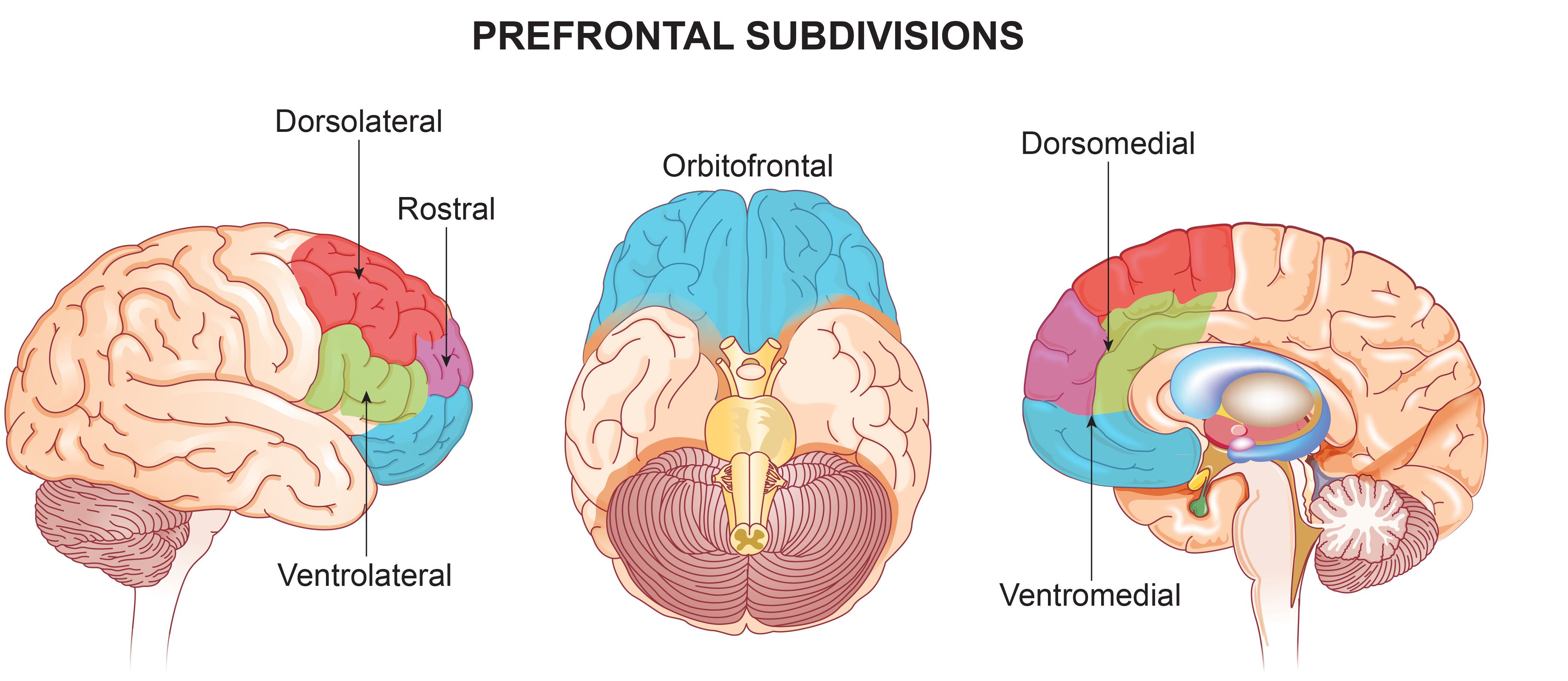
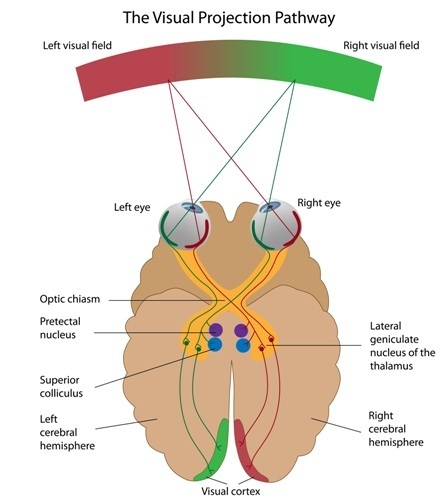
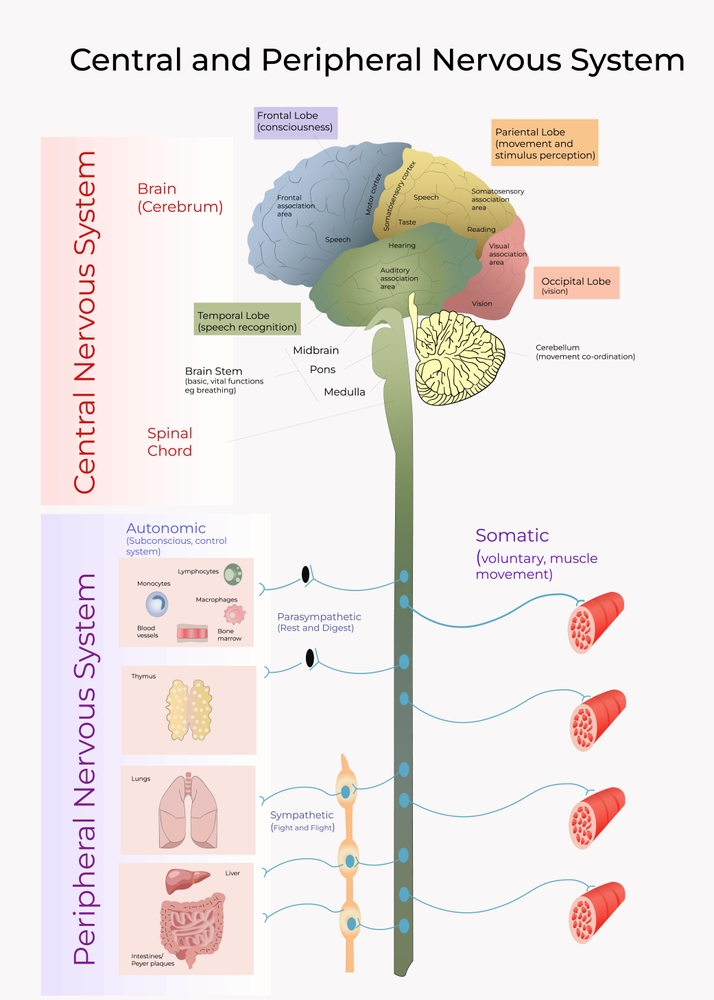
Visual System
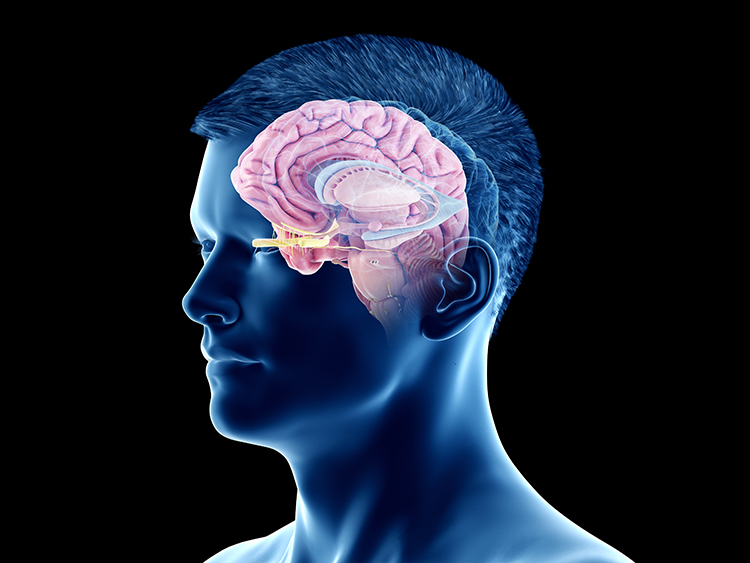
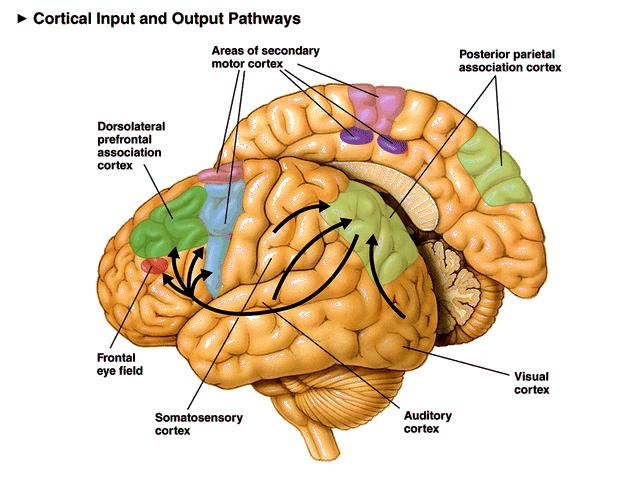
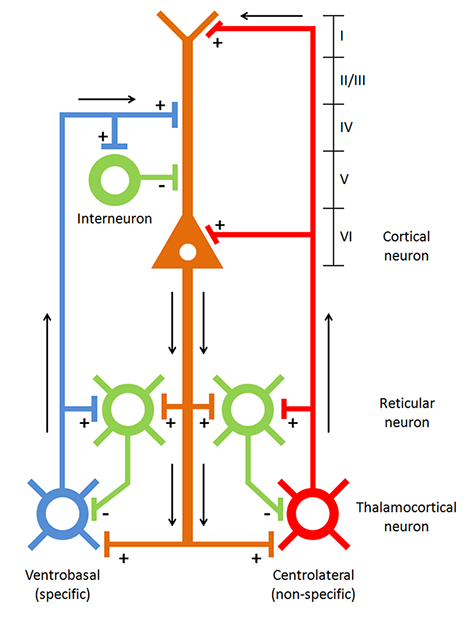
Retinal ganglion cells, which comprise the optic nerves, ascend to the midline optic chiasm where the two optic nerves meet. Here, temporal axons (toward the side of the head) continue as part of their own side's optic tract, and the nasal