https://doi.org/10.1146/annurev.neuro.30.051606.094328
Epstein R. A. (2008). Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences, 12(10), 388–396. https://doi.org/10.1016/j.tics.2008.07.004
Etkin, A., Egner, T., & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. https://doi.org/10.1016/j.tics.2010.11.004
Faraone, S. V., Biederman, J., & Mick, E. (2006). The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychological Medicine, 36(2), 159–165. https://doi.org/10.1017/S003329170500471X
A. Fornito, A. Zalesky, & E. T. Bullmore (Eds.) (2016). Fundamentals of brain network analysis. Elsevier. https://doi.org/10.1016/C2012-0-06036-X
Fox, D. (2008). The secret life of the brain. New Scientist, 2681.
Frank, G. K., Shott, M. E., Riederer, J., & Pryor, T. L. (2016). Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Translational Psychiatry, 6(11), e932. https://doi.org/10.1038/tp.2016.199
Frankowski, J. C., Tierno, A., Pavani, S., Cao, Q., Lyon, D. C., & Hunt, R. F. (2022). Brain-wide reconstruction of inhibitory circuits after traumatic brain injury. Nat Commun, 13, 3417. https://doi.org/10.1038/s41467-022-31072-2
Friederici A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. https://doi.org/10.1152/physrev.00006.2011
Fuster J. M. (2001). The prefrontal cortex--An update: Time is of the essence. Neuron, 30(2), 319–333. https://doi.org/10.1016/s0896-6273(01)00285-9
Gevensleben, H., Moll, G. H., Rothenberger, A., & Heinrich, H. (2014). Neurofeedback in attention-deficit/hyperactivity disorder - Different models, different ways of application. Frontiers in Human Neuroscience, 8, 846. https://doi.org/10.3389/fnhum.2014.00846
Gilmore, A. W., Nelson, S. M., Chen, H. Y., & McDermott, K. B. (2018). Task-related and resting-state fMRI identify distinct networks that preferentially support remembering the past and imagining the future. Neuropsychologia, 110, 180–189. https://doi.org/10.1016/j.neuropsychologia.2017.06.016
Gilmore, A. W., Nelson, S. M., & McDermott, K. B. (2015). A parietal memory network revealed by multiple MRI methods. Trends in Cognitive Sciences, 19(9), 534–543. https://doi.org/10.1016/j.tics.2015.07.004
Glasser, M. F., Coalson, T. S., Robinson, E. C., Hacker, C. D., Harwell, J., Yacoub, E., Ugurbil, K., Andersson, J., Beckmann, C. F., Jenkinson, M., Smith, S. M., & Van Essen, D. C. (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536(7615), 171–178. https://doi.org/10.1038/nature18933
Gordon, E. M., Chauvin, R. J., Van, A. N., Rajesh, A., Nielsen, A., Newbold, D. J., Lynch, C. J., Seider, N. A., Krimmel, S. R., Scheidter, K. M., Monk, J., Miller, R. L., Metoki, A., Montez, D. F., Zheng, A., Elbau, I., Madison, T., Nishino, T., Myers, M. J., Kaplan, S., … Dosenbach, N. U. F. (2023). A somato-cognitive action network alternates with effector regions in motor cortex. Nature, 617(7960), 351–359. https://doi.org/10.1038/s41586-023-05964-2
Gorges, M., Müller, H. P., & Kassubek, J. (2018). Structural and functional brain mapping correlates of impaired eye movement control in Parkinsonian Syndromes: A systems-based concept. Frontiers in Neurology, 9, 319. https://doi.org/10.3389/fneur.2018.00319
Gottfried J. A. (2010). Central mechanisms of odour object perception. Nature Reviews. Neuroscience, 11(9), 628–641. https://doi.org/10.1038/nrn2883
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., Reiss, A. L., & Schatzberg, A. F. (2007). Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62(5), 429–437. https://doi.org/10.1016/j.biopsych.2006.09.020
Griffin, D. M., Hoffman, D. S., & Strick, P. L. (2015). Corticomotoneuronal cells are "functionally tuned." Science, 350(6261), 667–670. https://doi.org/10.1126/science.aaa8035
Griffiths, T. D., & Warren, J. D. (2002). The planum temporale as a computational hub. Trends in Neurosciences, 25(7), 348–353. https://doi.org/10.1016/s0166-2236(02)02191-4
Grill-Spector, K., Knouf, N., & Kanwisher, N. (2004). The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience, 7(5), 555–562. https://doi.org/10.1038/nn1224
Gross J. J. (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39(3), 281–291. https://doi.org/10.1017/s0048577201393198
Hafting, T., Fyhn, M., Molden, S., Moser, M. B., & Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436(7052), 801–806. https://doi.org/10.1038/nature03721
Hamilton, J. P., Glover, G. H., Hsu, J. J., Johnson, R. F., & Gotlib, I. H. (2011). Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Human Brain Mapping, 32(1), 22–31. https://doi.org/10.1002/hbm.20997
Han, M., Jiang, G., Luo, H., & Shao, Y. (2021). Neurobiological bases of social networks. Frontiers in Psychology, 12, 626337. https://doi.org/10.3389/fpsyg.2021.626337
Heckers, S., Rauch, S. L., Goff, D., Savage, C. R., Schacter, D. L., Fischman, A. J., & Alpert, N. M. (1998). Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience, 1(4), 318–323. https://doi.org/10.1038/1137
Hoeft, F., Meyler, A., Hernandez, A., Juel, C., Taylor-Hill, H., Martindale, J. L., McMillon, G., Kolchugina, G., Black, J. M., Faizi, A., Deutsch, G. K., Siok, W. T., Reiss, A. L., Whitfield-Gabrieli, S., & Gabrieli, J. D. (2007). Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the United States of America, 104(10), 4234–4239. https://doi.org/10.1073/pnas.0609399104
Holmes, A. J., MacDonald, A., 3rd, Carter, C. S., Barch, D. M., Andrew Stenger, V., & Cohen, J. D. (2005). Prefrontal functioning during context processing in schizophrenia and major depression: An event-related fMRI study. Schizophrenia Research, 76(2-3), 199–206. https://doi.org/10.1016/j.schres.2005.01.021
Hoskinson, K. R., Bigler, E. D., Abildskov, T. J., Dennis, M., Taylor, H. G., Rubin, K., Gerhardt, C. A., Vannatta, K., Stancin, T., & Yeates, K. O. The mentalizing network and theory of mind mediate adjustment after childhood traumatic brain injury. Soc Cogn Affect Neurosci., 14(12), 1285-1295. https://doi.org/10.1093/scan/nsaa006. PMID: 31993655; PMCID: PMC7137721.
Hu, Y., Wang, J., Li, C., Wang, Y. S., Yang, Z., & Zuo, X. N. (2016). Segregation between the parietal memory network and the default mode network: Effects of spatial smoothing and model order in ICA. Science Bulletin, 61(24), 1844–1854. https://doi.org/10.1007/s11434-016-1202-z
Hyman, B. T., Van Hoesen, G. W., Damasio, A. R., & Barnes, C. L. (1984). Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science, 225(4667), 1168–1170. https://doi.org/10.1126/science.6474172
Jackson, D. C., Malmstadt, J. R., Larson, C. L., & Davidson, R. J. (2000). Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology, 37(4), 515–522.
Jasper, H. H. (1958). The Ten-Twenty Electrode System of the International Federation. Electroencephalography and Clinical Neurophysiology, 10, 371-375.
Kaufman, M. T., Churchland, M. M., Ryu, S. I., & Shenoy, K. V. (2014). Cortical activity in the null space: Permitting preparation without movement. Nature Neuroscience, 17(3), 440–448. https://doi.org/10.1038/nn.3643
Khan, U. A., Liu, L., Provenzano, F. A., Berman, D. E., Profaci, C. P., Sloan, R., Mayeux, R., Duff, K. E., & Small, S. A. (2014). Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nature Neuroscience, 17(2), 304–311. https://doi.org/10.1038/nn.3606
Kluetsch, R. C., Ros, T., Théberge, J., Frewen, P. A., Calhoun, V. D., Schmahl, C., Jetly, R., & Lanius, R. A. (2014). Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatrica Scandinavica, 130(2), 123–136. https://doi.org/10.1111/acps.12229
Kringelbach M. L. (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews. Neuroscience, 6(9), 691–702. https://doi.org/10.1038/nrn1747
Kroptov, J. D. (2009). Quantitative EEG, event-related potentials and neurotherapy. Academic Press, Elsevier.
Kumral, D., Schaare, H. L., Beyer, F., Reinelt, J., Uhlig, M., Liem, F., Lampe, L., Babayan, A., Reiter, A., Erbey, M., Roebbig, J., Loeffler, M., Schroeter, M. L., Husser, D., Witte, A. V., Villringer, A., & Gaebler, M. (2019). The age-dependent relationship between resting heart rate variability and functional brain connectivity. NeuroImage, 185, 521–533. https://doi.org/10.1016/j.neuroimage.2018.10.027
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure & Function, 214(5-6), 519–534. https://doi.org/10.1007/s00429-010-0255-z
Leech, R., & Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain: A Journal of Neurology, 137(Pt 1), 12–32. https://doi.org/10.1093/brain/awt162
Linden D. E. (2006). How psychotherapy changes the brain--The contribution of functional neuroimaging. Molecular Psychiatry, 11(6), 528–538. https://doi.org/10.1038/sj.mp.4001816
Liston, C., Malter Cohen, M., Teslovich, T., Levenson, D., & Casey, B. J. (2011). Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: Pathway to disease or pathological end point? Biological Psychiatry, 69(12), 1168–1177. https://doi.org/10.1016/j.biopsych.2011.03.022
Makovac, E., Meeten, F., Watson, D. R., Herman, A., Garfinkel, S. N., D Critchley, H., & Ottaviani, C. (2016). Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in Generalized Anxiety Disorder. Biological Psychiatry, 80(10), 786–795. https://doi.org/10.1016/j.biopsych.2015.10.013
Margulies, D. S., Kelly, A. M., Uddin, L. Q., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. NeuroImage, 37(2), 579–588. https://doi.org/10.1016/j.neuroimage.2007.05.019
Margulies, D. S., Vincent, J. L., Kelly, C., Lohmann, G., Uddin, L. Q., Biswal, B. B., Villringer, A., Castellanos, F. X., Milham, M. P., & Petrides, M. (2009). Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America, 106(47), 20069–20074. https://doi.org/10.1073/pnas.0905314106
Mayberg H. S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin, 65, 193–207. https://doi.org/10.1093/bmb/65.1.193
Mayberg, H. S., Lozano, A. M., Voon, V., McNeely, H. E., Seminowicz, D., Hamani, C., Schwalb, J. M., & Kennedy, S. H. (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45(5), 651–660. https://doi.org/10.1016/j.neuron.2005.02.014
Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483-506. https://doi.org/10.1016/j.tics.2011.08.003
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5-6), 655–667. https://doi.org/10.1007/s00429-010-0262-0
Mesa, N. (2023). New brain network connecting mind and body discovered. Retrieved from The Scientist.
