Montages and Spectral and Topographic Aspects of the EEG
Contamination of the EEG by physiological and exogeneous artifacts requires that clinicians take extensive precautions, examine the raw EEG record, and remove contaminated epochs through artifacting. Impedance tests and behavioral tests help ensure the fidelity of qEEG recording. Graphic © Medical-R/Shutterstock.com.
International QEEG Certification Board Blueprint Coverage
This unit addresses IV. Montages and Spectral and Topographic Aspects of the EEG (3 hours).

This unit covers Using a Limited Number of Electrodes, Montage Options and Their Consequences, Transforms and Power Displays, Filtering Methods, EEG Frequency Band Sources, and Connectivity, Phase, and Coherence.
Using a Limited Number of Electrodes
Peer-reviewed evidence suggests that more EEG channels provide a more accurate assessment and achieve superior clinical or performance outcomes than fewer channels (Lau et al., 2012).
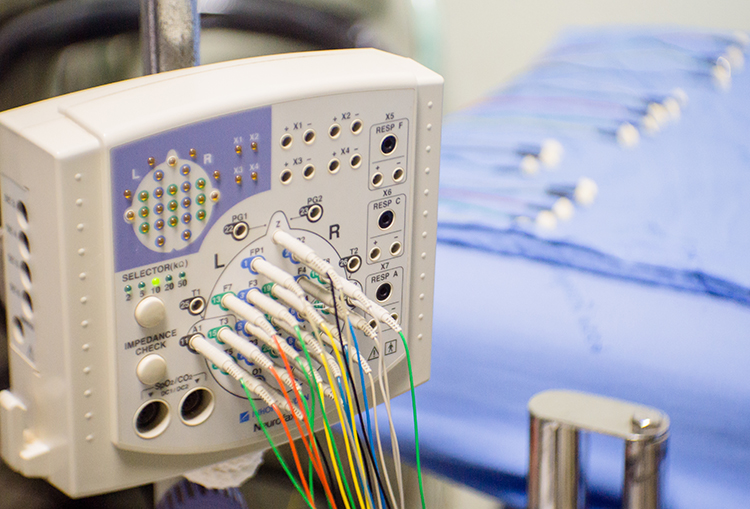
Although some practitioners conduct assessment and training with a single channel, assessments and training methods that use more than one channel have become more available. For example, the cost of a full 19-channel EEG assessment has decreased substantially. Such an assessment can provide not only EEG amplitude data from all sites in the 10-20 system but additionally makes calculations of metrics such as coherence and phase that give information on how well the 10-20 sites communicate with each other. These data from multi-channel methods are beneficial for complex symptom profiles like those associated with Autism Spectrum Disorders, epilepsy, and traumatic brain injury (Thompson & Thompson, 2016). Graphic © Chaikom/Shutterstock.com.

A channel is an EEG amplifier output resulting from scalp electrical activity from three electrode/sensor connections to the scalp. These sensors are commonly known as active, reference, and ground electrodes, though they are more appropriately called positive +, negative - and reference. They are placed on the head in the following manner: an active or positive electrode is placed over a known EEG generator like Cz. A reference or negative electrode may be located on the scalp, earlobe, or mastoid. A ground/reference electrode may also be placed on an earlobe or mastoid (Thompson & Thompson, 2015).
Active and reference sensors are identical balanced inputs and interchangeable. However, some neurofeedback data acquisition systems require the designation of a specific sensor as a "reference," as in a linked-ears reference.
A derivation is the assignment of two electrodes to an amplifier's inputs 1 and 2. For example, Fp1 to O2 means that Fp1 is placed in input 1 and O2 in input 2.
A montage groups electrodes together (combines derivations) to record EEG activity (Thomas, 2007).
All montages compare EEG activity between one or more pairs of electrode sites.
Modern amplifiers record all input sensors in reference to a common sensor - often Cz - and all montage (sensor comparison) changes are performed in the software. Amplifiers no longer require manual switching of electrodes between inputs.The narrated video below © John S. Anderson displays the same 21-channel recording viewed using different montages with a 60-Hz notch filter on and off.
Montage Options and Their Consequences
Referential (Monopolar) Montage
A referential (monopolar) montage places one active electrode (A) on the scalp and a "neutral" reference (R) and ground (G) on the ear or mastoid. Graphic © John S. Anderson.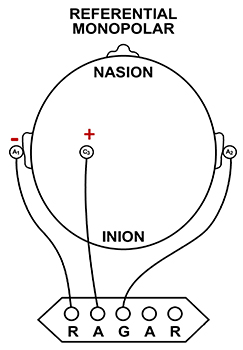
A referential montage assumes that the EEG activity seen on the computer screen represents the active (+) site because the reference (-) site is assumed to be neutral (i.e., producing no EEG activity) and because of the subtraction of signals produced by noise and artifacts that are common to both active and reference sites (common-mode rejection). In the photograph below, the blue cable would be used for the active electrode, the yellow cable with an ear clip for reference, and the black cable with an ear clip for the ground.

However, this montage is vulnerable to artifacts from the contraction of facial muscles (Demos, 2019). The ear reference is also known to produce reference contamination, where EEG signals from this electrode are contributed or added to other electrodes via the mechanism of the differential amplifier, where anything different between the "active" and "reference" sensors is retained. This commonly results in alpha activity produced by posterior alpha sources close to the ear.
Sequential (Bipolar) Montage
A sequential (bipolar) montage presents a sequence of comparisons of positive (+) and negative (-) electrodes (often called ‘active’ and ‘reference’) that are attached to sites on the scalp and therefore considers the reference electrode to be a second active electrode. The ground (G) electrode is attached to the scalp, to an earlobe, or over the mastoid process. Graphic © John S. Anderson.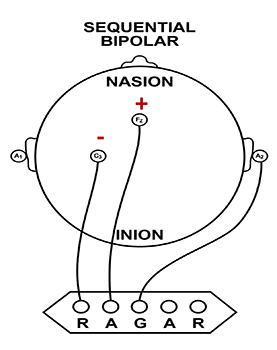
The sequential montage detects the difference in EEG between the positive and the negative electrodes (active and reference), as the referential montage does. Still, now the signal for the channel represents the difference between two sources of EEG activity. In cases when 19-channels are used, this montage is usually presented with electrode pairs shown in sequence. Note that only the black cable for the ground has an ear clip in the photograph below.

When used as only a single channel, this montage does not detect localized EEG activity well because it shows only the difference between the A and R signals. However, when used as part of a 19-channel assessment, it localizes EEG events related to epilepsy. This montage can also reduce artifacts when the A and R electrodes are relatively close.
A sequential montage is frequently used in neurofeedback and trains the difference between EEG activity at the A and R electrodes. However, when neurofeedback training produces a change, it remains uncertain whether it is because of a change in EEG at the A electrode, the R electrode, or both electrodes.
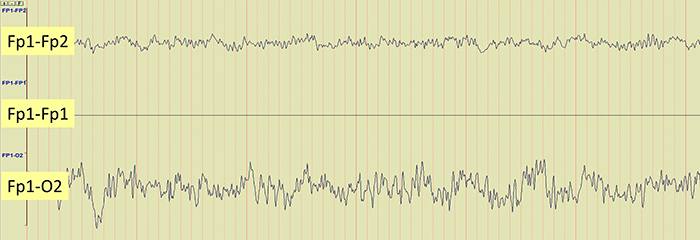
The EEG record appears more similar when sensors are closer together and less similar when they are farther apart (Fp1-O2). When electrodes are spaced close together (Fp1-Fp2), this montage may reject actual EEG activity (Fp1-Fp1). Graphic © John S. Anderson.
EEG activity ranges from DC (slow cortical potentials) to gamma (34-60+ Hz) (Collura, 2014). Hertz (Hz) is an abbreviation for cycles per second. The raw EEG signal consists of oscillating electrical potential differences detected from the scalp. Raw or wave displays plot voltage using a bipolar (positive/negative) scale with zero in the middle. This is the analog form of the signal in which voltage continuously varies instead of digital representation using 0s and 1s. The graphic © John S. Anderson shows the voltage as µV peak to peak.
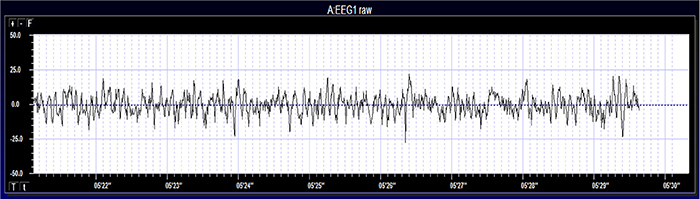
BASIC SIGNAL MEASUREMENT TERMS
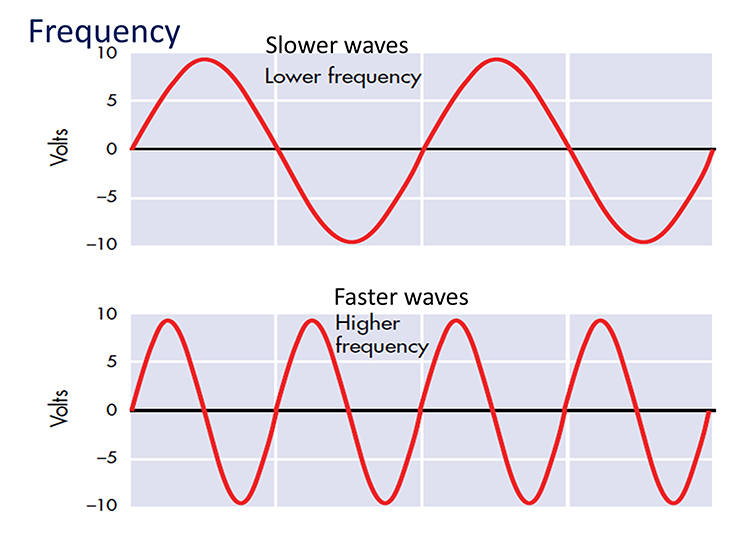
EEG waveforms share the features of frequency and shape (Libenson, 2010). Frequency measures the speed and is the number of cycles completed each second. The higher the frequency (f), the shorter the wavelength (λ). The mathematical relationship is f = 1/λ. To measure frequency in the raw waveform, count the number of peaks or zero crossings and divide by 2. Graphic © John S. Anderson.
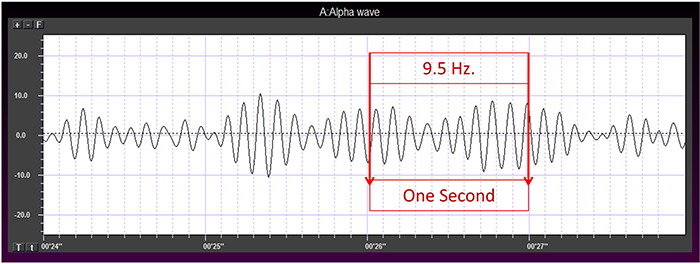
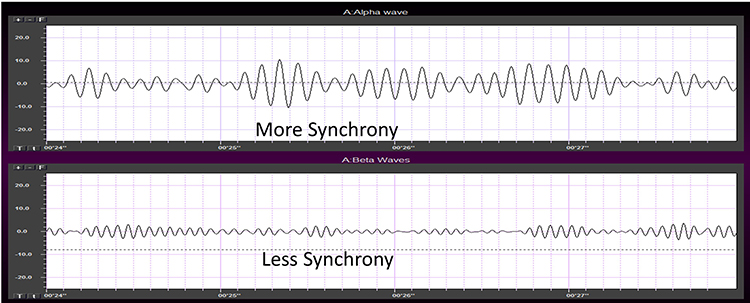
Amplitude measures size, which is the "amount" of energy within an EEG frequency band. The amplitude and morphology of any EEG frequency band reflect the number of neurons discharging simultaneously at that frequency. High amplitude means that many neurons are depolarizing and hyperpolarizing at the same time.
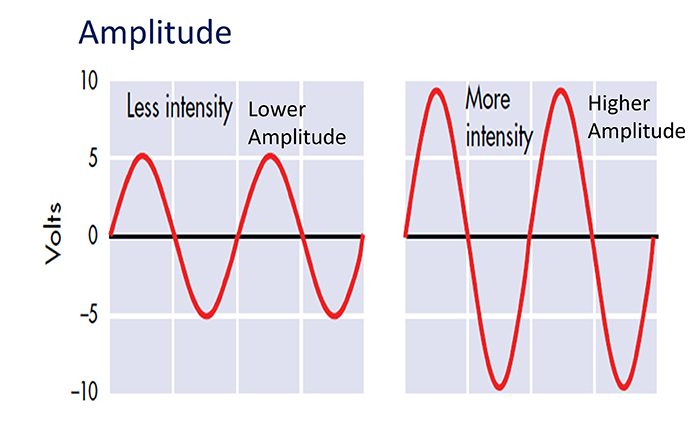
Greater synchrony among neurons firing results in higher amplitude (Demos, 2019). Graphic © John S. Anderson.
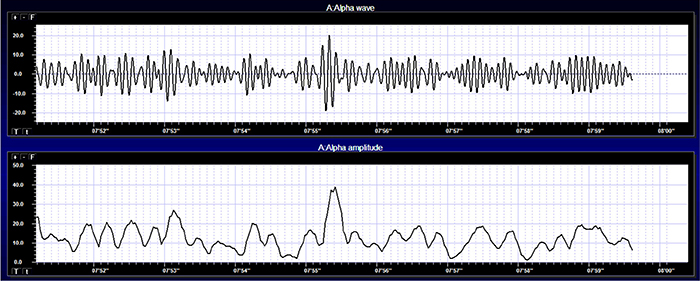
Amplitude displays show voltage using a scale where all values are positive (greater than zero). They only show voltage changes, not the signal waveform. Graphic © John S. Anderson shows the oscillating raw alpha waveform (top) and alpha amplitude (bottom).
