Key Studies
Clinical and performance applications of neurofeedback are supported by a growing body of research that supports its efficacy. Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.) provides a detailed review of these studies. This unit summarizes representative randomized controlled trials (RCTs) and quasi-experimental studies, which provide the strongest evidence of efficacy. Graphic © Zayabich/Shutterstock.com.

This unit addresses IV. Research Evidence Base for Neurofeedback - B. Key Research Studies.

This unit covers Attention Deficit Hyperactivity Disorder, Mild Closed Head Injuries and Traumatic Brain Injury, Substance Use Disorder, Epilepsy, Anxiety and Anxiety Disorders, Post-Traumatic Stress Disorder, Depression, and Tinnitus.
Please click on the podcast icon below to hear a full-length lecture.

We have updated the efficacy ratings for clinical applications covered in AAPB's Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.).

DSM-5 revised the diagnostic criteria for attention deficit hyperactivity disorder (ADHD) by providing examples of symptom presentation, increasing the age range for symptom onset, and reducing the required number of symptoms for adolescents and adults (Brown, 2013). Graphic © Oksana Mizina/Shutterstock.com.

Boys are diagnosed with ADHD 4-5 times more often than girls (Costello et al., 2003). Their parents are more likely to pursue treatment due to their more severe symptoms or greater degree of impairment (Beidel et al., 2014). Girls are more likely to show inattention and are less likely to be diagnosed with a learning disability, comorbid depression, oppositional defiant disorder, or conduct disorder than their male classmates (Biederman et al., 2002; Spencer et al., 2007).
Almost half of the children diagnosed with ADHD exhibit difficulty learning, communicating, and interacting with their classmates. Roughly 80% misbehave, frequently very seriously (Goldstein, 2011; Mash & Wolfe, 2010).
Lubar, Swartwood, Swartwood, and O'Donnell (1995) reported that training to reduce slow EEG activity increased WISC-R and Test of Variables of Attention (TOVA) scores. Full-scale WISC-R scores increased about 12 points. The increase in TOVA scores correlated with decreased slow EEG activity.
Lubar (1995) followed 52 patients treated with NF for as long as 10 years. Their improvement on the Connors scale, used to measure attention, remained stable at follow-up.

Rossiter and La Vaque (1995) matched and randomly assigned 46 participants to either Ritalin or NF. Both groups improved on TOVA measures of inattention, impulsivity, information processing, and response variability.
Linden, Habib, and Radojevic's (1996) controlled study of 18 children demonstrated that NF to increase beta and suppress theta activity increased intelligence scores and reduced inattention rated by their parents compared to a wait-list control group.
Thompson and Thompson (1998) reported the successful treatment of 98 children and 13 adults over 40 50-minute sessions using Lubar's ADHD protocol. The percentage of children using Ritalin declined from 30% at the start of the study to 6% post-treatment. Theta/beta ratios significantly declined for children but not for adults. Study participants achieved impressive pre-treatment to post-treatment gains on intelligence, TOVA, and Wide Range Achievement Test scores. Lynda and Michael Thompson are pictured below.

Case studies by Ramos (1998) and Wadhwani, Radvanski, and Carmody (1998) support the efficacy of NF for ADD and ADHD.
Camp (1999) reported that theta suppression biofeedback training compared favorably with cognitive behavior modification, based on pre-treatment to post-treatment changes in 48 children on TOVA, parent and teacher ratings, and ADHD scales.
The Kaiser and Othmer (2000) multi-center study involved 1,089 patients aged 5 to 67 years. It demonstrated that SMR-beta NF training produced significant gains on TOVA measures of attentiveness, impulse control, and response variability.
Carmody and colleagues (2001) randomly assigned 16 children (ages 8-10) to either EEG biofeedback or a wait-list control condition. Eight of the 16 children were diagnosed with ADHD, and 8 had received no diagnosis of any disorder. In the EEG biofeedback condition, participants received 3-4 weekly sessions of EEG biofeedback (using a synthesis of Protocols 1 and 3) for 6 months and completed 36-48 sessions.
The children diagnosed with ADHD who received EEG biofeedback decreased impulsivity as measured by the TOVA, and their teachers' ratings of attentiveness on the School Version of the Attention Deficit Disorders Evaluation Scale (ADDES) improved. Selected qEEG measures did not consistently validate improvement by participants in the EEG biofeedback condition.
Monastra, Monastra, and George (2002) compared 49 children diagnosed with ADHD who participated in 1-year multimodal program (Ritalin, parent counseling, and academic consultation) with 51 children who participated in the multimodal program combined with NF (weekly 30 to 40-min sessions using the Lubar protocol with a cash reward for increased frontal cortical arousal).
Both groups significantly improved performance on TOVA and the Attention Deficit Disorders Evaluation Scale when medicated with Ritalin, but only the group that received NF maintained performance gains when unmedicated. A qEEG scan only showed reduced cortical slowing in children who received NF. Parenting style moderated behavioral symptoms at home but not in the classroom. Vincent Monastra is pictured below.

Fuchs, Birbaumer, Lutzenberger, Gruzelier, and Kaiser (2003) compared the efficacy of 3 months of sensorimotor rhythm (12-15 Hz) and beta1 (15-18 Hz) NF against methylphenidate in 46 ADHD children. The children were assigned to the NF (22) and medication (12) based on their parents' preference (the assignment was nonrandom). Both treatment groups improved on all TOVA subscales and speed and accuracy on the d2 Attention Endurance Test. Teacher and parent ratings of ADHD behaviors on the IOWA-Conners Behavior Rating Scale also improved for both groups.
Monastra and colleagues (2005) assigned a more conservative rating of probably efficacious for EEG biofeedback for ADHD in an AAPB White Paper. Despite significant improvement in about 75% of patients in the published studies they examined, the authors concluded that more randomized, controlled group studies that control for therapist and patient characteristics are needed to calculate the percentage of patients diagnosed with ADHD who will achieve these gains in typical clinical settings.
NF appears to be superior to no treatment and equivalent to stimulant medication. Patients require at least 20 sessions and as many as 50 sessions to produce clinical improvement.
Gevensleben and colleagues (2009) conducted a multisite randomized controlled study of 102 children diagnosed with ADHD using NF training that combined blocks of theta/beta and slow cortical potential NF and computer-based attention skills training control. The combined NF group was superior to the control group on parent and teacher ratings, and both NF protocols produced comparable changes. These gains were maintained at a 6-month follow-up (Gevensleben et al., 2010).
Sherlin, Arns, Lubar, and Sokhadze (2010) argued that NF for ADHD is safe, should be reclassified as level 5: efficacious and specific, produces long-term effects that last from 3-6 months, and may produce clinical results like stimulant medications. While NF effectively treats inattention and impulsivity, they suggested that medication may be more appropriate when the primary symptom is hyperactivity and that NF may be successfully combined with medication. Leslie Sherlin is pictured below.

Duric and colleagues (2012) conducted a randomized controlled trial of 91 children and adolescents diagnosed with ADHD assigned to 30 NF sessions, methylphenidate, or NF with methylphenidate. Parent ratings of core ADHD symptoms improved for all three groups, and there were no group differences. NF achieved equal efficacy to methylphenidate.
The American Academy of Pediatrics (2012) rated biofeedback for child and adolescent attention and hyperactivity behaviors Level 1- Best support.
Pigott, De Biase, Bodenhamer-Davis, and Davis (2013) provided a comprehensive review of NF efficacy for ADHD and persuasively argued that it should receive a level-5 classification. The authors emphasized that compared with stimulant medication, only NF has demonstrated effectiveness at 2-year follow-up.
Meisel and colleagues (2013) reported a randomized controlled trial of 23 children diagnosed with ADHD who either completed 40 theta/beta NF sessions or received methylphenidate. While both groups improved on parent and teacher ratings of functioning and core ADHD symptoms, only the NF group improved on academic performance at 6-month follow-up.
In their review of recent studies, Arns and Strehl (2013) concluded that in randomized controlled trials where theta/beta or slow cortical potential NF were active treatments and either cognitive training or EMG biofeedback were controls, NF produced significant improvements in teacher ratings.
Steiner and colleagues (2014) conducted a randomized controlled study of 104 children assigned to either NF, cognitive training (CT), or control conditions. At a 6-months follow-up, the NF group sustained greater gains on the Conners 3-P, Executive Functioning, Hyperactivity/Impulsivity, and Behavior Rating Inventory of Executive Function (BRIEF) subscales than the CT or control groups. Moreover, the NF group maintained their stimulant dosage while the CT and control groups increased their dosage.
Below is a NeXus-10 ® BioTrace+ caterpillar game. The three caterpillars represent the theta, SMR, and beta frequency bands.
NFB interventions included training to decrease the theta/beta ratio and increase sensorimotor rhythm (SMR) activity or slow cortical potential (SCP) activity.
NFB training resulted in post-treatment gains in ADHD symptoms, including the Attention Deficit Disorders Evaluation Scale (ADDES). Participants also improved on intelligence, TOVA measures of attentiveness, impulse control, and response variability, and Wide Range Achievement Test scores.
Traumatic brain injury (TBI) results when an external force produces intracranial injury through acceleration or direct impact. Mild TBI symptoms that NF may treat include deficits in memory, attention, and decision-making (National Institute of Neurological Disorders and Stroke, 2008). Check out Siddharthan Chandran's TED Talk Can the Damaged Brain Repair Itself?
Twenty-five of 32 patients emerged from their comas after 1-6 treatments. NF for coma involved inhibiting 4-7 Hz activity and reinforcing the replacement of 4-7 Hz with 15-18 Hz activity.
Ayers and colleagues started NF for open head trauma at the somatosensory cortex. They trained these patients to decrease 4-7 Hz activity and increase 15-18 Hz activity.
Thornton (2000) reported that NF improves the memory of TBI patients. Tinius and Tinius (2000) found that it improves attention, problem-solving, and task performance. Keller's (2001) controlled study showed that beta training significantly improved attention compared to matched controls. Another controlled study by Schoenberger, Shif, Esty, Ochs, and Matheis (2001) showed that NF enhanced cognitive function and self-reported depression and fatigue.
Walker, Norman, and Weber (2002) reported that 88% of mild TBI patients achieved over 50% improvement in EEG coherence. All patients who had been previously employed resumed work after completing their training.
Thornton and Carmody (2013) reported auditory and visual memory gains in 15 TBI patients who received NF training for qEEG power and connectivity in an uncontrolled pre-test/post-test study.
HRV was the only BFB modality. NFB training involved operant conditioning utilizing real-time normative database comparisons (z-score training) and training several measures concurrently (e.g., inhibiting 4-7 Hz and rewarding 15-18 Hz activity). EEG tomography through Low-Resolution Electromagnetic Tomography Analysis (LORETA) offered greater training specificity and TBI treatment customization.
Participants reduced medication, the number of symptoms, symptom severity, and anxiety. They improved on attention, cognitive function, memory, and tandem gait time (walking in a straight line by placing one foot directly in front of the other, heel-to-toe).
Koob (2006) defined drug addiction as a: “Chronically relapsing disorder that is characterized by a compulsion to seek and take a drug, loss of control in limiting intake and the emergence of a negative emotional state (e.g., dysphoria, anxiety, irritability) when access to the drug is prevented (here, defined as the ‘dark side’ of addiction).”
In DSM-5, substance use disorders (SUDs) are seen as behavioral disorders consisting of three elements:
1. Loss of control (e.g., compulsive use)
2. Continued intake despite serious adverse consequences
3. Preoccupation with obtaining, using, and recovering from the substance (Julien et al., 2023)
Graphic © Jan H Anderson/Shutterstock.com.

Alcohol is another drug of abuse that, like opioids, can threaten health and safety. Graphic © medicalstocks/Shutterstock.com.
The Menninger ON-OFF-ON EEG protocol teaches a patient to increase the amplitude within a frequency band, reduce the amplitude, and then increase the amplitude again during 100- or 200- second segments. For example, a patient may increase 8-13 Hz alpha activity for 100 s, decrease it for 100 s, and then increase it for 100 seconds. This approach may produce superior control compared to procedures that only train alpha or theta increase (Norris, 1988).
The Menninger alpha-theta protocol places the active electrode 1 centimeter above and left of the inion (the bony prominence located on the back of the head) with a reference on the left earlobe. This protocol teaches EEG control using 100- or 200-seconds “ON-OFF-ON” exercises.
Temperature and frontal SEMG biofeedback precede alpha-theta training. Patients receive 3-4 weekly sessions of temperature biofeedback followed by 3-4 weekly sessions of frontal SEMG biofeedback. Judy Green has likened the temperature and SEMG biofeedback sessions to settling elementary school students in their seats so they can pay attention without distraction. These sessions also teach patients the strategy of passive volition (allowing), which is critical to alpha-theta training.
The patient then receives 10-12 bi-weekly sessions of alpha-theta biofeedback using ON-OFF-ON exercises. Training attempts to gradually slow the EEG until the patient can increase alpha and theta amplitude without falling asleep.
Egner, Strawson, and Gruzelier (2002) addressed whether the effects of alpha-theta NF depend on with-session EEG changes or are non-specific and shared with other relaxation procedures. They compared the effect of contingent and noncontingent alpha-theta NF on theta/alpha ratios within and across sessions.
The contingent group achieved increased within-session theta/alpha ratios, while the noncontingent group did not. The contingent group also achieved higher theta/alpha ratios than the noncontingent group during some training sessions. There were no group differences in subjective reports of activation since both groups reported significantly lower activation following training. Both contingent and noncontingent NF were relaxing. This study validated the assumption underlying alpha-theta NF that accurate feedback results in higher within-session theta/alpha ratios than does noncontingent NF.
The Peniston addiction protocol (1989) is a multimodal approach that incorporates biofeedback and non-biofeedback components. Patients start with visualization training, receive 6 temperature biofeedback sessions, learn rhythmic breathing techniques, participate in autogenic training exercises, learn to construct personalized imagery, and experience guided imagery (where the therapist directs the patient visualization). This training prepares patients for 30 alpha-theta sessions in which they learn to slow the EEG to increase alpha and theta amplitude using the Menninger alpha-theta training procedure. Following the alpha-theta biofeedback sessions, the supervising physician may need to adjust medication.
Experimental patients who received Peniston and Kulkosky’s alpha-theta protocol for alcoholism and control patients were assessed over a 24-month follow-up period. Across this period, 8 of the 10 experimental patients and none of the 10 controls maintained abstinence from alcohol.
Due to the Peniston-Kulkosky addiction protocol's multimodal nature, we cannot identify the components responsible for clinical improvement. Further clinical research that "dismantles" this protocol will be required to isolate the active treatment components.
Kaiser and Scott (Kaiser et al., 1999; Scott et al., 2002, 2005) modified the Peniston protocol to increase its success with patients dependent on cannabis and stimulants. The Kaiser-Scott protocol, which starts with NF ADHD training and then progresses to the Peniston protocol, has improved retention and abstinence in these hard-to-treat populations.
This video takes the viewer through an alpha-theta training demonstration using the Nexus/Biotrace system (Mind Media, Roermond, The Netherlands) using the training approach developed by John Anderson. The demonstration uses saved session data to illustrate the functions and discuss the threshold settings and training concepts.
Schneider et al. (1993) reported that 6 of 10 male alcoholics remained abstinent 4 months after completing slow cortical potential NF.
Taub and colleagues (1994) randomly assigned 118 chronic alcoholics to one of four treatments: Alcoholics Anonymous and counseling (RTT), RTT combined with Transcendental Meditation, RTT combined with SEMG biofeedback, or RTT plus sham neurotherapy. The sham NF condition involved "electrocranial stimulation" and not alpha-theta biofeedback. Rates of self-reported abstinence were 25%, 65%, 55%, and 28%, respectively. While the addition of Transcendental Meditation and EMG biofeedback seemed to increase abstinence, sham neurotherapy did not. The addition of Transcendental Meditation or EMG biofeedback to RTT produced abstinence rates comparable to those reported for the addition of alpha-theta biofeedback.
In a controlled study, Saxby and Peniston (1995) demonstrated that alpha-theta NF could reduce depression in alcoholics and increase the rate of abstinence assessed over a 21-month follow-up period.
Kelley’s (1997) 3-year follow-up study of 20 Native American alcoholic inpatients reported the following changes: increased EEG synchrony and alpha-theta amplitudes, extinction of drinking behavior, less personally damaging behavior (81%), and lower Beck Depression Inventory scores.
Burkett and colleagues (2003) conducted an open-label uncontrolled study of the Scott-Kaiser modification of the Peniston protocol with 270 male homeless patients addicted to crack cocaine who also received faith-based interventions within a residential setting. At 1-year follow-up, data from 94 patients who completed treatment showed abstinence from alcohol or other drugs in 53.2% and drug use only 1-3 times in 23.4%, corroborated by urinalysis. A follow-up report on 87 patients who completed treatment documented improvement on urinalysis, length of residential stay, and self-reported depression (Burkett et al., 2005).
Bodenhamer-Davis and Calloway (2004) conducted an uncontrolled trial of 16 outpatients diagnosed with chemical dependency in which they administered alpha-theta NF. At 74- to 98-month follow-up,
81.3% were abstinent, and rates for re-arrest and loss of probation were lower than for a comparison group.
Scott, Kaiser, Othmer, and Sideroff (2005) randomly assigned patients with mixed substance abuse to EEG biofeedback or a control group. The EEG biofeedback group stayed in treatment longer than the control group. Seventy-seven percent of patients who completed EEG training remained abstinent at 12 months compared with 44% of controls.
Sokhadze, Cannon, and Trudeau (2008) found that alpha-theta training for alcoholism and a combination of alpha-theta training, beta training for polydrug abuse (including stimulants), and residential treatment were probably effective.
The five other RCTs incorporated alpha and high beta regulation, alpha/theta with TEMP and guided imagery, SMR and beta upregulation with 1-13 Hz and high beta (18-22 Hz) suppression, SMR/theta followed by alpha-theta, and the Scott–Kaiser NFB protocol.
The Scott-Kaiser protocol, which starts with NF ADHD training and then progresses to the Peniston protocol, has improved retention and abstinence in these hard-to-treat populations. Participants increased abstinence, quality of life, self-efficacy, time in the program, and TOVA (continuous attention), and reduced addiction severity and craving.
The Scott-Kaiser modification of the Peniston Protocol can be classified as probably efficacious with residential or office-based rehabilitation and opioid replacement for alcohol, opioid, mixed-substance, and stimulant abusers.
Alpha-theta NF received a level-2 rating of possibly efficacious.
Epilepsy is a family of neurological disorders featuring short, periodic motor, sensory, or cognitive malfunction attacks. Epileptic seizures have been divided into (1) partial or focal seizures, (2) primary generalized seizures, (3) status epilepticus, and (4) recurrence patterns. Check out the Epilepsy Therapy Project video What is Epilepsy? -- Understanding Epilepsy animation. Graphic © Alila Medical Media/Shutterstock.com.
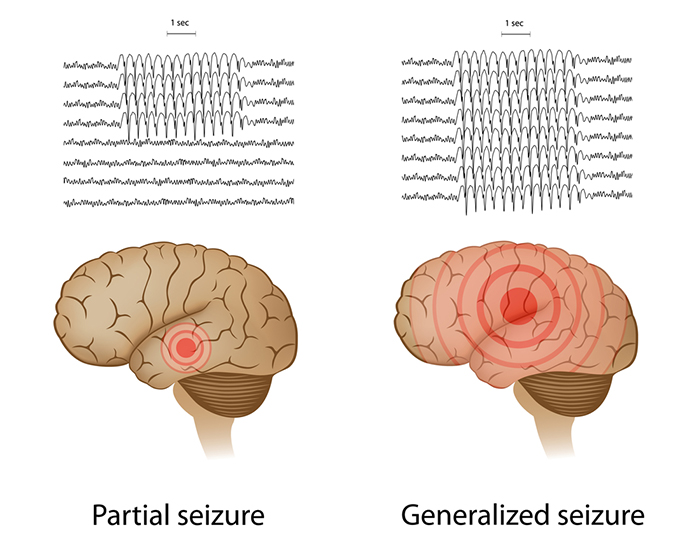
Petit mal seizures feature loss of consciousness without abnormal movement (patient appears to be daydreaming). The patient, typically a child, may suffer hundreds of these seizures daily for periods lasting up to 30 seconds. Check out the Epilepsy Therapy Project video Understanding Partial Seizures.
Tonic-clonic seizures (grand mal seizures) are primary generalized seizures featuring a peculiar cry, loss of consciousness, fall, tonic-clonic convulsions of all extremities, incontinence, and amnesia for the episode. These seizures are diagnosed in fewer than 20% of adult epileptics. Check out the Epilepsy Therapy Project video Understanding Generalized Seizures. Graphic © Natty_Blissful/Shutterstock.com.
 Graphic © Steve Buckley/Shutterstock.com shows a 2-year-old boy with 24-hour EEG electrodes to find the source of his seizures.
Graphic © Steve Buckley/Shutterstock.com shows a 2-year-old boy with 24-hour EEG electrodes to find the source of his seizures.

The sensorimotor cortex is a central cortical area defined by the central sulcus (fissure of Rolando) separating the frontal and parietal lobes. Sterman (1977) recorded the EEG over the left sensorimotor cortex from sites 10% and 30% lateral to the vertex (slightly medial to C3 and T3).
The sensorimotor rhythm (SMR) is an EEG rhythm from 12-14 Hz located over the sensorimotor cortex (central sulcus). This rhythm is associated with inhibition of movement and reduced muscle tone.
For example, Sterman's protocol trains an epileptic patient to increase SMR (12-14 Hz) amplitude and duration while theta (4-7 Hz), beta (20+ Hz), epileptiform spikes, and EMG artifact are suppressed during 36 sessions. The aim is to normalize the waking and sleep EEG with elevated SMR and suppressed theta and beta activity.