Morosan, P., Rademacher, J., Schleicher, A., Amunts, K., Schormann, T., & Zilles, K. (2001). Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage, 13(4), 684–701. https://doi.org/10.1006/nimg.2000.0715
Neef, N. E., Anwander, A., Bütfering, C., Schmidt-Samoa, C., Friederici, A. D., Paulus, W., & Sommer, M. (2018). Structural connectivity of right frontal hyperactive areas scales with stuttering severity. Brain: A Journal of Neurology, 141(1), 191–204. https://doi.org/10.1093/brain/awx316
Nelson, S. M., Savalia, N. K., Fishell, A. K., Gilmore, A. W., Zou, F., Balota, D. A., & McDermott, K. B. (2016). Default mode network activity predicts early memory decline in healthy young adults aged 18-31. Cerebral Cortex, 26(8), 3379–3389. https://doi.org/10.1093/cercor/bhv165
Neville, K. R., & Haberly, L. B. (2004). Olfactory cortex. The synaptic organization of the brain. In G. M. Shepherd (Ed.). Oxford University Press, 415–454. https://doi.org/10.1093/acprof:oso/9780195159561.003.0010
Newbold, D. J., & Dosenbach, N. U. F. (2021). Tracking plasticity of individual human brains. Current Opinion in Behavioral Sciences, 40, 161-168. https://doi.org/10.1016/j.cobeha.2021.04.018
Niendam, T. A., Laird, A. R., Ray, K. L., Dean, Y. M., Glahn, D. C., & Carter, C. S. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective & Behavioral Neuroscience, 12(2), 241–268. https://doi.org/10.3758/s13415-011-0083-5
Nikolin, S., Boonstra, T. W., Loo, C. K., & Martin, D. (2017). Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PloS one, 12(8), e0181833. https://doi.org/10.1371/journal.pone.0181833
Ogawa H. (1994). Gustatory cortex of primates: anatomy and physiology. Neuroscience Research, 20(1), 1–13. https://doi.org/10.1016/0168-0102(94)90017-5
Olson, I. R., Plotzker, A., & Ezzyat, Y. (2007). The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain: A Journal of Neurology, 130(Pt 7), 1718–1731. https://doi.org/10.1093/brain/awm052
Ongür, D., Ferry, A. T., & Price, J. L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology, 460(3), 425–449. https://doi.org/10.1002/cne.10609
Ottaviani, C., Watson, D. R., Meeten, F., Makovac, E., Garfinkel, S. N., & Critchley, H. D. (2016). Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biological Psychology, 119, 31–41. https://doi.org/10.1016/j.biopsycho.2016.06.009
Padmanabhan, A., Lynch, C. J., Schaer, M., & Menon, V. (2017). The Default Mode Network in autism. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 2(6), 476–486. https://doi.org/10.1016/j.bpsc.2017.04.004
Paus, T., Kalina, M., Patocková, L., Angerová, Y., Cerný, R., Mecir, P., Bauer, J., & Krabec, P. (1991). Medial vs lateral frontal lobe lesions and differential impairment of central-gaze fixation maintenance in man. Brain, 114, 2051-2067. https://doi.org/10.1093/brain/114.5.2051
Peng, K., Steele, S. C., Becerra, L., & Borsook, D. (2018). Brodmann area 10: Collating, integrating and high level processing of nociception and pain. Progress in Neurobiology, 161, 1–22. https://doi.org/10.1016/j.pneurobio.2017.11.004
Petersen, S. E., & Fiez, J. A. (1993). The processing of single words studied with positron emission tomography. Annual Review of Neuroscience, 16, 509-530. https://doi.org/10.1146/annurev.ne.16.030193.002453
Price, J. L., & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 192–216. https://doi.org/10.1038/npp.2009.104
Purves, D. (2018). Neuroscience (6th ed.). Oxford University Press.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. https://doi.org/10.1073/pnas.98.2.676
Rajkowska, G., & Goldman-Rakic, P. S. (1995). Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cerebral Cortex, 5(4), 323–337. https://doi.org/10.1093/cercor/5.4.323
Ramnani, N., & Owen, A. M. (2004). Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews. Neuroscience, 5(3), 184–194. https://doi.org/10.1038/nrn1343
Rogers, M. A., Kasai, K., Koji, M., Fukuda, R., Iwanami, A., Nakagome, K., Fukuda, M., & Kato, N. (2004). Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Rresearch, 50(1), 1–11. https://doi.org/10.1016/j.neures.2004.05.003
Rolls E. T. (2006). Brain mechanisms underlying flavour and appetite. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361(1471), 1123–1136. https://doi.org/10.1098/rstb.2006.1852
Rolls, E. T., Cheng, W., & Feng, J. (2020). The orbitofrontal cortex: Reward, emotion and depression. Brain Communications, 2(2), fcaa196. https://doi.org/10.1093/braincomms/fcaa196
Ros, T., J Baars, B., Lanius, R. A., & Vuilleumier, P. (2014). Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Frontiers in Human Neuroscience, 8, 1008. https://doi.org/10.3389/fnhum.2014.01008
Rosenberg, M. D., Finn, E. S., Scheinost, D., Constable, R. T., & Chun, M. M. (2017). Characterizing attention with predictive network models. Trends in Cognitive Sciences, 21(4), 290–302. https://doi.org/10.1016/j.tics.2017.01.011
Rosenberg, M. D., Finn, E. S., Scheinost, D., Papademetris, X., Shen, X., Constable, R. T., & Chun, M. M. (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19(1), 165–171. https://doi.org/10.1038/nn.4179
Roy, A. K., Shehzad, Z., Margulies, D. S., Kelly, A. M., Uddin, L. Q., Gotimer, K., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. https://doi.org/10.1016/j.neuroimage.2008.11.030
Roy, M., Shohamy, D., & Wager, T. D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. https://doi.org/10.1016/j.tics.2012.01.005
Rudebeck, P. H., Putnam, P. T., Daniels, T. E., Yang, T., Mitz, A. R., Rhodes, S. E., & Murray, E. A. (2014). A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proceedings of the National Academy of Sciences of the United States of America, 111(14), 5391–5396. https://doi.org/10.1073/pnas.1317695111
Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X., & Kastner, S. (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science, 337(6095), 753-756. https://doi.org/10.1126/science.1223082
Sadeghi, S., Schmidt, S., Mier, D., & Hass, J. (2022). Effective connectivity of the human mirror neuron system during social cognition. Social Cognitive and Affective Neuroscience, 17(8), 732–743. https://doi.org/10.1093/scan/nsab138
Sakaki, M., Yoo, H. J., Nga, L., Lee, T. H., Thayer, J. F., & Mather, M. (2016). Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage, 139, 44–52. https://doi.org/10.1016/j.neuroimage.2016.05.076
Sanders, R. H., & Levitin, D. J. (2020). Towards an understanding of control of complex rhythmical "wavelike" coordination in humans. Brain Sciences, 10(4), 215. https://doi.org/10.3390/brainsci10040215
Shoemaker, J. K., Norton, K. N., Baker, J., & Luchyshyn, T. (2015). Forebrain organization for autonomic cardiovascular control. Autonomic Neuroscience: Basic & Clinical, 188, 5–9. https://doi.org/10.1016/j.autneu.2014.10.022
Schumann, A., de la Cruz, F., Köhler, S., Brotte, L., & Bär, K. J. (2021). The influence of heart rate variability biofeedback on cardiac regulation and functional brain connectivity. Frontiers in Neuroscience, 15, 691988. https://doi.org/10.3389/fnins.2021.691988
Sedley, W., Parikh, J., Edden, R. A., Tait, V., Blamire, A., & Griffiths, T. D. (2015). Human auditory cortex neurochemistry reflects the presence and severity of tinnitus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(44), 14822–14828. https://doi.org/10.1523/JNEUROSCI.2695-15.2015
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., Reiss, A. L., & Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Seghier M. L. (2013). The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist: A Review Journal Bridging Neurobiology, Neurology and Psychiatry, 19(1), 43–61. https://doi.org/10.1177/1073858412440596
Sheline, Y. I., Price, J. L., Yan, Z., & Mintun, M. A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11020–11025. https://doi.org/10.1073/pnas.1000446107
Shepherd G. M. (2007). Perspectives on olfactory processing, conscious perception, and orbitofrontal cortex. Annals of the New York Academy of Sciences, 1121, 87–101. https://doi.org/10.1196/annals.1401.032
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex, 22. https://doi.org/158–165.10.1093/cercor/bhr099
Shofty, B., Gonent, T., Bergmann, E., Mayseless, N., Korn, A., Shamay-Tsoory, S., Grossman, R., Jalon, I., & Ram, Z. (2022). The default network is causally linked to creative thinking. Molecular Psychiatry. https://doi.org/10.1038/s41380-021-01403-8
Small, D. M., Zald, D. H., Jones-Gotman, M., Zatorre, R. J., Pardo, J. V., Frey, S., & Petrides, M. (1999). Human cortical gustatory areas: A review of functional neuroimaging data. Neuroreport, 10(1), 7–14. https://doi.org/10.1097/00001756-199901180-00002
Suzuki, W. A., & Amaral, D. G. (1994). Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. The Journal of Comparative Neurology, 350(4), 497–533. https://doi.org/10.1002/cne.903500402
Thayer, J. F., Hansen, A. L., Saus-Rose, E., & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 37(2), 141–153. https://doi.org/10.1007/s12160-009-9101-z
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. https://doi.org/10.1016/s0165-0327(00)00338-4
Thompson, M., & Thompson, L. (2015). The neurofeedback book (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Utevsky, A. V., Smith, D. V., & Huettel, S. A. (2014). Precuneus is a functional core of the default-mode network. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(3), 932–940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
van Ettinger-Veenstra, H., Lundberg, P., Alföldi, P., Södermark, M., Graven-Nielsen, T., Sjörs, A., Engström, M., & Gerdle, B. (2019). Chronic widespread pain patients show disrupted cortical connectivity in default mode and salience networks, modulated by pain sensitivity. Journal of pain research, 12, 1743–1755. https://doi.org/10.2147/JPR.S189443
Van Hoesen, G., & Pandya, D. N. (1975). Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Research, 95(1), 1–24. https://doi.org/10.1016/0006-8993(75)90204-8
van Strien, N. M., Cappaert, N. L., & Witter, M. P. (2009). The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nature Reviews. Neuroscience, 10(4), 272–282. https://doi.org/10.1038/nrn2614
Vann, S. D., Aggleton, J. P., & Maguire, E. A. (2009). What does the retrosplenial cortex do? Nature Reviews. Neuroscience, 10(11), 792–802. https://doi.org/10.1038/nrn2733
Vogt B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews. Neuroscience, 6(7), 533–544. https://doi.org/10.1038/nrn1704
Vogt, B. A., Finch, D. M., & Olson, C. R. (1992). Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex, 2(6), 435–443. https://doi.org/10.1093/cercor/2.6.435-a
Vossel, S., Geng, J. J., & Fink, G. R. (2014). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(2), 150–159. https://doi.org/10.1177/1073858413494269
Watkins, K. E., Vargha-Khadem, F., Ashburner, J., Passingham, R. E., Connelly, A., Friston, K. J., Frackowiak, R. S., Mishkin, M., & Gadian, D. G. (2002). MRI analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain: A Journal of Neurology, 125(Pt 3), 465–478. https://doi.org/10.1093/brain/awf057
Wesson, D. W., & Wilson, D. A. (2011). Sniffing out the contributions of the olfactory tubercle to the sense of smell: Hedonics, sensory integration, and more? Neuroscience and Biobehavioral Reviews, 35(3), 655–668. https://doi.org/10.1016/j.neubiorev.2010.08.004
Wiebking, C., & Northoff, G. (2014). Interoceptive awareness and the insula - Application of neuroimaging techniques in psychotherapy. GSTF International Journal of Psychology, 1(1), 53-60. https://doi.org/10.5176/0000-0002_1.1.8
Witter, M. P., Wouterlood, F. G., Naber, P. A., & Van Haeften, T. (2000). Anatomical organization of the parahippocampal-hippocampal network. Annals of the New York Academy of Sciences, 911, 1–24. https://doi.org/10.1111/j.1749-6632.2000.tb06716.x
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., Roffman, J. L., Smoller, J. W., Zöllei, L., Polimeni, J. R., Fischl, B., Liu, H., & Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. https://doi.org/10.1152/jn.00338.2011
Zerr, C. L., Berg, J. J., Nelson, S. M., Fishell A. K., Savalia, N. K., & McDermott, K. B. (2018). Learning efficiency: Identifying individual differences in learning rate and retention in healthy adults. Psychological Science, 956797618772540. PMID 29953332 https://doi.org/10.1177/0956797618772540
Zotev, V., Krueger, F., Phillips, R., Alvarez, R. P., Simmons, W. K., Bellgowan, P., Drevets, W. C., & Bodurka, J. (2011). Self-regulation of amygdala activation using real-time FMRI neurofeedback. PloS one, 6(9), e24522. https://doi.org/10.1371/journal.pone.0024522
E. BEHAVIORAL CORRELATES TO BRAIN REGIONS AND NETWORKS
Please click on the podcast icon below to hear a full-length lecture over Brodmann areas.

A Deep Dive into Brodmann Areas
Brodmann areas are a historically important division of the cerebral cortex into distinct regions based on their cytoarchitecture or neuronal arrangement and connections. The concept of Brodmann areas was introduced by the German neurologist Korbinian Brodmann in the early 20th century (Brodmann, 1909). Brodmann's work has significantly impacted the understanding of the functional organization of the cortex and remains influential in contemporary neuroscience research.
Brodmann's classification was based on his observations of differences in the cellular organization of the cortex across various mammalian species, including humans. He identified 43 numbered areas in the human brain by examining the cellular organization, cell types, and layer thickness (Zilles & Amunts, 2010). Although some refinements have been made since Brodmann's initial classification, many identified areas retain their original numbering.
