Evidence-Based Interventions
What You Will Learn
Heart rate variability biofeedback has emerged as a versatile intervention spanning an impressive range of clinical applications—but how do we separate genuine treatment effects from placebo responses? This chapter equips you with the critical evaluation skills to assess HRV biofeedback's efficacy across conditions from asthma and depression to anxiety disorders and chronic pain. You will master the criteria professional organizations use to rate treatment effectiveness, examine the mechanisms that may explain why breathing at your resonance frequency produces therapeutic benefits, and explore the evidence base for optimal performance applications with athletes and performing artists.
Whether you treat veterans at a VA hospital, manage chronic conditions in a community clinic, or train elite athletes for peak performance, understanding this evidence will sharpen your clinical decision-making about when and how HRV biofeedback can deliver meaningful outcomes for your clients.
The efficacy of HRV biofeedback (HRVB) ranges from probably efficacious to not empirically supported, depending on the condition being treated. Since clinical improvement does not always correlate with physiological change, the precise mechanisms by which HRVB improves health and performance remain an active area of investigation. Gevirtz (2013) proposes three candidate mechanisms that likely work in concert: restored autonomic balance, increased vagal tone, and modulation of the cholinergic anti-inflammatory system—a pathway we will explore in the cutting-edge topics section.

BCIA Blueprint Coverage
This unit addresses VI. HRV Applications: A. Clinical applications, and B. Optimal performance applications.
Topics include the clinical efficacy of established medical practices, criteria for clinical efficacy ratings, an overview of HRV biofeedback efficacy, and detailed coverage of asthma, chronic obstructive pulmonary disease (COPD), depression, fibromyalgia (FM), chronic muscle pain, coronary artery disease, essential hypertension, preeclampsia, anxiety disorders including phobia and post-traumatic stress disorder (PTSD), functional abdominal pain, irritable bowel syndrome, and optimal performance training.
🎧 Listen to the Full Chapter Lecture
The Clinical Efficacy of Established Medical Practices
Here is a sobering finding that should give us all pause. Prasad et al. (2013) examined 363 studies of an accepted drug or medical procedure published in The New England Journal of Medicine from 2001 to 2010. More than 40% were ineffective or harmful, 38% were beneficial, and 22% had uncertain value.
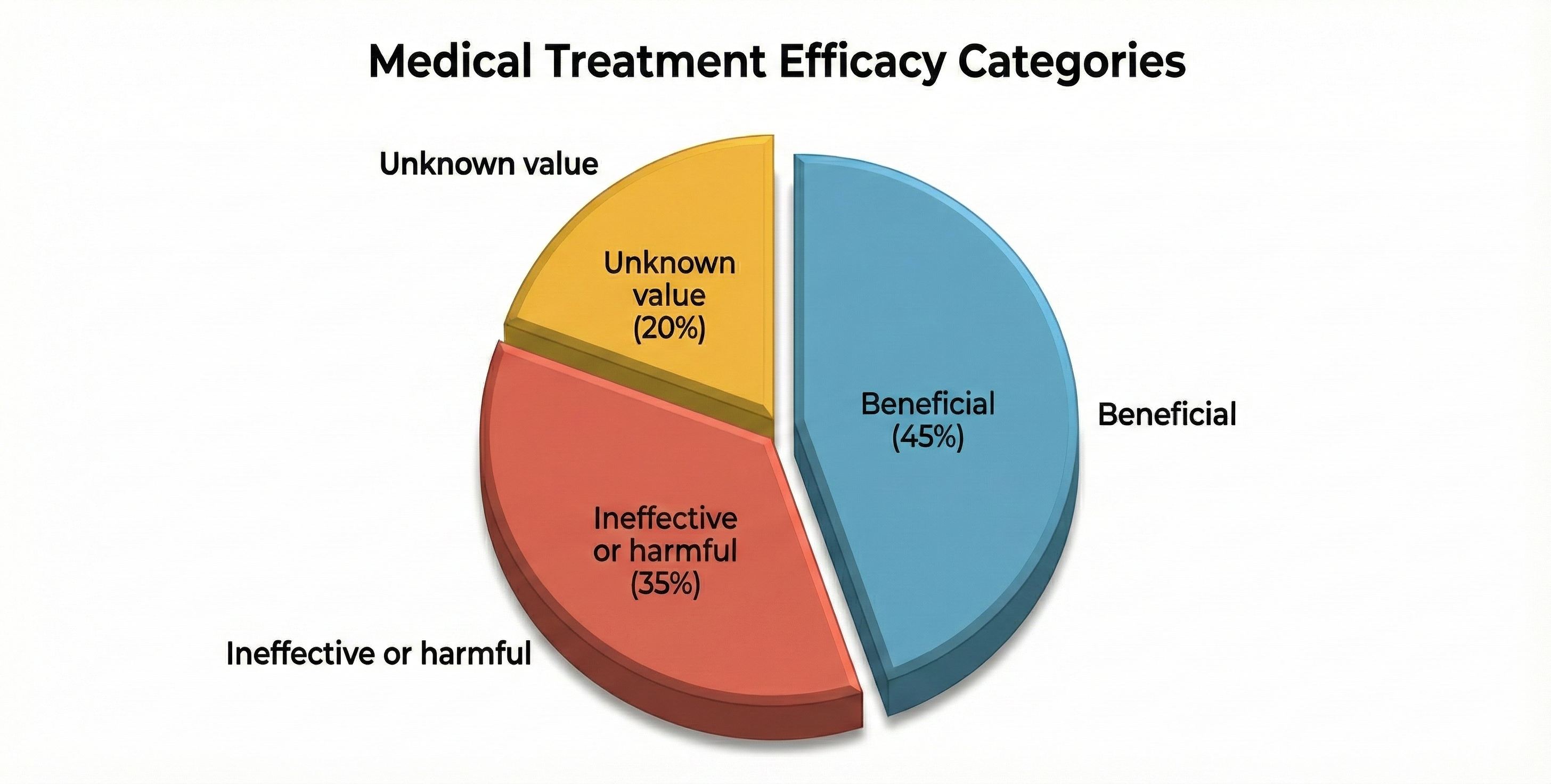
Examples of ineffective or harmful practices included hormone replacement therapy in postmenopausal women and aggressive blood sugar reduction in Type 2 diabetics treated in intensive care, which increased mortality rates.
The authors observed: "Nevertheless, the reversals we have identified at the very least call these practices into question. Some practices ought to be abandoned, whereas others warrant retesting in more powerful investigations. One of the greatest virtues of medical research is our continual quest to reassess it." (p. 796)
Criteria for Clinical Efficacy: The Five Levels
The following guidelines for evaluating the clinical efficacy of biofeedback and neurofeedback interventions were recommended by a joint Task Force and adopted by the Boards of Directors of the Association for Applied Psychophysiology (AAPB) and the International Society for Neuronal Regulation (ISNR) (LaVaque et al., 2002). The Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.) ratings are discussed in the Applications units.

Level 1: Not Empirically Supported
Supported only by anecdotal reports and/or case studies in non-peer reviewed venues.
Level 2: Possibly Efficacious
At least one study of sufficient statistical power with well-identified outcome measures but lacking randomized assignment to a control condition internal to the study.
Level 3: Probably Efficacious
Multiple observational studies, clinical studies, wait-list controlled studies, and within-subject and intrasubject replication studies that demonstrate efficacy.
Level 4: Efficacious
This level requires that, in comparison with a no-treatment control group, alternative treatment group, or sham (placebo) control utilizing randomized assignment, the investigational treatment is shown to be statistically significantly superior to the control condition or the investigational treatment is equivalent to a treatment of established efficacy in a study with sufficient power to detect moderate differences. Additionally, the studies must have been conducted with a population treated for a specific problem, for whom inclusion criteria are delineated in a reliable, operationally defined manner. The study must have used valid and clearly specified outcome measures related to the problem being treated. The data must be subjected to appropriate data analysis. The diagnostic and treatment variables and procedures must be clearly defined in a manner that permits replication of the study by independent researchers. Finally, the superiority or equivalence of the investigational treatment must have been shown in at least two independent research settings.
Level 5: Efficacious and Specific
The investigational treatment is statistically superior to credible sham therapy, pill, or alternative bona fide treatment in at least two independent research settings.

The Growing Evidence Base for HRV Biofeedback
The empirical support for HRVB has grown substantially and continues to strengthen with each passing year. Notably, unlike the medical practices reviewed by Prasad and colleagues (2013), no mainstream HRVB application has been shown to be harmful or ineffective. HRVB outcome research remains in its early stages, and researchers have yet to fully characterize its therapeutic potential. For clinicians, this means we can offer HRVB as a safe intervention with growing evidence while acknowledging that our understanding continues to evolve.
Lehrer and Colleagues' Systematic Review and Meta-Analysis

Lehrer and colleagues' (2020) systematic review and meta-analysis of 58 papers represents the most comprehensive evaluation of HRV biofeedback efficacy available. Their findings provide a solid empirical foundation for clinical practice:
A significant small to moderate effect size was found favoring HRVB, which does not differ from that of other effective treatments. With a small number of studies for each, HRVB has the largest effect sizes for anxiety, depression, anger and athletic/artistic performance and the smallest effect sizes on PTSD, sleep and quality of life. We found no significant differences for number of treatment sessions or weeks between pretest and post-test, whether the outcome measure was targeted to the population, or year of publication. Effect sizes are larger in comparison to inactive than active control conditions although significant for both. HRVB improves symptoms and functioning in many areas, both in the normal and pathological ranges. It appears useful as a complementary treatment.
This meta-analysis provides several clinically relevant insights: the intervention shows particular promise for anxiety, depression, and performance applications; effect sizes remain significant even when compared to active controls; and HRVB appears versatile enough to benefit both clinical and non-clinical populations.
Gevirtz's Hypothetical Mechanisms for HRV Biofeedback
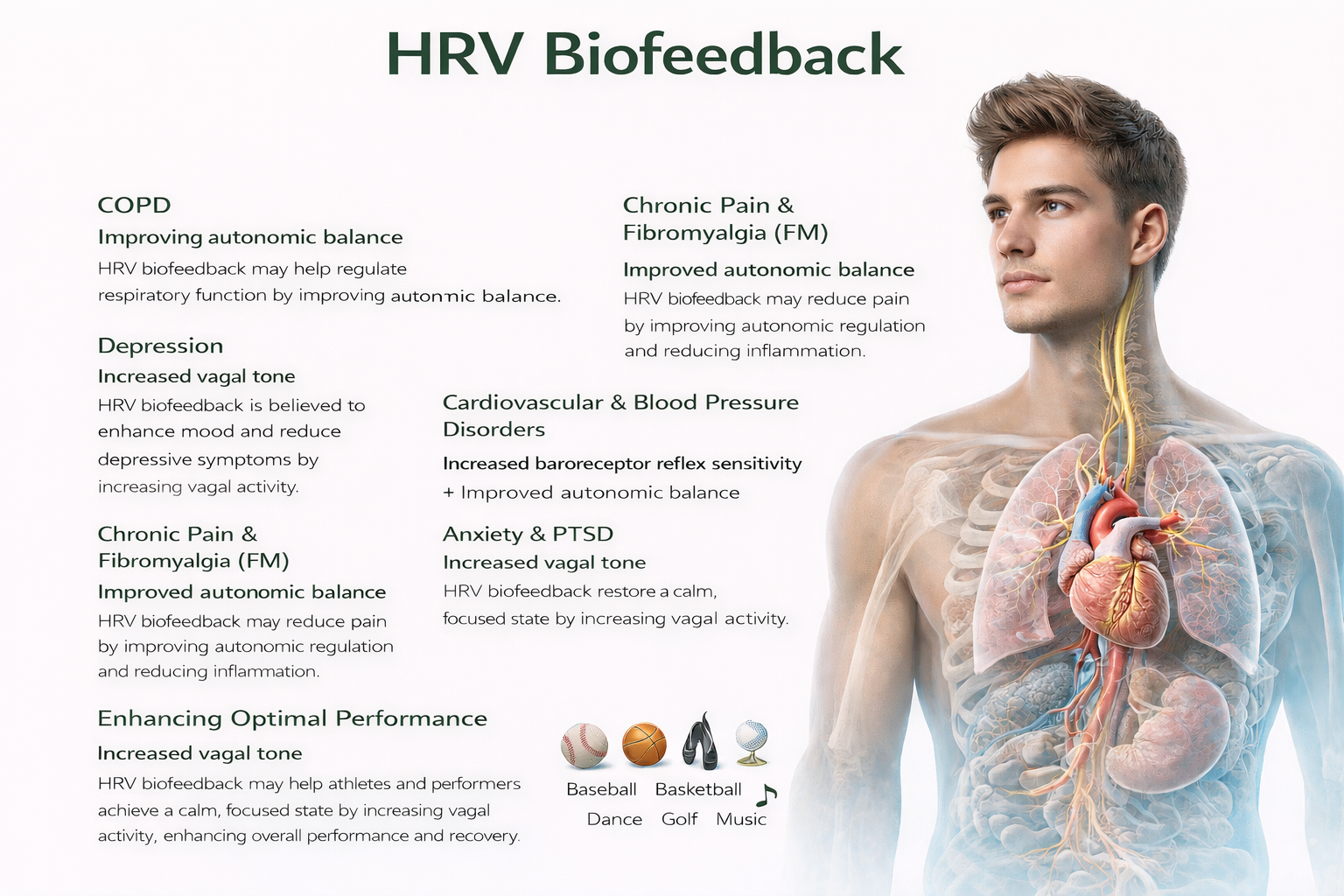
A Word of Caution: Biofeedback Is Not a Panacea
While the evidence for HRV biofeedback is encouraging, experienced clinicians know that not every client responds to treatment—a reality true of every therapeutic intervention. Dr. Saul Rosenthal organized the groundbreaking virtual symposium "Crappy Cases: Should I Zig, Zag, or Drive Off the Cliff?" to address this directly. His presentation explores what seasoned clinicians can learn from treatment failures and provides instructive case studies that illuminate the boundaries of our interventions.
Comprehension Questions: Clinical Efficacy Foundations
- What percentage of established medical practices were found to be ineffective or harmful in the Prasad et al. (2013) study, and why is this finding relevant to biofeedback practice?
- According to the AAPB/ISNR Task Force criteria, what evidence distinguishes a Level 5 (efficacious and specific) rating from a Level 4 (efficacious) rating?
- What three mechanisms does Gevirtz (2013) propose to explain how HRV biofeedback produces therapeutic benefits, and how might understanding these mechanisms inform your clinical practice?
- Based on Lehrer and colleagues' meta-analysis, for which conditions does HRV biofeedback show the largest effect sizes, and what does this suggest about treatment selection?
Asthma: A Success Story for HRV Biofeedback

The Global Initiative for Asthma (2018) defines asthma as a "heterogeneous disease, usually characterized by chronic airway inflammation." In clinical practice, this means respiratory symptoms—wheezing, shortness of breath, chest tightness, and cough—that vary in intensity over time, accompanied by reversible limitations in expiratory airflow. For practitioners in VA hospitals, respiratory clinics, or primary care settings, asthma represents one of the most evidence-supported applications for HRV biofeedback.
🎧 Mini-Lecture: Pathways to Asthma
Understanding the Cellular Cascade
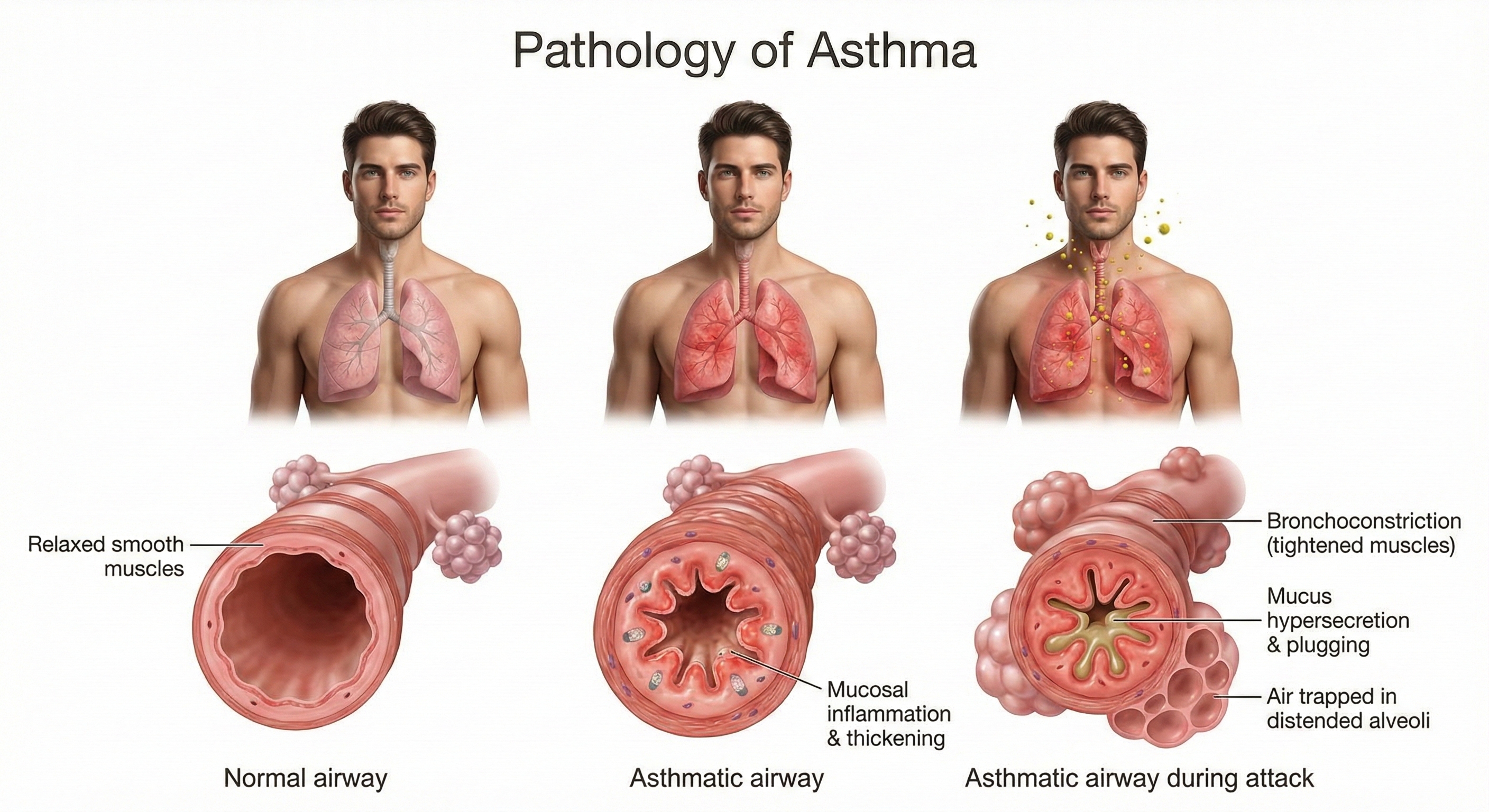
At the cellular level, asthma begins when the airway epithelium (the tissue lining the airways) releases signaling molecules called alarmins—including TSLP, IL-33, and IL-25—that initiate an inflammatory cascade affecting both structural and immune cells (Varricchi et al., 2024). This process involves T helper 2 (Th2) cells producing cytokines that drive eosinophilic inflammation (inflammation dominated by eosinophils, a type of white blood cell involved in allergic responses), mucus hypersecretion, and airway remodeling.
What is airway remodeling, and why should clinicians care? It refers to structural changes—epithelial damage, smooth muscle thickening, and subepithelial fibrosis (scarring beneath the airway lining)—that may become irreversible over time (Yamasaki, 2023). This progression underscores why early, effective asthma management matters: chronic inflammation can permanently alter airway structure, making future interventions less effective. The stress-asthma connection adds another dimension: both acute and chronic stress can precipitate attacks in children with asthma (Sandberg et al., 2000), creating a clear opening for biofeedback intervention.
How Common Is Asthma?
Recent surveillance data reveal the scope: approximately 25 million Americans currently have asthma—4.7 million children and 20.3 million adults (Pate & Zahran, 2024). That translates to roughly 1 in 13 people in your community, clinic, or hospital.
The epidemiological patterns have shifted over the past decade. Among children, asthma prevalence has actually decreased significantly since 2010. Among adults, it has increased since 2013 (Pate & Zahran, 2024). Current estimates show 8.6% of adults and 6.5% of children have asthma (National Center for Health Statistics, 2025). Women are more affected than men (9.7% versus 6.2%), and significant racial disparities persist—non-Hispanic Black individuals show the highest prevalence at 11.1% (Pate & Zahran, 2024). For practitioners serving diverse populations, these disparities matter for understanding patient needs and barriers to care.
Resonance Frequency Biofeedback Protocol
HRV biofeedback combined with breathing retraining has produced impressive results in asthma treatment: reduced symptom frequency and severity, improved pulmonary function, and decreased medication use. The key protocol was developed by Lehrer and colleagues (2000), combining resonance frequency HRV biofeedback with abdominal pursed-lips breathing.
What exactly is resonance frequency? Every oscillating system—including your cardiovascular system—has a frequency at which it responds most powerfully when stimulated. Think of pushing a child on a swing: push at the right moment and the swing soars higher with minimal effort; push at the wrong time and you fight against the swing's natural rhythm. In HRV biofeedback, the resonance frequency is the breathing rate at which an individual's cardiovascular system produces the greatest respiratory sinus arrhythmia (RSA)—the natural acceleration of heart rate during inhalation and deceleration during exhalation. By breathing at this personalized rate (typically around 6 breaths per minute, though it varies between individuals), clients can maximize the training effect.
A systematic review of HRV biofeedback in chronic disease management confirmed significant positive effects on asthma without adverse effects. Improvements in symptoms co-occurred with enhanced autonomic function (Laborde et al., 2022)—suggesting the training doesn't just mask symptoms but actually improves underlying regulatory capacity.

🎧 Mini-Lecture: HRV Biofeedback for Asthma
The Research Evidence
The evidence base for HRV biofeedback in asthma has grown substantially over two decades. Lehrer and colleagues (1997) conducted a controlled study comparing three biofeedback approaches in adults with asthma: RSA biofeedback, neck/trapezius SEMG biofeedback, and incentive inspirometry biofeedback. Only the RSA biofeedback group showed large-scale within-session decreases in respiratory impedance. This finding is clinically significant because pulmonary impedance—the resistance of the bronchioles to airflow—is precisely what needs to decrease for asthma patients to breathe more easily.
Subsequent research expanded these findings. Kern-Buell and colleagues (2000) found that SEMG biofeedback might reduce inflammation and asthma symptoms. Lehrer, Smetankin, and Potapova (2000) reported that the Smetankin method of RSA biofeedback reduced both asthma symptoms and airway resistance in 20 unmedicated children—demonstrating effects without the confound of medication changes.
Song and Lehrer (2003) provided insight into optimal breathing rates. They instructed five female volunteers to breathe at rates of 3, 4, 6, 8, 10, 12, and 14 breaths per minute while measuring HRV amplitude (peak-to-trough heart rate differences across the breathing cycle). The pattern was clear: slower breathing produced higher HRV amplitudes, with the peak occurring at 4 breaths per minute and declining slightly at 3 breaths per minute. This establishes a physiological basis for the "sweet spot" that maximizes cardiovascular oscillation during training.
The largest clinical trial came from Lehrer and colleagues (2004), who examined HRV biofeedback in 94 adult asthma patients. After stabilizing all participants on controller medication, researchers randomly assigned patients to one of four conditions: HRV biofeedback with abdominal breathing training, HRV biofeedback alone, placebo EEG biofeedback, or a waiting list control. Both HRV groups required less steroid medication and showed better pulmonary function than controls. Notably, the two HRV groups didn't differ significantly from each other—suggesting that the HRV component, not the breathing training per se, drove the benefits.
What about systematic reviews? Yorke, Fleming, and Shuldham's (2007) Cochrane Systematic Review examined 15 studies involving 687 participants. While limited data prevented definitive conclusions about psychological interventions overall, one finding stood out: biofeedback significantly increased forced expiratory volume (FEV1)—the amount of air a person can forcefully exhale in one second, a key measure of lung function.
Meuret and colleagues (2007) took a different approach, using capnometric biofeedback to raise end-tidal pCO2 (partial pressure of carbon dioxide) while reducing respiration rate. This intervention decreased both the frequency and severity of asthma symptoms—consistent with the idea that normalizing breathing chemistry, not just rate, matters for outcomes.
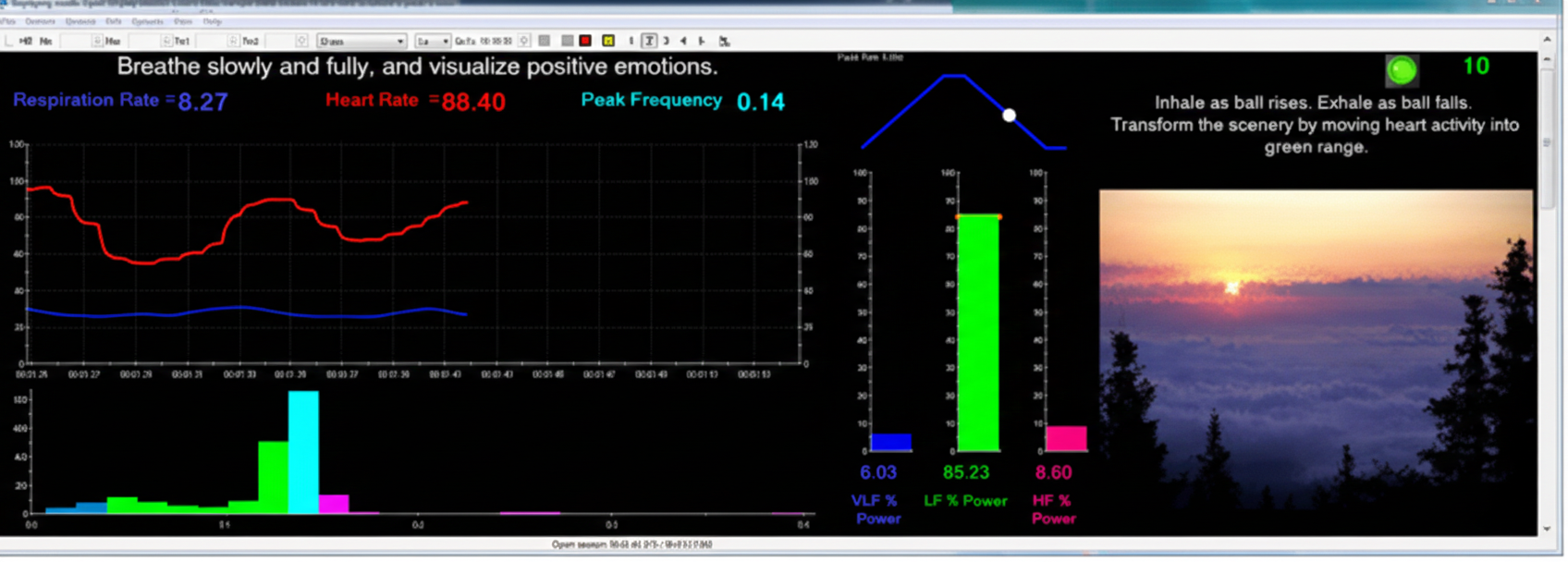
Clinical Efficacy Rating
Based on five randomized controlled trials (RCTs), Lehrer, Moritz, and Greenfield (2023) rated HRV biofeedback for asthma as level 5: efficacious and specific in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). This is the highest rating on the efficacy scale, indicating that HRV biofeedback for asthma has been shown superior to credible sham therapy and equivalent or superior to established treatments in studies with adequate statistical power.
Study participants demonstrated improvements across multiple domains: asthma severity and symptoms, pulmonary function measured objectively, and medication use. The impact of HRV biofeedback on airway inflammation remains under investigation, potentially revealing additional mechanisms that may expand clinical applications. For practitioners, asthma represents one of the strongest evidence-based indications for HRV biofeedback—a success story that demonstrates what rigorous research can establish about our interventions.
Asthma is a chronic inflammatory condition affecting 25 million Americans—about 1 in 13 people. Stress can trigger attacks, creating a clear role for biofeedback intervention. Significant racial disparities persist, with non-Hispanic Black individuals showing the highest prevalence (11.1%). The standout finding: resonance frequency HRV biofeedback combined with abdominal pursed-lips breathing has earned a level 5 rating (efficacious and specific)—the highest possible—based on five RCTs. This means it has been demonstrated superior to credible sham therapy. Clinical benefits include reduced medication dependence, improved pulmonary function, and decreased symptom severity. Optimal breathing rates for maximizing HRV amplitude are typically 4-6 breaths per minute, though individual resonance frequencies vary. The key mechanism appears to be HRV training itself, not just breathing modification—the two HRV biofeedback groups in Lehrer's large trial showed equivalent benefits regardless of whether they also received breathing training.
Chronic Obstructive Pulmonary Disease (COPD)

