Psychophysiology
What You Will Learn in This Chapter
Have you ever noticed your heart pounding during a suspenseful movie scene, even though you are perfectly safe in your seat? This unit explores the fascinating two-way street between your mind and body. You will discover how Green and Green's revolutionary psychophysiological principle explains why thoughts can make your palms sweat and why relaxing your muscles can calm your racing mind.
Along the way, you will explore the three branches of the autonomic nervous system, unpack Porges' intriguing polyvagal theory (which reveals that we have more options than just "fight or flight"), and learn why conducting a thorough psychophysiological stress profile is essential before designing a treatment plan. By the end of this unit, you will understand concepts like homeostasis, habituation, the law of initial values, and how placebo effects work at the neurological level.
You will also discover cutting-edge research frontiers that are reshaping how scientists and clinicians think about the mind-body connection. These include how your nervous system can actually help "brake" inflammation, why some people with Long COVID experience symptoms that feel like their body's automatic settings are off, how slow aging of the immune system connects to autonomic flexibility, and what happens when your brain misreads signals from inside your body.
This unit covers the Psychophysiological Principle, the Autonomic Nervous System (ANS), Sympathetic Division, Parasympathetic Division, Porges' Polyvagal Theory, Enteric Division, The Relationship Between the Sympathetic and Parasympathetic Branches, Homeostasis, Autonomic Balance, Psychophysiological Measurements, Orienting and Defensive Responses, Activation and Habituation, Situational Specificity, Law of Initial Values (LIV), Generalization of Biofeedback Training, and the Placebo Effect. This unit also explores cutting-edge research frontiers including the Inflammatory Reflex and Neuroimmune Regulation, Long COVID and Autonomic Dysfunction, Inflammaging and Healthy Aging, and Interoception and Body Signal Awareness.
BCIA Blueprint Coverage: This unit addresses Structure and function of the autonomic nervous system (V-A1-2) and Psychophysiological Concepts (V-B).

This powerful idea forms the foundation of Green and Green's psychophysiological principle.
Listen to a mini-lecture on PsychophysiologyPsychophysiology studies the interrelationship between psychological and physiological processes. As Andreassi (2007) explained, "Psychophysiology is the study of relations between psychological manipulations and resulting physiological responses, measured in the living organisms, to promote understanding of the relation between mental and bodily processes" (p. 2). The interrelationship between psychological and physiological processes is dynamic and bidirectional.

As a biofeedback practitioner, you study the basic principles of psychophysiology to understand disease processes and health and how clinical interventions achieve their results.
The idiographic approach, which emphasizes individual differences, is critical to biofeedback practice. Think of it this way: just as no two fingerprints are identical, each client's response to stressors is unique, specific, and often complex.
Clinical Application: Discovering Your Client's Unique Pattern
To effectively treat your client, you need to discover their characteristic responses to stressors during a psychophysiological profile administered during the assessment stage of treatment. Consider a client named Jack who comes to you for stress management. During his assessment, you notice that his hand temperature falls and his respiration rate increases during math and visual imagery stressors. Based on this unique pattern, you might consider training Jack to maintain hand temperature around 90°F (32.2°C) while breathing under 7 breaths per minute. This tailored approach may prove far more effective than using a standard treatment protocol that disregards his response stereotypy, his characteristic pattern of physiological responses to stress.

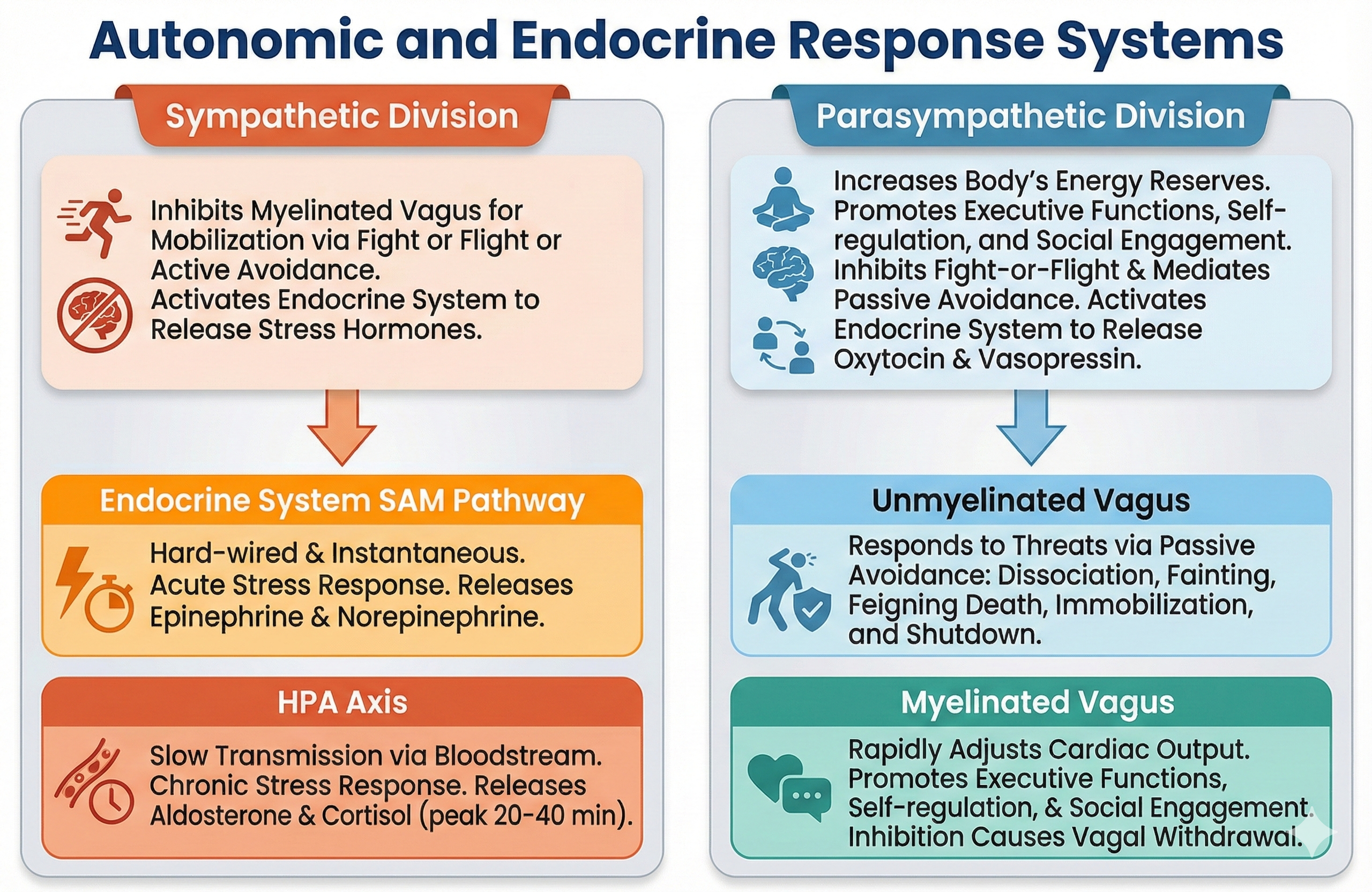
This unit will challenge the simplistic belief that the sympathetic division fight-or-flight response is our only means of responding to stressors. The parasympathetic branch expands our response options through immobilization, feigning death, passive avoidance, and shutdown, or through social engagement supported by the release of the hormone oxytocin.

The Revolutionary Psychophysiological Principle
Green, Green, and Walters' (1970) psychophysiological principle was revolutionary because it proposed a bidirectional relationship between physiological and psychological functioning:
Every change in the physiological state is accompanied by an appropriate change in the mental emotional state, conscious or unconscious, and conversely, every change in the mental emotional state, conscious or unconscious, is accompanied by an appropriate change in the physiological state.
Here is a compelling example of the psychophysiological principle in action: facial muscle contraction can influence emotion, and emotion can influence facial muscle contraction. Researchers have discovered that Botox injections, which paralyze facial muscles to treat wrinkles, actually reduce the intensity of a person's emotional experiences. When you cannot physically frown, the brain receives fewer signals associated with negative emotions.