The Importance of Brodmann Areas
The identification of Brodmann areas has facilitated the investigation of functional specialization within the cortex. Most cortical functions involve the networked activity of multiple Brodmann areas. Several Brodmann areas are now associated with specific functions, such as primary sensory and motor areas, as well as higher cognitive functions like language processing and decision-making (Glasser et al., 2016). For instance, Brodmann area 4 corresponds to the primary motor cortex, area 17 to the primary visual cortex, and areas 44 and 45 to Broca's area, which is crucial for speech production. While Brodmann areas provide a valuable framework for understanding cortical organization, they do not capture the full complexity of the brain's functional architecture. Advances in neuroimaging techniques have identified additional areas and functional networks, highlighting the intricate organization of the cortex beyond Brodmann's classification (Glasser et al., 2016).Areas 3, 1, and 2: Primary Somatosensory Cortex (S1)
The primary somatosensory cortex (S1) is a critical region for processing somatosensory information in the brain. It is involved in processing touch, proprioception, and temperature. These four cortical areas contain separate somatotopic maps (Purves, 2018). Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The S1 is located in the postcentral gyrus, mainly in Brodmann areas 3, 1, and 2. These areas have distinct functions; Brodmann area 3 receives and processes cutaneous and proprioceptive inputs, area 1 processes tactile stimuli, and area 2 integrates proprioceptive and tactile inputs (Kaas, 2008).Location
The S1 is located in the parietal lobe, immediately posterior to the central sulcus. It is bordered by the primary motor cortex (M1) anteriorly and the secondary somatosensory cortex (S2) posteriorly. The closest sites are C3 and C4, which overlie the central sulcus (Jasper, 1958).Connections
The S1 strongly connects with other cortical and subcortical areas, including the M1, premotor cortex, supplementary motor area, posterior parietal cortex, and thalamus (Lemon, 2008). These connections are essential for sensorimotor integration and control.Participation in brain networks
The S1 is a critical node in the somatosensory network, which includes other areas like the S2, insular cortex, and parietal operculum. It also participates in the sensorimotor network, interacting with the motor and premotor cortices (Sepulcre, 2012).Functions
The S1 is crucial for processing somatosensory information like touch, proprioception, and temperature. It plays a significant role in perceiving object features, body awareness, and sensorimotor integration.Role in clinical disorders
The altered functioning of the S1 has been implicated in various clinical conditions, including neuropathic pain (Baliki et al., 2011), phantom limb pain (Makin et al., 2013), and stroke-related sensory deficits (Carey et al., 2002).Area 4: Primary Motor Cortex (M1)
The primary motor cortex (M1) is a key region in the brain responsible for the execution of voluntary movements. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The M1 is located in the precentral gyrus, mainly in Brodmann area 4. It contains large pyramidal neurons, known as Betz cells, essential for motor control (Geyer et al., 1996).Location
The M1 is situated in the frontal lobe, immediately anterior to the central sulcus. It is bordered by the posteriorly primary somatosensory cortex (S1) and anteriorly premotor cortex. The closest sites are C3 and C4, which overlie the central sulcus (Jasper, 1958).Connections
The M1 strongly connects with various cortical and subcortical areas, including the S1, premotor cortex, supplementary motor area, posterior parietal cortex, and thalamus (Lemon, 2008). These connections are critical for sensorimotor integration and control.Participation in brain networks
The M1 is a central node in the sensorimotor network, interacting with the somatosensory cortex, premotor cortex, and other motor-related areas (Sepulcre, 2012).Functions
The primary function of the M1 is the execution of voluntary movements. M1 neurons primarily control movements rather than discrete muscles (Breedlove & Watson, 2023). It is critical in planning, controlling, and coordinating complex motor tasks.Role in clinical disorders
Alterations in M1 function have been implicated in various clinical conditions, including motor deficits following stroke (Ward, 2004), Parkinson's disease (Wu & Hallett, 2013), and motor neuron diseases like amyotrophic lateral sclerosis (ALS; Kew & Leigh, 1997).Areas 5 and 7: Somatosensory Association Cortex (SAC)
The somatosensory association cortex (SAC) is involved in the integration and interpretation of somatosensory information coming from the primary somatosensory cortex (S1). Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The SAC is mainly located in Brodmann areas 5 and 7 within the posterior parietal cortex (Culham & Kanwisher, 2001).Location
The SAC is situated in the parietal lobe, superior to the primary somatosensory cortex (S1), and posterior to the postcentral gyrus. The closest sites are likely P3 and P4, which overlie the parietal cortex.Connections
The SAC strong connects with various cortical and subcortical regions, including the S1, primary motor cortex (M1), premotor cortex, supplementary motor area, posterior parietal cortex, and thalamus (Cavada & Goldman-Rakic, 1989). These connections are critical for sensorimotor integration, spatial awareness, and higher-order sensory processing.Participation in brain networks
The SAC is a key node in the somatosensory network, including areas like the S1, S2, and insular cortex. Additionally, it is part of the dorsal attention network, which is involved in attentional control and spatial processing (Corbetta & Shulman, 2002).Functions
The SAC is essential for integrating and interpreting somatosensory information, including tactile and proprioceptive stimuli. It plays a significant role in sensorimotor integration, spatial awareness, and attention.Role in clinical disorders
Alterations in SAC function have been implicated in various clinical conditions, including somatosensory neglect (Vallar et al., 2003), spatial processing deficits (Whitlock et al., 2012), and somatosensory deficits in autism spectrum disorder (Cascio et al., 2012).Area 6: Supplementary Motor Cortex and Premotor Cortex
The supplementary motor cortex (SMA) and premotor cortex (PMC) are critical regions for planning and executing voluntary movements. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The SMA is primarily located in Brodmann area 6, on the medial aspect of the frontal lobe (Picard & Strick, 2001). The PMC is also predominantly found in Brodmann area 6 but is located on the lateral aspect of the frontal lobe (Wise et al., 1997).Location
The SMA is located in the medial part of the frontal lobe, superior to the cingulate sulcus and anterior to the paracentral lobule. The PMC is situated in the lateral part of the frontal lobe, anterior to the primary motor cortex (M1). The closest sites are likely FC3 and FC4, which overlie the dorsolateral prefrontal cortex.Connections
Both the SMA and PMC have strong connections with various cortical and subcortical areas, including the M1, primary somatosensory cortex (S1), posterior parietal cortex, and basal ganglia (Lemon, 2008; Nachev et al., 2008). These connections are critical for sensorimotor integration, movement planning, and execution.Participation in brain networks
The SMA and PMC are central nodes in the sensorimotor network, interacting with the M1, S1, and other motor-related areas (Sepulcre, 2012).Functions
The SMA and PMC are essential for motor planning, execution, and coordination of complex movements. The SMA is particularly involved in initiating and controlling internally generated movements, while the PMC is more concerned with the planning and executing visually-guided movements (Wise et al., 1997; Picard & Strick, 2001).Role in clinical disorders
Alterations in SMA and PMC function have been implicated in various clinical conditions, including movement disorders like Parkinson's disease (Wu & Hallett, 2013), apraxia (Haaland et al., 2000), and motor deficits following stroke (Ward, 2004).Area 8: Frontal Eye Field (FEF)
The frontal eye field (FEF) is essential for the control of eye movements and visual attention. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The FEF is predominantly in Brodmann area 8, located in the dorsolateral prefrontal cortex (Paus, 1996).Location
The FEF is located in the anterior bank of the precentral sulcus within the dorsolateral prefrontal cortex, close to the border with the primary motor cortex (M1) (Paus, 1996). The closest sites are likely F3 and F4, which overlie the dorsolateral prefrontal cortex.Connections
The FEF has extensive connections with other cortical and subcortical regions, including the parietal cortex, superior colliculus, thalamus, and extrastriate visual areas (Schall, 2002; Stanton et al., 2005). These connections are crucial for visual attention, saccadic eye movements, and smooth pursuit.Participation in brain networks
The FEF is a key node in the dorsal attention network responsible for goal-directed attention and eye movement control. This network also includes the intraparietal sulcus and superior parietal lobule (Corbetta & Shulman, 2002).Functions
The FEF is crucial in controlling saccadic eye movements, smooth pursuit, and visual attention. It is involved in the initiation, planning, and execution of eye movements, as well as the allocation of attention to relevant visual stimuli (Schall, 2002). The FEF is vital in cognitive functions, including attention orientation, visual consciousness, access to our conscious experience, perceptual performance, and decision-making (Vernet et al., 2014).Role in clinical disorders
Alterations in FEF function have been implicated in various clinical conditions, including attention deficit hyperactivity disorder (ADHD; Mahone et al., 2011), oculomotor apraxia (Rizzo et al., 1996), and progressive supranuclear palsy (Burrell et al., 2012).Areas 9 and 46: Dorsolateral Prefrontal Cortex (DLPFC)
The dorsolateral prefrontal cortex (DLPFC), which consists of distinct regions, is essential for higher-order cognitive functions, including working memory, executive control, and decision-making (Ahuja & Rodriguez, 2022). Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The DLPFC is primarily located in Brodmann areas 9 and 46 within the lateral aspect of the frontal lobe (Petrides, 2005).Location
The DLPFC is situated in the lateral portion of the frontal lobe, superior and anterior to the premotor and primary motor cortex (M1). The closest sites are likely F3 and F4.Connections
The DLPFC has extensive connections with other cortical and subcortical regions, including the parietal cortex, medial prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex, thalamus, and basal ganglia (Petrides & Pandya, 2002). These connections are critical for cognitive control, working memory, and decision-making.Participation in brain networks
The DLPFC is a key node in the frontoparietal control network, which is responsible for executive control, and the working memory network, which maintains and manipulates information (Cabeza & Nyberg, 2000; Vincent et al., 2008).Functions
The DLPFC plays a crucial role in higher-order cognitive functions such as working memory, executive control, and decision-making. It allocates cognitive resources, goal-directed behavior, task switching, and the flexible adaptation of behavior in response to changing demands (Breedlove & Watson, 2023; Petrides, 2005).Role in clinical disorders
Alterations in DLPFC function have been implicated in various clinical conditions, including schizophrenia (Barch, 2005), attention deficit hyperactivity disorder (ADHD; Cortese et al., 2012), and major depressive disorder (MDD; Drevets et al., 2008).Area 10: Anterior Prefrontal Cortex (aPFC)
The anterior prefrontal cortex (aPFC), also referred to as the frontopolar cortex, is involved in higher-order cognitive processes such as decision-making, planning, and reasoning. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The aPFC is primarily located in Brodmann area 10, at the most anterior part of the frontal lobe (Ramnani & Owen, 2004).Location
The aPFC is located at the most rostral part of the frontal lobe, anterior to the dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex. The closest sites are likely Fp1 and Fp2, which overlie the frontal pole.Connections
The aPFC has extensive connections with other cortical and subcortical regions, including the DLPFC, orbitofrontal cortex, medial prefrontal cortex, posterior parietal cortex, temporal cortex, and thalamus (Burgess et al., 2007). These connections are essential for complex cognitive tasks, multitasking, and mentalizing.Participation in brain networks
The aPFC is a key node in the frontoparietal control network, which is responsible for executive control, as well as the default mode network (DMN), which is involved in self-referential processing and mentalizing (Vincent et al., 2008; Spreng et al., 2009).Functions
The aPFC involves higher-order cognitive processes such as decision-making, planning, reasoning, multitasking, and mentalizing. It is crucial in coordinating and integrating information from various cognitive domains and is responsible for goal-directed behavior and social cognition (Ramnani & Owen, 2004). The aPFC is engaged in various tasks, such as problem-solving, memory recall, future-oriented memory, source and context memory, task-switching, and attention reallocation (Ramnani & Owen, 2004). The aPFC contributes to high-level nociception and pain processing (Peng et al., 2018).Role in clinical disorders
Alterations in aPFC function have been implicated in various clinical conditions, including autism spectrum disorder (ASD; Gilbert et al., 2008), schizophrenia (Perlstein et al., 2001), and major depressive disorder (MDD; Drevets et al., 2008).Areas 11, 12, 13, and 47: Orbitofrontal Cortex (OFC)
The orbitofrontal cortex (OFC) processes reward, emotion, and decision-making and integrates sensory information with emotional valence. Graphics © Big8/Shutterstock.com..jpg)
.jpg)
Brodmann areas
The OFC primarily encompasses Brodmann areas 11, 12, 13, and 47, located in the ventral portion of the frontal lobe (Kringelbach, 2005).Location
The OFC is situated in the ventral part of the frontal lobe, just above the orbits (eye sockets). The anterior prefrontal cortex and the medial prefrontal cortex border it. The closest sites are likely Fp1 and Fp2, which overlie the ventral and rostral portions of the frontal lobe.Connections