Magnitude represents the average amplitude over a unit of time using quantification methods like peak-to-peak (P-P) and root mean square (RMS). The peak-to-peak method measures waveform "height" from peak to trough. In contrast, the root mean square method calculates the area under the EEG waveform and is analogous to the weight of an object (Collura, 2014). The graphic below that illustrates EEG spectrum magnitude © John S. Anderson.
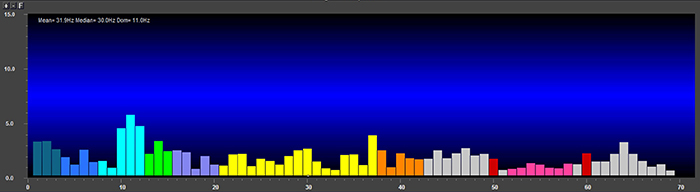
EEG signal power is magnitude squared and may be expressed as microvolts squared or picowatts/resistance. Most qEEG databases convert power into standard deviations, whereas the Jewel database transforms amplitudes into standard deviations or Z-scores (Demos, 2019). The graphic below © John S. Anderson shows the EEG power spectrum instead of the magnitude spectrum.
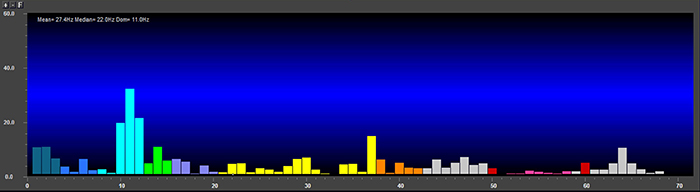
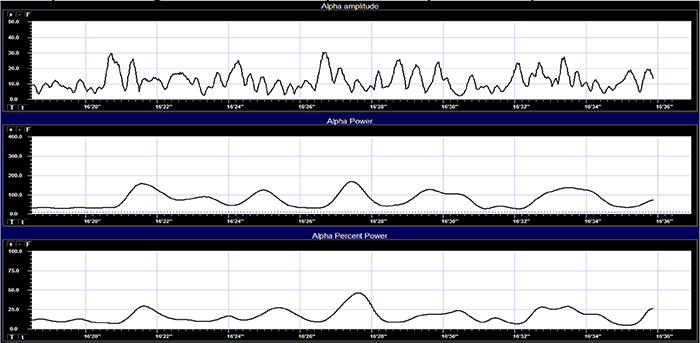
Percent power is the power within a frequency band expressed as a percentage of total EEG power. The graphic below © John S. Anderson shows alpha amplitude, alpha power, and alpha percent power.
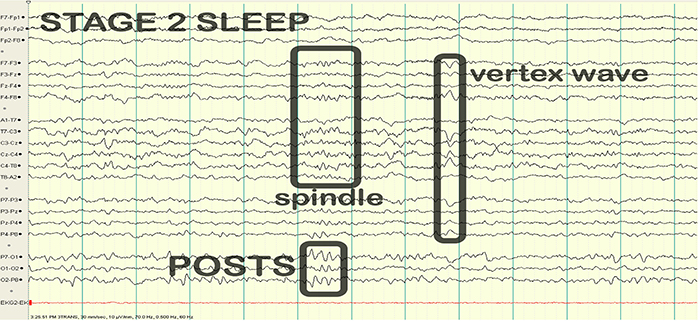
EEG waveforms may assume a distinctive shape or morphology like POSTs (positive occipital sharp transients of sleep), spindles (oscillations), and vertex (V) waves that appear over Cz during stage 2 sleep. The graphic below © eegatlas-online.com shows stage 2 sleep.
FILTERING METHODS
Sampling
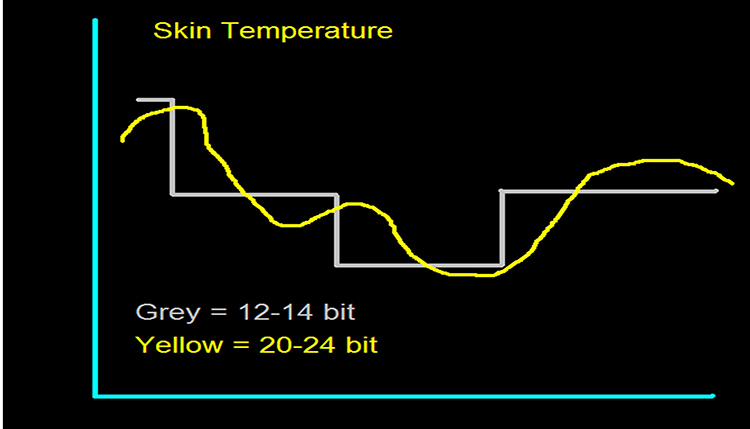
Data acquisition systems digitize and process the raw EEG signal. Digitization transforms the raw signal into a digital form. An analog-to-digital converter (ADC) samples the analog signal (transforms it into numerical values) with a sampling resolution, sampling rate, and epoch length.The sampling resolution is the number of digital bits used to represent a signal. Each bit (binary digit) is assigned a binary value of 0 or 1. Systems that sample the EEG signal utilize from 8-24 bits. The advantages of 24-bit sampling are an accurate sampling of the EEG signal's DC and AC components and the ability to sample a wide range of signal voltages called the dynamic range (Collura, 2014). Graphic © John S. Anderson.
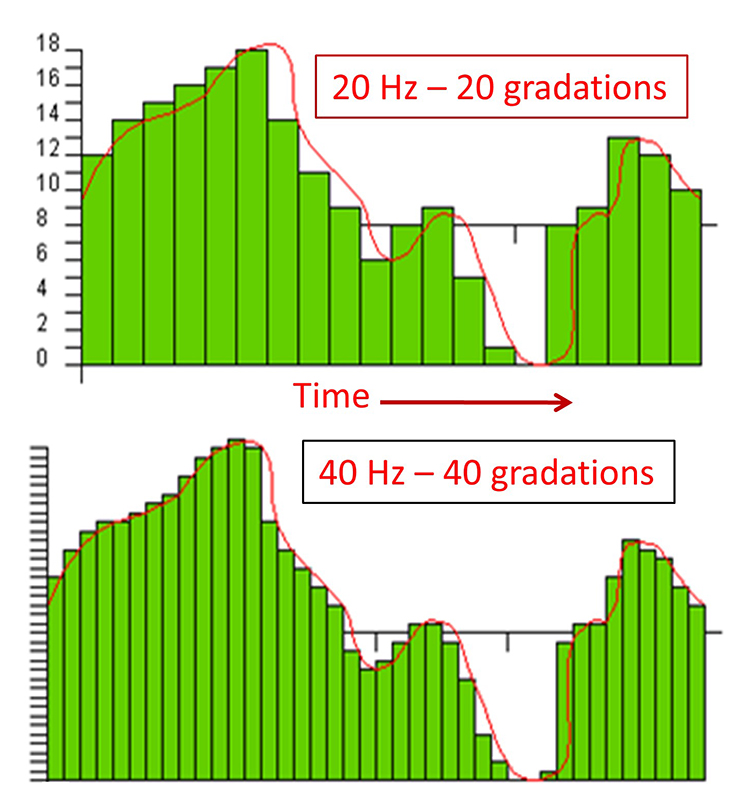
The sampling rate is the number of times that the ADC samples the EEG signal per second. A rate of twice the highest frequency is the minimum acceptable sampling rate when performing Fast Fourier Transform (FFT) analysis. Graphic © John S. Anderson.
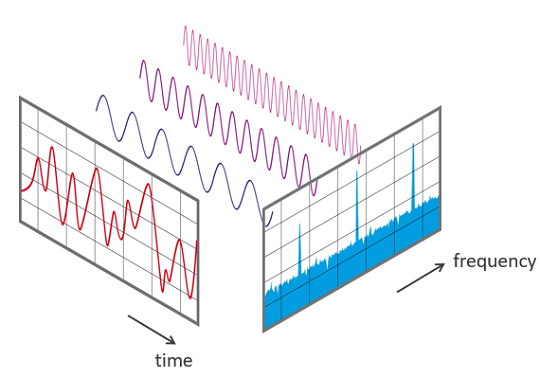
A FFT is a mathematical transformation that converts a complex signal into component sine waves whose amplitude can be calculated. The graphic below shows the decomposition of the original signal (left) into its sinewaves of different frequencies (center) and is courtesy of NTi Audio.
A rate of twice the highest frequency is insufficient to visually represent the EEG signal since it only samples the highest frequency twice per cycle. This low rate also allows the harmonics of 50/60 Hz noise (which can extend to several hundred hertz) to contaminate the EEG signal. For example, when there is a 240-Hz harmonic of 60Hz noise, sampling at 256 samples per second (sps) can result in a spurious 16-Hz waveform (Collura, 2014). Faster sampling rates are desirable, particularly when resolving high-frequency signals. A sampling rate of 512 sps is a good choice for frequencies up to 64 Hz, and 1024 sps is suitable for frequencies up to 128 Hz. However, a sampling rate of 256 sps is considered adequate for most purposes
FFT analysis breaks the EEG signal into 1- to 2-s chunks called epochs. Epoch length sets the lowest and highest frequencies that the FFT can represent (Collura, 2014). Graphic © John S. Anderson shows the conversion of a complex signal using FFT.
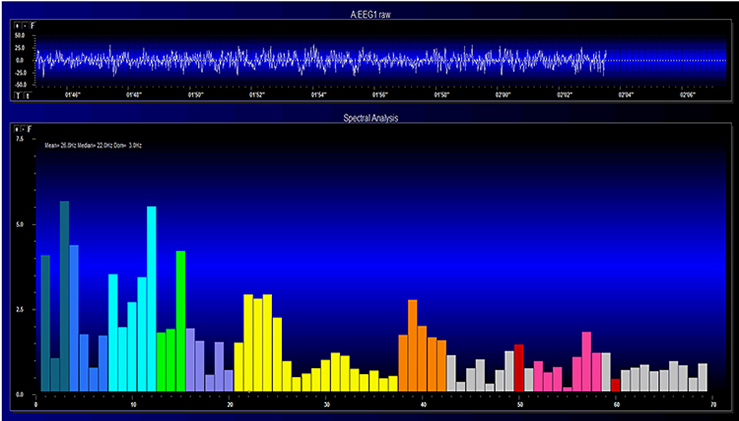
The FFT power spectral analysis video below © John S. Anderson. The top window shows the raw EEG signal, and the bottom window features a spectral display created using FFT analysis.
Joint time-frequency analysis (JTFA) computes values on each data point at rates up to 256 times per second without using a fixed epoch length. Where FFT simultaneously calculates amplitudes for all frequency bands, JTFA analyzes preselected bands. The JFTA graphic © John S. Anderson.
EEG Frequency Band Sources
Thalamic Generators
Anderson and Anderson (1968) advanced the facultative pacemaker theory that thalamic neurons activate cortical neurons and thalamic inhibitory interneurons via recurrent collaterals. While these thalamocortical neurons only excite a limited number of cortical neurons, thalamic interneurons inhibit a large pool of thalamocortical relay neurons. When the inhibition ends after one-tenth of a second, the thalamocortical neurons experience rebound excitation. This synchronized depolarization excites both cortical neurons and thalamic inhibitory interneurons, which will inhibit a more extensive pool of thalamocortical relay neurons and initiate another cycle of excitation and inhibition that produces EEG rhythms (Fisch, 1999). Graphic courtesy of Zachary Barry and featured in Wikipedia's article Recurrent ThalamoCortical Resonance.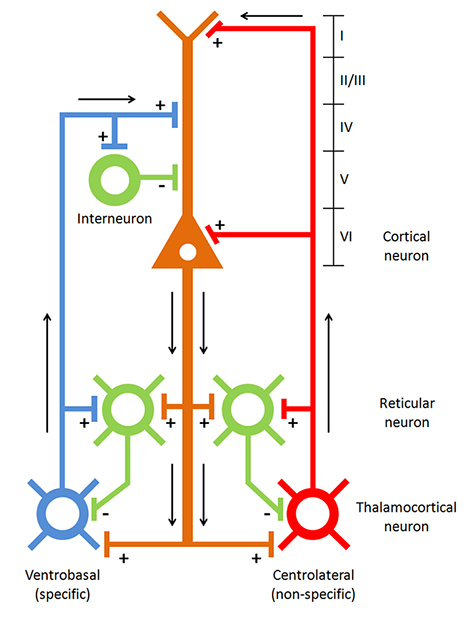
Caption: Thalamocortical circuit diagram depicting specific/sensory and non-specific intralaminar thalamocortical systems.
The networking of excitatory and inhibitory thalamic neurons imposes a group rhythm on its members that is transmitted to cortical macrocolumns by thalamocortical neurons (Bear, Connors, & Paradiso, 2020).
The nucleus reticularis of the thalamus may function as a pacemaker by releasing the inhibitory transmitter GABA at synapses with thalamocortical neurons. These neurons depolarize cortical neurons and thalamic inhibitory interneurons via burst discharges when their inhibition ends.
As discussed in the Neurophysiology unit, oscillatory activity may involve an interaction between thalamocortical relay neurons (TCR), nucleus reticularis neurons (RE), and interneurons. These interactions are mediated by diverse neurotransmitters, including acetylcholine and GABA.
