Psychopharmacology
Psychopharmacology studies the mechanisms by which drugs impact the human brain and behavior (Julien, Advokat, & Comaty, 2023). An understanding of psychopharmacology is important to neurofeedback because drugs can affect a client's clinical presentation, EEG, assessment, and training success.
A single dose of a prescription psychotropic drug can markedly change the EEG within 1-3 hours of administration. Families of psychotropic drugs that share therapeutic equivalence (e.g., chlorpromazine-like neuroleptics and haloperidol-like neuroleptics) produce similar systematic EEG changes (Knott, 2000). A drug's plasma level, which depends on the dose, distribution volume, and metabolism, influences the magnitude of EEG alterations, which should be symmetrical and often widespread.
Common EEG changes include a slowing of background activity, increased beta activity, epileptiform activity, triphasic waves, and widespread delta and increased theta activity (Blume, 2006).
Epileptiform activity graphic © Chaikom/Shutterstock.com.
This unit addresses V. Psychopharmacological Considerations.

This unit covers Relationships of Drugs and Neurotransmitter Modulation, Potential Effects of Prescribed and Non-Prescribed Drugs on Clinical Presentation, Potential Effects of Prescribed and Non-Prescribed Drugs on EEG Measures, Potential Effects of Different Drugs on Neurofeedback Assessment and Training, Recommended Medication Management Approaches, and Drug Implications for Assessment and Neurofeedback.
Please click on the podcast icon below to hear a full-length lecture.

Neurons synthesize chemicals called neurotransmitters (NTs), which bind to a neuron's receptors to alter their activity. Neurotransmitter release animation (no sound) © Madrock24/Shutterstock.com.
Drugs are molecules that modify physiological processes. Psychoactive drugs alter brain functions to influence mood (e.g., anxiety) and behavior (e.g., attention). Psychopharmacology studies drug effects on our nervous system, psychological states, and behavior (Julien, Advokat, & Comaty, 2023).
NTs and drugs are ligands, molecules that form covalent bonds with receptors. Pharmacodynamics studies how drug interactions with receptors affect the body.
Drug binding to receptors is characterized by the properties of specificity and affinity. Specificity refers to the degree to which a drug only binds to a given receptor. Affinity describes the strength of a drug's covalent bond with its receptor. Stronger affinity results in greater drug effects.
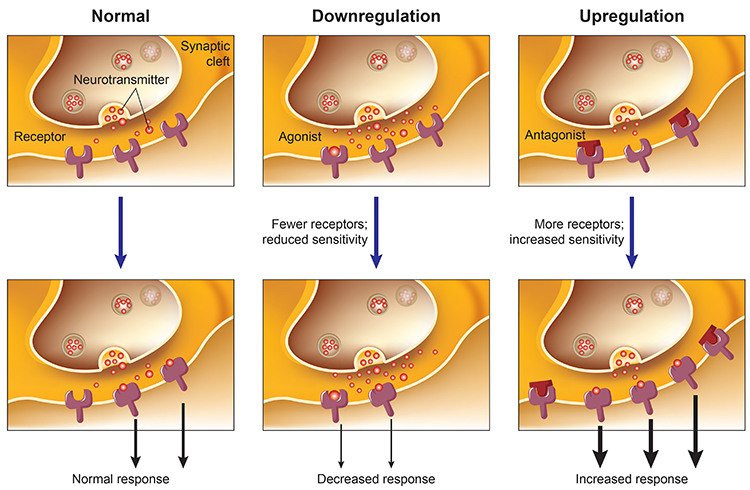
NTs and psychoactive drugs exert acute and chronic effects when they bind to receptors. Acute effects are the immediate changes produced by receptor binding. Receptor activation may last from several to hundreds of milliseconds (Julien et al., 2023). For example, serotonin-selective reuptake inhibitors (SSRIs) prolong serotonin availability within the synaptic cleft.
In contract, chronic effects are long-term alterations in receptors and the functioning of neural networks. Months of SSRI treatment can reduce the number of serotonin receptors and increase mood and social engagement. This phenomenon is called downregulation. Graphic adapted from Julien et al. (2023).
Psychoactive drugs cannot confer "superpowers" on receptors like Peter Parker's dramatic transformation after being bitten by a radioactive spider. Instead, drugs can only modulate, promote or inhibit, a receptor's normal response to a NT.
The term agonist describes the many ways a drug can promote a NT's actions. In contrast, the term antagonist characterizes how a drug can interfere with a NT's actions. We caution against limiting agonism to NT imitation and antagonism to receptor blockade because drugs can promote or interfere with NT action in diverse ways.
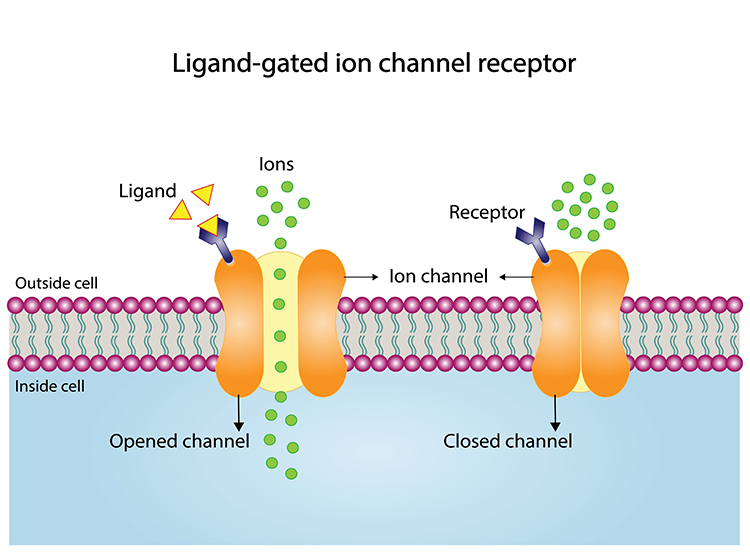
Consider an ionotropic receptor. NT binding alters the shape of its central ion channel, allowing ion movement. Ionotropic receptor graphic © Ph-HY/Shutterstock.com.
For example, the site where the amino acid GABA binds is called its orthosteric site. GABA ionotropic receptor graphic © Designua/Shutterstock.com.
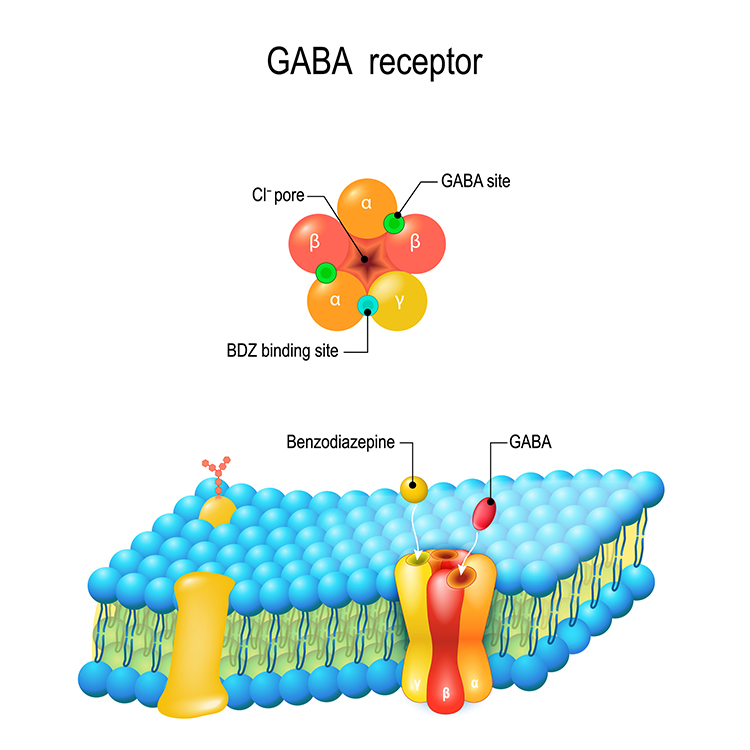
When GABA binds to inhibitory GABA-A receptors, it triggers the inward movement of negative chloride ions, increasing inhibition. The separate subunits where drugs like ethanol bind are called allosteric sites. Ethanol modulates GABA's action, increasing chloride ion entry and neuronal inhibition (Förstera et al., 2016). In this example, ethanol functions as a GABA agonist.
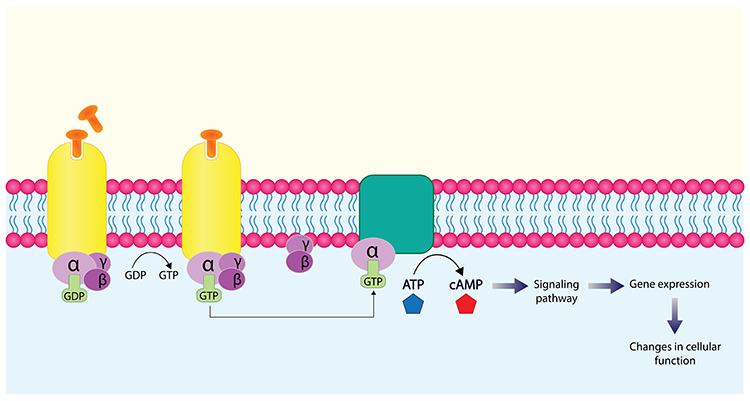
G-protein-coupled receptors (also called metabotropic receptors) are proteins that convert signals from NTs and drugs into intracellular messages. Activation of G-proteins within a neuron's interior can result in the synthesis of second messengers that modulate its actions. Second messengers can alter cell division and differentiation, energy conversion and utilization, membrane potential, and ion channel Graphic © Ph-HY/Shutterstock.com.
.jpg)
A drug's half-life is the time required to eliminate 50% of a drug. Following redistribution from the blood plasma to body tissues, there is a linear rate of decline for all drugs with the exception of ethanol (Julien et al., 2023).
A client is not drug-free (e.g., 98% eliminated) until six half-lives have passed.
DRUG CLASS EFFECTS ON EEQ/QEEG MEASUREMENTS
Caption: The authors largely based this table on the excellent Aiyer et al. (2016) systematic review.
Dr. Ron Swatzyna and colleagues (2024) emphasized the importance of using routine EEGs in cases where psychiatric medications have failed to provide relief. They argued that the traditional symptom-based approach in psychiatry, as outlined by the Diagnostic and Statistical Manual (DSM), is insufficient and often leads to multiple medication trials that do not address the underlying causes of psychiatric symptoms.

The authors proposed that EEGs can reveal brain abnormalities not detectable through standard psychiatric assessments. They identified four key EEG biomarkers—focal slowing (FS), spindling excessive beta (SEB), encephalopathy (EN, diffuse slowing), and isolated epileptiform discharges (IEDs)—that are associated with medication failure in refractory patients. These biomarkers suggest specific types of brain dysregulation, such as localized cerebral pathology, cortical irritability, or underlying encephalopathy, which can manifest as complex behavioral or psychiatric issues.

Tricyclic antidepressants (TCAs) like imipramine (Tofranil) can produce anxiety, blurred vision, dizziness, fatigue and weakness, psychotic symptoms (rare), sedation, seizures (rare), sexual dysfunction, and suicidality (rare) (Stahl, 2021).
Selective serotonin reuptake inhibitors (SSRIs) like fluoxetine (Prozac) can produce agitation, anxiety, headache, insomnia, mania (rare), sedation, seizures (rare), sexual dysfunction, suicidality (rare), and tremors (Stahl, 2021).
Dual-action antidepressants like duloxetine (Cymbalta) can produce hypomania (rare), hypertension, insomnia, sedation, sexual dysfunction, and suicidality (rare) (Stahl, 2021).
Irreversible MAO inhibitors like selegeline (Emsam, Eldepryl) can produce confusion, dizziness, dyskinesia, hallucinations, headache, hypertensive crisis, mania (rare), seizures (rare), and
suicidality (rare) (Stahl, 2021).
Selective norepinephrine reuptake inhibitors (SNRIs) like atomoxetine (Strattera) can produce abdominal pain, anxiety, agitation, aggression, dizziness, dysmenorrhea, dyspepsia, elevated heart rate, fatigue (especially in children), hypertension, hypomania and mania (rare), orthostatic hypotension, priapism, sedation, sexual dysfunction, and suicidality (rare) (Stahl, 2021).
At higher doses, sedating TCAs can increase delta and widespread theta power (Bauer & Bauer, 2005; Van Cott & Brenner, 2003).
TCAs can cause asynchronous slow waves and increase sleep spindles (Thompson & Thompson, 2015). TCAs and SSRIs can provoke spikes or polyspikes. Excessive TCA doses can increase delta and theta power, where theta appears diffusely (Blume, 2006).
MAO inhibitors like isocarboxazid (Marplan) increase 20-30 Hz power while decreasing power in slower and high frequencies like CNS stimulants (Thompson & Thompson, 2015).
The nonsedating SSRI citalopram (Celexa) decreases total, delta, theta, and alpha power, while it increases beta and gamma power (Bauer & Bauer, 2005; Nissen et al., 2020; Saletu, 2010; Van Cott & Brenner, 2003).
Vortioxetine (Trintellix) decreases theta band (4-8 Hz) power and increases beta (12-32 Hz) and gamma (32-45 Hz) band power (Nissen et al., 2020).
High SSRI doses may produce bisynchronous spikes or polyspikes. Serotonin syndrome is associated with triphasic waves, which signal toxic encephalopathy (Blume, 2006).
ANTIPSYCHOTICS

First-generation agents (FGAs) like haloperidol (Haldol) can produce akathisia (restless movement disorder), extrapyramidal symptoms (pseudo-Parkinsonism, tardive dyskinesia, and tardive dystonia), blurred vision, death and stroke in elderly with dementia-related psychosis, dizziness, hypertension, hypotension, neuroleptic malignant syndrome (rare), neuroleptic-induced deficit syndrome (analogous to the negative symptoms of schizophrenia), seizures (rare), and tachycardia.
Second-generation (atypical) agents (SGAs) like clozaril (Clozapine) can produce death and stroke in the elderly with dementia-related psychosis, neuroleptic malignant syndrome (when used with another agent), sedation, seizures, tachycardia, and tardive dyskinesia (rare).
Third-generation agents (TGAs) show mixed effects. Drugs like aripiprazole (Abilify) can produce activation, akathisia, death and stroke in the elderly with dementia-related psychosis, dizziness, headache, impaired impulse control (rare), insomnia, loss of energy, neuroleptic malignant syndrome, sedation, seizures (rare), and tardive dyskinesia (theoretical risk).
Chlorpromazine (Thorazine) can increase sharp theta transients at frontal and temporal sites. Chlorpromazine can significantly slow the posterior dominant rhythm (PDR) (Demos, 2019).
Chlorpromazine reduces amplitude and increasing variability in evoked responses (Laurian et al., 1981).
The attenuation of alpha-blocking in response to sensory stimuli may be associated with memory deficits produced by this drug (Saletu, 2010; Thompson & Thompson, 2016). High phenothiazine doses may produce bisynchronous spikes or polyspikes (Blume, 2006).
Haloperidol (Haldol), a nonsedating FGA, increased alpha 1 and beta 1 power in human EEG bands and tended to shift the beta 1 centroid posterior (Merlotti et al., 2007; Yoshimura et al., 2007).
Clozapine (Clozaril) decreases alpha (Baik et al., 2011; MacCrimmon et al., 2012) and beta, and increases delta and theta power (Knott et al., 2001). Excessive doses of the SGA clozapine can increase delta and theta power, where theta appears diffusely (Blume, 2006).
Clozapine produces specific topographic EEG changes, marked slowing in frontal, central, and parietal scalp areas, distinct from those of other neuroleptics (Joutsiniemi et al., 2001).
Where dopamine receptor hypersensitivity produces extrapyramidal side effects like tardive dyskinesia, FGAs may cause extended trains of mixed fast/sharp transients, EEG slowing, and potentiation of latent epileptiform activity (J. Gunkelman cited by Thompson & Thompson, 2016).
BENZODIAZEPINES

Long-acting agents like diazepam (Valium) can produce ataxia, confusion, depression, dizziness, fatigue, forgetfulness, hallucinations (rare), hyperexcitability, hypotension (rare), mania (rare), nervousness, respiratory depression (overdose with respiratory depressants), sedation, slurred speech, and weakness (Stahl, 2017).
Intermediate-acting agents like lorazepam (Ativan) can produce the same side effects as their long-acting counterparts (Stahl, 2017).
Short-acting agents like alprazolam (Xanax) can also produce the same side effects as their long and intermediate-acting counterparts (Stahl, 2017).
Benzodiazepine receptor agonist (BZRA) hypnotics like zolpidem (Ambien) can produce amnesia (dose-dependent), ataxia, dizziness, hallucinations (rare), headache, hyperexcitability, nervousness,
respiratory depression (overdose with respiratory depressants), and sedation.
Benzodiazepines may increase spindling beta and inhibit epileptiform activity (Bauer & Bauer, 2005; Blume, 2006; Julien et al., 2023; Knott, 2000; Thompson & Thompson, 2015; Van Cott & Brenner, 2003).
Benzodiazepines like haloxazolam (Somelin), flunitrazepam (Rohypnol), and triazolam (Halcion) increase higher frequency activity in the sigma and beta bands and reduce lower frequency activity in sleep EEG spectra (Tan et al., 2003).
Diazepam (Valium) decreases EEG power in 1- to 6-, 8- to 12-, and 19- to 35-Hz bands and disrupts right intrahemispheric temporal coupling in the 8-12 Hz range (Muñoz-Torres et al., 2011).
Midazolam (Versed) and its active metabolite, alpha-hydroxy-midazolam, increased EEG beta activity and decreased alpha activity in healthy participants. These changes were associated with sedation and persisted for hours after administration, even at low plasma levels (Hotz et al., 2000).
Alprazolam (Xanax) administration led to increased higher frequency activity in the sigma and beta bands, while lower frequency activity reduced in sleep EEG spectra (Tan et al., 2003).