Key Takeaways: The Psychophysiological Principle
The psychophysiological principle tells us that mind and body form a continuous loop. When your client learns to change their physiology through biofeedback, they are simultaneously changing their psychological state. Conversely, cognitive and emotional interventions produce measurable physiological changes. This bidirectional relationship is the foundation of biofeedback practice.
The Autonomic Nervous System: Your Body's Control Center
The central nervous system (CNS) includes the brain, spinal cord, and retina. The peripheral nervous system consists of the somatic nervous system and the three branches of the autonomic nervous system (Breedlove & Watson, 2023).
Listen to a mini-lecture on the Central and Peripheral Nervous Systems
The somatic nervous system controls the contraction of skeletal muscles and transmits somatosensory information to the CNS. The autonomic nervous system (ANS) regulates cardiac and smooth muscle and glands, transmits sensory information to the CNS, and innervates muscle spindles.
The ANS is divided into three main systems: sympathetic, parasympathetic, and enteric. Check out the YouTube video The Autonomic Nervous System.
The Sympathetic Division: Your Emergency Response System
Think of the sympathetic nervous system (SNS) as your body's alarm system. It readies you for action and regulates activities that expend stored energy. A simple way to remember this system is that sympathetic activity prepares the body for "fight or flight" (Gentile et al., 2024; Karemaker, 2017).
Listen to a mini-lecture on the Sympathetic Nervous System Function
The SNS, in concert with the endocrine system, responds to threats to our safety through mobilization, fight-or-flight, and active avoidance. The SNS responds more slowly (greater than 5 seconds) and for a more extended period than the more rapid (less than 1 second) parasympathetic vagus system (Nunan et al., 2010). Porges (2011) theorized that the SNS inhibits the unmyelinated vagus (dorsal vagal complex) to mobilize us for action.
When activated, the SNS produces mass activation, meaning multiple organs respond simultaneously. This coordinated response includes increased heart rate and respiration, dilated pupils, reduced digestive activity, and increased blood flow to skeletal muscles. The SNS achieves this through the release of norepinephrine and epinephrine from the adrenal medulla, arrector pili muscles of the skin, cutaneous sweat glands, and most blood vessels.
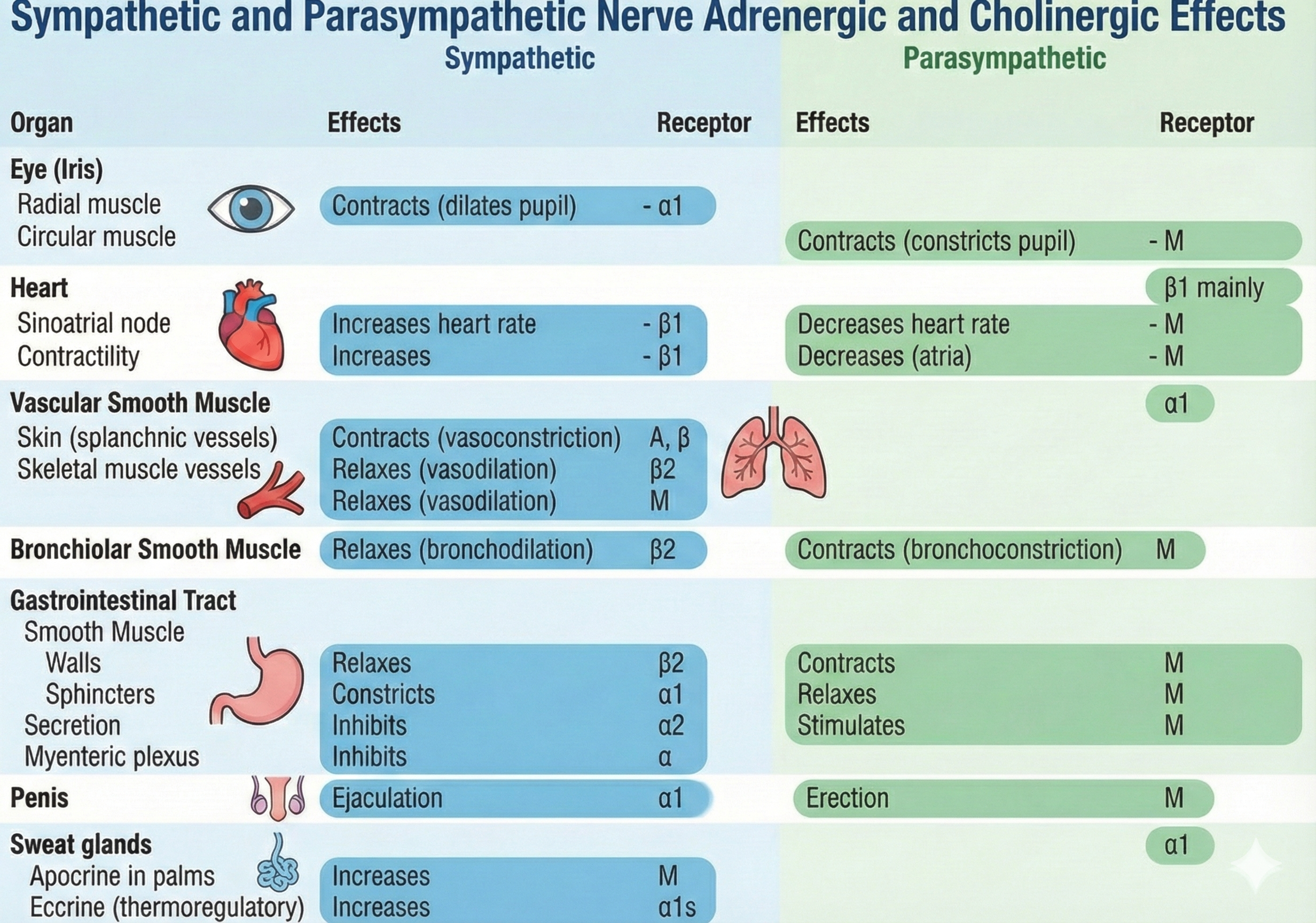
Adrenergic receptors produce changes through G-proteins. The following table is adapted from Fox and Rompolski (2022).
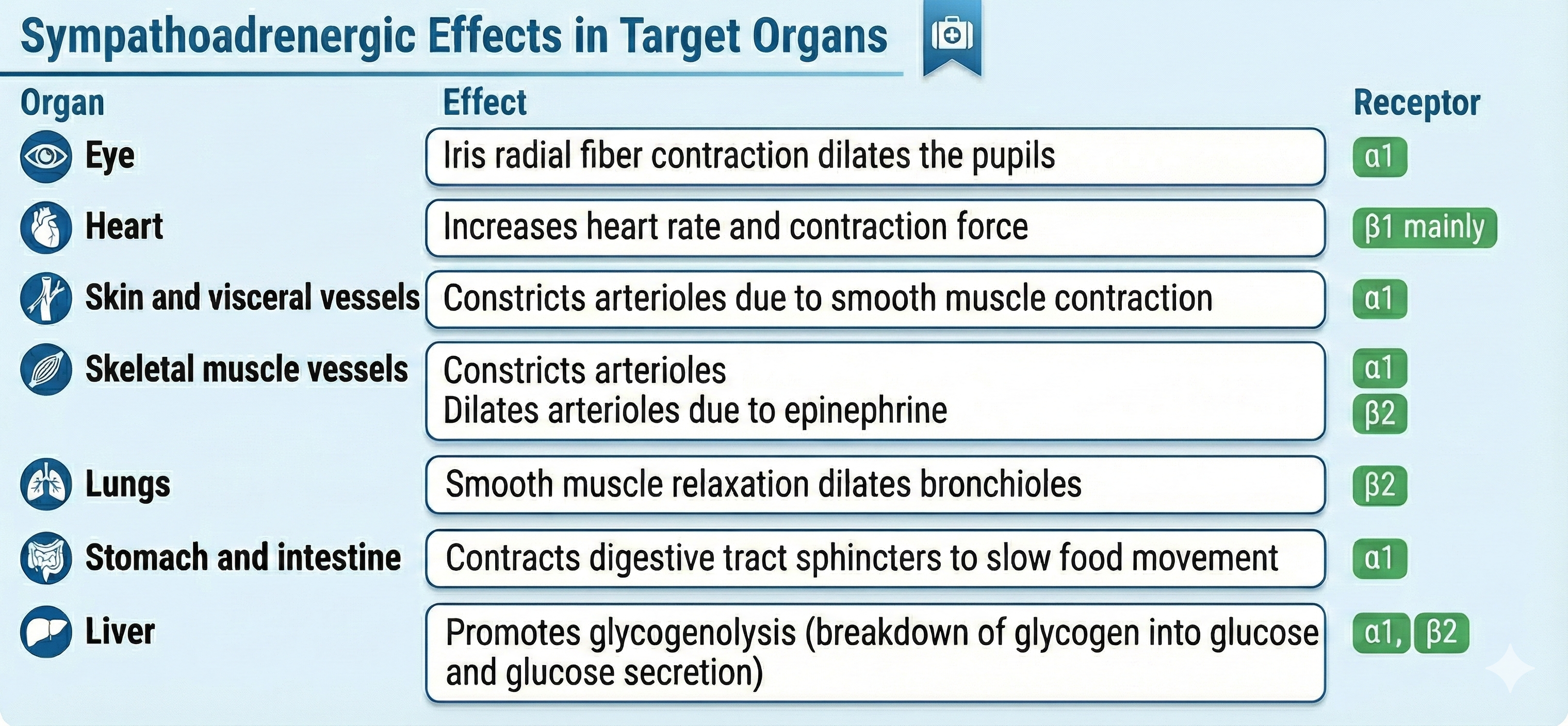
Organs With Sympathetic Innervation Only
The adrenal medulla, arrector pili muscles of the skin, cutaneous sweat glands, and most blood vessels receive sympathetic innervation exclusively (Fox & Rompolski, 2025).
Preganglionic and Postganglionic Neurotransmitters Are Different
SNS preganglionic and postganglionic axons release different neurotransmitters. SNS preganglionic axons secrete acetylcholine, while the postganglionic axons secrete norepinephrine. Sweat glands and skeletal muscle blood vessels are the exceptions to this rule. The postganglionic axons that innervate them release acetylcholine (Fox & Rompolski, 2025).
The Sympathetic Branch and Heart Rate Variability
Heart rate variability (HRV) is the natural beat-to-beat variation in the time between heartbeats, reflecting how flexibly the ANS is influencing the heart. A later section explores HRV in depth as a window into brain-heart communication. Here, we focus on a critical clinical point: the SNS does not appear to contribute significantly to the low-frequency (LF) component (0.04-0.15 Hz) of HRV under resting conditions, as was previously believed (Draghici & Taylor, 2016; Tiwari et al., 2020).
A healthy heart is not a metronome.
Most commonly used HRV measures, such as the root mean square of successive differences (RMSSD) and high-frequency (HF) power (0.15-0.40 Hz), are strongly driven by rapid vagus nerve effects on the heart and are therefore considered markers of cardiac vagal tone, or parasympathetic influence (Ernst, 2017; Thomas et al., 2019). Reviews and experimental work show that markers once thought to indicate sympathetic activity, such as LF power, actually reflect a mixture of influences and should not be treated as pure sympathetic measures (Ernst, 2017; Thomas et al., 2019). The broad consensus is that the main HRV indices used in research and clinical practice mostly reflect parasympathetic rather than sympathetic activity (Draghici & Taylor, 2016; Tiwari et al., 2020).
Stephen Porges has emphasized that most stressors do not require the sympathetic branch's intensive energy expenditure during fight-or-flight.
When we perceive daily stressors as challenging, this causes vagal withdrawal instead of sympathetic activation. In vagal withdrawal, we disengage parasympathetic control of our viscera (large internal organs) and reduce HRV.
We shift from a more calm, socially engaged state. We are now ready for mobilization, either fight or flight (mediated by the SNS) or immobilization.
Vagal withdrawal increases power in the very-low-frequency (VLF) band (0.04 Hz and below) and lowers it in the HF band.