halves cross over to join the opposite side's optic tract. Most optic tract axons project information to the lateral geniculate nucleus of the thalamus.While the lateral geniculate nucleus (LGN) relays visual information to the cortex, brainstem, and cortical neurons modulate its activity. Brainstem neurons that mediate alertness and attention can adjust the LGN's response to visual input. Moreover, the cortex can exert a top-down selection of visual input to increase attention to a salient region of the visual field at the expense of others (Bear, Connors, & Paradiso, 2020).
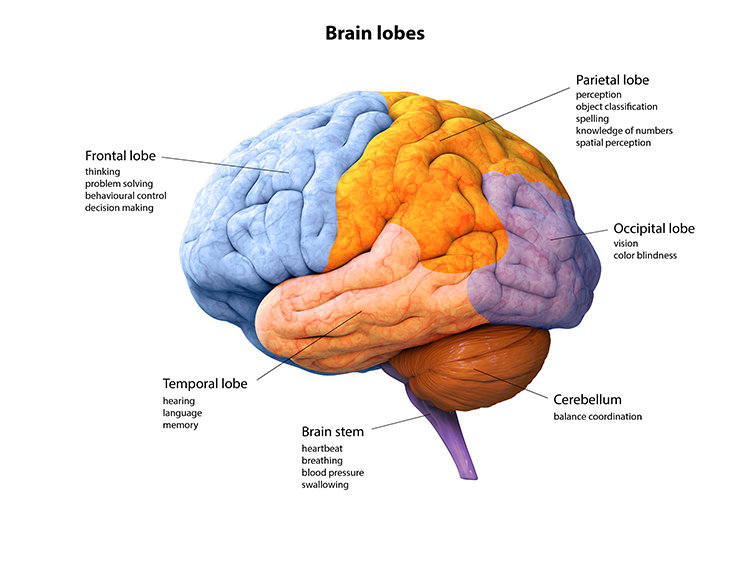
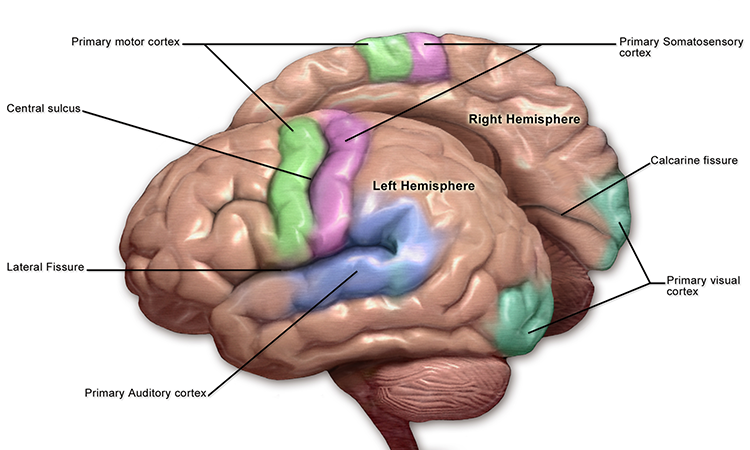
LGN neurons form the optic radiations and project to the primary visual cortex (V1) in the occipital lobe in cortical layer IV. A minority of retinal ganglion cell axons target the dorsal midbrain superior colliculus, which is concerned with visual gaze direction and attention to selected visual objects (Breedlove & Watson, 2023).
