Heart Rate Variability (HRV)
What You Will Learn
"HRV is the organized fluctuation of time intervals between successive heartbeats defined as interbeat intervals" (Shaffer et al., 2020). The oscillations of a healthy heart are complex, and HRV indexes how efficiently you mobilize and utilize limited self-regulatory resources to maintain homeostasis. In this chapter, you will discover why HRV plays a vital role in regulatory capacity, executive functions, health, and performance.
You will explore the meaning and sources of HRV, including respiratory sinus arrhythmia, the baroreceptor reflex, and vascular tone rhythms. You will learn how factors like age, heart rate, breathing rate, and resonance influence HRV. You will examine the correlates of low and normal HRV, understand the benefits of increased HRV, and see how HRV biofeedback works through Vaschillo's two closed-loop model. Whether you are a student learning psychophysiology or a clinician integrating biofeedback into practice, this chapter will give you the foundational understanding you need.
A healthy heart can rapidly adjust to sudden challenges because its interlocking control systems are well-calibrated and cooperative. HRV is central to health, performance, and resilience, and behavioral interventions such as aerobic exercise, healthy breathing, compassion, and mindfulness meditation offer powerful, evidence-based strategies for increasing it. For clinicians, HRV provides both a window into a client's autonomic flexibility and a measurable training target.

BCIA Blueprint Coverage
This unit addresses II. Heart Rate Variability (2 hours).
Professionals completing this module will be able to discuss the meaning of HRV, the sources of HRV, factors that influence HRV, correlates of low and normal HRV, and the benefits of increased HRV.
This unit covers The Meaning of HRV, The Sources of HRV, Factors that Influence HRV, Correlates of Low and Normal HRV, The Benefits of HRV, and Heart-Brain Interactions.
🎧 Listen to the Full Chapter Lecture
The Meaning of HRV
This section introduces the concept of heart rate variability and explains why it matters for clinical and optimal performance practice. You will learn how heart rate constrains HRV, why a healthy heart is not a metronome, and how key theoretical frameworks connect HRV to self-regulation and health. Understanding these fundamentals will shape how you assess clients, set training goals, and explain biofeedback rationale.
Heart Rate and Its Consequences
Heart rate is the number of heartbeats per minute. In clinical practice, a client's resting heart rate is often the first physiological metric you observe, and it carries more prognostic weight than many clinicians realize.

Elevated HR Is Associated with Dementia and Cognitive Decline
Imahori et al. (2021) conducted a cohort study of 2,147 adults aged 60 and older who were free of dementia at enrollment. Resting heart rates of 80 bpm or higher, compared with 60 to 69 bpm, were associated with a greater risk of dementia and more rapid cognitive decline, independent of cardiovascular disease. For practitioners working in VA settings or geriatric care, this finding suggests that elevated resting heart rate may be a modifiable risk factor worth monitoring alongside traditional cognitive screening.
Elevated HR Limits HRV
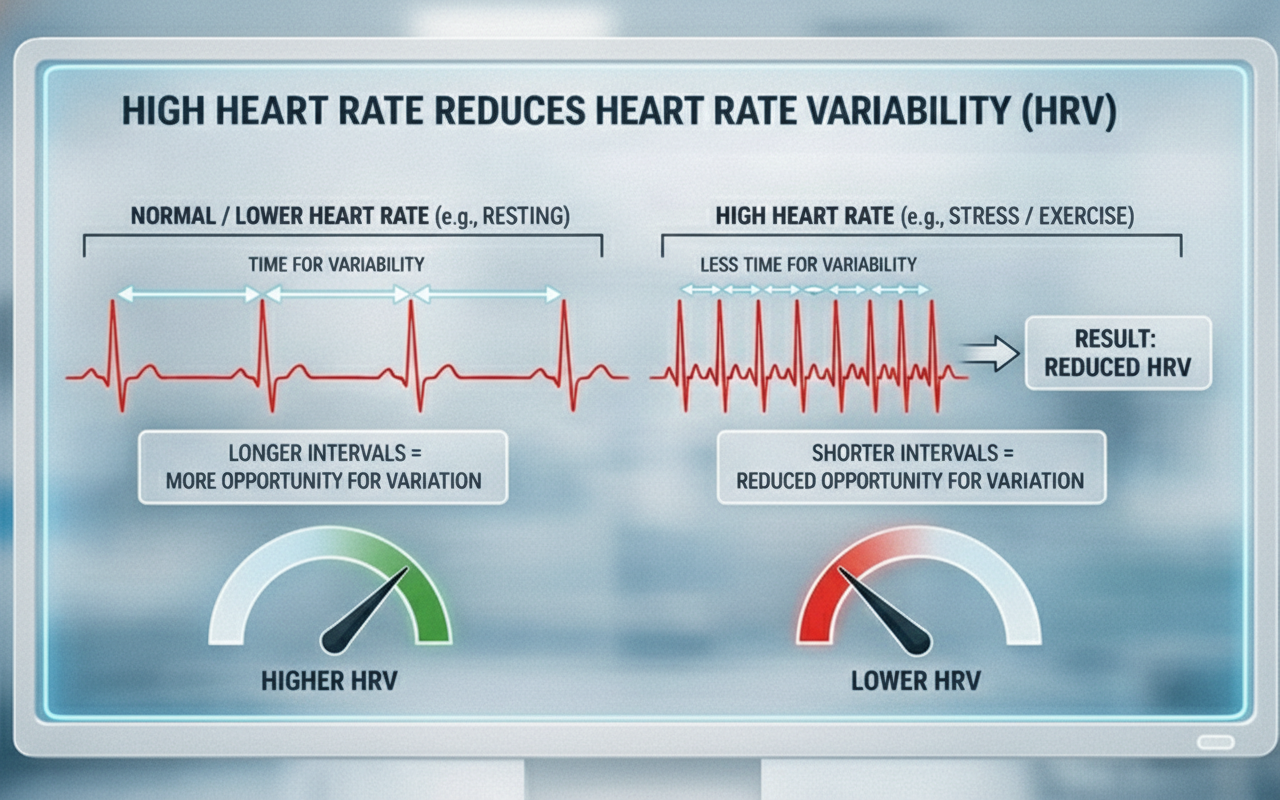
Heart rate matters for biofeedback practitioners because a high rate can reduce heart rate variability (HRV)—the changes in the time intervals between consecutive heartbeats (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). We measure these interbeat intervals (IBIs) in milliseconds, and the degree to which they fluctuate reflects the heart's capacity to adapt moment by moment.
🎧 Mini-Lecture: Heart Rate Variability Overview
Faster heart rates reduce the time between successive beats, leaving less opportunity for IBIs to vary. This is a straightforward but clinically important point: an anxious client presenting with a resting heart rate of 90+ bpm has limited room for beat-to-beat fluctuation, which constrains HRV before training even begins. Resting heart rates that exceed 90 bpm are also associated with an elevated risk of mortality (Zhang et al., 2016).
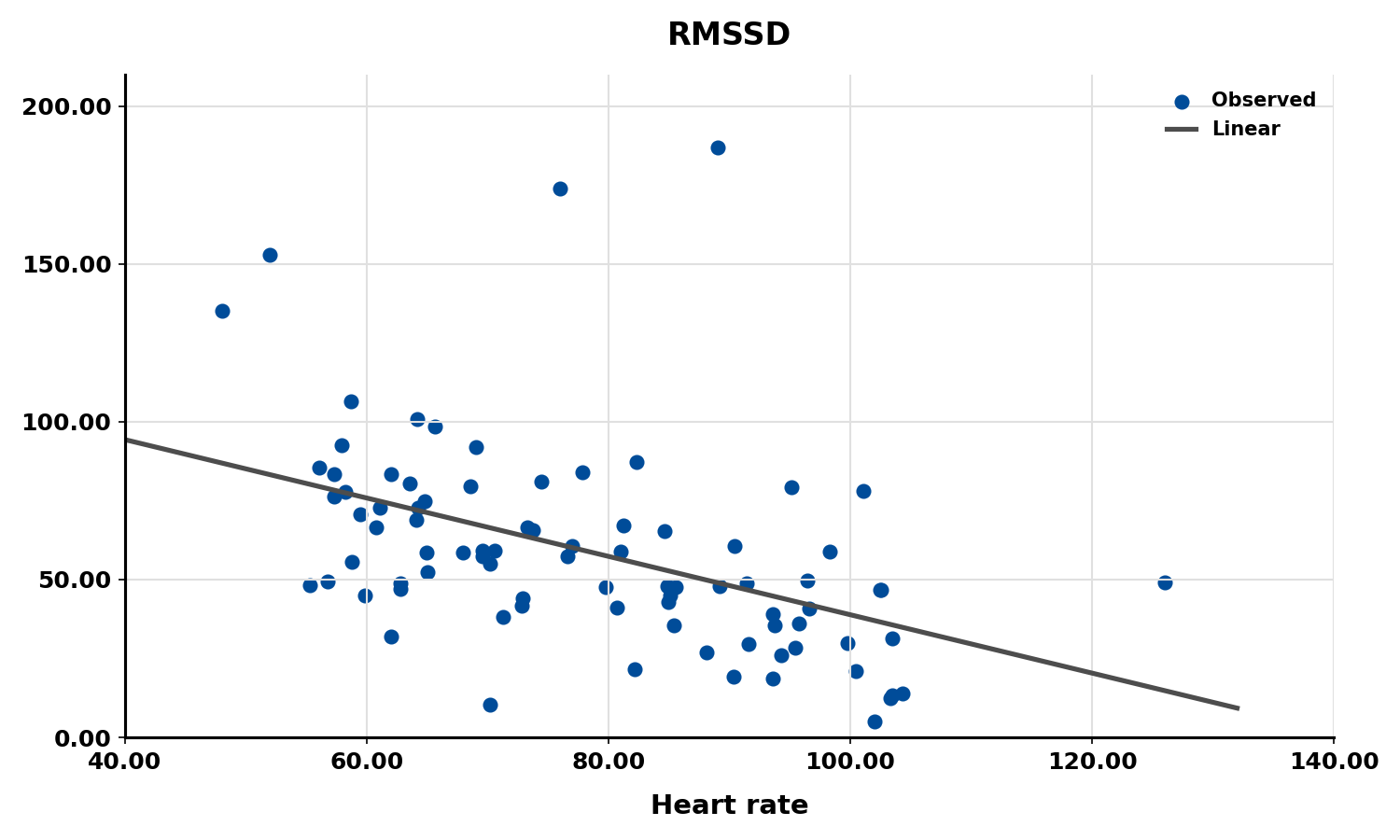
The next three scatterplots illustrate this inverse relationship between HR and three widely used HRV metrics: RMSSD, SDNN, and low-frequency power.
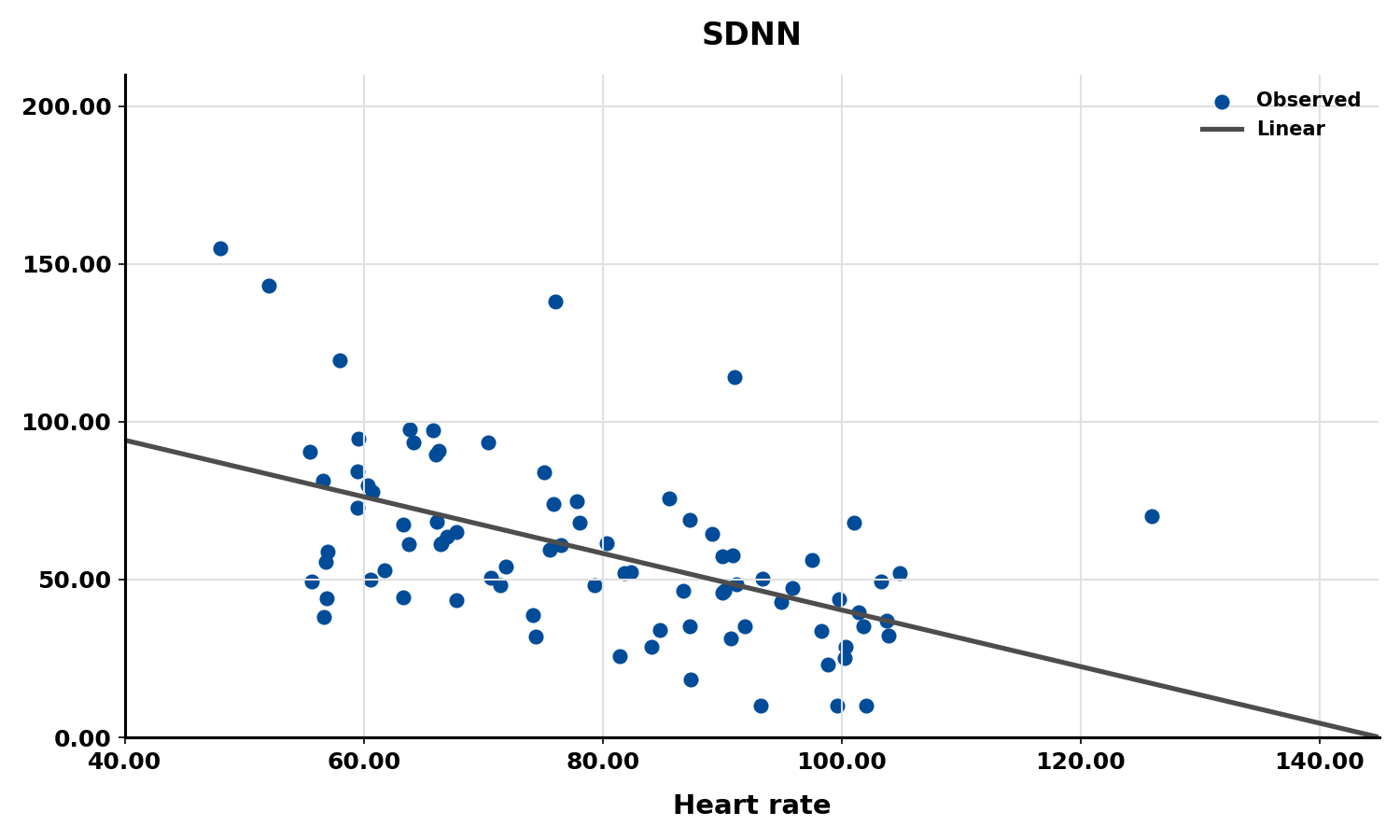
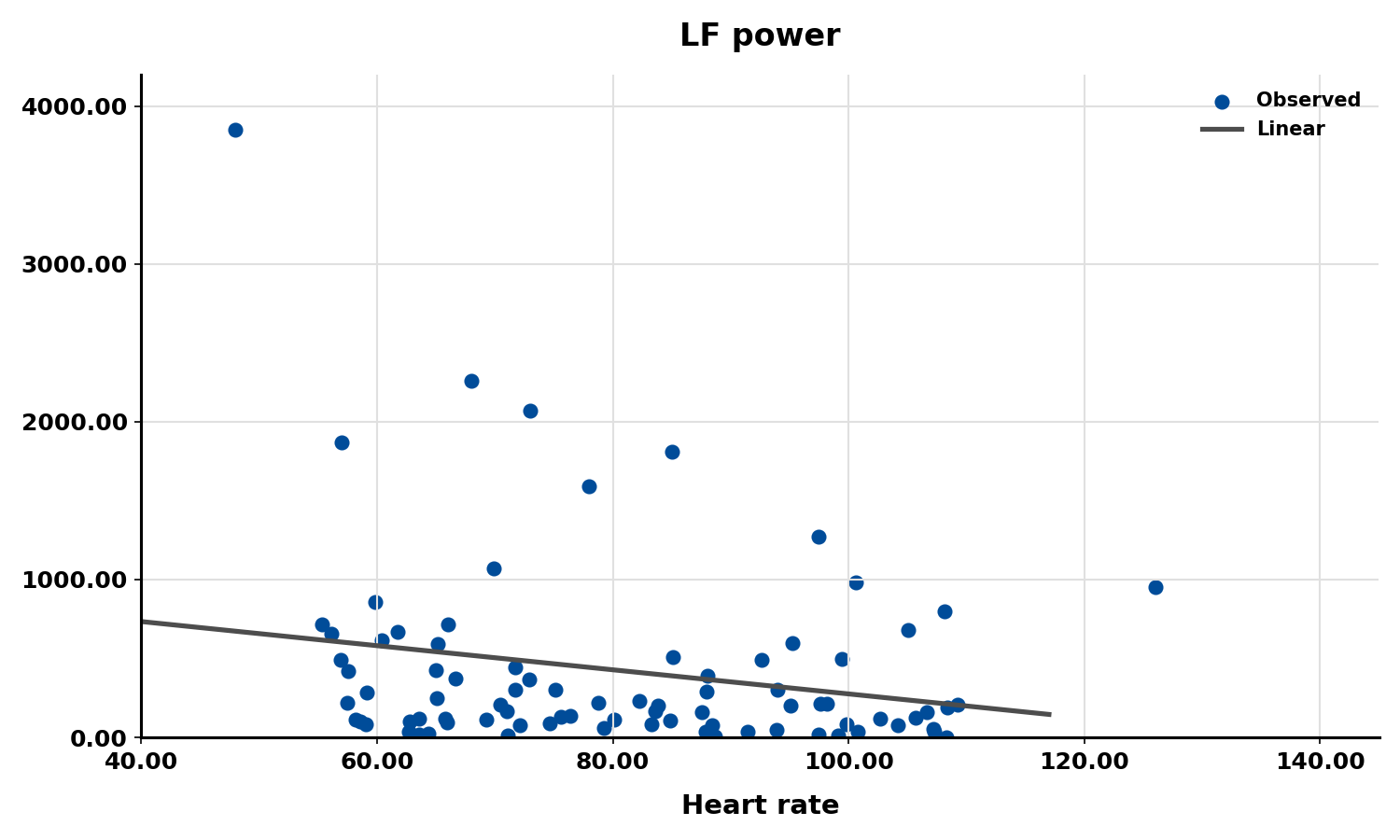
Conversely, the slower heart rates seen in endurance athletes—such as trail runners—increase the time between adjacent heartbeats, creating more opportunity for IBIs to vary and raising HRV. This phenomenon is called cycle length dependence (McCraty & Shaffer, 2015), and it explains why aerobic conditioning is one of the most reliable ways to improve HRV outside the clinic.

Typical non-athlete resting heart rates range from 60 to 80 bpm, while athletes may have resting rates between 40 and 60 bpm (Khazan, 2019). When you encounter an athlete with unusually low resting heart rate, expect correspondingly higher HRV values—an important consideration when establishing normative baselines for optimal performance training.

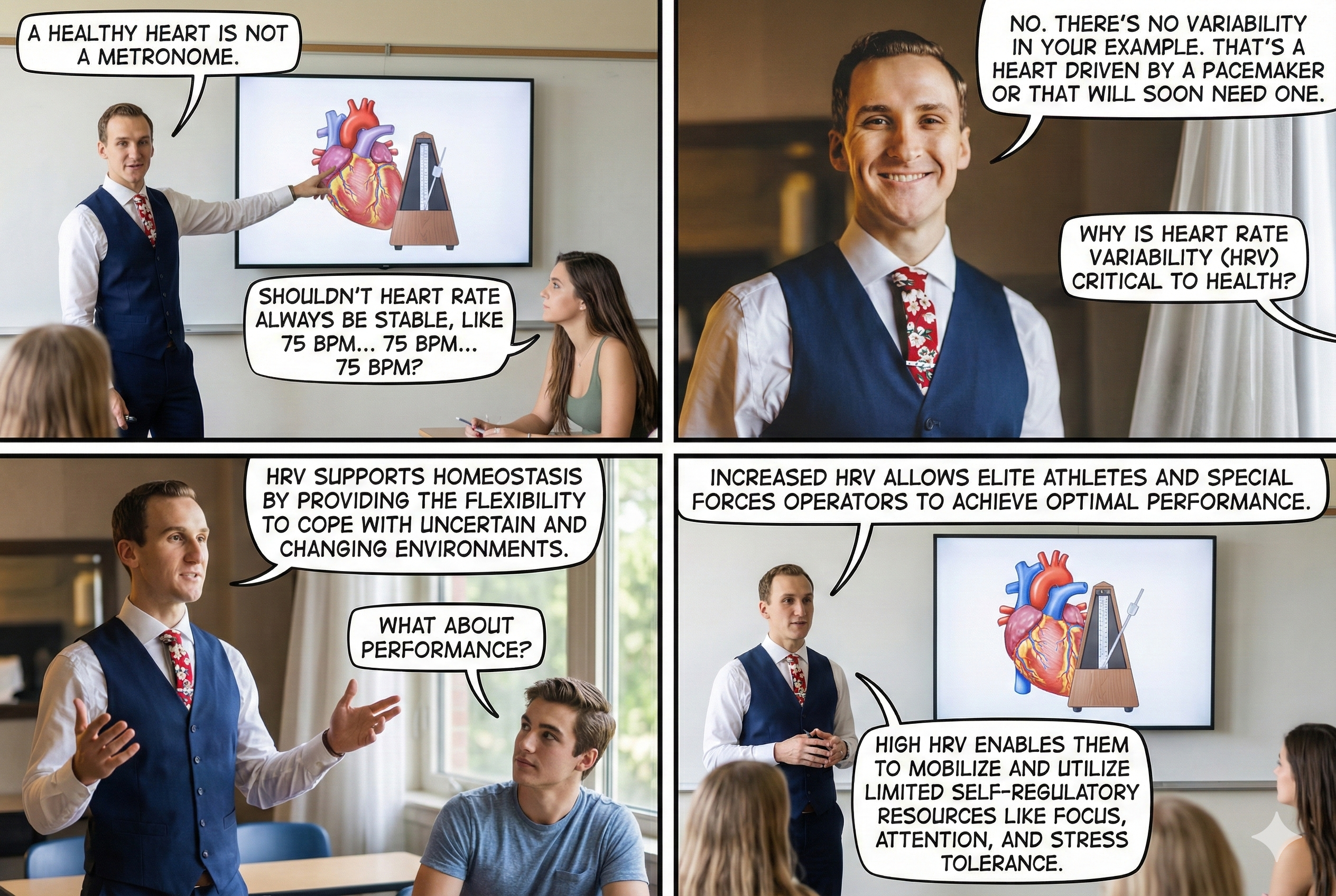
A Healthy Heart Is Not a Metronome
A healthy heart is not a metronome. This simple idea is one of the most important concepts in HRV biofeedback. When the time intervals between heartbeats change substantially across successive breathing cycles, it demonstrates that the cardiovascular center can effectively modulate vagal tone—the parasympathetic "brake" on the heart.
🎧 Mini-Lecture: Why Is Heart Rate Variability Important?
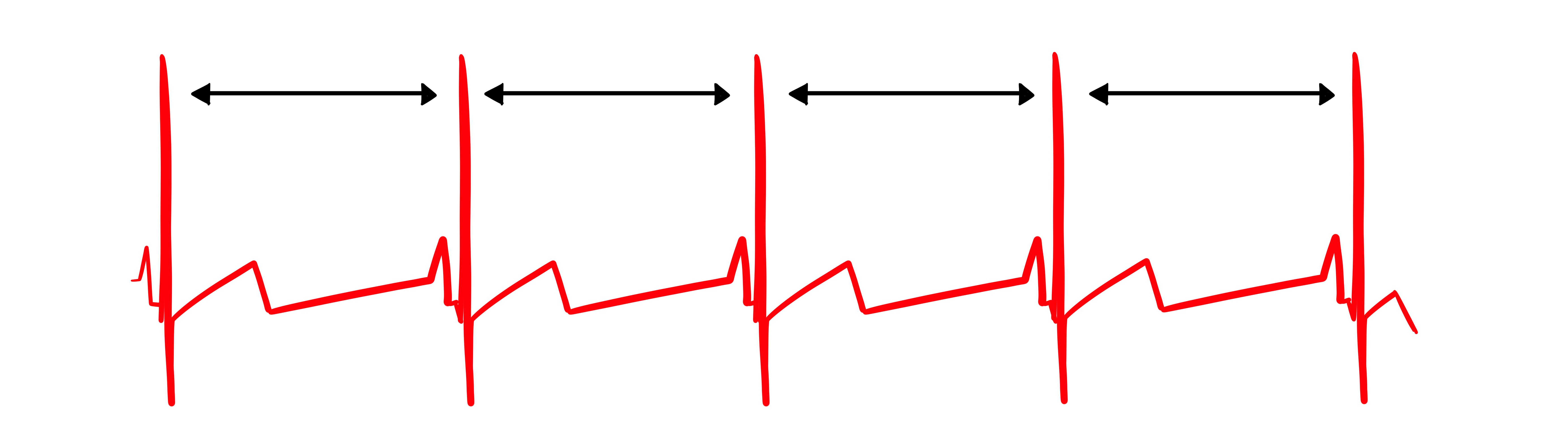
The record below shows healthy variability—the time intervals between successive heartbeats clearly differ. For your clients, this is the pattern you want to see and help them develop.
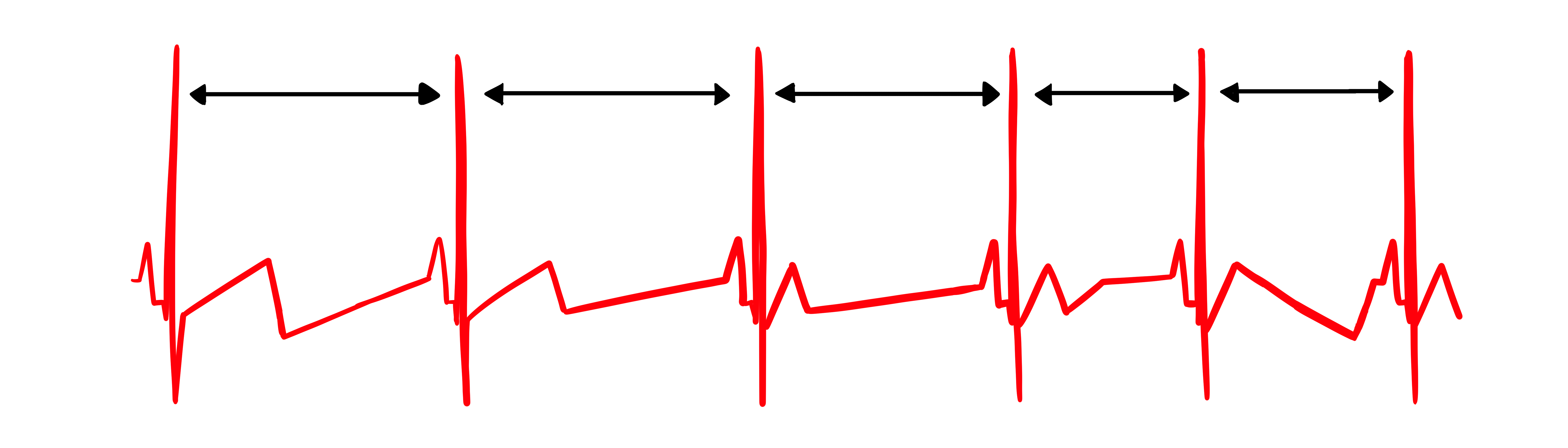
In contrast, this record shows no variability: the IBIs are identical. This display could represent a heart driven by a pacemaker or one that needs one. When you see this pattern in a client who is not pacemaker-dependent, it signals significantly compromised autonomic regulation.
"The complexity of a healthy heart rhythm is critical to the maintenance of homeostasis because it provides the flexibility to cope with an uncertain and changing environment. HRV metrics are important because they are associated with regulatory capacity, health, and performance and can predict morbidity and mortality" (Shaffer et al., 2020).
Check out the YouTube video HRV Training and its Importance.
"HRV is associated with executive function, regulatory capacity, and health. Cardiac vagal control indexes how efficiently we mobilize and utilize limited self-regulatory resources during resting, reactivity, and recovery conditions" (Shaffer et al., 2020).
HRV as a Multisystem Biomarker
Vagal tone modulation helps maintain the dynamic autonomic balance critical to cardiovascular health. Autonomic imbalance due to deficient vagal inhibition is implicated in increased morbidity and all-cause mortality (Thayer, Yamamoto, & Brosschot, 2010). For the clinician, this means that HRV is not merely a research metric—it is a practical indicator of how well a client's autonomic nervous system is functioning day to day.
HRV appears to index autonomic functioning, blood pressure, neurocardiac functioning, digestion, oxygen and carbon dioxide exchange, vascular tone (the diameter of resistance vessels), and possibly facial muscle regulation (Gevirtz et al., 2016). It also reflects the vagal contribution to executive functions, affective control, and social self-regulation (Byrd et al., 2015; Laborde et al., 2017; Mather & Thayer, 2018). This breadth of associations is why HRV biofeedback has applications across such diverse clinical populations—from cardiac rehabilitation patients to service members managing stress.
Influential HRV Theories
Two theoretical frameworks help clinicians understand why HRV matters and how to interpret it. The Vagal Tank Theory frames cardiac vagal control as a dynamic, depletable resource for self-regulation, while the Autonomic Space Theory reveals that sympathetic and parasympathetic activity interact more flexibly than older models assumed. Together, they provide a comprehensive framework for understanding HRV as a marker of physiological adaptability, emotional regulation, and cognitive flexibility.