Dr. Gevirtz provides an overview of the sympathetic branch © Association for Applied Psychophysiology and Biofeedback. You can enlarge this video by clicking on the bracket icon at the bottom right of the screen. When finished, click on the ESC key.
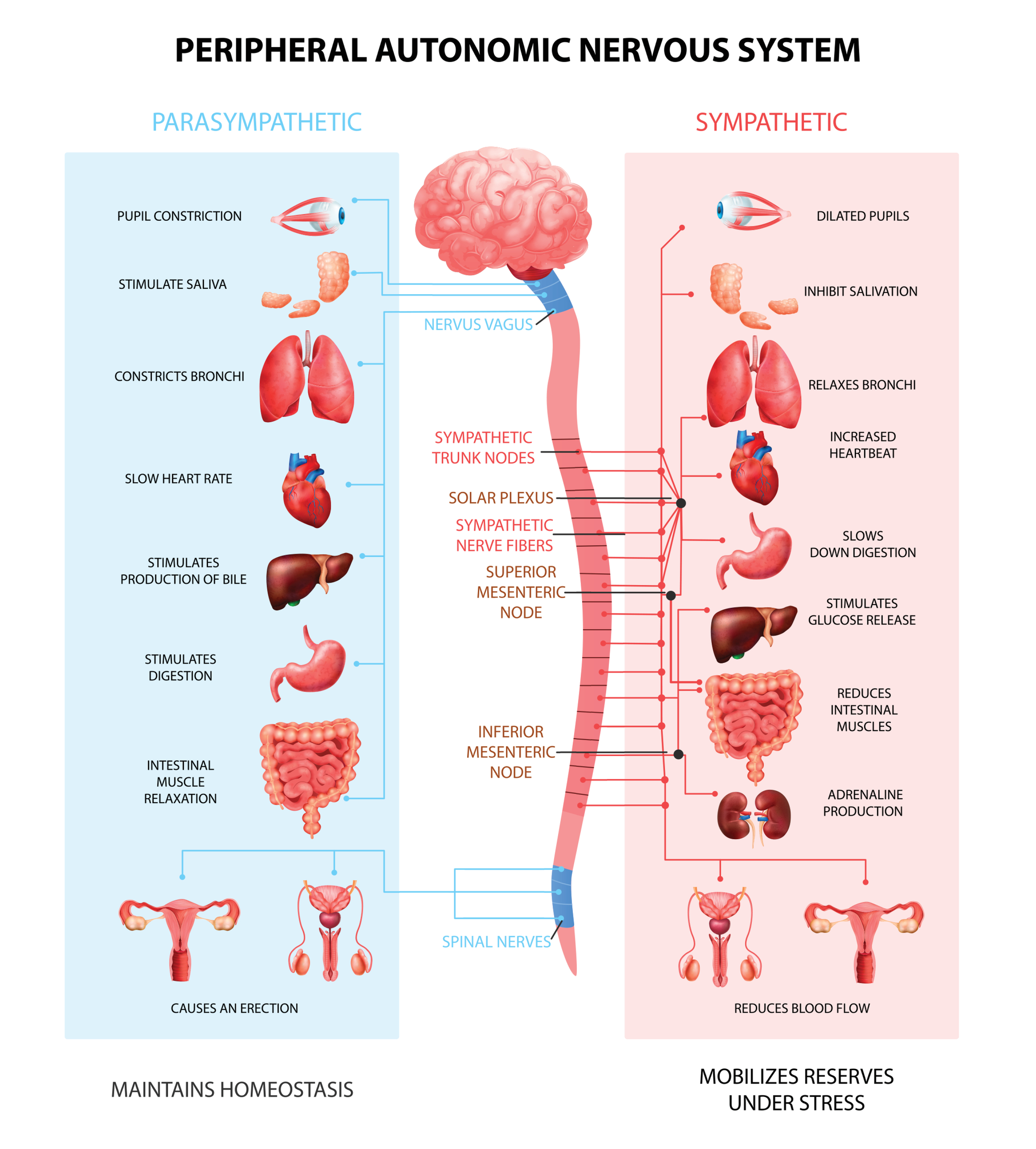
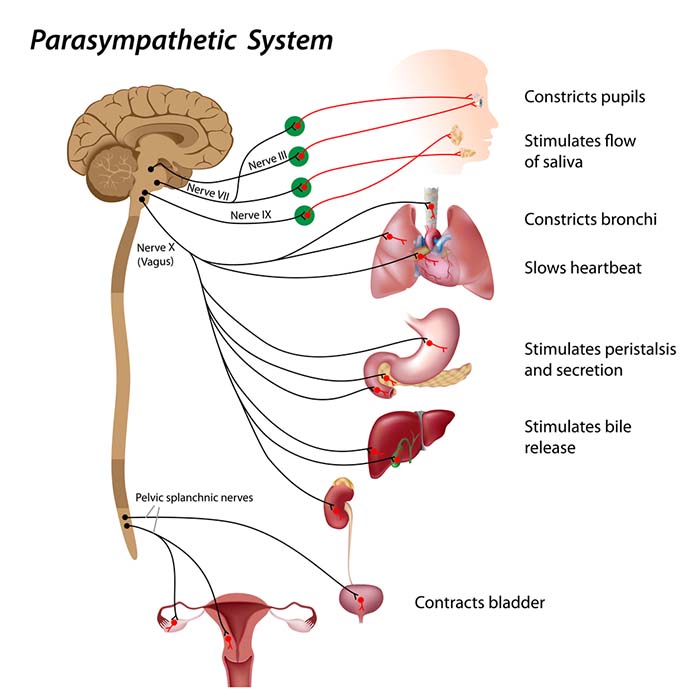
The Parasympathetic Division: Rest, Digest, and Connect
The parasympathetic division, or parasympathetic nervous system (PNS), regulates activities that increase the body's energy reserves, including salivation, gastric (stomach) and intestinal motility, gastric juice secretion, and increased blood flow to the gastrointestinal system. Think of it as your body's "rest and digest" system (Gentile et al., 2024; Karemaker, 2017). When we discuss Porges' polyvagal theory, we will learn that this system is also involved in self-regulation, social engagement, and passive responses to inescapable threats.
Listen to a mini-lecture on the Parasympathetic Nervous System FunctionHRV biofeedback uses slow-paced breathing and rhythmic skeletal muscle contraction to restore healthy parasympathetic activity.
PNS cell bodies are found in the nuclei of four cranial nerves (especially the vagus) and the spinal cord's sacral region (S2-S4). The parasympathetic division is craniosacral.
Listen to a mini-lecture on the Parasympathetic Nervous System OrganizationUnlike the sympathetic division, parasympathetic ganglia are located near their target organs. This arrangement means that preganglionic axons are relatively long, postganglionic axons are relatively short, and PNS changes can be selective.
Preganglionic neurons travel with the oculomotor, facial, glossopharyngeal, and vagus cranial nerves. Preganglionic neurons that exit the vagus (X) nerve at the medulla synapse with terminal ganglia within the heart, lungs, esophagus, stomach, pancreas, liver, and intestines. Both PNS preganglionic and postganglionic axons release acetylcholine (Fox & Rompolski, 2025).
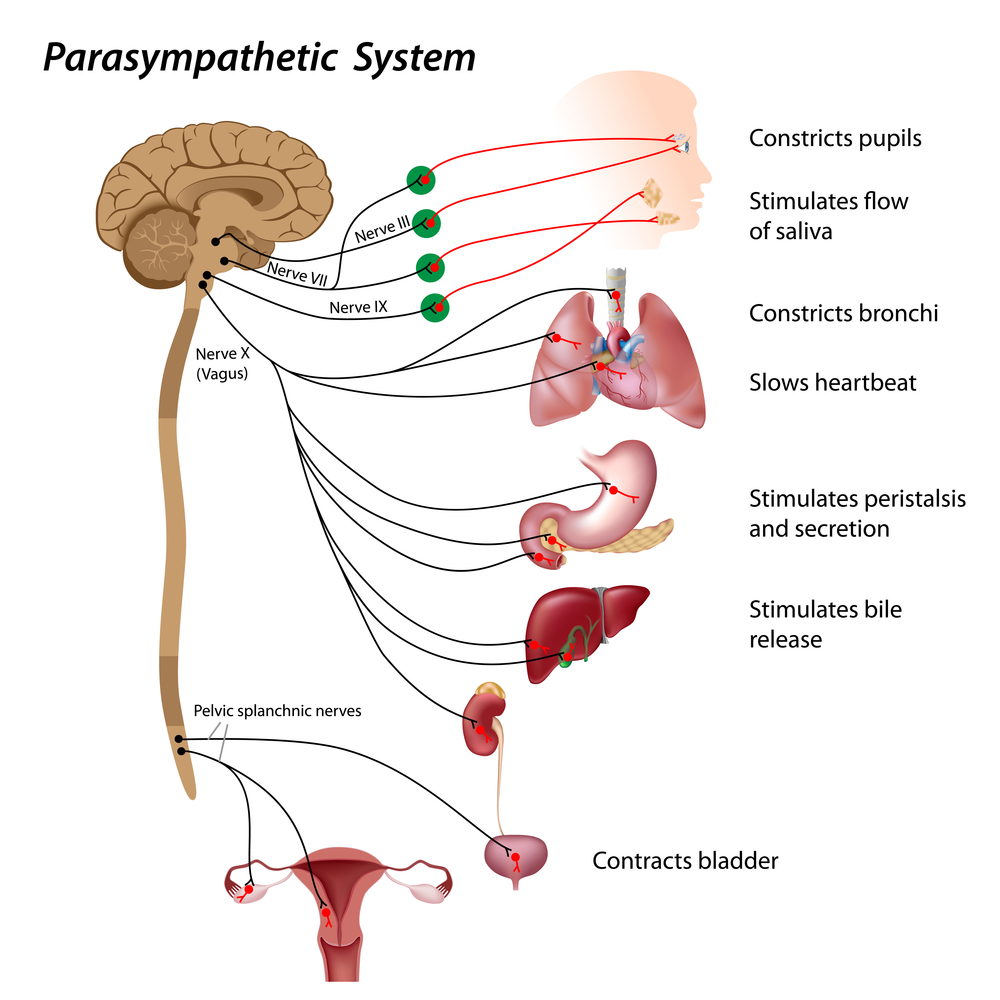
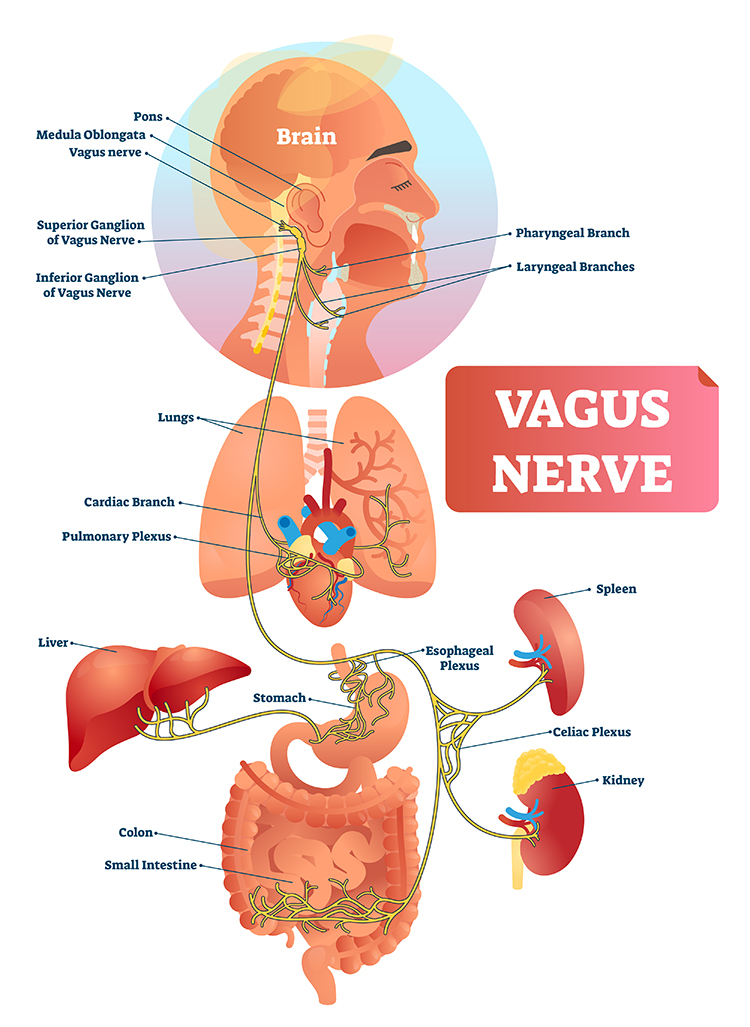
The Vagus Nerve: A Major Parasympathetic Pathway
The vagus nerve is the main parasympathetic nerve going from the brainstem to the heart, lungs, and digestive organs (Olshansky et al., 2008; Rajendran et al., 2023). Its efferent fibers (outgoing signals) release the chemical messenger acetylcholine at the heart, slowing the natural pacemaker and reducing heart rate, especially on a beat-to-beat time scale (Draghici & Taylor, 2016; Rajendran et al., 2018). Most vagus nerve fibers are actually afferent, meaning they carry sensory information from the heart and other organs back to the brain, where this information helps adjust both parasympathetic and sympathetic output (van Weperen & Vaseghi, 2023).
Because vagal signals can rapidly slow the heart and inhibit sympathetic drive, many therapies for heart disease and stress aim to enhance vagal activity, either with drugs, breathing and relaxation techniques, or electrical vagus nerve stimulation (Gentile et al., 2024; Pérez-Alcalde et al., 2024).
Sympathetic Fibers Inside the Vagus Nerve
Although the vagus nerve is usually taught as a parasympathetic nerve, human anatomical studies show it also contains a small but real sympathetic component. In carefully analyzed human cervical vagus nerves, about 13 to 14 percent of fibers had sympathetic characteristics, identified by a marker enzyme called tyrosine hydroxylase (Kronsteiner et al., 2024). Another study of more than 100 human vagus nerves found that sympathetic fibers made up around four to six percent of the total cross-sectional area, and that these fibers were scattered or clustered in different patterns from person to person (Seki et al., 2014).
This mixing means that stimulating the vagus in the neck, for example in implanted vagus nerve stimulation for epilepsy or heart failure, may activate not only parasympathetic fibers but also some sympathetic fibers, which could partially explain why heart rate responses and side effects vary between patients (Gentile et al., 2024; Seki et al., 2014).
Dr. Gevirtz provides an overview of the parasympathetic branch © Association for Applied Psychophysiology and Biofeedback.
Dampening Inflammation
The vagus nerve's sensory branch detects inflammation and infection via tissue necrosis factor (TNF) and interleukin-1 (IL-1). The motor branch of the vagus signals descending neurons to release norepinephrine, which prompts spleen immune cells to release acetylcholine to macrophages to dampen inflammation (Schwartz, 2015).
The vagal cholinergic cytokine control system may be influenced by resonance-frequency (RF) breathing (Gevirtz, 2013; Tracey, 2007).
Read the Science News article Viva vagus: Wandering nerve could lead to range of therapies.
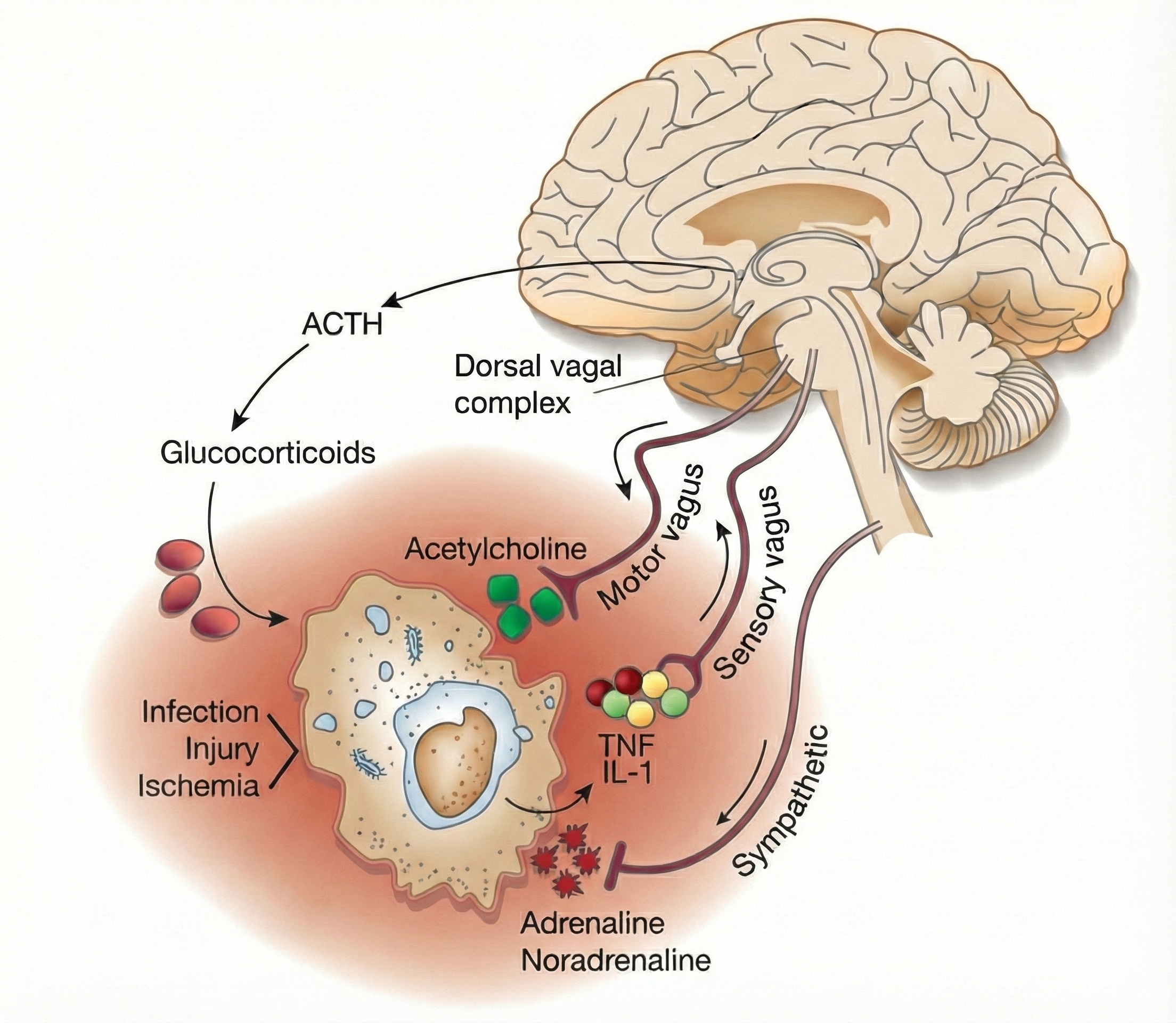
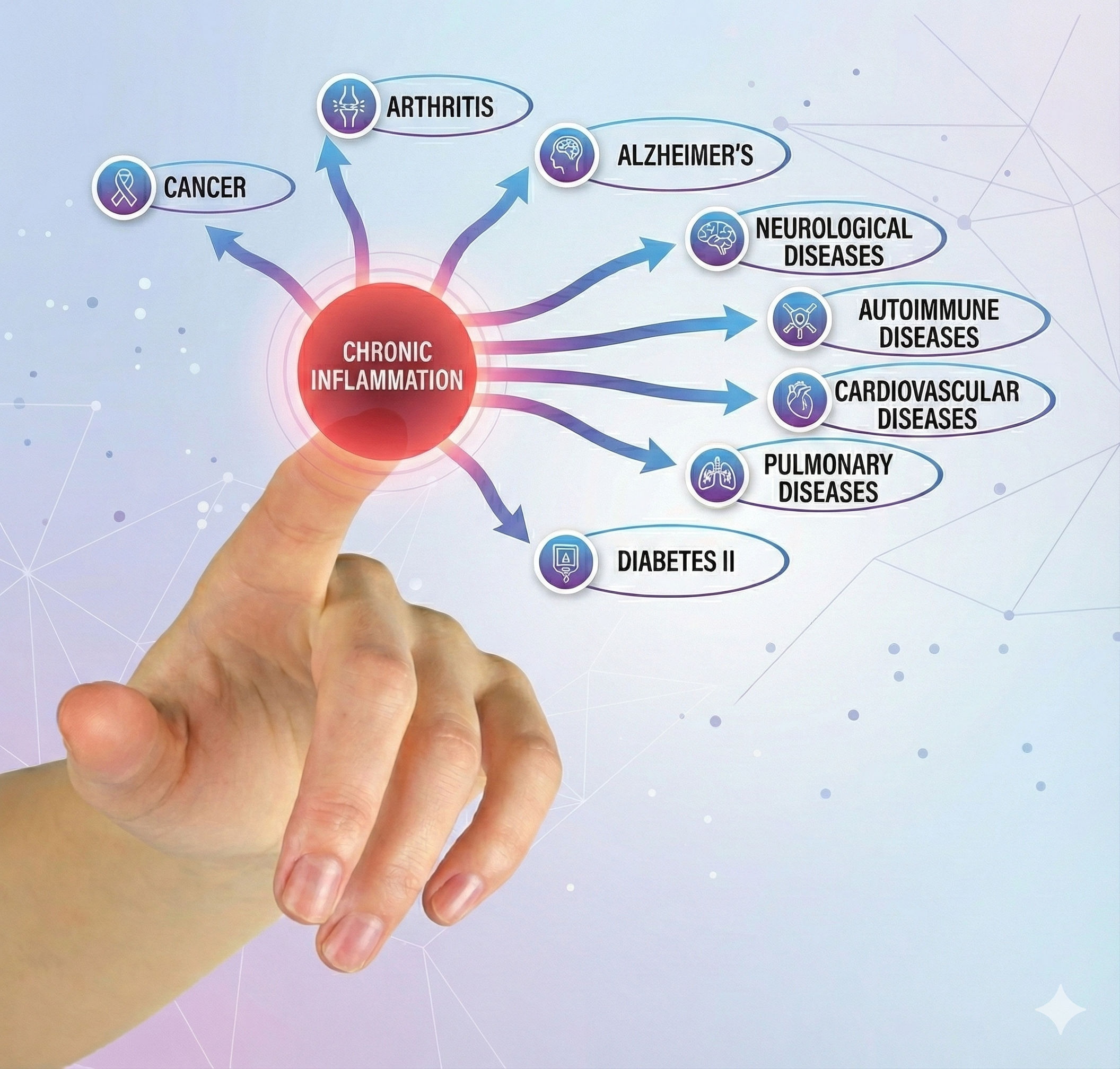
