Cardiovascular Anatomy
What You Will Learn
Your cardiovascular system is an engineering marvel that pumps 1,500 to 2,000 gallons of blood every single day. In this chapter, you will explore the anatomy and physiology of the blood vessels and the heart, and discover why these structures matter for biofeedback practice. You will learn how arteries, veins, capillaries, and arteriovenous anastomoses work together to regulate blood flow and temperature. You will examine the heart's conduction system, understand the ECG waveform, and see why heart rate variability reflects the dynamic interplay between sympathetic and parasympathetic control. Along the way, you will encounter clinical applications that connect cardiovascular anatomy to the modalities you will train, including temperature, blood volume pulse, and HRV biofeedback.
This section introduces the cardiovascular system and explains why it sits at the center of so many biofeedback interventions. You will see how clinicians use cardiovascular modalities to treat a wide range of disorders and enhance performance, and you will learn why the heart-brain connection has transformed how we think about autonomic regulation.
Biofeedback interventions like heart rate variability biofeedback (HRVB)—a training method that teaches clients to increase the healthy beat-to-beat variation in their heart rate—target the cardiovascular system to treat disorders as diverse as anxiety, depression, essential hypertension, migraine, post-traumatic stress disorder, Raynaud's disorder, and stress. In the clinic or performance lab, practitioners monitor blood pressure (BP), heart rate (HR), heart rate variability (HRV), pulse wave velocity, temperature, and blood volume pulse to track cardiovascular function in real time. Whether you work in a VA medical center, a rehabilitation hospital, or an elite training facility, these modalities give you a direct window into how your client's autonomic nervous system is performing.
A paradigm shift is reshaping how clinicians approach disorders like depression and heart failure. Rather than relying solely on pharmacological interventions, practitioners increasingly teach clients to enhance HRV through exercises that strengthen parasympathetic nervous system (PNS) tone. PNS activity is also called vagal tone because the vagus nerve is the primary component of this autonomic branch (Breit et al., 2018). For practitioners working with veterans dealing with PTSD or athletes seeking peak mental performance, strengthening vagal tone is a powerful, non-invasive strategy for improving self-regulation.
The brain receives more afferent (body-to-brain) projections from the heart than from any other organ. Emerging evidence suggests that the heart's intrinsic nervous system has extensive bidirectional connections with the brain, meaning the heart does not simply follow orders—it sends information back that shapes how we think and feel (MacKinnon et al., 2013; Shaffer et al., 2014). Researchers increasingly recognize HRV as an index of vulnerability to stressors and disease, making it a critical measure across clinical and performance settings.
The PNS and Baroreceptor System Produce Brief Resting HRV Without a Sympathetic Contribution
One of the most clinically actionable insights in cardiovascular biofeedback is that patients can learn to increase the healthy variability of their hearts. This capacity makes HRV biofeedback effective for disorders like anxiety, asthma, depression, hypertension, and irritable bowel syndrome. At its core, HRVB training helps patients restore a healthy dynamic balance between the sympathetic and parasympathetic branches of the autonomic nervous system.
Temperature is one of the most widely trained biofeedback modalities. While still incomplete, our understanding of the mechanisms underlying hand-warming and hand-cooling has radically changed due to landmark studies by Robert Freedman. These findings underscore the complexity of the cardiovascular system and have direct implications for how you design temperature training protocols for clients with Raynaud's disease, anxiety, or stress-related conditions.
Response Coupling and Fractionation
When monitoring clients, you will observe two important patterns that affect how you interpret multimodal recordings. In response coupling, physiological measures change together—for example, heart rate and blood pressure both rise during a stress response. In response fractionation, measures change independently, so heart rate might decrease while blood pressure increases. These patterns reflect the multiple, independent control processes that jointly produce cardiovascular measures.
Healthy physiological systems operate nonlinearly—that is, unpredictably—which enables them to adapt to rapidly changing demands. Whether responses couple or fractionate during a particular recording depends on a complicated interplay of client characteristics, task demands, and environmental variables. This is why relying on a single modality can be misleading: the best clinical practice is to monitor several cardiovascular signals simultaneously for a more complete picture of autonomic function.
🎧 Mini-Lecture: Cardiovascular Anatomy Overview
BCIA Blueprint Coverage
I. HRV Anatomy and Physiology: A. Cardiac Anatomy and Physiology.
Professionals completing this unit will be able to discuss how the ECG is generated, sympathetic and parasympathetic influences, and heart-brain interactions.
This unit covers arteries, three measures of peripheral blood flow, veins, capillaries, arteriovenous anastomoses (AVAs), blood pressure, the heart, and heart rate variability.
🎧 Chapter Lecture: Cardiovascular Anatomy and Physiology
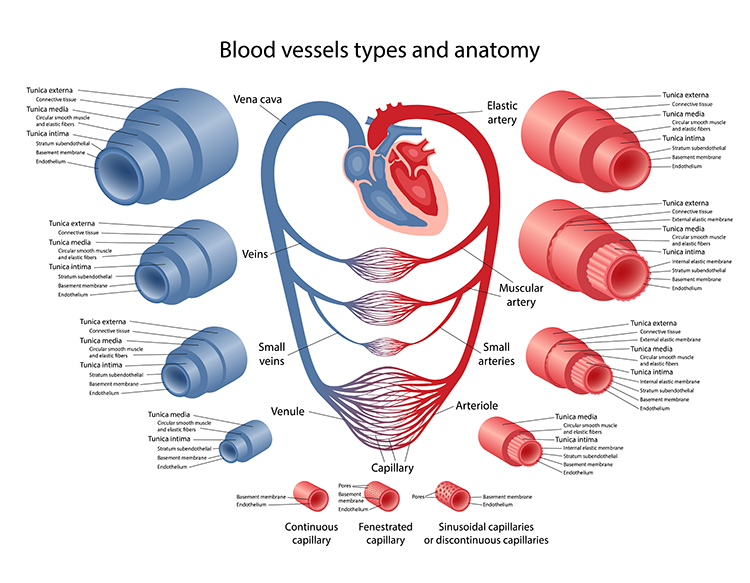
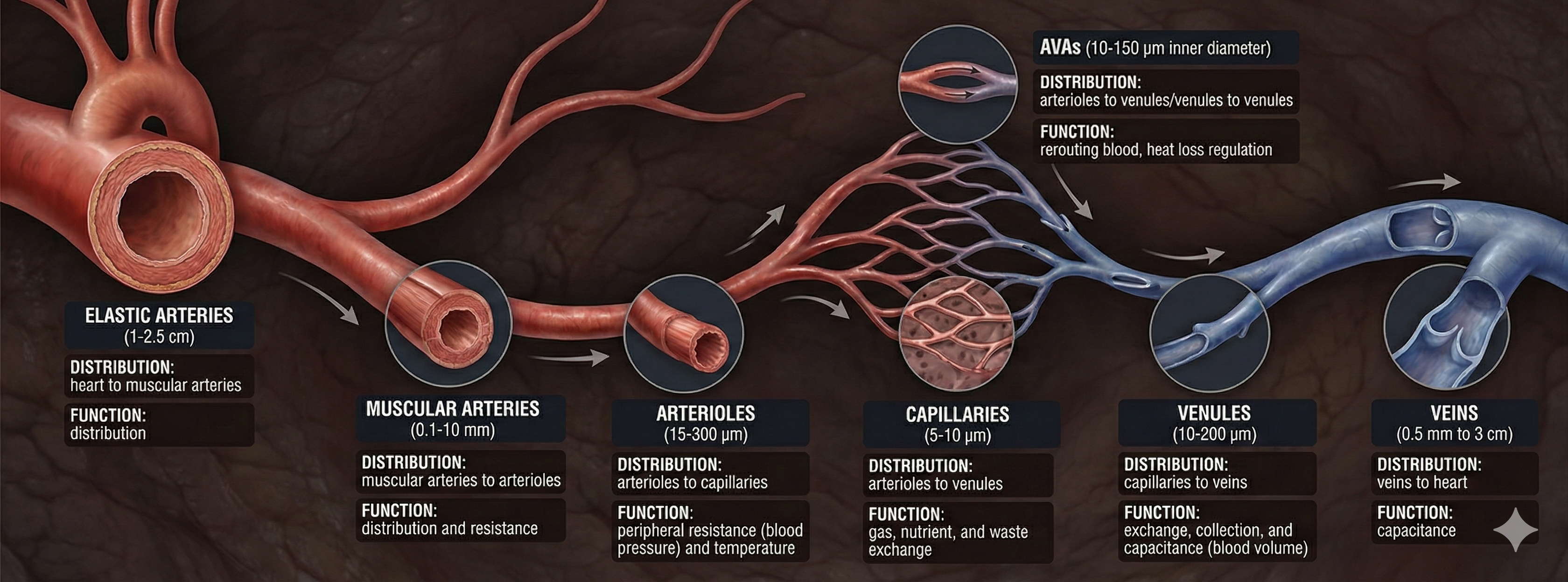
Arteries
This section covers the structure and function of arteries—the vessels that carry blood away from the heart. You will learn how different types of arteries contribute to blood flow regulation, why arterioles are central to peripheral resistance, and how the three arterial layers relate directly to temperature biofeedback training.
Arteries are blood vessels that carry blood away from the heart. They are divided into three main categories based on size and function: elastic arteries, muscular arteries, and arterioles. Understanding this hierarchy matters for biofeedback practice because the smallest arteries—arterioles—are the primary targets of the temperature and blood volume pulse modalities you will use with clients.
Check out the Khan Academy YouTube video Arteries vs. Veins - What's the Difference?
Types of Arteries
Elastic arteries are large vessels like the aorta that distribute blood from the heart to muscular arteries. Medium-sized muscular arteries, such as the brachial artery, circulate blood throughout the body. Arterioles—almost microscopic at just 8-50 microns in diameter—deliver blood to capillaries and anastomoses. These tiny vessels are clinically significant because they are responsible for roughly 50% of peripheral resistance (the opposition to blood flow in the vascular system) through their narrow diameter, contractility, and massive surface area.
The video below shows red blood cells traveling through a pulsating arteriole, illustrating how these tiny vessels actively regulate blood flow with each contraction cycle.
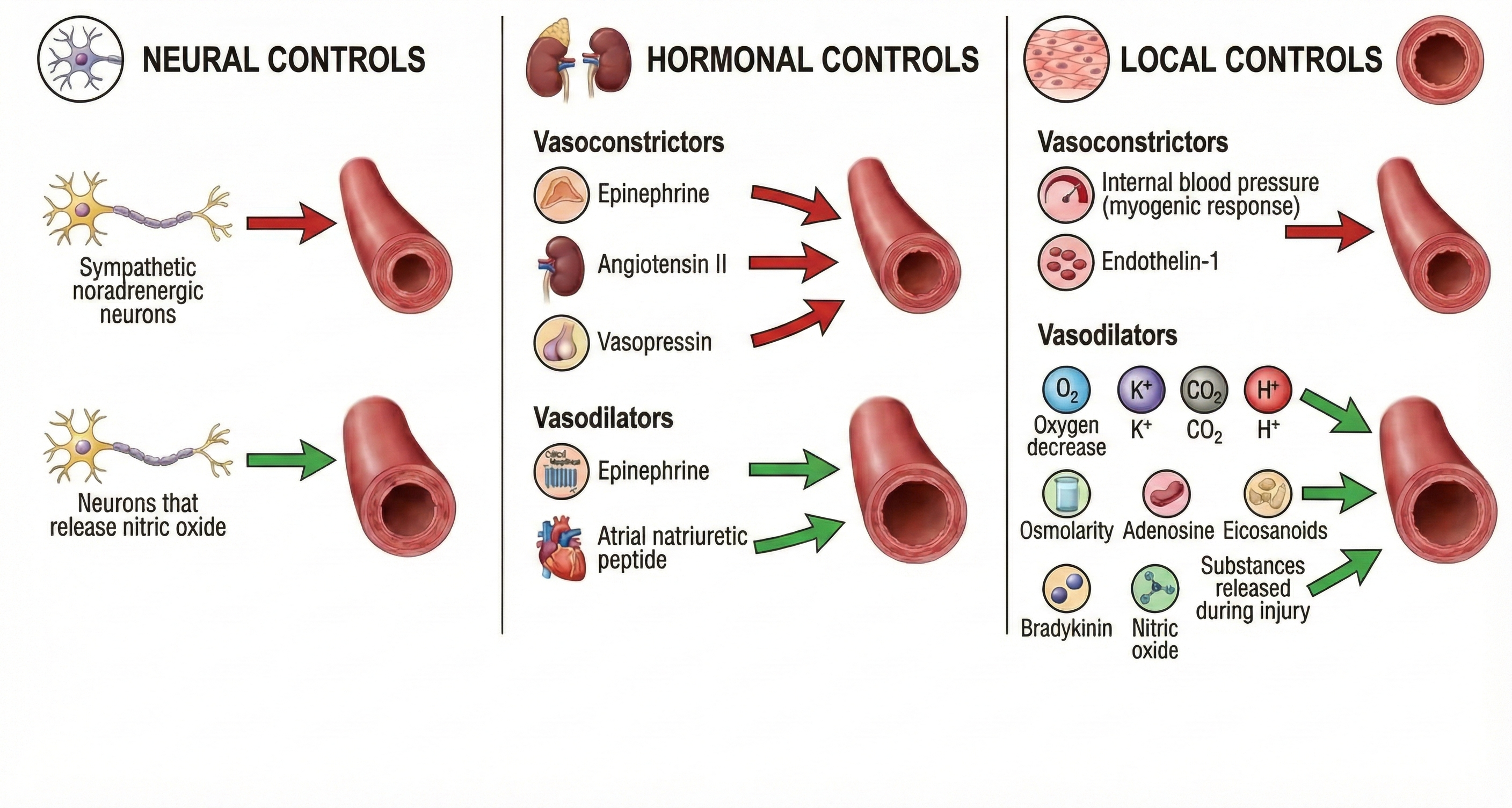
Arteriole Diameter and Its Control
The control of arteriole diameter is crucial for regulating both blood pressure and hand temperature—two measures you will encounter frequently in clinical practice. Neural, hormonal, and local controls cooperate to regulate blood flow through arterioles, and these control mechanisms play varying roles across different organs. For the biofeedback practitioner, this means that the mechanisms governing finger temperature differ from those controlling blood flow in the forearm or gut.
🎧 Mini-Lecture: Factors That Control Arteriole Diameter
The Three Arterial Layers (Tunics)
All arteries have three layers, or tunics, surrounding a hollow lumen (the open center through which blood flows). The tunica interna (innermost layer) responds to the hormones epinephrine (E) and norepinephrine (NE) with vasodilation—an increase in lumen diameter that allows more blood flow—in digits like the fingers. The tunica media (middle layer) is composed of smooth muscle and elastic fibers controlled by sympathetic constrictor fibers (C-fibers), making it the site of neurally-controlled vasoconstriction—a decrease in lumen diameter that restricts blood flow—in the digits. The tunica externa, or external layer, comprises a connective tissue sheath that provides structural support.
This three-layer architecture has a direct clinical implication that changes how you think about temperature biofeedback: hand-warming and hand-cooling are produced by separate mechanisms operating at different arterial layers. Hand-warming involves the release of a beta-adrenergic hormone and nitric oxide at the tunica interna. Hand-cooling, by contrast, is mediated by vasoconstrictor hormones and the firing of sympathetic C-fibers at the tunica media. This distinction explains why a client might successfully warm their hands using one strategy but need a completely different approach to prevent cooling.