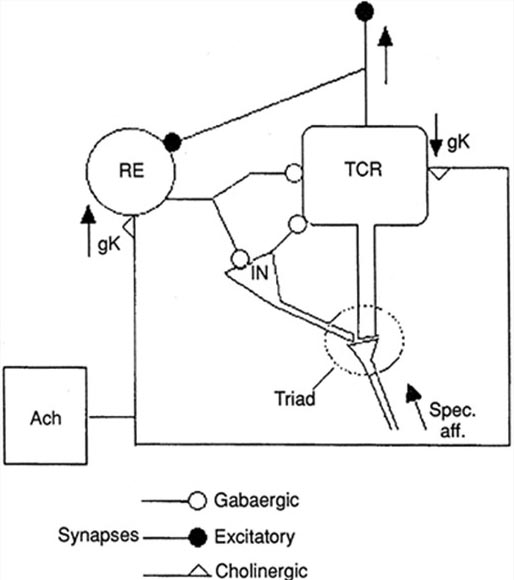
The thalamus is the dominant pacemaker for rhythmic EEG activity, including theta (3-8 Hz), alpha (8-12 Hz), and SMR (13-15 Hz) (Amzica & Lopes da Silva, 2018).
Cortical Generators
The cerebral cortex (gray matter) consists of neuronal cell bodies, glial cells, and blood vessels. White matter lies beneath the neocortex, which consists of myelinated nerves, nonmyelinated fibers, and glial cells. The EEG mainly originates from pyramidal neurons in layers 3, 5, and 6 of gray matter that is approximately 5-mm thick. Check out the Blausen Cerebrum: Gray Matter and White Matter animation.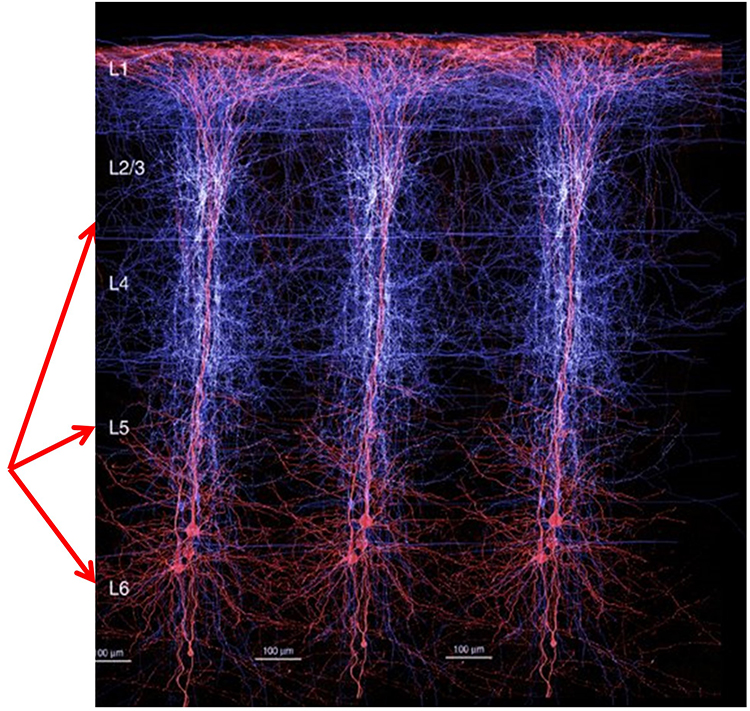
Vertical cortical macrocolumns contain hundreds of pyramidal neurons and supporting stellate and basket cells (Thompson & Thompson, 2016). Each pyramidal neuron may receive more than 100,000 synapses. These macrocolumns are positioned side by side and perpendicular to the cortical surface. Since neighboring macrocolumns often receive the same afferent messages, this increases the probability that they will fire together and generate a potential that we can detect from the scalp. A reliable scalp EEG requires a minimum of 6 cm² of synchronized cortex (Dyro, 1989).
Although thalamic pacemakers generate EEG rhythms, resonant loops between cortical macrocolumns may be another source (Traub et al., 1989). Over 97 percent of the conversations within the brain are cortical-to-cortical (Thompson & Thompson, 2016). This communication is primarily confined within the same hemisphere. A resonant loop develops when macrocolumns that share afferent input fire synchronously to generate an electrical potential. The distance between the cortical macrocolumns that participate in a resonant loop is one determinant of EEG frequency. The closer the macrocolumns in a resonant loop, the higher the frequency they can generate (Lubar, 1997).
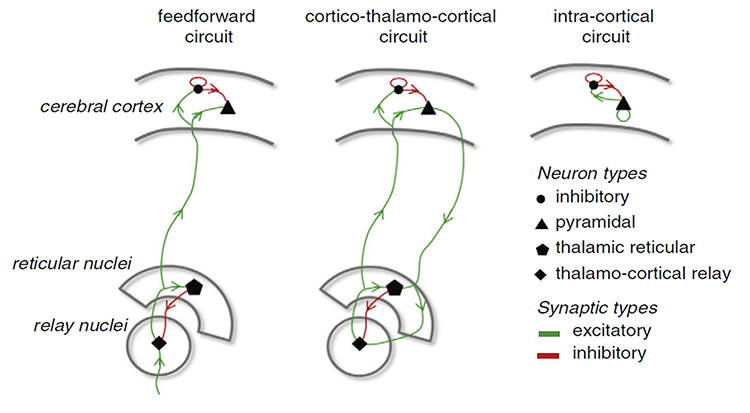
The graphic below illustrates thalamocortical and cortical-cortical EEG sources.
There are three types of resonant loops driven by afferent input or thalamic pacemakers: local, regional, and global. Local loops couple neighboring macrocolumns and may generate frequencies above 30 Hz in the high-beta and gamma ranges. Regional loops couple macrocolumns separated by several centimeters and may produce alpha and beta rhythms. Finally, global loops couple macrocolumns as distant as 7 cm (for example, between the frontal and parietal lobes) and may create delta and theta rhythms.
While only 3 percent of these linkages are thalamocortical, they greatly influence the EEG by subcortically connecting distant cortical regions and producing most synchronous activity (Steriade, 1990). Lubar (1997) proposed a violin analogy where the thalamic pacemakers that fire at varying frequencies are the strings, and the resonant loops that introduce different time delays are the instrument's resonant cavity. Graphic of circuits that contribute to the EEG by Hindriks and van Putten © 2013 NeuroImage.
Spindling is a synaptically generated oscillation in a circuit that includes the reticular nuclei (Steriade, 2005). The video of alpha spindling © John S. Anderson.
Different spindle frequencies are due to corresponding durations of thalamocortical neuron hyperpolarization. For example, longer hyperpolarizations associated with EEG synchronized states produce 7-Hz or lower-frequency spindles. In contrast, relatively short hyperpolarizations result in 14 Hz spindles (Steriade, 2005).
The thalamocortical model © 2008 National Academy of Sciences from Izhikevich and Edelman's Large-scale model of mammalian thalamocortical systems article in the Proceedings of the National Academy of Sciences of the United States of America.
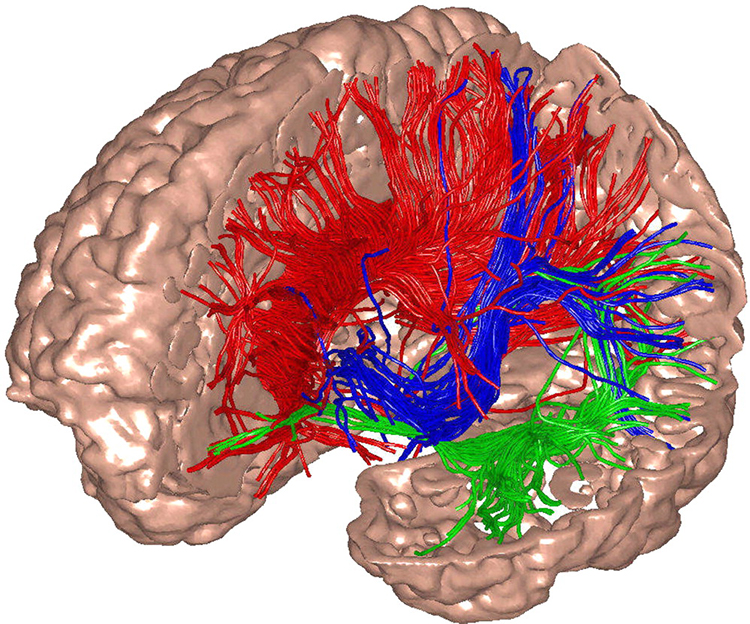
Caption: The researchers used diffusion tensor imaging to illustrate the targets of white-matter fibers.
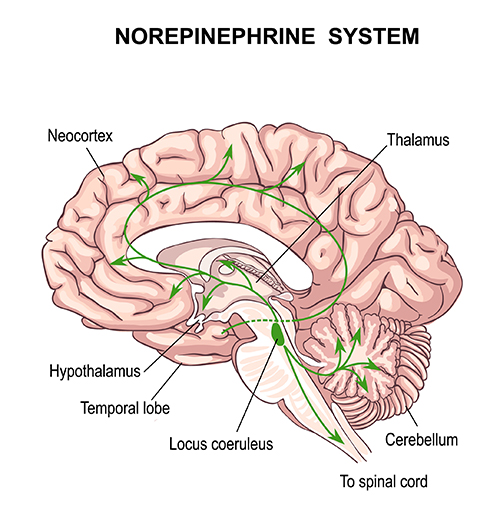
The electrical potentials generated by the thalamus can volume conduct near the speed of light through cerebrospinal fluid (CSF), brain tissue, the skull, and the scalp so that nearly identical waveforms can simultaneously appear at distant sites (Fisch, 1999; Thompson & Thompson, 2016).The Locus Coeruleus Inhibits Thalamic Alpha Generators
When we are inattentive, thalamic pacemakers generate the alpha rhythm. When we need to focus attention, we activate the brainstem noradrenergic locus coeruleus. The increased release of norepinephrine by this 15-millimeter network focuses attention and abolishes alpha oscillations. The locus coeruleus enhances the brain's sensory information processing by suppressing thalamic alpha generators. This may be an underlying mechanism of the phenomenon of alpha blocking. Although researchers cannot noninvasively monitor locus coeruleus activity in human participants, it is correlated with pupil dilation. In human studies, the greater the alpha blocking response and pupil dilation, the better the performance on demanding attention tasks (Dahl et al., 2020; Dahl et al., 2022). Graphic © Vasilisa Tsoy/Shutterstock.The alpha rhythm is not a cause but a sign that incoming stimulation is too weak to overcome inhibition by the reticular nucleus. EEG activity is not causal and reflects network activity that has already occurred.
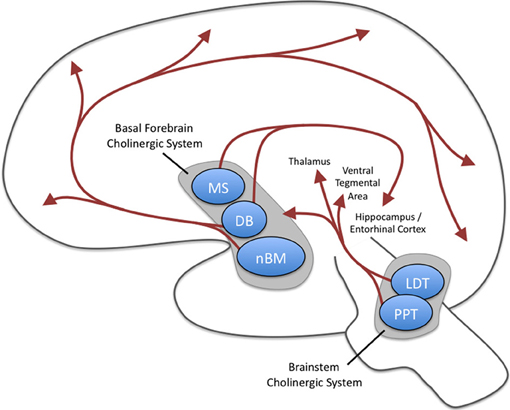
Additional Subcortical Generators
Ascending projections from the basal forebrain, reticular formation, locus coeruleus, and raphe systems disrupt brain rhythms.These neurons receive information from most sensory systems and cortical regions and directly desynchronize the EEG through synapses on cortical neurons and indirectly through innervation of thalamic pacemakers. Desynchronization shifts pyramidal neurons from burst firing to more continuous firing or the generation of single spikes (Fisch, 1999).The cholinergic basal forebrain, located in the ventral frontal lobe and anterior hypothalamus, influences cerebral blood flow and cognitive activity. Graphic courtesy of Newman et al. (2012), Cholinergic modulation of cognitive processing: Insights drawn from computation models.
The reticular activating system (RAS) includes a network of 90 nuclei within the central brainstem from the lower medulla through the thalamus that activates the brain to promote attention, consciousness, and wakefulness. This network receives input from ascending sensory tracts (auditory, olfactory, somatosensory, and visual systems). The RAS projects to the thalamus and diffusely to the cortex. The RAS also has diffuse cortical projections that bypass the thalamus.
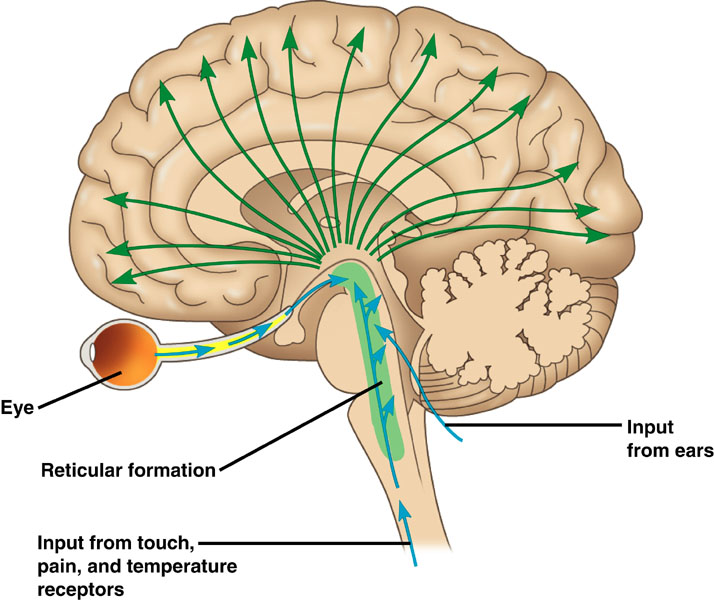
The noradrenergic brainstem locus coeruleus system, which projects to the thalamus, limbic system, and cerebral cortex, also contributes to wakefulness and vigilance for salient stimuli.