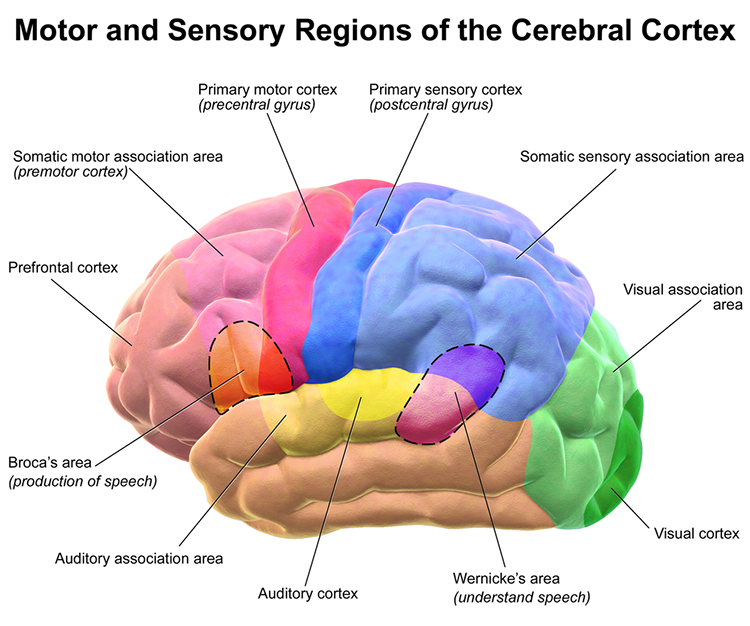
The cortex contains many specialist regions to process visual properties like color, shape, location, motion, and orientation. Evolution has organized these visual areas into dorsal and ventral streams that begin in the primary visual cortex. The dorsal stream, which projects from V1 to the parietal lobe, helps us localize objects and guide movements. There are neurons with visual and motor properties in the adjacent motor association cortex, called mirror neurons. Networks of mirror neurons may play a role in learning how to perform actions by observing others' movements, understanding others' actions and intentions, and empathy.
The lower ventral stream, which projects to inferior temporal and frontal areas, allows us to identify objects and faces. Visual pathway graphic © Alila Medical Media/Shutterstock.com.
Auditory System
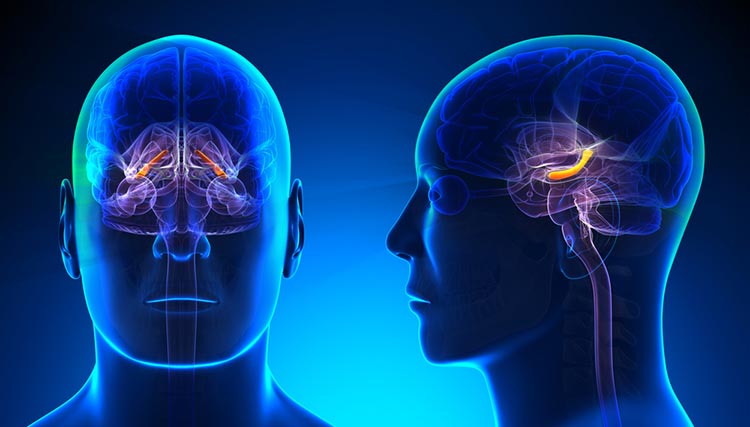
The cochlea's inner hair cells within the organ of Corti send 30,000 to 50,000 auditory fibers to several destinations: the midbrain superior olivary nuclei and inferior colliculi, and the medial geniculate nucleus of the thalamus. The superior olivary nuclei process binaural (two-ear) information to localize sound. All ascending auditory neurons innervate the inferior colliculi, some via intermediate relays.The inferior colliculi integrate information about spatial localization and multiple sensory modalities, including somatosensory information. They project to the thalamus' medial geniculate nucleus (MGN), which projects to several cortical auditory areas using two separate pathways. The MGN mainly relays frequency, amplitude, and binaural information to the auditory cortex in the temporal lobe. Auditory pathway graphic © medicalstocks/Shutterstock.com.