Three Measures of Peripheral Blood Flow
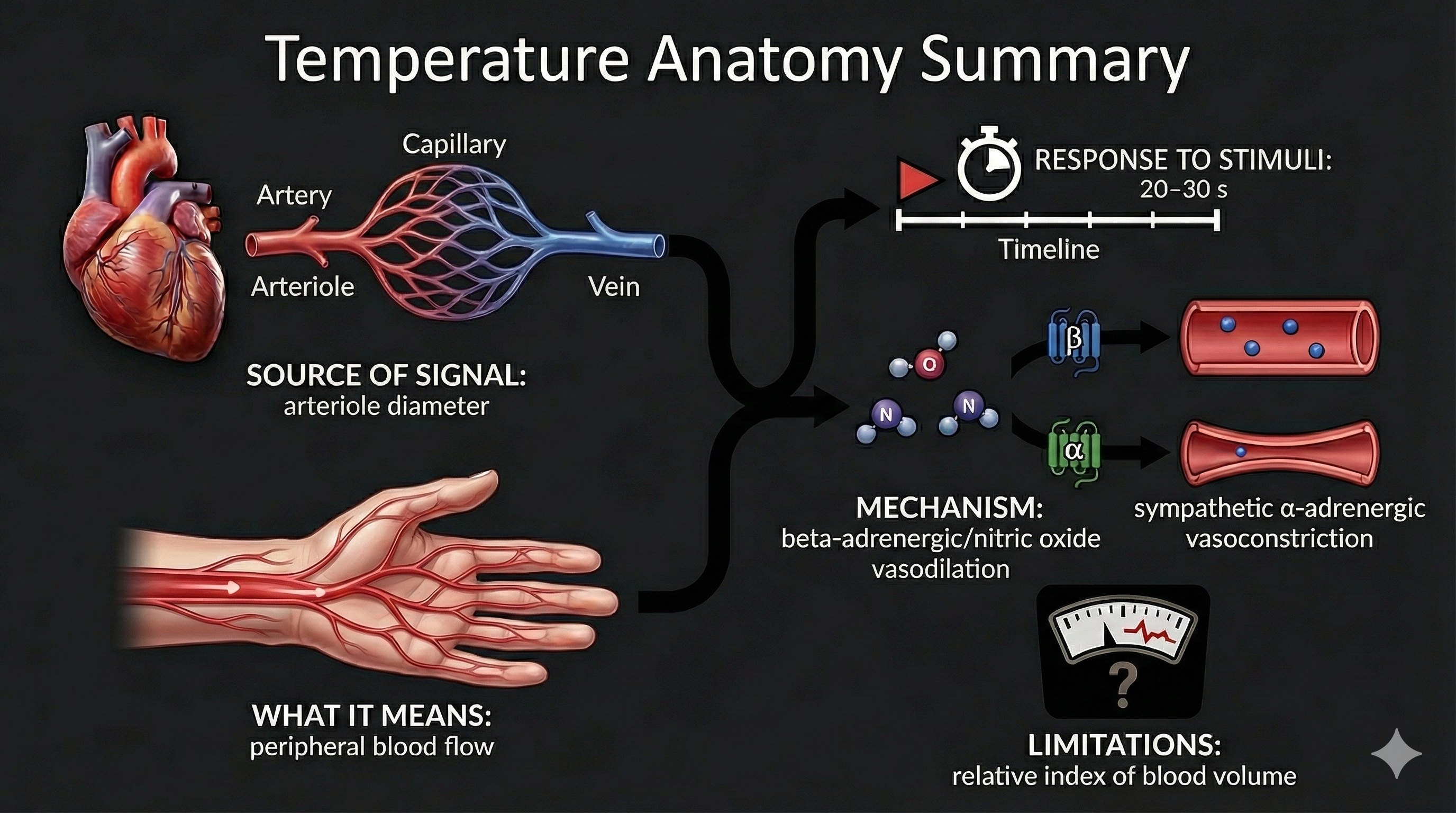
This section compares three biofeedback modalities that measure blood movement through the peripheral arteries: temperature, blood volume pulse (BVP), and pulse wave velocity (PWV). You will learn the clinical strengths of each, their response times, and when to choose one over another in practice.
Temperature, blood volume pulse, and pulse wave velocity all depend on blood movement through arteries, but they capture different aspects of that movement. Temperature and blood volume pulse provide relative measures of peripheral blood flow, and understanding the strengths and limitations of each will help you select the right modality for each client.
Temperature Responds to Stimuli in 20-30 Seconds, While Blood Volume Pulse Reacts in a Blazingly Fast 0.5-2 Seconds
Temperature
Skin temperature indirectly indexes peripheral blood flow, which is primarily regulated by cutaneous (skin-level) arterioles (Peek, 2016). Temperature is a gradual, tonic (sustained, slow-changing) index of blood flow. Following a stressor, it may take 20-30 seconds for a temperature drop to register because arterioles must first constrict, tissue perfusion with blood must decline, and the sensor (a thermistor—a temperature-sensitive resistor placed on the skin) must detect the change.
🎧 Mini-Lecture: Hand Temperature
Exposure to Cold, Overbreathing, Trying Too Hard, Stressors, and Worrying Can Trigger Hand-Cooling
"Temperature is the modality most vulnerable to effort" (Khazan, 2019, p. 90). When clients try too hard to warm their hands, the effort itself can trigger sympathetic activation and produce the opposite result. This vulnerability also applies to BVP since both modalities monitor the same underlying arteriolar physiology. Overbreathing contributes to hand-cooling because CO2 loss reduces nitric oxide release, which is needed to relax arteriole walls.
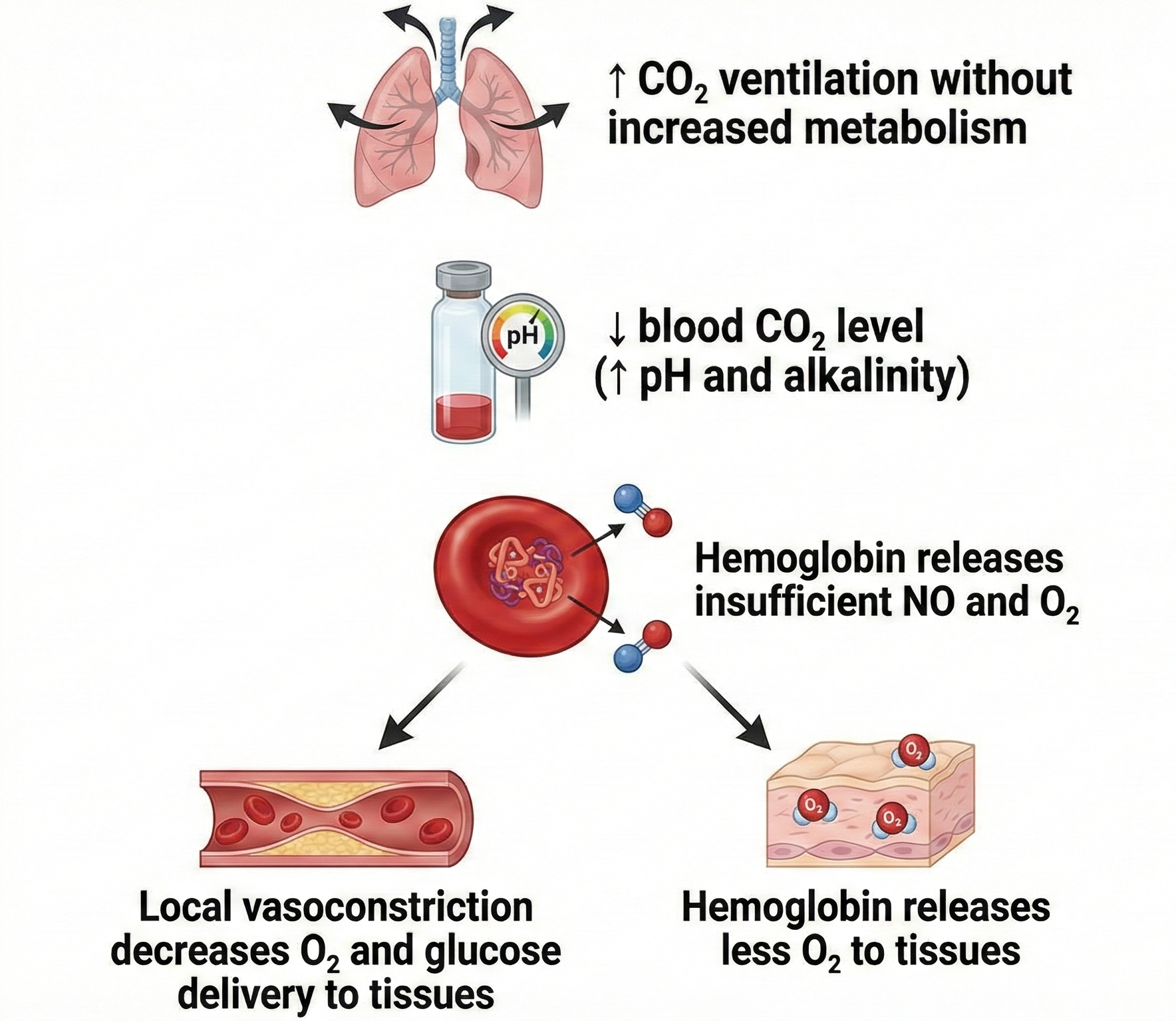
Blood Volume Pulse
Blood volume pulse (BVP) is the phasic (momentary, beat-to-beat) change in blood volume with each heartbeat. It is the vertical distance between the maximum value (peak) and the minimum value (trough) of a pulse wave, measured by a photoplethysmograph (PPG)—an optical sensor that shines infrared light into the skin and detects changes in light absorption as blood volume fluctuates. BVP responds to a stressor in a blazingly fast 0.5-2 seconds—far quicker than temperature—because it uses light-based detection rather than a sluggish temperature sensor.
🎧 Mini-Lecture: Blood Volume Pulse
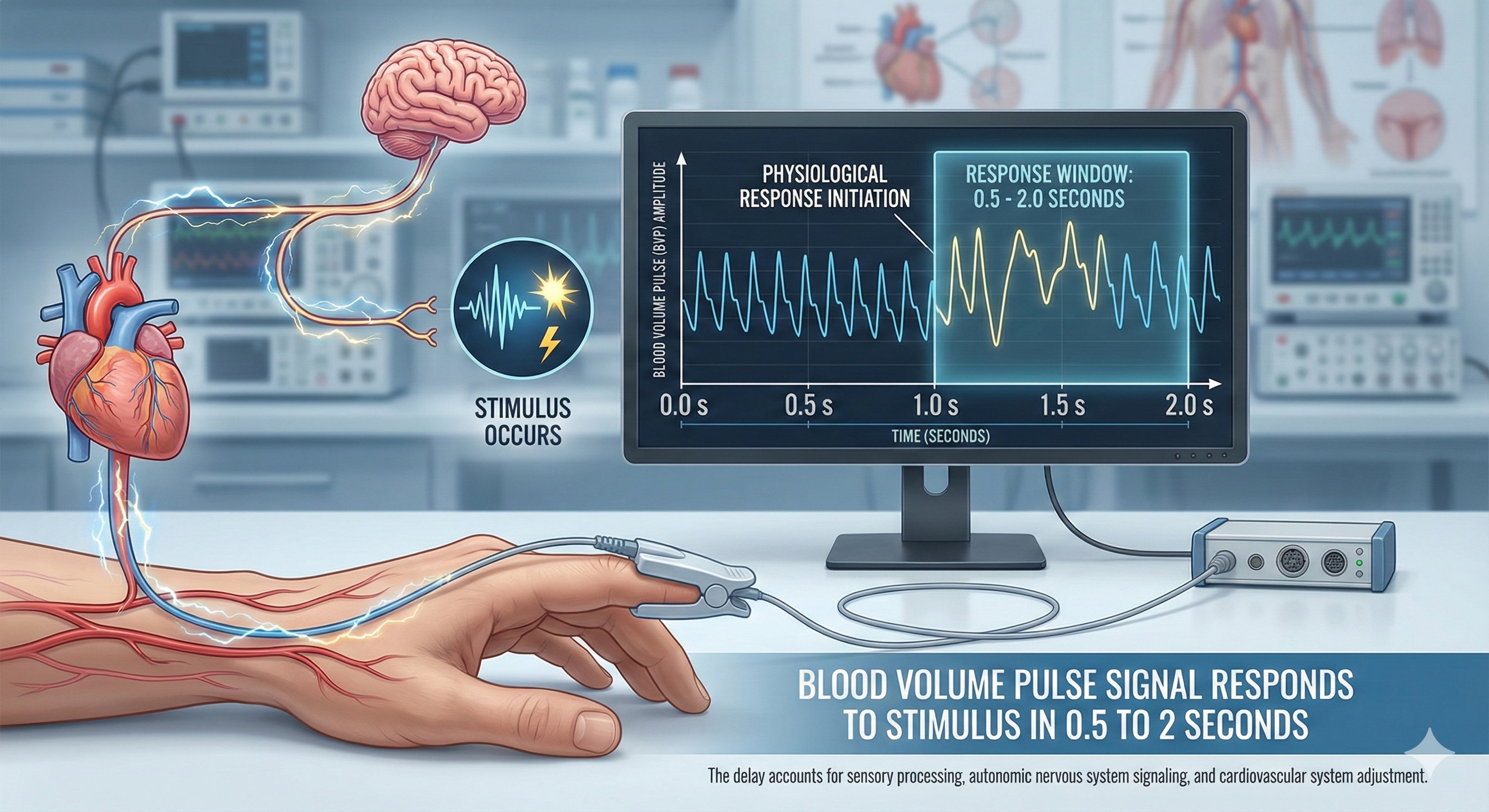
This speed advantage makes BVP a powerful complement to temperature biofeedback. When hand-warming stalls—a common frustration in clinical practice—the large-scale BVP changes visible in hands that are not cold can provide higher-resolution feedback that keeps clients engaged and progressing.
BVP Waveform Morphology
The shape of the BVP waveform itself carries diagnostic information. A decrease in pulse transit time (the interval between the heartbeat and the arrival of the pulse wave at the finger) and loss of the dicrotic notch (a small downward deflection after the peak of each pulse wave) are associated with aging, arteriosclerosis, and hypertension (Izzo & Shykoff, 2001). Peper et al. (2007) compared BVP waveforms and BP values for two parents and their teenage daughter in the recordings below.
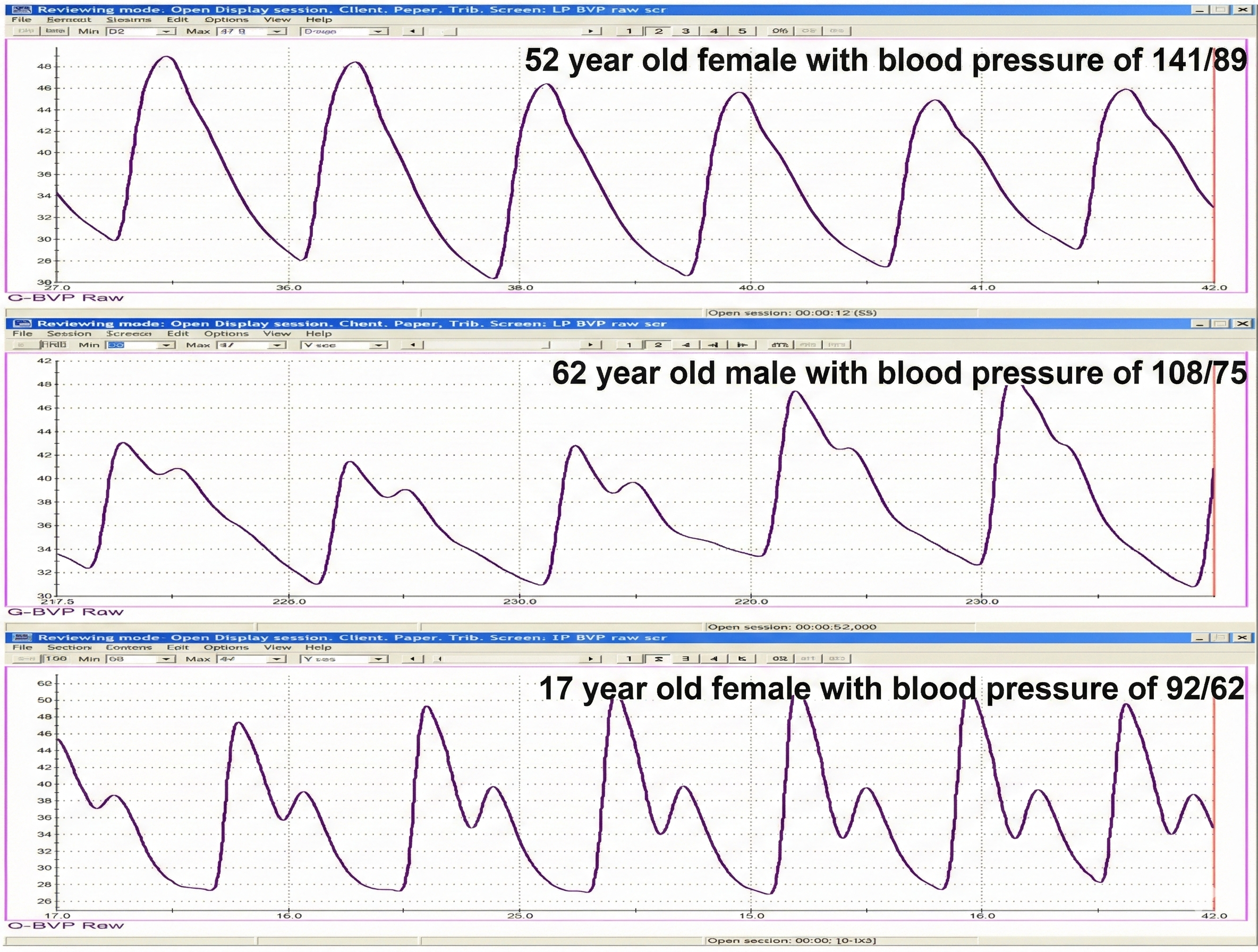
BVP amplitude can also reveal a client's cognitive and emotional responses in real time. The recording below from Peper et al. (2007) shows how BVP amplitude drops sharply in response to both external stimuli and internal emotional processing.