Chronic obstructive pulmonary disease (COPD) encompasses a family of lung diseases united by one feature: they all interfere with airflow. The Global Initiative for Chronic Obstructive Lung Disease (2019) defines it as "a common preventable and treatable disease characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases."
That final phrase—"noxious particles or gases"—points to the primary culprit: tobacco smoke. Unlike asthma, which typically involves reversible airway obstruction, COPD involves permanent structural damage—a distinction with important implications for treatment expectations and patient education. COPD is a progressive disorder with serious consequences: almost 50% of severe cases die within 10 years of initial diagnosis.
Two Pathways to Airflow Obstruction
Two main conditions comprise COPD: chronic obstructive bronchitis and emphysema. Most patients present with features of both, creating a complex clinical picture.
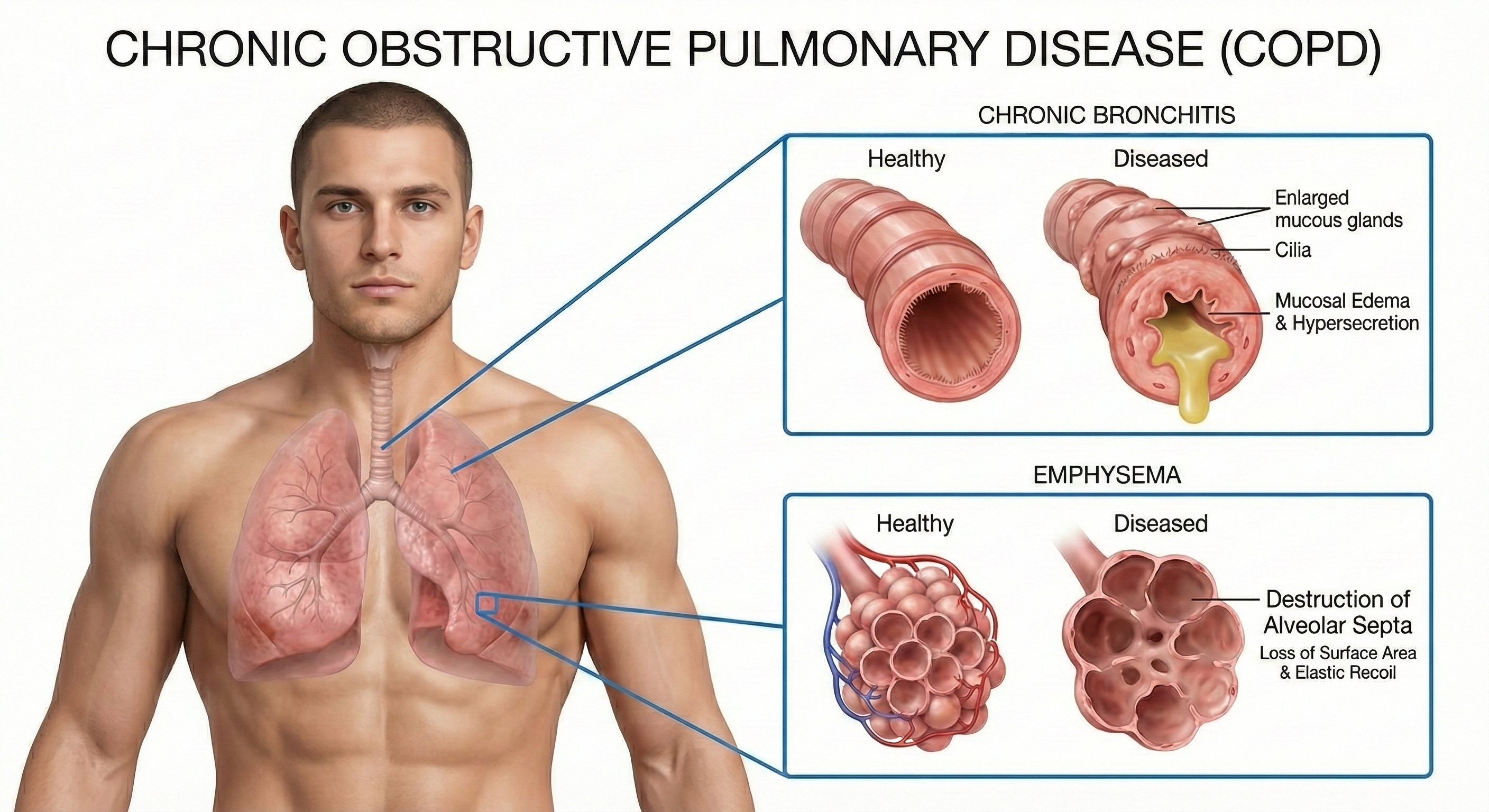
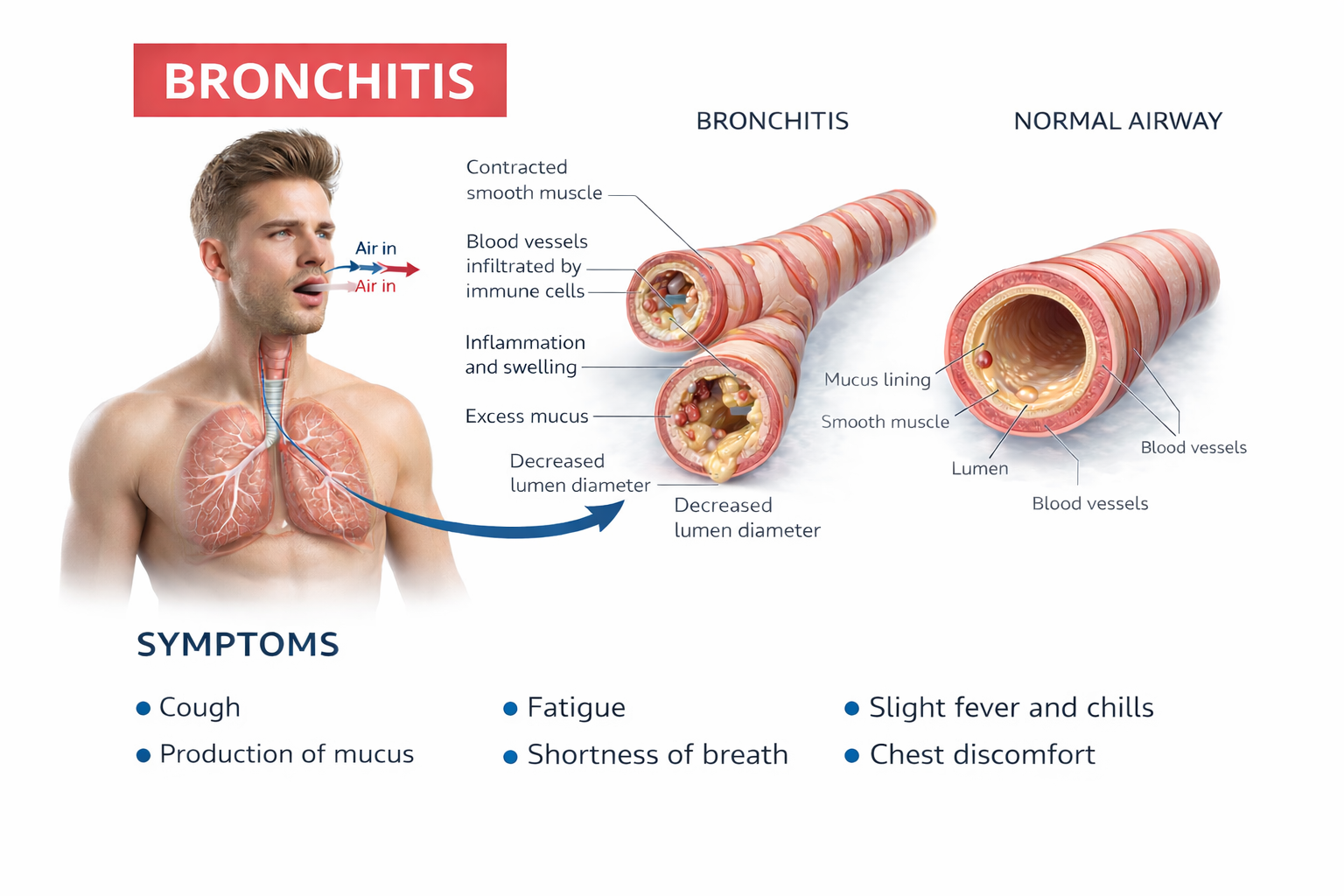
Chronic bronchitis involves mucus hypersecretion and chronic productive cough—not just a week of coughing, but at least 3 months for a minimum of 2 consecutive years (Huether & McCance, 2020). The airway obstruction results primarily from mucus plugging and inflammation. Think of it as airways clogged by their own secretions.
Emphysema works differently. Here, the problem is structural damage to the alveoli—the tiny air sacs where gas exchange occurs. Abnormal, irreversible expansion of these gas-exchange airways accompanies destruction of alveolar walls (Huether & McCance, 2020). Unlike chronic bronchitis where mucus buildup causes obstruction, in emphysema it's inflammation and lung damage that produce the obstruction.
The surviving air sacs are larger but less numerous and less effective—imagine trying to absorb oxygen through a few large balloons instead of millions of tiny ones. This structural destruction is why emphysema responds less dramatically to treatment than conditions involving only functional (rather than anatomical) changes.
Scope of the Problem
COPD represents a major public health burden—one of those conditions where the numbers alone tell a compelling story. According to recent National Health Interview Survey data, the age-adjusted prevalence of diagnosed COPD among U.S. adults is 3.8%, affecting approximately 16 million people (Manneh & Lucas, 2025).
The demographic patterns are notable and clinically relevant. Women are more likely than men to have COPD (4.1% versus 3.4%)—possibly related to changing smoking patterns over recent decades and potentially to increased susceptibility to tobacco-related lung damage. Prevalence increases dramatically with age, rising from just 0.4% among adults aged 18-24 to 10.5% among those 75 and older. This age gradient reflects COPD's progressive nature and the years of exposure typically required before symptoms emerge.
In 2023, COPD was the fifth leading cause of death in the United States, claiming 141,733 lives. The economic burden is substantial: annual medical costs reach an estimated $24 billion among adults 45 years and older (Manneh & Lucas, 2025). Racial disparities exist here too, though the pattern differs from asthma: White non-Hispanic adults show higher prevalence (4.4%) than Hispanic (2.0%) or Asian (1.0%) adults. COPD prevalence also tracks with socioeconomic factors—decreasing with higher family income and notably higher in rural communities where smoking rates tend to be elevated.
Multimodal Treatment Approach
COPD treatment requires combining multiple approaches—no single intervention is sufficient. Clinicians typically integrate HRV biofeedback with exercise and paced breathing instruction to increase ventilation and exercise tolerance. A network meta-analysis of breathing exercises for COPD found that various approaches improve exercise capacity, pulmonary function, and inspiratory muscle pressure, with diaphragmatic breathing and pursed-lip breathing showing particular benefits (Chen et al., 2023).
Esteve and colleagues (1996) demonstrated the potential of breathing pattern training: they randomly assigned COPD patients to either breathing training or a control group. The trained group showed remarkable improvements—FEV1 (forced expiratory volume in one second) increased by 22% and FVC (forced vital capacity)—the total amount of air a person can forcefully exhale—increased by 19%. The control group showed no improvement. These are clinically meaningful gains that translate to improved daily function.
Giardino and colleagues (2004) took a comprehensive approach, combining HRV biofeedback with exercise in 10 COPD patients. Participants received five HRV biofeedback sessions to increase HRV, combined with paced breathing instruction. They also walked four times a week, using their paced respiration skills to control breathing, and monitored their oxygen levels with a pulse oximeter—a device that measures dissolved oxygen in the bloodstream using a light sensor placed on the finger. Results were encouraging: patients improved on the six-minute walking distance test (6MWD), which measures functional capacity, and the St. George's Respiratory Questionnaire (SGRQ), which assesses overall quality of life. Eight of the ten participants achieved clinically significant gains on these measures.
Emerging protocols now address a critical challenge: COPD patients often experience both dyspnea (shortness of breath) and anxiety simultaneously, each feeding the other in a vicious cycle. The Capnography-Assisted Learned Monitored (CALM) Breathing therapy uses end-tidal CO2 biofeedback combined with slow nasal breathing exercises to target dysfunctional breathing patterns in COPD patients. Pilot studies show participants report reduced dyspnea intensity, less avoidance of physical activity, and improved well-being (Norweg et al., 2024). By targeting both the physical and psychological components together, these protocols may reach patients who haven't responded to either approach alone.
Clinical Efficacy Rating
Based on eight RCTs, Gilbert (2023) rated biofeedback for COPD as level 3: probably efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). Why the lower rating compared to asthma (which earned level 5)? The COPD studies had limitations in design and sample size that prevented a higher classification. This doesn't mean the treatment doesn't work—it means we need more rigorous research to establish efficacy with greater certainty.
For practitioners, level 3 suggests a reasonable evidence base for offering biofeedback to COPD patients, with appropriate informed consent about the current state of the evidence. Unlike asthma, where HRVB can modify the underlying pathophysiology, COPD treatment focuses more on optimizing function within the constraints of permanent structural damage. Setting realistic expectations with these patients is essential.
COPD—encompassing chronic bronchitis (mucus obstruction) and emphysema (alveolar destruction)—affects approximately 16 million Americans and ranks as the fifth leading cause of death. The condition shows highest prevalence among older adults (10.5% in those 75+), women, and rural communities. Treatment requires a multimodal approach: HRV biofeedback combined with exercise and paced breathing instruction. The evidence is encouraging—breathing pattern training can increase FEV1 by 22% and FVC by 19%—but study limitations leave biofeedback for COPD rated at level 3 (probably efficacious), lower than asthma's level 5. Emerging protocols like CALM Breathing target both dyspnea and anxiety simultaneously, recognizing that these often feed each other in a vicious cycle. For practitioners, the key takeaway: COPD patients can benefit from biofeedback as part of comprehensive pulmonary rehabilitation, but expectations should be calibrated to the current evidence base and the irreversible nature of structural lung damage.
Check Your Understanding: Respiratory Conditions
- What is the clinical efficacy rating for HRV biofeedback treatment of asthma, and what specific evidence supports this highest-level rating?
- Explain how resonance frequency biofeedback works. What is the resonance frequency, and why does breathing at this rate maximize the training effect?
- Through what mechanisms might HRV biofeedback improve asthma symptoms? Why did Lehrer's research suggest that HRV training—not breathing modification per se—is the key ingredient?
- What distinguishes chronic bronchitis from emphysema in COPD patients? How does this distinction affect treatment expectations?
- Why does COPD have a lower efficacy rating (level 3) than asthma (level 5) despite both being respiratory conditions? What does this difference suggest about patient education and informed consent?
- What is CALM Breathing therapy, and how does it address the dual challenge of dyspnea and anxiety in COPD patients?
Depression: Multiple Pathways to Treatment