Rockstroh et al. (1993) reported a pre-test/post-test study of 28 1-hour sessions of bi-directional SCP training for 25 uncontrolled epilepsy patients. Follow-up data from 18 participants, who monitored their seizure frequency through training and 1-year follow-up, showed reduced seizure frequency.
Kotchoubey et al. (1996) reported that SCP NF decreased the baseline seizure frequency in drug-resistant epileptics and Kotchoubey et al. (1997) showed that this improvement was maintained 6 months post-treatment.
Kotchoubey, Busch, Strehl, and Birbaumer (1999) concluded that SMR and SCP protocols improve epilepsy control in about 66% of patients. While the mechanism underlying SCP training remains unclear, it may involve increased 6.0-7.9 Hz theta activity during training trials without feedback.
Joy Andrews et al. (2000) found that a NF protocol involving five consecutive days of training enabled 79% of patients to control their seizures.
Sterman (2000) summarized 18 peer-reviewed studies in which 174 patients were trained using his SMR protocol. The outcome data were impressive; 82% clinically improved, reducing seizures by more than 30%. The average seizure reduction exceeded 50%. Many studies found decreased seizure severity. Five percent of patients remained seizure-free for as long as one year. In those studies where researchers recorded pre-treatment and post-treatment EEG amplitudes, 66% normalized their EEG power spectra.
Kotchoubey et al. (2001) treated patients with refractory epilepsy with an anti-epileptic drug and psychosocial counseling, a breathing training control group, or SCP NF in a non-randomized clinical study in which participants selected their treatment. Only the drug and SCP groups significantly reduced seizure frequency.
La Vaque (2003) considers slow cortical potential (SCP) training to be highly effective in controlling "drug-resistant" epilepsy.
Marson and Ramaratnam (2003) reviewed randomized controlled trial studies and reported a significant reduction in median seizure activity in one study.
Sheth, Stafstrom, and Hsu (2005) examined 16 studies of biofeedback treatment of refractory epilepsy, including contingent negative variation (CNV), slow cortical potential (SCP), and galvanic skin response (GSR). While most studies involved 1-8 patients, one enrolled 83 patients. Epilepsy symptoms improved in 82% of patients who received biofeedback training. Both the EEG and GSR treatments produced significant gains.
Strehl and colleagues (2005) reported that 70% of SCP training treatment success with drug-resistant patients could be predicted by initial cortical excitability (negative SCP amplitude), location of epileptic foci (sites that trigger seizures), and personality. Successful patients did not exhibit large negative SCP amplitudes at the start of training, did not have left temporal foci, and reported lower life satisfaction and high reactivity to stressors.
Ramaratnam, Baker, and Goldstein (2005), in a Cochrane Database Systematic Review, challenged the efficacy of NF for epilepsy due to methodological flaws and a small sample size.
A meta-analysis by Tan and colleagues (2009) reviewed nine studies that used SMR and one that used SCP neurofeedback. They found consistent reductions in seizure frequency for drug-resistant patients and successful reduction in seizure frequency by 79% of patients who received SMR training.
In a meta-analysis, SMR- and SCP-based treatments were associated with fewer seizures and improved quality of life in a RCT.

Generalized anxiety disorder (GAD) is defined by excessive anxiety and worry most of the time for at least 6 months (Beidel, Bulik, & Stanley, 2014). Chronic overarousal results in fatigue and insomnia, worsened by changes in their circadian rhythm due to their job or travel (McGrady & Moss, 2013).

Patients perceive their worrying as outside their control. They present with physical (muscle tension) and cognitive symptoms (the belief that worrying can prevent an adverse event). They are usually diagnosed with a second disorder (Beidel, Bulik, & Stanley, 2014).
Specific phobia involves significant emotional distress, excessive anxiety, or fear about an object or situation that disrupts everyday performance. DSM-5 lists five specifiers: animal phobias, natural environment phobias, blood/injection/ injury phobias, situational phobias, and other phobias (Beidel, Bulik, & Stanley, 2014).

DSM-5 classifies Post-traumatic stress disorder (PTSD) as one of the Trauma and Stress-Related Disorders. PTSD is a response to a traumatic event like assault, military combat, or rape that may be experienced firsthand or observed (American Psychiatric Association, 2013).
Only the alpha-increase condition decreased heart rate reactivity to stressors. Subjects in the frontal EMG, alpha-increase, and alpha suppression conditions maintained improvement in STAI-Trait Anxiety and PSC scores at 6 weeks post-treatment. The alpha-increase and alpha-suppression groups showed further improvement in Psychosomatic Symptom Checklist scores at 6 weeks post-treatment.
Vanathy, Sharma, and Kumar (1998) randomly assigned participants who met the diagnostic criteria for Generalized Anxiety Disorder (GAD) to a wait-list control, alpha-increase biofeedback, or theta-increase biofeedback. EEG biofeedback consisted of 15 sessions to enhance alpha or theta and suppress beta.
Both the alpha-increase and theta-increase groups reduced self-reported (STAI-State Anxiety) and blind observer-rated anxiety (Hamilton Anxiety Rating Scale) in comparison to the control group. These results may have been due to Type 1 error caused by multiple t-tests without statistical correction. The authors' failure to observe changes in EEG power in the alpha or theta bands following EEG training suggests that non-specific treatment components, and not EEG training, may have produced clinical improvement, assuming that the findings of symptom improvement were valid.
Agnihotry, Paul, and Sandhu (2007) conducted a randomized controlled study of 45 patients diagnosed with Generalized Anxiety Disorder, which compared frontalis SEMG relaxation, EEG training to increase alpha, and a control group. Both the SEMG and EEG groups reduced STAI-State Anxiety and Trait Anxiety scores. Galvanic skin resistance (more resistance means less SNS activation) increased for both groups, with more significant gains for the SEMG group. At a 2-week follow-up, the SEMG group maintained the largest gains on all three measures.
Coy, Cardenas, Cabrera, Zirot, and Claros (2005) found that combining the antidepressant imipramine and biofeedback resulted in more significant anxiety reduction than imipramine alone.
Reiner (2008) reported an uncontrolled pre-test/post-test study of 24 patients diagnosed with an anxiety disorder and receiving outpatient CBT who practiced at home using a portable HRVB device. Nineteen participants who completed the study reduced Spielberger State-Trait Anger Expression Inventory (STAEI) and Spielberger State-Trait Anxiety Inventory (STAI-Y) scores and increased Pittsburgh Sleep Quality Index (PSQI) scores and sleep duration. Participants who practiced more frequently achieved more significant improvements on these measures.
Mikosch and colleagues (2010) conducted a randomized controlled study of 212 patients scheduled for elective coronary angiography (CA), which compared an intervention group that received psychological support, abdominal breathing instruction, and one session of HRVB with a control group that received TAU and information. While both groups reduced Spielberger State-Trait Anxiety Inventory (STAI) scores after the CA, the intervention group showed the largest decrease, and its scores were normal.
Ratanasiripong, Sverduk, Prince, and Havashino (2012) reported a randomized controlled study of 30 undergraduates, which compared counseling plus HRVB with a counseling comparison group. While both groups reduced Beck Anxiety Inventory scores, the HRVB group showed more significant improvement.
Based on five RCTs, Donald Moss and Matthew Watkins rated BFB and NFB for GAD as efficacious. The BFB interventions included HR decrease, HRV increase, SCL decrease, SEMG decrease, and virtual reality (VRB). The NF interventions included alpha increase and alpha/theta increase.
Participants decreased HR, SCL, state and trait anxiety, and HR reactivity to stress. They increased HRV measures (HF and LF power) and theta power.
Panic Disorder (PD)
Based on five RCTs, Donald Moss and Matthew Watkins rated BFB for PD as efficacious and specific.
The BFB interventions included end-tidal CO2 increase, HRV increase, and SEMG decrease.
Participants reduced agoraphobic avoidance, anger, panic-related cognitions, PD frequency and severity, and state and trait anxiety. They increased end-tidal CO2 and sleep time.
Phobia
Donald Moss and Matthew Watkins rated BFB for phobia as possibly efficacious for biofeedback and neurofeedback.
Post-Traumatic Stress Disorder (PTSD)
Based on four RCTs, Donald Moss, Fred Shaffer, and Matthew Watkins rated BFB and NFB for PTSD as efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.).
The BFB interventions included HRV and TEMP increase, and respiration rate (RR) decrease. The NFB interventions included left amygdala fMRI decrease; 2-6 Hz and 22-36 Hz decrease, and 10-13 Hz increase; and theta and high-beta decrease and low-beta increase.
Participants reduced anxiety and depression scores and PTSD symptoms.

The National Survey on Drug Use and Health (NSDUH) defines a significant depressive episode as a minimum 2-week phase during which an individual encounters feelings of depression or a lack of enjoyment in their usual activities.
Major depressive disorder is diagnosed when five or more depressive symptoms, including sadness or loss of pleasure, are present for two weeks. Depressed patients may sleep too much or too little, display psychomotor retardation or agitation, show change in weight or appetite, experience loss of energy, feel worthless or guilty, are unable to concentrate, think, or make decisions, and repeatedly think about death or suicide (Beidel, Bulik, & Stanley, 2014). Check out the YouTube video The Science of Depression. Graphic © FANDESIGN/Shutterstock.com.
Multiple pathways to depression include polygenic dysfunction involving the lateral and medial orbitofrontal cortex and limbic system, and environmental factors (McGrady & Moss, 2013).
Depression is associated with activation of the lateral orbitofrontal cortex, which signals that our behavior has not been rewarded. This activation may be related to feelings of loss and disappointment. Since this region communicates with networks responsible for our self-concept, this may also lower self-esteem (Cheng et al., 2016).
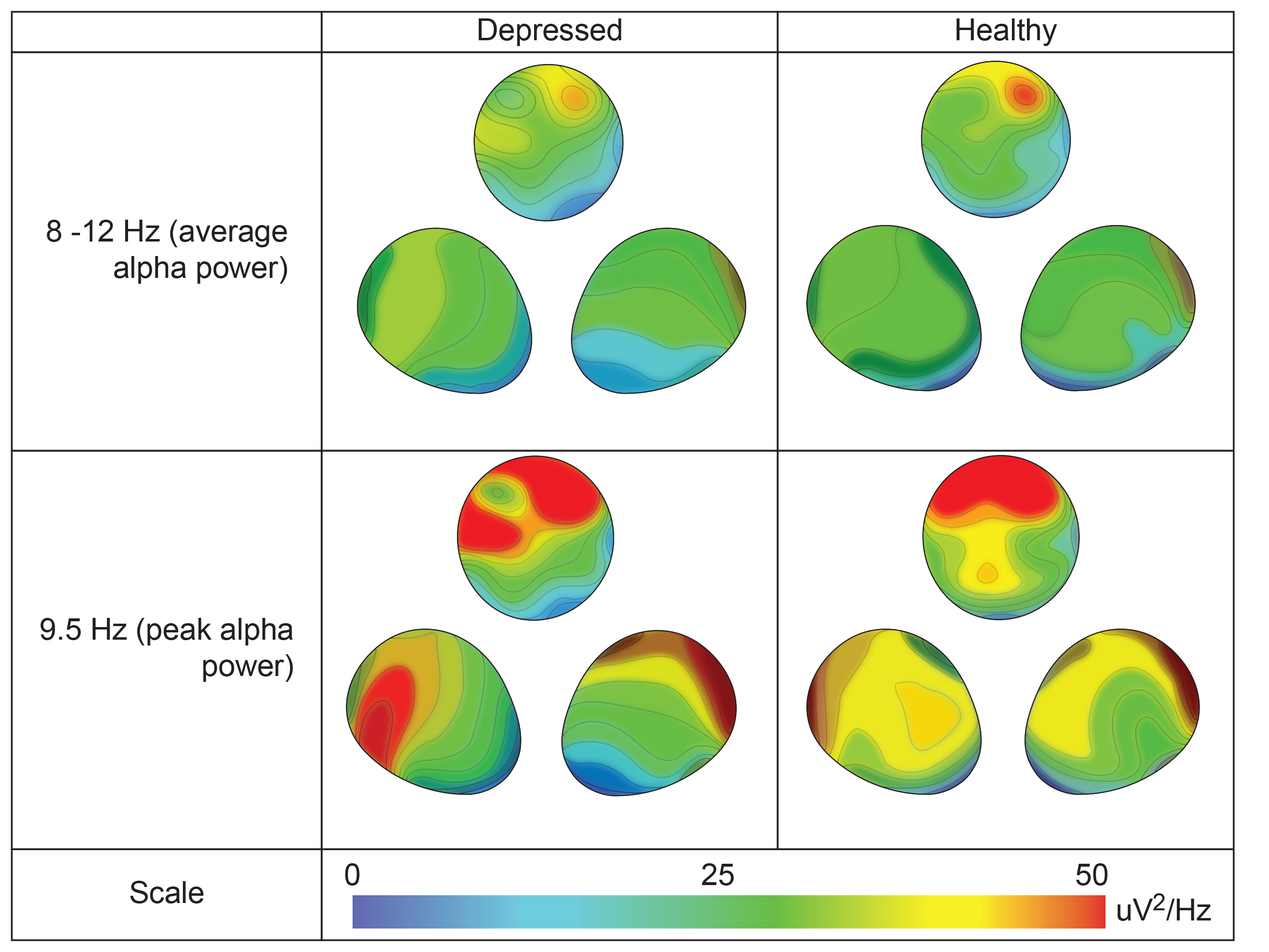
The frontal distribution of average and peak alpha power differs between depressed and healthy individuals. Graphic redrawn by minaanandag on Fiverr.com.
Depression also involves reduced activation of reward circuitry in the medial orbitofrontal cortex and its communication with autobiographical memory systems. These changes may contribute to depressed patients' lack of enjoyment of daily activities and difficulty remembering happy experiences. Billeke, P. and Aboitiz, F. (2013) Social cognition in schizophrenia: From social stimuli processing to social engagement. Front. Psychiatry, 4(4). http:10.3389/fpsyt.2013.00004
http://journal.frontiersin.org/article/10.3389/fpsyt.2013.00004/full
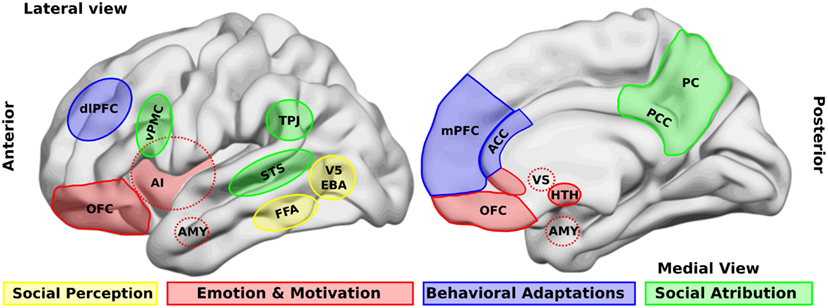
Caption: Brain areas that participate in social processing. A simple classification of brain areas involved in social processing differentiates regions that participate in four related processes. The first is the perception of basic social stimuli, such as biological motions (V5), part of the body (extra-striate body area, EBA), and faces (fusiform face area, FFA). Another process includes emotional and motivational appraisal, where the amygdala (AMY), the anterior insula (AI), the subgenual and perigenual anterior cingulate cortex (ACC), as well as the orbitofrontal cortex (OFC) participate.
These cortical structures are in interaction with subcortical structures as the ventral striatum (VS), and the hypothalamus (HTH). These structures in turn interact with other regions which participate in the goal-directed, adaptive behaviors, and the categorization processes, such as the dorsolateral and the medial prefrontal cortex (dlPFC, mPFC) and the ACC. Finally for social attribution, areas like the ventral premotor cortex (vPMC), the superior temporal sulcus (STS), the AI, the posterior cingulate cortex (PCC), and the precuneus (PC) participate in more automatic, bottom-up inferences of other people’s mental states; whereas structures like the mPFC and the temporo-parietal junction (TPJ) are involved in more cognitive theory of mind skills.
Depression also involves disrupting the habenular nucleus, which is adjacent to the pineal gland. Since the habenular nucleus helps us filter out negative cognitions and memories, disabling this function results in the triad (negative self-perception, view of your immediate situation, and expectation of the future) that characterizes depressive thinking (Lawson et al., 2016). Animal research reveals that cocaine withdrawal increases habenular nucleus anti-reward pathway activation (Clerke et al., 2021).
Insular cortex graphic Ide JS and Li C-S R (2011) Creative Commons Attribution 3.0. Error-related functional connectivity of the habenula in humans. Front. Hum. Neurosci. http://journal.frontiersin.org/article/10.3389/fnhum.2011.00025/fu
1. About 8.4% of U.S. adults experienced a MDD episode in the last year.
2. MDD was more prevalent in women (10.5%) than men (6.2%).
3. MDD prevalence was highest among those aged 18-25 (17.0%).
If untreated, 25-30% of adult depressive attempt or commit suicide. Most cases of major depression involve another comorbid psychological disorder that is primary (Zimmerman et al., 2002). Only 21% of annual cases of depression are adequately treated (Kessler et al., 2003).
Clinical depression is associated with less activation of the left frontal lobe than the right. Since alpha is an "idling frequency," this asymmetry is seen when the alpha amplitude is greater in the left (F3) than the right frontal lobe (F4). The goal of alpha asymmetry NF for depression is to correct this imbalance, decreasing left frontal alpha with respect to right frontal alpha.
Patients are seen once or twice a week for one-hour sessions, which consist of 30 minutes of EEG training followed by 30 minutes of psychotherapy. Scalp sites F3 and F4 are used and are referenced to CZ. A bell or clarinet tone reinforces behavior when the asymmetry score exceeds 0 (right alpha amplitude exceeds left).
Typically, it is desirable for 15-18 Hz amplitude to be higher and 8-11 Hz amplitude to be lower at F3 compared to F4.
fMRI protocols attempt to up-regulate the activity of targeted regions that mediate positive emotion.
Case studies reported by Kumano et al. (1996) and Rosenfeld (2000) and a pilot study by Waldkoetter and Sanders (1997) support the Baehr et al. (1999) finding that NF can reduce the symptoms of clinical depression.
Corrado and Gottlieb (1999) compared biofeedback-assisted relaxation with a wait-list control condition in chronic pain patients. The biofeedback-assisted relaxation group achieved improved Beck Depression Inventory scores.
Rosenfeld (2000) summarized a series of case studies involving patients diagnosed with depression. Before NF sessions, patients were trained to breathe diaphragmatically for 15-30 minutes and to warm their hands to a criterion of 95 degrees F. Active electrodes at F3 and F4 were both referenced to Cz. Training sessions conducted twice a week and were divided into 50% NF and 50% psychotherapy. As the alpha asymmetry score improved in four cases, Beck Depression Inventory (BDI) and Minnesota Multiphasic Personality Inventory (MMPI) depression scores declined.
Baehr, Rosenfeld, and Baehr (2001) reported follow-up data for three of six patients diagnosed with unipolar depression who had completed 27 sessions of alpha asymmetry training. The authors compared pre-treatment and follow-up alpha asymmetry and Beck Depression Inventory scores. All three patients had achieved normal right hemisphere alpha asymmetry scores and Beck scores by the completion of their training and maintained these gains at 1- to 5-year follow-up.
Choi et al. (2011) conducted a randomized controlled trial of 24 right-handed depressed patients who had not received psychoactive drugs within 2 months of the study. The researchers placed active electrodes at F3 and F4, referenced to Cz, and utilized Rosenfeld’s asymmetry protocol. Training sessions comprised six 4-minute trials separated by 30-second rest periods. They trained participants twice a week for 5 weeks. Following NF training, participants received self-training to reproduce the mental state they experienced during NF without equipment twice a week for 1 month. The psychotherapy placebo sessions were also conducted for 5 weeks. After these sessions, they were referred to other therapists who provided traditional psychotherapy for depression as required.
Only the NF group increased right frontal alpha power and asymmetry scores and a significant improvement on the HAM-D and BDI-II scales. Six (50%) of the NF participants and none of the psychotherapy placebo participants achieved a clinical response.