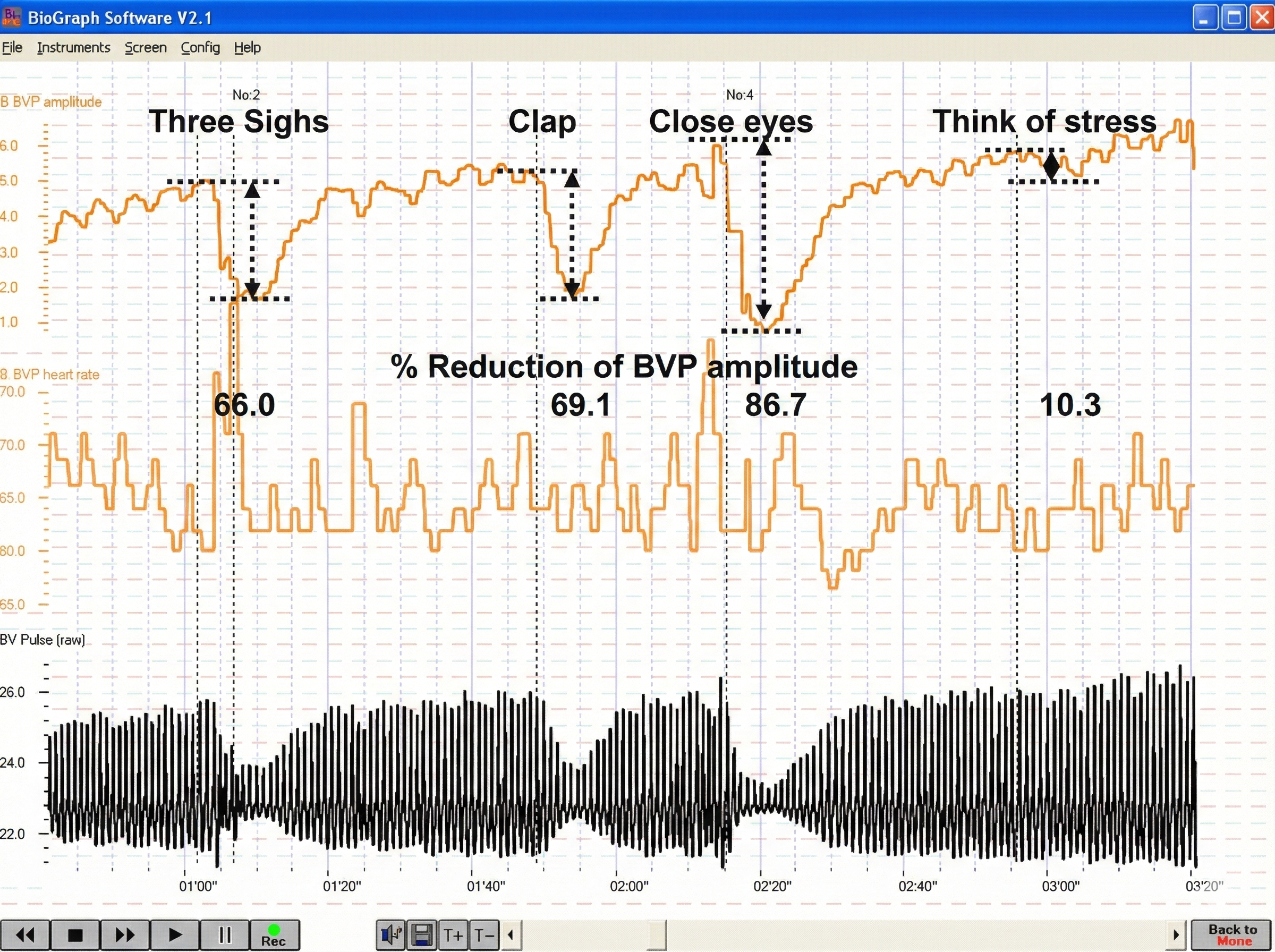
Below is a BioGraph ® Infiniti blood volume pulse (BVP) display. Note the small dicrotic notch following the peak of each waveform. The reduction or disappearance of a dicrotic notch may indicate the loss of arterial flexibility seen in arteriosclerosis.
Temperature vs. BVP: When to Use Which
When a client is successfully warming their hands, temperature has two advantages over BVP: it is measured in absolute units (degrees Fahrenheit or Celsius) and changes more gradually, making trends easier for clients to follow. However, BVP's speed makes it invaluable for detecting rapid sympathetic shifts that temperature simply cannot capture. In practice, the best approach is often to use both modalities together—temperature for tracking overall trends and BVP for catching moment-to-moment autonomic changes.
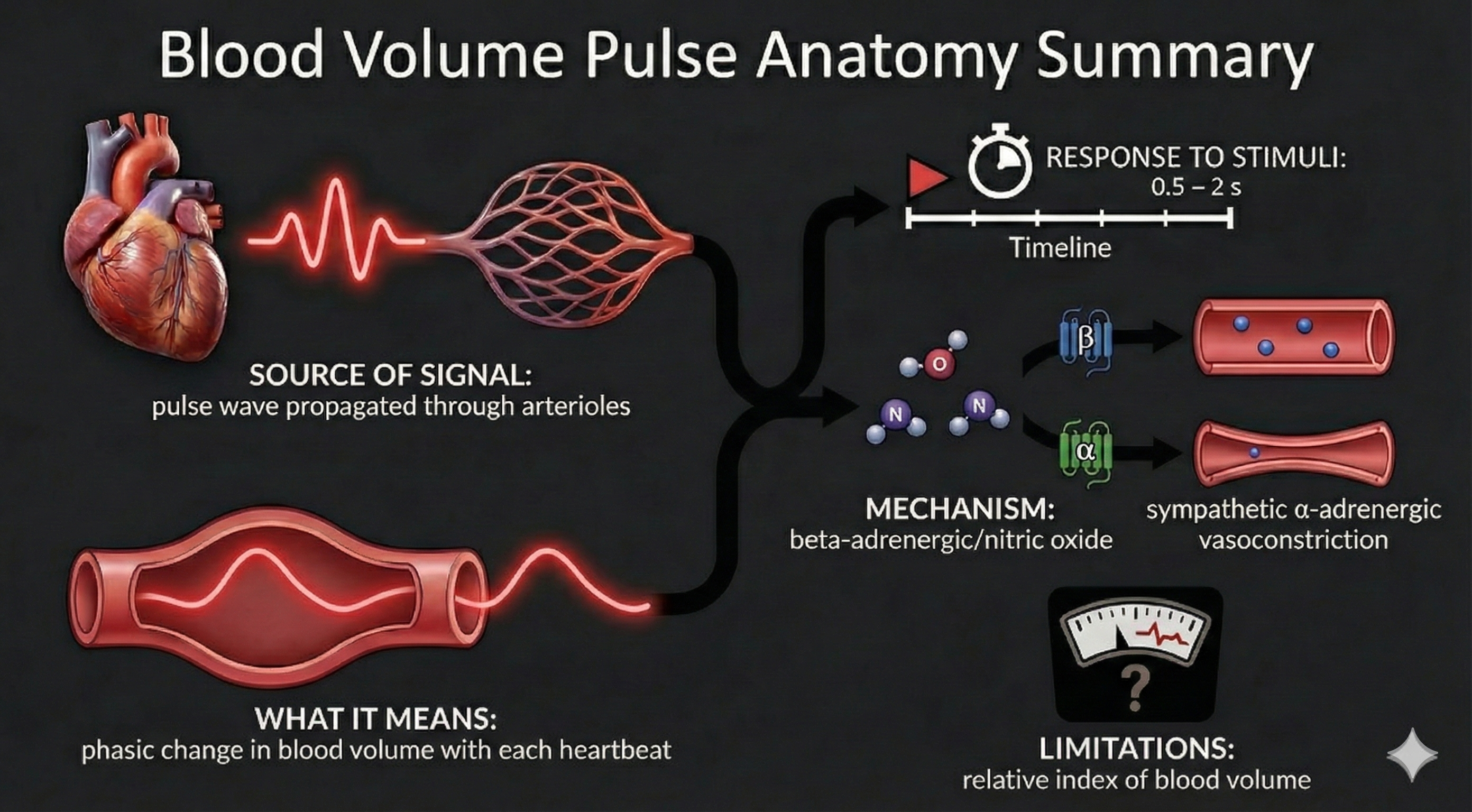
Pulse Wave Velocity
Ejection of blood from the left ventricle during systole produces a pulse wave that travels through the arterial system. Pulse wave velocity (PWV) is the rate of that movement, measured by placing pressure transducers (motion sensors) at two points along the arterial system, such as the brachial and radial arteries of the same arm. The interval required for the pulse wave to travel between these two points is called transit time (TT).
PWV serves as an indirect measure of blood pressure change. Researchers have reported correlations with average and systolic (but not diastolic) BP changes during stress tests, making it a useful complementary measure when direct BP monitoring is impractical during a training session.
Imagine you are working with a client, Maria, who has cold hands and anxiety. You place a temperature sensor on her finger and see a baseline of 78°F. When you ask her to recall a recent stressful meeting, her temperature drops by 3 degrees over 30 seconds as her arterioles constrict. Switching to BVP, you notice the amplitude change happens almost instantly. This is why BVP can be a powerful complement to temperature biofeedback: it gives your client real-time feedback about sympathetic activation that temperature simply cannot match in speed.
Comprehension Questions: Arteries and Peripheral Blood Flow
- What are the three tunics of an artery, and which layer is involved in hand-warming versus hand-cooling?
- Why is BVP faster than temperature at detecting a sympathetic stress response?
- What does the absence of a dicrotic notch in a BVP waveform suggest about a client's cardiovascular health?
- Explain why arterioles are responsible for approximately 50% of peripheral resistance.
Veins
This section covers the venous side of the circulation—the vessels that return blood to the heart. You will learn how veins and venules differ structurally from arteries, why valves are essential in low-pressure vessels, and how venous return contributes to overall cardiovascular function.
Veins are blood vessels that route blood from tissues back to the heart. They contain the same three tunics found in arteries, but these layers are thinner due to the much lower pressure in the venous system. This structural difference reflects the veins' role: they do not need the thick, muscular walls that arteries require to withstand the force of blood pumped directly from the heart.
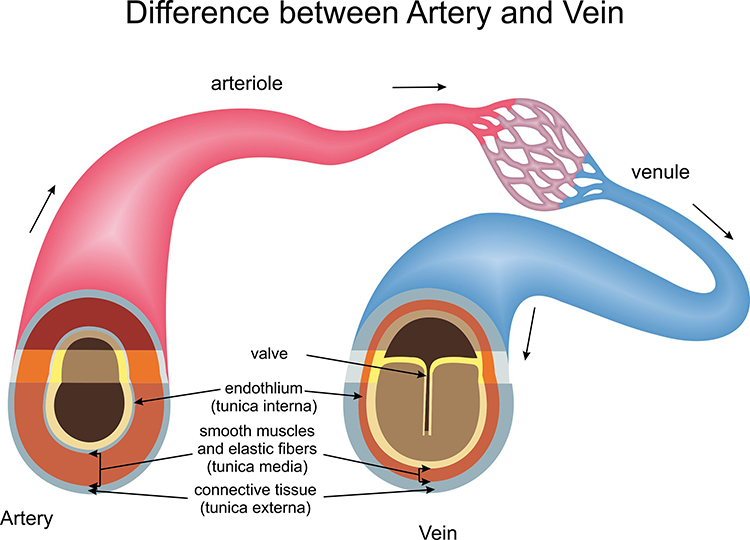
A venule is a small vein (less than 2 millimeters in diameter) that collects blood from capillaries and delivers it to a vein. Smooth muscle in venule walls allows them to actively adjust diameter. The low return pressure in these vessels requires valves that prevent backward blood flow—without them, gravity would cause blood to pool in the extremities. Venules are essential in controlling return blood flow to the heart due to their narrow diameter, contractility, and extensive surface area.

Capillaries
This section explains the structure and function of capillaries, the smallest blood vessels where the actual exchange of nutrients and waste between blood and tissues takes place. Understanding capillary function helps you appreciate why blood flow regulation at the arteriolar level has such far-reaching effects on tissue health.
Capillaries may directly connect arterioles with venules or form extensive networks for the rapid exchange of nutrients and waste products between blood and tissue cells. A capillary generally consists of a single layer of endothelium (the thin cell lining) and a basement membrane. Because capillaries lack a smooth muscle layer, changes in their diameter are passive rather than actively controlled. This exchange function is aided by walls only 1 micron thick, extensive branching, and massive surface area—capillary density is greatest where tissue metabolic activity is highest.

True capillaries extend from arterioles or metarterioles (small vessels between arterioles and capillaries). A precapillary sphincter functions as a valve that controls blood flow to the tissues at the arterial end of a capillary. When sympathetic activation constricts precapillary sphincters, tissue perfusion drops—a mechanism directly relevant to the temperature changes you observe during biofeedback sessions.
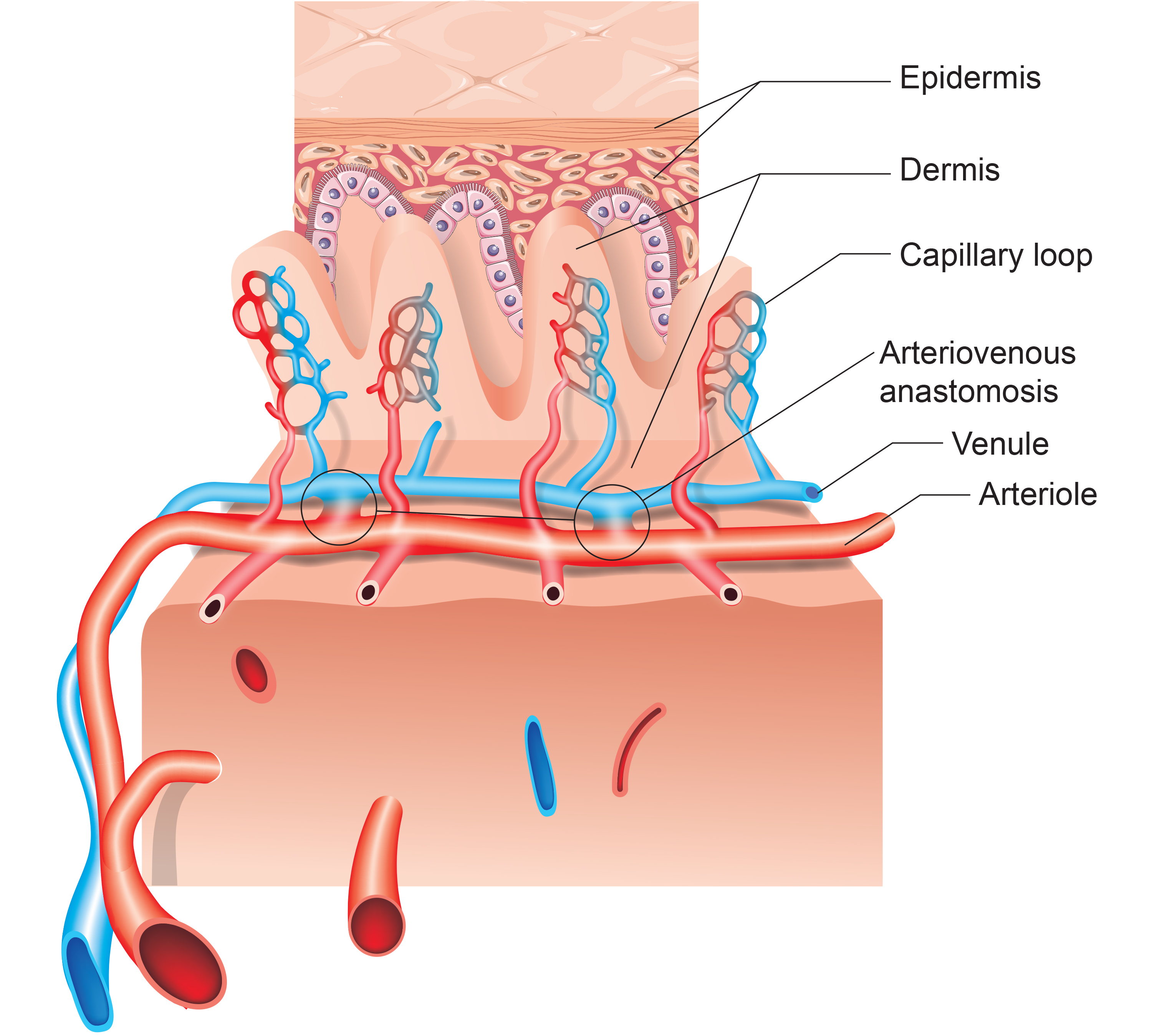
Arteriovenous Anastomoses (AVAs)
This section introduces arteriovenous anastomoses (AVAs), specialized vascular structures that bypass capillaries to directly connect arterioles and venules. AVAs play a central role in temperature regulation and are directly implicated in Raynaud's disease and Raynaud's phenomenon—conditions you may treat with temperature biofeedback.
Arteriovenous anastomoses (AVAs) are junctions of two or more vessels that supply the same region. Unlike capillaries, AVAs bypass the capillary bed to directly shunt blood from arterioles to venules, and they play a key role in temperature regulation (Walløe, 2016). These vessels contain all three tunics found in arterioles and venules, and their smooth muscle allows them to actively adjust diameter.