Major depressive disorder (MDD) is more than simply feeling sad or going through a difficult time. MDD is diagnosed when five or more depressive symptoms, including persistent sadness or loss of pleasure in activities that used to be enjoyable, persist for at least two weeks. Depressed patients may sleep excessively or insufficiently, display psychomotor retardation or agitation, show changes in weight or appetite, experience loss of energy, feel worthless or guilty, struggle to concentrate or make decisions, and in severe cases, repeatedly think about death or suicide (Beidel, Bulik, & Stanley, 2014). For clinicians working with this population in VA hospitals, community clinics, or private practice, understanding depression's neurobiological underpinnings helps explain how both neurofeedback and HRV biofeedback interventions target specific aspects of the disorder.
Check out the YouTube video The Science of Depression for an accessible overview of the neuroscience underlying this disorder.
Demographics
The scope of depression has expanded dramatically in recent years, creating substantial unmet treatment needs across all clinical settings. According to the most recent National Health and Nutrition Examination Survey (2021-2023), depression prevalence in the past two weeks was 13.1% among adolescents and adults aged 12 and older (Brody & Hughes, 2025). This represents a substantial increase compared to pre-pandemic levels. Nearly 21% of Americans will experience major depressive disorder at some point in their lifetime.
Age patterns reveal important clinical considerations. Depression prevalence is highest among adolescents aged 12-19, with a striking 26.5% of adolescent females reporting symptoms—more than one in four. Those aged 18 to 29 (34.3%) and 30 to 44 (34.9%) show markedly higher lifetime rates of depression diagnosis than those over 44, suggesting that younger cohorts may face either greater risk or greater willingness to seek diagnosis.
Gender differences are pronounced: more than a third of women (36.7%) report being diagnosed with depression at some point in their lifetime, significantly exceeding the 20.4% of men. The rate of diagnosis for women has increased at nearly twice the pace of men since 2017. The stakes of untreated depression are severe: 25-30% of adults diagnosed with depression attempt or commit suicide. Most cases of major depression involve another primary comorbid psychological disorder (Zimmerman et al., 2002), complicating treatment. Alarmingly, only 21% of annual depression cases receive adequate treatment (Kessler et al., 2003)—creating substantial unmet need that biofeedback and neurofeedback practitioners can help address.
Neurophysiological Basis: Frontal Alpha Asymmetry
Multiple pathways converge in depression, including polygenic inheritance, dysfunction involving the frontal cortex and limbic system, and environmental factors (McGrady & Moss, 2013). Understanding these pathways helps clinicians appreciate why different treatment approaches may target different aspects of the disorder—and why HRV biofeedback and neurofeedback often work synergistically.
Richard Davidson proposed the theory of frontal alpha asymmetry (FAA) as a neurophysiological marker for depression (Davidson, 1992). This theory distinguishes between two complementary brain systems. The behavioral activation system (BAS), mediated primarily by the left frontal cortex, drives approach behavior and positive emotions—the motivation to engage with rewarding activities and experiences. The behavioral inhibition system (BIS), mediated by the right frontal cortex, drives withdrawal motivation and negative affect—the tendency to avoid threatening or unpleasant situations.
Depression is associated with reduced left frontal activity relative to right frontal activity, reflecting diminished approach motivation and positive affect. In other words, the depressed brain shows the signature of withdrawal. Clinically, this manifests as the anhedonia, social withdrawal, and loss of motivation that characterize the disorder.
Here is a crucial point that initially seems counterintuitive but is essential for understanding neurofeedback treatment: because alpha power is inversely related to cortical activity (more alpha means less neural firing), higher right alpha relative to left alpha actually represents a healthier pattern. When we see greater alpha amplitude over the right frontal region compared to the left, this indicates reduced right-hemisphere activity—less withdrawal motivation—relative to left-hemisphere activity—more approach motivation.
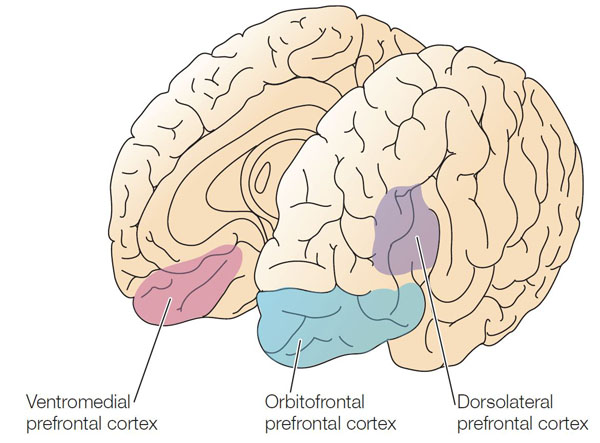
Depression is also associated with increased activation of the lateral orbitofrontal cortex, which signals when behavior has not been rewarded. This activation may underlie the persistent feelings of loss and disappointment characteristic of depression. Because this region communicates with networks responsible for self-concept, excessive lateral orbitofrontal activation may also lower self-esteem (Cheng et al., 2016). When patients describe pervasive feelings of failure or worthlessness, these neural circuits may be driving their experience.
Simultaneously, depression involves reduced activation of reward circuitry in the medial orbitofrontal cortex and its communication with autobiographical memory systems. These changes may explain why depressed patients lose enjoyment of previously pleasurable activities (anhedonia) and have difficulty recalling happy experiences—symptoms that profoundly impact quality of life and treatment engagement.
Depression also involves disruption of the habenular nucleus, located adjacent to the pineal gland. The habenula normally helps filter out negative cognitions and memories; when this function is impaired, patients experience the cognitive triad that characterizes depressive thinking: negative perceptions of self, the immediate situation, and the future (Lawson et al., 2016). Interestingly, animal research reveals that cocaine withdrawal increases habenular nucleus anti-reward pathway activation (Clerke et al., 2021), suggesting connections between depression and addiction pathways that may be relevant for VA and substance abuse treatment settings.
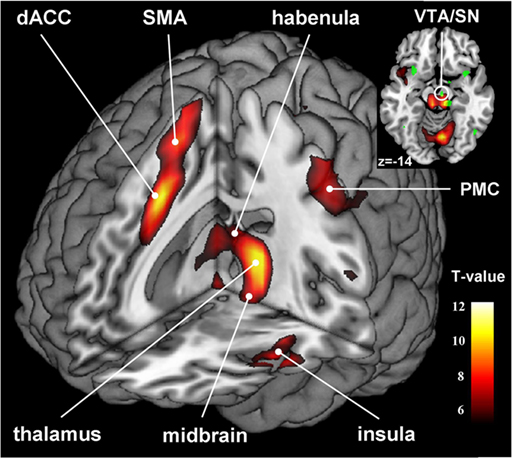
Autonomic Dysregulation in Depression
Beyond cortical dysfunction, depression is also characterized by autonomic nervous system imbalance—reduced heart rate variability and parasympathetic withdrawal. In plain terms, the nervous system in depression often mirrors what we see in anxiety: it is shifted toward sympathetic dominance with reduced vagal tone. This autonomic profile connects depression to increased cardiovascular risk and provides the rationale for HRV biofeedback intervention. By restoring autonomic balance, HRV biofeedback may address both the physiological dysregulation and, through bottom-up pathways, the mood symptoms of depression.
Neurofeedback and Biofeedback Protocols
EEG and functional MRI (fMRI) are the primary neurofeedback interventions for depression, while EMG and HRV are the central biofeedback modalities. Each approach targets different aspects of the disorder, and clinicians may combine them based on individual patient presentations.
Alpha asymmetry neurofeedback for mood disorders attempts to correct frontal asymmetry by training clients to increase right frontal alpha relative to left frontal alpha. This intervention rests on the frontal alpha asymmetry model and aims to reduce right frontal activity (withdrawal) or increase left frontal activity (approach). Training protocols typically place active electrodes at F3 and F4 to monitor alpha power at both sites simultaneously.
Baehr and colleagues (1997) used an AAPB task force approach to treat participants in an A1 score-guided protocol. The A1 score is calculated by subtracting log left-alpha power from log right-alpha power. The larger the A1 score, the more left frontal activity (approach behavior) relative to right frontal activity (withdrawal behavior). Patients showed significant improvement in depression symptoms as their A1 scores normalized.
fMRI Neurofeedback
Real-time fMRI neurofeedback allows patients to observe and regulate activity in specific brain regions with high spatial precision. While EEG-based neurofeedback can localize activity only roughly, fMRI can target structures deep in the brain with millimeter accuracy—including the amygdala and other emotion-processing regions implicated in depression.
Young and colleagues (2017) conducted a double-blind, placebo-controlled RCT on MDD patients, targeting the amygdala or a control region. The experimental group showed increased amygdala response and significant depression symptom decreases, with 32% meeting remission criteria—compared to only 8% symptom decrease and 6% remission in the control group. These results provide strong evidence for specific neural effects and suggest that fMRI neurofeedback may become an important treatment option as the technology becomes more accessible.
HRV Biofeedback Studies
Heart rate variability biofeedback (HRVB) targets the autonomic dysregulation component of depression by training patients to breathe at their resonance frequency—typically around 6 breaths per minute—which maximizes respiratory sinus arrhythmia and strengthens vagal tone. This bottom-up approach may influence mood through interoceptive feedback and by restoring the calming parasympathetic activity that is diminished in depression.
Siepmann and colleagues (2008) conducted a randomized trial comparing HRV biofeedback to a placebo condition in patients with major depression. The HRV biofeedback group showed significant reductions in both depression and anxiety symptoms, supporting the intervention's efficacy for mood disorders.
Caldwell and Steffen (2018) found that HRV biofeedback combined with psychotherapy produced larger decreases in depression symptoms than psychotherapy alone. This finding has immediate clinical relevance: adding HRVB to standard treatment may enhance outcomes without requiring specialized neurofeedback equipment.
Meta-analysis of HRV biofeedback for depression found a medium effect size (g = 0.38) for reducing depressive symptoms, comparable to other established treatments like cognitive-behavioral therapy (Pizzoli et al., 2021). This effect size means that the average person receiving HRVB improves more than approximately 65% of those in control conditions—a clinically meaningful benefit.
Clinical Efficacy Rating
Based on 10 RCTs, Zachary Meehan, Fred Shaffer, and Christopher Zerr rated biofeedback and neurofeedback for major depressive disorder as efficacious and specific in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). This is the highest efficacy rating, indicating that the intervention has been shown superior to credible placebo controls and alternative treatments. Both alpha asymmetry neurofeedback and HRV biofeedback have demonstrated efficacy, giving clinicians flexibility in treatment selection based on available equipment and patient characteristics.
For practitioners, this efficacious and specific rating means that biofeedback and neurofeedback represent evidence-based treatment options that can be offered with confidence, either as standalone interventions or as adjuncts to psychotherapy and medication. The combination of neurofeedback addressing cortical dysregulation and HRVB addressing autonomic imbalance may provide particularly comprehensive treatment.
Depression involves dysfunction in multiple systems: cortical imbalance (reduced left frontal activity relative to right, reflecting diminished approach motivation), subcortical disruption (orbitofrontal and habenular dysfunction affecting reward processing and negative thought filtering), and autonomic dysregulation (reduced HRV and parasympathetic withdrawal). Treatment approaches include alpha asymmetry neurofeedback targeting frontal asymmetry, fMRI neurofeedback targeting emotion-processing regions, and HRV biofeedback targeting autonomic regulation. Biofeedback and neurofeedback for depression are rated efficacious and specific—the highest evidence rating. HRV biofeedback shows a medium effect size comparable to CBT, and combining HRVB with psychotherapy enhances outcomes. The dramatic increase in depression prevalence—over 13% currently experiencing symptoms—underscores the urgent need for accessible, effective treatments.
Fibromyalgia: A Pain Amplification Disorder
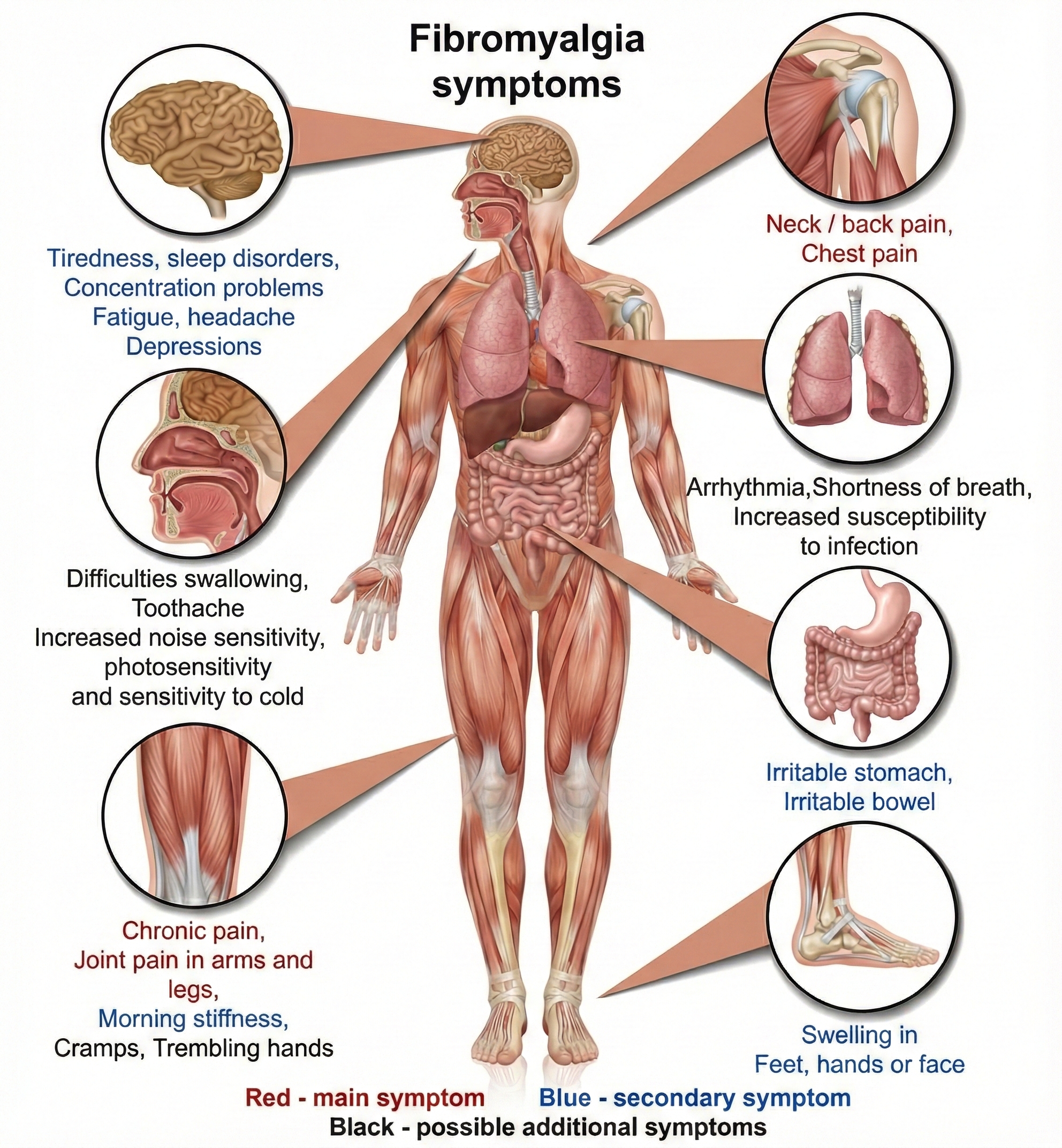
Fibromyalgia (FM) is a chronic benign pain disorder characterized by widespread musculoskeletal pain, tenderness, stiffness, and fatigue. What sets FM apart from most pain conditions is the absence of identifiable tissue damage or inflammation at pain sites—making diagnosis challenging and frustrating for both clinicians and patients. For practitioners in VA hospitals, pain clinics, or primary care settings, FM patients often arrive after years of inconclusive workups, dismissed by providers who could not identify structural pathology. Understanding FM as a central sensitization syndrome helps explain why autonomic interventions may succeed where peripheral treatments have failed.
McGrady and Moss (2013) conceptualize FM as a "pain amplification disorder" produced by the twin mechanisms of allodynia and hyperalgesia. Allodynia means that patients experience previously benign stimuli as painful—a light touch that would be imperceptible to healthy individuals causes significant discomfort. Hyperalgesia means patients experience mildly painful stimuli as severely painful—minor bumps or pressure produce disproportionate pain responses. These phenomena reflect dysfunction in central nervous system pain processing rather than peripheral tissue damage.
Understanding Nociplastic Pain
The International Association for the Study of Pain has introduced the term nociplastic pain to describe this third category of pain, distinct from nociceptive pain (caused by tissue damage) and neuropathic pain (caused by nervous system lesions). Nociplastic pain involves altered nociception despite no clear evidence of actual or threatened tissue damage or nervous system disease (Nijs, Malfliet, & Nishigami, 2023). This conceptual advance helps clinicians explain to patients why their pain is real even when imaging and laboratory tests are normal.
Evidence suggests that FM involves defective descending pain modulation, where normal inhibitory pathways that would suppress pain signals are impaired, contributing to widespread pain hypersensitivity (Almeida Silva & Pinto, 2025). The brainstem and cortical structures that normally filter and dampen ascending pain signals function abnormally, allowing pain signals that would normally be suppressed to reach conscious awareness.
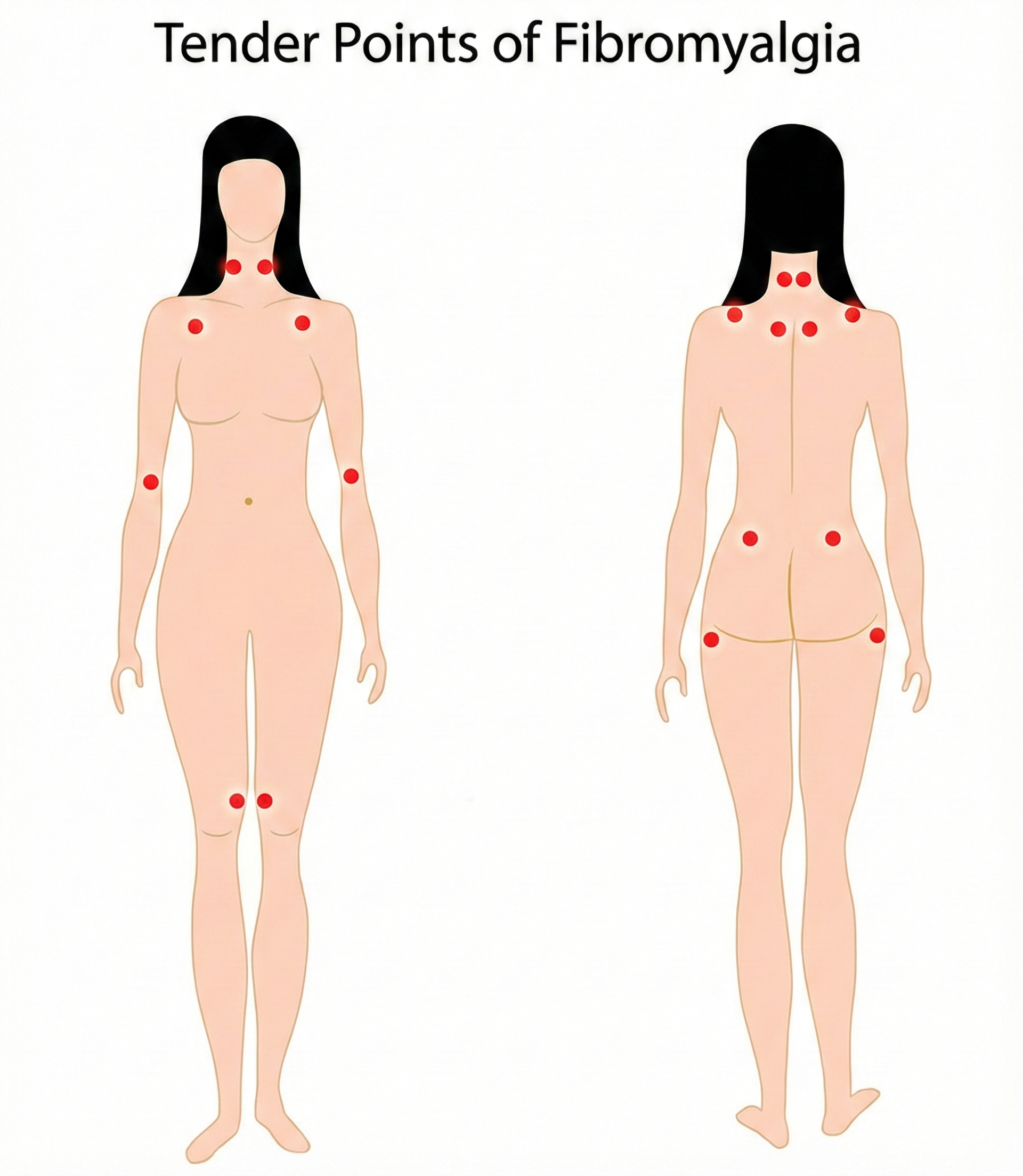
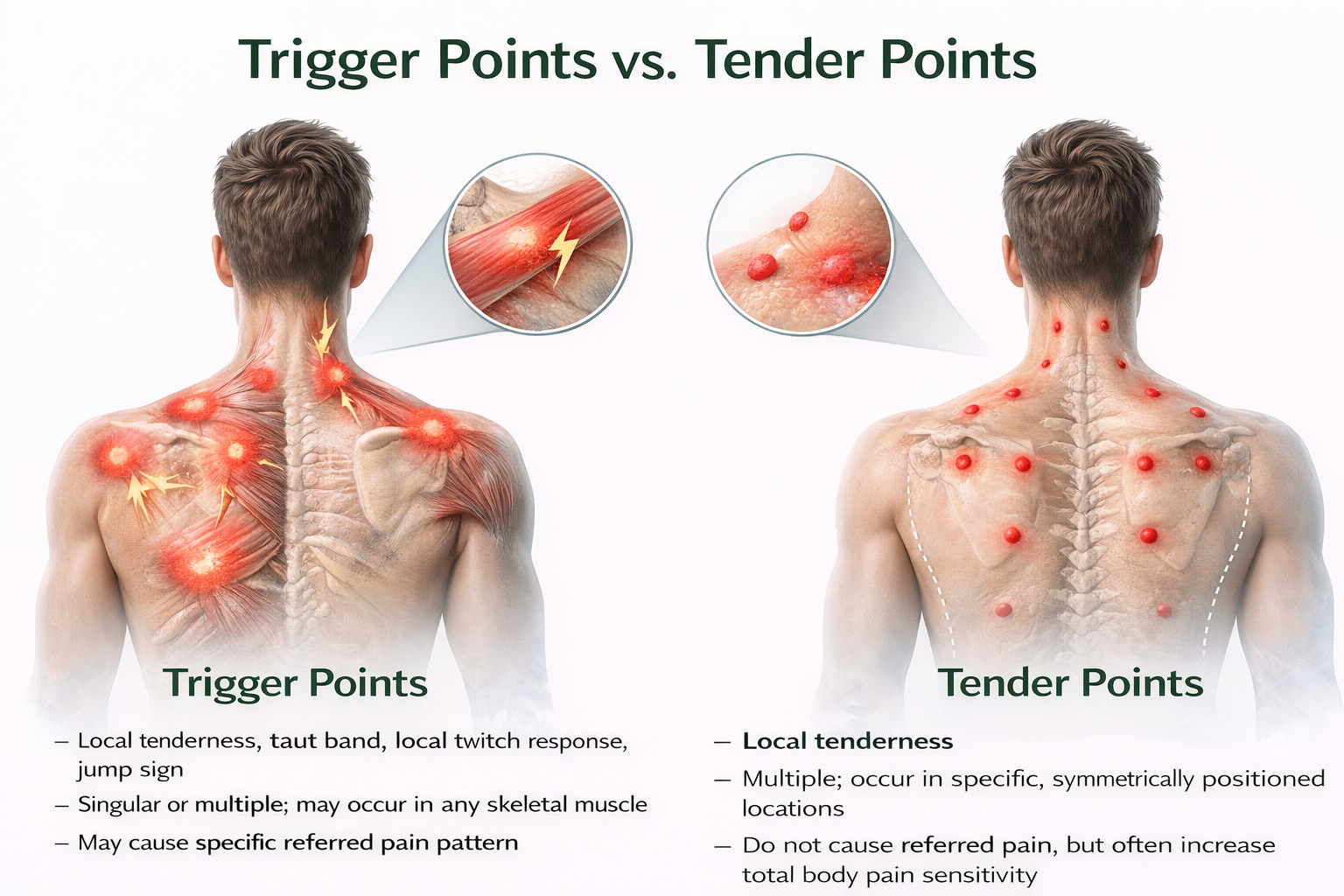
Differentiating Tender Points from Trigger Points
Patients may present with both fibromyalgia and myofascial pain syndrome (MPS) and exhibit both tender points and trigger points—making careful differential diagnosis essential. Tender points are specific locations where light pressure causes pain and are the hallmark of fibromyalgia; they represent generalized hypersensitivity rather than local pathology and are located at a muscle's insertion (the tendinous attachment to a movable bone) instead of the muscle belly. When compressed, they produce local pain but not the referred pain pattern associated with trigger points. Pressure on tender points may increase overall pain sensitivity.
In contrast, trigger points are hyperirritable spots in taut muscle bands that refer pain to distant locations and characterize myofascial pain syndrome—they represent localized muscle dysfunction that can be directly treated. Accurate diagnosis requires careful examination by an experienced clinician and determines which treatment approach is likely to succeed (Alvarez & Rockwell, 2002).
Diagnostic Criteria Evolution
The American College of Rheumatology (ACR) adult criteria include widespread pain for at least 3 months on both sides of the body and pain during gentle palpation on 11 of 18 tender points on the neck, shoulder, chest, back, arm, hip, and knee sites. However, the 2010 criteria eliminated tender point evaluation since many physicians could not perform the examination reliably (Goldenberg, 2010)—a practical acknowledgment that diagnostic validity requires consistent application across clinicians. Changes in diagnostic criteria over the past decade have resulted in more patients with chronic pain meeting fibromyalgia criteria (American Academy of Family Physicians, 2023).
Patients also present with attentional deficits, depression, severe fatigue, headaches, impaired multitasking, irritable bowel syndrome, memory deficits, sleep disturbance, and temporomandibular muscle and joint pain (Donaldson & Sella, 2003; Tortora & Derrickson, 2021). This constellation of symptoms reflects the central nature of the disorder—affecting multiple systems that share common neural pathways.
Demographics
Fibromyalgia affects approximately 2-4% of the global population, with estimates ranging from 4 million to 10 million people in the United States alone (American Academy of Family Physicians, 2023). The global prevalence is approximately 2.7%, with the U.S. prevalence at approximately 3.1% and Europe at 2.5% (Soroosh & Farbod, 2024).
The condition predominantly affects women, who comprise 75-90% of diagnosed cases, with a female-to-male ratio of approximately 3:1. The diagnosis is usually made between ages 20 and 50, though the incidence increases with age such that by age 80, approximately 8% of adults meet diagnostic criteria. Peak prevalence in women occurs between ages 60 and 70. For practitioners serving diverse populations, understanding these demographic patterns helps identify at-risk patients who may benefit from early intervention.
Etiology and Pathophysiology
The etiology of fibromyalgia appears to involve a central hypersensitivity to heat, cold, and electrical stimulation (Desmeules et al., 2003). Fibromyalgia patients may have low levels of serotonin, amino acids like tryptophan, and insulin-like growth factor (IGF-1), and high levels of substance P and ACTH.
Critically for HRV biofeedback rationale, Meeus and colleagues (2013) reviewed 16 controlled studies of HRV levels in FM and chronic fatigue syndrome (CFS). The findings indicated that FM patients demonstrate higher sympathetic and lower parasympathetic activity—precisely the autonomic imbalance that HRVB targets. This autonomic dysregulation provides a biological rationale for intervention that connects the patient's experience to a treatable mechanism.
Post-COVID Syndrome and Fibromyalgia
Emerging evidence suggests that post-COVID syndrome (PCS), also known as long COVID, frequently includes chronic musculoskeletal pain resembling fibromyalgia. A 2024 study found that 72.2% of PCS patients with musculoskeletal pain met American College of Rheumatology criteria for fibromyalgia (Khoja et al., 2024). The proposed mechanisms include neuroinflammation, small fiber neuropathy, and central sensitization—pathways that overlap with established fibromyalgia pathophysiology.
Patients with pre-existing chronic pain conditions appear more likely to develop long COVID symptoms. These findings underscore the importance of comprehensive pain assessment in post-COVID patients and suggest that biofeedback interventions targeting autonomic dysregulation may be particularly relevant for this population.
Sleep Disturbance in Fibromyalgia
Sleep disturbance is a hallmark of fibromyalgia, with 60-80% of patients reporting poor sleep quality. Non-restorative sleep associated with frequent awakenings is characteristic of the disorder and is linked to symptom severity. The relationship between disordered sleep and fibromyalgia is bidirectional—sleep problems increase the risk of developing chronic widespread pain, while pain disrupts sleep architecture (Lawson, 2020).
A 2025 systematic review and meta-analysis found that cognitive behavioral therapy for insomnia (CBT-I) significantly improved sleep quality, while also reducing pain, anxiety, and depression (Ho et al., 2025). In contrast, pharmacological approaches showed mixed results. The authors concluded that CBT-I should be considered a first-line treatment for addressing insomnia in individuals with fibromyalgia.
A double-blind crossover study found that suvorexant, an orexin receptor antagonist approved for insomnia, improved sleep time and reduced next-day pain sensitivity in fibromyalgia patients with comorbid insomnia (Roehrs et al., 2020). These findings support the hypothesis that improving sleep quality may directly reduce pain sensitivity in this population.
Biofeedback Treatment of Fibromyalgia
At this point, there is no evidence that biofeedback is superior to other mind-body therapies for fibromyalgia when administered alone. However, biofeedback shows promise as part of comprehensive multimodal treatment approaches.
Glombiewski, Bernardy, and Häuser's (2013) meta-analysis of seven RCTs with 321 patients diagnosed with fibromyalgia found that EMG biofeedback was superior to control groups in reducing pain severity, with a large effect size. This finding suggests that biofeedback can meaningfully address the pain component of FM, though effects on fatigue, depression, and sleep were less consistent.
A 2024 RCT by Sancassiani and colleagues found that heart rate variability biofeedback (HRV-BF) improved perceived energy and functional ability in 64 fibromyalgia patients when added to standard pharmacotherapy (Sancassiani et al., 2024). These improvements in energy and function—often more debilitating than pain itself for FM patients—highlight HRV biofeedback's potential contribution to comprehensive treatment.
Reneau (2020) reviewed research on HRVB for FM, suggesting beneficial effects of HRV training on chronic pain through multiple pathways including improved sleep, reduced stress reactivity, and possibly direct anti-inflammatory effects through the cholinergic anti-inflammatory pathway.
Research increasingly incorporates HRV biofeedback as one component of multimodal treatment packages alongside exercise, cognitive therapies (Acceptance and Commitment Therapy and Cognitive Behavioral Therapy), and interventions to improve sleep habits. The putative mechanism involves improved autonomic balance through enhanced vagal tone (Gevirtz, 2013), which could reduce central sensitization and improve the sleep disturbances that perpetuate FM symptoms.
Babu and colleagues (2007) conducted a study of 30 FM patients, comparing SEMG biofeedback to sham biofeedback. Both groups showed symptom improvement, but the biofeedback group improved more—suggesting specific effects beyond placebo while acknowledging that attention and expectation also play therapeutic roles.
Wu and colleagues (2021) extended earlier neurofeedback research, showing significant improvements in pain severity, pain interference, FM symptom severity, sleep latency, and sustained attention in the NFB group. This multi-domain improvement pattern suggests neurofeedback may address FM's central mechanisms more directly than peripheral biofeedback approaches.
Clinical Efficacy Rating
Based on one RCT using SEMG biofeedback and two RCTs using theta/SMR neurofeedback, Christopher Gilbert (2023) rated biofeedback for fibromyalgia at level 3, probably efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). For clinicians, this suggests biofeedback is a reasonable component of comprehensive FM treatment, though it should not be offered as a standalone cure. Setting appropriate expectations with these patients is essential—explaining that biofeedback targets the autonomic dysregulation underlying their condition while acknowledging the need for multimodal approaches.
Fibromyalgia is a pain amplification disorder involving allodynia (innocuous stimuli perceived as painful) and hyperalgesia (mild pain perceived as severe), now understood as nociplastic pain—altered nociception without tissue damage. Tender points, distinct from trigger points, are located at muscle insertions and produce local rather than referred pain. FM patients demonstrate autonomic dysregulation with elevated sympathetic and reduced parasympathetic activity, providing a rationale for HRV biofeedback intervention. SEMG biofeedback has shown large effect sizes for reducing pain severity, while HRV biofeedback improves energy and functional ability. Sleep disturbance affects 60-80% of FM patients, with CBT-I emerging as a first-line treatment. Post-COVID syndrome frequently presents with FM-like symptoms. A comprehensive treatment approach combining biofeedback with exercise, cognitive therapy, and sleep interventions produces the best outcomes. Biofeedback for FM is rated Level 3 (probably efficacious).
Comprehension Questions: Fibromyalgia
- What is nociplastic pain and how does it differ from nociceptive and neuropathic pain? Why is this distinction clinically important when explaining fibromyalgia to patients?
- Explain the differences between tender points and trigger points. Why does misidentifying these lead to inappropriate treatment?
- What autonomic abnormalities have been documented in fibromyalgia patients, and how do these findings support the rationale for HRV biofeedback intervention?
- Describe the bidirectional relationship between sleep disturbance and fibromyalgia. What treatment approaches have shown promise for addressing this comorbidity?
- Why might post-COVID syndrome patients benefit from comprehensive pain assessment, and what biofeedback interventions might be relevant for this population?
Myofascial Pain: Understanding Trigger Points
Myofascial pain syndrome (MPS) is a regional musculoskeletal condition characterized by the presence of trigger points, which are hyperirritable regions of taut bands of skeletal muscle in the muscle belly or associated fascia. Pressure on these areas is painful, and they can produce referred pain and tenderness, motor dysfunction, and autonomic changes.
Travell and Simons (1992) created the definitive trigger point reference. Trigger points are associated with palpable nodules in taut bands of muscle fibers and produce local and referred pain.
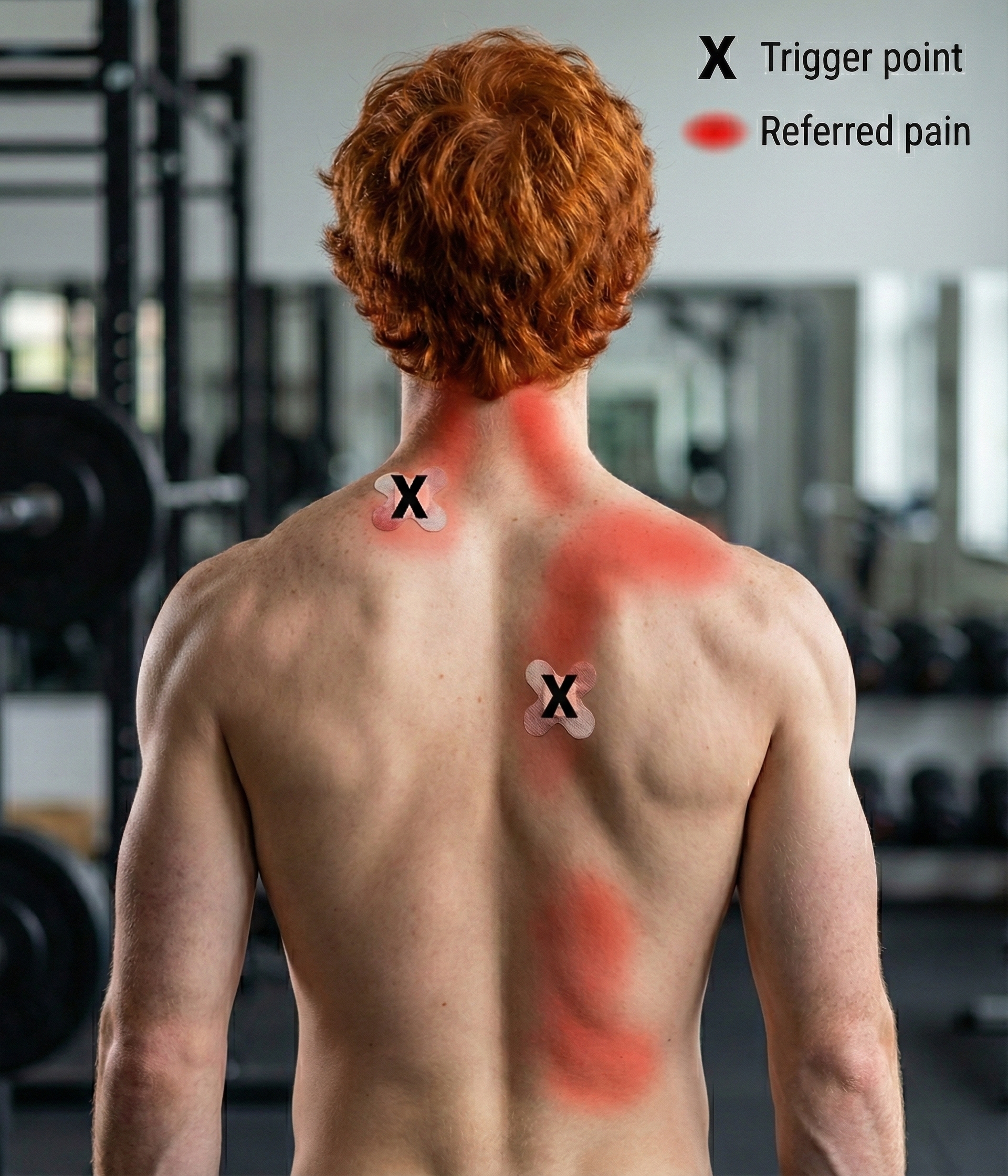
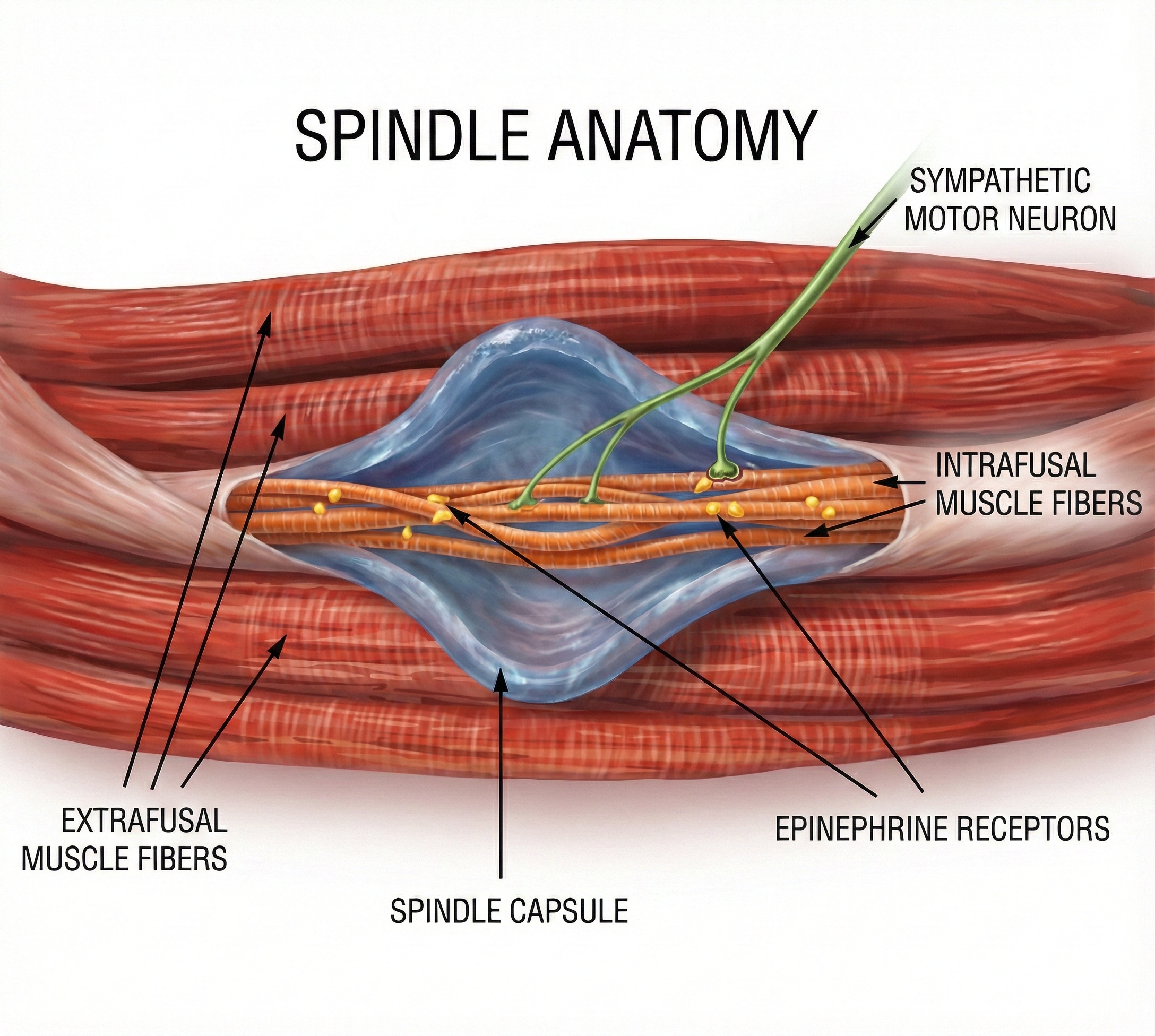
Gevirtz (2013) hypothesizes that SNS innervation of muscles spindles may result in trigger points.
Clinical Efficacy
Sherman, Tan, and Wei (2016) rated biofeedback for myofascial pain syndrome as level 2 possibly efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (3rd ed.).
Chronic Neck Pain
Chronic neck pain exemplifies the multifactorial nature of pain conditions and illustrates how biofeedback fits within comprehensive treatment. Mechanical or musculoskeletal factors include cervical spondylosis (age-related wear) and cervical degenerative disc disease. Poor posture—especially related to workplace ergonomics or prolonged use of digital devices (commonly termed "text neck")—has become increasingly prevalent in modern clinical populations.
Psychological factors including stress, anxiety, and depression have been associated with chronic neck pain. Studies suggest that these factors contribute to both the onset and persistence of neck pain (Shahidi et al., 2017)—making psychological intervention, including biofeedback, directly relevant rather than merely supportive.
Accidents or injuries, particularly whiplash from motor vehicle accidents, can lead to chronic neck pain. In whiplash, soft tissues in the neck are strained or torn (Spitzer et al., 1995), but psychological factors often determine whether acute injury becomes chronic disability.
Research Evidence
Hallman and colleagues (2011) conducted a study examining the impact of HRVB training on chronic neck pain in stressed patients. The results showed that the group receiving HRVB training reported significant improvements in pain, vitality, and social functioning compared to the no-treatment control group. This multi-domain improvement supports the Hubbard-Gevirtz model's prediction that addressing autonomic dysregulation should affect both physical and functional outcomes.
Clinical Efficacy Rating
Based on five RCTs, Rosenthal (2023) rated biofeedback for chronic neck pain as level 4, efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). This higher rating compared to fibromyalgia suggests that conditions with clearer local pathology may respond more consistently to biofeedback intervention.
Fibromyalgia and chronic myofascial pain involve distinct pathophysiology but may both benefit from autonomic interventions. FM involves central sensitization without identifiable tissue damage, while myofascial pain involves localized trigger points with clear local pathology. The Hubbard-Gevirtz model proposes that sympathetically-mediated muscle spindle spasm contributes to trigger point development—a mechanism that HRVB may interrupt through accentuated antagonism. HRV biofeedback is rated Level 3 for fibromyalgia and Level 4 for chronic neck pain, suggesting that conditions with more localized pathology may respond more consistently to treatment.
Comprehension Questions: Pain Conditions
- What distinguishes tender points in fibromyalgia from trigger points in myofascial pain syndrome, and why does this distinction matter for treatment selection?
- According to the Hubbard-Gevirtz model, what is the proposed mechanism for trigger point development, and how might HRVB interrupt this pathway?
- Why might HRV biofeedback be helpful for chronic pain conditions based on the autonomic findings reviewed (e.g., Meeus et al., 2013)?
- What psychological factors have been associated with chronic neck pain, and how does this support biofeedback's role in comprehensive treatment?
Cardiovascular Disorders
A sobering reality confronts anyone who thinks heart health is someone else's problem: O'Hearn and colleagues (2022) reported that cardiometabolic health among American adults actually declined between 1999 and 2018. By the end of that period, fewer than 7% of US adults qualified as being in optimal cardiometabolic shape. Part of what drove those dismal numbers was a major 2017 guideline change: normal blood pressure is now defined as below 120/80 mmHg, down from the previous threshold of 140/90 mmHg. That single diagnostic shift instantly expanded the population eligible for behavioral and medical intervention by tens of millions of people.
The way clinicians think about treating cardiovascular disorders has undergone a fundamental shift. The older model focused almost exclusively on calming down an overactive sympathetic nervous system—as if the only problem were a car with a stuck accelerator. The newer model recognizes that many cardiovascular patients also have "bad brakes," meaning their parasympathetic system is not providing enough counterbalance. Instead of just reducing sympathetic overdrive, clinicians now increasingly explore interventions that boost vagal tone (the strength of the parasympathetic influence on the heart) and increase heart rate variability. This dual approach—addressing both the accelerator and the brakes—represents a more complete strategy for restoring cardiovascular balance.
A landmark 2020 systematic review and meta-analysis by Lehrer and colleagues examined 58 randomized controlled trials of HRV biofeedback across a wide range of conditions. The verdict? Small-to-moderate effect sizes favoring HRV biofeedback for both cardiovascular and psychological outcomes, with particularly robust effects for anxiety, depression, and anger regulation (Lehrer et al., 2020). For many patients, the most evidence-supported cardiovascular interventions include weight loss, stress management training, and surface electromyographic (SEMG) and temperature biofeedback—often used in combination.
🎧 Mini-Lecture: Cardiovascular Interventions Overview
Heart Failure and Coronary Artery Disease: Restoring Balance
Heart failure and coronary artery disease represent some of the most serious cardiovascular conditions, affecting millions and often limiting both quality and length of life. Can HRV biofeedback help these patients? An emerging body of research suggests it can, though the evidence is still developing.
🎧 Mini-Lecture: Heart Failure and Coronary Artery Disease
To understand why HRV biofeedback might help, you first need to understand these conditions. Heart failure occurs when one or both of the ventricles (the heart's lower chambers, which do the heavy lifting of pumping blood) cannot keep up with the body's demands. Either cardiac output becomes insufficient to properly perfuse (deliver blood to) the tissues, or the left ventricle cannot fill properly, causing pressure to back up into the lungs (Huether et al., 2020). When the left ventricle fails, blood backs up into the lungs, causing shortness of breath and fatigue. When the right ventricle fails, blood backs up into the body, causing fluid to accumulate in the legs (edema) and abdomen. Many patients eventually develop failure of both sides.
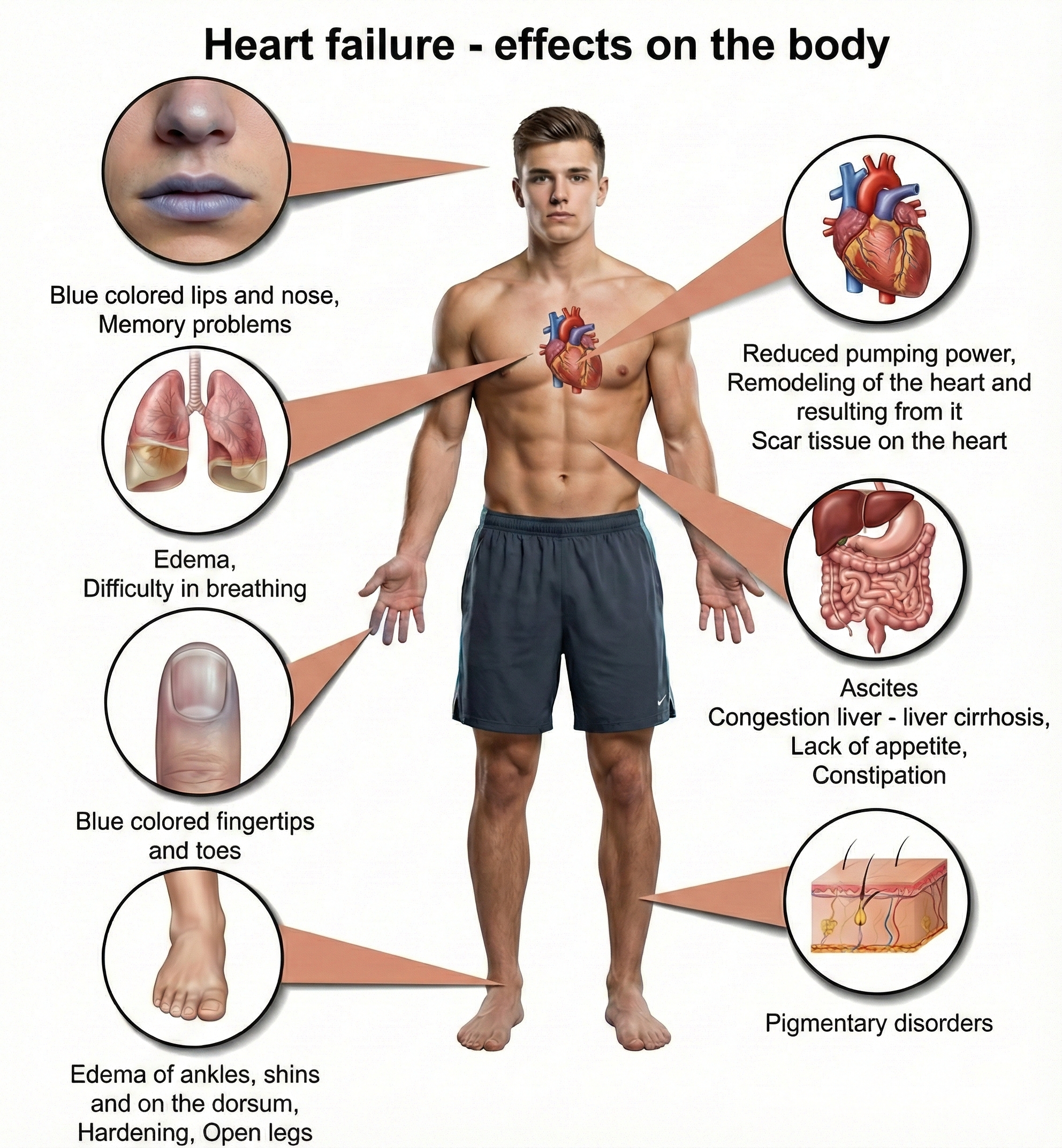
Here is where the story connects to biofeedback: a critical driver of heart failure progression is autonomic nervous system dysregulation (Beghini et al., 2024). In a healthy cardiovascular system, the sympathetic and parasympathetic branches work in dynamic balance, adjusting cardiac output moment-to-moment to match the body's needs. In heart failure, this balance breaks down. Patients develop sympathetic overdrive, meaning sustained overactivation of the fight-or-flight response even at rest, combined with parasympathetic withdrawal, a reduction in the calming vagal influence on the heart.
This autonomic imbalance is not just a symptom; it actively worsens the disease. Chronic sympathetic overdrive promotes adverse cardiac remodeling (structural changes to the heart muscle that reduce function) and metabolic dysfunction. Clinicians have long known that patients with the highest levels of neurohumoral activation, estimated by measuring plasma norepinephrine levels, have the worst prognosis. This understanding provides a strong rationale for HRV biofeedback: if you can restore autonomic balance, you may be able to slow or even partially reverse heart failure progression.
Coronary artery disease (CAD) involves a different but related problem: reduced blood flow through the coronary arteries that supply the heart muscle itself. The usual culprit is atheromas, deposits of lipid-containing plaques that build up on the inner walls of arteries, gradually narrowing the channel through which blood flows. As long as the narrowing is partial, the patient may have no symptoms at rest but experience chest pain (angina) during exertion when the heart needs more oxygen than the narrowed arteries can deliver.
The danger comes when an atheroma's fibrous outer layer ruptures. The body responds to this rupture as it would to any wound, initiating a clotting cascade that can form a thrombus (blood clot). If that clot completely blocks a coronary artery, the result is a myocardial infarction (MI), commonly called a heart attack, meaning death of heart muscle tissue downstream from the blockage. Like heart failure, CAD is associated with autonomic dysregulation, making HRV biofeedback a logical intervention target.
How Common Is Heart Failure?
Heart failure is alarmingly common and getting worse. Currently, about 6.7 million Americans over age 20 live with heart failure, and projections suggest this number will rise to 8.7 million by 2030 (Bozkurt et al., 2024). The lifetime risk, meaning the probability of developing the condition at some point during one's life, has increased to 24%. That means roughly 1 in 4 people will eventually develop heart failure.
Perhaps most concerning, the proportion of younger patients (ages 35-64) is increasing, and mortality rates have been rising since 2012 with a sharp acceleration during 2020-2021. Heart failure remains the leading cause of hospitalization for patients over 65. These statistics underscore the urgent need for effective interventions at all stages of the disease, from prevention to advanced heart failure management.
Biofeedback Research for Heart Failure and CAD
HRV biofeedback may improve cardiac function by restoring autonomic balance (Gevirtz, 2013). This represents a paradigm shift from the earlier model of simply reducing SNS activation to actively restoring balance between sympathetic and parasympathetic branches (Moravec & McKee, 2013). Decreased HRV is a significant independent risk factor for cardiac patient morbidity and mortality, making interventions that restore HRV clinically valuable.
Emerging evidence suggests that GLP-1 receptor agonists may complement biofeedback approaches in heart failure management. The landmark SELECT trial demonstrated that semaglutide reduced major adverse cardiovascular events by 20% in patients with obesity and established cardiovascular disease, with benefits extending to those with heart failure (Lincoff et al., 2023).
A prespecified analysis found that among patients with heart failure at enrollment, semaglutide reduced the composite heart failure endpoint by 21% compared to placebo, with benefits observed in both heart failure with preserved ejection fraction and heart failure with reduced ejection fraction (Deanfield et al., 2024). The mechanisms underlying these benefits likely include weight reduction, blood pressure lowering, anti-inflammatory effects, and improved metabolic function, all of which address key pathways contributing to heart failure progression.
Exercise training also plays a crucial role in heart failure rehabilitation. A 2024 systematic review and meta-analysis found that exercise training significantly improves peak oxygen consumption in patients with heart failure with preserved ejection fraction by approximately 2.0 mL/kg/min, along with meaningful improvements in quality of life (Baral et al., 2024).
A 2025 multicenter randomized trial of 322 patients found that combined endurance and resistance training over 12 months produced clinically meaningful improvements, with 20.5% of exercising patients showing improvement on a composite outcome compared to only 8.1% of usual care controls (Edelmann et al., 2025). These findings support integrating structured exercise programs with HRV biofeedback training for heart failure patients.
Del Pozo, Gevirtz, Scher, and Guarneri (2004) randomly assigned 63 CAD patients to traditional cardiac rehabilitation or six biofeedback sessions including abdominal breathing training and cardiorespiratory biofeedback. The biofeedback group increased HRV from baseline (mean SDNN rose from 28 to 42 ms) while controls deteriorated, demonstrating that CAD patients can meaningfully increase HRV through training.
Lin and colleagues (2015) randomly assigned 154 CAD patients to HRV biofeedback plus standard care or standard care alone. The biofeedback group showed significantly increased low-frequency HRV and SDNN along with decreased hostility. A landmark 2018 follow-up tracked 210 patients for one year and found dramatically fewer hospital readmissions (12% versus 25%) and emergency visits (13% versus 36%) in the biofeedback group. These findings provide compelling evidence that HRV biofeedback may improve cardiovascular prognosis (Lin et al., 2018).
Swanson and colleagues (2009) found that HRV biofeedback increased exercise tolerance in heart failure patients with a left ventricular ejection fraction (LVEF) of 31% or higher, suggesting the intervention may be most beneficial for patients with moderately reduced cardiac function.
Clinical Efficacy Rating
Christine Moravec and Michael McKee (2016) initially rated biofeedback for heart failure and coronary artery disease as level 2: possibly efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (3rd ed.). However, the field has advanced considerably since that rating. Subsequent RCTs by Lin and colleagues (2015, 2018) have substantially strengthened the evidence base, demonstrating not only short-term autonomic improvements but also meaningful one-year cardiovascular prognosis benefits including reduced hospitalizations and emergency visits. These newer findings suggest the evidence may now support a higher efficacy rating, though formal reassessment awaits future editions of the efficacy guidelines.
Heart failure affects 6.7 million Americans, with a lifetime risk of 24%, meaning roughly 1 in 4 people will develop this condition. The disease is driven in large part by autonomic dysregulation: chronic sympathetic overdrive combined with parasympathetic withdrawal. This creates a vicious cycle where autonomic imbalance promotes adverse cardiac remodeling, which further worsens autonomic function. HRV biofeedback represents a paradigm shift toward actively restoring autonomic balance rather than simply suppressing sympathetic activation.
The research is encouraging: Del Pozo et al. (2004) showed that CAD patients can increase their SDNN from 28 to 42 ms through cardiorespiratory biofeedback. Lin et al. (2018) demonstrated that HRV biofeedback cut hospital readmissions roughly in half at one-year follow-up. Swanson et al. (2009) found improved exercise tolerance in heart failure patients with LVEF of 31% or higher, suggesting the intervention works best for those with moderately impaired rather than severely impaired cardiac function.
Check Your Understanding
- Explain how autonomic dysregulation contributes to heart failure progression. Why is this relationship described as a "vicious cycle"?
- Why is decreased HRV considered a significant independent risk factor in cardiac patients, and what does this suggest about the potential value of HRV biofeedback?
- What specific HRV improvements did Del Pozo et al. (2004) observe in CAD patients who received cardiorespiratory biofeedback, and why was the comparison to the control group particularly striking?
- Based on Swanson et al. (2009), which heart failure patients appear most likely to benefit from HRV biofeedback, and what might explain this pattern?
Essential Hypertension: When Blood Pressure Stays Too High