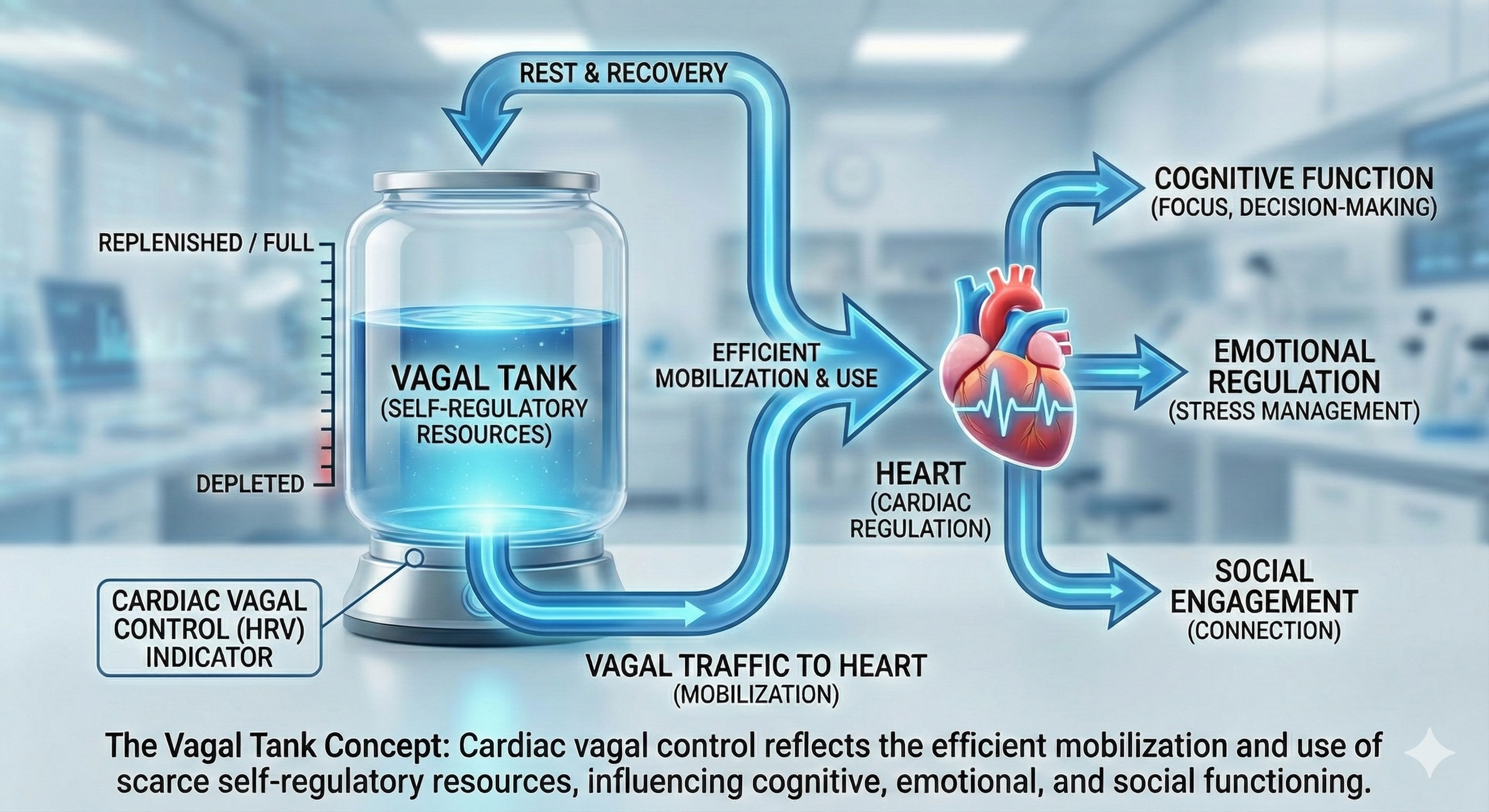
Vagal Tank Theory
The Vagal Tank Theory (Laborde et al., 2018) conceptualizes cardiac vagal control as a dynamic resource that can be depleted or replenished—much like a fuel tank. It consists of three Rs: (1) Resting vmHRV, reflecting baseline self-regulation capacity; (2) Reactivity, the vagal withdrawal response to stressors; and (3) Recovery, the ability to restore autonomic balance post-stressor. A higher vagal "tank" is associated with better stress resilience, emotional regulation, and health outcomes. In practice, this means you need to assess clients at rest, during challenge, and during recovery to get a complete picture of their autonomic resources. A single resting vmHRV measurement tells only part of the story.
From Laborde and colleagues' perspective, vagal traffic to the heart indicates how efficiently we mobilize and use scarce self-regulatory resources.
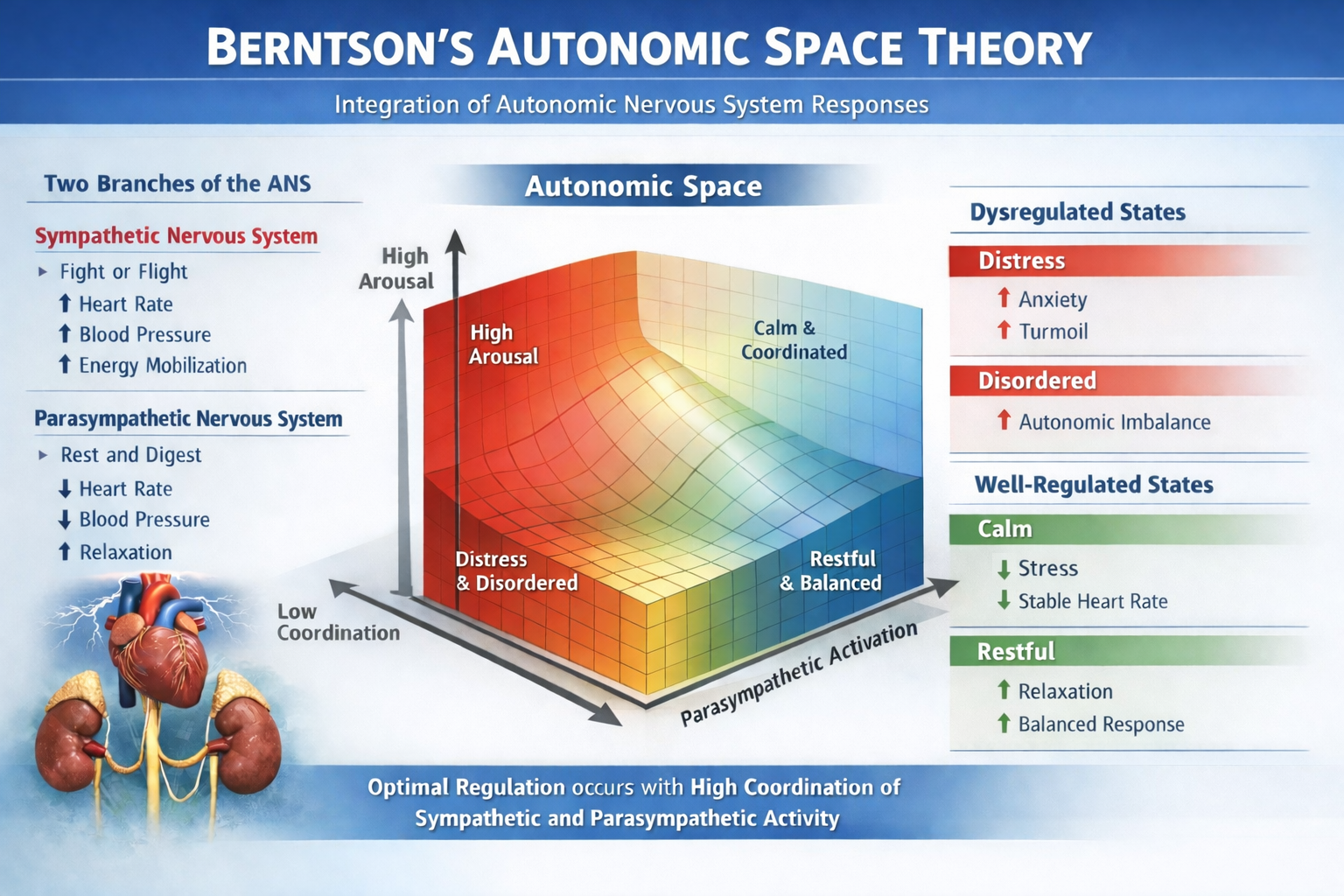
Autonomic Space Theory
The Autonomic Space Theory (Berntson et al., 1994) challenges the simplistic reciprocal model of autonomic control by proposing three modes: (1) Reciprocal Activation, where one branch is active and the other is suppressed; (2) Coactivation, where both sympathetic and parasympathetic systems are active simultaneously; and (3) Coinhibition, where both are suppressed. This framework matters clinically because it explains why some clients show paradoxical physiological patterns—such as elevated heart rate alongside high HRV—that a simple "seesaw" model of autonomic balance cannot explain.
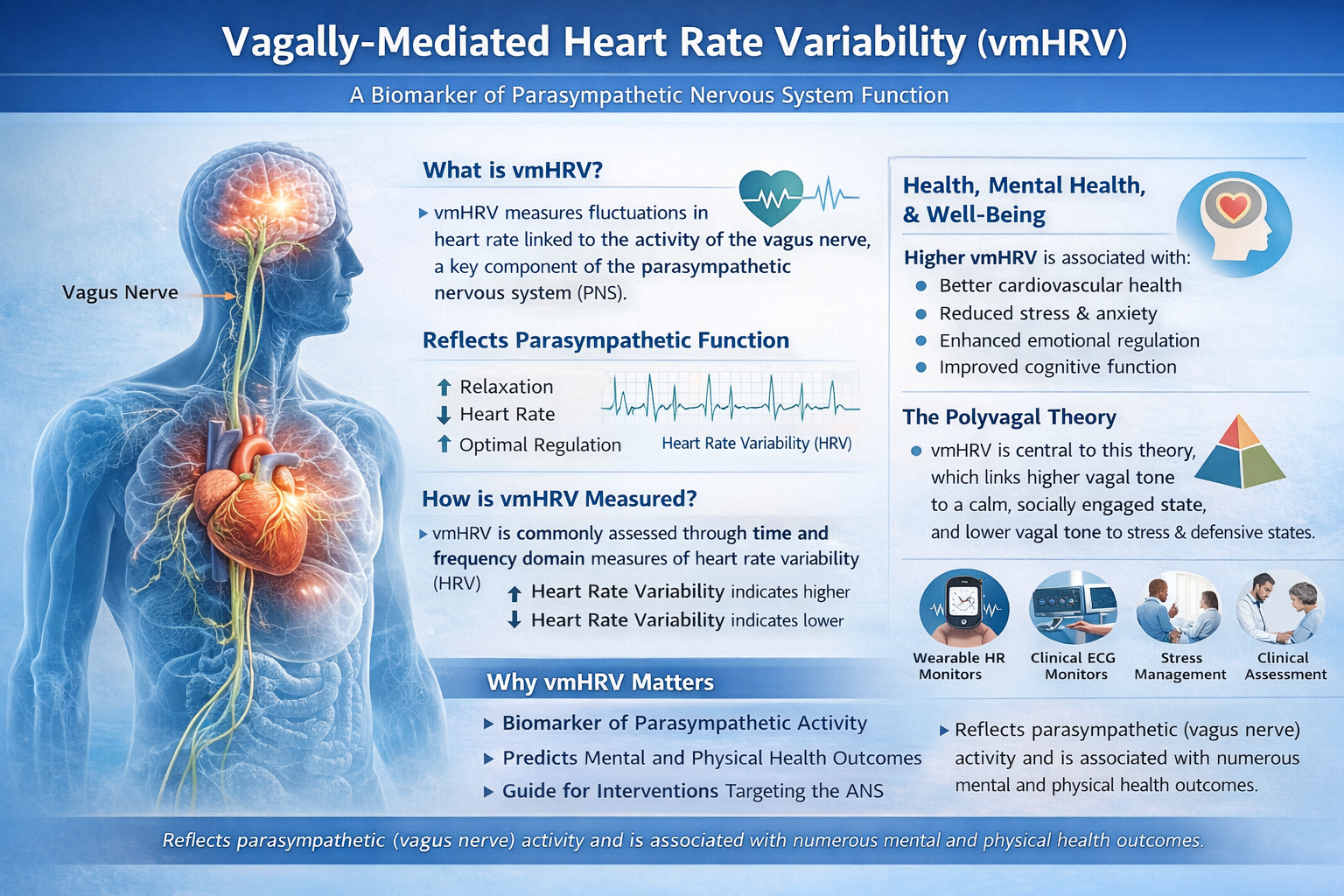
Vagally-Mediated HRV (vmHRV)
This subsection covers vagally-mediated HRV (vmHRV) as a clinical biomarker, its companion concept of sympathetically-mediated HRV (smHRV), and the health implications of vmHRV across multiple body systems. Understanding vmHRV is essential because it provides the clearest autonomic signal you can track with standard biofeedback equipment.
Vagally-mediated heart rate variability (vmHRV) has emerged as a critical biomarker for self-regulation and health, offering insights into psychological and physiological adaptation. As a noninvasive and cost-effective tool, vmHRV serves as an actionable measure in physical and mental health, social interactions, stress regulation, and performance optimization (Laborde et al., 2023). For clinicians and performance coaches alike, vmHRV is one of the most practical psychophysiological metrics available.
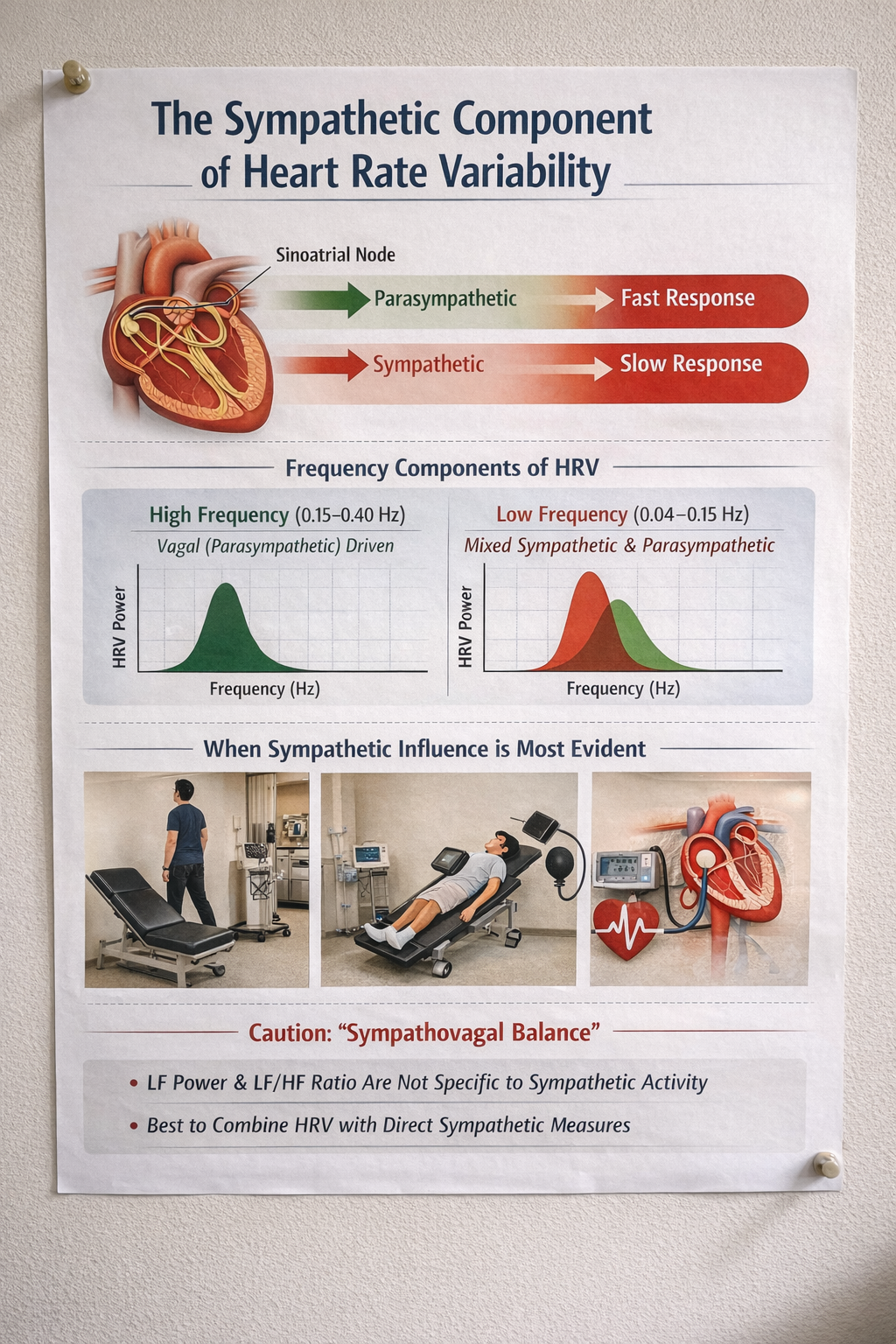
The sympathetic nervous system is another source of HRV. Sympathetically-mediated HRV (smHRV) refers to HRV components influenced by the sympathetic nervous system, typically assessed through measures such as low-frequency (LF) power in HRV analysis. However, the interpretation of LF power as a direct marker of sympathetic activity remains debated—a nuance that matters when you interpret spectral analysis results for clients.
How the Autonomic Nervous System Shapes Heart Rate Variability
HRV captures the moment-to-moment push and pull of the autonomic nervous system on the heart's pacemaker, the sinoatrial node. The two branches of this system operate on very different timelines. Parasympathetic (vagal) signals act almost instantaneously, modulating heart rate on a beat-to-beat basis through acetylcholine release. Sympathetic signals, by contrast, work more slowly, taking several seconds to exert their effects through norepinephrine. This speed difference is why high-frequency HRV (roughly 0.15–0.40 Hz), and especially the familiar breathing-linked rhythm known as respiratory sinus arrhythmia, is understood to be primarily a vagal phenomenon in healthy adults (Berntson et al., 1997; Malik et al., 1996).
Slower Rhythms Tell a More Complex Story
The question of whether sympathetic activity leaves a detectable signature in HRV has been explored for decades. Foundational spectral analysis work by Akselrod and colleagues (1981) first demonstrated that HRV contains separable frequency components tied to distinct autonomic control processes. Later, Pomeranz et al. (1985) used pharmacologic blockade combined with postural changes to show that low-frequency oscillations (roughly 0.04–0.15 Hz) shift from predominantly parasympathetic mediation when a person is lying down to a mixed sympathetic-parasympathetic pattern when standing. Perhaps the most compelling evidence comes from microneurography studies, in which researchers directly record sympathetic nerve traffic in peripheral nerves. Pagani et al. (1997) showed that as sympathetic drive increases, LF oscillations become more prominent and track closely with sympathetic nerve firing patterns and arterial pressure fluctuations, pointing to a shared sympathetic rhythm during activation.
When Sympathetic Signatures Emerge Most Clearly
A sympathetic contribution to HRV appears most consistently under conditions that ramp up sympathetic drive and strongly engage the baroreflex, the negative-feedback loop in which blood pressure changes trigger reflex autonomic adjustments. Classic examples include standing up (orthostatic stress), head-up tilt testing, and experimentally induced blood pressure changes using vasoactive drugs (Pomeranz et al., 1985; Pagani et al., 1997). In these situations, vagal withdrawal typically reduces HF power and short-term time-domain measures like RMSSD, while spectral energy shifts toward the LF band. Tilt experiments illustrate this dramatically: LF power becomes dominant, and the LF/HF ratio rises markedly; when beta-adrenergic blockers are administered, this LF predominance is blunted, supporting the case for sympathetic involvement (Pagani et al., 1986). For longer recordings, broader measures like SDNN capture total variance across many time scales, including slower sympathetically linked and circadian influences, so they may include sympathetic contributions without being specific to sympathetic cardiac control (Berntson et al., 1997; Malik et al., 1996).
Why "Sympathovagal Balance" Remains Contentious
A heated debate persists over whether standard HRV metrics can validly quantify cardiac sympathetic tone or so-called sympathovagal balance in any straightforward, context-independent way. Critics point out that LF power is heavily shaped by baroreflex modulation of both autonomic branches and often contains substantial parasympathetic contributions, making it an ambiguous stand-in for sympathetic control (Eckberg, 1997; Goldstein et al., 2011). Adding to the complexity, LF power and the LF/HF ratio can shift simply because vagal tone drops, even when direct sympathetic measures show no parallel increase. Reviews synthesizing blockade studies and cross-method comparisons have concluded that LF and LF/HF do not reliably track sympathetic cardiac activity across different conditions and should not be treated as direct sympathetic measures (Billman, 2013; Reyes del Paso et al., 2013). Instead, clinicians and researchers who need to draw inferences about sympathetic function are best served by triangulating HRV data with independent measures such as blood pressure variability, pre-ejection period, microneurography, or catecholamine levels, while carefully controlling for respiration and signal nonstationarity.
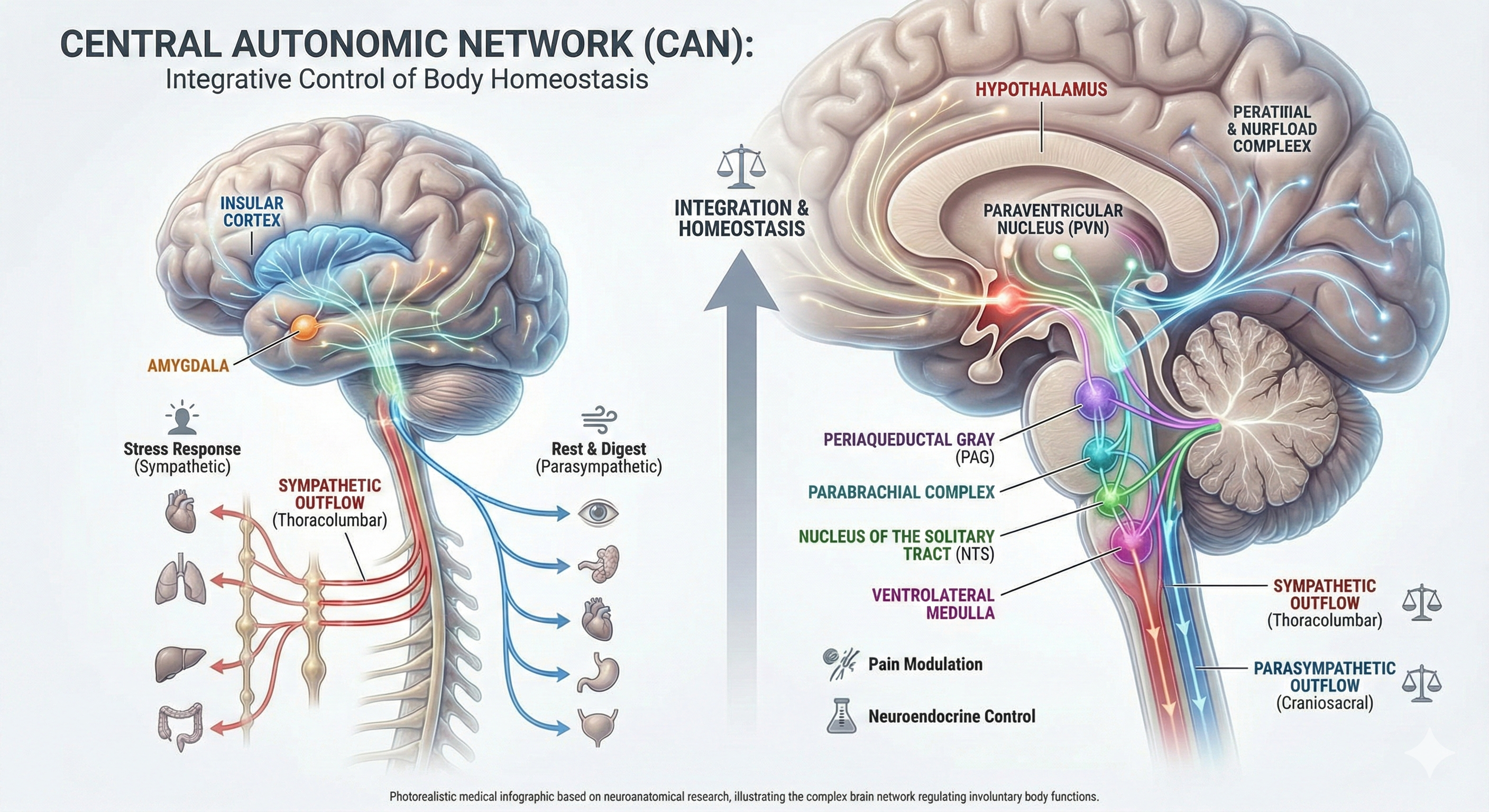
vmHRV and Self-Regulation
Heart rate variability, particularly its vagally mediated component, reflects the dynamic interplay between the autonomic nervous system and cognitive-emotional regulation. The neurovisceral integration model (Thayer et al., 2009) posits that vmHRV is linked to self-regulation processes via prefrontal cortical control over the vagus nerve, facilitating adaptive responses to environmental demands. In plain terms, the prefrontal cortex acts as a top-down regulator of the heart through vagal pathways, and vmHRV is the measurable signal of that regulation.
Health Implications of vmHRV
Cardiovascular Health
The autonomic nervous system (ANS) plays a critical role in maintaining physiological stability, and vmHRV is an essential biomarker of its function. Higher vmHRV indicates robust parasympathetic activity, which supports greater adaptability to environmental and internal stressors. Lower vmHRV, on the other hand, signals autonomic dysregulation and increased health risks.
Numerous studies have demonstrated that lower vmHRV predicts cardiovascular disease (Hillebrand et al., 2013), including hypertension, atherosclerosis, and heart failure. Individuals with higher vmHRV tend to exhibit greater cardiovascular efficiency, characterized by better baroreceptor sensitivity, improved endothelial function, and enhanced myocardial perfusion. In contrast, diminished vmHRV is often accompanied by increased arterial stiffness, reduced heart rate recovery following exertion, and an overall heightened risk for adverse cardiac events, including myocardial infarction and stroke.
Metabolic Regulation
Beyond cardiovascular health, vmHRV has significant implications for metabolic regulation. Research has consistently linked low vmHRV to metabolic disorders such as diabetes mellitus (Benichou et al., 2018), insulin resistance, and obesity. The autonomic imbalance observed in individuals with lower vmHRV contributes to dysregulated glucose metabolism, increased systemic inflammation, and greater susceptibility to metabolic syndrome.
High vmHRV, conversely, is associated with better glycemic control and improved pancreatic beta-cell function. The modulation of insulin sensitivity via vagal pathways suggests that vmHRV-enhancing interventions—such as slow-paced breathing and vagus nerve stimulation—could serve as valuable therapeutic strategies for individuals with metabolic disorders. For clinicians treating diabetic veterans or hospital patients with metabolic syndrome, HRV biofeedback offers a complementary approach that targets an upstream regulatory mechanism.
Immune Function
Another critical dimension of vmHRV's health implications is its connection to immune function. The cholinergic anti-inflammatory pathway, mediated by vagal activity, plays a pivotal role in regulating inflammatory responses (Williams et al., 2019). Lower vmHRV is associated with increased levels of pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), which are implicated in the pathogenesis of numerous chronic diseases, including autoimmune conditions, neurodegenerative diseases, and cancer.
Conversely, higher vmHRV correlates with an anti-inflammatory state that facilitates faster recovery from infections and injuries while mitigating chronic inflammation-related pathologies. This has led to growing interest in using vmHRV as a biomarker for immune resilience, particularly in conditions such as rheumatoid arthritis, lupus, and inflammatory bowel disease. The clinical implication is clear: by training clients to improve vagal tone, you may help modulate their inflammatory burden as well.
Stress and Sleep Regulation
The relationship between vmHRV and stress-related health outcomes is particularly compelling. Chronic stress and prolonged exposure to psychological stressors contribute to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis—the body's central stress response system—and sustained autonomic imbalance. Individuals with lower vmHRV exhibit heightened sympathetic activity and reduced parasympathetic regulation, leading to persistently elevated cortisol levels, increased oxidative stress, and greater allostatic load (the cumulative wear and tear from chronic stress).
This autonomic dysfunction is linked to conditions including irritable bowel syndrome, chronic fatigue syndrome, and fibromyalgia—conditions frequently seen in VA, hospital, and clinic settings. Individuals with higher vmHRV demonstrate more efficient stress recovery mechanisms, allowing for better emotional and physiological resilience.
The significance of vmHRV in sleep regulation further highlights its broad health implications. Sleep disturbances, including insomnia, sleep apnea, and restless leg syndrome, have been associated with reduced vmHRV, indicating impaired autonomic balance during sleep (Chouchou & Desseilles, 2014). Poor sleep quality triggers a cascade of negative health outcomes, including impaired cognitive function, weakened immune defense, and increased cardiovascular and metabolic risk.
Individuals with higher vmHRV tend to experience more stable sleep patterns, enhanced slow-wave sleep, and improved nocturnal autonomic regulation, which collectively support overall well-being and cognitive performance. For clinicians, this connection between vmHRV and sleep quality provides a strong rationale for incorporating HRV biofeedback into treatment plans for clients with insomnia or other sleep disorders.