🎧 Mini-Lecture: Arteriovenous Anastomoses (AVAs)
AVAs Bypass Capillaries, Directly Shunt Blood from Arterioles to Venules, and Help Regulate Temperature

AVAs are all closed when a naked human body is exposed to ambient temperatures around 79°F and progressively open as body surface temperature approaches 97°F. When AVAs dilate, they transfer blood from the epidermis to the interior, cooling the skin. This mechanism is directly implicated in both Raynaud's disease (a primary condition with no known cause) and Raynaud's phenomenon (a secondary condition associated with connective tissue disorders), where AVAs respond abnormally to mild cold-related stimuli. For the biofeedback practitioner treating Raynaud's clients, understanding AVA function explains why hand temperature can drop so dramatically in response to seemingly minor cold exposure.
Blood Pressure
This section covers the fundamentals of blood pressure—how it is generated, measured, and regulated. Understanding blood pressure mechanics is essential because hypertension is one of the most common conditions you will encounter, and biofeedback training can reduce BP by targeting both cardiac output and peripheral resistance.
Blood pressure (BP) is the force exerted by blood as it presses against blood vessel walls. In clinical practice, BP specifically refers to the pressure in arteries. Two factors determine BP: cardiac output—the amount of blood the heart pumps per minute—and peripheral resistance. Cardiac output is calculated by multiplying stroke volume (the amount of blood ejected by the left ventricle during one contraction, typically about 70 milliliters) by heart rate (the number of contractions per minute). A typical resting adult produces a cardiac output of about 5.25 liters per minute (70 ml × 75 bpm).
🎧 Mini-Lecture: Cardiac Measurements
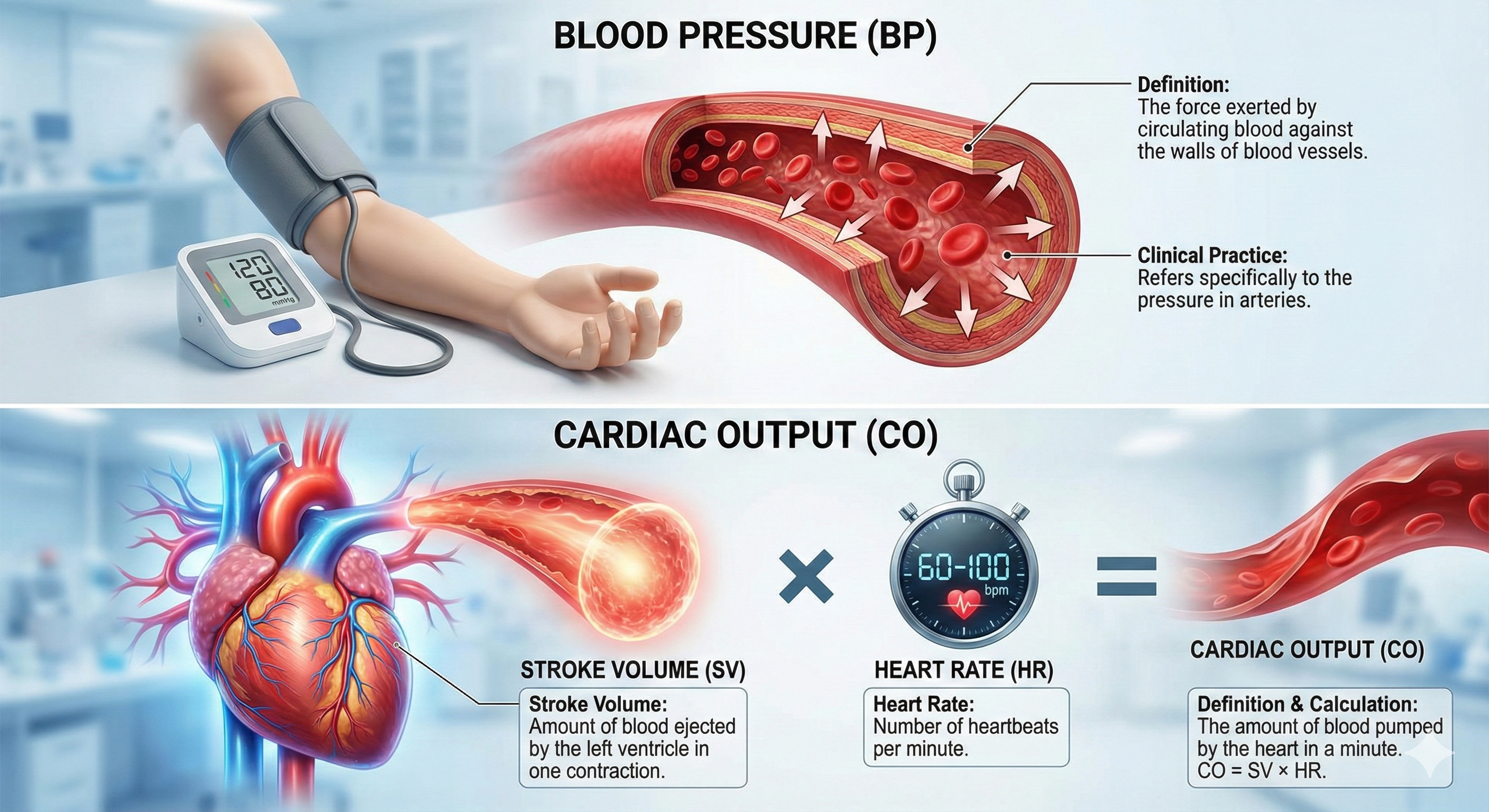
Blood leaving the left ventricle meets resistance due to blood viscosity (thickness), blood vessel length, and blood vessel radius. The fundamental relationship is straightforward: blood pressure equals cardiac output times resistance. Self-regulation skills that lower BP work by reducing cardiac output, resistance, or both—which is exactly what biofeedback modalities like HRVB and temperature training can accomplish.
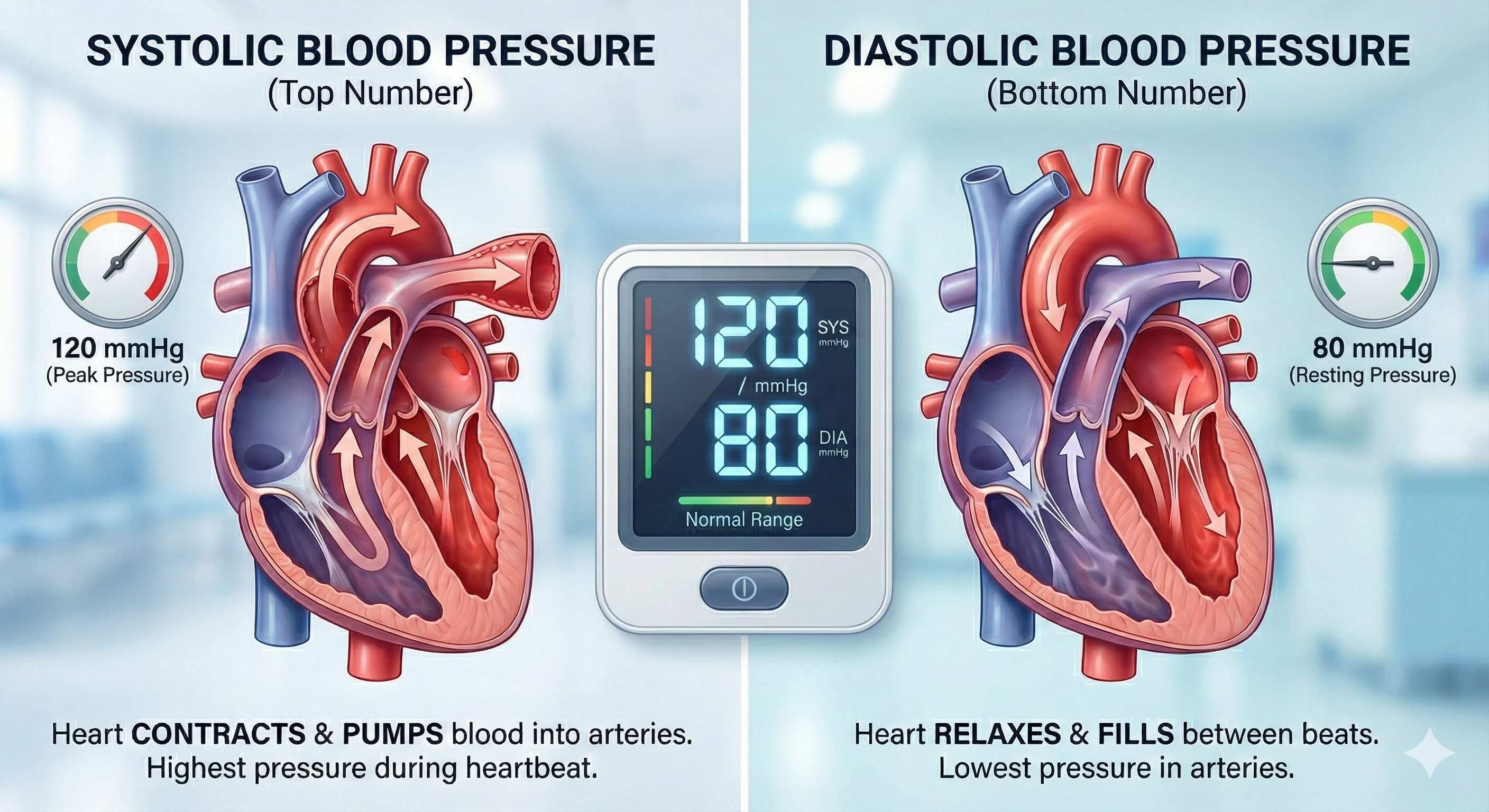
Systolic and Diastolic Blood Pressure
Clinicians measure both systolic and diastolic BP. Systolic blood pressure (SBP) is the force exerted by blood on arterial walls during contraction of the left ventricle (called systole). SBP is the upper value when BP is reported and averages about 120 mmHg in young adult males at rest. Diastolic blood pressure (DBP) is the force applied against arteries during ventricular relaxation (called diastole) and is the lower value, averaging about 80 mmHg at rest.
Comprehension Questions: Blood Vessels and Blood Pressure
- How do veins differ from arteries structurally, and why?
- What role do AVAs play in temperature regulation, and at what body surface temperatures do they open and close?
- Write out the formula for blood pressure and explain how self-regulation skills can reduce it.
The Heart
This section covers the structure and electrical activity of the heart—from its four chambers to the conduction system that generates the ECG waveform. Understanding cardiac anatomy and the ECG is essential for HRV biofeedback practice because the interbeat intervals you train clients to modulate originate in the heart's pacemaker cells.
The heart is a hollow muscular organ about the size of a closed fist that pumps 1,500 to 2,000 gallons of blood each day. It beats around 100,000 times daily and 2.5 billion times during a typical lifetime. The heart contains four chambers: two atria (upper chambers) that receive returning venous blood, and two ventricles (lower chambers) that pump blood from the heart into the arteries (Tortora & Derrickson, 2021).
🎧 Mini-Lecture: Overview of the Heart

Review the External Structure of the Heart
Click on the link below to review an interactive diagram created by raymondmitchelafrica.
Review External Heart Structure on Quizlet
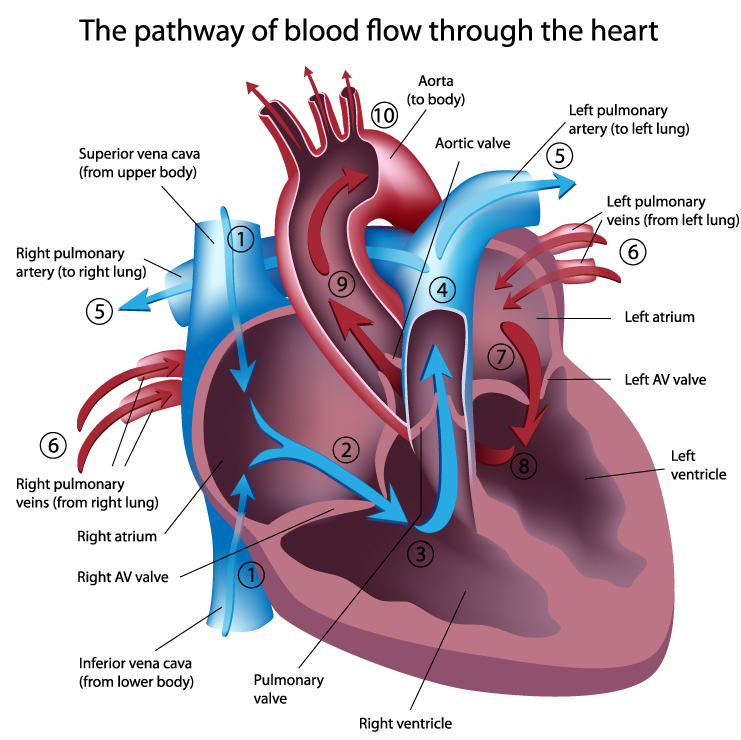
Blood Flow Through the Heart
Deoxygenated blood enters the right atrium through the superior and inferior vena cava. After passing through the right atrioventricular orifice (tricuspid valve), blood flows into the right ventricle and is pumped to the lungs via the pulmonary arteries, where waste gases are removed and oxygen is replenished. Oxygenated blood returns through the pulmonary veins to the left atrium, passes through the left atrioventricular orifice (mitral valve) into the left ventricle, and during contraction is ejected through the aorta to supply the entire arterial system (Tortora & Derrickson, 2021).
You can label the heart's arteries, chambers, and valves on the back of your hand and then place it over your chest to display the relative positions of these structures—a portable study aid you can also use with clients during patient education.

The Cardiac Cycle
The cardiac cycle consists of systole (ventricular contraction) and diastole (ventricular relaxation). During systole (about 0.3 seconds), BP peaks as left ventricle contraction ejects blood from the heart—this is when systolic BP is measured. BP is lowest during diastole (about 0.4 seconds) as the left ventricle relaxes, and this is when diastolic BP is measured (Tortora & Derrickson, 2021).
Pacemakers
The heart contains autorhythmic fibers—specialized cardiac cells that spontaneously generate the electrical impulses (pacemaker potentials) that initiate each heartbeat. These fibers are so self-sufficient that they continue to initiate contractions even after surgeons sever all cardiac nerves and remove a heart from the chest for transplantation. The two internal pacemakers responsible for heart rhythm are the sinoatrial (SA) node and the atrioventricular (AV) node. The electrocardiogram (ECG) records the activity of this conduction system (Tortora & Derrickson, 2021).
🎧 Mini-Lecture: Cardiac Conduction
Check out the Blausen Conduction System animation.