Linden et al. (2012) reported an open-label pilot study of fMRI NF for 16 participants diagnosed with Recurrent Depressive Disorder. In the fMRI NF condition, the researchers trained 8 participants during four sessions to up-regulate brain regions responsive to positive emotions using a visual display updated every 2 seconds. Each session consisted of three 7-minute trials. In the control condition, the researchers instructed 8 participants to utilize positive imagery techniques employed by the fMRI NF participants during four sessions conducted outside the scanner. The fMRI NF group successfully up-regulated the target areas (left or right ventromedial prefrontal cortex, insula, dorsolateral prefrontal cortex, medial temporal lobe, or the orbitofrontal cortex). While the fMRI NF group improved on the Hamilton Depression Rating Scale (HDRS), the control group did not change.
Young et al. (2014) randomly assigned unmedicated participants diagnosed with major depressive disorder to either receive rtfMRI-nf from the left amygdala (experimental; n = 14) or the left intraparietal sulcus (control; n = 7). Training sessions consisted of seven 8-minute 30-second runs. The experimental group increased left amygdala activation when recalling positive autobiographical memories within the first training session. This training effect was maintained during transfer runs in which participants did not receive NF. In contrast, the control group did not increase the activation of the intraparietal sulcus. While the experimental group decreased Profile of Mood States (POMS) depression scores, it was not superior to the control group. The experimental group decreased State-Trait Anxiety Inventory (STAI) state and trait scores, and the two groups only differed on state anxiety. Finally, the experimental group increased Visual Analog Scale (VAS) happiness ratings, and this gain was more significant than in the control group.
Karavidas and colleagues (2007) administered 10 weekly sessions of heart rate variability (HRV) biofeedback to 11 patients diagnosed with major depressive disorder (MDD). By session 4, these patients showed significant improvement on the Hamilton Depression Scale (HAM-D) and the Beck Depression Inventory (BDI-II). They maintained these changes over the remaining 6 weeks of training.
Siepmann et al. (2008) conducted an open-label controlled pilot study with 38 participants. They placed 14 depressed individuals in the HRVB condition. Additionally, they randomly assigned 12 healthy individuals to HRVB and active control (watch the same HRVB display without instructions) conditions. The decision to assign all depressed individuals to the HRVB condition and none to the active control condition undermined the internal validity of this design for these participants. All participants received three treatment sessions per week for 2 weeks. While depressed individuals significantly reduced Beck Depression Inventory (BDI) scores, decreased Spielberger State-Trait Anxiety Inventory (STAI) scores and heart rate, and increased HRV compared to baseline, healthy participants who received HRVB or the active control treatment did not change.
Zucker et al. (2009) conducted a controlled pilot study with 38 participants recruited from a residential therapeutic community for substance use disorder e diagnosed with PTSD symptoms. They randomly assigned individuals to either HRVB (StressEraser) or progressive muscle relaxation (PMR) recording. They instructed participants to practice 20 minutes per day and complete weekly logs. The HRVB group achieved lower Beck Depression Inventory (BDI-II) scores and increased HRV (SDNN) compared to the PMR group. Both groups significantly reduced PTSD symptoms on the Posttraumatic Stress-Total (PTS-T) scale and PTSD Checklist-Civilian Version (PCL-C). Increased HRV predicted improvement, even when respiration rate was statistically controlled.
Patron et al. (2013) studied 26 individuals with depressive symptoms following cardiac surgery. They randomly assigned participants to either five 45-minute sessions of HRVB or treatment as usual (TAU). HRVB was superior to TAU in increasing respiratory sinus arrhythmia (RSA), heart rate speeding and slowing across the breathing cycle, and decreasing the Centre for Epidemiologic Studies of Depression (CES-D) values from pre- to post-treatment. Improvement was correlated with increased RSA.
BFB treatments included HRV increase. NFB interventions included alpha asymmetry reduction, alpha/theta increase, and real-time functional MRI (rtfMRI) to increase left or right ventromedial prefrontal cortex, insula, dorsolateral prefrontal cortex, medial temporal lobe, or orbitofrontal cortex activity.
Participants achieved decreased behavioral inhibition, depression symptoms and severity, and state and trait anxiety.
Tinnitus involves ringing in the ear when noise is absent and is associated with hearing loss. This symptom can be debilitating when it interferes with speech comprehension. Tinnitus affects over 45 million Americans. Conventional medical treatments include background music, masking sounds, and antidepressants (Lalwani, 2014). Graphic © Dora Zett/Shutterstock.com.



alpha asymmetry neurofeedback for mood disorders: a protocol that trains depressed clients to relax and warm their hands using diaphragmatic breathing and autogenic phrases and then decrease left frontal alpha with respect to the right frontal alpha.
amplitude control: a protocol that trains patients to increase EEG amplitude (signal strength) instead of frequency. This strategy can raise the amplitude of both a target rhythm (beta) and an unwanted rhythm (alpha) since it does not teach frequency discrimination.
connectivity training: a strategy designed to correct deficient or excessive communication between two brain sites measured by indices like coherence and comodulation.
frequency control: a protocol that trains patients to increase the amplitude of one frequency (SMR) while suppressing another (theta). This procedure refines EEG control and may result in better clinical outcomes than amplitude control.
Generalized Anxiety Disorder (GAD): a disorder characterized by excessive anxiety and worry, which frequently interfere with daily functioning.
hyperactivity: excessive motor activity for a situation like a classroom, including fidgeting, leaving a seat, and climbing or running.
impulsivity: a failure to restrain behavior, including answering before a question is completed, interrupting others' conversations or play, and an inability to wait for one's turn.
inattention: difficulty sustaining attention, including distractibility, insufficient attention to detail, careless mistakes, forgetfulness, and loss of assignments and toys.
International 10-20 system: a standardized procedure for placing 21 recording and 1 ground electrode on adults.
Kaiser-Scott protocol (Scott-Kaiser modification): an intervention that starts with NF ADHD training and then progresses to the Peniston protocol.
Menninger alpha-theta protocol: a procedure that helps clients increase their attention through temperature and frontal SEMG training and then teaches clients to gradually slow the EEG until they can increase alpha and theta amplitude without falling asleep.
Menninger ON-OFF-ON EEG training: a procedure that teaches clients to increase the amplitude within a frequency band, reduce the amplitude, and then increase the amplitude again during 100- or 200-second segments. For example, clients may be instructed to increase 8-13 Hz alpha activity for 100 seconds, decrease it for 100 seconds, and then increase it for 100 seconds.
passive volition: in autogenic training, visualizing the desired change and then allowing the body to make the change at its own pace.
Peniston-Kulkosky addiction protocol: a multi-modal approach that incorporates visualization training, temperature biofeedback, rhythmic breathing techniques, autogenic training exercises, construction of personalized imagery, guided imagery, and 30 alpha-theta sessions.
performance-based protocols: the use of tasks and neurofeedback training to correct symptoms and improve performance. This approach compares clients to themselves and not a clinical database.
petit mal seizures: a type of epilepsy characterized by the loss of consciousness without abnormal movement during which a client appears to be daydreaming. A child may suffer hundreds of these seizures daily for periods lasting up to 30 seconds.
sensorimotor cortex: the central cortical area defined by the central sulcus (fissure of Rolando) separating the frontal and parietal lobes.
sensorimotor rhythm (SMR): an EEG rhythm from 12-14 Hz located over the sensorimotor cortex (central sulcus) that is associated with inhibition of movement and reduced muscle tone.
slow cortical potential (SCP) training: neurofeedback to increase the gradual negative changes in the membrane potentials of cortical dendrites that last from 300 milliseconds to several seconds to reduce neuronal excitability in conditions like grand mal epilepsy and migraines.
SMR training: a procedure that up-trains SMR activity and possibly down-trains theta, beta, and epileptiform activity.
Sterman's grand mal epilepsy protocol: a procedure that trains an epileptic patient to increase SMR (12-14 Hz) amplitude and duration while theta (4-7 Hz), beta (20+ Hz), epileptiform spikes, and EMG artifact are suppressed during 36 sessions. The aim is to normalize the waking and sleep EEG with elevated SMR and suppressed theta and beta activity.
stroke: cerebrovascular accident (CVA), destruction of brain tissue (infarction) due to cerebral hemorrhage, and cerebral ischemia affecting blood vessels that supply the brain. CVAs show abrupt onset and involve temporary or permanent neurological symptoms like aphasia, paralysis, or loss of sensation.
theta/beta training: a procedure that down-trains theta and up-trains beta.
tinnitus: ringing in the ear when noise is absent.
tonic-clonic seizures (grand mal seizures): primary generalized seizures featuring a peculiar cry, loss of consciousness, fall, tonic-clonic convulsions of all extremities, incontinence, and amnesia for the episode. These seizures are diagnosed in fewer than 20% of adult epileptics.
traumatic brain injury: intracranial insult due to acceleration or direct impact that may cause permanent or temporary cognitive, physical, and psychosocial deficits and impaired or altered consciousness.
Z-score training: a strategy that attempts to normalize brain function with respect to mean values in a clinical database. EEG amplitudes that are 2 or more standard deviations above or below the database means are down-trained or up-trained to treat symptoms and improve performance.
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Click on the Quizlet logo to review our chapter flashcards.

BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Now that you have completed this module, consider the elements that neurofeedback training shares with modalities like EMG and temperature. How is neurofeedback training different?
Adamolekun, B. (2013). Seizure disorders. The Merck manual.
Agnihotry, H., Paul, M., & Sandhu, J. S. (2007). Biofeedback approach in the treatment of generalized anxiety disorder. Iranian Journal of Psychiatry, 2, 90-95.
American Academy of Pediatrics (2010). Evidence-based child and adolescent psychosocial interventions.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Author.
Andreassi, J. L. (2000). Psychophysiology: Human behavior and physiological response. Lawrence Erlbaum and Associates, Inc.
Andrews, D. J., Reiter, J. M., Schonfeld, W., Kastl, A., & Denning, P. (2000). A neurobehavioral treatment for unilateral complex partial seizure disorders: A comparison of right- and left-hemisphere patients. Seizure, 9(3), 189-197. https://doi.org/10.1053/seiz.1999.0375
Arns, M., Heinrich, H., & Strehl, U. (2013). Evaluation of neurofeedback in ADHD: The long and winding road. Biological Psychology, 95, 108-115. https://doi.org/10.1016/j.biopsycho.2013.11.013
Aronson, S. C. (2005). Depression. eMedicine.
Ayers, M. E. (1995). EEG neurofeedback to bring individuals out of level 2 coma [Abstract]. Biofeedback and Self-Regulation, 20(3), 304-305.
Ayers, M. E. (1995). Long-term follow-up of EEG neurofeedback with absence seizures. Biofeedback & Self-Regulation, 20(3), 309-310.
Baehr, E., Miller, E., Rosenfeld, J. P., & Baehr, R. (2004). Changes in frontal brain asymmetry associated with premenstrual dysphoric disorder: A single case study. Journal of Neurotherapy, 8(1), 29-42. https://doi.org/10.1300/J184v08n01_03
Baehr, E., Rosenfeld, J. P., & Baehr, R. (1997). The clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression: Two case studies. Journal of Neurotherapy, 2(3), 10-23. https://doi.org/10.1300/J184v02n03_02
Baehr, E., Rosenfeld, J. P., & Baehr, R. (2001). Clinical use of an alpha asymmetry neurofeedback protocol in the treatment of mood disorders: Follow-up study one to five years post therapy. Journal of Neurotherapy, 4(4), 11-18. https://doi.org/10.1300/J184v04n04_03
Beauregard, M., & Levesque, J. (2006). Functional magnetic resonance imaging investigation of the effects of neurofeedback training on the neural bases of selective attention and response inhibition in children with attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback, 31(1), 2-20. https://doi.org/10.1007/s10484-006-9001-y
Becerra, J., Fernandez, T., Harmony, T., Caballero, M. I., Garcia, F., Fernandez-Bouzas, A., . . . Prado-Alcala, R. A. (2006). Follow-up study of learning-disabled children treated with neurofeedback or placebo. Clin EEG Neurosci, 37(3), 198-203. https://doi.org/10.1177/155005940603700307
Beidel, D. C., Bulik, C. M., & Stanley, M. A. (2014). Abnormal psychology (3rd ed.). Pearson Education, Inc.
Belanger, H. G., Tate, D. F., & Vanderploog, R. D. (2018). Concussion and mild traumatic brain injury. In J. E. Morgan & J. H. Ricker (Eds.), Textbook of clinical neuropsychology (2nd ed., pp. 411-448). Routledge.
Biederman, J., Mick, E., Faraone, S. V., Braaten, E., Doyle, A., Spencer, T., Wilens, T. E., Frazier, E., & Johnson, M. A. (2002). Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. American Journal of Psychiatry, 159, 36–42. https://doi.org/10.1176/appi.ajp.159.1.36
Bodenhamer-Davis, E., & Callaway, T. (2004). Extended follow-up of Peniston Protocol results with chemical dependency. Journal of Neurotherapy, 8(2), 135-148.
Bray, S., Shimojo, S., & O'Doherty, J. P. (2007). Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J Neurosci, 27(28), 7498-74507. https://doi.org/10.1523/jneurosci.2118-07.2007
Brown, P. (2013). ADHD and the DSM-5: Update on revision to diagnostic criteria. Consultant for Pediatricians, 12(10), 453-454.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. H. (2004). Treatment effects related to EEG- biofeedback for crack cocaine dependency in a faith-based homeless mission. Journal of Neurotherapy, 8(2), 138-140.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. (2005). An open clinical trial utilizing real-time EEG operant conditioning as an adjunctive therapy in the treatment of crack cocaine dependence. Journal of Neurotherapy, 9(2), 27-47. https://doi.org/10.1300/J184v09n02_03
Camp, B. W. (1999). Attention deficit hyperactivity disorders: Studies of EEG patterns and biofeedback training (Final Report, Grant No. H113G40130), U.S. Office of Education.
Cannon, R., Lubar, J., Congedo, M., Thornton, K., Towler, K., & Hutchens, T. (2007). The effects of neurofeedback training in the cognitive division of the anterior cingulate gyrus. Int J Neurosci, 117(3), 337-357. https://doi.org/10.1080/00207450500514003
Caria, A., Veit, R., Sitram, R., Lotze, M., Weiskopf, N., Grodd, W., & Birbaumer, N. (2007). Regulation of anterior insular cortex activity using real-time fMRI. NeuroImage, 35(3), 1238-1246. https://doi.org/10.1016/j.neuroimage.2007.01.018
Carmody, D. P., Radvanski, D. C., Wadhwani, S., Sabo, M. J., & Vergara, L. (2001). EEG biofeedback training and attention-deficit/hyperactivity disorder in an elementary school setting. Journal of Neurotherapy, 4(3), 5-27. https://doi.org/10.1300/J184v04n03_02
Cheng, W., Rolls, E. T., Qiu, W., Tang, Y., Huang, C. C., Wang, X., Zhang, J., Lin, W., Zheng, L., Pu, J., Tsai, S.-J., Yang, A. C., Lin, C.-P., Wang, F., Xoe, P., & Feng, J. (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain, 139(Pt 12), 3296-3309. https://doi.org/10.1093/brain/aww255
Corrado, P., & Gottlieb, H. (1999). The effect of biofeedback and relaxation training on depression in chronic pain patients. American Journal of Alternative Medicine, 9, 18-21.
Corrado, P., Gottlieb, H., & Abdelhamid, M. H. (2003). The effect of biofeedback and relaxation training on anxiety and somatic complaints in chronic pain patients. American Journal of Pain Management, 13(4), 133-139.
Costello, E. J., Mustillo, S., Erkanli, A., Keeler, G., & Angold, A. (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry, 60(8), 837-844. https://doi.org/10.1001/archpsyc.60.8.837
Coy, P. C., Cardenas, S. J., Cabrera, D. M., Zirot, G. Z., & Claros, M. S. (2005). Psychophysiological and behavioral treatment of anxiety disorder. Salud Mental, 28(1), 28-37.
Dohrmann, K., Elbert, T., Schlee, W., & Weisz, N. (2007). Tuning the tinnitus percept by modification of synchronous brain activity. Restor Neurol Neursci, 25(3-4), 371-378. PMID: 17943012
Dreschler, R., Straub, M., Doehnert, M., Heinrich, H., Steinhausen, H. C., & Brandeis, D. (2007). Controlled evaluation of a neurofeedback training of slow cortical potentials in children with Attention Deficit/Hyperactivity Disorder (ADHD). Behav Brain Funct, 3(1), 35. https://doi.org/10.1186/1744-9081-3-35
Duric, N. S., Assmus, J., Gundersen, D. I., & Elgen, I. B. (2012). Neurofeedback for the treatment of children and adolescents with ADHD: A randomized and controlled clinical trial using parental reports. BMC Psychiatry, 12(1), 107. https://doi.org/10.1186/1471-244X-12-107
Egner, T., & Sterman, M. B. (2006). Neurofeedback treatment of epilepsy: From basic rationale to practical application. Expert Rev Neurother, 6(2), 247-257. https://doi.org/10.1586/14737175.6.2.247
Egner, T., Strawson, E., & Gruzelier, J. H. (2002). EEG signature and phenomenology of alpha/theta neurofeedback training versus mock feedback. Applied Psychophysiology and Biofeedback, 27(4), 261-270. https://doi.org/10.1023/a:1021063416558
Elbert, T., Rockstroh, B., Canavan, A., Birbaumer, N., Lutzenberger, W., von Billow, I., & Linden, A. (1991). Self-regulation of slow cortical potentials and its role in epileptogenesis. In J. Carlson & R. Seifert (Eds.), Biobehavioral self-regulation and health. Plenum Press.
Enriquez-Geppert, S., Brown, T., Henrich, H., Arns, M., & Pimenta, M. G. (2023). Attention Deficit Hyperactivity Disorder. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Evans, J. R., & Abarbanel, A. (1999). Introduction to quantitative EEG and neurofeedback. Academic Press.
Foster, D. S., & Thatcher, R. W. (2016). Traumatic Brain Injury (TBI) and Post Traumatic Stress Disorder (PTSD). In G. Tan, F. Shaffer, R. R. Lyle, & I. Teo (Eds.). Evidence-based practice in biofeedback and neurofeedback (3rd ed.). AAPB.
Friel, P. N. (2007). EEG biofeedback in the treatment of attention deficit hyperactivity disorder. Altern Med Rev, 12(2), 146-151. PMID: 17604459
Fuchs, T., Birbaumer, N., Lutzenberger, W., Gruzelier, J. H., & Kaiser, J. (2003). Neurofeedback treatment for Attention-Deficit/Hyperactivity Disorder in children: A comparison with methylphenidate. Applied Psychophysiology and Biofeedback, 28(1), 1-12. https://doi.org/10.1023/a:1022353731579
Gevensleben, H., Holl, B., Albrecht, B., Schlamp, D., Kratz, O., Studer, P., . . . Heinrich, H. (2010). Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. European Child & Adolescent Psychiatry, 19(9), 715-24. doi:10.1007/s00787-010-0109-5
Gevensleben, H., Holl, B., Albrecht, B., Vogel, C., Schlamp, D., Kratz, O., . . . Heinrich, H. (2009). Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 50(7), 780-9. https://doi.org/10.1111/j.1469-7610.2008.02033.x
Gevensleben, H., Kleemeyer, M., Rothenberger, L. G., Studer, P., Flaig-Röhr, A., Moll, G. H., . . . Heinrich, H. (2013). Neurofeedback in ADHD: Further pieces of the puzzle. Brain Topography. https://doi.org/10.1007/s10548-013-0285-y
Gevirtz, R. (2013). The promise of heart rate variability biofeedback: Evidence-based applications. Biofeedback, 41(3), 110-120. https://doi.org/10.5298/1081-5937-41.3.01
Goldstein, S. (2011). Attention-Deficit/ Hyperactivity disorder. In S. Goldstein, & C. R. Reynolds (Eds.), Handbook of neurodevelopmental and genetic disorders in children (2nd ed.). Guilford Press.
Gruzelier, J., Thompson, T., Brandt, R., & Steffert, T. (2014). Application of alpha/theta neurofeedback and heart rate variability training to contemporary dancers: State anxiety and creativity. International Journal of Psychophysiology, 93(1), 105-111. https://doi.org/10.1016/j.ijpsycho.2013.05.004
Halverson, J. L. (2014). Depression. eMedicine.
Hammond, D. C. (2001). Neurofeedback training for anger control. Journal of Neurotherapy, 5(4), 98-103.
Hammond, D. C. (2001). Neurofeedback treatment of depression with the Roshi. Journal of Neurotherapy, 4(2), 45-56. https://doi.org/10.1300/J184v04n02_06
Hammond, D. C. (2005). Neurofeedback with anxiety and affective disorders. Child & Adolescent Psychiatric Clinics of North America, 14(1), 105-123. https://doi.org/10.1016/j.chc.2004.07.008
Holtmann, M., & Stadler, C. (2006). Electroencephalographic biofeedback for the treatment of attention-deficit hyperactivity disorder in childhood and adolescence. Expert Rev Neurother, 6(4), 533-540. https://doi.org/10.1586/14737175.6.4.533
Kaiser, D. A., & Othmer, S. (2000). Effect of neurofeedback on variables of attention in a large multi-center trial. Journal of Neurotherapy, 4(1), 5-15. http://dx.doi.org/10.1300/J184v04n01_02
Kaiser, D. A., Othmer, S., & Scott, B. (1999). Effect of neurofeedback on chemical dependency treatment. Biofeedback & Self-Regulation, 20(3), 304–305.
Karavidas, M. K., Lehrer, P. M., Vaschillo, E., Vaschillo, B., Marin, H., Buyske, S., Malinovsky, I., Radvanski, D., & Hassett, A. (2007). Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback, 32(1), 19-30. https://doi.org/10.1007/s10484-006-9029-z
Keller, I. (2001). Neurofeedback therapy of attention deficits in patients with traumatic brain injury. Journal of Neurotherapy, 5(1-2), 19-32. https://doi.org/10.1300/J184v05n01_03
Kelley, M. J. (1997). Native Americans, neurofeedback, and substance abuse theory. Three year outcome of alpha/theta neurofeedback training in the treatment of problem drinking among Dine’ (Navajo) people. Journal of Neurotherapy, 2, 24–60. https://doi.org/10.1300/J184v02n03_03
Kessler, R. C., Adler, L., Barkley, R., Biederman, J., Conners, C. K., Demler, O., Faraone, S. V., Greenhill, L. L., Howes, M. J., Secnik, K., Spencer, T., Ustun, T. B., Walters, E. E., & Zaslavsky, A. M. (2006). The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. The American Journal of Psychiatry, 163(4), 716–723. https://doi.org/10.1176/ajp.2006.163.4.716
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K. R., Rush, J., Walters, E. E., & Wang, P. S. (2003). The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289, 3095-3105. https://doi.org/10.1001/jama.289.23.3095
Kessler, R. C., McGonagle, K. A., Zhao, S., Nelson, C. B., Hughes, M., Eshleman, S., Wittchen, H. U., & Kendler, K. S. (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry, 51(1), 8-19. https://doi.org/10.1001/archpsyc.1994.03950010008002
Koob, G. F. (2006). The neurobiology of addiction: A neuroadaptational view relevant for diagnosis. Addiction, 101, 23-30. https://doi.org/10.1111/j.1360-0443.2006.01586.x
Kotchoubey, B., Blankenhorn, V., Froscher, W., Strehl, U., & Birbaumer, N. (1997). Stability of cortical self-regulation in epilepsy patients. Neuroreport, 27(8), 1867-1870. https://doi.org/10.1097/00001756-199705260-00015
Kotchoubey, B., Schneider, D., Schleichert, H., Strehl, U., Uhlmann, C., Blankenhorn, V., . . . Birbaumer, N. (1996). Self-regulation of slow cortical potentials in epilepsy: A retrial with analysis of influencing factors. Epilepsy Research, 25(3), 269-276. https://doi.org/10.1016/S0920-1211(96)00082-4
Kotchoubey, B., Busch, S., Strehl, U., & Birbaumer, N. (1999). Changes in EEG power spectra during biofeedback of slow cortical potentials in epilepsy. Applied Psychophysiology and Biofeedback, 24(4), 213-233. https://doi.org/10.1023/a:1022226412991
Kotchoubey, B., Blankenhorn, V., Froscher, W., Strehl, U., & Birbaumer, N. (1996). Stability of cortical self-regulation in epilepsy patients. Neuroreport, 27(8), 1867-1870.
Kotchoubey, B., Strehl, U., Holzapfel, S., Blankenhorn, V., Froscher, W., & Birbaumer, N. (1999). Negative potential shifts and the prediction of the outcome of neurofeedback therapy in epilepsy. Clinical Neurophysiology, 110(4), 683-686. https://doi.org/10.1016/s1388-2457(99)00005-x
Kotchoubey, B., Strehl, U., Uhlmann, C., Holzapfel, S., Konig, M., Fröscher, W., Blankenhorn, V., & Birbaumer, N. (2001). Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia, 42(3), 406-416. https://doi.org/10.1046/j.1528-1157.2001.22200.x
Kumano, H., Horie, H., Shidara, T., Kuboki, T., Suematsu, H., & Yasushi, M. (1996). Treatment of a depressive disorder patient with EEG-driven photic stimulation. Biofeedback and Self-Regulation, 21(4), 323-334. https://doi.org/10.1007/bf02214432
Kushner, D. (1998). Mild traumatic brain injury: Toward understanding manifestations and treatment. Archives of Internal Medicine, 158(15), 1617-1624. https://doi.org/10.1001/archinte.158.15.1617
La Vaque, T. J. (2003). Neurofeedback, neurotherapy, and quantitative EEG. In D. Moss, A. McGrady, T. Davies, and I. Wickramasekera (Eds.), Handbook of mind-body medicine for primary care. Sage Publications, Inc.
Lagos, L., Vaschillo, B., Vaschillo, E., Lehrer, P. M., & Bates, M. (2008). Heart rate variability biofeedback as a strategy for dealing with competitive anxiety: A case study. Biofeedback. 36(3), 109–115.
Langlois, J. A., Rutland-Brown, W., & Wald, M. M. (2006). The epidemiology and impact of traumatic brain injury: A brief overview. Journal of Head Trauma Rehabilitation, 21(5), 375–378. https://doi.org/10.1097/00001199-200609000-00001
Lawson, R. P., Nord, C. L., Seymour, D. L., Thomas, D. L., Dayan, P., Pilling, S., & Roiser, J. P. (2016). Disrupted habenula function in major depression. Molecular Psychiatry. https://doi.org/10.1038/mp.2016.81
Leins, U., Goth, G., Hinterberger, T., Klinger, C., Rumpf, N., & Strehl, U. (2007). Neurofeedback for Children with ADHD: A Comparison of SCP and Theta/Beta Protocols. Applied Psychophysiology and Biofeedback, 32(2), 73-88. PMID: 16869483
Levesque, J., Beauregard, M., & Mensour, B. (2006). Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study. Neurosci Lett, 394(3), 216-221. https://doi.org/10.1016/j.neulet.2005.10.100
Lubar, J. F. (1985). EEG biofeedback and learning disabilities. Theory into Practice, 26, 106-111. https://doi.org/10.1080/00405848509543156
Lubar, J. F. (1995). Neurofeedback for the management of attention-deficit/hyperactivity disorder. In M. S. Schwartz & Associates (Eds.), Biofeedback: A practitioner's guide (2nd ed.). Guilford.
Lubar, J. F. (2003). Neurofeedback for the management of attention‑deficit/hyperactivity disorders. In M. S. Schwartz, & F. Andrasik (Eds.), Biofeedback: A practitioner's guide (3rd ed.). Guilford.
Lubar, J. O., & Lubar, J. F. (1984). Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback and Self-Regulation, 9, 1-23. https://doi.org/10.1007/bf00998842
Lubar, J. F., Swartwood, M. O., Swartwood, J. N., & O'Donnell, P. (1995). Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in TOVA scores, behavioral ratings, and WISC-R performance. Biofeedback and Self-Regulation, 20, 83-99. https://doi.org/10.1007/bf01712768
Mann, C., Lubar, J., Zimmerman, A., Miller, C., & Nuenchen, R. (1992). Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatric Neurology, 8, 30-36. https://doi.org/10.1016/0887-8994(92)90049-5
Marson, A., & Ramaratnam, S. (2003). Epilepsy. Clinical Evidence, (9), 1403-1420. PMID: 15366192
Mash, E. J., & Wolfe, D. A. (2010). Abnormal child psychology (4th ed.). Wadsworth/Cengage Learning.
McGinty, E. E., & Barry, C. L. (2020). Stigma reduction to combat the addiction crisis—Developing an evidence base. The New England Journal of Medicine, 382, 1291–1292. https://doi.org/10.1056/NEJMp2000227
McGrady, A., & Moss, D. (2013). Pathways to illness, pathways to health. Springer.
Meehan, Z. M., Shaffer, F., & Zerr, C. L. (2023). Depression. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Meisel, V., Servera, M., Garcia-Banda, G., Cardo, E., & Moreno, I. (2013). Neurofeedback and standard pharmacological intervention in ADHD: A randomized controlled trial with six-month follow-up. Biol Psychol, 94(1), 12-21. https://doi.org/10.1016/j.biopsycho.2013.04.015
Michael, A. J., Krishnaswamy, S., Mohamed, J. (2008). An open label study of the use of EEG biofeedback using beta training to reduce anxiety for patients with cardiac events. Neuropsychiatr Dis Treat, 1(4), 357-63. PMCID: PMC2424123
Mikosch, P., Hadrawa, T., Laubreiter, K., Brandl, J., Pilz, J., Stettner, H., & Grimm, G. (2010). Effectiveness of respiratory-sinus-arrhythmia biofeedback on state anxiety in patients undergoing coronary angiography. Journal of Advanced Nursing, 66(5), 1101-1110. https://doi.org/10.1111/j.1365-2648.2010.05277.x
Monastra, V. J., Lynn, S., Linden, M., Lubar, J. F., Gruzelier, J., & LaVaque, T. J. (2005). Electroencephalographic biofeedback in the treatment of Attention Deficit/Hyperactivity Disorder. Applied Psychophysiology and Biofeedback, 30(2), 95-114. https://doi.org/10.1007/s10484-005-4305-x
Monastra, V. J., Monastra, D. M., & George S. (2002). The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of Attention-Deficit/Hyperactivity Disorder. Applied Psychophysiology and Biofeedback, 27(4), 231-249. https://doi.org/10.1023/a:1021018700609
Moss, D., Shaffer, F., & Watkins, M. (2023). Posttraumatic Stress Disorder (PTSD). In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Moss, D., & Watkins, M. (2023). Anxiety and anxiety disorders. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
National Survey on Drug Use and Health (NSDUH). Retrieved from https://www.samhsa.gov/data/release/2020-national-survey-drug-use-and-health-nsduh-releases
Paul, M., & Garg, K. (2012). The effect of heart rate variability biofeedback on performance psychology of basketball players. Applied Psychophysiology and Biofeedback, 37, 131-144. https://doi.org/10.1007/s10484-012-9185-2
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13(2), 271-279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy, 3, 37-55.
Peniston, E. G., Marrinan, D. A., Deming, W. A., & Kulkosky, P. J. (1993). EEG alpha-theta brainwave synchronization in Vietnam theater veterans with combat-related post-traumatic stress disorder and alcohol abuse. Advances in Medical Psychotherapy, 6, 37-50.
Pigott, H. E., De Biase, L., Bodenhamer-Davis, E., & Davis, R. E. (2013). The evidence-base for neurofeedback as a reimbursable healthcare service to treat Attention Deficit/Hyperactivity Disorder.
Ramaratnam, S., Baker, G. A., & Goldstein, L. (2001). Psychological treatments for epilepsy. Cochrane Database of Systematic Reviews Online Update Software, 4, CD002029. Ramaratnam, S., Baker, G. A., & Goldstein, L. H. (2008). Psychological treatments for epilepsy. Cochrane Database Syst Rev, (3), CD002029. https://doi.org/10.1002/14651858.cd002029
Ramos, F. (1998). Frequency band interaction in ADD/ADHD neurotherapy. Journal of Neurotherapy, 3(1), 26-41. https://doi.org/10.1300/J184v03n01_04
Ratanasiripong, P., Sverduk, K., Prince, J., & Hayashino, D. (2012). Biofeedback and Counseling for stress and anxiety among college students. Journal of College Student Development, 53(5), 742-749. http://dx.doi.org/10.1353/csd.2012.0070
Raymond, J., Sajid, I., Parkinson, L. A., & Gruzelier, J. H. (2005). Biofeedback and dance performance: A preliminary investigation. Applied Psychophysiology and Biofeedback, 30(1), 64-73. https://doi.org/10.1007/s10484-005-2175-x
Reiner, R. (2008). Integrating a portable biofeedback device into clinical practice for patients with anxiety disorders: Results of a pilot study. Applied Psychophysiology and Biofeedback, 33(1), 55-61. https://doi.org/10.1007/s10484-007-9046-6
Rice, K. M., Blanchard, E. B., & Purcell, M. (1993). Biofeedback treatments of generalized anxiety disorder: Preliminary results. Biofeedback and Self-Regulation, 18(2), 93-105. https://doi.org/10.1007/bf01848110
Rockstroh, B., Elbert, T., Birbaumer, N., Wolf, P., Düchting-Röth, A., Reker, M., Daum, I., Lutzenberger, W., & Dichgans, J. (1993). Cortical self-regulation in patients with epilepsies. Epilepsy Research, 14, 63-72. https://doi.org/10.1016/0920-1211(93)90075-i
Rosenfeld, J. P. (2000). An EEG Biofeedback protocol for affective disorders. Clinical Electroencephalography, 31(1), 7-12. https://doi.org/10.1177/155005940003100106
Rosenfeld, J. P., Baehr, E., Baehr, R., Gotlib, I. H., & Ranganath, C. (1996). Preliminary evidence that daily changes in frontal alpha asymmetry correlate with changes in affect in therapy sessions. International Journal of Psychophysiology, 23, 137-141. https://doi.org/10.1016/0167-8760(96)00037-2
Rossiter, T. R. (1998). Patient-directed neurofeedback for AD/HD. Journal of Neurotherapy, 2(4), 54-63. https://doi.org/10.1300/J184v02n04_04
Rossiter, T. R., & La Vaque, T. J. (1995). A comparison of EEG biofeedback and psychostimulants in treating attention deficit/hyperactivity disorders. Journal of Neurotherapy, 1(1), 48-59. https://doi.org/10.1300/J184v01n01_07
Rota, G., Sitaram, R.,Veit, R., Erb, M., Weiskopf, M., Dogil, G., & Birbaumer, N. (2009). Self-regulation of regional cortical activity using real-time fMRI: The right inferior frontal gyrus and linguistic processing. Hum Brain Mapp, 30(5), 1605-1614. https://doi.org/10.1002/hbm.20621
Saxby, E., & Peniston, E. G. (1995). Alpha-theta brainwave neurofeedback training: An effective treatment for male and female alcoholics with depressive symptoms. Journal of Clinical Psychology, 51(5), 685-693. https://doi.org/10.1002/1097-4679(199509)51:5<685::aid-jclp2270510514>3.0.co;2-k
Schneider, F., Elbert, T., Heimann, H., Welker, A., Stetter, F., Mattes, R., Birbaumer, N., & Mann, K. (1993). Self-regulation of slow cortical potentials in psychiatric patients: Alcohol dependency. Biofeedback and Self-Regulation, 18(1), 23-32. https://doi.org/10.1007/bf00999511
Scott, W., & Kaiser, D. (1998). Augmenting chemical dependency treatment with neurofeedback training. Journal of Neurotherapy, 3(1), 66.
Scott, W. C., Kaiser, D., Othmer, S., & Sideroff, S. I. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455-469. https://doi.org/10.1081/ada-200056807
Schwartz, A., & Cohen, S. (March 31, 2013). A.D.H.D. see in 11% of U.S. children as diagnoses rise. New York Times.
Shaffer, F., & Mannion, M. (2016). Tinnitus. In G. Tan, F. Shaffer, R. R. Lyle, & I. Teo (Eds.). Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Sherlin, L., Arns, M., Lubar, J., & Sokhadze, E. (2010). A position paper on neurofeedback for the treatment of ADHD. Journal of Neurotherapy, 14(2), 66-78. https://doi.org/10.1080/10874201003773880
Sheth, R. D., Stafstrom, C. E., & Hsu, D. (2005). Nonpharmacological treatment options for epilepsy. Seminars in Pediatric Neurology, 12(2), 106-113. https://doi.org/10.1016/j.spen.2005.03.005
Sokhadze, T. M., Cannon, R. L., & Trudeau, D. L. (2008). EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Applied Psychophysiology and Biofeedback, 33(1), 1-28. https://dx.doi.org/10.1007%2Fs10484-007-9047-5
Sokhadze, E. M., & Trudeau, D. (2023). Alcohol and drug dependence. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Spencer, T. J., Biederman, J., & Mick, E. (2007). Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. Ambulatory Pediatrics, 7, 73–81. https://doi.org/10.1016/j.ambp.2006.07.006
Steiner, N. J., Frenette, E. C., Rene, K. M., Brennan, R. T., & Perrin, E. C. (2014). In-School neurofeedback training for ADHD: Sustained improvements from a randomized control trial. Pediatrics. https://doi.org/10.1542/peds.2013-2059
Sterman, M. B. (1973). Neurophysiological and clinical studies of sensorimotor EEG biofeedback training: Some effects on epilepsy. L. Birk (Ed.), Biofeedback: Behavioral Medicine. Grune & Stratton, pp. 147-165.
Sterman, M. B. (1973). Neurophysiological and clinical studies of sensorimotor EEG biofeedback training: Some effects on epilepsy. Seminars in Psychiatry, 5(4), 507-525. PMID: 4770578
Sterman, M. B. (1977). Sensorimotor EEG operant conditioning: Experimental and clinical effects. Pavlovian Journal of Biological Sciences, 12(2), 63-92. https://doi.org/10.1007/bf03004496
Sterman, M. B. (1986). Epilepsy and its treatment with EEG feedback therapy. Annals of Behavioral Medicine, 8, 21-25. https://doi.org/10.1207/s15324796abm0801_4
Sterman, M. B. (1997). The challenge of EEG biofeedback in the treatment of epilepsy: A view from the trenches. Biofeedback, 25(1), 6-7, 20-21, 23.
Sterman, M. B. (2000). Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clinical Electroencephalography, 31(1), 45-55. https://doi.org/10.1177/155005940003100111
Sterman, M. B., & Egner, T. (2006). Foundation and practice of neurofeedback for the treatment of epilepsy. Applied Psychophysiology and Biofeedback, 31(1), 21-35. https://doi.org/10.1007/s10484-006-9002-x
Sterman, M. B., & Friar, L. (1972). Suppression of seizures in epileptics following sensorimotor EEG feedback training. Electroencephalography & Clinical Neurophysiology, 33, 89-95. https://doi.org/10.1016/0013-4694(72)90028-4
Sterman, M. B., & Lantz, D. (2001). Changes in lateralized memory performance in subjects with epilepsy following neurofeedback training. Journal of Neurotherapy, 5, 63-72. https://doi.org/10.1300/J184v05n01_06
Sterman, M. B., & Macdonald, L. R. (1978). Effects of central cortical EEG feedback training on incidence of poorly controlled seizures. Epilepsia, 19(3), 207-222. https://doi.org/10.1111/j.1528-1157.1978.tb04483.x
Sterman, M. B., Macdonald, L. R., & Stone, R. K. (1974). Biofeedback training of the sensorimotor electroencephalogram rhythm in man: Effects on epilepsy. Epilepsia, 15(3), 395-416. https://doi.org/10.1111/j.1528-1157.1974.tb04016.x
Sterman, M. B., & Shouse, M. N. (1980). Quantitative analysis of training, sleep EEG and clinical response to EEG operant conditioning in epileptics. Electroencephalography & Clinical Neurophysiology, 49, 558-576. https://doi.org/10.1016/0013-4694(80)90397-1
Strack, B., & Gevirtz, R. (2011). Getting to the heart of the matter: Heart rate variability biofeedback for enhanced performance. In B. Strack, M. Linden & V. Wilson (Eds.), Biofeedback and neurofeedback applications in sport psychology (pp. 145-174). Association for Applied Psychophysiology and Biofeedback.
Strehl, U., Kotchoubey, B., Trevorrow, T., & Birbaumer, N. (2005). Predictors of seizure reduction after self-regulation of slow cortical potentials as a treatment of drug-resistant epilepsy. Epilepsy & Behavior, 6, 156-166. https://doi.org/10.1016/j.yebeh.2004.11.004
Strehl, U., Leins, U., Groth, G., Klinger, C., Hinterberger, T., & Birbaumer, N. (2006). Self-regulation of slow cortical potentials: A new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics, 118(5): e1530-40. https://doi.org/10.1542/peds.2005-2478
Tan, G., Thornby, J., Hammond, D. C., Strehl, U., Canady, B., Arnemann, K., & Kaiser, D. A. (2009). Meta-analysis of EEG biofeedback in treating epilepsy. Clinical EEG and Neuroscience, 40(3), 173–179.
Taub, E., Steiner, S. S., Weingarten, E., & Walton, K. G. (1994). Effectiveness of broad spectrum approaches to relapse prevention in severe alcoholism: A long-term, randomized, controlled trial of Transcendental Meditation, EMG biofeedback and electronic neurotherapy. Alcoholism Treatment Quarterly, 11(1-2), 187-220. https://doi.org/10.1300/J020v11n01_06
Thompson, L., & Thompson, M. (1998). Neurofeedback combined with training in metacognitive strategies: Effectiveness in students with ADD. Applied Psychophysiology and Biofeedback, 23(4), 243-263. https://doi.org/10.1023/a:1022213731956
Thompson, W. (2014). Alcoholism. eMedicine.
Thornton, K. (2000). Improvement/rehabilitation of memory functioning with neurotherapy/QEEG biofeedback. Journal of Head Trauma Rehabilitation, 15(6), 1285-1296. https://doi.org/10.1097/00001199-200012000-00008
Thornton, K. E., & Carmody, D. P. (2005). Electroencephalogram biofeedback for reading disability and traumatic brain injury. Child and Adolescent Psychiatric Clinics of North America, 14(1), 137-162. https://doi.org/10.1016/j.chc.2004.07.001
Thornton, K., & Carmody, D. (2013). The relation between memory improvement and QEEG changes in three clinical groups as a result of EEG biofeedback treatment. Journal of Neurotherapy, 17(2), 116-131. doi:10.1080/10874208.2013.785183
Thurber, M. R. (2006). Effects of heart-rate variability biofeedback training and emotional regulation on music performance anxiety in university students. University of North Texas Press.
Tinius, T. P., & Tinius, K. A. (2000). Changes after EEG biofeedback and cognitive retraining in adults with mild traumatic brain injury and attention deficit hyperactivity disorder. Journal of Neurotherapy, 4(2), 27-44. https://doi.org/10.1300/J184v04n02_05
Vanathy, S., Sharma, P. S. V. N., & Kumar, K. B. (1998). The efficacy of alpha and theta neurofeedback training in treatment of generalized anxiety disorder. Indian Journal of Clinical Psychology, 25(2), 136-143.
Vinas, F. C. (2013). Penetrating head trauma. eMedicine.
Volkow, N. D., & McLellan, A. T. (2016). Opioid abuse in chronic pain--Misconceptions and mitigation strategies. The New England Journal of Medicine, 374(13), 1253–1263. https://doi.org/10.1056/NEJMra1507771
Wadhwani, S., Radvanski, D. C., & Carmody, D. P. (1998). Neurofeedback training in a case of attention deficit hyperactivity disorder. Journal of Neurotherapy, 3(1), 42-49.
Waldkoetter, R. O., & Sanders, G. O. (1997). Auditory brainwave stimulation in treating alcoholic depression. Perceptual and Motor Skills, 84(1), 226. https://doi.org/10.2466/pms.1997.84.1.226
Walker, J. E., Norman, C. A., & Weber, R. K. (2002). Impact of qEEG-guided coherence training for patients with a mild closed head injury. Journal of Neurotherapy, 6(2), 31-43.
Yates, W. R. (2014). Anxiety disorders. eMedicine.
Zimmerman, M., Chelminksi, I., & McDermut, W. (2002). Major depressive disorder and Axis I diagnostic comorbidity. Journal of Clinical Psychiatry, 63, 187-193. https://doi.org/10.4088/jcp.v63n0303

BCIA Blueprint Coverage
This unit addresses IV. Research Evidence Base for Neurofeedback - B. Key Research Studies.

This unit covers Attention Deficit Hyperactivity Disorder, Mild Closed Head Injuries and Traumatic Brain Injury, Substance Use Disorder, Epilepsy, Anxiety and Anxiety Disorders, Post-Traumatic Stress Disorder, Depression, and Tinnitus.
Please click on the podcast icon below to hear a full-length lecture.

EVIDENCE-BASED PRACTICE (4TH ED.)
We have updated the efficacy ratings for clinical applications covered in AAPB's Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.).

Attention Deficit Hyperactivity Disorder (ADHD)
DSM-5 revised the diagnostic criteria for attention deficit hyperactivity disorder (ADHD) by providing examples of symptom presentation, increasing the age range for symptom onset, and reducing the required number of symptoms for adolescents and adults (Brown, 2013). Graphic © Oksana Mizina/Shutterstock.com.

Demographics
The Centers for Disease Control and Prevention (Schwartz & Cohen, 2013) estimates that 11% of school-aged children and almost 20% of high school boys are medically diagnosed with ADHD in the US.Boys are diagnosed with ADHD 4-5 times more often than girls (Costello et al., 2003). Their parents are more likely to pursue treatment due to their more severe symptoms or greater degree of impairment (Beidel et al., 2014). Girls are more likely to show inattention and are less likely to be diagnosed with a learning disability, comorbid depression, oppositional defiant disorder, or conduct disorder than their male classmates (Biederman et al., 2002; Spencer et al., 2007).
Almost half of the children diagnosed with ADHD exhibit difficulty learning, communicating, and interacting with their classmates. Roughly 80% misbehave, frequently very seriously (Goldstein, 2011; Mash & Wolfe, 2010).
Neurofeedback Studies
Theta/beta ratio (TBR), SMR, and SCP interventions have been widely investigated using randomized controlled trials. The aim of theta/beta training is to down-train theta and up-train beta amplitude. SMR training attempts to increase SMR amplitude. SCP training seeks to increase the amplitude of positive SCPs.Lubar, Swartwood, Swartwood, and O'Donnell (1995) reported that training to reduce slow EEG activity increased WISC-R and Test of Variables of Attention (TOVA) scores. Full-scale WISC-R scores increased about 12 points. The increase in TOVA scores correlated with decreased slow EEG activity.
Lubar (1995) followed 52 patients treated with NF for as long as 10 years. Their improvement on the Connors scale, used to measure attention, remained stable at follow-up.