Understanding How Blood Pressure Works
Before diving into what goes wrong in hypertension, you need to understand the basic physics of blood pressure. Think of your cardiovascular system as a sophisticated plumbing network with a pump (your heart) pushing fluid (blood) through a complex system of pipes (blood vessels). Blood pressure (BP) is determined by two main factors: how hard the pump is working and how much the pipes resist the flow.
In physiological terms, BP equals the product of cardiac output (the amount of blood the heart pumps each minute) multiplied by systemic vascular resistance (how much the blood vessels resist blood flow). Systemic vascular resistance depends on three things: blood viscosity (how thick or "syrupy" the blood is), total blood vessel length, and most critically, the radius of the blood vessels.
Cardiac output is itself determined by two factors: stroke volume (how much blood the heart ejects with each beat) and stroke rate (how many times per minute the heart beats). Here is an everyday analogy: imagine a garden hose connected to an outdoor faucet. You can increase the water pressure coming out of the hose in two ways. You can turn up the faucet to push more water through (analogous to increasing cardiac output), or you can partially pinch the hose opening to create more resistance (analogous to increasing vascular resistance). Your cardiovascular system works the same way, but the "pinching" happens at thousands of tiny vessels throughout your body.
The real workhorses of blood pressure control are your arterioles, tiny arteries (8 to 50 microns in diameter, roughly the width of a human hair) scattered throughout your body. These small vessels are remarkably powerful regulators because of a principle from physics: resistance to flow increases exponentially as a tube's diameter decreases.
A seemingly minor adjustment in arteriole diameter can dramatically change systemic vascular resistance, which in turn shifts blood pressure and tissue perfusion (Tortora & Derrickson, 2021). This is why chronic tension in the smooth muscle lining these tiny vessels can lead to sustained high blood pressure, and why relaxation techniques that reduce that tension can be so effective.
🎧 Mini-Lecture: Blood Pressure and Peripheral Resistance
What Counts as Hypertension?
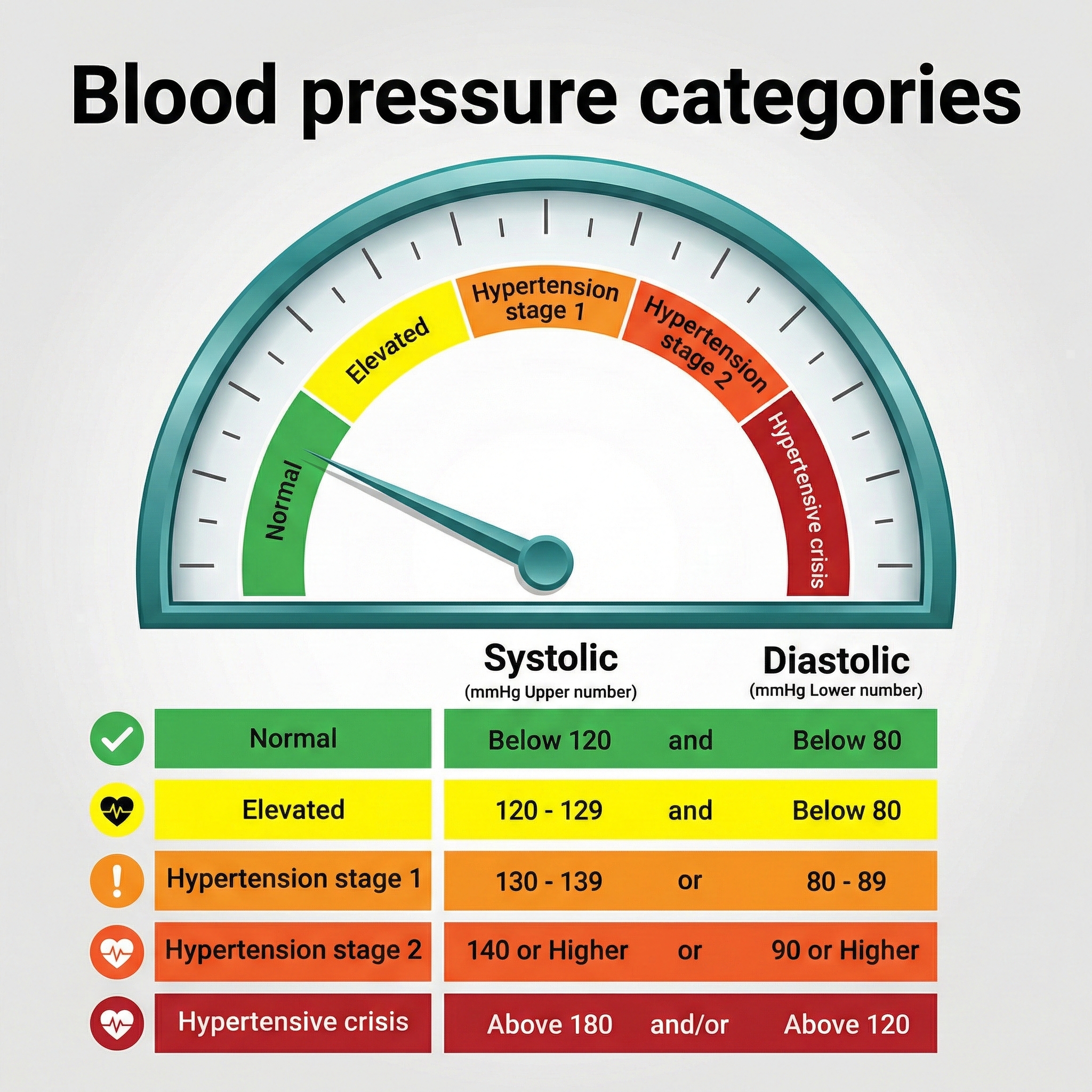
The goalposts for what counts as "high blood pressure" moved significantly in 2017, and understanding those new thresholds matters for clinical practice. Stage 1 hypertension is now defined as a systolic BP (SBP, the top number when blood pressure is measured) of 130 mmHg or higher and/or a diastolic BP (DBP, the bottom number) of 80 mmHg or higher.
This redefinition by the 2017 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (Whelton et al., 2017) was not just an academic exercise. Under the old 2003 National Heart, Lung, and Blood Institute (NHLBI) guidelines, about 72 million Americans were classified as hypertensive. Under the 2017 guidelines, that number jumped to 103 million. In practical terms, 50% of American men and 38% of women now meet criteria for hypertension. If you are doing cardiovascular biofeedback, your potential client base just expanded dramatically.
Why did the guidelines change? The NIH Systolic Blood Pressure Intervention Trial (SPRINT) provided compelling evidence that lower is better, at least for high-risk patients. This landmark study found that treating hypertensive patients 50 years or older to a SBP target of 120 mmHg reduced cardiovascular events by 30% and all-cause mortality by nearly 25%, compared to the traditional target of 140 mmHg (Medscape, 2015). Those are substantial benefits that justify the more aggressive treatment thresholds.

However, the story is not as simple as "lower is always better." McEvoy and colleagues (2016) sounded an important warning: when aggressively lowering SBP below 140 mmHg, clinicians should not to let DBP fall below 70 mmHg, and especially not below 60 mmHg.
The danger is that very low diastolic pressure can threaten blood delivery to the heart itself, since the coronary arteries primarily fill during diastole (when the heart is relaxing). In their study, low DBP values were independently associated with progressive heart damage, coronary heart disease, and death.
The clinical takeaway? Aim for the sweet spot: low enough to protect against stroke and heart attack, but not so low that you starve the heart of blood.
Who Gets Hypertension?
Hypertension is so common that calling it an "epidemic" barely captures the scope. Nearly half of all American adults (47.7%) have hypertension, and the rates climb steeply with age: 23.4% of those aged 18-39, 52.5% of those 40-59, and a striking 71.6% of adults 60 and older (Fryar et al., 2024).
Men (50.8%) have slightly higher prevalence than women (44.6%), though this gap narrows after menopause. Racial disparities are substantial: Black Americans have the highest prevalence of any racial or ethnic group at approximately 46%, compared to 39% for White Americans (Fan et al., 2023; Siddiqui et al., 2024). People with diabetes face elevated rates as well.
Perhaps the most alarming statistic is the treatment gap: only about 21% of hypertensive adults have their blood pressure controlled below 130/80 mmHg (Fryar et al., 2024). Without lifestyle changes or drug intervention, patients with elevated BP frequently progress to full-blown hypertension (Huether et al., 2020). While elevated BP is often called a "silent killer" because it produces no obvious symptoms, some patients do experience signs like blurred vision and swelling ankles (edema).
Primary Versus Secondary Hypertension
When clinicians talk about hypertension, they distinguish between two fundamentally different categories. The vast majority, about 92 to 95% of all cases, are classified as primary hypertension (also called essential hypertension). Primary hypertension means chronically elevated BP that cannot be traced to any single identifiable cause (Huether et al., 2020). Instead, it emerges from a complex interplay of genetic predispositions, lifestyle factors, and physiological processes that we will explore shortly.
The remaining 5% of cases are classified as secondary hypertension, which means the elevated BP has a specific, identifiable cause like kidney disease, adrenal gland tumors, or obstructive sleep apnea (Huether et al., 2020). Secondary hypertension is important to identify because treating the underlying cause may resolve the high blood pressure entirely. For biofeedback practitioners, the key point is that primary hypertension, with its multiple contributing factors and no single "cure," is where behavioral interventions can make the biggest difference.
What Goes Wrong in Essential Hypertension?
Essential hypertension is not a single disease but rather a heterogeneous disorder, meaning different patients develop it through different pathways involving various combinations of genetic and environmental factors. What these pathways share is that they ultimately increase either circulating blood volume, peripheral vascular resistance, or both.
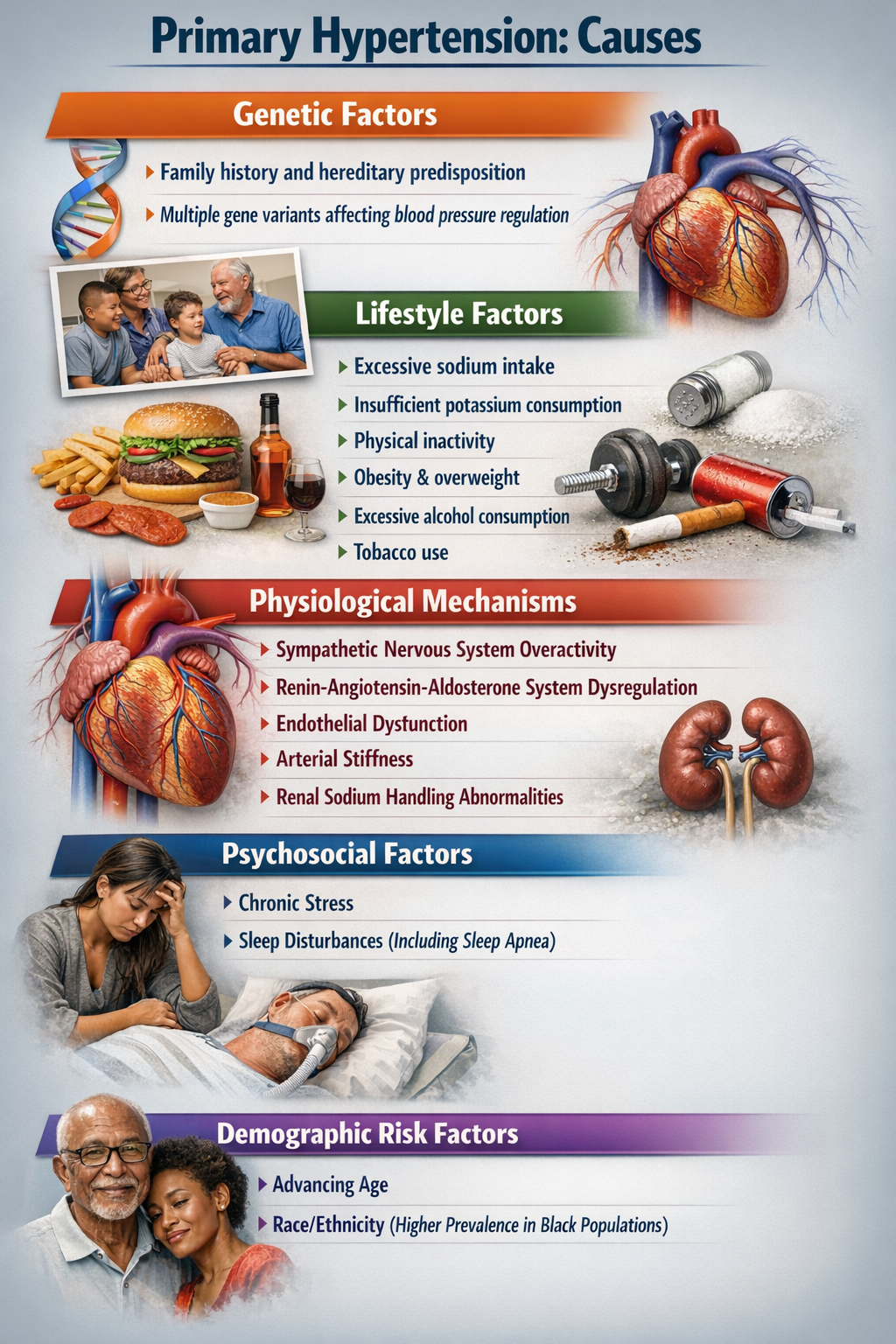
Current research increasingly points to autonomic dysregulation as a central driver in many patients. This dysregulation involves the sympathetic nervous system (your "fight or flight" branch) being chronically overactive while the parasympathetic system (your "rest and digest" branch) is underactive (Harrison et al., 2021). The sympathetic branch increases heart rate and cardiac contractility while constricting blood vessels, all of which raise blood pressure. Normally, parasympathetic activity counterbalances these effects, but in essential hypertension, that balance is disrupted. This insight has direct clinical implications: it explains why biofeedback interventions that restore parasympathetic tone can effectively lower blood pressure.
🎧 Mini-Lecture: Primary Hypertension Mechanisms
Hypertension Symptoms
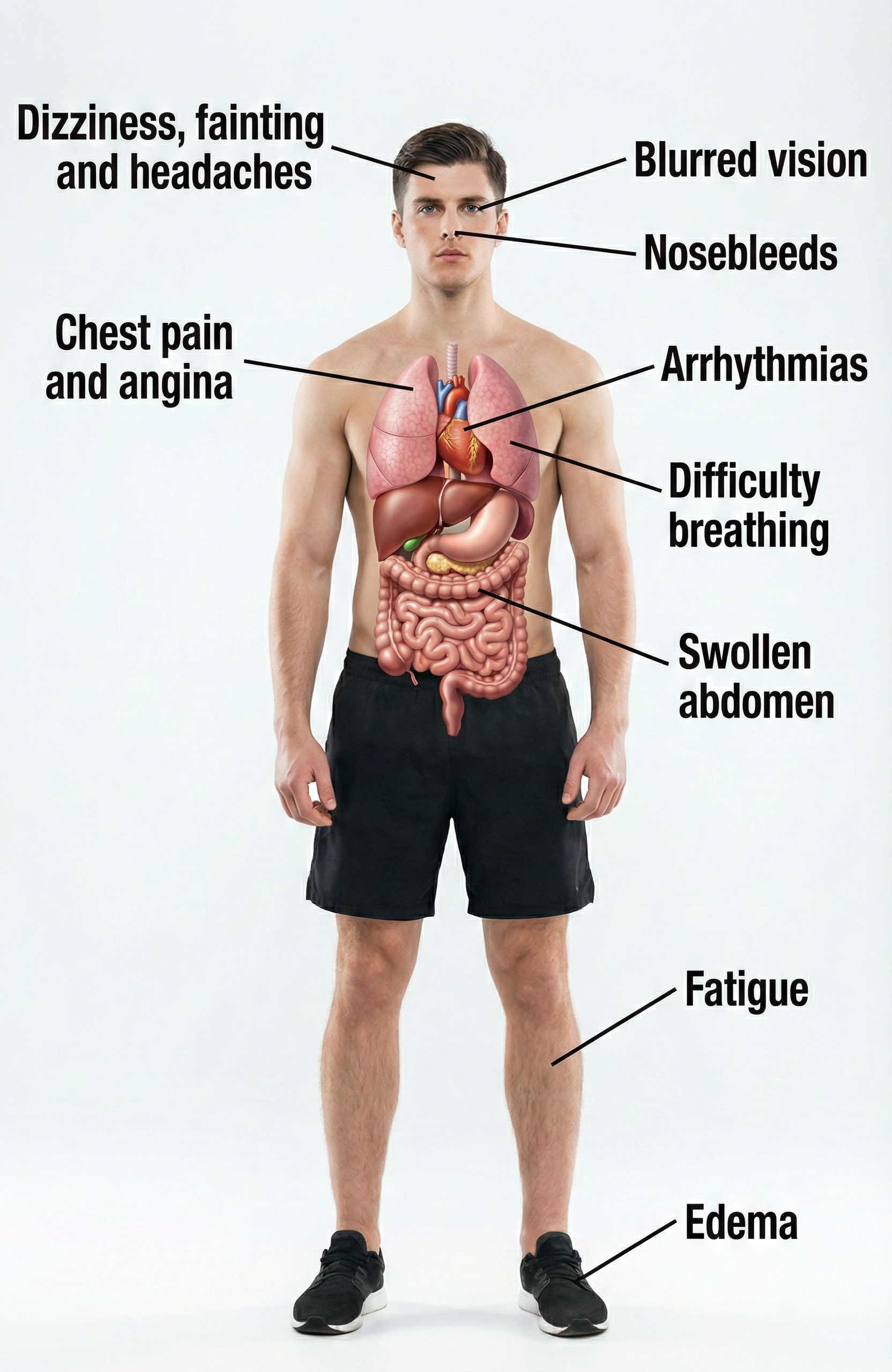
While elevated BP can be "silent," diverse symptoms like blurred vision and swelling ankles (edema) may accompany this chronic health condition.
Hypertension Complications
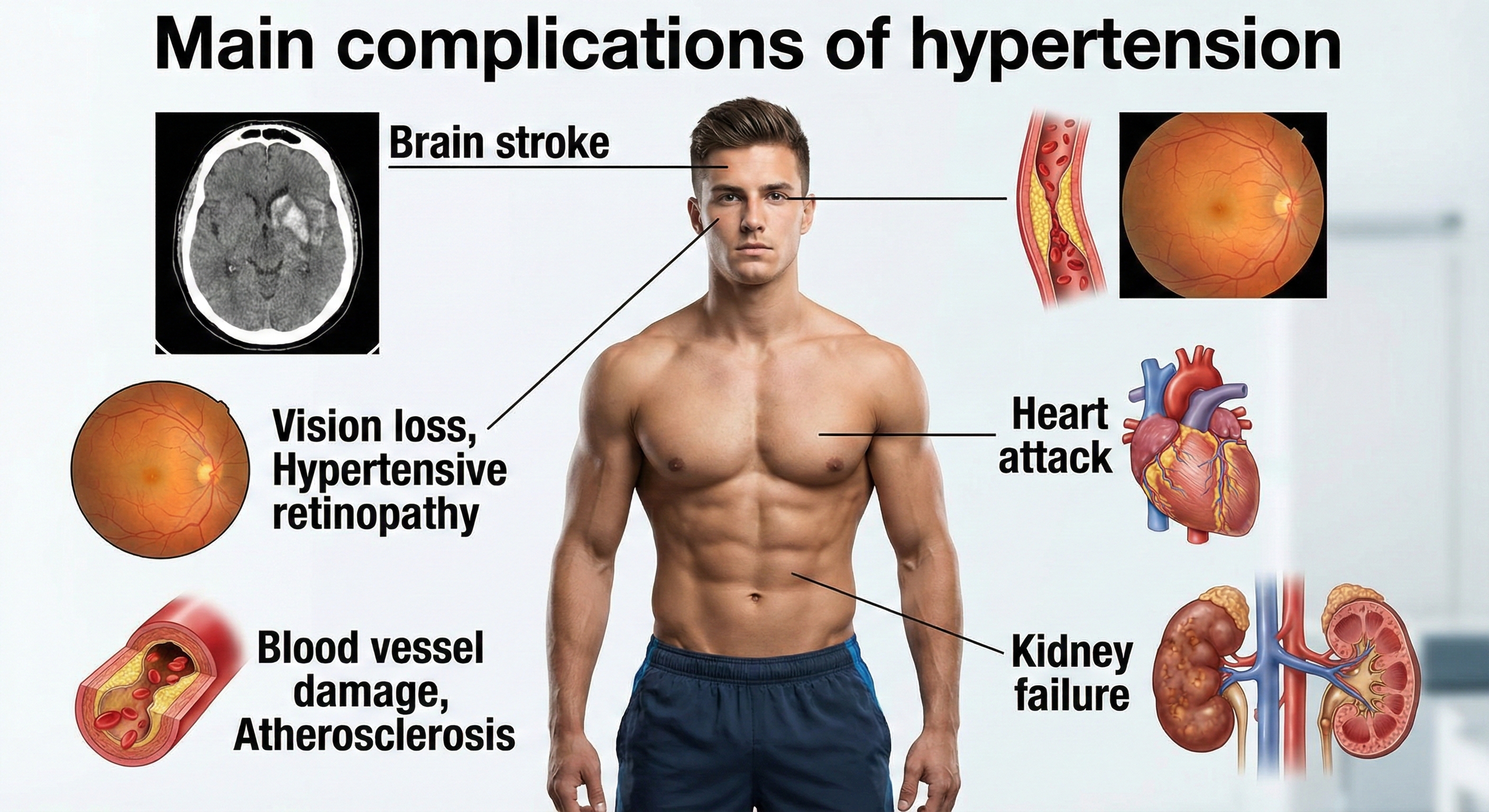
Researchers have reported that slight blood pressure elevations in middle age are associated with a 30% greater risk of dementia or cognitive impairment in two decades. Medication that lowers BP can reduce this risk (Hughes et al., 2020).
🎧 Mini-Lecture: Hypertension Symptoms
Three major mechanisms contribute to essential hypertension at the physiological level.
First, increased salt absorption leads to volume expansion, meaning more fluid in the blood vessels and higher pressure against vessel walls.
Second, an impaired response of the renin-angiotensin-aldosterone system (RAAS) disrupts normal blood pressure regulation.
The RAAS is a hormone system that acts like a thermostat for blood pressure and fluid balance. When activated, it triggers blood vessel constriction and signals the kidneys to retain sodium and water, both of which raise blood pressure. In essential hypertension, this system often remains chronically overactive even when blood pressure is already elevated.
Third, increased sympathetic nervous system activation directly raises heart rate and vascular resistance (Iqbal & Jamal, 2023).
Exciting new research also implicates the gut microbiome, the trillions of microorganisms living in your intestines, as a potential player. High-salt diets appear to alter the composition of gut bacteria in ways that promote inflammation and hypertension, opening a fascinating new avenue for future interventions (Wei et al., 2022).
Beyond physiology, demographic factors like age, gender, and ethnicity, along with psychological factors including depression, anger, and anxiety, all influence blood pressure (McGrady & Moss, 2013). Twin studies and the landmark Framingham Heart Study suggest that the heritable component of BP ranges from 33 to 57% (Madhur, 2014).
In other words, genes load the gun, but lifestyle pulls the trigger. This understanding of autonomic imbalance provides the scientific rationale for why biofeedback interventions that restore parasympathetic tone can effectively lower blood pressure in many patients.
Lifestyle Changes That Make a Difference
Before reaching for biofeedback equipment, it is worth noting that some of the most powerful blood pressure interventions involve no technology at all.
Potential SBP reductions from lifestyle changes vary by individual, but weight loss consistently emerges as the most effective lifestyle modification. This makes sense when you consider that obesity raises blood pressure through multiple pathways: it increases blood volume, promotes sympathetic activation, contributes to insulin resistance, and strains the heart.
Addressing obesity attacks the problem from several angles simultaneously.
Salt sensitivity is another important individual factor. About 60% of hypertensive patients and 25% of normotensive individuals are salt-sensitive, meaning their blood pressure rises significantly when they consume salt. Interestingly, biology is not uniform: 4 to 5% of individuals actually experience lower BP when they ingest salt (Harvard Heart Letter, August 2019).
Stress management deserves special mention because it represents a natural bridge to biofeedback. Chronic stress contributes to hypertension through sustained sympathetic activation and elevated cortisol, making relaxation training a logical complement to other lifestyle changes.
🎧 Mini-Lecture: Steps to Reduce Hypertension
Drug Treatments for Hypertension
As a biofeedback practitioner, you will often work with clients already taking antihypertensive medications. Understanding how these drugs work helps you anticipate their effects on biofeedback measurements and coordinate care with prescribing physicians. The main drug classes target different parts of the blood pressure equation.
Diuretics (sometimes called "water pills") reduce blood volume by causing the kidneys to excrete more water and salt in urine, essentially reducing the amount of fluid the heart has to pump.
ACE inhibitors block the formation of angiotensin II, a powerful vasoconstrictor in the RAAS system. By blocking this hormone, ACE inhibitors cause blood vessels to relax (vasodilation, which reduces systemic vascular resistance) and reduce aldosterone secretion (which decreases sodium and water retention).
Beta-blockers work on the heart side of the equation: they inhibit renin secretion and decrease both heart rate and the force of cardiac contraction, reducing cardiac output.
Finally, calcium channel blockers slow the entry of calcium ions (Ca2+) into heart muscle cells and smooth muscle in blood vessel walls. Less calcium means weaker contractions, reducing both the heart's workload and vascular resistance.
A newer class of medications deserves attention from biofeedback practitioners: GLP-1 receptor agonists (GLP-1 RAs). These drugs, originally developed for diabetes management, have demonstrated significant blood pressure-lowering effects.
GLP-1, or glucagon-like peptide-1, is a naturally occurring hormone released by intestinal cells after eating that regulates appetite, blood sugar, and cardiovascular function. GLP-1 receptor agonists mimic this hormone's effects. A 2024 individual patient data meta-analysis found that semaglutide, a commonly prescribed GLP-1 RA, reduces systolic blood pressure by approximately 4.8 mmHg, with much of this effect mediated through weight loss (Kennedy et al., 2024).
Tirzepatide, a newer dual-action medication that activates both GLP-1 and GIP receptors, produces even larger blood pressure reductions of 7 to 11 mmHg in patients with obesity (de Lemos et al., 2024). For biofeedback practitioners, these medications represent both an opportunity and a consideration: clients on GLP-1 RAs may show improved baseline blood pressure readings, and the combined effects of medication, weight loss, and biofeedback training could produce synergistic benefits.
A crucial clinical point: biofeedback interventions can interact synergistically with anti-hypertensive medication, sometimes producing larger BP reductions than either approach alone. This is generally good news, but it means patients must monitor their BP daily and discuss medication adjustments with their physician before making changes independently. The last thing you want is a client becoming hypotensive (blood pressure that is too low) because their combined interventions are working too well.
Richard's Journey: A Pathways Model Intervention
Richard, a 45-year-old mildly overweight Caucasian male, had been living with stage 1 hypertension for a decade. His blood pressure was well controlled using a combination of an ACE inhibitor and beta-blocker, but he wanted to reduce his dependence on medication. His treatment followed a tiered approach, starting with the least intensive interventions and adding components as needed.
His Level One interventions focused on self-monitoring and basic lifestyle adjustments: tracking his daily steps and gradually building up to 10,000 per day, logging his alcohol and food intake, weighing himself weekly, recording his morning BP readings, and taking a movement break every hour during work.
After one month without significant BP improvement, Richard moved to Level Two interventions that added skill-building components. He recruited an exercise partner to walk with him for at least 35 minutes five times per week (leveraging social support to boost motivation) and began practicing slow abdominal breathing using the Optimal HRV smartphone application. He also added a new Level One intervention: listening to classical music for at least 30 minutes daily at home.
Two months in, his blood pressure was gradually declining, but his weight remained stubbornly stable. Richard added another Level One intervention by using an activity tracker to monitor his sleep and adjusted his work schedule to ensure 8 hours nightly. His single Level Three intervention involved consulting twice with a licensed dietician to overhaul his eating patterns. The dietician guided him toward a DASH diet, reducing saturated fats and simple carbohydrates.
By the 6-month mark, the results were impressive: improved diet, better sleep, more energy, and blood pressure trending toward normal. His physician encouraged him to discontinue the beta-blocker and halve his ACE inhibitor dosage while continuing daily BP monitoring. At the end of 6 months, Richard's BP had fallen to the normotensive range, his blood lipids were normal, and he had lost 15 pounds. At one-year follow-up, his BP remained well controlled on reduced medication. He had regained about half the lost weight, but his sleep quality remained excellent and his DASH diet was now a habit.
Richard's case illustrates several key principles. First, biofeedback success often depends on the foundation provided by Level One and Level Two interventions; the HRV biofeedback training was not a magic bullet but one component of a comprehensive program. Second, self-monitoring of BP, diet, activity, sleep, and weight helped him develop the mindfulness needed to sustain behavior change. Third, social support matters: having an exercise partner made him more likely to actually exercise. Finally, he ensured safety by partnering with his physician to taper medication gradually rather than making changes on his own.
Six Factors That Predict Successful Blood Pressure Reduction
Not all hypertension treatment programs are created equal. In a landmark analysis, McGrady (1996) identified six specific factors that separated the efficacy studies reporting the largest and most consistent BP reductions from less successful attempts.