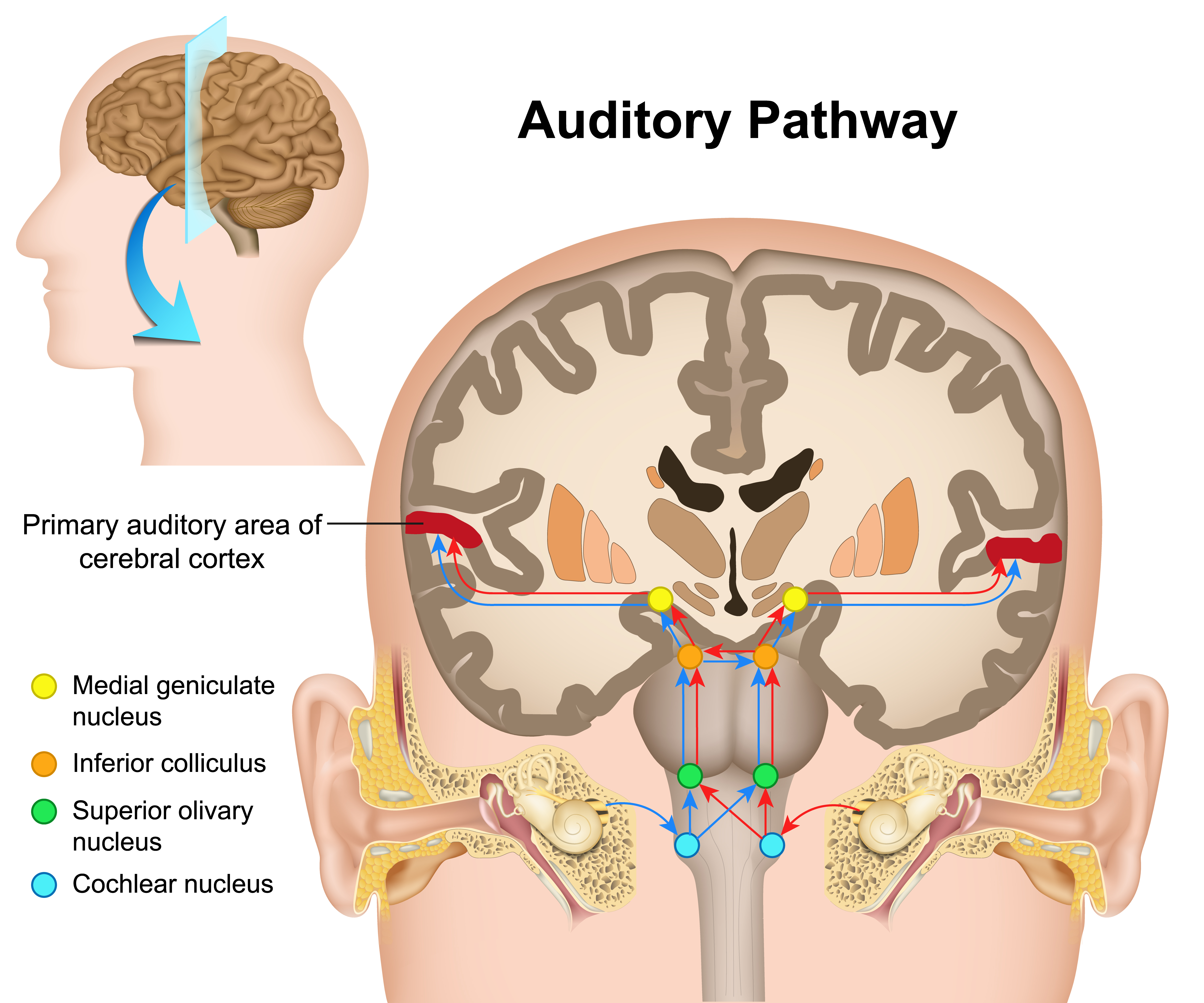
The auditory cortex processes auditory information within dorsal and ventral streams. The dorsal stream, which extends to the parietal lobe, helps us spatially localize sounds. The lower ventral stream, which projects to the temporal lobe, appears to analyze sound components, perhaps including speech sounds (Breedlove & Watson, 2023).