Clinical and Psychological Relevance
Mental Health Disorders
In clinical psychology and psychiatry, vmHRV is increasingly recognized as a transdiagnostic biomarker—a marker that cuts across traditional diagnostic categories rather than being specific to any single disorder. Individuals with low vmHRV consistently exhibit greater vulnerability to psychiatric disorders, including anxiety disorders, major depressive disorder (MDD), posttraumatic stress disorder (PTSD), and bipolar disorder (Beauchaine & Thayer, 2015). Reduced vmHRV in these populations reflects diminished vagal regulation of emotional responses, heightened sympathetic arousal, and impaired top-down control from prefrontal cortical regions.
For anxiety disorders, lower vmHRV is associated with excessive autonomic arousal, persistent worry, and hypervigilance (Wang et al., 2023). Individuals with generalized anxiety disorder (GAD) often show reduced parasympathetic tone, leading to increased heart rate, respiratory irregularities, and prolonged physiological recovery from stressors. This is precisely the pattern you will observe on biofeedback screens during intake assessments.
Similarly, individuals with PTSD exhibit pronounced autonomic dysregulation, with lower vmHRV reflecting impaired fear extinction and heightened amygdala reactivity to trauma-related cues. Targeted interventions such as HRV biofeedback and vagal nerve stimulation have shown promise in improving autonomic regulation and reducing symptoms of hyperarousal in these populations. For practitioners working with military service members and veterans, HRV biofeedback offers an evidence-based approach to addressing the autonomic roots of trauma-related symptoms.
Comprehension Questions: The Meaning of HRV
- How does cycle length dependence explain the relationship between heart rate and HRV?
- What are the three Rs of the Vagal Tank Theory, and why are they clinically relevant?
- Explain how vmHRV serves as a biomarker across cardiovascular, metabolic, and immune function.
- Why is a heart with no variability between beats a cause for clinical concern?
The Sources of HRV
Now that you understand what HRV is and why it matters, this section examines where HRV comes from. You will learn about the three most important sources of short-term HRV, how Vaschillo's two closed-loop model explains the mechanisms behind HRV biofeedback, and how slow-paced breathing and slow-paced contraction stimulate the cardiovascular system to produce larger HR oscillations. These mechanisms form the scientific foundation for every HRV biofeedback protocol you will use in practice.
HRV is produced by interacting regulatory mechanisms that operate on different time scales (Moss, 2004). Circadian rhythms, core body temperature, and metabolism contribute to 24-hour HRV recordings, which represent the "gold standard" for clinical HRV assessment. The parasympathetic, cardiovascular, and respiratory systems produce short-term (approximately 5-minute) HRV measurements—the type most commonly used in biofeedback sessions.
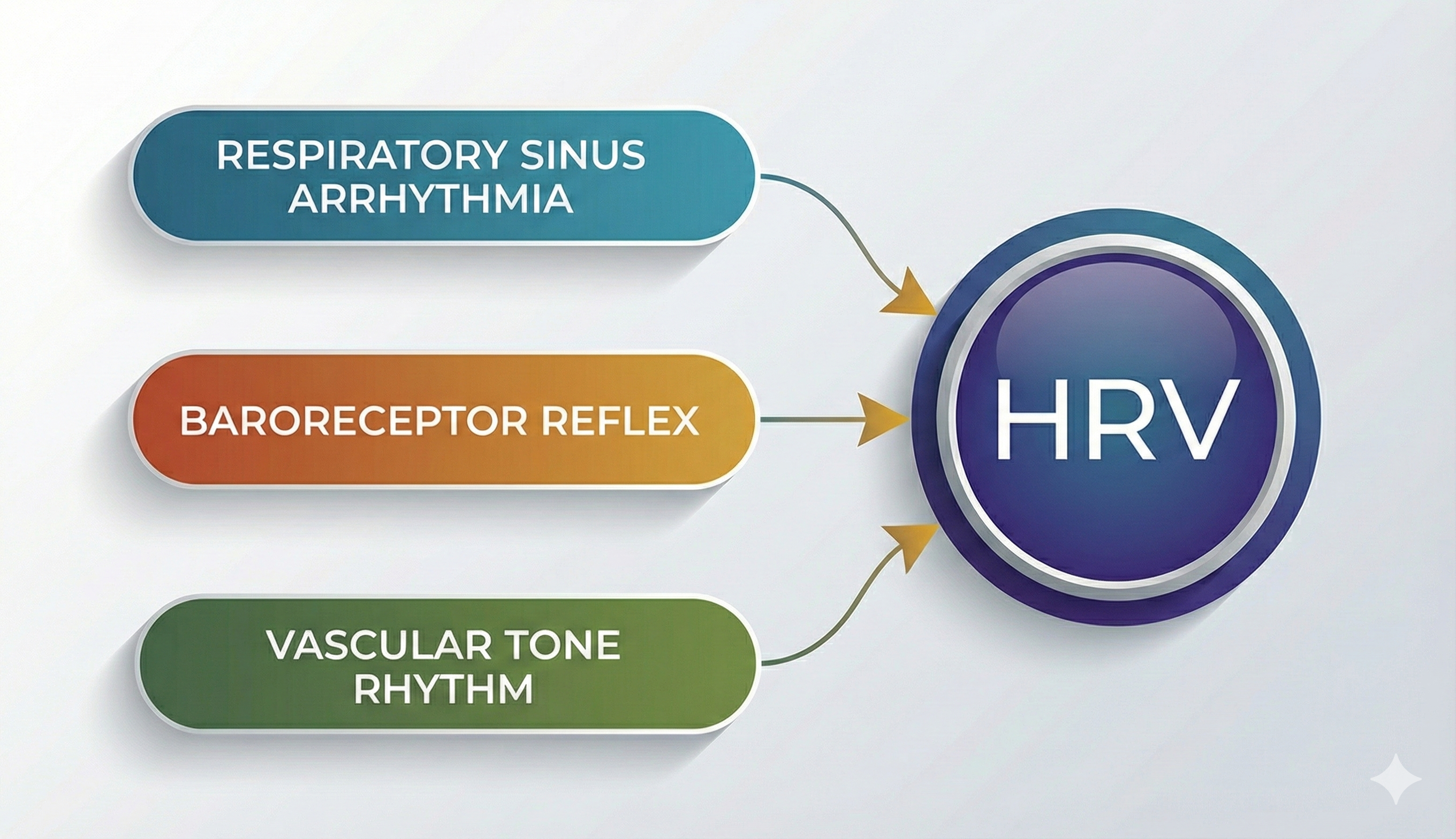
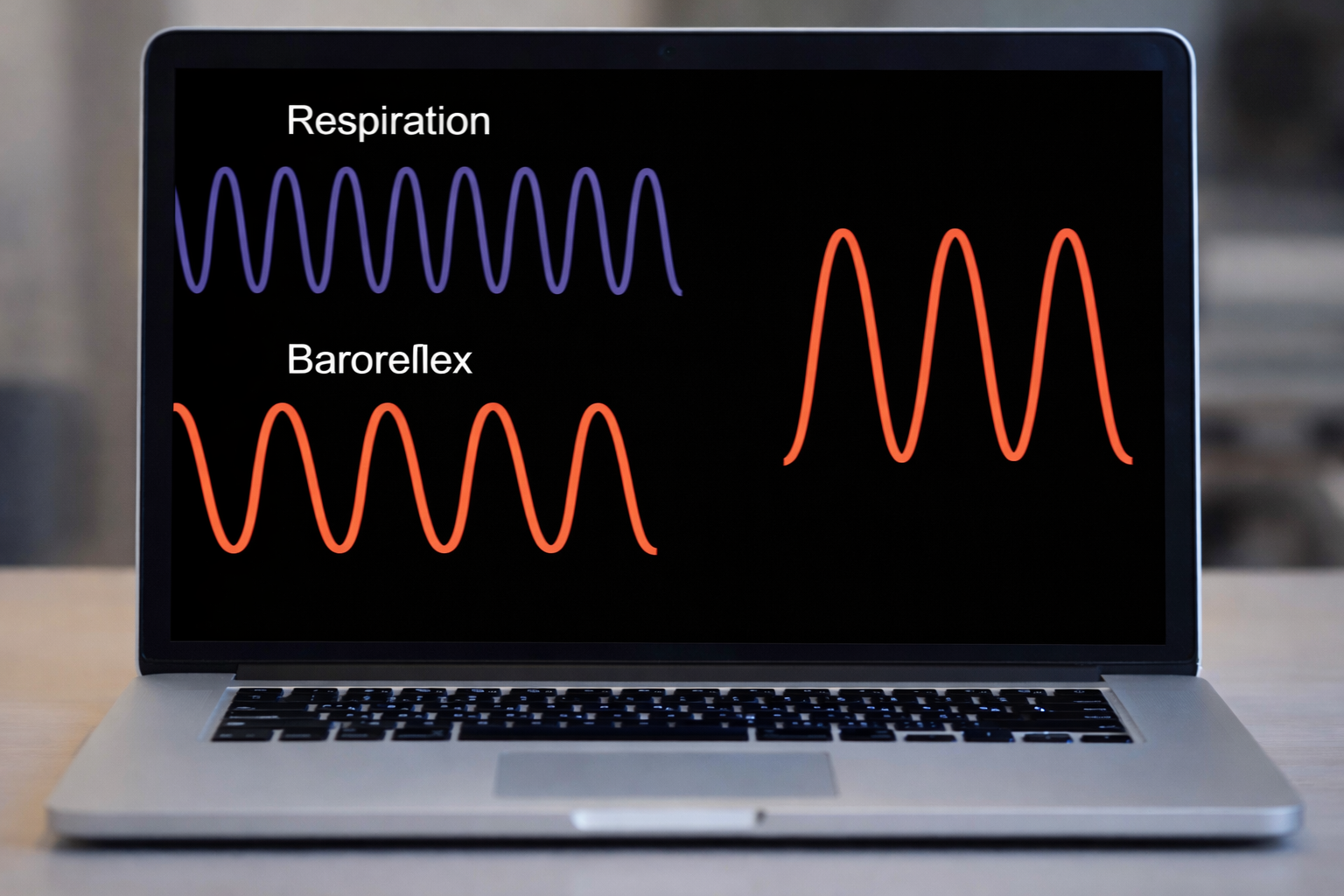
Respiratory sinus arrhythmia, the baroreceptor reflex, and the vascular tone rhythm are the most important sources of HRV (Hayano & Yuda, 2019; Vaschillo et al., 2002).
🎧 Mini-Lecture: Sources of Heart Rate Variability
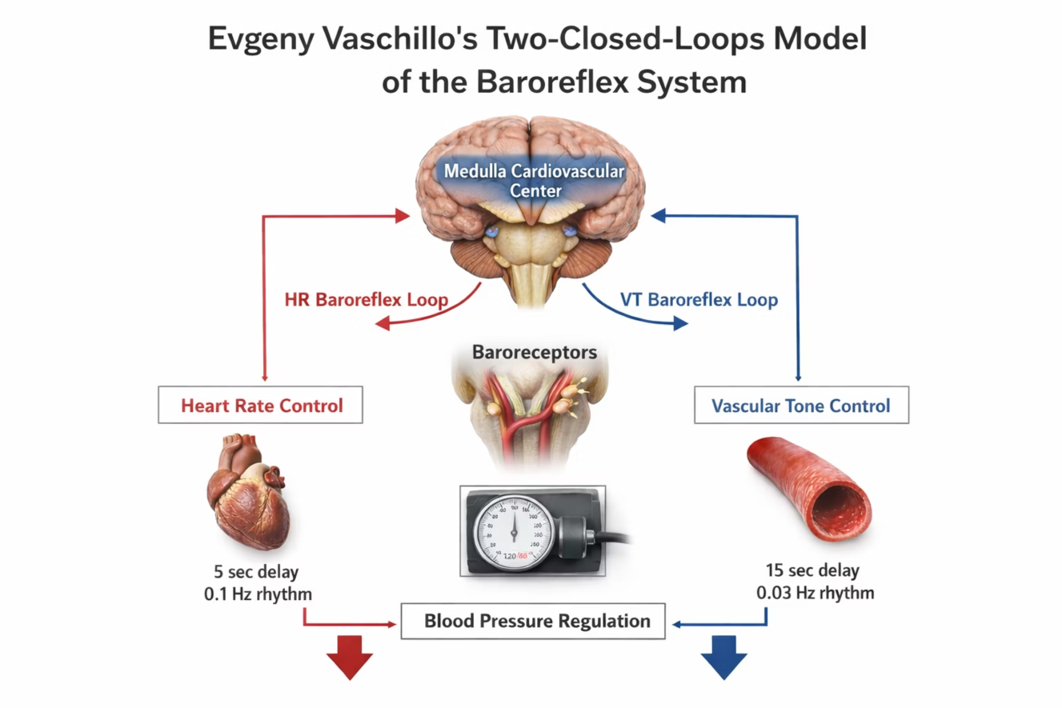
Vaschillo's Two Closed-Loop Model
This section explains the mechanistic model that underlies HRV biofeedback. Vaschillo's two closed-loop model is not just an academic theory—it is the reason slow-paced breathing and slow-paced contraction work. Vaschillo et al. (2002) described the heart rate (HR) and vascular tone (VT) baroreflexes as closed loops and proposed that stimulating one closed loop activates its counterpart. Understanding this model will help you explain to clients why the breathing and contraction exercises they practice actually improve their cardiovascular regulation.
Each baroreflex is a potential target for HRV biofeedback training. SPB and SPC at approximately 6 bpm/cpm can stimulate the HR and VT baroreflex loops, separately or synergistically. SPC at approximately 1 cpm can activate the VT baroreflex.
Heart Rate Baroreceptor Reflex
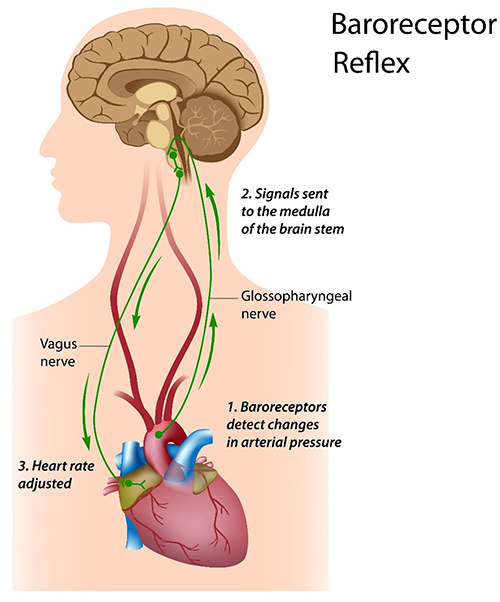
Although HRV promotes health and performance, blood pressure variability can endanger each. The HR baroreflex regulates acute BP changes to ensure stability—it is essentially the body's built-in blood pressure thermostat. This reflex exhibits resonance because it is a feedback system with a fixed delay, and inertia due to blood volume in the vascular tree accounts for most of that delay.
The HR baroreflex has an approximately 5-second delay with a resonance frequency of approximately 0.1 Hz. This 5-second delay is a critical number in HRV biofeedback: it determines the breathing rate at which the baroreflex can be most effectively stimulated.
🎧 Mini-Lecture: The Baroreceptor Reflex
Vascular Tone Baroreceptor Reflex
The vascular tone (VT) baroreflex regulates resistance blood vessel diameter. It has a 15-second delay and 0.03 Hz resonance frequency—much slower than the HR baroreflex. Vaschillo and colleagues (2002) proposed that the VT and HR baroreflexes work together to regulate BP and HR. Think of them as two interconnected thermostats: one adjusting the heart's rate and the other adjusting the diameter of blood vessels, both responding to blood pressure changes but at different speeds.
.jpg)
Respiratory Sinus Arrhythmia
Respiratory sinus arrhythmia (RSA)—the speeding and slowing of heart rate across each breathing cycle—is the primary and entirely parasympathetic source of HRV (Gevirtz, 2020). RSA is the signal you will most directly observe and train during HRV biofeedback sessions.
In the graphic below, the blue waveform represents the breathing cycle, and the red signals are heartbeats. Note that the heartbeats are spaced more closely (HR speeds) during inhalation and farther apart (HR slows) during exhalation.
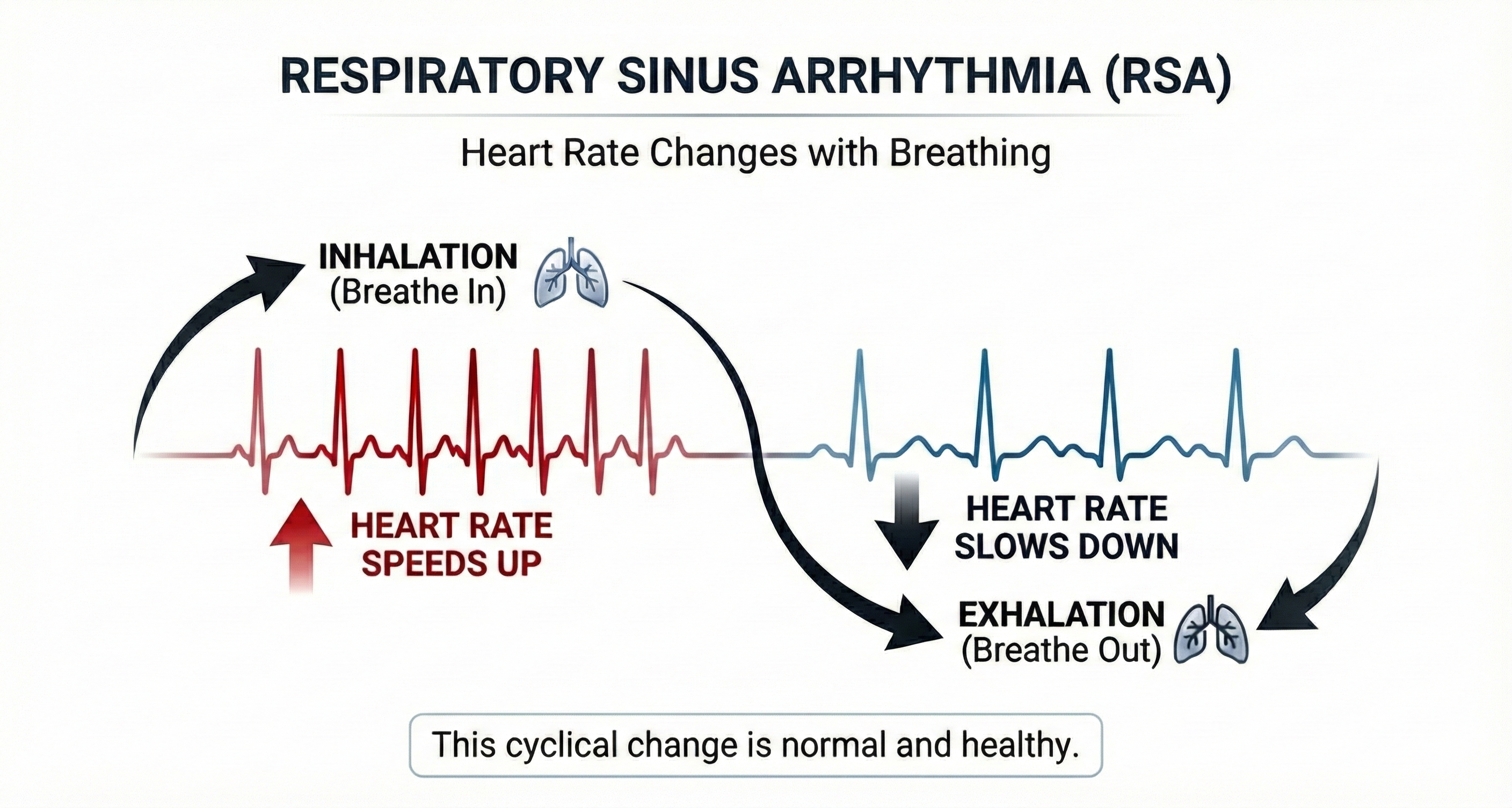
Inhalation disengages the vagal brake—the tonic parasympathetic inhibition of heart rate—speeding HR. This is a purely parasympathetic process. Exhalation reapplies the vagal brake, slowing HR. Understanding this mechanism is essential for coaching clients: when you cue longer exhalations, you are directly promoting vagal engagement.
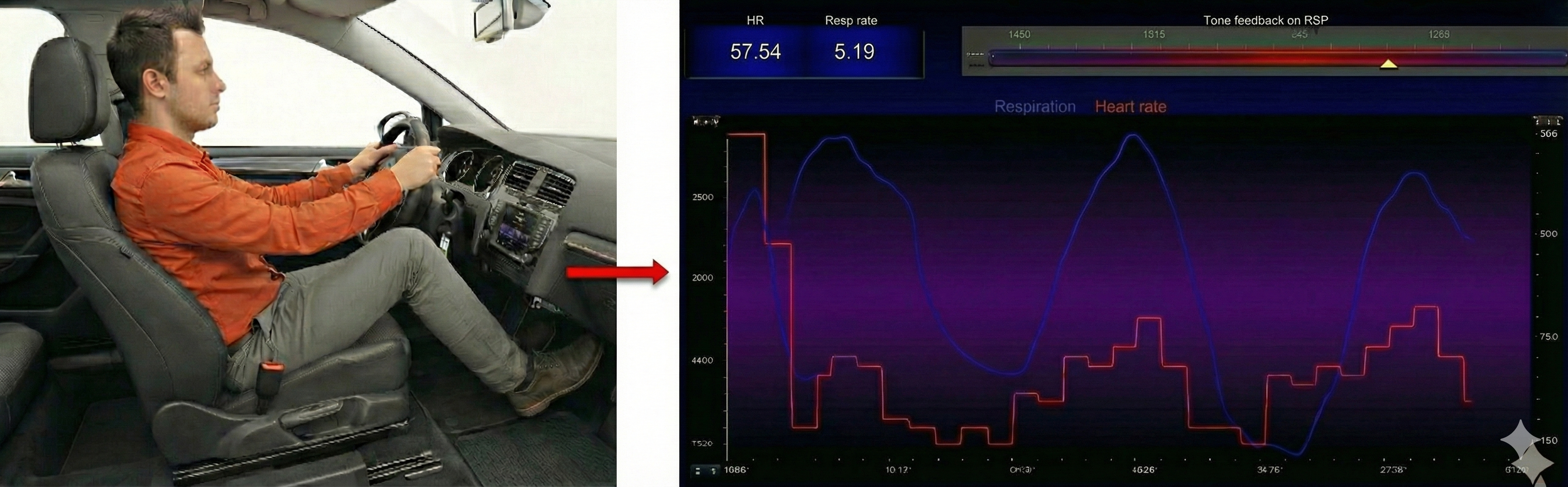
Exhalation reapplies the vagal brake, slowing heart rate.
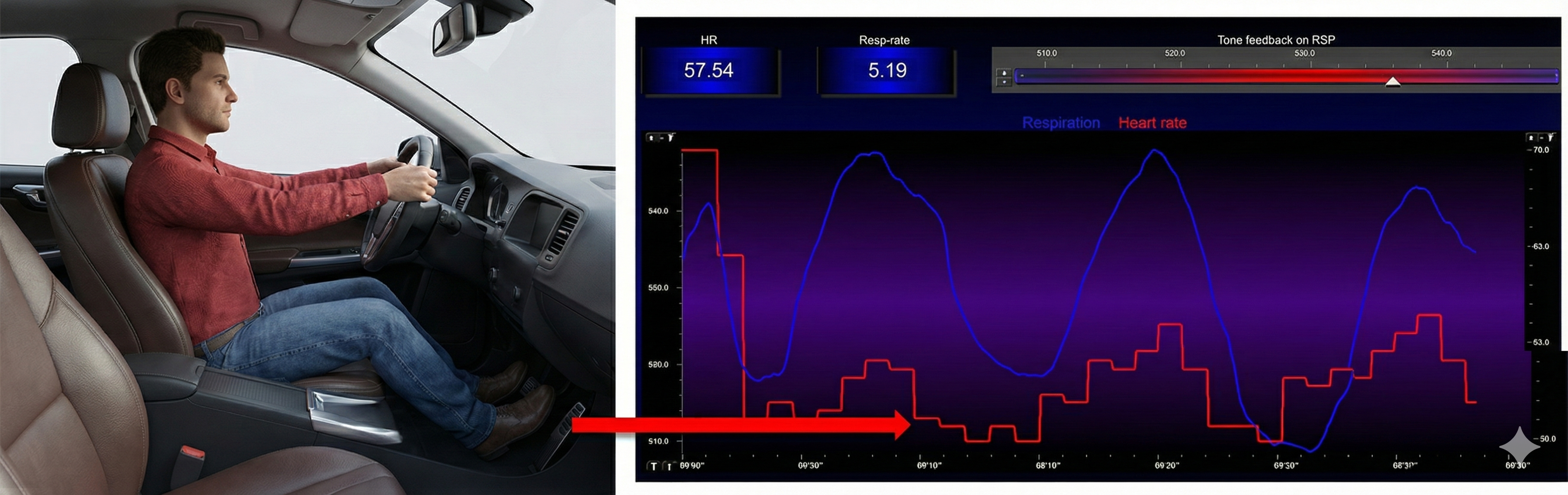
Inhalation speeds the heart, and about 5 seconds later, BP falls. During exhalation, the heart slows, and about 5 seconds later, BP increases. This 5-second lag connects RSA to the baroreflex and is the reason that breathing rate around 6 bpm produces the strongest resonance effects.
Slow-Paced Breathing and Slow-Paced Muscle Contraction Stimulate Vaschillo's Two Closed Loops
This section explains how the two primary HRV biofeedback techniques—slow-paced breathing and slow-paced contraction—activate the baroreflexes described in Vaschillo's model. You will learn the specific rates that target each baroreflex, the immediate versus long-term effects of training, and the research evidence supporting these protocols.
Respiratory sinus arrhythmia, the baroreceptor reflex, and the vascular tone rhythm are the most important sources of short-term (approximately 5-minute) HRV. These processes are exclusively parasympathetic (Hayano & Yuda, 2019; Vaschillo et al., 2002).
We can stimulate the HR and VT baroreflexes using slow-paced breathing (SPB) and slow-paced contraction (SPC) protocols. These are the two primary tools in your HRV biofeedback toolkit.
Targeting the Heart Rate Baroreceptor Reflex
Slow-paced breathing (SPB) (approximately 6 bpm) and slow-paced contraction (SPC) (approximately 6 cpm) can increase HR oscillations and HRV, separately or synergistically. This means clinicians can choose or combine techniques based on the client's needs and abilities.
Slow-Paced Breathing
SPB works because respiration produces blood pressure oscillations via changes in thoracic pressure (Pinsky, 2018) that stimulate the baroreflexes. Before HRV biofeedback training, respiration and the baroreflex are usually out of phase, resulting in weak resonance effects—that is, small HR changes.
HRV biofeedback training slows breathing to the baroreflex's rhythm, aligning these processes and significantly increasing resonance effects. This alignment is the core therapeutic mechanism: you are teaching the client's respiratory system to "push the swing" at exactly the right moment.
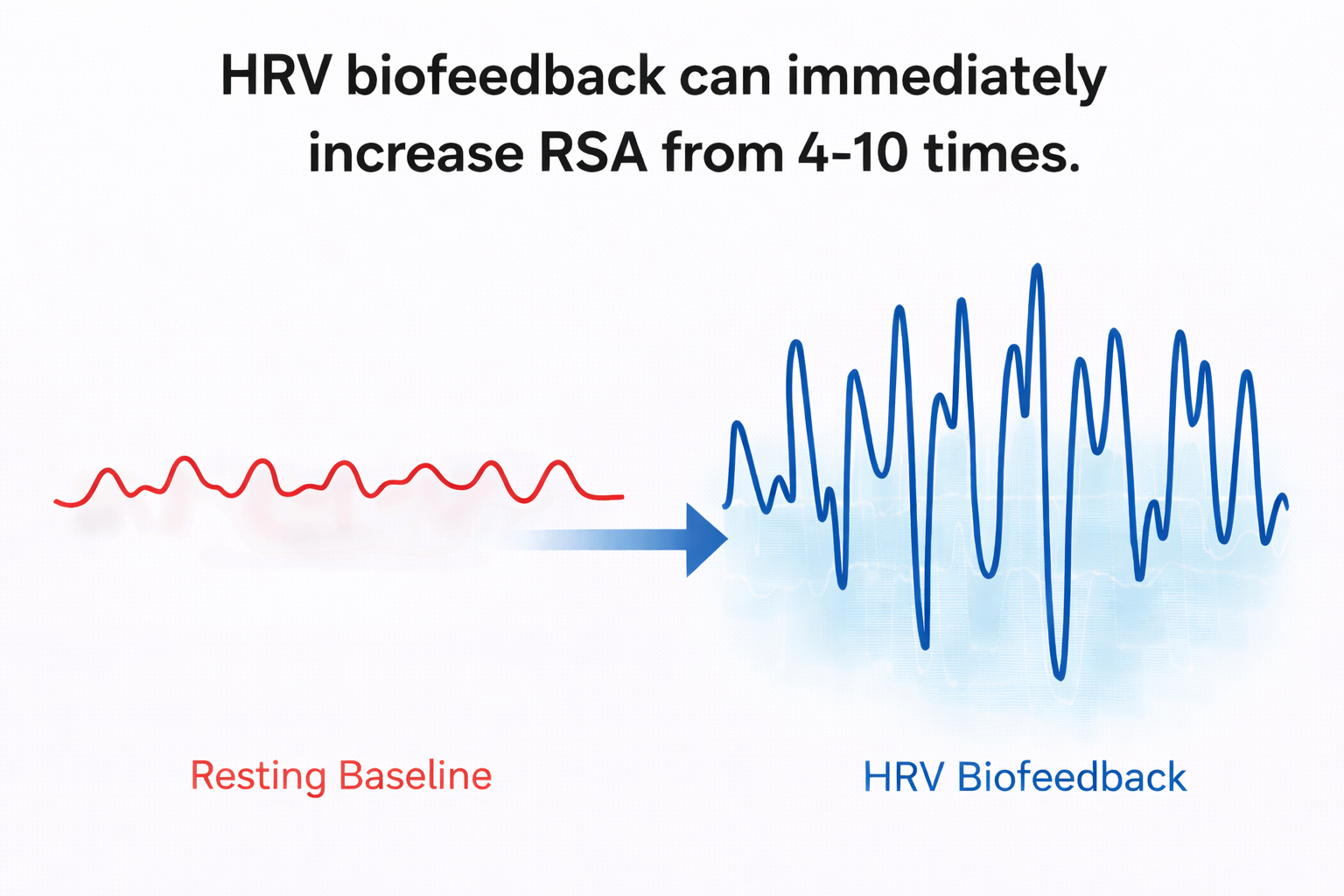
Slowing breathing to rates between 4.5 and 6.5 bpm for adults and 6.5 to 9.5 bpm for children increases RSA (Lehrer & Gevirtz, 2014). Increased RSA immediately "exercises" the baroreflex without changing vagal tone or tightening BP regulation—those changes require weeks to months of practice. HRV biofeedback can immediately increase RSA 4 to 10 times compared to a resting baseline (Lehrer et al., 2020b; Vaschillo et al., 2002). This distinction between immediate and long-term effects is critical for setting client expectations: they will see dramatic changes on the screen during their first session, but lasting autonomic improvements require consistent home practice.