These factors serve as a practical checklist when designing interventions.
🎧 Mini-Lecture: Six Evidence-Based Factors for Blood Pressure Reduction
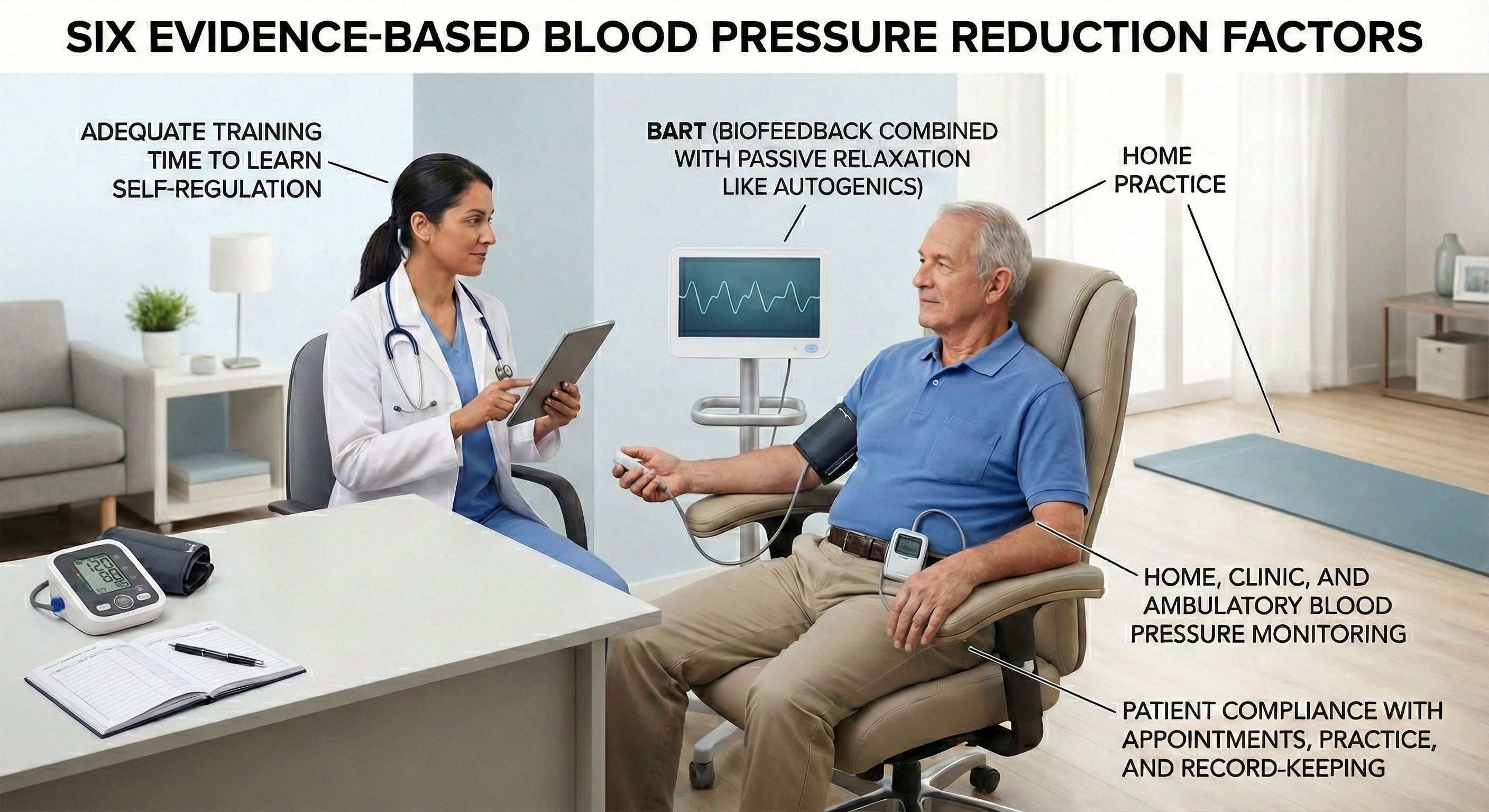
Who Responds Best to Biofeedback?
Biofeedback works better for some hypertensive patients than others, and research has identified predictors of treatment response. McGrady and Higgins (1989) and Weaver and McGrady (1995) studied unmedicated white adult males with mild to moderate hypertension who received a comprehensive treatment package: SEMG and temperature biofeedback, autogenic training, home practice, and BP monitoring.
The patients who showed the greatest response shared a physiological profile suggesting chronic sympathetic overactivation: elevated resting heart rate, cool hands (indicating peripheral vasoconstriction), high SEMG readings (reflecting muscle tension), and elevated plasma renin activity (a marker of RAAS activation).
The best predictors of how much BP actually decreased were high heart rate, cool hands, high trait anxiety, and high-normal cortisol. These findings make intuitive sense: patients whose hypertension is driven primarily by sympathetic overactivation are the ones most likely to benefit from interventions that reduce that activation.
A multi-modal strategy that combines biofeedback, relaxation training, and medical management appears most effective for patients whose BP shows marked reactivity to stressors (Frank et al., 2010). If a patient's blood pressure spikes when they are stressed but normalizes when they are calm, they are likely a good candidate for biofeedback intervention.
Multiple Pathways to Lower Blood Pressure
Because BP is determined by multiple factors (cardiac output, vascular resistance, blood volume), patients can lower their blood pressure through several different biological pathways. This is actually good news clinically: if one pathway is not available or responsive in a particular patient, others may still be trainable.
The main pathways include reducing cardiac output, lowering stress hormones like cortisol and norepinephrine, decreasing skeletal muscle tension (which affects vascular resistance), reducing systemic vascular resistance directly through vasodilation, and stimulating the baroreflex (a feedback loop that senses BP changes and adjusts heart rate accordingly).
Different biofeedback modalities appear to work through different mechanisms. Temperature biofeedback, which trains patients to warm their hands by dilating peripheral blood vessels, may reduce systemic vascular resistance and decrease plasma norepinephrine levels (McCoy et al., 1988).
Relaxation training appears to reduce cardiac output (Gervino & Veazey, 1984), while deep muscle relaxation may lower both cardiac output and plasma norepinephrine. The multi-modal therapy used by McGrady and colleagues, combining multiple biofeedback modalities with relaxation techniques, reduced plasma cortisol levels (McGrady et al., 1987), suggesting stress hormone reduction as another active mechanism.
Aerobic exercise represents another powerful, evidence-based pathway for blood pressure reduction that complements biofeedback training. A 2024 dose-response meta-analysis of 34 trials found that aerobic exercise reduces blood pressure in a dose-dependent manner, with the greatest reductions occurring at 150 minutes per week, producing SBP decreases of 7.2 mmHg and DBP decreases of 5.6 mmHg (Jabbarzadeh Ganjeh et al., 2024).
A comprehensive 2023 network meta-analysis of 270 randomized controlled trials found that isometric exercise training, surprisingly, produces the largest blood pressure reductions of all exercise modalities, with SBP decreases of 8.2 mmHg and DBP decreases of 4.0 mmHg (Edwards et al., 2023). These findings suggest that combining biofeedback with structured exercise programs may produce additive blood pressure benefits, particularly when exercise prescriptions are individualized based on patient preferences and capabilities.
One particularly interesting pathway involves the baroreflex. Gevirtz (2005) observed that when individuals breathe at their resonance frequency (typically around 6 breaths per minute), heart rate and blood pressure become synchronized but 180 degrees out of phase, meaning heart rate peaks when BP is lowest and vice versa. This breathing pattern appears to stimulate and strengthen the baroreflex. He proposed that resonance frequency biofeedback may lower BP precisely by enhancing this built-in pressure regulation system.


How to Measure Your HRV
As biofeedback practitioners increasingly use heart rate variability to assess autonomic function and guide treatment, teaching clients how to measure their HRV accurately at home has become a critical clinical skill. Unlike a single blood pressure reading that captures a snapshot in time, HRV tells a more nuanced story about how your autonomic nervous system is adapting to life's demands. But here is the catch: HRV is exquisitely sensitive to how, when, and under what conditions you measure it. Get the protocol wrong, and you will be chasing noise rather than signal.