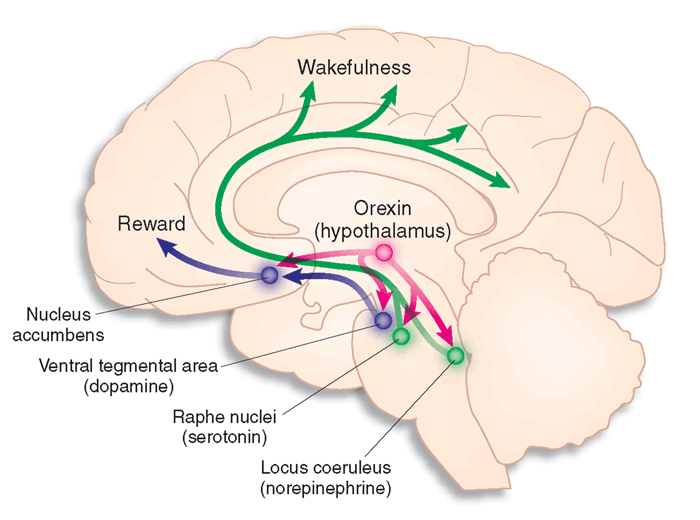
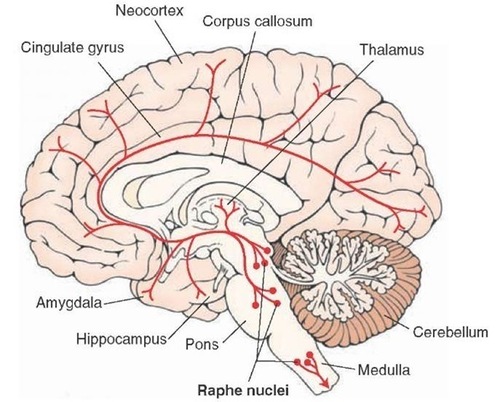
Finally, the serotonergic raphe system is a midline network of cell bodies within the brainstem and midbrain that may influence alertness and vigilance through reciprocal connections with the suprachiasmatic nucleus of the hypothalamus (Monti & Jantos, 2008).
Cortical and Subcortical Generators of Specific EEG Rhythms
Slow Cortical Potentials (0-1 Hz)
Slow cortical potentials (SCPs) have been identified in cortical neurons, the thalamus, and glial cells. Cortical neurons in layers II to VI generate slow oscillations when the thalamus is removed or when cortical tissue is studied in vitro (in an artificial environment) or in vivo (within a living organism). Thalamic reticular neurons exhibit similar slow spontaneous oscillations when studied in vitro, and synchronized intracortical oscillations may depend on a corticothalamic network that targets these thalamic neurons.Glial cells generate slow SCPs when they burn sugar, producing negatively charged bicarbonate ions. Unlike EEG rhythms like delta, SCPs are not the summation of dendritic potentials. SCPs are associated with glial cells and gap junctions. Glial cells chemically communicate among themselves and with neurons. The slow oscillations of glial cells may influence the timing of neuronal firing through their control of potassium ion outflow (Steriade, 2005). These slow oscillations appear to organize the generation of other brain rhythms.
"The concept of a unified corticothalamic network that generates diverse types of brain rhythms grouped by the cortical slow oscillation (Steriade, 2001a,b) is supported by EEG studies in humans" (Molle et al., 2002).
Caton (1875) observed that the cortex's direct current baseline becomes negative whenever it is more active. The voltage gradients range from 150-200 μV. Underlying "tone" or valence factors determine the firing characteristics of neurons within a network. When SCPs are more positive, there is reduced firing of cortical neurons due to hypopolarization. When SCPs are more negative, there is increased firing due to depolarization.
The following 19-channel BioTrace+ /NeXus-32 display of 0.1-1 Hz SCP activity © John S. Anderson.
Perspective on Fast Cortical Potentials
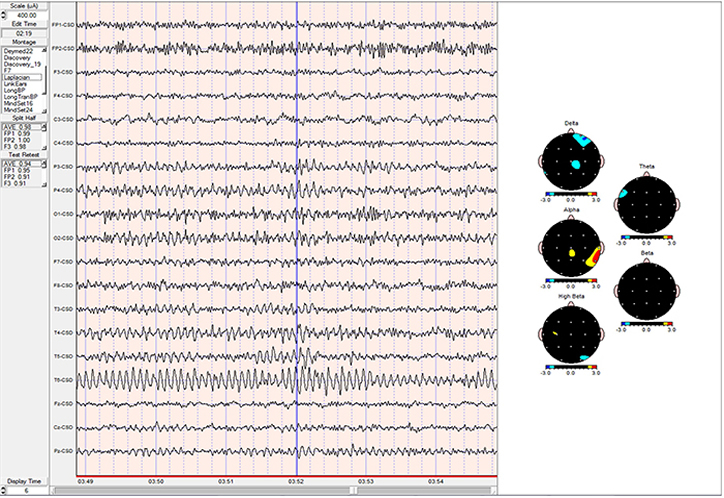
EEG "bands" are somewhat arbitrary ranges of frequencies that have evolved from observation and usage. The following BioTrace+ /NeXus-32 video of raw and spectral EEG displays © John S. Anderson. Frequency is plotted along the horizontal axis, and amplitude is shown on the vertical axis.While frequency band labels are helpful descriptors, they can also be misleading. Classification of an EEG rhythm is based on context (measurement conditions and EEG activity during the specific epoch), frequency, and waveform morphology.
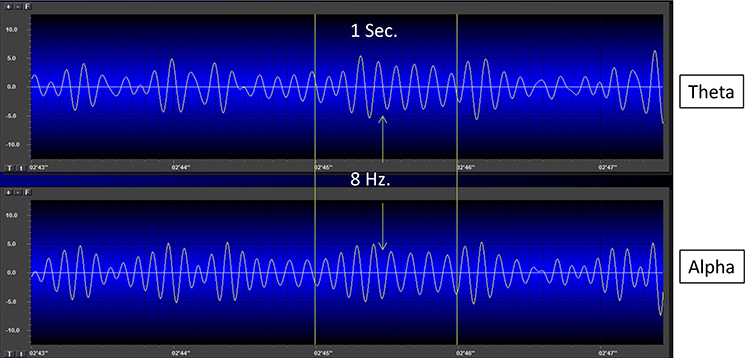
The process of up-training or down-training signal amplitude in one or more of the EEG bands using an EEG is called EEG biofeedback or neurofeedback. Minimum EEG voltages of 20-30 μV are seen in children and adults (Kraus et al., 2011).
Brainwaves Reflect Behavior
The ratio of slow (theta) to faster (beta) brainwaves shows how alert you are. This is the theta/beta ratio.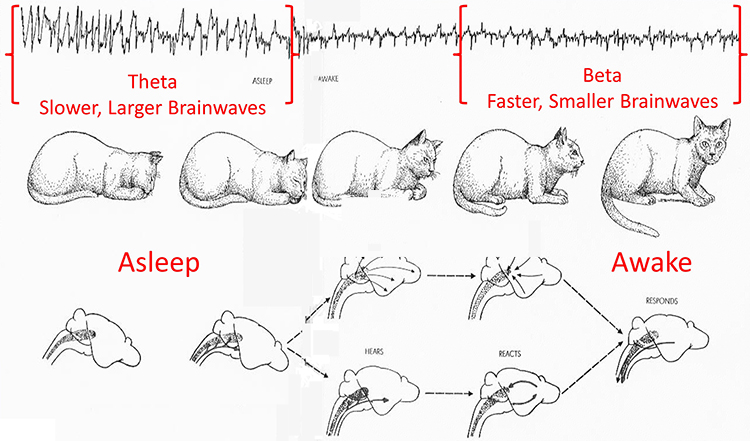
In the next section, we will examine delta, theta, rhythmic slow-wave, alpha, mu, synchronous "alpha," SMR, beta, high or fast beta, and gamma activity.
Local Versus Global Decision-making
The short time windows of fast oscillators facilitate local integration and decision-making, primarily because of the limitations of the axon conduction delays. The long time windows of slow oscillators can involve many neurons in large or distant brain areas and favor complex, global decisions.Delta (0.5-3 Hz)
There are two delta rhythms, a slow oscillation under 1 Hz and a traditional 1-4 Hz oscillation. The slow 0.3-0.4 Hz oscillation originates in the neocortex and persists when the thalamus is removed. Thalamo-cortical neurons generate the 1-4 Hz oscillations observed during human stage-3 sleep. Slow neocortical oscillations may synchronize the thalamic delta rhythm (Steriade, 2005).Delta activity is generated by cortical neurons when other connections do not activate them and is found predominantly in frontal areas. Delta is associated with sleep and infancy. Delta is associated with sleep and infancy. During stage-3 sleep, delta allows astrocytes to rebuild their stores of glycogen. Clinicians observe delta in clients diagnosed with ADHD, brain tumors, learning disorders, and traumatic brain injury (TBI). Rhythmic high-amplitude delta is associated with TBI, mainly if localized. Diffuse delta may be found in ADHD and learning disorders.
Normal Amplitudes
Delta should not be present in significant amounts in the awake adult EEG. "Apparent" delta is usually an eye movement artifact. Some delta activity probably occurs in the waking adult EEG.Delta bands are inhibited or down-trained but rarely rewarded. Delta desynchronization can be rewarded. The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 1-4 Hz activity from a 10-year-old male © John S. Anderson.
Theta (3-8 Hz)
The mechanisms that generate the theta rhythm are poorly understood. Theta differs depending on location and source. Amzica and Lopes da Silva (2011) consider the classic septal/diagonal band pacemaker model incomplete. Hippocampal interneurons, which innervate the hypothetical medial septum pacemaker, exercise top-down control. The hypothalamic supramammillary nucleus, with extensive connections to the brainstem, diencephalon, and medial septum, may also pace and modulate hippocampal theta. Further, a non-cholinergic theta source has been found within the entorhinal cortex of the hippocampus.Theta is associated with creativity, global synchronization, memory formation, and recall. Increased theta amplitudes correspond with hypo-perfusion and decreased glucose metabolism. Excessive frontal theta is linked with depression, daydreaming, distractibility, and inattention. A theta/beta (T/B) ratio of 3.0 may indicate ADHD depending on age, as T/B ratios are developmentally mediated (Monastra et al., 1999).
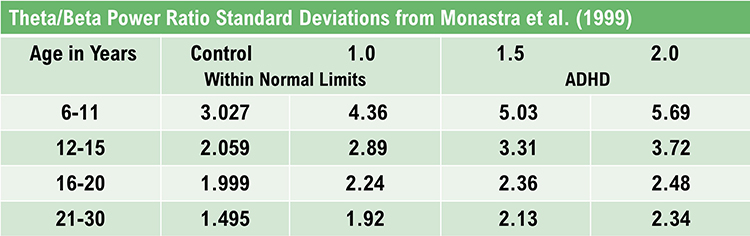
Normal Amplitudes
Theta voltage is age-related in the awake EEG. Voltage diminishes from age 8-30 with minimal amounts over age 30. A typical 6-7 Hz rhythm in the frontal midline (FCz) is associated with mental activity such as problem-solving and a wide variety of other functions. This rhythm appears to be limbic in origin. It is higher in amplitude and more synchronous when processing the feedback that an error has occurred. The 4-Hz rhythm is associated with childhood pleasurable experiences and memory searches in adults.Rhythmic Slow Wave (RSW or Theta)
Inhibit theta to remediate symptoms. Reward posterior RSW in alpha/theta training for addictions, global synchronization, optimal performance, and PTSD. RSW is generally not increased frontally. Clinicians may train for increases or decreases in phase synchrony. RSW is mainly seen in the frontal-midline (FCz) when awake with eyes open. The limbic system and thalamus generate RSW. Depending on location, RSW may be slowed-alpha as thalamic output slows.The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 4-8 Hz activity from a 10-year-old boy © John S. Anderson.
Alpha (8-13 Hz)
The 8-13-Hz alpha rhythm differs from spindle waves in both its source and the activity during which it is observed. Alpha 1 (low alpha) ranges from 8-10 Hz and alpha 2 (high alpha) from 10-13 Hz (Thompson & Thompson, 2016). Alpha rhythms depend on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons (Hughes & Crunelli, 2005). The alpha rhythm is maintained and propagated by cortical networks (Amzica & Lopes da Silva, 2018). Graphic of thalamocortical architecture courtesy of the Laboratory of Neuro Imaging and Martinos Center for Biomedical Imaging, Consortium of the Human Connectome Project.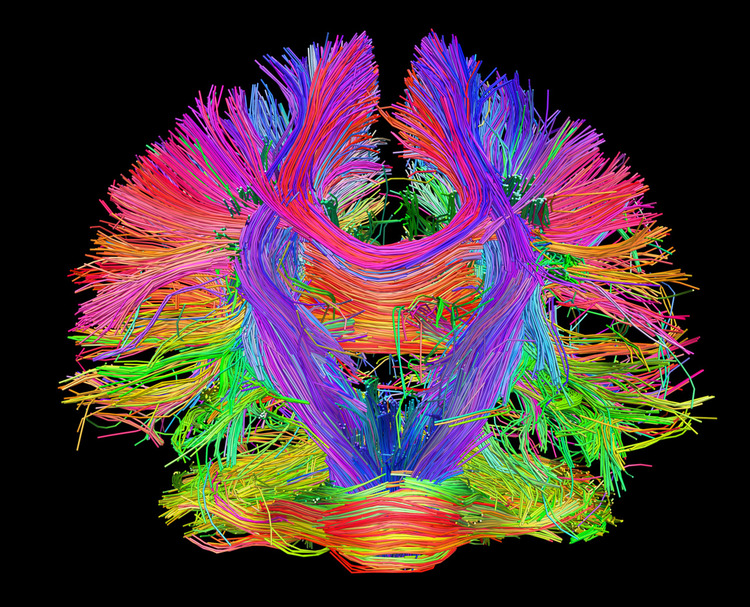
Researchers have correlated the alpha rhythm with relaxed wakefulness. There are age- and function-related differences. Spindle waves, in contrast, originate in the thalamus and occur during unconsciousness and stage 2 sleep (Steriade, 2005).