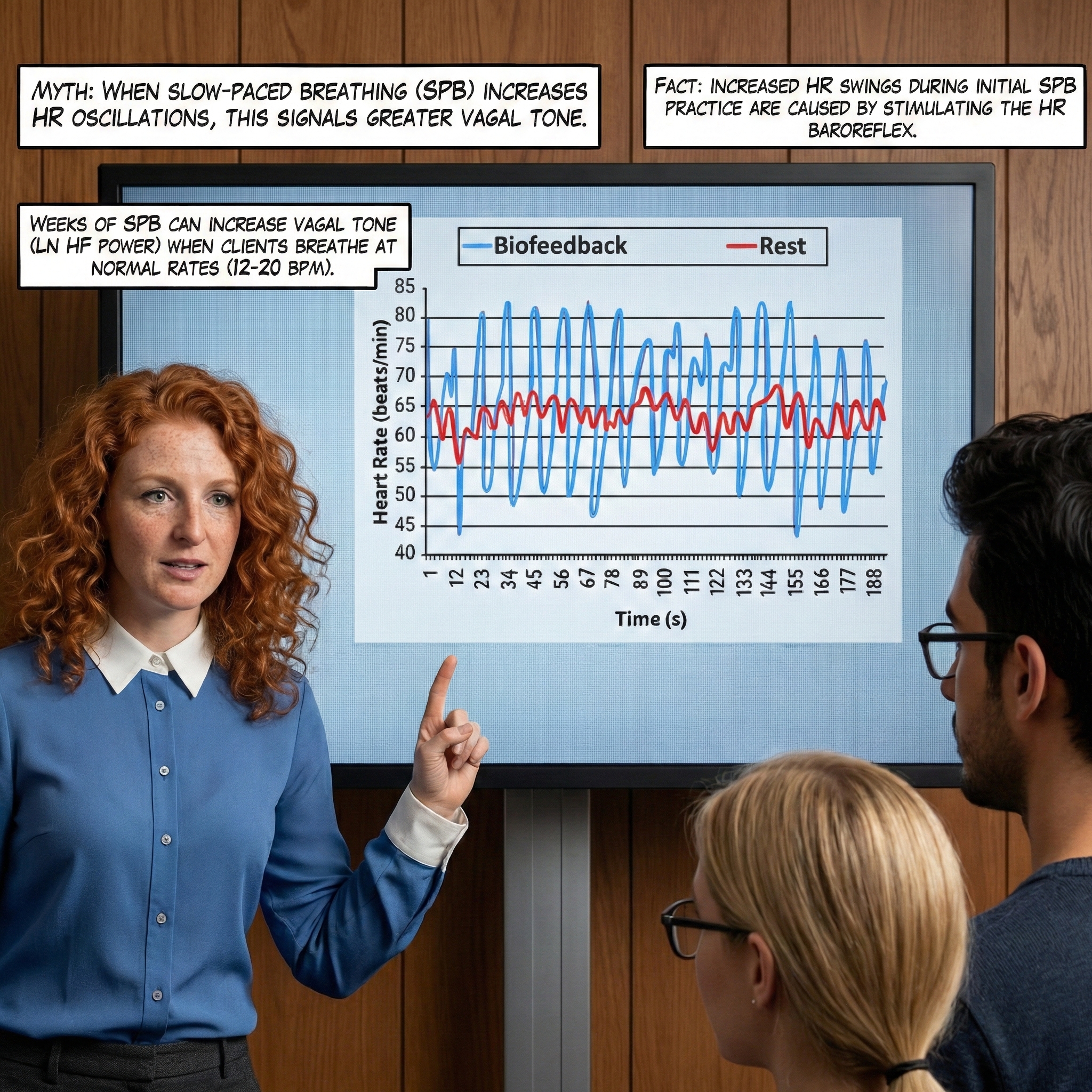
Slow-Paced Contraction
Slow-paced contraction (SPC) involves rhythmic wrists-core-ankles contraction with legs crossed. SPC at approximately 6 and 2 cpm may stimulate blood pressure, HR, and VT control systems without requiring the client to slow their respiration (Vaschillo et al., 2011). This makes SPC an important alternative for clients who find slow breathing uncomfortable, anxiety-provoking, or physiologically contraindicated (such as those with certain respiratory conditions).

Like slow-paced breathing, slow-paced contraction amplifies heart rate oscillations. SPC stimulates the HR baroreflex at approximately 6 cpm and the VT baroreflex at approximately 2 cpm to immediately increase HRV. As with SPB, months of practice are required to increase vagal tone.
Baroreflex Resonance Through Slow-Paced Contraction
Every time you rhythmically squeeze a skeletal muscle, you are doing more than just moving a limb. Your contracting muscle acts as a mechanical pump. It compresses the blood vessels running through it and pushes surges of venous blood (blood returning to the heart through the veins) back toward the heart. Each surge temporarily increases venous return, meaning more blood flows into the heart's chambers between beats. That extra filling stretches the heart wall. Through a principle called the Frank-Starling mechanism, this stretch causes a stronger contraction. The heart then ejects a larger volume of blood (stroke volume) into the arteries, briefly raising arterial blood pressure. When the muscle relaxes, the pumping effect pauses and pressure falls back down.
The net effect is a train of rhythmic pressure waves layered on top of your baseline circulation. Your nervous system does not ignore these waves. Arterial baroreceptors are stretch-sensitive nerve endings embedded in two key locations: the carotid sinus (a small dilation at the base of the internal carotid artery in the neck) and the aortic arch (the curved top of the aorta just above the heart). These sensors detect the rhythmic pressure swings and convert them into volleys of electrical signals sent toward the brain (Vaschillo et al., 2002).
From these peripheral sensors, the signal travels along well-mapped neural pathways into the central nervous system. Afferent (sensory) nerve fibers within two cranial nerves carry the information: the glossopharyngeal nerve (cranial nerve IX) and the vagus nerve (cranial nerve X). These fibers deliver baroreceptor discharge into the nucleus tractus solitarius. This is a cluster of neurons in the medulla oblongata (the lowest part of the brainstem) that serves as the primary integration center for cardiovascular reflexes. Here, the brainstem coordinates a moment-to-moment rebalancing of autonomic outflow. It adjusts heart rate through vagal and sympathetic efferent (motor) pathways. The technical term for this rate control is cardiac chronotropy. At the same time, it fine-tunes the degree of contraction in the smooth muscle lining blood vessel walls. This property is known as vascular tone.
The entire circuit forms a closed-loop negative-feedback system. It runs from the initial stretch of the arterial wall to the cardiac and vascular adjustments and back again. "Negative feedback" means the system works to oppose whatever change triggered it. If pressure rises, the reflex brings it back down. If pressure falls, the reflex pushes it back up. This loop is called the baroreflex. Its purpose is to buffer blood pressure against sudden disturbances and keep it relatively stable.
What makes this loop especially interesting from a biofeedback perspective is that it is not instantaneous. Each stage of the process takes a finite amount of time. Signal transduction occurs at the baroreceptor. Central processing takes place in the brainstem. Efferent nerves then conduct impulses outward. Finally, the heart and blood vessels respond.
The complete round trip around the loop occupies several seconds. Because of this built-in delay, the baroreflex has what engineers and physiologists call a natural resonant frequency. This term refers to the specific rate of stimulation at which a system responds with maximum amplitude rather than dampening the input. The system's internal timing makes this possible. For the human baroreflex, this resonant frequency is typically near 0.1 Hz in adults. That corresponds to a roughly 10-second oscillatory cycle, or one full wave every ten seconds.
When an outside perturbation, such as a rhythmic muscle squeeze, arrives at or near this intrinsic frequency, the feedback loop cannot fully cancel one disturbance before the next one arrives. Instead of damping out the pressure oscillations, the system amplifies them. Small, periodic nudges in arterial pressure are progressively magnified into large-amplitude swings in heart period (the time between heartbeats) and vascular tone. This phenomenon is known as cardiovascular resonance (Lehrer et al., 2020; Vaschillo et al., 2002).
The empirical fingerprint of this resonance is unmistakable in laboratory recordings. When muscle tension is cycled at approximately 0.1 Hz (one contraction-relaxation cycle every ten seconds), a technique called spectral analysis reveals the effect clearly. Spectral analysis is a mathematical method that breaks a complex physiological signal into its component frequencies. It shows a sharp, concurrent spike in power at the 0.1 Hz frequency across several channels. These channels include interbeat intervals (the time between successive heartbeats), systolic blood pressure (the peak pressure during each heartbeat), and pulse transit time (the time it takes a pressure wave to travel from the heart to a peripheral site like a fingernail bed).
One noteworthy detail is that the pressure-related oscillations often exceed the cardiac oscillations in size. This pattern points toward a pressure-driven reflex origin. The brain is not simply commanding the heart to follow a rhythm on its own. Instead, the rhythmic mechanical forcing of the blood vessels entrains (synchronizes) the baroreflex loop first. The heart then follows as one component of the integrated regulatory response (Vaschillo et al., 2002).
Vaschillo's broader theoretical framework helps explain why these effects propagate so widely across the cardiovascular system. Heart rate and blood pressure, vascular tone and blood pressure: these are not isolated pairings. They are coupled, interlocking closed loops that share common nodes. A common node is a variable that appears in more than one feedback circuit. Driving any single variable rhythmically can therefore engage the entire regulatory network.
The rhythmic input could be paced breathing, deliberate muscle contraction, or another periodic stimulus. The perturbation reverberates through the coupled loops. Coherent oscillations, meaning oscillations that are synchronized in frequency and phase, amplify across multiple physiological channels at the same time. What begins as a simple, voluntary squeeze of muscle cascades into a system-wide resonance event. This event offers both a powerful window into autonomic function and a potent lever for therapeutic intervention.
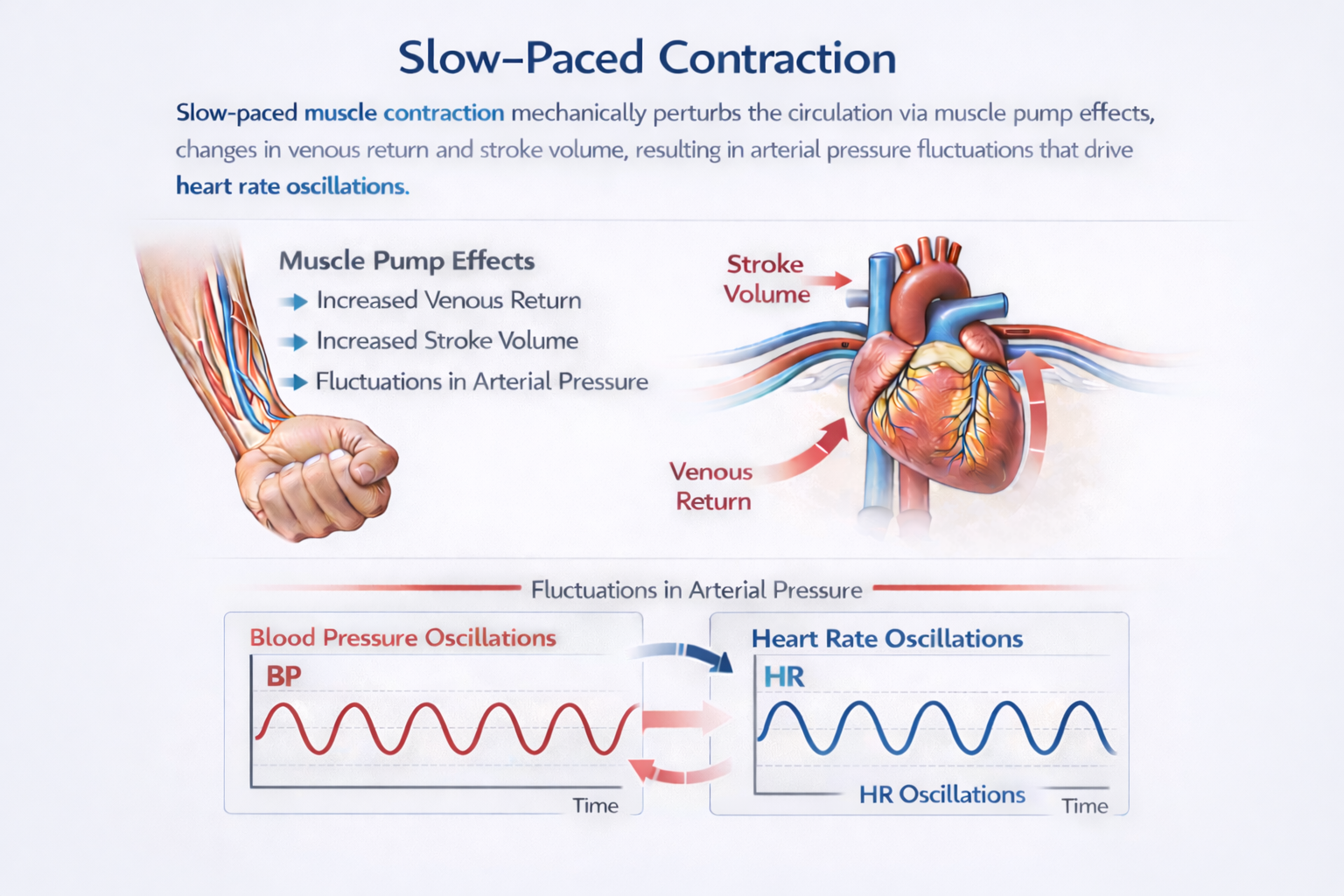
Maximum-Minimum HR is not RSA because it is driven by muscle contraction instead of breathing. The peak frequency is the HRV frequency with the greatest power. In the screen captures below, SPC stimulated the baroreceptor reflex at the intended frequency (0.2 Hz for 12 cpm and 0.1 Hz for 6 cpm).
Below is a BioGraph Infiniti display of 12-cpm SPC. At the top right, note that the Maximum-Minimum heart rate is 5 bpm. At the left, the peak frequency is 0.2 Hz.
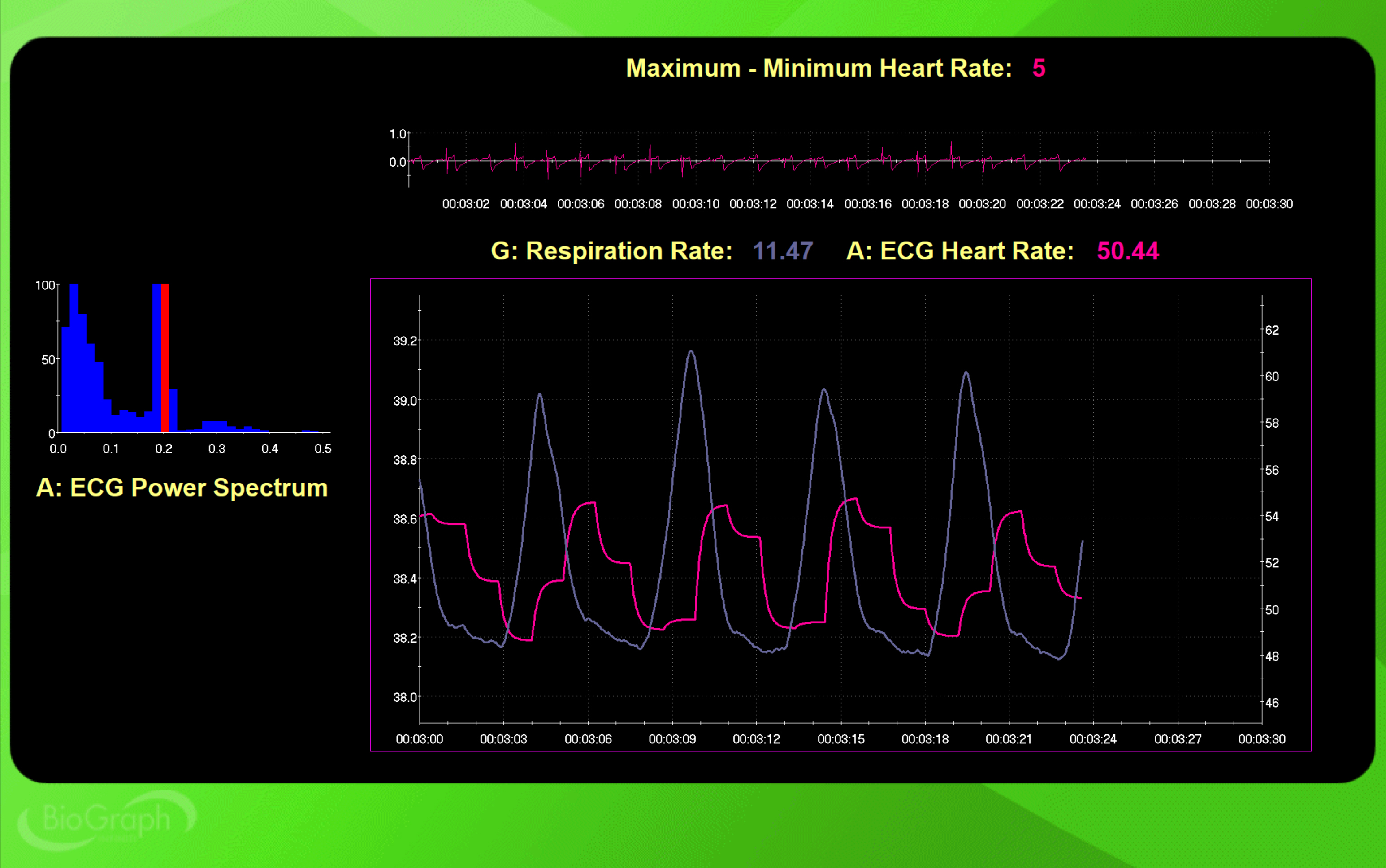
Next is a 6-cpm SPC display. The Maximum-Minimum heart rate is 13 bpm—a substantial increase. At the left, the peak frequency is 0.1 Hz. The difference between 5 bpm at 12 cpm and 13 bpm at 6 cpm powerfully demonstrates how slowing the contraction rate to the baroreflex's resonance frequency amplifies HR oscillations.
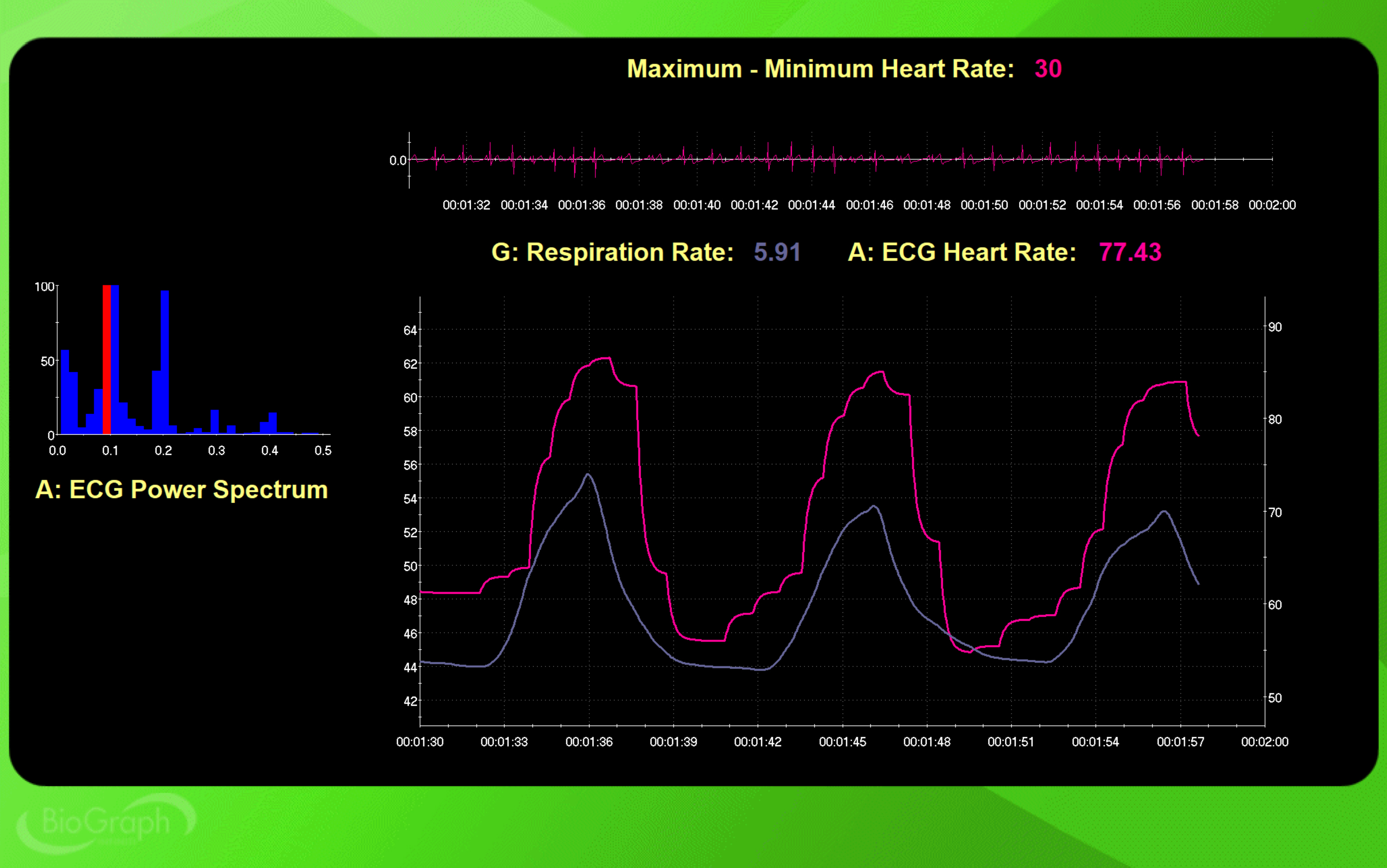
Targeting the Vascular Tone Baroreceptor Reflex
Shaffer, Moss, and Meehan (2022) reported that slow-paced contraction at 1 and 6 cpm increased five time-domain metrics (HR Max-HR Min, RMSSD, SDNN, TI, and TINN), one frequency-domain metric (LF power), and three nonlinear metrics (D2, SD1, SD2) to a greater degree than slow-paced contraction at 12 cpm. There were no differences between the 1 and 6 cpm conditions, suggesting that clinicians can use either rate effectively.
Meehan and Shaffer (2023) compared 6-cpm wrist-ankle slow-paced contraction with 6-cpm wrist-core-ankle slow-paced contraction. Both conditions produced greater HR, HR Max-HR Min, and LF power than the control condition. The wrist-core-ankle method yielded greater HR and HR Max-HR Min than wrist-ankle slow-paced contraction, providing clinicians with evidence to prefer the more comprehensive contraction technique.
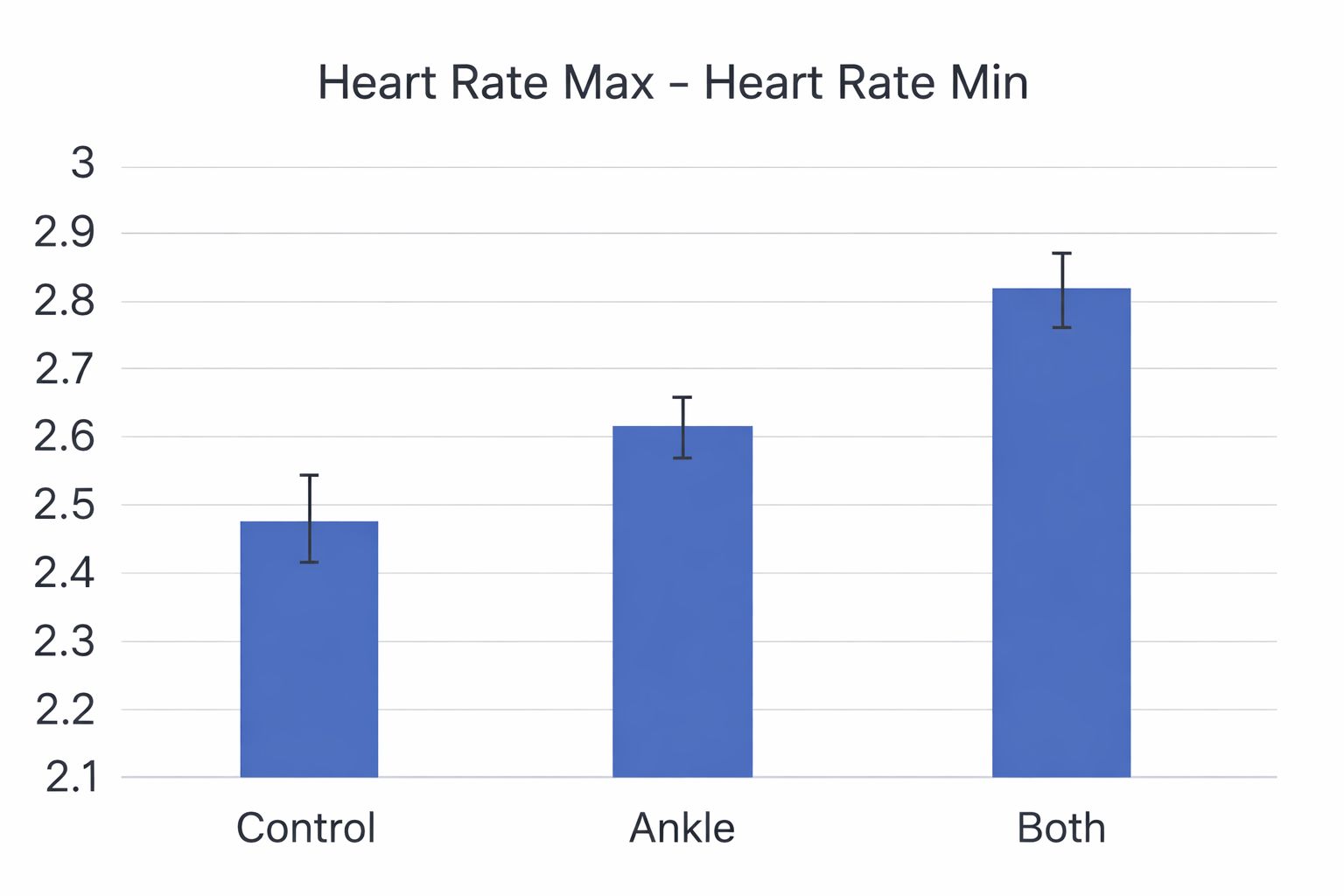
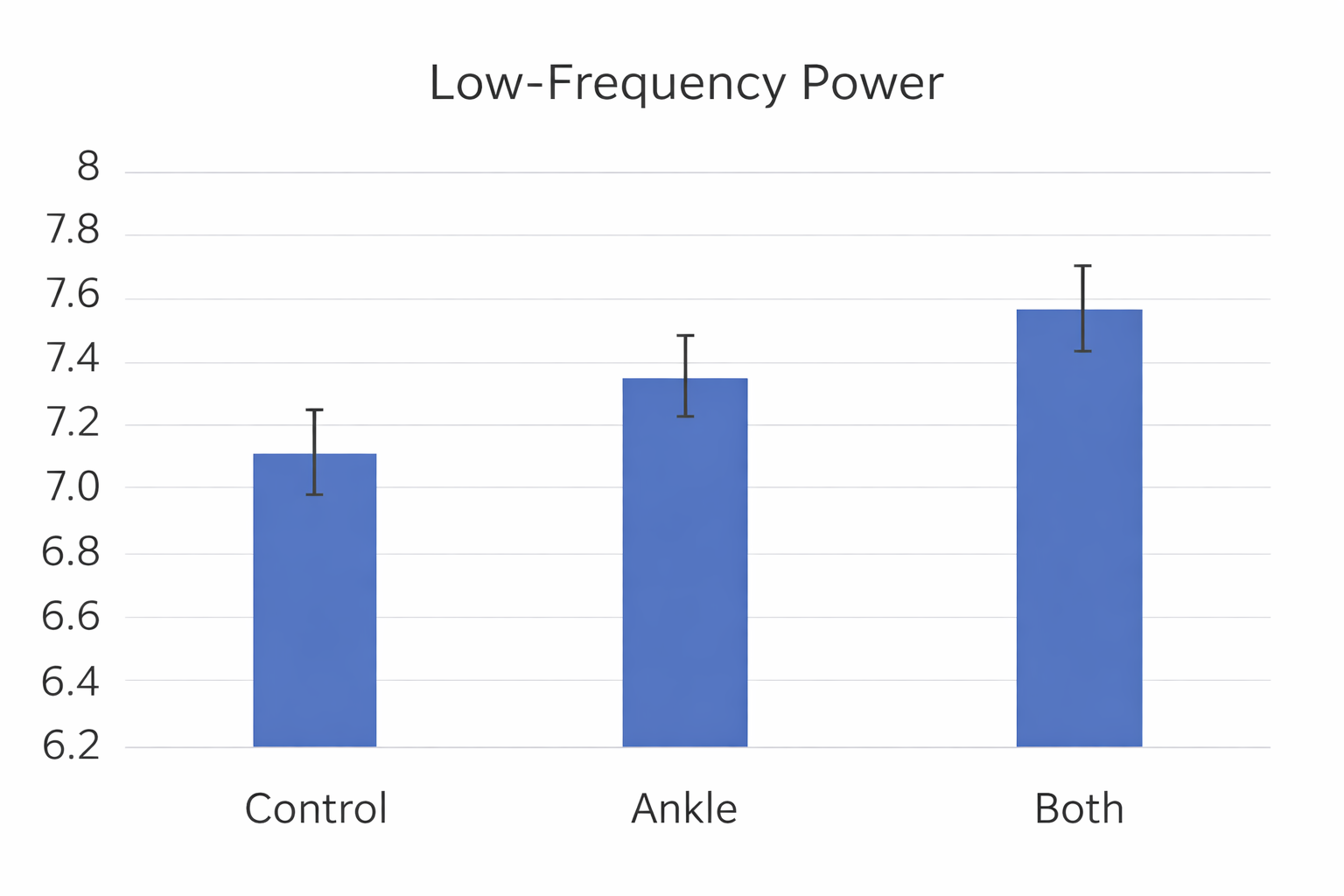
Comprehension Questions: Sources of HRV and Biofeedback Mechanisms
- What are the three most important sources of short-term HRV, and what do they have in common?
