Heart Rate Variability Assessment
Clinicians can measure the
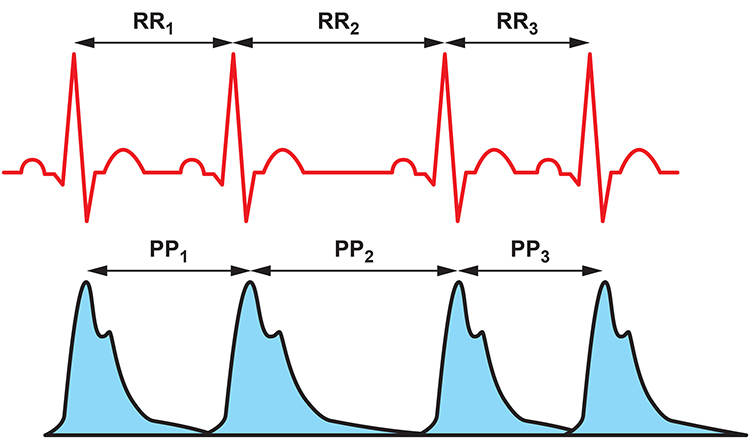
interbeat interval (IBI) using
electrocardiography (ECG) to find the R-spike and
photoplethysmography (PPG) to find the peak of the blood volume pulse (BVP) signal. The R-spike is volume conducted by the heart, while the pulse wave travels down the arterial tree to an earlobe or finger.
The ECG method calculates
heart rate variability (HRV), while the PPG approach measures
pulse rate variability (PRV). Clinicians and consumers use PRV to estimate HRV parameters like RMSSD.
Short-term ECG and PPG IBI measurements are highly correlated at rest. However, this relationship breaks down when clients engage in paced breathing (PB) or standing. HRV and PRV metrics also diverge when clients move or are challenged by stressors because sympathetic nervous system (SNS) activation changes the stiffness of the blood vessels and reduces pulse transit time (the time it takes for the blood pressure wave to travel through the arteries).
Although 24-hour recordings remain the "gold standard" for health risk stratification, there is growing evidence that brief measurements (e.g., 5-minute) can predict mortality. Bittium Faros™ 360 ambulatory ECG.

Clinicians should consider context and client characteristics when interpreting HRV time- and frequency-domain measurements. Both sets of HRV metrics must be referenced to age- and gender-related norms for the appropriate monitoring period.
We cannot compare HRV time-domain values (e.g., RMSSD and SDNN) obtained during slow-paced breathing to resting norms. Moreover, very-low-frequency and high-frequency measurements are meaningless during slow-paced breathing.
This
Real Genius episode was drawn by
Dani S@unclebelang.
BCIA Blueprint Coverage
This unit addresses I
V. HRV Measurements: C. Brief versus 24-hour monitoring and D. How to interpret HRV Measurements.
Professionals completing this unit will be able to discuss:
A. The advantages of 24-hour monitoring over brief measurement epochs
B. How to interpret HRV brief and 24-hour measurements
C.
HRVB training goals

This unit covers Brief Versus 24-Hour Monitoring, The Validity of Ultra-Short-Term Measurements, 24-Hour Monitoring Captures Slower Rhythms, How To Interpret HRV Measurements, HRV Time-Domain, and Frequency-Domain Norms.
Please click on the podcast icon below to hear a full-length lecture.

Brief Versus 24-hour Monitoring
HRV
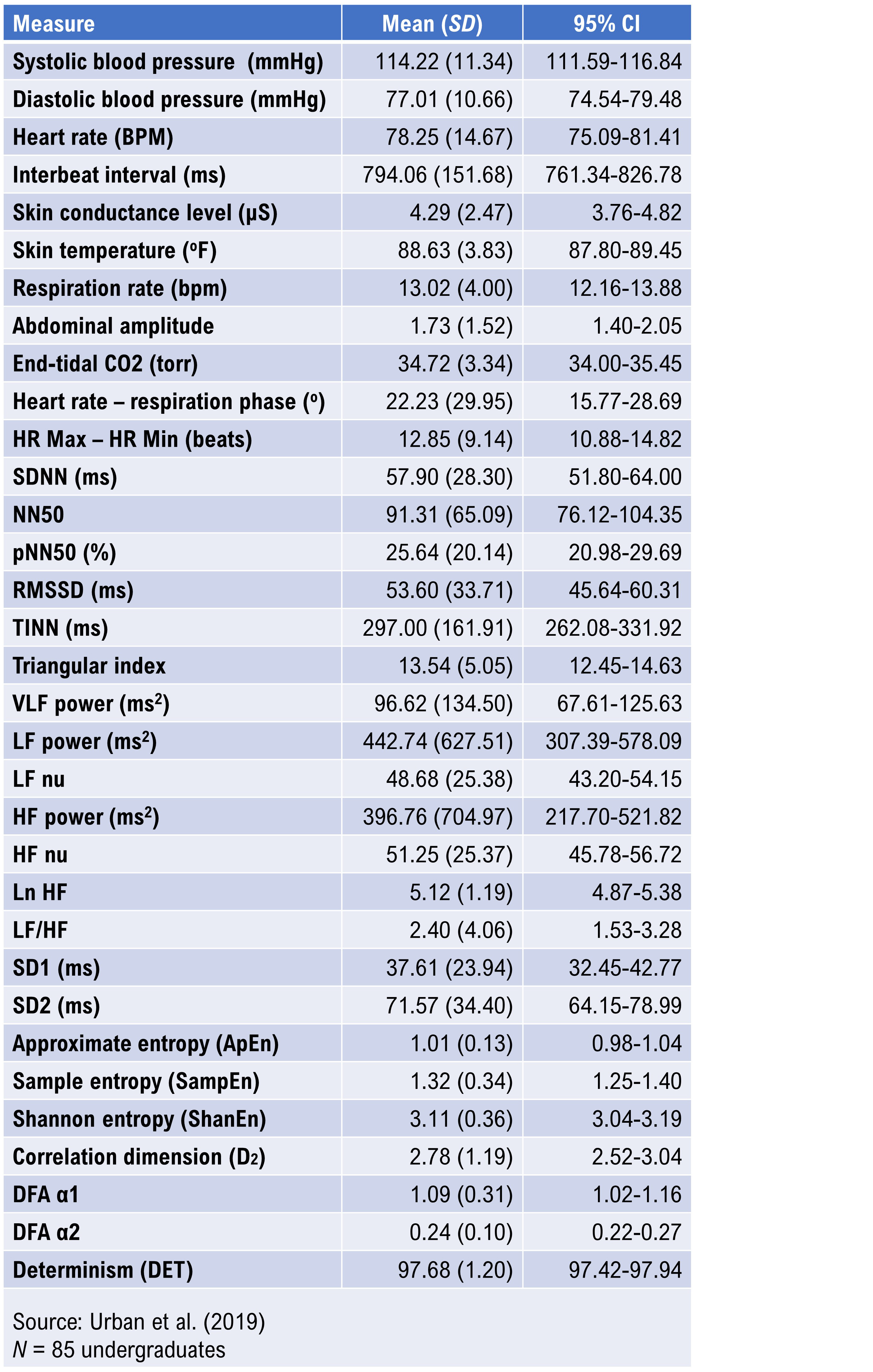
time-domain measures quantify the degree of variability in the interbeat intervals (IBIs) between adjacent heartbeats observed during monitoring periods ranging from 60 seconds to 24 hours (Shaffer, McCraty, & Zerr, 2014). Brief resting measurement periods underestimate time-domain values. Nunan et al. (2010) found that published brief values for healthy adults were lower than Task Force (1996) norms.
HRV
frequency-domain measures use power spectral analysis to separate HRV into its component frequency bands, including ultra-low-frequency (ULF), very-low-frequency (VLF), low-frequency (LF), and high-frequency (HF). Brief monitoring cannot accurately measure ULF power since processes generate it with periods that range from 5 minutes to 24 hours. These oscillations are so gradual that clinicians cannot provide real-time ULF biofeedback. Likewise, slow fluctuations contribute to VLF activity, so brief monitoring periods produce values of questionable validity.
The Validity of Ultra-Short-Term Measurements
The explosion in ambulatory heart rate monitoring has increased interest in
ultra-short-term (UST) HRV measurements based on less than 5 minutes of data. The Fitbit Sense 2 is shown below.

Evaluate HRV UST Studies with Caution
The HRV UST literature is in its infancy. UST studies compare samples as brief as 10 seconds with conventional 5-minute samples to evaluate their equivalence. Several studies have mistakenly assumed that the correlation between two measurements denotes agreement. Correlation doesn't imply equality since it does not control measurement bias (Bland & Altman, 1986).
Eight of eleven studies reviewed by Shaffer, Meehan, and Zerr (2020) did not address measurement bias, rendering their findings
Best Statistical Practices
Researchers should determine
a priori the largest acceptable difference between an UST and 300-second HRV value. Next, they should prepare difference plots like Bland–Altman using a 95% confidence interval and conduct an equality test (e.g., Student’s
t-test) to confirm that the UST and 300-second values are identical. When an UST measurement passes the quality test, it is a surrogate (Shaffer, Meehan, & Zerr, 2020).
Summary of HRV UST Studies
Click on the Read More button to review several influential HRV UST studies.
McNames and Aboy (2006) compared 10-second to 10-minute resting ECG recordings with 5-minute recordings using archival data from PhysioNet. HF, the standard deviation of successive NN interval differences (SDSD), and RMSSD achieved the strongest correlations. Fleming and DeMets (1996) observed, "A correlate does not a surrogate make" (p. 605).
Salahuddin et al. (2007) obtained 5 minutes of resting ECG data from 24 healthy students. Mean heart rate and RMSSD required 10 seconds, PNN50, HF, LF/HF, LFnu, and HFnu required 20 seconds, LF required 30 seconds, VLF required 50 seconds, SDNN and the coefficient of variance (CV) needed 60 seconds, HRV Index and TINN required 90 seconds, and the Stress Index (SI) required 100 seconds, to estimate 150-second values.
Nussinovitch et al. (2011) compared 10-second and 1-minute resting ECG recordings with 5-minute recordings from 70 healthy volunteers. While ultra-short-term RMSSD measurements achieved acceptable correlations, SDNN did not.
Esco and Flatt (2014) acquired ECG measurements from 23 male collegiate athletes (aged 19–21 years) for 10 minutes while supine before a treadmill test and 30 minutes post-exercise. They analyzed the last 5 minutes of each rest period and compared log-transformed 10-, 30-, and 60-seconds with 300-second root mean square of the successive differences (RMSSD) values. They compared intra-class correlations (ICCs) and Bland–Altman plots (mean difference ± 1.96
SD) across the three UST periods and concluded that 60 seconds yielded the largest ICC and most stringent LOA. Whereas the ICC test identified 60 seconds as a potential surrogate, a Bland–Altman plot confirmed its criterion validity with respect to 300-second RMSSD measurements.
Baek et al. (2015) recorded 5 minutes of resting PPG data from 467 healthy volunteers. Heart rate required 10 seconds, HF required 20 seconds, RMSSD required 30 seconds, pNN50 required 60 seconds, LF, LFnu, HFnu, and LF/HF required 90 seconds, SDNN required 240 seconds, and VLF needed 270 seconds to estimate 5-minute values. These minimum periods differed by age group.