Chronic inflammation is implicated in a wide range of disorders, including Alzheimer's, cancer, cardiovascular diseases, depression, and diabetes (Dhar, Lambert, & Barton, 2016; Poole, Dickens, & Steptoe, 2011). See the Cutting Edge section later in this unit for more on the inflammatory reflex and how HRV biofeedback may influence neuroimmune regulation.
A Modern View of Sympathetic-Parasympathetic Interaction
Instead of one branch turning fully on while the other turns fully off, both branches are usually active at the same time, with their levels constantly adjusted to keep blood pressure, heart rate, and other functions in a healthy range (Karemaker, 2017; Khan et al., 2019). The heart receives input from both systems simultaneously, and their combined signals set the actual heart rate from moment to moment (Draghici & Taylor, 2016; Rajendran et al., 2018). In many situations both branches change together, rather than in a simple seesaw pattern, so researchers now talk less about a single "sympatho-vagal balance" and more about a flexible, context-dependent interaction (Ernst, 2017; Karemaker, 2017).
Putting this together, current perspectives see the sympathetic and parasympathetic branches as partners in a coordinated control system rather than simple rivals. The sympathetic branch provides energy and support for challenge, while the parasympathetic branch, especially through the vagus nerve, provides rapid braking and fine-tuning, with HRV serving mainly as a window into this vagal control (Ernst, 2017; Gentile et al., 2024). The discovery that the human vagus nerve contains a noticeable minority of sympathetic fibers adds nuance: even "parasympathetic" pathways carry mixed information, and therapies that target the vagus may influence both branches at once (Kronsteiner et al., 2024; Seki et al., 2014). For students and clinicians, this means thinking less in terms of a simple on-off switch and more in terms of a flexible, bidirectional conversation between sympathetic and parasympathetic influences that continuously shapes heart function and overall health (Karemaker, 2017; Khan et al., 2019).
Key Takeaways: Sympathetic and Parasympathetic Divisions
The SNS mobilizes your body for action, expending stored energy during emergencies. The PNS restores and conserves energy, promoting "rest and digest" functions. Rather than working like a simple seesaw, both branches are usually active simultaneously, with their levels constantly adjusted to maintain homeostasis. The vagus nerve plays a particularly important role in rapid heart rate control and dampening inflammation, which has implications for treating chronic conditions. Most HRV indices primarily reflect parasympathetic rather than sympathetic activity.
Comprehension Questions: The Autonomic Nervous System
- How does the psychophysiological principle explain the relationship between Botox injections and emotional experience?
- Your client Sarah shows decreased hand temperature and increased respiration rate during stress. How would you use this response stereotypy information in treatment planning?
- Why does the SNS produce "mass activation" while the PNS can produce more selective responses?
- Explain how vagal withdrawal differs from sympathetic activation in response to everyday stressors.
- How might HRV biofeedback influence the cholinergic anti-inflammatory pathway?
- Why is the vagus nerve not a purely parasympathetic pathway?
Porges' Polyvagal Theory: Beyond Fight or Flight
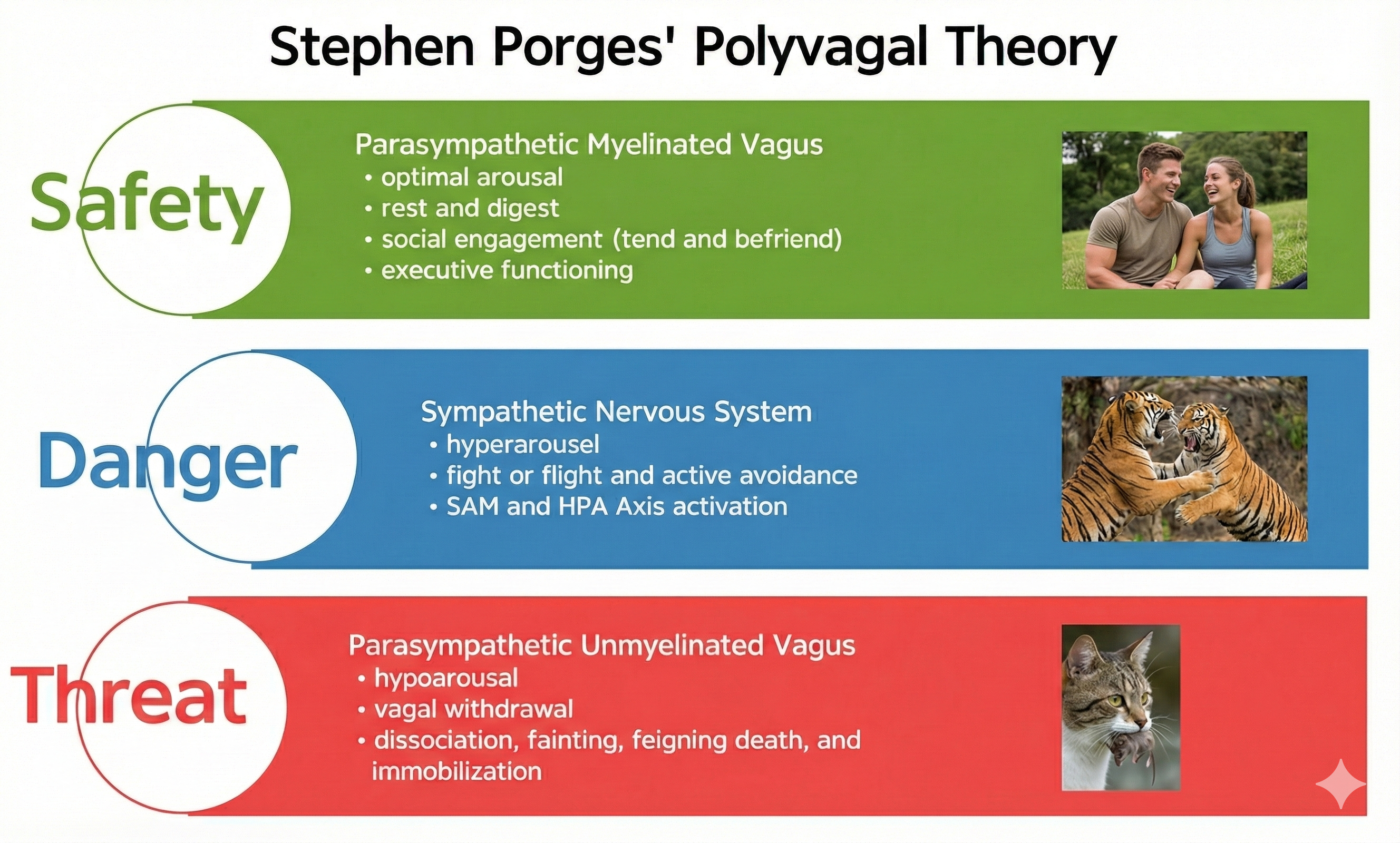
Polyvagal theory tells a story about how our bodies react to safety and danger. Instead of seeing the ANS as a simple "on/off" switch between rest-and-digest and fight-or-flight, Stephen Porges argues that mammals have three main response systems, arranged in a rough hierarchy (Porges, 1995, 2007, 2021). The oldest system, linked to an unmyelinated vagus nerve, supports shutdown and "freeze." The sympathetic system supports fight-or-flight. The newest system, the ventral vagal complex, is said to connect the heart with the face and voice muscles, supporting social engagement, calm states, and feelings of safety (Porges, 2003, 2007, 2021).
The vagus, the 10th cranial nerve, inhibits the heart and increases bronchial tone in the lungs. The vagus contains specialized subsystems that control competing adaptive responses, such as tend-and-befriend versus freezing.
According to Porges' (2011) fascinating and controversial theory, the ANS must be considered a "system," with the vagal nerve containing specialized subsystems that regulate competing adaptive responses. His theory proposes competing roles for the unmyelinated fibers in the vagus, which originate in the dorsal motor complex, and newer myelinated nerves which originate in the nucleus ambiguus.
The Ventral Vagal Complex: Our Social Engagement System
Porges theorizes that the evolution of the ANS was central to developing emotional experience and affective processes involved in social behavior. As human beings, we are not limited to fight, flight, or freezing behavioral responses. When we encounter stressors, we can self-regulate and initiate pro-social behaviors, including the tend-and-befriend response.