- Explain how Vaschillo's two closed-loop model describes the relationship between slow-paced breathing and the baroreflexes.
- Why does HRV biofeedback produce immediate RSA increases but require months of practice for lasting vagal tone changes?
- How do slow-paced breathing and slow-paced contraction differ in targeting the HR and VT baroreflexes?
Factors That Influence HRV
This section covers the critical factors that shape a client's HRV values: age, time of day, heart rate, resonance, and breathing rate and depth. Understanding these influences is essential for accurate assessment, realistic goal-setting, and effective training. Without accounting for these factors, you risk misinterpreting a client's HRV data or setting inappropriate benchmarks.
Age
In a cross-sectional study of 8,203,261 participants using 24-hour Fitbit photoplethysmographic recordings, all HRV time- and frequency-domain metrics declined between ages 20 and 60 (Natarajan et al., 2020). This is the single largest HRV normative dataset ever published, and its message for clinicians is unambiguous: always interpret HRV values in the context of a client's age.
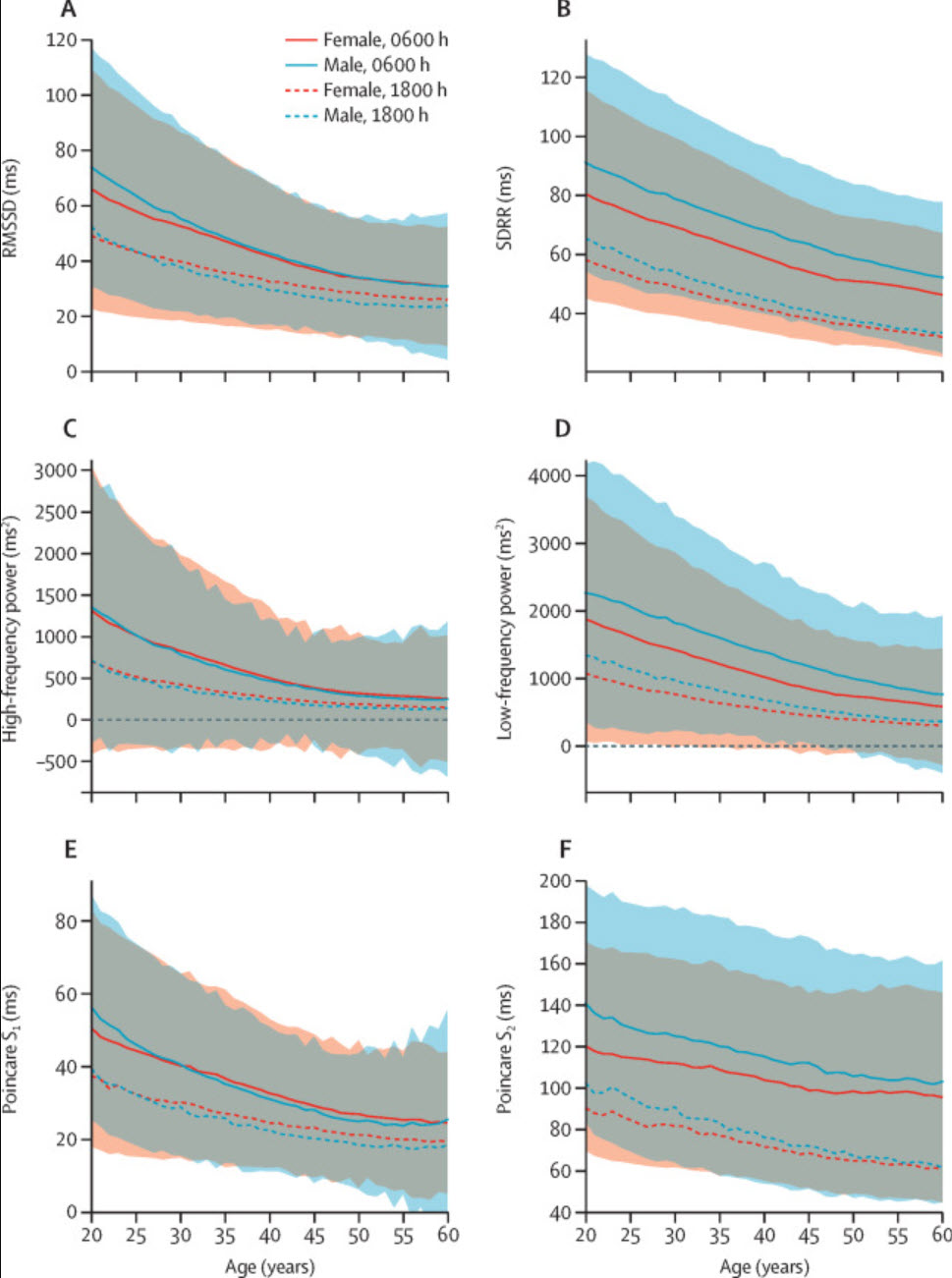
Almeida-Santos et al. (2016) obtained 24-hour ECG recordings of 1,743 subjects aged 40 to 100. They found a linear decline in SDNN, the standard deviation of the average NN intervals for each 5-minute segment (SDANN), and the standard deviation of NN intervals (SDNN index). However, they found a U-shaped pattern for the RMSSD and pNN50 with aging, decreasing from ages 40 to 60 and then increasing after age 70. This U-shaped recovery is noteworthy: it suggests that some vagal metrics may partially rebound in older adults, though the mechanisms remain under investigation. The age groups were 1 (40 to 49 years), 2 (50 to 59 years), 3 (60 to 69 years), 4 (70 to 79 years), and 5 (80 years and older).
.jpg)
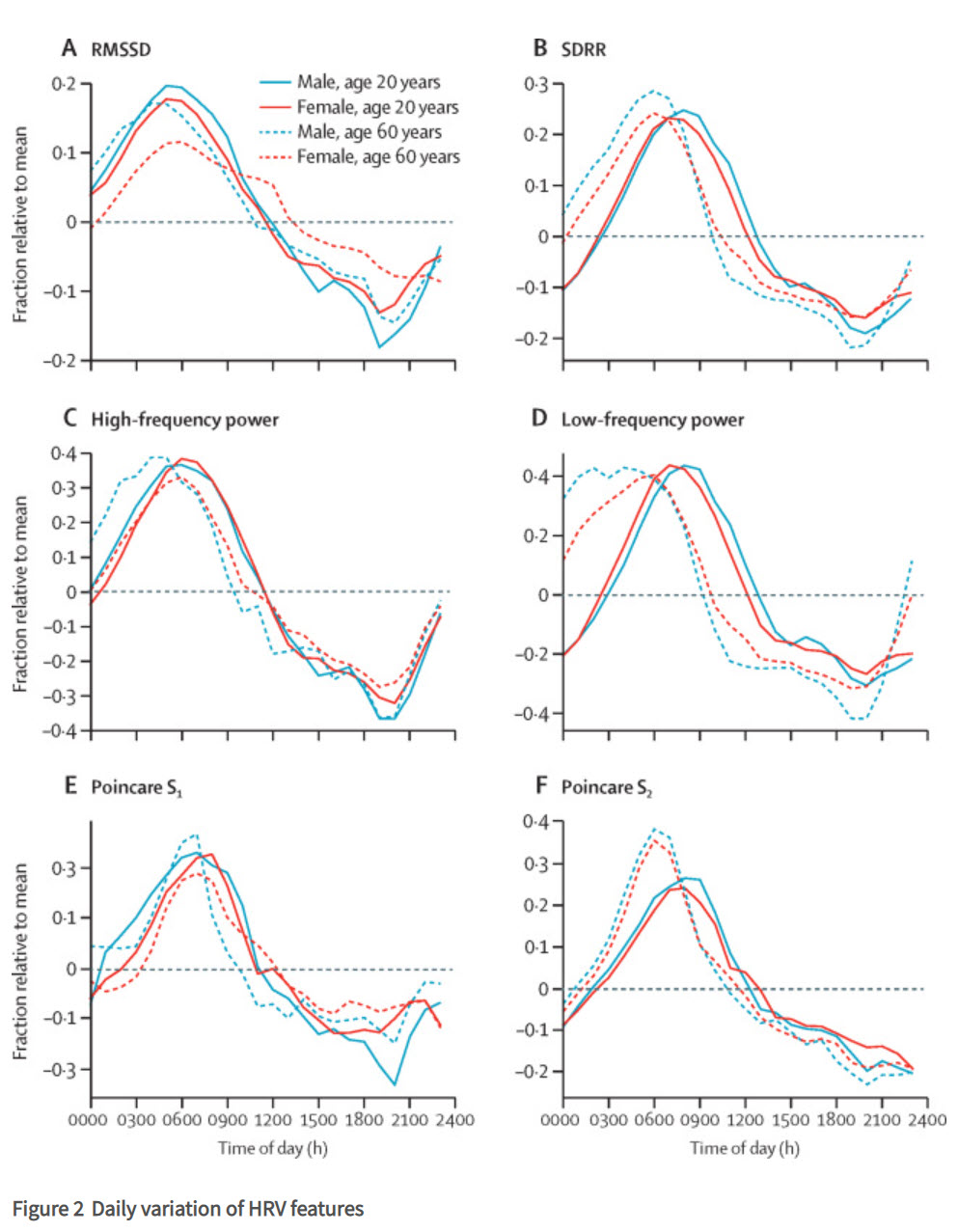
Time of Day
All HRV metrics reached their maximum values between 5 and 8 am and minimum values between 7 and 10 pm. This circadian pattern has direct implications for scheduling assessments and comparing session data: a client assessed at 8 am will likely show higher HRV than the same client assessed at 8 pm, independent of any training effect. For consistent tracking, try to schedule sessions at the same time of day.
HR Limits HRV
As discussed earlier, cycle length dependence means that faster heart rates decrease the opportunity for IBIs to vary in length, whereas slower heart rates increase the chance of beat-to-beat differences.
This is worth revisiting because it has a practical consequence: interventions that lower resting heart rate—such as aerobic exercise and stress management—can indirectly improve HRV by creating more "room" for variability between beats.
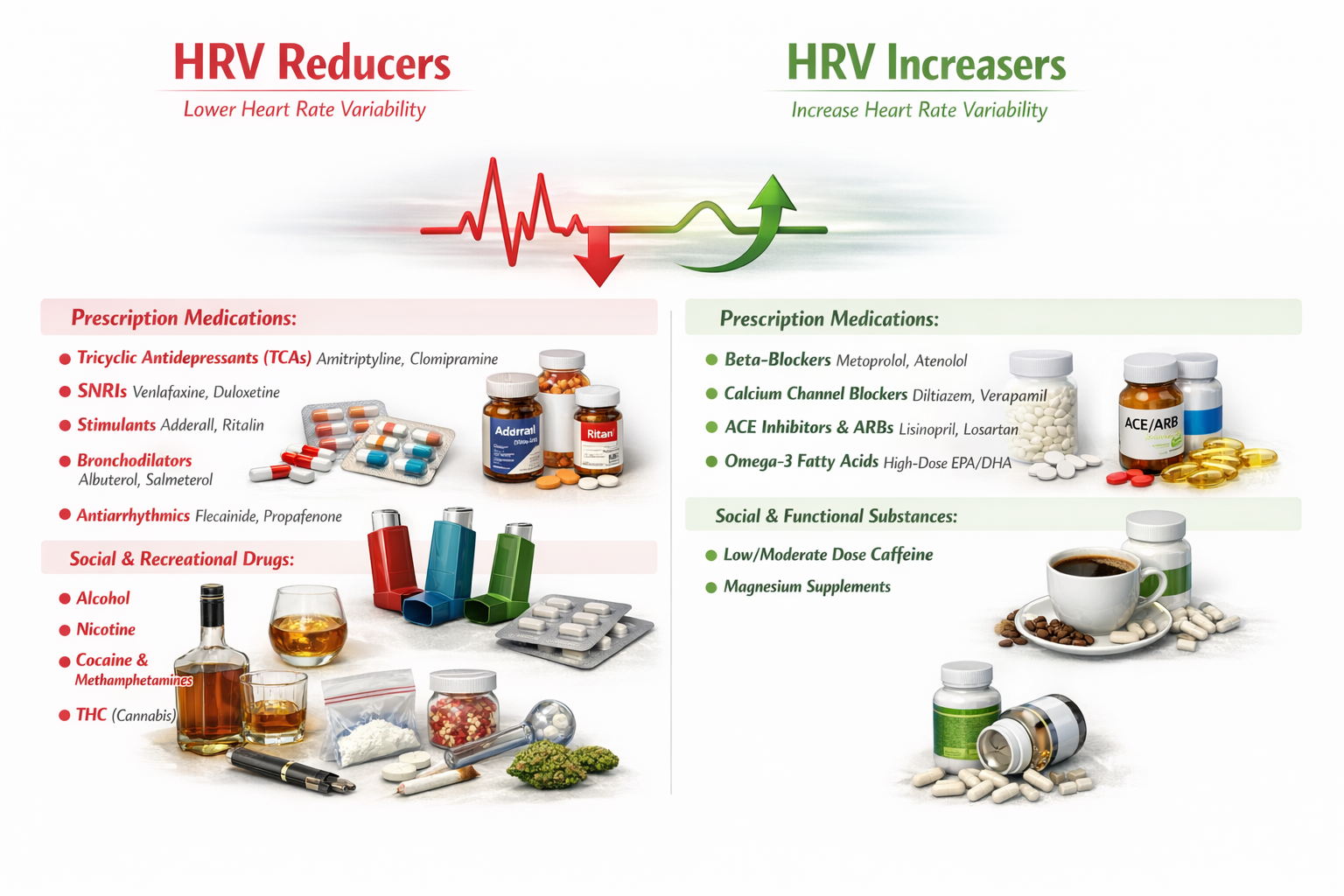
When Medications and Substances Shift Your Heart's Rhythm
What many professionals find surprising is just how powerfully common medications and everyday substances can push HRV in one direction or the other.
Substances That Dampen Heart Rate Variability
A wide range of prescription medications are known to reduce HRV. Tricyclic antidepressants such as amitriptyline and clomipramine exert strong anticholinergic effects that blunt vagal tone and suppress the parasympathetic input that drives healthy variability. Serotonin-norepinephrine reuptake inhibitors (SNRIs) like venlafaxine and duloxetine similarly tend to decrease HRV, likely through their noradrenergic activity that tips the autonomic balance toward sympathetic dominance. Stimulant medications, including well-known drugs like Adderall and Ritalin, ramp up sympathetic nervous system activity and, in doing so, tend to compress the beat-to-beat variability that clinicians look for as a marker of cardiac health. Bronchodilators such as albuterol and salmeterol, commonly prescribed for asthma, activate beta-adrenergic receptors and can reduce parasympathetic modulation of heart rhythm. Antiarrhythmic agents like flecainide and propafenone, somewhat paradoxically, may also lower HRV by altering the electrical conduction pathways in the heart
Beyond prescription drugs, several social and recreational substances are also associated with reduced HRV. Alcohol consumption suppresses vagal tone and disrupts autonomic regulation, particularly with heavier or chronic use. Nicotine activates the sympathetic nervous system and has been consistently linked to diminished HRV in both acute and long-term studies. Cocaine and methamphetamines produce intense sympathetic surges that dramatically reduce variability and place enormous strain on the cardiovascular system. THC, the primary psychoactive compound in cannabis, also appears to reduce HRV, likely through its complex interactions with autonomic regulation and cardiovascular reflexes.
Substances That Enhance Heart Rate Variability
On the other side of the equation, certain prescription medications are associated with increases in HRV. Beta-blockers such as metoprolol and atenolol reduce sympathetic input to the heart, effectively "making room" for greater parasympathetic influence and thereby increasing beat-to-beat variability. Calcium channel blockers like diltiazem and verapamil similarly shift the autonomic balance in a direction that favors higher HRV. ACE inhibitors and angiotensin receptor blockers (ARBs), including lisinopril and losartan, have been shown to improve HRV as part of their broader cardioprotective effects, possibly by reducing the neurohormonal stress on the cardiovascular system. High-dose omega-3 fatty acids (EPA and DHA), while technically a supplement rather than a traditional prescription medication, have accumulated enough research support to be recognized as a meaningful contributor to improved HRV, likely through anti-inflammatory pathways and favorable effects on cell membrane function.
Among social and functional substances, low to moderate doses of caffeine appear to support or modestly increase HRV, a finding that often surprises students who assume all stimulants would decrease variability. The key distinction is dosage: moderate caffeine intake does not produce the same degree of sympathetic overdrive seen with stronger stimulants. Magnesium supplements round out the list of HRV-increasing substances, which makes physiological sense given magnesium's well-established role in supporting parasympathetic nervous system function and healthy cardiac electrical activity.
Why This Matters for Biofeedback Practice
For professionals working in biofeedback, understanding these pharmacological influences on HRV is essential. When a client's HRV readings seem unusually low or fail to respond to training protocols like slow-paced breathing, the explanation may lie not in poor technique but in the medications or substances that client is using. Conversely, a client taking beta-blockers may show higher baseline HRV that partly reflects pharmacological rather than purely autonomic improvement. Effective biofeedback practice demands that practitioners account for these chemical influences when interpreting data, designing training protocols, and setting realistic goals with their clients.
Resonance
Resonance is an amplification process that relies on simple physics (Lehrer, 2020). An external force causes a closed-loop (negative feedback) system to oscillate with greater amplitude at its inherent resonance frequency (RF). This concept is the scientific foundation of HRV biofeedback, and four everyday examples make it intuitive.
First, striking one tuning fork causes the second to vibrate in unison, causing the ball to swing.
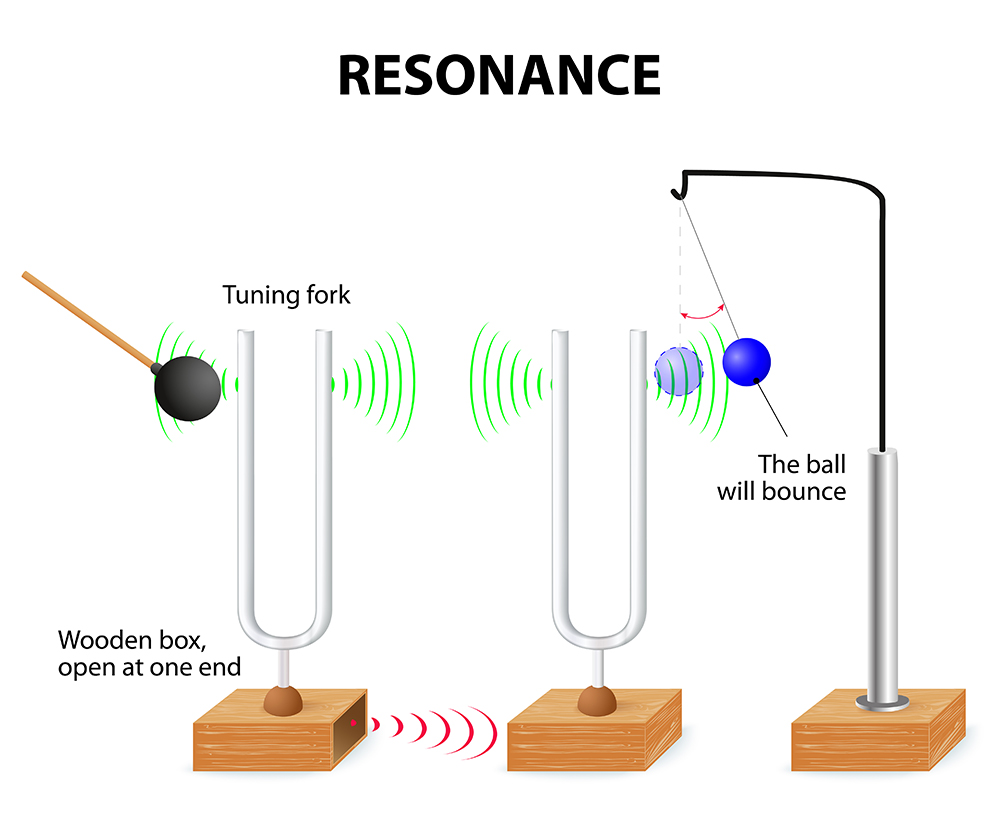
Second, a bell struck by a Buddhist monk for prayer time resonates after the initial strike. Third, think of pushing a child on a swing: there is a single frequency that moves the child the highest, and the best pushing rate is analogous to the system's RF (Khazan, 2020). This is the analogy most useful for explaining biofeedback to clients—you are helping them find the "pushing rate" that makes their heart rate oscillations the largest.


Finally, overloading a wine glass with sound at its RF can cause it to shatter because it cannot withstand the vibrational energy.

The baroreflex system exhibits resonance because it is a feedback system with a fixed delay. Inertia due to blood volume in the vascular tree accounts for most of this delay. An important clinical detail: taller people and men have lower resonance frequencies than women and shorter people because they have larger blood volumes (Lehrer, 2020). This means the ideal breathing rate varies from client to client, which is why individualized resonance frequency assessment is a cornerstone of best practice.

Resonance Frequency Breathing and Its Effects
When clients breathe at their RF, HR and respiration are in perfect phase (0°); their peaks and valleys coincide. This phase alignment is one of the key indicators you use during resonance frequency assessment to identify the optimal breathing rate for each client.
🎧 Mini-Lecture: The Resonance Frequency
🎧 Mini-Lecture: HRV Changes During and After Training
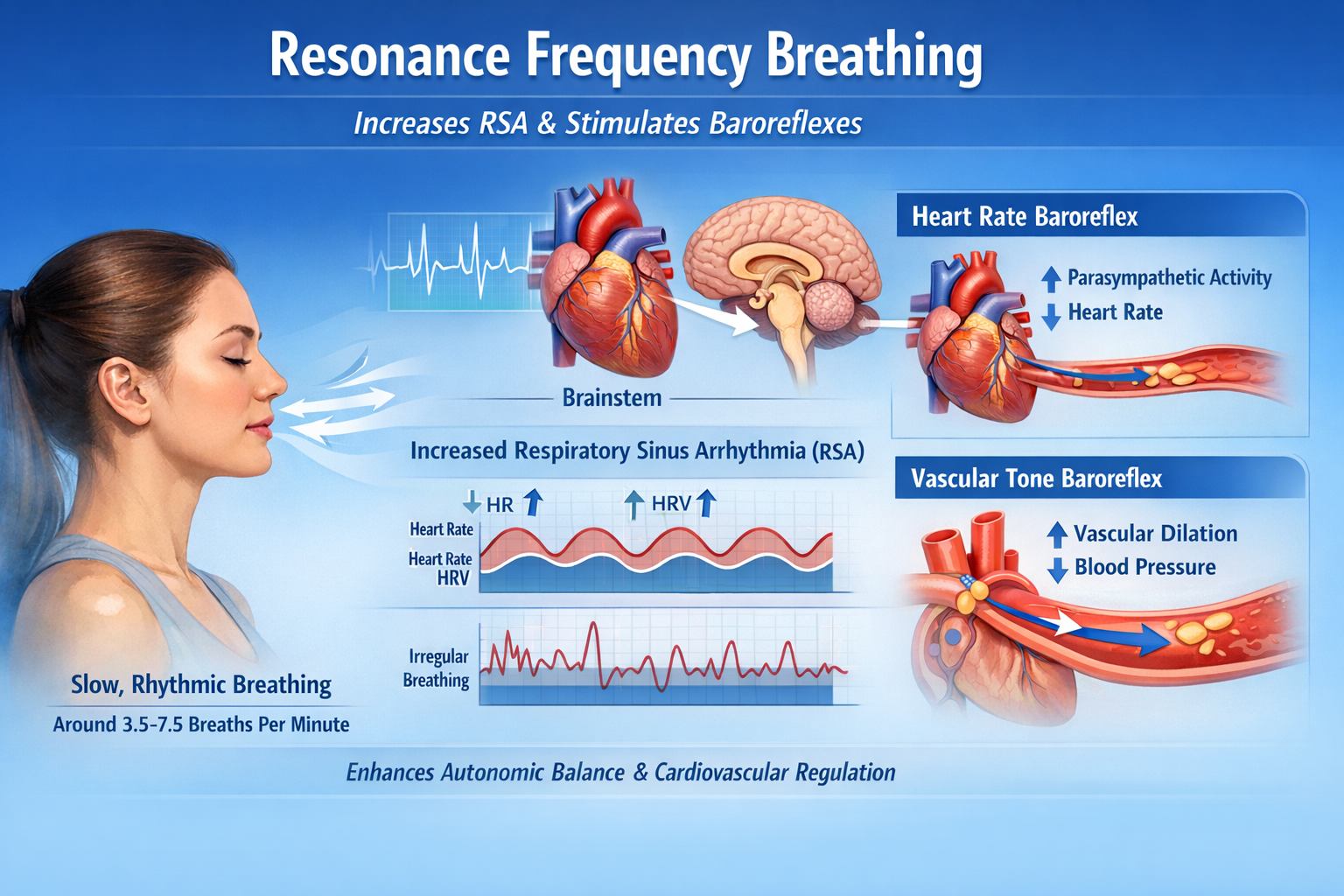
You can observe the effect of breathing rate on RSA during paced breathing and select the rate that produces the largest HR oscillations. Adult breathing at 4.5 to 6.5 bpm shifts the ECG peak frequency from the high-frequency band (approximately 0.20 Hz) to the cardiovascular system's RF (approximately 0.10 Hz), more than doubling the energy in the low-frequency band of the ECG (0.04 to 0.15 Hz).
We train clients to increase low-frequency power and RSA during biofeedback sessions so that high-frequency (HF) power and time-domain measures like the RMSSD and the SDNN might increase during baselines when they breathe at typical rates (Lehrer, 2020).