Rossiter and La Vaque (1995) matched and randomly assigned 46 participants to either Ritalin or NF. Both groups improved on TOVA measures of inattention, impulsivity, information processing, and response variability.
Linden, Habib, and Radojevic's (1996) controlled study of 18 children demonstrated that NF to increase beta and suppress theta activity increased intelligence scores and reduced inattention rated by their parents compared to a wait-list control group.
Thompson and Thompson (1998) reported the successful treatment of 98 children and 13 adults over 40 50-minute sessions using Lubar's ADHD protocol. The percentage of children using Ritalin declined from 30% at the start of the study to 6% post-treatment. Theta/beta ratios significantly declined for children but not for adults. Study participants achieved impressive pre-treatment to post-treatment gains on intelligence, TOVA, and Wide Range Achievement Test scores. Lynda and Michael Thompson are pictured below.

Case studies by Ramos (1998) and Wadhwani, Radvanski, and Carmody (1998) support the efficacy of NF for ADD and ADHD.
Camp (1999) reported that theta suppression biofeedback training compared favorably with cognitive behavior modification, based on pre-treatment to post-treatment changes in 48 children on TOVA, parent and teacher ratings, and ADHD scales.
The Kaiser and Othmer (2000) multi-center study involved 1,089 patients aged 5 to 67 years. It demonstrated that SMR-beta NF training produced significant gains on TOVA measures of attentiveness, impulse control, and response variability.
Carmody and colleagues (2001) randomly assigned 16 children (ages 8-10) to either EEG biofeedback or a wait-list control condition. Eight of the 16 children were diagnosed with ADHD, and 8 had received no diagnosis of any disorder. In the EEG biofeedback condition, participants received 3-4 weekly sessions of EEG biofeedback (using a synthesis of Protocols 1 and 3) for 6 months and completed 36-48 sessions.
The children diagnosed with ADHD who received EEG biofeedback decreased impulsivity as measured by the TOVA, and their teachers' ratings of attentiveness on the School Version of the Attention Deficit Disorders Evaluation Scale (ADDES) improved. Selected qEEG measures did not consistently validate improvement by participants in the EEG biofeedback condition.
Monastra, Monastra, and George (2002) compared 49 children diagnosed with ADHD who participated in 1-year multimodal program (Ritalin, parent counseling, and academic consultation) with 51 children who participated in the multimodal program combined with NF (weekly 30 to 40-min sessions using the Lubar protocol with a cash reward for increased frontal cortical arousal).
Both groups significantly improved performance on TOVA and the Attention Deficit Disorders Evaluation Scale when medicated with Ritalin, but only the group that received NF maintained performance gains when unmedicated. A qEEG scan only showed reduced cortical slowing in children who received NF. Parenting style moderated behavioral symptoms at home but not in the classroom. Vincent Monastra is pictured below.

Fuchs, Birbaumer, Lutzenberger, Gruzelier, and Kaiser (2003) compared the efficacy of 3 months of sensorimotor rhythm (12-15 Hz) and beta1 (15-18 Hz) NF against methylphenidate in 46 ADHD children. The children were assigned to the NF (22) and medication (12) based on their parents' preference (the assignment was nonrandom). Both treatment groups improved on all TOVA subscales and speed and accuracy on the d2 Attention Endurance Test. Teacher and parent ratings of ADHD behaviors on the IOWA-Conners Behavior Rating Scale also improved for both groups.
Monastra and colleagues (2005) assigned a more conservative rating of probably efficacious for EEG biofeedback for ADHD in an AAPB White Paper. Despite significant improvement in about 75% of patients in the published studies they examined, the authors concluded that more randomized, controlled group studies that control for therapist and patient characteristics are needed to calculate the percentage of patients diagnosed with ADHD who will achieve these gains in typical clinical settings.
NF appears to be superior to no treatment and equivalent to stimulant medication. Patients require at least 20 sessions and as many as 50 sessions to produce clinical improvement.
Gevensleben and colleagues (2009) conducted a multisite randomized controlled study of 102 children diagnosed with ADHD using NF training that combined blocks of theta/beta and slow cortical potential NF and computer-based attention skills training control. The combined NF group was superior to the control group on parent and teacher ratings, and both NF protocols produced comparable changes. These gains were maintained at a 6-month follow-up (Gevensleben et al., 2010).
Sherlin, Arns, Lubar, and Sokhadze (2010) argued that NF for ADHD is safe, should be reclassified as level 5: efficacious and specific, produces long-term effects that last from 3-6 months, and may produce clinical results like stimulant medications. While NF effectively treats inattention and impulsivity, they suggested that medication may be more appropriate when the primary symptom is hyperactivity and that NF may be successfully combined with medication. Leslie Sherlin is pictured below.

Duric and colleagues (2012) conducted a randomized controlled trial of 91 children and adolescents diagnosed with ADHD assigned to 30 NF sessions, methylphenidate, or NF with methylphenidate. Parent ratings of core ADHD symptoms improved for all three groups, and there were no group differences. NF achieved equal efficacy to methylphenidate.
The American Academy of Pediatrics (2012) rated biofeedback for child and adolescent attention and hyperactivity behaviors Level 1- Best support.
Pigott, De Biase, Bodenhamer-Davis, and Davis (2013) provided a comprehensive review of NF efficacy for ADHD and persuasively argued that it should receive a level-5 classification. The authors emphasized that compared with stimulant medication, only NF has demonstrated effectiveness at 2-year follow-up.
Meisel and colleagues (2013) reported a randomized controlled trial of 23 children diagnosed with ADHD who either completed 40 theta/beta NF sessions or received methylphenidate. While both groups improved on parent and teacher ratings of functioning and core ADHD symptoms, only the NF group improved on academic performance at 6-month follow-up.
In their review of recent studies, Arns and Strehl (2013) concluded that in randomized controlled trials where theta/beta or slow cortical potential NF were active treatments and either cognitive training or EMG biofeedback were controls, NF produced significant improvements in teacher ratings.
Steiner and colleagues (2014) conducted a randomized controlled study of 104 children assigned to either NF, cognitive training (CT), or control conditions. At a 6-months follow-up, the NF group sustained greater gains on the Conners 3-P, Executive Functioning, Hyperactivity/Impulsivity, and Behavior Rating Inventory of Executive Function (BRIEF) subscales than the CT or control groups. Moreover, the NF group maintained their stimulant dosage while the CT and control groups increased their dosage.
Below is a NeXus-10 ® BioTrace+ caterpillar game. The three caterpillars represent the theta, SMR, and beta frequency bands.
Clinical Efficacy
Based on six RCTs, Stefanie Enriquez-Geppert and colleagues rated NFB for ADHD as efficacious and specific in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.).NFB interventions included training to decrease the theta/beta ratio and increase sensorimotor rhythm (SMR) activity or slow cortical potential (SCP) activity.
NFB training resulted in post-treatment gains in ADHD symptoms, including the Attention Deficit Disorders Evaluation Scale (ADDES). Participants also improved on intelligence, TOVA measures of attentiveness, impulse control, and response variability, and Wide Range Achievement Test scores.
Mild Closed Head Injuries and Traumatic Brain Injury (TBI)
Traumatic brain injury (TBI) results when an external force produces intracranial injury through acceleration or direct impact. Mild TBI symptoms that NF may treat include deficits in memory, attention, and decision-making (National Institute of Neurological Disorders and Stroke, 2008). Check out Siddharthan Chandran's TED Talk Can the Damaged Brain Repair Itself?
Demographics
There are about 1.9 million cases of skull fracture or intracranial injury in the United States each year. In 1992, firearms caused the most deaths from TBI (Vinas, 2013). Falls account for 28%, motor vehicle accidents 20%, injury by an object 19%, and violence 11% of TBI (Langlois, 2006). Graphic © ESB Professional/Shutterstock.com.
Neurofeedback Studies
Ayers (1995) reported treating 32 level-two coma patients, who were comatose for more than 2 months, noninvasively with NF. There is a generalized response where patients move aimlessly and inconsistently in a level-two coma on the Rancho Los Amigos Cognitive Scale. If they open their eyes, patients do not focus on objects.Twenty-five of 32 patients emerged from their comas after 1-6 treatments. NF for coma involved inhibiting 4-7 Hz activity and reinforcing the replacement of 4-7 Hz with 15-18 Hz activity.
Ayers and colleagues started NF for open head trauma at the somatosensory cortex. They trained these patients to decrease 4-7 Hz activity and increase 15-18 Hz activity.
Thornton (2000) reported that NF improves the memory of TBI patients. Tinius and Tinius (2000) found that it improves attention, problem-solving, and task performance. Keller's (2001) controlled study showed that beta training significantly improved attention compared to matched controls. Another controlled study by Schoenberger, Shif, Esty, Ochs, and Matheis (2001) showed that NF enhanced cognitive function and self-reported depression and fatigue.
Walker, Norman, and Weber (2002) reported that 88% of mild TBI patients achieved over 50% improvement in EEG coherence. All patients who had been previously employed resumed work after completing their training.
Thornton and Carmody (2013) reported auditory and visual memory gains in 15 TBI patients who received NF training for qEEG power and connectivity in an uncontrolled pre-test/post-test study.
Clinical Efficacy
Anne Ward Stevens and Kori Trotter rated NFB for concussion as probably efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.).HRV was the only BFB modality. NFB training involved operant conditioning utilizing real-time normative database comparisons (z-score training) and training several measures concurrently (e.g., inhibiting 4-7 Hz and rewarding 15-18 Hz activity). EEG tomography through Low-Resolution Electromagnetic Tomography Analysis (LORETA) offered greater training specificity and TBI treatment customization.
Participants reduced medication, the number of symptoms, symptom severity, and anxiety. They improved on attention, cognitive function, memory, and tandem gait time (walking in a straight line by placing one foot directly in front of the other, heel-to-toe).
Substance Use Disorders
Koob (2006) defined drug addiction as a: “Chronically relapsing disorder that is characterized by a compulsion to seek and take a drug, loss of control in limiting intake and the emergence of a negative emotional state (e.g., dysphoria, anxiety, irritability) when access to the drug is prevented (here, defined as the ‘dark side’ of addiction).”
In DSM-5, substance use disorders (SUDs) are seen as behavioral disorders consisting of three elements:
1. Loss of control (e.g., compulsive use)
2. Continued intake despite serious adverse consequences
3. Preoccupation with obtaining, using, and recovering from the substance (Julien et al., 2023)
Graphic © Jan H Anderson/Shutterstock.com.

Alcohol is another drug of abuse that, like opioids, can threaten health and safety. Graphic © medicalstocks/Shutterstock.com.

Demographics
The U.S. National Longitudinal Alcohol Epidemiologic Study estimated that the lifetime prevalence for adult alcoholism ranges between 13.5-23.5%. In the past year, 7.5-9.5% of adults experienced alcohol abuse and dependency. Twenty percent of hospitalized adults are alcoholics. The CDC estimates that over 38 million adults engage in binge drinking four times a month (Thompson, 2014).Neurofeedback Protocols
Researchers often observe deficient slow-wave activity (delta, theta, and alpha) and excessive beta activity in alcoholics. Alcohol consumption slows their alpha frequency and increases its amplitude (Peniston & Kulkosky, 1990). The Menninger Clinic ON-OFF-ON training and alpha-theta protocol provided components of the Peniston Protocol, which has been effectively used to treat alcoholism.The Menninger ON-OFF-ON EEG protocol teaches a patient to increase the amplitude within a frequency band, reduce the amplitude, and then increase the amplitude again during 100- or 200- second segments. For example, a patient may increase 8-13 Hz alpha activity for 100 s, decrease it for 100 s, and then increase it for 100 seconds. This approach may produce superior control compared to procedures that only train alpha or theta increase (Norris, 1988).
The Menninger alpha-theta protocol places the active electrode 1 centimeter above and left of the inion (the bony prominence located on the back of the head) with a reference on the left earlobe. This protocol teaches EEG control using 100- or 200-seconds “ON-OFF-ON” exercises.
Temperature and frontal SEMG biofeedback precede alpha-theta training. Patients receive 3-4 weekly sessions of temperature biofeedback followed by 3-4 weekly sessions of frontal SEMG biofeedback. Judy Green has likened the temperature and SEMG biofeedback sessions to settling elementary school students in their seats so they can pay attention without distraction. These sessions also teach patients the strategy of passive volition (allowing), which is critical to alpha-theta training.
The patient then receives 10-12 bi-weekly sessions of alpha-theta biofeedback using ON-OFF-ON exercises. Training attempts to gradually slow the EEG until the patient can increase alpha and theta amplitude without falling asleep.
Egner, Strawson, and Gruzelier (2002) addressed whether the effects of alpha-theta NF depend on with-session EEG changes or are non-specific and shared with other relaxation procedures. They compared the effect of contingent and noncontingent alpha-theta NF on theta/alpha ratios within and across sessions.
The contingent group achieved increased within-session theta/alpha ratios, while the noncontingent group did not. The contingent group also achieved higher theta/alpha ratios than the noncontingent group during some training sessions. There were no group differences in subjective reports of activation since both groups reported significantly lower activation following training. Both contingent and noncontingent NF were relaxing. This study validated the assumption underlying alpha-theta NF that accurate feedback results in higher within-session theta/alpha ratios than does noncontingent NF.
The Peniston addiction protocol (1989) is a multimodal approach that incorporates biofeedback and non-biofeedback components. Patients start with visualization training, receive 6 temperature biofeedback sessions, learn rhythmic breathing techniques, participate in autogenic training exercises, learn to construct personalized imagery, and experience guided imagery (where the therapist directs the patient visualization). This training prepares patients for 30 alpha-theta sessions in which they learn to slow the EEG to increase alpha and theta amplitude using the Menninger alpha-theta training procedure. Following the alpha-theta biofeedback sessions, the supervising physician may need to adjust medication.
Experimental patients who received Peniston and Kulkosky’s alpha-theta protocol for alcoholism and control patients were assessed over a 24-month follow-up period. Across this period, 8 of the 10 experimental patients and none of the 10 controls maintained abstinence from alcohol.
Due to the Peniston-Kulkosky addiction protocol's multimodal nature, we cannot identify the components responsible for clinical improvement. Further clinical research that "dismantles" this protocol will be required to isolate the active treatment components.
Kaiser and Scott (Kaiser et al., 1999; Scott et al., 2002, 2005) modified the Peniston protocol to increase its success with patients dependent on cannabis and stimulants. The Kaiser-Scott protocol, which starts with NF ADHD training and then progresses to the Peniston protocol, has improved retention and abstinence in these hard-to-treat populations.
This video takes the viewer through an alpha-theta training demonstration using the Nexus/Biotrace system (Mind Media, Roermond, The Netherlands) using the training approach developed by John Anderson. The demonstration uses saved session data to illustrate the functions and discuss the threshold settings and training concepts.
Neurofeedback Studies
Peniston and Kulkosky (1990) reported that patients who received alpha-theta NF achieved significantly greater decreases on Millon Clinical Multiaxial Inventory factors than those who received conventional medical treatment. Alcoholics who received alpha-theta NF improved on schizoid, avoidant, passive-aggressive, schizotypal, borderline, paranoid, anxiety, somatoform, dysthymic, alcohol abuse, psychotic thinking, psychotic depression, and psychotic delusional factors.Schneider et al. (1993) reported that 6 of 10 male alcoholics remained abstinent 4 months after completing slow cortical potential NF.
Taub and colleagues (1994) randomly assigned 118 chronic alcoholics to one of four treatments: Alcoholics Anonymous and counseling (RTT), RTT combined with Transcendental Meditation, RTT combined with SEMG biofeedback, or RTT plus sham neurotherapy. The sham NF condition involved "electrocranial stimulation" and not alpha-theta biofeedback. Rates of self-reported abstinence were 25%, 65%, 55%, and 28%, respectively. While the addition of Transcendental Meditation and EMG biofeedback seemed to increase abstinence, sham neurotherapy did not. The addition of Transcendental Meditation or EMG biofeedback to RTT produced abstinence rates comparable to those reported for the addition of alpha-theta biofeedback.
In a controlled study, Saxby and Peniston (1995) demonstrated that alpha-theta NF could reduce depression in alcoholics and increase the rate of abstinence assessed over a 21-month follow-up period.
Kelley’s (1997) 3-year follow-up study of 20 Native American alcoholic inpatients reported the following changes: increased EEG synchrony and alpha-theta amplitudes, extinction of drinking behavior, less personally damaging behavior (81%), and lower Beck Depression Inventory scores.
Burkett and colleagues (2003) conducted an open-label uncontrolled study of the Scott-Kaiser modification of the Peniston protocol with 270 male homeless patients addicted to crack cocaine who also received faith-based interventions within a residential setting. At 1-year follow-up, data from 94 patients who completed treatment showed abstinence from alcohol or other drugs in 53.2% and drug use only 1-3 times in 23.4%, corroborated by urinalysis. A follow-up report on 87 patients who completed treatment documented improvement on urinalysis, length of residential stay, and self-reported depression (Burkett et al., 2005).
Bodenhamer-Davis and Calloway (2004) conducted an uncontrolled trial of 16 outpatients diagnosed with chemical dependency in which they administered alpha-theta NF. At 74- to 98-month follow-up,
81.3% were abstinent, and rates for re-arrest and loss of probation were lower than for a comparison group.
Scott, Kaiser, Othmer, and Sideroff (2005) randomly assigned patients with mixed substance abuse to EEG biofeedback or a control group. The EEG biofeedback group stayed in treatment longer than the control group. Seventy-seven percent of patients who completed EEG training remained abstinent at 12 months compared with 44% of controls.
Sokhadze, Cannon, and Trudeau (2008) found that alpha-theta training for alcoholism and a combination of alpha-theta training, beta training for polydrug abuse (including stimulants), and residential treatment were probably effective.
Clinical Efficacy
Estate M. Sokhadze and David Trudeau rated NFB for SUD as probably efficacious based on an RCT (N = 121) using the Scott–Kaiser NFB protocol.The five other RCTs incorporated alpha and high beta regulation, alpha/theta with TEMP and guided imagery, SMR and beta upregulation with 1-13 Hz and high beta (18-22 Hz) suppression, SMR/theta followed by alpha-theta, and the Scott–Kaiser NFB protocol.
The Scott-Kaiser protocol, which starts with NF ADHD training and then progresses to the Peniston protocol, has improved retention and abstinence in these hard-to-treat populations. Participants increased abstinence, quality of life, self-efficacy, time in the program, and TOVA (continuous attention), and reduced addiction severity and craving.
The Scott-Kaiser modification of the Peniston Protocol can be classified as probably efficacious with residential or office-based rehabilitation and opioid replacement for alcohol, opioid, mixed-substance, and stimulant abusers.
Alpha-theta NF received a level-2 rating of possibly efficacious.
Epilepsy
Epilepsy is a family of neurological disorders featuring short, periodic motor, sensory, or cognitive malfunction attacks. Epileptic seizures have been divided into (1) partial or focal seizures, (2) primary generalized seizures, (3) status epilepticus, and (4) recurrence patterns. Check out the Epilepsy Therapy Project video What is Epilepsy? -- Understanding Epilepsy animation. Graphic © Alila Medical Media/Shutterstock.com.

Petit mal seizures feature loss of consciousness without abnormal movement (patient appears to be daydreaming). The patient, typically a child, may suffer hundreds of these seizures daily for periods lasting up to 30 seconds. Check out the Epilepsy Therapy Project video Understanding Partial Seizures.
Tonic-clonic seizures (grand mal seizures) are primary generalized seizures featuring a peculiar cry, loss of consciousness, fall, tonic-clonic convulsions of all extremities, incontinence, and amnesia for the episode. These seizures are diagnosed in fewer than 20% of adult epileptics. Check out the Epilepsy Therapy Project video Understanding Generalized Seizures. Graphic © Natty_Blissful/Shutterstock.com.
 Graphic © Steve Buckley/Shutterstock.com shows a 2-year-old boy with 24-hour EEG electrodes to find the source of his seizures.
Graphic © Steve Buckley/Shutterstock.com shows a 2-year-old boy with 24-hour EEG electrodes to find the source of his seizures.
The sensorimotor cortex is a central cortical area defined by the central sulcus (fissure of Rolando) separating the frontal and parietal lobes. Sterman (1977) recorded the EEG over the left sensorimotor cortex from sites 10% and 30% lateral to the vertex (slightly medial to C3 and T3).
The sensorimotor rhythm (SMR) is an EEG rhythm from 12-14 Hz located over the sensorimotor cortex (central sulcus). This rhythm is associated with inhibition of movement and reduced muscle tone.
Demographics
In the United States, about 2% of adults experience a seizure, and two-thirds of these patients do not have additional episodes. Those who experience two or more seizures are diagnosed with epilepsy, which is frequently idiopathic (no identified cause) or symptomatic (identified cause like a brain tumor). Age of seizure onset provides a guide to its likely cause (Adamolekun, 2013).Neurofeedback Protocols
The two main neurofeedback protocols to treat uncontrolled generalized seizures are sensorimotor rhythm (SMR) up-training and slow cortical potential (SCP) training.SMR Up-training
SMR up-training, which may include inhibition of theta, beta, and epileptiform spikes, is the most frequently used protocol to manage generalized seizures (Tan et al., 2009). The graphic below shows a NeXus-10 SMR display.
For example, Sterman's protocol trains an epileptic patient to increase SMR (12-14 Hz) amplitude and duration while theta (4-7 Hz), beta (20+ Hz), epileptiform spikes, and EMG artifact are suppressed during 36 sessions. The aim is to normalize the waking and sleep EEG with elevated SMR and suppressed theta and beta activity.