The two main classes of CNS stimulants include amphetamines and nonamphetamine behavioral stimulants.
Amphetamines like amphetamine D, L (Adderall) can produce adverse cardiovascular effects, cardiac arrhythmia, dizziness, headache, hypertension, hypomania, insomnia, irritability, mania, nervousness, overstimulation, psychotic episodes, sexual dysfunction (long-term), suicidality, and worsened tics (Stahl, 2017).
Nonamphetamine behavioral stimulants like methylphenidate (Ritalin) can produce adverse cardiovascular effects, cardiac arrhythmia, dizziness, headache, hypertension, hypomania, insomnia, irritability, mania, nervousness, overstimulation, priapism (rare), psychotic episodes,
suicidality, and worsened tics (Stahl, 2017).
Dexamphetamine (Vyvanse) and methylphenidate (Ritalin) reduced theta and increased beta power (Clarke et al., 2002).
Methylphenidate (Ritalin) reduces delta and theta power, increasing posterior alpha and low beta power for up to 6 hours following drug administration (Blume, 2006; Thompson & Thompson, 2015).
Methylphenidate decreases EEG mu power in the right primary motor cortex during motor imagery and execution tasks, compared to risperidone (Aprigio et al., 2021).
Methylphenidate effectively increases the P3 ERP response in ADHD subjects, potentially reducing mental fatigue-related decreases in response time (Rubinson et al., 2019).
A client's level of arousal modulates the EEG response to a CNS stimulant. Stimulants increase alpha power in under-aroused, decrease alpha power in typically aroused, and do not alter alpha in anxious (fast-EEG) clients (J. Gunkelman, cited by Thompson & Thompson, 2015).

Lithium can produce arrhythmia, ataxia, bradycardia, cardiovascular changes, delirium, forgetfulness, hypotension, and lithium toxicity (Stahl, 2017).
First-generation anticonvulsants like phenobarbital (Phenobarbital) can produce aggression, confusion, depression, dizziness, drowsiness, excitement, forgetfulness, hallucinations (rare), headache, insomnia, nightmares, and respiratory depression (in overdose or with other CNS depressants) (Stahl, 2017).
Second-generation anticonvulsants like valproate (Depakene) can produce ataxia, bradycardia, dizziness, headache, sedation, suicidality, tachycardia, and weakness (Stahl, 2017).
Atypical antipsychotics like olanzapine (Zyprexa) can produce ✽ death and stroke in elderly with dementia-related psychosis, diabetes, dizziness, neuroleptic malignant syndrome (rare), orthostatic hypotension, pain (back, chest, extremity, joint), sedation, seizures (rare), and tardive dyskinesia (rare) (Stahl, 2017).
Omega-3 fatty acids do not produce significant side effects that would affect clinical presentation.
Gabapentin (Neurontin) increases delta and theta power and decreases alpha power (Saletu et al., 1996). Its effects on beta power are inconclusive (Saletu et al., 1996).
Lamotrigine (Lamictal) decreases delta (Wu & Xiao, 1996), theta, alpha, and beta power (Clemens et al., 2008; Neufeld et al., 1999; Wu & Xiao, 1996).
Lithium can cause generalized asynchronous slowing that reduces the peak alpha frequency. Lithium may increase delta, theta, alpha, and beta power (Henninger, 1978) and greatly potentiate latent epileptiform activity. High lithium doses may produce bisynchronous spikes or polyspikes and increase delta and theta power, where theta appears diffusely (Blume, 2006). Lithium toxicity dramatically slows the EEG and causes triphasic discharges (Thompson & Thompson, 2016).
Oxcarbamazepine (Trileptal) decreases alpha power (Clemens et al., 2006). There are no data on its effects on delta, theta, and beta band power.
Phenobarbital (Luminal) can induce rhythmic 18-26 Hz activity that starts in the frontal lobe and can progressively extend to the whole cortex. Progressively higher doses promote EEG slowing and reduced beta activity until slow-wave activity eclipses beta activity. Voltage can decrease until the brain enters an iso-electric state like a medically induced coma (Blume, 2006; Thompson & Thompson, 2016). Barbiturate withdrawal may increase beta activity. Pentobarbital intoxication can result in triphasic waves. In general, antiepileptic drug reduction may increase the frequency of focal spikes or spike waves (Blume, 2006).
Barbiturate withdrawal may increase beta activity. Pentobarbital (Nembutol) intoxication can result in triphasic waves.
Antiepileptic drug reduction may increase the frequency of focal spikes or spike waves (Blume, 2006).
Topiramate (Topamax) increases delta and theta and decreases alpha power (Mercarelli et al., 2001). Topiramate increases the absolute beta and theta activity diffusely and decreased relative alpha activity over the left hemisphere (Neufeld et al., 1999).
Valproate (Depakote) decreases alpha (Clemens et al., 2014; Guo et al., 2014) and increases beta power (Clemens et al., 2014). Valproic acid intoxication can produce triphasic waves (Blume, 2006).
Vigabatrin (Sabril) administration decreased absolute alpha and beta activity and decreased absolute theta in the frontal and parieto-occipital regions (Neufeld et al., 1999).
Neurotoxicity caused by high levels of antiepileptic drugs may cause diffuse delta and increased theta power. Valproic acid (Depakene) intoxication can produce triphasic waves (Blume, 2006).
OPIOID ANALGESICS
Pure agonists like morphine (MS-IR) can produce agitation, confusion, dizziness, drowsiness, hallucinations, respiratory depression, and weakness (Advokat et al., 2019).
Partial agonists like buprenorphine (Subutex) can produce headache, insomnia, mood swings, orthostatic hypotension, respiratory depression, and sedation (Advokat et al., 2019; Stahl, 2017).
Mixed agonist-antagonists like pentazocine (Talwin) can produce confusion (rare), depression, double vision (rare), drowsiness, excitement, insomnia, irregular heartbeat, irritability, sleepiness, very slow or very rapid breathing, and weakness (Advokat et al., 2019).
Opioids such as remifentanil (Ultiva) and fentanyl (Duragesic) increase delta band power (0.5-4 Hz) during wakefulness and analgesia (Garcia et al., 2021; Graversen et al., 2015).
Remifentanil decreases theta (4-8 Hz) and alpha (8-13 Hz) band power, which correlates with its analgesic effects (Garcia et al., 2021; Graversen et al., 2015).
CAFFEINE

Caffeine acutely reduces theta and alpha power with rebound increases in these bands (Thompson & Thompson, 2016). Decreased theta activity (5-8 Hz) occurs during wakefulness and sleep deprivation. This reduction is observed both under relaxed conditions and during mental tasks (Landolt et al., 2004; Pollock et al., 1981).
Caffeine leads to a global reduction in alpha power (8-14 Hz) and an increase in alpha frequency, indicating heightened arousal (Barry et al., 2005; Foxe et al., 2012). This effect is more pronounced with eyes open compared to eyes closed (Siepmann & Kirch, 2002).
Caffeine diminishes the absolute power of slow and fast alpha and slow beta activities in various brain regions (Siepmann & Kirch, 2002). It also increases beta power (12-40 Hz) in frontal and central brain areas, particularly in sleep-deprived individuals (Drapeau et al., 2006; Patat et al., 2000).

Cannabis intake disrupts theta oscillations, leading to decreased theta power, which is correlated with impaired working memory and attentional performance (Böcker et al., 2010; Ilan et al., 2004; Morrison et al., 2011).
Cannabis acutely increases frontal alpha and chronically promotes frontal interhemispheric connectivity (hypercoherence and phase synchrony) (Thompson & Thompson, 2015).
THC has dose-dependent effects on beta power, with higher doses leading to increased beta activity, which may be related to muscle activity or heightened arousal (Böcker et al., 2010; Volavka et al., 1971). Chronic cannabis users exhibit beta power during resting states (Prashad et al., 2018).
Cannabis disrupts gamma oscillations, particularly during tasks requiring complex perceptual processing, such as coherent motion perception. Reduced gamma power is associated with perceptual alterations and may contribute to the cognitive impairments observed in heavy cannabis users (Skosnik et al., 2014).

Cocaine significantly increases beta power, particularly in the frontal and central areas of the brain (Herning et al., 1994, 1985; Noldy et al., 1994).
During cocaine withdrawal, there is a significant decrease in beta-2 power, indicating long-term neuroadaptive changes, especially in intravenous users (Noldy et al., 1994).

Individuals diagnosed with alcohol use disorder or who are vulnerable to developing this disorder frequently present with elevated beta (> 20 Hz and between 24-26 Hz) and decreased 6-10 Hz and alpha power (Thompson & Thompson, 2015). Alcohol withdrawal may increase beta activity, spikes, and polyspikes (Blume, 2006).
NICOTINE

Nicotine increases beta band power, which is associated with increased alertness and cognitive processing (Lindgren et al., 1999; Pickworth et al., 1986).
Nicotine reduced power in the 12.5-18.4 Hz band in the left middle frontal gyrus during eyes-open conditions. During eyes-closed conditions, nicotine reduced power across 8.5-18.4 Hz in areas spanning the superior frontal gyri to the supplementary motor areas (Ranzi et al., 2016).

Clinicians must understand their scope-of-practice restrictions when discussing medication issues. Clients often seek alternative treatments when medication management is ineffective or has undesirable side effects. Medications are prescribed for presenting symptoms related to atypical causal factors.
A careful and comprehensive assessment may reveal a more appropriate medication approach. Best case, neurofeedback may correct underlying causal factors, resulting in general improvement and reduced need for further medication.
Discuss the scope and limitations of your professional license and your ability to address medication issues. Review your client's plans regarding medication.
Continue current prescriptions? If not, have they consulted with their prescribing physician?
Decrease or eliminate current medications? Your client's best choice is to discuss medication adjustment with the prescribing physician. If they wish to proceed without physician consultation, explain your limitations and ethical concerns about proceeding with neurofeedback training under these circumstances.
Medications often need to be adjusted due to the effects of training. The clinician or client must interact with the prescribing physician regarding these changes. As neurofeedback changes brain function and structure, medications may need to be titrated or withdrawn. Standardized psychological (Beck Depression Inventory) and performance (Computerized Continuous Performance Test) instruments may help inform the physician's decision.
Wherever possible, involve your client in this process since physicians may not welcome your involvement in medication decisions. Clients may find it easier to interact with the prescribing physician when given accurate information.
When interpreting your initial assessment battery and subsequent reassessment testing, take drug effects into account. Develop training goals based on initial testing with medication. Retest with the same medication unless withdrawn to ensure a valid comparison.
Develop a personalized neurofeedback training strategy that does not attempt to train against a drug's principal effects on the EEG.

activation: the process by which a drug binds to a receptor and initiates a cellular response.
acute effects: immediate or short-term physiological or psychological effects of a drug following administration.
affinity: the strength of binding between a drug and its receptor, determining how well the drug will bind to its target.
agonst: a substance that promotes the action of a naturally occurring neurotransmitter.
alpha blocking: arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it entirely while increasing EEG power in the beta range.
alpha rhythm: 8-12-Hz and 8-13-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely-distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly.
alpha spindles: regular bursts of alpha activity.
amplitude: the energy or power contained within the EEG signal measured in microvolts or picowatts.
antagonist: a substance that interferes with the action of agonists or naturally occurring neurotransmitters.
anterior: near or toward the front of the head, for example, the anterior cingulate.
anterior cingulate: the division of the prefrontal cortex that plays a vital role in attention and is activated during working memory. It mediates emotional and physical pain and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components.
arousal: a process that combines alertness and wakefulness, produced by at least five neurotransmitters, including acetylcholine, histamine, hypocretin, norepinephrine, and serotonin.
asynchronous waves: neurons depolarize and hyperpolarize independently.
benzodiazepine receptor agonist (BZRA) hypnotics: nonbenzodiazepine BZRAs like zolpidem (Ambien) that are prescribed to treat insomnia.
beta rhythm: 12-36 Hz rhythm associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges.
bilateral synchronous slow waves: a pathological sign observed in drowsy children. When detected in alert adults, intermittent bursts of high amplitude slow waves may signify gray matter lesions in deep midline structures.
cerebral cortex: the layer of gray matter that covers the cerebral hemispheres. The cerebral cortex consists of gray matter and white matter.
chronic effects: long-term physiological or psychological effects that occur with prolonged use of a drug.
complex: a sequence of waves.
continuous irregular delta: slow waves produced by white matter lesions seen in disorders like multiple sclerosis.
delta rhythm: 0-4 Hz, 0.5-3.5 Hz, and 1-4 Hz oscillations generated by thalamocortical neurons during stage-3 sleep.
diphasic wave: a wave that contains both a negative and positive deflection from the baseline.
dominant frequency: the EEG frequency with the greatest amplitude.
downregulation: the decrease in receptor number or sensitivity due to prolonged exposure to an agonist, leading to reduced response.
drugs: chemical substances used to diagnose, treat, or prevent disease, or to relieve symptoms.
dual-action antidepressants: medications like duloxetine (Cymbalta) that activate 5-HT1 receptors to produce antidepressant and anxiolytic effects, while they blockade 5-HT2 (agitation, restlessness, and sexual dysfunction) and 5-HT3 (nausea, headache, and vomiting) receptors to minimize their side effects.
EEG activity: a single wave or successive waves.
EEG coherence: shared oscillatory activity (identical waveform morphology) between two sites expressed as the square of the correlation coefficient between their frequencies.
EEG power: signal energy in the EEG spectrum. Most EEG power falls within the 0-20 Hz frequency range. EEG power is measured in microvolts or picowatts.
electroencephalogram (EEG): the voltage difference between at least two electrodes, where at least one electrode is located on the scalp or inside the brain. The EEG is a recording of EPSPs and IPSPs that occur primarily in dendrites in pyramidal cells located in macrocolumns, several millimeters in diameter, in the upper cortical layers.
elimination half-life: the time required for the concentration of a drug in the bloodstream to decrease by half.
evoked potential: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). An evoked potential has a negative peak around 80-90 ms and a positive peak about 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
fast cortical potentials: EEG rhythms that range from 0.5 Hz-100 Hz. The main frequency ranges include delta, theta, alpha, sensorimotor rhythm, and beta.
first-generation antipsychotics (FGAs): FGAs like chlorpromazine (Thorazine) are prescribed to treat the positive symptoms of schizophrenia and exert their effects through D2 receptor blockade.
focal waves: EEG waves detected within a limited area of the scalp, cerebral cortex, or brain.
frequency: the number of cycles completed each second expressed in hertz (Hz).
frequency synchrony: when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
frontal lobes: the most anterior cortical lobes of the brain that are divided into the motor cortex, premotor cortex, and prefrontal cortex.
G-protein-coupled receptor (GPCR): a large family of receptors that activate intracellular signaling pathways via G-proteins upon ligand binding.
gamma rhythm: 25-75 Hz, 35-45 Hz, 38-42 Hz, 40 Hz oscillations that may speed information distribution and processing. Gamma bursts occur during problem-solving, and the absence of gamma is associated with cognitive deficits and learning disorders. Gamma is theorized as a "binding rhythm" that integrates sensory inputs into perception and consciousness.
generalized asynchronous slow waves: waves seen in sleepy children and those with elevated temperatures. This may indicate degenerative disease, dementia, encephalopathy, head injury, high fever, migraine, and Parkinson's disease in adults.
hertz (Hz): the unit of frequency, an abbreviation for cycles per second.
hypercoherence: abnormally-high functional connectivity between two sites.
intermediate-acting benzodiazepines: benzodiazepines with mean half-lives from 15-80 hours prescribed to manage anxiety.
ionotropic receptor: a receptor that forms an ion channel pore, allowing ions to pass through the membrane in response to ligand binding.
irregular waves: successive waves that constantly alter their shape and duration.
irreversible MAO inhibitors (MAOIs): MAOIs like selegeline (Emsam) that form permanent bonds with the MAO enzyme and are prescribed for major depressive disorder (MDD).
kappa rhythm: bursts of alpha or theta and is detected over the temporal lobes of subjects during cognitive activity.
lambda waves: saw-toothed transient waves from 20-50 μV in amplitude and 100-250 ms in duration detected over the occipital cortex during wakefulness. These positive deflections are time-locked to saccadic movements and observed during visual scanning, as during reading.
lateralized waves: waves that are primarily detected on one side of the scalp and may indicate pathology.
ligands: molecules that bind to specific sites on a target protein, such as receptors, to exert their biological effect.
local synchrony: synchrony that occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals.
localized slow waves: waves that may indicate a transient ischemic attack (TIA) or stroke, migraine, mild head injury, or tumors above the tentorium. Deep lesions result in bilateral or unilateral delta.
long-acting benzodiazepines: benzodiazepines like diazepam (Valium) with mean half-lives ranging from 10-80 hours prescribedto manage anxiety.
metabotropic receptor: a receptor that indirectly influences ion channels through signal transduction mechanisms, often involving G-proteins.
mixed opioid agonist-antagonists: drugs like pentazocine (Talwin) that are kappa agonists and weak mu antagonists that are prescribed for the management of pain.
monoamine oxidase (MAO): an enzyme that degrades and inactivates the monoamine neurotransmitters dopamine, norepinephrine, and serotonin.
monoamine oxidase inhibitors (MAOIs): antidepressant drugs that interfere with MAO's breakdown of monoamines and increase monoamine availability and are prescribed to manage major depressive disorder (MDD).
monophasic wave: either a single negative (upward) or positive (downward) deflection from baseline.
mu rhythm: arch-shaped waves that range from 7-11 Hz with amplitudes typically below 50 μV detected over Cz and Pz in waking subjects. These waves are seen in the healthy EEG records of 7% of the population. While these waves resemble alpha, they contain sharp positive transients and curved negative segments. Mu waves are blocked or reduced by exposure to a tactile stimulus, planning to move, readiness to move, or moving a contralateral limb (making a fist).
multiple spike-and-slow-wave complex: multiple spikes associated with at least one slow wave.
neurotransmitter (NT): a chemical messenger that transmits signals across a synapse from one neuron to another target neuron, muscle cell, or gland cell. Neurotransmitters are crucial for various bodily functions, including mood regulation, sleep, cognition, and muscle movement. They are released from synaptic vesicles in the presynaptic neuron into the synaptic cleft, where they bind to specific receptors on the postsynaptic cell, initiating a response.
partial opioid agonists: drugs like buprenorphine (Subutex) that produce less-than-maximal analgesia and are prescribed to manage pain.
peak alpha frequency: the highest-amplitude alpha frequency (8-12, 8-13 Hz) within an epoch.
pharmacodynamics: he study of the biochemical and physiological effects of drugs and their mechanisms of action in the body.
phase: the degree to which the peaks and valleys of EEG waveforms coincide.
phase synchrony: synchrony when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
polyphasic (multiphasic) wave: a wave that contains two or more deflections of opposite polarity from baseline.
polyspikes: a series of three or more consecutive spikes (≥ 10 Hz) that last a minimum of 300 milliseconds.
posterior: near or toward the back of the head.
psychoactive drugs: substances that affect the central nervous system, altering brain function and resulting in changes in perception, mood, consciousness, cognition, or behavior.
psychopharmacology: the study of the use of medications in treating mental disorders, focusing on the effects of psychoactive drugs on mood, sensation, thinking, and behavior.
pure opioid agonists: drugs like morphine that produce maximal analgesia and are prescribed for to manage pain.
regular or monomorphic waves: successive waves with identical shapes. Regular waves may resemble sine waves (sinusoidal) or maybe arched (resembling wickets) or saw-toothed (asymmetrical and triangular).
second messenger: intracellular signaling molecule released by the cell in response to receptor activation, which amplify and propagate the signal within the cell.
second-generation antipsychotics: SGAs like clozapine (Clozaril) are prescribed to treat the positive symptoms of schizophrenia and antagonize D2 receptors less effectively than D1 receptors and significantly less than 5-HT2 receptors.
selective norepinephrine reuptake inhibitors (SNRIs): drugs like atomoxetine (Strattera) that specifically interfere with norepinephrine reuptake for the management of major depressive disorder (MDD).
selective serotonin reuptake inhibitors (SSRIs): drugs like fluoxetine (Prozac) that specifically interfere with serotonin reuptake for the management of major depressive disorder (MDD).
sharp transients: sequences that contain several sharp waves.
sharp waves: waves that resemble spikes with a pointed peak with a longer 70-200-ms duration.
short-acting benzodiazepines: benzodiazepines like alprazolam (Xanax) with mean half-lives that range from 2.5-12 hours prescribed to manage anxiety.
specificity: the ability of a drug to bind to a particular receptor site or target, reducing off-target effects.
spike: a negative transient with a pointed peak at conventional paper speeds, 20-70-ms duration, and 40-100 μV amplitude.
spike-and-slow-wave complex: a spike followed by a higher amplitude slow wave at 3 Hz. In an absence seizure, the amplitudes are very high (e.g., 160 μV).
spindle waves: waves that originate in the thalamus and occur during unconsciousness and stage II sleep.
synchronous: adverb meaning that groups of neurons depolarize and hyperpolarize simultaneously.
synchrony: the coordinated firing of pools of neurons. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
theta rhythm: 4-7 Hz rhythm generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
third-generation antipsychotics (TGAs): drugs like aripiprazole (Abilify) that is a partial agonist at D2 and 5-HT1A receptors and an antagonist at 5-HT2 receptors prescribed for the management of the positive and negative symptoms of schizophrenia.
total power: the sum of the voltages in all EEG frequency bands.
transient: a single wave or sequence of regular waves, called a complex, distinguishable from background EEG activity.
triphasic waves (TWs): medium-to-high-amplitude sharp transients that often involve a negative-positive-negative sequence. TWs are distributed diffusely and symmetrically with frontal predominance.
upregulation: increase in receptor number or sensitivity due to prolonged exposure to an antagonist or low levels of agonist, leading to an enhanced response.
waveform: the shape and form of an EEG signal.
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