Porges calls this the social engagement system, and the theory suggests that this system depends upon the healthy functioning of the myelinated vagus, a vagal brake. From Porges' perspective, we only activate the myelinated vagus when our nervous system perceives that we are safe. Social engagement is a mutual process and may be integral to playing with others.

Social engagement often involves eye contact.

Social engagement also includes voluntary close physical proximity.

The myelinated vagus enables us to self-regulate, calm ourselves, and inhibit sympathetic outflow to the heart. It allows us to engage prefrontal cortex executive functions such as attention and mindfulness.


Professionals increasingly provide HRV biofeedback training before starting neurofeedback to activate the prefrontal cortex.

Daily stressors inhibit the myelinated vagus, producing vagal withdrawal, interfering with attention and social engagement.

In contrast, positive events may produce happiness that facilitates the myelinated vagus while increasing sympathetic activation.

The SNS in Polyvagal Context
The SNS, in concert with the endocrine system, responds to threats to our safety through mobilization, which includes fight-or-flight and active avoidance. The SNS responds more slowly and for a more extended period (more than a few seconds) than the parasympathetic vagus system.
The SNS inhibits the unmyelinated vagus to mobilize us for action instead of fainting or freezing. In contrast, the parasympathetic myelinated vagus rapidly adjusts cardiac output, promotes social engagement (the tend-and-befriend response), and enables self-regulation. These three changes promote biofeedback training.
According to this theory, quality communication and pro-social behaviors are possible only when defensive circuits are inhibited (Shaffer, McCraty, & Zerr, 2014).

The Unmyelinated Vagus: When Fight and Flight Are Not Options
Porges hypothesizes that the unmyelinated fibers regulate the freeze response and respond to threats through immobilization, feigning death, passive avoidance, and shutdown. Check out the YouTube video The Polyvagal Theory and PTSD with Stephen Porges, PhD.