Baek et al. (2015) obtained resting PPG measurements from 467 healthy participants (249 men and 218 women; aged 8–69). They compared 10-, 20-, 30-, 60-, 90-, 180-, 210-, 240-, and 270-second values with 300-second measurements. Their criteria for selecting the shortest UST period were a significant Pearson
r and non-significant (
p > 0.05) Kruskal–Wallis statistic. Although they illustrated their results with Bland–Altman plots (mean difference ± 1.96
SD), the authors did not use them to draw conclusions.
Munoz et al. (2015) recorded beat-to-beat middle finger pressure using a Portapres® device from 3387 participants (1660 men and 1727 women; aged 44–63 years) in the Prevention of Renal and Vascular End-Stage Disease study. They obtained recordings over 15 minutes while resting in the supine position. The authors analyzed the last 4–5 minutes of data that exhibited a stationarity pattern and compared the log-transformed 10-, 30-, and 120-second with 300-second RMSSD and SDNN values. They compared ICC, Pearson
r values, and Bland–Altman plots across the three UST periods. The authors concluded that a minimum of 10 seconds was required to measure RMSSD and 30 seconds to calculate SDNN.
Shaffer et al. (2019) obtained 5-minute EEG recordings from 38 healthy undergraduates (20 men and 18 women; aged 18–23 years) while sitting upright under resting conditions with their eyes open. They acquired 10-, 20-, 30-, 60-, 90-, 120-, 180-, and 240-s epochs from the 5-min recordings. They calculated the time domain, frequency domain, and non-linear HRV metrics following the manual removal of artifacts. The authors identified potential surrogates using a Pearson
r with a conservative criterion (
r ≥ 0.90). They applied Bland–Altman’s LOA technique using an allowable difference of ±5% of the range of the 5-min value and a Student’s
t-test to confirm the equality of UST and ST values. The minimum UST values are shown in the table below.
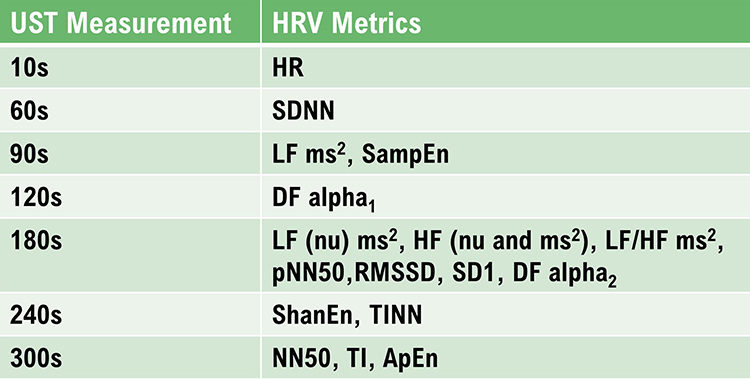
Caption: DFA a1, detrended fluctuation analysis, which describes short-term fluctuations; DFA a2, detrended fluctuation analysis, which describes long-term fluctuations; ECG, electrocardiogram; HF ms2 absolute power of the HF band; HFnu, relative power of the HF band in normal units; HF peak, highest amplitude frequency in the HF band; HF%, HF power as a percentage of total power; HR, heart rate; HTI, HRV triangular index or integral of the density of the NN interval histogram divided by its height; limits of agreement, criterion that two methods are equivalent if there is an acceptable a priori difference between their values in absolute units; LF ms2, absolute power of the LF band; LFnu, relative power of the LFy band in normal units; LF peak, highest amplitude frequency in the LF band; LF%, LF power as a percentage of total power; LF/HF, ratio of LF-to-HF power; NN interval, time between adjacent normal heartbeats; nu, normal units calculated by dividing the absolute power for a specific frequency band by the summed absolute power of the LF and HF bands; pNN50, percentage of successive interbeat intervals that differ by more than 50 ms; RMSSD, root mean square of successive R–R interval differences; R–R interval, time between all adjacent heartbeats; SampEn, sample entropy, which measures signal regularity and complexity; SD1, Poincaré plot standard deviation perpendicular to the line of identity; SD2, Poincaré plot standard deviation along the line of identity; SD1/SD2, ratio of SD1 to SD2 that measures the unpredictability of the R–R time series and autonomic balance under appropriate monitoring conditions; SDNN, standard deviation of NN intervals; TINN, triangular interpolation of the R–R interval histogram or baseline width of the RR interval histogram; total power, sum of power (ms2) in VLF, LF, and HF bands; UST, ultra-short-term (< 5 min).
Perspective
The varying minimum recording periods reported by the reviewed studies may reflect differences in recording method (BVP or ECG), age, health, measurement conditions, artifacting procedures, and the equivalence criteria used. Discount any study that did not specify a minimum acceptable difference a priori and conduct a Bland-Altman difference plot with an equality test.
For healthy individuals, resting baselines as brief as 1 minute should be sufficient to measure heart rate and SDNN, and 3 minutes to measure RMSSD as long as the data are carefully artifacted. Clinicians should not use these measurements instead of conventional 5-minute and 24-hour metrics until measurement protocols are standardized and normative values for healthy nonathletes, optimal performance, and clinical populations are established.
24-Hour Monitoring Detects Slower Rhythms
Twenty-four-hour monitoring detects slower sources of variability and a wider range of activity than brief recording. Circadian
rhythms, core body temperature, metabolism, the renin-angiotensin system, and parasympathetic and sympathetic activity may contribute to 24-hour HRV (Shaffer, McCraty, & Zerr, 2014).
Services like the
HeartMath Institute's Autonomic Assessment Report ® can artifact and analyze uploaded 24-hour data to provide more a comprehensive evaluation than is possible in a clinical setting.
The following profile assesses a 51-year-old male at elevated risk for heart attack © Institute of HeartMath.
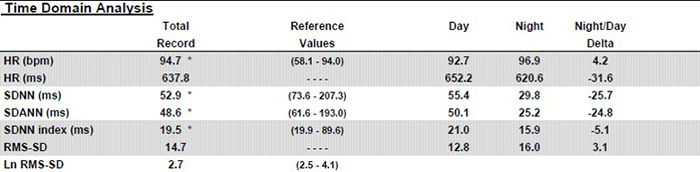
Caption: Note the dramatic difference of almost 26 milliseconds in SDNN between day and night values. Twenty-four-hour monitoring provides a more complete assessment of time-domain measures.
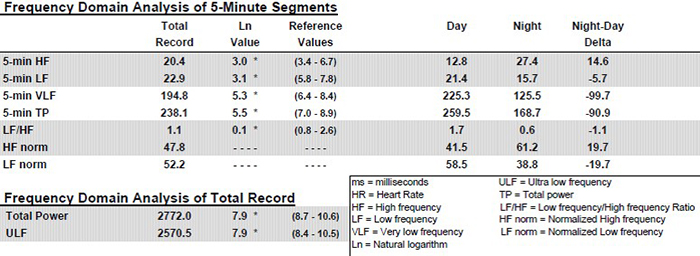
Twenty-four-hour monitoring permits the calculation of ULF power and includes sleep data in calculating the power in all four frequency bands. Again, see the striking differences between day and night values in all the bands. Twenty-four-hour monitoring also provides a more complete assessment of frequency domain measures.
Clinicians should consider supplementing short-term pre- and post-assessment with 24-hour
monitoring, particularly for clients with elevated heart attack risk. This model is analogous to the increased administration of a
quantitative EEG (qEEG) to guide and evaluate improvement in neurofeedback. Below is a BrainAvatar Brain Map from BrainMaster Technologies, Inc.
How To Interpret HRV Measurements
Measurement context and client characteristics are crucial to interpreting HRV time domain and frequency domain measurements.
Measurement Context
Contextual factors include:
1. monitoring period length (e.g., brief or 24-hour)
2. detection method
3. presence or absence of feedback and pacing
4. ambulatory or stationary monitoring
5. position (e.g., supine or sitting upright)
6. intensity of physical activity
7. tasks performed during measurement
8. social demand characteristics within the monitoring situation
9. relationship with staff
Recording period length, detection method, feedback, breathing pacing, movement, position, the intensity of physical activity, tasks, demand characteristics, and relationship variables can affect both sets of measurements by changing ANS activation, breathing mechanics, and emotions.
Recording Period Length
The length of the recording period significantly affects both HRV time- and frequency-domain measurements. This
Real Genius episode was drawn by
Dani S@unclebelang.
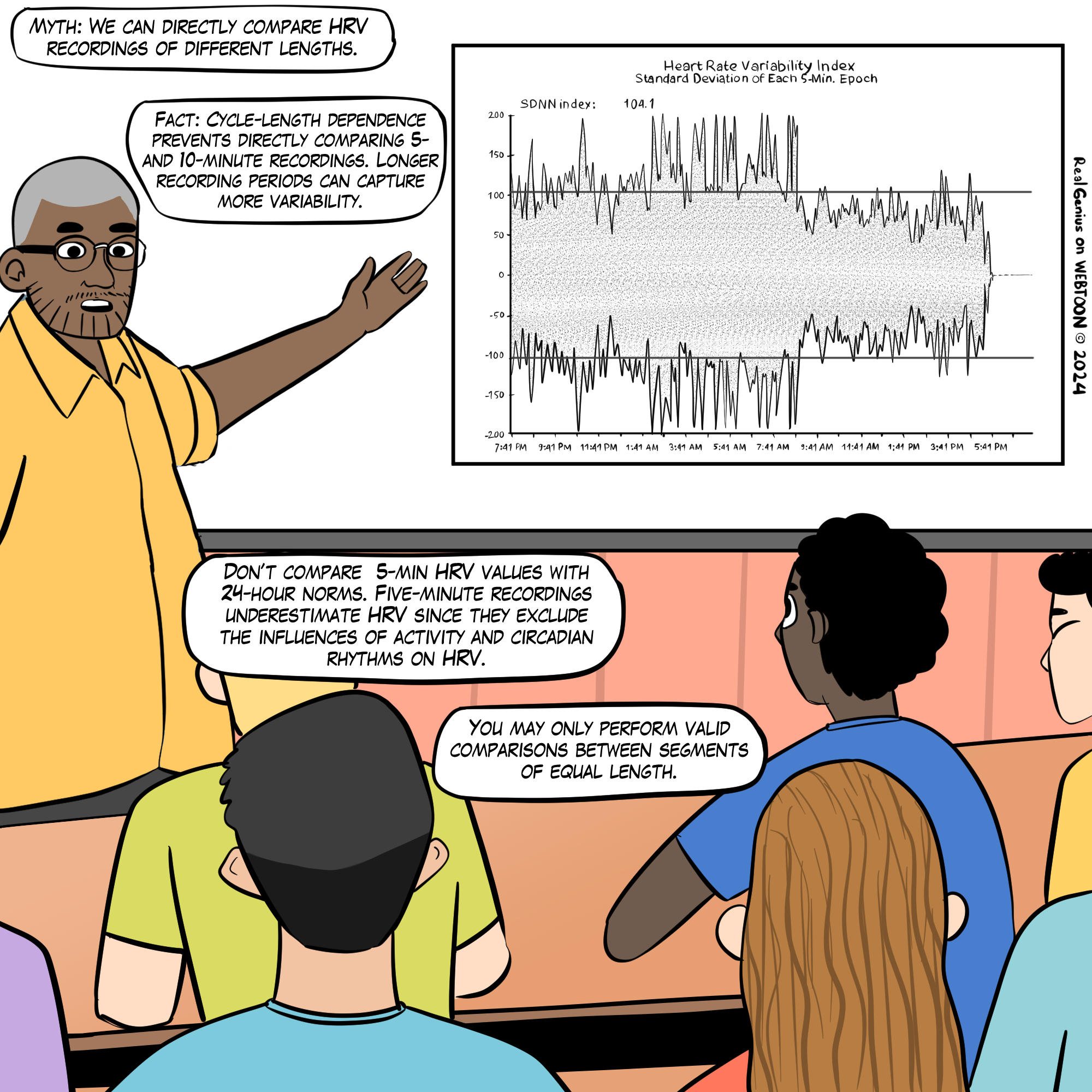
Resting values obtained from brief monitoring periods can dramatically underestimate HRV, correlating poorly with 24-hour indices.