Why Measurement Consistency Matters
HRV fluctuates throughout the day based on countless factors: circadian rhythms, core body temperature, metabolism, sleep cycles, and the renin-angiotensin system all contribute to these natural variations (Shaffer & Ginsberg, 2017). The gold standard for clinical HRV assessment remains the 24-hour recording, which captures this full range of physiological variation (Task Force, 1996). However, for practical home monitoring, shorter recordings work well when collected under standardized conditions.
The European Society of Cardiology and North American Society of Pacing and Electrophysiology established that short-term 5-minute recordings provide reliable data for most clinical applications (Task Force, 1996).
More recently, research has shown that even ultra-short recordings of 1 minute can yield valid RMSSD values when using appropriate protocols (Esco & Flatt, 2014).
The key principle is consistency. Whatever protocol you choose, stick with it. Comparing a measurement taken lying in bed right after waking to one taken standing after coffee is comparing apples to oranges, and the difference will tell you nothing useful about your actual autonomic state.
When to Measure: Optimal Timing
Morning measurements, taken immediately after waking while still in a rested state, have emerged as the gold standard for practical HRV monitoring. This timing captures your baseline autonomic function before the day's stressors have influenced your physiology. Research with athletes and healthy populations consistently shows that morning HRV measurements provide the most sensitive indicator of recovery status and response to training loads (Plews et al., 2013). The morning routine works because you can replicate it daily in essentially the same physiological state: rested, fasted, and before engaging with the demands of the day.
Nocturnal HRV monitoring offers an alternative approach that many wearable devices now support. By averaging HRV across a sleep period, nocturnal recordings capture autonomic function during the body's natural recovery phase. Both morning and nighttime measurements are valid approaches, but once you pick a protocol, maintain consistency over time (Plews et al., 2014).
The Standard Protocol: Four Essential Steps
Whether you are measuring a client in the clinic or teaching them to measure at home, follow this four-step protocol for reliable results:
Step 1: Use the bathroom first. A full bladder activates the sympathetic nervous system, which will artificially lower your HRV reading. This mirrors the blood pressure measurement principle that a full bladder can increase readings by 10 to 15 mmHg (Muntner et al., 2019).
Step 2: Sit up straight. Body position profoundly affects HRV measurements. In the supine (lying down) position, the cardiovascular system experiences minimal gravitational stress, generally resulting in higher parasympathetic activity. Moving to seated or standing positions requires orthostatic adaptation and typically increases sympathetic outflow to maintain blood pressure (Buchheit, 2014).
The seated position represents an excellent compromise: it provides enough physiological challenge to detect meaningful changes while remaining comfortable and practical for daily use. Research shows moderate to good reproducibility for time-domain HRV metrics like RMSSD in both supine and seated positions, with ICCs ranging from 0.65 to 0.89 (Dantas et al., 2019). Most importantly, whichever position you choose, use it consistently.
Step 3: Measure for at least 1 minute. While the traditional standard recommends 5-minute recordings (Task Force, 1996), research has validated that RMSSD can be accurately measured in as little as 60 seconds when following proper protocols (Esco & Flatt, 2014; Nussinovitch et al., 2011). For other HRV metrics like SDNN or frequency-domain measures, longer recordings of 2 to 5 minutes may be needed (Shaffer & Ginsberg, 2017). If your app or device specifies a particular duration, follow its recommendations.
Step 4: Breathe normally. Do not try to slow your breathing or control it in any special way during measurement unless your device specifically instructs otherwise. Natural, uncontrolled breathing allows the recording to capture your genuine resting autonomic state. Controlled breathing during measurement will shift your HRV values and make day-to-day comparisons meaningless.
Choosing Your Sensor: ECG vs. PPG
Two main technologies dominate consumer HRV measurement: electrocardiography (ECG) and photoplethysmography (PPG). Understanding the difference helps you choose the right tool and interpret results appropriately.
ECG-based devices, including chest straps like the Polar H10, detect the heart's electrical activity directly. They measure the precise timing between R-waves in the ECG signal, providing gold-standard accuracy for HRV analysis. Validation studies consistently show excellent agreement between chest strap ECG devices and clinical multi-lead ECG systems, with correlations often exceeding 0.99 (Gilgen-Ammann et al., 2019). For biofeedback practitioners requiring research-grade accuracy, ECG chest straps remain the preferred option.
PPG-based devices, found in smartwatches, fitness bands, and smart rings, use optical sensors to detect blood volume changes in peripheral circulation. While convenient, PPG does not directly measure the heart's electrical activity. PPG estimates HRV from pulse rate variability (PRV), the variation in pulse arrival times at the wrist or finger (Charlton et al., 2025).
Research shows that PPG-derived HRV correlates well with ECG-derived HRV during rest and stationary conditions, but accuracy declines progressively during movement and physical activity (Georgiou et al., 2018). Time-domain variables like RMSSD show acceptable agreement with ECG in resting conditions, while frequency-domain parameters exhibit lower reliability (Morelli et al., 2025).
Interpreting Your Results: The Normal Range
Perhaps the most important thing to understand about HRV is that it is profoundly individual. Population averages exist, but they are less useful than tracking your own personal baseline over time. RMSSD values in healthy adults typically range from 19 to 107 ms, though published ranges vary considerably across studies based on age, fitness level, and measurement conditions (Nunan et al., 2010).
Younger individuals and athletes generally show higher HRV values than older or sedentary populations. HRV also declines with age as autonomic function naturally attenuates (Task Force, 1996; Dantas et al., 2024).
Rather than comparing yourself to population norms, focus on establishing your personal baseline over 1 to 2 weeks of consistent daily measurements. Once you have a baseline, you can interpret deviations meaningfully. A reading within your normal range generally signals adequate recovery and readiness for physical or mental demands. A reading significantly below your normal range may indicate incomplete recovery, elevated stress, illness onset, or overtraining in athletes (Plews et al., 2013).
When NOT to Measure Your HRV
Just as certain conditions invalidate blood pressure readings, specific circumstances will produce misleading HRV values that should not be compared to your baseline:
After alcohol consumption. Acute alcohol intake produces dose-dependent reductions in parasympathetic HRV. A large Finnish study of over 4,000 individuals found that high alcohol intake decreased RMSSD by an average of 12.9 ms during the first hours of sleep, while also reducing recovery time by nearly 40 percentage points (Pietila et al., 2018).
Even moderate drinking reduces HRV, with one study finding that Oura ring users showed a mean decrease of 10.8 milliseconds (about 15.6%) on nights after drinking alcohol. The effects can persist for 4 to 5 days following heavy consumption, making post-drinking HRV measurements unreliable indicators of true autonomic function.
After caffeine consumption. While caffeine's effects on HRV are more complex than alcohol's, most research shows that caffeine stimulates the autonomic nervous system and can alter both time-domain and frequency-domain HRV metrics (Koenig et al., 2013). Morning measurements should occur before your first cup of coffee. If you must consume caffeine, wait at least 30 minutes and ideally several hours before measuring.
After late exercise. Intense exercise significantly affects HRV for hours afterward as your body recovers. Evening workouts will still influence your next morning's HRV reading. A quantitative analysis found that parasympathetic reactivation following exercise depends on intensity, duration, and fitness level, with full recovery sometimes requiring extended periods (Dantas et al., 2024).
For the most stable baseline readings, avoid strenuous exercise within 24 hours of measurement when possible, or at least maintain consistent exercise timing relative to your HRV measurements.
After large meals. Digestion activates the autonomic nervous system and diverts blood flow to the gastrointestinal tract. Measuring HRV immediately after eating will not reflect your true resting autonomic state. Wait at least 2 hours after a substantial meal.
During acute illness or stress. While HRV can detect illness and stress, measurements taken during these periods should be interpreted cautiously rather than compared to your healthy baseline. Your immune system's activation during illness naturally suppresses HRV as part of the inflammatory response.
Choosing a Home Monitor
For clients interested in daily HRV tracking, several categories of devices merit consideration. ECG chest straps like the Polar H10 or Garmin HRM-Pro offer research-grade accuracy at relatively low cost, but require putting on a strap each morning.
Smart rings like the Oura Ring provide overnight HRV tracking with minimal inconvenience but use PPG technology. Smartwatches from manufacturers like Apple, Garmin, and Fitbit can measure HRV through either PPG (continuous) or ECG (on-demand spot checks on some models).
Validation studies have found wide variation in consumer device accuracy. Research comparing popular devices to clinical ECG found that ECG-based apps like HRV4Training achieved the highest accuracy (mean absolute percentage error below 7%), while camera-based smartphone apps showed the poorest performance (Stone et al., 2021). Encourage clients to choose devices with published validation studies and to interpret trends rather than individual readings.
Key Takeaways for HRV Measurement
Accurate HRV measurement requires consistency above all else. Measure at the same time daily, ideally first thing in the morning before getting out of bed or consuming anything. Use the bathroom first, sit up straight, measure for at least 60 seconds, and breathe normally without trying to control your breath.
Choose one sensor type and stick with it. Avoid measuring after alcohol, caffeine, intense exercise, or large meals. Interpret your readings against your own personal baseline rather than population averages. A reading within your normal range signals good recovery; a reading significantly below baseline may indicate stress, incomplete recovery, or early illness. Track trends over weeks rather than reacting to single measurements.
Check Your Understanding
- Why is consistency in HRV measurement protocol more important than achieving any single "correct" method?
- What four steps comprise the standard protocol for morning HRV measurement?
- How do ECG-based and PPG-based HRV monitors differ in what they actually measure, and when might each be preferred?
- Explain why alcohol consumption affects HRV and how long these effects may persist after drinking.
Watch Out for Medication Effects
Prescription medications and social drugs can significantly affect biofeedback measurements, potentially masking problems or creating misleading baselines. A savvy clinician needs to know what their client is taking and how it might influence readings.
Inderal (propranolol), a beta-blocker, produces peripheral vasodilation that can artificially warm the hands. A patient on Inderal might show a seemingly healthy hand temperature of 92 degrees F (33.3 degrees C) when their true baseline without medication might be a cool 88 degrees F (31.1 degrees C).
The practical implication? When medication artificially raises hand temperature, set a higher training target; the client has more room to improve than their initial reading suggests.
Caffeine presents the opposite challenge. Medications containing caffeine (such as Cafergot and Fiorinal, used for headaches) or caffeine-containing beverages like coffee and energy drinks can increase heart rate and constrict peripheral blood vessels. These effects directly oppose what most cardiovascular biofeedback protocols are trying to achieve. If a client is loading up on caffeine before sessions, they are essentially fighting their own training. The clinician should consult with the patient's physician about gradually eliminating or restricting caffeine intake during the treatment period.
Nicotine may be the most problematic substance for cardiovascular biofeedback. Nicotine raises heart rate and is a potent vasoconstrictor, meaning it directly counteracts efforts to lower blood pressure. For patients who smoke or use nicotine products, smoking cessation treatment should be considered as part of, or prior to, biofeedback intervention. Without addressing nicotine use, other interventions may be swimming upstream.
Designing Your Client's Treatment Program
When designing a treatment program for essential hypertension, McGrady's (1996) six factors should serve as your foundation. But effective treatment also requires individualization based on thorough medical and behavioral assessment. Biofeedback for hypertension should never be attempted as a standalone intervention without a recent medical workup and ongoing communication with the patient's physician. This is not just good practice; it is essential for patient safety. Blood pressure changes during treatment can necessitate medication adjustments, and only the prescribing physician can make those calls. Medical oversight is particularly critical when a client's BP begins dropping significantly, as medication doses may need to be reduced to prevent hypotension.
After medical clearance, conduct a psychophysiological profile to identify which response systems are dysregulated and need training. Does the client show elevated frontalis EMG suggesting chronic muscle tension? Cool peripheral temperature indicating vasoconstriction? Poor HRV suggesting autonomic imbalance? The answers guide which modalities to include.
A multi-modal strategy that retrains the specific systems showing dysregulation, promotes relevant lifestyle changes (like exercise, diet modification, and sleep improvement), and teaches stress management when indicated shows the most clinical promise. One-size-fits-all approaches are rarely optimal.
What the Research Shows
The evidence base for biofeedback in hypertension has grown substantially, with recent systematic reviews and meta-analyses providing increasingly compelling support. Vital and colleagues (2021) analyzed nine randomized controlled trials involving 462 participants and found that biofeedback significantly improved diastolic blood pressure control compared to control conditions. Notably, interventions that combined biofeedback with complementary elements like relaxing music, breathing exercises, and muscle relaxation produced the greatest blood pressure reductions, supporting the multi-modal approach discussed earlier.
An even more comprehensive 2024 meta-analysis by Jenkins and colleagues examined 20 RCTs and found clinically meaningful reductions in both systolic (4.52 mmHg) and diastolic blood pressure (5.19 mmHg) following biofeedback interventions (Jenkins et al., 2024). Six different biofeedback modalities were represented across these studies, with HRV biofeedback being the most commonly used approach.
Earlier meta-analyses laid the groundwork for these findings. Yucha and colleagues (2001) compared biofeedback training against active comparison treatments like meditation and found that biofeedback achieved greater reductions in both SBP (6.7 mmHg) and DBP (3.8 mmHg) than inactive control treatments.
Another important finding: thermal and electrodermal biofeedback appear to outperform direct BP biofeedback (Linden & Moseley, 2006). This is a somewhat counterintuitive finding, but it makes sense when you consider that peripheral temperature and skin conductance provide more immediate, controllable feedback than BP readings, which fluctuate considerably and are harder to learn to control directly.
Wang and colleagues (2010) conducted an RCT specifically with prehypertensive post-menopausal women, a population at high risk for progression to full hypertension. They compared slow abdominal breathing combined with frontal SEMG biofeedback against breathing training alone.
The combined SEMG biofeedback group showed impressive results: SBP dropped 8.4 mmHg and DBP dropped 3.9 mmHg, compared to only 4.3 mmHg SBP reduction in the control group. Training participants to breathe at approximately six breaths per minute with daily home practice enhanced outcomes, consistent with Gevirtz's work on resonance frequency breathing.
Clinical Efficacy Rating
Based on three RCTs for essential hypertension and five for pre-hypertension treatments, Angele McGrady (2023) rated biofeedback for essential hypertension as level 4: efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). This is a meaningful rating; level 4 requires that the treatment be superior to a no-treatment control group, a wait-list, or a credible placebo in at least two independent research settings.
The biofeedback interventions studied trained participants to lower electrodermal and SEMG activity and SBP, and to increase HRV. The outcomes included reductions in both DBP and SBP, along with increased baroreflex sensitivity (BRS). Think of BRS as a measure of how "tuned up" your blood pressure regulation system is: if the baroreflex responds strongly to a small BP change, BRS is high, meaning the system is working efficiently. Low BRS means the system responds sluggishly, allowing BP to drift higher without appropriate correction. Biofeedback appears to improve this regulatory sensitivity.
Weight loss remains the single most effective lifestyle change for reducing blood pressure because obesity contributes to hypertension through multiple pathways simultaneously. McGrady's six factors provide a blueprint for successful interventions: biofeedback for hand warming and frontalis EMG reduction, autogenic training, consistent home practice, progressive muscle relaxation, and relaxation recordings.
The best candidates for biofeedback treatment show physiological signs of sympathetic overactivation: elevated resting heart rate, cool hands, high anxiety levels, and high-normal cortisol. A multi-modal strategy that combines biofeedback with relaxation training and medical management produces the best outcomes, particularly for patients whose blood pressure is stress-reactive.
Different biofeedback modalities work through different mechanisms: temperature biofeedback appears to reduce systemic vascular resistance by promoting peripheral vasodilation, while resonance frequency breathing at approximately 6 breaths per minute may lower blood pressure by stimulating and strengthening the baroreflex. Understanding these multiple pathways allows clinicians to tailor interventions to individual patient profiles.
Check Your Understanding
- What are the two primary factors that determine blood pressure, and how does each contribute to hypertension?
- Why might a client with cool hands and high heart rate be an especially good candidate for biofeedback treatment of hypertension?
- How do the mechanisms of temperature biofeedback and resonance frequency breathing differ in lowering blood pressure?
- What precautions should a biofeedback practitioner take when working with a client taking beta-blockers like Inderal?
- Why is a multi-modal treatment strategy recommended over single-modality approaches for essential hypertension?
How to Measure Your Blood Pressure
Accurate blood pressure measurement is essential for diagnosing and managing hypertension (Muntner et al., 2019). A single 5 mmHg error can misclassify millions of people (Stergiou et al., 2021), and when multiple errors stack up, readings can run 20 to 30 mmHg higher than true blood pressure. The good news: most errors are preventable.
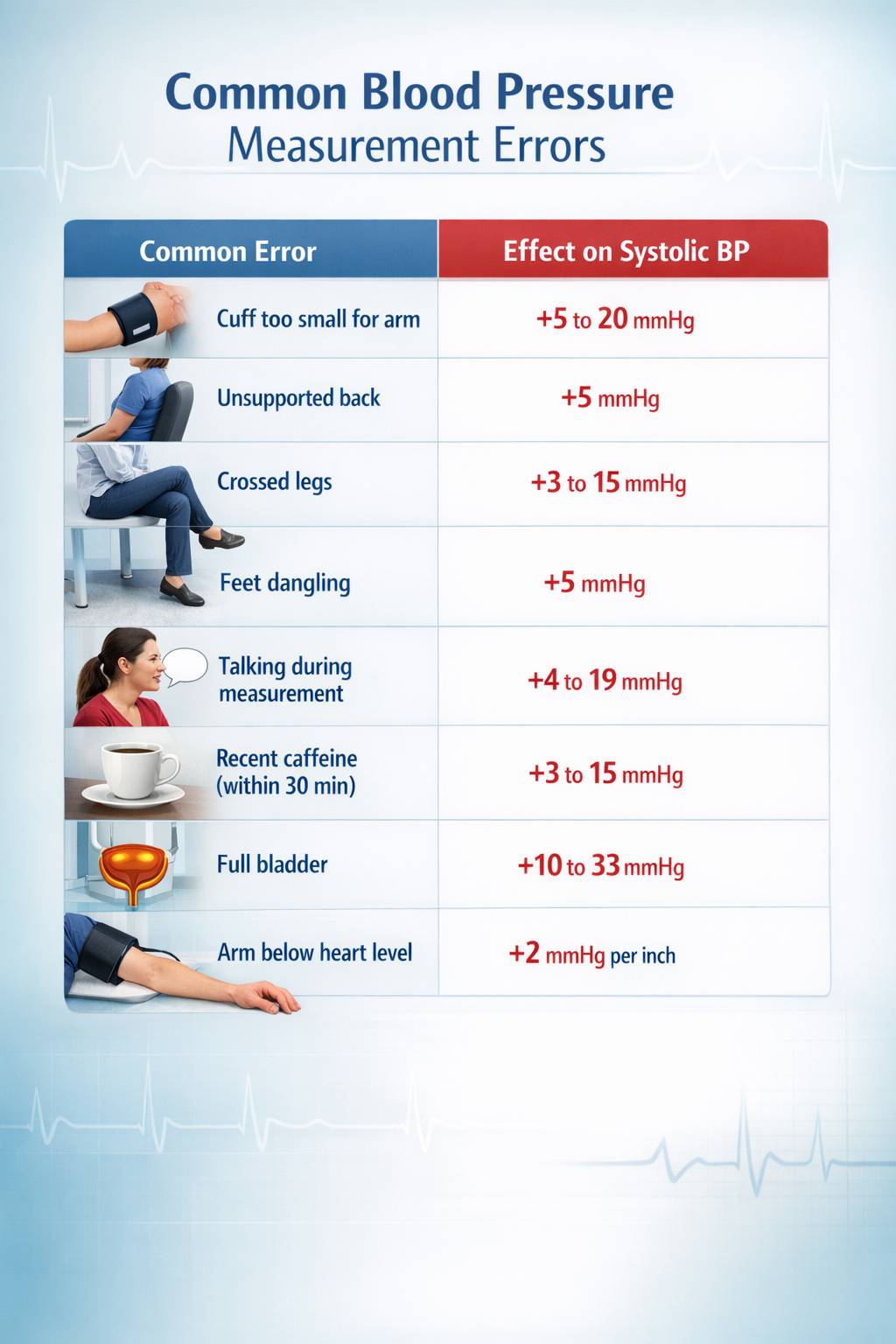
Preparation and Positioning
Avoid caffeine, exercise, and smoking for at least 30 minutes before measurement (Jones et al., 2025). Empty the bladder, then sit quietly for 5 minutes in a chair with back support, feet flat on the floor, legs uncrossed. Support the arm on a flat surface with the cuff at heart level. Place the cuff on bare skin or over only a thin sleeve.
Cuff size is the most common source of error. The inflatable bladder should encircle at least 80% of arm circumference (Muntner et al., 2019). A too-small cuff produces falsely elevated readings; one study found nearly 20 mmHg overestimation when a regular cuff was used on someone needing an extra-large (Juraschek et al., 2023). Over 17 million American adults have arm circumferences outside the 22 to 42 cm range of standard home monitor cuffs (Matsushita et al., 2024).
Taking and Timing Measurements
Use a validated oscillometric device (check validatebp.org); avoid wrist and finger monitors (Muntner et al., 2019). Remain silent during measurement, as conversation can raise systolic BP by 10 to 19 mmHg (Long et al., 1982). Take at least two readings one to two minutes apart and average them; discard the first reading if substantially higher (Jones et al., 2025).
For home monitoring, measure twice daily: morning (after waking and emptying the bladder, before medications or breakfast) and evening (before bed). Take two readings at each session. Continue for 3 to 7 days when establishing a baseline (Shimbo et al., 2020). Avoid measuring during acute illness, unusual stress, or immediately after large meals.
Home Versus Office Measurement
Home measurements eliminate white-coat effects, which cause 25 to 30% of patients with elevated office readings to show normal BP at home (Mancia et al., 2006). Home BP better predicts cardiovascular outcomes than office readings (Shimbo et al., 2020). However, masked hypertension, where BP appears normal at home but runs elevated during daily activities, affects 10 to 15% of the population and carries similar cardiovascular risk to sustained hypertension. Some clients benefit from 24-hour ambulatory monitoring to capture both patterns.
Check Your Understanding
- A client's blood pressure reading is 148/92 mmHg. You notice they are using a standard cuff despite having a large arm circumference, their legs are crossed, and they were talking to you during the measurement. Approximately how much might these errors have inflated the systolic reading?
- Why is home blood pressure monitoring considered superior to office measurement for predicting cardiovascular outcomes?
- What is the difference between white-coat hypertension and masked hypertension, and why does each matter clinically?
Vasovagal Syncope: When the Vagus Nerve Goes Too Far
Hypertension involves too little vagal influence on the heart. Now we turn to a condition where the problem is too much vagal activity at the wrong time. Syncope is the medical term for a temporary loss of consciousness, what most people call fainting. Vasovagal syncope (VVS), also called neurocardiogenic syncope, is a specific type featuring a brief loss of consciousness that typically occurs while standing. It is the classic "fainting spell" that might happen when someone sees blood, stands too long in a hot room, or experiences sudden emotional stress.
🎧 Mini-Lecture: Vasovagal Syncope Triggers and Symptoms

Here is what happens physiologically: during a stressful event, the vagus nerve (the main parasympathetic pathway to the heart) becomes dramatically overactive. This surge of vagal activity slows the heart rate and dilates blood vessels, causing cardiac output to drop and blood to pool in the legs and lower body. With less blood reaching the brain, the person becomes light-headed and may lose consciousness entirely.
It is essentially the opposite problem from hypertension: instead of the sympathetic system being chronically overactive, the parasympathetic system overreacts acutely. The episode typically resolves quickly once the person lies down or falls, which allows blood to flow more easily back to the brain.

Who Experiences Vasovagal Syncope?
If you have ever fainted or come close, you are far from alone. Vasovagal syncope is remarkably common. A recent global meta-analysis pooling data from 36,156 individuals estimated the worldwide prevalence at approximately 16.4% (Salari et al., 2024). That means roughly one in six people will experience at least one VVS episode in their lifetime.
Women are disproportionately affected, with some studies finding prevalence rates more than twice as high in females compared to males (Tajdini et al., 2023).
Classic vasovagal syncope typically first appears during adolescence, with the most common age of onset around 13 years, though episodes can begin at any age. By the time people reach middle age, about 35% of those between 35 and 60 years have experienced at least one episode of syncope.
From a healthcare system perspective, syncope accounts for 1 to 3% of emergency room visits and 6% of hospital admissions each year (Morag, 2015). While most episodes are benign and self-limiting, the potential for injury during a fall and the anxiety surrounding unpredictable episodes make this condition worth treating.
Biofeedback Research for Vasovagal Syncope
You might wonder: if VVS involves excessive parasympathetic activity, why would relaxation techniques that increase parasympathetic tone help? The answer lies in improving overall autonomic regulation rather than simply shifting the balance in one direction. Biofeedback-assisted relaxation therapy (BART) has been used to treat VVS, typically combining SEMG and thermal biofeedback with autogenic training and progressive relaxation.
The goal is not to further increase vagal tone but to help patients develop better overall autonomic flexibility and to reduce the stress and anxiety that often trigger VVS episodes in the first place.
McGrady, Bush, and Grubb (1997) reported a case series of 10 consecutive VVS patients who received comprehensive treatment: EMG and temperature biofeedback, diaphragmatic breathing instruction, and both autogenic training and progressive relaxation. Patients received an average of 8.5 fifty-minute training sessions. The results were encouraging: by the end of training, 6 of 7 patients diagnosed with syncope had reduced their episodes by at least 50%, and half of all patients reported improvement on each of their symptoms.
McGrady et al. (2003) followed up with a more rigorous test of BART for VVS. Subjects were evaluated during a 2-week pretest period, then randomly assigned to either treatment or a wait-list control condition. The treatment consisted of 10 fifty-minute sessions incorporating SEMG and thermal biofeedback, autogenic and progressive relaxation training, and guidance on applying techniques like progressive relaxation to their specific symptoms. Patients practiced at home using provided scripts and audiotapes for 10 to 15 minutes twice daily.
The results showed that the BART group achieved greater reductions in both headache index and loss of consciousness than the wait-list control group. Both groups improved on measures of state anxiety and depression, but the BART group showed superior outcomes on the primary VVS symptoms.
Clinical Efficacy Rating
Based on one RCT, Fred Shaffer (2023) rated biofeedback for vasovagal syncope as level 2: possibly efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). This relatively modest rating reflects the limited number of studies and small sample sizes rather than negative findings. The existing research is promising, but more and larger RCTs are needed to establish biofeedback as a definitive treatment for VVS.
Vasovagal syncope affects approximately one in six people worldwide, with women experiencing episodes at more than twice the rate of men. The mechanism involves a paradoxical overactivation of the vagus nerve during stress, causing blood vessels to dilate and blood to pool in the lower body. With less blood reaching the brain, the person feels light-headed and may faint. BART protocols using SEMG and thermal biofeedback combined with autogenic and progressive relaxation training show promise for reducing syncope episodes by at least 50% in most treated patients.
The intervention works not by further increasing vagal tone but by improving overall autonomic regulation and reducing stress-related triggers.
Check Your Understanding
- What is the physiological sequence of events that causes loss of consciousness in vasovagal syncope, and how does it differ from the autonomic pattern seen in hypertension?
- Given that VVS involves excessive parasympathetic activity, why might relaxation training still be effective rather than making the problem worse?
- What specific components did McGrady et al. (2003) include in their BART protocol for VVS, and what outcomes did they measure?
Preeclampsia: A Serious Pregnancy Complication
Imagine being pregnant and suddenly developing dangerously high blood pressure—a condition that threatens both your life and your baby's. That's preeclampsia, also called pregnancy-induced hypertension (PIH). It's defined by elevated blood pressure and protein in the urine (indicating kidney stress) appearing after the 20th week of pregnancy. While it might sound like "just high blood pressure," preeclampsia can rapidly become life-threatening.
Scientists now understand preeclampsia through a two-stage model. In Stage 1, during the first trimester, the placenta doesn't attach properly. Specifically, the spiral arteries that should remodel to deliver blood to the placenta don't develop correctly, leading to reduced blood flow.
In Stage 2, the poorly functioning placenta releases an imbalance of proteins into the mother's bloodstream: too many anti-angiogenic factors (like sFlt-1 and soluble endoglin, which block blood vessel formation) and too few pro-angiogenic factors (like VEGF and PlGF, which promote blood vessel growth). This imbalance damages blood vessel linings throughout the body, causing widespread constriction and immune system dysfunction (Liu et al., 2024; Osman et al., 2024). The result? Hypertension, organ damage, and in severe cases, seizures or death.

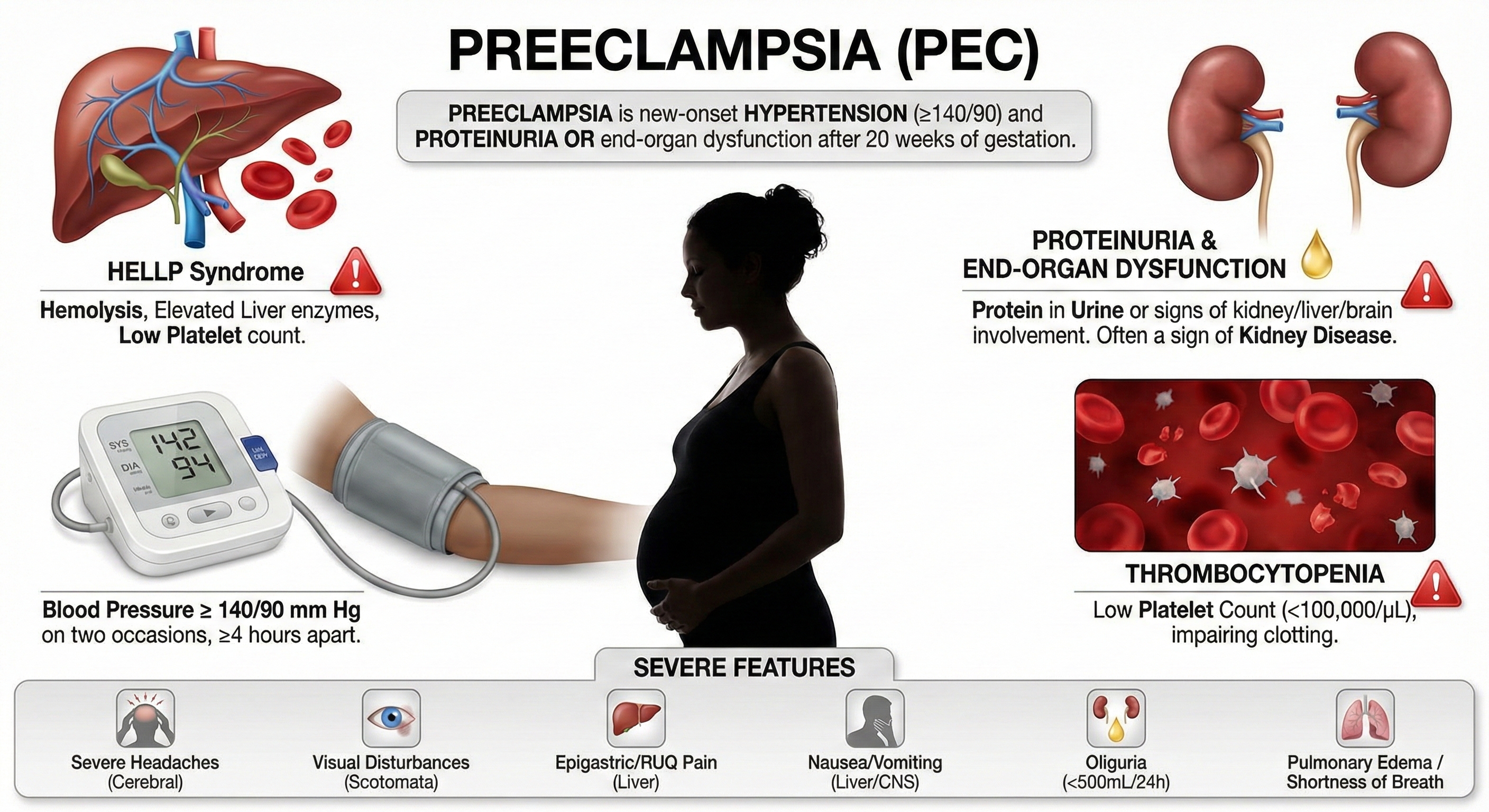
The Scope of the Problem
Preeclampsia affects 2-8% of pregnancies worldwide—which translates to millions of women each year—and remains a leading cause of maternal and fetal death (Johnson et al., 2022). The global toll is sobering: over 70,000 maternal deaths and 500,000 fetal deaths annually. These aren't just statistics; they represent families devastated by a condition we still can't fully prevent.
The burden of preeclampsia falls unequally across racial groups, and this disparity demands attention. In the United States, Black women experience significantly higher rates of preeclampsia than White women—one study found 12.4% prevalence in Black women compared to 7.1% in White women (Sharma et al., 2021).
But here's the crucial finding: these disparities aren't simply biological. Research shows that Black women born in the U.S. face higher preeclampsia risk than Black immigrant women, strongly suggesting that environmental and social factors—chronic stress, healthcare access, systemic racism—play significant roles (Johnson et al., 2022). American Indian and Alaskan Native women also experience disproportionately elevated rates (Johnson et al., 2022).
Understanding these disparities matters for biofeedback practitioners because stress-reduction interventions may be particularly valuable for populations facing chronic stressors.
Biofeedback Research Shows Strong Results
This is where biofeedback shines. Three randomized controlled trials (El Kosery et al., 2005; Little et al., 1984; Somers et al., 1989) and a multi-group study with a historical control (Cullins et al., 2013) tested whether biofeedback-assisted relaxation could help manage preeclampsia. The modalities used included electrodermal biofeedback (measuring galvanic skin response and skin conductance), heart rate variability biofeedback, and temperature biofeedback.
Why these modalities? Each provides a window into autonomic nervous system activity and can be used to train patients toward parasympathetic dominance—a calmer, less reactive physiological state.
Clinical Efficacy
The results are impressive. Based on three RCTs, Fred Shaffer rated electrodermal and temperature BART as efficacious and specific for preeclampsia—the highest rating in the evidence-based practice hierarchy. What does "efficacious and specific" mean? It indicates that biofeedback showed benefits superior to a credible placebo treatment or alternative therapy, ruling out the possibility that improvement was simply due to attention or expectation.
Participants showed improvements across multiple measures: less frequent hospitalizations, shorter hospital stays, lower diastolic blood pressure (DBP), lower mean arterial pressure (MAP), lower systolic blood pressure (SBP), reduced need for antihypertensive medication (methyldopa), and less protein in the urine (proteinuria). These aren't just numbers—they represent safer pregnancies and better outcomes for mothers and babies.
Sarah, a 32-year-old pregnant woman, developed elevated blood pressure at 24 weeks. Her obstetrician referred her for biofeedback-assisted relaxation training as an adjunct to medical monitoring. Over six sessions, Sarah learned to use temperature biofeedback and diaphragmatic breathing to activate her parasympathetic nervous system. Her blood pressure stabilized, and she was able to reduce her antihypertensive medication dosage while maintaining close medical supervision throughout the remainder of her pregnancy.
Biofeedback-assisted relaxation training for preeclampsia has achieved the highest efficacy rating based on multiple RCTs. Effective modalities include electrodermal and temperature biofeedback. Participants show improvements across multiple measures including blood pressure, hospitalization rates, medication needs, and proteinuria levels.
Comprehension Questions
- At what point in pregnancy does preeclampsia typically develop?
- What efficacy rating did biofeedback for preeclampsia receive, and why?
- Which populations face disproportionately higher risks from preeclampsia?
Check Your Understanding: Cardiovascular Disorders
- Explain how autonomic dysregulation contributes to both coronary artery disease and essential hypertension. Why does this shared mechanism make HRV biofeedback a logical intervention for both conditions?
- What specific HRV improvements did Del Pozo et al. (2004) observe in CAD patients who received cardiorespiratory biofeedback? What happened to the control group, and why is this comparison particularly striking?
- According to McGrady's research, what patient characteristics predict the best response to biofeedback for hypertension? What does this suggest about the mechanism of action?
- The Lin et al. (2018) follow-up study tracked CAD patients for one year. What outcomes did they measure, and what do the results suggest about biofeedback's potential to affect disease prognosis rather than just symptoms?
- Blood pressure is determined by cardiac output times systemic vascular resistance. Explain how arterioles—despite being tiny vessels—play such a powerful role in blood pressure regulation. What implications does this have for relaxation-based interventions?
- Why is biofeedback for preeclampsia rated only Level 2 despite the clear relevance of stress reduction during complicated pregnancies? What does this rating reflect?
- Compare the clinical efficacy ratings for coronary artery disease, essential hypertension, and preeclampsia. What factors account for the differences in evidence strength across these cardiovascular conditions?
Anxiety Disorders: When Worry Takes Over
Anxiety disorders represent the most common mental health conditions encountered in clinical practice, and HRV biofeedback has demonstrated strong evidence for their treatment—making this a high-value application for biofeedback practitioners across all settings.
Generalized Anxiety Disorder (GAD) is defined by excessive anxiety and worry occurring more days than not for at least 6 months. Unlike normal worry that comes and goes with life circumstances, GAD involves persistent, hard-to-control worry about multiple areas of life—work, health, family, finances—even when there is little or nothing to provoke it. The worry is disproportionate to actual circumstances and difficult to control. Accompanying symptoms may include restlessness, fatigue, difficulty concentrating, irritability, muscle tension, and sleep disturbances—symptoms that often respond well to autonomic regulation training.
DSM-5 classifies specific phobia as one of the Anxiety Disorders. Specific phobias include animal phobias (e.g., spiders, dogs), natural environment phobias (e.g., heights, storms), blood/injection/injury phobias, situational phobias (e.g., flying, enclosed spaces), and other phobias (Beidel, Bulik, & Stanley, 2014). While specific phobias are often treated with exposure therapy, biofeedback can help patients manage the intense physiological arousal that makes exposure so aversive—essentially providing a tool to regulate their own nervous system during therapeutic confrontation with feared stimuli.


DSM-5 classifies Post-traumatic stress disorder (PTSD) as one of the Trauma and Stress-Related Disorders. PTSD develops in some people following exposure to a traumatic event like assault, military combat, rape, or witnessing death or serious injury. The disorder is characterized by intrusive re-experiencing of the trauma (flashbacks, nightmares), avoidance of trauma-related stimuli, negative alterations in cognition and mood, and marked changes in arousal and reactivity (American Psychiatric Association, 2013). PTSD is particularly prevalent in VA settings, where biofeedback practitioners often work with veterans who have experienced combat trauma and may be hesitant to engage in traditional talk therapy.