Click on the Quizlet Plus logo to review our chapter flashcards.

BioSource Software offers Functional Neuroanatomy, which reviews critical behavioral networks, and qEEG100, which provides extensive multiple-choice testing over the IQCB Blueprint.


Now that you have completed this unit, explain how an SSRI like fluoxetine (Prozac) could affect the EEG? How would you adjust neurofeedback training for depression for clients using Prozac. Why shouldn't you train against a drug's effects on the EEG?
Arzy, S., Allali, G., Brunet, D., Michel, C. M., Kaplan, P. W., & Seeck, M. (2010). Antiepileptic drugs modify power of high EEG frequencies and their neural generators. European Journal of Neurology, 17(10), 1308–1312. https://doi.org/10.1111/j.1468-1331.2010.03018.x
Banoczi, W. R. (2005). How some drugs affect the electroencephalogram (EEG). Am J END Technol, 45, 118-129. PMID: 15989074
Aiyer, R., Novakovic, V., & Barkin, R. L. (2016). A systematic review on the impact of psychotropic drugs on electroencephalogram waveforms in psychiatry. Postgraduate Medicine, 128(7), 656–664. https://doi.org/10.1080/00325481.2016.1218261
Barry, R. J., Clarke, A. R., Hajos, M., McCarthy, R., Selikowitz, M., & Bruggemann, J. M. (2009). Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology, 57(7-8), 702–707. https://doi.org/10.1016/j.neuropharm.2009.08.003
Bauer, G., & Bauer, R. (2005). EEG drug effects and central nervous system poisoning. In E. Niedermeyer & F. Lopes da Silva (Eds.). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Blume, W. T. (2006). Drug effects on EEG. Journal of Clinical Neurophysiology, 23(4), 306-311. https://doi.org/10.1097/01.wnp.0000229137.94384.fa
Bos, D. J., Oranje, B., Veerhoek, E. S., Van Diepen, R. M., Weusten, J. M., Demmelmair, H., Koletzko, B., de Sain-van der Velden, M. G., Eilander, A., Hoeksma, M., & Durston, S. (2015). Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(10), 2298–2306. https://doi.org/10.1038/npp.2015.73
Bromm, B., Ganzel, R., Herrmann, W. M., Meier, W., & Scharein, E. (1986). Pentazocine and flupirtine effects on spontaneous and evoked EEG activity. Neuropsychobiology, 16(2-3), 152–156. https://doi.org/10.1159/000118317
Brunner, D. P., Dijk, D. J., Münch, M., & Borbély, A. A. (1991). Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology, 104(1), 1–5. https://doi.org/10.1007/BF02244546
Cajochen, C., Kräuchi, K., von Arx, M. A., Möri, D., Graw, P., & Wirz-Justice, A. (1996). Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neuroscience Letters, 207(3), 209–213. https://doi.org/10.1016/0304-3940(96)12517-9
Carley, D. W., & Farabi, S. S. (2016). Physiology of sleep. Diabetes Spectrum: A Publication of the American Diabetes Association, 29(1), 5–9. https://doi.org/10.2337/diaspect.29.1.5
Clarke, A. R., Barry, R. J., Bond, D., McCarthy, R., & Selikowitz, M. (2002). Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology, 164(3), 277–284. https://doi.org/10.1007/s00213-002-1205-0
Clemens, B., Ménes, A., Piros, P., Bessenyei, M., Altmann, A., Jerney, J., Kollár, K., Rosdy, B., Rózsavölgyi, M., Steinecker, K., & Hollódy, K. (2006). Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Research, 70(2-3), 190–199. https://doi.org/10.1016/j.eplepsyres.2006.05.003
Clemens, B., Piros, P., Bessenyei, M., Tóth, M., Hollódy, K., & Kondákor, I. (2008). Imaging the cortical effect of lamotrigine in patients with idiopathic generalized epilepsy: A low-resolution electromagnetic tomography (LORETA) study. Epilepsy Research, 81 (2-3), 204–210. https://doi.org/10.1016/j.eplepsyres.2008.06.002
Clemens, B., Puskás, S., Besenyei, M., Kovács, N. Z., Spisák, T., Kis, S. A., Emri, M., Hollódy, K., Fogarasi, A., Kondákor, I., & Fekete, I. (2014). Valproate treatment normalizes EEG functional connectivity in successfully treated idiopathic generalized epilepsy patients. Epilepsy Research, 108(10), 1896–1903. https://doi.org/10.1016/j.eplepsyres.2014.09.032
Cohen, H., Porjesz, B., & Begleiter, H. (1993). Ethanol-induced alterations in electroencephalographic activity in adult males. Neuropsychopharmacology, 8, 365-370. https://doi.org/10.1038/npp.1993.36
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Dias Alves, M., Micoulaud-Franchi, J. A., Simon, N., & Vion-Dury, J. (2018). Electroencephalogram modifications associated with atypical strict antipsychotic monotherapies. Journal of Clinical Psychopharmacology, 38(6), 555–562. https://doi.org/10.1097/JCP.0000000000000953
Dijk, D. J., & Cajochen, C. (1997). Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. Journal of Biological Rhythms, 12(6), 627–635. https://doi.org/10.1177/074873049701200618
Dimpfel, W., Schober, F., & Spüler, M. (1993). The influence of caffeine on human EEG under resting condition and during mental loads. The Clinical Investigator, 71, 197-207. https://doi.org/10.1007/BF00180102
Drapeau, C., Hamel-Hébert, I., Robillard, R., Selmaoui, B., Filipini, D., & Carrier, J. (2006). Challenging sleep in aging: The effects of 200 mg of caffeine during the evening in young and middle‐aged moderate caffeine consumers. Journal of Sleep Research, 15. https://doi.org/10.1111/j.1365-2869.2006.00518.x
Eschmann, G., Irrgang, V., & Rüther, E. (1983). Effects of beta-receptor blockers in pharmacology EEG. Neuropsychobiology, 10(2-3), 190–192. https://doi.org/10.1159/000118008
Flachenecker P. (2013). A new multiple sclerosis spasticity treatment option: Effect in everyday clinical practice and cost-effectiveness in Germany. Expert Review of Neurotherapeutics, 13(3 Suppl 1), 15–19. https://doi.org/10.1586/ern.13.1
Fontani, G., Corradeschi, F., Felici, A., Alfatti, F., Migliorini, S., & Lodi, L. (2005). Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. European Journal of Clinical Investigation, 35(11), 691–699. https://doi.org/10.1111/j.1365-2362.2005.01570.x
Foxe, J., Morie, K., Laud, P., Rowson, M., Bruin, E., & Kelly, S. (2012). Assessing the effects of caffeine and theanine on the maintenance of vigilance during a sustained attention task. Neuropharmacology, 62, 2320-2327. https://doi.org/10.1016/j.neuropharm.2012.01.020
Galderisi, S., Mucci, A., Volpe, U., & Boutros, N. (2009). Evidence-based medicine and electrophysiology in schizophrenia. Clinical EEG and Neuroscience, 40(2), 62–77. https://doi.org/10.1177/155005940904000206
García, P., Kreuzer, M., Hight, D., & Sleigh, J. (2021). Effects of noxious stimulation on the electroencephalogram during general anaesthesia: A narrative review and approach to analgesic titration. British Journal of Anaesthesia, 126(2), 445-457 . https://doi.org/10.1016/j.bja.2020.10.036
Goldstein, L., Murphree, H. B., & Pfeiffer, C. C. (1968). Comparative study of EEG effects of antihistamines in normal volunteers. The Journal of Clinical Pharmacology and the Journal of New Drugs, 8(1), 42–53. https://doi.org/10.1002/j.1552-4604.1968.tb00091.x
Graversen, C., Malver, L., Kurita, G., Staahl, C., Christrup, L., Sjøgren, P., & Drewes, A. (2015). Altered frequency distribution in the electroencephalogram is correlated to the analgesic effect of Remifentanil. Basic & Clinical Pharmacology & Toxicology, 116. https://doi.org/10.1111/bcpt.12330
Guo, J., Wang, D., Ren, M., Xiong, B., Li, Z., Wang, X., & Zeng, K. (2014). QEEG analysis of the effects of sodium valproate on adult Chinese patients with generalized tonic-clonic seizures. Metabolic Brain Disease, 29(3), 801–807. https://doi.org/10.1007/s11011-014-9561-0
Han, G., Matsumoto, S., Diaz, J., Greene, R. W., & Vogt, K. E. (2022). Dihydropyridine calcium blockers do not interfere with non-rapid eye movement sleep. Frontiers in Neuroscience, 16, 969712. https://doi.org/10.3389/fnins.2022.969712
Hasan, M., Pulman, J., & Marson, A. G. (2013). Calcium antagonists as an add-on therapy for drug-resistant epilepsy. The Cochrane Database of Systematic Reviews, 2013(3), CD002750. https://doi.org/10.1002/14651858.CD002750.pub2
Heninger G. R. (1978). Lithium carbonate and brain function. I. Cerebral-evoked potentials, EEG, and symptom changes during lithium carbonate treatment. Archives of General Psychiatry, 35(2), 228–233. https://doi.org/10.1001/archpsyc.1978.01770260106013
Herning, R. I., Glover, B. J., Koeppl, B., Phillips, R. L., & London, E. D. (1994). Cocaine-induced increases in EEG alpha and beta activity: Evidence for reduced cortical processing. Neuropsychopharmacology, 11(1), 1-9. https://doi.org/10.1038/npp.1994.30
Herning, R. I., Jones, R. T., Hooker, W. D., Mendelson, J., & Blackwell, L. (1985). Cocaine increases EEG beta: A replication and extension of Hans Berger's historic experiments. Clinical Neurophysiology, 60(6), 470-477. https://psycnet.apa.org/doi/10.1016/0013-4694(85)91106-X
Hotz, M., Ritz, R., Linder, L., Scollo-Lavizzari, G., & Haefeli, W. (2000). Auditory and electroencephalographic effects of midazolam and alpha-hydroxy-midazolam in healthy subjects. British Journal of Clinical Pharmacology, 49(1), 72-9. https://doi.org/10.1046/J.1365-2125.2000.00104.X.
Hughes, A., Lynch, P., Rhodes, J., Ervine, C., & Yates, R. (2001). Electroencephalographic and psychomotor effects of chlorpromazine and risperidone relative to placebo in normal healthy volunteers. British Journal of Clinical Pharmacology, 48(3), 323-30. https://doi.org/10.1046/J.1365-2125.1999.00021.X.
Hyun, J., Baik, M. J., & Kang, U. G. (2011). Effects of psychotropic drugs on quantitative EEG among patients with schizophrenia-spectrum disorders. Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology, 9(2), 78–85. https://doi.org/10.9758/cpn.2011.9.2.78
Joutsiniemi, S., Gross, A., & Appelberg, B. (2001). Marked Clozapine-induced slowing of EEG background over frontal, central, and parietal scalp areas in schizophrenic patients. Journal of Clinical Neurophysiology, 18, 9-13. https://doi.org/10.1097/00004691-200101000-00003
Julien, R. M., Advokat, C. D., & Comaty, J. E. (2023). Julien's primer of drug action: A comprehensive guide to the actions, uses, and side effects of psychoactive drugs (15th ed.). Worth Publishers.
Khajehpour, H., Mohagheghian, F., Ekhtiari, H., Makkiabadi, B., Jafari, A. H., Eqlimi, E., & Harirchian, M. H. (2019). Computer-aided classifying and characterizing of methamphetamine use disorder using resting-state EEG. Cognitive Neurodynamics, 13(6), 519–530. https://doi.org/10.1007/s11571-019-09550-z
Knott, V. J. (200). Quantitative EEG methods and measures in human psychopharmacological research. Human Psychopharmacology, 15, 479-498. https://doi.org/10.1002/1099-1077(200010)15:7<479::AID-HUP206>3.0.CO;2-5
Knott, V., Labelle, A., Jones, B., & Mahoney, C. (2001). Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophrenia Research, 50(1-2), 41–53. https://doi.org/10.1016/s0920-9964(00)00165-1
Knott, V., Mahoney, C., Kennedy, S., & Evans, K. (2000). Pre-treatment EEG and it's relationship to depression severity and paroxetine treatment outcome. Pharmacopsychiatry, 33(6), 201–205. https://doi.org/10.1055/s-2000-8356
Landolt, H., Rétey, J., Tönz, K., Gottselig, J., Khatami, R., Buckelmüller, I., & Achermann, P. (2004). Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in hHumans. Neuropsychopharmacology, 29, 1933-1939. https://doi.org/10.1038/sj.npp.1300526
Laurian, S., Le, P., Baumann, P., Perey, M., Gaillard, J., & Müller, P. (1981). Relationship between plasma-levels of Chlorpromazine and effects on EEG and evoked potentials in healthy volunteers. Pharmacopsychiatry, 14, 199 - 204. https://doi.org/10.1055/s-2007-1019598
Lindgren, M., Molander, L., Verbaan, C., Lunell, E., & Rosén, I. (1999). Electroencephalographic effects of intravenous nicotine – A dose-response study. Psychopharmacology, 145, 342-350. https://doi.org/10.1007/s002130051067
MacCrimmon, D., Brunet, D., Criollo, M., Galin, H., & Lawson, J. S. (2012). Clozapine augments delta, theta, and right frontal EEG alpha power in schizophrenic patients. ISRN Psychiatry, 2012, 596486. https://doi.org/10.5402/2012/596486
Malver, L., Brokjær, A., Staahl, C., Graversen, C., Andresen, T., & Drewes, A. (2014). Electroencephalography and analgesics. British Journal of Clinical Pharmacology, 77. https://doi.org/10.1111/bcp.12137
Marciani, M. G., Gigli, G. L., Maschio, M. C., Stefani, N., Stefanini, F., & Bernardi, G. (1992). EEG changes induced by carbamazepine therapy at rest and during mental processes. Italian Journal of Neurological Sciences, 13(9), 729–733. https://doi.org/10.1007/BF02229157
Matousek M. (1987). EEG assessment of the sedative and excitatory properties of CNS-active compounds in the patients with depression. Neuropsychobiology, 17(1-2), 118–120. https://doi.org/10.1159/000118348
McClelland, G., Cooper, S., & Pilgrim, A. (1990). A comparison of the central nervous system effects of haloperidol, chlorpromazine and sulpiride in normal volunteers. British Journal of Clinical Pharmacology, 30(6), 795-803. https://doi.org/10.1111/J.1365-2125.1990.TB05444.X
Mecarelli, O., Piacenti, A., Pulitano, P., Vicenzini, E., Rizzo, C., Rinalduzzi, S., de Feo, M. R., & Accornero, N. (2001). Clinical and electroencephalographic effects of topiramate in patients with epilepsy and healthy volunteers. Clinical Neuropharmacology, 24(5), 284–289. https://doi.org/10.1097/00002826-200109000-00005
Mecarelli, O., Vicenzini, E., Pulitano, P., Vanacore, N., Romolo, F. S., Di Piero, V., Lenzi, G. L., & Accornero, N. (2004). Clinical, cognitive, and neurophysiologic correlates of short-term treatment with carbamazepine, oxcarbazepine, and levetiracetam in healthy volunteers. The Annals of Pharmacotherapy, 38(11), 1816–1822. https://doi.org/10.1345/aph.1E136
Merlotti, E., Mucci, A., Bucci, P., Volpe, U., Montefusco, V., Galderisi, S., & Maj, M. (2007). Topographic and tomographic EEG changes after a single oral dose of antipsychotic drugs in healthy young subjects. European Psychiatry, 22, S160 - S161. https://doi.org/10.1016/j.eurpsy.2007.01.521
Montgomery, P., Burton, J. R., Sewell, R. P., Spreckelsen, T. F., & Richardson, A. J. (2013). Low blood long chain omega-3 fatty acids in UK children are associated with poor cognitive performance and behavior: a cross-sectional analysis from the DOLAB study. PLoS ONE, 8(6), e66697. https://doi.org/10.1371/journal.pone.0066697
Muñoz-Torres, Z., Río-Portilla, Y., & Corsi-Cabrera, M. (2011). Diazepam-induced changes in EEG oscillations during performance of a sustained attention task. Journal of Clinical Neurophysiology, 28, 394–399. https://doi.org/10.1097/WNP.0b013e318227323a
Neufeld, M., Kogan, E., Chistik, V., & Korczyn, A. (1999). Comparison of the effects of vigabatrin, lamotrigine, and topiramate on quantitative EEGs in patients with epilepsy. Clinical Neuropharmacology, 22(2), 80-6 . https://doi.org/10.1097/00002826-199903000-00003
Newton, T. F., Cook, I. A., Kalechstein, A. D., Duran, S., Monroy, F., Ling, W., & Leuchter, A. F. (2003). Quantitative EEG abnormalities in recently abstinent methamphetamine dependent individuals. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 114(3), 410–415. https://doi.org/10.1016/s1388-2457(02)00409-1
Noldy, N., Santos, C., Politzer, N., Blair, R., & Carlen, P. (1994). Quantitative EEG changes in cocaine withdrawal: Evidence for long-term CNS effects. Neuropsychobiology, 30(4), 189-196. https://doi.org/10.1159/000119160
Ott, G. E., Rao, U., Lin, K. M., Gertsik, L., & Poland, R. E. (2004). Effect of treatment with bupropion on EEG sleep: Relationship to antidepressant response. The International Journal of Neuropsychopharmacology, 7(3), 275–281. https://doi.org/10.1017/S1461145704004298
Paes, F., Machado, S., Arias-Carrión, O., Domingues, C. A., Teixeira, S., Velasques, B., Cunha, M., Minc, D., Basile, L. F., Budde, H., Cagy, M., Piedade, R., Kerick, S., Menéndez-González, M., Skaper, S. D., Norwood, B. A., Ribeiro, P., & Nardi, A. E. (2011). Effects of Methylphenidate on performance of a practical pistol shooting task: a quantitative electroencephalography (qEEG) study. International Archives of Medicine, 4(1), 6. https://doi.org/10.1186/1755-7682-4-6
Patat, A., Rosenzweig, P., Enslen, M., Trocherie, S., Miget, N., Bozón, M., Allain, H., & Gandon, J. (2000). Effects of a new slow release formulation of caffeine on EEG, psychomotor and cognitive functions in sleep‐deprived subjects. Human Psychopharmacology: Clinical and Experimental, 15. 3.0.CO;2-C" target="_blank">https://doi.org/10.1002/(SICI)1099-1077(200004)15:3<153::AID-HUP154>3.0.CO;2-C
Patrick, R. P., & Ames, B. N. (2015). Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 29(6), 2207–2222. https://doi.org/10.1096/fj.14-268342
Pickworth, W., Herning, R., & Henningfield, J. (1986). Electroencephalographic effects of nicotine chewing gum in humans. Pharmacology Biochemistry and Behavior, 25, 879-882. https://doi.org/10.1016/0091-3057(86)90401-6
Pollock, V., Teasdale, T., Stern, J., & Volavka, J. (1981). Effects of caffeine on resting EEG and response to sine wave modulated light. Electroencephalography and Clinical Neurophysiology, 51(5), 470-6. https://doi.org/10.1016/0013-4694(81)90223-6
Prashad, S., Dedrick, E., & Filbey, F. (2018). Cannabis users exhibit increased cortical activation during resting state compared to non-users. NeuroImage, 179, 176-186. https://doi.org/10.1016/j.neuroimage.2018.06.031
Ranzi, P., Thiel, C., & Herrmann, C. (2016). EEG source reconstruction in male nonsmokers after nicotine administration during the resting state. Neuropsychobiology, 73, 191 - 200. https://doi.org/10.1159/000445481
Rubinson, M., Horowitz, I., Naim-Feil, J., Gothelf, D., Levit-Binnun, N., & Moses, E. (2019). Effects of methylphenidate on the ERP amplitude in youth with ADHD: A double-blind placebo-controlled cross-over EEG study. PLoS ONE, 14. https://doi.org/10.1371/journal.pone.0217383
Saletu, B., Anderer, P., & Saletu-Zyhlarz, G. M. (2006). EEG topography Neuroscience, 37(2), 66–80. https://doi.org/10.1177/155005940603700205
Saletu, B., Anderer, P., Saletuzyhlarz, G. M. (2010). EEG mapping and tomography in drug evaluation. Medicographia, 32, 190-200. https://doi.org/10.1177/155005940603700205
Saletu, B., Grünberger, J., & Linzmayer, L. (1986). Evaluation of encephalotropic and psychotropic properties of gabapentin in man by pharmaco-EEG and psychometry. International Journal of Clinical Pharmacology, Therapy, and Toxicology, 24(7), 362–373.
Siepmann, M., & Kirch, W. (2002). Effects of caffeine on topographic quantitative EEG. Neuropsychobiology, 45, 161 - 166. https://doi.org/10.1159/000054958
Simeon, J. G., Ferguson, H. B., & Van Wyck Fleet, J. (1986). Bupropion effects in attention deficit and conduct disorders. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 31(6), 581–585. https://doi.org/10.1177/070674378603100617
Skosnik, P., Krishnan, G., D’Souza, D., Hetrick, W., & O’Donnell, B. (2014). Disrupted gamma-band neural oscillations during coherent motion perception in heavy cannabis users. Neuropsychopharmacology, 39, 3087-3099. https://doi.org/10.1038/npp.2014.166
Stahl, S. M. (2017). Stahl's essential psychopharmacology prescriber's guide (6th ed.). Cambridge University Press.
Swatzyna, R. J., Morrow, L. M., Collins, D. M., Barr, E. A., Roark, A. J., & Turner, R. P. (2024). Evidentiary significance of routine EEG in refractory cases: A paradigm shift in psychiatry. Clinical EEG and neuroscience, 15500594231221313. Advance online publication. https://doi.org/10.1177/15500594231221313
Tan, X., Uchida, S., Matsuura, M., Nishihara, K., & Kojima, T. (2003). Long‐, intermediate‐ and short‐acting benzodiazepine effects on human sleep EEG spectra. Psychiatry and Clinical Neurosciences, 57. https://doi.org/10.1046/j.1440-1819.2003.01085.x
Tashkin D. P. (2013). Effects of marijuana smoking on the lung. Annals of the American Thoracic Society, 10(3), 239–247. https://doi.org/10.1513/AnnalsATS.201212-127FR
Thanacoody, H. K., & Thomas, S. H. (2005). Tricyclic antidepressant poisoning: Cardiovascular toxicity. Toxicological Reviews, 24(3), 205–214. https://doi.org/10.2165/00139709-200524030-00013
Thomaides, T., Tagaris, G., & Karageorgiou, C. (1996). EEG and topographic frequency analysis in migraine attack before and after sumatriptan infusion. Headache, 36(2), 111–114. https://doi.org/10.1046/j.1526-4610.1996.3602111.x
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Van Cott, A., & Brenner, R. P. (2003). Drug effects and toxic encephalopathies. In J. S. Ebersole & T. A. Pedley (Eds.). Current practice of clinical electroencephalography (3rd ed.). Lippincott Williams & Wilkins.
van der Post, J., Schram, M. T., Schoemaker, R. C., Pieters, M. S., Fuseau, E., Pereira, A., Baggen, S., Cohen, A. F., & van Gerven, J. M. (2002). CNS effects of sumatriptan and rizatriptan in healthy female volunteers. Cephalalgia: An International Journal of Headache, 22(4), 271–281. https://doi.org/10.1046/j.1468-2982.2002.00344.x
Volavka, J., Dornbush, R., Feldstein, S., Clare, G., Zaks, A., Fink, M., & Freedman, A. (1971). Marijuana, EEG, and behavior.Annals of the New York Academy of Sciences, 191. https://doi.org/10.1111/j.1749-6632.1971.tb13999.x
Wang, W. W., Li, J. C., & Wu, X. (2003). Quantitative EEG effects of topiramate. Clinical EEG (Electroencephalography), 34(2), 87–92. https://doi.org/10.1177/155005940303400208
Wu, X., & Xiao, C. H. (1996). Quantitative pharmaco-EEG of carbamazepine in volunteers and epileptics. Clinical EEG (Electroencephalography), 27(1), 40–45. https://doi.org/10.1177/155005949602700107
Yoshimura, M., Koenig, T., Irisawa, S., Isotani, T., Yamada, K., Kikuchi, M., Okugawa, G., Yagyu, T., Kinoshita, T., Strik, W., & Dierks, T. (2007). A pharmaco-EEG study on antipsychotic drugs in healthy volunteers. Psychopharmacology, 191, 995-1004. https://doi.org/10.1007/s00213-007-0737-8
Zajicek, J., Fox, P., Sanders, H., Wright, D., Vickery, J., Nunn, A., Thompson, A., & UK MS Research Group (2003). Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet, 362(9395), 1517–1526. https://doi.org/10.1016/S0140-6736(03)14738-1
Zhu, J., Coppens, R. P., Rabinovich, N. E., & Gilbert, D. G. (2017). Effects of bupropion sustained release on task-related EEG alpha activity in smokers: Individual differences in drug response. Experimental and Clinical Psychopharmacology, 25(1), 41–49. https://doi.org/10.1037/pha0000109
A single dose of a prescription psychotropic drug can markedly change the EEG within 1-3 hours of administration. Families of psychotropic drugs that share therapeutic equivalence (e.g., chlorpromazine-like neuroleptics and haloperidol-like neuroleptics) produce similar systematic EEG changes (Knott, 2000). A drug's plasma level, which depends on the dose, distribution volume, and metabolism, influences the magnitude of EEG alterations, which should be symmetrical and often widespread.
Common EEG changes include a slowing of background activity, increased beta activity, epileptiform activity, triphasic waves, and widespread delta and increased theta activity (Blume, 2006).
Epileptiform activity graphic © Chaikom/Shutterstock.com.