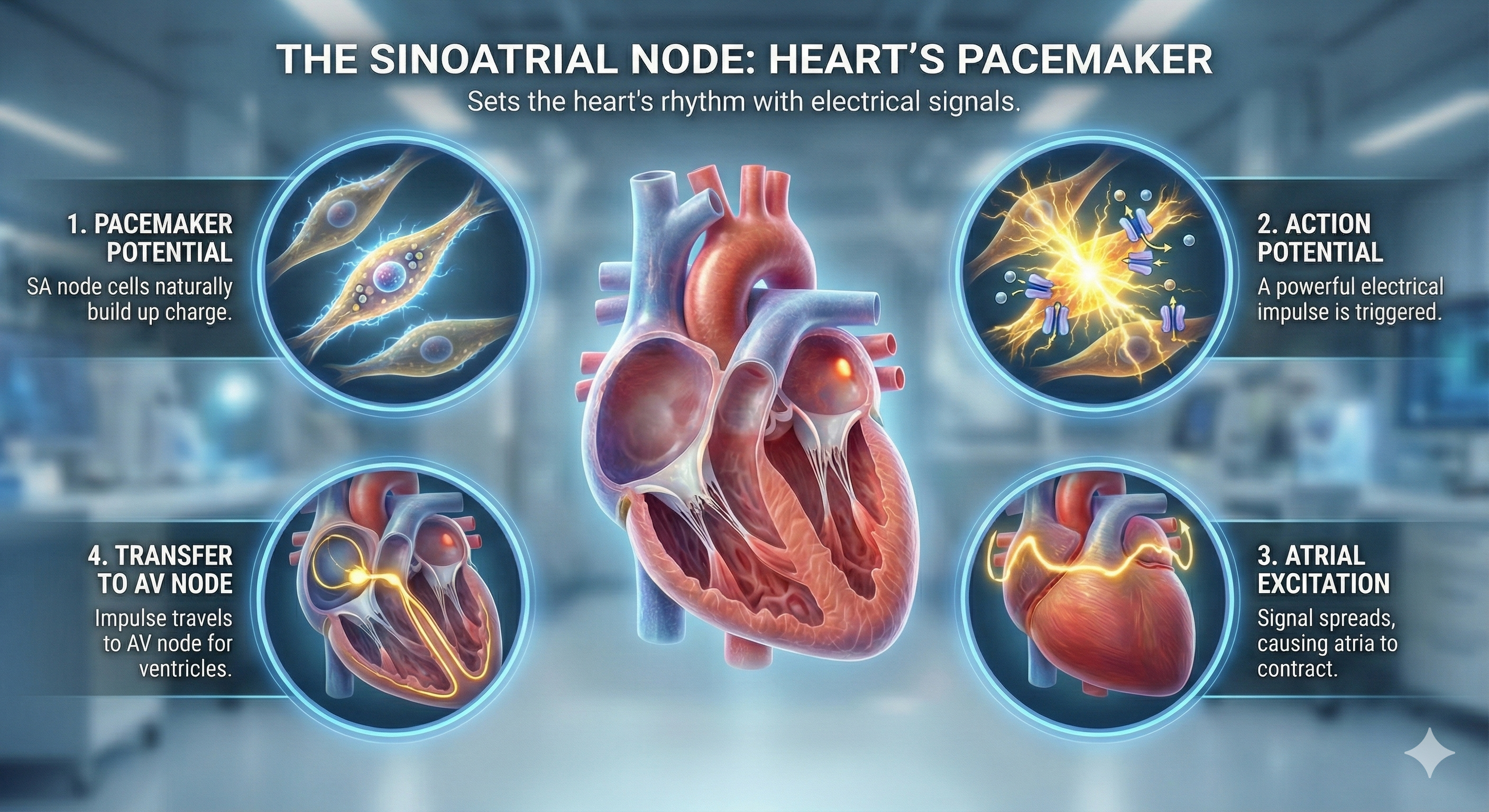
Cardiac Conduction and the ECG Waveform
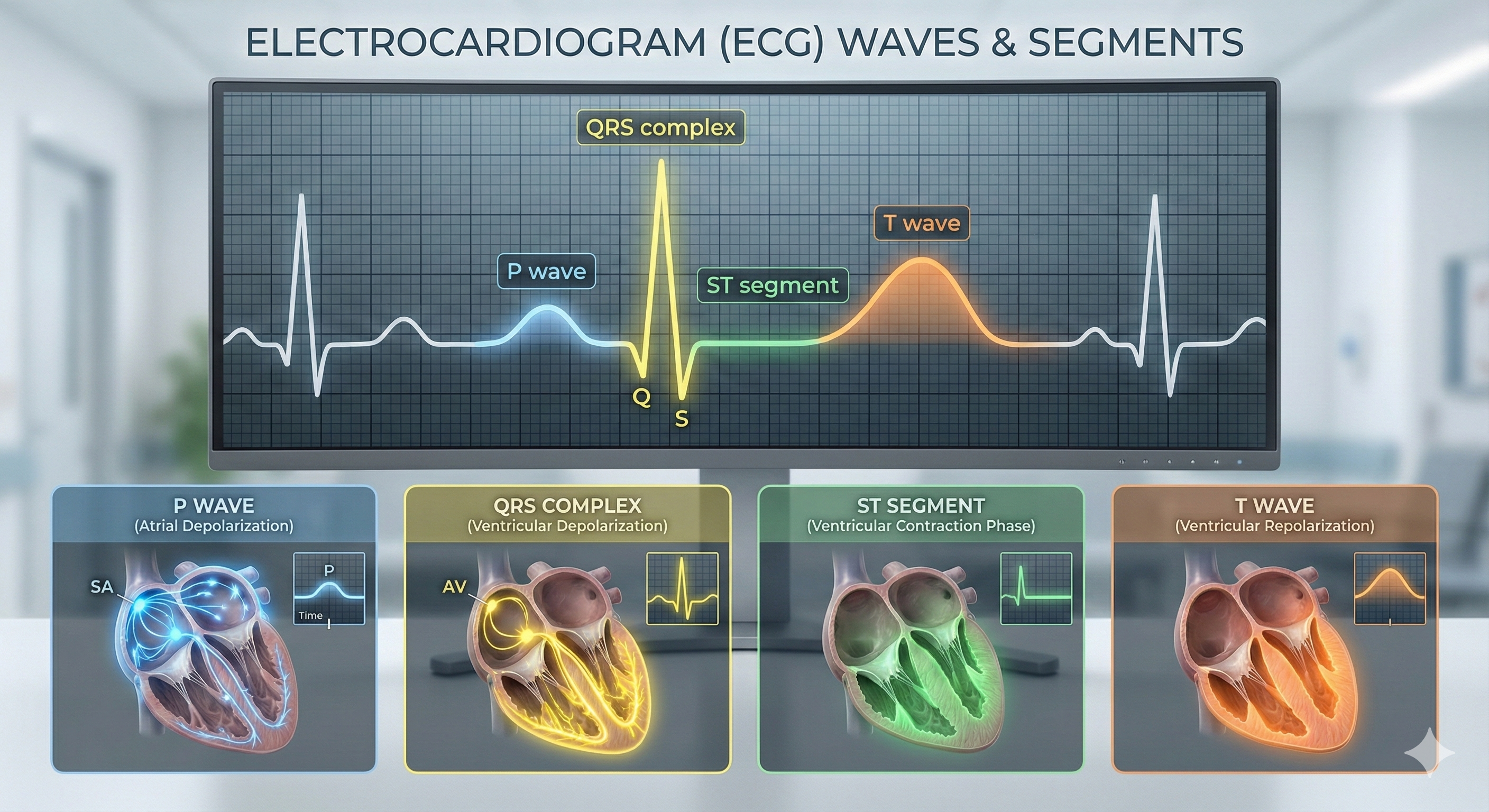
The SA node initiates each cardiac cycle in a healthy heart by firing 60-100 action potentials per minute, which usually prevents slower parts of the conduction system from generating competing potentials. The impulse travels through the atria to the AV node in about 0.03 seconds. As contractile fibers in the atria depolarize, they produce the P wave of the ECG, which culminates in atrial contraction (atrial systole).
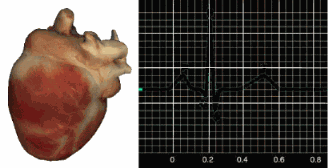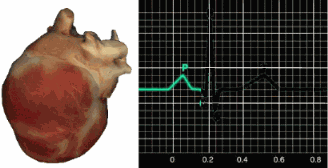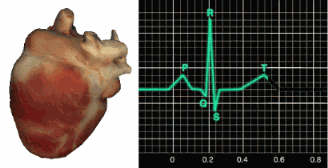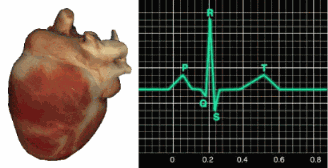
The AV node can replace an injured or diseased SA node as a backup pacemaker, spontaneously depolarizing 40-60 times per minute. From the AV node, the signal rapidly spreads through the atrioventricular (AV) bundle to the top of the septum. Descending right and left bundle branches conduct the action potential over the ventricles about 0.2 seconds after the P wave appears.
Conduction myofibers extend from the bundle branches into the myocardium (heart muscle), depolarizing contractile fibers in the ventricles. This ventricular depolarization generates the QRS complex, and ventricular contraction (ventricular systole) follows shortly after. Contraction continues through the S-T segment, and the T wave—representing ventricular repolarization (the electrical resetting of ventricular cells)—appears about 0.4 seconds following the P wave. The ventricles relax (ventricular diastole) 0.6 seconds after the P wave begins (Tortora & Derrickson, 2021).
Check out the YouTube video 15 Second EKG.
Considerations for HRV Biofeedback Training
Before beginning any HRV biofeedback protocol, clinicians should examine the ECG tracing for evidence of arrhythmias, ischemia, and prolonged Q-T intervals that could endanger client safety (Drew et al., 2004). This brief morphology screen is a best-practice safety step—not a diagnostic assessment, but a precaution that could identify clients who need medical clearance before training. It takes only moments but could prevent a serious adverse event.
Regulation by the Cardiovascular Center
While the SA node generates the intrinsic heartbeat rhythm, the rate and force of each contraction are continuously adjusted by autonomic motor neurons, circulating hormones, and ions. Understanding this regulation is the foundation for all HRV biofeedback work, because HRV itself is produced by the dynamic interplay between these control systems acting on the interbeat interval—the time between adjacent heartbeats.
The cardiovascular center, located in the medulla of the brainstem, serves as the brain's command center for cardiac regulation. It integrates sensory information from proprioceptors (limb position), chemoreceptors (blood chemistry), and baroreceptors (blood pressure), as well as input from the cerebral cortex and limbic system. Based on this integration, the cardiovascular center adjusts autonomic balance by modulating sympathetic and parasympathetic motor neuron output (Tortora & Derrickson, 2021). This means that your client's thoughts, emotions, and physical activity all converge on a single brainstem structure that tunes cardiac function in real time.
Sympathetic Control
Sympathetic cardiac accelerator nerves target the SA node, AV node, and the bulk of the myocardium. When these motor neurons fire, they release norepinephrine (NE) and epinephrine (E), which bind to beta-adrenergic (β1) receptors on cardiac muscle fibers. This binding speeds spontaneous SA and AV node depolarization (increasing heart rate) and strengthens the contractility of the atria and ventricles (increasing stroke volume). For practitioners working with athletes, this is the system that enables the rapid cardiovascular scaling needed during intense physical performance.
In failing hearts, the number of beta-adrenergic receptors is reduced, and their contractile response to NE and E is weakened (Ogletree-Hughes et al., 2001). This downregulation helps explain why heart failure patients show diminished HRV and reduced capacity to respond to physical and emotional demands—a finding with important implications for cardiac rehabilitation programs that incorporate biofeedback.
Parasympathetic Control
Like cardiac accelerator nerves, the left and right parasympathetic vagus (X) nerves also innervate the SA node, AV node, and atrial cardiac muscle. When these motor neurons fire, they trigger acetylcholine release, which binds to muscarinic (mainly M2) receptors. This cholinergic binding decreases the rate of spontaneous depolarization in the SA and AV nodes, slowing heart rate. Because there is sparse vagal innervation of the ventricles, vagal tone minimally affects ventricular contractility (Tortora & Derrickson, 2021). The vagus nerve's ability to rapidly slow the heart is what makes it the primary target of HRV biofeedback training.
🎧 Mini-Lecture: Autonomic Control of the Heart
Autonomic Balance
A healthy heart depends on a dynamic balance between the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS). The synergistic relationship between these autonomic branches is complex: sometimes reciprocal (when one goes up, the other goes down), sometimes additive (both increase together), or subtractive (both decrease together) (Gevirtz et al., 2016). This complexity is why a simple "sympathetic equals bad, parasympathetic equals good" framework is insufficient for clinical practice.
PNS control predominates at rest, resulting in an average resting heart rate of 75 bpm—significantly slower than the SA node's intrinsic rate, which decreases with age from an average of 107 bpm at age 20 to 90 bpm at age 50 (Opthof, 2000). The fact that resting HR is well below the intrinsic rate tells you that the vagus nerve is actively applying the brakes, even at rest.
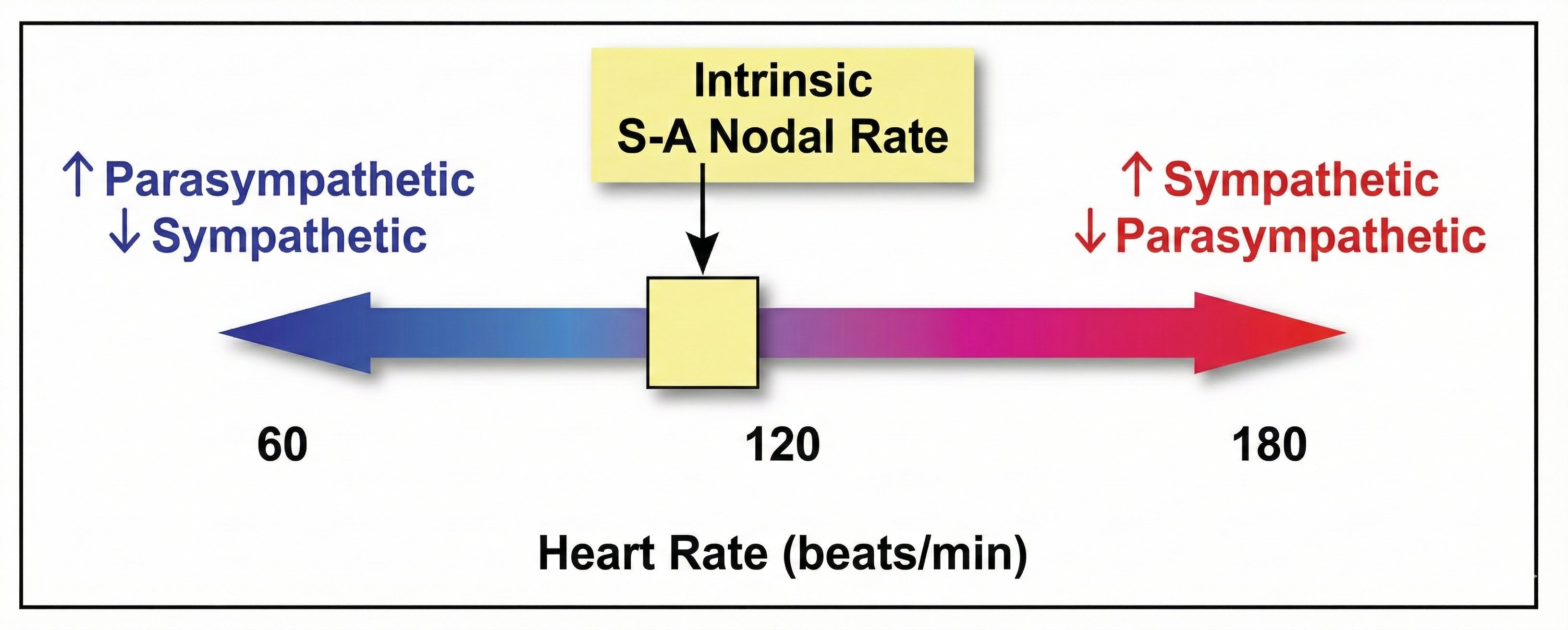
The PNS can slow the heart by 20-30 beats per minute or even briefly stop it (Tortora & Derrickson, 2021)—a dramatic demonstration of accentuated antagonism, the phenomenon in which parasympathetic effects overwhelm sympathetic influences (Olshansky et al., 2011). Crucially for biofeedback practice, parasympathetic nerves exert their effects in less than 1 second, while sympathetic nerves take more than 5 seconds to respond (Nunan et al., 2010; Shaffer et al., 2014; Tortora & Derrickson, 2021). This speed difference is why rapid beat-to-beat changes in heart rate primarily reflect vagal activity—the very signal you train in HRV biofeedback.
While the SNS can suppress PNS activity, it can also increase PNS reactivity (Gellhorn, 1957). Parasympathetic rebound may occur following high stress, resulting in increased nighttime gastric activity (Nada et al., 2001) and worsened asthma symptoms (Ballard, 1999). This rebound effect is clinically relevant: clients who experience intense daytime stress may report unexpected symptoms at night as their parasympathetic system overcompensates.
Cardiac Regulation by Hormones and Ions
Beyond autonomic neural control, circulating hormones and ions also influence the heart. Epinephrine, norepinephrine, and thyroid hormones all increase heart rate and contractility. The cations (positive ions) K+, Ca2+, and Na+ significantly affect cardiac function. Elevated plasma levels of K+ and Na+ decrease heart rate and contraction force, while high intracellular Ca2+ levels have the opposite effect (Tortora & Derrickson, 2021). These hormonal and ionic influences help explain why factors like thyroid disorders, electrolyte imbalances, and medication side effects can alter HRV independently of autonomic tone.
Consider a new client, James, who arrives for his first HRV biofeedback session. You attach ECG sensors and notice an unusually fast resting heart rate of 105 bpm. Before beginning any biofeedback protocol, you examine the ECG morphology. Is the rhythm regular? Are the P waves, QRS complexes, and T waves normal? Could there be a prolonged Q-T interval? This quick screen, recommended by Drew and colleagues (2004), is an essential safety step before training. You also note that James's intrinsic SA node rate at his age should be around 100 bpm, so the fact that parasympathetic control is not slowing his heart below that rate tells you something important about his autonomic balance.
Heart Rate
This section covers heart rate and its relationship to heart rate variability. You will learn what resting heart rate values are normal versus concerning, why faster heart rates reduce HRV, and why a healthy heart produces unpredictable, chaotic beat-to-beat variation.
Heart rate (also called stroke rate) is the number of heartbeats per minute. A typical resting value for a young adult male is 75 bpm. Resting rates slower than 60 bpm (bradycardia) and faster than 100 bpm (tachycardia) may indicate a cardiovascular disorder. Typical non-athlete resting heart rates range from 60-80 bpm, while well-conditioned athletes may have resting rates between 40-60 bpm due to cardiovascular adaptations that increase stroke volume (Khazan, 2019). Whether you work with a veteran being treated for PTSD or an elite athlete pursuing optimal performance, resting HR provides your first clue about autonomic balance.

Abnormal or irregular rhythms are called arrhythmias or dysrhythmias (Tortora & Derrickson, 2021). For the biofeedback practitioner, arrhythmias are important to identify because they can confound HRV measurements and may require medical referral before training begins.
Why Heart Rate Matters for HRV
Heart rate is clinically significant for HRV biofeedback because a faster rate mechanically reduces heart rate variability. Faster heartbeats allow less time between successive beats for heart rate to vary, compressing the interbeat interval and lowering HRV. This is why clients with elevated resting heart rates often show depressed HRV even before accounting for any autonomic dysfunction.