Summary of Polyvagal Theory's Three Systems
When our nervous system perceives safety, we activate the ventral vagal complex (myelinated vagus) to conserve and rebuild energy stores (rest and digest), socially bond with others (tend and befriend), and engage in executive functions like self-regulation and planning.
When our nervous system perceives danger, we activate the SNS and the endocrine system's SAM pathway and HPA axis and inhibit the unmyelinated vagus, for fight or flight or active avoidance.
When our nervous system perceives that our life is threatened and that fight, flight, or active avoidance will not succeed (like a mouse in the jaws of a cat), we activate the unmyelinated vagus (dorsal vagal complex). This results in passive avoidance through dissociation, fainting, feigning death, immobilization, and shutdown.
Neuroception: The Body's Safety Scanner
A key idea in polyvagal theory is neuroception: the nervous system is constantly and automatically "scanning" for cues of safety or threat (tone of voice, facial expression, posture), and then shifting bodily state in ways that shape emotion, attention, digestion, and how open or defensive we feel in relationships (Porges, 2007; Porges, 2022). Because of this, the theory has become popular in trauma therapy, developmental psychology, and creative arts therapies as a way to explain why warm relationships, prosodic voice, music, and movement can help people feel safer and more regulated (Beauchaine et al., 2007; Fox, 2025; Haeyen, 2024; Porges, 2017).
Scientific Status of Polyvagal Theory
The status of polyvagal theory in science is more complicated than its clinical popularity might suggest. On the supportive side, many studies link measures thought to reflect vagal regulation, especially respiratory sinus arrhythmia (RSA), a pattern in heart rate tied to breathing, to social behavior, emotion regulation, and mental health problems. Some reviews argue that new work in neuroscience and clinical research fits reasonably well with core polyvagal ideas (Beauchaine et al., 2007; Manzotti et al., 2023; Porges, 2022, 2025). Clinicians also report that polyvagal language about "states," "safety," and "co-regulation" helps them design and explain body-based, relational interventions (Fox, 2025; Haeyen, 2024; Koenig, 2025; Schroeter, 2016).
On the critical side, recent papers argue that several basic biological claims are wrong or overstated. For example, critics contend that certain vagal pathways do not cleanly map onto specific behaviors, that RSA is not uniquely mammalian, and that reptiles are not essentially "asocial" compared to mammals as the theory suggests. Critics also argue that relying on RSA as a one-to-one measure of "vagal tone" is a conceptual mistake (Doody et al., 2023; Grossman, 2023). Even authors generally sympathetic to the framework describe it as a useful, integrative model that still needs revision to match what is known from comparative anatomy and neurobiology (Manzotti et al., 2023).
Right now, polyvagal theory is highly influential in clinical practice and as a metaphor for safety and connection, while its detailed evolutionary and anatomical story remains debated and only partly supported.
Clinical Application: Understanding Your Client's Nervous System State
Consider a client named Elena who comes to you after experiencing trauma. She describes feeling "checked out" during stressful situations and has difficulty connecting with others. Through the lens of polyvagal theory, you recognize that Elena's nervous system may be defaulting to unmyelinated vagal responses (shutdown, dissociation) rather than engaging the social engagement system. Your treatment approach might focus on helping Elena's nervous system recognize safety cues, perhaps starting with HRV biofeedback to strengthen myelinated vagal function before addressing more complex therapeutic goals.
Key Takeaways: Polyvagal Theory
Polyvagal theory proposes three hierarchically arranged response systems rather than a simple on/off switch. The ventral vagal complex (myelinated vagus) supports social engagement and self-regulation when we feel safe. The sympathetic system mobilizes us for active responses to danger. The dorsal vagal complex (unmyelinated vagus) produces shutdown responses when escape seems impossible. The concept of neuroception explains how our nervous system automatically scans for safety and threat cues. Understanding which system your client's nervous system defaults to can guide treatment planning. While polyvagal theory is highly influential clinically, its detailed biological claims remain debated in the scientific literature.
The Enteric Division: Your Second Brain
The story of the gut-brain axis starts with the enteric nervous system (ENS), sometimes called the "second brain." Wrapped in layers along the gastrointestinal tract, the ENS contains hundreds of millions of neurons, comparable to the spinal cord (Boron & Boulpaep, 2005), organized into two main networks: the myenteric plexus and submucosal plexus. These networks can sense what is in the gut and coordinate motility, secretion, blood flow, and local immune responses, often without direct orders from the brain (Carabotti et al., 2015; Kulkarni et al., 2018). The ENS is now widely accepted as a major division of the peripheral nervous system with broad influence on digestion, metabolism, and inflammation (Hajjeh et al., 2025; O'Riordan et al., 2025).
Here is a surprising fact: the gut contains more than 90% of the body's serotonin (Khazan, 2013) and about 50% of its dopamine. The ENS releases a comparable range of neurotransmitters as the central nervous system (Gershon, 1999) and contains interneurons, sensory neurons, autonomic motor neurons, and neuroglial cells. A subset of intestinal sensory neurons travels in the vagus nerve to influence ANS activity (Fox & Rompolski, 2025). Check out the Khan Academy YouTube video Control of the GI Tract.
Modern imaging and genetic tools show that enteric neurons and glial cells constantly communicate with epithelial cells, immune cells, and gut microbes, forming a local control center rather than a passive relay (Dowling et al., 2022; Kulkarni et al., 2018). While the ENS locally regulates intestinal functions, sympathetic and parasympathetic efferents can override its activity during intense emotions like anger and fear (Fox & Rompolski, 2025). During physical activity and stress, sympathetic firing inhibits intestinal motility. During rest, parasympathetic firing promotes motility by exciting enteric preganglionic neurons (Khazan, 2013).
The Gut-Brain Axis
The gut-brain axis describes the two-way communication between this "second brain," the central nervous system, and the gut environment, including the microbiota. Signals travel along several routes at once: fast neural pathways (vagus nerve, spinal afferents, ENS circuits), hormonal systems such as the hypothalamic-pituitary-adrenal (HPA) stress axis, and immune and metabolic signaling through cytokines and microbial metabolites (Carabotti et al., 2015; Hattori & Yamashiro, 2021; Rusch et al., 2023).
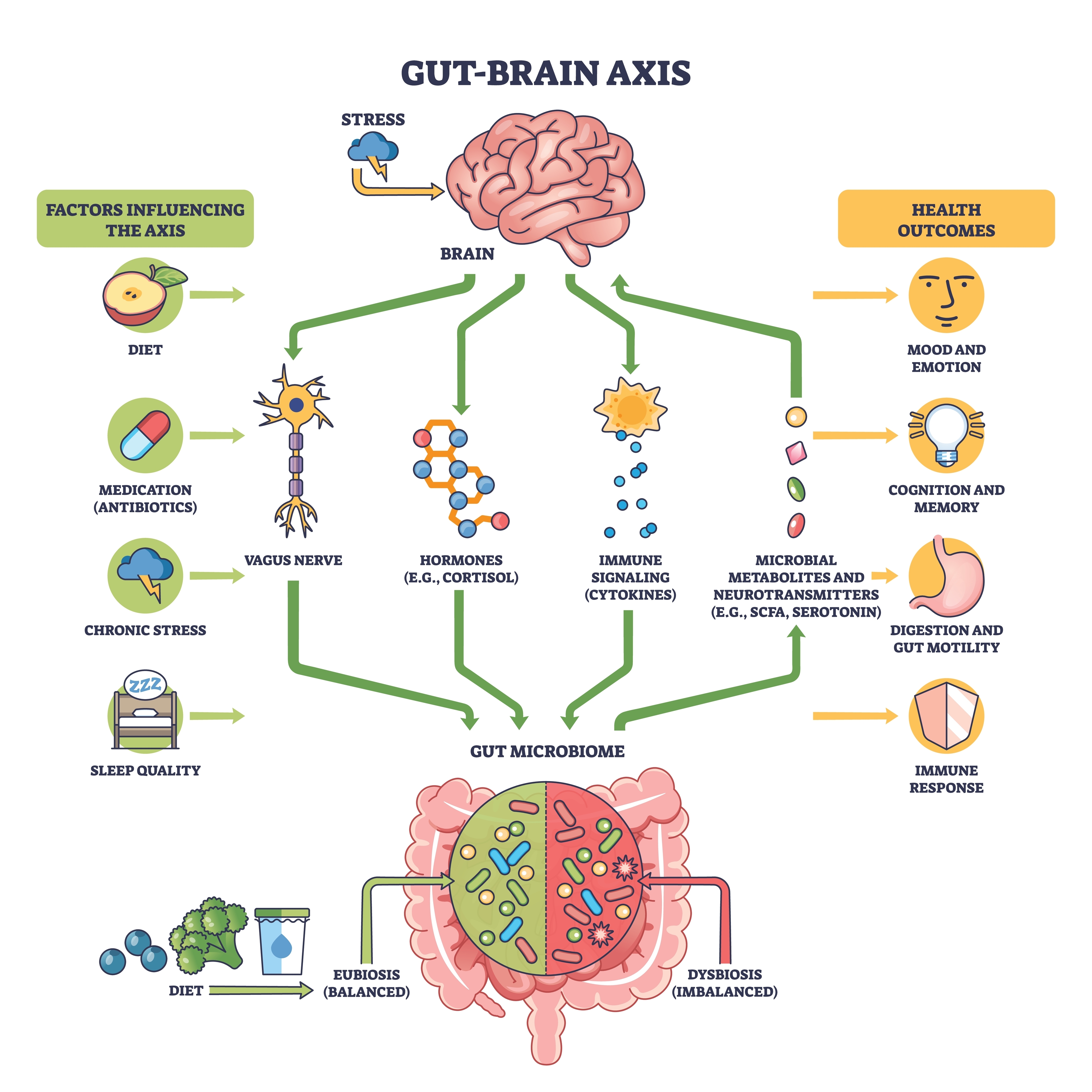
Trillions of microbes in the gut add another layer, producing short-chain fatty acids, neurotransmitter-like chemicals, and other metabolites that can act on enteric neurons, endocrine cells, and immune cells, indirectly shaping brain function and behavior (Chen et al., 2021; Cryan et al., 2019; Schneider et al., 2024). The current consensus is not that the microbiota "controls your mind," but that there is a real, biologically grounded microbiota-gut-brain axis influencing stress reactivity, mood, and cognition, especially under conditions of imbalance or disease (Cryan et al., 2019; Mohajeri et al., 2018; Petrut et al., 2025).
Research Evidence
Evidence for this axis comes from multiple directions. In animals, raising mice without microbes or disrupting their gut flora with antibiotics changes gut metabolites, alters expression of brain signaling molecules, and impairs certain types of memory, supporting a causal link from gut to brain (Fröhlich et al., 2016; Rusch et al., 2023). Germ-free or dysbiotic animals also show abnormal stress responses and altered neurogenesis, which can sometimes be normalized by restoring microbes (Cryan et al., 2019; Schneider et al., 2024).
In humans, gut dysbiosis is associated with irritable bowel syndrome, anxiety and depression, autism spectrum conditions, and neurodegenerative diseases such as Parkinson's and Alzheimer's disease. Specific microbial profiles correlate with cognitive markers and Alzheimer's biomarkers in people with mild cognitive impairment (Carabotti et al., 2015; Fan et al., 2025; Hajjeh et al., 2025). These associations do not prove direct causation in all cases, but together with mechanistic animal work, they strongly support the idea that gut microbes and the ENS help tune brain circuits involved in mood, stress, and cognition (Chen et al., 2021; Mohajeri et al., 2018).
Stress, Diet, and the Microbiome
This gut-brain conversation has practical consequences for health and performance. Stress signals from the brain can alter gut motility, barrier function, and microbiota composition via the HPA axis and ANS, sometimes promoting overgrowth of harmful bacteria and inflammation (Carabotti et al., 2015; Hattori & Yamashiro, 2021; Ramadan et al., 2025). In the other direction, microbial metabolites and ENS activity influence appetite, energy use, and glucose handling, affecting body weight and metabolic risk (Nikolla et al., 2025; Schneider et al., 2024).
Diet emerges as a key lever: what people eat reshapes the microbiota and its metabolites, which can in turn modulate stress resilience, learning, and emotional regulation through immune, endocrine, and neural pathways (Cryan et al., 2019; Schneider et al., 2024; Wójcik et al., 2025). Early work on psychobiotics (probiotics or prebiotics aimed at mental health) and dietary interventions suggests modest but promising benefits for mood and cognitive performance in some groups, though effects are strain- and context-dependent and far from a universal "fix" (Mohajeri et al., 2018; Petrut et al., 2025; Schneider et al., 2024).
Clinical Implications
Current thinking views the ENS and gut-brain axis as central regulators of whole-body homeostasis rather than curiosities on the side. ENS dysfunction is now proposed as a driver, not just a marker, of neurological and neurodevelopmental disorders, helping to explain why gastrointestinal problems are so common in conditions like Parkinson's disease, Alzheimer's disease, and autism spectrum disorder (Cryan et al., 2019; Dowling et al., 2022; Hajjeh et al., 2025). At the same time, clinical translation is still in early stages: many striking findings come from animal models, human data are often correlational, and individual responses vary widely (Chen et al., 2021; O'Riordan et al., 2025; Schneider et al., 2024).
Even with these caveats, the gut-brain axis offers a powerful framework for understanding how stress, diet, infection, and microbiota shape mental and physical performance, and hints that future therapies may target not just the brain, but also the "second brain" and the microbes that live alongside it.
The Relationship Between the Sympathetic and Parasympathetic Branches
Berntson, Cacioppo, and Quigley (1993) challenge the concept of a continuum ranging from SNS to PNS dominance. They argue that the two autonomic branches do not only act antagonistically (reciprocally). They also exert complementary, cooperative, and independent actions.
Antagonistic Actions
The SNS and PNS branches compete to control target organs such as the heart. For example, the PNS can slow the heart by 20 to 30 beats per minute or briefly stop it (Tortora & Derrickson, 2023). Since these divisions generally produce contradictory actions, like speeding and slowing the heart, their effect on an organ depends on their current balance of activity. This competitive relationship is called accentuated antagonism (Olshansky et al., 2011).
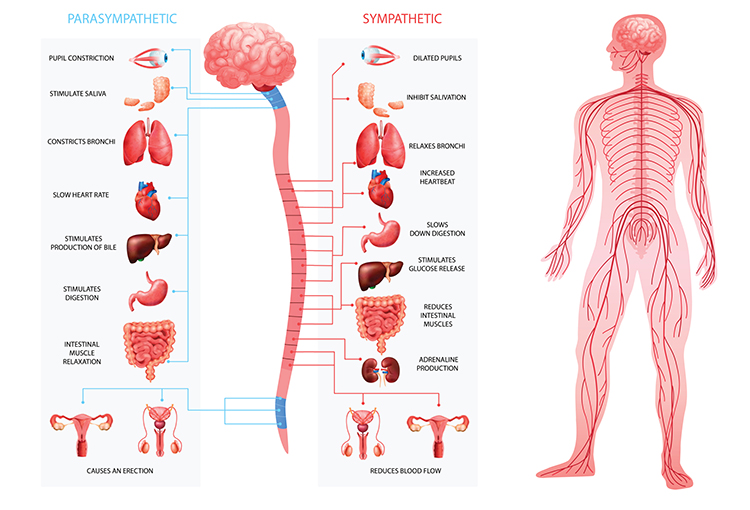
This adrenergic and cholinergic effects table was adapted from Fox and Rompolski (2022). Adrenergic receptors are alpha (α) or beta (β), and cholinergic receptors are muscarinic (M).
Complementary Actions
SNS and PNS actions are complementary when they produce similar changes in the target organ. Saliva production serves as an example. PNS activation produces watery saliva, and SNS activation constricts salivary gland blood vessels producing thick saliva.
Cooperative Actions
SNS and PNS actions are cooperative when their different effects result in a single action. Sexual function provides an example. PNS activation produces erection and vaginal secretions, while SNS activation produces ejaculation and orgasm.
Exclusive Sympathetic Control
The SNS exclusively innervates several organs. They are controlled by increasing or decreasing the firing of SNS postganglionic fibers: adrenal medulla, arrector pili muscle, sweat glands, and most blood vessels.
Higher Brain Center Control
The brainstem's medulla regulates blood pressure, defecation, heart rate, respiration, and vomiting, distributes signals between the brain and spinal cord, and directly controls the ANS. The medulla is influenced by sensory input and the hypothalamus (Breedlove & Watson, 2023).
The hypothalamus is a forebrain structure located below the thalamus. The hypothalamus is the body's homeostat (a device that maintains homeostasis) and dynamically maintains balance through its control of the ANS, endocrine system, survival behaviors, and interconnections with the immune system. The hypothalamus consists of specialized nuclei.
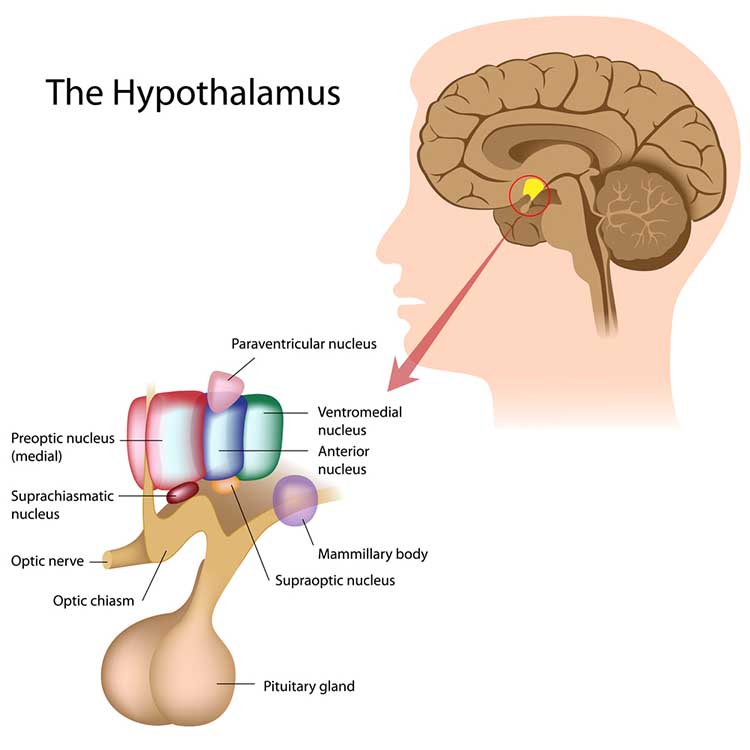
The hypothalamus is influenced by the cerebellum, cerebral cortex, and limbic system.
Comprehension Questions: ANS Branches and Their Relationships
- Explain why the enteric nervous system is sometimes called the "second brain."
- Give an example of how the sympathetic and parasympathetic divisions work cooperatively rather than antagonistically.
- According to polyvagal theory, what must happen for a client to engage in effective social communication during therapy?
- How does the hypothalamus function as a "homeostat"?
Homeostasis: Maintaining Your Internal Balance
Homeostasis maintains the body's internal environment within healthy physiological limits. Think of it like a thermostat in your home. Claude Bernard introduced the basic concept of homeostasis and stated, "The stability of the internal environment is the condition of a healthy life." Cannon (1939) introduced the term homeostasis in 1932 and elaborated on this concept in The Wisdom of the Body.
The body achieves homeostasis for specific variables, like blood pressure, through interlocking negative and positive feedback systems. Check out the Bozeman Science YouTube video Positive and Negative Feedback Loops.
Two important modifications to the principle of homeostasis are the boundary model and allostasis.
The boundary model challenges the maintenance of set points as rigid and proposes that physiological processes are maintained within acceptable ranges.
Allostasis means the maintenance of stability through change and is a process that complements homeostasis. Allostasis is achieved by anticipating challenges and adapting through behavior (including learning) and physiological change. Therapists utilize allostasis when they teach patients to use an abbreviated relaxation exercise (low-and-slow breathing) when they anticipate stressors like traffic jams.
The Allostatic Load Model: When Stress Takes Its Toll
The allostatic load model begins with a familiar experience: life keeps throwing demands at the body, and the body must constantly adjust to stay in balance. These adjustments, like speeding up the heart, raising blood pressure, or releasing stress hormones so we can cope, are called allostasis. Allostatic load is the "wear and tear" that builds up when these stress responses are activated too often, for too long, or do not shut off properly (Guidi et al., 2020; Juster et al., 2010; Logan & Barksdale, 2008). Instead of focusing on a single organ, researchers measure allostatic load with a bundle of markers from several systems, such as blood pressure, cholesterol, blood sugar, inflammatory markers, and sometimes stress hormones, to capture how strained the body has become (Juster et al., 2010; Rodriguez et al., 2019).