The OFC has extensive connections with other cortical and subcortical regions, including the amygdala, insula, cingulate cortex, hippocampus, thalamus, striatum, and sensory cortices (Kringelbach, 2005; Price, 2007). These connections are essential for emotion processing, reward-based decision-making, and social cognition.Participation in brain networks
The OFC is a key node in the salience network, which is responsible for detecting and integrating emotionally and motivationally salient stimuli, and the default mode network (DMN), which is involved in self-referential processing and social cognition (Seeley et al., 2007; Spreng et al., 2009).Functions
The OFC is crucial in processing reward, emotion, and decision-making. It integrates sensory information with emotional valence, evaluates outcomes and actions, and represents social and emotional information (Kringelbach, 2005).Role in clinical disorders
Alterations in OFC function have been implicated in various clinical conditions, including obsessive-compulsive disorder (OCD) (Menzies et al., 2008), major depressive disorder (MDD) (Drevets, 2007), bipolar disorder (BD) (Blumberg et al., 2003), and addiction (Volkow & Fowler, 2000). Depression may be associated with heightened responsiveness and increased connectivity in the lateral orbitofrontal cortex (not linked to rewards), while it is connected to reduced responsiveness and connectivity in the medial orbitofrontal cortex (related to rewards; Rolls, Cheng, & Feng, 2020).Areas 13-16 and 52: Insular Cortex (Insula)
The insular cortex, or insula, is involved in diverse functions, including interoception, emotion processing, pain perception, and cognitive control. Graphics © Big8/Shutterstock.com..jpg)
.jpg)
Brodmann areas
The insular cortex comprises Brodmann areas 13, 14, 15, 16, and parts of area 52. These include sensorimotor, central-olfactogustatory, socio-emotional, and cognitive anterior-dorsal regions (Kurth et al., 2010).Location
The insular cortex is situated deep within the lateral sulcus, which separates the frontal and parietal lobes from the temporal lobe. The opercula of the frontal, parietal, and temporal lobes cover it.Connections
The insular cortex has extensive connections with various cortical and subcortical regions, including the amygdala, anterior cingulate cortex (ACC), prefrontal cortex, primary and secondary somatosensory cortices, orbitofrontal cortex (OFC), and thalamus (Nieuwenhuys, 2012). These connections contribute to the diverse functions of the insula.Participation in brain networks
The insular cortex is a key node in the salience network, which is responsible for detecting and integrating emotionally and motivationally salient stimuli, and the central autonomic network (CAN), which is involved in autonomic regulation (Seeley et al., 2007; Thayer et al., 2012).Functions
The insular cortex is crucial in interoception, emotion processing, pain perception, and cognitive control. It represents internal bodily states, integrates sensory and emotional information, and modulates cognitive and affective processes (Craig, 2009).Role in clinical disorders
Alterations in insular cortex function have been implicated in various clinical conditions, including anxiety disorders (Paulus & Stein, 2006), major depressive disorder (MDD; Sliz & Hayley, 2012), addiction (Naqvi & Bechara, 2010), and autism spectrum disorder (ASD; Di Martino et al., 2009).Area 17: Primary Visual Cortex (V1)
The primary visual cortex (V1), or the striate cortex, is responsible for processing basic visual information, such as orientation, spatial frequency, and color. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The primary visual cortex is primarily located in Brodmann area 17, in the occipital lobe (Horton & Adams, 2005).Location
The primary visual cortex is located in the occipital lobe, along the calcarine sulcus, which runs horizontally through the medial part of the lobe. The closest sites are likely O1 and O2, which overlie the occipital lobe.Connections
The primary visual cortex receives input from the lateral geniculate nucleus (LGN) of the thalamus and sends output to the secondary visual cortex (V2) and other extrastriate areas (V3, V4, V5/MT). These connections are essential for the hierarchical processing of visual information (Felleman & Van Essen, 1991).Participation in brain networks
The primary visual cortex is a key node in the visual processing network responsible for processing and interpreting visual information from the retina. This network includes other areas of the occipital lobe and extends to the parietal and temporal cortices (Nassi & Callaway, 2009).Functions
The primary visual cortex processes basic visual information, such as orientation, spatial frequency, and color. It forms the initial stage of the hierarchical processing of visual information and is critical for visual perception (Horton & Adams, 2005).Role in clinical disorders
Alterations in primary visual cortex function have been implicated in various clinical conditions, including amblyopia (lazy eye; Hess et al., 2010), cortical blindness (Celesia, 2005), and visual hallucinations in conditions like Charles Bonnet syndrome (Griffiths, 2000).Areas 18 and 19: Secondary Visual Cortex (V2)
The secondary visual cortex (V2), also known as the prestriate cortex, is involved in the further processing and integration of visual information received from the primary visual cortex (V1). Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The secondary visual cortex is primarily located in Brodmann areas 18 and 19, in the occipital lobe (Tootell et al., 1998).Location
The secondary visual cortex is located in the occipital lobe, surrounding the primary visual cortex along the calcarine sulcus, extending to the lateral parts of the occipital lobe. The closest sites are likely O1 and O2.Connections
The V2 receives input from the primary visual cortex (V1). It sends output to higher-order extrastriate areas (V3, V4, V5/MT) and other cortical regions involved in visual processing, including the parietal and temporal cortices (Felleman & Van Essen, 1991).Participation in brain networks
The secondary visual cortex is a key node in the visual processing network responsible for processing and interpreting visual information from the retina. This network includes other areas of the occipital lobe and parietal and temporal cortices (Nassi & Callaway, 2009).Functions
The secondary visual cortex is involved in further processing and integrating visual information from the primary visual cortex. It is crucial in processing complex visual attributes, such as form, color, and motion (Tootell et al., 1998).Role in clinical disorders
Alterations in secondary visual cortex function have been implicated in various clinical conditions, including visual agnosia, characterized by the inability to recognize objects despite normal visual acuity and intact primary visual cortex function (Milner & Goodale, 2008).Areas 18, 19, 37, 21, and 22: Visual Association Cortex (V3, V4, V5)
The visual association cortex, also known as the higher-order extrastriate cortex, is responsible for the advanced processing of visual information, such as object recognition, face perception, and processing of complex visual scenes. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The visual association cortex comprises several Brodmann areas, including areas 18, 19, 37, 21, and 22, mainly in the occipital and temporal lobes (Tootell et al., 1998; Kanwisher & Yovel, 2006).Location
The visual association cortex is located primarily in the occipital and temporal lobes, surrounding the primary (V1) and secondary (V2) visual cortices. It includes regions such as the fusiform face area (FFA), the parahippocampal place area (PPA), and the lateral occipital complex (LOC; Epstein & Kanwisher, 1998; Kanwisher & Yovel, 2006; Malach et al., 1995). The closest sites are likely O1, O2, T5, and T6, which overlie the occipital and temporal lobes.Connections
The visual association cortex receives input from the primary (V1) and secondary (V2) visual cortices and has extensive connections with other cortical and subcortical regions, including the parietal lobe, prefrontal cortex, hippocampus, and amygdala (Felleman & Van Essen, 1991; Kravitz et al., 2013).Participation in brain networks
The visual association cortex is a key component of the ventral visual processing stream, also known as the "what" pathway, responsible for object recognition and processing of complex visual scenes (Kravitz et al., 2011).Functions
The visual association cortex is involved in advanced visual processing, including object recognition, face perception, processing of complex visual scenes, and integration of visual information with other sensory modalities (Kanwisher & Yovel, 2006; Tootell et al., 1998).Role in clinical disorders
Alterations in visual association cortex function have been implicated in various clinical conditions, including prosopagnosia (face blindness; Duchaine & Nakayama, 2006), visual agnosia (Milner & Goodale, 2008), and higher-order visual processing deficits in conditions such as autism spectrum disorder (ASD; Simmons et al., 2009).Areas 20 and 37: Inferior Temporal Gyrus (ITG)
The inferior temporal gyrus (ITG) is a part of the temporal lobe involved in high-level visual processing and object recognition. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The inferior temporal gyrus primarily includes Brodmann areas 20 and 37 (Amunts et al., 2000).Location
The inferior temporal gyrus is located in the ventral part of the temporal lobe, below the middle temporal gyrus and superior temporal sulcus, and above the fusiform gyrus. The closest sites are likely T5 (or TP7) and T6 (or TP8), which overlie the temporal lobes.Connections
The ITG has extensive connections with other cortical and subcortical regions, including the primary and secondary visual cortices, fusiform gyrus, parahippocampal gyrus, hippocampus, amygdala, and prefrontal cortex (Kravitz et al., 2013; Felleman & Van Essen, 1991).Participation in brain networks
The ITG is a key component of the ventral visual processing stream, also known as the "what" pathway, responsible for object recognition and processing of complex visual scenes (Kravitz et al., 2011).Functions
The ITG is involved in high-level visual processing, object recognition, semantic processing, and the integration of visual information with other sensory modalities (Kanwisher & Yovel, 2006).Role in clinical disorders
Alterations in ITG function have been implicated in various clinical conditions, including visual agnosia, prosopagnosia (face blindness), and higher-order visual processing deficits in conditions such as autism spectrum disorder (ASD; Duchaine & Nakayama, 2006; Simmons et al., 2009).Areas 21 and 39: Middle Temporal Gyrus (MTG)
The middle temporal gyrus (MTG) is a part of the temporal lobe involved in various functions, such as semantic processing, language, and high-level visual processing. Graphics © Big8/Shutterstock.com..jpg)
.jpg)
Brodmann areas
The MTG primarily includes Brodmann areas 21 and 39 (Amunts et al., 2000).Location
The MTG is located in the lateral part of the temporal lobe, between the superior temporal gyrus (above) and the inferior temporal gyrus (below), and adjacent to the superior temporal sulcus.Connections
The MTG has extensive connections with other cortical and subcortical regions, including the primary and secondary visual cortices, the angular gyrus, the fusiform gyrus, the parahippocampal gyrus, the hippocampus, the amygdala, and the prefrontal cortex (Kravitz et al., 2013; Felleman & Van Essen, 1991).Participation in brain networks
The MTG participates in various brain networks, including the ventral visual processing stream ("what" pathway) for object recognition and processing of complex visual scenes (Kravitz et al., 2011), and the language network for semantic processing and word retrieval (Binder et al., 2009).Functions
The MTG is involved in various functions, such as semantic processing, language comprehension, word retrieval, and high-level visual processing, including object and face recognition (Binder et al., 2009; Kanwisher & Yovel, 2006).Role in clinical disorders
Alterations in MTG function have been implicated in various clinical conditions, including semantic dementia (Hodges et al., 1992), language impairments in aphasia (Dronkers et al., 2004), and higher-order visual processing deficits in conditions such as autism spectrum disorder (ASD; Simmons et al., 2009).Areas 22, 39, and 40: Superior Temporal Gyrus (STG)
The superior temporal gyrus (STG), including Wernicke's area, is a part of the temporal lobe involved in various functions such as language comprehension, auditory processing, and social cognition. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
Wernicke's area primarily includes Brodmann area 22 and, to some extent, areas 39 and 40 (Amunts et al., 2000).Location
Wernicke's area is located in the posterior part of the superior temporal gyrus, usually in the left hemisphere, near the lateral sulcus. The STG runs laterally along the temporal lobe, above the middle temporal gyrus. The closest site is likely T5 (or TP7) for the left hemisphere, where Wernicke's area is typically located.Connections
Wernicke's area has extensive connections with other language-related regions, including Broca's area (via the arcuate fasciculus), the angular gyrus, and other parts of the superior temporal gyrus (Friederici, 2009). The STG also connects with the primary and secondary auditory cortices, social cognition, and memory regions.Participation in brain networks
Wernicke's area participates in the language network, playing a crucial role in language comprehension and semantic processing (Binder et al., 2009). The STG is also involved in the auditory processing network and the social cognition network.Functions
Wernicke's area involves language comprehension, semantic processing, and integrating auditory information into meaningful speech (Price, 2012). The STG also plays a role in auditory processing, social cognition, and memory.Role in clinical disorders
Alterations in the function of Wernicke's area and the STG have been implicated in various clinical conditions, such as Wernicke's aphasia, characterized by impaired language comprehension and fluent but nonsensical speech (Dronkers et al., 2004). The STG has also been implicated in auditory processing deficits and social cognition impairments in conditions such as autism spectrum disorder (ASD; Boddaert et al., 2004).Area 23: Ventral Posterior Cingulate Cortex (vPCC)
The ventral posterior cingulate cortex (vPCC) is a region within the posterior cingulate cortex (PCC), a part of the limbic system involved in various functions such as memory, emotion, and self-referential processing. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The vPCC primarily includes Brodmann area 23 (Vogt et al., 2006).Location
