Signal Processing
EEG waveforms may be described by their frequency, shape, and amplitude. The amount of energy within an EEG frequency band may be quantified using peak-to-peak and root mean square methods. Data acquisition systems transform the raw analog signal into a digital form. High sampling resolutions measured in digital bits are required to accurately sample DC and AC components and a wide range of signal voltages. A minimum sampling rate of twice the highest frequency is necessary when performing Fast Fourier Transform analysis. After the EEG signal has passed through several amplification stages, it is filtered to exclude unwanted frequencies and minimize artifact and distortion.
The EEG spectrum is composed of frequency bands which are further subdivided. EEG frequency bands are correlated with unique subjective states like internal focus and conscious problem-solving. Clinicians and researchers use LORETA, sLORETA, eLORETA, and surface Laplacian analysis to localize the cortical source of the scalp EEG.
Finally, professionals need to recognize and understand the significance of clinically significant raw waveforms like the kappa rhythm, lambda waves, vertex sharp transients, mu waves, spike and wave, SMR, sleep spindles, and K-complexes. The graphic below is courtesy of Deymed.
BCIA Blueprint Coverage
This unit addresses III. Instrumentation and Electronics - C. Signal Processing.

This unit covers Analog, Raw EEG, Basic Signal Measurement Terms, Filtering Methods, Subjective Characteristics of Frequency Bands, Waveform Morphology, Source Localization, and Clinically Significant Waveforms.
Please click on the podcast icon below to hear a full-length lecture.

ANALOG, RAW EEG
EEG activity ranges from DC (slow cortical potentials) to gamma (34-60+ Hz) (Collura, 2014). Hertz (Hz) is an abbreviation for cycles per second. The raw EEG signal consists of oscillating electrical potential differences detected from the scalp. Raw or wave displays plot voltage using a bipolar (positive/negative) scale with zero in the middle. This is the analog form of the signal in which voltage continuously varies instead of digital representation using 0s and 1s.
It is always important to view EEG activity as the result of brain functions and behaviors rather than their cause. For example, alpha activity does not cause a relaxed state but is simply a reflection of that relaxed state having already occurred. Rewarding an increase in alpha amplitude is indirectly rewarding the brain for producing the state or states that result in increased alpha synchronization among the neurons being recorded. Another example is gamma activity, which is a result of glial cells organizing networks of neurons to accomplish a task or tasks. Thus, gamma emerges from a network of neurons bound together via the glial system. The graphic © John S. Anderson shows the voltage as µV peak to peak.
BASIC SIGNAL MEASUREMENT TERMS
EEG waveforms share the features of frequency and shape (Libenson, 2024). Click on the Read More button to review frequency and amplitude.
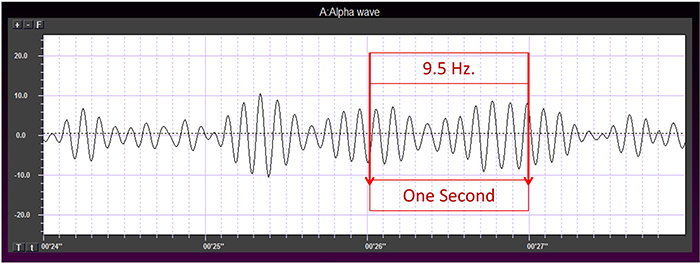
Frequency measures the speed and is the number of cycles completed each second. The higher the frequency (f), the shorter the wavelength (λ). The mathematical relationship is f = 1/λ. To measure frequency in the raw waveform, count the number of peaks or zero crossings and divide by 2. Graphic © John S. Anderson.
Frequency graphic © Bany's beautiful art/Shutterstock.com.
Amplitude measures size, which is the "amount" of energy within an EEG frequency band. The amplitude and morphology of any EEG frequency band reflect the number of neurons discharging simultaneously at that frequency. High amplitude means that many neurons are depolarizing and hyperpolarizing at the same time. Amplitude graphic © petrroudny43/Shutterstock.com.
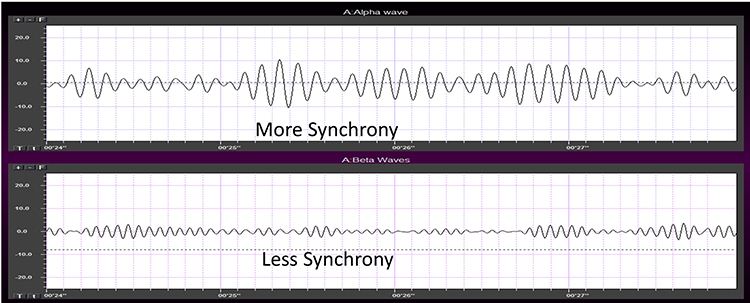
Greater synchrony among neurons firing results in higher amplitude (Demos, 2019). Graphic © John S. Anderson.
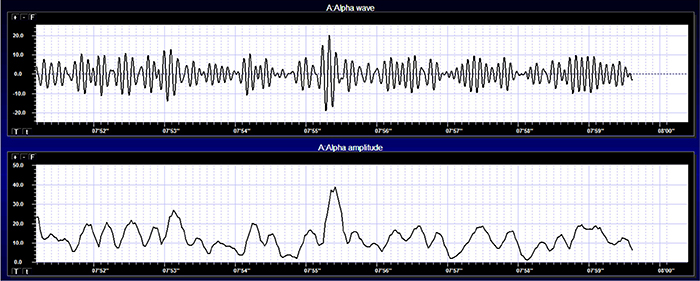
Amplitude displays show voltage using a scale where all values are positive (greater than zero). They only show voltage changes, not the signal waveform. Graphic © John S. Anderson shows the oscillating raw alpha waveform (top) and alpha amplitude (bottom).
Magnitude represents the average amplitude over a unit of time using quantification methods like peak-to-peak (P-P) and root mean square (RMS). The peak-to-peak method measures waveform "height" from peak to trough. In contrast, the root mean square method calculates the area under the EEG waveform and is analogous to the weight of an object (Collura, 2014). The graphic below that illustrates EEG spectrum magnitude © John S. Anderson.
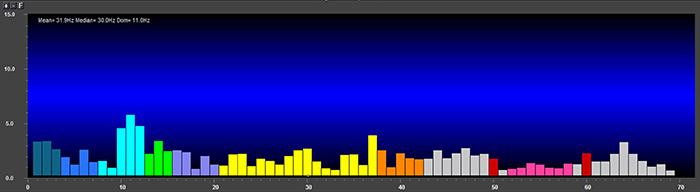
EEG signal power is magnitude squared and may be expressed as microvolts squared or picowatts/resistance. Most qEEG databases convert power into standard deviations, whereas the Jewel database transforms amplitudes into standard deviations or Z-scores (Demos, 2019). The graphic below © John S. Anderson shows the EEG power spectrum instead of the magnitude spectrum.
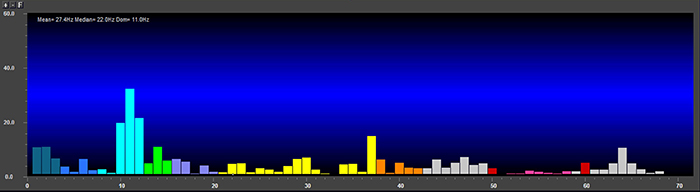
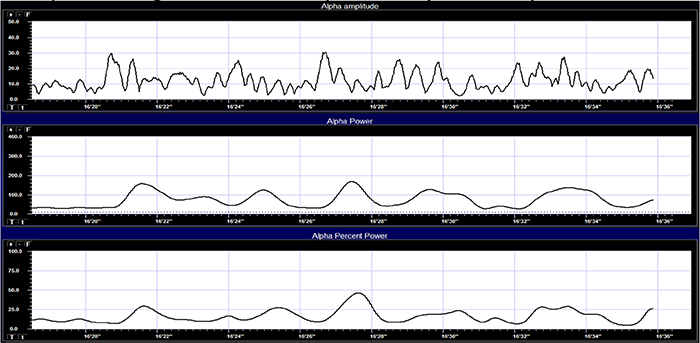
Percent power is the power within a frequency band expressed as a percentage of total EEG power. The graphic below © John S. Anderson shows alpha amplitude, alpha power, and alpha percent power.
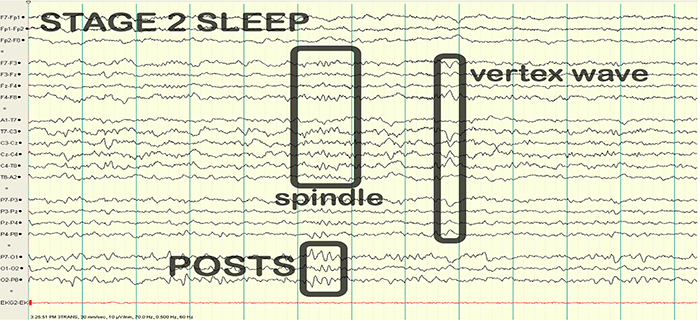
EEG waveforms may assume a distinctive shape or morphology like POSTs (positive occipital sharp transients of sleep), spindles (oscillations), and vertex (V) waves that appear over Cz during stage 2 sleep. The graphic below © eegatlas-online.com shows stage 2 sleep.
FILTERING METHODS AND SUBJECTIVE CHARACTERISTICS OF FREQUENCY BANDS
Sampling
Data acquisition systems digitize and process the raw EEG signal. Digitization transforms the raw signal into a digital form. An analog-to-digital converter (ADC) samples the analog signal (transforms it into numerical values) with a sampling resolution, sampling rate, and epoch length. Graphic courtesy of Deymed,
The sampling resolution is the number of digital bits used to represent a signal. Each bit (binary digit) is assigned a binary value of 0 or 1. Systems that sample the EEG signal utilize from 8-24 bits. The advantages of 24-bit sampling are an accurate sampling of the EEG signal's DC and AC components and the ability to sample a wide range of signal voltages called the dynamic range (Collura, 2014). However, the ability to utilize these finer resolutions depends upon the quality of the video processing system and the resolution of the visual display (monitor) of the computer used to display the data.
The sampling rate is the number of times that the ADC samples the EEG signal per second. A rate of twice the highest frequency is the minimum acceptable sampling rate when performing Fast Fourier Transform (FFT) analysis.
A FFT is a mathematical transformation that converts a complex signal into component sine waves whose amplitude can be calculated. The graphic below shows the decomposition of the original signal (left) into its sinewaves of different frequencies (center). This graphic is © Garegin Melkonyan, who uploaded it to ResearchGate.
.jpg)
A rate of twice the highest frequency is insufficient to visually represent the EEG signal since it only samples the highest frequency twice per cycle. This low rate also allows the harmonics of 50/60 Hz noise (which can extend to several hundred hertz) to contaminate the EEG signal. For example, when there is a 240-Hz harmonic of 60Hz noise, sampling at 256 samples per second (sps) can result in a spurious 16-Hz waveform (Collura, 2014). Faster sampling rates are desirable, particularly when resolving high-frequency signals. A sampling rate of 512 sps is a good choice for frequencies up to 64 Hz, and 1024 sps is suitable for frequencies up to 128 Hz. However, a sampling rate of 256 sps is considered adequate for most purposes
FFT analysis breaks the EEG signal into 1- to 2-s chunks called epochs. Epoch length sets the lowest and highest frequencies that the FFT can represent (Collura, 2014). Graphic © John S. Anderson shows the conversion of a complex signal using FFT.
The FFT power spectral analysis video below © John S. Anderson. The top window shows the raw EEG signal, and the bottom window features a spectral display created using FFT analysis.
Joint time-frequency analysis (JTFA) computes values on each data point at rates up to 256 times per second without using a fixed epoch length. Where FFT simultaneously calculates amplitudes for all frequency bands, JTFA analyzes preselected bands. The JFTA graphic © John S. Anderson.
Filtering the Data
After a differential amplifier boosts the EEG signal, it is filtered and then amplified by a second single-ended amplifier. Click on the Read More button to review how filters work.Filters exclude unwanted EEG frequencies to detect activity of clinical interest and minimize artifact and distortion. Clinical EEG analysis uses low-frequency, high-frequency, bandpass, and notch filters (Libenson, 2024). Clinicians must disable filters to examine EEG morphology (Demos, 2019).

A low-frequency filter (high-pass filter) filters out low-frequency activity and passes only the frequencies above a set value (e.g., 1.6 Hz).
A high-frequency filter (low-pass filter) filters out high-frequency activity and passes only the frequencies lower than the set value (e.g.,15 Hz). This filter can help reduce the distortion that EMG artifact causes to the raw EEG waveform (Thompson & Thompson, 2015).
"However, use of filters to remove EMG artifact must be used with care because muscle artifact is broadband so that the remaining signal might well contain subtle but significant muscle artifact in roughly the 15-30 Hz range, where genuine EEG power is typically low. Such subtle artifact could substantially reduce signal-to-noise ratio in the beta band” (Nunez & Srinivasan, 2006).
A bandpass filter passes the frequencies between the set values, which constitute the "band" of the filter.
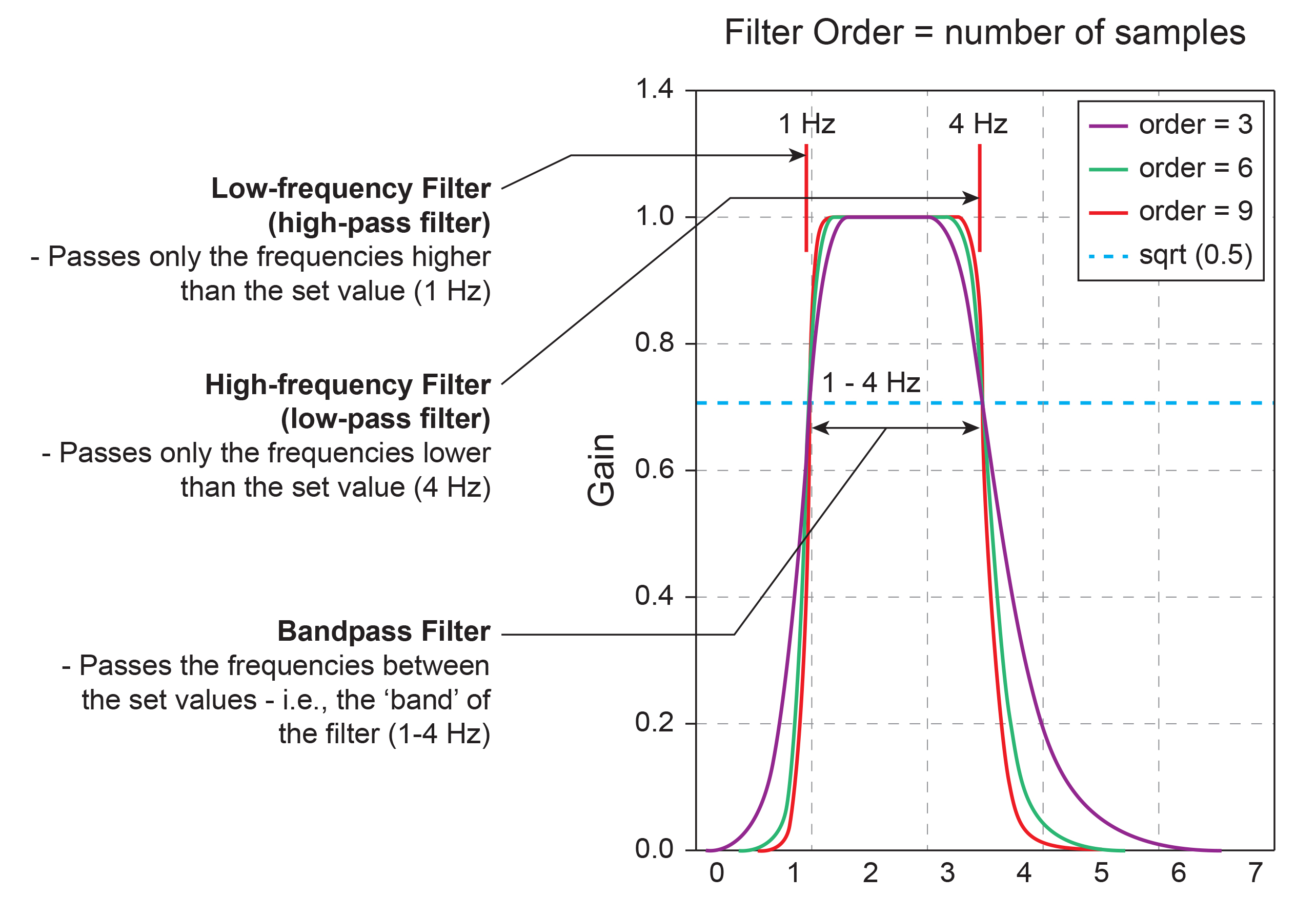
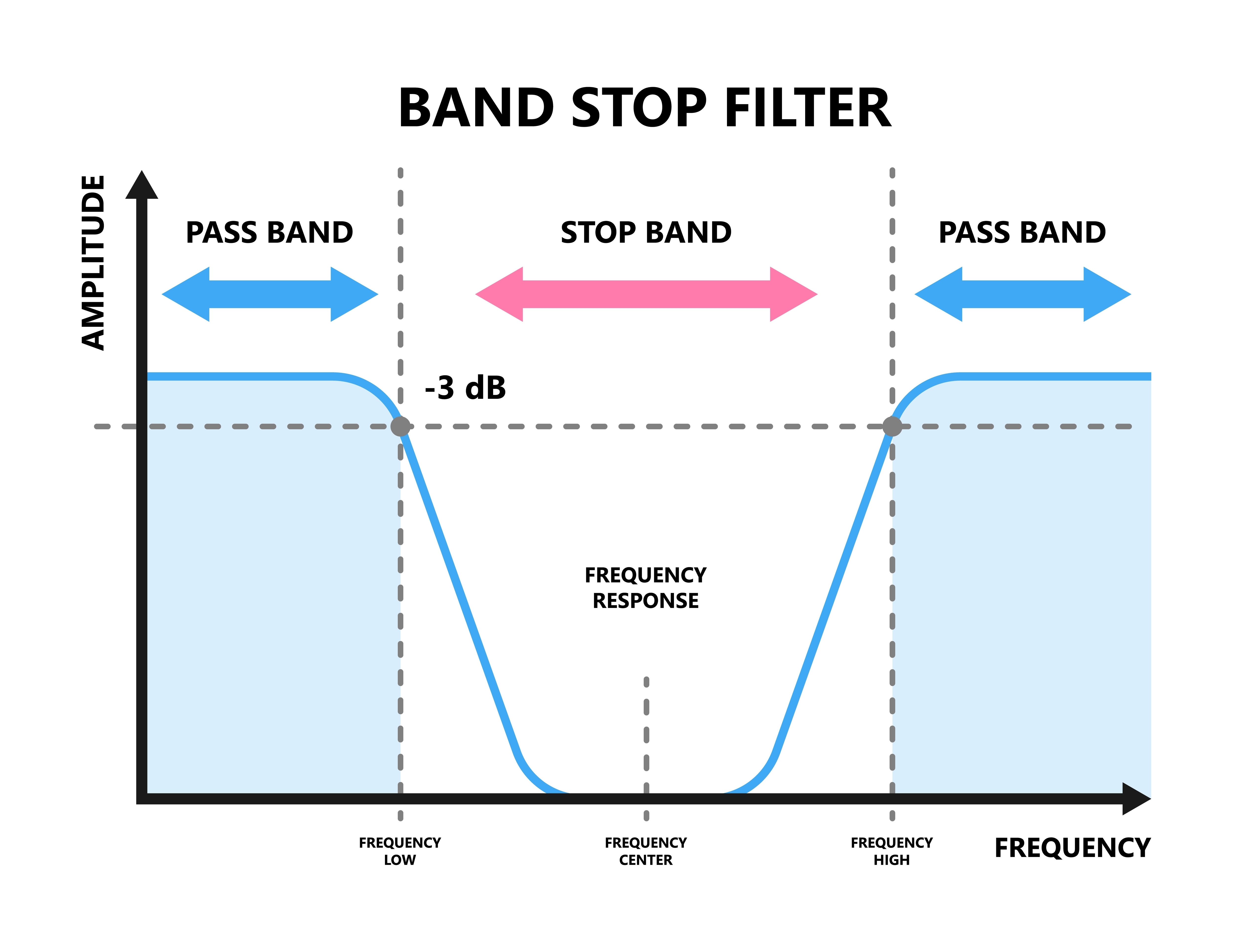
A notch filter excludes a narrow frequency band to control 50/60Hz artifact produced by line current (Libenson, 2024). The stop band is the range of frequencies attenuated by a notch filter. Use notch filters as a last resort. Stop band graphic © Pepermpron/Shutterstock.com.
Analog and Digital Filters
Analog filters contain analog circuits designed using components like capacitors, resistors, and operational amplifiers. Analog filters represent voltage as continuously varying. Most analog filters use the infinite impulse response (IIR) approach, described below.Digital filters use digital processors, like a digital signal processing (DSP) chip, to exclude unwanted frequencies. First, an analog-to-digital converter (ADC) samples and digitizes the analog signal, representing signal voltages as binary numbers. Second, a DSP chip performs calculations on the binary numbers. Third, a digital-to-analog converter (DAC) may transform the sampled, digitally filtered signal back to analog form. Graphic © Foud A. Saad/Shutterstock.com.
Three main methods of digital filtering are finite impulse response (FIR), infinite impulse response (IIR), and Fast Fourier Transformation (FFT). Digital filters primarily sample data within a bandpass (e.g., 13-15 Hz) but do not wholly exclude frequencies above and below this frequency range. These frequencies are attenuated to varying degrees (Thompson & Thompson, 2015).
FIR filters continuously update their averaging of EEG voltage with new data points. A filter's order is determined by the number of data points that it averages. A higher-order filter more sharply attenuates frequencies outside the bandpass. Software may allow you to select both filter type and order. FIR filters attenuate frequencies above and below the bandpass more gradually than IIR and FFT filters.
Higher-order filters trade off precision for speed. The output of a higher-order filter provides a more accurate picture of the power in a specified band but does so more slowly than a lower-order filter. Recall that the order of a filter refers to the number of data samples used to calculate the output. A higher-order filter computes an output using more samples and is more accurate but introduces a longer delay, as each sample represents a period.
IIR filters assign frequency components to discrete bins as the signal is amplified. Demos (2019) likens these filters to a sieve that admits the signal of interest while discarding frequencies that won't be trained or measured. IIR filters are recursive because they use part of their output as input. IIR filters attenuate frequencies outside the bandpass more sharply than FIR filters with the same order, have greater time delay (that depends on frequency) due to greater filter sharpness, achieve faster computation due to their lower order, and are less stable than FIR filters.
FFT filters use Fast Fourier transforms to calculate the average voltage of an EEG signal's component frequencies for a specified time. This period must be at least as long as the most extended frequency period or wavelength. Therefore, to adequately represent 1 Hz (wavelength of 1000 ms) activity in the EEG using a FFT, at least 1 s of data must be used. This results in a too-slow response to provide the optimal representation of the data for real-time training - generally considered to be 250 ms or less. Because of this, FFTs are used for offline signal analysis and processing (qEEG) but not for either amplitude or z-score neurofeedback training.