Research Support for the Allostatic Load Model
Over the last 30 years, research has largely supported the core idea that higher allostatic load reflects harmful "biological wear and tear" from chronic stress. People with higher scores are more likely to have heart disease, cognitive problems, pain conditions, mental health issues, disability, and higher risk of death, even after controlling for standard medical risk factors (Guidi et al., 2020; Juster et al., 2010; Rabey & Moloney, 2022). Large analyses show that a relatively small set of markers, such as inflammation (C-reactive protein), resting heart rate, good cholesterol, waist-to-height ratio, and long-term blood sugar, can already predict slower walking, weaker grip strength, poorer self-rated health, and higher mortality (McCrory et al., 2023).
Studies also find that allostatic load tends to be higher in people facing long-term adversity, such as low income, discrimination, or demanding jobs, and in some student groups with more stress and sleep problems, helping explain why some social groups have worse health than others (Christensen et al., 2022; Igboanugo & Mielke, 2023; Liu et al., 2025; Rodriguez et al., 2019).
Refining the Allostatic Load Model
Current thinking is not that the model is perfect, but that it is useful and needs refining. One major debate is how best to measure allostatic load: different studies use different biomarkers, cutoffs, and scoring rules, which makes it hard to compare results or set clear clinical thresholds (Carbone et al., 2022; Guidi et al., 2020). Newer work is pushing toward a shared definition and simpler, standardized scores that clinicians could actually use with patients (Fava et al., 2022; McCrory et al., 2023).
Researchers are also expanding the model. For example, some frame allostatic load as an energetic cost problem, where chronic stress forces the body to spend so much energy on survival that it has less left for repair and healthy aging (Bobba-Alves et al., 2022). Others apply the idea to relationships and groups, talking about social allostatic load when stressed groups get stuck in unhealthy interaction patterns (Saxbe et al., 2019).
Overall, the allostatic load model is widely accepted as a powerful way to connect chronic stress with multi-system bodily change, even as scientists work to clarify exactly how to define, measure, and apply it.
Autonomic Balance: Finding the Middle Ground
Wenger's idea of autonomic balance centers on the relationship between the sympathetic ("fight or flight") and parasympathetic ("rest and digest") branches of the autonomic nervous system. In his classic view, healthy functioning depends on a dynamic balance between these two systems; too much sympathetic activity or too little parasympathetic tone leads to autonomic imbalance and vulnerability to disease.
Wenger (1972) proposed that resting individuals are located along a continuum ranging from SNS to PNS dominance. Normally distributed A-bar scores, which measure location on this continuum, are calculated from a battery of autonomic measurements. Low A-bar scores indicate SNS dominance, while high A-bar scores suggest PNS dominance. Most subjects show intermediate scores. Wenger and colleagues reported that individuals with low A-bar scores, whom he called sympathicotonics, suffered an elevated incidence of neurotic, psychotic, psychosomatic, and medical disorders.
Generally, low and high A-bar scores seem to predispose individuals to develop psychological and medical disorders. Where SNS dominance (low A-bar scores) may result in cardiac arrhythmia and essential hypertension, PNS dominance (high A-bar scores) may be associated with asthma, colitis, and hypotension.
Modern Support for Autonomic Balance
Modern research broadly supports the intuition that chronic sympathetic dominance with reduced parasympathetic activity is harmful. This pattern is linked to cardiovascular disease, increased mortality, and multiple risk factors such as hypertension, obesity, and chronic stress, often indexed by reduced HRV as a marker of low parasympathetic tone and autonomic imbalance (Thayer et al., 2010; Zoccali et al., 2025). Similar imbalances have been implicated in chronic kidney disease, where sympathetic overactivity and diminished vagal (parasympathetic) influence promote inflammation and cardiovascular complications (Zoccali et al., 2025).
Beyond the Simple Seesaw Model
However, the simple "seesaw" version of autonomic balance, where sympathetic and parasympathetic activity are treated as exact opposites on a single line, is now seen as too crude. Work on autonomic "modes of control" shows that the two branches can change reciprocally, independently, or even rise and fall together, creating a richer autonomic space than a one-dimensional balance score can capture (Berntson, 2018; Quigley & Moore, 2018).
This has led to more nuanced composite measures such as cardiac autonomic balance (CAB) and cardiac autonomic regulation (CAR), which distinguish relative dominance of one branch from overall co-activation or co-inhibition and better predict outcomes like myocardial infarction, neuroinflammation, and emotional functioning (Berntson, 2018; Stone et al., 2020).
Limitations of the LF/HF Ratio
HRV remains a central noninvasive tool for assessing autonomic function, but influential reviews argue that LF HRV and the LF/HF ratio do not reliably reflect sympathetic tone or true "sympathovagal balance," and that much of the HRV spectrum is actually shaped by the parasympathetic system (Billman, 2013; Hayano & Yuda, 2021; Reyes del Paso et al., 2013; von Rosenberg et al., 2017).
Contemporary Understanding of Autonomic Balance
Today, Wenger's core insight, that coordinated sympathetic and parasympathetic activity, rather than either system alone, is crucial for health, still guides research, but the measurement and theory have grown more sophisticated. Instead of treating "autonomic balance" as a single ratio like LF/HF, current models emphasize multi-dimensional patterns across organs and time, grounded in brain-body networks that integrate emotion, cognition, and physiology (Ernst, 2017; Quigley & Moore, 2018; Thayer & Brosschot, 2005).
Autonomic imbalance is now framed as a common pathway linking chronic negative affect, inflammation, and cardiovascular risk, with HRV and related indices used as biomarkers of this broader regulatory system (Olivieri et al., 2024; Thayer et al., 2010; Zoccali et al., 2025). In this sense, Wenger's concept survives as a powerful metaphor and starting point, but contemporary science treats "balance" as a flexible, brain-embedded control strategy rather than a simple numerical score.
Heart Rate Variability: A Window into Brain-Heart Communication
HRV is the natural variation in the time between consecutive heartbeats, usually measured from the R waves on an electrocardiogram and expressed as changing interbeat intervals over time (Acharya et al., 2006; Shaffer & Ginsberg, 2017). A healthy heart is not a perfect metronome but speeds up and slows down slightly from beat to beat, allowing rapid adjustment to physical and emotional demands (Kara et al., 2003; Shaffer & Ginsberg, 2017). Higher HRV at rest is generally associated with better cardiovascular health and resilience, while very low HRV often appears in serious heart disease and is linked to higher risk of death after events such as heart attacks (Acharya et al., 2006; Tiwari et al., 2020). Modern reviews emphasize that HRV should be viewed as a dynamic signal that reflects how flexibly the heart and the nervous system can respond to changing situations across seconds, hours, and even years (Ernst, 2017; Kemp et al., 2017).
Autonomic and Physiological Sources of HRV
HRV mainly arises from the ANS, which includes the sympathetic branch that prepares the body for action and the parasympathetic branch that supports rest and recovery (Acharya et al., 2006; Tiwari et al., 2020). Fast beat-to-beat changes are driven largely by the vagus nerve, the main parasympathetic nerve to the heart, which can quickly slow the heart in response to breathing and other reflexes (Ernst, 2017; Shaffer & Ginsberg, 2017). This vagal influence shows up strongly in time-domain measures such as RMSSD and in HF power in frequency-domain analysis (Shaffer & Ginsberg, 2017; Tiwari et al., 2020).
Slower oscillations in HRV reflect a mix of baroreflex activity, sympathetic influences, thermoregulation, and other processes, which makes their interpretation more complex (Ernst, 2017; Stauss, 2003). Recent work also notes that especially in older adults, some increased variability can come from disorganized or fragmented sinus rhythm that is not purely autonomic in origin, reminding researchers to interpret HRV with care (Arakaki et al., 2023).
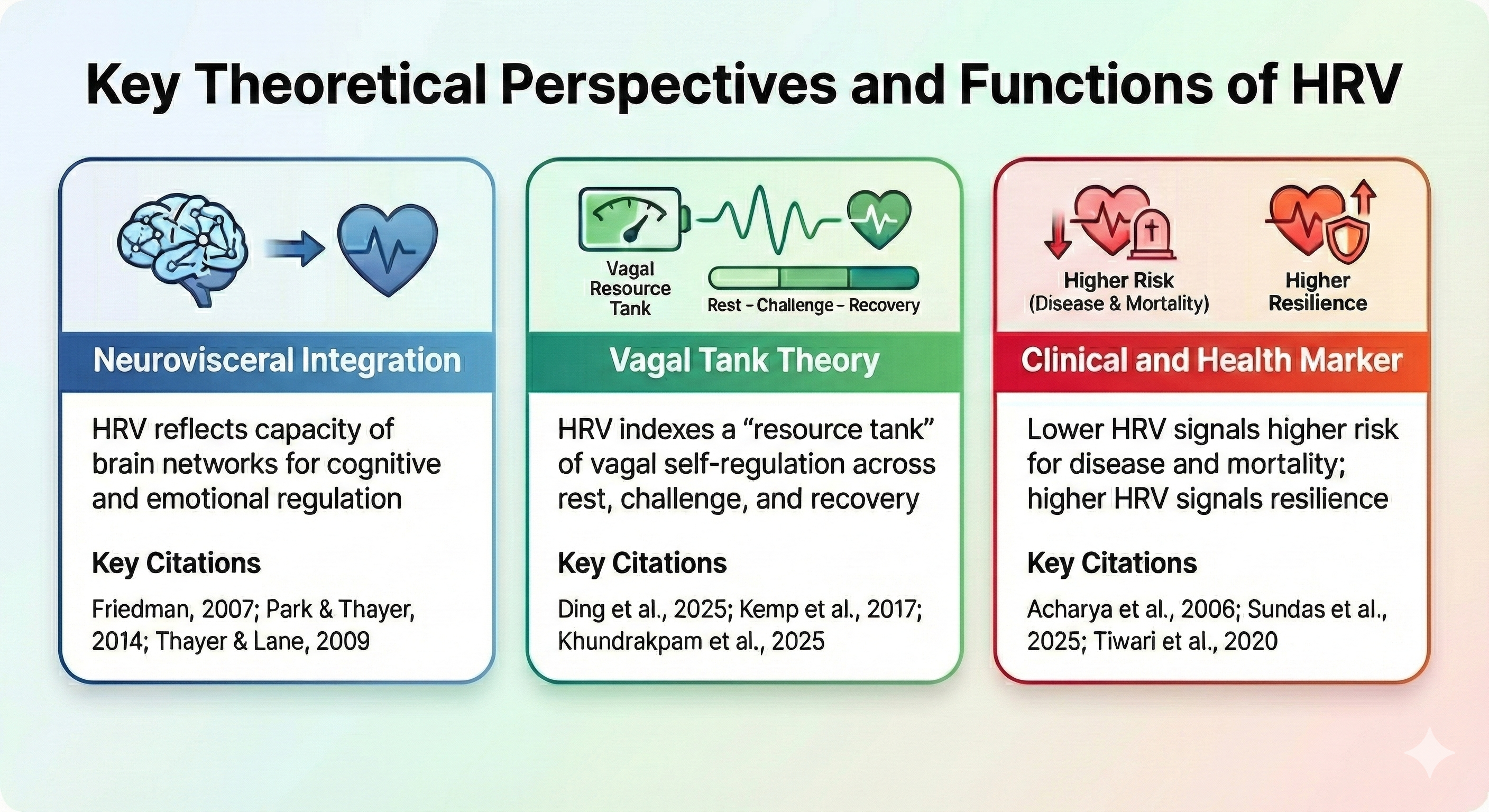
The Neurovisceral Integration Model
Modern theories see HRV not only as a cardiac signal but also as an indirect reflection of brain-heart communication. The neurovisceral integration model proposes that vagally mediated HRV, especially HF HRV, indexes the functional capacity of a network of brain regions called the central autonomic network (CAN), which links areas involved in attention, emotion, and autonomic control (Thayer & Lane, 2000, 2009). Higher resting HRV tends to be associated with better executive functions, such as working memory, cognitive flexibility, and inhibition, as well as more effective emotion regulation (Park & Thayer, 2014; Thayer & Lane, 2009).
In this view, the same prefrontal brain circuits that help a person shift attention or control impulses also send inhibitory signals down to autonomic centers, using the vagus nerve to "brake" the heart and dampen excessive stress responses (Friedman, 2007; Thayer & Lane, 2009). HRV therefore acts as a convenient peripheral marker of how well these brain networks can support self-regulation, adaptation, and health.
Vagal Tank Theory
Vagal tank theory offers a more metaphorical but intuitive way to think about HRV. It suggests that cardiac vagal activity functions like a "tank" of regulatory resources that can be filled, used, and refilled over time, much like an energy reserve for self-control and coping (Ernst, 2017; Kemp et al., 2017). In this framework, resting HRV reflects the current "level" in the tank, phasic changes in HRV during a challenge reflect how much of that resource is recruited, and recovery HRV shows how well the tank is refilled after the task ends (Ding et al., 2025; Khundrakpam et al., 2025).
Experimental work using anxiety induction and HRV biofeedback training in college students has shown that higher resting HRV is associated with stronger vagal responses during stress and better recovery afterward, supporting the idea that a fuller vagal tank allows more flexible engagement and disengagement of regulation when needed (Ding et al., 2025). This theory has inspired interventions such as slow-paced breathing and HRV biofeedback that aim to "top up" vagal resources to improve stress management and emotional resilience (Khundrakpam et al., 2025; Moss, 2025).
HRV, Health, and Cognition
Across many studies, lower HRV has been linked to a wide range of problems including cardiovascular disease, anxiety, depression, and cognitive decline, whereas higher HRV is generally associated with better emotional well-being and cognitive performance (Friedman, 2007; Kemp et al., 2017; Tiwari et al., 2020). From a neurovisceral integration and vagal tank perspective, HRV helps indicate how flexibly a person can shift between states, such as from rest to focused effort and back to recovery, without getting "stuck" in chronic stress responses (Friedman, 2007; Park & Thayer, 2014).
For example, higher resting HRV has been associated with more adaptive attention to emotional stimuli and better affective flexibility, while lower HRV is linked to hypervigilance and less flexible responses to negative information (Grol & De Raedt, 2020; Park & Thayer, 2014). In psychotherapy and stress management, HRV training is being explored as a way to strengthen this flexibility, potentially improving affect regulation and social engagement by enhancing vagal function (Moss, 2025; Shaffer & Ginsberg, 2017).
HRV as a Dynamic Biomarker
Current thinking treats HRV as a dynamic biomarker, meaning a measurable signal that tracks ongoing changes in autonomic and brain function over time rather than a fixed trait (Ernst, 2017; Sundas et al., 2025). Reviews describe how HRV can connect "psychological moments" such as daily stress or brief emotional experiences with long-term outcomes like healthy aging and mortality, by capturing how the vagus nerve and CAN manage repeated challenges across the lifespan (Kemp et al., 2017; Sundas et al., 2025).
At the same time, researchers warn that HRV is influenced by age, posture, breathing, measurement length, and even fragmented sinus rhythm, so careful protocols and interpretation are essential (Arakaki et al., 2023; Shaffer & Ginsberg, 2017; Stauss, 2003). Overall, the latest models agree that HRV is more than just a simple index of "sympathetic versus parasympathetic balance." It is a rich signal reflecting how heart, brain, and body work together to support flexibility, self-regulation, and health across time (Ernst, 2017; Thayer & Lane, 2009).
Key Takeaways: Heart Rate Variability
HRV reflects the flexible, moment-to-moment adjustments of heart rate driven primarily by the vagus nerve. The neurovisceral integration model proposes that HRV indexes the brain's capacity for cognitive and emotional regulation through the CAN. Vagal tank theory offers an intuitive metaphor: HRV reflects a "resource tank" that can be depleted during stress and replenished during recovery. Higher resting HRV is generally associated with better cardiovascular health, cognitive flexibility, and emotional resilience, while low HRV signals higher risk for disease and mortality.
Psychophysiological Measurements: What We Monitor and Why
Psychophysiologists monitor tonic, phasic, and spontaneous psychophysiological activity.
Listen to a mini-lecture on Tonic and Phasic ActivityTonic activity measures background activity and is the magnitude of physiological activity over a specified period before stimulating the subject. A resting baseline, where a participant sits quietly without breathing or relaxation instructions or feedback for 3 or 5 minutes, measures tonic activity. Clinicians obtain pre- and post-baselines to measure the psychophysiological change in variables like HRV or hand temperature within and across training sessions.
Phasic activity is a discrete response evoked by a specific stimulus, like being surprised. Since subjects continuously react to environmental and internal stimuli that we cannot directly observe, interpreting phasic activity can be challenging.