Dr. Inna Khazan (2022) invites us to consider the SDNN. The mean short-term value for healthy adults was 50 milliseconds (Nunan et al., 2010). For athletes, it was 70 milliseconds (Berkoff et al., 2007). However, for healthy 20-29-year-olds, it was 153 milliseconds (Umetani et al., 1998).
Detection Method
Under resting conditions, ECG and PPG methods yielded errors of less than 6% for most HRV measures and 29.9% for pNN50 in one study (Jeyhani et al., 2015). However, the PPG method may inflate HRV values and be a poor surrogate for ECG when participants stand, perform slow-paced breathing, or have low HRV (Constant et al., 1999; Hemon & Phillips, 2016; Jan et al., 2019; Medeiros et al., 2011).
Client Characteristics
Client characteristics include:
1. age
2. sex
3. health
4. aerobic fitness
5. medication
6. recent or immediate physical activity
7. breathing pattern (e.g., respiration rate and inhalation-to-exhalation ratio)
8. cognitive and emotional activity (e.g., affect, expectancies, imagery, and self-statements)
Age
HRV time-domain measurements decline with age (Nunan et al., 2010; Abhishekh et al., 2013) and decreased health (Agelink, Box, Ullrich, & Andrich, 2002; Bigger et al., 1995).
Almedia-Santos et al. (2016) obtained 24-hour ECG recordings of 1743 subjects 40-100 years of age. They found a linear decline in SDNN, SDANN, and SDNN index. However, they found a U-shaped pattern for RMSSD and pNN50 with aging, decreasing from 40-60 and then increasing after age 70.
Bonnemeier et al. (2003) obtained 24-hour recordings from 166 healthy volunteers (85 men and 81 women) ages 20-70. They found the most dramatic HRV parameter decrease between the second and third decades.
Sex
A meta-analysis of 296,247 healthy participants examined 50 HRV measures (Koenig & Thayer, 2016). Women had higher mean HR (smaller RR intervals) and lower SDNN and SDNN index values, especially in 24-hour studies, compared to men. They showed lower total, VLF, and LF power but greater HF power.
While women showed relative vagal dominance, despite higher mean HR, men showed relative SNS dominance, despite their lower HR.
Health
Cardiovascular disorders, disorders with autonomic dysregulation (anxiety and depression), and asthma are associated with reduced HRV (Shaffer & Venner, 2013).
Aerobic Fitness
Time-domain measurements rise with increased aerobic fitness (Aubert, Seps, & Beckers, 2003;
De Meersman, 1993).
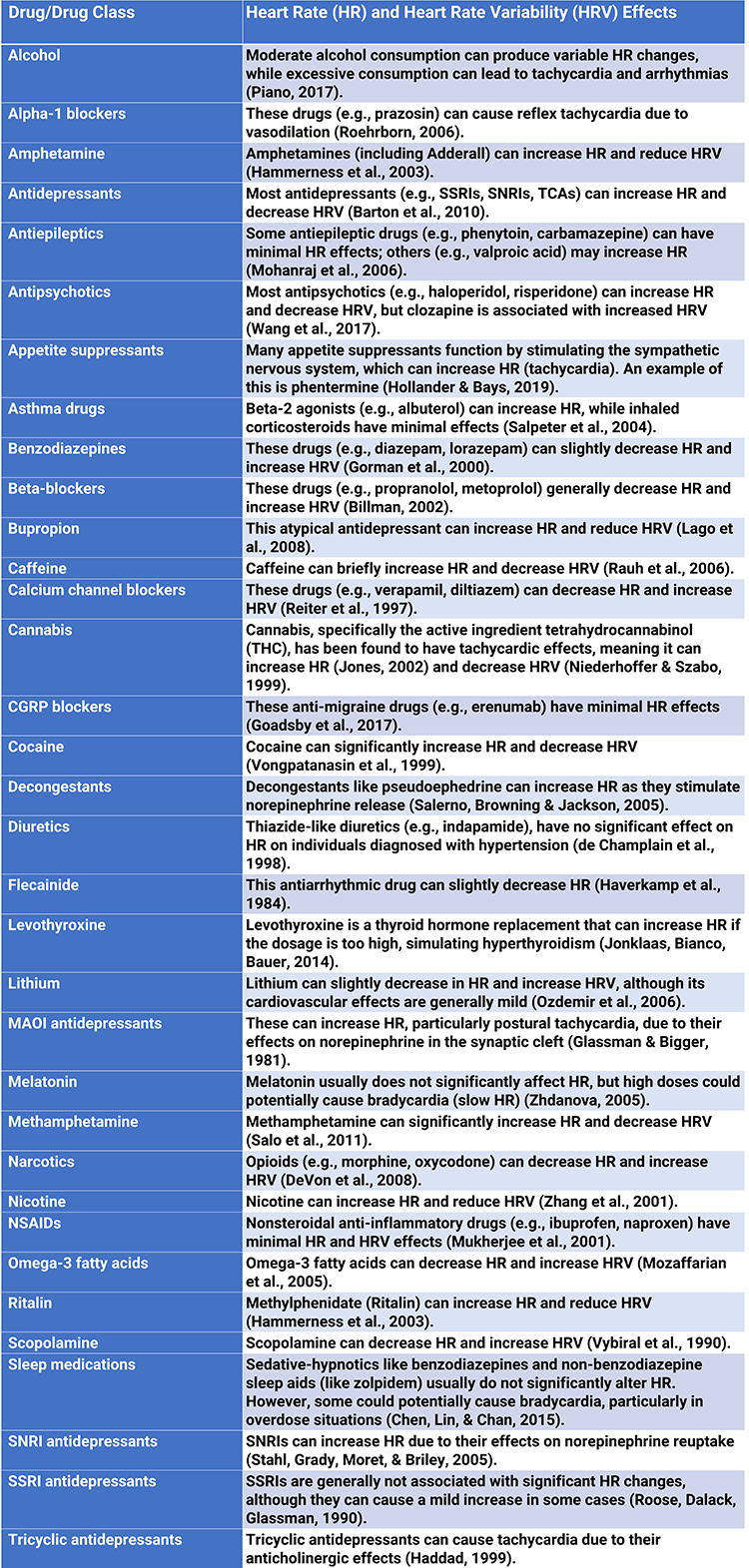
Medication
Medication can affect both time domain and frequency domain measurements. Reviewing a list of all the medications your client is currently taking is essential. In some cases, different members of a drug class can produce different effects, like clozapine among antipsychotics.
Breathing
Compared with breathing at typical rates, slow-paced breathing can increase time-domain measurements and render frequency-domain calculations meaningless (Gevirtz, 2021; Nunan et al., 2010).
Paced breathing in the LF range increases RSA, exercises the baroreflex, and maximizes time-domain and LF band (Shaffer & Meehan, 2020). Breathing at rates
significantly above or below an individual's RF may diminish time-domain and LF band values. Overbreathing is often associated with shallow thoracic breathing at rates that are multiples of the RF (Khazan, 2019).
Emotions
HRV measurements are state-dependent. HRV is lowered by stress, difficult emotions (e.g., anger and anxiety), and higher cognitive loads (McCraty, 2012).
Sympathetic nervous system activation may increase power in the ULF, VLF, and LF bands, resulting in a high LF/HF ratio (Lehrer, 2012).
HRV Time-Domain and Frequency-Domain Norms
After you have artifacted client HRV data, you may compare these values to appropriate short-term or 24-hour norms. Remember that short-term and 24-hour values are not interchangeable.
Prediction of Mortality
The predictive power of 24-hour recordings to predict mortality is well-established.
Kleiger and colleagues (1987) demonstrated the 24-hour VLF and LF power predicted mortality years after myocardial infarction. Moreover, the Task Force report (1996) stratified heart attack risk using 24-hour SDNN measurements. These norms are reproduced later in this unit.
Researchers have also shown that selected brief measures can also predict mortality.
ECG frequency-domain (VLF, LF, HF, and LF/HF) values obtained from 2- to 15-minute recordings predicted death from all causes, myocardial infarction, arrhythmia, and sudden death over a 31-month follow-up (Bigger, Fleiss, & Steinman, 1993).
Five-minute ECG HRV triangular index (HTI) recordings predicted cardiovascular and all-cause death in atrial fibrillation patients with a mean follow-up from 1.6 to 3.6 years (Hämmerle et al., 2020).
Compare Apples with Apples
To compare client data to short-term norms, you must
use the same participant characteristics (e.g., age, fitness, and sex), recording method (e.g., ECG or PPG), position, tasks, feedback, and breathing rates) to ensure valid conclusions.
Brief-Measurement Norms
Click the Read More button to see brief-measurement norms for healthy adults, children, undergraduates, and elite athletes.
Nunan et al. (2010) reviewed normative data from short-term HRV studies published after the Task Force report (1996). The 44 selected studies meeting their criteria involved 21,438 healthy adult participants. The authors reported HRV values according to whether breathing was free or paced, sex, and spectral power analysis (autoregression or Fast Fourier transformation).
Recall that
LFnu and
HFnu are normalized values calculated for brief measurements by dividing LF power or HF power by the sum of LF power + HF power.