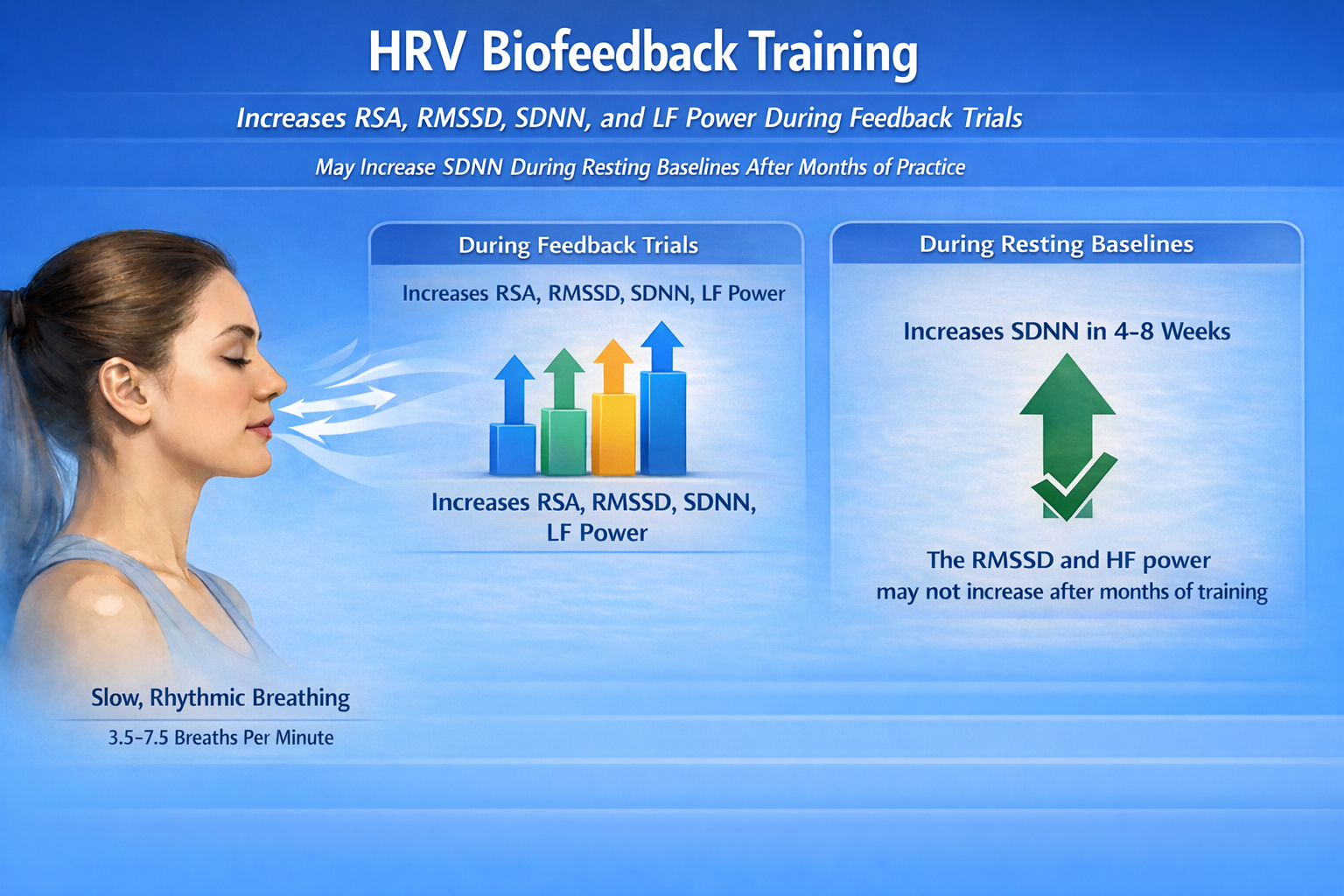
In other words, the goal is not to have clients breathe slowly all day; it is to build underlying autonomic fitness that manifests when they breathe normally.
Short-Term Resting HRV Measurements
Across studies, short-term resting SDNN is the most likely resting index to show a detectable increase within 4–8 weeks, while the RMSSD and HF power are less consistent, sometimes showing no clear resting change even after 4–8 weeks and only emerging (if at all) over longer intervals such as ~12 weeks in clinical samples (Herhaus et al., 2022; Limmer et al., 2021; Schumann et al., 2021).
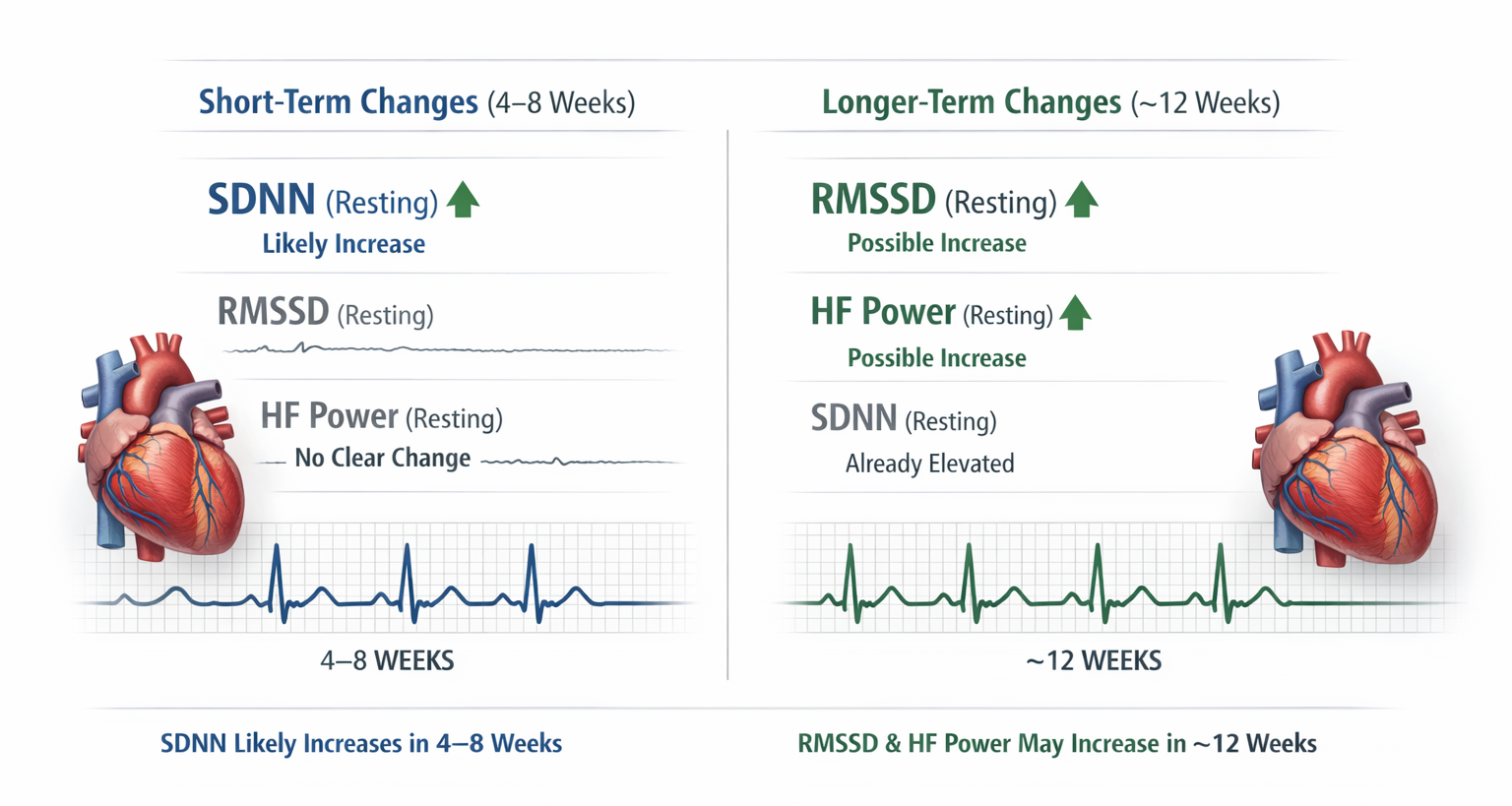
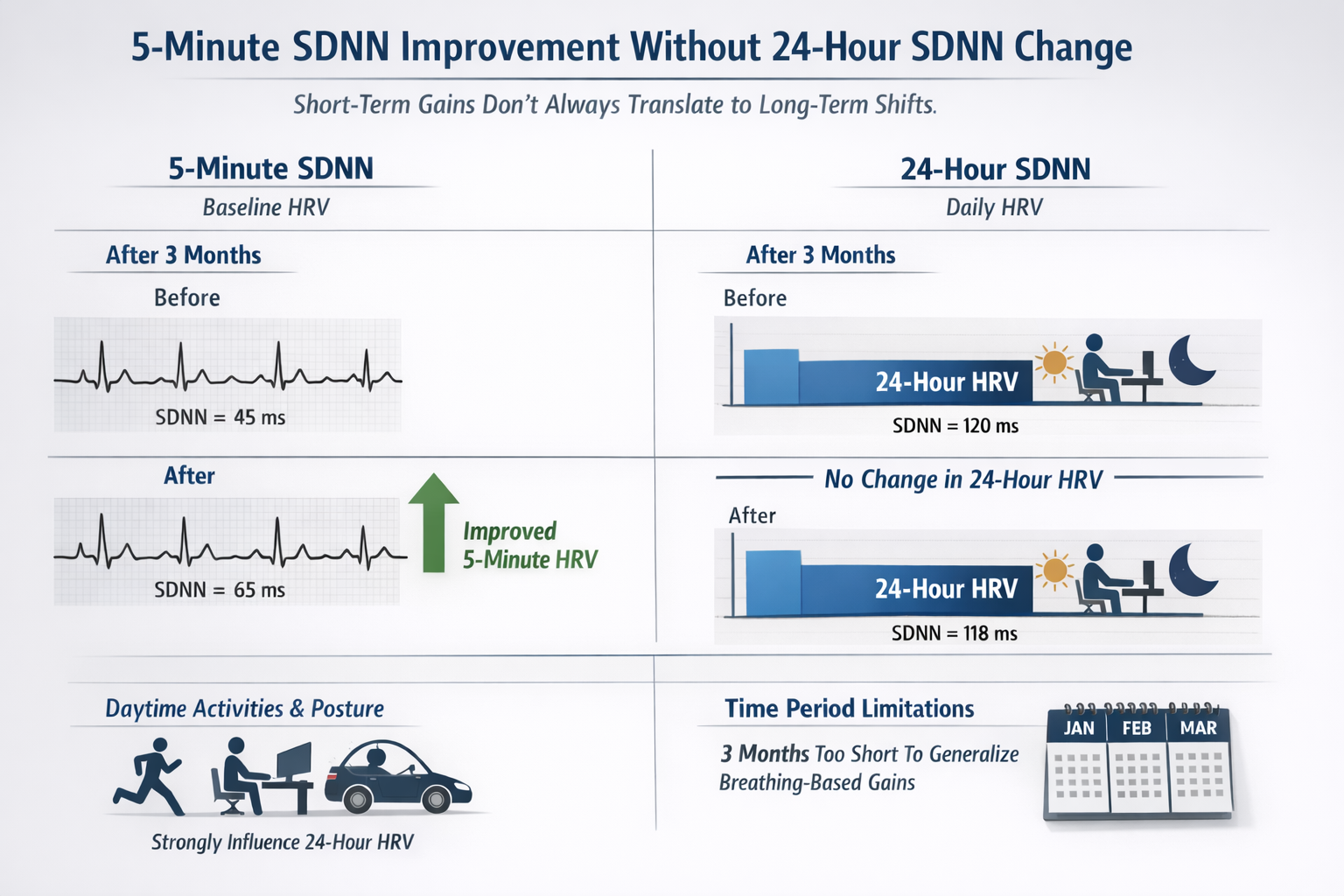
24-Hour HRV Changes Lag Behind Short-Term Changes
Limmer et al. (2022) reported 5-minute baseline SDNN improvements over weeks without seeing a corresponding 24-hour SDNN shift over the same interval. They cautioned that 24-hour HRV is heavily influenced by daytime activities and posture, and that a three-month window may be too short for breathing-based gains to fully generalize into day-long habitual patterns captured by 24-hour recordings.
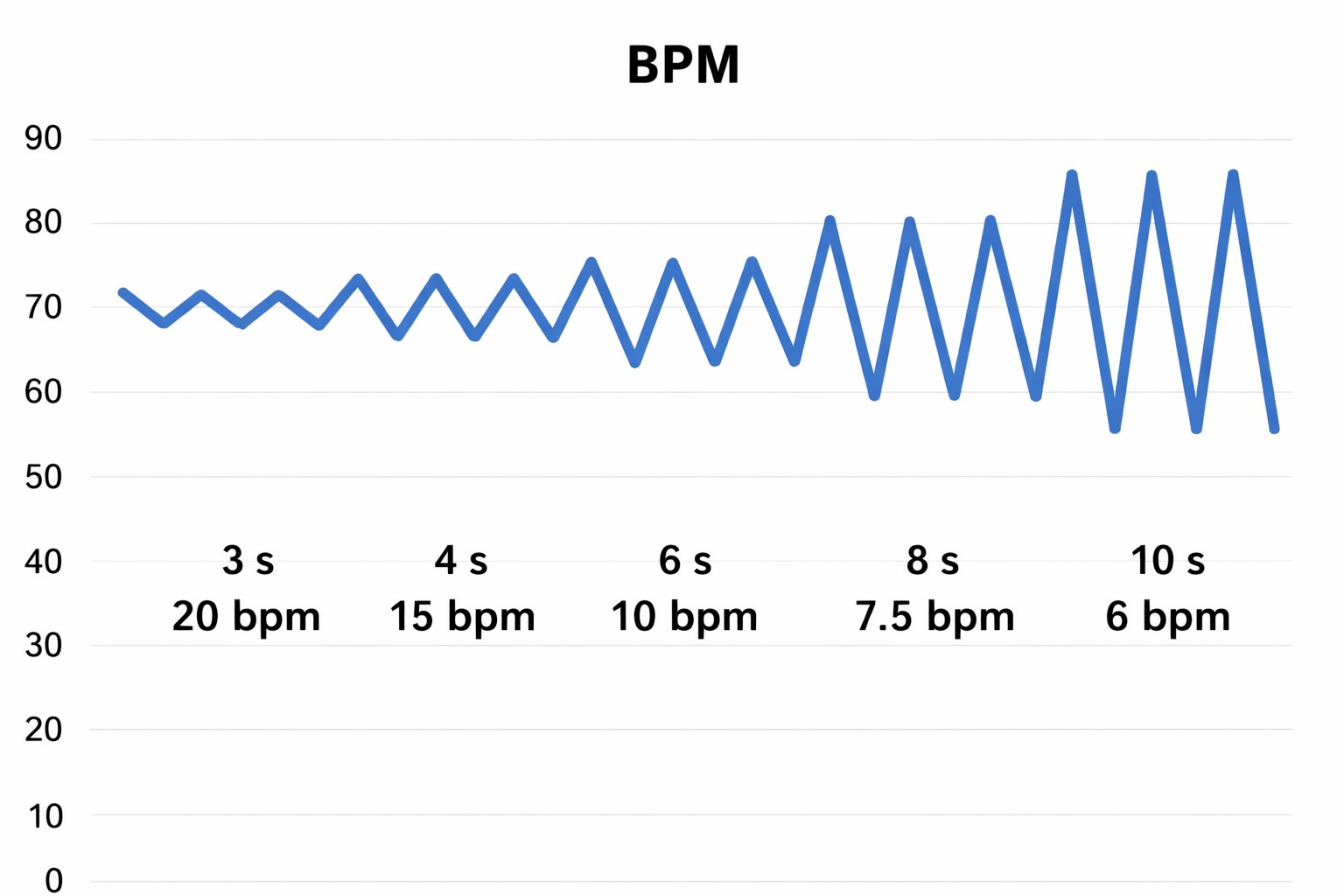
Breathing Rate and Depth
RSA increases with greater respiratory depth (Hirsch & Bishop, 1981) and lower respiration rate (Brown et al., 1993). A 10-second breathing cycle corresponds to a 6-bpm respiration rate in the graphic below, which falls within the 4.5 to 6.5 adult RF range. Both rate and depth matter: coaching clients to breathe slowly but shallowly will not produce optimal results.
The graphic below was adapted from Grossman and Kollai (1993). RSA, shown as a change in heart rate from inhalation to exhalation, increases as the respiration rate approaches 6 bpm.
Comprehension Questions: Factors That Influence HRV
- How do age-related changes in HRV differ between time-domain metrics like SDNN and metrics like RMSSD?
- Explain resonance using one of the everyday analogies presented in this section.
- Why do taller individuals tend to have lower resonance frequencies?
- How does breathing rate interact with respiratory depth to influence RSA?
Correlates of Low and Normal HRV
This section examines two sides of the same coin: the risks associated with low HRV and the clinical benefits of increasing it. You will learn why HRV is desirable while blood pressure variability is dangerous, how reduced HRV predicts disease and mortality, and why Friedman's "bad brakes" metaphor captures the mechanism of HRV biofeedback. These findings form the evidence base for recommending HRV biofeedback to clients and referring providers.
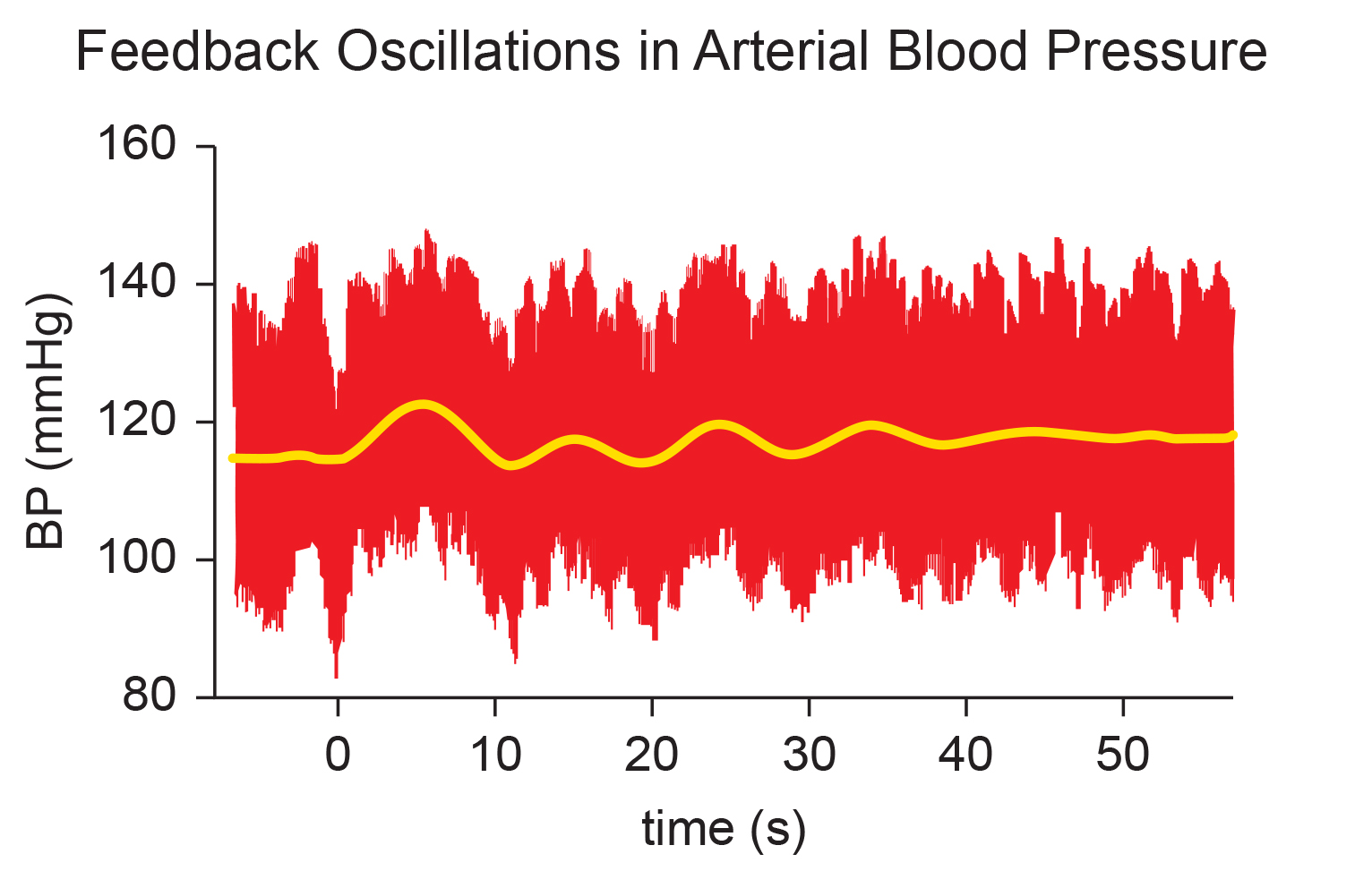
Heart Rate Variability Is Desirable, While Blood Pressure Variability Is Dangerous
Heart rate variability biofeedback is extensively used to treat various disorders (e.g., asthma and depression) and enhance performance across diverse contexts (e.g., sports; Gevirtz, 2013; Lehrer et al., 2020a; Tan et al., 2016). Lehrer et al. (2020) observed that HRV biofeedback has the largest effect sizes on anxiety, depression, anger, and athletic/artistic performance and the smallest effect sizes on PTSD, sleep, and quality of life.
Although the final targets of these applications may differ, HRV biofeedback increases vagal tone (Vaschillo et al., 2006) and stimulates the negative feedback loops responsible for homeostasis (Lehrer & Eddie, 2013). Whereas HRV is desirable, BP variability can endanger health—we require BP stability under constant workloads (Gevirtz, 2020). This distinction is worth emphasizing to clients: the goal of training is to increase the heart's flexibility while keeping blood pressure steady.
Reduced HRV Is Associated with Disease and Loss of Adaptability
The clinical significance of low HRV has been recognized for decades. In the early 1960s, researchers found that changes in HRV preceded fetal distress (Hon & Lee, 1963). Reduced HRV is associated with vulnerability to physical and psychological stressors and disease (Lehrer, 2007), and prospective studies have shown that decreased HRV is the strongest independent predictor of coronary atherosclerosis progression (McCraty & Shaffer, 2015).
🎧 Mini-Lecture: Reduced HRV Is Associated with Disease and Loss of Adaptability
Low HRV is a marker for cardiovascular disorders, including hypertension (especially with left ventricular hypertrophy), ventricular arrhythmia, chronic heart failure, and ischemic heart disease (Bigger et al., 1995; Casolo et al., 1989; Maver et al., 2004; Nolan et al., 1992; Roach et al., 2004). Low HRV predicts sudden cardiac death, especially due to arrhythmia following myocardial infarction and post-heart attack survival (Bigger et al., 1993; Bigger et al., 1992; Kleiger et al., 1987).
Depression, HRV, and Cardiac Risk
Depression in myocardial infarction (MI) patients increases mortality. Depressed patients are twice as likely as non-depressed individuals to have lower HRV (16% vs. 7%), and lower HRV is a strong independent predictor of post-MI death (Carney et al., 2001). HRV biofeedback might reduce anxiety and depression—both associated with low vagal activity—because it increases vagal tone. From Friedman's (2007) perspective, the problem is not "a sticky accelerator." HRV biofeedback may fix "bad brakes" (p. 186). This metaphor is clinically useful: rather than merely dampening the sympathetic nervous system, HRV biofeedback strengthens the parasympathetic system's ability to regulate the heart.
Reduced HRV may predict disease and mortality because it indexes reduced regulatory capacity—the ability to adaptively surmount challenges like exercise and stressors. Patient age may be an essential link between reduced HRV and regulatory capacity since HRV and nervous system function decline with age (Shaffer et al., 2014).
Reduced HRV is also seen in disorders with autonomic dysregulation, including anxiety and depressive disorders, asthma, and vulnerability to sudden infant death (Agelink et al., 2002; Carney et al., 2001; Cohen & Benjamin, 2006; Giardino et al., 2004; Kazuma et al., 1997). Lehrer (2007) believes that HRV indexes adaptability and marshals evidence that increased RSA represents more efficient regulation of BP, HR, and gas exchange by synergistic control systems.
The Benefits of Increased HRV
This section translates the research on HRV correlates into concrete clinical benefits. You will learn how HRV biofeedback enhances RSA, increases low-frequency band power, improves gas exchange, strengthens baroreflex gain, modulates immunity, and may even remodel failing hearts. Each benefit connects to specific mechanisms that explain why HRV biofeedback works across such diverse clinical populations.
RSA
When clients breathe at their resonance frequency, HR and respiration are in perfect phase (0°); their peaks and valleys coincide. This frequency in adults varies from 4.5 to 6.5 breaths per minute (Gevirtz et al., 2016). When clients breathe at this rate, they "exercise" the baroreflex.
Resonance frequency (RF) breathing amplifies the swings in HR produced by the baroreflex, increasing baroreflex gain and RSA. RF breathing also modulates blood pressure changes since HR and BP oscillations are 180° out of phase (DeBoer et al., 1987; Vaschillo et al., 2002). This out-of-phase relationship is protective: as HR rises, BP falls, and vice versa, preventing dangerous spikes in either direction.
Low-frequency Band Power
RF breathing shifts the peak frequency from the high-frequency band (approximately 0.20 Hz) to the cardiovascular system's RF (approximately 0.10 Hz). RF breathing more than doubles the energy in the low-frequency band of the ECG (0.04 to 0.15 Hz). This corresponds to the Institute of HeartMath's concept of coherence, in which a client produces a "narrow, high-amplitude, easily visualized peak" from 0.09 to 0.14 Hz (Ginsberg et al., 2010, p. 54; McCraty et al., 2009). On your biofeedback display, this appears as a tall, sharp spike in the power spectrum—a visual confirmation that the client has achieved resonance.
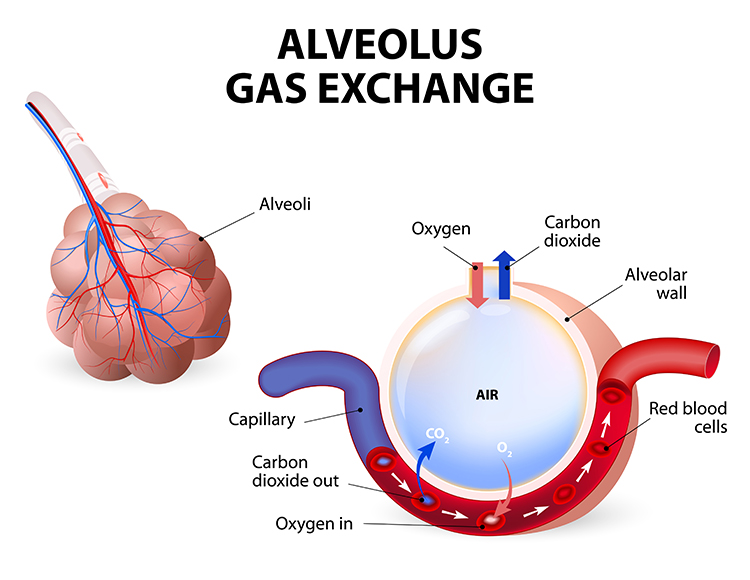
Regulation of Carbon Dioxide and Oxygen
The perfect phase relationship between HR and respiration rate results in the most efficient gas exchange and optimal oxygen saturation (Bernardi et al., 2001; Vaschillo et al., 2004; Yasuma & Hayano, 2004). When the heart beats faster during inhalation (when fresh oxygen is available) and slower during exhalation, blood flow is optimally matched to ventilation.
Resonance frequency training also improves oxygen regulation by increasing the sensitivity of chemoreceptors—sensors that detect oxygen and carbon dioxide levels in the blood. More sensitive chemoreceptors mean tighter regulation of blood gases, which benefits clients with respiratory conditions like asthma and COPD.
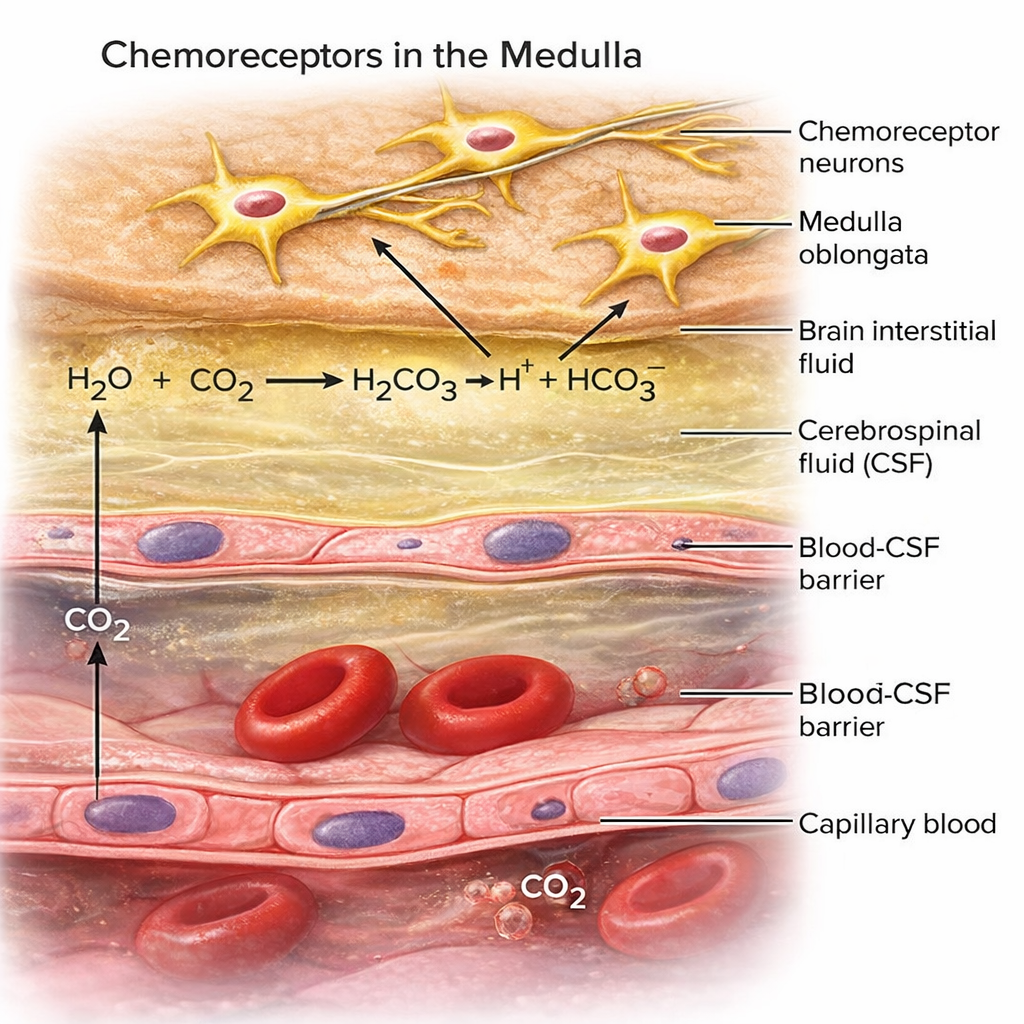
Baroreflex Gain and BP Regulation
While HRV biofeedback training immediately produces large-scale RSA increases, months of practice can increase baroreflex gain when clients are not receiving feedback (Lehrer, 2013; Lehrer et al., 2003). Increased baroreceptor gain is analogous to a more sensitive thermostat—the body detects smaller BP changes and corrects them more quickly. This is one of the most important long-term benefits of HRV biofeedback: the client's cardiovascular system becomes fundamentally better at self-regulation.