Demographics
Anxiety disorders are the most common mental disorders in the United States. According to the National Institute of Mental Health, approximately 19.1% of U.S. adults—nearly one in five—experience an anxiety disorder in any given year (NIMH, 2023). Lifetime prevalence rates are substantial: panic disorder (2.3-2.7%), generalized anxiety disorder (4.1-6.6%), OCD (2.3-2.6%), PTSD (1-9.3%), and social phobia (2.6-13.3%). The wide range for PTSD reflects variation across populations, with much higher rates among combat veterans and survivors of sexual assault.
Post-traumatic stress disorder affects approximately 6% of Americans at some point in their lives (Goldstein, Smith, & Chou, 2016). The male-to-female ratio for a lifetime anxiety disorder is 3 to 2 (Yates, 2014)—meaning women are significantly more likely to experience anxiety disorders, a pattern that should inform clinical outreach and screening. However, PTSD rates are elevated in male veterans, making it a common presenting concern in VA clinical settings.
Neurophysiological Basis: Autonomic Dysregulation
Anxiety disorders are associated with autonomic nervous system dysregulation, characterized by reduced heart rate variability and parasympathetic withdrawal. In plain terms, the nervous system is stuck in "fight-or-flight" mode, with the calming branch (parasympathetic) unable to adequately counterbalance the arousal branch (sympathetic). This creates a chronic state of hypervigilance that maintains anxiety symptoms even in the absence of actual threat.
GAD is linked to abnormal functioning within several brain structures, including the amygdala (the brain's threat detection center), prefrontal cortex (involved in executive control and worry regulation), and anterior cingulate cortex (which monitors for errors and conflicts). These areas play critical roles in fear response, emotion regulation, and cognitive processing of uncertainty and worry. Neurotransmitter systems, particularly serotonin, norepinephrine, and GABA systems, are also implicated in GAD pathophysiology (Bandelow & Michaelis, 2015).
Family and twin studies suggest a moderate genetic contribution to GAD, with heritability estimates ranging from 15% to 50%. However, the specific genes contributing to this disorder remain largely unknown (Hettema, 2008)—suggesting that environmental factors, including learned autonomic patterns, play a substantial role and may be modifiable through biofeedback training.
Environmental stressors, particularly traumatic or stressful events in childhood, can increase the risk of developing GAD. This might reflect the impact of chronic stress on the brain's stress response systems, including the hypothalamic-pituitary-adrenal (HPA) axis. Early stress may essentially "tune" the autonomic nervous system toward hypervigilance—a pattern that HRV biofeedback may help recalibrate by strengthening vagal tone and restoring flexibility in autonomic responses.
Cognitive theories suggest that individuals with GAD have heightened sensitivity to uncertainty and a propensity to perceive ambiguous situations as threatening. This cognitive bias may result in persistent worry and fear (Dugas, Gagnon, Ladouceur, & Freeston, 1998). The physiological arousal that accompanies threat perception may reinforce these cognitive patterns through interoceptive feedback—creating a vicious cycle where anxious thoughts trigger physical symptoms, and physical symptoms convince the brain that threat is present. Biofeedback can interrupt this cycle by giving patients direct control over their physiological arousal.
Treatment Approaches and Rationale
Heart rate variability biofeedback (HRVB) targets autonomic dysregulation directly by training patients to breathe at their resonance frequency—typically around 6 breaths per minute—which maximizes the amplitude of respiratory sinus arrhythmia and strengthens baroreflex function (Lehrer et al., 2020). This slow, paced breathing activates the parasympathetic nervous system and helps shift autonomic balance away from chronic sympathetic dominance. Meta-analyses confirm that HRVB produces large effect sizes for reducing self-reported stress and anxiety (Goessl, Curtiss, & Hofmann, 2017).
Most controlled, randomized experiments have found that neurofeedback and biofeedback (electrodermal, SEMG, and temperature) produce anxiety reductions comparable to relaxation procedures like meditation and progressive relaxation. Biofeedback and relaxation procedures may achieve equivalent results because anxiety involves disordered attention and cognition in addition to abnormal physiological arousal—both approaches address the arousal component. However, biofeedback offers unique advantages: objective, real-time feedback on physiological states; quantifiable progress that motivates patients; and a technology-based framework that appeals to patients who may be skeptical of purely psychological approaches. In some cases, biofeedback may be superior to relaxation procedures and produce additive effects when combined with medication or psychotherapy.
Research Evidence
Generalized Anxiety Disorder
Murphy (2009) conducted a study on 79 adults diagnosed with Generalized Anxiety Disorder, randomly assigning 40 to receive either Cognitive Behavioral Therapy with heart rate variability training or CBT with Progressive Muscle Relaxation (PMR). The HRV training involved home practice using a personal HRV training device, the StressEraser. Both groups showed significant reductions in anxiety, with the percentage of participants meeting GAD diagnostic criteria decreasing from 100% to 42% post-intervention. There were no significant differences between the HRV and PMR groups—suggesting that both approaches effectively address anxiety's physiological component, though HRV biofeedback offers the advantages of objective feedback and quantifiable progress.
Anxiety as a Life Problem
This category includes anxiety in students, the workplace, gifted children, medical settings, and in sport and the performing arts—contexts where clinical diagnosis may not be present but anxiety still impairs function and quality of life. These populations are particularly relevant for biofeedback practitioners working in wellness, corporate, educational, and performance enhancement settings.
Henriques and colleagues (2011) conducted two studies using HRVB in college students and found significant reductions in anxiety and depression symptoms, though changes were not strongly related to HRV magnitude. This dissociation between physiological change and symptom improvement suggests that learning to attend to and regulate internal states may be therapeutic independent of the magnitude of physiological change achieved—the process of developing interoceptive awareness and self-regulation skills may itself be beneficial.
In the Ratanasiripong and colleagues (2012) study, 30 undergraduates assigned to a counseling plus HRVB group showed significantly larger improvements in anxiety scores than the counseling-only group—demonstrating additive effects when biofeedback augments traditional counseling approaches.
Aritzeta and colleagues (2017) found that students who underwent biofeedback intervention significantly reduced anxiety and improved exam performance, in addition to showing increased HRV. This dual improvement in both physiological markers and functional outcomes strengthens the case for biofeedback in academic settings and suggests that autonomic regulation skills transfer to real-world performance situations.
Prinsloo and colleagues (2013) found that a single 10-minute HRV training session in adult males with work-related stress resulted in increased relaxation and a significant reduction in state anxiety, with a larger effect size than a control group using sham feedback. Interestingly, the control group also showed reduced state anxiety, suggesting that focused attention alone might have some relaxation benefits—though real biofeedback provided significantly greater effects. This finding has practical implications: even brief biofeedback sessions can produce measurable benefits, making the intervention feasible for busy clinical settings.
Clinical Efficacy Rating
Fred Shaffer, Christopher Zerr, and Zachary Meehan rated biofeedback for anxiety as level 4, efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). Based on 5 and 15 RCTs for GAD and anxiety as a life problem respectively, this rating makes anxiety one of the strongest evidence-based applications for biofeedback.
The biofeedback modalities demonstrating effectiveness include HRV increase (addressing autonomic dysregulation), thermal/temperature increase (promoting peripheral relaxation), SEMG relaxation (releasing muscle tension associated with anxiety), and EDA/SCL decrease (reducing electrodermal arousal). Neurofeedback interventions showing benefit include alpha increase and alpha-theta training. Participants in these studies demonstrated decreased HR, SCL, state and trait anxiety, and HR reactivity to stress, along with increased HRV measures (HF and LF power) and theta power—a pattern consistent with reduced sympathetic arousal and enhanced vagal tone.
For clinicians, this efficacious rating means that biofeedback—particularly HRV biofeedback—represents a well-established treatment option for anxiety that can be offered with confidence. Multiple modalities show benefit, giving practitioners flexibility to address the specific symptom profile each patient presents: HRV biofeedback for pervasive autonomic dysregulation, temperature biofeedback for patients with cold extremities indicating peripheral vasoconstriction, SEMG biofeedback for those with prominent muscle tension, and alpha-theta neurofeedback for patients with central nervous system hyperarousal.
Anxiety disorders are the most common mental disorders, affecting nearly one in five U.S. adults annually. Biofeedback for anxiety is rated level 4 (efficacious), making it one of the strongest evidence-based applications. Anxiety disorders involve autonomic dysregulation—the nervous system stuck in fight-or-flight mode with parasympathetic withdrawal and reduced HRV. HRV biofeedback trains patients to breathe at their resonance frequency (typically around 6 breaths per minute), maximizing respiratory sinus arrhythmia and strengthening vagal tone. Multiple modalities show benefit: HRV biofeedback addresses autonomic dysregulation directly, temperature biofeedback promotes peripheral relaxation, SEMG biofeedback releases muscle tension, and alpha-theta neurofeedback reduces central nervous system hyperarousal. Even single 10-minute sessions produce measurable anxiety reduction, and combining biofeedback with CBT or counseling enhances outcomes beyond either intervention alone.
Clinical Case Presentations
Dr. Inna Khazan describes her treatment of anxiety and pain. You can enlarge the video by clicking on the bracket icon at the bottom right of the screen. When finished, click on the bracket icon or ESC key.
Dr. Inna Khazan describes her multimodal treatment of developmental trauma.
Dr. Donald Moss describes his integrative treatment of a young girl and her mother.
Dr. Donald Moss describes his integrative treatment of Jorge using the Pathways framework.
Comprehension Questions: Anxiety and Stress-Related Disorders
- What is the clinical efficacy rating for HRV biofeedback treatment of anxiety, and what does this suggest about treatment selection for anxious patients?
- What is autonomic dysregulation and how does it manifest in anxiety disorders? How does HRV biofeedback address this underlying mechanism?
- Why might biofeedback and relaxation procedures produce equivalent results for anxiety reduction? What unique advantages does biofeedback offer despite this equivalence?
- What did Prinsloo and colleagues (2013) find regarding a single 10-minute HRV training session, and what are the clinical implications of these findings for busy practitioners?
- Explain the concepts of behavioral activation system (BAS) and behavioral inhibition system (BIS). How do these concepts relate to understanding both anxiety and depression?
Functional Abdominal Pain: When the Gut-Brain Connection Goes Awry
You've probably heard the phrase "gut feeling"—and there's real neuroscience behind it. Your gut contains over 100 million neurons (more than your spinal cord!), and it communicates constantly with your brain through what scientists call the gut-brain axis. When this communication system malfunctions, the result can be chronic pain with no obvious physical cause.
Functional gastrointestinal disorders are diagnosed by their symptoms rather than visible abnormalities. Doctors examine these patients, run tests, perform scans—and find nothing structurally wrong. Yet the pain is very real. These conditions include cyclic vomiting, irritable bowel syndrome (IBS), and functional abdominal pain.