Demos (2019) likens these filters to slicing a pie. FFT filters attenuate frequencies outside the bandpass more sharply than FIR filters and require greater computing power (Fisch, 1999; Thompson & Thompson, 2015).
Digital filtering methods enjoy four advantages over analog filters. First, clinicians can retrospectively adjust filter settings when reviewing the EEG record since digital filters are programmable. Second, digital filters can be designed to minimize phase distortion (displacement of the EEG waveform in time). Third, digital filters can achieve greater stability over time and across various frequencies. Fourth, digital filters can more accurately process low-frequency signals.
Since different digital filters can produce widely different statistics for the same frequency range, use the same filter for all statistical calculations (Thompson & Thompson, 2015).
Single-Hertz Bins
Neurofeedback providers using products like the Neuroguide LifeSpan database use single-hertz bins to determine the best training range for each client. They locate the highest amplitude bin (highest z-score) and select a range centered on that bin. Deviations from normal are correlated with clinically significant conditions. For example, lower than normal PDRs may index cognitive decline. For example, if the highest amplitude bin was 9 Hz, you could choose an 8-10 Hz training range. The wider the range, the less specific the training is to the highest amplitude bin (Demos, 2019).Single-hertz bins also help identify the posterior dominant rhythm (PDR), the highest amplitude frequency detected at the posterior scalp. The PDR is measured with eyes closed, and interpretation is based on age. Normal values are 6 Hz for 1-year-olds, 8 Hz for 8-year-olds, 9 Hz for 10-12-year-olds, and 10 Hz for 13-14 year-olds. For adults, normal values are between 9 and 11 Hz (Demos, 2019).
Subjective Characteristics of Frequency Bands
Most EEG power or signal energy falls within the 0-20 Hz frequency range. You may recall that hertz (Hz) is an abbreviation for cycles per second. The dominant frequency is the frequency with the greatest amplitude. It is at least 13 Hz in awake adults. EEG power is measured in microvolts or picowatts.
Higher frequencies reflect cognitive activity and active processing of sensory input. They involve relatively desynchronized activity like alert wakefulness and REM sleep. Lower frequencies reflect strongly synchronized activity like interactive neuronal communication, control of network activity, nondreaming sleep, and coma.
The table below is adapted from Wilson et al. (2011) and based on Thompson and Thompson (2015). Different authors define frequency bandpasses differently. For example, delta 0.5-3 Hz or 1-4 Hz.
DELTA (0.5-3 or 4 HZ)
Delta EEG activity, characterized by low-frequency oscillations (0.5 to 3 or 4 Hz), plays a crucial role in various brain functions, particularly during sleep and in pathological conditions.
We will explore the generators of delta activity, its significance in brain function, and its association with disease. Public Domain, https://commons.wikimedia.org/w/index.php?curid=453193
.jpg)
Synchronous means that groups of neurons depolarize and hyperpolarize at the same time. Delta comprises less than 5% of a healthy adult's percent of amplitude compared with 70% for occipital alpha (Thatcher, 1999). The greatest amplitude or signal strength is found in the central region of the scalp. The delta rhythm is the dominant frequency from ages 1-2 and is associated in adults with deep sleep and brain pathologies like trauma and tumors, and learning disability (Hugdahl, 1995; Thompson & Thompson, 2015).
Sleep deprivation can increase delta amplitude. Adult high-amplitude rhythmic delta indicates pathology like traumatic brain injury (TBI). This is often due to white matter damage blocking signals that would otherwise activate the damaged brain regions.
Undergraduates performing problem-solving tasks exhibit arrhythmic delta (Lubar et al., 2001). Children diagnosed with ADHD or learning disabilities may present with diffuse delta and theta. When this occurs, clinicians may inhibit 2-7 Hz instead of 4-7 Hz. Amplitude training is appropriate for inhibiting but not rewarding delta (Demos, 2019).
Low-amplitude delta may be associated with ADHD, anxiety, insomnia, and TBI. Z-score training is safest for uptraining delta (Demos, 2019).
The movie below is a 19-channel BioTrace+ /NeXus-32 display of delta activity © John S. Anderson. Brighter colors represent higher delta amplitudes. Higher peaks represent higher delta amplitudes in the graphs at the end of each line. Frequency histograms are displayed for each channel.
Delta Rhythm Generators
Delta waves are primarily associated with deep stages of sleep, especially slow-wave sleep (SWS), and are generated by the thalamocortical network. This network's synchronization of neuronal activity is critical for generating delta rhythms. Several mechanisms contribute to the generation of delta waves.Thalamic Pacemaker Neurons
Thalamic neurons, particularly those in the thalamic reticular nucleus (TRN), act as pacemakers for delta activity. These neurons exhibit rhythmic burst firing patterns propagated to the cortex, resulting in synchronized delta oscillations (Steriade et al., 1993).Cortical Neurons
Cortical neurons, especially those in the neocortex, also play a significant role in generating delta waves. The interplay between excitatory pyramidal neurons and inhibitory interneurons within cortical columns contributes to the rhythmicity observed in delta activity (Destexhe et al., 1999). Pyramidal neuron graphic © Juan Gaertner/Shutterstock.com.
Thalamocortical Interactions
The reciprocal connections between the thalamus and cortex are essential for generating and maintaining delta rhythms. These interactions facilitate the synchronization of neuronal firing across large cortical areas, leading to the widespread presence of delta waves during sleep (Amzica & Steriade, 1998).The Meaning of Delta EEG Activity
Delta activity is most prominently observed during the deep stages of non-REM sleep, which are believed to play several critical roles.
Sleep and Restoration
Delta waves are a hallmark of slow-wave sleep, a phase critical for physical and cognitive restoration. During this phase, the brain undergoes synaptic pruning, and memory consolidation.During SWS, the body undergoes several restorative processes, including tissue repair, muscle growth, and the release of growth hormones. Delta waves facilitate these processes by ensuring deep, uninterrupted sleep, which is necessary for effective physical recovery (Tononi & Cirelli, 2014). Healthy adult hypnogram from Spieshoeffer et al. (2019).
.jpg)
Caption: representative polysomnogram showing healthy sleep architecture with characteristic and repetitive passage through sleep cycles. N3 represents slow-wave sleep, which occurs more during the first half of the night, whereas REM sleep is more common during the second half. Pathological sleep is characterized by reduced slow-wave sleep and/or REM sleep and/or sleep fragmentation. W: awake; N1: non-REM I sleep; N2: non-REM II sleep; N3: non-REM III sleep; R: REM sleep.
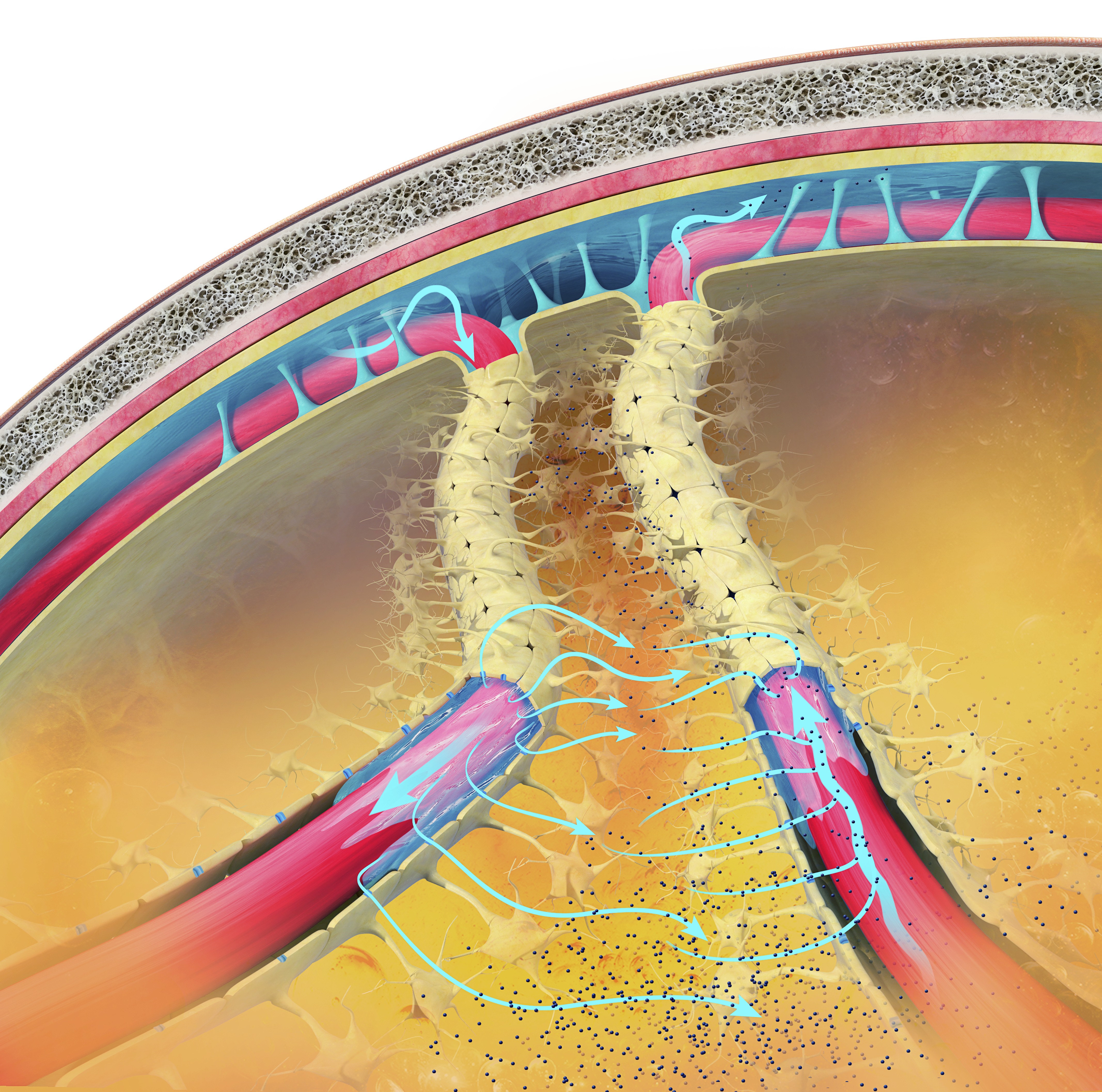
Delta activity helps in clearing metabolic waste products from the brain, such as beta-amyloid, which, if accumulated, can contribute to neurodegenerative diseases like Alzheimer's. This glymphatic clearance system is more active during SWS, supported by delta oscillations (Xie et al., 2013).
The glymphatic system is a newly discovered lymphatic system in the brain. It provides a flow of CSF through the brain's interior that helps clear cellular debris, proteins, and other wastes. Glymphatic system graphic © Claus Lunau/Science Photo Library.
Delta EEG activity during sleep, particularly in the prefrontal cortex, is associated with better performance on neuropsychological tasks specific to the left prefrontal cortex in healthy older adults (Anderson & Horne, 2003). Sleep deprivation can increase delta waking amplitude.
Cognitive Performance
Delta EEG activity increases during mental tasks requiring attention to internal processing, such as difficult mental calculations and short-term memory tasks. This suggests that delta activity is related to the cognitive effort involved in internal processing (Harmony et al., 1996).Memory Consolidation
Delta oscillations are associated with the consolidation of declarative memory. The synchronous activity of delta waves helps to transfer information from the hippocampus to the neocortex, facilitating long-term memory storage (Marshall & Born, 2007).Homeostatic Regulation
Delta activity reflects the homeostatic regulation of sleep. Higher amounts of delta activity indicate a higher sleep pressure, which is the body's way of balancing sleep and wakefulness to maintain overall health (Achermann & Borbély, 2003).Neuroprotection
Delta activity supports the maintenance and health of neurons, potentially protecting against the accumulation of neurotoxic substances. Regular deep sleep with sufficient delta activity is linked to a lower risk of developing neurodegenerative diseases (Varga et al., 2016).Emotional Regulation
Adequate delta activity during deep sleep contributes to emotional stability and resilience.Deep sleep, characterized by delta waves, helps regulate mood and emotional responses. Disruptions in delta sleep are associated with mood disorders such as depression and anxiety (Goldstein & Walker, 2014).
The Delta Rhythm in Disease
Neurodegenerative Disorders
Alterations in delta activity have been observed in neurodegenerative diseases such as Alzheimer's and Parkinson's disease. Patients with Alzheimer's disease, for example, exhibit disrupted delta oscillations, which correlate with cognitive decline and memory impairment (Varga et al., 2016).In nondemented, amyloid-positive subjects, higher delta power is associated with clinical progression from subjective cognitive decline to mild cognitive impairment or dementia. This indicates that delta activity may be a prognostic marker for cognitive decline (Gouw et al., 2017).
Sleep Disorders
Changes in delta activity are also linked to sleep disorders like insomnia and sleep apnea. Reduced delta power during sleep is often associated with poor sleep quality and increased daytime fatigue (Chokroverty, 2017).Psychological Disorders
Delta activity is implicated in various psychiatric disorders, including depression and schizophrenia. Abnormal delta oscillations are often observed in these conditions, suggesting a link between disrupted delta activity and the pathophysiology of these disorders (Gardner et al., 2014).Increased delta activity, along with decreased alpha activity, differentiates psychotic disorders such as schizophrenia, bipolar disorder with psychotic features, and methamphetamine-induced psychosis. This pattern indicates dysfunctional thalamocortical connectivity and may serve as a neurophysiological biomarker for these conditions (Howells et al., 2018).
Children diagnosed with ADHD or learning disabilities may present with diffuse delta and theta. When this occurs, clinicians may inhibit 2-7 Hz instead of 4-7 Hz. Amplitude training is appropriate for inhibiting but not rewarding delta (Demos, 2019).
Low-amplitude delta may be associated with ADHD, anxiety, insomnia, and TBI. Z-score training is the safest way to uptrain delta (Demos, 2019).
Encephalopathy
Specific delta EEG patterns, such as continuous slowing and frontal intermittent delta activity (FIRDA), are associated with different pathological conditions and outcomes in encephalopathic patients. For example, delta activity is linked to alcohol/drug abuse and HIV infection, while FIRDA is associated with past cerebrovascular accidents (Sirin et al., 2019; Sutter, Stevens, & Kaplin, 2012). Three-per-second patterns, previously known as absence seizures, occur within the delta band and are markers of epileptiform activity.Brain Lesions
Focal delta activity on EEG is significantly associated with structural brain lesions, such as those caused by strokes, tumors, and trauma. This correlation highlights the importance of delta activity in identifying underlying brain abnormalities (Gilmore & Brenner, 1981; Nazish, 2020).Amnesic Mild Cognitive Impairment
In patients with amnesic mild cognitive impairment not due to Alzheimer's disease, those with epileptiform EEG activity show higher temporal delta source activities. This suggests the role of neural hypersynchronization in their brain dysfunctions (Babiloni et al., 2020).Conclusion
Delta EEG activity, generated primarily by the thalamocortical network and cortical neurons, is essential for several critical brain functions, especially during slow-wave sleep (SWS). It facilitates physical and cognitive restoration, including synaptic pruning, memory consolidation, and the clearance of metabolic waste. Delta activity is also linked to cognitive performance, homeostatic sleep regulation, neuroprotection, and emotional stability.
Recent research highlights the significance of delta activity in various health and disease contexts. In neurodegenerative disorders like Alzheimer's and Parkinson's disease, disrupted delta oscillations correlate with cognitive decline. Similarly, sleep disorders such as insomnia and sleep apnea are associated with reduced delta power, leading to poor sleep quality and increased fatigue. Psychiatric conditions, including depression and schizophrenia, often exhibit abnormal delta activity, suggesting a role in their pathophysiology. Specific delta patterns are also indicative of encephalopathy, brain lesions, and amnesic mild cognitive impairment, serving as potential neurophysiological biomarkers.
THETA (3-8 HZ)
The theta rhythm ranges from 3-7 Hz, 4-7 Hz, or 4-8 Hz with 20-100 microvolts (Thompson & Thompson, 2015). Theta may be arrhythmic or rhythmic (Demos, 2019). Theta is seen during drowsiness or starting to sleep, hypnagogic imagery (intense imagery experienced before sleep onset), and hypnosis (Libenson, 2024).
The greatest amplitude is found in the frontal and temporal regions of the scalp. Since there may be several theta generators, the theta rhythm is associated with different behavioral processes. The theta rhythm is associated with creativity, but also with anxiety, daydreaming, depression, inattention, and minor TBI. Excessive left hemisphere (LH) theta may be associated with depression, and right hemisphere (RH) theta may be linked to anxiety (Demos, 2019).
EEG activity in the theta frequency band is quite specific to the location where it is recorded. 4-8 Hz activity in temporal areas has different functional and behavioral correlates from the same frequency activity in frontal midline or posterior areas. This, again, reaffirms that location and behavior are essential components when analyzing scalp EEG.
Below is an example of filtered (4-8 Hz) theta activity.
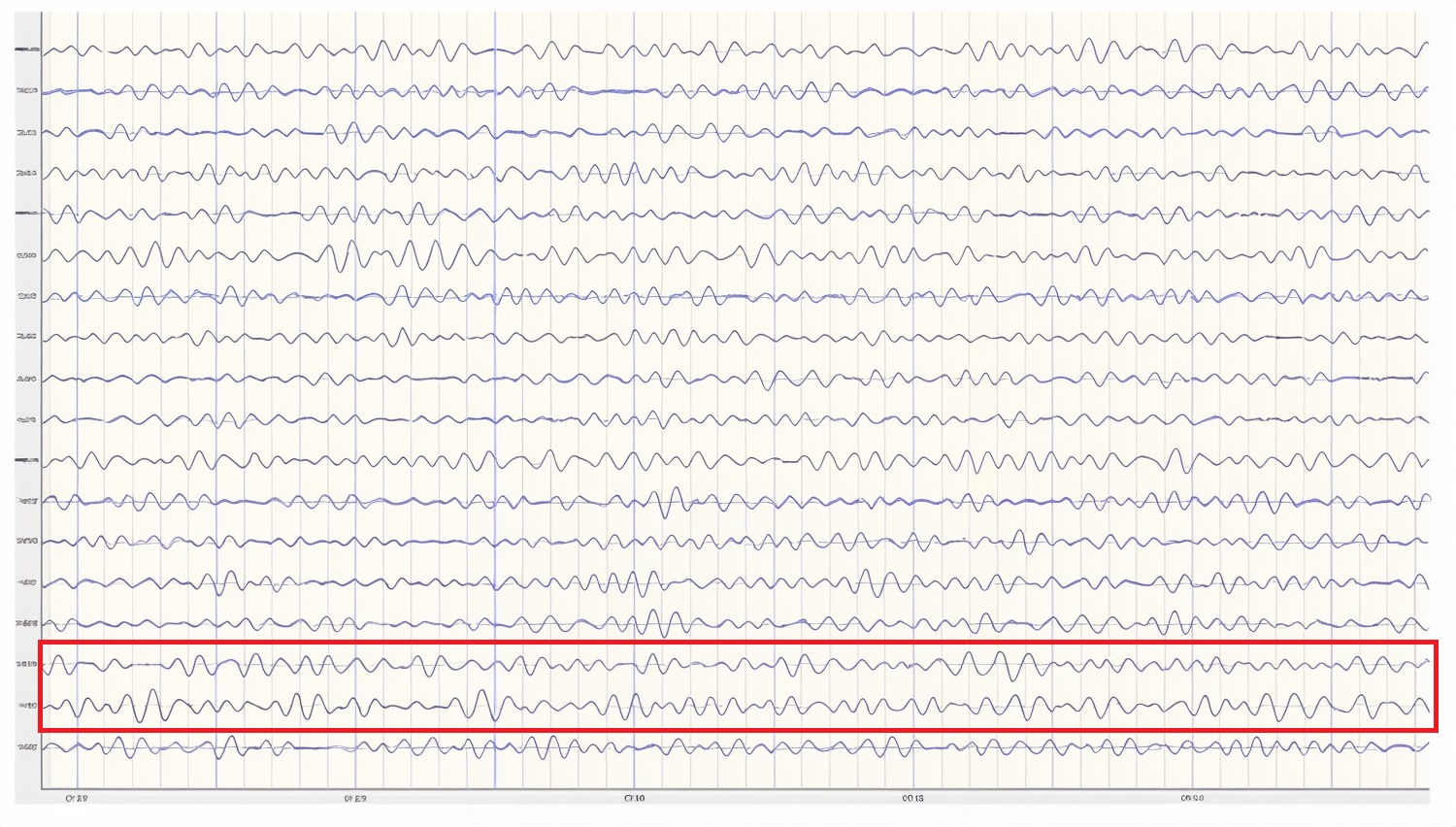
Caption: This is an eyes-closed recording in the longitudinal bipolar montage with a 20-µV scale with dark vertical lines showing the beginning of each new 1-second segment. The EEG is displayed using a 4-8 Hz filter to isolate that frequency band from the full band EEG. Observe the slightly greater amplitude and rhythmicity in temporal derivations.
Below is a 1-45 Hz display of the same EEG recording at the same time location.
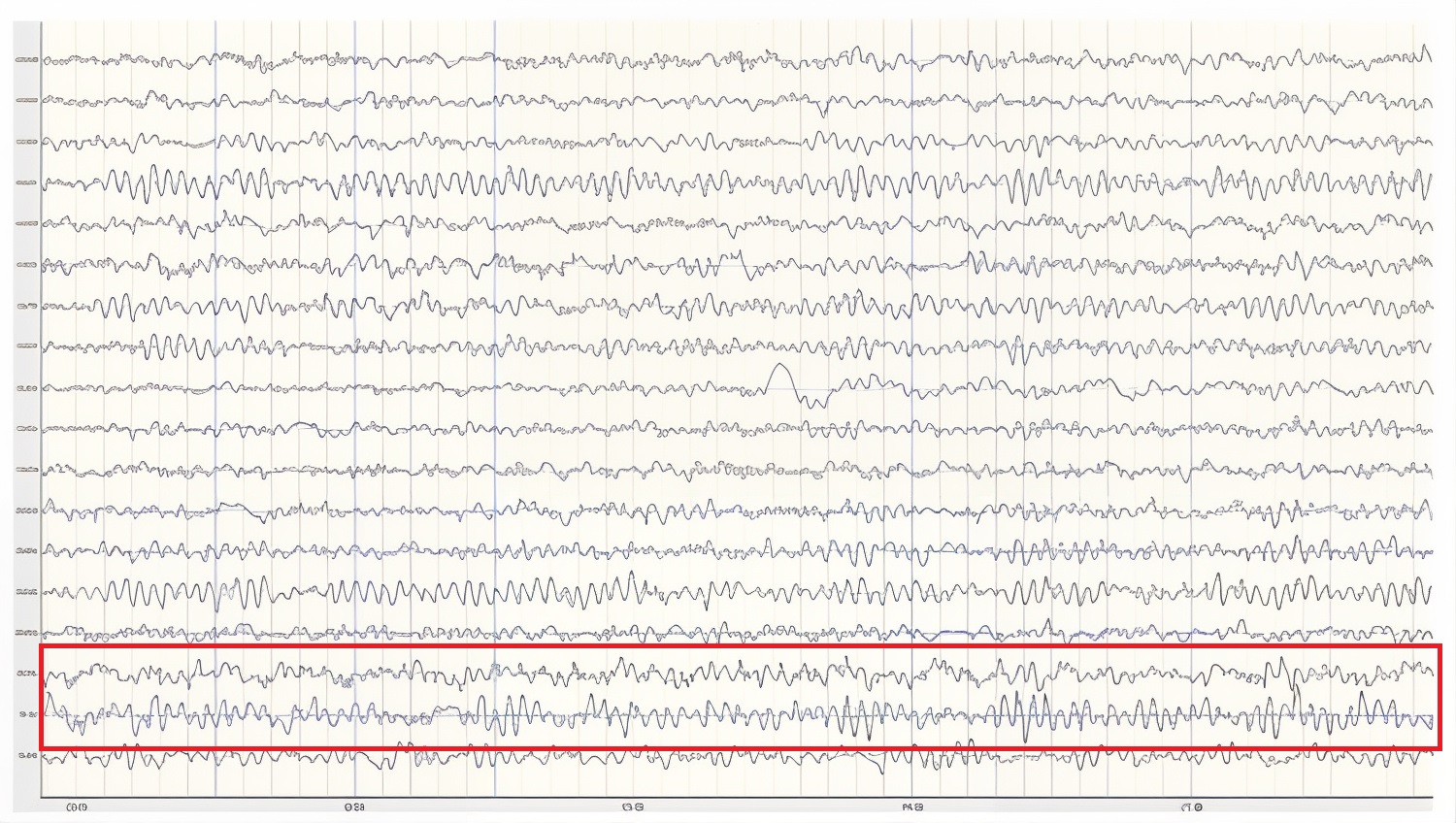
Caption: This is an eyes-closed recording in the longitudinal bipolar montage with a 50-µV scale. The EEG is displayed using a 1-45 Hz filter to show the relatively full EEG band.