BCIA Blueprint Coverage
This unit addresses V. Psychopharmacological Considerations.

This unit covers Relationships of Drugs and Neurotransmitter Modulation, Potential Effects of Prescribed and Non-Prescribed Drugs on Clinical Presentation, Potential Effects of Prescribed and Non-Prescribed Drugs on EEG Measures, Potential Effects of Different Drugs on Neurofeedback Assessment and Training, Recommended Medication Management Approaches, and Drug Implications for Assessment and Neurofeedback.
Please click on the podcast icon below to hear a full-length lecture.

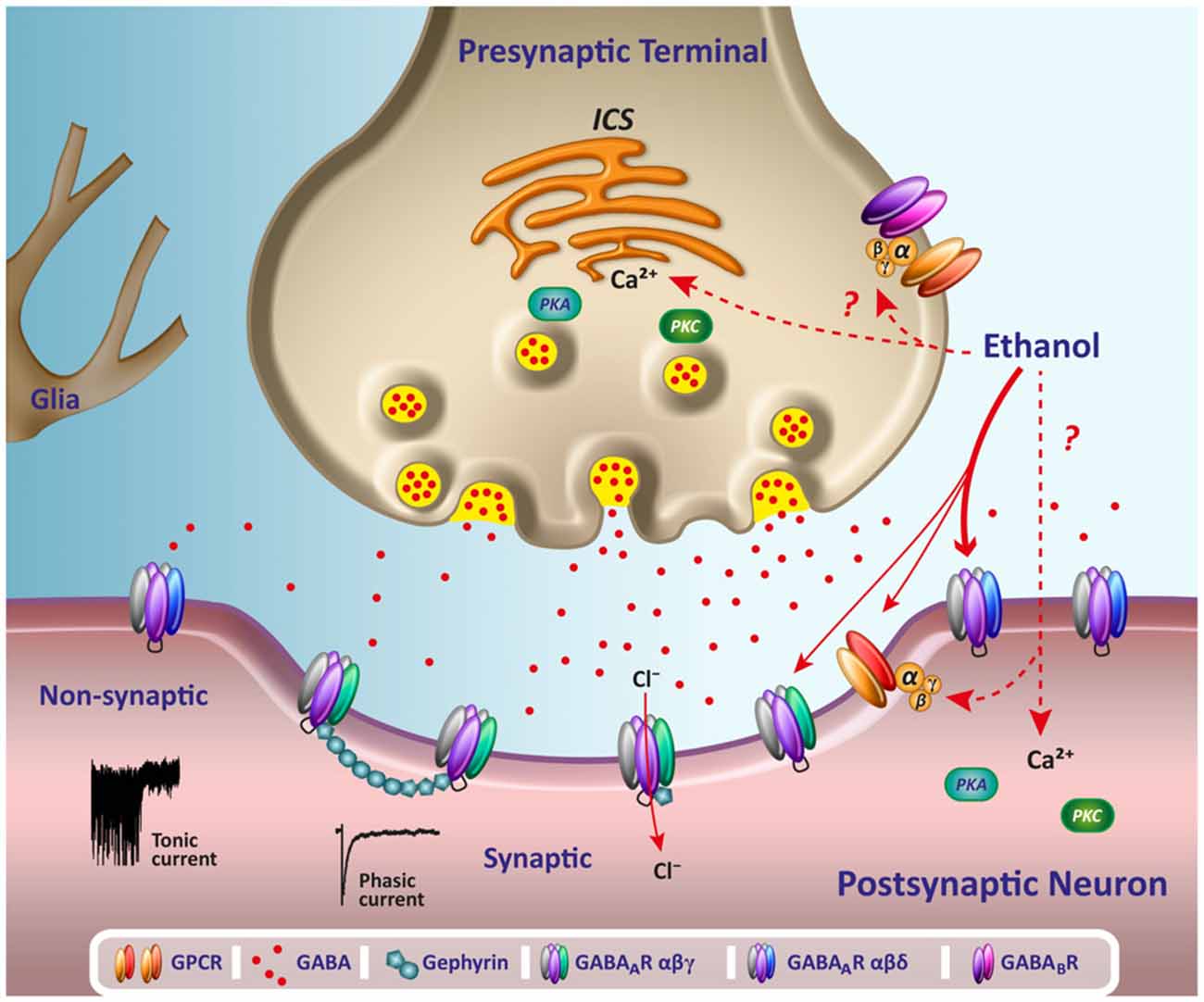
RELATIONSHIPS OF DRUGS AND NEUROTRANSMITTER MODULATION
Neurons synthesize chemicals called neurotransmitters (NTs), which bind to a neuron's receptors to alter their activity. Neurotransmitter release animation (no sound) © Madrock24/Shutterstock.com.
Caption: The arrival of an action potential depolarizes the axon terminal, allowing calcium ions to enter. Calcium activates molecules that transport vesicles to the presynaptic membrane, creating fusion pores that allow neurotransmitter molecules to enter the fluid-filled synaptic cleft and bind to postsynaptic receptors. The neurotransmitteer release process is called exocytosis.
Drugs are molecules that modify physiological processes. Psychoactive drugs alter brain functions to influence mood (e.g., anxiety) and behavior (e.g., attention). Psychopharmacology studies drug effects on our nervous system, psychological states, and behavior (Julien, Advokat, & Comaty, 2023).
NTs and drugs are ligands, molecules that form covalent bonds with receptors. Pharmacodynamics studies how drug interactions with receptors affect the body.
It is a basic principle of pharmacology that the physiological and behavioral effects induced by a drug result from the drug’s interactions with receptors (Julien et al., 2023, p. 59).
Drug binding to receptors is characterized by the properties of specificity and affinity. Specificity refers to the degree to which a drug only binds to a given receptor. Affinity describes the strength of a drug's covalent bond with its receptor. Stronger affinity results in greater drug effects.
NTs and psychoactive drugs exert acute and chronic effects when they bind to receptors. Acute effects are the immediate changes produced by receptor binding. Receptor activation may last from several to hundreds of milliseconds (Julien et al., 2023). For example, serotonin-selective reuptake inhibitors (SSRIs) prolong serotonin availability within the synaptic cleft.
In contract, chronic effects are long-term alterations in receptors and the functioning of neural networks. Months of SSRI treatment can reduce the number of serotonin receptors and increase mood and social engagement. This phenomenon is called downregulation. Graphic adapted from Julien et al. (2023).