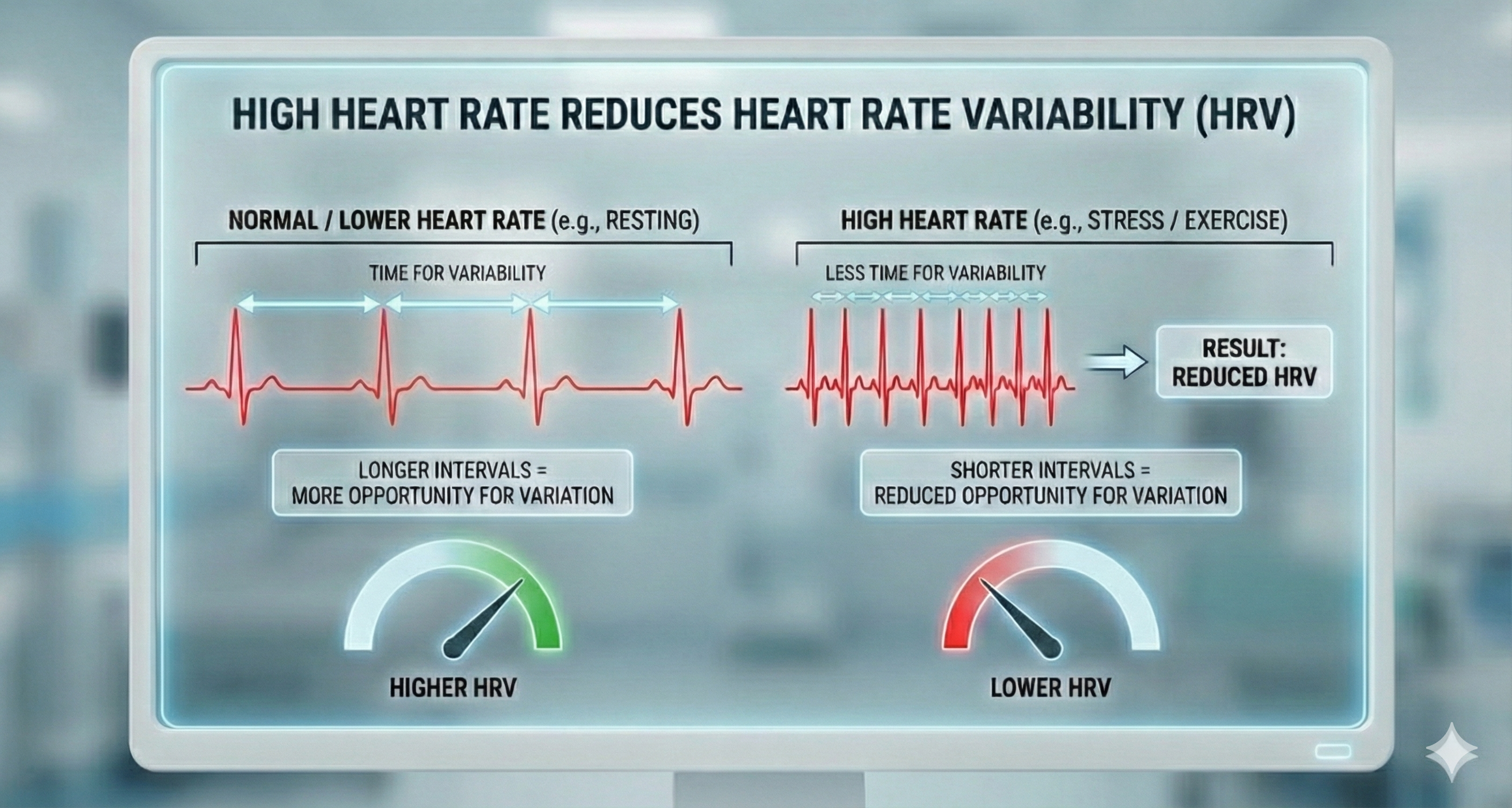
Analysis of heart rates in healthy individuals reveals a chaotic, unpredictable pattern. Successive heart rate values might be 65, 78, 72, and 86 bpm, illustrating the variability of a healthy heart that can rapidly adapt to changing workloads. This variability is severely reduced in hearts damaged by cardiovascular disease, where the heart loses its ability to flexibly respond to demands—a hallmark of poor regulatory capacity (the ability to adaptively respond to challenges like exercise and stressors).
Below is a three-dimensional BioGraph ® Infiniti HRV display of the ECG power spectrum. HRV biofeedback training aims at increasing the power at 0.1 Hz (corresponding to about 6 breaths per minute) to maximize healthy variability.
Heart Rate Variability (HRV)
This section introduces heart rate variability—the core measure targeted by HRV biofeedback. You will learn what HRV is, how it is produced, and why it matters for both clinical and performance applications.
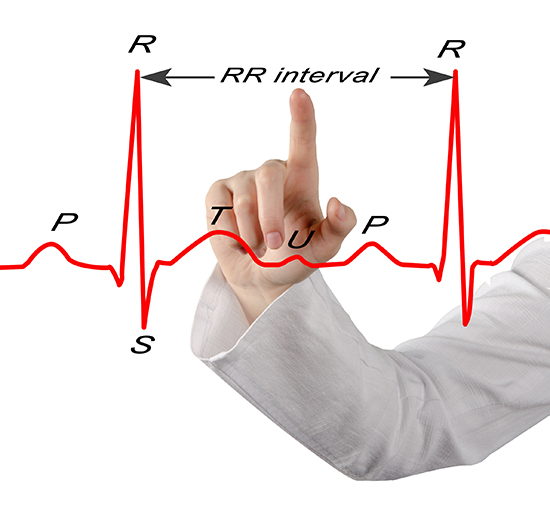
Heart rate variability (HRV) consists of changes in the time intervals between consecutive heartbeats (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). More precisely, "HRV is the organized fluctuation of time intervals between successive heartbeats defined as interbeat intervals" (Shaffer et al., 2020). We measure these interbeat intervals in milliseconds, and it is the pattern of variation in these intervals—not the heart rate itself—that provides the richest information about autonomic function.
🎧 Mini-Lecture: Heart Rate Variability Overview
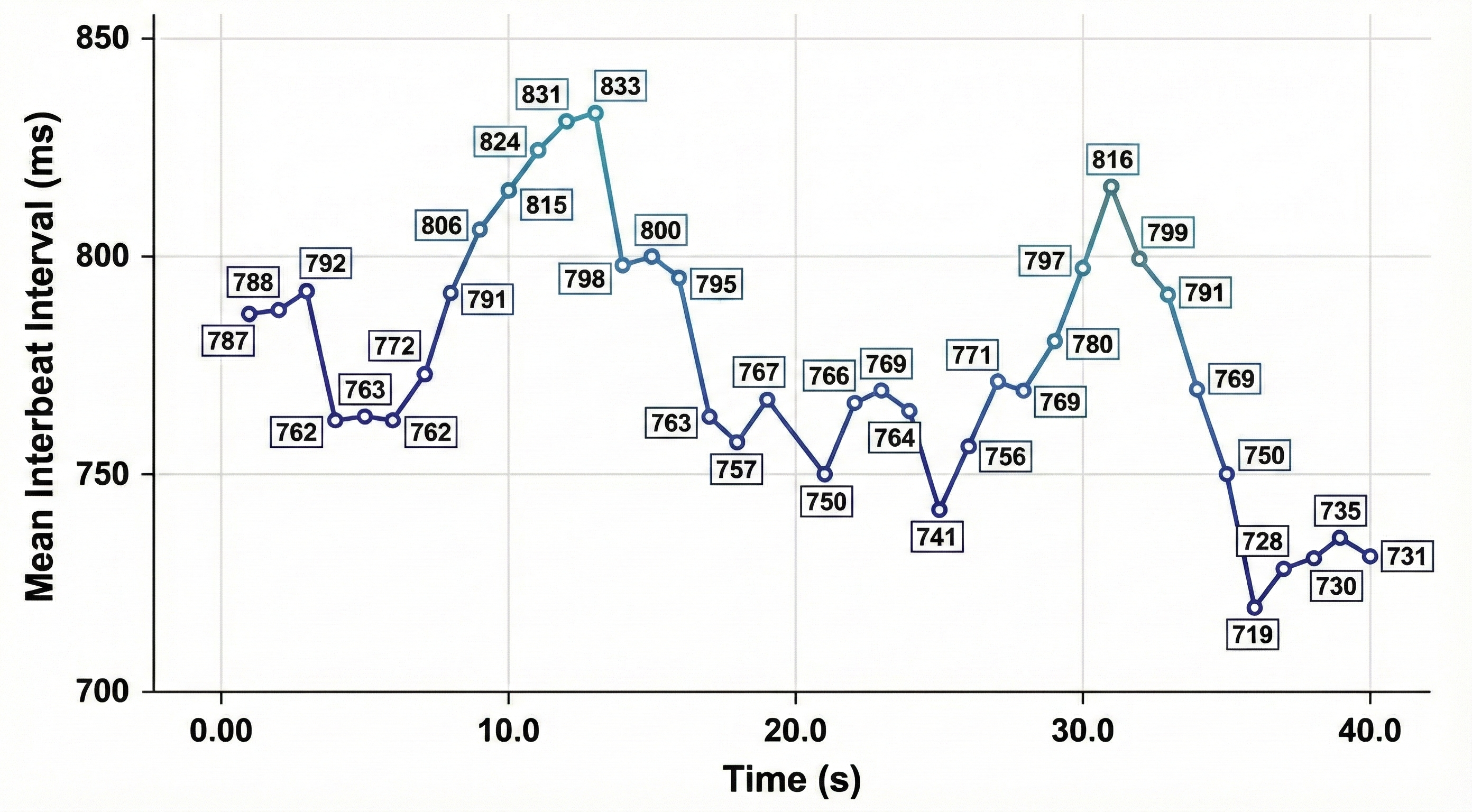
HRV is produced by interacting regulatory mechanisms that operate on different time scales (Moss, 2004). Over 24 hours, circadian rhythms, core body temperature, and metabolism shape HRV—which is why 24-hour recordings represent the "gold standard" for clinical HRV assessment. Over shorter periods (e.g., 5-minute recordings), the parasympathetic, cardiovascular, and respiratory systems are the primary drivers of HRV. In your daily practice, you will most often work with these short-term recordings, which capture the autonomic dynamics most directly influenced by biofeedback training.
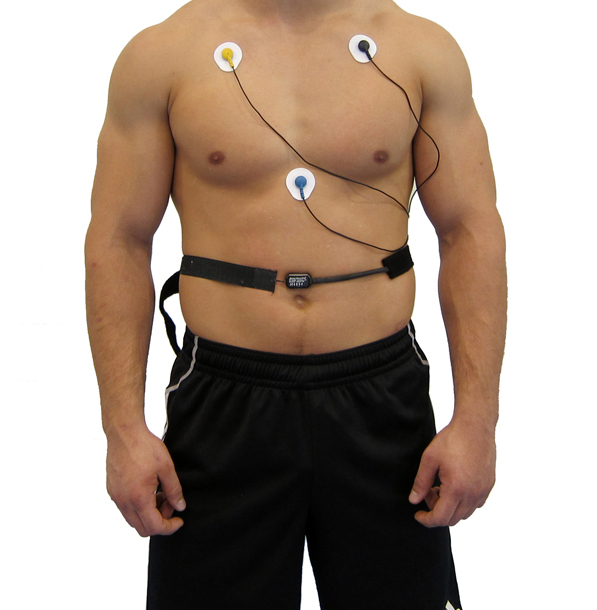
Clinicians can monitor HRV using ECG and respiration sensors simultaneously, as shown below. This combined approach allows you to observe how breathing patterns influence beat-to-beat heart rate changes in real time.
Heart-Brain Interactions
This section explores the bidirectional communication between the heart and brain. Understanding these pathways explains why HRV is more than just a cardiac metric—it is a window into how the brain regulates emotion, attention, and threat processing.
Thayer and Lane (2000) proposed a neurovisceral integration model that describes how a central autonomic network (CAN)—a set of interconnected brain structures including the anterior cingulate, insula, ventromedial prefrontal cortex, amygdala, and hypothalamus—links with the brainstem's nucleus of the solitary tract (NST) through feedback and feed-forward loops. They speculated that a breakdown in negative feedback within this network may produce the increased SNS arousal that characterizes anxiety disorders. Thayer et al. (2012, p. 754) further contended that regions including the amygdala and medial prefrontal cortex, which evaluate "threat and safety," help regulate HRV through their connections with the NST. For clinicians treating anxiety, PTSD, or panic disorder, this model explains why enhancing HRV through biofeedback may improve emotional regulation at the brain level.
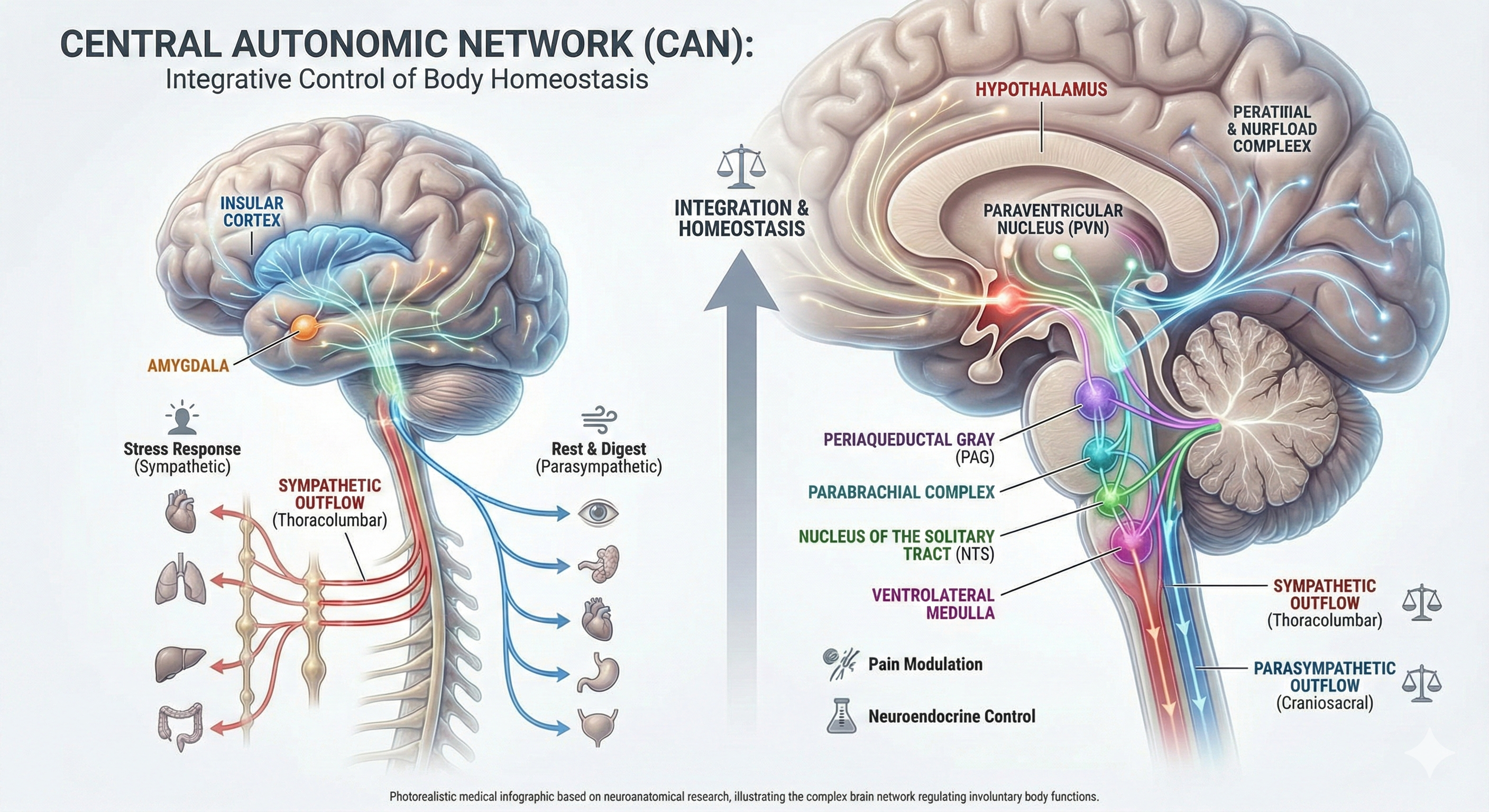
The Heart's "Little Brain"
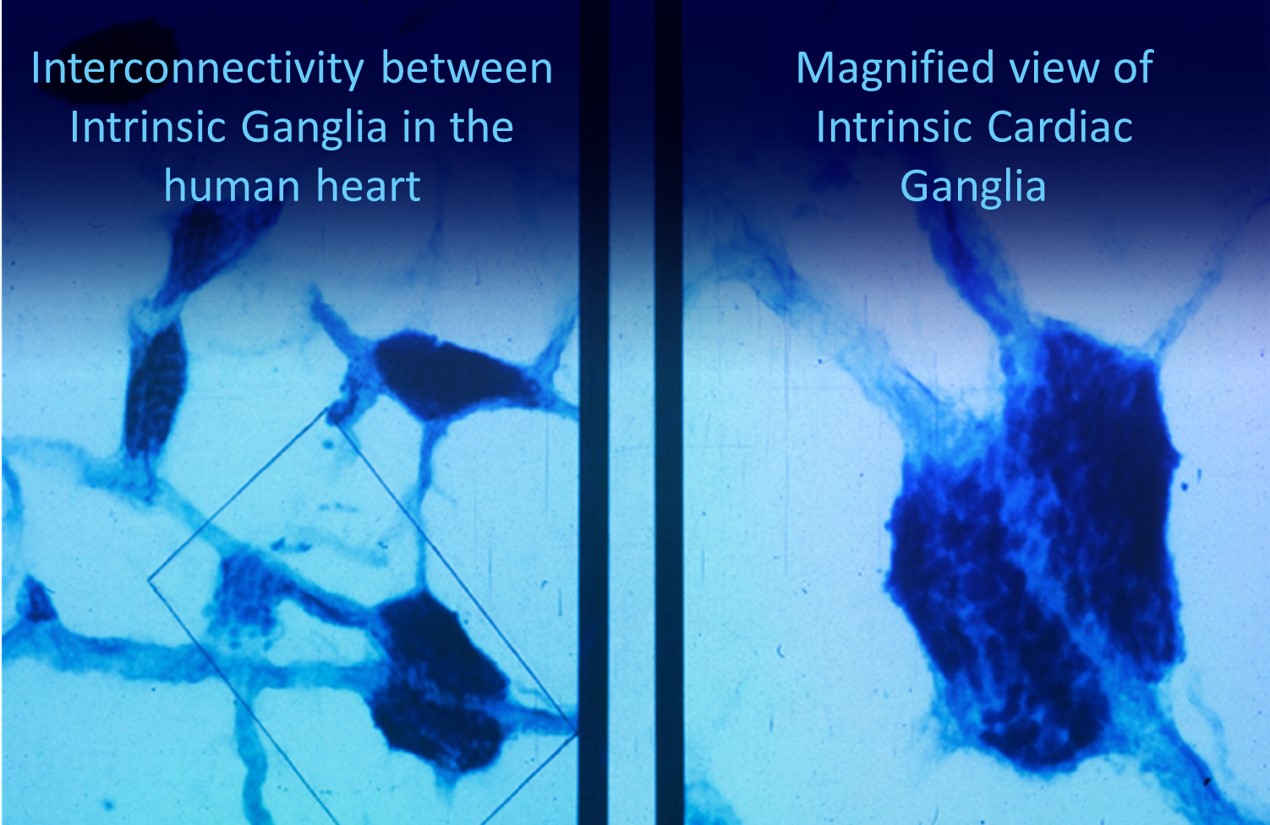
Shaffer et al. (2014) proposed that interconnected cardiac ganglia create an intrinsic nervous system within the heart that influences the SA and AV node pacemakers and forms reciprocal connections with the extrinsic cardiac ganglia found in the chest cavity and the medulla. The sensory, interconnecting, afferent, and motor neurons within the heart can function independently, constituting what researchers call a "little brain" on the mammalian heart.