Functional abdominal pain (FAP), also called recurrent abdominal pain (RAP), features episodes of abdominal pain without detectable pathology—no tumors, no obstruction, no inflammatory bowel disease (IBD). "Functional" doesn't mean the pain is imaginary; it means the problem lies in how the nervous system is functioning rather than in visible tissue damage. An estimated 15-20% of school-age children and adolescents experience at least one episode of RAP each year, with girls affected 1.5 times more often than boys (Greenberger, 2013).
For these children, stomach aches mean missed school, social isolation, and frustrated parents wondering why their child keeps complaining about pain that tests can't explain.
Cyclic vomiting syndrome is another distressing functional disorder: severe episodes of nausea and vomiting lasting hours to days, with complete recovery between episodes. Imagine being perfectly fine one day, then suddenly vomiting uncontrollably for 24 hours, then recovering completely—only to have it happen again weeks or months later. This condition primarily affects children between ages 3-7, though adults can develop it too (Greenberger, 2013).
HRV Biofeedback Addresses Autonomic Imbalance
Here's where the gut-brain connection becomes therapeutically useful. Richard Gevirtz, a pioneering researcher in this area, proposed that FAP is associated with vagal deficiency—essentially, the parasympathetic "rest and digest" branch of the autonomic nervous system isn't active enough. When vagal tone is low, the gut becomes hypersensitive and overreactive. A series of three uncontrolled studies (Humphreys & Gevirtz, 2000; Sowder et al., 2010; Stern et al., 2014) tested whether heart rate variability biofeedback could restore balance.
How might HRV biofeedback help? Researchers propose multiple pathways (Lehrer & Gevirtz, 2014). First, it stimulates the baroreflex—a feedback loop that helps stabilize blood pressure and, more broadly, autonomic function. Second, vagal afferent signals travel up to brain regions (the limbic system and prefrontal cortex) where pain perception is processed and can be modulated. Third, remember that cholinergic anti-inflammatory pathway we discussed with arthritis? It applies here too—enhanced vagal tone can reduce gut inflammation. The overall effect is like resetting a dysfunctional communication system: the gut and brain start "talking" normally again.
Clinical Efficacy
Based on three uncontrolled studies, Richard Gevirtz (2023) rated HRV biofeedback as level 3 - probably efficacious for functional abdominal pain in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). Why "probably" rather than definitely? The studies lacked control groups, so we can't rule out placebo effects or spontaneous improvement. Still, participants showed meaningful improvements: higher vagal tone (measured through HRV metrics), fewer GI symptoms like nausea, diarrhea, and constipation, reduced functional disability, and better quality of life.
These converging improvements across physiological and symptomatic measures strengthen the case that HRV biofeedback is doing something real.
Ten-year-old Jason missed so much school due to stomach pain that his grades suffered and he felt isolated from friends. Medical workups found no physical cause. His pediatrician referred him for HRV biofeedback training. Over eight sessions, Jason learned resonance frequency breathing and practiced with a portable device at home. His stomach pain episodes decreased from daily to once weekly, and he regained confidence in his ability to manage symptoms when they occurred.
Irritable Bowel Syndrome: The Most Common Functional GI Disorder
If you've ever had butterflies in your stomach before a presentation, you've experienced a mild version of what IBS patients live with constantly. Irritable bowel syndrome (IBS) is the most common functional GI disorder, accounting for 12% of primary care visits and 28% of gastroenterological visits (Chiarioni & Whitehead, 2008). That's an enormous healthcare burden for a condition with no visible abnormality.
IBS features abdominal pain or discomfort combined with altered bowel habits—constipation, diarrhea, or maddening alternation between both. Current research views IBS as a disorder of the gut-brain axis, involving bidirectional miscommunication between the central nervous system and the digestive tract (Mayer et al., 2023).
What goes wrong? Several things: the gut microbiome becomes unbalanced (dysbiosis), the intestinal lining becomes hypersensitive to normal stimuli (visceral hypersensitivity), low-grade inflammation develops in the gut wall, and the stress-response system (hypothalamic-pituitary-adrenal or HPA axis) becomes dysregulated (Palaniswamy, 2025). Notice how these mechanisms involve both physical changes in the gut and nervous system dysfunction—which explains why IBS is so frustrating for patients whose doctors keep saying "nothing is wrong."
Who Develops IBS?
IBS is remarkably common. A 2024 meta-analysis across 52 countries estimated the global prevalence at approximately 14%—higher than previous estimates (Alqahtani et al., 2024). However, the numbers vary dramatically depending on which diagnostic criteria are used. The Rome Foundation Global Study reported 3.8% prevalence using the stricter Rome IV criteria, compared to 10.1% using the older Rome III criteria (Huang et al., 2023). This difference matters clinically: Rome IV requires more frequent symptoms, so it identifies more severely affected patients.
Women are about 1.5 times more likely to be diagnosed with IBS than men (Alqahtani et al., 2024)—though whether this reflects true prevalence differences or differential help-seeking behavior remains debated. Psychological factors significantly influence IBS development, with research showing increased risk associated with stress, anxiety, and depression.
These aren't just correlations; the relationship is likely bidirectional, with gut dysfunction worsening mood and psychological distress worsening gut symptoms. Most IBS patients develop symptoms before age 35. When IBS-like symptoms first appear in older adults, clinicians should investigate carefully to rule out more serious conditions like inflammatory bowel disease or colon cancer.
Biofeedback Helps Restore Normal Function
Given that IBS involves gut-brain miscommunication and stress system dysregulation, it makes sense that biofeedback might help—and research supports this. A series of uncontrolled studies (Leahy et al., 1998; Schwartz et al., 1990) and randomized controlled trials (Blanchard et al., 1992; Dobbin et al., 2013) examined whether BART, often combined with cognitive-behavioral therapy (CBT) and hypnotherapy, could reduce IBS symptoms. These multicomponent approaches target different aspects of the disorder: biofeedback addresses autonomic dysregulation, CBT addresses maladaptive thoughts and behaviors, and hypnotherapy can modulate gut sensitivity.
🎧 Listen to a Mini-Lecture on IBS
Clinical Efficacy
Based on five RCTs, Donald Moss and Mark Watkins rated rectosigmoid and temperature biofeedback with Progressive Relaxation for IBS as level 4 - efficacious in Evidence-Based Practice in Biofeedback and Neurofeedback (4th ed.). What's "rectosigmoid biofeedback"? It trains patients to normalize activity in the lower colon—the rectosigmoid region—where many IBS symptoms originate. Combined with temperature biofeedback (addressing overall autonomic balance) and Progressive Relaxation (reducing muscle tension and overall stress), this multimodal approach produced significant reductions in GI and IBS symptoms.
Functional gastrointestinal disorders share a common thread: disrupted gut-brain communication that biofeedback can help restore. HRV biofeedback is rated probably efficacious (level 3) for functional abdominal pain, working through baroreflex stimulation, vagal afferent pathways, and anti-inflammatory effects. For IBS, rectosigmoid and temperature biofeedback with Progressive Relaxation achieves efficacious status (level 4). The gut-brain axis provides a compelling rationale for these interventions—by normalizing autonomic function, biofeedback helps the gut and brain "talk" properly again.
Comprehension Questions
- What distinguishes functional abdominal pain from other GI conditions?
- How might HRV biofeedback help with functional abdominal pain?
- What efficacy rating did biofeedback for IBS receive?
- Why might symptoms in older adults warrant additional medical investigation?
Optimal Performance: Beyond Clinical Applications
HRV biofeedback is not limited to clinical populations. Athletes, performers, and professionals seeking peak performance have embraced this approach to enhance their capabilities—expanding biofeedback practice beyond traditional healthcare settings.
Optimal performance refers to the highest level of functioning an individual is capable of achieving, often used in the context of athletic or cognitive performance. For biofeedback practitioners working with military personnel, elite athletes, or performing artists, this application offers opportunities to help healthy individuals achieve their potential.
Athletic Performance
Athletes at all levels use HRV monitoring to optimize training loads and recovery. When resting HRV decreases from baseline, this may indicate overtraining or insufficient recovery—a signal that training intensity should be reduced. Conversely, improvements in resting HRV often correlate with improved fitness and readiness to perform. This monitoring function provides immediate practical value independent of biofeedback training.
Beyond monitoring, HRVB training helps athletes achieve the physiological state associated with peak performance. Strack and Gevirtz (2011) described how HRV biofeedback can be integrated into sport psychology practice, helping athletes achieve the calm focus and controlled arousal that characterize optimal performance states.
Performing Arts
Musicians, dancers, and actors often experience performance anxiety that can interfere with their abilities—sometimes ending promising careers. HRV biofeedback offers a tool for managing arousal levels and achieving optimal performance states that complements traditional performance psychology approaches.
Raymond and colleagues (2005) conducted a preliminary investigation of biofeedback and dance performance, finding that biofeedback training improved performance quality—suggesting that autonomic regulation contributes to artistic expression.
Thurber (2006) studied the effects of heart-rate variability biofeedback training and emotional regulation on music performance anxiety in university students. The intervention helped reduce anxiety and improve performance—outcomes with practical significance for music programs and performing arts schools.
Research Evidence
Lehrer and colleagues' (2020) meta-analysis found that HRVB shows its largest effect sizes for athletic and artistic performance applications—larger even than for most clinical conditions. This finding suggests that the intervention may be particularly well-suited for enhancing performance in healthy populations, where ceiling effects might be expected to limit gains. For practitioners seeking to expand beyond clinical populations, optimal performance represents an evidence-based growth area.
Clinical Efficacy Rating
Based on multiple studies, HRV biofeedback for optimal performance applications is rated level 3, probably efficacious. The evidence is promising but more controlled studies with specific performance populations are needed. Given the large effect sizes observed in meta-analysis, this application merits continued investigation and clinical implementation.
HRV biofeedback shows promise for functional gastrointestinal disorders by addressing the gut-brain connection and restoring autonomic balance. Visceral hypersensitivity and heightened stress reactivity characterize these conditions, providing a clear rationale for autonomic intervention. The intervention has also found robust applications in optimal performance, with Lehrer's meta-analysis showing the largest effect sizes for athletic and artistic performance. Treatment of FAP is rated Level 2, while IBS and optimal performance applications are rated Level 3. For practitioners, these applications extend biofeedback's reach beyond traditional clinical populations.
Comprehension Questions: GI Disorders and Performance
- What is visceral hypersensitivity and how does it contribute to functional abdominal pain? Why does this mechanism suggest autonomic intervention may help?
- What did the Humphreys and Gevirtz (2000) study find when comparing HRVB to other treatments for pediatric abdominal pain, and why is this finding clinically significant?
- According to Lehrer's meta-analysis, for which applications does HRVB show the largest effect sizes? What implications does this have for expanding biofeedback practice?
- How might athletes use HRV monitoring to optimize their training, and how does this monitoring function differ from biofeedback training?
Glossary
A1 score: frontal alpha asymmetry score calculated by subtracting log left-alpha power from log right-alpha power to assess hemispheric activation balance.
accentuated antagonism: the principle that increasing parasympathetic activity can suppress sympathetic effects; proposed as a mechanism by which HRV biofeedback may reduce sympathetic innervation of trigger points.
ACE inhibitors: drugs that block angiotensin II formation, resulting in vasodilation (reducing systemic vascular resistance) and reduced aldosterone secretion; used to lower blood pressure.
airway remodeling: structural changes in the airways caused by chronic inflammation in asthma, including thickening of the airway wall and increased smooth muscle mass.
alarmins: signaling molecules, including TSLP, IL-33, and IL-25, released by the airway epithelium that initiate the inflammatory cascade in asthma.
allodynia: a type of pain hypersensitivity where typically non-painful stimuli, such as touch or temperature changes, are perceived as painful.
alpha asymmetry neurofeedback for mood disorders: a protocol that trains depressed clients to increase right frontal alpha relative to left frontal alpha, thereby reducing right frontal activity or increasing left frontal activity.
angina pectoris: severe chest pain caused by myocardial ischemia when reduced oxygen delivery impairs cardiac muscle function.
arterioles: the almost microscopic (8 to 50 microns in diameter) blood vessels that deliver blood to capillaries; these vessels play a crucial role in the regulation of blood pressure and blood flow through their narrow diameter and contractility.
asthma: a chronic respiratory condition where the bronchial tubes become inflamed and constrict, leading to episodes of wheezing, coughing, and shortness of breath.
atheroma: a deposit or plaque within the intima of the arterial wall, consisting of lipids, cholesterol, cells (including smooth muscle cells and macrophages), and connective tissue.
autonomic dysregulation: an imbalance between the sympathetic and parasympathetic branches of the autonomic nervous system, typically involving sympathetic overactivity paired with reduced parasympathetic activity; a central driver of essential hypertension and heart failure progression.
baroreceptor: stretch receptors in the walls of blood vessels, particularly in the carotid sinus and aortic arch, that detect changes in blood pressure and help maintain it within normal ranges.
baroreflex: the body's automatic blood pressure regulation system; resonance frequency biofeedback may lower blood pressure by stimulating and strengthening this mechanism.
behavioral activation system (BAS): brain system associated with approach motivation and positive emotions, mediated primarily by left frontal cortex.
behavioral inhibition system (BIS): brain system associated with withdrawal motivation and negative affect, mediated primarily by right frontal cortex.
beta-blockers: drugs like Inderal (propranolol) that block the action of epinephrine and norepinephrine on beta-adrenergic receptors, inhibit renin secretion, and decrease heart rate and cardiac contractibility; used to lower blood pressure and prevent cardiac arrhythmia.
blood pressure: the force exerted by circulating blood on the walls of the body's arteries, measured in millimeters of mercury (mmHg); the product of cardiac output and systemic vascular resistance.
bronchoconstriction: the dramatic tightening of smooth muscle surrounding the airways that restricts airflow in asthma.
calcium channel blockers: drugs like nifedipine (Procardia, Adalat) that slow calcium entry into myocardial fibers, reducing the heart's workload, contraction force, and blood pressure.
Capnography-Assisted Learned Monitored (CALM) Breathing: a therapy that uses end-tidal CO2 biofeedback combined with slow nasal breathing exercises to target dysfunctional breathing patterns in COPD patients.
cardiac output: the amount of blood pumped by the heart; the product of stroke volume and stroke rate.
chronic bronchitis: mucus hypersecretion and chronic productive cough for at least 3 months for a minimum of 2 consecutive years.
chronic functional abdominal pain: functional abdominal pain occurring continuously or periodically for more than 3 months.
chronic myofascial pain syndrome: a regional pain disorder characterized by trigger points—hyperirritable regions of taut bands of skeletal muscle in the muscle belly or associated fascia.
chronic obstructive pulmonary disease (COPD): a progressive lung disease characterized by increasing breathlessness due to obstructed airflow from the lungs; encompasses chronic bronchitis and emphysema.
clinical efficacy: the ability of an intervention to produce beneficial effects under ideal conditions, typically assessed in controlled clinical trial settings.
cognitive behavioral therapy for insomnia (CBT-I): a structured, evidence-based nonpharmacological treatment for insomnia that addresses the psychological and behavioral factors perpetuating sleep problems; meta-analyses show CBT-I improves both sleep quality and pain outcomes in patients with comorbid insomnia and chronic pain.
coronary artery disease (CAD): a cardiovascular condition characterized by reduced coronary artery circulation due to atherosclerotic plaque buildup.
descending pain modulation: CNS pathways that modulate pain signals, including inhibitory pathways that normally suppress pain transmission; impairment of these pathways contributes to central sensitization in conditions like fibromyalgia.
diastolic blood pressure (DBP): the lower number in a blood pressure reading, representing the force exerted on artery walls when the heart is at rest between beats.
diuretics: drugs that reduce blood volume by removing water and salt in urine; used to treat hypertension.
dyspnea: shortness of breath; a common symptom in COPD that often co-occurs with anxiety in a self-reinforcing cycle.
eclampsia: a serious complication of preeclampsia involving seizures; can be life-threatening for both mother and baby.
emphysema: a form of COPD involving abnormal, irreversible expansion of the gas-exchange airways associated with destruction of alveolar walls.
endothelial dysfunction: compromised function of the inner lining of blood vessels, often triggered by risk factors including hypertension, hyperlipidemia, diabetes, smoking, sedentary lifestyle, and chronic stress.
endothelium: the inner lining of blood vessels that normally maintains balance between vasodilators and vasoconstrictors, prevents thrombosis, and inhibits smooth muscle proliferation and inflammation.
eosinophilic inflammation: inflammation dominated by eosinophils, a type of white blood cell involved in allergic responses; characteristic of asthma pathophysiology.
fibromyalgia (FM): a chronic benign pain disorder characterized by widespread musculoskeletal pain, tenderness, stiffness, and fatigue, conceptualized as a pain amplification disorder produced by allodynia and hyperalgesia.
forced expiratory volume (FEV1): the amount of air a person can forcefully exhale in one second; a key measure of lung function.
frontal alpha asymmetry (FAA): the difference in alpha power between left and right frontal regions, proposed as a neurophysiological marker for depression.
functional abdominal pain (FAP): abdominal pain in patients who show heightened visceral pain receptor sensitivity and increased sensitivity to stressors, without identifiable structural abnormalities.
functional gastrointestinal disorders (FGIDs): disorders characterized by chronic or recurrent gastrointestinal symptoms that cannot be explained by structural or biochemical abnormalities; includes irritable bowel syndrome, functional dyspepsia, and functional constipation.
FVC (forced vital capacity): the total amount of air a person can forcefully exhale after taking the deepest breath possible.
generalized anxiety disorder (GAD): excessive anxiety and worry occurring more days than not for at least 6 months, with accompanying symptoms including restlessness, fatigue, difficulty concentrating, irritability, muscle tension, and sleep disturbances.
gut microbiome: the community of microorganisms (bacteria, viruses, and fungi) living in the intestines; recent research shows that high-salt diets can alter gut bacteria composition in ways that promote inflammation and hypertension.
HELLP syndrome: a life-threatening complication of preeclampsia characterized by Hemolysis, Elevated Liver enzymes, and Low Platelet count.
heterogeneous disorder: a condition with multiple different underlying causes or pathways; essential hypertension is heterogeneous because different patients develop it through different combinations of genetic and environmental factors.
high-impact chronic pain: chronic pain that significantly limits daily activities; reported by approximately 6.9% of the U.S. population.
hyperalgesia: the perception of mildly painful stimuli as severely painful.
hypertension: a chronic medical condition characterized by persistently elevated blood pressure, usually defined as a reading of 130/80 mmHg or higher.
hypoxia: a state in which the oxygen level available to tissues is below physiological needs, leading to cellular injury and potentially death.
irritable bowel syndrome (IBS): a chronic functional gastrointestinal disorder typified by recurrent abdominal pain related to defecation or accompanied by changes in bowel habits; subtypes include IBS with predominant constipation (IBS-C), diarrhea (IBS-D), and mixed (IBS-M).
major depressive disorder (MDD): a mood disorder defined by persistent sadness, loss of interest or pleasure, feelings of guilt or low self-worth, disturbed appetite and sleep, difficulty concentrating, and in severe cases, suicidal ideation.
mediational model of muscle pain: hypothesis that lack of assertiveness and resultant worry each trigger sympathetic activation, leading to muscle tension and trigger point formation.
myocardial infarction (MI): death of heart muscle occurring when atherosclerosis completely obstructs a coronary artery; commonly called a heart attack.
myocardial ischemia: reduced perfusion (blood flow) of the heart muscle caused by obstructed blood flow.
nociplastic pain: a third category of pain distinct from nociceptive and neuropathic pain, arising from altered nociception despite no clear evidence of tissue damage or nervous system lesion; recognized as the mechanism underlying conditions such as fibromyalgia.
optimal performance: the highest level of functioning an individual is capable of achieving, often used in the context of athletic or cognitive performance.
palpation: examination by feeling or pressing with the hand; essential for identifying trigger points, which cannot be detected using SEMG electrodes.
parasympathetic withdrawal: a reduction in the calming vagal influence on the heart, often accompanying sympathetic overdrive in conditions like heart failure; contributes to autonomic dysregulation.
post-COVID syndrome (PCS): also known as long COVID; a condition in which symptoms persist for weeks to months following acute COVID-19 infection, frequently including chronic musculoskeletal pain resembling fibromyalgia.
post-traumatic stress disorder (PTSD): a trauma and stress-related disorder following exposure to traumatic events, characterized by intrusive re-experiencing, avoidance, negative alterations in cognition and mood, and marked changes in arousal and reactivity.
preeclampsia: a potentially dangerous pregnancy complication characterized by high blood pressure and signs of damage to another organ system, most often the liver and kidneys; typically develops after 20 weeks of pregnancy.
primary hypertension: chronically elevated blood pressure not due to an identifiable cause; accounts for 92 to 95% of hypertension cases. Also called essential hypertension.
proteinuria: protein leakage into urine; a characteristic finding in preeclampsia resulting from endothelial dysfunction.
pulmonary impedance: the resistance of the bronchioles to airflow; decreasing pulmonary impedance allows asthma patients to breathe more easily.
pulse oximeter: a device that measures dissolved oxygen in the bloodstream using a light sensor placed on the finger.
recurrent abdominal pain (RAP): intermittent functional abdominal pain with at least 3 episodes over 3 months; seen in up to 10% of children and 2% of adults.
renin-angiotensin-aldosterone system (RAAS): a hormone system that regulates blood pressure and fluid balance; when activated, it causes blood vessels to constrict and signals the kidneys to retain sodium and water, both of which raise blood pressure.
resonance frequency: the breathing rate, typically around 6 breaths per minute, at which an individual's cardiovascular system produces the greatest respiratory sinus arrhythmia; breathing at this rate maximizes the training effect in HRV biofeedback.
respiratory sinus arrhythmia (RSA): the natural acceleration of heart rate during inhalation and deceleration during exhalation.
salt-sensitive: describes individuals whose blood pressure increases with salt intake; approximately 60% of hypertensive patients and 25% of normotensive individuals.
secondary hypertension: elevated blood pressure with an identifiable cause, such as kidney disease, adrenal gland tumors, or obstructive sleep apnea; accounts for approximately 5% of hypertension cases.
specific phobia: a type of anxiety disorder characterized by a pronounced fear or anxiety about a specific object or situation, leading to avoidance behavior; examples include fear of flying, spiders, or blood.
stroke rate: the number of heartbeats per minute; also referred to as heart rate.
stroke volume: the amount of blood pumped from the left ventricle of the heart with each beat.
sympathetic overdrive: sustained overactivation of the sympathetic nervous system even at rest, which worsens cardiac remodeling and metabolic dysfunction when combined with parasympathetic withdrawal.
systemic vascular resistance: the resistance to blood flow offered by all of the systemic vasculature, excluding the pulmonary vasculature; influenced by blood viscosity and blood vessel length and radius.
systolic blood pressure (SBP): the higher number in a blood pressure reading, representing the force exerted on artery walls when the heart contracts.
tender points: places on the body where light pressure causes pain, located at a muscle's insertion rather than the muscle belly; produce local pain but not referred pain. Distinct from trigger points.
thrombus: a solid or semi-solid blood clot that forms within the vascular system, often at sites of blood vessel injury or turbulence.
trigger points: hyperirritable spots in taut muscle bands that refer pain to distant locations and characterize myofascial pain syndrome; represent localized muscle dysfunction that can be directly treated. Distinct from tender points.
vagal tone: the strength of parasympathetic (vagus nerve) influence on the heart; higher vagal tone is associated with better cardiovascular health, greater stress resilience, and higher heart rate variability.
visceral hypersensitivity: increased sensitivity to normal gut sensations, resulting in pain perception even with normal digestive functions.
References
Almeida Silva, M., & Pinto, P. R. (2025). Descending pain modulation in fibromyalgia: A short review of mechanisms and biomarkers. Diagnostics, 15(21), 2702. https://doi.org/10.3390/diagnostics15212702
Alonso-Blanco, C., Fernandez-de-las-Penas, C., Morales-Cabezas, M., Zarco-Moreno, P., Ge, H. Y., & Florez-Garcia, M. (2011). Multiple active myofascial trigger points reproduce the overall spontaneous pain pattern in women with fibromyalgia and are related to widespread mechanical hypersensitivity. The Clinical Journal of Pain, 27(5), 405-413. https://doi.org/10.1097/AJP.0b013e318210110a
Alvarez, D. J., & Rockwell, P. G. (2002). Trigger points: Diagnosis and management. American Family Physician, 65(4), 653-661.
American Academy of Family Physicians. (2023). Fibromyalgia: Diagnosis and management. American Family Physician, 107(2), 137-144. PMID: 36791447
Aritzeta, A., Soroa, G., Balluerka, N., Muela, A., Gorostiaga, A., & Aliri, J. (2017). Reducing anxiety and improving academic performance through a biofeedback relaxation training program. Applied Psychophysiology and Biofeedback, 42(3), 193-202. https://doi.org/10.1007/s10484-017-9367-z
Babu, A. S., Mathew, E., Danda, D., & Prakash, H. (2007). Management of patients with fibromyalgia using biofeedback: A randomized control trial. Indian Journal of Medical Sciences, 61(8), 455-461.
Baehr, E., Rosenfeld, J. P., & Baehr, R. (1997). The clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression. Journal of Neurotherapy, 2(3), 10-23.
Bandelow, B., & Michaelis, S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327-335.
Beghini, A., Sammartino, A. M., Papp, Z., von Haehling, S., Bhatt, D. L., Aziz, E. F., & Bayes-Genis, A. (2024). 2024 update in heart failure. ESC Heart Failure, 11(2), 1-13. https://doi.org/10.1002/ehf2.14549
Beidel, D. C., Bulik, C. M., & Stanley, M. A. (2014). Abnormal psychology (3rd ed.). Pearson.
Brody, D. J., & Hughes, J. P. (2025). Symptoms of depression among adults: United States, 2021-2023. NCHS Data Brief, (521), 1-8.
Caldwell, Y. T., & Steffen, P. R. (2018). Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. International Journal of Psychophysiology, 131, 96-101.
CDC. (2023). Chronic pain and high-impact chronic pain among U.S. adults, 2019. National Center for Health Statistics.
Chen, Y. W., Camp, P. G., Coxson, H. O., Road, J. D., Guenette, J. A., & Hunt, M. A. (2023). A systematic review and network meta-analysis of breathing exercises for chronic obstructive pulmonary disease. Respiratory Medicine, 207, 107080.
Cheng, W., Rolls, E. T., Qiu, J., Liu, W., Tang, Y., Huang, C., . . . Feng, J. (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain, 139(12), 3296-3309.
Clerke, J., Preston-Ferrer, P., Zouridis, I. S., Tissot, A., Batti, L., Voigt, F. F., Pagès, S., Burgalossi, A., & Mameli, M. (2021). Output-specific adaptation of habenula-midbrain excitatory synapses during cocaine withdrawal. Frontiers in Synaptic Neuroscience, 13, 643138. https://doi.org/10.3389/fnsyn.2021.643138
Davidson, R. J. (1992). Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition, 20(1), 125-151.
Del Pozo, J. M., Gevirtz, R. N., Scher, B., & Guarneri, E. (2004). Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. American Heart Journal, 147(3), E11.
Desmeules, J. A., Cedraschi, C., Rapiti, E., Baumgartner, E., Finckh, A., Cohen, P., . . . Vischer, T. L. (2003). Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis and Rheumatism, 48(5), 1420-1429.
Donaldson, C. C. S., & Sella, G. E. (2003). The fibromyalgia syndrome: A comprehensive neuromuscular rehabilitation perspective. In D. Moss, A. McGrady, T. Davies, & I. Wickramasekera (Eds.), Handbook of mind-body medicine for primary care (pp. 277-295). Sage Publications.
Dugas, M. J., Gagnon, F., Ladouceur, R., & Freeston, M. H. (1998). Generalized anxiety disorder: A preliminary test of a conceptual model. Behaviour Research and Therapy, 36(2), 215-226.
Esper, R. J., Nordaby, R. A., Vilarino, J. O., Paragano, A., Cacharron, J. L., & Machado, R. A. (2006). Endothelial dysfunction: A comprehensive appraisal. Cardiovascular Diabetology, 5, 4.
Esteve, F., Blanc-Gras, N., Gallego, J., & Benchetrit, G. (1996). The effects of breathing pattern training on ventilatory function in patients with COPD. Biofeedback and Self-Regulation, 21(4), 311-321.
Fan, W., Fang, X., Chen, L., Wang, Q., & Qin, T. (2023). Hypertension prevalence, awareness, treatment, and control in adults: A multicountry cross-sectional analysis. BMC Public Health, 23, 508.
Finley, J. (2013). Myofascial pain. In D. Moss, F. Shaffer, R. Lyle, & S. Rosenberg (Eds.), Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Frank, D. L., Khorshid, L., Kiffer, J. F., Moravec, C. S., & McKee, M. G. (2010). Biofeedback in medicine: Who, when, why and how? Mental Health in Family Medicine, 7(2), 85-91.
Fryar, C. D., Ostchega, Y., Hales, C. M., Zhang, G., & Kruszon-Moran, D. (2024). Hypertension prevalence, awareness, treatment, and control among adults aged 18 and over: United States, August 2021-August 2023. NCHS Data Brief, (487), 1-8.
Gevirtz, R. N. (2003). The promise of HRV biofeedback: Some preliminary results and speculations. Biofeedback, 31(3), 18-19.
Gevirtz, R. N. (2005). Resonant frequency training to restore autonomic homeostasis for treatment of psychophysiological disorders. Biofeedback, 27(1), 7-9.
Gevirtz, R. N. (2013). The promise of heart rate variability biofeedback: Evidence-based applications. Biofeedback, 41(3), 110-120.
Giardino, N. D., Chan, L., & Borson, S. (2004). Combined heart rate variability and pulse oximetry biofeedback for chronic obstructive pulmonary disease: Preliminary findings. Applied Psychophysiology and Biofeedback, 29(2), 121-133.
Gilbert, C. (2023). Fibromyalgia. In I. Kazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenberg (Eds.). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Global Initiative for Asthma. (2018). Global strategy for asthma management and prevention. Retrieved from https://ginasthma.org
Global Initiative for Chronic Obstructive Lung Disease. (2019). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Retrieved from https://goldcopd.org
Glombiewski, J. A., Bernardy, K., & Häuser, W. (2013). Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: A meta-analysis and a systematic review of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine, 962741.
Goedde, M. G., Kabins, S. S., & Koenig, M. E. (2007). The effects of patient speech on blood pressure. Journal of Undergraduate Research, 8(1), 1-4.
Goessl, V. C., Curtiss, J. E., & Hofmann, S. G. (2017). The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychological Medicine, 47(15), 2578-2586.
Goldenberg, D. L. (2009). Diagnosis and differential diagnosis of fibromyalgia. The American Journal of Medicine, 122(12 Suppl), S14-S21. https://doi.org/10.1016/j.amjmed.2009.09.007
Goldstein, R. B., Smith, S. M., & Chou, S. P. (2016). The epidemiology of DSM-5 posttraumatic stress disorder in the United States. Journal of Clinical Psychiatry, 77(10), 1439-1450.
Greenberger, N. J. (2013). Chronic and recurrent abdominal pain. Merck Manual.
Guiles, R. F., Stern, M. J., & Gevirtz, R. N. (2014). HRV biofeedback for pediatric irritable bowel syndrome and functional abdominal pain. Applied Psychophysiology and Biofeedback, 39(3-4), 291-296.
Guiles, R. F., Stern, M. J., Huntley, S. D., & Gevirtz, R. N. (2016). Functional abdominal pain. In D. Moss & F. Shaffer (Eds.), Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Hallman, D. M., Olsson, E. M., von Scheele, B., Melin, L., & Lyskov, E. (2011). Effects of heart rate variability biofeedback in subjects with stress-related chronic neck pain: A pilot study. Applied Psychophysiology and Biofeedback, 36(2), 71-80.
Harrison, D. G., Coffman, T. M., & Wilcox, C. S. (2021). Pathophysiology of hypertension: The mosaic theory and beyond. Circulation Research, 128(7), 847-863.
Henriques, G., Keffer, S., Abrahamson, C., & Horst, S. J. (2011). Exploring the effectiveness of a computer-based heart rate variability biofeedback program in reducing anxiety in college students. Applied Psychophysiology and Biofeedback, 36(2), 101-112.
Hettema, J. M. (2008). What is the genetic relationship between anxiety and depression? American Journal of Medical Genetics Part C, 148(2), 140-146.
Ho, F. Y., Chan, C. S., Lo, W., & Leung, J. C. (2025). Cognitive behavioral therapy for insomnia in fibromyalgia: A systematic review and meta-analysis. Sleep Medicine Reviews, 67, 101788.
Huether, S. E., & McCance, K. L. (2020). Understanding pathophysiology (7th ed.). Mosby Elsevier.
Hughes, D., Judge, C., Murphy, R., Loughlin, E., Costello, M., Whiteley, W., . . . O'Donnell, M. J. (2020). Association of blood pressure lowering with incident dementia or cognitive impairment: A systematic review and meta-analysis. JAMA, 323(19), 1934-1944.
Humphreys, P. A., & Gevirtz, R. N. (2000). Treatment of recurrent abdominal pain: Components analysis of four treatment protocols. Journal of Pediatric Gastroenterology and Nutrition, 31(1), 47-51.
Iqbal, A. M., & Jamal, S. F. (2023). Essential hypertension. In StatPearls. StatPearls Publishing.
Kaplan, G. A., & Keil, J. E. (1993). Socioeconomic factors and cardiovascular disease: A review of the literature. Circulation, 88(4), 1973-1998.
Kern-Buell, C. L., McGrady, A. V., Conran, P. B., & Nelson, L. A. (2000). Asthma severity, psychophysiological indicators of arousal, and immune function in asthma patients undergoing biofeedback-assisted relaxation. Applied Psychophysiology and Biofeedback, 25(2), 79-91.
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K. R., . . . Wang, P. S. (2003). The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289(23), 3095-3105.
Khoja, O., Sherif, S., & Sherif, Y. (2024). Fibromyalgia and long COVID syndrome. Pain Medicine, 25(1), 34-42.
Korterink, J. J., Diederen, K., Benninga, M. A., & Tabbers, M. M. (2015). Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PloS One, 10(5), e0126982.
Laborde, S., Allen, M. S., Borges, U., Dosseville, F., Hosang, T. J., Iskra, M., . . . Javelle, F. (2022). Effects of voluntary slow breathing on heart rate and heart rate variability: A systematic review and a meta-analysis. Neuroscience & Biobehavioral Reviews, 138, 104711.
LaVaque, T. J., Hammond, D. C., Trudeau, D., Monastra, V., Perry, J., Lehrer, P., . . . Sherman, R. (2002). Template for developing guidelines for the evaluation of the clinical efficacy of psychophysiological interventions. Applied Psychophysiology and Biofeedback, 27(4), 273-281.
Lawson, K. (2020). Sleep dysfunction in fibromyalgia and therapeutic approaches. OA Arthritis, 2(1), 4.
Lawson, R. P., Nord, C. L., Seymour, B., Thomas, D. L., Dayan, P., Pilling, S., & Roiser, J. P. (2016). Disrupted habenula function in major depression. Molecular Psychiatry, 22(2), 202-208.
Lehrer, P., Vaschillo, E., Vaschillo, B., Lu, S. E., Scardella, A., Siddique, M., & Habib, R. H. (2004). Biofeedback treatment for asthma. Chest, 126(2), 352-361.
Lehrer, P., Kaur, K., Sharma, A., Shah, K., Huseby, R., Bhavsar, J., & Zhang, Y. (2020). Heart rate variability biofeedback improves emotional and physical health and performance: A systematic review and meta-analysis. Applied Psychophysiology and Biofeedback, 45(3), 109-129.
Lehrer, P. M., Hochron, S. M., Mayne, T., Isenberg, S., Carlson, V., Lasoski, A. M., . . . Rausch, L. (1994). Relaxation and music therapies for asthma among patients prestabilized on asthma medication. Journal of Behavioral Medicine, 17(1), 1-24. https://doi.org/10.1007/BF01856879
Lehrer, P. M., Smetankin, A., & Potapova, T. (2000). Respiratory sinus arrhythmia biofeedback therapy for asthma: A report of 20 unmedicated pediatric cases using the Smetankin method. Applied Psychophysiology and Biofeedback, 25(3), 193-200.
Lehrer, P. M., Vaschillo, E., Vaschillo, B., Lu, S. E., Eckberg, D. L., Edelberg, R., . . . Hamer, R. M. (2003). Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine, 65(5), 796-805.
Lehrer, P. M., Moritz, C., & Greenfield, N. S. (2023). Asthma. In I. Kazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenberg (Eds.). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Lin, G., Xiang, Q., Fu, X., Wang, S., Wang, S., Chen, S., . . . Wang, T. (2012). Heart rate variability biofeedback decreases blood pressure in prehypertensive subjects by improving autonomic function and baroreflex. Journal of Alternative and Complementary Medicine, 18(2), 143-152. https://doi.org/10.1089/acm.2010.0607
Lin, I. M., Fan, S. Y., Yen, C. F., Yeh, Y. C., Tang, T. C., Huang, M. F., . . . Tsai, H. Y. (2019). Heart rate variability biofeedback increased autonomic activation and improved symptoms of depression and insomnia among patients with major depressive disorder. Clinical Psychopharmacology and Neuroscience, 17(2), 222-232. https://doi.org/10.9758/cpn.2019.17.2.222
Linden, W., & McGrady, A. (2016). Mechanisms of biofeedback therapy for blood pressure. In D. Moss & F. Shaffer (Eds.), Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Madhur, M. S. (2014). Hypertension. Medscape.
Manneh, R., & Lucas, J. W. (2025). Chronic obstructive pulmonary disease among adults aged 18 and over, United States, 2022. NCHS Data Brief, (495), 1-8.
McGrady, A. (1996). Good news-bad news: Applied psychophysiology in cardiovascular disorders. Biofeedback and Self-Regulation, 21(4), 335-346.
McGrady, A., & Higgins, J. T. (1989). Prediction of response to biofeedback-assisted relaxation in hypertensives: Development of a hypertensive predictor profile (HYPP). Psychosomatic Medicine, 51(3), 277-284.
McGrady, A., & Moss, D. (2013). Pathways to illness, pathways to health. Springer.
Meeus, M., Goubert, D., De Backer, F., Struyf, F., Hermans, L., Coppieters, I., . . . Nijs, J. (2013). Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: A systematic review. Seminars in Arthritis and Rheumatism, 43(2), 279-287.
Meuret, A. E., Ritz, T., Wilhelm, F. H., & Roth, W. T. (2007). Targeting pCO2 in asthma: Pilot evaluation of a capnometry-assisted breathing training. Applied Psychophysiology and Biofeedback, 32(3-4), 99-109.
Moravec, C. S., & McKee, M. G. (2016). Heart failure. In D. Moss & F. Shaffer (Eds.), Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Moss, D., Shaffer, F., & White, P. (2009). Heart rate variability suite. Thought Technology Ltd.
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., . . . Turner, M. B. (2016). Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation, 133(4), e38-e360.
Murphy, A. (2009). A preliminary investigation of stress reduction techniques and a heart rate variability training program for the treatment of generalized anxiety disorder. Dissertation, University of Missouri-Columbia.
Naghavi, M., Libby, P., Falk, E., Casscells, S. W., Litovsky, S., Rumberger, J., . . . Willerson, J. T. (2003). From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation, 108(14), 1664-1672.
National Center for Health Statistics. (2025). Asthma. Centers for Disease Control and Prevention.
Nijs, J., Malfliet, A., & Nishigami, T. (2023). Nociplastic pain: The missing piece of the chronic pain puzzle. Pain, 164(6), 1171-1176.
NIMH. (2023). Anxiety disorders. National Institute of Mental Health.
Nolan, R. P., Floras, J. S., Harvey, P. J., Kamath, M. V., Picton, P. E., Chessex, C., . . . Chen, M. H. (2010). Behavioral neurocardiac training in hypertension: A randomized, controlled trial. Hypertension, 55(4), 1033-1039.
Norweg, A., Hofferber, B., Maguire, S., Oh, C., Raveis, V. H., & Simon, N. M. (2024). Breathing on the mind: Treating dyspnea and anxiety symptoms with biofeedback in chronic lung disease—A qualitative analysis. Respiratory Medicine, 221, 107505. https://doi.org/10.1016/j.rmed.2023.107505
Nurmagambetov, T., Kuwahara, R., & Garbe, P. (2018). The economic burden of asthma in the United States, 2008-2013. Annals of the American Thoracic Society, 15(3), 348-356.
O'Hearn, M., Lauren, B. N., Wong, J. B., Kim, D. D., & Mozaffarian, D. (2022). Trends and disparities in cardiometabolic health among U.S. adults, 1999-2018. Journal of the American College of Cardiology, 80(2), 138-151. https://doi.org/10.1016/j.jacc.2022.04.046
O'Donnell, D. E., Laveneziana, P., Webb, K., & Neder, J. A. (2008). Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax, 64(3), 216-223.
Olshansky, B., Sabbah, H. N., Hauptman, P. J., & Colucci, W. S. (2008). Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation, 118(8), 863-871.
Overhaus, S., Ruddel, H., Curio, I., Mussgay, L., & Scholz, O. B. (2003). Biofeedback of baroreflex sensitivity in patients with mild essential hypertension. International Journal of Behavioral Medicine, 10(2), 151-158.
Pate, C. A., & Zahran, H. S. (2024). The status of asthma in the United States. Preventing Chronic Disease, 21, E53. https://doi.org/10.5888/pcd21.240005
Pizzoli, S. F. M., Marzorati, C., Gatti, D., Monzani, D., Mazzocco, K., & Pravettoni, G. (2021). A meta-analysis on heart rate variability biofeedback and depressive symptoms. Scientific Reports, 11, 6650.
Prasad, V., Vandross, A., Toomey, C., Cheung, M., Rho, J., Quinn, S., . . . Cifu, A. (2013). A decade of reversal: An analysis of 146 contradicted medical practices. Mayo Clinic Proceedings, 88(8), 790-798.
Prinsloo, G. E., Derman, W. E., Lambert, M. I., & Rauch, H. G. L. (2013). The effect of a single episode of short duration heart rate variability biofeedback on measures of anxiety and relaxation states. International Journal of Stress Management, 20(4), 391-411. https://doi.org/10.1037/a0034777
Rahman, I., & Adcock, I. M. (2006). Oxidative stress and redox regulation of lung inflammation in COPD. The European Respiratory Journal, 28(1), 219-242. https://doi.org/10.1183/09031936.06.00053805
Ratanasiripong, P., Sverduk, K., Prince, J., & Hayashino, D. (2012). Biofeedback and counseling for stress and anxiety among college students. Journal of College Student Development, 53(5), 742-749.
Raymond, J., Sajid, I., Parkinson, L. A., & Gruzelier, J. H. (2005). Biofeedback and dance performance: A preliminary investigation. Applied Psychophysiology and Biofeedback, 30(1), 64-73.
Reneau, M. (2020). Heart rate variability biofeedback to treat fibromyalgia: An integrative literature review. Pain Management Nursing, 21(3), 225-232. https://doi.org/10.1016/j.pmn.2019.08.001
Reyes del Paso, G. A., Cea, J. I., Gonzalez-Pinto, A., Cabo, O. M., Caso, R., Brazal, J., . . . Gonzalez, M. I. (2006). Short-term effects of a brief respiratory training on baroreceptor cardiac reflex function in normotensive and mild hypertensive subjects. Applied Psychophysiology and Biofeedback, 31(1), 37-49.
Roehrs, T. A., Roth, T., Tang, N. K. Y., & Smith, M. T. (2020). Sleep and pain in humans with fibromyalgia and comorbid insomnia: Double-blind, crossover study of suvorexant 20 mg versus placebo. SLEEP Advances, 1(1), zpaa003. https://doi.org/10.1093/sleepadvances/zpaa003
Rosenthal, S. (2023). Chronic neck pain. In I. Kazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenberg (Eds.). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Ross, R. (1993). The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature, 362(6423), 801-809. https://doi.org/10.1038/362801a0
Sancassiani, F., Machado, S., Ruggiero, V., Cacace, E., Carmassi, C., Gesi, C., Dell'Osso, L., & Carta, M. G. (2024). Heart rate variability biofeedback efficacy on fatigue and energy levels in fibromyalgia: A secondary analysis of RCT NCT0412183. Journal of Clinical Medicine, 13(14), 4008. https://doi.org/10.3390/jcm13144008
Sandberg, S., Paton, J. Y., Ahola, S., McCann, D. C., McGuinness, D., Hillary, C. R., & Oja, H. (2000). The role of acute and chronic stress in asthma attacks in children. Lancet, 356, 982-987. https://doi.org/10.1016/s0140-6736(00)02715-x
Saps, M., Velasco-Benitez, C. A., Langshaw, A. H., & Ramírez-Hernández, C. R. (2016). Prevalence of functional gastrointestinal disorders in children and adolescents: Comparison between Rome III and Rome IV criteria. Journal of Pediatrics, 199, 212-216.
Shaffer, F. (2023). Preeclampsia. In I. Kazan, F. Shaffer, D. Moss, R. Lyle, & S. Rosenberg (Eds.). Evidence-based practice in biofeedback and neurofeedback (4th ed.). Association for Applied Psychophysiology and Biofeedback.
Shahidi, B., Curran-Everett, D., & Maluf, K. S. (2015). Psychosocial, physical, and neurophysiological risk factors for chronic neck pain: A prospective inception cohort study. The Journal of Pain, 16(12), 1288-1299. https://doi.org/10.1016/j.jpain.2015.09.002
Siddiqui, M. J., Ahmad, M., Ahmad, A., & Khan, M. A. (2024). Bridging the gap: Tackling racial and ethnic disparities in hypertension management. Cureus, 16(9), e68651. https://doi.org/10.7759/cureus.68651
Siepmann, M., Aykac, V., Unterdorfer, J., Petrowski, K., & Mueck-Weymann, M. (2008). A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Applied Psychophysiology and Biofeedback, 33(4), 195-201.
Somers, P. J., Gevirtz, R. N., Jasin, S. E., & Chin, H. G. (1989). The efficacy of biobehavioral and compliance interventions in the adjunctive treatment of mild pregnancy-induced hypertension. Biofeedback and Self-Regulation, 14(4), 309-318. https://doi.org/10.1007/BF00999122
Song, H., & Lehrer, P. M. (2003). The effects of specific respiratory rates on heart rate and heart rate variability. Applied Psychophysiology and Biofeedback, 28(1), 13-23. https://doi.org/10.1023/a:1022312815649
Soroosh, S. G., & Farbod, A. (2024). Epidemiology of fibromyalgia: East versus West. International Journal of Rheumatic Diseases, 27(12), e15428. https://doi.org/10.1111/1756-185X.15428
Sowder, E., Gevirtz, R., Shapiro, W., & Ebert, C. (2010). Restoration of vagal tone: A possible mechanism for functional abdominal pain. Applied Psychophysiology and Biofeedback, 35(3), 199-206.
Spitzer, W. O., Skovron, M. L., Salmi, L. R., Cassidy, J. D., Duranceau, J., Suissa, S., & Zeiss, E. (1995). Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: Redefining "whiplash" and its management. Spine, 20(8 Suppl), 1S-73S.
Stern, M. J., Guiles, R. F., & Gevirtz, R. (2014). HRV biofeedback for pediatric irritable bowel syndrome and functional abdominal pain: A clinical replication series. Applied Psychophysiology and Biofeedback, 39(3-4), 287-291. https://doi.org/10.1007/s10484-014-9261-x
Strack, B., & Gevirtz, R. (2011). Getting to the heart of the matter: Heart rate variability biofeedback for enhanced performance. In B. Strack, M. Linden & V. Wilson (Eds.), Biofeedback and neurofeedback applications in sport psychology (pp. 145-174). Association for Applied Psychophysiology and Biofeedback.
Swanson, K. S., Gevirtz, R. N., Brown, M., Spira, J., Guarneri, E., & Stoletniy, L. (2009). The effect of biofeedback on function in patients with heart failure. Applied Psychophysiology and Biofeedback, 34(2), 71-91.
Taghizadeh, N., Eslaminejad, A., & Raoufy, M. R. (2019). Protective effect of heart rate variability biofeedback on stress-induced lung function impairment in asthma. Respiratory Physiology and Neurobiology, 262, 49-56. https://doi.org/10.1016/j.resp.2019.01.011
Thurber, M. R. (2006). Effects of heart-rate variability biofeedback training and emotional regulation on music performance anxiety in university students. University of North Texas Press.
Tortora, G. J., & Derrickson, B. H. (2021). Principles of anatomy and physiology (16th ed.). John Wiley & Sons, Inc.
Tracey, K. J. (2002). The inflammatory reflex. Nature, 420(6917), 853-859.
Tsai, P. S., Chang, N. C., Chang, W. Y., Lee, P. H., & Wang, M. Y. (2007). Blood pressure biofeedback exerts intermediate-term effects on blood pressure and pressure reactivity in individuals with mild hypertension: A randomized controlled study. Journal of Alternative and Complementary Medicine, 13(5), 547-554. https://doi.org/10.1089/acm.2007.6289
Van Gestel, A. J., Kohler, M., Steier, J., Teschler, S., Russi, E. W., & Teschler, H. (2012). The effects of controlled breathing during pulmonary rehabilitation in patients with COPD. Respiration, 83(2), 115-124. https://doi.org/10.1159/000324449
Varricchi, G., Brightling, C. E., Grainge, C., Lambrecht, B. N., & Chanez, P. (2024). Airway remodelling in asthma and the epithelium: On the edge of a new era. European Respiratory Journal, 63(4), 2301619. https://doi.org/10.1183/13993003.01619-2023
Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., . . . Tsao, C. W. (2020). Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation, 141(9), e139-e596.
Volgman, A. S., Palaniappan, L. S., Aggarwal, N. T., Gupta, M., Khandelwal, A., Krishnan, A. V., . . . Wong, N. D. (2018). Atherosclerotic cardiovascular disease in South Asians in the United States: Epidemiology, risk factors, and treatments. Circulation, 138(1), e1-e34.
Walling, A. D. (2004). Management of gestational hypertension-preeclampsia. American Family Physician, 69(4), 979.
Weaver, M. T., & McGrady, A. (1995). A provisional model to predict blood pressure response to biofeedback-assisted relaxation. Biofeedback and Self-Regulation, 20(3), 229-240.
Wei, S., Bahl, M. I., Trost, K., Gao, K., Licht, T. R., & Schwab, C. (2022). Diet, microbiome, and host metabolism: The link to salt-sensitive hypertension. Gut Microbes, 14(1), 2116266. https://doi.org/10.1080/19490976.2022.2116266
Wheat, A. L., & Larkin, K. T. (2010). Biofeedback of heart rate variability and related physiology: A critical review. Applied Psychophysiology and Biofeedback, 35(3), 229-242.
Wu, Y. L., Fang, S. C., Chen, S. C., Tai, C. J., & Tsai, P. S. (2021). Effects of neurofeedback on fibromyalgia: A randomized controlled trial. Pain Management Nursing, 22(6), 755-763. https://doi.org/10.1016/j.pmn.2021.01.004
Yamasaki, A. (2023). Editorial: Airway remodelling in asthma—What is new? Frontiers in Allergy, 4, 1129840. https://doi.org/10.3389/falgy.2023.1129840
Yates, W. R. (2014). Anxiety disorders. Medscape.
Yorke, J., Fleming, S. L., & Shuldham, C. M. (2007). Psychological interventions for adults with asthma. Cochrane Database of Systematic Reviews, (1), CD002982. https://doi.org/10.1002/14651858.cd002982.pub3
Young, K. D., Zotev, V., Phillips, R., Misaki, M., Yuan, H., Drevets, W. C., & Bodurka, J. (2014). Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PloS One, 9(2), e88785.
Yu, L. C., Fan, S. Y., Chien, C. L., & Lin, T. H. (2018). One year cardiovascular prognosis of the randomized, controlled, short-term heart rate variability among patients with coronary artery disease. International Journal of Behavioral Medicine, 25(3), 271-282. https://doi.org/10.1007/s12529-017-9707-7
Yusuf, S., Reddy, S., Ounpuu, S., & Anand, S. (2001). Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation, 104(22), 2746-2753.
Zimmerman, M., Chelminksi, I., & McDermut, W. (2002). Major depressive disorder and Axis I diagnostic comorbidity. Journal of Clinical Psychiatry, 63, 187-193.
Return to Top