Theta activity in an awake adult is considered abnormal (Libenson, 2024).
Amzica and Lopes da Silva (2018) cite various studies regarding the theta rhythm, which they identify as 4-7 Hz. As discussed earlier, they note that normal theta activity should not be confused with pathologic theta, which represents a slowing of the alpha frequency band into the theta range. They suggest that this slowing of alpha may result from reducing cerebral blood flow or metabolic encephalopathies. Metabolic encephalopathies can result from chemical imbalances due to various causal factors, from kidney or liver dysfunction, diabetes, or a variety of other health issues.
Arnolds et al. (1980) found significant differences between behavioral conditions when viewing hippocampal theta recorded with depth electrodes. Writing resulted in faster frequency and greater rhythmicity but lower amplitude than sitting or walking. In contrast, a word association task resulted in faster frequency, greater rhythmicity, and increased amplitude in the period of silence immediately following the question but before the answer was given.
Ekstrom and colleagues (2005) studied hippocampal and neocortical theta activity during a virtual driving task that involved location finding. They found that both areas increased theta during all tasks associated with the driving simulation. A significant correlation between all areas showed increased coordination between multiple areas while accomplishing the tasks. They concluded that cortical and hippocampal theta oscillations and coordination between these areas are associated with attention and sensorimotor integration.
Childhood Disorders
The theta rhythm is the dominant frequency in healthy young children (Thompson & Thompson, 2015). Theta amplitudes and normative theta-to-beta ratios are higher in children than in older adults. Children diagnosed with ADHD often have higher ratios than children without ADHD. Theta-to-beta ratios greater than 3:1 may indicate a slow-wave disorder, and children with a slow-wave disorder may have ratios as high as 6:1 (Demos, 2019). Excessive theta graphic retrieved from ADDYSSEY.
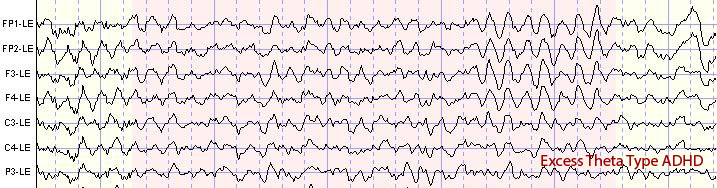
Historically, the study of attention disorders has focused on excess frontal theta activity in individuals with inattentive ADHD. The ratio of theta (4-8 Hz) activity to beta (13-21 Hz) activity, or the theta/beta ratio (T/B ratio), was developed to make the analysis of this metric easier. It was initially calculated using a single channel vertex location at Cz (Monastra et al., 1999). Other studies compared multiple locations and found that the Cz location was accurate and represented the location of the largest deviation of the ratio between previously diagnosed ADHD clients and typical controls (Lubar, 1991). Identifying an elevated T/B ratio (meaning more than typical theta compared to the amount of beta) compared to typical controls appeared to be an accurate way to assess attention disorders.
In a large, blinded, multi-center validation of the theta/beta ratio compared to rating scales for assessing ADHD, the researchers calculated the sensitivity and specificity of measures. They found that the T/B ratio achieved superior results than commonly used rating scales (Snyder et al., 2008). With a sample size of 159 individuals, the EEG assessment showed a sensitivity of 87% and a specificity of 94%, for an overall accuracy of 89% compared to the next closest measure, the Connors’ Rating Scale – Teacher version (CRS – Teacher), with a sensitivity of 67% and specificity of 41%, for an overall accuracy of 58%.
The sensitivity of an assessment measure determines how accurately it identifies individuals known to have a particular condition. Specificity measures how accurately the measure correctly eliminates individuals without the condition from being identified as having the condition.
For example, the Conners Parent Rating Scale-Revised (CPRS-R) shows a sensitivity of 77%, correctly identifying 77 percent of clients with ADHD. It has a specificity of 73%, correctly identifying 73 percent of clients known not to have ADHD. The theta/beta ratio biomarker described above outperforms the CPRS-R by correctly identifying more true positive ADHD and negative non-ADHD clients (Chang et al., 2016).
However, Chang and colleagues note that the Child Behavior Checklist (CBCL), also included in their study, provides a more comprehensive analysis of the client and is more effective at identifying possible comorbidities often mistaken for the different subtypes of ADHD. They also note that using such a checklist approach provides the clinician with information they may not otherwise be able to identify in the clinical setting. It appears that a combined approach using a well-validated checklist in combination with objective measures such as the theta/beta ratio or other quantitative EEG assessment tools and a continuous performance test such as the Test of Variables of Attention (TOVA) would provide a comprehensive assessment for childhood disorders of attention.
In the research of Snyder and colleagues (2008), only 6% of typical children were incorrectly identified using the T/B ratio. This suggests that, at a minimum, a simple, single-channel EEG assessment should be included as a component of a comprehensive approach to identifying ADHD children before prescribing medication.
Interestingly, Van Son and colleagues (2019) expanded the associations that could be identified with the T/B ratio. They determined that a higher T/B ratio was negatively correlated with prefrontal executive control, including response inhibition and negative affect control. This suggests that excess theta in relation to beta activity could lead to greater impulsivity and to a lack of control of negative behaviors. They also found an association between higher T/B ratios and reward-motivated decision-making, possibly selecting immediate gratification at the expense of long-term benefit. Finally, they correlated the T/B ratio with increased mind wandering, decreased executive network functions, and increased default mode network (DMN) activity.
As with all the previous EEG frequencies and assessment measures, the correct amount of a particular frequency activity is important. For example, someone who lacks appropriate default mode functioning may experience a lack of the type of resting-state activity that appears to have a therapeutic effect. The DMN has also been called the resting state network (RSN) due to its functions that differ from task-oriented behaviors. It is thought to be important for a variety of reasons. Therefore, a person with a lower-than-typical T/B ratio may benefit from increased theta voltage and some training in activating the DMN. In contrast, an individual diagnosed with ADHD may benefit from training to reduce or inhibit excess theta voltage.
Temporal lobe theta likely reflects the hippocampal theta identified using depth electrodes. It appears to be associated with route finding and navigation, both hippocampal functions. Differential, interhemispheric (bipolar montage) training of temporal lobe areas in the theta frequency range has been a component of certain approaches to neurofeedback for some time (Othmer, 2007). This approach is used for various conditions, including migraine, tinnitus, PMS, and many others. Frequencies are adjusted to facilitate the optimal response and may range from the alpha frequencies through the theta frequencies down to the infra-low frequencies below 1 Hz.
Again, the analysis of theta activity is often aided by using a normative database. Otherwise, determining whether an amount is too high or too low in amplitude is difficult. Fortunately, for the T/B ratio assessment, Monastra (2001) has provided a table of values with three age ranges: 6-11 yrs, 12-15 yrs, and 16-20 yrs. The table of mean T/B power ratios is below.
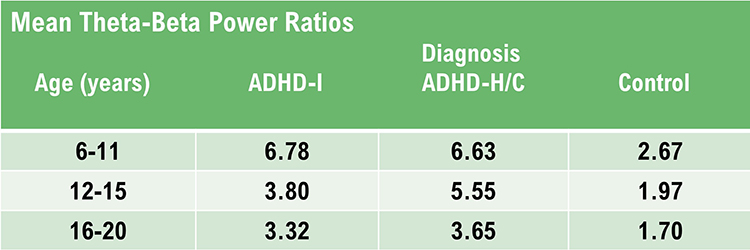
Caption: ADHD-I = attention deficit-hyperactivity disorder, inattentive type; ADHD-H/C = attention deficit-hyperactivity disorder, hyperactive-combined type.
Assessment of theta activity, more generally, beyond the T/B ratio in the central midline, is more challenging. As van Son (2019) noted, assessment under task may be essential to determine these results more accurately.
Two strategies to reduce high theta-to-beta ratios are amplitude training (down-training theta) and ratio training (rewarding decreases in the theta-to-beta ratio; Demos, 2019).
Cognitive Decline
Brief memory lapses (senior moments) are associated with LH bursts of rhythmic temporal theta (BORTTs) due to sleepiness or reduced hippocampal perfusion. Assessment and training should incorporate memory tasks. The protocol should inhibit LH temporal lobe theta, particularly at T3.
Where BORTTs are produced by drowsiness, training should include behavioral interventions to reduce insomnia (Demos, 2019).
While alpha/theta protocols to treat substance use disorders up-train theta, this should be proscribed in epilepsy in the frontal lobes, where it could impair attention or decisions, or in PSTD due to the risk of provoking flashbacks (Demos, 2019).
The movie below is a 19-channel BioTrace+ /NeXus-32 display of theta activity © John S. Anderson. Brighter colors represent higher theta amplitudes. Frequency histograms are displayed for each channel.
Substance Use Disorders
While alpha/theta protocols to treat substance use disorders up-train theta, this should be proscribed in epilepsy in the frontal lobes, where it could impair attention or decisions, or in PSTD due to the risk of provoking flashbacks (Demos, 2019). Using alpha-theta training protocols has generated caution from some practitioners and instructors, as indicated above in Demos (2019) and in conference presentations (personal experience). These cautions may result from a misunderstanding of the mechanism of alpha-theta training. In an upcoming post, John S. Anderson will address some of these issues and questions.
Final Notes
Recognition of standard EEG frequencies, analysis of such activity, and use of this information for training and re-assessment are important parts of neurofeedback practice. This section has attempted to provide an overview of this area to aid new practitioners and experienced clinicians in furthering their understanding of this complex study area.
One of the greatest benefits of neuroscience in general and electroencephalography and neurofeedback specifically is that they encourage lifelong learning. Suppose this section appeared overwhelming, with few hard and fast rules or concrete facts to hold on to. In that case, it is important to remember that one can do useful and effective neurofeedback without an in-depth understanding of this area. Understanding the EEG comes gradually through regular exposure to educational materials, lectures, and workshops, working with an experienced mentor, and regular interaction with clients and their EEG recordings. Pursuing and maintaining certification with the Biofeedback Certification International Alliance (BCIA) is a good way to engage in continuing professional education and lifelong learning.
There is no substitute for viewing large numbers of EEG recordings. As mentioned in the beginning, this can start with an EEG atlas and then progress to examining your own recordings, whether a single channel, a couple of channels, or multiple channels.
Patience with one’s process is an integral part of any learning experience. Think of the time it takes to learn any worthwhile skill, from swimming to learning a musical instrument to learning a new computer or phone operating system. Managing one’s expectations is crucial.
ALPHA (8-12 or 13 HZ)
Does the Alpha Rhythm Range From 8-12 or 8-13 Hz?
In electroencephalography (EEG), the alpha rhythm is generally defined as having a frequency range of 8-12 Hz or 8-13 Hz. This discrepancy arises from variations in historical definitions, regional practices, and updates in scientific standards.
Many sources traditionally define the alpha rhythm within the 8-12 Hz range. This is common in older literature and is used in some clinical contexts to describe the typical frequency band associated with a relaxed, awake state. For example, Jadeja (2021) describes clinically relevant EEG frequency bands, including the alpha rhythm, as 8-12 Hz.
More recent or alternative standards may extend this range to 8-13 Hz to accommodate a broader spectrum of alpha activity observed in various populations and conditions. For example, Kane and colleagues (2017) offered the following revised definition:
Rhythm at 8–13 Hz inclusive occurring during wakefulness over the posterior regions of the head, generally with maximum amplitudes over the occipital areas. Amplitude varies but is mostly below 50 µV in the adult, but often much higher in children. Best seen with the eyes closed, during physical relaxation and relative mental inactivity. Blocked or attenuated by attention, especially visual, and mental effort. Comment: use of term rhythm must be restricted to those rhythms that fulfill these criteria. Activities in the alpha band which differ from the alpha rhythm as regards their topography and/or reactivity, should either have specific appellations (for instance: the mu rhythm and alpha coma) or should be referred to as rhythms of alpha frequency or alpha activity.
Summary
The slight variation in definitions typically does not impact the practical applications significantly but reflects different methodological approaches or updates in EEG research standards. The choice of definition can depend on the specific requirements of a study or the preference of a clinical guideline being followed. Both ranges are correct.The Source of the Alpha Rhythm
Alpha waves are also integral to emotional regulation. Studies have shown that increased Alpha power in the right frontal cortex is associated with positive affect, while reduced alpha activity in this region correlates with negative emotions such as anxiety and depression. This lateralization of alpha activity reflects the brain’s ability to balance emotional responses, with implications for understanding mood disorders. Conversely, excess alpha activity in the left frontal cortex is linked to depression and lack of engagement in everyday life tasks.
Activity in the 8-12 Hz frequency range appears associated with reduced sensory and cognitive activity. Why is this so? What mechanism is responsible for this rhythmic activity?
One of the main communication pathways between the external world, the senses that perceive and transmit this information, and the cortical neurons that receive it is the thalamic-cortical relay system, often designated the TCR system. Remember that alpha activity is also associated with anterior/posterior interactive communication, which integrates posterior sensory processing and integration with anterior cognitive processing, decision making and executive functions.
The thalamus receives incoming sensory input (a paired structure in the brain's center. The individual nuclei of the thalamus transmit that sensory information to appropriate areas of the cortex. The occipital and parietal areas of the cortex are the primary visual processing areas, just as the temporal areas process most of the auditory information. In contrast, central Rolandic areas process tactile and other signals from the skin and muscles. Of course, current findings indicate that brain activity is associated with coordination within and between cortical networks and influences from subcortical structures and local and TCR influences. Still, the TCR system is a primary pathway for determining which cortex areas receive each type of sensory input.
When the eyes close, the neurons responsible for processing incoming visual information no longer have “ work” to do, and so they respond to another signal coming through the TCR system. This is a rhythmic signal mediated by a membrane of inhibitory GABAergic neurons that surround most of the thalamus and provide inhibitory regulation of the signals traveling to the cortex. This is called the reticular nucleus of the thalamus (TRN) or nucleus reticularis of the thalamus (NRT). The function of this system is much too complex for this section, but a good treatment is available in Crabtree (2018), and an examination of the role of the TCR and TRN systems in consciousness is found in Min (2010).
The rhythmic signal from the TCR and NRT interaction produces a 10-Hz (8-12 Hz range) input to the visual processing neurons when visual sensory input is withdrawn (eyes closing), resulting in those neurons firing synchronously in that frequency in response to this input. The voltage of a specific EEG frequency, measured at the scalp surface, is directly proportional to the number of cortical neurons firing synchronously in that frequency (Nunez & Srinivasan, 2006). Therefore, when the eyes are closed, and the signal from the TCR system changes from sensory input to a rhythmic 10-Hz input, visual neurons respond to that input, and the voltage of alpha, and most specifically in adults, 10-Hz activity increases in voltage. The image below shows a spectral display of an eyes-closed EEG. Graphic redrawn by minaanandag on Fiverr.com.
.jpg)
Caption: An eyes-closed EEG in a longitudinal bipolar montage is represented as a spectral display. The x-axis shows frequency from 0-30 Hz, and the y-axis shows absolute power (uV Sq). Note the peak at about 10 Hz. Voltage is higher in the P3-O1 derivation than in the P4-O2 derivation, revealing a small asymmetry. The highest power is in the T4-T6 derivation.
Identifying alpha activity in the scalp's parietal and/or occipital areas is usually quite easy, particularly in the eyes-closed condition. The image below shows an eyes-closed alpha pattern from a 15 -year-old male.
.jpg)
Caption: eyes-closed EEG filtered to 1-45 Hz in the longitudinal bipolar montage with a 50-uV scale. Boxes indicate the most prominent 8-12 Hz activity. This montage represents a series of adjacent electrode comparisons or derivations, as each signal tracing is derived from each pair of electrode comparisons.
A longitudinal bipolar montage is displayed below.
.jpg)
Caption: Longitudinal bipolar montage (commonly known as the “double banana” montage).
Observe that the rhythmic activity is well-defined and has the typical bursting or spindling pattern of the alpha rhythm, resulting from the input of the TCR and NRT systems. Spindling consists of a series of distinct oscillations of a particular frequency that begin with relatively low amplitude, increase in amplitude, and then decrease in amplitude, giving the appearance of a spindle such as one used in spinning, with fiber wound around it. Graphic © New Africa/Shutterstock.com.

The waves are quite sinusoidal (waving up and down in a smooth rhythm similar to a sine curve) and continue throughout the recording with minimal disruption. The voltage indicator shows that the maximum voltage at the moment of the line placement was from about 20 to 30 uV at the peak of the waveform in this montage.
One can determine the wave's frequency by counting the number of peaks in a one-second segment or counting the number of times the wave crossed the zero line and dividing by 2 (zero crossings/2). Either method gives an 8-9 Hz value when multiple one-second epochs are counted. This is somewhat slow for a 15-year-old, although the voltage appears to be within normal limits. When compared to a normative database, we can see that, indeed, it is a slow peak alpha when compared to other 15-year-old males, as indicated by the chart below from the NeuroGuide™ database.
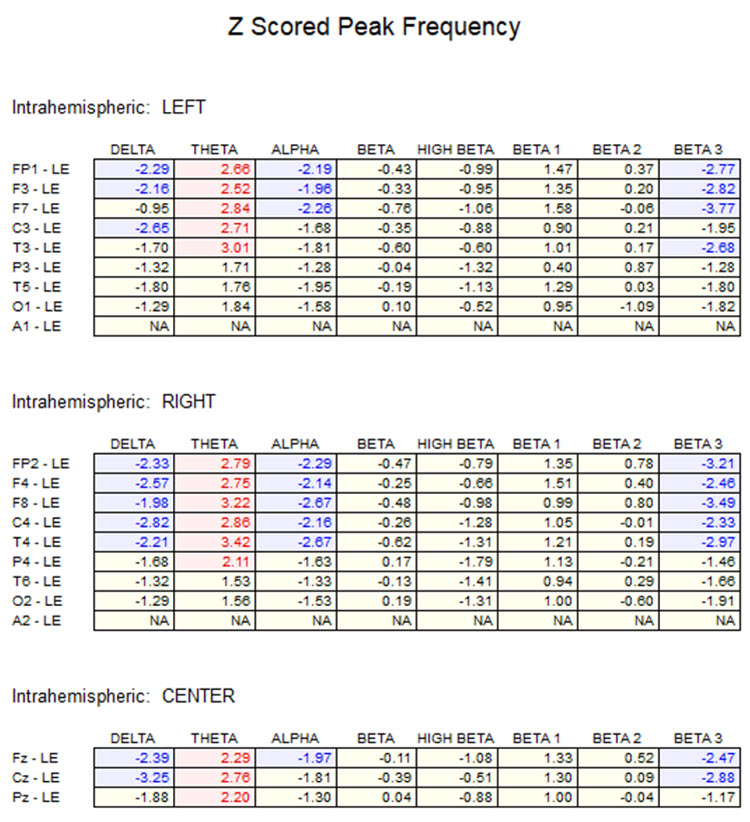
Caption: the alpha peak frequency z-scores are at least 1 SD below expected values (> -1.0 SD) in all locations, and many areas show deviations exceeding the significance cutoff of -1.96 SD (blue highlight). The database also plots the theta peak frequency as fast (red highlight) due to slow alpha in the 8-Hz frequency bin or segment. There are likely other slow components of the dominant rhythm that contribute to this incorrect plot of the frequency information. Ideally, the peak frequency of the EEG should be calculated within a broad range from approximately 6 Hz to about 14-16 Hz to avoid this type of error. This is another problem with the somewhat arbitrary designation of frequency bands using set values.
The same data processed by the iSynchBrain database show similar findings for O1 and O2 below.
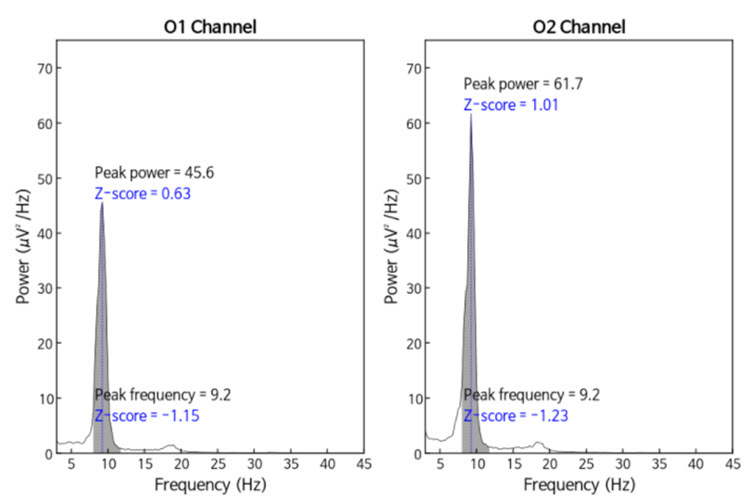
Caption: Peak frequency power and frequency comparisons at O1 and O2 show a slow peak frequency at 9.2 Hz bilaterally, resulting in z-scores of -1.15 and -1.23, respectively. Voltage (power) on the left is 0.63 SD, and on the right is 1.01 SD, showing slightly elevated values. This database provides single-Hz calculations for frequency and amplitude rather than using an arbitrary band to define alpha.
The alpha peak frequency is age-dependent, though not called "alpha" until the frequency reaches 8 Hz. It is designated as the posterior basic rhythm or posterior dominant rhythm (PDR) in early infancy. It appears around 4 months with a frequency of about 4 cycles per second (c/s) or Hz (Schomer & Lopes da Silva, 2017). The PDR increases (speeds up) during maturation and is approximately 6 c/s at 1 year and up to 8 c/s at around 3 years of age. This is when it can be called the alpha rhythm.