SCP Training
SCP training has emerged as an alternative to SMR training for generalized tonic-clonic seizures, especially in Europe. SCPs consist of positive and negative event-related EEG waveforms that last several seconds (slower than 1 Hz). Positive SCPs are associated with cortical hyperpolarization and inhibited neuronal firing. Conversely, negative shifts reflect cortical depolarization and increased neuronal firing. SCP protocols involve bi-directional training. Clinicians use operant conditioning to teach patients to increase positive shifts and reduce negative shifts to raise neuron firing thresholds and reduce epileptiform activity. This strategy has also been successfully applied to treat migraines. The graphic below shows a NeXus-10 SCP display.
Neurofeedback Studies
Elbert et al. (1991) conducted a double-blind, randomized controlled study of 14 patients with seizures not controlled by medication. They assigned patients to either 28 1-hour sessions of bidirectional SCP or alpha training. At 1-year follow-up, all patients in the SCP group and only 1 in the alpha group reduced seizure frequency.Rockstroh et al. (1993) reported a pre-test/post-test study of 28 1-hour sessions of bi-directional SCP training for 25 uncontrolled epilepsy patients. Follow-up data from 18 participants, who monitored their seizure frequency through training and 1-year follow-up, showed reduced seizure frequency.
Kotchoubey et al. (1996) reported that SCP NF decreased the baseline seizure frequency in drug-resistant epileptics and Kotchoubey et al. (1997) showed that this improvement was maintained 6 months post-treatment.
Kotchoubey, Busch, Strehl, and Birbaumer (1999) concluded that SMR and SCP protocols improve epilepsy control in about 66% of patients. While the mechanism underlying SCP training remains unclear, it may involve increased 6.0-7.9 Hz theta activity during training trials without feedback.
Joy Andrews et al. (2000) found that a NF protocol involving five consecutive days of training enabled 79% of patients to control their seizures.
Sterman (2000) summarized 18 peer-reviewed studies in which 174 patients were trained using his SMR protocol. The outcome data were impressive; 82% clinically improved, reducing seizures by more than 30%. The average seizure reduction exceeded 50%. Many studies found decreased seizure severity. Five percent of patients remained seizure-free for as long as one year. In those studies where researchers recorded pre-treatment and post-treatment EEG amplitudes, 66% normalized their EEG power spectra.
Kotchoubey et al. (2001) treated patients with refractory epilepsy with an anti-epileptic drug and psychosocial counseling, a breathing training control group, or SCP NF in a non-randomized clinical study in which participants selected their treatment. Only the drug and SCP groups significantly reduced seizure frequency.
La Vaque (2003) considers slow cortical potential (SCP) training to be highly effective in controlling "drug-resistant" epilepsy.
Marson and Ramaratnam (2003) reviewed randomized controlled trial studies and reported a significant reduction in median seizure activity in one study.
Sheth, Stafstrom, and Hsu (2005) examined 16 studies of biofeedback treatment of refractory epilepsy, including contingent negative variation (CNV), slow cortical potential (SCP), and galvanic skin response (GSR). While most studies involved 1-8 patients, one enrolled 83 patients. Epilepsy symptoms improved in 82% of patients who received biofeedback training. Both the EEG and GSR treatments produced significant gains.
Strehl and colleagues (2005) reported that 70% of SCP training treatment success with drug-resistant patients could be predicted by initial cortical excitability (negative SCP amplitude), location of epileptic foci (sites that trigger seizures), and personality. Successful patients did not exhibit large negative SCP amplitudes at the start of training, did not have left temporal foci, and reported lower life satisfaction and high reactivity to stressors.
Ramaratnam, Baker, and Goldstein (2005), in a Cochrane Database Systematic Review, challenged the efficacy of NF for epilepsy due to methodological flaws and a small sample size.
A meta-analysis by Tan and colleagues (2009) reviewed nine studies that used SMR and one that used SCP neurofeedback. They found consistent reductions in seizure frequency for drug-resistant patients and successful reduction in seizure frequency by 79% of patients who received SMR training.
Clinical Efficacy
Lauren Frey rated SMR-based and SCP-based NFB as efficacious for seizures, SMR-based NFB as probably efficacious for non-seizure manifestations of epilepsy, and connectivity-based NFB as not empirically supported for seizures.In a meta-analysis, SMR- and SCP-based treatments were associated with fewer seizures and improved quality of life in a RCT.
Anxiety and Anxiety Disorders

Generalized anxiety disorder (GAD) is defined by excessive anxiety and worry most of the time for at least 6 months (Beidel, Bulik, & Stanley, 2014). Chronic overarousal results in fatigue and insomnia, worsened by changes in their circadian rhythm due to their job or travel (McGrady & Moss, 2013).

Patients perceive their worrying as outside their control. They present with physical (muscle tension) and cognitive symptoms (the belief that worrying can prevent an adverse event). They are usually diagnosed with a second disorder (Beidel, Bulik, & Stanley, 2014).
Specific phobia involves significant emotional distress, excessive anxiety, or fear about an object or situation that disrupts everyday performance. DSM-5 lists five specifiers: animal phobias, natural environment phobias, blood/injection/ injury phobias, situational phobias, and other phobias (Beidel, Bulik, & Stanley, 2014).

DSM-5 classifies Post-traumatic stress disorder (PTSD) as one of the Trauma and Stress-Related Disorders. PTSD is a response to a traumatic event like assault, military combat, or rape that may be experienced firsthand or observed (American Psychiatric Association, 2013).
Demographics
Estimated lifetime prevalence rates for anxiety disorders in the United States are panic disorder (2.3-2.7%), generalized anxiety disorder (4.1-6.6%), OCD (2.3-2.6%), PTSD (1-9.3%), and social phobia (2.6-13.3%). The male-to-female ratio for a lifetime anxiety disorder is 3 to 2 (Yates, 2014).Overview
Most controlled, randomized experiments have found that neurofeedback and biofeedback (electrodermal, SEMG, and temperature) produce comparable anxiety reductions to relaxation procedures like meditation and progressive relaxation. Biofeedback and relaxation procedures may achieve equivalent results because anxiety involves disordered attention and cognition in addition to abnormal physiological arousal. In some cases, biofeedback may be superior to relaxation procedures and may produce additive effects when combined with medication.Neurofeedback Studies
Rice, Blanchard, and Purcell (1993) studied 45 patients with generalized anxiety. Thirty-eight of these patients satisfied the DSM-III criteria for Generalized Anxiety Disorder (GAD) and 7 were subclinical for GAD and only met 2 of these criteria. They randomly assigned patients to one of five conditions: frontal EMG biofeedback, EEG biofeedback to increase alpha, EEG biofeedback to decrease alpha, pseudomeditation, or a wait-list control. For the two EEG biofeedback conditions, the electrodes were placed at Oz, the right mastoid process, and the forehead. All four treatment groups received eight 60-minute sessions and achieved significant reductions on STAI-Trait Anxiety scores and Psychosomatic Symptom Checklist (PSC) scores.Only the alpha-increase condition decreased heart rate reactivity to stressors. Subjects in the frontal EMG, alpha-increase, and alpha suppression conditions maintained improvement in STAI-Trait Anxiety and PSC scores at 6 weeks post-treatment. The alpha-increase and alpha-suppression groups showed further improvement in Psychosomatic Symptom Checklist scores at 6 weeks post-treatment.
Vanathy, Sharma, and Kumar (1998) randomly assigned participants who met the diagnostic criteria for Generalized Anxiety Disorder (GAD) to a wait-list control, alpha-increase biofeedback, or theta-increase biofeedback. EEG biofeedback consisted of 15 sessions to enhance alpha or theta and suppress beta.
Both the alpha-increase and theta-increase groups reduced self-reported (STAI-State Anxiety) and blind observer-rated anxiety (Hamilton Anxiety Rating Scale) in comparison to the control group. These results may have been due to Type 1 error caused by multiple t-tests without statistical correction. The authors' failure to observe changes in EEG power in the alpha or theta bands following EEG training suggests that non-specific treatment components, and not EEG training, may have produced clinical improvement, assuming that the findings of symptom improvement were valid.
Agnihotry, Paul, and Sandhu (2007) conducted a randomized controlled study of 45 patients diagnosed with Generalized Anxiety Disorder, which compared frontalis SEMG relaxation, EEG training to increase alpha, and a control group. Both the SEMG and EEG groups reduced STAI-State Anxiety and Trait Anxiety scores. Galvanic skin resistance (more resistance means less SNS activation) increased for both groups, with more significant gains for the SEMG group. At a 2-week follow-up, the SEMG group maintained the largest gains on all three measures.
Biofeedback Studies
Corrado, Gottlieb, and Abdelhamid (2003) reported that chronic pain patients who received a combination of finger temperature and SEMG biofeedback showed reduced anxiety and physical complaints compared to those in a pain education control group.Coy, Cardenas, Cabrera, Zirot, and Claros (2005) found that combining the antidepressant imipramine and biofeedback resulted in more significant anxiety reduction than imipramine alone.
Reiner (2008) reported an uncontrolled pre-test/post-test study of 24 patients diagnosed with an anxiety disorder and receiving outpatient CBT who practiced at home using a portable HRVB device. Nineteen participants who completed the study reduced Spielberger State-Trait Anger Expression Inventory (STAEI) and Spielberger State-Trait Anxiety Inventory (STAI-Y) scores and increased Pittsburgh Sleep Quality Index (PSQI) scores and sleep duration. Participants who practiced more frequently achieved more significant improvements on these measures.
Mikosch and colleagues (2010) conducted a randomized controlled study of 212 patients scheduled for elective coronary angiography (CA), which compared an intervention group that received psychological support, abdominal breathing instruction, and one session of HRVB with a control group that received TAU and information. While both groups reduced Spielberger State-Trait Anxiety Inventory (STAI) scores after the CA, the intervention group showed the largest decrease, and its scores were normal.
Ratanasiripong, Sverduk, Prince, and Havashino (2012) reported a randomized controlled study of 30 undergraduates, which compared counseling plus HRVB with a counseling comparison group. While both groups reduced Beck Anxiety Inventory scores, the HRVB group showed more significant improvement.
Clinical Efficacy
Generalized Anxiety Disorder (GAD)Based on five RCTs, Donald Moss and Matthew Watkins rated BFB and NFB for GAD as efficacious. The BFB interventions included HR decrease, HRV increase, SCL decrease, SEMG decrease, and virtual reality (VRB). The NF interventions included alpha increase and alpha/theta increase.
Participants decreased HR, SCL, state and trait anxiety, and HR reactivity to stress. They increased HRV measures (HF and LF power) and theta power.
Panic Disorder (PD)
Based on five RCTs, Donald Moss and Matthew Watkins rated BFB for PD as efficacious and specific.
The BFB interventions included end-tidal CO2 increase, HRV increase, and SEMG decrease.
Participants reduced agoraphobic avoidance, anger, panic-related cognitions, PD frequency and severity, and state and trait anxiety. They increased end-tidal CO2 and sleep time.
Phobia
Donald Moss and Matthew Watkins rated BFB for phobia as possibly efficacious for biofeedback and neurofeedback.
Post-Traumatic Stress Disorder (PTSD)
Based on four RCTs, Donald Moss, Fred Shaffer, and Matthew Watkins rated BFB and NFB for PTSD as efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.).
The BFB interventions included HRV and TEMP increase, and respiration rate (RR) decrease. The NFB interventions included left amygdala fMRI decrease; 2-6 Hz and 22-36 Hz decrease, and 10-13 Hz increase; and theta and high-beta decrease and low-beta increase.
Participants reduced anxiety and depression scores and PTSD symptoms.
Depression

The National Survey on Drug Use and Health (NSDUH) defines a significant depressive episode as a minimum 2-week phase during which an individual encounters feelings of depression or a lack of enjoyment in their usual activities.
Major depressive disorder is diagnosed when five or more depressive symptoms, including sadness or loss of pleasure, are present for two weeks. Depressed patients may sleep too much or too little, display psychomotor retardation or agitation, show change in weight or appetite, experience loss of energy, feel worthless or guilty, are unable to concentrate, think, or make decisions, and repeatedly think about death or suicide (Beidel, Bulik, & Stanley, 2014). Check out the YouTube video The Science of Depression. Graphic © FANDESIGN/Shutterstock.com.

Multiple pathways to depression include polygenic dysfunction involving the lateral and medial orbitofrontal cortex and limbic system, and environmental factors (McGrady & Moss, 2013).
Depression is associated with activation of the lateral orbitofrontal cortex, which signals that our behavior has not been rewarded. This activation may be related to feelings of loss and disappointment. Since this region communicates with networks responsible for our self-concept, this may also lower self-esteem (Cheng et al., 2016).
The frontal distribution of average and peak alpha power differs between depressed and healthy individuals. Graphic redrawn by minaanandag on Fiverr.com.

Authors' caption: Topographical distributions of 8–12 Hz band average alpha power (top) and 9.5 Hz peak alpha power (bottom) for depressed (left) and healthy (right) participants, showing a top view stretched across all recording sites, along with two 3D-rotated views to display frontal laterality. Depressed participants assessed at pretreatment show a more diffuse anterior-to-posterior pattern of alpha activation (note contour lines), unlike healthy participants who retain focused right frontal activity. Both groups have a peak frequency at 9.5 Hz, at which the noted contrast is particularly evident. Contour lines are spaced at intervals of 5 μV2/Hz.
Depression also involves reduced activation of reward circuitry in the medial orbitofrontal cortex and its communication with autobiographical memory systems. These changes may contribute to depressed patients' lack of enjoyment of daily activities and difficulty remembering happy experiences. Billeke, P. and Aboitiz, F. (2013) Social cognition in schizophrenia: From social stimuli processing to social engagement. Front. Psychiatry, 4(4). http:10.3389/fpsyt.2013.00004
http://journal.frontiersin.org/article/10.3389/fpsyt.2013.00004/full

Caption: Brain areas that participate in social processing. A simple classification of brain areas involved in social processing differentiates regions that participate in four related processes. The first is the perception of basic social stimuli, such as biological motions (V5), part of the body (extra-striate body area, EBA), and faces (fusiform face area, FFA). Another process includes emotional and motivational appraisal, where the amygdala (AMY), the anterior insula (AI), the subgenual and perigenual anterior cingulate cortex (ACC), as well as the orbitofrontal cortex (OFC) participate.
These cortical structures are in interaction with subcortical structures as the ventral striatum (VS), and the hypothalamus (HTH). These structures in turn interact with other regions which participate in the goal-directed, adaptive behaviors, and the categorization processes, such as the dorsolateral and the medial prefrontal cortex (dlPFC, mPFC) and the ACC. Finally for social attribution, areas like the ventral premotor cortex (vPMC), the superior temporal sulcus (STS), the AI, the posterior cingulate cortex (PCC), and the precuneus (PC) participate in more automatic, bottom-up inferences of other people’s mental states; whereas structures like the mPFC and the temporo-parietal junction (TPJ) are involved in more cognitive theory of mind skills.
Depression also involves disrupting the habenular nucleus, which is adjacent to the pineal gland. Since the habenular nucleus helps us filter out negative cognitions and memories, disabling this function results in the triad (negative self-perception, view of your immediate situation, and expectation of the future) that characterizes depressive thinking (Lawson et al., 2016). Animal research reveals that cocaine withdrawal increases habenular nucleus anti-reward pathway activation (Clerke et al., 2021).
Insular cortex graphic Ide JS and Li C-S R (2011) Creative Commons Attribution 3.0. Error-related functional connectivity of the habenula in humans. Front. Hum. Neurosci. http://journal.frontiersin.org/article/10.3389/fnhum.2011.00025/fu

Demographics
The 2020 National Survey on Drug Use and Health (NSDUH) found:1. About 8.4% of U.S. adults experienced a MDD episode in the last year.
2. MDD was more prevalent in women (10.5%) than men (6.2%).
3. MDD prevalence was highest among those aged 18-25 (17.0%).

If untreated, 25-30% of adult depressive attempt or commit suicide. Most cases of major depression involve another comorbid psychological disorder that is primary (Zimmerman et al., 2002). Only 21% of annual cases of depression are adequately treated (Kessler et al., 2003).
Neurofeedback and Biofeedback Protocols
EEG and functional MRI (fMRI) are the primary NF interventions, and EMG and HRV are the central biofeedback interventions for depression. NF EEG protocols attempt to correct frontal alpha asymmetry or enhance parietal-occipital upper alpha. The rationale for alpha asymmetry neurofeedback for mood disorders is that the left frontal cortex mediates approach behavior while the right mediates negative affect.Clinical depression is associated with less activation of the left frontal lobe than the right. Since alpha is an "idling frequency," this asymmetry is seen when the alpha amplitude is greater in the left (F3) than the right frontal lobe (F4). The goal of alpha asymmetry NF for depression is to correct this imbalance, decreasing left frontal alpha with respect to right frontal alpha.
Successful NF training increases the activation of the left hemisphere with respect to the right.
Before EEG asymmetry training, patients are trained using diaphragmatic breathing and autogenic phrases to teach them to relax and warm their hands. The hand-warming criterion is 95 degrees F.Patients are seen once or twice a week for one-hour sessions, which consist of 30 minutes of EEG training followed by 30 minutes of psychotherapy. Scalp sites F3 and F4 are used and are referenced to CZ. A bell or clarinet tone reinforces behavior when the asymmetry score exceeds 0 (right alpha amplitude exceeds left).
Typically, it is desirable for 15-18 Hz amplitude to be higher and 8-11 Hz amplitude to be lower at F3 compared to F4.
fMRI protocols attempt to up-regulate the activity of targeted regions that mediate positive emotion.
Neurofeedback Studies
EEG Studies
Baehr et al. (1999) reported that 3 of 6 patients discontinued antidepressant medication before the close of the fourth treatment quarter and that their proportion of A (alpha asymmetry) scores remained stable.Case studies reported by Kumano et al. (1996) and Rosenfeld (2000) and a pilot study by Waldkoetter and Sanders (1997) support the Baehr et al. (1999) finding that NF can reduce the symptoms of clinical depression.
Corrado and Gottlieb (1999) compared biofeedback-assisted relaxation with a wait-list control condition in chronic pain patients. The biofeedback-assisted relaxation group achieved improved Beck Depression Inventory scores.
Rosenfeld (2000) summarized a series of case studies involving patients diagnosed with depression. Before NF sessions, patients were trained to breathe diaphragmatically for 15-30 minutes and to warm their hands to a criterion of 95 degrees F. Active electrodes at F3 and F4 were both referenced to Cz. Training sessions conducted twice a week and were divided into 50% NF and 50% psychotherapy. As the alpha asymmetry score improved in four cases, Beck Depression Inventory (BDI) and Minnesota Multiphasic Personality Inventory (MMPI) depression scores declined.
Baehr, Rosenfeld, and Baehr (2001) reported follow-up data for three of six patients diagnosed with unipolar depression who had completed 27 sessions of alpha asymmetry training. The authors compared pre-treatment and follow-up alpha asymmetry and Beck Depression Inventory scores. All three patients had achieved normal right hemisphere alpha asymmetry scores and Beck scores by the completion of their training and maintained these gains at 1- to 5-year follow-up.
Choi et al. (2011) conducted a randomized controlled trial of 24 right-handed depressed patients who had not received psychoactive drugs within 2 months of the study. The researchers placed active electrodes at F3 and F4, referenced to Cz, and utilized Rosenfeld’s asymmetry protocol. Training sessions comprised six 4-minute trials separated by 30-second rest periods. They trained participants twice a week for 5 weeks. Following NF training, participants received self-training to reproduce the mental state they experienced during NF without equipment twice a week for 1 month. The psychotherapy placebo sessions were also conducted for 5 weeks. After these sessions, they were referred to other therapists who provided traditional psychotherapy for depression as required.
Only the NF group increased right frontal alpha power and asymmetry scores and a significant improvement on the HAM-D and BDI-II scales. Six (50%) of the NF participants and none of the psychotherapy placebo participants achieved a clinical response.
Functional MRI Studies
Real-time functional MRI NF (rtfMRI-nf) interventions are designed to increase the metabolism of brain regions, like the ventromedial prefrontal cortex, that mediate positive affect. Graphic @copy: Gorondenkoff/shutterstock.com.
Linden et al. (2012) reported an open-label pilot study of fMRI NF for 16 participants diagnosed with Recurrent Depressive Disorder. In the fMRI NF condition, the researchers trained 8 participants during four sessions to up-regulate brain regions responsive to positive emotions using a visual display updated every 2 seconds. Each session consisted of three 7-minute trials. In the control condition, the researchers instructed 8 participants to utilize positive imagery techniques employed by the fMRI NF participants during four sessions conducted outside the scanner. The fMRI NF group successfully up-regulated the target areas (left or right ventromedial prefrontal cortex, insula, dorsolateral prefrontal cortex, medial temporal lobe, or the orbitofrontal cortex). While the fMRI NF group improved on the Hamilton Depression Rating Scale (HDRS), the control group did not change.
Young et al. (2014) randomly assigned unmedicated participants diagnosed with major depressive disorder to either receive rtfMRI-nf from the left amygdala (experimental; n = 14) or the left intraparietal sulcus (control; n = 7). Training sessions consisted of seven 8-minute 30-second runs. The experimental group increased left amygdala activation when recalling positive autobiographical memories within the first training session. This training effect was maintained during transfer runs in which participants did not receive NF. In contrast, the control group did not increase the activation of the intraparietal sulcus. While the experimental group decreased Profile of Mood States (POMS) depression scores, it was not superior to the control group. The experimental group decreased State-Trait Anxiety Inventory (STAI) state and trait scores, and the two groups only differed on state anxiety. Finally, the experimental group increased Visual Analog Scale (VAS) happiness ratings, and this gain was more significant than in the control group.
Biofeedback Studies
Surface EMG Biofeedback
Durmus, Alayli, and Canturk (2005) randomly assigned 50 female patients diagnosed with knee osteoarthritis to biofeedback-assisted isometric exercise or electrical stimulation. For both groups, 20 minutes of therapy was applied 5 days a week for 4 weeks. Patients were evaluated before and after treatment. Both groups showed significant improvements in pain, measured by the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), and physical function scores. They demonstrated significant improvements in anxiety and depression scores on the Hospital Anxiety Depression (HAD).Heart Rate Variability Biofeedback
HRVB is possibly efficacious for depression, and its effects may be mediated by the diaphragm's stimulation of vagal afferent nerves (Gevirtz, 2013). This hypothesis is supported by findings that vagal nerve stimulation in some studies improved intractable depression. Vagal stimulation graphic Manu5, CC BY-SA 4.0, via Wikimedia Commons.
Karavidas and colleagues (2007) administered 10 weekly sessions of heart rate variability (HRV) biofeedback to 11 patients diagnosed with major depressive disorder (MDD). By session 4, these patients showed significant improvement on the Hamilton Depression Scale (HAM-D) and the Beck Depression Inventory (BDI-II). They maintained these changes over the remaining 6 weeks of training.
Siepmann et al. (2008) conducted an open-label controlled pilot study with 38 participants. They placed 14 depressed individuals in the HRVB condition. Additionally, they randomly assigned 12 healthy individuals to HRVB and active control (watch the same HRVB display without instructions) conditions. The decision to assign all depressed individuals to the HRVB condition and none to the active control condition undermined the internal validity of this design for these participants. All participants received three treatment sessions per week for 2 weeks. While depressed individuals significantly reduced Beck Depression Inventory (BDI) scores, decreased Spielberger State-Trait Anxiety Inventory (STAI) scores and heart rate, and increased HRV compared to baseline, healthy participants who received HRVB or the active control treatment did not change.
Zucker et al. (2009) conducted a controlled pilot study with 38 participants recruited from a residential therapeutic community for substance use disorder e diagnosed with PTSD symptoms. They randomly assigned individuals to either HRVB (StressEraser) or progressive muscle relaxation (PMR) recording. They instructed participants to practice 20 minutes per day and complete weekly logs. The HRVB group achieved lower Beck Depression Inventory (BDI-II) scores and increased HRV (SDNN) compared to the PMR group. Both groups significantly reduced PTSD symptoms on the Posttraumatic Stress-Total (PTS-T) scale and PTSD Checklist-Civilian Version (PCL-C). Increased HRV predicted improvement, even when respiration rate was statistically controlled.
Patron et al. (2013) studied 26 individuals with depressive symptoms following cardiac surgery. They randomly assigned participants to either five 45-minute sessions of HRVB or treatment as usual (TAU). HRVB was superior to TAU in increasing respiratory sinus arrhythmia (RSA), heart rate speeding and slowing across the breathing cycle, and decreasing the Centre for Epidemiologic Studies of Depression (CES-D) values from pre- to post-treatment. Improvement was correlated with increased RSA.
Clinical Efficacy
Based on 10 RCTs, Zachary Meehan, Fred Shaffer, and Christopher Zerr rated BFB and NFB for MDD as efficacious and specific in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.).BFB treatments included HRV increase. NFB interventions included alpha asymmetry reduction, alpha/theta increase, and real-time functional MRI (rtfMRI) to increase left or right ventromedial prefrontal cortex, insula, dorsolateral prefrontal cortex, medial temporal lobe, or orbitofrontal cortex activity.
Participants achieved decreased behavioral inhibition, depression symptoms and severity, and state and trait anxiety.
Tinnitus
Tinnitus involves ringing in the ear when noise is absent and is associated with hearing loss. This symptom can be debilitating when it interferes with speech comprehension. Tinnitus affects over 45 million Americans. Conventional medical treatments include background music, masking sounds, and antidepressants (Lalwani, 2014). Graphic © Dora Zett/Shutterstock.com.