Alpha is the dominant rhythm in adults and is located posteriorly. The 8-10 Hz range is associated with ADHD, daydreaming, fogginess, OCD, and TBI. Frontal asymmetry is associated with depression. The 10-12 Hz range is seen with inner calm (calm and alert) and meditation. Clinicians train alpha amplitude and phase synchrony up or down for remediation of symptoms, depending on location.
Posterior Dominant Rhythm (PDR)
The posterior alpha rhythm is visible at about 4 months with a frequency of around 4 Hz. Between 3-5 years, this rhythm is approximately 8 Hz with amplitudes as high as 100 μV. From 6-15 years, this rhythm is 9 Hz by age 7 and 10 Hz by ages 10-15 with a mean amplitude of 50-60 μV. Girls show a statistically faster acceleration of posterior alpha frequency than boys. From 13-21 years, the mean alpha frequency is 10 Hz, and amplitudes decline throughout this period. Faster alpha frequencies are associated with higher IQ and better memory performance.The following 19-channel BioTrace+ /NeXus-32 display of the response of the posterior dominant rhythm to eyes opening and closing © John S. Anderson.
Normal Amplitudes
The typical adult alpha frequency ranges from 9.5-10.5 Hz. Alpha below 8 Hz is considered abnormal. There are age-related differences. Alpha frequency declines after age 70. Adult amplitudes are 50 μV or less:60% have ~ 20-60 μV
28% have < 25 μV
6% have > 60 μV
Higher alpha amplitudes are observed over the non-dominant (right) hemisphere (alpha asymmetry). Most studies show no effect of handedness. Asymmetry is generally no more than 20 μV or 20% of the greater of the two amplitudes (Amzica & Lopes da Silva, 2018).
Causes of Excessive Alpha Amplitudes
Sleep deprivation or metabolic exhaustion can result in high amplitude and slowing of the peak frequency and persistent alpha during an eyes-open condition. Meditation practices can cause increased amplitudes and slowing, a faster alpha response to an eyes-closed condition, and persistent alpha in an eyes-open condition. Marijuana use and abuse can cause increased amplitudes and slowing, persistent alpha in an eyes-open condition, depending on the type of marijuana. These effects can persist for many years following abstinence.The following 19-channel BioTrace+ /NeXus-32 display of eyes-closed 8-12 Hz activity from a 13-year-old girl © John S. Anderson.
Mu Rhythm (7-11 Hz)
While the 7-11-Hz mu rhythm usually overlaps with the alpha range, its morphology deviates from the alpha waveform as one end is pointed. The mu rhythm can be recorded at C3 and C4 in a minority of subjects and may represent suppression of hand movement or imagining hand movement (Thompson & Thompson, 2016). Mu rhythms appear to regulate motor cortex activities via prefrontal cortical mirror neurons. These circuits may play a critical role in imitation learning and our ability to understand the actions of others. Mu rhythms facilitate the conversion of visual and auditory input into integrated skill-building functions. Attenuation of the mu rhythm appears to be associated with the activation of this function (Pineda, n.d.). The mu rhythm is highlighted below.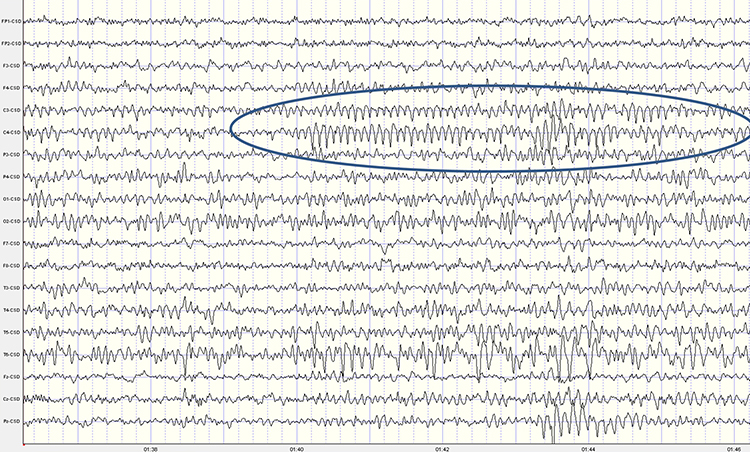
This second example of the mu rhythm shows a classic 10-11 Hz and 19-20 Hz "Owl Eye" presentation.
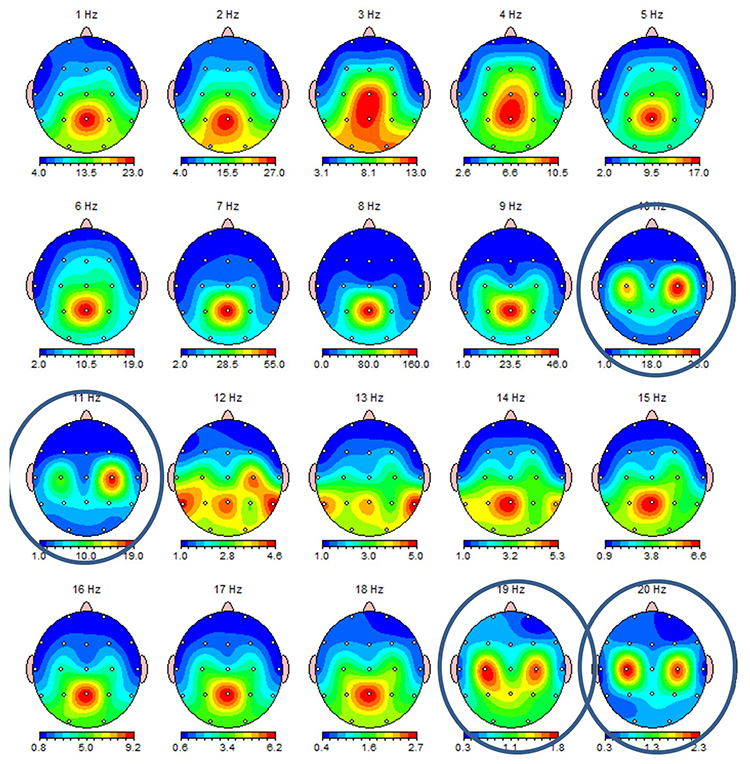
Synchronous "Alpha"
Various sensory systems such as our auditory, somatosensory, and visual systems produce localized and semi-independent "alpha" activity. Synchronous, distributed alpha integrates perception and facilitates action. Synchronous "alpha" appears to block the localized alpha-like patterns such as mu and the posterior rhythm in favor of more broadly distributed network integration.Sensorimotor Rhythm (13-15 Hz)
The sensorimotor rhythm (SMR) is beta 1 located on the sensorimotor strip (C3, Cz, C4). SMR amplitude increases when the motor circuitry is idle. SMR increases with stillness and decreases with movement. Deficient SMR may be observed in movement spectrum complaints like hyperactivity and tics. SMR appears as sleep spindles during stage-2 sleep. SMR is associated with neutral blood perfusion of the brain and resting levels of glucose metabolism. Clinicians typically reward increased SMR amplitude to calm hyperactivity and during theta/beta ratio training.The following 19-channel BioTrace+ /NeXus-32 display of 12-15 Hz activity © John S. Anderson.
Beta (over 12Hz)
Beta consists of rhythmic activity between 13-38 Hz. There are four beta ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz). Beta is located mainly in the frontal lobes. Beta is associated with focus, analysis, and relaxed thinking (Thompson & Thompson, 2015).Excessive beta is observed in anxiety, depression (asymmetry), insomnia, OCD, and sleep disorders. Deficient beta is seen in ADHD, cognitive decline, and learning disorders.
Since beta overlaps with the EMG range, clinicians must be careful when up-training this rhythm and use an EMG inhibit. Beta is generated by the brainstem and cortex and is associated with hyper-perfusion and increased glucose metabolism.
Normal 16-20+ Hz Beta Amplitudes
Beta amplitudes are minimal in children up to 12 years. There is a significant increase in beta amplitude and organization between 12-30 years. Beta is commonly seen in nearly all adults with amplitudes of 20 μV or less. Interhemispheric amplitude asymmetries exceeding 35% are abnormal. The following 19-channel BioTrace+ /NeXus-32 display of 13-21 Hz activity © John S. Anderson.Fast or High Beta Rhythms (20-35 Hz)
Fast 20-35-Hz oscillations are generated by activation of the mesencephalic reticular formation. Thalamocortical, rostral thalamic intralaminar, and cortical neurons spontaneously oscillate in this range. This activity is primarily seen in the frontal lobes and is associated with hyper-perfusion and increased glucose metabolism. Persistent excessive activity can lead to metabolic exhaustion.This activity may be associated with peak performance and cognitive processing and related to specificity and precision in information processing. Excessive high beta is associated with alcoholism, anxiety, OCD, rumination, and worry. Clinicians often inhibit high beta activity but rarely reward it.
The following 19-channel BioTrace+ /NeXus-32 display of eyes-closed ~ 25 Hz fast beta activity © John S. Anderson.
Gamma Rhythms (28-80 Hz)
Amzica and Lopes da Silva (2011) concluded that gamma oscillations might speed information distribution and processing. Gamma bursts occur during problem-solving, and the absence of gamma is associated with cognitive deficits and learning disorders. Gamma synchrony is related to cognitive processing and is essential in coding by contributing specificity and precision to information processing. Gamma is theorized to serve as a "binding rhythm" that integrates sensory inputs into perception and consciousness.The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 36-44 Hz activity in a 10-year-old boy © John S. Anderson.
Gamma rhythms are linked with SCPs. The following BioTrace+ /NeXus-32 display of SCP and gamma activity © John S. Anderson.
CONNECTIVITY, PHASE, AND COHERENCE
Neural networks are systems of interconnected ensembles of neurons that collaborate to achieve a goal (Thompson & Thompson, 2016). Networks communicate and perform functions via hub- or node-based communication systems. Connectome graphic from van den Heuvel and Sporns (2011).
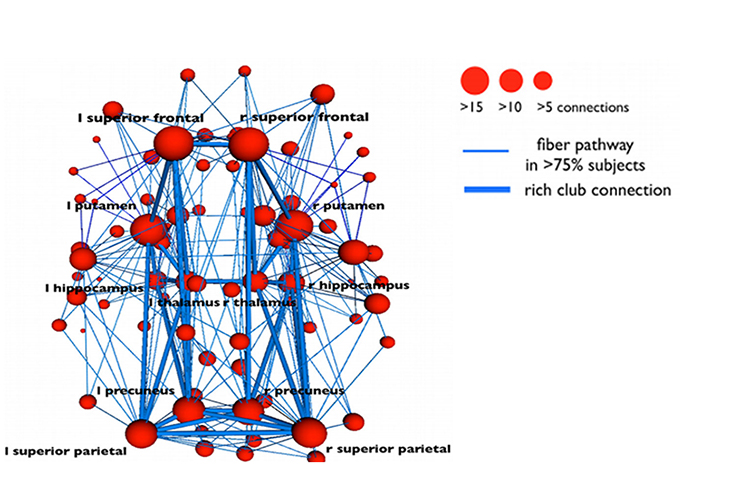
Connectivity
Networks like the Affect, Attention, Default, Executive, and Salience systems synchronize the activity of cortical and subcortical regions to perform functions. Connectivity is the degree of synchrony between the oscillations of specialized brain regions (nodes) within a network (Bastos & Schoffelen, 2016). Strongly connected brain regions are called hubs. Hubs can be primarily connected to nodes (vertices) within their local modules (sets of interconnected nodes) or nodes in more distant modules (Bullmore & Sporns, 2009).Neurofeedback training can increase or decrease connectivity using a normative database. For example, BrainMaster's BrainAvatar software allows clinicians to train specific networks, like the Default Mode Network (DMN).
See the Assumptions unit for an in-depth discussion of the Affective, Default Mode, Executive, Motor, Network, Oculomotor, Salience, and Social Networks.
Phase Reset Coordination of Neural Networks
Phase refers to the degree to which the peaks and valleys of EEG waveforms coincide. Phase measures the time shift between EEG activity in two brain regions. Phase represents the number of elements in a network times the delay in that network. The graphic below shows in-phase and out-of-phase waveforms.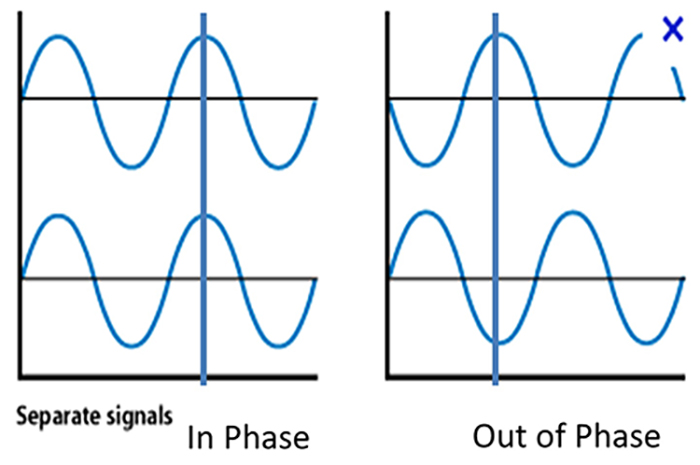
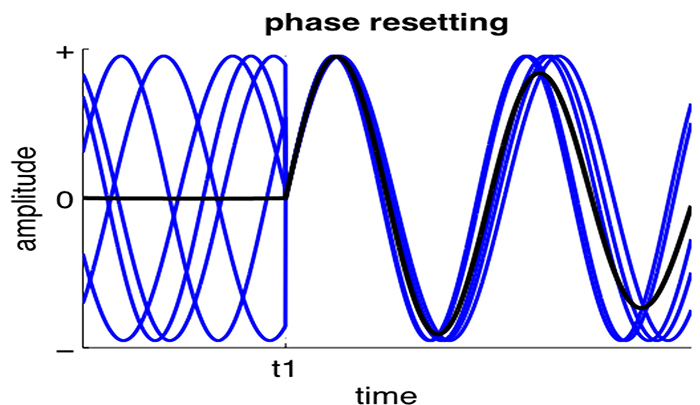
Phase reset (PR) is defined by a sudden change in phase difference (phase shift duration or SD) followed by a period of phase locking (lock duration or LD). PR = SD + LD (Thatcher et al., 2009). The graphic below shows multiple signals phase locking.