The frequency reaches 10 Hz by approximately 10 years of age, and that (10.2 ± 0.9/sec) is the peak frequency of adulthood (Petersén & Eeg-Olofsson, 1971). The previous example shows why a peak frequency between 8-9 Hz is slow for a 15-year-old.
The Alpha Frequency's Meaning and Importance
The speed or frequency of the alpha peak frequency is not often mentioned, even in a neurologist’s report. For the neurofeedback practitioner, it is helpful to understand the factors associated with different alpha frequencies. The alpha peak frequency measures the frequency of the rhythmic pattern of the posterior rhythm (generally called alpha). This has traditionally been an important measure. Although it has recently been somewhat de-emphasized in some EEG circles, it remains an interesting measure. A great deal of research supports it as a useful metric for assessment.Researchers such as Klimesch and others (Haegens et al., 2014; Hanslmayr et al., 2005; Mierau et al., 2017) have found it important to first assess the individual peak alpha frequency for normal subjects before investigating the cognitive effects of alpha neurofeedback.
The speed of the alpha frequency is also dynamic and reflects the type of activity. Increased cognitive load, particularly related to complex memory tasks, will briefly speed up the alpha frequency. Metabolic and gender factors also affect the speed of the alpha frequency.
During the menstrual cycle, women experience hormonal fluctuations, which directly affect this measure, alternately speeding and slowing the peak alpha frequency. The speed of the alpha frequency is also dynamic and reflects the type of activity. Increased cognitive load, particularly related to complex memory tasks, will briefly speed up the alpha frequency. Metabolic and gender factors also affect the speed of the alpha frequency. During the menstrual cycle, women experience hormonal fluctuations, which directly affect this measure, alternately speeding and slowing the peak alpha frequency.
A slow peak alpha frequency has been associated with some forms of cognitive decline and memory impairment (López-Sanz et al., 2016), as well as mild traumatic brain injury (mTBI; Jabbari et al., 1985; Williams, 1941). A fast peak alpha frequency has been associated with improved scores on timed IQ tests. It has also been associated with enhanced memory and cognitive performance in various age groups (Grandy et al., 2013). A faster peak alpha frequency is associated with advanced reading skills in precocious children (Suldo et al., 2001).
Negative correlations of a peak alpha frequency faster than 10.5 Hz, possibly associated with an overly activated central nervous system, may include sleep initiation problems, anxiety, intrusive thoughts, and difficulty with self-soothing and self-calming skills.
The peak alpha frequency changes throughout the lifespan. Therefore, age-normed values for the peak alpha frequency are important for assessment purposes. The normal adult peak alpha frequency is 9.5-10.5 Hz (Jabbari et al., 1985). Scholarly literature states that the peak alpha frequency is not abnormal until it is below 8 Hz (Schomer & Lopes da Silva, 2017). However, it is commonly thought to be potentially meaningful when the frequency is below 9 Hz for an adult.
Angelakis et al. (2004) stated that slowing the alpha peak frequency by more than 1 Hz (9 Hz for an adult) is generally a sign of pathology. A slow alpha frequency can be associated with fatigue, cognitive decline, and memory impairment. Slowing of the background alpha rhythm is also a sign of generalized cerebral dysfunction (Nayak & Anilkumar, 2021). Rathee and colleagues (2020) related the speed of the peak alpha frequency to reading comprehension. They found that a slower peak alpha frequency is associated with poor comprehension.
Asymmetries in the posterior rhythm are common, and some are expected. Typically, the posterior rhythm has a higher voltage over the right, nondominant hemisphere. Surprisingly, a complete absence of the posterior rhythm can occur in a small percentage of otherwise normal individuals. While the absence of the posterior rhythm can also be seen in individuals with brain injuries or other abnormalities, such cases usually exhibit additional EEG abnormalities. Therefore, an EEG that only shows the absence of the posterior rhythm without other abnormalities should be considered normal (Libenson, 2024).
However, other sources correlate the absence of the posterior alpha rhythm with a variety of other indicators, even when additional abnormal EEG patterns are not present.
Niedermeyer (1997) discusses the absence of the posterior alpha rhythm and points out correlations between chronic alcoholism and vertebrobasilar artery insufficiency. He suggests that a lack of posterior alpha may be associated with dysfunctional synchronization mechanisms and states:
The crucial factor in these considerations might be the question: is absence of the alpha rhythms in the scalp EEG (and even in recordings from deeper structures) synonymous with alpha absence in the microstructure? In other words: is alpha rhythm a truly universal phenomenon in healthy persons regardless of EEG-alpha-absence caused by lack of synchronizing mechanisms? Accordingly, persons with an inherited low voltage fast pattern do have a posterior alpha but are unable to show it in their EEG records.Other conditions that show an absence of the posterior alpha rhythm include seizure disorders. Aich (2014) and colleagues found a significant correlation (<0.01) between the presence of seizure activity and the absence of the alpha rhythm. This study identified 48.3% of their identified seizure disorder participants as having no visible alpha rhythm.
Eyes-Closed Alpha Response Voltage Meaning and Importance
The amplitude of the activity can also be meaningful in addition to the peak frequency within the 8-12 or 8-13 Hz alpha band. Anxiety and TBI can reduce alpha activity during baselines. Since concentration and thinking can suppress alpha, instruct clients to refrain from these activities during recording (Demos, 2019).The alpha voltage will be partially affected by the montage that is used. For example, considering the discussion of differential amplifiers in the Instrumentation and Electronics section, the closer two electrodes are to each other, the more the rhythmic, synchronous patterns will be attenuated. Common-mode rejection (CMR) is most sensitive to frequency synchronization, so waves that are the same frequency and also synchronous (e.g., 10 Hz waves, waving up and down at the same time at the two sensor locations + and – [also commonly called “active” and “ reference”]) will be rejected. Comparing the O1 and O2 occipital electrodes to each other will result in a lower apparent alpha voltage if the two waveforms are synchronous, which is quite likely.
Conversely, comparing either O1 or O2 to an ear reference or possibly to a forehead reference would result in almost no rejection of alpha activity. Therefore, the rhythmic patterns are unlikely to be similar at these distant locations and will be retained. When viewing standard voltage information in an atlas, a research paper, or a textbook, try to identify the montage used when those standards were developed.
Simonova et al. (1967) found amplitudes between 20 and 60 μV in 66% of their subjects, while values below 20 μV were found in 28% and above 60 μV in only 6%. Schomer and Lopes da Silva (2017) suggest that values between 10 and 60 μV are typical. However, other sources such as the John Hopkins Atlas of EEG (2011) and Libenson’s (2024) Practical Approach to Electroencephalography (2nd ed.) cite 20 μV as the minimum voltage for adults. These differences may seem insignificant but can represent the difference between a low voltage fast EEG finding and a typical assessment.
Rhythmic alpha activity represents the synchronization of the EEG. It represents part of the excitation/inhibition cycle. When either large or small groups of neurons perform tasks, this results in the desynchronization of the EEG during work, as each group of neurons performs its function locally and independently. This is followed by a resting or inhibitory phase that results in the synchronization of the EEG and, hence, an increase in alpha amplitude as many neurons fire synchronously. This is seen in the shift from active visual processing when the eyes are open to a synchronous pattern of oscillatory activity when neurons do not have incoming visual input to process and can rest. This measure of alpha voltage change from eyes open to eyes closed, known as the alpha response, and the decrease of alpha with eyes opening, called alpha blocking, helps identify if the work/rest cycle is occurring correctly.
Someone with an eyes-closed posterior dominant rhythm voltage below 20 μV suggests to the neurofeedback assessor that the person does not easily shift to a state of decreased arousal/alertness necessary for the alpha amplitude to increase. The disconnection from the outside world upon eyes closing should result in decreased sensory processing of vision and other senses and decreased cognitive activity, leading to an increase in 8-12 Hz amplitude or power.
Typically, alpha activity voltage should increase as more neurons fire synchronously in this frequency. When this increase is less than 50% above the resting baseline eyes-open alpha voltage, it usually indicates some difficulty turning off the mind, meaning that neurons remain activated and working and thus prevent them from entering a resting state. The lack of a typical alpha increase may be associated with heightened states of alertness and vigilance, meaning that these clients maintain their external perceptive focus and/or cognitive activity, even when the eyes are closed, likely inhibiting the ability to achieve global synchronous activity. Resulting behavioral consequences can include fatigue, as the neurons are constantly engaged and are not allowed to rest. This pattern may be associated with a history of trauma and/or a history of hypervigilance for various reasons.
Example of a typical alpha response upon eyes closing.
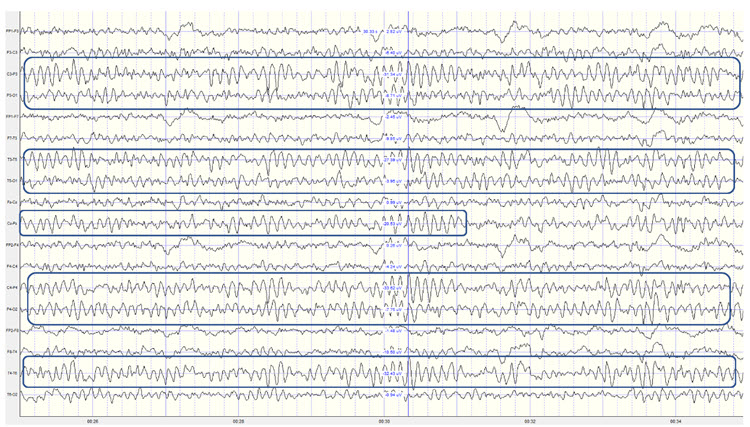
Caption: This is a 19-channel recording of the transition from eye-open to eye-closed conditions. It is a longitudinal bipolar montage, and the scale is 50 μV. Note that the eyes close at minute 2:40 (indicated by the eye movement), and the immediate response of the alpha rhythm appears.
The movie below is a 19-channel BioTrace+ /NeXus-32 display of alpha activity under eyes-closed and eyes-open conditions © John S. Anderson.
Alpha-Blocking Response Meaning and Importance
Conversely, the continued presence of alpha once the eyes are open suggests a lack of appropriate alpha blocking. This appears to result from a lack of inhibition of synchronous generator mechanisms. Hartoyo and colleagues (2020) showed a simple mechanism: excitatory input to inhibitory cortical neurons. This differs from the excitatory cortical neurons that typically reduce synchronous cortical firing in favor of local responses to incoming stimuli when the eyes are opened.
Note that activation of inhibitory mechanisms results in increased inhibition, even though the function is initially excitatory. Conversely, activation of excitatory mechanisms results in greater activation. This can seem confusing, and it may help to focus on the result, whether excitatory or inhibitory, rather than the initial behavior.
There should be a dynamic balance between excitation and inhibition in the human neocortex (Dehghani et al., 2016). When this balance is disrupted, we see the behavioral effects noted here. Alpha blocking represents the re-activation of visual processing neurons when visual input returns. Typically, as these neurons are no longer in a common or general resting state but are involved in task-oriented behaviors that are more localized, synchronous activity should decrease (be inhibited). Therefore, the overall voltage will decrease because of less synchronization. This does not imply that more neurons are firing when the alpha amplitude is higher; it means less synchronization and, hence, lower voltage.
Imagine an auditorium where everyone claps in unison (eyes-closed alpha rhythm). The noise is loud because the claps are synchronized (higher amplitude), with silence in between. Now, picture everyone clapping independently, perhaps in sync with immediate neighbors but not the whole audience. This resembles the eyes-open condition when recording the alpha rhythm: there is continuous noise because someone is always clapping, resulting in a faster frequency of claps. However, it’s never as loud as synchronized clapping, leading to lower overall voltage despite the higher frequency. Thus, alpha amplitude decreases quickly when eyes open, within 1-2 seconds, and certainly within 10-15 seconds. Delays in alpha blocking suggest difficulty returning to the task. Desynchronization occurs in posterior areas as visual processing begins when the eyes are opened.
The most common reasons for the lack of appropriate alpha blocking (meaning alpha activity persists after eyes are opened) are:
1. Fatigue, including sleep deprivation
2. Long-term meditation practice, particularly mantra meditation
3. Marijuana use and abuse, generally long-term, chronic
4. Cerebral dysfunction due to disease, injury, or possibly chemical exposure
Below is an example of correct alpha-blocking following the eyes opening.
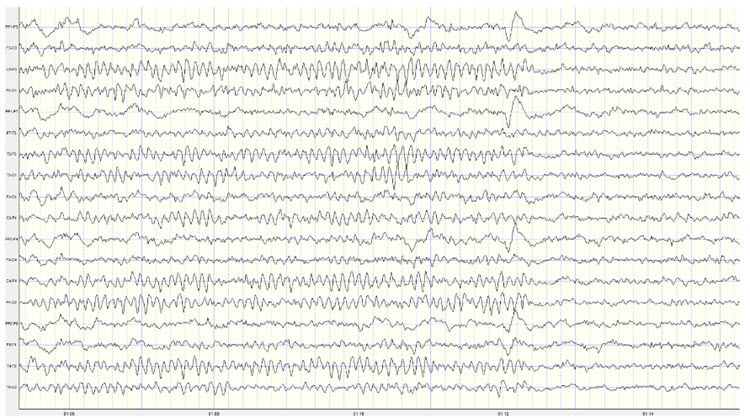
Caption: This is a 19-channel recording of the transition from eyes-closed to eyes-open conditions. This is a longitudinal bipolar montage, and the scale is 50 μV. Note the eyes opening at minute 1:12 (indicated by the eye movement) and the immediate blocking of the alpha rhythm.
Clearly, the response of 8-12 Hz EEG activity can be quite revealing and provides the clinician with helpful information about the client. However, it is important to note that other factors can affect the EEG recording. We have already noted the effects of artifacts on the EEG in general. Additionally, the client’s state of mind, level of anxiety, comfort with the application of sensors to the scalp, level of trust of the practitioner conducting the recording, amount of sleep, the use of caffeine and other stimulants and common medications can all affect the results of the recording.
Once an assessment is made of excess or deficient alpha activity, lack of an alpha response or persistent alpha following the eyes opening, or a slow or fast peak alpha frequency, the clinician can proceed with training to address these findings.
In neurofeedback training, Alpha waves are a primary target for interventions aimed at improving relaxation, focus, and emotional regulation. Alpha neurofeedback involves training individuals to enhance or suppress Alpha activity in specific brain regions based on their unique needs. For example, increasing posterior Alpha activity is commonly used to promote relaxation and reduce stress, while suppressing excessive frontal Alpha may help alleviate symptoms of depression and improve motivation. The speed of the peak alpha frequency can be trained as well, increasing a slow frequency to improve cognitive performance or slowing an excessively fast frequency to reduce anxiety and hypervigilance.
There are multiple approaches to training the 8-12 Hz frequency band, including training specific segments of that band to achieve training goals. For example, if a lack of an alpha response to eyes closing is associated with anxiety and possibly insomnia, training for an increase in the 8-10 Hz portion of the posterior alpha rhythm in the eyes-closed condition may be an effective intervention. If the peak alpha frequency is slow, training for increases in the 10-12 Hz portion may help speed up this frequency. If there is persistent alpha in the eyes-open condition, inhibiting or downtraining 8-12 Hz generally may be helpful.
Of course, with any intervention, other causal factors must be addressed as well. Persistent alpha and/or frontal alpha can signify fatigue secondary to a sleep disorder such as sleep apnea. Therefore, a referral to a physician for a sleep study may be helpful. Over-arousal patterns that correspond to a lack of alpha response can be associated with a history of emotional, psychological, physical, or sexual trauma. The neurofeedback clinician may need to address these issues or refer the client to an appropriate therapist.
Summary
The alpha rhythm is characterized by a frequency range of 8-12 Hz or 8-13 Hz, with the discrepancy arising from variations in historical definitions, regional practices, and scientific standards. Traditionally, the 8-12 Hz range is used in older literature and clinical contexts. However, more recent standards extend this to 8-13 Hz to include a broader spectrum of alpha activity.
The alpha rhythm is primarily observed when a person is awake, relaxed, and with their eyes closed. This activity is mediated by the thalamic-cortical relay (TCR) system, involving the thalamus and cortical neurons. When visual input ceases (e.g., eyes closed), the reticular nucleus of the thalamus (TRN) sends rhythmic signals to the visual processing neurons, producing the alpha waves detected in EEG recordings.
Clinically, the alpha rhythm is crucial for diagnosing sleep disorders, cognitive function, and various neurological conditions. A slow peak alpha frequency is linked to cognitive decline and memory impairment, while a faster peak frequency correlates with better cognitive performance and higher IQ scores. Neurofeedback protocols often use individual peak alpha frequencies to enhance cognitive functions. Deviations from the normal alpha frequency range can indicate neurological or psychiatric conditions, such as generalized cerebral dysfunction or persistent alpha activity with eyes open.
The analysis of alpha rhythms in EEG is essential for clinical and research purposes, aiding in diagnosing and understanding various conditions and developing targeted treatments. The amplitude and frequency of alpha activity provide insights into a patient's cognitive and emotional state, supporting personalized therapeutic interventions. The importance of alpha rhythms underscores their role in cognitive neuroscience and clinical neurophysiology.
SENSORIMOTOR RHYTHM (12 or 13-15 HZ)
Sensorimtor rhythm (SMR) activity falls within the frequency range of 12 to 15 Hz, overlapping with the beta band's lower and alpha bands' higher ends. SMR waves are defined by their frequency and location, and are distinct from other EEG rhythms due to their association with a calm, alert state.SMR is typically recorded over the sensorimotor cortex and characterized by rhythmic oscillations distinct from the higher-frequency beta and lower-frequency alpha waves (Sterman, 1996). Activity within this 12-15 Hz frequency band occurring in regions other than the sensory motor cortex is considered low beta or beta1 and is not defined as the sensory motor rhythm.
The SMR rhythm is generated by thalamic ventrobasal relay cells and reentrant thalamocortical loops (Thompson & Thompson, 2015). These circuits involve the thalamus sending sensory information to the cortex, where it is processed and sent back to the thalamus, creating rhythmic oscillations (Lopes da Silva, 1991). The sensorimotor cortex, particularly the primary motor cortex (M1) and the primary somatosensory cortex (S1), contains neurons that contribute to the generation of SMR. The synchronized activity of these neurons results in the rhythmic patterns observed in SMR (Pfurtscheller & Lopes da Silva, 1999).
The movie below is a 19-channel BioTrace+ /NeXus-32 display of SMR activity © John S. Anderson. Brighter colors represent higher SMR amplitudes. Frequency histograms are displayed for each channel. Notice the runs of high-amplitude SMR activity.
The Meaning of the Sensorimotor Rhythm
SMR activity is associated with a state of relaxed wakefulness and motor inhibition.Please click on the podcast icon below to hear a lecture over the second half of this unit.

The sensorimotor rhythm may signal an internal focus (Demos, 2019). It is believed to reflect the brain's ability to maintain a calm, focused state without engaging in motor activity. SMR is often considered a marker of optimal sensorimotor integration linked to several cognitive and motor functions.
SMR is related to the suppression of motor activity, indicating a state where the brain is inhibiting unnecessary movements. This is important for stillness and precision tasks (Sterman, 1996). Increased SMR activity is associated with improved motor performance and reduced motor activity (Corsi-Cabrera et al., 2001; Sazgar & Young, 2019).
Increased SMR activity is associated with improved attention and focus, as it reflects a state where the brain is not distracted by extraneous motor activity and instead concentrates on sensory input and cognitive tasks (Egner & Gruzelier, 2001).
Psychological and Medical Disorders
Attention-Deficit Hyperactivity Disorder (ADHD)
Individuals with ADHD often exhibit reduced SMR activity. Neurofeedback training to increase SMR can improve attention and behavioral control (Lubar, 1991). Based on six RCTs, Stefanie Enriquez-Geppert and colleagues (2023) rated neurofeedback for ADHD as efficacious and specific in Evidence-Based Practice in Biofeedback and Neurofeedback(4th ed.).Generalized Seizures
Abnormal SMR activity can be indicative of dysfunctional thalamocortical circuits, which are often implicated in the generation of epileptic seizures. Modulating SMR activity through neurofeedback or other interventions has been explored as a potential therapeutic approach for reducing seizure frequency and severity (Moffet et al., 2017).Frey (2023) rated SMR-based and slow cortical potential (SCP)-based neurofeedback as efficacious for seizures, SMR-based NFB as probably efficacious for non-seizure manifestations of epilepsy, and connectivity-based NFB as not empirically supported for seizures.
In two meta-analyses (Soroush et al., 2023; Tan et al., 2009), SMR- and SCP-based treatments were associated with fewer seizures. In a sham-controlled RCT, neurofeedback for pediatric focal epilepsy was associated with improved quality of life, but only SMR neurofeedback improved cognition (Morales-Quezada et al., 2019).
Anxiety and Depression
SMR neurofeedback has also been explored as a treatment for anxiety and depression. Increasing SMR activity can help alleviate symptoms by promoting a state of calm and reducing hyperarousal (Hammond, 2005).Sleep
During sleep, particularly in non-rapid eye movement (NREM) sleep, SMR activity is less prominent than during wakefulness. However, the broader beta band, which includes SMR frequencies, shows distinct patterns across different sleep stages. For instance, beta activity is generally reduced during NREM sleep but can show transient increases during sleep spindles, which are bursts of oscillatory brain activity during stage 2 NREM sleep (Krystal et al., 2002; Merica & Fortune, 2005).SMR activity plays a role in sleep regulation and quality. Higher levels of SMR activity are associated with better sleep onset and maintenance. Individuals with higher SMR activity tend to fall asleep more easily and have more stable sleep patterns (Hoedlmoser et al., 2008). SMR neurofeedback training has been shown to improve sleep quality by increasing SMR activity, leading to deeper and more restorative sleep (Cortoos et al., 2010).
Performance
Enhancing SMR activity through neurofeedback training has been shown to improve cognitive performance, including memory, attention, and executive function. This is likely due to the increased focus and reduced distractibility associated with higher SMR levels (Vernon et al., 2003).Increased SMR activity is associated with improved motor performance, particularly in tasks requiring fine motor control and precision. This is because SMR reflects a state of motor inhibition, allowing for more controlled and deliberate movements (Gruzelier et al., 2014).
The Sensorimotor Rhythm and Sleep Spindles
Sleep spindles and sensory-motor rhythm (SMR) share several characteristics and underlying mechanisms, highlighting their interconnected roles in brain function, particularly in relation to sleep. The sleep spindle graphic © eegatlas-online.com..png)
Similarities and Overlapping Features
Both SMR and sleep spindles operate within overlapping frequency ranges. SMR typically ranges from 12 to 15 Hz, while sleep spindles are generally observed in the 12 to 16 Hz range. This overlap suggests a possible functional and mechanistic connection between the two types of brain activity (De Gennaro & Ferrara, 2003; Sterman, 1996).Thalamocortical circuits generate both SMR and sleep spindles. The thalamus, particularly the thalamic reticular nucleus, is crucial in generating sleep spindles by interacting with the cortex to produce rhythmic bursts of activity. Similarly, the thalamocortical loops involved in SMR generation facilitate synchronized oscillations in the sensorimotor cortex (Steriade et al., 1993; Lopes da Silva, 1991).