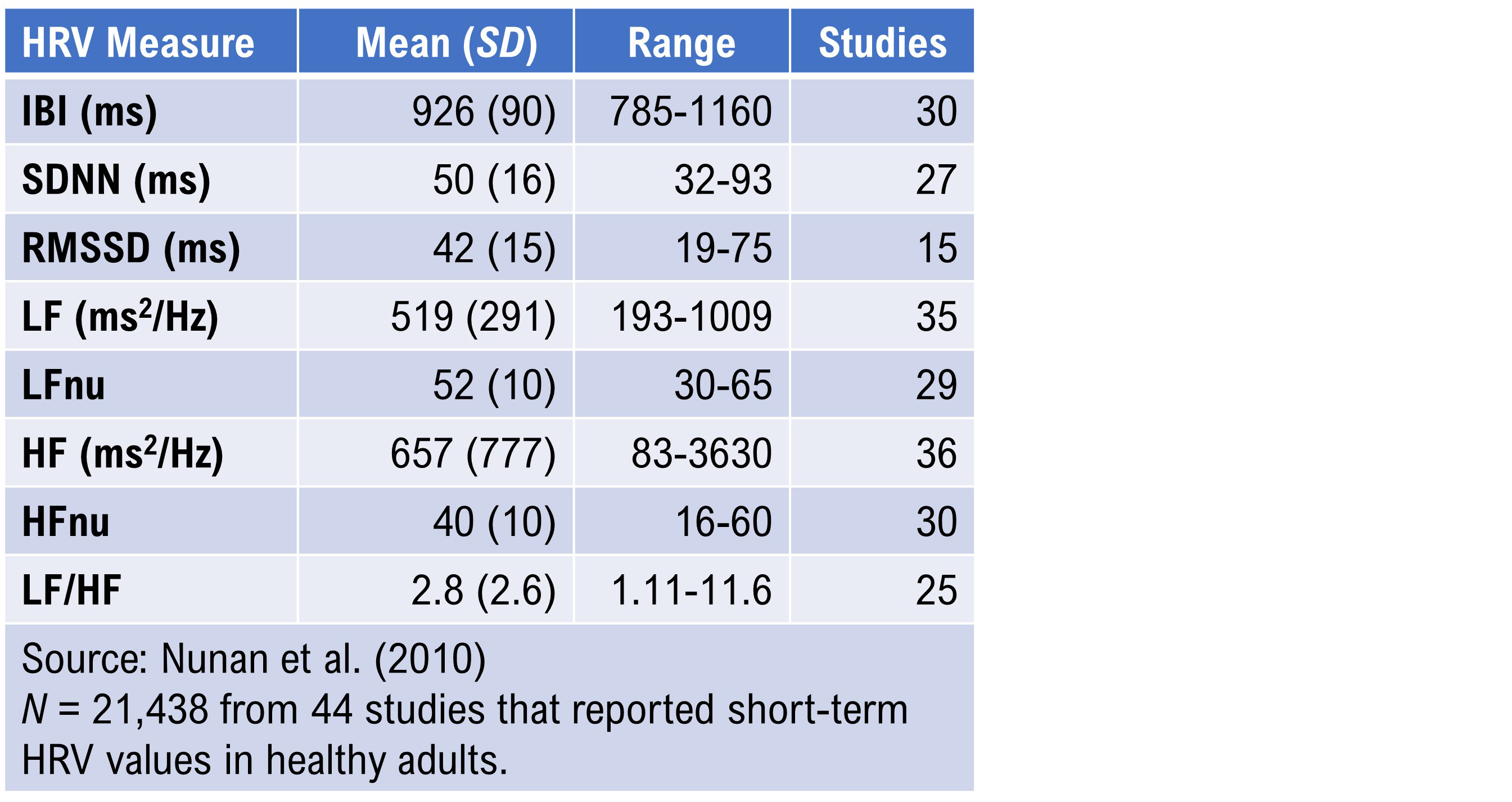
Voss et al. (2015) reported HRV metrics from 5-minute recordings from 1906 healthy adults, 782 women and 1124 men, 25-49 and 50-74.
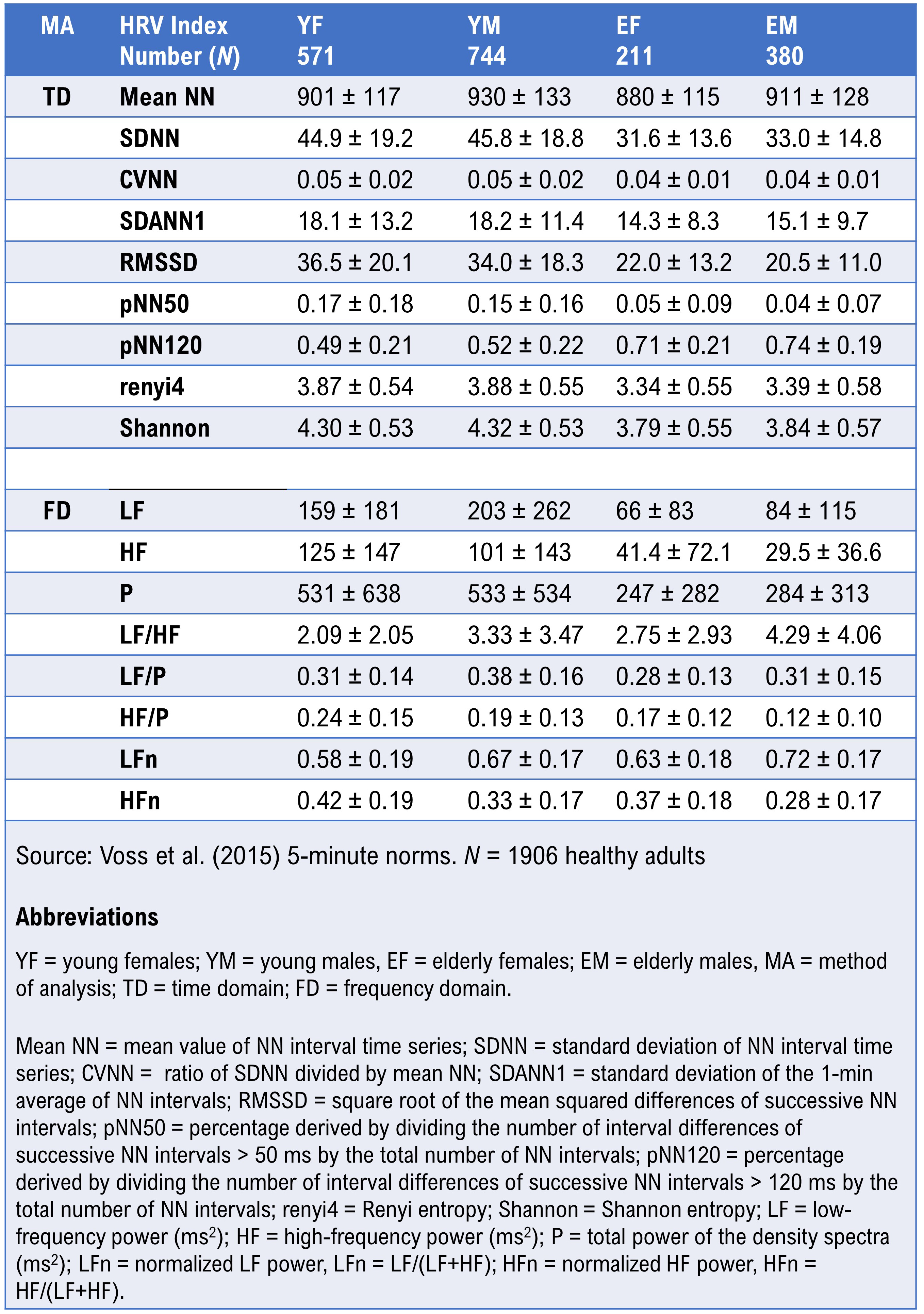
Children 6-8 Years of Age
Seppälä et al. (2014) reported HRV metrics from 1- and 5-minute ECG recordings from 465 children ages 6-8. The table reproduces the 5-minute percentiles for a majority of the parameters.
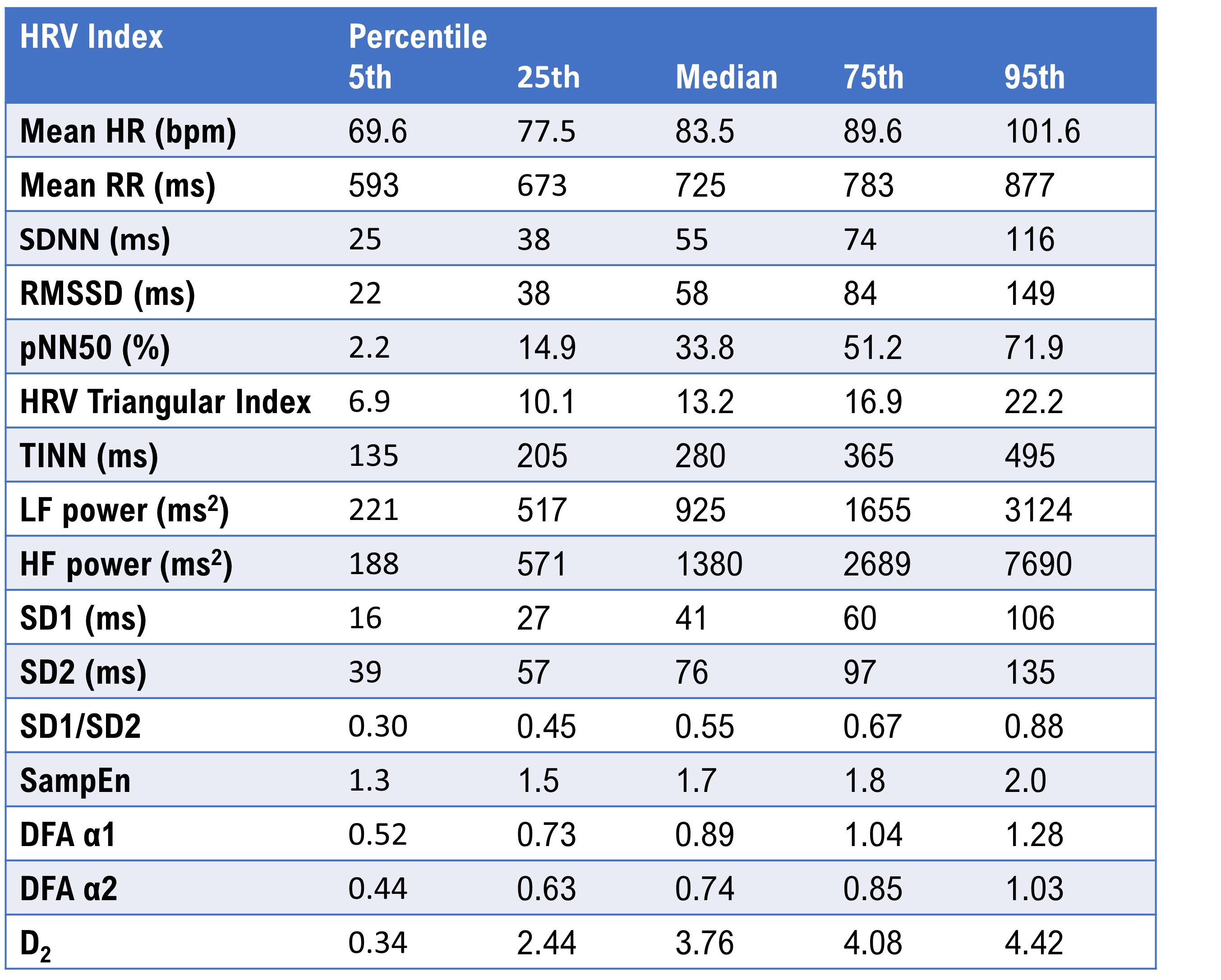
Undergraduate Norms
Urban et al. (2019) reported 5-minute baseline measurements on 85 undergraduates (59 women and 26 men), 18-28 years of age. Participants sat upright with eyes open, no feedback, and with instructions to breathe normally. HRV data were obtained using ECG. Data were detrended using a smoothness priors procedure. The frequency-domain analysis utilized Welch's periodogram (FFT) technique.