🎧 Mini-Lecture: Heart-Brain Interactions
The ascending afferent nerves from the heart help regulate cardiac rhythms and influence efferent SNS and PNS activity. A remarkable 85-90% of vagus nerve fibers are afferents (carrying information toward the brain), and more afferent projections from the heart target the brain than from any other major organ. These afferent signals from the intrinsic cardiac nervous system appear to affect attention, motivation, perceptual sensitivity, and emotional processing (Shaffer et al., 2014). This finding has profound implications for performance practitioners: optimizing cardiac coherence through HRV biofeedback may enhance cognitive function by improving the quality of signals the heart sends to the brain.
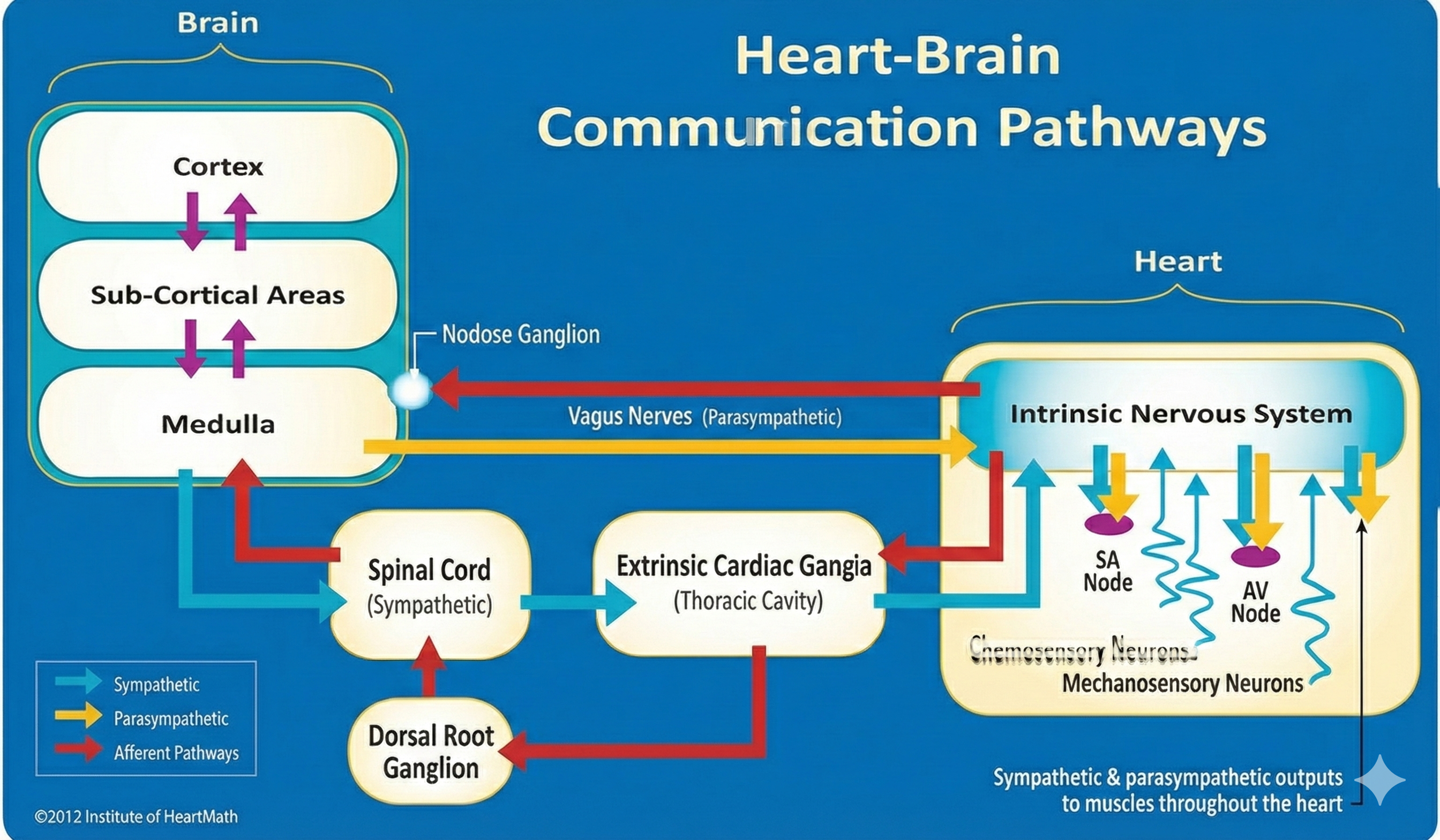
Resonance Frequency Breathing and Cardiac-Brain Communication
MacKinnon et al. (2013) provided compelling evidence for how HRV biofeedback strengthens heart-brain communication. They found that HRV influences the amplitude of heartbeat event-related potentials (HERPs)—negative EEG potentials that appear about 200-300 milliseconds after each R-spike and index cardiac afferent communication with the brain. Both negative and positive emotion conditions reduced HRV and HERP amplitude, suggesting that emotional states can disrupt heart-to-brain signaling. In contrast, resonance frequency breathing increased HRV above baseline and increased HERP amplitude.
The authors speculated that resonance frequency breathing reduces interference with vagal afferent signal transmission from the heart to the cerebral cortex. For practitioners, this finding offers a compelling mechanism to share with clients: resonance frequency breathing does not just calm the body—it may actually enhance the clarity of the information your heart sends to your brain, improving emotional regulation and cognitive performance.
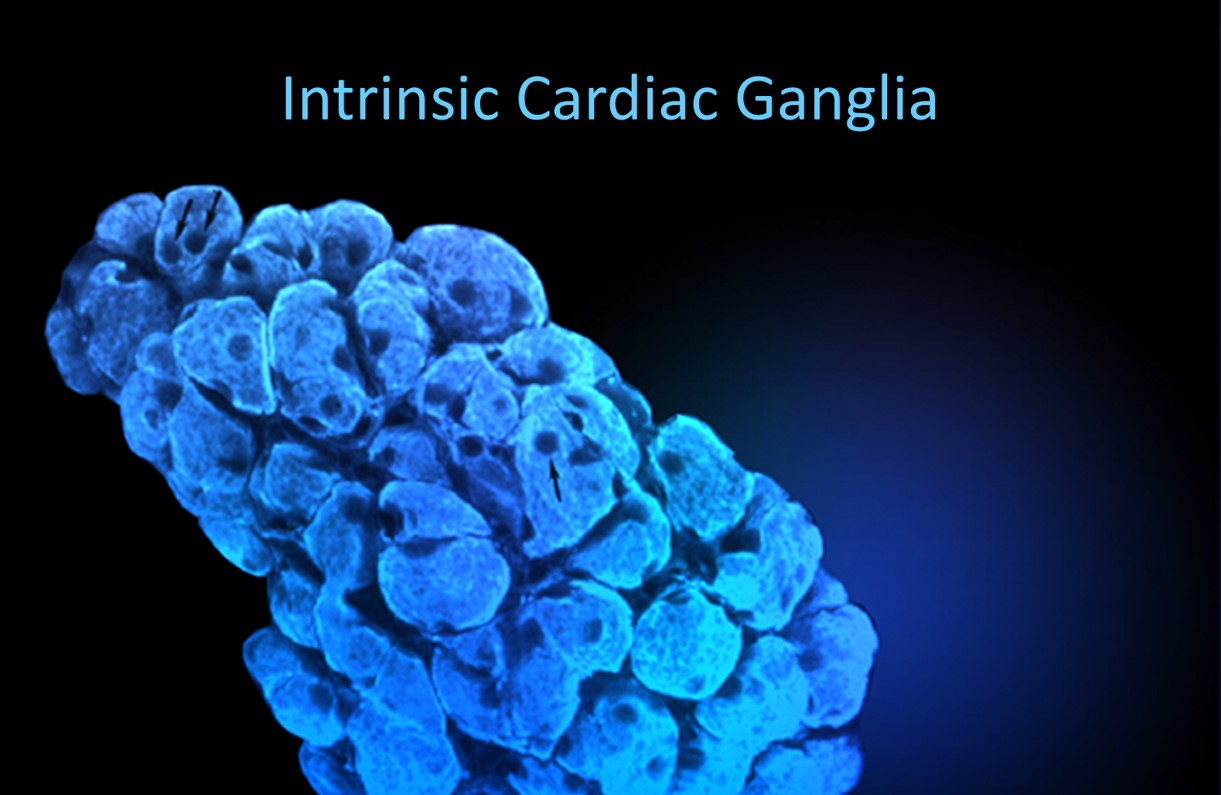
Comprehension Questions: The Heart and HRV
- Trace the path of blood through the heart from the vena cava to the aorta, naming each chamber and valve.
- Why does a faster heart rate tend to reduce HRV?
- Compare the speed at which parasympathetic and sympathetic nerves exert their effects on heart rate, and explain why this difference matters for biofeedback.
- What is the neurovisceral integration model, and how does it connect brain structures to HRV regulation?
- What evidence supports the concept of the heart as having a "little brain"?
Cutting Edge Topics
The Heart's Intrinsic Nervous System and Cardiac Neuroscience
Research into the heart's intrinsic nervous system continues to reveal surprises. The "little brain on the heart" contains approximately 40,000 neurons organized into interconnected ganglia. These neurons process sensory information locally before relaying it to the brain, suggesting the heart plays an active role in information processing rather than merely responding to commands from the central nervous system. Ongoing studies are exploring whether therapies that enhance vagal tone, including HRV biofeedback, might influence cardiac ganglia function and improve outcomes in conditions like heart failure and arrhythmias.
Inflammation, HRV, and Cardiovascular Disease Risk
The link between chronic inflammation and cardiovascular disease has become a major focus of research. Markers like C-reactive protein (CRP) and interleukin-6 (IL-6) are now recognized as independent risk factors for heart attack (Ridker et al., 2000). What makes this especially relevant for biofeedback practitioners is emerging evidence that HRV indexes the body's ability to regulate inflammation through the vagal anti-inflammatory pathway. Researchers are investigating whether HRV biofeedback training could reduce inflammatory markers by strengthening parasympathetic tone.
Advances in Blood Volume Pulse and Wearable Technology
The photoplethysmograph (PPG) technology behind BVP has undergone a revolution through consumer wearable devices. Smartwatches and fitness trackers now use PPG to estimate heart rate, detect irregular rhythms, and even approximate blood oxygen levels. These developments raise exciting possibilities for extending biofeedback practice beyond the clinic. Researchers are exploring whether wearable PPG devices can provide clinically meaningful HRV data for home-based training programs, potentially making cardiovascular biofeedback more accessible to a broader population.
Assignment
Now that you have completed this module, describe how this module has changed your understanding of hand-warming. Also, explain when blood volume pulse feedback could be more useful than temperature biofeedback.
Glossary
0.1 Hz biofeedback: training to concentrate ECG power around 0.1 Hz in the low frequency (LF) band by teaching patients to breathe diaphragmatically at their resonance frequency around 6 breaths per minute and experience positive emotional tone to maximize HR variability.
accentuated antagonism: the phenomenon in which the PNS can slow the heart by 20 or 30 beats per minute or briefly stop it, illustrating the dominance of parasympathetic control at rest.
alpha-adrenergic receptors: G protein-coupled receptors for the catecholamines epinephrine and norepinephrine. The binding of these catecholamines to arteriole alpha-adrenergic receptors can produce hand-cooling.
arrhythmias: abnormal or irregular rhythms, also called dysrhythmias.
arteries: blood vessels that carry blood away from the heart and that are divided into elastic and muscular arteries and arterioles.
arteriole vasoconstriction: the decreased diameter of an arteriole's lumen due to activation of vasoconstricting sympathetic nerves that act on alpha-adrenergic receptors, circulating hormones, and local chemical factors.
arteriole vasodilation: the increased diameter of an arteriole's lumen due to the circulation of a beta-adrenergic agent in the blood. There are no vasodilating nerves in the fingers.
arterioles: the almost-microscopic (8-50 microns in diameter) blood vessels that deliver blood to capillaries and anastomoses. Arterioles may control up to 50% of peripheral resistance through their narrow diameter, contractility, and massive surface area that follows a fractal pattern.
arteriovenous anastomoses (AVAs): junctions of two or more vessels that supply the same region, directly shunt blood from arterioles to venules, and help to regulate temperature.
atrioventricular (AV) bundle: cardiac cells that conduct electrical impulses from the AV node to the top of the septum.
atrioventricular (AV) node: one of two internal pacemakers primarily responsible for the heart rhythm, located between the atria and the ventricles.
autorhythmic fibers: cardiac fibers that spontaneously generate the pacemaker potentials that initiate cardiac contractions.
baroreflex: baroreceptor reflex that provides negative feedback control of BP. Elevated BP activates the baroreflex to lower BP, and low BP suppresses the baroreflex to raise BP.
beta-adrenergic agent: a molecule that binds to a beta-adrenergic receptor to start the cascade that causes hand-warming.
beta-adrenergic receptors: G protein-coupled receptors for the catecholamines epinephrine and norepinephrine. Catecholamine binding to the lumen of an arteriole is responsible for hand-warming.
blood pressure: the force exerted by blood as it presses against arteries.
blood volume pulse (BVP): the phasic change in blood volume with each heartbeat. It is the vertical distance between the minimum value (trough) of one pulse wave and the maximum value (peak) of the next measured using a photoplethysmograph (PPG).
bundle branches: fibers that descend along both sides of the septum (right and left bundle branches) and conduct the action potential over the ventricles about 0.2 seconds after the appearance of the P wave.
capillaries: blood vessels that are 7-9 microns in diameter, found near almost all cells, and may directly connect arterioles with venules or form extensive networks for rapid exchange of a large volume of substances (nutrients and waste products).
cardiac accelerator nerves: sympathetic nerves that arise from the medulla's cardiovascular center that increase the rate of spontaneous depolarization in the SA and AV nodes and increase stroke volume by strengthening the contractility of the atria and ventricles.
cardiac cycle: one cycle consists of systole (ventricular contraction) and diastole (ventricular relaxation).