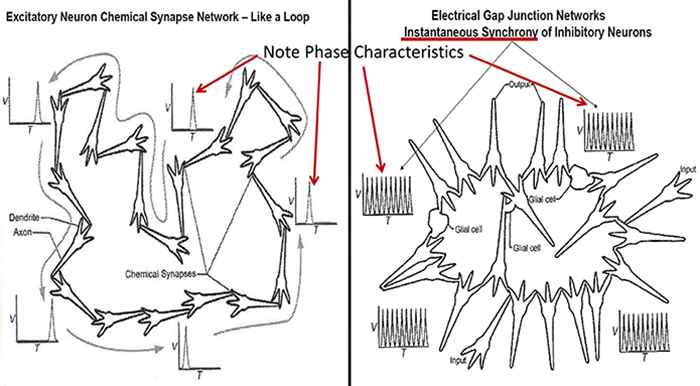
Gap junction coupling explains phase locking and phase shifting (Hughes & Crunelli, 2007).
Resetting the phase of ongoing oscillatory activity to endogenous (internal) or exogenous (environmental) cues facilitates coordinated information transfer within circuits and between distributed brain areas. Phase resetting is a critical marker of dynamic state changes within functional networks (Voloh & Womelsdorf, 2016).
Phase rests create a neural context, a narrow band of frequencies that uniquely characterize the activated circuits. They impose coherent low-frequency phases to which high-frequency activations can synchronize. These are identifiable as cross-frequency correlations that span large distances. Phase rests are critical for neural coding models that depend on phase, increasing the informational content of neural representations. They likely originate from the dynamics of canonical E-I circuits that are anatomically ubiquitous.
Phase resets reorganize oscillations in diverse task contexts: attentional stimulus selection, classical conditioning, cross-modal integration, sensory perception, and spatial navigation. Phase resets can drive changes in ensemble organization, functional networks, neural excitability, and overt behavior.
The graphic below shows low- and high-frequency oscillations resulting from phase reset decreases and increases in neural activity, respectively.
EEG Synchrony and Phase
Networks of neurons generating the EEG activity at different sites can produce signals that are identical in amplitude, frequency, and phase or that are entirely unrelated.Synchrony means that the firing of pools of neurons is coordinated. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
Local synchrony occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals. For example, an alpha amplitude of 20-60 μV detected at O1-A1 is produced by the synchronous firing of pools of neurons. This site's beta activity amplitude is lower due to desynchronized firing. This is analogous to the volume generated by a choir. When performers sing in unison, they produce a louder sound than singing separately.
Frequency synchrony occurs when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
Phase synchrony occurs when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
Coherence
Coherence represents the degree of coupling between separate cortical
regions and reflects neural network connectivity and dynamics
(Bullmore & Sporns, 2009). Coherence evaluates the linear association or correlation between the EEG waveforms recording from two different scalp locations (two referential montages). A two-channel referential montage is shown below.
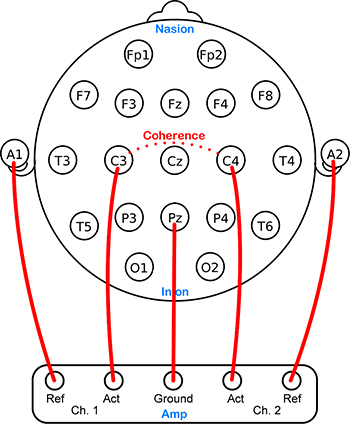
Coherence measures the degree to which two areas have consistent phase relationships at a designated frequency. When the phase difference between two signals is constant, coherence = 1. When the phase difference is random, coherence = 0.
Hyper-coherence means too much coupling and involves a failure to activate cortical regions selectively and may interfere with multitasking and rapid decision-making. Hypo-coherence, which often results from traumatic brain injuries, means too little coupling and involves a breakdown in
communication between regions that should normally communicate with each other (Wilson et al., 2011).
Coherence is a correlation coefficient (squared) that estimates relative amplitude and phase consistency between any pair of signals in each frequency band (Bendat & Piersol, 2000).
The key component of that explanation is the word consistency. Coherence is based on phase, and phase measures the relationship between two signals (two separate electrode locations), generally relating to a specific EEG frequency band when discussing EEG phase and coherence. Phase measures the timing relationship of the two waveforms.
The images below show five identical wave patterns that are shifted in time compared to each other. If we start with the first waveform at the bottom of the image, the subsequent waves are shifted in time. This is known as phase shift or phase angle. The angle is represented as degrees, so a wave that begins one-fourth of the way through the original wave’s cycle is said to have a 90-degree phase angle (example B). The same waveform beginning at the halfway point has a phase angle of 180 degrees, which means the waves are exactly opposite (example C). When the original wave is in the upward part of its cycle, the 180 degrees out of phase wave is in the downward part of its cycle.
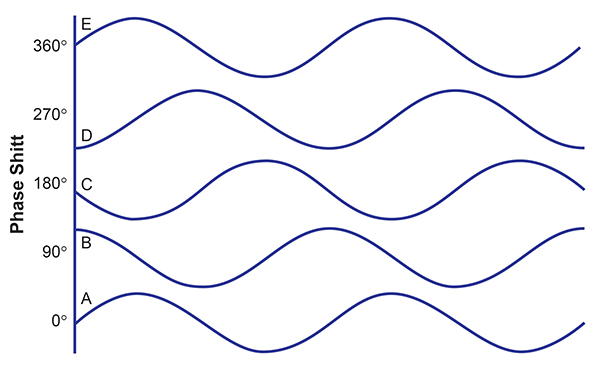
As previously noted, coherence is a measure of the consistency of these phase relationships. Wave patterns do not have to be in phase (zero phase angle – A compared to E above to be coherent. They only need to be in the same phase relationship consistently to be highly coherent.
Coherence values range from 0-1, with zero representing a random relationship between the two signals and 1 representing the signals remaining in the same phase relationship for the entire period being measured (usually a minimum of 2 seconds).
Because signals can be out of phase and remain coherent, the term coherence should not be confused with phase synchrony, which describes two waves oscillating with the same time value (zero phase angle). For example, coherence is often incorrectly applied to heart rate and respiration. Generally, one of the goals for heart rate variability training (HRV) is to synchronize the respiration and heart rate waveforms. This is an example of phase synchrony.
When this is consistently the case, the two waves are highly coherent. However, suppose a true coherence calculation is used. In that case, the two waveforms could be significantly offset from one another and still show a high degree of coherence, as long as they remained consistently in the same phase relationship. This may confuse the outcome measures.
In truth, all HRV measures use a calculation of phase angle, the Pearson’s correlation coefficient, to determine their relative phase synchrony rather than the more complex coherence calculation, which is a combination of the cross-spectral density and the auto-spectral density between the two signals.
EEG coherence estimates interaction between neural systems, measured by amplitude and phase consistency in each EEG frequency band between two distinct scalp sensor locations. This is not the same as a synchrony measure between locations. However, as noted above, high coherence can correspond to increased phase synchrony between two locations at a given EEG frequency. The key factor in the coherence measure is consistency.
Two signals can be asynchronous but can have high coherence if consistently in the same asynchronous relationship. This consistency implies that there is some coordinating mechanism between the two locations that is related to communication and/or the interaction between the two locations (Nunez, 2006).
The history of coherence measures being applied to the EEG began with Donald Walter (Walter, 1963), and since that time, there have been many studies published regarding the use of EEG coherence to identify factors in cognition, brain maturation, heritability, gender differences, and a variety of clinical disorders (Thatcher, 2012).
There are a variety of methods for estimating coherence. Typically coherence was measured using the linked ears montage or a common reference.
Thatcher (2012) states that this approach is necessary to correctly calculate phase differences because other montage arraignments distort the original time series and thus the relationships between individual sensor locations. Thatcher (2004) claims that the reliability of coherence measures using common average reference or Laplacian montages, though generally quite high, is irrelevant because these montaging methods render the resulting calculations uninterpretable.
He argues that these montages involve the “mixing” of signals from multiple electrodes (all 19 channels in the case of common average and the electrodes immediately surrounding the electrode of interest in Laplacian). He provides examples using artificially-generated signals and compares the accuracy and sensitivity of response of the various montages, and determines that only the linked-ear or common reference (such as Cz or Fcz) correctly represents the changes occurring in the source signals as greater amounts of noise are added.
Unfortunately, the linked ear and common references in this study were necessarily neutral, without signal content, and therefore do not reflect real-world examples.
On the other hand, Nunez and Srinivasan (2006) demonstrate that coherence using a common reference (such as a linked-ear or linked-mastoid sensors) elevates all coherence measures for all frequencies and electrode combinations because this method adds a common signal to each channel.
The idea of a “neutral” reference without any EEG or other electrical activity is not valid in real-world examples. Ear and mastoid reference channels frequently show rhythmic alpha activity, particularly in the eyes-closed condition, and are also often contaminated by EMG artifacts from masseter muscle contractions.
Similarly, a common reference such as Cz or Fcz would add real EEG signal characteristics to the resulting calculation. These factors influence the phase and coherence calculations, particularly for frontal electrode locations when using the linked ear or linked mastoid references, because of the function of the differential amplifier.
For a complete discussion of differential amplifier functions, please see the Instrumentation and Electronics section. For our purposes in this discussion, it is sufficient to understand the concept of common-mode rejection.
Common-mode rejection eliminates those common or similar signals between two inputs, often called the active and reference but more correctly known as positive and negative. The initial purpose of this method was to eliminate “mains” artifact, commonly known as 60 Hz artifact in North America, and 50 Hz artifacts in most of the rest of the world, due to the standard oscillating frequency of the alternating current in electrical wiring.
Common mode rejection also rejects any other electrical signal, including EEG, from active/reference pairs when the signals are the same in frequency and, to a lesser extent, amplitude. However, if the active and the reference inputs contain signals that are different, then those different signals are retained in the resulting signal.
When the “reference” channel derived from the ear or mastoid references that contain substantial EEG activity, such as alpha, is compared to the frontal “active” channels that generally do not contain substantial alpha activity, even in the eyes closed condition, the resulting signal will retain the alpha activity from the reference channel. This creates the appearance of rhythmic, highly synchronous alpha activity in frontal sensors and therefore results in high alpha coherence values in comparisons of those sensor pairs.
Compared to a normative database, these high coherence values will generate falsely elevated z-score values compared to the normative population, and neurofeedback training may be implemented to “correct” this high coherence.
Nunez and Srinivasan (2006) suggest that the common average reference method shows coherence results more similar to experimental, reference-independent coherence calculations. However, they note that many sensors are needed to generate an effective average reference value. Their example used 111 scalp electrode locations rather than the typical 19 electrode locations used in most normative databases.
Another method for calculating coherence is the use of the surface Laplacian method. This method removes some of the issues associated with reference contamination though it does tend to increase noise at each electrode location (Nunez & Srinivasan, 2006). It also removes the problem of volume conduction.
Volume conduction represents the conduction of bioelectric signals (brainwaves) through neural tissues. They show up in adjacent sensor locations that are not directly above the signal generator in the cortex. Volume conduction decreases with distance, so closely spaced electrode pairs can be expected to be more coherent, and the greater coherence will be disproportionally affected by common sources and volume conduction.
In contrast, widely spaced electrode pairs can be expected to reflect actual communication variables between the two areas more accurately. Because the Laplacian operator utilizes the average of the immediately surrounding electrodes as the reference, the common signals are minimized, and the coherence may provide a more accurate reflection of coherence in closely spaced electrode pairs.
The eventual answer to which coherence measure is better must await further studies, ideally using real-world models. Current practice utilizing either method will produce results that require an understanding of the benefits and limitations of each approach.
Excess frontal alpha coherence derived from linked ear reference data should be reviewed cautiously. The raw EEG signal should always be viewed using multiple montages to determine the accuracy of the frontal alpha activity displays.
Two examples of the same recording are shown below. The top example shows the linked ears montage with clear frontal alpha activity. Note the alpha activity present in the A1 and A2 electrode tracings at the bottom of the image and also note the alpha activity at T5 and T6, which are adjacent to the ears. The second image shows the longitudinal bipolar montage with a more typical posterior distribution of alpha activity and very little frontal alpha. The electrode locations and/or derivations (comparisons) are shown on the left side of each image. Note also the elevated global alpha coherence derived from the linked ears montage. No coherence calculation is possible for the longitudinal bipolar montage.