Drugs Modulate NT Actions at Their Receptors
Psychoactive drugs cannot confer "superpowers" on receptors like Peter Parker's dramatic transformation after being bitten by a radioactive spider. Instead, drugs can only modulate, promote or inhibit, a receptor's normal response to a NT.
The term agonist describes the many ways a drug can promote a NT's actions. In contrast, the term antagonist characterizes how a drug can interfere with a NT's actions. We caution against limiting agonism to NT imitation and antagonism to receptor blockade because drugs can promote or interfere with NT action in diverse ways.
Drugs and NTs Target Extracellular Receptors
Extracellular binding sites, which are exposed to ligands (e.g., drug and NT molecules) in the synaptic cleft and extracellular fluid, are among the best-recognized drug targets. They include families of ionotropic and metabotropic receptors.Consider an ionotropic receptor. NT binding alters the shape of its central ion channel, allowing ion movement. Ionotropic receptor graphic © Ph-HY/Shutterstock.com.

For example, the site where the amino acid GABA binds is called its orthosteric site. GABA ionotropic receptor graphic © Designua/Shutterstock.com.

Caption: The five subunits form a chloride ion channel.
When GABA binds to inhibitory GABA-A receptors, it triggers the inward movement of negative chloride ions, increasing inhibition. The separate subunits where drugs like ethanol bind are called allosteric sites. Ethanol modulates GABA's action, increasing chloride ion entry and neuronal inhibition (Förstera et al., 2016). In this example, ethanol functions as a GABA agonist.

Caption: Ethanol binds to its allosteric site on the GABA-A receptor, modulating GABA's action when it binds to its orthosteric site.
G-protein-coupled receptors (also called metabotropic receptors) are proteins that convert signals from NTs and drugs into intracellular messages. Activation of G-proteins within a neuron's interior can result in the synthesis of second messengers that modulate its actions. Second messengers can alter cell division and differentiation, energy conversion and utilization, membrane potential, and ion channel Graphic © Ph-HY/Shutterstock.com.

Caption: NT binding to a G-protein-coupled receptor activates an alpha-subunit inside the cell, transforming GDP to GTP and ATP into the second-messenger cyclic AMP.
Drugs Modulate NT Systems Through Actions At Diverse Targets
Drugs modulate NT systems by altering their production, release, and clearance. Graphic © rob9000/Shutterstock.com..jpg)
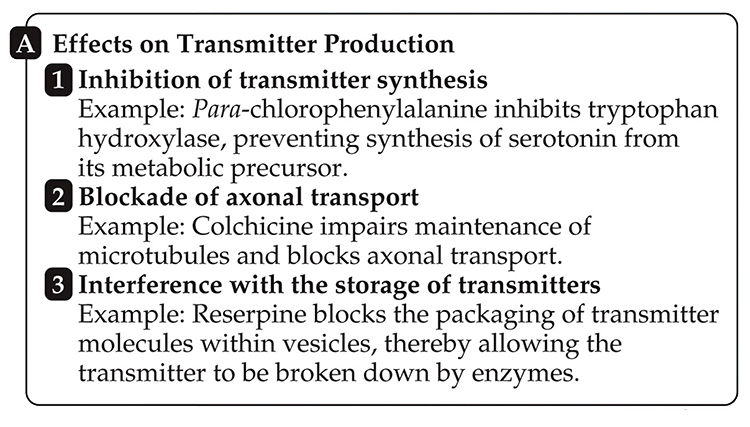
NT Production
Drugs can (1) target enzymes to prevent NT synthesis (e.g., para-chlorophenylalanine), (2) disrupt axonal transport (e.g., colchicine), and (3) interfere with NT storage (e.g., reserpine).
NT Release
Drugs can prevent NT release by blocking (4) sodium channels (e.g., tetrodotoxin) or (5) calcium channels (e.g., verapamil), (6) they can compete for occupancy on presynaptic receptors (e.g., caffeine), and (7) can alter NT release (e.g., amphetamines and Botox).
NT Clearance
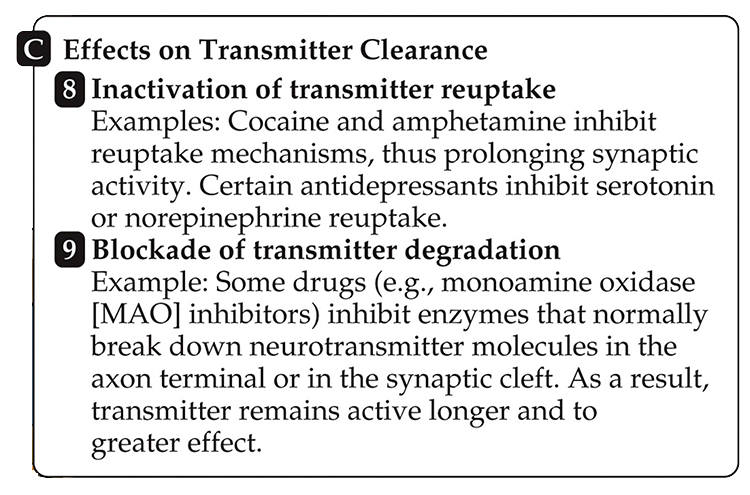
Drugs can interfere with (8) NT reuptake (e.g., cocaine) and (9) NT degradation (e.g., MAO inhibitors).
HOW DRUG HALF-LIVES INFLUENCE OUR INTERPRETATION OF EEG/QEEG AND BEHAVIOR
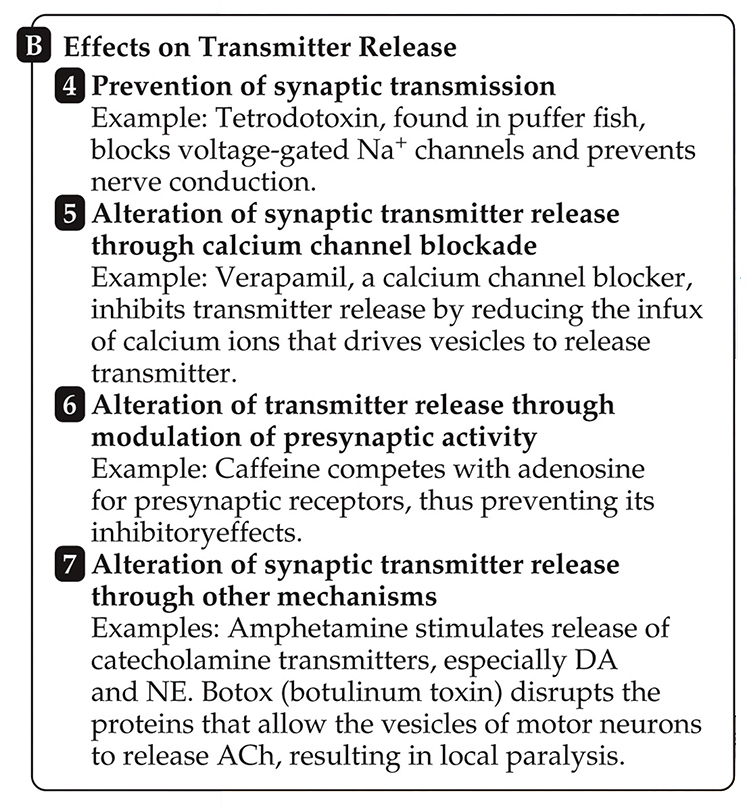
A drug's half-life is the time required to eliminate 50% of a drug. Following redistribution from the blood plasma to body tissues, there is a linear rate of decline for all drugs with the exception of ethanol (Julien et al., 2023).

Note: After intravenous injection, the plasma concentration of the drug rises rapidly to about 31 micrograms/milliliter (μg/mL). The concentration is measured every 30 minutes for the first 2 hours and every 2 hours thereafter until 12 hours after injection. Redistribution occurs over the first 2 hours, as the drug leaves plasma, enters body tissues, and equilibrates with those tissues. After redistribution, the fall in plasma concentration is linear, decreasing by half every 4 hours. Thus, the half-life of the drug is 4 hours.
EEG/qEEG professionals should know the half-lives of their clients' psychoactive drugs to estimate how long they will remain in the body, affecting measurements, behavior, and training.
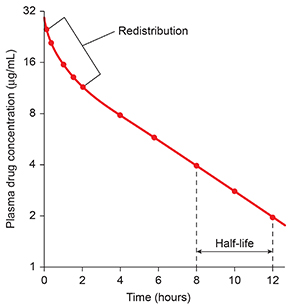
A client is not drug-free (e.g., 98% eliminated) until six half-lives have passed.

DRUG CLASS EFFECTS ON EEQ/QEEG MEASUREMENTS

Caption: The authors largely based this table on the excellent Aiyer et al. (2016) systematic review.
A Psychopharmacology Paradigm Shift
Dr. Ron Swatzyna and colleagues (2024) emphasized the importance of using routine EEGs in cases where psychiatric medications have failed to provide relief. They argued that the traditional symptom-based approach in psychiatry, as outlined by the Diagnostic and Statistical Manual (DSM), is insufficient and often leads to multiple medication trials that do not address the underlying causes of psychiatric symptoms.

The authors proposed that EEGs can reveal brain abnormalities not detectable through standard psychiatric assessments. They identified four key EEG biomarkers—focal slowing (FS), spindling excessive beta (SEB), encephalopathy (EN, diffuse slowing), and isolated epileptiform discharges (IEDs)—that are associated with medication failure in refractory patients. These biomarkers suggest specific types of brain dysregulation, such as localized cerebral pathology, cortical irritability, or underlying encephalopathy, which can manifest as complex behavioral or psychiatric issues.
The use of EEG biomarker identification represents a positive step toward personalized medicine. By directly testing the organ being treated, practitioners can gain a deeper understanding of the underlying brain activity and create treatment plans tailored to the specific needs of each patient. (Swatzyna et al., 2024, p. 9)Incorporating routine EEGs into psychiatric practice could significantly enhance treatment precision by identifying these biomarkers, thereby moving toward personalized medicine. This approach aligns with the goals of the National Institute of Mental Health's Research Domain Criteria (RDoC) project, which advocates for a shift away from symptom-based diagnosis toward a more biologically grounded understanding of mental disorders.
Antidepressants

Clinical Presentation
The major antidepressant classes include tricyclic antidepressants (TCAs), second-generation atypical antidepressants, selective serotonin reuptake inhibitors (SSRIs), dual-action antidepressants, irreversible MAO inhibitors, and selective norepinephrine reuptake inhibitors (SNRIs).Tricyclic antidepressants (TCAs) like imipramine (Tofranil) can produce anxiety, blurred vision, dizziness, fatigue and weakness, psychotic symptoms (rare), sedation, seizures (rare), sexual dysfunction, and suicidality (rare) (Stahl, 2021).
Selective serotonin reuptake inhibitors (SSRIs) like fluoxetine (Prozac) can produce agitation, anxiety, headache, insomnia, mania (rare), sedation, seizures (rare), sexual dysfunction, suicidality (rare), and tremors (Stahl, 2021).
Dual-action antidepressants like duloxetine (Cymbalta) can produce hypomania (rare), hypertension, insomnia, sedation, sexual dysfunction, and suicidality (rare) (Stahl, 2021).
Irreversible MAO inhibitors like selegeline (Emsam, Eldepryl) can produce confusion, dizziness, dyskinesia, hallucinations, headache, hypertensive crisis, mania (rare), seizures (rare), and
suicidality (rare) (Stahl, 2021).
Selective norepinephrine reuptake inhibitors (SNRIs) like atomoxetine (Strattera) can produce abdominal pain, anxiety, agitation, aggression, dizziness, dysmenorrhea, dyspepsia, elevated heart rate, fatigue (especially in children), hypertension, hypomania and mania (rare), orthostatic hypotension, priapism, sedation, sexual dysfunction, and suicidality (rare) (Stahl, 2021).
EEG Measures
TCAs
Sedating TCAs like amitriptyline (Elavil) and imipramine (Tofranil) increase theta and fast-beta power and decrease alpha and total power (Knott, 2000; Saletu, 2010; Thompson & Thompson, 2016). However, nonsedating TCAs that resemble desipramine (Norpramin) increase alpha and fast beta (Knott, 2000).At higher doses, sedating TCAs can increase delta and widespread theta power (Bauer & Bauer, 2005; Van Cott & Brenner, 2003).
TCAs can cause asynchronous slow waves and increase sleep spindles (Thompson & Thompson, 2015). TCAs and SSRIs can provoke spikes or polyspikes. Excessive TCA doses can increase delta and theta power, where theta appears diffusely (Blume, 2006).
MAOIs
The nonselective, irreversible MAO inhibitor iproniazid (Marsalid) increases theta to a smaller degree than amitryptiline (Elavil), while it increases fast-beta power to a greater degree.MAO inhibitors like isocarboxazid (Marplan) increase 20-30 Hz power while decreasing power in slower and high frequencies like CNS stimulants (Thompson & Thompson, 2015).
SSRIs
The SSRIs fluoxetine (Prozac), paroxetine HCl (Paxil), and sertraline HCl (Zoloft) modestly increase 18-25 Hz frontocentral beta and decrease anterior alpha power (Thompson & Thompson, 2016).The nonsedating SSRI citalopram (Celexa) decreases total, delta, theta, and alpha power, while it increases beta and gamma power (Bauer & Bauer, 2005; Nissen et al., 2020; Saletu, 2010; Van Cott & Brenner, 2003).
Vortioxetine (Trintellix) decreases theta band (4-8 Hz) power and increases beta (12-32 Hz) and gamma (32-45 Hz) band power (Nissen et al., 2020).
High SSRI doses may produce bisynchronous spikes or polyspikes. Serotonin syndrome is associated with triphasic waves, which signal toxic encephalopathy (Blume, 2006).
ANTIPSYCHOTICS

Clinical Presentation
The major antipsychotic classes include first-generation, second-generation (atypical), and third-generation agents.First-generation agents (FGAs) like haloperidol (Haldol) can produce akathisia (restless movement disorder), extrapyramidal symptoms (pseudo-Parkinsonism, tardive dyskinesia, and tardive dystonia), blurred vision, death and stroke in elderly with dementia-related psychosis, dizziness, hypertension, hypotension, neuroleptic malignant syndrome (rare), neuroleptic-induced deficit syndrome (analogous to the negative symptoms of schizophrenia), seizures (rare), and tachycardia.
Second-generation (atypical) agents (SGAs) like clozaril (Clozapine) can produce death and stroke in the elderly with dementia-related psychosis, neuroleptic malignant syndrome (when used with another agent), sedation, seizures, tachycardia, and tardive dyskinesia (rare).
Third-generation agents (TGAs) show mixed effects. Drugs like aripiprazole (Abilify) can produce activation, akathisia, death and stroke in the elderly with dementia-related psychosis, dizziness, headache, impaired impulse control (rare), insomnia, loss of energy, neuroleptic malignant syndrome, sedation, seizures (rare), and tardive dyskinesia (theoretical risk).
EEG Measures
All FGAs do not produce identical EEG changes. Chlorpromazine (Thorazine), a sedating FGA, increases slow wave activity (delta and theta) and decreases alpha (8-14 Hz) and faster (beta) wave activity in human EEG (Hughes et al., 2001; McClelland et al., 1990). FGAs may slow the peak alpha frequency and produce synchronous slow-wave activity.Chlorpromazine (Thorazine) can increase sharp theta transients at frontal and temporal sites. Chlorpromazine can significantly slow the posterior dominant rhythm (PDR) (Demos, 2019).
Chlorpromazine reduces amplitude and increasing variability in evoked responses (Laurian et al., 1981).
The attenuation of alpha-blocking in response to sensory stimuli may be associated with memory deficits produced by this drug (Saletu, 2010; Thompson & Thompson, 2016). High phenothiazine doses may produce bisynchronous spikes or polyspikes (Blume, 2006).
Haloperidol (Haldol), a nonsedating FGA, increased alpha 1 and beta 1 power in human EEG bands and tended to shift the beta 1 centroid posterior (Merlotti et al., 2007; Yoshimura et al., 2007).
Clozapine (Clozaril) decreases alpha (Baik et al., 2011; MacCrimmon et al., 2012) and beta, and increases delta and theta power (Knott et al., 2001). Excessive doses of the SGA clozapine can increase delta and theta power, where theta appears diffusely (Blume, 2006).
Clozapine produces specific topographic EEG changes, marked slowing in frontal, central, and parietal scalp areas, distinct from those of other neuroleptics (Joutsiniemi et al., 2001).
Where dopamine receptor hypersensitivity produces extrapyramidal side effects like tardive dyskinesia, FGAs may cause extended trains of mixed fast/sharp transients, EEG slowing, and potentiation of latent epileptiform activity (J. Gunkelman cited by Thompson & Thompson, 2016).
BENZODIAZEPINES