cardiac output: the amount of blood pumped by the heart in a minute calculated by multiplying stroke volume times HR. This is 5.25 liters/minute (70 milliliters x 75 beats/minute) in a normal, resting adult.
cardiotachometer: device that measures the frequency of ventricular contraction beat-to-beat.
cardiovascular center: a structure located in the medulla of the brainstem that integrates sensory information and adjusts autonomic balance via sympathetic and parasympathetic motor neurons.
cations: positive ions like K+, Ca2+, and Na+.
chaos: unpredictability due to non-linear dynamics.
conduction myofibers: fibers that extend from the bundle branches into the myocardium, depolarizing contractile fibers in the ventricles.
diastole: the period when the ventricles or atria relax.
diastolic blood pressure (DBP): the force applied against arteries during ventricular relaxation.
dilation: increased lumen diameter.
dysrhythmias: an arrhythmia.
elastic arteries: large arteries like the aorta that distribute blood from the heart to muscular arteries.
electrocardiogram (ECG): a recording of the heart's electrical activity using an electrocardiograph.
frequency domain measures of HRV: the calculation of the absolute or relative power of the HRV signal within four frequency bands.
hand-cooling: reduced peripheral blood flow mainly controlled by vasoconstricting sympathetic nerves that act on alpha-adrenergic receptors. Circulating hormones and local factors also reduce the arteriolar diameter.
hand-warming: increased peripheral blood flow primarily due to circulating hormones and local vasodilators. There are no vasodilating nerves in the fingers, although they exist in the forearm.
heart: a hollow, muscular organ about the size of a closed fist that contains four chambers (two ventricles and two atria) that function as two pumps.
heart rate: the number of heartbeats per minute, also called stroke rate.
heart rate variability (HRV): beat-to-beat changes in HR, including changes in the RR intervals between consecutive heartbeats.
high coherence: a single high amplitude peak in the 0.09-0.14 Hz range.
high-frequency (HF) band: ECG frequency range from 0.15-0.40 Hz representing the inhibition and activation of the vagus nerve by breathing (RSA).
interbeat interval (IBI): the time interval between the peaks of successive R-spikes (initial upward deflections in the QRS complex). This period is also called the NN (normal-to-normal) interval.
left atrium: the upper chamber of the heart that receives oxygenated blood from the pulmonary veins and pumps it to the left ventricle.
left ventricle: the bottom chamber of the heart that receives oxygenated blood from the left atrium and pumps it through the aorta.
low-frequency (LF) band: the ECG frequency range of 0.04-0.15 Hz that may represent the influence of PNS, SNS, and baroreflex activity (when breathing at resonance frequency).
medium-sized muscular arteries: arteries like the brachial artery that receive blood from elastic arteries and distribute blood throughout the body.
neurovisceral integration model: Thayer and Lane's (2000) model describing how a central autonomic network links brainstem and forebrain structures through feedback and feed-forward loops to regulate HRV.
nonlinearly: unpredictably, reflecting the complex adaptive behavior of healthy physiological systems.
nucleus ambiguus system: the nucleus dorsal to the inferior olivary nucleus of the upper medulla that gives rise to vagus nerve motor fibers.
P wave: an ECG structure produced as contractile fibers in the atria depolarize and culminates in the atria's contraction (atrial systole).
parasympathetic nervous system (PNS): the autonomic branch that predominates at rest, slowing heart rate and promoting restorative functions through the vagus nerve.
parasympathetic vagus (X) nerves: cranial nerves that arise from the medulla's cardiovascular center, decrease the rate of spontaneous depolarization in SA and AV nodes, and slow the HR from the SA node's intrinsic rate of 100 beats per minute.
person effect: Taub and School's (1978) observation that biofeedback training is a social situation and that a client's relationship with the therapist may be the most critical aspect of training.
photoplethysmograph (PPG): a device that measures the relative amount of blood flow through tissue using a photoelectric transducer.
precapillary sphincter: in capillaries, a valve at the arterial end of a capillary that controls blood flow to the tissues.
pulse wave velocity (PWV): the rate of pulse wave movement through the arteries that is measured by placing pressure transducers (motion sensors) at two points along the arterial system (like the brachial and radial arteries of the same arm).
QRS complex: an ECG structure that corresponds to the depolarization of the ventricles.
R-spike: the initial upward deflection in the QRS complex of the ECG.
Raynaud's patients: medical patients diagnosed with Raynaud's disease or Raynaud's phenomenon who exhibit abnormal anastomoses dilation in response to mild cold-related stimuli.
regulatory capacity: the ability to adaptively respond to challenges like exercise and stressors.
response coupling: responses change together (HR up, BP up).
response fractionation: responses change independently (HR down, BP up).
resonance frequency: the frequency at which a system, like the cardiovascular system, can be activated or stimulated.
respiratory sinus arrhythmia (RSA): respiration-driven heart rhythm that contributes to the high frequency (HF) component of HRV. Inhalation inhibits vagal nerve slowing of the heart (increasing HR), while exhalation restores vagal slowing (decreasing HR).
right atrium: the upper chamber of the heart that receives deoxygenated blood and pumps it into the right ventricle.
right ventricle: the lower chamber of the heart that receives deoxygenated blood from the right atrium and pumps it into the pulmonary artery.
S-T segment: an ECG structure that connects the QRS complex and the T wave. Ventricular contraction continues through the S-T segment.
sinoatrial (SA) node: the node of the heart that initiates each cardiac cycle through spontaneous depolarization of its autorhythmic fibers.
skin temperature: an indirect index of peripheral blood flow, which is primarily regulated by cutaneous arterioles.
spectral analysis: the division of HRV into its component rhythms that operate within different frequency bands.
stroke volume: the amount of blood ejected by the left ventricle during one contraction.
sympathetic cardiac accelerator nerves: nerves that arise from the medulla's cardiovascular center that increase the rate of spontaneous depolarization in the SA and AV nodes and increase stroke volume by strengthening the contractility of the atria and ventricles.
sympathetic nervous system (SNS): the autonomic branch that mobilizes the body's resources for action, increasing heart rate and contractility through norepinephrine and epinephrine release.
systole: the contraction of the left ventricle.
systolic blood pressure (SBP): the force exerted by blood on arterial walls during contraction of the left ventricle.
T wave: ECG structure that represents ventricular repolarization.
transit time (TT): in pulse wave velocity, the interval required for the pulse wave to move between two points along the arterial system.
tunica externa: the external layer of an artery composed of a connective tissue sheath.
tunica interna: the innermost layer of an artery that responds to epinephrine and norepinephrine with vasodilation in digits like the fingers.
tunica media: the middle layer of an artery composed of smooth muscle and elastic fibers and controlled by sympathetic constrictor fibers (C-fibers). This layer is a site of neurally-controlled vasoconstriction (decrease in lumen diameter and blood flow) in the digits.
ultra-low-frequency (ULF) band: the ECG frequency range below 0.003 Hz. Very-slow biological processes that may contribute to this band include circadian rhythms, core body temperature, metabolism, and the renin-angiotensin system. There may also be PNS and SNS contributions.
vagal tone: parasympathetic nervous system activity, named for the vagus nerve which is the primary component of this autonomic branch.
vagal withdrawal: sympathetic suppression of parasympathetic activity associated with anxiety, effort, and fear.
vagus nerve: the parasympathetic vagus (X) nerve decreases the rate of spontaneous depolarization in the SA and AV nodes and slows the HR. Heart rate increases often reflect reduced vagal inhibition.
vasodilation: increase in lumen diameter and blood flow.
veins: blood vessels that route blood from tissues back to the heart and contain the same three layers found in arteries. These layers are thinner in veins due to lower pressure.
venule: a small vein (less than 2 millimeters in diameter) that collects blood from capillaries and delivers it to a vein. The low return pressure in these vessels requires valves that prevent backward blood flow.
very-low-frequency (VLF): the ECG frequency range of 0.003-0.04 Hz may represent temperature regulation, gastric, plasma renin fluctuations, endothelial, physical activity influences, possible intrinsic cardiac nervous system, PNS, and SNS contributions.
References
Agelink, M., Boz, C., Ullrich, H., & Andrich, J. (2002). Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry Research, 113(1-2), 139-149. https://doi.org/10.1016/s0165-1781(02)00225-1
Ballard, R. D. (1999). Sleep, respiratory physiology, and nocturnal asthma. Chronobiology International, 16(5), 565-580. https://doi.org/10.3109/07420529908998729
Breit, S., Kupferberg, A., Rogler, G., & Hasler, G. (2018). Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Frontiers in Psychiatry, 9, 44. https://doi.org/10.3389/fpsyt.2018.00044
Drew, B. J., Califf, R. M., Funk, M., Kaufman, E. S., Krucoff, M. W., Laks, M. M., Macfarlane, P. W., Sommargren, C., Swiryn, S., & Van Hare, G. F. (2004). Practice standards for electrocardiographic monitoring in hospital settings: An American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Circulation, 110(17), 2721-2746. https://doi.org/10.1161/01.CIR.0000145144.56673.59
Gellhorn, E. (1957). Autonomic imbalance and the hypothalamus. University of Minnesota Press.
Gevirtz, R., Schwartz, M. S., & Lehrer, P. M. (2016). Cardiorespiratory biofeedback. In M. S. Schwartz & F. Andrasik (Eds.), Biofeedback: A practitioner's guide (4th ed.). The Guilford Press.
Izzo, J. L., & Shykoff, B. E. (2001). Arterial stiffness: Clinical relevance, measurement, and treatment. Reviews in Cardiovascular Medicine, 2(1), 29-40.
Khazan, I. (2019). Biofeedback and mindfulness in everyday life: Practical solutions for improving your health and performance. W. W. Norton & Company.
MacKinnon, S., Gevirtz, R., McCraty, R., & Brown, M. (2013). Utilizing heartbeat evoked potentials to identify cardiac regulation of vagal afferents during emotion and resonance breathing. Applied Psychophysiology and Biofeedback, 38(4), 241-255. https://doi.org/10.1007/s10484-013-9226-5
MacLean, B. (2004). The heart and the breath of love. Biofeedback, 32(4), 21-25.
Maver, J., Strucl, M., & Accetto, R. (2004). Autonomic nervous system activity in normotensive subjects with a family history of hypertension. Clinical Autonomic Research, 14(6), 369-375. https://doi.org/10.1007/s10286-004-0185-z
McCraty, R., Atkinson, M., Tiller, W. A., Rein, G., & Watkins, A. D. (1995). The effects of emotions on short term power spectrum analysis of heart rate variability. American Journal of Cardiology, 76(14), 1089-1093. https://doi.org/10.1016/s0002-9149(99)80309-9
McCraty, R., Atkinson, M., Tomasino, D., & Bradley, R. T. (2006). The coherent heart. Institute of HeartMath.
McCraty, R., Atkinson, M., Tomasino, D., & Bradley, R. T. (2009). The coherent heart: Heart-brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral Review, 5(2), 10-115.
McCraty, R., & Shaffer, F. (2015). Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Global Advances in Health and Medicine, 4(1), 46-61. https://doi.org/10.7453/gahmj.2014.073
Moravec, C. S., & McKee, M. G. (2013). Psychophysiologic remodeling of the failing human heart. Biofeedback, 41(1), 7-12. https://doi.org/10.5298/1081-5937-41.1.04
Moss, D. (2004). Heart rate variability (HRV) biofeedback. Psychophysiology Today, 1, 4-11.
Nada, T., Nomura, M., Iga, A., Kawaguchi, R., Ochi, Y., Saito, K., Nakaya, Y., & Ito, S. (2001). Autonomic nervous function in patients with peptic ulcer studied by spectral analysis of heart rate variability. Journal of Medicine, 32(5-6), 333-347. PMID: 11958279
Nolan, J., Flapan, A. D., Capewell, S., MacDonald, T. M., Neilson, J. M., & Ewing, D. J. (1992). Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. British Heart Journal, 67(6), 482-485. https://doi.org/10.1136/hrt.67.6.482
Nunan, D., Sandercock, G. R. H., & Brodie, D. A. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology, 33(11), 1407-1417. https://doi.org/10.1111/j.1540-8159.2010.02841.x
Ogletree-Hughes, M. L., Stull, L. B., Sweet, W. E., Smedira, N. G., McCarthy, P. M., & Moravec, C. S. (2001). Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor down-regulation in the failing human heart. Circulation, 104, 881-886. https://doi.org/10.1161/hc3301.094911
Olshansky, B., Sabbah, H. N., Hauptman, P. J., & Colucci, W. S. (2008). Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation, 118, 863-871. https://doi.org/10.1161/circulationaha.107.760405
Opthof, T. (2000). The normal range and determinants of the intrinsic heart rate in man. Cardiovascular Research, 45, 177-184. PMID: 10728332
Papillo, J. F., & Shapiro, D. (1990). The cardiovascular system. In J. T. Cacioppo & L. G. Tassinary (Eds.), Principles of psychophysiology: Physical, social, and inferential elements (pp. 456-512). Cambridge University Press.
Peek, C. J. (2016). A primer of traditional biofeedback instrumentation. In M. S. Schwartz & F. Andrasik (Eds.), Biofeedback: A practitioner's guide (4th ed.). The Guilford Press.
Peper, E., Harvey, R., Lin, I., Tylova, H., & Moss, D. (2007). Is there more to blood volume pulse than heart rate variability, respiratory sinus arrhythmia, and cardio-respiratory synchrony? Biofeedback, 35(2), 54-61.
Porges, S. W. (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. W. W. Norton & Company.
Ridker, P. M., Rifai, N., Stampfer, M. J., & Hennekens, C. H. (2000). Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation, 101(15), 1767-1772. https://doi.org/10.1161/01.CIR.101.15.1767
Roach, D., Wilson, W., Ritchie, D., & Sheldon, R. (2004). Dissection of long-range heart rate variability: Controlled induction of prognostic measures by activity in the laboratory. Journal of the American College of Cardiology, 43(12), 2271-2277. https://doi.org/10.1016/j.jacc.2004.01.050
Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Frontiers in Psychology, 5, Article 1040. https://doi.org/10.3389/fpsyg.2014.01040
Shaffer, F., Meehan, Z. M., & Zerr, C. L. (2020). A critical review of ultra-short-term heart rate variability norms research. Frontiers in Neuroscience, 14, Article 594880. https://doi.org/10.3389/fnins.2020.594880
Shaffer, F., & Moss, D. (2006). Biofeedback. In Y. Chun-Su, E. J. Bieber, & B. Bauer (Eds.), Textbook of complementary and alternative medicine (2nd ed.). Informa Healthcare.
Stauss, H. M. (2003). Heart rate variability. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 285, R927-R931. https://doi.org/10.1152/ajpregu.00452.2003
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043-1065. PMID: 8598068
Taylor, J. A., Carr, D. L., Myers, C. W., & Eckberg, D. L. (1998). Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation, 98, 547-555. https://doi.org/10.1161/01.cir.98.6.547
Taylor, S. E. (2006). Tend and befriend: Biological bases of affiliation under stress. Current Directions in Psychological Science, 15(6), 273-277. https://doi.org/10.1111/j.1467-8721.2006.00451.x
Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J., & Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36, 747-756. https://doi.org/10.1016/j.neubiorev.2011.11.009
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201-216. https://doi.org/10.1016/s0165-0327(00)00338-4
Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122-131. https://doi.org/10.1016/j.ijcard.2009.09.543
Tortora, G. J., & Derrickson, B. H. (2021). Principles of anatomy and physiology (16th ed.). John Wiley & Sons, Inc.
Umetani, K., Singer, D. H., McCraty, R., & Atkinson, M. (1998). Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. Journal of the American College of Cardiology, 31(2), 593-601. https://doi.org/10.1016/s0735-1097(97)00554-8
Vaschillo, E., Lehrer, P., Rishe, N., & Konstantinov, M. (2002). Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback, 27, 1-27. https://doi.org/10.1023/a:1014587304314
Vaschillo, E., Vaschillo, B., & Lehrer, P. (2004). Heartbeat synchronizes with respiratory rhythm only under specific circumstances. Chest, 126, 1385-1386. https://doi.org/10.1016/S0012-3692(15)31329-5
Vaschillo, E. G., Vaschillo, B., & Lehrer, P. M. (2006). Characteristics of resonance in heart rate variability stimulated by biofeedback. Applied Psychophysiology and Biofeedback, 31(2), 129-142. https://doi.org/10.1007/s10484-006-9009-3
Vaschillo, E. G., Vaschillo, B., Pandina, R. J., & Bates, M. E. (2011). Resonances in the cardiovascular system caused by rhythmical muscle tension. Psychophysiology, 48, 927-936. https://doi.org/10.1111/j.1469-8986.2010.01156.x
Walløe, L. (2016). Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature (Austin), 3(1), 92-103. https://doi.org/10.1080/23328940.2015.1088502
Widmaier, E. P., Raff, H., & Strang, K. T. (2019). Vander's human physiology: The mechanisms of body function (15th ed.). McGraw-Hill.
Yasuma, F., & Hayano, J. (2004). Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm? Chest, 125(2), 683-690. https://doi.org/10.1378/chest.125.2.683
Return to Top