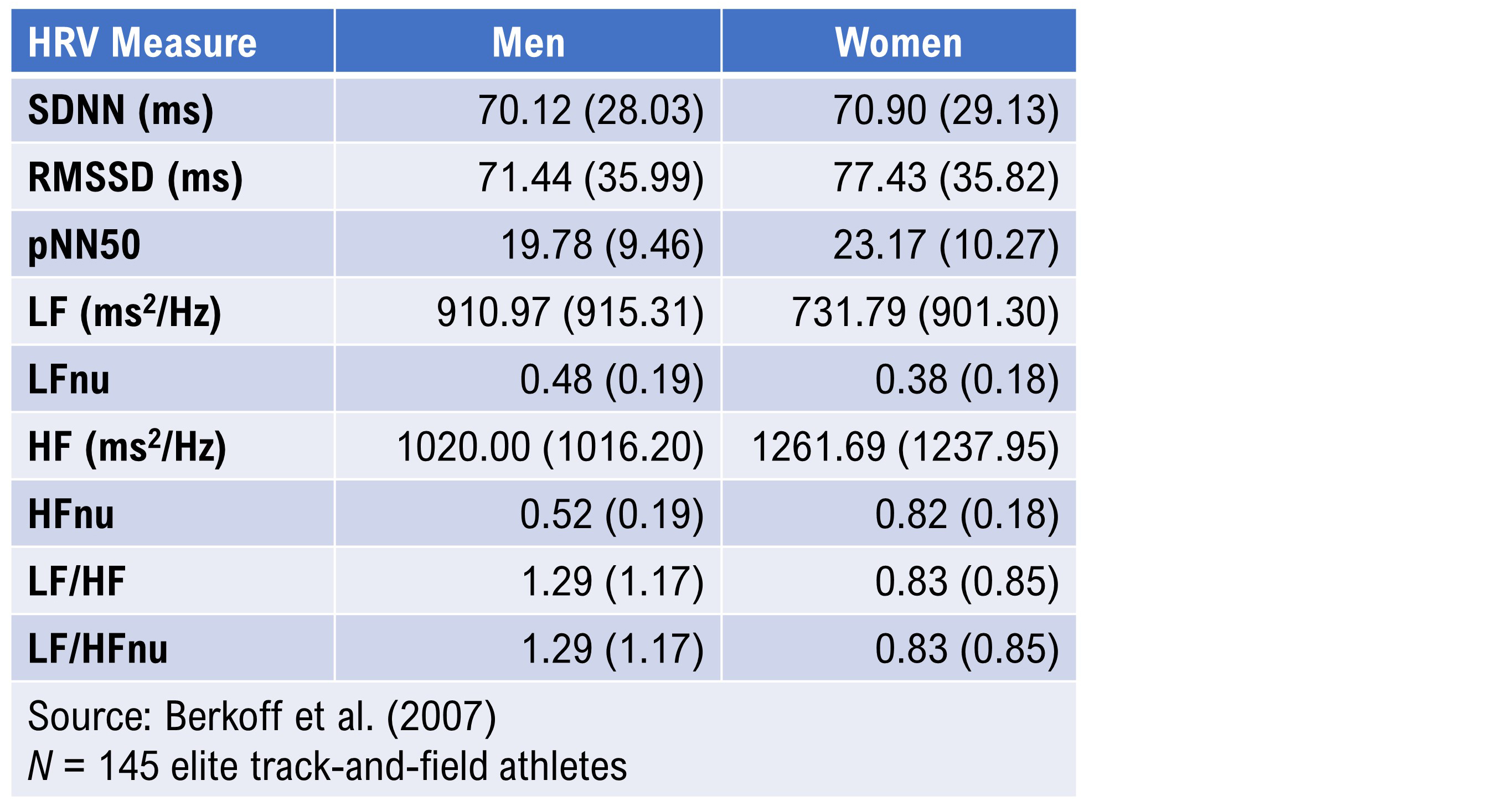
Elite Athletes
Optimal performance professionals should be interested in the Berkoff et al. (2007) short-term norms from 145 elite track-and-field athletes measured before the 2004 U.S.A. Olympic Trials.
The investigators monitored the athletes in the supine position using ECG after up to 5 minutes of rest to stabilize HR.
24-Hour Measurement Norms
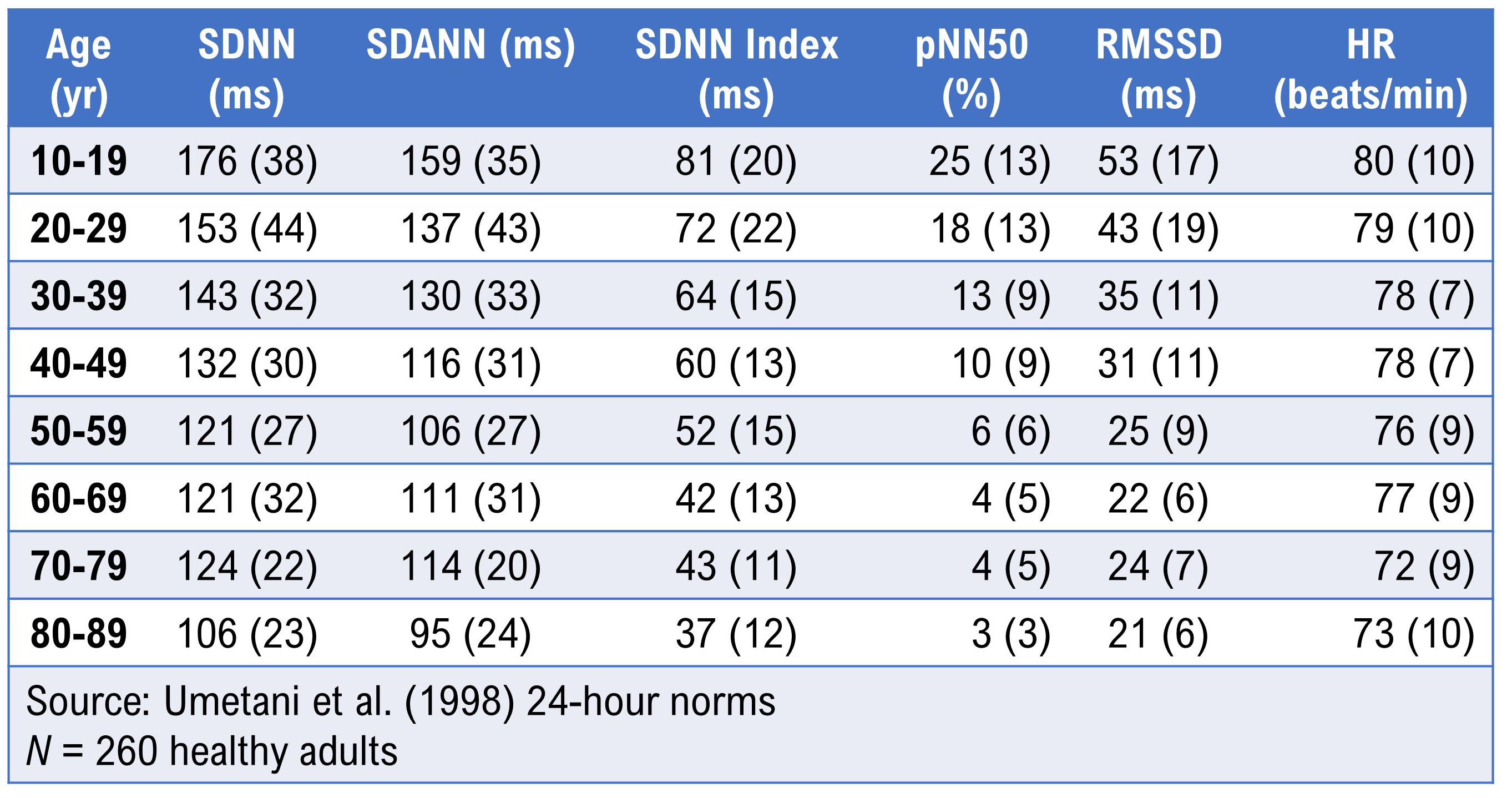
Umetani et al. (1998) published 24-hour norms for 260 healthy participants aged 10-99 years old. They reported that several HRV time-domain indices declined
with age. After age 65, subjects fell below cutoffs for increased threat of mortality. Before age 30, female subjects had lower HRV measurements than their male counterparts. This gender difference vanished after 50 years of age.
Institute of HeartMath Autonomic Assessment Reports
The Institute of HeartMath's Autonomic Assessment Reports evaluate 24-hour recordings using age-related norms. They compare a client's time-domain and normalized frequency-domain measurements to age-related reference values.
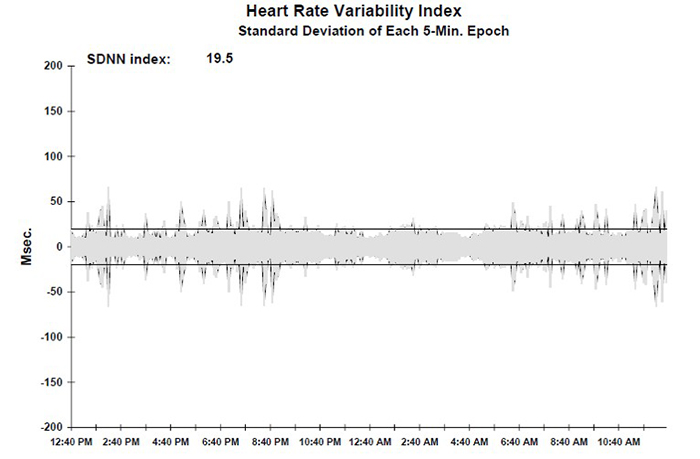
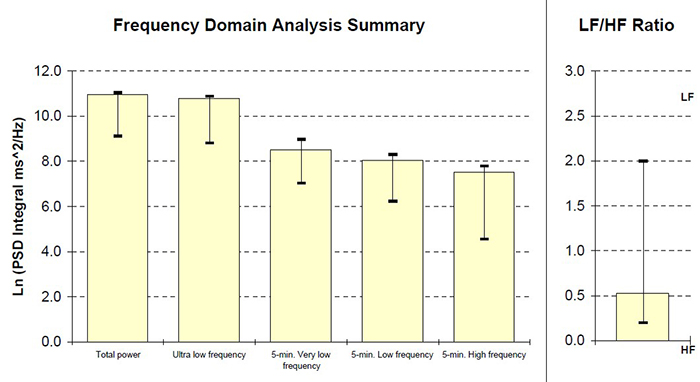
In the frequency-domain analysis summary below of a 51-year-old male with elevated heart attack risk, all indices fell significantly below the natural log values for his age. See the diminished 24-hour SDNN index time-domain plot. The graphics below are © Institute of HeartMath.
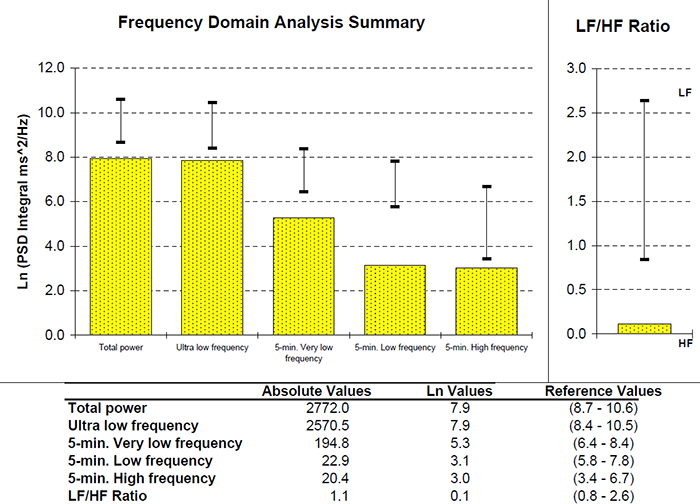
Low VLF power is an important finding since it is associated with an elevated heart attack risk (Bernardi, Valle, Calciati, & Sleight, 1996).