Clinical Presentation
The major benzodiazepine classes include long-acting agents, intermediate-acting agents, short-acting agents, and benzodiazepine receptor agonist (BZRA) hypnotics.Long-acting agents like diazepam (Valium) can produce ataxia, confusion, depression, dizziness, fatigue, forgetfulness, hallucinations (rare), hyperexcitability, hypotension (rare), mania (rare), nervousness, respiratory depression (overdose with respiratory depressants), sedation, slurred speech, and weakness (Stahl, 2017).
Intermediate-acting agents like lorazepam (Ativan) can produce the same side effects as their long-acting counterparts (Stahl, 2017).
Short-acting agents like alprazolam (Xanax) can also produce the same side effects as their long and intermediate-acting counterparts (Stahl, 2017).
Benzodiazepine receptor agonist (BZRA) hypnotics like zolpidem (Ambien) can produce amnesia (dose-dependent), ataxia, dizziness, hallucinations (rare), headache, hyperexcitability, nervousness,
respiratory depression (overdose with respiratory depressants), and sedation.
EEG Measures
Benzodiazepines reduce alpha and increase beta power, especially over 20 Hz (high-beta). They can reduce the PDR (Demos, 2019).Benzodiazepines may increase spindling beta and inhibit epileptiform activity (Bauer & Bauer, 2005; Blume, 2006; Julien et al., 2023; Knott, 2000; Thompson & Thompson, 2015; Van Cott & Brenner, 2003).
Benzodiazepines like haloxazolam (Somelin), flunitrazepam (Rohypnol), and triazolam (Halcion) increase higher frequency activity in the sigma and beta bands and reduce lower frequency activity in sleep EEG spectra (Tan et al., 2003).
Diazepam (Valium) decreases EEG power in 1- to 6-, 8- to 12-, and 19- to 35-Hz bands and disrupts right intrahemispheric temporal coupling in the 8-12 Hz range (Muñoz-Torres et al., 2011).
Midazolam (Versed) and its active metabolite, alpha-hydroxy-midazolam, increased EEG beta activity and decreased alpha activity in healthy participants. These changes were associated with sedation and persisted for hours after administration, even at low plasma levels (Hotz et al., 2000).
Alprazolam (Xanax) administration led to increased higher frequency activity in the sigma and beta bands, while lower frequency activity reduced in sleep EEG spectra (Tan et al., 2003).
CNS STIMULANTS

The two main classes of CNS stimulants include amphetamines and nonamphetamine behavioral stimulants.
Amphetamines like amphetamine D, L (Adderall) can produce adverse cardiovascular effects, cardiac arrhythmia, dizziness, headache, hypertension, hypomania, insomnia, irritability, mania, nervousness, overstimulation, psychotic episodes, sexual dysfunction (long-term), suicidality, and worsened tics (Stahl, 2017).
Nonamphetamine behavioral stimulants like methylphenidate (Ritalin) can produce adverse cardiovascular effects, cardiac arrhythmia, dizziness, headache, hypertension, hypomania, insomnia, irritability, mania, nervousness, overstimulation, priapism (rare), psychotic episodes,
suicidality, and worsened tics (Stahl, 2017).
EEG Measures
Amphetamine (Adderall) reduces total power and reduces absolute delta, theta, alpha, and beta power (Saletu, 2010).Dexamphetamine (Vyvanse) and methylphenidate (Ritalin) reduced theta and increased beta power (Clarke et al., 2002).
Methylphenidate (Ritalin) reduces delta and theta power, increasing posterior alpha and low beta power for up to 6 hours following drug administration (Blume, 2006; Thompson & Thompson, 2015).
Methylphenidate decreases EEG mu power in the right primary motor cortex during motor imagery and execution tasks, compared to risperidone (Aprigio et al., 2021).
Methylphenidate effectively increases the P3 ERP response in ADHD subjects, potentially reducing mental fatigue-related decreases in response time (Rubinson et al., 2019).
A client's level of arousal modulates the EEG response to a CNS stimulant. Stimulants increase alpha power in under-aroused, decrease alpha power in typically aroused, and do not alter alpha in anxious (fast-EEG) clients (J. Gunkelman, cited by Thompson & Thompson, 2015).
MOOD STABILIZERS

Clinical Presentation
The major mood stabilizer classes include lithium, first-generation anticonvulsants, second-generation anticonvulsants, atypical antipsychotics, and omega-3 fatty acids.Lithium can produce arrhythmia, ataxia, bradycardia, cardiovascular changes, delirium, forgetfulness, hypotension, and lithium toxicity (Stahl, 2017).
First-generation anticonvulsants like phenobarbital (Phenobarbital) can produce aggression, confusion, depression, dizziness, drowsiness, excitement, forgetfulness, hallucinations (rare), headache, insomnia, nightmares, and respiratory depression (in overdose or with other CNS depressants) (Stahl, 2017).
Second-generation anticonvulsants like valproate (Depakene) can produce ataxia, bradycardia, dizziness, headache, sedation, suicidality, tachycardia, and weakness (Stahl, 2017).
Atypical antipsychotics like olanzapine (Zyprexa) can produce ✽ death and stroke in elderly with dementia-related psychosis, diabetes, dizziness, neuroleptic malignant syndrome (rare), orthostatic hypotension, pain (back, chest, extremity, joint), sedation, seizures (rare), and tardive dyskinesia (rare) (Stahl, 2017).
Omega-3 fatty acids do not produce significant side effects that would affect clinical presentation.
EEG Measures
Carbamazepine (Tegretol) decreases alpha (Marciani et al., 1992) and increases delta and theta (Wu & Xiao, 1996), and beta power (Marciani et al., 1992). Carbamazepine and lamotrigine, which are sodium-channel blockers, altered gamma power in the 50–60 Hz range, while valproate affected gamma power in the 30–40 Hz range (Arzy et al., 2010).Gabapentin (Neurontin) increases delta and theta power and decreases alpha power (Saletu et al., 1996). Its effects on beta power are inconclusive (Saletu et al., 1996).
Lamotrigine (Lamictal) decreases delta (Wu & Xiao, 1996), theta, alpha, and beta power (Clemens et al., 2008; Neufeld et al., 1999; Wu & Xiao, 1996).
Lithium can cause generalized asynchronous slowing that reduces the peak alpha frequency. Lithium may increase delta, theta, alpha, and beta power (Henninger, 1978) and greatly potentiate latent epileptiform activity. High lithium doses may produce bisynchronous spikes or polyspikes and increase delta and theta power, where theta appears diffusely (Blume, 2006). Lithium toxicity dramatically slows the EEG and causes triphasic discharges (Thompson & Thompson, 2016).
Oxcarbamazepine (Trileptal) decreases alpha power (Clemens et al., 2006). There are no data on its effects on delta, theta, and beta band power.
Phenobarbital (Luminal) can induce rhythmic 18-26 Hz activity that starts in the frontal lobe and can progressively extend to the whole cortex. Progressively higher doses promote EEG slowing and reduced beta activity until slow-wave activity eclipses beta activity. Voltage can decrease until the brain enters an iso-electric state like a medically induced coma (Blume, 2006; Thompson & Thompson, 2016). Barbiturate withdrawal may increase beta activity. Pentobarbital intoxication can result in triphasic waves. In general, antiepileptic drug reduction may increase the frequency of focal spikes or spike waves (Blume, 2006).
Barbiturate withdrawal may increase beta activity. Pentobarbital (Nembutol) intoxication can result in triphasic waves.
Antiepileptic drug reduction may increase the frequency of focal spikes or spike waves (Blume, 2006).
Topiramate (Topamax) increases delta and theta and decreases alpha power (Mercarelli et al., 2001). Topiramate increases the absolute beta and theta activity diffusely and decreased relative alpha activity over the left hemisphere (Neufeld et al., 1999).
Valproate (Depakote) decreases alpha (Clemens et al., 2014; Guo et al., 2014) and increases beta power (Clemens et al., 2014). Valproic acid intoxication can produce triphasic waves (Blume, 2006).
Vigabatrin (Sabril) administration decreased absolute alpha and beta activity and decreased absolute theta in the frontal and parieto-occipital regions (Neufeld et al., 1999).
Neurotoxicity caused by high levels of antiepileptic drugs may cause diffuse delta and increased theta power. Valproic acid (Depakene) intoxication can produce triphasic waves (Blume, 2006).
OPIOID ANALGESICS

Clinical Presentation
The major opioid analgesic classes include pure agonists, partial agonists, and mixed agonist-antagonists.Pure agonists like morphine (MS-IR) can produce agitation, confusion, dizziness, drowsiness, hallucinations, respiratory depression, and weakness (Advokat et al., 2019).
Partial agonists like buprenorphine (Subutex) can produce headache, insomnia, mood swings, orthostatic hypotension, respiratory depression, and sedation (Advokat et al., 2019; Stahl, 2017).
Mixed agonist-antagonists like pentazocine (Talwin) can produce confusion (rare), depression, double vision (rare), drowsiness, excitement, insomnia, irregular heartbeat, irritability, sleepiness, very slow or very rapid breathing, and weakness (Advokat et al., 2019).
EEG Measures
Immediately after morphine administration, during the euphoric high, alpha power increases, and the peak alpha frequency slows. Delta and theta power may increase, as well as time spent in REM sleep (Malver et al., 2014). Increasing the dose causes EEG slowing and may progress to an iso-electric brain like a barbiturate-induced coma (Thompson & Thompson, 2015).Opioids such as remifentanil (Ultiva) and fentanyl (Duragesic) increase delta band power (0.5-4 Hz) during wakefulness and analgesia (Garcia et al., 2021; Graversen et al., 2015).
Remifentanil decreases theta (4-8 Hz) and alpha (8-13 Hz) band power, which correlates with its analgesic effects (Garcia et al., 2021; Graversen et al., 2015).
RECREATIONAL DRUGS
CAFFEINE

Clinical Presentation
Caffeine increases alertness and reduces fatigue. In vulnerable individuals, caffeine can promote anxiety and nervousness, cardiac arrhythmia, hypertension, and insomnia (Advokat et al., 2019).EEG Measures
Caffeine reduces delta power under resting conditions (Dimpfel et al., 1993; Pollock et al., 1981).Caffeine acutely reduces theta and alpha power with rebound increases in these bands (Thompson & Thompson, 2016). Decreased theta activity (5-8 Hz) occurs during wakefulness and sleep deprivation. This reduction is observed both under relaxed conditions and during mental tasks (Landolt et al., 2004; Pollock et al., 1981).
Caffeine leads to a global reduction in alpha power (8-14 Hz) and an increase in alpha frequency, indicating heightened arousal (Barry et al., 2005; Foxe et al., 2012). This effect is more pronounced with eyes open compared to eyes closed (Siepmann & Kirch, 2002).
Caffeine diminishes the absolute power of slow and fast alpha and slow beta activities in various brain regions (Siepmann & Kirch, 2002). It also increases beta power (12-40 Hz) in frontal and central brain areas, particularly in sleep-deprived individuals (Drapeau et al., 2006; Patat et al., 2000).
CANNABIS

Clinical Presentation
Cannabis can produce increased blood pressure and heart rate; cerebral artery constriction; impaired attention, coordination, memory, perception, and reaction time; mild euphoria; reduced anxiety; relaxation; acute depressive reactions, panic attacks, and mild paranoia at very high doses; and altered sensory perception, confusion, delusions, depersonalization, disorientation, hallucinations, and paranoia at massive doses (Advokat et al., 2019).EEG Measures
Cannabis users exhibit decreased delta power during resting states, indicating increased cortical activation and potential disinhibition of inhibitory function (Prashad et al., 2018).Cannabis intake disrupts theta oscillations, leading to decreased theta power, which is correlated with impaired working memory and attentional performance (Böcker et al., 2010; Ilan et al., 2004; Morrison et al., 2011).
Cannabis acutely increases frontal alpha and chronically promotes frontal interhemispheric connectivity (hypercoherence and phase synchrony) (Thompson & Thompson, 2015).
THC has dose-dependent effects on beta power, with higher doses leading to increased beta activity, which may be related to muscle activity or heightened arousal (Böcker et al., 2010; Volavka et al., 1971). Chronic cannabis users exhibit beta power during resting states (Prashad et al., 2018).
Cannabis disrupts gamma oscillations, particularly during tasks requiring complex perceptual processing, such as coherent motion perception. Reduced gamma power is associated with perceptual alterations and may contribute to the cognitive impairments observed in heavy cannabis users (Skosnik et al., 2014).
COCAINE

Clinical Presentation
Cocaine can produce increased blood pressure, heart rate, and temperature; increased alertness and energy; reduced fatigue; mood elevation; insomnia; and mild depression during withdrawal. Higher plasma levels can be associated with agitation, anxiety, impulsivity, suspiciousness, paranoia, and paranoid psychosis; repetitive behavior; and cardiac arrhythmias, cardiorespiratory arrest, and strokes (Advokat et al., 2019).EEG Measures
Cocaine decreases delta and theta and increases alpha and beta power (Bauer & Bauer, 2005; Herning et al., 1985). Cocaine enhances alpha power in the frontal and temporal regions (Herning et al., 1994).Cocaine significantly increases beta power, particularly in the frontal and central areas of the brain (Herning et al., 1994, 1985; Noldy et al., 1994).
During cocaine withdrawal, there is a significant decrease in beta-2 power, indicating long-term neuroadaptive changes, especially in intravenous users (Noldy et al., 1994).
ETHANOL

Clinical Presentation
Ethanol can produce short-term psychological effects of aggressiveness and violence; behavioral disinhibition; impaired attention, memory, motor coordination, and problem-solving; reduced anxiety and fear; respiratory depression; seizure following abrupt withdrawal; shortsightedness and tunnel vision; and slowed perceptual speed (Advokat et al., 2019).EEG Measures
Ethanol significantly affects EEG activity at anterior sites, causing increased activity in the slow alpha frequency band (Cohen et al., 1993).Individuals diagnosed with alcohol use disorder or who are vulnerable to developing this disorder frequently present with elevated beta (> 20 Hz and between 24-26 Hz) and decreased 6-10 Hz and alpha power (Thompson & Thompson, 2015). Alcohol withdrawal may increase beta activity, spikes, and polyspikes (Blume, 2006).
NICOTINE

Clinical Presentation
Nicotine increases alertness and may improve cognitive performance in smokers (and possibly nonsmokers). Nicotine can produce side effects of dizziness and headache. Nicotine withdrawal may result in anxiety, depression, and insomnia. Tobacco tars are a primary contributing factor in cancer and cardiovascular disorders linked to long-term tobacco consumption (Advokat et al., 20194).EEG Measures
Like caffeine, nicotine acutely reduces theta and alpha power with rebound increases in these bands (Pickworth et al., 1986; Thompson & Thompson, 2016).Nicotine increases beta band power, which is associated with increased alertness and cognitive processing (Lindgren et al., 1999; Pickworth et al., 1986).
Nicotine reduced power in the 12.5-18.4 Hz band in the left middle frontal gyrus during eyes-open conditions. During eyes-closed conditions, nicotine reduced power across 8.5-18.4 Hz in areas spanning the superior frontal gyri to the supplementary motor areas (Ranzi et al., 2016).
CLINICAL ISSUES

Clinicians must understand their scope-of-practice restrictions when discussing medication issues. Clients often seek alternative treatments when medication management is ineffective or has undesirable side effects. Medications are prescribed for presenting symptoms related to atypical causal factors.
A careful and comprehensive assessment may reveal a more appropriate medication approach. Best case, neurofeedback may correct underlying causal factors, resulting in general improvement and reduced need for further medication.
Recommended Medication Management Approaches
Discuss the scope and limitations of your professional license and your ability to address medication issues. Review your client's plans regarding medication.
Continue current prescriptions? If not, have they consulted with their prescribing physician?
Decrease or eliminate current medications? Your client's best choice is to discuss medication adjustment with the prescribing physician. If they wish to proceed without physician consultation, explain your limitations and ethical concerns about proceeding with neurofeedback training under these circumstances.
Medications often need to be adjusted due to the effects of training. The clinician or client must interact with the prescribing physician regarding these changes. As neurofeedback changes brain function and structure, medications may need to be titrated or withdrawn. Standardized psychological (Beck Depression Inventory) and performance (Computerized Continuous Performance Test) instruments may help inform the physician's decision.
Wherever possible, involve your client in this process since physicians may not welcome your involvement in medication decisions. Clients may find it easier to interact with the prescribing physician when given accurate information.
Drug Implications for Assessment and Neurofeedback
When interpreting your initial assessment battery and subsequent reassessment testing, take drug effects into account. Develop training goals based on initial testing with medication. Retest with the same medication unless withdrawn to ensure a valid comparison.
Develop a personalized neurofeedback training strategy that does not attempt to train against a drug's principal effects on the EEG.