Increased baroreflex gain means that the cardiovascular system produces large-scale HR increases and decreases when a client inhales and exhales, which translates into greater HRV even during normal breathing.
Modulation of Immunity
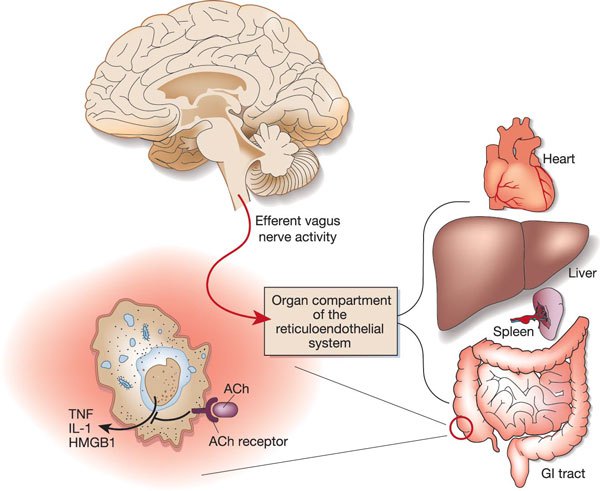
Like vagal nerve stimulation (VNS), resonance frequency breathing may also influence the parasympathetic cholinergic cytokine control system that modulates immunity through interleukins and interferons (Gevirtz, 2013; Tracey, 2007). This connection between vagal tone and immune function is one of the most exciting frontiers in HRV biofeedback research.
The sensory vagus detects inflammation and infection via tissue necrosis factor (TNF) and interleukin-1 (IL-1). The motor vagus signals descending neurons to release norepinephrine to spleen T cells, prompting these cells to release acetylcholine to macrophages to dampen inflammation (Schwartz, 2015). This is the cholinergic anti-inflammatory pathway—a dedicated neural circuit that links vagal tone directly to inflammatory control.
Chronic inflammation is implicated in diverse disorders, including Alzheimer's disease, cancer, cardiovascular diseases, depression, and diabetes (Dhar et al., 2016; Poole et al., 2011). For clinicians, this means that HRV biofeedback may offer benefits that extend well beyond what clients initially seek treatment for.
Lehrer et al. (2010) demonstrated that subjects trained to breathe at their RF minimized the reduction of HRV, headache, and eye photosensitivity following an injection of lipopolysaccharide (LPS), an inflammatory cytokine. This finding provides experimental evidence that RF breathing can actively modulate the body's inflammatory response.
Remodeling Failing Hearts
Moravec and McKee (2013) reported preliminary evidence that HRV biofeedback may act like a left ventricular assist device (LVAD)—a mechanical pump used to support failing hearts—to help remodel failing hearts. Examination of harvested hearts revealed that transplant candidates trained to slow their breathing to 8 to 9 breaths per minute increased the numbers of β1 receptors on cardiac muscle fibers and cardiac muscle contraction in response to norepinephrine (NE) and epinephrine (E).
HRV biofeedback for heart failure patients represents a paradigm shift. Instead of only targeting sympathetic activation, HRV biofeedback teaches patients to restore autonomic balance by decreasing SNS arousal while increasing PNS activity. For clinicians working in cardiac rehabilitation, this finding suggests a biofeedback application with potentially life-saving implications.
Comprehension Questions: Correlates and Benefits of HRV
- Why is HRV desirable while blood pressure variability is dangerous?
- How does the concept of "bad brakes" versus a "sticky accelerator" help explain the mechanism of HRV biofeedback for depression?
- Describe the cholinergic anti-inflammatory pathway and its relevance to HRV biofeedback.
- How might HRV biofeedback act like a left ventricular assist device in heart failure patients?
HRV Biofeedback Is Like Weightlifting
This brief section addresses one of the most common misconceptions clients have about HRV biofeedback training. Understanding this analogy will help you set appropriate expectations and prevent clients from overusing slow-paced breathing outside of practice sessions.
Slow-paced breathing used in HRV biofeedback is like weightlifting. Just as we only expect athletes to lift weights during workouts, we do not expect clients to walk around breathing at 6 bpm all day. They do not have to continuously breathe at their RF to benefit from improved homeostatic regulation, regulatory capacity, and executive function.
.jpg)
Continuous RF breathing would actually jeopardize homeostasis since breathing rate and volume should adjust to changing daily physical workloads. A runner needs to breathe faster; a sleeper needs to breathe more slowly. The benefits of HRV biofeedback come from regular "workouts" that strengthen the baroreflex and vagal tone over time, not from maintaining a slow breathing rate around the clock.

HRV Myths
This section addresses common misconceptions about HRV that circulate among practitioners and clients alike. Being able to identify and correct these myths will strengthen your credibility and help clients develop realistic expectations about HRV biofeedback.
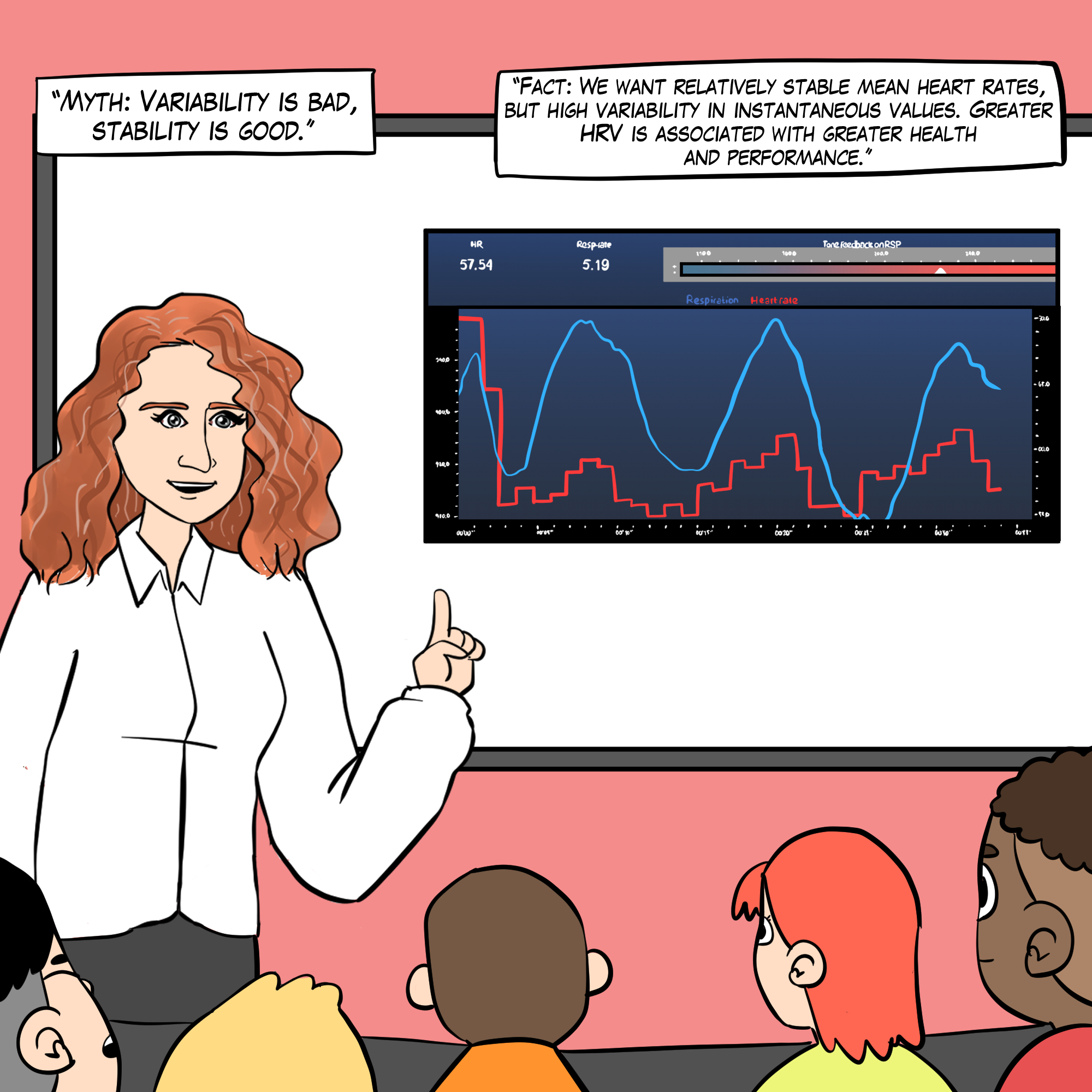
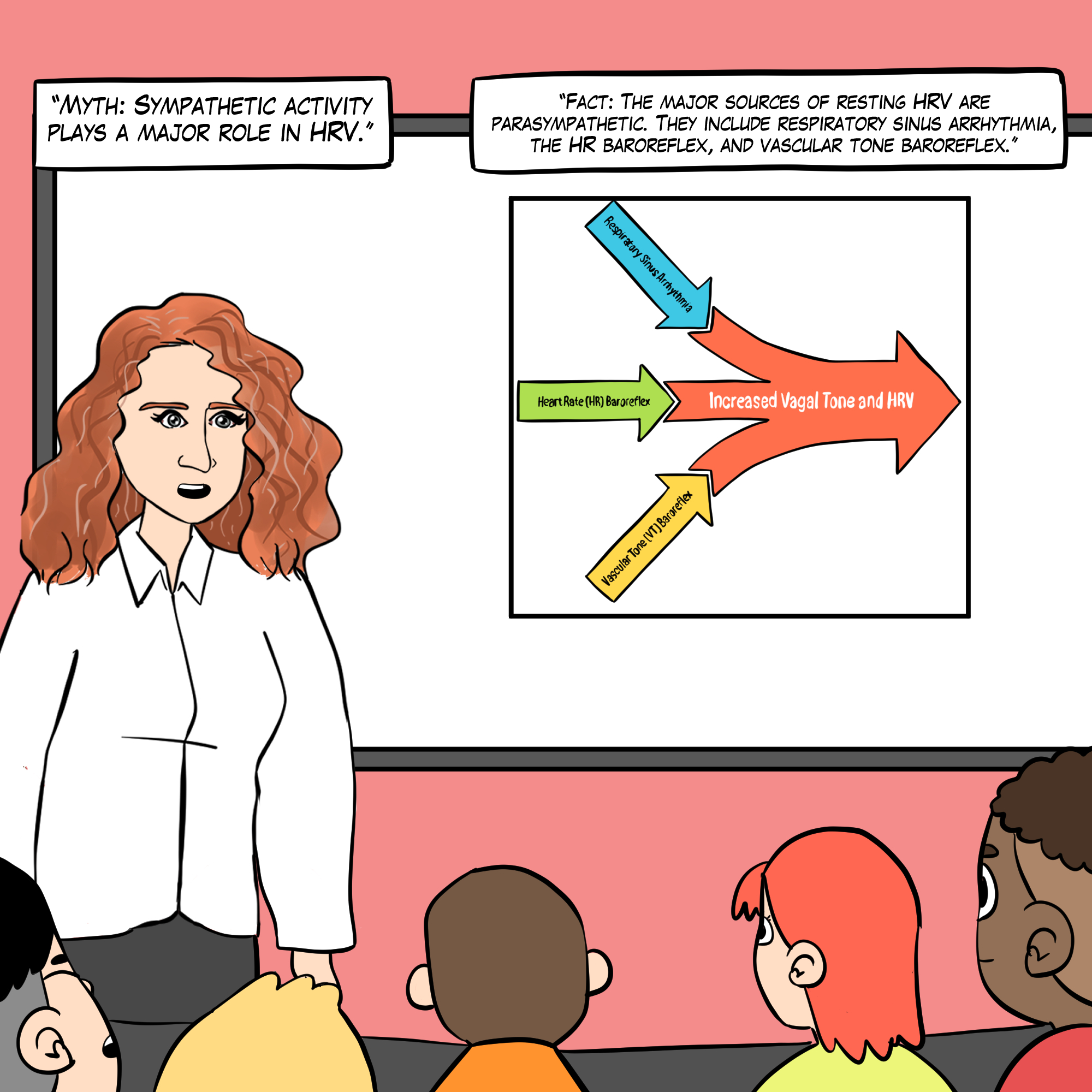
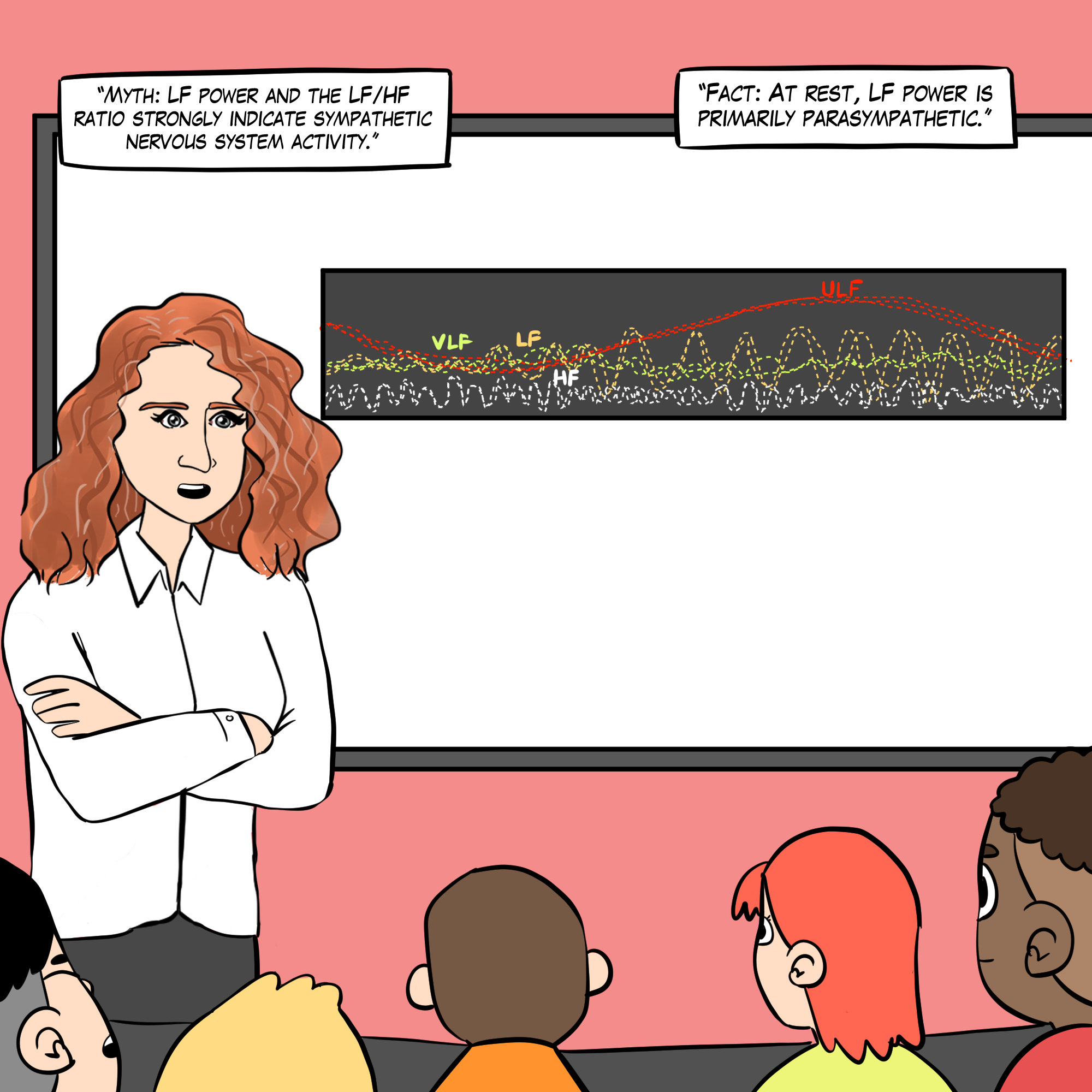
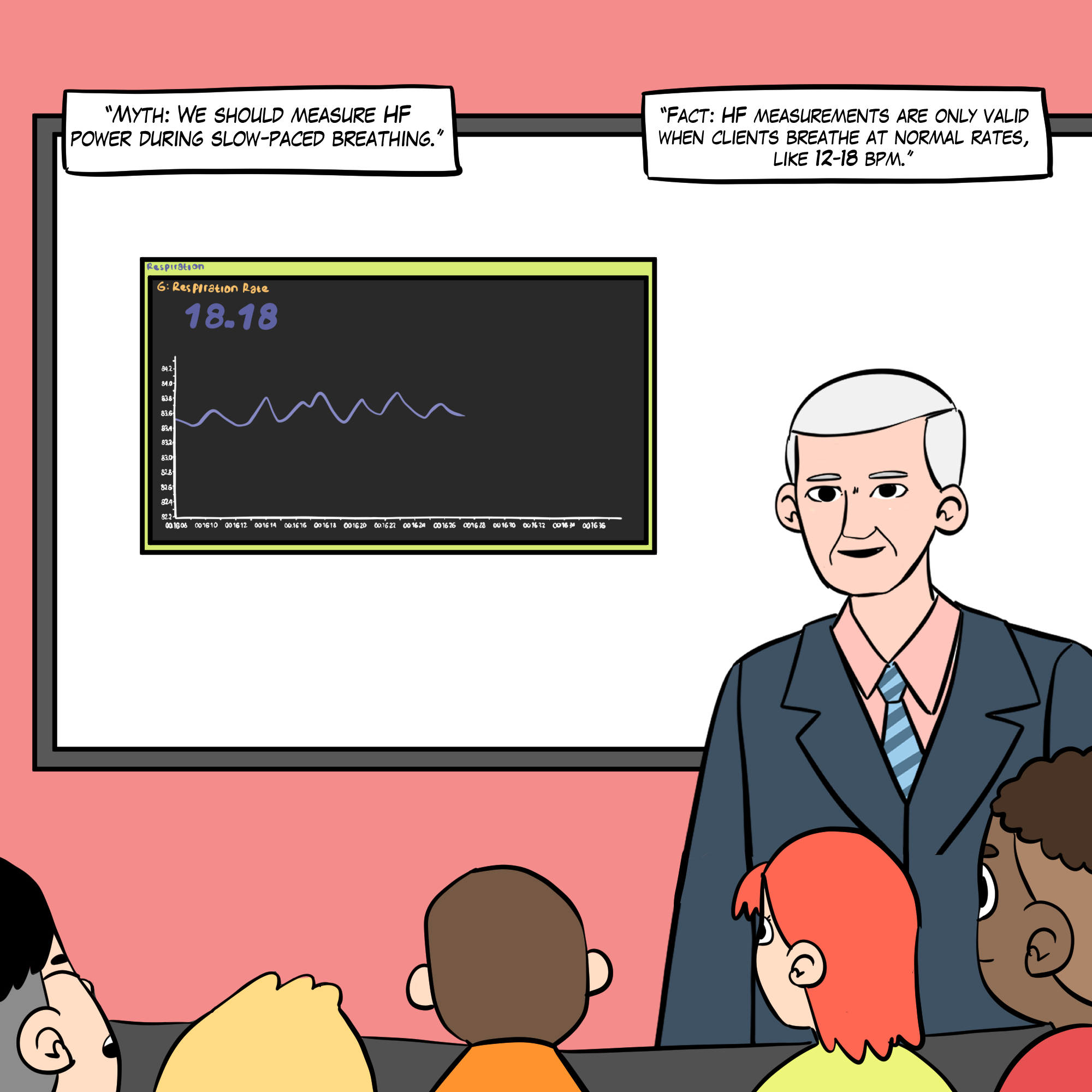
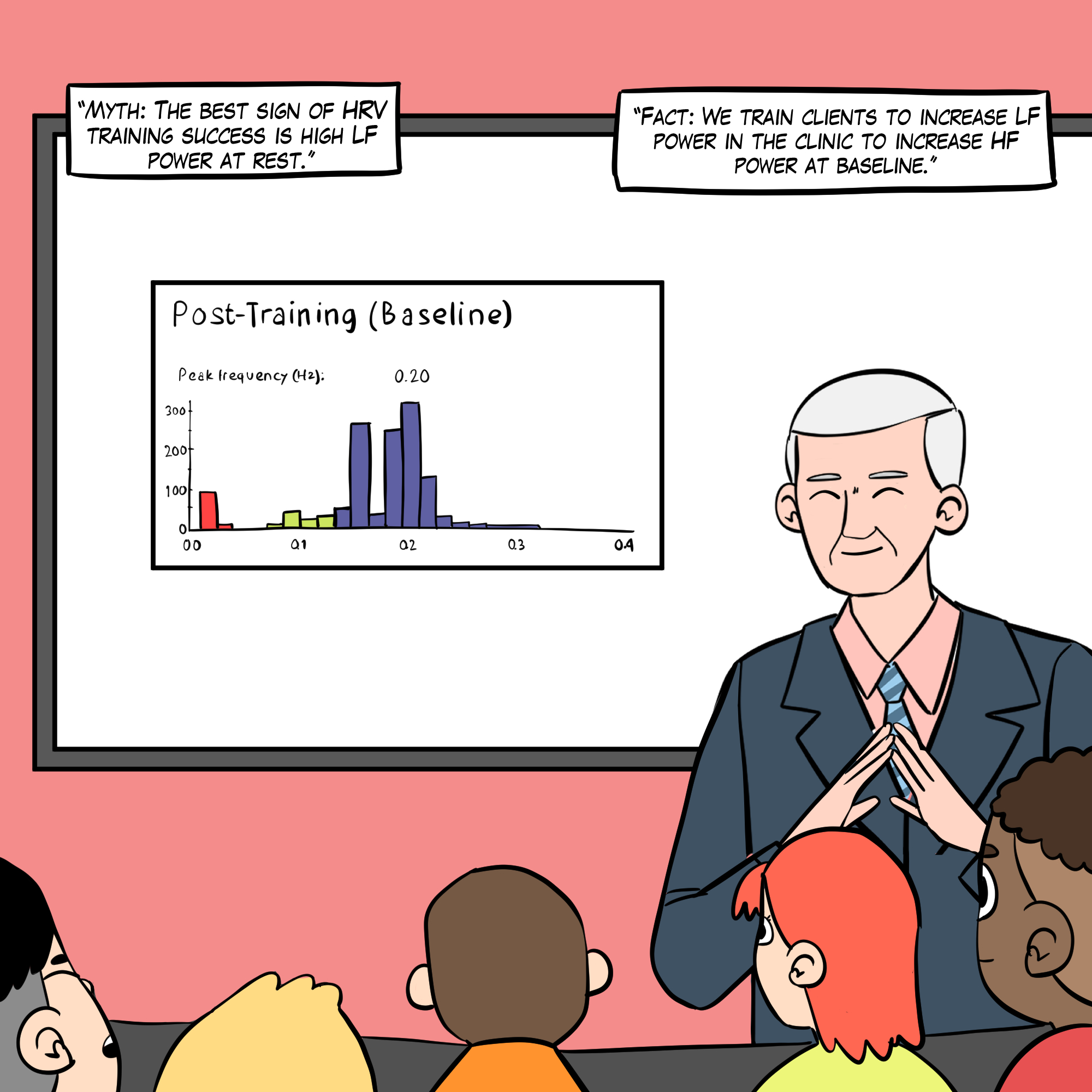
See A Comprehensive Guide to HRV Myths for more detailed explanations.
Cutting Edge Topics
Elevated Resting Heart Rate and Cognitive Decline
Emerging evidence links elevated resting heart rate to accelerated cognitive decline and dementia risk in older adults. Imahori et al. (2021) found that resting heart rates of 80 bpm or higher are associated with a greater risk of dementia, independent of cardiovascular disease, suggesting that heart rate management may be an underappreciated modifiable risk factor for cognitive health in aging populations.
vmHRV as a Transdiagnostic Biomarker
Research increasingly positions vagally-mediated HRV as a transdiagnostic biomarker that cuts across traditional psychiatric diagnostic categories. Rather than being specific to any single disorder, low vmHRV appears to index a shared vulnerability to emotional dysregulation that manifests differently across anxiety, depression, PTSD, and bipolar disorder (Beauchaine & Thayer, 2015). This perspective has implications for developing unified treatment approaches that target autonomic regulation as a common therapeutic mechanism.
The Gut-Brain-Heart Axis and HRV
Exciting new research explores how the vagus nerve connects gut microbiome health, brain function, and cardiac regulation in an integrated axis. Since the vagus nerve is the primary neural pathway between the gut and the brain, interventions that enhance vagal tone through HRV biofeedback may have broader systemic effects than previously understood, potentially influencing gut health, inflammatory responses, and even neurotransmitter production.
Assignment
Now that you have completed this module, monitor your HR as you inhale and exhale to observe your own RSA. What is the average difference between your fastest and slowest HRs across several breathing cycles? How has this unit changed how you might explain HRV and its potential benefits to a client?
Glossary
0.1 Hz biofeedback: training to concentrate ECG power around 0.1 Hz in the low frequency (LF) band by teaching patients to breathe diaphragmatically at their RF around 6 breaths per minute and to experience positive emotional tone to maximize heart rate variability.
abdominal excursion: the degree of outward and inward stomach movement across the breathing cycle.
Autonomic Space Theory: Berntson and colleagues challenged the reciprocal model of autonomic control, proposing that sympathetic and parasympathetic systems can function independently. It identifies three modes: reciprocal activation, coactivation, and coinhibition, highlighting the complex interplay of autonomic regulation in stress, cognition, and physiological adaptability.
baroreceptor gain: increased baroreceptor sensitivity to BP changes.
baroreceptor reflex (baroreflex): a mechanism that provides negative feedback control of BP. Elevated BP activates the baroreflex to lower BP and low BP suppresses the baroreflex to raise BP.
baroreceptors: BP sensors located in the aortic arch and internal carotid arteries.
chaos: unpredictability due to nonlinear dynamics.
cardiac chronotropy: the rate of cardiac contraction, essentially how fast or slow the heart beats. Positive chronotropic effects increase heart rate (e.g., sympathetic activation), while negative chronotropic effects decrease it (e.g., vagal tone).
cardiovascular resonance: the phenomenon in which heart rate oscillations and blood pressure waves become maximally synchronized, typically occurring when breathing rate matches the ~0.1 Hz resonant frequency of the baroreflex loop (roughly 6 breaths per minute). This produces the largest possible heart rate variability amplitude and is the physiological basis for resonance frequency biofeedback.
chemoreceptor: sensors that detect oxygen and carbon dioxide in the blood to regulate gas concentration.
cycle length dependence: the phenomenon where faster HRs reduce the time between successive beats and the opportunity for the interbeat intervals (IBIs) to vary, resulting in lower HRV.
D2: correlation dimension estimates the minimum number of variables required to construct a system dynamics model.
epinephrine (E): an adrenal medullary hormone that increases muscle blood flow, converts stored nutrients into glucose for use by skeletal muscles, and initiates cardiac muscle contraction when it binds to β1 receptors.
fractals: infinitely complex geometric patterns that are self-similar across different scales.
Frank-Starling mechanism: the intrinsic property of the heart whereby increased venous return stretches the ventricular myocardium during diastole, resulting in a more forceful contraction and greater stroke volume. In short, the heart automatically adjusts its output to match the volume of blood flowing into it — the more the ventricle fills, the harder it pumps.
frequency-domain measures of HRV: the absolute or relative power of the HRV signal within four frequency bands.
heart rate baroreflex: the closed-loop encompassing the cardiovascular control center, heart rate control system, and blood pressure control system.
heart rate variability (HRV): the beat-to-beat changes in HR involving changes in the RR intervals between consecutive heartbeats.
high-frequency (HF) band: an ECG frequency range from 0.15-0.40 Hz that represents the inhibition and activation of the vagus nerve by breathing (RSA).
homeostasis: a state of dynamic constancy achieved by stabilizing conditions about a setpoint, whose value may change over time.