Compare the previous record with the 24-hour frequency domain summary
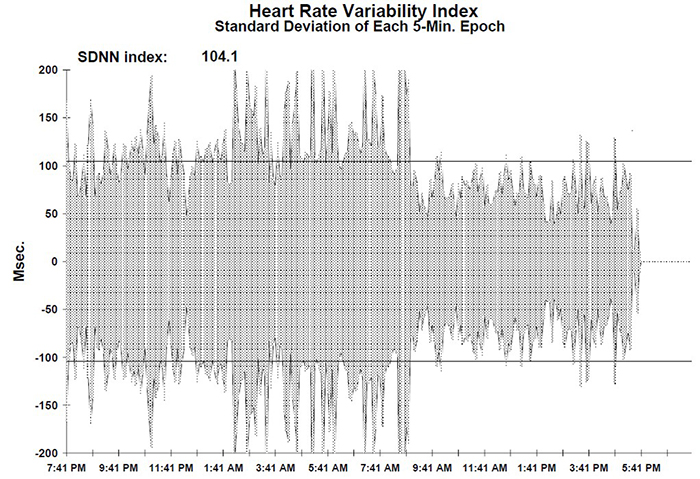
and SDNN index plot for a 25-year-old aerobically-fit client. The younger, healthier client exhibited greater total power and power in each of the four bands. The SDNN Index value of 104.1 eclipsed the older client's SDNN Index of 19.5. Graphics © the Institute of HeartMath.
HRV Values During Baselines and HRV Biofeedback
Baseline Values
Baselines can be 3-5 minutes of resting activity without PB or feedback. Since baseline length can affect HRV values, use identical epochs for pre-session and post-session measurements. Most signal power should fall in the HF band (green) instead of the LF band (salmon) since your client is breathing at typical rates.
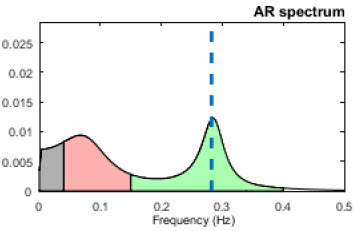
Distract clients (e.g., reading or video) during post-baselines to prevent their continuation of PB (Gevirtz, 2020).
HRV measurements will be invalid if clients don’t breathe at normal rates.
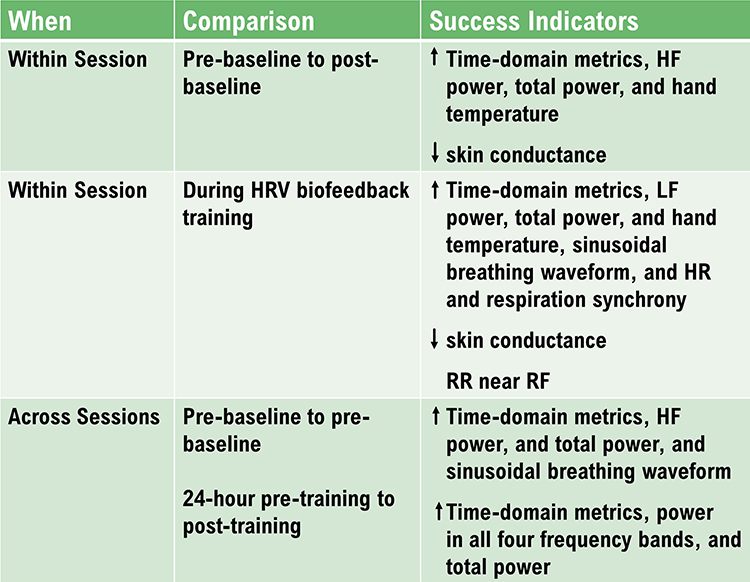
Comparing Pre-Session to Post-Session Baseline Values
You can compare pre-baseline to post-baseline values
within a session to evaluate progress. Look for increases in time domain measures (HR Max-HR Min and RMSSD), HF power, and total power (VLF+LF+HF). You will only see greater LF power if breathing has slowed below 8 bpm.
Look for these same changes when you compare pre-baselines
across sessions. These changes may reflect learning due to training in the clinic and practice.
HRV Biofeedback Training Measurements
During HRV training, increased VLF can serve as a “red flag” like increased skin conductance level and decreased finger temperature. Greater VLF power may signal anxiety, effort, or vagal withdrawal. A mindfulness approach that encourages passive volition can reduce VLF power due to these causes.
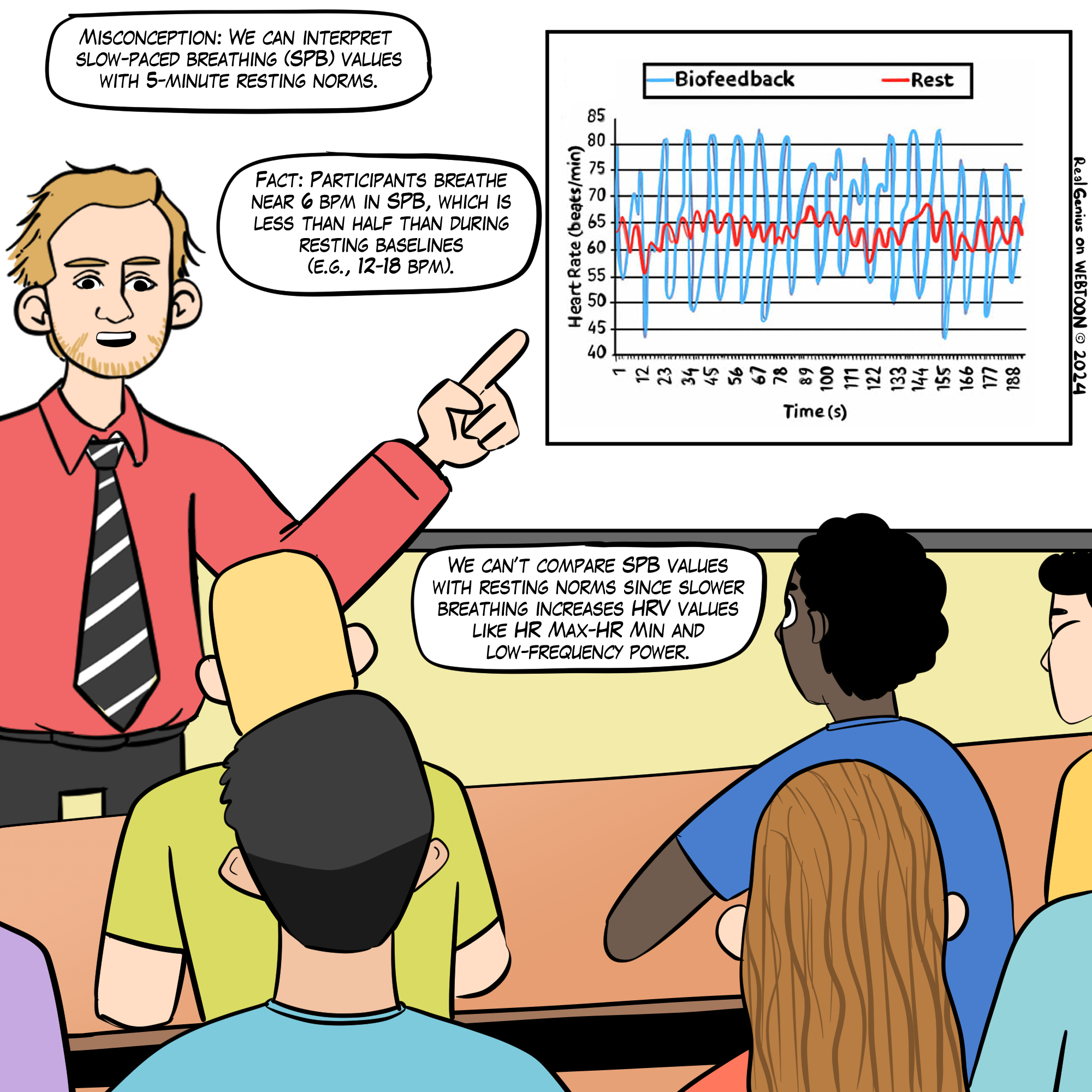
RSA
RSA should increase when your clients breathe near their resonance frequency (Vaschillo et al., 2002). Graphic adapted from Elite Academy.
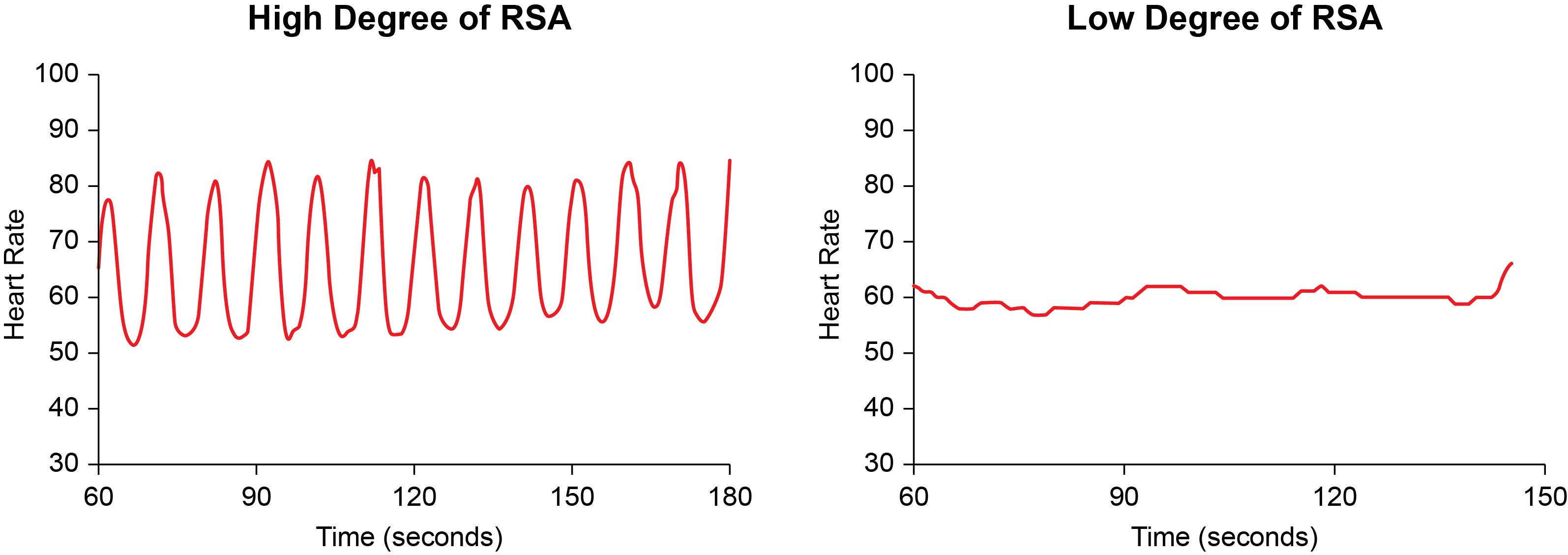
Increased RSA immediately “exercises” the baroreflex without changing vagal tone or tightening BP regulation. Those changes require weeks of practice. HRV biofeedback can increase RSA 4-10 times compared to a resting baseline (Lehrer et al., 2020b; Vaschillo et al., 2002).