Biofeedback and Neurofeedback Studies
The biofeedback studies (House, 1978; Walsh & Gerley, 1985; Weise et al., 2008) provided surface EMG and finger temperature biofeedback. The neurofeedback studies (Crocetti, Forti, & Del Bo, 2010; Dohrmann, Elbert, Schlee, & Weisz, 2007) provided feedback to modify power in the delta and tau bands of the EEG and to down-regulate real-time functional MRI (rtfMRI) activity in cortical auditory processing regions.Clinical Efficacy
Shaffer and Mannion (2016) rated biofeedback for tinnitus as level 3 - probably efficacious and neurofeedback for tinnitus as level 2 - possibly efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (3rd ed.). These ratings were limited by the reliance of many of the reviewed studies on a single-group pre-test/post-test design, which precluded drawing causal conclusions.

Glossary
alpha asymmetry neurofeedback for mood disorders: a protocol that trains depressed clients to relax and warm their hands using diaphragmatic breathing and autogenic phrases and then decrease left frontal alpha with respect to the right frontal alpha.
amplitude control: a protocol that trains patients to increase EEG amplitude (signal strength) instead of frequency. This strategy can raise the amplitude of both a target rhythm (beta) and an unwanted rhythm (alpha) since it does not teach frequency discrimination.
connectivity training: a strategy designed to correct deficient or excessive communication between two brain sites measured by indices like coherence and comodulation.
frequency control: a protocol that trains patients to increase the amplitude of one frequency (SMR) while suppressing another (theta). This procedure refines EEG control and may result in better clinical outcomes than amplitude control.
Generalized Anxiety Disorder (GAD): a disorder characterized by excessive anxiety and worry, which frequently interfere with daily functioning.
hyperactivity: excessive motor activity for a situation like a classroom, including fidgeting, leaving a seat, and climbing or running.
impulsivity: a failure to restrain behavior, including answering before a question is completed, interrupting others' conversations or play, and an inability to wait for one's turn.
inattention: difficulty sustaining attention, including distractibility, insufficient attention to detail, careless mistakes, forgetfulness, and loss of assignments and toys.
International 10-20 system: a standardized procedure for placing 21 recording and 1 ground electrode on adults.
Kaiser-Scott protocol (Scott-Kaiser modification): an intervention that starts with NF ADHD training and then progresses to the Peniston protocol.
Menninger alpha-theta protocol: a procedure that helps clients increase their attention through temperature and frontal SEMG training and then teaches clients to gradually slow the EEG until they can increase alpha and theta amplitude without falling asleep.
Menninger ON-OFF-ON EEG training: a procedure that teaches clients to increase the amplitude within a frequency band, reduce the amplitude, and then increase the amplitude again during 100- or 200-second segments. For example, clients may be instructed to increase 8-13 Hz alpha activity for 100 seconds, decrease it for 100 seconds, and then increase it for 100 seconds.
passive volition: in autogenic training, visualizing the desired change and then allowing the body to make the change at its own pace.
Peniston-Kulkosky addiction protocol: a multi-modal approach that incorporates visualization training, temperature biofeedback, rhythmic breathing techniques, autogenic training exercises, construction of personalized imagery, guided imagery, and 30 alpha-theta sessions.
performance-based protocols: the use of tasks and neurofeedback training to correct symptoms and improve performance. This approach compares clients to themselves and not a clinical database.
petit mal seizures: a type of epilepsy characterized by the loss of consciousness without abnormal movement during which a client appears to be daydreaming. A child may suffer hundreds of these seizures daily for periods lasting up to 30 seconds.
sensorimotor cortex: the central cortical area defined by the central sulcus (fissure of Rolando) separating the frontal and parietal lobes.
sensorimotor rhythm (SMR): an EEG rhythm from 12-14 Hz located over the sensorimotor cortex (central sulcus) that is associated with inhibition of movement and reduced muscle tone.
slow cortical potential (SCP) training: neurofeedback to increase the gradual negative changes in the membrane potentials of cortical dendrites that last from 300 milliseconds to several seconds to reduce neuronal excitability in conditions like grand mal epilepsy and migraines.
SMR training: a procedure that up-trains SMR activity and possibly down-trains theta, beta, and epileptiform activity.
Sterman's grand mal epilepsy protocol: a procedure that trains an epileptic patient to increase SMR (12-14 Hz) amplitude and duration while theta (4-7 Hz), beta (20+ Hz), epileptiform spikes, and EMG artifact are suppressed during 36 sessions. The aim is to normalize the waking and sleep EEG with elevated SMR and suppressed theta and beta activity.
stroke: cerebrovascular accident (CVA), destruction of brain tissue (infarction) due to cerebral hemorrhage, and cerebral ischemia affecting blood vessels that supply the brain. CVAs show abrupt onset and involve temporary or permanent neurological symptoms like aphasia, paralysis, or loss of sensation.
theta/beta training: a procedure that down-trains theta and up-trains beta.
tinnitus: ringing in the ear when noise is absent.
tonic-clonic seizures (grand mal seizures): primary generalized seizures featuring a peculiar cry, loss of consciousness, fall, tonic-clonic convulsions of all extremities, incontinence, and amnesia for the episode. These seizures are diagnosed in fewer than 20% of adult epileptics.
traumatic brain injury: intracranial insult due to acceleration or direct impact that may cause permanent or temporary cognitive, physical, and psychosocial deficits and impaired or altered consciousness.
Z-score training: a strategy that attempts to normalize brain function with respect to mean values in a clinical database. EEG amplitudes that are 2 or more standard deviations above or below the database means are down-trained or up-trained to treat symptoms and improve performance.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Assignment
Now that you have completed this module, consider the elements that neurofeedback training shares with modalities like EMG and temperature. How is neurofeedback training different?
References
Adamolekun, B. (2013). Seizure disorders. The Merck manual.
Agnihotry, H., Paul, M., & Sandhu, J. S. (2007). Biofeedback approach in the treatment of generalized anxiety disorder. Iranian Journal of Psychiatry, 2, 90-95.
American Academy of Pediatrics (2010). Evidence-based child and adolescent psychosocial interventions.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Author.
Andreassi, J. L. (2000). Psychophysiology: Human behavior and physiological response. Lawrence Erlbaum and Associates, Inc.
Andrews, D. J., Reiter, J. M., Schonfeld, W., Kastl, A., & Denning, P. (2000). A neurobehavioral treatment for unilateral complex partial seizure disorders: A comparison of right- and left-hemisphere patients. Seizure, 9(3), 189-197. https://doi.org/10.1053/seiz.1999.0375
Arns, M., Heinrich, H., & Strehl, U. (2013). Evaluation of neurofeedback in ADHD: The long and winding road. Biological Psychology, 95, 108-115. https://doi.org/10.1016/j.biopsycho.2013.11.013
Aronson, S. C. (2005). Depression. eMedicine.
Ayers, M. E. (1995). EEG neurofeedback to bring individuals out of level 2 coma [Abstract]. Biofeedback and Self-Regulation, 20(3), 304-305.
Ayers, M. E. (1995). Long-term follow-up of EEG neurofeedback with absence seizures. Biofeedback & Self-Regulation, 20(3), 309-310.
Baehr, E., Miller, E., Rosenfeld, J. P., & Baehr, R. (2004). Changes in frontal brain asymmetry associated with premenstrual dysphoric disorder: A single case study. Journal of Neurotherapy, 8(1), 29-42. https://doi.org/10.1300/J184v08n01_03
Baehr, E., Rosenfeld, J. P., & Baehr, R. (1997). The clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression: Two case studies. Journal of Neurotherapy, 2(3), 10-23. https://doi.org/10.1300/J184v02n03_02
Baehr, E., Rosenfeld, J. P., & Baehr, R. (2001). Clinical use of an alpha asymmetry neurofeedback protocol in the treatment of mood disorders: Follow-up study one to five years post therapy. Journal of Neurotherapy, 4(4), 11-18. https://doi.org/10.1300/J184v04n04_03
Beauregard, M., & Levesque, J. (2006). Functional magnetic resonance imaging investigation of the effects of neurofeedback training on the neural bases of selective attention and response inhibition in children with attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback, 31(1), 2-20. https://doi.org/10.1007/s10484-006-9001-y
Becerra, J., Fernandez, T., Harmony, T., Caballero, M. I., Garcia, F., Fernandez-Bouzas, A., . . . Prado-Alcala, R. A. (2006). Follow-up study of learning-disabled children treated with neurofeedback or placebo. Clin EEG Neurosci, 37(3), 198-203. https://doi.org/10.1177/155005940603700307
Beidel, D. C., Bulik, C. M., & Stanley, M. A. (2014). Abnormal psychology (3rd ed.). Pearson Education, Inc.
Belanger, H. G., Tate, D. F., & Vanderploog, R. D. (2018). Concussion and mild traumatic brain injury. In J. E. Morgan & J. H. Ricker (Eds.), Textbook of clinical neuropsychology (2nd ed., pp. 411-448). Routledge.
Biederman, J., Mick, E., Faraone, S. V., Braaten, E., Doyle, A., Spencer, T., Wilens, T. E., Frazier, E., & Johnson, M. A. (2002). Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. American Journal of Psychiatry, 159, 36–42. https://doi.org/10.1176/appi.ajp.159.1.36
Bodenhamer-Davis, E., & Callaway, T. (2004). Extended follow-up of Peniston Protocol results with chemical dependency. Journal of Neurotherapy, 8(2), 135-148.
Bray, S., Shimojo, S., & O'Doherty, J. P. (2007). Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J Neurosci, 27(28), 7498-74507. https://doi.org/10.1523/jneurosci.2118-07.2007
Brown, P. (2013). ADHD and the DSM-5: Update on revision to diagnostic criteria. Consultant for Pediatricians, 12(10), 453-454.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. H. (2004). Treatment effects related to EEG- biofeedback for crack cocaine dependency in a faith-based homeless mission. Journal of Neurotherapy, 8(2), 138-140.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. (2005). An open clinical trial utilizing real-time EEG operant conditioning as an adjunctive therapy in the treatment of crack cocaine dependence. Journal of Neurotherapy, 9(2), 27-47. https://doi.org/10.1300/J184v09n02_03
Camp, B. W. (1999). Attention deficit hyperactivity disorders: Studies of EEG patterns and biofeedback training (Final Report, Grant No. H113G40130), U.S. Office of Education.
Cannon, R., Lubar, J., Congedo, M., Thornton, K., Towler, K., & Hutchens, T. (2007). The effects of neurofeedback training in the cognitive division of the anterior cingulate gyrus. Int J Neurosci, 117(3), 337-357. https://doi.org/10.1080/00207450500514003
Caria, A., Veit, R., Sitram, R., Lotze, M., Weiskopf, N., Grodd, W., & Birbaumer, N. (2007). Regulation of anterior insular cortex activity using real-time fMRI. NeuroImage, 35(3), 1238-1246. https://doi.org/10.1016/j.neuroimage.2007.01.018
Carmody, D. P., Radvanski, D. C., Wadhwani, S., Sabo, M. J., & Vergara, L. (2001). EEG biofeedback training and attention-deficit/hyperactivity disorder in an elementary school setting. Journal of Neurotherapy, 4(3), 5-27. https://doi.org/10.1300/J184v04n03_02
Cheng, W., Rolls, E. T., Qiu, W., Tang, Y., Huang, C. C., Wang, X., Zhang, J., Lin, W., Zheng, L., Pu, J., Tsai, S.-J., Yang, A. C., Lin, C.-P., Wang, F., Xoe, P., & Feng, J. (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain, 139(Pt 12), 3296-3309. https://doi.org/10.1093/brain/aww255
Corrado, P., & Gottlieb, H. (1999). The effect of biofeedback and relaxation training on depression in chronic pain patients. American Journal of Alternative Medicine, 9, 18-21.
Corrado, P., Gottlieb, H., & Abdelhamid, M. H. (2003). The effect of biofeedback and relaxation training on anxiety and somatic complaints in chronic pain patients. American Journal of Pain Management, 13(4), 133-139.
Costello, E. J., Mustillo, S., Erkanli, A., Keeler, G., & Angold, A. (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry, 60(8), 837-844. https://doi.org/10.1001/archpsyc.60.8.837
Coy, P. C., Cardenas, S. J., Cabrera, D. M., Zirot, G. Z., & Claros, M. S. (2005). Psychophysiological and behavioral treatment of anxiety disorder. Salud Mental, 28(1), 28-37.
Dohrmann, K., Elbert, T., Schlee, W., & Weisz, N. (2007). Tuning the tinnitus percept by modification of synchronous brain activity. Restor Neurol Neursci, 25(3-4), 371-378. PMID: 17943012
Dreschler, R., Straub, M., Doehnert, M., Heinrich, H., Steinhausen, H. C., & Brandeis, D. (2007). Controlled evaluation of a neurofeedback training of slow cortical potentials in children with Attention Deficit/Hyperactivity Disorder (ADHD). Behav Brain Funct, 3(1), 35. https://doi.org/10.1186/1744-9081-3-35
Duric, N. S., Assmus, J., Gundersen, D. I., & Elgen, I. B. (2012). Neurofeedback for the treatment of children and adolescents with ADHD: A randomized and controlled clinical trial using parental reports. BMC Psychiatry, 12(1), 107. https://doi.org/10.1186/1471-244X-12-107
Egner, T., & Sterman, M. B. (2006). Neurofeedback treatment of epilepsy: From basic rationale to practical application. Expert Rev Neurother, 6(2), 247-257. https://doi.org/10.1586/14737175.6.2.247
Egner, T., Strawson, E., & Gruzelier, J. H. (2002). EEG signature and phenomenology of alpha/theta neurofeedback training versus mock feedback. Applied Psychophysiology and Biofeedback, 27(4), 261-270. https://doi.org/10.1023/a:1021063416558
Elbert, T., Rockstroh, B., Canavan, A., Birbaumer, N., Lutzenberger, W., von Billow, I., & Linden, A. (1991). Self-regulation of slow cortical potentials and its role in epileptogenesis. In J. Carlson & R. Seifert (Eds.), Biobehavioral self-regulation and health. Plenum Press.
Enriquez-Geppert, S., Brown, T., Henrich, H., Arns, M., & Pimenta, M. G. (2023). Attention Deficit Hyperactivity Disorder. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Evans, J. R., & Abarbanel, A. (1999). Introduction to quantitative EEG and neurofeedback. Academic Press.
Foster, D. S., & Thatcher, R. W. (2016). Traumatic Brain Injury (TBI) and Post Traumatic Stress Disorder (PTSD). In G. Tan, F. Shaffer, R. R. Lyle, & I. Teo (Eds.). Evidence-based practice in biofeedback and neurofeedback (3rd ed.). AAPB.
Friel, P. N. (2007). EEG biofeedback in the treatment of attention deficit hyperactivity disorder. Altern Med Rev, 12(2), 146-151. PMID: 17604459
Fuchs, T., Birbaumer, N., Lutzenberger, W., Gruzelier, J. H., & Kaiser, J. (2003). Neurofeedback treatment for Attention-Deficit/Hyperactivity Disorder in children: A comparison with methylphenidate. Applied Psychophysiology and Biofeedback, 28(1), 1-12. https://doi.org/10.1023/a:1022353731579
Gevensleben, H., Holl, B., Albrecht, B., Schlamp, D., Kratz, O., Studer, P., . . . Heinrich, H. (2010). Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. European Child & Adolescent Psychiatry, 19(9), 715-24. doi:10.1007/s00787-010-0109-5
Gevensleben, H., Holl, B., Albrecht, B., Vogel, C., Schlamp, D., Kratz, O., . . . Heinrich, H. (2009). Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 50(7), 780-9. https://doi.org/10.1111/j.1469-7610.2008.02033.x
Gevensleben, H., Kleemeyer, M., Rothenberger, L. G., Studer, P., Flaig-Röhr, A., Moll, G. H., . . . Heinrich, H. (2013). Neurofeedback in ADHD: Further pieces of the puzzle. Brain Topography. https://doi.org/10.1007/s10548-013-0285-y
Gevirtz, R. (2013). The promise of heart rate variability biofeedback: Evidence-based applications. Biofeedback, 41(3), 110-120. https://doi.org/10.5298/1081-5937-41.3.01
Goldstein, S. (2011). Attention-Deficit/ Hyperactivity disorder. In S. Goldstein, & C. R. Reynolds (Eds.), Handbook of neurodevelopmental and genetic disorders in children (2nd ed.). Guilford Press.
Gruzelier, J., Thompson, T., Brandt, R., & Steffert, T. (2014). Application of alpha/theta neurofeedback and heart rate variability training to contemporary dancers: State anxiety and creativity. International Journal of Psychophysiology, 93(1), 105-111. https://doi.org/10.1016/j.ijpsycho.2013.05.004
Halverson, J. L. (2014). Depression. eMedicine.
Hammond, D. C. (2001). Neurofeedback training for anger control. Journal of Neurotherapy, 5(4), 98-103.
Hammond, D. C. (2001). Neurofeedback treatment of depression with the Roshi. Journal of Neurotherapy, 4(2), 45-56. https://doi.org/10.1300/J184v04n02_06
Hammond, D. C. (2005). Neurofeedback with anxiety and affective disorders. Child & Adolescent Psychiatric Clinics of North America, 14(1), 105-123. https://doi.org/10.1016/j.chc.2004.07.008
Holtmann, M., & Stadler, C. (2006). Electroencephalographic biofeedback for the treatment of attention-deficit hyperactivity disorder in childhood and adolescence. Expert Rev Neurother, 6(4), 533-540. https://doi.org/10.1586/14737175.6.4.533
Kaiser, D. A., & Othmer, S. (2000). Effect of neurofeedback on variables of attention in a large multi-center trial. Journal of Neurotherapy, 4(1), 5-15. http://dx.doi.org/10.1300/J184v04n01_02
Kaiser, D. A., Othmer, S., & Scott, B. (1999). Effect of neurofeedback on chemical dependency treatment. Biofeedback & Self-Regulation, 20(3), 304–305.
Karavidas, M. K., Lehrer, P. M., Vaschillo, E., Vaschillo, B., Marin, H., Buyske, S., Malinovsky, I., Radvanski, D., & Hassett, A. (2007). Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback, 32(1), 19-30. https://doi.org/10.1007/s10484-006-9029-z
Keller, I. (2001). Neurofeedback therapy of attention deficits in patients with traumatic brain injury. Journal of Neurotherapy, 5(1-2), 19-32. https://doi.org/10.1300/J184v05n01_03
Kelley, M. J. (1997). Native Americans, neurofeedback, and substance abuse theory. Three year outcome of alpha/theta neurofeedback training in the treatment of problem drinking among Dine’ (Navajo) people. Journal of Neurotherapy, 2, 24–60. https://doi.org/10.1300/J184v02n03_03
Kessler, R. C., Adler, L., Barkley, R., Biederman, J., Conners, C. K., Demler, O., Faraone, S. V., Greenhill, L. L., Howes, M. J., Secnik, K., Spencer, T., Ustun, T. B., Walters, E. E., & Zaslavsky, A. M. (2006). The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. The American Journal of Psychiatry, 163(4), 716–723. https://doi.org/10.1176/ajp.2006.163.4.716
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K. R., Rush, J., Walters, E. E., & Wang, P. S. (2003). The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289, 3095-3105. https://doi.org/10.1001/jama.289.23.3095
Kessler, R. C., McGonagle, K. A., Zhao, S., Nelson, C. B., Hughes, M., Eshleman, S., Wittchen, H. U., & Kendler, K. S. (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry, 51(1), 8-19. https://doi.org/10.1001/archpsyc.1994.03950010008002