The vPCC is located in the medial part of the brain, in the posterior cingulate cortex, and ventral to the dorsal posterior cingulate cortex (dPCC). It is positioned between the precuneus and the corpus callosum. The closest sites are likely Pz and CPz, located over the midline parietal and central regions, respectively.Connections
The vPCC connects with various brain regions, including the medial prefrontal cortex (mPFC), hippocampus, parahippocampal gyrus, and lateral parietal regions (Leech & Sharp, 2014; Utevsky et al., 2014).Participation in brain networks
The vPCC is a key component of the default mode network (DMN), which is active during rest and involved in self-referential thinking, autobiographical memory, and social cognition (Raichle et al., 2001; Buckner et al., 2008).Functions
The vPCC is involved in various functions, such as self-referential thinking, autobiographical memory, social cognition, and emotional processing (Leech & Sharp, 2014; Utevsky et al., 2014).Role in clinical disorders
Alterations in vPCC function have been implicated in various clinical conditions, including Alzheimer's disease (Buckner et al., 2005), major depressive disorder (Sheline et al., 2010), and autism spectrum disorder (ASD; Padmanabhan et al., 2017).Areas 24 and 25: Ventral Anterior Cingulate Cortex (vACC)
The ventral anterior cingulate cortex (vACC) is a region within the anterior cingulate cortex (ACC), which is part of the limbic system and involved in various functions, such as emotion processing, reward-based learning, and decision-making. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The vACC primarily includes Brodmann areas 24 and 25 (Vogt, 2005).Location
The vACC is located in the medial part of the brain, in the anterior cingulate cortex, ventral to the dorsal anterior cingulate cortex (dACC). It is positioned anterior to the genu of the corpus callosum. The closest sites are likely FCz and Cz, located over the midline frontal and central regions, respectively.Connections
The vACC has connections with various brain regions, including the amygdala, hippocampus, medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), and nucleus accumbens (Bush et al., 2000; Etkin et al., 2011).Participation in brain networks
The vACC is a key component of the salience network, which detects and integrates salient emotional and sensory stimuli and modulates attention and cognitive control (Menon, 2011; Seeley et al., 2007).Functions
The vACC is implicated in various cognitive and emotional functions, including error detection, conflict monitoring, emotion regulation, empathy, and social cognition (Bush et al., 2000; Etkin et al., 2011).Role in clinical disorders
Abnormalities in the vACC have been implicated in several psychiatric and neurological disorders, such as depression, anxiety, schizophrenia, bipolar disorder, attention deficit hyperactivity disorder (ADHD), and autism spectrum disorders (Drevets et al., 2008; Etkin et al., 2010).Areas 25 and 24b: Subgenual Ventromedial Prefrontal Cortex (vmPFC)
The subgenual region of the ventromedial prefrontal cortex (vmPFC) is an important brain region involved in various cognitive and emotional processes. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The subgenual region of the vmPFC primarily consists of Brodmann areas 25 and 24b (Ongür et al., 2003).Location
The subgenual region of the vmPFC is located in the medial prefrontal cortex, ventral to the genu of the corpus callosum, and adjacent to the anterior cingulate cortex (Mayberg, 2003). Its nearby EEG electrode positions include Fp1, Fp2, Fz, and AFz, located along the scalp's midline (Jasper, 1958).Connections
The subgenual region of the vmPFC has extensive connections with other brain regions, including the amygdala, hippocampus, hypothalamus, nucleus accumbens, thalamus, and other prefrontal areas (Ongür et al., 2003; Price & Drevets, 2010).Participation in brain networks
The subgenual vmPFC is a key component of the default mode network (DMN) and the affective network, involved in self-referential processing, emotion regulation, and decision-making (Buckner et al., 2008; Rudebeck et al., 2014).Functions
The subgenual vmPFC is implicated in various cognitive and emotional functions, including value-based decision-making, emotion regulation, self-referential processing, and social cognition (Rudebeck et al., 2014; Roy et al., 2012).Role in clinical disorders
Abnormalities in the subgenual vmPFC have been implicated in several psychiatric disorders, such as major depressive disorder, bipolar disorder, anxiety disorders, and post-traumatic stress disorder (Mayberg, 2003; Price & Drevets, 2010).Areas 29 and 30: Ectosplenial Retrosplenial Cerebral Cortex
The ectosplenial region is not a widely recognized or well-established region within the human retrosplenial cortex. However, the retrosplenial cortex is a crucial brain area involved in various cognitive processes, particularly spatial memory, and navigation. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The retrosplenial cortex mainly comprises Brodmann areas 29 and 30, in the posterior cingulate cortex (Vogt et al., 2006).Location
The retrosplenial cortex is situated in the medial parietal lobe, posterior to the splenium of the corpus callosum, and adjacent to the posterior cingulate cortex (Vann et al., 2009). Its nearby EEG electrode positions include Pz, CPz, and Oz, located along the midline of the scalp (Jasper, 1958).Connections
The retrosplenial cortex has extensive connections with other brain regions, including the hippocampus, parahippocampal cortex, thalamus, anterior cingulate cortex, and other parietal and frontal areas (Vann et al., 2009).Participation in brain networks
The retrosplenial cortex is a key component of the default mode network (DMN) and is involved in spatial memory, episodic memory, and self-referential processing (Buckner et al., 2008).Functions
The retrosplenial cortex is implicated in various cognitive functions, including spatial memory, navigation, episodic memory, and scene construction (Epstein, 2008; Vann et al., 2009).Role in clinical disorders
Abnormalities in the retrosplenial cortex have been implicated in several neurological and psychiatric disorders, such as Alzheimer's disease, amnesia, and schizophrenia, which often involve impairments in spatial memory and navigation (Maguire, 2001; Mendez & Cherrier, 2003).Areas 29 and 30: Retrosplenial Cingulate Cortex
The retrosplenial cingulate cortex is an important brain region involved in various cognitive processes, particularly related to spatial memory and navigation. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The retrosplenial cortex mainly comprises Brodmann areas 29 and 30, which are located in the posterior cingulate cortex (Vogt et al., 2006).Location
The retrosplenial cortex is situated in the medial parietal lobe, posterior to the splenium of the corpus callosum, and adjacent to the posterior cingulate cortex (Vann et al., 2009). Its nearby EEG electrode positions include Pz, CPz, and Oz, located along the midline of the scalp (Jasper, 1958).Connections
The retrosplenial cortex has extensive connections with other brain regions, including the hippocampus, parahippocampal cortex, thalamus, anterior cingulate cortex, and other parietal and frontal areas (Vann et al., 2009).Participation in brain networks
The retrosplenial cortex is a key component of the default mode network (DMN) and is involved in spatial memory, episodic memory, and self-referential processing (Buckner et al., 2008).Functions
The retrosplenial cortex is implicated in various cognitive functions, including spatial memory, navigation, episodic memory, and scene construction (Vann et al., 2009; Epstein, 2008).Role in clinical disorders
Abnormalities in the retrosplenial cortex have been implicated in several neurological and psychiatric disorders, such as Alzheimer's disease, amnesia, and schizophrenia, which often involve impairments in spatial memory and navigation (Maguire, 2001; Mendez & Cherrier, 2003).Areas 23, 24, and 31: Dorsal Posterior Cingulate Cortex (dPCC)
The dorsal posterior cingulate cortex (dPCC) is an important brain region involved in various cognitive processes, particularly related to attention and memory. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The dPCC is primarily composed of Brodmann areas 23 and 31. These areas are associated with the cingulate cortex and form part of the limbic system, which plays a crucial role in emotion formation, processing, learning, and memory (Vogt, Finch, & Olson, 1992).Location
The dPCC is located in the medial aspect of the brain, towards the back. It's located directly above the corpus callosum, a nerve fiber bundle connecting the left and right cerebral hemispheres.Connections
The dPCC has numerous connections with other areas of the brain. It connects with other regions of the cingulate cortex, the medial prefrontal cortex, the parahippocampal gyrus, and the precuneus. It also connects with the thalamus and various parts of the temporal and parietal lobes. These connections make the dPCC a central hub for information processing and transfer (Margulies et al., 2009).Participation in brain networks
The dPCC is part of several crucial brain networks. It is an integral part of the default mode network (DMN), which is most active when the brain is at rest and not focused on the outside world. The dPCC also interacts with the salience network, which is crucial for determining the sensory or emotional inputs most relevant to our goals and current situation (Leech & Sharp, 2014).Functions
The functions of the dPCC are diverse and complex due to its involvement in various brain networks and its wide-ranging connections. These functions include self-referential thought, episodic memory retrieval, and consciousness. It also plays a role in internally directed thought, such as daydreaming, future planning, and moral reasoning (Andrews-Hanna et al., 2010).Role in clinical disorders
Abnormalities or dysfunction in the dPCC have been linked to several clinical disorders. These include Alzheimer's disease, where decreased activity in the dPCC has been associated with the early stages of the disease (Buckner, R. L., et al., 2005). The dPCC has also been implicated in various psychiatric disorders, such as depression, anxiety, and schizophrenia, where altered connectivity within and between networks involving the dPCC is often seen (Greicius et al., 2007).Areas 24, 25, 32, and 33: Anterior Cingulate Cortex (ACC)
The anterior cingulate cortex (ACC) is a crucial brain region involved in various cognitive, emotional, and regulatory processes. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The ACC is divided into several subregions, including the dorsal ACC (dACC; Brodmann areas 24 and 32) and the ventral ACC (vACC; Brodmann areas 25 and 33; Vogt, 2009).Location
The ACC is located in the medial aspect of the cerebral cortex, surrounding the corpus callosum, with the dACC situated dorsally and the vACC situated ventrally (Vogt, 2009).Connections
The ACC has extensive connections with other brain regions, including the prefrontal cortex, parietal cortex, amygdala, hippocampus, thalamus, and other limbic areas (Devinsky et al., 1995).Participation in brain networks
The ACC is a key component of several brain networks, including the default mode network (DMN), the salience network, and the executive control network, which are involved in cognitive, emotional, and behavioral processing (Bressler & Menon, 2010).Functions
The ACC is implicated in various cognitive functions, including attention, error detection, conflict monitoring, emotion regulation, and decision-making (Bush et al., 2000).Role in clinical disorders
Abnormalities in the ACC have been implicated in several neurological and psychiatric disorders, such as Alzheimer's disease, depression, anxiety, and schizophrenia, which often involve impairments in cognitive, emotional, and behavioral processing (Vogt, 2005).Areas 24, 32, and 33: Cingulate Cortex
The cingulate cortex is an important brain region involved in various cognitive, emotional, and behavioral processes. Graphic © Big8/Shutterstock.com..jpg)
.jpg)
Brodmann areas
The cingulate cortex is divided into several subregions, including the anterior cingulate cortex (ACC; Brodmann areas 24, 32, and 33) and the posterior cingulate cortex (PCC; Brodmann areas 23, 29, and 30; Vogt, 2009).Location
The cingulate cortex is located in the medial aspect of the cerebral cortex, surrounding the corpus callosum, with the ACC situated anteriorly and the PCC situated posteriorly (Vogt, 2009). EEG electrode positions near the cingulate cortex include Fz, FCz, and Cz, located along the scalp's midline (Jasper, 1958).Connections
The cingulate cortex has extensive connections with other brain regions, including the prefrontal cortex, parietal cortex, amygdala, hippocampus, thalamus, and other limbic areas (Devinsky et al., 1995).Participation in brain networks
The cingulate cortex is a key component of several brain networks, including the default mode network (DMN), the salience network, and the executive control network, which are involved in cognitive, emotional, and behavioral processing (Bressler & Menon, 2010).Functions
The cingulate cortex is implicated in various cognitive functions, including attention, error detection, conflict monitoring, emotion regulation, and decision-making (Bush et al., 2000).Role in clinical disorders
Abnormalities in the cingulate cortex have been implicated in several neurological and psychiatric disorders, such as Alzheimer's disease, depression, anxiety, and schizophrenia, which often involve impairments in cognitive, emotional, and behavioral processing (Vogt, 2005).Area 27: Pyriform (Piriform) Cortex
The pyriform cortex, also known as the primary olfactory cortex, is a crucial brain region that processes olfactory information. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The pyriform cortex is not typically associated with specific Brodmann areas. It is part of the allocortex, which has a simpler organization than the isocortex, where Brodmann areas are usually defined (Shepherd, 2007).Location
The pyriform cortex is in the medial temporal lobe, anterior to the perirhinal cortex and lateral to the amygdala (Neville & Haberly, 2004).Connections
The pyriform cortex has extensive connections with other brain regions, including the olfactory bulb, amygdala, thalamus, orbitofrontal cortex, and hippocampus, which are involved in processing and integrating olfactory information (Gottfried, 2010).Participation in brain networks
The pyriform cortex is a key component of the olfactory network, which processes and integrates olfactory information from the environment and plays a role in memory, emotion, and decision-making (Gottfried, 2010).Functions