As with the visual pathways, the auditory system involves extensive feedback. Neurons in the brainstem innervate the outer hair cells that adjust the sensitivity of the basilar membrane within the organ of Corti to specific frequencies. Also, auditory cortex axons innervate both the inferior colliculi and MGN to exercise top-down control (Bear, Connors, & Paradiso, 2020).
Somatosensory System
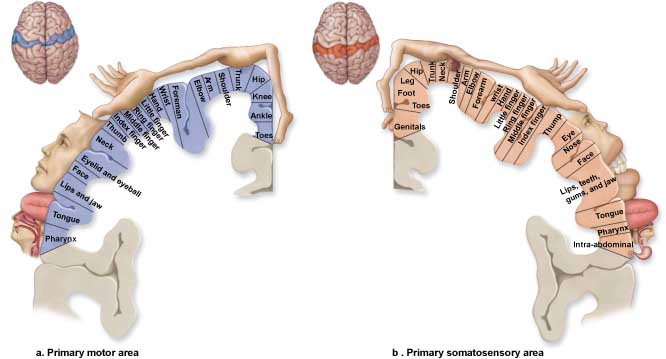
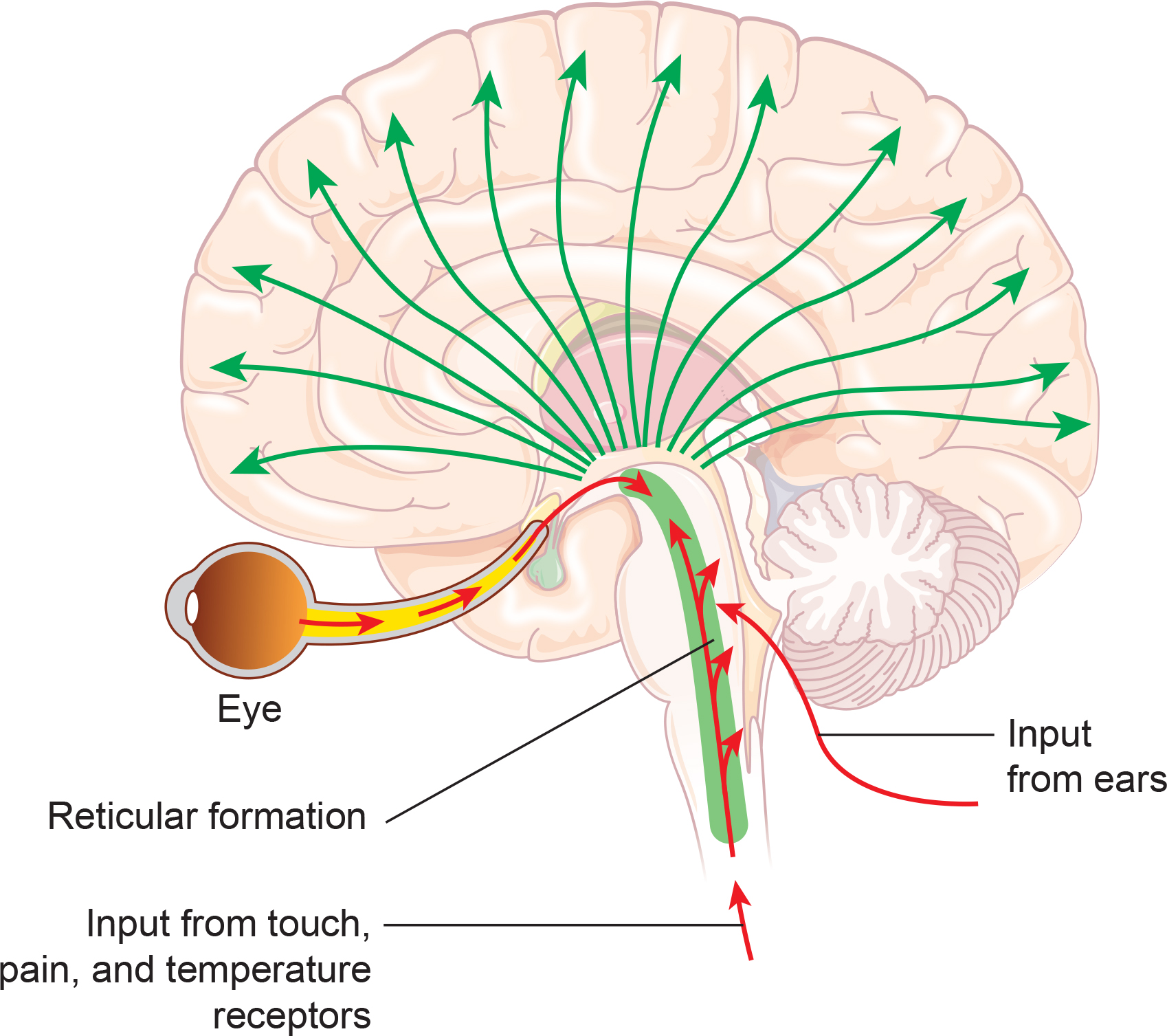
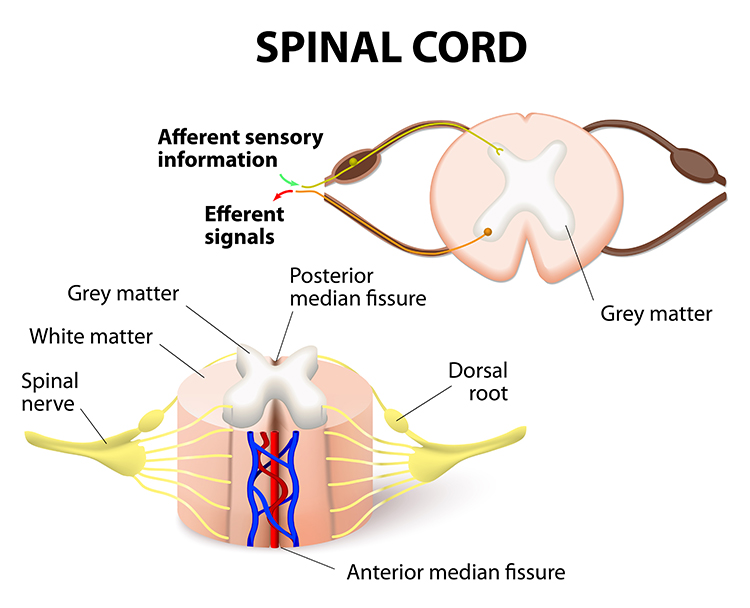
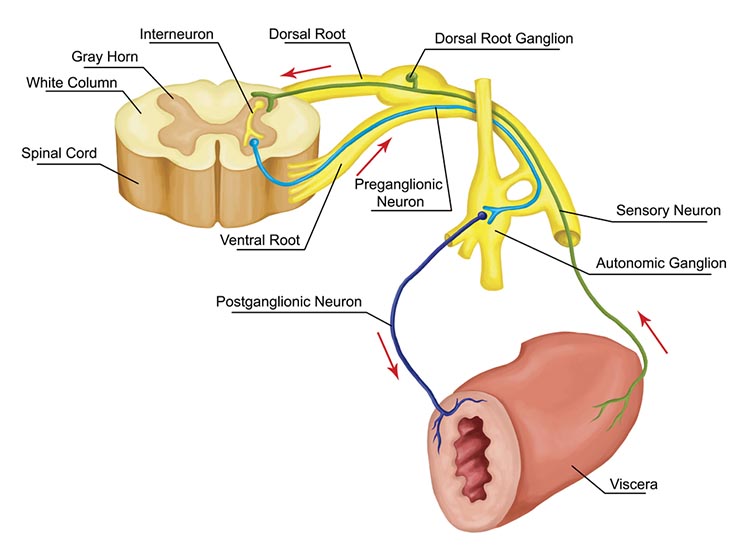