SMR is associated with relaxed wakefulness and motor inhibition, which can facilitate the transition to sleep. Sleep spindles occur during stage 2 of non-REM sleep and are associated with maintaining sleep, particularly by protecting the sleeper from external stimuli and aiding sleep consolidation (De Gennaro & Ferrara, 2003; Hammond, 2005).
Functional Roles
Sleep spindles play a critical role in maintaining sleep stability by reducing the brain’s responsiveness to external stimuli. SMR, by promoting a calm and relaxed state, may facilitate the onset of sleep and enhance the stability of the sleep cycle, thereby supporting the generation of sleep spindles (Hoedlmoser et al., 2008).SMR and sleep spindles are implicated in memory consolidation processes. Sleep spindles are known to be involved in consolidating declarative and procedural memories during sleep. SMR, by supporting focused attention and cognitive control during wakefulness, may indirectly enhance memory processes by optimizing brain function before sleep (Marshall & Born, 2007; Vernon et al., 2003).
Neurofeedback training targeting SMR has been shown to improve sleep quality and cognitive performance. Similarly, interventions aimed at enhancing sleep spindle activity have been explored for their potential to improve memory and cognitive function. The shared thalamocortical mechanisms suggest that training in one of these rhythms could potentially influence the other, offering combined benefits for sleep and cognitive health (Cortoos et al., 2010; Hoedlmoser et al., 2008).
Summary
SMR EEG activity, occurring in the frequency range of 12 to 15 Hz, is primarily generated by neurons in the sensorimotor cortex, particularly those involved in motor control and sensory processing. Thalamic ventrobasal relay cells and reentrant thalamocortical loops are crucial in generating SMR. The thalamus sends sensory information to the cortex, which processes it and sends it back to the thalamus, creating rhythmic oscillations. The synchronized activity of neurons in the primary motor cortex (M1) and primary somatosensory cortex (S1) results in the rhythmic patterns observed in SMR.SMR activity is associated with relaxed wakefulness and motor inhibition, reflecting the brain's ability to maintain a calm, focused state without engaging in motor activity. It is considered a marker of optimal sensorimotor integration linked to several cognitive and motor functions, such as improved attention, focus, and motor performance.
In psychological and medical disorders, SMR activity is often reduced in individuals with ADHD, but neurofeedback training can improve attention and behavioral control. Abnormal SMR activity is also implicated in generalized seizures, anxiety, and depression, with neurofeedback training showing potential therapeutic benefits.
SMR plays a role in sleep regulation and quality. Higher SMR levels are associated with better sleep onset and maintenance, and SMR neurofeedback training has been shown to improve sleep quality, leading to deeper and more restorative sleep.
Enhancing SMR activity through neurofeedback training improves cognitive performance, including memory, attention, and executive function, as well as motor performance, particularly in tasks requiring fine motor control and precision.
SMR and sleep spindles share several characteristics and underlying mechanisms, particularly their generation by thalamocortical circuits. Both play critical roles in maintaining sleep stability and are involved in memory consolidation processes. Neurofeedback training targeting SMR can improve sleep quality and cognitive performance, and interventions to enhance sleep spindle activity show similar benefits.
BETA (over 12 HZ)
The beta rhythm exceeds 12 Hz with 2-20 microvolts asynchronous waves. Asynchronous means the neurons depolarize and hyperpolarize independently.
The beta rhythm in EEG is typically defined within the frequency range of 13-30 Hz. However, some sources may use slightly different ranges. The variation in definitions can be attributed to differences in historical research, regional practices, and specific research contexts (Jadeja, 2021).
The beta rhythm is distributed across the scalp, with the highest amplitude in the frontal and precentral areas.
Beta Rhythm Generators
Whereas the sensorimotor cortex generates the sensorimotor rhythm, beta is generated in the brainstem and cortex. Ascending sensory traffic from the brainstem can override thalamic pacemakers (Andreassi, 2000), and specific cortical regions can produce localized beta activity beneath active electrodes (Thompson & Thompson, 2015). Reticular activating system graphic redrawn by minaanandag.
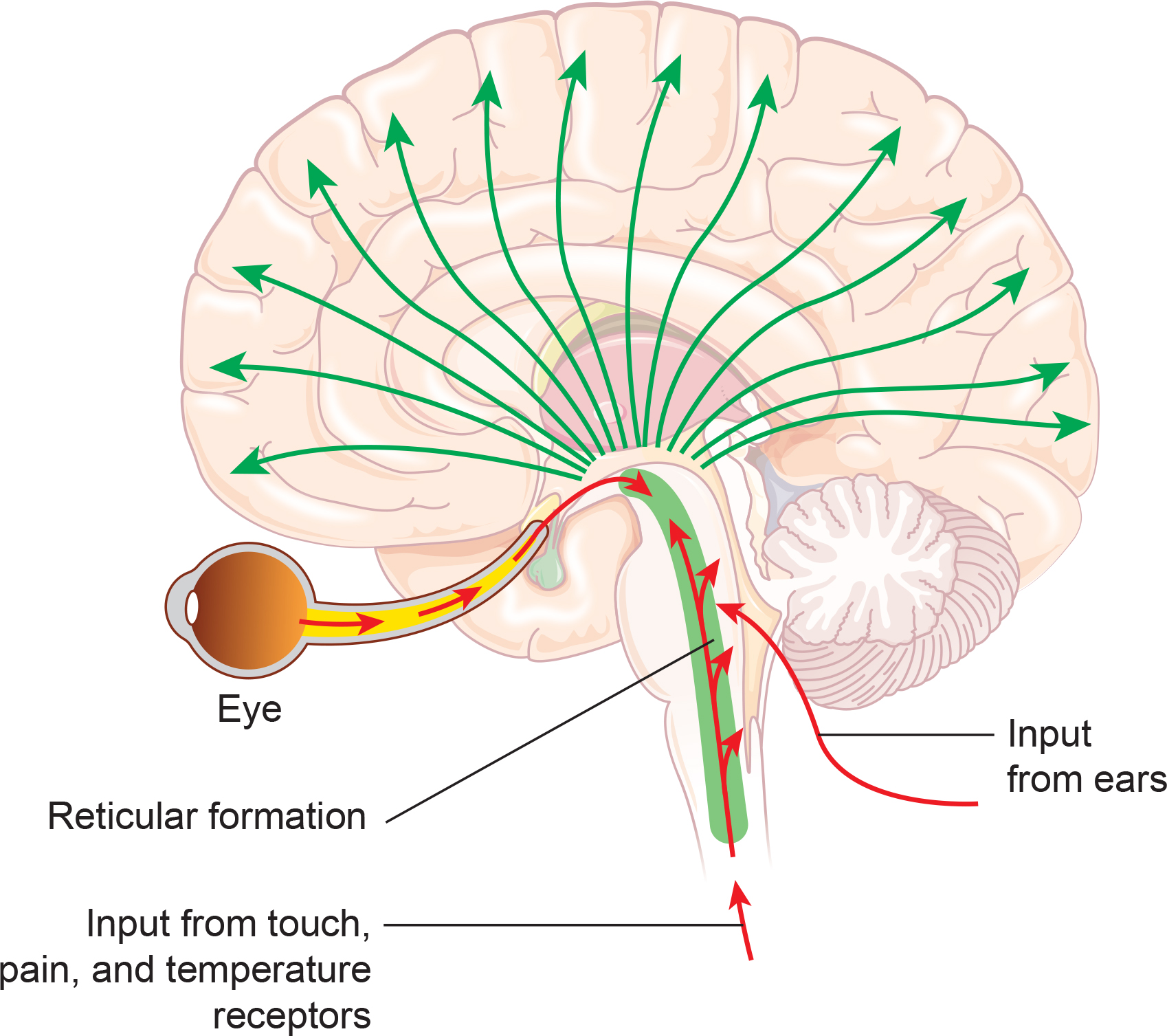
Kane and colleagues (2017) offered the following revised definition:
Any EEG rhythm between 14 and 30 Hz (wave duration 33–72 ms). Most characteristically recorded over the fronto-central regions of the head during wakefulness. Amplitude of fronto-central beta rhythm varies but is mostly below 30 µV. Blocking or attenuation of the beta rhythm by contralateral movement or tactile stimulation is especially obvious in electrocorticograms. Other beta rhythms are most prominent in other locations or are diffuse, and may be drug-induced (for example alcohol, barbiturates, benzodiazepines and intravenous anaesthetic agents).
Beta is also associated with the negative shift of the DC gradient (Speckmann et al., 2017). The DC gradient, only measurable by a DC-coupled EEG amplifier, measures the overall electrical gradient of cortical areas under the recording sensors. This gradient shows a slow oscillation of less than 1 second and is usually in the 0.1-0.2 Hz range. A shift of the gradient from its current state, becoming more electrically positive or negative, occurs every 5-10 seconds and sometimes quite a bit less often.
Buzsaki (2011) states that there is a progression of frequencies whose bandwidths overlap and interact with each other, from frequencies that take 15-40 seconds to complete each cycle up to those that oscillate at 200-600 cycles per second. Gunkelman (2005) called beta and gamma activity “emergent properties of bound networks.” This means that, as slower frequencies of EEG synchronize across networks, beta and gamma emerge in bursts of activity in coordination with that synchrony.
Beta activity is associated with "work" and reflects the ongoing excitatory/inhibitory cycles occurring on multiple time scales. While the work/rest cycle mentioned in relation to the alpha response and alpha blocking is one type of excitatory/inhibitory cycle on a broader scale, beta activity reflects actions that occur millisecond by millisecond and appear to be more locally generated.
Below is an example of eyes-open beta activity.
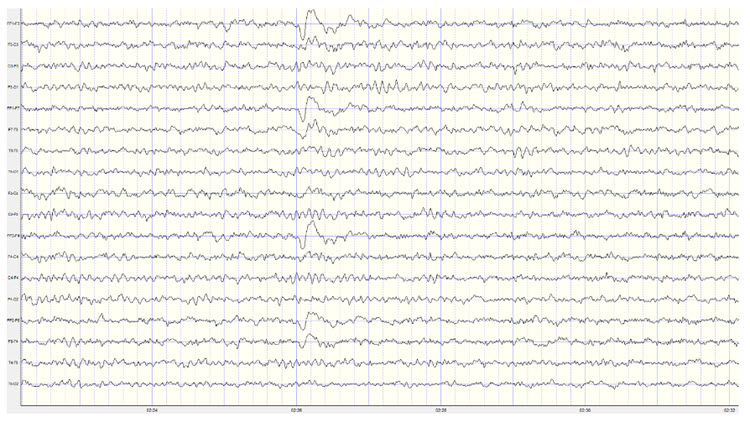
Caption: This is a 19-channel EEG recording in the eyes-open condition. This is a longitudinal bipolar montage, and the scale is 50 μV. Observe the alpha activity continuing at a much lower voltage in parietal and occipital derivations, compared to the previous eyes-closed examples, resulting from the attenuation of alpha with the opening of the eyes. There is beta activity in the 20-30 Hz range at less than 10 μV, seen mostly in frontal, central, parietal, and temporal derivations (comparisons between adjacent sensors) and somewhat slower 15-20 Hz patterns.
Kropotov (2016) describes the existence of several beta rhythms with different frequencies, various locations, and distinct functions. From this information, he states there is likely no single neuronal mechanism for generating localized beta activity. This fits the understanding that beta activity is associated with local tasks and, therefore, is mediated more by local mechanisms, with overall coordination from network systems and other rhythmic activity.
Beta is divided into narrower bands associated with cortical performance and subjective experience. These bands include low beta, high beta, and gamma. Demos (2019) advises that you should specify the actual range of interest.
Low Beta (16-20+ Hz, also known as beta2)
The cortex produces low beta when we solve problems like multiplication. When a child correctly answers a math problem, the 17-Hz amplitude may increase while theta and 8-10 Hz alpha amplitude simultaneously decrease. While detected between 12-15 Hz, it is less often seen above 20 Hz (Thompson & Thompson, 2015).High Beta (20-35 Hz, also known as beta3 or fast beta)
High beta is correlated with multi-tasking and optimal performance and anxiety, migraine, obsessive-compulsive disorder (OCD), rumination, and worrying. While elevations may indicate a range of disorders, they may represent the brain's compensation for elevated theta. Clinicians rarely reinforce high beta. Instead, they may inhibit high beta and theta (Demos, 2019).Beta Spindles
Beta spindles are trains of spindle-like waveforms with frequencies lower than 20 Hz but more often fall between 22 and 25 Hz. Beta spindles may signal ADHD, especially with tantrums, anxiety, autism spectrum disorders (ASD), epilepsy, and insomnia (Arns et al., 2015; Demos, 2019; Thompson & Thompson, 2015).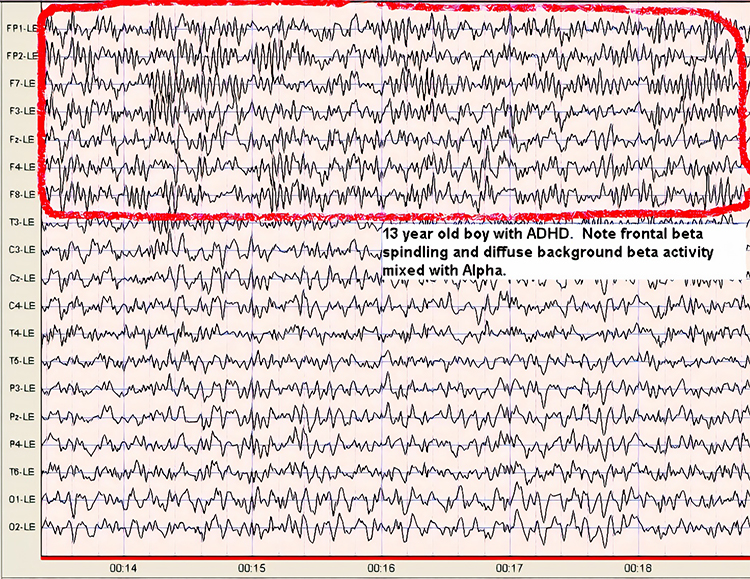
19-21 Hz or 20-23 Hz
Clients diagnosed with anxiety disorder frequently show increased power in the 19-21 Hz or 20-23 Hz range compared with 16-18 Hz. Elevations in these ranges may be associated with emotional intensity. The Thompsons (2015) advise clinicians to use open-ended questions to question clients about their mental activity and emotional state when these bands are elevated without telegraphing their expectations.24-36 Hz
Clients who are distressed, hypervigilant, and who overthink, worry, and ruminate may show marked elevations in this range. A peak in this range may be associated with family or personal substance use disorder and may indicate the instrumental use of drugs to control anxiety. High-amplitude beta in this range is not always a negative indicator since it is also observed when highly intelligent individuals multi-task (Thompson & Thompson, 2015).The movie below is a 19-channel BioTrace+ /NeXus-32 display of low beta (13-21 Hz) and high beta (22-34 Hz) activity © John S. Anderson. Brighter colors represent higher beta amplitudes. Frequency histograms are displayed for each channel.
Beta Rhythm Abnormalities
High voltage or abundant beta activity is the most common fast activity abnormality in EEGs due to pharmacologic effects. Benzodiazepines (e.g., diazepam, lorazepam) and barbiturates (e.g., phenobarbital) often cause this increase, as they are used for seizure management. This drug-induced beta activity is usually diffuse or frontally predominant and can also appear transiently during drowsiness, subsiding with deeper sleep (Libenson, 2024).
When beta activity is typical for the client based on age, state (eyes-open, eyes-closed, under task, etc.), and location, it suggests those areas are functioning as expected. If beta activity is deficient, that may mean that the site is under-functioning or under-activated for some reason. Reasons may include damage of some sort, metabolic deficits, fatigue, or other factors. An area that is consistently over-functioning may, in time, because of overuse and fatigue, end up with a lower level of functioning and, hence, less beta activity.
Higher-than-typical beta amplitude at a given location may mean the area is over-functioning or overly activated. Whether the beta amplitude is higher or lower than average, the clinician will want to understand the underlying functional neuroanatomy of the area to aid in the assessment process. For example, suppose the right posterior temporal/parietal junction that roughly underlies the area between T4 and T6 (TP8 in the 10-10 system) shows excess beta amplitude. In that case, it may be associated with heightened sensitivity to or attention to non-verbal communication, such as tone of voice, facial expression, and body language associated with angry outbursts or that may signal danger. If this area is under-activated, it may represent a self-protective “disconnection” from these same signals (Gunkelman, 2021).
Individuals who typically show excess beta activity at sleep onset and during sleep stages have a higher incidence of insomnia (Perlis et al., 2001). Meier and colleagues (2014) found a correlation between excess beta power in frontal, central, and temporal areas with delinquent behavior in adult men with concurrent ADHD symptomatology. In Rowan’s Primer of EEG (2nd ed.), Marcuse and colleagues (2016) identify excess interhemispheric beta asymmetry as an important diagnostic tool. The side with reduced relative beta power points to the pathological hemisphere. They identify brain abscesses, stroke, tumors, vascular malformations, and cortical dysplasia as associated with a focal decrease or enhancement of beta activity.
A reference to a normative database can be useful when assessing beta activity, particularly if a traumatic brain injury is suspected. Comparison with other EEG frequencies is also important, as an excess or lack of beta activity often accompanies differences from expected values for other frequencies.
Significant cerebral dysgenesis, like lissencephaly, can cause increased beta activity, typically accompanied by other EEG abnormalities and intellectual disability. In such cases, the beta activity is often slower. Increased beta activity can also appear in normal EEGs without explanation, potentially due to incomplete medication lists or residual drug effects.
Reductions in beta activity frequency (18-30 Hz) often indicate diffuse cortical injury, such as post-anoxic episodes, or the effects of sedative medications. Severe cortical injuries may show an absence of beta activity, though some patients naturally exhibit less beta activity as a normal variant. Low amplifier gains can obscure normal fast activity in EEG traces with high-voltage slow activity.
A true asymmetry in fast activity between brain regions is a significant abnormality, suggesting potential cortical damage. Fast waves are generated at the cortical level, reflecting the activity of circuits near the scalp surface rather than deeper or more medial regions of the brain. Asymmetry in fast activity often indicates cortical damage, with the area of lower voltage marking the abnormality, such as in the case of a cortical stroke where beta activity is reduced. Rarely, an abnormal cortical area may show increased fast activity.
Spindling Excessive Beta (SEB)
Spindling excessive beta (SEB) refers to a specific pattern observed in electroencephalogram (EEG) recordings characterized by high-frequency beta waves (13-30 Hz) that display a spindle-like appearance. These beta spindles often exceed 20 μV in amplitude and can occur in both frontal and central regions of the brain. SEB is distinguished from typical beta activity by its spindling morphology and higher amplitude (Arns et al., 2015; Krepel et al., 2021). Beta spindle graphic retrieved from ADDYSSEY.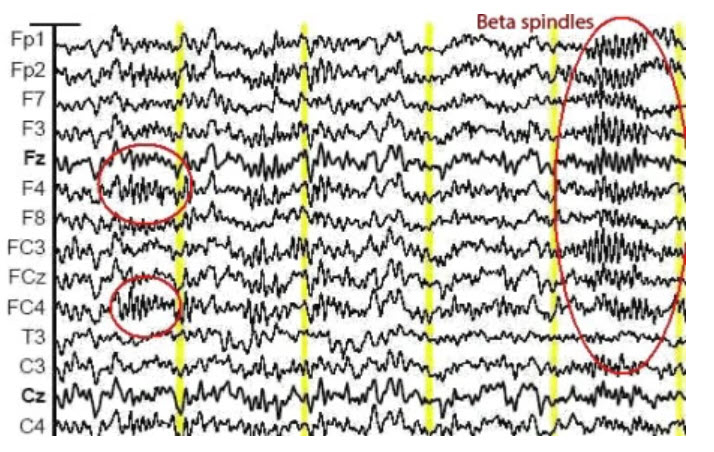
SEB appears to be a transdiagnostic EEG feature, meaning it is observed across various psychiatric disorders and is not limited to a single diagnosis (Arns et al., 2015; Krepel et al., 2021). SEB has been associated with a range of psychiatric and neurological conditions, making it a critical biomarker in clinical neurophysiology. Its clinical importance is primarily seen in sleep disorders, Attention-Deficit Hyperactivity Disorder, medication resistance, and epilepsy.
Sleep Disorders
SEB is often linked with sleep disturbances, particularly insomnia. Insomnia complaints are more prevalent in individuals with frontal SEB patterns (Arns et al., 2015). Studies indicate that individuals with SEB may experience difficulties in maintaining sleep, leading to chronic insomnia (Swatzyna et al., 2022). SEB may be a marker of hypoarousal or sub-vigil states.Autism Spectrum Disorder (ASD)
In ASD, SEB can reflect dysregulation in the mirror neuron system that subsequently fails to modulate this bursting pattern.Attention-Deficit/Hyperactivity Disorder (ADHD)
There is a notable association between SEB and ADHD. Patients exhibiting SEB often report symptoms of inattention, hyperactivity, and impulsivity. Children with excess beta activity in their EEGs are a small subset of ADHD children, similar to other ADHD children but more prone to temper tantrums and moodiness (Clarke et al., 2001). This association suggests that SEB can be used as an objective marker for ADHD diagnosis and management (Swatzyna et al., 2022).Medication Resistance
SEB is frequently observed in patients who have failed multiple medication trials for various psychiatric conditions. This resistance to medication highlights the need for alternative therapeutic approaches for individuals exhibiting SEB (Swatzyna et al., 2022).Epilepsy
Beta spindles can co-occur with seizure disorders, although patients with a history of seizures were excluded from some SEB studies to isolate its effects in other conditions. The presence of SEB in epilepsy patients can complicate the clinical picture, necessitating careful EEG interpretation (Swatzyna et al., 2022).Mechanisms and Hypotheses
SEB might reflect a sub-vigil state, which is an intermediate state between wakefulness and sleep. This state can lead to cognitive impairments and behavioral disturbances observed in conditions like ADHD and insomnia (Swatzyna et al., 2022). Benzodiazepines and other substances affecting GABAergic transmission can induce SEB, suggesting a neurochemical basis for its occurrence. This insight provides avenues for pharmacological interventions targeting the GABA system (Swatzyna et al., 2022).Treatment Implications
Recognizing SEB in patients can influence treatment strategies. For instance, behavioral interventions targeting sleep hygiene may benefit those with SEB-related insomnia. Similarly, neurofeedback and other non-pharmacological treatments might offer alternative approaches for ADHD patients exhibiting SEB, especially those resistant to conventional medications (Swatzyna et al., 2022).Conclusion
SEB is a distinctive EEG pattern with significant clinical implications across various psychiatric and neurological disorders. Its presence can aid in the diagnosis, understanding, and treatment of conditions such as insomnia, ADHD, and medication-resistant psychiatric disorders. Continued research into SEB will further elucidate its mechanisms and optimize therapeutic strategies.Summary
The beta rhythm, typically defined within the 13-30 Hz range, is characterized by asynchronous waves with amplitudes between 2-20 microvolts. Asynchronous in this context means that neurons depolarize and hyperpolarize independently. The brainstem and cortex generate the beta rhythm, with the highest amplitude in the frontal and precentral areas. Beta activity reflects ongoing excitatory and inhibitory cycles, correlates with tasks and states such as attention, problem-solving, and anxiety, and is sensitive to pharmacological influences. Beta abnormalities can indicate various clinical conditions, including insomnia, ADHD, and epilepsy.