The pyriform cortex primarily processes olfactory information, including odor discrimination, odor memory, and odor-guided behavior (Neville & Haberly, 2004).Role in clinical disorders
Abnormalities in the piriform cortex have been implicated in several neurological and psychiatric disorders, such as Alzheimer's disease, Parkinson's disease, and schizophrenia, which often involve impairments in olfactory function (Doty, 2008).Area 28: Ventral Entorhinal Cortex (vEC)
The ventral entorhinal cortex (vEC) is an important brain region for various cognitive processes, particularly memory and spatial navigation. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The entorhinal cortex is not typically associated with specific Brodmann areas, as it is part of the allocortex, which has a simpler organization than the isocortex, where Brodmann areas are usually defined (Witter et al., 2000).Location
The ventral entorhinal cortex is located in the medial temporal lobe, anterior to the hippocampus and posterior to the perirhinal cortex (Witter et al., 2000).Connections
The ventral entorhinal cortex has extensive connections with other brain regions, including the hippocampus, perirhinal cortex, parahippocampal cortex, and prefrontal cortex, which are involved in memory and spatial navigation (van Strien et al., 2009; Witter et al., 2000).Participation in brain networks
The ventral entorhinal cortex is a key component of the medial temporal lobe memory system, crucial for episodic memory and spatial navigation (Eichenbaum et al., 2007).Functions
The ventral entorhinal cortex is implicated in various cognitive functions, including episodic memory and spatial navigation (Eichenbaum et al., 2007; Hafting et al., 2005).Role in clinical disorders
Abnormalities in the ventral entorhinal cortex have been implicated in several neurological and psychiatric disorders, such as Alzheimer's disease, temporal lobe epilepsy, and schizophrenia, which often involve impairments in memory and spatial navigation (Braak & Braak, 1991; Du et al., 2017).Areas 28 and 34: Dorsal Entorhinal Cortex (dEC)
The dorsal entorhinal cortex (dEC) is an important brain region in spatial memory and navigation. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
Brodmann areas do not easily define the entorhinal cortex (EC), as it is an evolutionarily conserved structure that does not map neatly onto the cytoarchitectonic divisions. However, it is often associated with Brodmann areas 28 and 34 (Van Strien et al., 2009).Location
The dEC is located in the medial temporal lobe, situated dorsal to the ventral entorhinal cortex (vEC) (Van Strien et al., 2009).Connections
The dEC has extensive connections with other brain regions, including the hippocampus, perirhinal cortex, parahippocampal cortex, and other medial temporal lobe structures (Witter et al., 2000).Participation in brain networks
The dEC is involved in the medial temporal lobe memory system, which plays a crucial role in spatial memory and navigation (Eichenbaum, 2000).Functions
The dEC is implicated in various cognitive functions, including spatial memory, navigation, and contextual processing (Hafting et al., 2005).Role in clinical disorders
Abnormalities in the dEC have been implicated in several neurological disorders, such as Alzheimer's, which involves memory and navigation impairments (Khan et al., 2014).Areas 29 and 30: Ectosplenial Retrosplenial Cerebral Cortex
The ectosplenial region is not a widely recognized or well-established region within the human retrosplenial cortex. However, the retrosplenial cortex is a crucial brain area involved in various cognitive processes, particularly spatial memory and navigation. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The retrosplenial cortex mainly comprises Brodmann areas 29 and 30, located in the posterior cingulate cortex (Vogt et al., 2006).Location
The retrosplenial cortex is situated in the medial parietal lobe, posterior to the splenium of the corpus callosum, and adjacent to the posterior cingulate cortex (Vann et al., 2009). Its nearby EEG electrode positions include Pz, CPz, and Oz, located along the midline of the scalp (Jasper, 1958).Connections
The retrosplenial cortex has extensive connections with other brain regions, including the hippocampus, parahippocampal cortex, thalamus, anterior cingulate cortex, and other parietal and frontal areas (Vann et al., 2009).Participation in brain networks
The retrosplenial cortex is a key component of the default mode network (DMN) and is involved in spatial memory, episodic memory, and self-referential processing (Buckner et al., 2008).Functions
The retrosplenial cortex is implicated in various cognitive functions, including spatial memory, navigation, episodic memory, and scene construction (Epstein, 2008; Vann et al., 2009).Role in clinical disorders
Abnormalities in the retrosplenial cortex have been implicated in several neurological and psychiatric disorders, such as Alzheimer's disease, amnesia, and schizophrenia, which often involve impairments in spatial memory and navigation (Maguire, 2001; Mendez & Cherrier, 2003).Areas 35 and 36: Perirhinal Cortex (PRC)
The perirhinal cortex (PRC) is a significant brain region involved in various cognitive processes, such as object recognition and memory. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The PRC is associated with Brodmann areas 35 and 36, located within the medial temporal lobe (Van Hoesen & Pandya, 1975).Location
The PRC is located in the medial temporal lobe, adjacent to the entorhinal and parahippocampal cortex (Van Hoesen & Pandya, 1975).Connections
The PRC has extensive connections with other brain regions, including the entorhinal cortex, hippocampus, parahippocampal cortex, amygdala, and other medial temporal lobe structures (Suzuki & Amaral, 1994).Participation in brain networks
The PRC is a crucial component of the medial temporal lobe memory system, playing an essential role in object recognition, associative memory, and familiarity-based recognition (Eichenbaum et al., 2007).Functions
The PRC is implicated in various cognitive functions, including object recognition, associative memory, and familiarity-based recognition (Eichenbaum et al., 2007).Role in clinical disorders
Abnormalities in the PRC have been implicated in several neurological disorders, such as Alzheimer's disease and other memory-related disorders (Khan et al., 2014).Areas 37 and 19: Fusiform Gyrus
The fusiform gyrus is a key brain region involved in various cognitive processes, such as face and object recognition. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The fusiform gyrus is associated with Brodmann areas 37 and 19, located on the ventral surface of the temporal and occipital lobes (Grill-Spector et al., 2001).Location
The fusiform gyrus is located on the ventral surface of the temporal and occipital lobes, medial to the inferior temporal gyrus, and lateral to the parahippocampal gyrus (Grill-Spector et al., 2001).Connections
The fusiform gyrus has extensive connections with other brain regions, including the inferior temporal cortex, occipital cortex, parietal cortex, amygdala, and other medial temporal lobe structures (Catani et al., 2003).Participation in brain networks
The fusiform gyrus is involved in the ventral visual processing stream, playing a crucial role in object and face recognition and other high-level visual processes (Grill-Spector et al., 2001).Functions
The fusiform gyrus is implicated in various cognitive functions, including object recognition, face recognition, and high-level visual processing (Grill-Spector et al., 2001).Role in clinical disorders
Abnormalities in the fusiform gyrus have been implicated in several neurological disorders, such as prosopagnosia, autism spectrum disorders, and Alzheimer's disease (Avidan & Behrmann, 2009).Area 38: Temporopolar Area (Temporal Pole)
The temporopolar area, also known as the temporal pole, involves various cognitive and emotional processes. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The temporopolar area is associated with Brodmann area 38, located at the most anterior part of the temporal lobe (Öngür et al., 2003).Location
The temporopolar area is located at the most anterior part of the temporal lobe, anterior to the superior, middle, and inferior temporal gyri (Öngür et al., 2003).Connections
The temporopolar area has extensive connections with other brain regions, including the amygdala, hippocampus, orbitofrontal cortex, insula, and other temporal lobe structures (Olson et al., 2007).Participation in brain networks
The temporopolar area is involved in various brain networks, including the default mode and salience networks, playing crucial roles in social cognition, emotional processing, and semantic memory (Roy et al., 2009).Functions
The temporopolar area is implicated in various cognitive functions, including social cognition, emotional processing, and semantic memory (Roy et al., 2009).Role in clinical disorders
Abnormalities in the temporopolar area have been implicated in several neurological disorders, such as frontotemporal dementia, Alzheimer's disease, and other memory-related disorders (Seeley et al., 2009).Area 39: Angular Gyrus
The angular gyrus is involved in various cognitive processes, such as language, attention, and spatial cognition. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The angular gyrus is associated with Brodmann area 39 in the parietal lobe (Caspers et al., 2006).Location
The angular gyrus is located in the parietal lobe, at the junction of the superior temporal and occipital lobes, and is bordered by the supramarginal gyrus and the occipital cortex (Caspers et al., 2006). The angular gyrus is near the P3 and P4 electrode sites of the International 10-20 system (Jasper, 1958).Connections
The angular gyrus has extensive connections with other brain regions, including the prefrontal cortex, posterior cingulate cortex, superior temporal sulcus, and other regions within the parietal lobe (Seghier, 2013).Participation in brain networks
The angular gyrus plays crucial roles in attention, memory, and language processing in several brain networks, such as the default mode and frontoparietal control networks (Seghier, 2013).Functions
The angular gyrus is implicated in various cognitive functions, including language processing, attention, spatial cognition, and mathematical processing (Seghier, 2013).Role in clinical disorders
Abnormalities in the angular gyrus have been implicated in several neurological disorders, such as dyslexia, aphasia, and Gerstmann syndrome, which involve impairments in language, calculation, and other cognitive processes (Hoeft et al., 2007).Area 40: Supramarginal Gyrus
The supramarginal gyrus is a brain region involved in various cognitive processes, such as language, attention, and sensorimotor integration. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The supramarginal gyrus is associated with Brodmann area 40, located in the parietal lobe (Caspers et al., 2006).Location
The supramarginal gyrus is located in the parietal lobe at the posterior end of the Sylvian fissure and is bordered by the angular gyrus and the postcentral gyrus (Caspers et al., 2006). It is situated near the P3 and P4 electrode sites of the International 10-20 system (Jasper, 1958).Connections
The supramarginal gyrus is involved in several brain networks, such as the frontoparietal control and dorsal attention networks, playing crucial roles in attention, language processing, and sensorimotor integration (Caspers et al., 2011).Participation in brain networks
The supramarginal gyrus is involved in several brain networks, such as the frontoparietal control network and the dorsal attention network, playing crucial roles in attention, language processing, and sensorimotor integration (Caspers et al., 2011).Functions
The supramarginal gyrus is implicated in various cognitive functions, including language processing, attention, sensorimotor integration, and working memory (Caspers et al., 2011).Role in clinical disorders
Abnormalities in the supramarginal gyrus have been implicated in several neurological disorders, such as dyslexia, apraxia, and other cognitive impairments involving language and sensorimotor processing (Hoeft et al., 2007).Areas 41 and 42: Auditory Cortex
The auditory cortex is a brain region involved in processing auditory information. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The auditory cortex comprises several Brodmann areas, including the primary auditory cortex (Brodmann areas 41 and 42) and the surrounding secondary auditory cortex (Brodmann area 22; Morosan et al., 2001).Location
The auditory cortex is located in the superior temporal gyrus within the Sylvian fissure, extending into the lateral sulcus in the temporal lobe (Morosan et al., 2001).Connections
The auditory cortex has extensive connections with other brain regions, including the thalamus (specifically, the medial geniculate nucleus), inferior colliculus, and other cortical regions involved in language, attention, and multisensory integration (Bizley & Cohen, 2013).Participation in brain networks
The auditory cortex participates in several brain networks, such as the auditory processing, language, and attention networks, playing crucial roles in sound processing, speech perception, and auditory attention (Griffiths & Warren, 2002).Functions
The auditory cortex involves various functions, including sound processing, speech perception, scene analysis, and attention (Griffiths & Warren, 2002).Role in clinical disorders
Abnormalities in the auditory cortex have been implicated in several neurological disorders, such as tinnitus, auditory processing disorders, and language-related impairments like dyslexia (Sedley et al., 2015).Area 43: Primary Gustatory Cortex (PGC)
The primary gustatory cortex (PGC) is a brain region that processes taste information. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The PGC is associated with Brodmann area 43, also known as the opercular part of the inferior frontal gyrus and part of the insular cortex (Brodmann area 13; Ogawa, 2012; Small et al., 1999).Location
The PGC is situated in the insular cortex, specifically in the anterior insula, and extends into the adjacent opercular part of the inferior frontal gyrus (Small et al., 1999).Connections
The PGC connects with various brain regions, including the thalamus (specifically, the ventroposteromedial nucleus), orbitofrontal cortex, amygdala, and other cortical regions involved in multisensory integration, emotion, and memory (Rolls, 2006).Participation in brain networks
The PGC is part of the gustatory processing network, which involves taste perception and associated emotional and cognitive processes (Rolls, 2006).Functions
The PGC processes taste information, including taste perception, taste discrimination, and integration with other sensory modalities (Small et al., 1999).Role in clinical disorders