Listen to a mini-lecture on Increased RSA © BioSource Software LLC. Graphic adapted from Gevirtz et al., 2016).
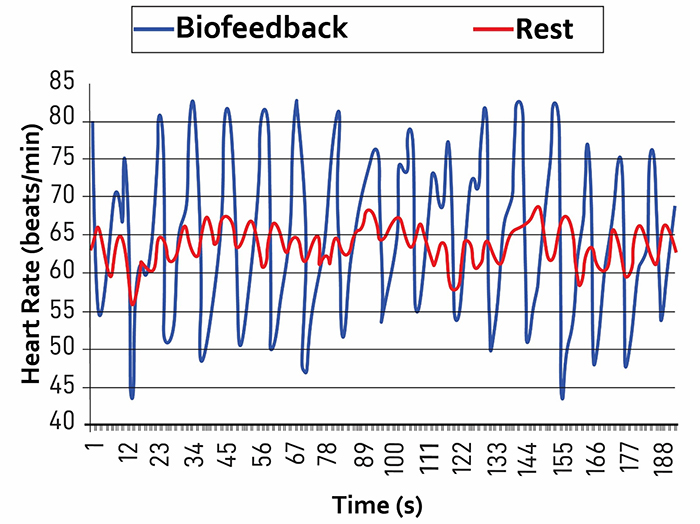
Caption: The red waveform shows HR oscillations while resting without breathing instructions or feedback. The blue waveform shows HR oscillations with HRV biofeedback and breathing from 4.5-6.5 bpm.
Time-Domain Measurements
Time-domain metrics (pNN50, RMSSD, SDNN) should increase from baseline to training trials. They may increase during the first minutes of training and then stabilize throughout the session. Troubleshoot if the time-domain values decline or wildly fluctuate across the session. Baseline mean time-domain values should also increase across a series of training sessions.
HRV time- and frequency-domain values obtained from smartphone apps may be invalid due to their failure to control artifacts. This Real Genius episode was drawn by Dani S@unclebelang.

Click on the Read More button is see sample Elite Academy displays.
In this Elite HRV screenshot, the bright blue line shows that LnRMSSD increased across the session. Note the breathing pacer at the top of the screen. Graphic © Elite Academy.
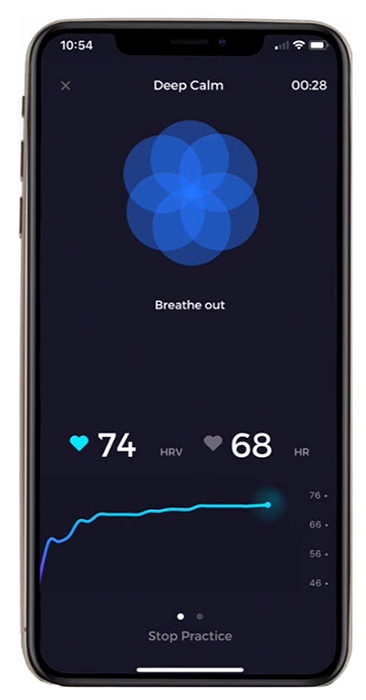
In this Elite HRV screenshot, the left display shows that the average LnRMSSD increased 10% across sessions. Graphic © Elite Academy.
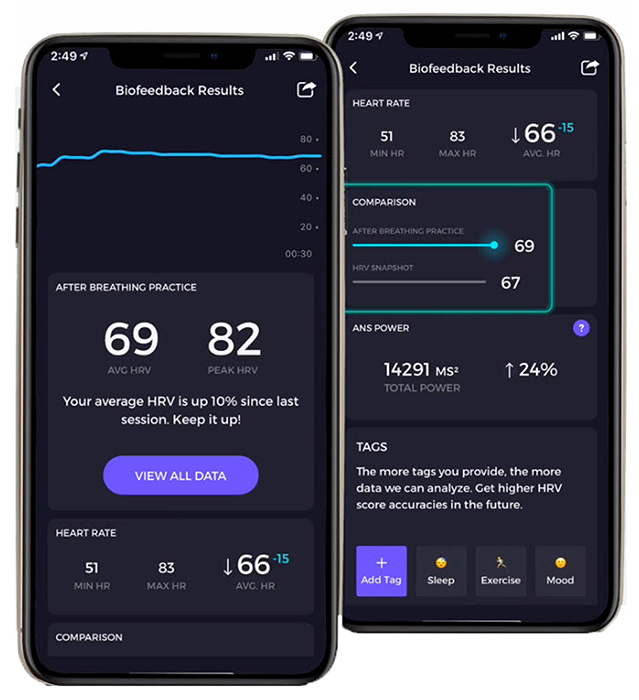
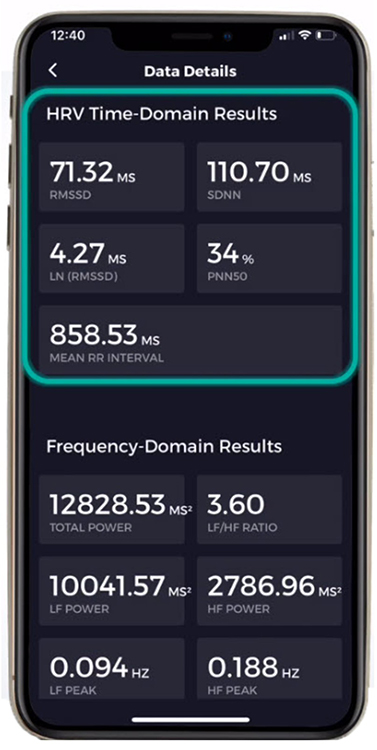
This Elite HRV screenshot shows the average RMSSD, LnRMSSD, SDNN, pNN50, and interbeat interval duration.
Graphic © Elite Academy.
Frequency-Domain Measurements
For healthy clients, we should only expect to see high LF power during PB. The rest of the time, when they breathe at typical rates, most power should fall in the HF range. Slow-paced breathing used in HRV biofeedback is like weightlifting. Graphic © pixelheadphoto digital skillet/Shutterstock.com.
.jpg)
Just as we only expect athletes to confine weighlifting to their workouts, we don’t expect clients to walk around breathing at 6 bpm constantly. They do not have to continuously breathe at their RF to benefit from improved homeostatic regulation, regulatory capacity, and executive function. Continuous RF breathing would jeopardize homeostasis since breathing rate and volume should adjust to changing physical workloads.
HF power should increase across pre-training baselines over training sessions when clients breathe at normal rates.
Graphic © Elite Academy.
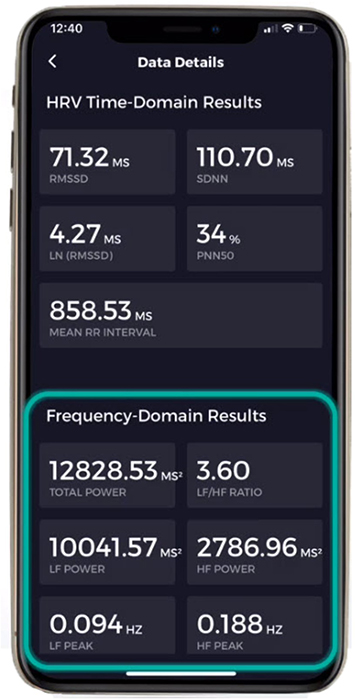
Autonomic Changes
Although autonomic change may depend on the training protocol and relationship with the practitioner, successful HRV biofeedback may increase hand temperature and reduce skin conductance level. Zerr et al. (2014) reported that four 30-minute HRVB training sessions lowered SCL from 5.8 µS to 2.3 µS and raised hand temperature from 90.2 to 93.9 °F (32.3 to 34.4 °C) from session 1 to session 4.
Frequency-Domain Changes Across a Training Session
During pre-training and post-training baselines, the bulk of power should fall in the HF range when the client breaths at typical rates. Only during HRV biofeedback training when a client breathes within the RF range should most power fall in the LF range.
The graphic below shows HF power in blue during a pre-training baseline, HRVB training, and a post-training baseline.
Note the greater LF power concentration post-training than pre-training, during which the client breathed at typical 15 and 14 bpm rates. Graphic © Inna Khazan.
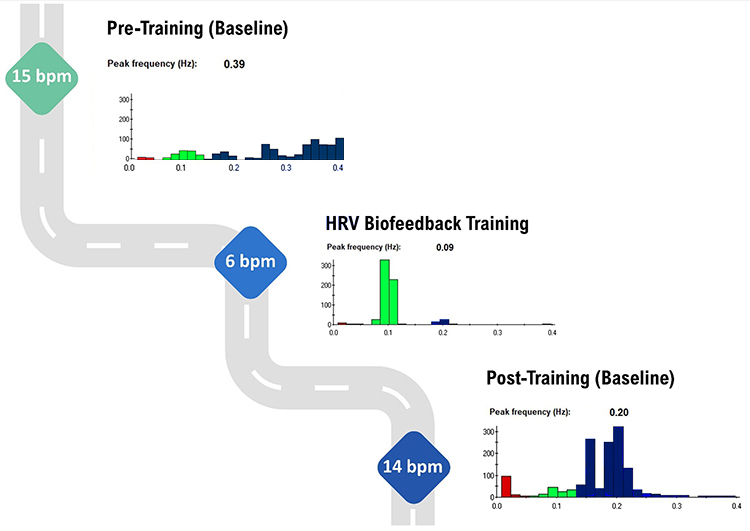
Caption: HF % power is 77% during pre-training and 87% post-training. LF % power is only elevated at 89% during RF breathing training.
A HRV Biofeedback Koan
HRV biofeedback trains clients in the LF range to increase HF power at baseline (Khazan, 2021).
Practitioners train clients to breathe at 6 bpm or their RF to increase LF power during HRV biofeedback trials. However, PB increases HF power during baselines when they breathe above 9 bpm. It doesn't increase LF power during baselines because 12-14 bpm is faster than the 8.5-bpm upper cutoff of the LF range (Shaffer, McCraty, & Zerr, 2014).
24-Hour Monitoring Values
You can compare 24-hour HRV values obtained before the first training session and after the end of training. Successful HRV biofeedback training should
increase 24-hour time-domain indices, power within all four frequency bands, and total power.
VLF power should be low during a training session and moderate-to-high during 24-hour monitoring.
Although we want to avoid increased VLF power during RF biofeedback sessions since this may signal increased SNS activity, we want to increase 24-hour VLF values because the VLF rhythm may be fundamental to health (Shaffer, McCraty, & Zerr, 2014).
Training Outcomes Summary
Glossary
absolute power: the magnitude of HRV within a frequency band measured in milliseconds squared divided by cycles per second (ms2/Hz).
approximate entropy (ApEn): a nonlinear index of HRV that measures the regularity and complexity of a time series.
concurrent validity: the degree to which a measurement procedure yields comparable results to an established procedure.
detrended fluctuation analysis (DFA): a nonlinear index of HRV that extracts the correlations between successive R-R intervals over different time scales and yields estimates of short-term (α1) and long-term (α2) fluctuations.
frequency-domain measures of HRV: the calculation of the absolute or relative power of the HRV signal within four frequency bands.
heart rate variability (HRV): the beat-to-beat changes in HR involving changes in the RR intervals between consecutive heartbeats.
high-frequency (HF) band: a HRV frequency range from 0.15-0.40 Hz that represents the inhibition and activation of the vagus nerve by breathing (respiratory sinus arrhythmia).