Glossary
activation: the process by which a drug binds to a receptor and initiates a cellular response.
acute effects: immediate or short-term physiological or psychological effects of a drug following administration.
affinity: the strength of binding between a drug and its receptor, determining how well the drug will bind to its target.
agonst: a substance that promotes the action of a naturally occurring neurotransmitter.
alpha blocking: arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it entirely while increasing EEG power in the beta range.
alpha rhythm: 8-12-Hz and 8-13-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely-distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly.
alpha spindles: regular bursts of alpha activity.
amplitude: the energy or power contained within the EEG signal measured in microvolts or picowatts.
antagonist: a substance that interferes with the action of agonists or naturally occurring neurotransmitters.
anterior: near or toward the front of the head, for example, the anterior cingulate.
anterior cingulate: the division of the prefrontal cortex that plays a vital role in attention and is activated during working memory. It mediates emotional and physical pain and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components.
arousal: a process that combines alertness and wakefulness, produced by at least five neurotransmitters, including acetylcholine, histamine, hypocretin, norepinephrine, and serotonin.
asynchronous waves: neurons depolarize and hyperpolarize independently.
benzodiazepine receptor agonist (BZRA) hypnotics: nonbenzodiazepine BZRAs like zolpidem (Ambien) that are prescribed to treat insomnia.
beta rhythm: 12-36 Hz rhythm associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges.
bilateral synchronous slow waves: a pathological sign observed in drowsy children. When detected in alert adults, intermittent bursts of high amplitude slow waves may signify gray matter lesions in deep midline structures.
cerebral cortex: the layer of gray matter that covers the cerebral hemispheres. The cerebral cortex consists of gray matter and white matter.
chronic effects: long-term physiological or psychological effects that occur with prolonged use of a drug.
complex: a sequence of waves.
continuous irregular delta: slow waves produced by white matter lesions seen in disorders like multiple sclerosis.
delta rhythm: 0-4 Hz, 0.5-3.5 Hz, and 1-4 Hz oscillations generated by thalamocortical neurons during stage-3 sleep.
diphasic wave: a wave that contains both a negative and positive deflection from the baseline.
dominant frequency: the EEG frequency with the greatest amplitude.
downregulation: the decrease in receptor number or sensitivity due to prolonged exposure to an agonist, leading to reduced response.
drugs: chemical substances used to diagnose, treat, or prevent disease, or to relieve symptoms.
dual-action antidepressants: medications like duloxetine (Cymbalta) that activate 5-HT1 receptors to produce antidepressant and anxiolytic effects, while they blockade 5-HT2 (agitation, restlessness, and sexual dysfunction) and 5-HT3 (nausea, headache, and vomiting) receptors to minimize their side effects.
EEG activity: a single wave or successive waves.
EEG coherence: shared oscillatory activity (identical waveform morphology) between two sites expressed as the square of the correlation coefficient between their frequencies.
EEG power: signal energy in the EEG spectrum. Most EEG power falls within the 0-20 Hz frequency range. EEG power is measured in microvolts or picowatts.
electroencephalogram (EEG): the voltage difference between at least two electrodes, where at least one electrode is located on the scalp or inside the brain. The EEG is a recording of EPSPs and IPSPs that occur primarily in dendrites in pyramidal cells located in macrocolumns, several millimeters in diameter, in the upper cortical layers.
elimination half-life: the time required for the concentration of a drug in the bloodstream to decrease by half.
evoked potential: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). An evoked potential has a negative peak around 80-90 ms and a positive peak about 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
fast cortical potentials: EEG rhythms that range from 0.5 Hz-100 Hz. The main frequency ranges include delta, theta, alpha, sensorimotor rhythm, and beta.
first-generation antipsychotics (FGAs): FGAs like chlorpromazine (Thorazine) are prescribed to treat the positive symptoms of schizophrenia and exert their effects through D2 receptor blockade.
focal waves: EEG waves detected within a limited area of the scalp, cerebral cortex, or brain.
frequency: the number of cycles completed each second expressed in hertz (Hz).
frequency synchrony: when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
frontal lobes: the most anterior cortical lobes of the brain that are divided into the motor cortex, premotor cortex, and prefrontal cortex.
G-protein-coupled receptor (GPCR): a large family of receptors that activate intracellular signaling pathways via G-proteins upon ligand binding.
gamma rhythm: 25-75 Hz, 35-45 Hz, 38-42 Hz, 40 Hz oscillations that may speed information distribution and processing. Gamma bursts occur during problem-solving, and the absence of gamma is associated with cognitive deficits and learning disorders. Gamma is theorized as a "binding rhythm" that integrates sensory inputs into perception and consciousness.
generalized asynchronous slow waves: waves seen in sleepy children and those with elevated temperatures. This may indicate degenerative disease, dementia, encephalopathy, head injury, high fever, migraine, and Parkinson's disease in adults.
hertz (Hz): the unit of frequency, an abbreviation for cycles per second.
hypercoherence: abnormally-high functional connectivity between two sites.
intermediate-acting benzodiazepines: benzodiazepines with mean half-lives from 15-80 hours prescribed to manage anxiety.
ionotropic receptor: a receptor that forms an ion channel pore, allowing ions to pass through the membrane in response to ligand binding.
irregular waves: successive waves that constantly alter their shape and duration.
irreversible MAO inhibitors (MAOIs): MAOIs like selegeline (Emsam) that form permanent bonds with the MAO enzyme and are prescribed for major depressive disorder (MDD).
kappa rhythm: bursts of alpha or theta and is detected over the temporal lobes of subjects during cognitive activity.
lambda waves: saw-toothed transient waves from 20-50 μV in amplitude and 100-250 ms in duration detected over the occipital cortex during wakefulness. These positive deflections are time-locked to saccadic movements and observed during visual scanning, as during reading.
lateralized waves: waves that are primarily detected on one side of the scalp and may indicate pathology.
ligands: molecules that bind to specific sites on a target protein, such as receptors, to exert their biological effect.
local synchrony: synchrony that occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals.
localized slow waves: waves that may indicate a transient ischemic attack (TIA) or stroke, migraine, mild head injury, or tumors above the tentorium. Deep lesions result in bilateral or unilateral delta.
long-acting benzodiazepines: benzodiazepines like diazepam (Valium) with mean half-lives ranging from 10-80 hours prescribedto manage anxiety.
metabotropic receptor: a receptor that indirectly influences ion channels through signal transduction mechanisms, often involving G-proteins.
mixed opioid agonist-antagonists: drugs like pentazocine (Talwin) that are kappa agonists and weak mu antagonists that are prescribed for the management of pain.
monoamine oxidase (MAO): an enzyme that degrades and inactivates the monoamine neurotransmitters dopamine, norepinephrine, and serotonin.
monoamine oxidase inhibitors (MAOIs): antidepressant drugs that interfere with MAO's breakdown of monoamines and increase monoamine availability and are prescribed to manage major depressive disorder (MDD).
monophasic wave: either a single negative (upward) or positive (downward) deflection from baseline.
mu rhythm: arch-shaped waves that range from 7-11 Hz with amplitudes typically below 50 μV detected over Cz and Pz in waking subjects. These waves are seen in the healthy EEG records of 7% of the population. While these waves resemble alpha, they contain sharp positive transients and curved negative segments. Mu waves are blocked or reduced by exposure to a tactile stimulus, planning to move, readiness to move, or moving a contralateral limb (making a fist).
multiple spike-and-slow-wave complex: multiple spikes associated with at least one slow wave.
neurotransmitter (NT): a chemical messenger that transmits signals across a synapse from one neuron to another target neuron, muscle cell, or gland cell. Neurotransmitters are crucial for various bodily functions, including mood regulation, sleep, cognition, and muscle movement. They are released from synaptic vesicles in the presynaptic neuron into the synaptic cleft, where they bind to specific receptors on the postsynaptic cell, initiating a response.
partial opioid agonists: drugs like buprenorphine (Subutex) that produce less-than-maximal analgesia and are prescribed to manage pain.
peak alpha frequency: the highest-amplitude alpha frequency (8-12, 8-13 Hz) within an epoch.
pharmacodynamics: he study of the biochemical and physiological effects of drugs and their mechanisms of action in the body.
phase: the degree to which the peaks and valleys of EEG waveforms coincide.
phase synchrony: synchrony when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
polyphasic (multiphasic) wave: a wave that contains two or more deflections of opposite polarity from baseline.
polyspikes: a series of three or more consecutive spikes (≥ 10 Hz) that last a minimum of 300 milliseconds.
posterior: near or toward the back of the head.
psychoactive drugs: substances that affect the central nervous system, altering brain function and resulting in changes in perception, mood, consciousness, cognition, or behavior.
psychopharmacology: the study of the use of medications in treating mental disorders, focusing on the effects of psychoactive drugs on mood, sensation, thinking, and behavior.
pure opioid agonists: drugs like morphine that produce maximal analgesia and are prescribed for to manage pain.
regular or monomorphic waves: successive waves with identical shapes. Regular waves may resemble sine waves (sinusoidal) or maybe arched (resembling wickets) or saw-toothed (asymmetrical and triangular).
second messenger: intracellular signaling molecule released by the cell in response to receptor activation, which amplify and propagate the signal within the cell.
second-generation antipsychotics: SGAs like clozapine (Clozaril) are prescribed to treat the positive symptoms of schizophrenia and antagonize D2 receptors less effectively than D1 receptors and significantly less than 5-HT2 receptors.
selective norepinephrine reuptake inhibitors (SNRIs): drugs like atomoxetine (Strattera) that specifically interfere with norepinephrine reuptake for the management of major depressive disorder (MDD).
selective serotonin reuptake inhibitors (SSRIs): drugs like fluoxetine (Prozac) that specifically interfere with serotonin reuptake for the management of major depressive disorder (MDD).
sharp transients: sequences that contain several sharp waves.
sharp waves: waves that resemble spikes with a pointed peak with a longer 70-200-ms duration.
short-acting benzodiazepines: benzodiazepines like alprazolam (Xanax) with mean half-lives that range from 2.5-12 hours prescribed to manage anxiety.
specificity: the ability of a drug to bind to a particular receptor site or target, reducing off-target effects.
spike: a negative transient with a pointed peak at conventional paper speeds, 20-70-ms duration, and 40-100 μV amplitude.
spike-and-slow-wave complex: a spike followed by a higher amplitude slow wave at 3 Hz. In an absence seizure, the amplitudes are very high (e.g., 160 μV).
spindle waves: waves that originate in the thalamus and occur during unconsciousness and stage II sleep.
synchronous: adverb meaning that groups of neurons depolarize and hyperpolarize simultaneously.
synchrony: the coordinated firing of pools of neurons. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
theta rhythm: 4-7 Hz rhythm generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
third-generation antipsychotics (TGAs): drugs like aripiprazole (Abilify) that is a partial agonist at D2 and 5-HT1A receptors and an antagonist at 5-HT2 receptors prescribed for the management of the positive and negative symptoms of schizophrenia.
total power: the sum of the voltages in all EEG frequency bands.
transient: a single wave or sequence of regular waves, called a complex, distinguishable from background EEG activity.
triphasic waves (TWs): medium-to-high-amplitude sharp transients that often involve a negative-positive-negative sequence. TWs are distributed diffusely and symmetrically with frontal predominance.
upregulation: increase in receptor number or sensitivity due to prolonged exposure to an antagonist or low levels of agonist, leading to an enhanced response.
waveform: the shape and form of an EEG signal.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET PlUS
Click on the Quizlet Plus logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Functional Neuroanatomy, which reviews critical behavioral networks, and qEEG100, which provides extensive multiple-choice testing over the IQCB Blueprint.