Abnormalities in the PGC have been implicated in several neurological disorders, such as taste-related disorders (ageusia) and eating disorders (anorexia nervosa; Frank et al., 2016).Area 44: Pars Opercularis (inferior temporal gyrus and part of Broca's area)
The pars opercularis is a brain region involved in language processing and motor control. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The pars opercularis is part of Brodmann area 44, also known as the opercular part of the inferior frontal gyrus (Amunts et al., 1999).Location
The pars opercularis is situated in the inferior frontal gyrus, posterior to the pars triangularis, and anterior to the precentral gyrus in the frontal lobe (Amunts et al., 1999). It is located near the F7 and F8 electrode sites of the International 10-20 system (Jasper, 1958).Connections
The pars opercularis connects with various brain regions, including the posterior superior temporal gyrus (Wernicke's area), precentral gyrus, supplementary motor area, and other cortical regions involved in language processing and motor control (Friederici, 2011).Participation in brain networks
The pars opercularis is part of the language and motor networks, playing crucial roles in speech production, syntactic processing, and motor control (Friederici, 2011).Functions
The pars opercularis is involved in various functions, including speech production, syntactic processing, and motor control (Friederici, 2011).Role in clinical disorders
Abnormalities in the pars opercularis have been implicated in several neurological disorders, such as developmental language disorders, stuttering, and apraxia of speech (Neef et al., 2018; Watkins et al., 2002).Area 45: Pars Triangularis (inferior temporal gyrus and part of Broca's area)
The pars triangularis is a brain region involved in language processing and executive functions. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The pars triangularis is part of Brodmann area 45, also known as the triangular part of the inferior frontal gyrus (Amunts et al., 1999).Location
The pars triangularis is situated in the inferior frontal gyrus, anterior to the pars opercularis, and posterior to the pars orbitalis in the frontal lobe (Amunts et al., 1999). It is located near the F7 and F8 electrode sites of the International 10-20 system (Jasper, 1958).Connections
The pars triangularis connects with various brain regions, including the posterior superior temporal gyrus (Wernicke's area), dorsolateral prefrontal cortex, anterior cingulate cortex, and other cortical regions involved in language processing and executive functions (Friederici, 2011).Participation in brain networks
The pars triangularis is part of the language and executive control networks, playing crucial roles in semantic processing, working memory, and cognitive control (Friederici, 2011).Functions
The pars triangularis is involved in various functions, including semantic processing, working memory, and cognitive control (Friederici, 2011).Role in clinical disorders
Abnormalities in the pars triangularis have been implicated in several neurological disorders, such as developmental language disorders, aphasia, and ADHD (Booth et al., 2005; Watkins et al., 2002).Areas 9, 46, 8, and 10: Dorsolateral Prefrontal Cortex (DLPFC)
The dorsolateral prefrontal cortex (DLPFC) is a critical brain region involved in various cognitive and executive functions. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The DLPFC mainly includes Brodmann areas 9 and 46 and parts of areas 8 and 10 (Rajkowska & Goldman-Rakic, 1995).Location
The DLPFC is situated in the lateral and superior part of the frontal lobe, encompassing the middle and superior frontal gyri (Rajkowska & Goldman-Rakic, 1995). The DLPFC is located near the F3 and F4 electrode sites of the International 10-20 system (Jasper, 1958).Connections
The DLPFC connects with various brain regions, including the parietal cortex, anterior cingulate cortex, thalamus, and striatum, forming key nodes within the fronto-parietal and cingulo-opercular networks (Fuster, 2001; Dosenbach et al., 2007).Participation in brain networks
The DLPFC is part of the central executive network, playing crucial roles in cognitive control, working memory, decision-making, and goal-directed behavior (Fuster, 2001; Niendam et al., 2012).Functions
The DLPFC is involved in various functions, including cognitive control, working memory, decision-making, and goal-directed behavior (Fuster, 2001; Niendam et al., 2012).Role in clinical disorders
Abnormalities in the DLPFC have been implicated in several neurological disorders, such as schizophrenia, depression, and ADHD (Broyd et al., 2009; Cao et al., 2021; Liston et al., 2011).Area 47: Pars Orbitalis (part of the inferior frontal gyrus)
The pars orbitalis is a brain region involved in various cognitive and emotional processes. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The pars orbitalis is part of Brodmann area 47, located in the orbital part of the inferior frontal gyrus (Amunts et al., 1999).Location
The pars orbitalis is situated in the inferior frontal gyrus, anterior to the pars triangularis, and posterior to the lateral orbital gyrus in the frontal lobe (Amunts et al., 1999). It is located near the Fp1 and Fp2 electrode sites.Connections
The pars orbitalis connects with various brain regions, including the amygdala, insula, anterior cingulate cortex, and other cortical regions involved in emotional processing, decision-making, and social cognition (Barbas, 2007; Ongür & Price, 2000).Participation in brain networks
The pars orbitalis is part of the salience network and other networks associated with emotional processing, decision-making, and social cognition (Seeley et al., 2007).Functions
The pars orbitalis involves various functions, including emotional processing, decision-making, and social cognition (Barbas, 2007; Ongür & Price, 2000).Role in clinical disorders
Abnormalities in the pars orbitalis have been implicated in several neurological disorders, such as mood disorders, anxiety disorders, and autism spectrum disorders (Phillips et al., 2003; Di Martino et al., 2009).Area 48: Retrosubicular Area (small medial temporal lobe area)
The retrosubicular area, also called the presubiculum, is a part of the hippocampal formation involved in various cognitive processes, particularly spatial navigation and memory. Graphic © Big8/Shutterstock.com..jpg)
Brodmann areas
The retrosubicular area is not directly associated with a specific Brodmann area, as it is part of the hippocampal formation, a medial temporal lobe structure not included in Brodmann's original cytoarchitectonic maps.Location
The retrosubicular area, or presubiculum, is situated in the medial temporal lobe between the subiculum and parasubiculum, forming part of the hippocampal formation (Amaral & Witter, 1995).Connections
The retrosubicular area connects with various brain regions, including the entorhinal cortex, other hippocampal subregions (e.g., subiculum, CA1), and the mammillary bodies via the fornix (Witter et al., 2000).Participation in brain networks
The retrosubicular area is part of the medial temporal lobe memory system and the Papez circuit, which are involved in memory processing, spatial navigation, and emotional processing (Aggleton & Brown, 1999; Eichenbaum, 2000).Functions
The retrosubicular area involves various functions, including spatial navigation, memory, and emotional processing (Eichenbaum, 2000).Role in clinical disorders
Abnormalities in the retrosubicular area have been implicated in several neurological disorders, such as Alzheimer's disease, temporal lobe epilepsy, and schizophrenia (Du et al., 1993; Heckers et al., 1998; Hyman et al., 1984).Areas 13, 14, and 52: Parainsular Area (junction of the temporal lobe and insula)
The parainsular area is not a well-defined or widely recognized region in the human brain, and limited information is available on this specific area. Graphics © Science and Fascija/Shutterstock.com.
.jpg)
Brodmann areas
The insular cortex is associated with Brodmann areas 13, 14, and 52.Location
The insular cortex is located deep within the lateral sulcus, separating the frontal and parietal lobes from the temporal lobe.Connections
The insular cortex has widespread connections with various brain regions, including the prefrontal cortex, parietal cortex, temporal cortex, and limbic structures (Augustine, 1996).Participation in brain networks
The insular cortex is involved in multiple brain networks, including the salience network, and is responsible for detecting and responding to salient stimuli (Menon & Uddin, 2010).Functions
The insular cortex involves various functions, including interoception, emotional processing, pain perception, and cognitive control (Craig, 2009).Role in clinical disorders
Abnormalities in the insular cortex have been implicated in several neurological and psychiatric disorders, such as anxiety, depression, autism, and schizophrenia (Menon, 2011).Glossary
anterior cingulate cortex (ACC): located in the medial portion of the frontal lobes, the anterior cingulate cortex encompasses Brodmann areas 24, 25, 32, and 33. It plays a role in executive function, emotional regulation, attention, conflict monitoring, and error detection.
anterior prefrontal cortex (aPFC): found in the most anterior region of the prefrontal cortex, the anterior prefrontal cortex includes Brodmann areas 10 and 11. It involves complex cognitive processes such as planning, decision-making, working memory, and abstract reasoning.
angular gyrus: located in the parietal lobe near the junction of the temporal and occipital lobes, the angular gyrus corresponds to Brodmann area 39. It plays a role in language processing, attention, spatial cognition, and integration of sensory information.
auditory cortex: situated in the superior temporal gyrus, the auditory cortex encompasses Brodmann areas 41 and 42. It is responsible for processing and interpreting auditory information.
cingulate cortex: a part of the limbic system, the cingulate cortex is situated in the medial aspects of the frontal and parietal lobes, covering Brodmann areas 23, 24, 30, 31, and 33. It involves emotion processing, memory, attention, and cognitive control.
dorsal anterior cingulate cortex (dACC): located in the dorsal region of the anterior cingulate cortex, the dorsal anterior cingulate cortex includes Brodmann areas 24 and 32. It plays a role in cognitive control, decision-making, and conflict monitoring.
dorsal entorhinal cortex (DEC): situated in the medial temporal lobe, the dorsal entorhinal cortex comprises parts of Brodmann area 28. It is involved in spatial memory and navigation.
dorsolateral prefrontal cortex (DLPFC): found in the lateral region of the prefrontal cortex, the dorsolateral prefrontal cortex includes Brodmann areas 9, 46, and parts of 8 and 10. It involves working memory, cognitive flexibility, planning, and abstract reasoning.
dorsal posterior cingulate cortex (dPCC): located in the posterior part of the cingulate cortex, the dorsal posterior cingulate cortex covers Brodmann area 31. It is involved in self-referential thought, memory, and spatial awareness.
ectosplenial cerebral cortex: part of the retrosplenial cortex, the ectosplenial cerebral cortex is situated within the cingulate cortex and covers Brodmann area 29. It plays a role in spatial memory, navigation, and contextual processing.
frontal eye field (FEF): located in the anterior part of the middle frontal gyrus, the frontal eye field corresponds to Brodmann area 8. It is involved in voluntary eye movement control and visual attention.
fusiform gyrus: situated in the ventral region of the temporal and occipital lobes, the fusiform gyrus includes Brodmann areas 37 and parts of 19 and 20. It is involved in face recognition, object recognition, and color and visual form processing.
inferior temporal gyrus (ITG): located in the inferior temporal lobe region, the inferior temporal gyrus encompasses Brodmann areas 20 and 21. It plays a role in visual object recognition and semantic memory.
insular cortex (insula): situated within the lateral sulcus, the insular cortex covers Brodmann areas 13, 14, 15, and 16. It is involved in processing emotions, interoception, self-awareness, pain perception, and taste sensation.
middle temporal gyrus (MTG): located in the middle region of the temporal lobe, the middle temporal gyrus encompasses Brodmann areas 21 and 37. It plays a role in language processing, semantic memory, and visual motion processing.
orbitofrontal cortex (OFC): found in the ventral part of the frontal lobes, the orbitofrontal cortex includes Brodmann areas 10, 11, 12, 13, 14, and 47. It involves decision-making, reward processing, emotional regulation, and social cognition.
pars opercularis: located in the inferior frontal gyrus, the pars opercularis corresponds to Brodmann area 44. It plays a role in language production and is part of Broca's area.
pars orbitalis: situated in the ventral part of the inferior frontal gyrus, the pars orbitalis covers Brodmann area 47. It is involved in language processing, social cognition, and emotional regulation.
pars triangularis: located in the anterior part of the inferior frontal gyrus, the pars triangularis corresponds to Brodmann area 45. It is involved in language processing and is part of Broca's area.
parainsular area: found adjacent to the insular cortex, the parainsular area comprises parts of Brodmann areas 13 and 52. It involves auditory and somatosensory integration and processing pain and temperature sensations.
perirhinal cortex: situated in the medial temporal lobe, the perirhinal cortex encompasses Brodmann areas 35 and 36. It plays a role in object recognition, associative memory, and contextual processing.
primary gustatory cortex: located within the insular cortex, the primary gustatory cortex corresponds to Brodmann area 43. It is responsible for processing taste information.
primary motor cortex (M1): situated in the precentral gyrus, the primary motor cortex corresponds to Brodmann area 4. It is responsible for voluntary movement control.
primary somatosensory cortex (S1): located in the postcentral gyrus, the primary somatosensory cortex covers Brodmann areas 1, 2, and 3. It is responsible for processing somatosensory information, including touch, pain, temperature, and proprioception.
primary visual cortex (V1): situated in the calcarine sulcus within the occipital lobe, the primary visual cortex corresponds to Brodmann area 17. It is responsible for processing basic visual information.
pyriform cortex: located in the ventral part of the temporal lobe, the pyriform cortex (also known as the primary olfactory cortex) includes parts of Brodmann areas 27, 28, and 34. It is responsible for processing olfactory information.