HR Max - HR Min: an index of heart rate variability that calculates the difference between the highest and lowest heart rates during each respiratory cycle.
HRV triangular index (HTI): a geometric measure based on 24-hour recordings, which calculates the integral of the RR interval histogram's density divided by its height.
interbeat interval (IBI): the time interval between the peaks of successive R-spikes (initial upward deflections in the QRS complex).
low-frequency (LF) band: a HRV frequency range of 0.04-0.15 Hz that may represent the influence of PNS and baroreflex activity (when breathing at the RF).
natural logarithm (Ln): the logarithm to the base e of a numeric value.
normal units (nu): the division of the absolute power for a specific frequency band by the summed absolute power of the LF and HF bands.
pNN50: the percentage of adjacent NN intervals that differ by more than 50 milliseconds.
pulse rate variability (PRV): a proxy of HRV derived from the BVP signal.
quantitative EEG (QEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
relative power: the percentage of total HRV.
resonance frequency: the frequency at which a system, like the cardiovascular system, can be activated or stimulated.
RMSSD: the square root of the mean squared difference of adjacent NN
intervals.
sample entropy (SampEn): a nonlinear index of HRV that was designed to provide a less-biased measure of signal regularity and complexity than ApEn.
SD1: the standard deviation of the distance of each point from the y = x-axis that measures short-term HRV.
SD2: the standard deviation of each point from the y = x + average RR interval that measures short- and long-term HRV.
SD1/SD2: a ratio that measures the unpredictability of the R-R time series and autonomic balance under appropriate monitoring conditions.
SDANN: the standard deviation of the average NN intervals (mean heart rate) for each of the 5-minute segments during a 24-hour recording.
SDNN: the standard deviation of the normal (NN) sinus-initiated IBI measured in milliseconds.
SDNN index: the mean of the standard deviations of all the NN intervals for each 5-minute segment of a 24-hour HRV recording.
spectral analysis: the division of heart rate variability into component rhythms that operate within different frequency bands.
time-domain measures of HRV: indices like SDNN that measure the degree to which the IBIs between successive heartbeats vary.
total power: the sum of power (ms2) in the ULF, VLF, LF, and HF bands for 24-hour recording and the VLF, LF, and HF bands for brief recording.
ultra-low-frequency (ULF) band: an ECG frequency range below 0.003 Hz that may represent very slow biological processes that include circadian rhythms, core body temperature, metabolism, the renin-angiotensin system, and possible PNS and SNS contributions.
ultra-short-term HRV measurements: HRV metrics based on recording periods shorter than 5 minutes.
very-low-frequency (VLF) band: a HRV frequency range of 0.003-0.04 Hz that may represent temperature regulation, plasma renin fluctuations, endothelial and physical activity influences, and possible intrinsic cardiac, PNS, and SNS contributions.
Test Yourself
Click on the ClassMarker logo to take 10-question tests over this unit without an exam password.

REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BCIA offers two HRV Biofeedback Certification paths: Biofeedback and Neurofeedback.
For Biofeedback, BioSource Software offers Human Physiology to satisfy BCIA's Human Anatomy & Physiology requirement. For Neurofeedback, BioSource provides Physiological Psychology to satisfy BCIA's Physiological Psychology requirement.
BCIA has accredited each course, and they combine affordable pricing ($150) with industry-leading content.

Assignment
Now that you have completed this module, does your clinic use 24-hour monitoring to assess HRV? Does it use age-related norms to interpret HRV measurements?
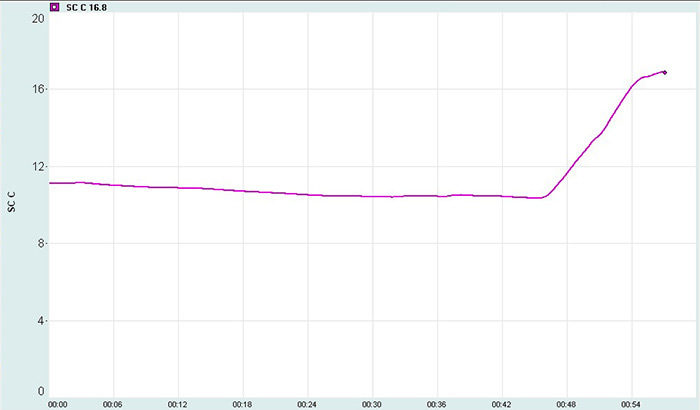
Skin conductance can provide invaluable information about a client's cognitive activity during HRV assessment. The clinician
captured this skin conductance response (SCR) as the client began thinking about his mother, who had died 3 months earlier.
Which modalities do you monitor in addition to heart rate and breathing? What do they contribute to your understanding of your clients?
References
Abhishekh, H. A., Nisarga, P., Kisan, R., Meghana, A., Chandran, S., Raju, T., & Satyaprabha, T. N. (2013). Influence of age and gender on autonomic regulation of heart. J Clin Monit Comput, 27, 259-264. https://doi.org/10.1007/s10877-012-9424-3
Agelink, M., Boz, C., Ullrich, H., & Andrich, J. (2002). Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry Research, 113(1), 139-149. https://doi.org/10.1016/s0165-1781(02)00225-1
Aubert, A. E., Seps, B., & Beckers, F. (2003). Heart rate variability in athletes. Sports Medicine, 33(12), 889-919. https://doi.org/10.2165/00007256-200333120-00003
Baek, H. J., Cho, C. H., Cho, J., & Woo, J. M. (2015). Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemedicine and e-Health, 21(5), 404-414. https://doi.org/10.1089/tmj.2014.0104
Berkoff, D. J., Cairns, C. B., Sanchez, L. D., & Moorman, C. T. (2007). Heart rate variability in elite American track-and-field athletes. Journal of Strength and Conditioning Research, 21(1), 227-231. https://doi.org/10.1519/00124278-200702000-00041
Bernardi, L., Valle, F. Coco, M., & Sleight, P. (1996). Physical activity influences heart rate variability and very-low-frequency components in Holter electrocardiograms. Cardiovascular Research, 32(2), 234-7. https://doi.org/10.1016/0008-6363(96)00081-8
Bigger, J. Y., Fleiss, J. L., Rolnitzky, L. M., & Steinman, R. C. (1993). The ability of several short-term measures of RR variability to predict mortality after myocardial infarction.
Circulation, 88, 927-934. https://doi.org/10.1161/01.CIR.88.3.927
De Meersman, R. E. (1993). Heart rate variability and aerobic fitness. American Heart Journal, 125(3). 726-731. https://doi.org/10.1016/0002-8703(93)90164-5
Gevirtz, R. N. (2017). Cardio-respiratory psychophysiology: Gateway to mind-body medicine.
Hämmerle, P., Eick, C., Blum, S.,
Schlageter, V., Bauer, A., Rizas, K. D., Eken, C., Coslovsky, M., Aeschbacher, S., Krisai, P., Meyre, P., Vesin, J.-M., Rodondi, N., Moutzouri, E., Beer, J., Moschovitis, G., Kobza, R., Di Valentino, M., Corino, V. D. A., Laureanti, R., . . . Swiss‐AF Study Investigators (2020). Heart rate variability triangular index as a predictor of cardiovascular mortality in patients with atrial fibrillation. Journal of the American Heart Association, 9(15). https://doi.org/10.1161/JAHA.120.016075
Jeyhani, V., Mahdiani, S., Peltokangas, M., & Vehkaoja, A. (2015). Comparison of HRV parameters derived from photoplethysmography and electrocardiography signals. Cong Proc IEEE Eng Med Biol Soc, 2015, 5952-5955. https://doi.org/10.1109/EMBC.2015.7319747
Khazan, I. Z. (2022). HRV biofeedback bootcamp. Association for Applied Psychophysiology and Biofeedback.
Khazan, I. Z., & Shaffer, F. (2019). Practical strategies for teaching your clients to breathe. Association for Applied Psychophysiology and Biofeedback 50th Annual Meeting, Denver, CO.
Lehrer, P. M. (2012). Personal communication.
Lehrer, P. M., Vaschillo, E., & Vaschillo, B. (2000). Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback, 25(3),
177-191.
McCraty, R. (2013). Personal communication regarding HRV assessment.
McNames, J., & Aboy, M. (2006). Reliability and accuracy of heart rate variability metrics versus ECG segment duration. Med Biol Eng Comput, 44(9), 747-756.
https://doi.org/10.1007/s11517-006-0097-2
Nunan, D., Sandercock, G. R. H., & Brodie, D. A. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology, 33, 1407–1417. https://doi.org/10.1111/j.1540-8159.2010.02841.x
Nussinovitch, U., Elishkevitz, K. P., Nussinovitch, M., Segev, S., Volovitz, B., & Nussinovitch, N. (2011).
Reliability of ultra-short ECG indices for heart rate variability. Ann Noninvasive Electrocardiol, 16(2), 117-122. https://doi.org10.1111/j.1542-474X.2011.00417.x
Salahuddin, L., Cho, J., Jeong, M. G., & Kim, D. (2007). Ultra short term analysis of heart rate variability for monitoring mental stress in mobile settings. Conf Proc IEEE Eng Med Biol Soc, 2007, 4656-4659.
https://doi.org/10.1109/iembs.2007.4353378
Shaffer, F. (in press). HRV metrics and norms. In D. Moss & F. Shaffer (Eds.). Physiological recording technology in biofeedback and neurofeedback. Association for Applied Psychophysiology and Biofeedback.
Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology. https://doi.org/10.3389/fpsyg.2014.01040
Shaffer, F., Shearman, S., & Meehan, Z.
M. (2016). The promise of ultra-short-term (UST) heart rate variability measurements. Biofeedback, 44, 229-233. http://dx.doi.org/10.5298/1081-5937-44.3.09
Task Force of the European Society of Cardiology and the North American
Society of Pacing and Electrophysiology (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043-1065. PMID: 8598068
Umetani, K., Singer, D. H., McCraty, R., & Atkinson, M. (1998). Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. Journal of the American College of
Cardiology, 31(2), 593-601. https://doi.org/10.1016/s0735-1097(97)00554-8
Urban, H.,
Gravett, N., Pecoraro, J., & Shaffer, F. (2019). Updated heart rate variability norms for healthy undergraduates. Association for Applied Psychophysiology and Biofeedback Annual Meeting, Denver, CO.
Vaschillo, E., Lehrer, P., Rishe, N., & Konstantinov, M. (2002). Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Applied
Psychophysiology and Biofeedback, 27, 1-27. https://doi.org/10.1023/a:1014587304314
Zerr, C., Kane, A., Vodopest, T., Allen, J., Fluty, E., Gregory, J., . . . Shaffer, F. (2014). HRV biofeedback training raises temperature and lowers skin conductance. Applied Psychophysiology and Biofeedback, 39(3), 299. https://doi.org/:10.1007/s10484-014-92549






























.jpg)