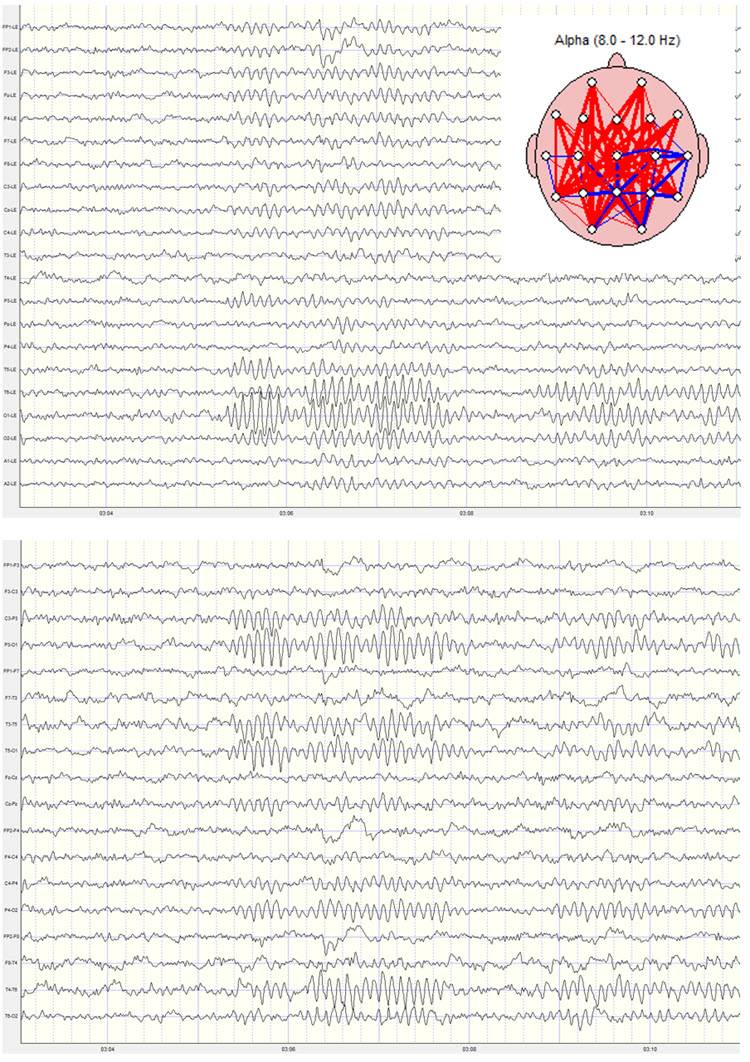
Other factors that can influence coherence measures include pervasive EMG artifacts that could not be removed with typical artifact removal methods. Sometimes this type of EMG artifact is not evident, particularly to beginning practitioners, and may be allowed to contaminate the record. When this happens, excessive hypercoherence in the beta and fast beta frequencies is sometimes seen in the example below. Note the excess beta and fast beta in the absolute power and relative power topographic maps and the global hypercoherence in the same frequency bands. This example also shows frontal delta and theta activity due to lateral eye movement artifact and the resulting hypercoherence in those frequencies in frontal comparisons.
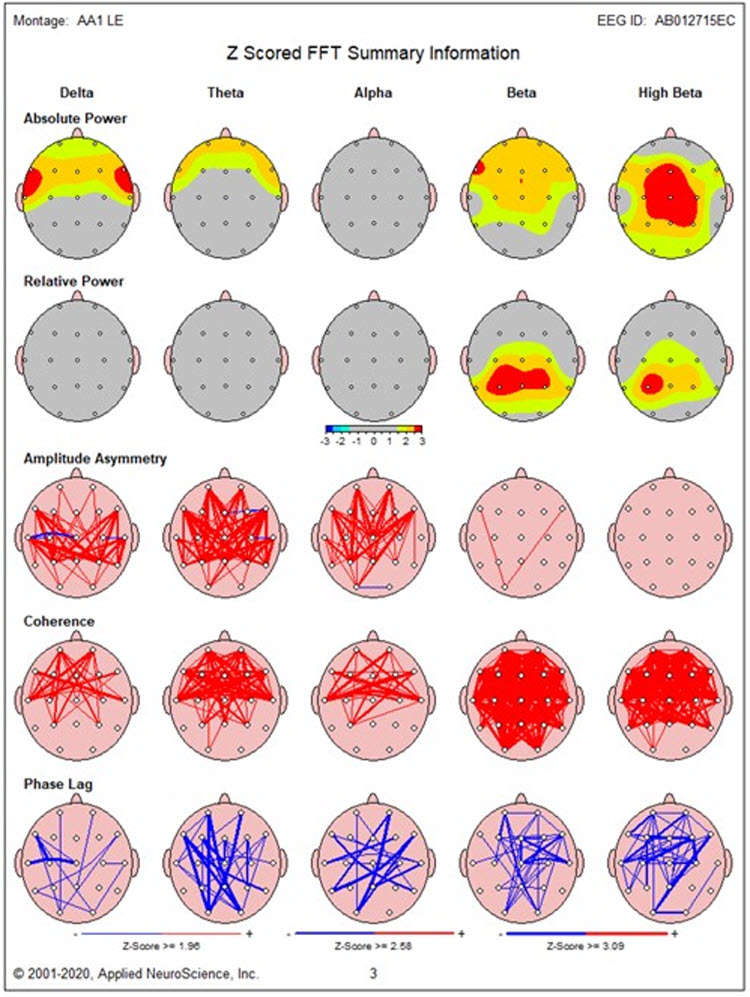
With these cautions in mind, coherence remains a useful tool, as it can be quite revealing, particularly in cases of traumatic brain injury, stroke, lesion, and other evaluations. With experience, it is usually possible to identify which coherence values represent actual findings and, more importantly, to correlate those findings with client history and presenting symptoms.
On the other hand, phase measurements alone are not particularly useful. For one thing, they are not directional. They simply show the timing relationship between two sensor locations. Even with statistical analysis, this does not reveal which location may be dysfunctional or what the causal factors may be (Nunez, 2006).
Additionally, a common reference distorts the phase in the same way mentioned in the coherence discussion because the phase is the source of the coherence calculation. Nunez (1974) shows that using a bipolar montage for phase calculation eliminates the problem of closely spaced electrodes (volume conduction) and the addition of the common signal (reference contamination). However, this isn’t suggested as a viable montage for the subsequent coherence calculation.
Values represent the measure of phase from -1 to +1, and statistical calculations can be made based on normative samples. However, experimental data (Nunez, 1995; Silberstein et al., 1993) show that only about 8% of the phase data collected could be identified as associated with traveling waves. The rest was too complex to identify source relationships. The data may represent valuable information, but interpretation using simple phase measures is not possible due to the complex nature of cortical communication systems.
Co-Modulation
Co-modulation is the degree of association in the magnitude of signals detected from two sources (sites). Co-modulation, which can be measured using the Pearson Product-Moment Correlation Coefficient, shows the degree to which signals strengthen and weaken in a correlated manner (Collura, 2009). Co-modulation does not measure phase or coherence, although it may reflect phase effects (Sterman & Kaiser, 2001).Glossary
50/60 Hz: external artifacts transmitted by nearby electrical sources.
acetylcholine: an amine neurotransmitter that binds to nicotinic and muscarinic ACh receptors.
acetylcholine esterase (AChE): the enzyme that deactivates ACh.
AChE-R: an abnormal form of acetylcholine esterase (AChE), which may render dendrites with acetylcholine receptors more excitable when stressed.
action potential: a propagated electrical signal that usually starts at a neuron’s axon hillock and travels to presynaptic axon terminals.
active electrode: an electrode placed over a site that is a known EEG generator like Cz.
adenylate cyclase: at a metabotropic receptor, an enzyme that transforms ATP into the second messenger cyclic AMP.
afferent: a neuron that transmits sensory information towards the central nervous system, or from one region to another.
all-or-none law: once an action potential is triggered in an axon, it is propagated, without decrement, to the end of the axon. The amplitude of the action potential is unrelated to the intensity of the stimulus that triggers it.
alpha-blocking: the replacement of the alpha rhythm by low-amplitude desynchronized beta activity during movement, attention, mental effort like complex problem-solving, and visual processing.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: regular bursts of alpha activity.
amplitude: the strength of the EEG signal measured in microvolt or picowatts.
amygdala: a limbic system structure plays a crucial role in learning about the consequences of our actions and creating declarative memories for events with emotional significance.
angular gyrus: the region located near the superior temporal lobe (BA 39) and involved in reading, math, and copying writing.
anterior: near or toward the front of the head, for example, the anterior cingulate.
anterior cingulate cortex (ACC): a division of the prefrontal cortex (Fpz, Fz, Cz, Pz) that plays a vital role in attention and is activated during working memory. The ACC mediates emotional and physical pain and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components.
anterior commissure: a bundle of nerve fibers that crosses the midline and connects the left and right temporal lobes, hippocampus, and amygdala.
apical dendrite: a dendrite that arises from the top of the pyramid and extends vertically to layer 1 of the neocortex.
arousal: a process that combines alertness and wakefulness, produced by at least five neurotransmitters, including acetylcholine, histamine, hypocretin, norepinephrine, and serotonin.
artifact: false signals like 50/60Hz noise produced by line current.
asynchronous waves: neurons depolarize and hyperpolarize independently.
basal forebrain: a cholinergic network located in the ventral frontal lobe and anterior hypothalamus that influences cerebral blood flow and cognitive activity.
basal ganglia: these forebrain structures consist of an egg-shaped nucleus that contains the putamen and globus pallidus and a tail-shaped structure called the caudate, which together are responsible for the production of movement. The basal ganglia have also been implicated in obsessive-compulsive disorder, Parkinson’s disease, and Huntington’s chorea.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
channel: an EEG amplifier output that is the result of scalp electrical activity from three electrode/sensor connections to the scalp.
cell body or soma: contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
central nervous system (CNS): the division of the nervous system that includes the brain, spinal cord, and retina.
classical routes for EEG activation: specific sensory pathways like the visual (retina to the visual cortex), auditory (cochlea to the auditory cortex), and somatosensory (chemoreceptors and mechanoreceptors to the somatosensory cortex) systems. Increased transmission of information through these pathways desynchronizes EEG activity in the cortical regions to which these afferent neurons project, as specialized circuits of neurons independently process this information.
coherence: the degree of coupling between separate cortical regions and reflects neural network connectivity and dynamics. Coherence evaluates the linear association or correlation between the EEG waveforms recording from two different scalp locations (two referential montages).
co-modulation: the degree of association in the magnitude of signals detected from two different sources (sites). Co-modulation, which can be measured using the Pearson Product-Moment Correlation Coefficient, shows the degree to which signals strengthen and weak in a correlated manner.
connectivity: the degree of synchrony between the oscillations of specialized brain regions (nodes) within a network.
contingent negative variation (CNV): a steady, negative shift in potential (15 μV in young adults) detected at the vertex. This slow cortical potential may reflect expectancy, motivation, intention to act, or attention. The CNV appears 200-400 ms after a warning signal (S1), peaks within 400-900 ms, and sharply declines after a second stimulus that requires the performance of a response (S2).
corticothalamic network: a unified network that generates diverse types of brain rhythms grouped by slow cortical oscillations.
delta rhythm: 0.05-3-Hz oscillations generated by thalamocortical neurons during stage-3 sleep.
depolarize: to make the membrane potential more positive by making the inside of the neuron more positive with respect to its outside.
derivation: the assignment of two electrodes to an amplifier's inputs 1 and 2.
desynchronization: the absence or loss of coordinated neuronal firing and synchronization of brain waves.
desynchrony: pools of neurons fire independently due to stimulation of specific sensory pathways up to the midbrain and high-frequency stimulation of the reticular formation and nonspecific thalamic projection nuclei.
differential amplifier (balanced amplifier): a device that boosts the difference between two inputs: the active (input 1) and reference (input 2).
dipole: the electrical field generated between the sink (where current enters the neuron) and the source (place at the other end of the neuron where current leaves) may be located anywhere along the dendrite.
EEG activity: a single wave or successive waves.
EEG artifacts: noncerebral electrical activity in an EEG recording can be divided into physiological and exogenous artifacts.
EEG power: the signal energy in the EEG spectrum. Most EEG power falls within the 0-20 Hz frequency range. EEG power is measured in microvolts or picowatts.
electrode: a specialized conductor that converts biological signals like the EEG into currents of electrons.
electroencephalogram (EEG): the voltage difference between at least two electrodes, where at least one electrode is located on the scalp or inside the brain. The EEG is a recording of both EPSPs and IPSPs that occur primarily in dendrites in pyramidal cells located in macrocolumns, several mm in diameter, in the upper cortical layers.
excitatory postsynaptic potential (EPSP): a brief positive shift in a postsynaptic neuron's potential produced when neurotransmitters bind to receptors and cause positive sodium ions to enter the cell. An EPSP pushes the neuron toward the excitation threshold when it can initiate an action potential.
extracellular dipole layers: macrocolumns of pyramidal cells, which lie parallel to the surface of the cortex, send opposite charges toward the surface and the deepest of the 5-7 layers of cortical neurons.
facultative pacemaker theory: Anderson and Anderson's (1968) theory that thalamic neurons activate cortical neurons and thalamic inhibitory interneurons via recurrent collaterals.
fast cortical potentials: EEG rhythms that range from 0.5 Hz-100 Hz. The main frequency ranges include delta, theta, alpha, sensorimotor rhythm, and beta.
frequency (Hz): the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
frequency synchrony: when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
gamma rhythms: a 28-80 Hz rhythm that includes the 38-42 Hz Sheer rhythm and is associated with learning and problem-solving, meditation, mental acuity, and peak brain function in children and adults.
global loops: cortical macrocolumns separated by as much as 7 cm and receive shared input fire synchronously to generate delta and theta rhythms.
gray matter: brain tissue that looks grayish brown and comprises cell bodies, dendrites, unmyelinated axons, glial cells, and capillaries.
ground electrode: a sensor placed on an earlobe, mastoid bone, or the scalp that is grounded to the amplifier.
hertz (Hz): unit of frequency measured in cycles per second.
high alpha (alpha 2): 10-12-Hz alpha associated with open awareness.
high beta (beta 4): 25-38-Hz activity mostly seen in the frontal lobes and is associated with hyper-perfusion and increased glucose metabolism. High or fast beta activity may be related to peak performance and cognitive processing and related to specificity and precision in information processing. Excessive high beta is associated with alcoholism, anxiety, OCD, rumination, and worry.
high-frequency filter (HFF): a filter that attenuates frequencies above a cutoff frequency.
hubs: highly centralized nodes through which other node pairs communicate; hubs allow efficient communication.
hyper-coherence: excessive coupling due to a failure to selectively activate cortical regions. Hyper-coherence may interfere with multitasking and rapid decision-making.
hyperpolarize: a negative shift in membrane potential (the inside becomes more negative with respect to the outside) due to the loss of positive ions or gain of negative ions.
hypnogogic hypersynchrony: the abrupt appearance of low-amplitude spikes that resembles epileptiform activity in an otherwise normal record.
hypo-coherence: deficient coupling due to a breakdown in communication between regions that should generally communicate with each other. Hypo-coherence often results from traumatic brain injuries.
impedance (Z): the complex opposition to an AC signal measured in Kohms.
impedance meter: device that uses an AC signal to measure impedance in an electric circuit, such as between active and reference electrodes.
impedance test: automated or manual measurement of skin-electrode impedance.
inion: the bony prominence on the back of the skull.