The somatosensory system employs specialized receptors to perceive itch, pain, temperature, and touch. For touch, the axon of a unipolar neuron enters the dorsal horn of the spinal cord and synapses with a dorsal column neuron in the medulla. Axons from this neuron decussate (cross the midline) and innervate the thalamus' ventral posterior nucleus (VPN). The VPN, in turn, distributes this information to the primary somatosensory cortex (S1). While each hemisphere's S1 maps touch information from the opposite side of the body, the secondary somatosensory cortex (S2) maps both sides. The maps are overlaid so that the left and right arms are represented in the same region of the body surface map (Breedlove & Watson, 2023). Spinothalamic graphic © VectorMine/Shutterstock.com.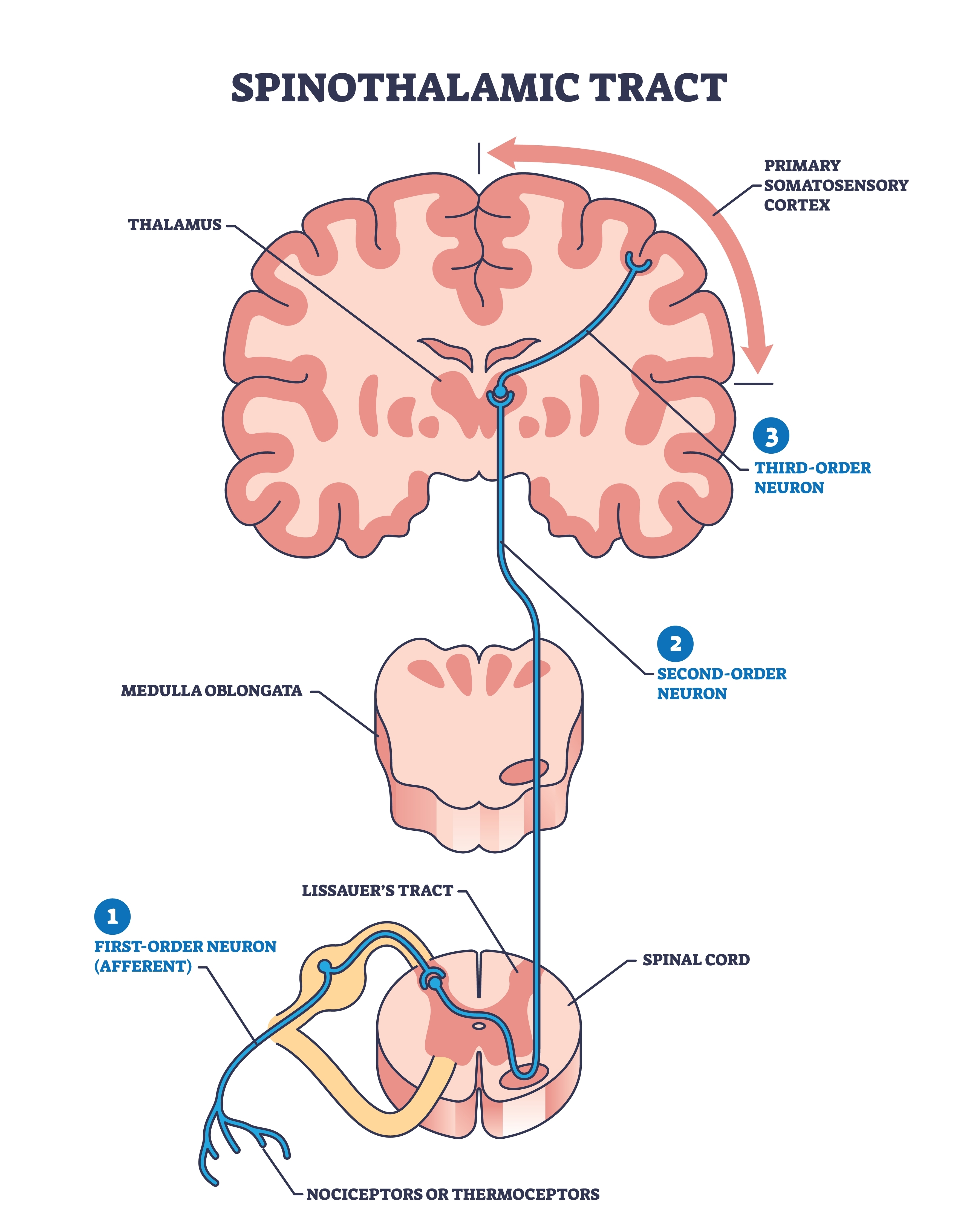
As with the visual and auditory systems, the ascending pathways do far more than relay information. These networks process and alter sensory information at each successive synapse. The cortex exercises top-down control over neurons in the dorsal column and VPN to dynamically adjust cortical inputs (Bear, Connors, & Paradiso, 2020).




.jpg)
.jpg)