retrosplenial cingulate cortex: found in the posterior part of the cingulate cortex, the retrosplenial cingulate cortex covers Brodmann areas 29 and 30. It is involved in spatial memory, navigation, and contextual processing.
retrosubicular area: located in the medial temporal lobe, the retrosubicular area is part of the parahippocampal gyrus and corresponds to Brodmann area 27. It is involved in spatial navigation and memory.
secondary visual cortex (V2): situated adjacent to the primary visual cortex in the occipital lobe, the secondary visual cortex corresponds to Brodmann area 18. It is involved in processing visual information, including recognition of shapes, colors, and spatial orientation.
somatosensory association cortex (SAC): located in the parietal lobe, the somatosensory association cortex encompasses Brodmann areas 5 and 7. It is involved in the integration and interpretation of somatosensory information, such as touch, pain, temperature, and proprioception.
subgenual ventromedial prefrontal cortex (vmPFC): situated in the ventral part of the medial prefrontal cortex, the subgenual ventromedial prefrontal cortex includes parts of Brodmann areas 25, 32, and 14. It is involved in emotional regulation, decision-making, and social cognition.
supplementary motor cortex (SMA): located in the medial part of the superior frontal gyrus, the supplementary motor cortex corresponds to Brodmann area 6. It is involved in planning and coordinating complex movements and motor learning.
supramarginal gyrus: situated in the parietal lobe, the supramarginal gyrus is part of the inferior parietal lobule and corresponds to Brodmann area 40. It is involved in language processing, attention, and spatial cognition.
superior temporal gyrus (STG): located in the superior region of the temporal lobe, the superior temporal gyrus encompasses Brodmann areas 22, 41, and 42. It plays a role in auditory processing, language comprehension, and social cognition.
temporopolar area: situated in the most anterior part of the temporal lobe, the temporopolar area corresponds to Brodmann area 38. It is involved in olfactory processing, social cognition, and semantic memory.
ventral anterior cingulate cortex (vACC): located in the ventral region of the anterior cingulate cortex, the ventral anterior cingulate cortex includes parts of Brodmann areas 24, 25, and 33. It is involved in emotional regulation, attention, and pain processing.
ventral entorhinal cortex (VEC): situated in the medial temporal lobe, the ventral entorhinal cortex covers parts of Brodmann area 28. It is involved in object recognition, memory, and contextual processing.
ventral posterior cingulate cortex (vPCC): located in the ventral part of the posterior cingulate cortex, the ventral posterior cingulate cortex includes parts of Brodmann areas 23 and 31. It involves self-referential thought, episodic memory retrieval, and emotional processing.
visual association cortex: found in the occipital and parietal lobes, the visual association cortex includes Brodmann areas 18, 19, and parts of 7. It is responsible for higher-level visual processing, including object recognition, motion perception, and spatial awareness.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Functional Neuroanatomy, which reviews critical behavioral networks, and qEEG100, which provides extensive multiple-choice testing over the IQCB Blueprint.


Assignment
Now that you have completed this unit, describe the adaptive functions of the Default Mode Network and the Affective Network.
References
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R., & Buckner, R. L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. https://doi.org/10.1016/j.neuron.2010.02.005
Arnsten A. F. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. https://doi.org/10.1038/nrn2648
Breedlove, S., M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Brodmann, K. (1909). Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth.
Caspers, S., Geyer, S., Schleicher, A., Mohlberg, H., Amunts, K., & Zilles, K. (2006). The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. NeuroImage, 33(2), 430–448. https://doi.org/10.1016/j.neuroimage.2006.06.054
Chau, W., & McIntosh, A. R. (2005). The Talairach coordinate of a point in the MNI space: How to interpret it. NeuroImage, 25(2), 408–416. https://doi.org/10.1016/j.neuroimage.2004.12.007
Cole, M. W., Reynolds, J. R., Power, J. D., Repovs, G., Anticevic, A., & Braver, T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16, 1348–1355. https://doi.org/10.1038/nn.3470
Corbetta, M., & Shulman, G. I. (1998). Human cortical mechanisms of visual attention during orienting and search. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 353(1373), 1353-1362. https://doi.org/10.1098/rstb.1998.0289
Craig A. D. (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10(1), 59–70. https://doi.org/10.1038/nrn2555
de la Cruz, F., Schumann, A., Köhler, S., Reichenbach, J. R., Wagner, G., & Bär, K. J. (2019). The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. NeuroImage, 196, 318–328. https://doi.org/10.1016/j.neuroimage.2019.04.014
A. Fornito, A. Zalesky, & E. T. Bullmore (Eds.) (2016). Fundamentals of brain network analysis. Elsevier. https://doi.org/10.1016/C2012-0-06036-X
Frank, G. K., Shott, M. E., Riederer, J., & Pryor, T. L. (2016). Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Translational Psychiatry, 6(11), e932. https://doi.org/10.1038/tp.2016.199
Glasser, M. F., Coalson, T. S., Robinson, E. C., Hacker, C. D., Harwell, J., Yacoub, E., Ugurbil, K., Andersson, J., Beckmann, C. F., Jenkinson, M., Smith, S. M., & Van Essen, D. C. (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536(7615), 171–178. https://doi.org/10.1038/nature18933
Gordon, E. M., Chauvin, R. J., Van, A. N., Rajesh, A., Nielsen, A., Newbold, D. J., Lynch, C. J., Seider, N. A., Krimmel, S. R., Scheidter, K. M., Monk, J., Miller, R. L., Metoki, A., Montez, D. F., Zheng, A., Elbau, I., Madison, T., Nishino, T., Myers, M. J., Kaplan, S., … Dosenbach, N. U. F. (2023). A somato-cognitive action network alternates with effector regions in motor cortex. Nature, 617(7960), 351–359. https://doi.org/10.1038/s41586-023-05964-2
Griffin, D. M., Hoffman, D. S., & Strick, P. L. (2015). Corticomotoneuronal cells are "functionally tuned." Science, 350(6261), 667–670. https://doi.org/10.1126/science.aaa8035
Hu, Y., Wang, J., Li, C., Wang, Y. S., Yang, Z., & Zuo, X. N. (2016). Segregation between the parietal memory network and the default mode network: Effects of spatial smoothing and model order in ICA. Science Bulletin, 61(24), 1844–1854. https://doi.org/10.1007/s11434-016-1202-z
Kaufman, M. T., Churchland, M. M., Ryu, S. I., & Shenoy, K. V. (2014). Cortical activity in the null space: Permitting preparation without movement. Nature Neuroscience, 17(3), 440–448. https://doi.org/10.1038/nn.3643
Kumral, D., Schaare, H. L., Beyer, F., Reinelt, J., Uhlig, M., Liem, F., Lampe, L., Babayan, A., Reiter, A., Erbey, M., Roebbig, J., Loeffler, M., Schroeter, M. L., Husser, D., Witte, A. V., Villringer, A., & Gaebler, M. (2019). The age-dependent relationship between resting heart rate variability and functional brain connectivity. NeuroImage, 185, 521–533. https://doi.org/10.1016/j.neuroimage.2018.10.027
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5-6), 655–667. https://doi.org/10.1007/s00429-010-0262-0
Mesa, N. (2023). New brain network connecting mind and body discovered. Retrieved from The Scientist.
Nelson, S. M., Savalia, N. K., Fishell, A. K., Gilmore, A. W., Zou, F., Balota, D. A., & McDermott, K. B. (2016). Default mode network activity predicts early memory decline in healthy young adults aged 18-31. Cerebral Cortex, 26(8), 3379–3389. https://doi.org/10.1093/cercor/bhv165
Newbold, D. J., & Dosenbach, N. U. F. (2021). Tracking plasticity of individual human brains. Current Opinion in Behavioral Sciences, 40, 161-168. https://doi.org/10.1016/j.cobeha.2021.04.018
Nikolin, S., Boonstra, T. W., Loo, C. K., & Martin, D. (2017). Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PloS one, 12(8), e0181833. https://doi.org/10.1371/journal.pone.0181833
Ongür, D., Ferry, A. T., & Price, J. L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology, 460(3), 425–449. https://doi.org/10.1002/cne.10609
Paus, T., Kalina, M., Patocková, L., Angerová, Y., Cerný, R., Mecir, P., Bauer, J., & Krabec, P. (1991). Medial vs lateral frontal lobe lesions and differential impairment of central-gaze fixation maintenance in man. Brain, 114, 2051-2067. https://doi.org/10.1093/brain/114.5.2051
Petersen, S. E., & Fiez, J. A. (1993). The processing of single words studied with positron emission tomography. Annual Review of Neuroscience, 16, 509-530. https://doi.org/10.1146/annurev.ne.16.030193.002453
Price, J. L., & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 192–216. https://doi.org/10.1038/npp.2009.104
Rosenberg, M. D., Finn, E. S., Scheinost, D., Constable, R. T., & Chun, M. M. (2017). Characterizing attention with predictive network models. Trends in Cognitive Sciences, 21(4), 290–302. https://doi.org/10.1016/j.tics.2017.01.011
Rosenberg, M. D., Finn, E. S., Scheinost, D., Papademetris, X., Shen, X., Constable, R. T., & Chun, M. M. (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19(1), 165–171. https://doi.org/10.1038/nn.4179
Roy, A. K., Shehzad, Z., Margulies, D. S., Kelly, A. M., Uddin, L. Q., Gotimer, K., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. https://doi.org/10.1016/j.neuroimage.2008.11.030
Roy, M., Shohamy, D., & Wager, T. D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. https://doi.org/10.1016/j.tics.2012.01.005
Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X., & Kastner, S. (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science, 337(6095), 753-756. https://doi.org/10.1126/science.1223082
Sadeghi, S., Schmidt, S., Mier, D., & Hass, J. (2022). Effective connectivity of the human mirror neuron system during social cognition. Social Cognitive and Affective Neuroscience, 17(8), 732–743. https://doi.org/10.1093/scan/nsab138
Sakaki, M., Yoo, H. J., Nga, L., Lee, T. H., Thayer, J. F., & Mather, M. (2016). Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage, 139, 44–52. https://doi.org/10.1016/j.neuroimage.2016.05.076
Shoemaker, J. K., Norton, K. N., Baker, J., & Luchyshyn, T. (2015). Forebrain organization for autonomic cardiovascular control. Autonomic Neuroscience: Basic & Clinical, 188, 5–9. https://doi.org/10.1016/j.autneu.2014.10.022
Schumann, A., de la Cruz, F., Köhler, S., Brotte, L., & Bär, K. J. (2021). The influence of heart rate variability biofeedback on cardiac regulation and functional brain connectivity. Frontiers in Neuroscience, 15, 691988. https://doi.org/10.3389/fnins.2021.691988
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex, 22. https://doi.org/158–165.10.1093/cercor/bhr099
Shofty, B., Gonent, T., Bergmann, E., Mayseless, N., Korn, A., Shamay-Tsoory, S., Grossman, R., Jalon, I., & Ram, Z. (2022). The default network is causally linked to creative thinking. Molecular Psychiatry. https://doi.org/10.1038/s41380-021-01403-8
Small, D. M., Zald, D. H., Jones-Gotman, M., Zatorre, R. J., Pardo, J. V., Frey, S., & Petrides, M. (1999). Human cortical gustatory areas: A review of functional neuroimaging data. Neuroreport, 10(1), 7–14. https://doi.org/10.1097/00001756-199901180-00002
Thayer, J. F., Hansen, A. L., Saus-Rose, E., & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 37(2), 141–153. https://doi.org/10.1007/s12160-009-9101-z
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. https://doi.org/10.1016/s0165-0327(00)00338-4
Thompson, M., & Thompson, L. (2015). The neurofeedback book (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
van Ettinger-Veenstra, H., Lundberg, P., Alföldi, P., Södermark, M., Graven-Nielsen, T., Sjörs, A., Engström, M., & Gerdle, B. (2019). Chronic widespread pain patients show disrupted cortical connectivity in default mode and salience networks, modulated by pain sensitivity. Journal of pain research, 12, 1743–1755. https://doi.org/10.2147/JPR.S189443
Wiebking, C., & Northoff, G. (2014). Interoceptive awareness and the insula - Application of neuroimaging techniques in psychotherapy. GSTF International Journal of Psychology, 1(1), 53-60. https://doi.org/10.5176/0000-0002_1.1.8
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., Roffman, J. L., Smoller, J. W., Zöllei, L., Polimeni, J. R., Fischl, B., Liu, H., & Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. https://doi.org/10.1152/jn.00338.2011
Zerr, C. L., Berg, J. J., Nelson, S. M., Fishell A. K., Savalia, N. K., & McDermott, K. B. (2018). Learning efficiency: Identifying individual differences in learning rate and retention in healthy adults. Psychological Science, 956797618772540. PMID 29953332 https://doi.org/10.1177/0956797618772540