HR Max-HR Min: an HRV index that calculates the average difference between the highest and lowest HRs during each respiratory cycle.
HRV frequency-domain measurements: metrics that quantify absolute or relative power distribution into four frequency bands, revealing the sources of HRV.
HRV nonlinear measurements: metrics that quantify the unpredictability of a time series, resulting from the complexity of the mechanisms that regulate the measured variable.
HRV time-domain measurements: metrics that quantify the total amount of HRV.
interbeat interval (IBI): the time interval between the peaks of successive R-spikes (initial upward deflections in the QRS complex). This is also called the NN (normal-to-normal) interval after removing artifacts.
low-frequency (LF) band: an HRV frequency range of 0.04-0.15 Hz that may represent the influence of PNS and baroreflex activity when breathing or contracting muscles between 4.5-6.5 times a minute.
Maximum-Minimum HR: see HR Max-HR Min.
neurovisceral integration model: Thayer and Lane's theoretical framework describing the role of the central autonomic network (CAN) in regulating autonomic, cognitive, and emotional processes. It emphasizes the prefrontal cortex's top-down control over heart rate variability (HRV) via the vagus nerve, linking HRV to self-regulation and mental health.
norepinephrine (NE): an adrenal medullary hormone that initiates cardiac muscle contraction when it binds to β1 receptors.
nucleus ambiguus system: the nucleus dorsal to the inferior olivary nucleus of the upper medulla that gives rise to vagus nerve motor fibers.
peak frequency: the HRV frequency with the greatest power.
regulatory capacity: the ability to adaptively surmount challenges like exercise and stressors.
resonance: an amplification process in which an external force causes a closed-loop (negative feedback) system to oscillate with greater amplitude at its inherent resonance frequency (RF).
resonance frequency: the frequency at which a system, like the cardiovascular system, can be activated or stimulated.
respiratory sinus arrhythmia (RSA): the respiration-driven heart rhythm that contributes to the high frequency (HF) component of heart rate variability. Inhalation inhibits vagal nerve slowing of the heart (increasing HR), while exhalation restores vagal slowing (decreasing HR).
resting baseline: breathing at typical rates without pacing or feedback.
RMSSD: the square root of the mean squared difference of adjacent NN intervals in milliseconds.
SD1: the standard deviation of the distance of each point from the y = x-axis that measures short-term HRV.
SD2: the standard deviation of each point from the y = x + average RR interval that measures short- and long-term HRV.
SDNN: the standard deviation of the normal (NN) sinus-initiated IBI measured in milliseconds.
slow-paced breathing (SPB): low-and-slow breathing at approximately 6 bpm for adults with longer exhalation than inhalation.
slow-paced contraction (SPC): wrist-core-ankle contraction with legs supported and crossed at rates of approximately 1 or approximately 6 cpm.
spectral analysis: the division of heart rate variability into its component rhythms that operate within different frequency bands.
sympathetically-mediated HRV (smHRV): HRV components influenced by the sympathetic nervous system, typically assessed through measures such as low-frequency (LF) power in HRV analysis. However, the interpretation of LF power as a direct marker of sympathetic activity remains debated.
time-domain measures of HRV: indices like SDNN that quantify the total amount of heart rate variability.
triangular index (TI): a geometric measure based on 24-hour recordings, which calculates the integral of the RR interval histogram's density divided by its height.
triangular interpolation of the NN interval histogram (TINN): the baseline width of a histogram displaying NN intervals.
ultra-low-frequency (ULF) band: an ECG frequency range below 0.003 Hz. Very slow biological processes that include circadian rhythms, core body temperature, metabolism, and the renin-angiotensin system, and possibly the PNS and SNS, generate ULF activity.
Vagal Tank Theory: Laborde and colleagues view cardiac vagal control as a dynamic resource for self-regulation, comprising resting vagal tone, reactivity to stress, and recovery. A higher vagal tank supports stress resilience, cognitive flexibility, and emotional regulation, while depletion leads to dysregulation and health risks.
vagally-mediated HRV (vmHRV): the high-frequency component of HRV, controlled by the parasympathetic nervous system via the vagus nerve. Indexed by the RMSSD and LF power during normal breathing, vmHRV serves as a biomarker of autonomic flexibility, with higher vmHRV indicating better cognitive control, emotional regulation, and stress resilience, while lower vmHRV is associated with poor health outcomes.
vagus nerve: the parasympathetic vagus (X) nerve decreases the rate of spontaneous depolarization in the SA and AV nodes and slows HR. Heart rate increases often reflect reduced vagal inhibition.
vascular tone (VT) baroreflex: the closed-loop encompassing the cardiovascular control center, vascular tone control system, and blood pressure control system.
very-low-frequency (VLF): an ECG frequency range of 0.003-0.04 Hz that may represent temperature regulation, plasma renin fluctuations, endothelial, physical activity influences, possible intrinsic cardiac nervous system, and PNS contributions.
References
Agelink, M., Boz, C., Ullrich, H., & Andrich, J. (2002). Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry Research, 113(1), 139-149. https://doi.org/10.1016/s0165-1781(02)00225-1
Akselrod, S., Gordon, D., Ubel, F. A., Shannon, D. C., Berger, A. C., & Cohen, R. J. (1981). Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science, 213, 220-222. https://doi.org/10.1126/science.6166045
Almeida-Santos, M. A., Barreto-Filho, J. A., Oliveira, J. L., Reis, F. P., da Cunha Oliveira, C. C., & Sousa, A. C. (2016). Aging, heart rate variability and patterns of autonomic regulation of the heart. Archives of Gerontology and Geriatrics, 63, 1-8. https://doi.org/10.1016/j.archger.2015.11.011
Balzarotti, S., Biassoni, F., Colombo, B., & Ciceri, M. R. (2017). Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biological Psychology, 130, 54-66. https://doi.org/10.1016/j.biopsycho.2017.10.008
Beauchaine, T. P., & Thayer, J. F. (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2 Pt 2), 338-350. https://doi.org/10.1016/j.ijpsycho.2015.08.004
Beckers, F., Verheyden, B., & Aubert, A. E. (2006). Aging and nonlinear heart rate control in a healthy population. American Journal of Physiology-Heart and Circulatory Physiology, 290, H2560-H2570. https://doi.org/10.1152/ajpheart.00903.2005
Benichou, T., Pereira, B., Mermillod, M., Tauveron, I., Pfabigan, D., Maqdasy, S., & Dutheil, F. (2018). Heart rate variability in type 2 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE, 13(4), e0195166. https://doi.org/10.1371/journal.pone.0195166
Bernardi, L., Gabutti, A., Porta, C., & Spicuzza, L. (2001). Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. Journal of Hypertension, 19(12), 2221-2229. https://doi.org/10.1097/00004872-200112000-00016
Bernardi, L., Valle, F., Coco, M., Calciati, A., & Sleight, P. (1996). Physical activity influences heart rate variability and very-low-frequency components in Holter electrocardiograms. Cardiovascular Research, 32, 234-237. https://doi.org/10.1016/0008-6363(96)00081-8
Berntson, G. G., Bigger, J. T., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., Nagaraja, H. N., Porges, S. W., Saul, J. P., Stone, P. H., & van der Molen, M. W. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623-648. https://doi.org/10.1111/j.1469-8986.1997.tb02140.x
Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), 459-487. https://doi.org/10.1037/0033-295X.98.4.459
Berntson, G. G., Quigley, K. S., & Lozano, D. (2007). Cardiovascular psychophysiology. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (3rd ed.). Cambridge University Press.
Bigger, J., Fleiss, J., Rolnitzky, L., & Steinman, R. (1993). The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation, 88, 927-934. https://doi.org/10.1161/01.cir.88.3.927
Bigger, J., Fleiss, J., Steinman, R., Rolnitzky, L., Kleiger, R., & Rottman, J. (1992). Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation, 85, 164-171. https://doi.org/10.1161/01.cir.85.1.164
Bigger, J. T., Fleiss, J. L., Steinman, R. C., Rolnitzky, L. M., Schneider, W. J., & Stein, P. K. (1995). RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation, 91(7), 1936-1943. https://doi.org/10.1161/01.cir.91.7.1936
Carney, R. M., Blumenthal, J. A., Stein, P. K., Watkins, L., Catellier, D., Berkman, L. F., Czajkowski, S. M., O'Connor, C., Stone, P. H., & Freedland, K. E. (2001). Depression, heart rate variability, and acute myocardial infarction. Circulation, 104(17), 2024-2028. https://doi.org/10.1161/hc4201.097834
Casolo, G., Balli, E., Taddei, T., Amuhasi, J., & Gori, C. (1989). Decreased spontaneous heart rate variability in congestive heart failure. The American Journal of Cardiology, 64(18), 1162-1167. https://doi.org/10.1016/0002-9149(89)90871-0
Cheng, Y. C., Huang, Y. C., & Huang, W. L. (2020). Heart rate variability in individuals with autism spectrum disorders: A meta-analysis. Neuroscience & Biobehavioral Reviews, 118, 463-471. https://doi.org/10.1016/j.neubiorev.2020.08.007
Cohen, H., & Benjamin, J. (2006). Power spectrum analysis and cardiovascular morbidity in anxiety disorders. Autonomic Neuroscience: Basic and Clinical, 128, 1-8. https://doi.org/10.1016/j.autneu.2005.06.007
DeBoer, R. W., Karemaker, J. M., & Strackee, J. (1987). Hemodynamic fluctuations and baroreflex sensitivity in humans: A beat-to-beat model. American Journal of Physiology-Heart and Circulatory Physiology, 253(22), H680-H689. https://doi.org/10.1152/ajpheart.1987.253.3.h680
Del Pozo, J. M., & Gevirtz, R. N. (2003). Complementary and alternative care for heart disease. Biofeedback, 31(3), 16-17.
Del Pozo, J. M., Gevirtz, R. N., Scher, B., & Guarneria, E. (2004). Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. American Heart Journal, 147(3), G1-G6. https://doi.org/10.1016/j.ahj.2003.08.013
Dhar, A. K., Lambert, G. W., & Barton, D. A. (2016). Depression and cardiovascular disease: Psychobiological mechanisms. In M. E. Alvarenga & D. Byrne (Eds.), Handbook of psychocardiology. Springer Singapore.
Ewing, D. J., Campbell, I. W., & Clarke, B. F. (1976). Mortality in diabetic autonomic neuropathy. Lancet, 1, 601-603. https://doi.org/10.1016/S0140-6736(76)90413-X
Gass, J. J., & Glaros, A. G. (2013). Autonomic dysregulation in headache patients. Applied Psychophysiology & Biofeedback, 38, 257-263. https://doi.org/10.1007/s10484-013-9231-8
Gevirtz, R. (2013). The nerve of that disease: The vagus nerve and cardiac rehabilitation. Biofeedback, 41(1), 32-38. http://dx.doi.org/10.5298/1081-5937-41.1.01
Gevirtz, R. N. (2000). Resonant frequency training to restore homeostasis for treatment of psychophysiological disorders. Biofeedback, 27, 7-9.
Gevirtz, R. N. (2003). The promise of HRV biofeedback: Some preliminary results and speculations. Biofeedback, 31(3), 18-19.
Gevirtz, R. N. (2005). Heart rate variability biofeedback in clinical practice. AAPB Fall workshop.
Gevirtz, R. N. (2017). Cardio-respiratory psychophysiology: Gateway to mind-body medicine.
Gevirtz, R. N., & Lehrer, P. (2003). Resonant frequency heart rate biofeedback. In M. S. Schwartz & F. Andrasik (Eds.), Biofeedback: A practitioner's guide (3rd ed.). The Guilford Press.
Gevirtz, R. N., Lehrer, P. M., & Schwartz, M. S. (2016). Cardiorespiratory biofeedback. In M. S. Schwartz & F. Andrasik (Eds.), Biofeedback: A practitioner's guide (4th ed.). The Guilford Press.
Giardino, N. D., Chan, L., & Borson, S. (2004). Combined heart rate variability and pulse oximetry biofeedback for chronic obstructive pulmonary disease. Applied Psychophysiology and Biofeedback, 29(2), 121-133. https://doi.org/10.1023/b:apbi.0000026638.64386.89
Ginsberg, J. P., Berry, M. E., & Powell, D. A. (2010). Cardiac coherence and posttraumatic stress disorder in combat veterans. Alternative Therapies, 16(4), 52-60.
Goldstein, D. S., Bentho, O., Park, M. Y., & Sharabi, Y. (2011). Low frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Experimental Physiology, 96(12), 1255-1261. PMID: 21642439
Herbs, D., Gevirtz, R. N., & Jacobs, D. (1994). The effect of heart rate pattern biofeedback for the treatment of essential hypertension [Abstract]. Biofeedback and Self-Regulation, 19(3), 281.
Herhaus, B., Kalin, A., Gouveris, H., & Petrowski, K. (2022). Mobile heart rate variability biofeedback improves autonomic activation and subjective sleep quality of healthy adults – A pilot study. Frontiers in Physiology, 13, 821741. https://doi.org/10.3389/fphys.2022.821741
Herhaus, B., Peter, A., Hummel, J., Kubiak, T., Heni, M., & Petrowski, K. (2025). Effect of 4 weeks resonance frequency breathing on glucose metabolism and autonomic tone in healthy adults. Diabetes & Metabolism Journal, 49(6), 1219–1228. https://doi.org/10.4093/dmj.2024.0647
Hillebrand, S., Gast, K. B., de Mutsert, R., Swenne, C. A., Jukema, J. W., Middeldorp, S., & Dekkers, O. M. (2013). Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. EP Europace, 15(5), 742-749. https://doi.org/10.1093/europace/eus341
Imahori, Y., Vetrano, D. L., Xia, X., Grande, G., Ljungman, P., Fratiglioni, L., & Qiu, C. (2021). Association of resting heart rate with cognitive decline and dementia in older adults: A population-based cohort study. Alzheimer's & Dementia. https://doi.org/10.1002/alz.12495
Karemaker, J. M. (2009). Counterpoint: Respiratory sinus arrhythmia is due to the baroreflex mechanism. Journal of Applied Physiology, 106(5), 1742-1743. https://doi.org/10.1152/japplphysiol.91107.2008a
Kazuma, N., Otsuka, K., Matsuoka, I., & Murata, M. (1997). Heart rate variability during 24 hours in asthmatic children. Chronobiology International, 14, 597-606. https://doi.org/10.3109/07420529709001450
Kleiger, R. E., Miller, J. P., Bigger, J. T., & Moss, A. J. (1987). Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. American Journal of Cardiology, 59, 256-262. https://doi.org/10.1016/0002-9149(87)90795-8
Laborde, S., Ackermann, S., Borges, U., D'Agostini, M., Giraudier, M., Iskra, M., Mosley, E., Ottaviani, C., Salvotti, C., Schmaußer, M., Szeska, C., Van Diest, I., Ventura-Bort, C., Voigt, L., Wendt, J., & Weymar, M. (2023). Leveraging vagally mediated heart rate variability as an actionable, noninvasive biomarker for self-regulation: Assessment, intervention, and evaluation. Policy Insights from the Behavioral and Brain Sciences, 10(2), 212-220. https://doi.org/10.1177/23727322231196789
Lehrer, P. (2022). My life in HRV biofeedback research. Applied Psychophysiology and Biofeedback, 1-10. https://doi.org/10.1007/s10484-022-09535-5
Lehrer, P., Karavidas, M. K., Lu, S. E., Coyle, S. M., Oikawa, L. O., Macor, M., Calvano, S. E., & Lowry, S. F. (2010). Voluntarily produced increases in heart rate variability modulate autonomic effects of endotoxin induced systemic inflammation: An exploratory study. Applied Psychophysiology and Biofeedback, 35(4), 303-315. https://doi.org/10.1007/s10484-010-9139-5
Lehrer, P., Kaur, K., Sharma, A., Shah, K., Huseby, R., Bhavsar, J., Sgobba, P., & Zhang, Y. (2020). Heart rate variability biofeedback improves emotional and physical health and performance: A systematic review and meta-analysis. Applied Psychophysiology and Biofeedback, 45, 109-129. https://doi.org/10.1007/s10484-020-09466-z
Lehrer, P., Vaschillo, B., Zucker, T., Graves, J., Katsamanis, M., Aviles, M., & Wamboldt, F. (2013). Protocol for heart rate variability biofeedback training. Biofeedback, 41(3), 98-109.
Lehrer, P., Vaschillo, E., Vaschillo, B., Lu, S-E., Scardella, A., Siddique, M., & Habib, R. (2004). Biofeedback treatment for asthma. Chest, 126, 352-361. https://doi.org/10.1378/chest.126.2.352
Lehrer, P. M. (2007). Biofeedback training to increase heart rate variability. In P. M. Lehrer, R. M. Woolfolk, & W. E. Sime (Eds.), Principles and practice of stress management (3rd ed.). The Guilford Press.
Lehrer, P. M. (2012). Personal communication.
Lehrer, P. M. (2013). How does heart rate variability biofeedback work? Resonance, the baroreflex, and other mechanisms. Biofeedback, 41(1), 26-31. https://doi.org/10.5298/1081-5937-41.1.02
Lehrer, P. M., & Eddie, D. (2013). Dynamic processes in regulation and some implications for biofeedback and biobehavioral interventions. Applied Psychophysiology & Biofeedback, 38, 143-155. https://doi.org/10.1007/s10484-013-9217-6
Lehrer, P. M., & Gevirtz, R. (2021). BCIA HRV Biofeedback didactic workshop. Association for Applied Psychophysiology and Biofeedback.
Lehrer, P. M., & Vaschillo, E. (2008). The future of heart rate variability biofeedback. Biofeedback, 36(1), 11-14.
Lehrer, P. M., Smetankin, A., & Potapova, T. (2000). Respiratory sinus arrhythmia biofeedback therapy for asthma: A report of 20 unmedicated pediatric cases using the Smetankin method. Applied Psychophysiology and Biofeedback, 25, 193-200. https://doi.org/10.1023/a:1009506909815
Lehrer, P. M., Vaschillo, E., Vaschillo, B., Lu, S. E., Eckberg, D. L., Edelberg, R., Shih, W. J., Lin, Y., Kuusela, T. A., Tahvanainen, K. U. O., & Hamer, R. M. (2003). Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine, 65, 796-805. https://doi.org/10.1097/01.psy.0000089200.81962.19
Lehrer, P. M., Vaschillo, E. V., & Vaschillo, B. (2004). Heartbeat synchronizes with respiratory rhythm only under specific circumstances. Chest, 126(4), 1385-1386. https://doi.org/10.1016/S0012-3692(15)31329-5
Limmer, A., Laser, M., & Schütz, A. (2021). Mobile heart rate variability biofeedback as a complementary intervention after myocardial infarction: A randomized controlled study. International Journal of Behavioral Medicine, 29(2), 230–239. https://doi.org/10.1007/s12529-021-10000-6
MacLean, B. (2004). The heart and the breath of love. Biofeedback, 32(4), 21-25.
Maver, J., Strucl, M., & Accetto, R. (2004). Autonomic nervous system activity in normotensive subjects with a family history of hypertension. Clinical Autonomic Research, 14(6), 369-375. https://doi.org/10.1007/s10286-004-0185-z
McCraty, R., & Shaffer, F. (2015). Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Global Advances in Health and Medicine, 4(1), 46-61. https://doi.org/10.7453/gahmj.2014.073
McCraty, R., Atkinson, M., & Tiller, W. A. (1995). The effects of emotions on short term power spectrum analysis of heart rate variability. American Journal of Cardiology, 76. https://doi.org/10.1016/s0002-9149(99)80309-9
McCraty, R., Atkinson, M., Tomasino, D., & Bradley, R. T. (2006). The coherent heart. Institute of HeartMath.
McCraty, R., Atkinson, M., Tomasino, D., & Bradley, R. T. (2009). The coherent heart: Heart-brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral Review, 5(2), 10-115.
Meehan, Z. M., & Shaffer, F. (2023a). Adding core muscle contraction to wrist-ankle rhythmical skeletal muscle tension increases respiratory sinus arrhythmia and low-frequency power. Applied Psychophysiology and Biofeedback, 48(1), 127-134. https://doi.org/10.1007/s10484-022-09568-w
Meehan, Z. M., & Shaffer, F. (2023b). Preeclampsia. In I. Z. Khazan, F. Shaffer, R. Lyle, D. Moss, & S. Rosenthal (Eds.), Evidence-based practice in biofeedback and neurofeedback (4th ed.).
Moravec, C. S., & McKee, M. G. (2013). Biofeedback in heart failure: Psychophysiologic remodeling of the failing heart. Biofeedback, 41(1), 7-12. http://dx.doi.org/10.5298/1081-5937-41.1.04
Moss, D. (2004). Heart rate variability (HRV) biofeedback. Psychophysiology Today, 1, 4-11.
Natarajan, A., Pantelopoulos, A., Emir-Farinas, H., & Natarajan, P. (2020). Heart rate variability with photoplethysmography in 8 million individuals: A cross-sectional study. The Lancet. Digital Health, 2(12), e650-e657. https://doi.org/10.1016/S2589-7500(20)30246-6
Nolan, J., Flapan, A. D., Capewell, S., MacDonald, T. M., Neilson, J. M., & Ewing, D. J. (1992). Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. British Heart Journal, 67(6), 482-485. https://doi.org/10.1136/hrt.67.6.482
Nunan, D., Sandercock, G. R. H., & Brodie, D. A. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology, 33(11), 1407-1417. https://doi.org/10.1111/j.1540-8159.2010.02841.x
Ogletree-Hughes, M. L., Stull, L. B., Sweet, W. E., Smedira, N. G., McCarthy, P. M., & Moravec, C. S. (2001). Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor down-regulation in the failing human heart. Circulation, 104, 881-886. https://doi.org/10.1161/hc3301.094911
Olshansky, B., Sabbah, H. N., Hauptman, P. J., & Colucci, W. S. (2008). Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation, 118, 863-871. https://doi.org/10.1161/circulationaha.107.760405
Opthof, T. (2000). The normal range and determinants of the intrinsic heart rate in man. Cardiovascular Research, 45, 177-184. PMID: 10728332
Pagani, M., Lombardi, F., Guzzetti, S., Rimoldi, O., Furlan, R., Pizzinelli, P., Sandrone, G., Malfatto, G., Dell'Orto, S., Piccaluga, E., Turiel, M., Baselli, G., Cerutti, S., & Malliani, A. (1986). Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research, 59(2), 178–193. doi:10.1161/01.RES.59.2.178. PMID: 2874900
Pagani, M., Montano, N., Porta, A., Malliani, A., Abboud, F. M., Birkett, C., & Somers, V. K. (1997). Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation, 95(6), 1441–1448. doi:10.1161/01.CIR.95.6.1441. PMID: 9118511
Papillo, J. F., & Shapiro, D. (1990). The cardiovascular system. In J. T. Cacioppo & L. G. Tassinary (Eds.), Principles of psychophysiology: Physical, social, and inferential elements (pp. 456-512). Cambridge University Press.
Peper, E., Harvey, R., Lin, I., Tylova, H., & Moss, D. (2007). Is there more to blood volume pulse than heart rate variability, respiratory sinus arrhythmia, and cardio-respiratory synchrony? Biofeedback, 35(2), 54-61.
Pomeranz, B., Macaulay, R. J., Caudill, M. A., Kutz, I., Adam, D., Gordon, D., Kilborn, K. M., Barger, A. C., Shannon, D. C., Cohen, R. J., & Benson, H. (1985). Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology, 248(1 Pt 2), H151–H153. doi:10.1152/ajpheart.1985.248.1.H151. PMID: 3970172
Poole, L., Dickens, C., & Steptoe, A. (2011). The puzzle of depression and acute coronary syndrome: Reviewing the role of acute inflammation. Journal of Psychosomatic Research, 71(2), 61-68.
Porges, S. W. (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. W. W. Norton & Company.
Reyes del Paso, G. A., Langewitz, W., Mulder, L. J. M., van Roon, A., & Duschek, S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology, 50(5), 477–487. doi:10.1111/psyp.12027. PMID: 23445494
Ridker, P. M., Rifai, N., Stampfer, M. J., & Hennekens, C. H. (2000). Plasma concentration interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation, 101(15), 1767-1772. https://doi.org/10.1161/01.CIR.101.15.1767
Roach, D., Wilson, W., Ritchie, D., & Sheldon, R. (2004). Dissection of long-range heart rate variability: Controlled induction of prognostic measures by activity in the laboratory. Journal of the American College of Cardiology, 43(12), 2271-2277. https://doi.org/10.1016/j.jacc.2004.01.050
Schmidt, J. E., & Carlson, C. R. (2009). A controlled comparison of emotional reactivity and physiological response in masticatory muscle pain patients. Journal of Orofacial Pain, 23, 230-242. PMID: 19639103
Schumann, A., de la Cruz, F., Köhler, S., Brotte, L., & Bär, K.-J. (2021). The influence of heart rate variability biofeedback on cardiac regulation and functional brain connectivity. Frontiers in Neuroscience, 15, 691988. https://doi.org/10.3389/fnins.2021.691988
Schwartz, S. (2015). Viva vagus: Wandering nerve could lead to range of therapies. Science News, 188(11), 18.
Shaffer, F., & Combatalade, D. C. (2013). Don't add or miss a beat: A guide to cleaner heart rate variability recordings. Biofeedback, 41(3), 121-130.
Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health. https://doi.org/10.3389/fpubh.2017.00258
Shaffer, F., & Moss, D. (2006). Biofeedback. In Y. Chun-Su, E. J. Bieber, & B. Bauer (Eds.), Textbook of complementary and alternative medicine (2nd ed.). Informa Healthcare.
Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Frontiers in Psychology. https://doi.org/10.3389/fpsyg.2014.01040
Shaffer, F., Meehan, Z. M., & Zerr, C. L. (2020). A critical review of ultra-short-term heart rate variability norms research. Frontiers in Neuroscience. https://doi.org/10.3389/fnins.2020.594880
Shaffer, F., Moss, D., & Meehan, Z. M. (2022). Rhythmic skeletal muscle tension increases heart rate variability at 1 and 6 contractions per minute. Applied Psychophysiology and Biofeedback. https://doi.org/10.1007/s10484-022-09541-7
Stauss, H. M. (2003). Heart rate variability. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 285, R927-R931. https://doi.org/10.1152/ajpregu.00452.2003
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043-1065. PMID: 8598068
Taylor, J. A., Carr, D. L., Myers, C. W., & Eckberg, D. L. (1998). Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation, 98, 547-555. https://doi.org/10.1161/01.cir.98.6.547
Taylor, S. E. (2006). Tend and befriend: Biological bases of affiliation under stress. Current Directions in Psychological Science, 15(6), 273-277. https://doi.org/10.1111/j.1467-8721.2006.00451.x
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201-216. https://doi.org/10.1016/s0165-0327(00)00338-4
Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J., & Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36, 747-756. https://doi.org/10.1016/j.neubiorev.2011.11.009
Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122-131. https://doi.org/10.1016/j.ijcard.2009.09.543
Umetani, K., Singer, D. H., McCraty, R., & Atkinson, M. (1998). Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. Journal of the American College of Cardiology, 31(2), 593-601. https://doi.org/10.1016/s0735-1097(97)00554-8
Vaillancourt, D. E., & Newell, K. M. (2002). Changing complexity in human behavior and physiology through aging and disease. Neurobiology of Aging, 23, 1-11. https://doi.org/10.1016/s0197-4580(01)00247-0
Vaschillo, E., Lehrer, P., Rishe, N., & Konstantinov, M. (2002). Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback, 27, 1-27. https://doi.org/10.1023/a:1014587304314
Vaschillo, E., Vaschillo, B., & Lehrer, P. (2004). Heartbeat synchronizes with respiratory rhythm only under specific circumstances. Chest, 126, 1385-1386. https://doi.org/10.1016/S0012-3692(15)31329-5
Vaschillo, E. G., Vaschillo, B., Pandina, R. J., & Bates, M. E. (2011). Resonances in the cardiovascular system caused by rhythmical muscle tension. Psychophysiology, 48, 927-936. https://doi.org/10.1111/j.1469-8986.2010.01156.x
Wang, Z., Luo, Y., Zhang, Y., Chen, L., Zou, Y., Xiao, J., & Zou, Z. (2023). Heart rate variability in generalized anxiety disorder, major depressive disorder and panic disorder: A network meta-analysis and systematic review. Journal of Affective Disorders, 330, 259-266. https://doi.org/10.1016/j.jad.2023.03.018
Williams, D. P., Koenig, J., Carnevali, L., Sgoifo, A., Jarczok, M. N., Sternberg, E. M., & Thayer, J. F. (2019). Heart rate variability and inflammation: A meta-analysis of human studies. Brain, Behavior, and Immunity, 80, 219-226. https://doi.org/10.1016/j.bbi.2019.03.009
Wolf, M. M., Varigos, G. A., Hunt, D., & Sloman, J. G. (1978). Sinus arrhythmia in acute myocardial infarction. Medical Journal of Australia, 2, 52-53. https://doi.org/10.5694/j.1326-5377.1978.tb131339.x
Yasuma, F., & Hayano, J. (2004). Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm? Chest, 125(2), 683-690. https://doi.org/10.1378/chest.125.2.683
Zhang, D., Shen, X., & Qi, X. (2016). Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. CMAJ, 188(3), E53-63. https://doi.org/10.1503/cmaj.150535
Return to Top