Koob, G. F. (2006). The neurobiology of addiction: A neuroadaptational view relevant for diagnosis. Addiction, 101, 23-30. https://doi.org/10.1111/j.1360-0443.2006.01586.x
Kotchoubey, B., Blankenhorn, V., Froscher, W., Strehl, U., & Birbaumer, N. (1997). Stability of cortical self-regulation in epilepsy patients. Neuroreport, 27(8), 1867-1870. https://doi.org/10.1097/00001756-199705260-00015
Kotchoubey, B., Schneider, D., Schleichert, H., Strehl, U., Uhlmann, C., Blankenhorn, V., . . . Birbaumer, N. (1996). Self-regulation of slow cortical potentials in epilepsy: A retrial with analysis of influencing factors. Epilepsy Research, 25(3), 269-276. https://doi.org/10.1016/S0920-1211(96)00082-4
Kotchoubey, B., Busch, S., Strehl, U., & Birbaumer, N. (1999). Changes in EEG power spectra during biofeedback of slow cortical potentials in epilepsy. Applied Psychophysiology and Biofeedback, 24(4), 213-233. https://doi.org/10.1023/a:1022226412991
Kotchoubey, B., Blankenhorn, V., Froscher, W., Strehl, U., & Birbaumer, N. (1996). Stability of cortical self-regulation in epilepsy patients. Neuroreport, 27(8), 1867-1870.
Kotchoubey, B., Strehl, U., Holzapfel, S., Blankenhorn, V., Froscher, W., & Birbaumer, N. (1999). Negative potential shifts and the prediction of the outcome of neurofeedback therapy in epilepsy. Clinical Neurophysiology, 110(4), 683-686. https://doi.org/10.1016/s1388-2457(99)00005-x
Kotchoubey, B., Strehl, U., Uhlmann, C., Holzapfel, S., Konig, M., Fröscher, W., Blankenhorn, V., & Birbaumer, N. (2001). Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia, 42(3), 406-416. https://doi.org/10.1046/j.1528-1157.2001.22200.x
Kumano, H., Horie, H., Shidara, T., Kuboki, T., Suematsu, H., & Yasushi, M. (1996). Treatment of a depressive disorder patient with EEG-driven photic stimulation. Biofeedback and Self-Regulation, 21(4), 323-334. https://doi.org/10.1007/bf02214432
Kushner, D. (1998). Mild traumatic brain injury: Toward understanding manifestations and treatment. Archives of Internal Medicine, 158(15), 1617-1624. https://doi.org/10.1001/archinte.158.15.1617
La Vaque, T. J. (2003). Neurofeedback, neurotherapy, and quantitative EEG. In D. Moss, A. McGrady, T. Davies, and I. Wickramasekera (Eds.), Handbook of mind-body medicine for primary care. Sage Publications, Inc.
Lagos, L., Vaschillo, B., Vaschillo, E., Lehrer, P. M., & Bates, M. (2008). Heart rate variability biofeedback as a strategy for dealing with competitive anxiety: A case study. Biofeedback. 36(3), 109–115.
Langlois, J. A., Rutland-Brown, W., & Wald, M. M. (2006). The epidemiology and impact of traumatic brain injury: A brief overview. Journal of Head Trauma Rehabilitation, 21(5), 375–378. https://doi.org/10.1097/00001199-200609000-00001
Lawson, R. P., Nord, C. L., Seymour, D. L., Thomas, D. L., Dayan, P., Pilling, S., & Roiser, J. P. (2016). Disrupted habenula function in major depression. Molecular Psychiatry. https://doi.org/10.1038/mp.2016.81
Leins, U., Goth, G., Hinterberger, T., Klinger, C., Rumpf, N., & Strehl, U. (2007). Neurofeedback for Children with ADHD: A Comparison of SCP and Theta/Beta Protocols. Applied Psychophysiology and Biofeedback, 32(2), 73-88. PMID: 16869483
Levesque, J., Beauregard, M., & Mensour, B. (2006). Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study. Neurosci Lett, 394(3), 216-221. https://doi.org/10.1016/j.neulet.2005.10.100
Lubar, J. F. (1985). EEG biofeedback and learning disabilities. Theory into Practice, 26, 106-111. https://doi.org/10.1080/00405848509543156
Lubar, J. F. (1995). Neurofeedback for the management of attention-deficit/hyperactivity disorder. In M. S. Schwartz & Associates (Eds.), Biofeedback: A practitioner's guide (2nd ed.). Guilford.
Lubar, J. F. (2003). Neurofeedback for the management of attention‑deficit/hyperactivity disorders. In M. S. Schwartz, & F. Andrasik (Eds.), Biofeedback: A practitioner's guide (3rd ed.). Guilford.
Lubar, J. O., & Lubar, J. F. (1984). Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback and Self-Regulation, 9, 1-23. https://doi.org/10.1007/bf00998842
Lubar, J. F., Swartwood, M. O., Swartwood, J. N., & O'Donnell, P. (1995). Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in TOVA scores, behavioral ratings, and WISC-R performance. Biofeedback and Self-Regulation, 20, 83-99. https://doi.org/10.1007/bf01712768
Mann, C., Lubar, J., Zimmerman, A., Miller, C., & Nuenchen, R. (1992). Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatric Neurology, 8, 30-36. https://doi.org/10.1016/0887-8994(92)90049-5
Marson, A., & Ramaratnam, S. (2003). Epilepsy. Clinical Evidence, (9), 1403-1420. PMID: 15366192
Mash, E. J., & Wolfe, D. A. (2010). Abnormal child psychology (4th ed.). Wadsworth/Cengage Learning.
McGinty, E. E., & Barry, C. L. (2020). Stigma reduction to combat the addiction crisis—Developing an evidence base. The New England Journal of Medicine, 382, 1291–1292. https://doi.org/10.1056/NEJMp2000227
McGrady, A., & Moss, D. (2013). Pathways to illness, pathways to health. Springer.
Meehan, Z. M., Shaffer, F., & Zerr, C. L. (2023). Depression. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Meisel, V., Servera, M., Garcia-Banda, G., Cardo, E., & Moreno, I. (2013). Neurofeedback and standard pharmacological intervention in ADHD: A randomized controlled trial with six-month follow-up. Biol Psychol, 94(1), 12-21. https://doi.org/10.1016/j.biopsycho.2013.04.015
Michael, A. J., Krishnaswamy, S., Mohamed, J. (2008). An open label study of the use of EEG biofeedback using beta training to reduce anxiety for patients with cardiac events. Neuropsychiatr Dis Treat, 1(4), 357-63. PMCID: PMC2424123
Mikosch, P., Hadrawa, T., Laubreiter, K., Brandl, J., Pilz, J., Stettner, H., & Grimm, G. (2010). Effectiveness of respiratory-sinus-arrhythmia biofeedback on state anxiety in patients undergoing coronary angiography. Journal of Advanced Nursing, 66(5), 1101-1110. https://doi.org/10.1111/j.1365-2648.2010.05277.x
Monastra, V. J., Lynn, S., Linden, M., Lubar, J. F., Gruzelier, J., & LaVaque, T. J. (2005). Electroencephalographic biofeedback in the treatment of Attention Deficit/Hyperactivity Disorder. Applied Psychophysiology and Biofeedback, 30(2), 95-114. https://doi.org/10.1007/s10484-005-4305-x
Monastra, V. J., Monastra, D. M., & George S. (2002). The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of Attention-Deficit/Hyperactivity Disorder. Applied Psychophysiology and Biofeedback, 27(4), 231-249. https://doi.org/10.1023/a:1021018700609
Moss, D., Shaffer, F., & Watkins, M. (2023). Posttraumatic Stress Disorder (PTSD). In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Moss, D., & Watkins, M. (2023). Anxiety and anxiety disorders. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
National Survey on Drug Use and Health (NSDUH). Retrieved from https://www.samhsa.gov/data/release/2020-national-survey-drug-use-and-health-nsduh-releases
Paul, M., & Garg, K. (2012). The effect of heart rate variability biofeedback on performance psychology of basketball players. Applied Psychophysiology and Biofeedback, 37, 131-144. https://doi.org/10.1007/s10484-012-9185-2
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13(2), 271-279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy, 3, 37-55.
Peniston, E. G., Marrinan, D. A., Deming, W. A., & Kulkosky, P. J. (1993). EEG alpha-theta brainwave synchronization in Vietnam theater veterans with combat-related post-traumatic stress disorder and alcohol abuse. Advances in Medical Psychotherapy, 6, 37-50.
Pigott, H. E., De Biase, L., Bodenhamer-Davis, E., & Davis, R. E. (2013). The evidence-base for neurofeedback as a reimbursable healthcare service to treat Attention Deficit/Hyperactivity Disorder.
Ramaratnam, S., Baker, G. A., & Goldstein, L. (2001). Psychological treatments for epilepsy. Cochrane Database of Systematic Reviews Online Update Software, 4, CD002029. Ramaratnam, S., Baker, G. A., & Goldstein, L. H. (2008). Psychological treatments for epilepsy. Cochrane Database Syst Rev, (3), CD002029. https://doi.org/10.1002/14651858.cd002029
Ramos, F. (1998). Frequency band interaction in ADD/ADHD neurotherapy. Journal of Neurotherapy, 3(1), 26-41. https://doi.org/10.1300/J184v03n01_04
Ratanasiripong, P., Sverduk, K., Prince, J., & Hayashino, D. (2012). Biofeedback and Counseling for stress and anxiety among college students. Journal of College Student Development, 53(5), 742-749. http://dx.doi.org/10.1353/csd.2012.0070
Raymond, J., Sajid, I., Parkinson, L. A., & Gruzelier, J. H. (2005). Biofeedback and dance performance: A preliminary investigation. Applied Psychophysiology and Biofeedback, 30(1), 64-73. https://doi.org/10.1007/s10484-005-2175-x
Reiner, R. (2008). Integrating a portable biofeedback device into clinical practice for patients with anxiety disorders: Results of a pilot study. Applied Psychophysiology and Biofeedback, 33(1), 55-61. https://doi.org/10.1007/s10484-007-9046-6
Rice, K. M., Blanchard, E. B., & Purcell, M. (1993). Biofeedback treatments of generalized anxiety disorder: Preliminary results. Biofeedback and Self-Regulation, 18(2), 93-105. https://doi.org/10.1007/bf01848110
Rockstroh, B., Elbert, T., Birbaumer, N., Wolf, P., Düchting-Röth, A., Reker, M., Daum, I., Lutzenberger, W., & Dichgans, J. (1993). Cortical self-regulation in patients with epilepsies. Epilepsy Research, 14, 63-72. https://doi.org/10.1016/0920-1211(93)90075-i
Rosenfeld, J. P. (2000). An EEG Biofeedback protocol for affective disorders. Clinical Electroencephalography, 31(1), 7-12. https://doi.org/10.1177/155005940003100106
Rosenfeld, J. P., Baehr, E., Baehr, R., Gotlib, I. H., & Ranganath, C. (1996). Preliminary evidence that daily changes in frontal alpha asymmetry correlate with changes in affect in therapy sessions. International Journal of Psychophysiology, 23, 137-141. https://doi.org/10.1016/0167-8760(96)00037-2
Rossiter, T. R. (1998). Patient-directed neurofeedback for AD/HD. Journal of Neurotherapy, 2(4), 54-63. https://doi.org/10.1300/J184v02n04_04
Rossiter, T. R., & La Vaque, T. J. (1995). A comparison of EEG biofeedback and psychostimulants in treating attention deficit/hyperactivity disorders. Journal of Neurotherapy, 1(1), 48-59. https://doi.org/10.1300/J184v01n01_07
Rota, G., Sitaram, R.,Veit, R., Erb, M., Weiskopf, M., Dogil, G., & Birbaumer, N. (2009). Self-regulation of regional cortical activity using real-time fMRI: The right inferior frontal gyrus and linguistic processing. Hum Brain Mapp, 30(5), 1605-1614. https://doi.org/10.1002/hbm.20621
Saxby, E., & Peniston, E. G. (1995). Alpha-theta brainwave neurofeedback training: An effective treatment for male and female alcoholics with depressive symptoms. Journal of Clinical Psychology, 51(5), 685-693. https://doi.org/10.1002/1097-4679(199509)51:5<685::aid-jclp2270510514>3.0.co;2-k
Schneider, F., Elbert, T., Heimann, H., Welker, A., Stetter, F., Mattes, R., Birbaumer, N., & Mann, K. (1993). Self-regulation of slow cortical potentials in psychiatric patients: Alcohol dependency. Biofeedback and Self-Regulation, 18(1), 23-32. https://doi.org/10.1007/bf00999511
Scott, W., & Kaiser, D. (1998). Augmenting chemical dependency treatment with neurofeedback training. Journal of Neurotherapy, 3(1), 66.
Scott, W. C., Kaiser, D., Othmer, S., & Sideroff, S. I. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455-469. https://doi.org/10.1081/ada-200056807
Schwartz, A., & Cohen, S. (March 31, 2013). A.D.H.D. see in 11% of U.S. children as diagnoses rise. New York Times.
Shaffer, F., & Mannion, M. (2016). Tinnitus. In G. Tan, F. Shaffer, R. R. Lyle, & I. Teo (Eds.). Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Sherlin, L., Arns, M., Lubar, J., & Sokhadze, E. (2010). A position paper on neurofeedback for the treatment of ADHD. Journal of Neurotherapy, 14(2), 66-78. https://doi.org/10.1080/10874201003773880
Sheth, R. D., Stafstrom, C. E., & Hsu, D. (2005). Nonpharmacological treatment options for epilepsy. Seminars in Pediatric Neurology, 12(2), 106-113. https://doi.org/10.1016/j.spen.2005.03.005
Sokhadze, T. M., Cannon, R. L., & Trudeau, D. L. (2008). EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Applied Psychophysiology and Biofeedback, 33(1), 1-28. https://dx.doi.org/10.1007%2Fs10484-007-9047-5
Sokhadze, E. M., & Trudeau, D. (2023). Alcohol and drug dependence. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Spencer, T. J., Biederman, J., & Mick, E. (2007). Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. Ambulatory Pediatrics, 7, 73–81. https://doi.org/10.1016/j.ambp.2006.07.006
Steiner, N. J., Frenette, E. C., Rene, K. M., Brennan, R. T., & Perrin, E. C. (2014). In-School neurofeedback training for ADHD: Sustained improvements from a randomized control trial. Pediatrics. https://doi.org/10.1542/peds.2013-2059
Sterman, M. B. (1973). Neurophysiological and clinical studies of sensorimotor EEG biofeedback training: Some effects on epilepsy. L. Birk (Ed.), Biofeedback: Behavioral Medicine. Grune & Stratton, pp. 147-165.
Sterman, M. B. (1973). Neurophysiological and clinical studies of sensorimotor EEG biofeedback training: Some effects on epilepsy. Seminars in Psychiatry, 5(4), 507-525. PMID: 4770578
Sterman, M. B. (1977). Sensorimotor EEG operant conditioning: Experimental and clinical effects. Pavlovian Journal of Biological Sciences, 12(2), 63-92. https://doi.org/10.1007/bf03004496
Sterman, M. B. (1986). Epilepsy and its treatment with EEG feedback therapy. Annals of Behavioral Medicine, 8, 21-25. https://doi.org/10.1207/s15324796abm0801_4
Sterman, M. B. (1997). The challenge of EEG biofeedback in the treatment of epilepsy: A view from the trenches. Biofeedback, 25(1), 6-7, 20-21, 23.
Sterman, M. B. (2000). Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clinical Electroencephalography, 31(1), 45-55. https://doi.org/10.1177/155005940003100111
Sterman, M. B., & Egner, T. (2006). Foundation and practice of neurofeedback for the treatment of epilepsy. Applied Psychophysiology and Biofeedback, 31(1), 21-35. https://doi.org/10.1007/s10484-006-9002-x
Sterman, M. B., & Friar, L. (1972). Suppression of seizures in epileptics following sensorimotor EEG feedback training. Electroencephalography & Clinical Neurophysiology, 33, 89-95. https://doi.org/10.1016/0013-4694(72)90028-4
Sterman, M. B., & Lantz, D. (2001). Changes in lateralized memory performance in subjects with epilepsy following neurofeedback training. Journal of Neurotherapy, 5, 63-72. https://doi.org/10.1300/J184v05n01_06
Sterman, M. B., & Macdonald, L. R. (1978). Effects of central cortical EEG feedback training on incidence of poorly controlled seizures. Epilepsia, 19(3), 207-222. https://doi.org/10.1111/j.1528-1157.1978.tb04483.x
Sterman, M. B., Macdonald, L. R., & Stone, R. K. (1974). Biofeedback training of the sensorimotor electroencephalogram rhythm in man: Effects on epilepsy. Epilepsia, 15(3), 395-416. https://doi.org/10.1111/j.1528-1157.1974.tb04016.x
Sterman, M. B., & Shouse, M. N. (1980). Quantitative analysis of training, sleep EEG and clinical response to EEG operant conditioning in epileptics. Electroencephalography & Clinical Neurophysiology, 49, 558-576. https://doi.org/10.1016/0013-4694(80)90397-1
Strack, B., & Gevirtz, R. (2011). Getting to the heart of the matter: Heart rate variability biofeedback for enhanced performance. In B. Strack, M. Linden & V. Wilson (Eds.), Biofeedback and neurofeedback applications in sport psychology (pp. 145-174). Association for Applied Psychophysiology and Biofeedback.
Strehl, U., Kotchoubey, B., Trevorrow, T., & Birbaumer, N. (2005). Predictors of seizure reduction after self-regulation of slow cortical potentials as a treatment of drug-resistant epilepsy. Epilepsy & Behavior, 6, 156-166. https://doi.org/10.1016/j.yebeh.2004.11.004
Strehl, U., Leins, U., Groth, G., Klinger, C., Hinterberger, T., & Birbaumer, N. (2006). Self-regulation of slow cortical potentials: A new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics, 118(5): e1530-40. https://doi.org/10.1542/peds.2005-2478
Tan, G., Thornby, J., Hammond, D. C., Strehl, U., Canady, B., Arnemann, K., & Kaiser, D. A. (2009). Meta-analysis of EEG biofeedback in treating epilepsy. Clinical EEG and Neuroscience, 40(3), 173–179.
Taub, E., Steiner, S. S., Weingarten, E., & Walton, K. G. (1994). Effectiveness of broad spectrum approaches to relapse prevention in severe alcoholism: A long-term, randomized, controlled trial of Transcendental Meditation, EMG biofeedback and electronic neurotherapy. Alcoholism Treatment Quarterly, 11(1-2), 187-220. https://doi.org/10.1300/J020v11n01_06
Thompson, L., & Thompson, M. (1998). Neurofeedback combined with training in metacognitive strategies: Effectiveness in students with ADD. Applied Psychophysiology and Biofeedback, 23(4), 243-263. https://doi.org/10.1023/a:1022213731956
Thompson, W. (2014). Alcoholism. eMedicine.
Thornton, K. (2000). Improvement/rehabilitation of memory functioning with neurotherapy/QEEG biofeedback. Journal of Head Trauma Rehabilitation, 15(6), 1285-1296. https://doi.org/10.1097/00001199-200012000-00008
Thornton, K. E., & Carmody, D. P. (2005). Electroencephalogram biofeedback for reading disability and traumatic brain injury. Child and Adolescent Psychiatric Clinics of North America, 14(1), 137-162. https://doi.org/10.1016/j.chc.2004.07.001
Thornton, K., & Carmody, D. (2013). The relation between memory improvement and QEEG changes in three clinical groups as a result of EEG biofeedback treatment. Journal of Neurotherapy, 17(2), 116-131. doi:10.1080/10874208.2013.785183
Thurber, M. R. (2006). Effects of heart-rate variability biofeedback training and emotional regulation on music performance anxiety in university students. University of North Texas Press.
Tinius, T. P., & Tinius, K. A. (2000). Changes after EEG biofeedback and cognitive retraining in adults with mild traumatic brain injury and attention deficit hyperactivity disorder. Journal of Neurotherapy, 4(2), 27-44. https://doi.org/10.1300/J184v04n02_05
Vanathy, S., Sharma, P. S. V. N., & Kumar, K. B. (1998). The efficacy of alpha and theta neurofeedback training in treatment of generalized anxiety disorder. Indian Journal of Clinical Psychology, 25(2), 136-143.
Vinas, F. C. (2013). Penetrating head trauma. eMedicine.
Volkow, N. D., & McLellan, A. T. (2016). Opioid abuse in chronic pain--Misconceptions and mitigation strategies. The New England Journal of Medicine, 374(13), 1253–1263. https://doi.org/10.1056/NEJMra1507771
Wadhwani, S., Radvanski, D. C., & Carmody, D. P. (1998). Neurofeedback training in a case of attention deficit hyperactivity disorder. Journal of Neurotherapy, 3(1), 42-49.
Waldkoetter, R. O., & Sanders, G. O. (1997). Auditory brainwave stimulation in treating alcoholic depression. Perceptual and Motor Skills, 84(1), 226. https://doi.org/10.2466/pms.1997.84.1.226
Walker, J. E., Norman, C. A., & Weber, R. K. (2002). Impact of qEEG-guided coherence training for patients with a mild closed head injury. Journal of Neurotherapy, 6(2), 31-43.
Yates, W. R. (2014). Anxiety disorders. eMedicine.
Zimmerman, M., Chelminksi, I., & McDermut, W. (2002). Major depressive disorder and Axis I diagnostic comorbidity. Journal of Clinical Psychiatry, 63, 187-193. https://doi.org/10.4088/jcp.v63n0303