International 10-10 system: a modified combinatorial system for electrode placement that expands the 10-20 system to 75 electrode sites to increase EEG spatial resolution and improve detection of localized evoked potentials.
International 10-20 system: a standardized procedure for placing 21 recording and one ground electrode on adults.
kappa rhythm: bursts of alpha or theta and is detected over the temporal lobes of subjects during cognitive activity.
lambda waves: saw-toothed transient waves from 20-50 mV in amplitude and 100-250 ms in duration detected over the occipital cortex during wakefulness. These positive deflections are time-locked to saccadic movements and observed during visual scanning, as during reading.
Layers I-III: cortical layers that receive corticocortical afferent fibers that connect the left and right hemispheres.
Layer III: the cortical layer that is the primary source of efferent corticocortical fibers.
Layer IV: the cortical layer that is the primary destination of thalamocortical afferents and intra-hemispheric corticocortical afferents.
Layer V: the cortical layer that is the primary origin of efferent fibers that target subcortical structures that have motor functions.
Layer VI: the cortical layer that projects cortico-thalamic efferent fibers to the thalamus, which, together with the thalamocortical afferents, creates a dynamic and reciprocal relationship between these two structures.
local loops: neighboring cortical macrocolumns that share input generate frequencies above 30 Hz in the high-beta and gamma ranges.
local synchrony: synchrony that occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals.
locus coeruleus system: the noradrenergic branch of the ascending reticular activating system that projects to the thalamus, limbic system, and cerebral cortex, and contributes to wakefulness and vigilance for salient stimuli. Subnormal norepinephrine transmission may contribute to ADHD.
low alpha (alpha 1): 8-10-Hz alpha below a client's peak alpha frequency when eyes are closed.
macrocolumns: circuits of cortical pyramidal neurons several millimeters in diameter that create extracellular dipole layers parallel to the surface of the cortex, that send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons. Since the pyramidal neurons are all aligned with the cortical surface, the postsynaptic potentials at cells within the same macrocolumn add together because they have the same positive or negative charge. The macrocolumns fire synchronously.
mastoid bone: the bony prominence behind the ear.
microvolt (μV): the unit of amplitude (signal strength) that is one-millionth of a volt.
module: a set of interconnected nodes in a neural network.
monopolar recording: a recording method that uses one active and one reference electrode.
montage: a grouping of electrodes (combining derivations) to record EEG activity.
motor unit: an alpha motor neuron and the skeletal muscle fibers it innervates.
mu rhythm: 7-11-Hz waves resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
nasion: the depression at the bridge of the nose.
neural network: a system of interconnected ensembles of neurons that collaborate to achieve a goal. These networks communicate and perform functions via hub- or node-based communication systems.
node: a vertex within a neural network.
notch filter: a filter that suppresses a narrow band of frequencies, such as those produce by line current at 50/60Hz.
ohm (Ω): the unit of impedance or resistance.
phase: the degree to which the peaks and valleys of EEG waveforms coincide. Phase measures the time shift between EEG activity in two brain regions.
phase reset: a sudden change in phase difference (phase shift duration or SD) followed by a period of phase locking (lock duration or LD). PR = SD + LDs.
phase synchrony: synchrony when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
preauricular point: the slight depression located in front of the ear and above the earlobe.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
raphe system: the midline network of cell bodies within the brainstem and midbrain that may influence alertness and vigilance through reciprocal connections with the suprachiasmatic nucleus of the hypothalamus.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
referential (monopolar) montage: the placement of one active electrode (A) on the scalp and a neutral reference (R) and ground (G) on the ear or mastoid.
regional loops: cortical macrocolumns that share input and are separated by several centimeters generate alpha and beta rhythms.
resonant loops: the synchronous firing by macrocolumns that share afferent input to generate an electrical potential.
sequential (bipolar) montage: placement of active (A) and reference (R) sensors on active scalp sites and the ground (G) to an earlobe or mastoid.
slow cortical potentials (SCPs): gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials. SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms.
synchronous: adverb meaning that groups of neurons depolarize and hyperpolarize simultaneously.
synchronous "alpha": network-wide "alpha" that integrates perception and facilitates action. This distributed activity appears to block localized alpha-like patterns such as mu and the posterior rhythm in favor of more broadly distributed network integration.
synchrony: the coordinated firing of pools of neurons. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
thalamus: the forebrain structure above the hypothalamus that consists of specialized nuclei that process and relay data to and from the telencephalon (cerebral cortex, basal ganglia, and limbic system). The thalamus analyzes all sensory data except olfaction before distributing this information to the cortex via thalamocortical afferent fibers. The thalamus contributes to SCPs, delta, theta, alpha, SMR activity, and beta-gamma activity.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
tragus: the flap at the opening of the ear.
+ve: the source is the place at the other end of the neuron where the current leaves. The source is symbolized by +ve.
-ve: a sink is where the current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, symbolized by -ve.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
vigilance system: in Sterman’s model, a system that consists of both specific brainstem nuclei (e.g., locus coeruleus and raphe nuclei) and their diffuse connections with the thalamus and other subcortical structures, and the cortex. Several neurotransmitter systems mediate vigilance, including cholinergic/glutamatergic (reticular formation), noradrenergic (locus coeruleus), and serotonergic (raphe) neurons.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET PLUS
Click on the Quizlet Plus logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Functional Neuroanatomy, which reviews critical behavioral networks, and qEEG100, which provides extensive multiple-choice testing over the IQCB Blueprint.


Assignment
Now that you have completed this module, explain why low-and-balanced skin-electrode impedances are important in neurofeedback training. Describe the precautions you take to achieve acceptable impedance values. How do you measure impedance with your neurofeedback system?
References
Aggleton, J. P., O'Mara, S. M., Vann, S. D., Wright, N. F., Tsanov, M., & Erichsen, J. T. (2010). Hippocampal-anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. Eur J Neurosci, 31(12), 2292-22307. https://doi.org/10.1111/j.1460-9568.2010.07251.x
Andreassi, J. L. (2000). Psychophysiology: Human behavior and physiological response. Lawrence Erlbaum and Associates, Inc.
Basmajian, J. V. (Ed.). (1989). Biofeedback: Principles and practice for clinicians. Williams & Wilkins.
Bastos, A. M., & Schoffelen, J-M. (2016). A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front Syst Neurosci. https://doi.org/10.3389/fnsys.2015.00175
Bendat, J. S., & Bendat, A. G. (2011). Random data: Analysis and measurement procedures (4th ed.). Wiley.
Breedlove, S. M., & Watson, N. V. (2020). Behavioral neuroscience (9th ed.). Sinauer Associates, Inc.
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature, 10, 186-198. https://doi.org/10.1038/nrn2575
Cacioppo, J. T., & Tassinary, L. G. (Eds.). (1990). Principles of psychophysiology. Cambridge University Press.
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Dahl, M. J., Mather, M., & Werkle-Bergner, M. (2022). Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends in Cognitive Sciences, 26(1), 38-52. https://doi.org/10.1016/j.tics.2021.10.009
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
Floyd, T. L. (1987). Electronics fundamentals: Circuits, devices, and applications. Columbus: Merrill Publishing Company.
Grant, A. (2015). Four elements earn permanent seats on the periodic table. Science News.
Halford, J. J., Sabau, D., Drislane, F. W., Tsuchida, T. N., & Sinha, S. R. (2016). American Clinical Society Guideline 4: Recording clinical EEG on digital media. Journal of Clinical Neurophysiology, 33(4), 317-319. https://doi.org/10.1080/21646821.2016.1245563
Hindriks, R., & van Putten, M. J. A. M. (2013). Thalamo-cortical mechanisms underlying changes in amplitude and frequency of human alpha oscillations. NeuroImage, 70, 150-163. https://doi.org/10.1016/j.neuroimage.2012.12.018
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Hughes, J. R. (1994). EEG in clinical practice (2nd ed.). Butterworth-Heinemann.
Hughes, S. W., & Crunelli, V. (2005). Thalamic mechanisms of EEG alpha rhythms and their pathological implications. The Neuroscientist, 11(4), 357-372. https://doi.org/10.1177/1073858405277450
Hughes S. W., & Crunelli, V. (2007). Just a phase they're going through: The complex interaction of intrinsic high-threshold bursting and gap junctions in the generation of thalamic alpha and theta rhythms. Int J Psychophysiol, 64(1), 3-17. https://doi.org/10.1016/j.ijpsycho.2006.08.004
Khazan, I. Z. (2019). Biofeedback and mindfulness in everyday life: Practical solutions for improving your health and performance. W. W. Norton & Company.
Izhikevich, E. M., & Edelman, G. M. (2008). Large-scale model of mammalian thalamocortical systems. Proceedings of the National Academy of Sciences of the United States of America, 105(9), 3593-3598. https://doi.org/10.1073/pnas.0712231105
Klass, D. W. (2008). The continuing challenge of artifacts in the EEG. EEG artifacts. American Society of Electroneurodiagnostic Technologists, Inc. https://doi.org/10.1080/00029238.1995.11080524
Kubala, T. (2009). Electricity 1: Devices, circuits, and materials (9th ed.). Cengage Learning.
Lau, T. M., Gwin, J. T., & Ferris, D. P. (2012). How many electrodes are really needed for EEG-based mobile brain imaging? Journal of Behavioral and Brain Science, 2(3), 387-393. https://doi.org/10.4236/jbbs.2012.23044
Lebby, P. C. (2013). Brain imaging: A guide for clinicians. Oxford University Press.
Libenson, M. H. (2010). Practical approach to electroencephalography. Saunders Elsevier.
Lin, F., Witzel, T., Hamalainen, M. S., Dale, A. M., Belliveau, J. W., & Stufflebeam, S. M. (2004). Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage, 2(3), 582-595. https://dx.doi.org/10.1016%2Fj.neuroimage.2004.04.027
Montgomery, D. (2004). Introduction to biofeedback. Module 3: Psychophysiological recording. Association for Applied Psychophysiology and Biofeedback.
Nilsson, J. W., & Riedel, S. A. (2008). Electric circuits (8th ed.). Pearson Prentice-Hall.
Nunez, P. (1995). Neocortical dynamics and human EEG rhythms. Oxford University Press.
Nunez, P. (2006). Electrical fields of the brain. Oxford University Press.
Ongür, D., & Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex, 10(3), 206-219. https://doi.org/10.1093/cercor/10.3.206
Ongür, D., & Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex, 10(3), 206-219. https://doi.org/10.1093/cercor/10.3.206
Peek, C. J. (2016). A primer of traditional biofeedback instrumentation. In M. S. Schwartz, & F. Andrasik (Eds.). (2016). Biofeedback: A practitioner's guide (4th ed.). The Guilford Press.
Picton, T. W., & Hillyard, S. A. (1972). Cephalic skin potentials in electroencephalography. Encephalogr Clin Neurophysiol, 33, 419-424. https://doi.org/10.1016/0013-4694(72)90122-8
Pfister, H., Kaynig, V., Botha, C. P., Bruckner, S., Dercksen, V., & Hege, H.-C. (2012). Visualization in connectomics. Mathematics and Visualization, 37. https://doi.org/10.1007/978-1-4471-6497-5_21
Silberstein, R. et al. (1993) unpublished data cited in Nunez, Neocortical dynamics and human EEG rhythms. Oxford University Press.
Steriade, M., & Llinás (1988). The functional states of the thalamus and the associated neuronal interplay. Physiol Rev, 68(3), 649-742. https://doi.org/10.1152/physrev.1988.68.3.649
Sterman, M. B., & Kaiser, D. A., (2001). Comodulation: A new QEEG analysis metric for assessment of structural and functional disorders of the CNS. Journal of Neurotherapy, 4(3), 73-83. https://doi.org/10.1300/J184v04n03_05
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Thatcher, R. W. (2012). Coherence, phase differences, phase shift, and phase lock in EEG/ERP analyses. Developmental Neuropsychology, 37(6), 476-496. https://doi.org/10.1080/87565641.2011.619241
Thatcher, R. W., Biver, C. J., & North, D. M. (2009). EEG and brain connectivity: A tutorial. Unpublished manuscript.
Thomas, C. (2007). What is a montage? EEG instrumentation. American Society of Electroneurodiagnostic Technologists, Inc.
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
van den Heuvel, M. P., & Sporns, O. (2011). Rich-club organization of the human connectome. J Neurosci, 31(44), 15775-15786. https://doi.org/10.1523/JNEUROSCI.3539-11.2011
Voloh, B., & Womelsdorf, T. (2016). A role of phase-resetting in coordinating large scale neural networks during attention and goal-directed behavior. Front Syst Neurosci, 10, doi:10.3389/fnsys.2016.00018
Zhang, H., Watrous, A., Patel, A., & Jacobs, J. (2018). Theta and alpha oscillations are traveling waves in the human neocortex. Neuron. https://doi.org/10.1016/j.neuron.2018.05.019