Spindling Excessive Beta (SEB) is a distinct EEG pattern characterized by high-frequency beta waves (13-30 Hz) with a spindle-like appearance, often exceeding 20 μV in amplitude. SEB is a transdiagnostic feature associated with psychiatric and neurological conditions such as insomnia, ADHD, medication resistance, and epilepsy. SEB is thought to reflect sub-vigil states, which are intermediate between wakefulness and sleep, potentially leading to cognitive impairments and behavioral disturbances. Recognizing SEB can influence treatment strategies, including behavioral interventions for sleep and neurofeedback for ADHD. Continued research is essential to further elucidate the mechanisms and therapeutic approaches for SEB.
GAMMA (28-80 Hz)
Gamma ranges from 30-100 Hz and includes the Sheer rhythm, which extends from 38-42 Hz (Buzsáki & Wang, 2012; Fries, 2015; Jensen et al., 2007). The difference in reported gamma frequency bands may reflect methodological differences and biological variability.
Methodological Differences
Different EEG systems and setups can have varying sensitivities and resolutions, affecting the frequency range they can accurately capture. High-density EEG systems may detect higher frequencies more reliably than standard setups (Hari & Puce, 2017; Nunez & Srinivasan, 2006).Data processing variations, such as filters, window lengths, and analytical algorithms, can influence the detection and categorization of gamma frequencies. High-frequency oscillations can be more susceptible to noise and artifacts, such as muscle activity (EMG) or electrical interference. Different studies may apply varying degrees of filtering and artifact rejection, affecting the upper limit of detectable gamma frequencies (Niedermeyer & da Silva, 2004).
For these reasons, different studies might use different criteria for identifying gamma oscillations.
Biological Variability
Due to genetic, developmental, and health-related factors, the EEG patterns of different individuals can be significantly variable. This can lead to different observed upper limits for gamma oscillations (Başar, 2012; Ulhaas & Singer, 2010).The cognitive or sensory task during EEG recording can affect the frequency and power of gamma oscillations. Different tasks may induce gamma activity in different frequency ranges.
Gamma oscillations can vary across different regions of the brain. For instance, the gamma frequency range observed in the visual cortex during visual processing tasks may differ from that observed in the hippocampus during memory tasks.
The overlap between gamma (30-100 Hz) and surface EMG (SEMG) (13-200 Hz) requires that clinicians examine the raw EEG to ensure that training does not reward muscle contraction. The movie below is a 19-channel BioTrace+ /NeXus-32 display of gamma activity © John S. Anderson. . Brighter colors represent higher gamma amplitudes. Frequency histograms are displayed for each channel. Notice that the mean frequency falls between 38 and 39 Hz.
This section delves into the generators of gamma activity, its meaning in the context of brain function, and recent findings in the field. Research has linked gamma activity to cortical activation, cognitive processes, attention, emotion, and motor functions, with implications for conditions like ADHD, GAD, and epilepsy.
Gamma Rhythm Generators
Gamma oscillations are primarily generated by the synchronized activity of excitatory and inhibitory neurons in the brain, particularly within the cortical regions. The interplay between pyramidal neurons and interneurons, especially those expressing parvalbumin, is crucial for generating these high-frequency oscillations. These interneurons provide rhythmic inhibitory input to pyramidal cells, creating a network capable of producing gamma waves (Buzsáki & Wang, 2012).Thalamocortical interactions also play a role in gamma generation. The thalamus, acting as a relay station for sensory information, can influence cortical gamma activity through its connections with the cortex. This interaction is particularly evident in sensory processing areas, where gamma oscillations are modulated by sensory input (Fries, 2015).
Furthermore, gamma activity is not uniformly distributed across the brain. Still, it is particularly prominent in regions involved in higher-order cognitive functions, such as the prefrontal cortex, hippocampus, and visual cortex. These areas utilize gamma oscillations for attention, memory encoding, and sensory perception (Bosman et al., 2014).
The Meaning of Gamma EEG Activity
Gamma oscillations have been implicated in various cognitive, perceptual, and motor functions, emotions, epilepsy, schizophrenia, Alzheimer's disease, and ADHD.Binding Sensory Information
One of the primary roles of gamma activity is in binding sensory information. This concept, known as the binding problem, refers to how the brain integrates information from different sensory modalities to create a coherent perceptual experience. Gamma synchrony is thought to facilitate this process by providing a temporal framework that binds disparate neural signals (Singer, 2013).This binding across distant networks is the result of glial cells such as astrocytes and their interconnections known as gap junctions. These gap junctions allow the nearly instantaneous synchronization of neurons, both local and distant, into organized networks, allowing Gamma and other EEG rhythms to emerge as a function of coordinated tasks such as sensory processing, communication and executive functions.
Attention
In the context of attention, gamma oscillations have been shown to enhance the processing of relevant stimuli while suppressing irrelevant information. This selective attention mechanism is believed to be mediated by synchronizing gamma activity between different brain regions, thereby enhancing communication and processing efficiency (Jensen et al., 2007). Enhanced gamma activity is associated with better performance on cognitive tasks (Hasib et al., 2023).Working and Long-Term Memory
Gamma activity is crucial for working memory and long-term memory encoding. Studies have demonstrated that gamma oscillations in the hippocampus and prefrontal cortex are involved in maintaining and manipulating information in working memory and encoding and retrieving long-term memories (Colgin, 2016).Motor Performance
Gamma event-related synchronization (ERS) is linked to motor performance, with distinct low (35-50 Hz) and high (75-100 Hz) gamma bands showing different temporal and spatial characteristics during motor tasks (Amo et al., 2016, 2017; Crone et al., 1998). Enhanced gamma activity is observed during voluntary movements (Amo et al., 2016).The Sheer rhythm is also associated with peak performance (Spydell, Ford, & Sheer, 1979). For example, 40-Hz activity is generated when athletes correct their balance after leaning forward on a balance board. Athletes who have sustained a concussion and suffer impaired balance do not increase 40-Hz power (Demos, 2019; Thompson & Thompson, 2015).
Emotions
Gamma fluctuations are associated with emotional experiences, with higher gamma activity linked to difficult emotions during worry inductions in GAD patients (Oathes, 2008).Epilepsy
Increased gamma activity is noted in patients with primary generalized epilepsy, potentially serving as a marker for underlying dysfunctions and a prerequisite for seizures (Willoughby et al., 2003).Schizophrenia
In schizophrenia, for example, deficits in gamma oscillations are thought to contribute to the cognitive impairments and sensory processing anomalies observed in patients (Uhlhaas & Singer, 2010).Alzheimer's Disease
Recent studies have suggested that gamma oscillations might play a role in clearing amyloid-beta plaques, a hallmark of the disease. Optogenetic stimulation of gamma oscillations in mouse models has reduced amyloid-beta levels, pointing to potential therapeutic avenues (Iaccarino et al., 2016).ADHD
Reduced gamma activity is observed in adults with ADHD, particularly in the right centroparietal region, correlating with symptom severity and suggesting altered network function (Tombor et al., 2019).Recent Findings
Advancements in neuroimaging and electrophysiological techniques have allowed for more precise mapping of gamma activity and its sources. Magnetoencephalography (MEG) and high-density EEG have provided more detailed spatial and temporal resolution, enhancing our understanding of gamma oscillations in health and disease (Hari & Puce, 2017).Conclusion
Gamma oscillations in EEG, ranging from 30-100 Hz, are critical for numerous brain functions, including cognitive processes, attention, memory, motor functions, and emotional experiences. This range includes the Sheer rhythm (38-42 Hz). Variations in reported gamma frequency bands stem from differences in EEG methodologies, such as system sensitivity and data processing techniques, and biological variability among individuals. High-density EEG systems can capture higher frequencies more reliably than standard setups. Gamma oscillations are generated by the synchronized activity of excitatory and inhibitory neurons, particularly in cortical regions, and their interplay is essential for producing these high-frequency waves.Gamma oscillations play a vital role in binding sensory information, a process known as the binding problem, which integrates information from different sensory modalities to create a coherent perceptual experience. In the context of attention, gamma oscillations enhance the processing of relevant stimuli while suppressing irrelevant information, thereby improving cognitive task performance. Gamma activity is crucial for both working memory and long-term memory encoding, particularly in the hippocampus and prefrontal cortex.
Motor performance is also linked to gamma oscillations, with distinct low (35-50 Hz) and high (75-100 Hz) gamma bands showing different characteristics during motor tasks. The Sheer rhythm is associated with peak performance, such as athletes correcting their balance, where increased 40-Hz activity is observed. Emotional experiences are also linked to gamma fluctuations, with higher gamma activity correlating with difficult emotions in conditions like generalized anxiety disorder (GAD).
Gamma oscillations are implicated in several neurological and psychiatric conditions. In epilepsy, increased gamma activity is observed and may serve as a marker for underlying dysfunctions. In schizophrenia, deficits in gamma oscillations contribute to cognitive impairments and sensory processing anomalies. Alzheimer's disease research suggests that gamma oscillations might help clear amyloid-beta plaques, pointing to potential therapeutic avenues. In ADHD, reduced gamma activity is noted, particularly in the right centroparietal region, correlating with symptom severity.
Recent advancements in neuroimaging, such as magnetoencephalography (MEG) and high-density EEG, have improved the spatial and temporal resolution of gamma activity mapping, enhancing the understanding of gamma oscillations in both healthy and diseased states. These technologies allow for more precise identification of gamma rhythm sources and their roles in brain function.
Gamma oscillations' importance in various brain functions and their implications in several conditions highlight the need for continued research to fully elucidate their mechanisms and therapeutic potential.
Waveform Morphology
A wave is a change in the potential difference between two EEG electrodes. Waveform and morphology refer to the shape of the signal generated by oscillating potential differences. Neurofeedback professionals examine the raw waveform's morphology before considering the filtered and quantified EEG (Demos, 2019). EEG activity means a single wave or series of waves (Fisch,1999).
We can distinguish between regular and irregular activity. A regular or monomorphic series of waves are rhythmic with the same frequency and morphology. Rhythmic waves that resemble sine waves are called sinusoidal.
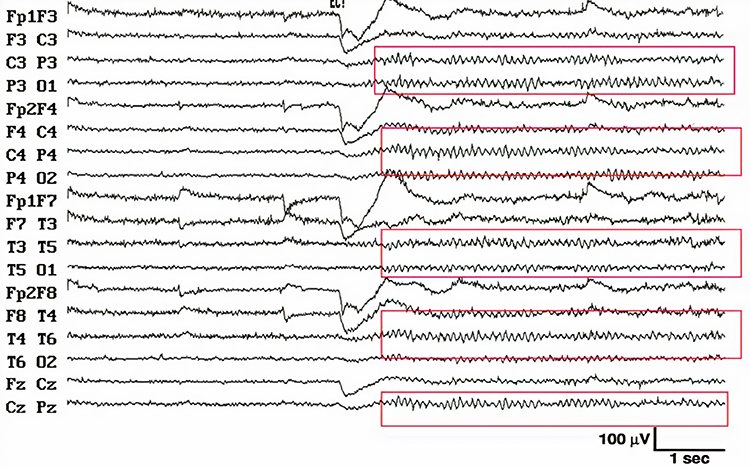
Regular waves may be arch-shaped and resemble wickets. Graphic © eegatlas-online.com.
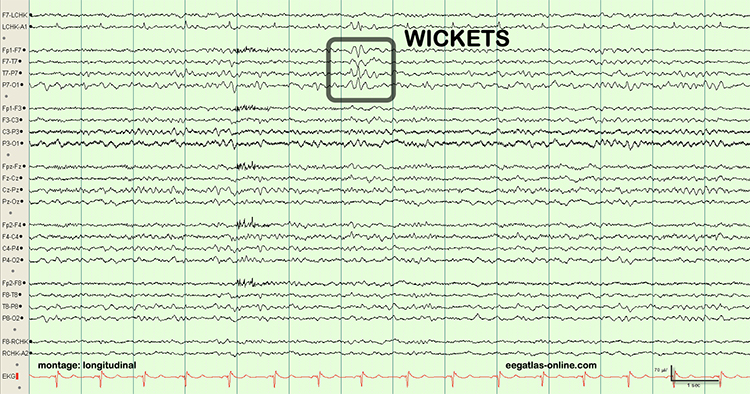
Saw-toothed waves resemble asymmetrical triangles. Graphic © eegatlas-online.com.
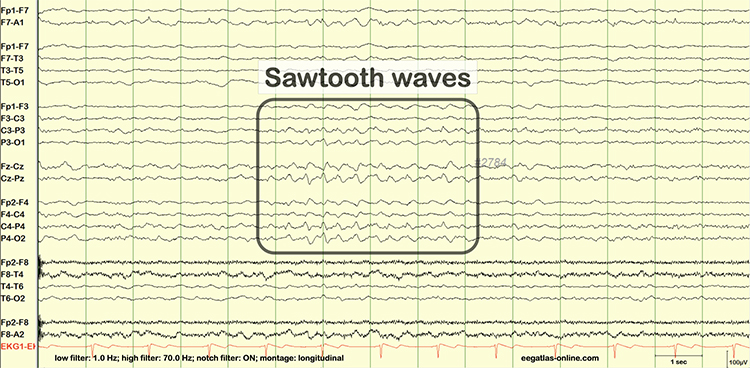
Irregular waves continuously change shape and duration. The graphic below shows runs of irregular high-amplitude delta waves.
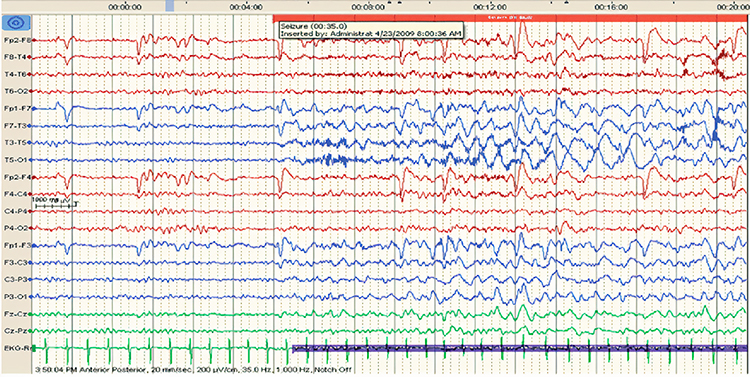
EEG waves may be monophasic or polyphasic. Monophasic waves possess a single upward or downward deflection. Two polyphasic waveforms containing waveforms with two or more elements are diphasic and triphasic waves. Diphasic waves have two elements--one positive and one negative. Triphasic waveforms contain three elements with alternating directions.
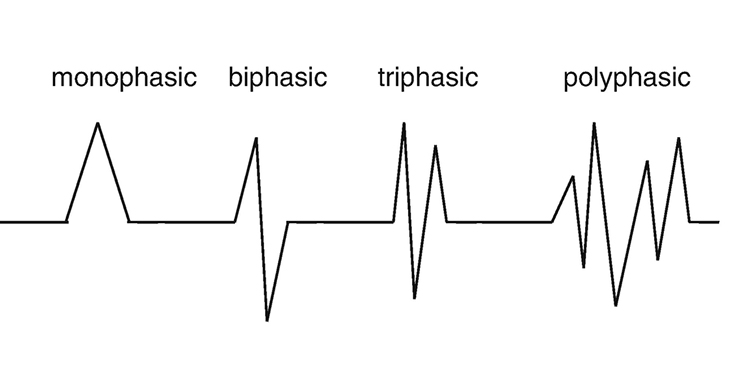
A transient is a single wave or series of waves distinct from background EEG activity.
Sharp transients are waves with steep peaks that are not produced by epilepsy.

In contrast, epileptiform activity consists of spikes and sharp waves. An epileptiform spike has a sharp appearance and lasts 20-70 ms. Watch the Blausen Epilepsy and Seizure animations. Graphic © eegatlas-online.com.
Less steeply shaped sharp waves last 70-200 ms. Complex denotes a series of waves that share a similar shape. See the K-complex in the EEG record below. Graphic © eegatlas-online.com.
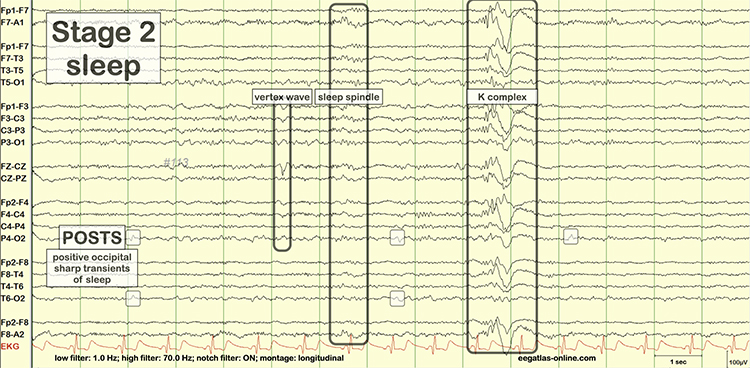
Source Localization
LORETA, sLORETA, eLORETA, and swLORETA
Low resolution electromagnetic tomography (LORETA) is Pascual-Marqui, Michel, and Lehman's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp. In this context, tomography refers to two-dimensional coronal, horizontal, and sagittal brain slices. LORETA does not identify subcortical sources like the amygdala or thalamus located below the cortical hemispheres (Thompson & Thompson, 2015).LORETA represents cortical sites using three-dimensional voxels, which are volumetric units. While its original voxels had a 7-mm spatial resolution (7 mm x 7 mm x 7 mm), the spatial resolution has increased to 5 mm (5 mm x 5 mm x 5 mm). LORETA values are expressed in amperes per cubic centimeter.
LORETA assigns each voxel x, y, and z Talairach coordinates referencing the original Talairach atlas and subsequent atlases like the Montreal Neurological Institute (MNI) atlas. Talairach coordinate assignment is based on vertical distance from a horizontal line from the anterior commissure (origin) to the posterior commissure. For example, x46, y33, z40 corresponds to Brodmann area 8.
These stereotaxic coordinates are primarily independent of brain shape and volume, which has permitted their use in other imaging methods (e.g., positron emission tomography (PET) and magnetic resonance imaging (MRI). Graphic courtesy of BrainMaster Technologies.
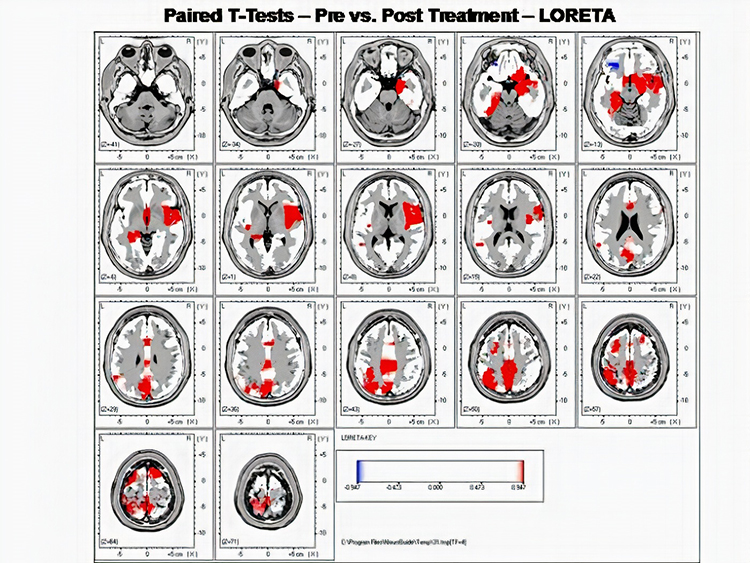
Standardized LORETA (sLORETA) achieves a resolution of 1 cubic centimeter. Smaller voxels more precisely localize cortical EEG sources of surface potentials. sLORETA estimates individual voxel's electrical potentials without regard to their frequency. sLORETA values are expressed in normalized F values. This refinement of LORETA trades absolute units of current density for reduced noise and more precise source localization. This is important because LORETA's "three-sphere model," which assumes different cortex, skull, and skin conductivity, suffers from artifacts ("ghost images") and limited source localization (Thompson & Thompson, 2015). Graphic courtesy of BrainMaster Technologies.
eLORETA, exact low resolution brain electromagnetic tomography, claims no localization error (Thompson & Thompson, 2015).
swLORETA stands for standardized, weighted LORETA, indicating the algorithms weigh the distances involved when assigning values to each region. Also, this method utilizes the information from a normative database to provide values reflecting statistical differences in standard deviations between the client and a typical population of age matched individuals.
Laplacian Analysis
Surface Laplacian (SL) analysis, which is also called current source density (CSD) and scalp current density (SCD), is a family of mathematical algorithms that provide two-dimensional images of radial current flow from cortical dipoles to the scalp. Positive values represent the current flow from the cortex to the scalp (sources). Negative values represent the current flow from the scalp to the brain (sinks).Unlike the LORETA family of inverse solutions, SL analysis is independent of reference recording procedures--all reference schemes will yield the same current flow estimates and polarity. SL analysis better localizes the EEG signal than surface potentials because it minimizes scalp EEG blurring produced by volume conduction. Finally, unlike inverse solutions, SL makes no assumptions about different tissue conductivity, functional neuroanatomy, cortical geometry and shape, and EEG sources (Kayser & Tenke, 2015). This figure was uploaded by Zoltan Juhasz to ResearchGate.
Clinically Significant Raw Waveforms
Clinically significant raw waveforms include kappa rhythm, lambda waves, vertex sharp transients, mu waves, spike and wave, isolated epileptiform discharges, SMR, sleep spindles, and K-complexes.
Kappa Rhythm
The kappa rhythm consists of very low amplitude activity in the alpha or theta range detected over temporal sites during mental activity. Their source (cortical effort or eyelid flutter) is controversial (Fisch, 1999).Lambda Waves
Lambda waves are positive sawtooth-shaped sharp transients detected from occipital sites when individuals view detailed images. Lambda waves last about 200-250 ms and appear as positive occipital sharp transients (POSTs) detected during sleep. These waves won't be observed in clinical EEGs unless the assessment includes viewing complex images. While the presence or absence of lambda waves is not abnormal, striking asymmetry may indicate an abnormality located on the lower amplitude side (Fisch, 1999). Graphic © eegatlas-online.com.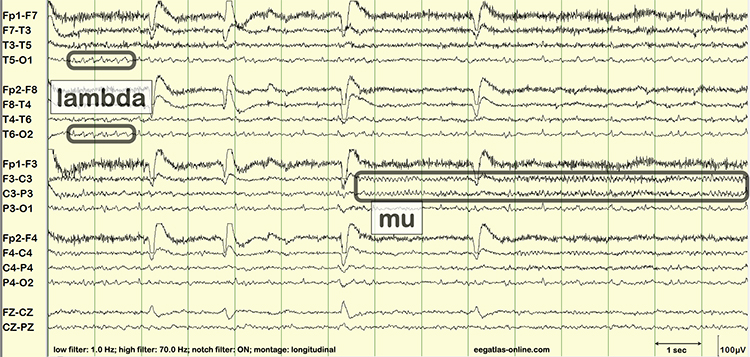
Vertex Sharp Transients
Negative polarity vertex sharp transients (V waves) are detected at the vertex in sleep records but are not usually observed during wakefulness. Rare in adults and more easily elicited in children, V waves may be evoked by unexpected stimuli like clapping. They may be a late evoked potential component independent of sensory modality and not confined to a specific sensory region. Their amplitude is greater during sleep than wakefulness (Fisch, 1999). Graphic © eegatlas-online.com.