Assignment
Now that you have completed this unit, explain how an SSRI like fluoxetine (Prozac) could affect the EEG? How would you adjust neurofeedback training for depression for clients using Prozac. Why shouldn't you train against a drug's effects on the EEG?
References
Arzy, S., Allali, G., Brunet, D., Michel, C. M., Kaplan, P. W., & Seeck, M. (2010). Antiepileptic drugs modify power of high EEG frequencies and their neural generators. European Journal of Neurology, 17(10), 1308–1312. https://doi.org/10.1111/j.1468-1331.2010.03018.x
Banoczi, W. R. (2005). How some drugs affect the electroencephalogram (EEG). Am J END Technol, 45, 118-129. PMID: 15989074
Aiyer, R., Novakovic, V., & Barkin, R. L. (2016). A systematic review on the impact of psychotropic drugs on electroencephalogram waveforms in psychiatry. Postgraduate Medicine, 128(7), 656–664. https://doi.org/10.1080/00325481.2016.1218261
Barry, R. J., Clarke, A. R., Hajos, M., McCarthy, R., Selikowitz, M., & Bruggemann, J. M. (2009). Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology, 57(7-8), 702–707. https://doi.org/10.1016/j.neuropharm.2009.08.003
Bauer, G., & Bauer, R. (2005). EEG drug effects and central nervous system poisoning. In E. Niedermeyer & F. Lopes da Silva (Eds.). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Blume, W. T. (2006). Drug effects on EEG. Journal of Clinical Neurophysiology, 23(4), 306-311. https://doi.org/10.1097/01.wnp.0000229137.94384.fa
Bos, D. J., Oranje, B., Veerhoek, E. S., Van Diepen, R. M., Weusten, J. M., Demmelmair, H., Koletzko, B., de Sain-van der Velden, M. G., Eilander, A., Hoeksma, M., & Durston, S. (2015). Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(10), 2298–2306. https://doi.org/10.1038/npp.2015.73
Bromm, B., Ganzel, R., Herrmann, W. M., Meier, W., & Scharein, E. (1986). Pentazocine and flupirtine effects on spontaneous and evoked EEG activity. Neuropsychobiology, 16(2-3), 152–156. https://doi.org/10.1159/000118317
Brunner, D. P., Dijk, D. J., Münch, M., & Borbély, A. A. (1991). Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology, 104(1), 1–5. https://doi.org/10.1007/BF02244546
Cajochen, C., Kräuchi, K., von Arx, M. A., Möri, D., Graw, P., & Wirz-Justice, A. (1996). Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neuroscience Letters, 207(3), 209–213. https://doi.org/10.1016/0304-3940(96)12517-9
Carley, D. W., & Farabi, S. S. (2016). Physiology of sleep. Diabetes Spectrum: A Publication of the American Diabetes Association, 29(1), 5–9. https://doi.org/10.2337/diaspect.29.1.5
Clarke, A. R., Barry, R. J., Bond, D., McCarthy, R., & Selikowitz, M. (2002). Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology, 164(3), 277–284. https://doi.org/10.1007/s00213-002-1205-0
Clemens, B., Ménes, A., Piros, P., Bessenyei, M., Altmann, A., Jerney, J., Kollár, K., Rosdy, B., Rózsavölgyi, M., Steinecker, K., & Hollódy, K. (2006). Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Research, 70(2-3), 190–199. https://doi.org/10.1016/j.eplepsyres.2006.05.003
Clemens, B., Piros, P., Bessenyei, M., Tóth, M., Hollódy, K., & Kondákor, I. (2008). Imaging the cortical effect of lamotrigine in patients with idiopathic generalized epilepsy: A low-resolution electromagnetic tomography (LORETA) study. Epilepsy Research, 81 (2-3), 204–210. https://doi.org/10.1016/j.eplepsyres.2008.06.002
Clemens, B., Puskás, S., Besenyei, M., Kovács, N. Z., Spisák, T., Kis, S. A., Emri, M., Hollódy, K., Fogarasi, A., Kondákor, I., & Fekete, I. (2014). Valproate treatment normalizes EEG functional connectivity in successfully treated idiopathic generalized epilepsy patients. Epilepsy Research, 108(10), 1896–1903. https://doi.org/10.1016/j.eplepsyres.2014.09.032
Cohen, H., Porjesz, B., & Begleiter, H. (1993). Ethanol-induced alterations in electroencephalographic activity in adult males. Neuropsychopharmacology, 8, 365-370. https://doi.org/10.1038/npp.1993.36
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Dias Alves, M., Micoulaud-Franchi, J. A., Simon, N., & Vion-Dury, J. (2018). Electroencephalogram modifications associated with atypical strict antipsychotic monotherapies. Journal of Clinical Psychopharmacology, 38(6), 555–562. https://doi.org/10.1097/JCP.0000000000000953
Dijk, D. J., & Cajochen, C. (1997). Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. Journal of Biological Rhythms, 12(6), 627–635. https://doi.org/10.1177/074873049701200618
Dimpfel, W., Schober, F., & Spüler, M. (1993). The influence of caffeine on human EEG under resting condition and during mental loads. The Clinical Investigator, 71, 197-207. https://doi.org/10.1007/BF00180102
Drapeau, C., Hamel-Hébert, I., Robillard, R., Selmaoui, B., Filipini, D., & Carrier, J. (2006). Challenging sleep in aging: The effects of 200 mg of caffeine during the evening in young and middle‐aged moderate caffeine consumers. Journal of Sleep Research, 15. https://doi.org/10.1111/j.1365-2869.2006.00518.x
Eschmann, G., Irrgang, V., & Rüther, E. (1983). Effects of beta-receptor blockers in pharmacology EEG. Neuropsychobiology, 10(2-3), 190–192. https://doi.org/10.1159/000118008
Flachenecker P. (2013). A new multiple sclerosis spasticity treatment option: Effect in everyday clinical practice and cost-effectiveness in Germany. Expert Review of Neurotherapeutics, 13(3 Suppl 1), 15–19. https://doi.org/10.1586/ern.13.1
Fontani, G., Corradeschi, F., Felici, A., Alfatti, F., Migliorini, S., & Lodi, L. (2005). Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. European Journal of Clinical Investigation, 35(11), 691–699. https://doi.org/10.1111/j.1365-2362.2005.01570.x
Foxe, J., Morie, K., Laud, P., Rowson, M., Bruin, E., & Kelly, S. (2012). Assessing the effects of caffeine and theanine on the maintenance of vigilance during a sustained attention task. Neuropharmacology, 62, 2320-2327. https://doi.org/10.1016/j.neuropharm.2012.01.020
Galderisi, S., Mucci, A., Volpe, U., & Boutros, N. (2009). Evidence-based medicine and electrophysiology in schizophrenia. Clinical EEG and Neuroscience, 40(2), 62–77. https://doi.org/10.1177/155005940904000206
García, P., Kreuzer, M., Hight, D., & Sleigh, J. (2021). Effects of noxious stimulation on the electroencephalogram during general anaesthesia: A narrative review and approach to analgesic titration. British Journal of Anaesthesia, 126(2), 445-457 . https://doi.org/10.1016/j.bja.2020.10.036
Goldstein, L., Murphree, H. B., & Pfeiffer, C. C. (1968). Comparative study of EEG effects of antihistamines in normal volunteers. The Journal of Clinical Pharmacology and the Journal of New Drugs, 8(1), 42–53. https://doi.org/10.1002/j.1552-4604.1968.tb00091.x
Graversen, C., Malver, L., Kurita, G., Staahl, C., Christrup, L., Sjøgren, P., & Drewes, A. (2015). Altered frequency distribution in the electroencephalogram is correlated to the analgesic effect of Remifentanil. Basic & Clinical Pharmacology & Toxicology, 116. https://doi.org/10.1111/bcpt.12330
Guo, J., Wang, D., Ren, M., Xiong, B., Li, Z., Wang, X., & Zeng, K. (2014). QEEG analysis of the effects of sodium valproate on adult Chinese patients with generalized tonic-clonic seizures. Metabolic Brain Disease, 29(3), 801–807. https://doi.org/10.1007/s11011-014-9561-0
Han, G., Matsumoto, S., Diaz, J., Greene, R. W., & Vogt, K. E. (2022). Dihydropyridine calcium blockers do not interfere with non-rapid eye movement sleep. Frontiers in Neuroscience, 16, 969712. https://doi.org/10.3389/fnins.2022.969712
Hasan, M., Pulman, J., & Marson, A. G. (2013). Calcium antagonists as an add-on therapy for drug-resistant epilepsy. The Cochrane Database of Systematic Reviews, 2013(3), CD002750. https://doi.org/10.1002/14651858.CD002750.pub2
Heninger G. R. (1978). Lithium carbonate and brain function. I. Cerebral-evoked potentials, EEG, and symptom changes during lithium carbonate treatment. Archives of General Psychiatry, 35(2), 228–233. https://doi.org/10.1001/archpsyc.1978.01770260106013
Herning, R. I., Glover, B. J., Koeppl, B., Phillips, R. L., & London, E. D. (1994). Cocaine-induced increases in EEG alpha and beta activity: Evidence for reduced cortical processing. Neuropsychopharmacology, 11(1), 1-9. https://doi.org/10.1038/npp.1994.30
Herning, R. I., Jones, R. T., Hooker, W. D., Mendelson, J., & Blackwell, L. (1985). Cocaine increases EEG beta: A replication and extension of Hans Berger's historic experiments. Clinical Neurophysiology, 60(6), 470-477. https://psycnet.apa.org/doi/10.1016/0013-4694(85)91106-X
Hotz, M., Ritz, R., Linder, L., Scollo-Lavizzari, G., & Haefeli, W. (2000). Auditory and electroencephalographic effects of midazolam and alpha-hydroxy-midazolam in healthy subjects. British Journal of Clinical Pharmacology, 49(1), 72-9. https://doi.org/10.1046/J.1365-2125.2000.00104.X.
Hughes, A., Lynch, P., Rhodes, J., Ervine, C., & Yates, R. (2001). Electroencephalographic and psychomotor effects of chlorpromazine and risperidone relative to placebo in normal healthy volunteers. British Journal of Clinical Pharmacology, 48(3), 323-30. https://doi.org/10.1046/J.1365-2125.1999.00021.X.
Hyun, J., Baik, M. J., & Kang, U. G. (2011). Effects of psychotropic drugs on quantitative EEG among patients with schizophrenia-spectrum disorders. Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology, 9(2), 78–85. https://doi.org/10.9758/cpn.2011.9.2.78
Joutsiniemi, S., Gross, A., & Appelberg, B. (2001). Marked Clozapine-induced slowing of EEG background over frontal, central, and parietal scalp areas in schizophrenic patients. Journal of Clinical Neurophysiology, 18, 9-13. https://doi.org/10.1097/00004691-200101000-00003
Julien, R. M., Advokat, C. D., & Comaty, J. E. (2023). Julien's primer of drug action: A comprehensive guide to the actions, uses, and side effects of psychoactive drugs (15th ed.). Worth Publishers.
Khajehpour, H., Mohagheghian, F., Ekhtiari, H., Makkiabadi, B., Jafari, A. H., Eqlimi, E., & Harirchian, M. H. (2019). Computer-aided classifying and characterizing of methamphetamine use disorder using resting-state EEG. Cognitive Neurodynamics, 13(6), 519–530. https://doi.org/10.1007/s11571-019-09550-z
Knott, V. J. (200). Quantitative EEG methods and measures in human psychopharmacological research. Human Psychopharmacology, 15, 479-498. https://doi.org/10.1002/1099-1077(200010)15:7<479::AID-HUP206>3.0.CO;2-5
Knott, V., Labelle, A., Jones, B., & Mahoney, C. (2001). Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophrenia Research, 50(1-2), 41–53. https://doi.org/10.1016/s0920-9964(00)00165-1
Knott, V., Mahoney, C., Kennedy, S., & Evans, K. (2000). Pre-treatment EEG and it's relationship to depression severity and paroxetine treatment outcome. Pharmacopsychiatry, 33(6), 201–205. https://doi.org/10.1055/s-2000-8356
Landolt, H., Rétey, J., Tönz, K., Gottselig, J., Khatami, R., Buckelmüller, I., & Achermann, P. (2004). Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in hHumans. Neuropsychopharmacology, 29, 1933-1939. https://doi.org/10.1038/sj.npp.1300526
Laurian, S., Le, P., Baumann, P., Perey, M., Gaillard, J., & Müller, P. (1981). Relationship between plasma-levels of Chlorpromazine and effects on EEG and evoked potentials in healthy volunteers. Pharmacopsychiatry, 14, 199 - 204. https://doi.org/10.1055/s-2007-1019598
Lindgren, M., Molander, L., Verbaan, C., Lunell, E., & Rosén, I. (1999). Electroencephalographic effects of intravenous nicotine – A dose-response study. Psychopharmacology, 145, 342-350. https://doi.org/10.1007/s002130051067
MacCrimmon, D., Brunet, D., Criollo, M., Galin, H., & Lawson, J. S. (2012). Clozapine augments delta, theta, and right frontal EEG alpha power in schizophrenic patients. ISRN Psychiatry, 2012, 596486. https://doi.org/10.5402/2012/596486
Malver, L., Brokjær, A., Staahl, C., Graversen, C., Andresen, T., & Drewes, A. (2014). Electroencephalography and analgesics. British Journal of Clinical Pharmacology, 77. https://doi.org/10.1111/bcp.12137
Marciani, M. G., Gigli, G. L., Maschio, M. C., Stefani, N., Stefanini, F., & Bernardi, G. (1992). EEG changes induced by carbamazepine therapy at rest and during mental processes. Italian Journal of Neurological Sciences, 13(9), 729–733. https://doi.org/10.1007/BF02229157
Matousek M. (1987). EEG assessment of the sedative and excitatory properties of CNS-active compounds in the patients with depression. Neuropsychobiology, 17(1-2), 118–120. https://doi.org/10.1159/000118348
McClelland, G., Cooper, S., & Pilgrim, A. (1990). A comparison of the central nervous system effects of haloperidol, chlorpromazine and sulpiride in normal volunteers. British Journal of Clinical Pharmacology, 30(6), 795-803. https://doi.org/10.1111/J.1365-2125.1990.TB05444.X
Mecarelli, O., Piacenti, A., Pulitano, P., Vicenzini, E., Rizzo, C., Rinalduzzi, S., de Feo, M. R., & Accornero, N. (2001). Clinical and electroencephalographic effects of topiramate in patients with epilepsy and healthy volunteers. Clinical Neuropharmacology, 24(5), 284–289. https://doi.org/10.1097/00002826-200109000-00005
Mecarelli, O., Vicenzini, E., Pulitano, P., Vanacore, N., Romolo, F. S., Di Piero, V., Lenzi, G. L., & Accornero, N. (2004). Clinical, cognitive, and neurophysiologic correlates of short-term treatment with carbamazepine, oxcarbazepine, and levetiracetam in healthy volunteers. The Annals of Pharmacotherapy, 38(11), 1816–1822. https://doi.org/10.1345/aph.1E136
Merlotti, E., Mucci, A., Bucci, P., Volpe, U., Montefusco, V., Galderisi, S., & Maj, M. (2007). Topographic and tomographic EEG changes after a single oral dose of antipsychotic drugs in healthy young subjects. European Psychiatry, 22, S160 - S161. https://doi.org/10.1016/j.eurpsy.2007.01.521
Montgomery, P., Burton, J. R., Sewell, R. P., Spreckelsen, T. F., & Richardson, A. J. (2013). Low blood long chain omega-3 fatty acids in UK children are associated with poor cognitive performance and behavior: a cross-sectional analysis from the DOLAB study. PLoS ONE, 8(6), e66697. https://doi.org/10.1371/journal.pone.0066697
Muñoz-Torres, Z., Río-Portilla, Y., & Corsi-Cabrera, M. (2011). Diazepam-induced changes in EEG oscillations during performance of a sustained attention task. Journal of Clinical Neurophysiology, 28, 394–399. https://doi.org/10.1097/WNP.0b013e318227323a
Neufeld, M., Kogan, E., Chistik, V., & Korczyn, A. (1999). Comparison of the effects of vigabatrin, lamotrigine, and topiramate on quantitative EEGs in patients with epilepsy. Clinical Neuropharmacology, 22(2), 80-6 . https://doi.org/10.1097/00002826-199903000-00003
Newton, T. F., Cook, I. A., Kalechstein, A. D., Duran, S., Monroy, F., Ling, W., & Leuchter, A. F. (2003). Quantitative EEG abnormalities in recently abstinent methamphetamine dependent individuals. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 114(3), 410–415. https://doi.org/10.1016/s1388-2457(02)00409-1
Noldy, N., Santos, C., Politzer, N., Blair, R., & Carlen, P. (1994). Quantitative EEG changes in cocaine withdrawal: Evidence for long-term CNS effects. Neuropsychobiology, 30(4), 189-196. https://doi.org/10.1159/000119160
Ott, G. E., Rao, U., Lin, K. M., Gertsik, L., & Poland, R. E. (2004). Effect of treatment with bupropion on EEG sleep: Relationship to antidepressant response. The International Journal of Neuropsychopharmacology, 7(3), 275–281. https://doi.org/10.1017/S1461145704004298
Paes, F., Machado, S., Arias-Carrión, O., Domingues, C. A., Teixeira, S., Velasques, B., Cunha, M., Minc, D., Basile, L. F., Budde, H., Cagy, M., Piedade, R., Kerick, S., Menéndez-González, M., Skaper, S. D., Norwood, B. A., Ribeiro, P., & Nardi, A. E. (2011). Effects of Methylphenidate on performance of a practical pistol shooting task: a quantitative electroencephalography (qEEG) study. International Archives of Medicine, 4(1), 6. https://doi.org/10.1186/1755-7682-4-6
Patat, A., Rosenzweig, P., Enslen, M., Trocherie, S., Miget, N., Bozón, M., Allain, H., & Gandon, J. (2000). Effects of a new slow release formulation of caffeine on EEG, psychomotor and cognitive functions in sleep‐deprived subjects. Human Psychopharmacology: Clinical and Experimental, 15. 3.0.CO;2-C" target="_blank">https://doi.org/10.1002/(SICI)1099-1077(200004)15:3<153::AID-HUP154>3.0.CO;2-C
Patrick, R. P., & Ames, B. N. (2015). Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 29(6), 2207–2222. https://doi.org/10.1096/fj.14-268342
Pickworth, W., Herning, R., & Henningfield, J. (1986). Electroencephalographic effects of nicotine chewing gum in humans. Pharmacology Biochemistry and Behavior, 25, 879-882. https://doi.org/10.1016/0091-3057(86)90401-6
Pollock, V., Teasdale, T., Stern, J., & Volavka, J. (1981). Effects of caffeine on resting EEG and response to sine wave modulated light. Electroencephalography and Clinical Neurophysiology, 51(5), 470-6. https://doi.org/10.1016/0013-4694(81)90223-6
Prashad, S., Dedrick, E., & Filbey, F. (2018). Cannabis users exhibit increased cortical activation during resting state compared to non-users. NeuroImage, 179, 176-186. https://doi.org/10.1016/j.neuroimage.2018.06.031
Ranzi, P., Thiel, C., & Herrmann, C. (2016). EEG source reconstruction in male nonsmokers after nicotine administration during the resting state. Neuropsychobiology, 73, 191 - 200. https://doi.org/10.1159/000445481
Rubinson, M., Horowitz, I., Naim-Feil, J., Gothelf, D., Levit-Binnun, N., & Moses, E. (2019). Effects of methylphenidate on the ERP amplitude in youth with ADHD: A double-blind placebo-controlled cross-over EEG study. PLoS ONE, 14. https://doi.org/10.1371/journal.pone.0217383
Saletu, B., Anderer, P., & Saletu-Zyhlarz, G. M. (2006). EEG topography Neuroscience, 37(2), 66–80. https://doi.org/10.1177/155005940603700205
Saletu, B., Anderer, P., Saletuzyhlarz, G. M. (2010). EEG mapping and tomography in drug evaluation. Medicographia, 32, 190-200. https://doi.org/10.1177/155005940603700205
Saletu, B., Grünberger, J., & Linzmayer, L. (1986). Evaluation of encephalotropic and psychotropic properties of gabapentin in man by pharmaco-EEG and psychometry. International Journal of Clinical Pharmacology, Therapy, and Toxicology, 24(7), 362–373.
Siepmann, M., & Kirch, W. (2002). Effects of caffeine on topographic quantitative EEG. Neuropsychobiology, 45, 161 - 166. https://doi.org/10.1159/000054958
Simeon, J. G., Ferguson, H. B., & Van Wyck Fleet, J. (1986). Bupropion effects in attention deficit and conduct disorders. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 31(6), 581–585. https://doi.org/10.1177/070674378603100617
Skosnik, P., Krishnan, G., D’Souza, D., Hetrick, W., & O’Donnell, B. (2014). Disrupted gamma-band neural oscillations during coherent motion perception in heavy cannabis users. Neuropsychopharmacology, 39, 3087-3099. https://doi.org/10.1038/npp.2014.166
Stahl, S. M. (2017). Stahl's essential psychopharmacology prescriber's guide (6th ed.). Cambridge University Press.
Swatzyna, R. J., Morrow, L. M., Collins, D. M., Barr, E. A., Roark, A. J., & Turner, R. P. (2024). Evidentiary significance of routine EEG in refractory cases: A paradigm shift in psychiatry. Clinical EEG and neuroscience, 15500594231221313. Advance online publication. https://doi.org/10.1177/15500594231221313
Tan, X., Uchida, S., Matsuura, M., Nishihara, K., & Kojima, T. (2003). Long‐, intermediate‐ and short‐acting benzodiazepine effects on human sleep EEG spectra. Psychiatry and Clinical Neurosciences, 57. https://doi.org/10.1046/j.1440-1819.2003.01085.x
Tashkin D. P. (2013). Effects of marijuana smoking on the lung. Annals of the American Thoracic Society, 10(3), 239–247. https://doi.org/10.1513/AnnalsATS.201212-127FR
Thanacoody, H. K., & Thomas, S. H. (2005). Tricyclic antidepressant poisoning: Cardiovascular toxicity. Toxicological Reviews, 24(3), 205–214. https://doi.org/10.2165/00139709-200524030-00013
Thomaides, T., Tagaris, G., & Karageorgiou, C. (1996). EEG and topographic frequency analysis in migraine attack before and after sumatriptan infusion. Headache, 36(2), 111–114. https://doi.org/10.1046/j.1526-4610.1996.3602111.x
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Van Cott, A., & Brenner, R. P. (2003). Drug effects and toxic encephalopathies. In J. S. Ebersole & T. A. Pedley (Eds.). Current practice of clinical electroencephalography (3rd ed.). Lippincott Williams & Wilkins.
van der Post, J., Schram, M. T., Schoemaker, R. C., Pieters, M. S., Fuseau, E., Pereira, A., Baggen, S., Cohen, A. F., & van Gerven, J. M. (2002). CNS effects of sumatriptan and rizatriptan in healthy female volunteers. Cephalalgia: An International Journal of Headache, 22(4), 271–281. https://doi.org/10.1046/j.1468-2982.2002.00344.x
Volavka, J., Dornbush, R., Feldstein, S., Clare, G., Zaks, A., Fink, M., & Freedman, A. (1971). Marijuana, EEG, and behavior.Annals of the New York Academy of Sciences, 191. https://doi.org/10.1111/j.1749-6632.1971.tb13999.x
Wang, W. W., Li, J. C., & Wu, X. (2003). Quantitative EEG effects of topiramate. Clinical EEG (Electroencephalography), 34(2), 87–92. https://doi.org/10.1177/155005940303400208
Wu, X., & Xiao, C. H. (1996). Quantitative pharmaco-EEG of carbamazepine in volunteers and epileptics. Clinical EEG (Electroencephalography), 27(1), 40–45. https://doi.org/10.1177/155005949602700107
Yoshimura, M., Koenig, T., Irisawa, S., Isotani, T., Yamada, K., Kikuchi, M., Okugawa, G., Yagyu, T., Kinoshita, T., Strik, W., & Dierks, T. (2007). A pharmaco-EEG study on antipsychotic drugs in healthy volunteers. Psychopharmacology, 191, 995-1004. https://doi.org/10.1007/s00213-007-0737-8
Zajicek, J., Fox, P., Sanders, H., Wright, D., Vickery, J., Nunn, A., Thompson, A., & UK MS Research Group (2003). Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet, 362(9395), 1517–1526. https://doi.org/10.1016/S0140-6736(03)14738-1
Zhu, J., Coppens, R. P., Rabinovich, N. E., & Gilbert, D. G. (2017). Effects of bupropion sustained release on task-related EEG alpha activity in smokers: Individual differences in drug response. Experimental and Clinical Psychopharmacology, 25(1), 41–49. https://doi.org/10.1037/pha0000109