Mu Waves
The mu rhythm is observed in less than 7% of EEG records, more often in younger adults (Thompson & Thompson, 2015). These 7- to 11-Hz waves resemble wickets and appear as several-second trains over sensorimotor, and less commonly, parietal sites. View the raw EEG at C3 and C4. Mu waves have been associated with mirror neurons and may be observed in children and adults on the autism spectrum. Clinicians inhibit mu activity during training and do not reinforce mu amplitude (Demos, 2019).Clinicians must distinguish the mu rhythm from alpha since they share common frequencies. Alpha waves are sinusoidal instead of wicket-shaped. Mu activity increases when clients reduce motor activity. Mainly contralateral mu activity is suppressed by making a fist, while alpha is not. The alpha rhythm is blocked when clients open their eyes; the mu rhythm is unaffected. The mu rhythm may be evoked by visual scanning tasks (Demos, 2019; Fisch, 1999). Graphic © eegatlas-online.com.
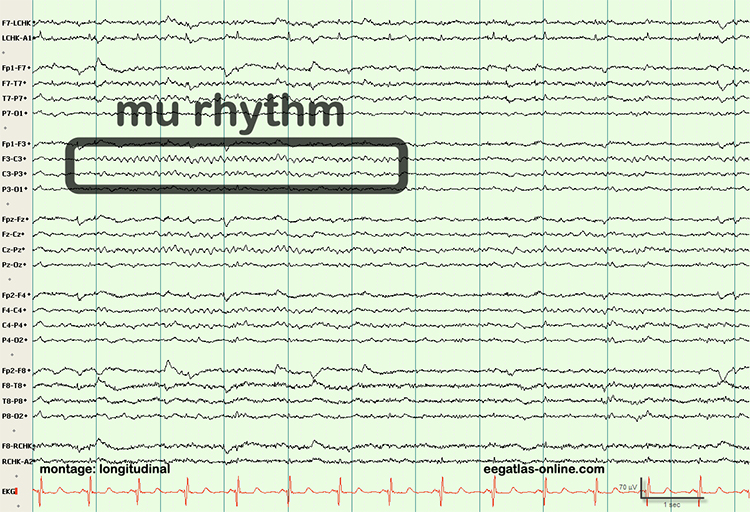
Spike-and-Wave Complexes
Spike-and-wave complexes consist of a spike that is succeeded by a slow wave. In the graphic below,A shows spikes, B represents sharp waves, and C is a spike-and-wave complex. Spike-and-wave complexes have a frequency of 3 Hz with amplitudes over 160 microvolts. They are observed in absence seizures (Thompson & Thompson, 2015). This figure was uploaded by Tshilidzi Marwala to ResearchGate.
Isolated Epileptiform Discharges
Swatzyna et al. (2022) addresses the prevalence of isolated epileptiform discharges (IEDs) in children and adolescents with psychiatric disorders, emphasizing the potential benefits of EEG in predicting medication response.A systematic literature review was conducted using the PubMed/Medline database, focusing on publications from 1994 onwards, which align with DSM-IV criteria. The search included terms related to epileptiform discharges and various psychiatric disorders, excluding studies not in English, those with non-human subjects, and those involving epilepsy. Additionally, a cross-sectional data review was performed using an IRB-approved archive from a psychiatric practice in Houston, Texas, encompassing EEG data from 722 children and adolescents aged 4-18.
Their systematic review identified 18 studies, revealing varying prevalence rates of IEDs across psychiatric disorders. For ADHD, the prevalence ranged from 16.1% to 34.8%, with a weighted mean prevalence of 25.2%. For ASD, prevalence rates were consistently high, ranging from 23.5% to 85.8%, with a weighted mean of 63.3%. Mood disorders had a lower prevalence, approximately 3% for non-psychotic mood disorders. The cross-sectional analysis showed similar prevalence rates for ADHD (36.9%) and ASD (34.8%), but higher rates for mood (38.9%) and anxiety disorders (39.1%), likely due to selection bias in the clinical sample.
The high prevalence of IEDs in ADHD and ASD suggests the need for more research to understand their role in these disorders' pathophysiology and treatment. The identification of IEDs could help in avoiding inappropriate medications that might exacerbate symptoms. The study highlights the importance of using EEG to guide medication selection, particularly in refractory psychiatric cases, to improve treatment outcomes and reduce the trial-and-error approach.
Research has also explored the implications of isolated epileptiform discharges in non-epileptic conditions. For example, these discharges have been observed in individuals with cognitive impairments, such as those with Alzheimer’s disease or developmental disorders. Understanding the broader impact of these discharges on brain function remains an active area of investigation, with potential implications for both diagnosis and intervention. An example is the use of EEG characteristics to predict the driving safety of individuals with isolated spike-wave discharges, as these transients can be associated with slow response times and/or missed responses.
Sensorimotor Rhythm (SMR)
The 13-15 Hz spindle-shaped sensorimotor rhythm (SMR) is detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity. Where we observe these frequencies at other scalp locations, they appear as desynchronized beta without spindling. The spindle shape is better visualized in microelectrode recordings than scalp EEGs. Since SMR is associated with mental calm and thinking before acting, clinicians may uptrain this rhythm in clients diagnosed with ADHD (Thompson & Thompson, 2015).
Sleep Spindles
Sleep spindles range from 12-15 Hz and last from 0.5 to several seconds. They resemble SMR spindles, occupy the same frequencies, and are concentrated at central sites. Unlike SMR, which is confined to the sensorimotor strip, sleep spindles are widely distributed over the scalp and are observed during Stage 2 and 3 sleep (Thompson & Thompson, 2015). Graphic © eegatlas-online.com.
K-Complexes
K-complexes are also observed in Stage 2 sleep. These sharp negative waveforms reach amplitudes over microvolts, succeeded by a more prolonged moderate-to-high-amplitude positive wave. Graphic © eegatlas-online.com.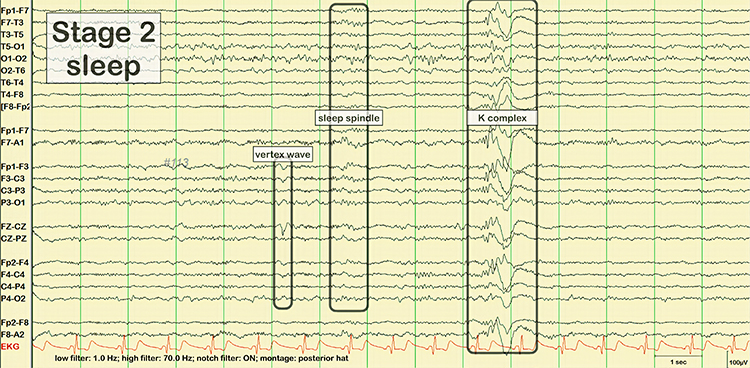

Glossary
alpha blocking: the replacement of the alpha rhythm by low-amplitude desynchronized beta activity during movement, attention, mental effort like complex problem-solving, and visual processing. alpha blocking normally occurs when eyes have just been opened. Arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it while increasing EEG power in the beta range.
alpha response: increased alpha amplitude.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: trains of alpha waves that are visible in the raw EEG and are observed during drowsiness, fatigue, and meditative practice
amplitude: the height of a wave, indicating the strength or intensity of a signal.
amyloid-beta: a protein fragment that accumulates in the brains of individuals with Alzheimer's disease, forming plaques.
analog: the representation of a signal by a continuously variable physical property like voltage.
analog filter: analog circuits designed using components like capacitors, resistors, and operational amplifiers designed to remove or enhance signal components.
analog-to-digital (A/D) converter: an electronic device that converts continuous signals to discrete digital values.
asynchronous waves: EEG activity where neurons depolarize and hyperpolarize independently.
attention: the cognitive process of selectively concentrating on one aspect of the environment while ignoring others.
attention-deficit hyperactivity disorder (ADHD): a neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
beta spindles: trains of spindle-like waveforms with frequencies that can be lower than 20 Hz but more often fall between 22 and 25 Hz. They may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia.
binding problem: the question of how the brain integrates information from different sensory modalities to create a coherent perceptual experience.
bit: binary digit: the smallest measurement unit for quantifying information that assumes a value of 0 or 1.
bit number: the number of voltage levels that an A/D converter can discern. A resolution of 16 bits means that the converter can discriminate among 65,536 voltage levels.
brain lesions: abnormal tissue in the brain resulting from injury or disease, such as strokes, tumors, or trauma.
bursts of rhythmic temporal theta (BORTTs): transient periods of rhythmic theta activity observed in the temporal regions of the brain during EEG recording. These bursts typically manifest as short-lived increases in theta oscillations and are often associated with cognitive processes such as memory encoding and retrieval.
cognitive performance: the ability to use mental processes to perform tasks, including memory, attention, and executive function.
cognitive processes: mental activities involved in acquiring, storing, and using knowledge. electrophysiological techniques: methods used to study the electrical properties of biological cells and tissues.
cognitive restoration: the process during sleep where the brain undergoes various activities that help improve mental functions, including memory consolidation and the clearance of metabolic waste.
complex: a series of waves that share a similar shape.
cortical neurons: neurons located in the brain's cortex that play a key role in various brain functions, including the generation of delta waves.
declarative memory: a type of long-term memory involving facts and information that can be consciously recalled.
delta waves: low-frequency brain oscillations ranging from 0.5 to 4 Hz, typically observed during deep stages of non-REM sleep.
desynchrony: pools of neurons fire independently due to stimulation of specific sensory pathways up to the midbrain and high-frequency stimulation of the reticular formation and nonspecific thalamic projection nuclei.
digital: representation of a signal property like voltage using a series of the digits 0 and 1.
digital filter: a circuit that uses digital processors, like a digital signal processing (DSP) chip, to remove or enhance signal components.
digitization: encoding analog information like continuously changing voltage into a series of the digits 0 and 1.
diphasic waves: waves that possess two elements--one positive and one negative.
dominant frequency: the frequency with the greatest amplitude; at least 13 Hz in awake adults.
dynamic range: the ability to sample a wide range of signal voltages.
EEG activity: a single wave or series of waves.
encephalopathy: a broad term for any diffuse disease of the brain that alters brain function or structure, often characterized by altered mental states and various neurological symptoms.
epileptiform activity: abnormal, paroxysmal EEG patterns that resemble those seen in epilepsy, often indicating a predisposition to seizures.
epoch: signal sampling period; commonly, a 1-s sample of EEG activity.
exact low resolution brain electromagnetic tomography (eLORETA): a version of LORETA that claims no localization error.
excitatory neurons: neurons that increase the likelihood of a firing action potential in the recipient neuron.
Fast Fourier Transform (FFT): a mathematical transformation that converts a complex signal into component sine waves whose amplitude can be calculated.
FFT filter: a filter that uses Fast Fourier transforms to calculate the average voltage of an EEG signal's component frequencies for a specified period.
filter: an electronic circuit that removes or enhances signal components.
fine motor control: the ability to make small, precise movements, often involving the coordination of muscles and nerves.
FIR filter: a filter that continuously updates its averaging of EEG voltage with new data points.
frequency: the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
gamma: 28-80 Hz rhythm that includes the 38-42 Hz Sheer rhythm and is associated with learning and problem-solving, meditation, mental acuity, and peak brain function in children and adults.
gamma event-related synchronization (ERS): a phenomenon where gamma oscillations (30-100 Hz) in the brain become more synchronized in response to a specific event or stimulus, often associated with increased neural processing and cognitive functions such as attention and memory encoding.
gamma oscillations: brain waves in the frequency range of approximately 30 to 100 Hz, associated with various cognitive functions.
glymphatic clearance system: a system in the brain responsible for removing waste products, which is more active during sleep.
hertz (Hz): unit of frequency measured in cycles per second.
high beta: 20-35 Hz rhythm correlated with multi-tasking and optimal performance and anxiety, migraine, obsessive-compulsive disorder (OCD), rumination, and worry.
high-frequency filter (HFF; low-pass filter): a filter that attenuates frequencies above a cutoff frequency.
hippocampus: a brain region involved in the formation and retrieval of memories.
hippocampal-neocortical transfer: the process during sleep where information is transferred from the hippocampus to the neocortex for long-term storage.
homeostatic sleep regulation: the process by which the body balances sleep and wakefulness to maintain overall health, often indicated by the amount of delta activity.
hyperarousal: a state of increased psychological and physiological tension, often associated with anxiety and stress.
IIR filter: a recursive filter that uses part of its output as input. IIR filters attenuate frequencies outside the bandpass more sharply than FIR filters with the same order, have greater time delay (that depends on frequency) due to greater filter sharpness, achieve faster computation due to their lower order, and are less stable than FIR filters.
inhibitory neurons: neurons that decrease the likelihood of a firing action potential in the recipient neuron.
irregular waves: EEG waves that continuously change shape and duration.
isolated epileptiform discharges (IEDs): spike and wave or sharp and slow wave patterns observed in EEG recordings of individuals who do not have clinical seizures. These discharges are subclinical, meaning they do not manifest as observable seizure activity but indicate underlying neuronal hyperexcitability.
Joint time-frequency analysis (JTFA): an algorithm that computes values on each data point at rates up to 256 times per second without using a fixed epoch length. Where FFT simultaneously calculates amplitudes for all frequency bands, JTFA analyzes preselected bands.
K-complex: sharp negative waveforms that reach amplitudes over microvolts succeeded by a longer moderate-to-high-amplitude positive wave observed in Stage 2 sleep.
kappa rhythm: very low amplitude activity in the alpha or theta range detected over temporal sites during mental activity.
long-term memory: the storage of information over an extended period.
low beta: 16-20+ Hz rhythm associated with successful problem-solving.
low resolution electromagnetic tomography (LORETA): Pascual-Marqui's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp.
low-frequency filter (high-pass filter): a circuit that filters out low-frequency activity and passes only the frequencies above a set value (e.g., 1.6 Hz).
magnetoencephalography (MEG): a neuroimaging technique for mapping brain activity by recording magnetic fields produced by electrical currents occurring naturally in the brain.
magnitude: the average amplitude over a unit of time using quantification methods like peak-to-peak (P-P) and root mean square (RMS).
memory consolidation: the process by which short-term memories are transformed into long-term memories during sleep.
microvolt: one-millionth of a volt.
mild cognitive impairment (MCI): a condition involving noticeable cognitive decline, greater than expected for a person's age, but not severe enough to interfere significantly with daily life or independent function.
monomorphic waves: series of waves that are rhythmic with the same frequency and morphology.
morphology: the shape of the signal generated by oscillating potential differences.
motor inhibition: suppressing motor activity, allowing for stillness and precision in movements.
mu rhythm: 7-11-Hz waves resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
neocortex: the part of the brain involved in higher-order brain functions, including sensory perception, cognition, and generation of delta waves.
neural oscillations: rhythmic or repetitive neural activity in the central nervous system.
neurodegenerative diseases: disorders characterized by the progressive degeneration of neurons, often associated with disrupted delta activity.
non-rapid eye movement (NREM) sleep: a phase of sleep that includes stages 1-3, with delta waves being most prominent in stage 3, also known as slow-wave sleep.
notch filter: a circuit that suppresses a narrow band of frequencies, such as those produced by line current at 50/60Hz.
optogenetic stimulation: a technique that uses light to control neurons genetically modified to express light-sensitive ion channels. This method allows precise control over the activity of specific neurons in the brain, enabling researchers to study the functions of neural circuits and their roles in behavior and neurological conditions.
order: the maximum delay in samples used in creating each output sample.
parvalbumin: a protein expressed in certain inhibitory interneurons.
peak-to-peak (p-p): a signal quantification method that measures waveform "height" from peak to trough.
percent power: the expression of power within a frequency band as a percentage of total EEG power.
physical restoration: the process during sleep where the body repairs tissues, grows muscles, and releases growth hormones.
polyphasic waves: waveforms with two or more elements; diphasic and triphasic waves.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
power: amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
prefrontal cortex: a part of the brain involved in complex cognitive behavior, decision-making, and moderating social behavior.
primary motor cortex (M1): a brain region involved in planning, controlling, and executing voluntary movements.
primary somatosensory cortex (S1): a brain region responsible for processing sensory information from the body.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
raw EEG signal: oscillating electrical potential differences detected from the scalp.
reference electrode: an electrode that is placed on the scalp, earlobe, or mastoid.
regular waves: rhythmic waves with the same frequency and morphology.
relaxed wavefulness: a state of being awake but calm and free from active motor activity.
Research Domain Criteria (RDoC): The Research Domain Criteria (RDoC) is a research framework developed by the National Institute of Mental Health (NIMH) that aims to integrate various domains of information (including genetics, neuroscience, and behavioral science) to understand mental disorders. It moves beyond traditional diagnostic categories to focus on fundamental biological and psychological processes that cut across different disorders.
reticular nucleus of the thalamus (TRN): GABAergic thalamic neurons that modulate signals from other thalamic nuclei and do not project to the cortex. Also called the nucleus reticularis of the thalamus (NRT).
root mean square (RMS): a signal quantification method that calculates the area under the EEG waveform and is analogous to the weight of an object.
sampling rate: the number of times per second that an ADC samples the EEG signal.
sampling resolution: a chronic brain disorder characterized by symptoms such as hallucinations, delusions, and cognitive impairments.
saw-toothed waves: waves that resemble asymmetrical triangles.
schizophrenia: a chronic brain disorder characterized by symptoms such as hallucinations, delusions, and cognitive impairments.
sensorimotor cortex: a region of the brain that processes and integrates sensory inputs and motor outputs.
sensorimotor rhythm (SMR): 12 to 15 Hz oscillations associated with motor inhibition and focused attention.
sensory perception: the process of recognizing and interpreting sensory stimuli.
sharp transients: waves with steep peaks that are not produced by epilepsy.
sharp waves: steeply-shaped waves with 70-200 ms duration.
Sheer rhythm: a brain oscillation characterized by high-frequency activity (38-42 Hz) that occurs in short, transient bursts. These bursts are typically synchronized across large neural populations and are thought to play a role in the timing and coordination of neuronal activity, particularly in relation to cognitive processes and sensory perception.
sinusoidal: rhythmic waves that resemble sine waves.
sleep pressure: the body's need for sleep, which increases with prolonged wakefulness and is reflected by the amount of delta activity during sleep.
sleep spindle: waves that range from 12-15 Hz and last from 0.5 to several seconds widely distributed over the scalp and are observed during Stage 2 and 3 sleep.
slow cortical potentials (SCPs): gradual voltage changes in the EEG that reflect changes in cortical excitability and are associated with various cognitive and motor processes.
slow-wave sleep (SWS): the deepest phase of non-REM sleep characterized by high amplitude, low-frequency delta waves.
spike: a waveform with a sharp appearance that lasts 20-70 ms.
spike-and-wave complexes: spikes that are succeeded by slow waves.
spinding excessive beta (SEB): an EEG pattern characterized by high-frequency beta waves (13-30 Hz) with a spindle-like appearance, often exceeding 20 μV in amplitude. It is associated with various psychiatric and neurological conditions, including ADHD, insomnia, and medication-resistant psychiatric disorders.
standardized LORETA (sLORETA): a refinement of LORETA that estimates each voxel's electrical potentials without regard to their frequency, expresses normalized F-values, and achieves a 1-cubic-cm resolution.
sub-vigil states: intermediate states between wakefulness and sleep, characterized by reduced sensory and cognitive activity. These states can lead to cognitive impairments and behavioral disturbances, often observed in conditions like ADHD and insomnia.
surface EMG (SEMG): a non-invasive technique used to measure and record the electrical activity produced by skeletal muscles. Sensors placed on the skin's surface detect the electrical potentials muscle fibers generate during contraction, providing information about muscle function, activation patterns, and fatigue.
surface Laplacian (SL) analysis: a family of mathematical algorithms that provide two-dimensional images of radial current flow from cortical dipoles to the scalp.
synaptic pruning: the process of eliminating weaker synaptic connections in the brain during sleep, which is believed to be facilitated by delta waves.
synchrony: the simultaneous occurrence of events or processes, in this context, the coordinated activity of neurons.
Talairach coordinate: coordinate assignment based on vertical distance from a horizontal line from the anterior commissure (origin) to the posterior commissure; referenced to the Talairach or Montreal atlases.
thalamic pacemaker neurons: neurons in the thalamus that generate rhythmic burst firing patterns, contributing to the synchronization of delta waves.
thalamic pacemaker neurons: neurons in the thalamus that generate rhythmic burst firing patterns, contributing to the synchronization of delta waves.
temporal framework: the timing structure that allows for the coordination of neural activity. thalamocortical interactions: the connections and communications between the thalamus and the cerebral cortex.
thalamic-cortical relay (TCR) system: the primary pathway for determining which cortical areas receive each type of sensory input.
thalamocortical circuits: neural pathways that connect the thalamus with the cortex, which generate rhythmic brain activity.
theta/beta ratio: the ratio between 4-7 Hz theta and 13-21 Hz beta, measured as amplitude squared, most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
transient: a single wave or series of waves distinct from background EEG activity.
triphasic wave: a wave that consists of three elements with alternating directions.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
vertex sharp transient (V wave): a negative-polarity waveform detected at the vertex in sleep records but are not usually observed during wakefulness.
voxel: a volumetric unit.
wave: a plot of voltage using a bipolar (positive/negative) scale with zero in the middle; the analog form of the signal in which voltage continuously varies.
waveform: the shape of the signal that is generated by oscillating potential differences between two electrodes.
working memory: a cognitive system responsible for temporarily holding information available for processing.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Physiological Psychology, which satisfies BCIA's Physiological Psychology requirement, and Neurofeedback100, which provides extensive multiple-choice testing over the Biofeedback Blueprint.

Assignment
Now that you have completed this module, write down the frequency bands you uptrain and downtrain in clinical or peak performance practice and explain the rationale for these choices.
References
Achermann, P., & Borbély, A. A. (2003). Mathematical models of sleep regulation. Frontiers in Bioscience, 8, s683-s693. https://doi.org/10.2741/1074
Aich T. K. (2014). Absent posterior alpha rhythm: An indirect indicator of seizure disorder? Indian Journal of Psychiatry, 56(1), 61–66. https://doi.org/10.4103/0019-5545.124715
Amo, C., Castillo, M., Barea, R., Santiago, L., Martínez-Arribas, A., Amo-López, P., & Boquete, L. (2016). Induced gamma-band activity during voluntary movement: EEG analysis for clinical purposes. Motor Control, 20(4), 409-28. https://doi.org/10.1123/mc.2015-0010
Amo, C., Santiago, L., Barea, R., López-Dorado, A., & Boquete, L. (2017). Analysis of Ggamma-band activity from human EEG using empirical mode decomposition. Sensors (Basel, Switzerland), 17. https://doi.org/10.3390/s17050989
Amzica, F., & Lopes da Silva, F. H. (2018). Cellular substrates of brain rhythms. In D. L. Schomer & F. H. Lopes da Silva (Eds.), Niedermeyer’s electroencephalography: Basic principles, clinical applications and related fields (7th ed., pp. 20–62). Oxford University Press.
Angelakis, E., Lubar, J. F., Stathopoulou, S., & Kounios, J. (2004). Peak alpha frequency: an electroencephalographic measure of cognitive preparedness. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(4), 887–897. https://doi.org/10.1016/j.clinph.2003.11.034
Anderson, C., & Horne, J. (2003). Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology, 40(3), 349-57. https://doi.org/10.1111/1469-8986.00038
Arns, M., Swatzyna, R., Gunkelman, J., & Olbrich, S. (2015). Sleep maintenance, spindling excessive beta and impulse control: an RDoC arousal and regulatory systems approach? Neuropsychiatric Electrophysiology, 1, 1-11. https://doi.org/10.1186/S40810-015-0005-9
Arnolds, D. E., Lopes da Silva, F. H., Aitink, J. W., Kamp, A., & Boeijinga, P. (1980). The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalography and Clinical Neurophysiology, 50(3-4), 324–328. https://doi.org/10.1016/0013-4694(80)90160-1
Babiloni, C., Noce, G., Bonaventura, C., Lizio, R., Pascarelli, M., Tucci, F., Soricelli, A., Ferri, R., Nobili, F., Famà, F., Palma, E., Cifelli, P., Marizzoni, M., Stocchi, F., Frisoni, G., & Percio, C. (2020). Abnormalities of cortical sources of resting state delta electroencephalographic rhythms are related to epileptiform activity in patients with amnesic mild cognitive impairment not due to Alzheimer's disease. Frontiers in Neurology, 11. https://doi.org/10.3389/fneur.2020.514136
Bosman, C. A., Lansink, C. S., & Pennartz, C. M. (2014). Functions of gamma-band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. European Journal of Neuroscience, 39(11), 1982-1999. https://doi.org/10.1111/ejn.12606
Buzsáki, G., & Wang, X. J. (2012). Mechanisms of gamma oscillations. Annual Review of Neuroscience, 35, 203–225. https://doi.org/10.1146/annurev-neuro-062111-150444
Chang, L. Y., Wang, M. Y., & Tsai, P. S. (2016). Diagnostic accuracy of rating scales for Attention-Deficit/Hyperactivity Disorder: A meta-analysis. Pediatrics, 137(3), e20152749. https://doi.org/10.1542/peds.2015-2749
Clarke, A., Barry, R., McCarthy, R., & Selikowitz, M. (2001). Excess beta activity in children with attention-deficit/hyperactivity disorder: an atypical electrophysiological group. Psychiatry Research, 103, 205-218. https://doi.org/10.1016/S0165-1781(01)00277-3
Colgin, L. L. (2016). Rhythms of the hippocampal network. Nature Reviews Neuroscience, 17(4), 239-249.
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Corsi-Cabrera, M., Pérez-Garci, E., Río-Portilla, Y., Ugalde, E., & Guevara, M. (2001). EEG bands during wakefulness, slow-wave, and paradoxical sleep as a result of principal component analysis in the rat. Sleep, 24(4), 374-80. https://doi.org/10.1093/sleep/23.6.1a.
Cortoos, A., De Valck, E., Arns, M., Breteler, M. H., & Cluydts, R. (2010). An exploratory study on the effects of tele-neurofeedback and tele-biofeedback on objective and subjective sleep in patients with primary insomnia. Applied Psychophysiology and Biofeedback, 35(2), 125-134. https://doi.org/10.1007/s10484-009-9116-z
Crabtree J. W. (2018). Functional diversity of thalamic reticular subnetworks. Frontiers in Systems Neuroscience, 12, 41. https://doi.org/10.3389/fnsys.2018.00041
Crone, N., Miglioretti, D., Gordon, B., & Lesser, R. (1998). Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain: A Journal of Neurology, 121( Pt 12), 2301-15. https://doi.org/10.1093/BRAIN/121.12.2301
De Gennaro, L., & Ferrara, M. (2003). Sleep spindles: An overview. Sleep Medicine Reviews, 7(5), 423-440. https://doi.org/10.1053/smrv.2002.0252
Dehghani, N., Peyrache, A., Telenczuk, B., Le Van Quyen, M., Halgren, E., Cash, S. S., Hatsopoulos, N. G., & Destexhe, A. (2016). Dynamic balance of excitation and inhibition in human and monkey neocortex. Scientific Reports, 6, 23176. https://doi.org/10.1038/srep23176
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Egner, T., & Gruzelier, J. H. (2001). Learned self-regulation of EEG frequency components affects attention and event-related brain potentials in humans. NeuroReport, 12(18), 4155-4159. https://doi.org/10.1097/00001756-200112210-00058
Ekstrom, A. D., Caplan, J. B., Ho, E., Shattuck, K., Fried, I., & Kahana, M. J. (2005). Human hippocampal theta activity during virtual navigation. Hippocampus, 15(7), 881–889. https://doi.org/10.1002/hipo.20109
Enriquez-Geppert, S., Brown, T., Henrich, H., Arns, M., & Pimenta, M. G. (2023). Attention Deficit Hyperactivity Disorder. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
Fries P. (2015). Rhythms for Cognition: Communication through coherence. Neuron, 88(1), 220–235. https://doi.org/10.1016/j.neuron.2015.09.034
Frey, L. (2023). Epilepsy. In I. Khazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenthal (Eds). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Gilmore, P., & Brenner, R. (1981). Correlation of EEG, computerized tomography, and clinical findings. Study of 100 patients with focal delta activity. Archives of Neurology, 38(6), 371-372. https://doi.org/10.1001/ARCHNEUR.1981.00510060073013.
Goldstein, A. N., & Walker, M. P. (2014). The role of sleep in emotional brain function. Annual Review of Clinical Psychology, 10, 679-708. https://doi.org/10.1146/annurev-clinpsy-032813-153716
Gouw, A., Alsema, A., Tijms, B., Borta, A., Scheltens, P., Stam, C., & Flier, W. (2017). EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiology of Aging, 57, 133-142. https://doi.org/10.1016/j.neurobiolaging.2017.05.017
Grandy, T. H., Werkle-Bergner, M., Chicherio, C., Schmiedek, F., Lövdén, M., & Lindenberger, U. (2013). Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology, 50(6), 570–582. https://doi.org/10.1111/psyp.12043
Gruzelier, J. H., Inoue, A., Smart, R. G., Steed, A., & Steffert, T. (2014). Acting performance and flow state enhanced with sensory-motor rhythm neurofeedback comparing ecologically valid immersive VR and training screen scenarios. Neuroscience Letters, 480(2), 112-116. https://doi.org/10.1016/j.neulet.2014.04.027
Haegens, S., Cousijnc, H., Wallis, G., Harrison, P. J., & Nobre, A. C. (2014). Inter- and intra-individual variability in alpha peak frequency. NeuroImage, 92, 46-55. doi.org/10.1016/j.neuroimage.2014.01.049
Hammond, D. C. (2005). Neurofeedback with anxiety and affective disorders. Child and Adolescent Psychiatric Clinics of North America, 14(1), 105-123. https://doi.org/10.1016/j.chc.2004.07.008
Hanslmayr, S., Sauseng, P., Doppelmayr, M., Schabus, M., & Klimesch, W. (2005). Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Applied Psychophysiology and Biofeedback, 30(1), 1–10. https://doi.org/10.1007/s10484-005-2169-8
Hari, R., & Puce, A. (2017). MEG-EEG primer. Oxford University Press.
Harmony, T., Fernández, T., Silva, J., Bernal, J., Díaz-Comas, L., Reyes, A., Marosi, E., Rodriguez, M., & Rodríguez, M. (1996). EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 24(1-2), 161-71. https://doi.org/10.1016/S0167-8760(96)00053-0
Hartoyo, A., Cadusch, P. J., Liley, D. T. J., & Hicks, D. G. (2020). Inferring a simple mechanism for alpha-blocking by fitting a neural population model to EEG spectra. PLoS Computational Biology, 16(4), e1007662. https://doi.org/10.1371/journal.pcbi.1007662
Hasib, T., & Vengadasalam, V. (2023). Analysing gamma frequency components in EEG signals: A comprehensive extraction approach. Journal of Informatics and Web Engineering. https://doi.org/10.33093/jiwe.2023.2.2.11
Hoedlmoser, K., Pecherstorfer, T., Gruber, G., Anderer, P., Doppelmayr, M., Klimesch, W., & Schabus, M. (2008). Instrumental conditioning of human sensorimotor rhythm (12-15 Hz) and its impact on sleep as well as declarative learning. Sleep, 31(10), 1401–1408.
Howells, F., Temmingh, H., Hsieh, J., Dijen, A., Baldwin, D., & Stein, D. (2018). Electroencephalographic delta/alpha frequency activity differentiates psychotic disorders: A study of schizophrenia, bipolar disorder and methamphetamine-induced psychotic disorder. Translational Psychiatry, 8. https://doi.org/10.1038/s41398-018-0105-y
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Iaccarino, H. F., Singer, A. C., Martorell, A. J., Rudenko, A., Gao, F., Gillingham, T. Z., Mathys, H., Seo, J., Kritskiy, O., Abdurrob, F., Adaikkan, C., Canter, R. G., Rueda, R., Brown, E. N., Boyden, E. S., & Tsai, L. H. (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature, 540(7632), 230–235. https://doi.org/10.1038/nature20587
Jadeja, N. M. (2021). Frequencies and rhythms. In How to read an EEG. Cambridge University Press. https://doi.org/10.1017/9781108918923.008
Jensen, O., Kaiser, J., & Lachaux, J. P. (2007). Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences, 30(7), 317–324. https://doi.org/10.1016/j.tins.2007.05.001
Kane, N., Acharya, J., Benickzy, S., Caboclo, L., Finnigan, S., Kaplan, P. W., Shibasaki, H., Pressler, R., & van Putten, M. J. A. M. (2017). A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clinical Neurophysiology Practice, 2, 170–185. https://doi.org/10.1016/j.cnp.2017.07.002
Kayser, J., & Tenke, C. E. (2015). On the benefits of using surface Laplacian (current source density) methodology in electrophysiology. Int J Psychophysiol, 97(3), 171-173. https://dx.doi.org/10.1016%2Fj.ijpsycho.2015.06.001
Krepel, N., van Dijk, H., Sack, A. T., Swatzyna, R. J., & Arns, M. (2021). To spindle or not to spindle: A replication study into spindling excessive beta as a transdiagnostic EEG feature associated with impulse control. Biological Psychology, 165, 108188. https://doi.org/10.1016/j.biopsycho.2021.108188
Libenson, M. H. (2024). Practical approach to electroencephalography (2nd ed.). Saunders Elsevier.
Lopes da Silva, F. H. (1991). Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalography and Clinical Neurophysiology, 79(2), 81-93. https://doi.org/10.1016/0013-4694(91)90044-5
López-Sanz, D., Bruña, R., Garcés, P., Cámara, C., Serrano, N., Rodríguez-Rojo, I., Delgado, M., Montenegro, M., López-Higes, R., Yus, M., & Maestú, F. (2016). Alpha band disruption in the AD-continuum starts in the Subjective Cognitive Decline stage: A MEG study.Scientific Reports, 6. https://doi.org/10.1038/srep37685.
Lubar, J. F. (1991). Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Biofeedback and Self-regulation, 16(3), 201–225. https://doi.org/10.1007/BF01000016
Lubar, J. F. (2001). Rationale for choosing bipolar versus referential training. Journal of Neurotherapy, 4(3), 94–97.
Marshall, L., & Born, J. (2007). The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences, 11(10), 442-450. https://doi.org/10.1016/j.tics.2007.09.001
Merica, H., & Fortune, R. (2005). Spectral power time-courses of human sleep EEG reveal a striking discontinuity at approximately 18 Hz marking the division between NREM-specific and wake/REM-specific fast frequency activity. Cerebral Cortex, 15(7), 877-884.
Mierau, M., Klimesch, W., & Lefebvre, J. (2017). State-dependent alpha peak frequency shifts: Experimental evidence, potential mechanisms and functional implications. Neuroscience, 360, 146-154. doi.org/10.1016/j.neuroscience.2017.07.037
Min, B. K., & Park, H. J. (2010). Task-related modulation of anterior theta and posterior alpha EEG reflects top-down preparation. BMC Neuroscience, 11, 79. https://doi.org/10.1186/1471-2202-11-79
Moffett, S., O’Malley, S., Man, S., Hong, D., & Martin, J. (2017). Dynamics of high frequency brain activity. Scientific Reports, 7. https://doi.org/10.1038/s41598-017-15966-6.
Monastra, V. J., Lubar, J. F., & Linden, M. (2001). The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: Reliability and validity studies. Neuropsychology, 15(1), 136–144. https://doi.org/10.1037//0894-4105.15.1.136
Monastra, V. J., Lubar, J. F., Linden, M., VanDeusen, P., Green, G., Wing, W., Phillips, A., & Fenger, T. N. (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: an initial validation study. Neuropsychology, 13(3), 424–433. https://doi.org/10.1037/0894-4105.13.3.424
Morales-Quezada, L., Martinez, D., El-Hagrassy, M. M., Kaptchuk, T. J., Sterman, M. B., & Yeh, G. Y. (2019). Neurofeedback impacts cognition and quality of life in pediatric focal epilepsy: An exploratory randomized double-blinded sham-controlled trial. Epilepsy & Behavior: E&B, 101(Pt A), 106570. https://doi.org/10.1016/j.yebeh.2019.106570
Nazish, S. (2020). Clinical and radiological correlates of different electroencephalographic patterns in hospitalized patients. Clinical EEG and Neuroscience, 52(4), 280-286. https://doi.org/10.1177/1550059420910
Neurophysiological parameters influencing sleep–wake discrepancy in insomnia disorder: A preliminary analysis on alpha rhythm during sleep onset. (2023). Brain Sciences. MDPI. https://www.mdpi.com/2076-3425/13/2/260
Niedermeyer E. (1997). Alpha rhythms as physiological and abnormal phenomena. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 26(1-3), 31–49. https://doi.org/10.1016/s0167-8760(97)00754-x
Niedermeyer, E., & da Silva, F. L. (2004). Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins.
Nunez, P. L., & Srinivasan, R. (2019). Electric fields of the brain: The neurophysics of EEG (2nd ed.). Oxford Academic. https://doi.org/10.1093/acprof:oso/9780195050387.001.0001
Oathes, D., Ray, W., Yamasaki, A., Borkovec, T., Castonguay, L., Newman, M., & Nitschke, J. (2008). Worry, generalized anxiety disorder, and emotion: Evidence from the EEG gamma band. Biological Psychology, 79, 165-170. https://doi.org/10.1016/j.biopsycho.2008.04.005
Othmer S. ( 2007). Progress in neurofeedback for the autism spectrum. Paper presented at the 38th Annual Meeting of the Association for Applied Psychophysiology & Biofeedback Monterey, Canada, 15–18 February 2007.
Petersén, I., & Eeg-Olofsson, O. (1971). The development of the electroencephalogram in normal children from the age of 1 through 15 years. Non-paroxysmal activity. Neuropadiatrie, 2(3), 247–304. https://doi.org/10.1055/s-0028-1091786
Pfurtscheller, G., & Lopes da Silva, F. H. (1999). Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110(11), 1842-1857. https://doi.org/10.1016/S1388-2457(99)00141-8
Rathee, S., Bhatia, D., Punia, V., & Singh, R. (2020). Peak alpha frequency in relation to cognitive performance. Journal of Neurosciences in Rural Practice, 11(3), 416–419. https://doi.org/10.1055/s-0040-1712585
Rowan, A. J., & Tolunsky, E. (2003). Primer of EEG with a mini-atlas. Butterworth-Heinemann.
Sazgar, M., & Young, M. (2019). Normal EEG awake and sleep. Absolute Epilepsy and EEG Rotation Review. https://doi.org/10.1007/978-3-030-03511-2_6
Schomer, D. L., & Lopes da Silva, F. H. (Eds.). (2017). Niedermeyer's electroencephalography: Basic principles, clinical applications, and related fields (7th ed.). Oxford Academic. https://doi.org/10.1093/med/9780190228484.001.0001
Simon, M., Schmidt, E. A., Kincses, W. E., Fritzsche, M., Bruns, A., Aufmuth, C., Bogdan, M., Rosenstiel, W., & Schrauf, M. (2011). EEG alpha spindle measures as indicators of driver fatigue under real traffic conditions. Clinical Neurophysiology, 122(6), 1168–1178. https://doi.org/10.1016/j.clinph.2010.10.044
Simonová, O., Roth, B., & Stein, J. (1967). EEG studies of healthy population--Normal rhythms of resting recording. Acta Universitatis Carolinae. Medica, 13(7), 543–551. PMID: 5620888
Singer W. (2013). Cortical dynamics revisited. Trends in Cognitive Sciences, 17(12), 616–626. https://doi.org/10.1016/j.tics.2013.09.006
Sirin, T., Şirinocak, P., Arkalı, B., Akıncı, T., & Yeni, S. (2019). Electroencephalographic features associated with intermittent rhythmic delta activity. Neurophysiologie Clinique, 49, 227-234. https://doi.org/10.1016/j.neucli.2019.01.036
Snyder, S. M., Quintana, H., Sexson, S. B., Knott, P., Haque, A. F., & Reynolds, D. A. (2008). Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatry Research, 159(3), 346–358. https://doi.org/10.1016/j.psychres.2007.05.00
Soroush-Vala, A., Rahmanian, M., Jadid, M., & Hassanvandi, S. (2023). Application of neurofeedback in treating epilepsy: A systematic review and meta-analysis. International Journal of Body, Mind and Culture, 10, 143-157. https://doi.org/10.22122/ijbmc.v10i2.506
Spiesshoefer, J., Linz, D., Skobel, E., Arzt, M., Stadler, S., Schoebel, C., Fietze, I., Penzel, T., Sinha, A. M., Fox, H., Oldenburg, O., & German Cardiac Society Working Group on Sleep Disordered Breathing (AG 35-Deutsche Gesellschaft für Kardiologie Herz und Kreislaufforschung e.V.) (2021). Sleep - the yet underappreciated player in cardiovascular diseases: A clinical review from the German Cardiac Society Working Group on Sleep Disordered Breathing. European Journal of Preventive Cardiology, 28(2), 189–200. https://doi.org/10.1177/2047487319879526
Spydell, J. D., Ford, M. R., & Sheer, D. E. (1979). Task dependent cerebral lateralization of the 40 Hertz EEG rhythm. Psychophysiology, 16(4), 347–350. https://doi.org/10.1111/j.1469-8986.1979.tb01474.x
Steriade, M., McCormick, D. A., & Sejnowski, T. J. (1993). Thalamocortical oscillations in the sleeping and aroused brain. Science, 262(5134), 679-685. https://doi.org/10.1126/science.8235588
Sterman, M. B. (1996). Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedback and Self-regulation, 21(1), 3-33. https://doi.org/10.1007/BF02214146
Sterman, M. B. (2000). Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clinical Electroencephalography, 31(1), 45-55. https://doi.org/10.1177/155005940003100111
Suldo, S. M., Olson, L. A., & Evans, J. R. (2002). Quantitative EEG Evidence of Increased Alpha Peak Frequency in Children with Precocious Reading Ability. XJournal of Neurotherapy, 5(3), 39–50. https://doi.org/10.1300/J184v05n03_0
Sutter, R., Stevens, R., & Kaplan, P. (2012). Clinical and imaging correlates of EEG patterns in hospitalized patients with encephalopathy. Journal of Neurology, 260, 1087-1098. https://doi.org/10.1007/s00415-012-6766-1
Swatzyna, R. J., Arns, M., Tarnow, J. D., Turner, R. P., Barr, E., MacInerney, E. K., Hoffman, A. M., & Boutros, N. N. (2022). Isolated epileptiform activity in children and adolescents: Prevalence, relevance, and implications for treatment. European Child & Adolescent Psychiatry, 31(4), 545–552. https://doi.org/10.1007/s00787-020-01597-2
Tan, G., Thornby, J., Hammond, D. C., Strehl, U., Canady, B., Arnemann, K., & Kaiser, D. A. (2009). Meta-analysis of EEG biofeedback in treating epilepsy. Clinical EEG and Neuroscience, 40(3), 173–179. https://doi.org/10.1177/155005940904000310
The Johns Hopkins atlas of digital EEG: An interactive training guide. (2011). Johns Hopkins University Press.
Thompson, M., & Thompson, L. (2015). The neurofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Tombor, L., Kakuszi, B., Papp, S., Réthelyi, J., Bitter, I., & Czobor, P. (2019). Decreased resting gamma activity in adult attention deficit/hyperactivity disorder. The World Journal of Biological Psychiatry, 20, 691 - 702. https://doi.org/10.1080/15622975.2018.1441547
Tononi, G., & Cirelli, C. (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron, 81(1), 12-34. https://doi.org/10.1016/j.neuron.2013.12.025
Uhlhaas, P. J., & Singer, W. (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews. Neuroscience, 11(2), 100–113. https://doi.org/10.1038/nrn2774
van Son, D., de Rover, M., De Blasio, F. M., van der Does, W., Barry, R. J., & Putman, P. (2019). Electroencephalography theta/beta ratio covaries with mind wandering and functional connectivity in the executive control network. Annals of the New York Academy of Sciences, 1452(1), 52–64. https://doi.org/10.1111/nyas.14180
Varga, A. W., Kishi, A., Mantua, J., Lim, J., Koushyk, V., Leiberg, S., Heintz, C., Naik, S., Rapoport, D. M., & Ayappa, I. (2016). Abnormal sleep spindles and slow waves in sleep apnea and Alzheimer disease. Sleep, 39(4), 775-787. https://doi.org/10.5665/sleep.5634
Vernon, D., Egner, T., Cooper, N., Compton, T., Neilands, C., Sheri, A., & Gruzelier, J. (2003). The effect of training distinct neurofeedback protocols on aspects of cognitive performance. International Journal of Psychophysiology, 47(1), 75-85. https://doi.org/10.1016/S0167-8760(02)00091-0
Williams D. (1941). The electro-encephalogram in acute injuries. Journal of Neurology and Psychiatry, 4(2), 107–130. https://doi.org/10.1136/jnnp.4.2.107
Willoughby, J., Fitzgibbon, S., Pope, K., Mackenzie, L., Medvedev, A., Clark, C., Davey, M., & Wilcox, R. (2003). Persistent abnormality detected in the non-ictal electroencephalogram in primary generalised epilepsy. Journal of Neurology, Neurosurgery & Psychiatry, 74, 51 - 55. https://doi.org/10.1136/jnnp.74.1.51.
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., O'Donnell, J., Christensen, D. J., Nicholson, C., Iliff, J. J., Takano, T., Deane, R., & Nedergaard, M. (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373-377. https://doi.org/10.1126/science.1241224