Spontaneous responses are physiological changes in the absence of detectable stimuli. These changes are important to researchers because they can confound the measurement of phasic activity. For example, suppose a spontaneous increase in skin conductance occurs as a subject is shown an emotionally-charged slide. In that case, a researcher could mistakenly conclude that the slide increased conductance more than it did (Stern, Ray, & Quigley, 2001).
Orienting and Defensive Responses: How We React to Our Environment
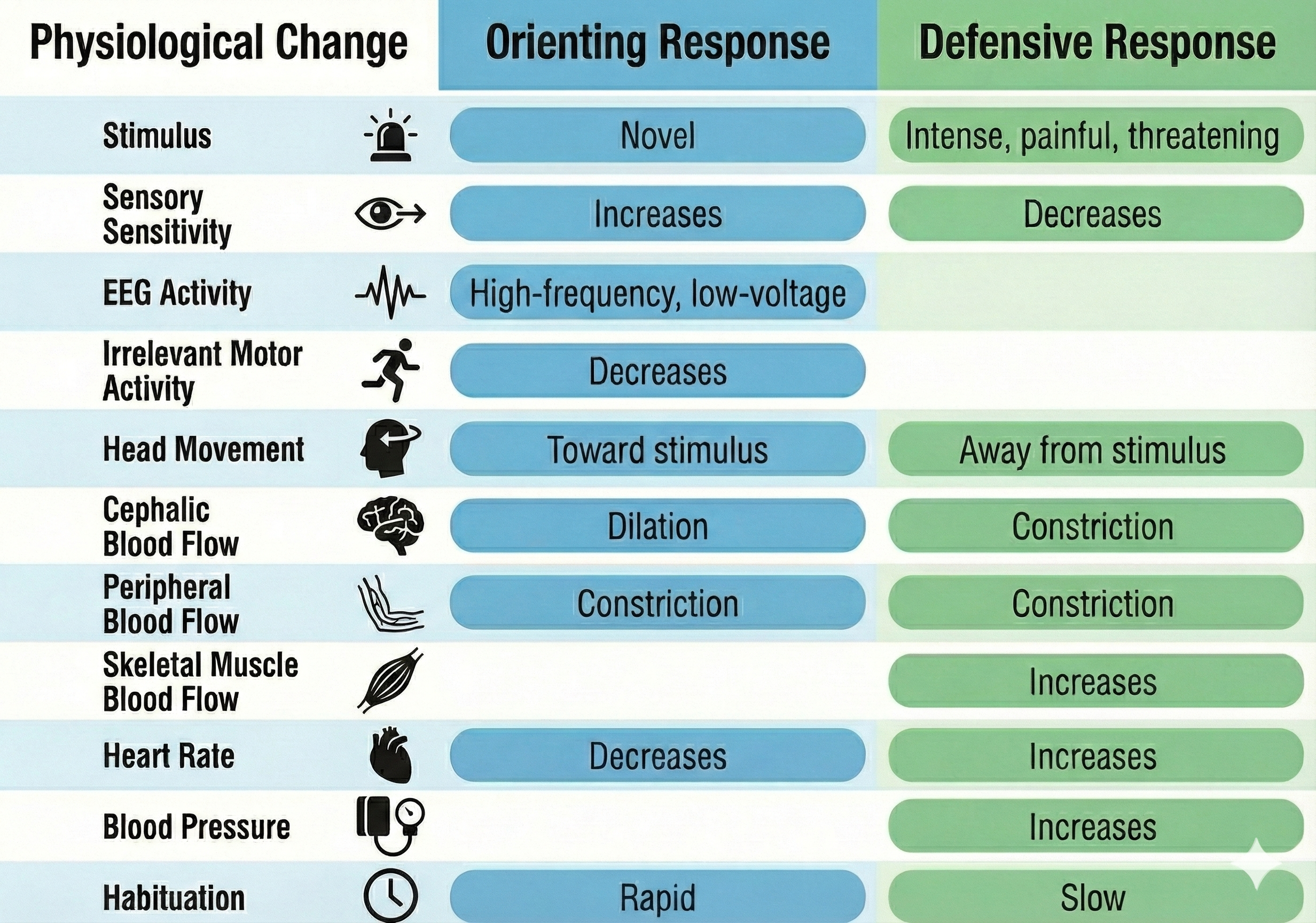
When exposed to a novel stimulus, an orienting response prepares us to deal with this challenge. Pavlov's (1927) orienting response is a "What is it?" reaction to stimuli like the sound of a vase crashing.
The orienting response includes increased sensory sensitivity, head (and ear) turning toward the stimulus, increased muscle tone (reduced movement), EEG desynchrony, peripheral constriction and cephalic vasodilation, a rise in skin conductance, heart rate slowing, and slower, deeper breathing. An orienting response rapidly habituates since it is no longer needed once we respond to a novel stimulus.
In contrast, a defensive response is a slowly habituating response pattern that limits harm from intense stimulation. This pattern includes reduced sensory sensitivity, a tendency to move away from the stimulus, heart rate increase, and both peripheral and cephalic vasoconstriction. For example, a loud noise in a workplace environment might elicit a defensive response.
Activation Theory: Understanding What Makes Behavior Intense
Duffy's activation theory starts from a simple question: what makes behavior intense? Instead of focusing on how strong an outward response looks (for example, how loudly someone yells), Elizabeth Duffy argued that psychologists should look at internal arousal processes, what she called activation (Duffy, 1957; Ehrlich, 1963; Pitz, 1964). She proposed that behavior varies along two basic dimensions: direction (what you do) and intensity (how strongly you do it), and that intensity is best understood as the degree to which the organism's stored energy is being released through metabolic and neural activity (Duffy, 1957; Ehrlich, 1963; Pitz, 1964). On this view, activation reflects the level of neural activity in central systems like the reticular activating system, influenced by external stimulation (noise, tasks), internal bodily states (heart rate), and thoughts, and is usually inferred from physiological measures such as brain waves or skin conductance rather than from behavior alone (Gardner, 1986).
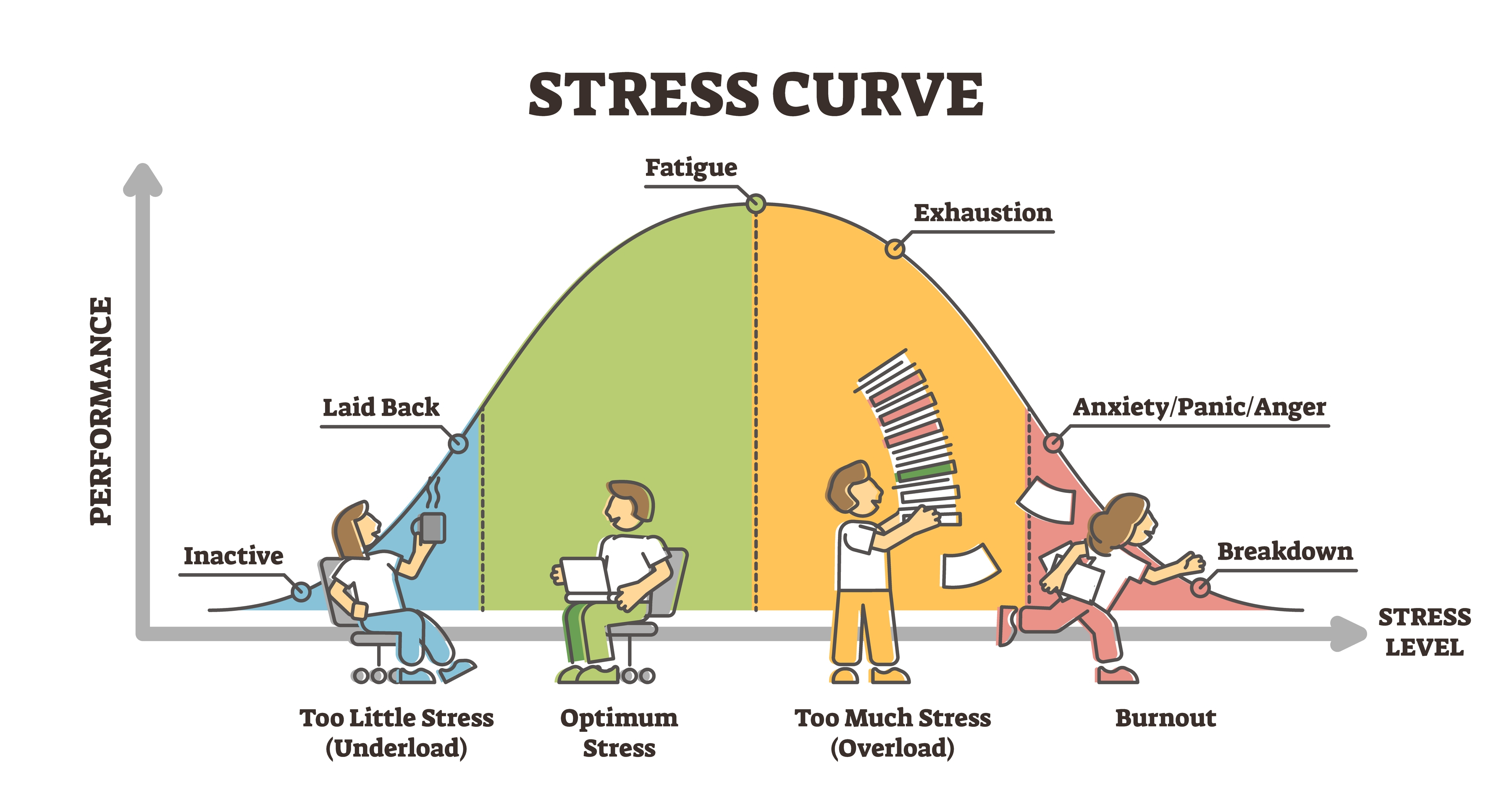
Activation and Performance: The Inverted-U
Research since Duffy's time has both extended and complicated this picture. Some work kept close to her single "activation" dimension, using it to explain how task design affects people's arousal, satisfaction, and performance at work. For example, Gardner (1986) tested activation theory in a lab setting and found that very boring, low-stimulation tasks could actually raise arousal and lower satisfaction more than moderately stimulating tasks, and that performance tended to follow an inverted-U curve, with both too little and too much activation hurting performance. From a biofeedback perspective, we want our clients to achieve optimal (not maximal) activation for any task. Think about a student taking an exam: too little activation means drowsiness and poor focus, while too much activation means anxiety that interferes with memory retrieval.
Multiple Dimensions of Activation
Other theorists split activation into multiple dimensions. Thayer (1978) proposed at least two types: energetic arousal (awake versus tired) and tense arousal (tense versus calm), and later work confirmed that these are not just mixtures of pleasantness and a single activation scale but genuinely distinct forms of activation (Schimmack & Reisenzein, 2002). Studies on attention similarly distinguish arousal (momentary reactions to input) from activation (ongoing readiness to respond), each tied to different brain circuits and functions (Pribram & McGuinness, 1975; VaezMousavi et al., 2007).
Current Status of Activation Theory
Because of these developments, Duffy's exact version of activation theory is no longer a major stand-alone theory, but its core ideas remain embedded in modern research. The general insight that internal activation underlies the intensity of behavior influenced later multidimensional models of arousal and activation and current work on the "energetics" of attention and performance (Pribram & McGuinness, 1975; Thayer, 1978; VaezMousavi et al., 2007). At the same time, the field has moved away from a single activation continuum toward more fine-grained systems: separate neural circuits for arousal, activation, and effort; different subjective forms of activation; and clear distinctions between baseline arousal and task-related activation (Pribram & McGuinness, 1975; Schimmack & Reisenzein, 2002; VaezMousavi et al., 2007). Empirically, this means Duffy's broad concept has been partly confirmed (internal arousal really does matter for intensity) but also revised and split into multiple components. Today, activation theory is best seen as a historically important starting point whose core themes survive inside more detailed neuropsychological and motivational models rather than as a current, unified theory being directly tested.
Problems with Activation Theory
Lacey (1967) and other researchers raised serious objections to activation theory, and their critiques reshaped how scientists think about physiological arousal. The core problem is that arousal is not a single dimension. Lacey argued that autonomic, behavioral, and cortical arousal are distinct phenomena that do not always move together, so measuring only one index can be misleading.
For instance, when heterosexual men view slides of nude women, heart rate may increase while finger blood flow remains unchanged (Stern, Ray, & Davis, 1980). If researchers relied solely on finger blood flow, they might wrongly conclude the men were not aroused at all. Adding to this complexity, individuals show unique response patterns called response stereotypy, meaning the same person tends to react in consistent ways to stimuli of similar intensity and emotional tone.
One patient might always respond to pressure with elevated heart rate and blood pressure, whether she is delivering a report at work or racing to meet a deadline. Because clients bring different vulnerabilities and learning histories into treatment, clinicians conduct a psychophysiological profile to map out each person's characteristic response pattern.
Beyond individual differences, activation theory stumbles on the finding that specific stimuli and emotions produce their own distinctive physiological signatures rather than simply turning a generic arousal dial up or down. This principle of stimulus-response specificity shows up when, for example, people reliably increase muscle tension during competitive challenges.
Similarly, emotional response specificity means that primary emotions like anger, fear, and happiness each come with unique physiological fingerprints. Research by Ekman and colleagues found that anger raises both heart rate and skin temperature, fear raises heart rate while lowering skin temperature, and happiness raises heart rate without affecting skin temperature. Schwartz, Weinberger, and Singer (1981) extended these findings, showing that cardiovascular patterns can distinguish anger from fear and separate both from happiness and sadness.
Finally, Lacey observed that responses are often complex, with some physiological indices increasing while others decrease at the same moment. He called this intricate pattern directional fractionation. When a police officer hears a sudden noise, for instance, EEG and skin conductance may spike while digestive activity simultaneously drops. Together, these critiques show that the body's stress responses are far more nuanced than a simple arousal thermometer would suggest.
Habituation
Habituation is the opposite of arousal. A person gradually ceases to respond or reduces their response to a constant stimulus. For example, after 15 trials of listening to a moderate intensity tone, heart rate might no longer increase. Predictable, low-intensity stimuli that convey no new information and require no response readily produce habituation. Habituation is inhibited when a stimulus is intense, complex, and unique, and the subject must respond to it (rate its unpleasantness).
The orienting response and defensive response differ in their speed of habituation. Orienting responses rapidly habituate, while defensive responses habituate very slowly.
Situational Specificity: Context Matters
Situational specificity means that a physiological response (blood pressure elevation) does not occur randomly and is most likely in situations with special characteristics.
A situation's properties may include the location (office), time of day (morning), activity (conference), individuals present (employer), and personal emotional state (anxiety). A client's hypertensive response may be classically or operantly conditioned, which means they may be unaware of learning this response since its symptoms are largely "silent," and both processes involve implicit learning. Due to associative learning, the presence of one or more situational cues may elicit a complete physiological response.
Clinicians investigate the situational specificity of presenting complaints when they conduct a history, perform a psychophysiological profile (PSP), ask the client to maintain a symptom journal, and monitor the client in multiple real-world settings (blood pressure measurement in the office and while stalled in traffic). Situational information is crucial in creating stimulus hierarchies when using systematic desensitization to treat a phobia.
Law of Initial Values: Why Starting Points Matter
Imagine giving the same cup of coffee to two people: one who just woke up groggy and one who is already buzzing with energy. Would you expect the same reaction? Joseph Wilder suspected not, and his law of initial values (LIV) attempts to capture this intuition in a formal principle. According to Wilder, when you administer the same stimulus to different people and measure their responses over the same time window, those who start at higher baseline levels tend to show smaller changes in the direction the stimulus would normally push them, and larger changes in the opposite direction, compared to those who start lower (Wilder, 1962, 1967/2014).
Consider a practical example. For function-raising stimuli (such as a drug designed to elevate blood pressure), the LIV predicts that individuals whose blood pressure is already high will show smaller increases, and paradoxically, more frequent drops. For function-depressing stimuli, the pattern flips to its mirror image. Wilder argued that this behavior reflects the basic self-regulatory machinery of the autonomic nervous system and claimed that it appears across many physiological systems. He pointed especially to cardiovascular measures like blood pressure and heart rate, as well as endocrine and pharmacologic responses to standard drug doses (Wilder, 1962, 1967/2014).
Early Evidence for the Law of Initial Values
Early psychophysiology research lent credibility to Wilder's claims. Investigators found LIV-like patterns for heart rate and respiration, including a striking phenomenon known as the crossover point, where responses flip from increases to paradoxical decreases once baselines climb high enough (Block & Bridger, 1962; Hord et al., 1964). These findings suggested that the body actively resists being pushed too far in any one direction.
Statistical Traps That Can Mimic Biology
Today, researchers view the LIV as conditional rather than a universal biological law. More recent analyses have revealed an uncomfortable truth: the familiar negative correlation between baseline and change can be partly a statistical illusion. Two culprits are mathematical coupling (when baseline values are embedded in the calculation of change scores) and regression to the mean (the tendency for extreme scores to drift toward average on retest). When investigators strip away these artifacts, some datasets actually show an anti-LIV pattern, where higher initial values are followed by larger, not smaller, responses (Geenen & van de Vijver, 1993; Jin, 1992; Myrtek & Foerster, 1986).
Where the LIV Holds and Where It Fails
Empirically, LIV-type effects receive their most consistent support for certain autonomic nervous system variables, especially heart rate and respiration rate measured under acute, standardized laboratory stressors. In these contexts, crossover points and paradoxical responses at high baselines do appear reliably (Block & Bridger, 1962; Hord et al., 1964). Other autonomic outputs tell a different story. Skin temperature, for instance, may show little evidence of the LIV. Skin conductance (a sweat-gland measure) can even display anti-LIV behavior, with higher baselines predicting larger responses (Hord et al., 1964). Stern, Ray, and Quigley (2001) similarly reported that studies support the LIV for heart rate and skin resistance but not for skin conductance.
Berntson and colleagues (1994) questioned the LIV's value and encouraged researchers to develop an "autonomic constraints" model tied to underpinning physiology.
Relevance of the LIV for Biofeedback Practice
The current status of the LIV is mixed. It captures a real, sometimes powerful baseline dependency in specific systems under specific conditions, but its reach is limited. Researchers studying phasic (rapidly changing) measures may consider employing statistical methods to control the influence of prestimulus values on the size of physiological responses. Careful statistical methods are essential before treating it as a general law of biological reactivity (Geenen & van de Vijver, 1993; Jin, 1992; Myrtek & Foerster, 1986; Wilder, 1962, 1967/2014).
Remember that the LIV is a principle, not a law, and does not apply to all subjects and response systems. Researchers can determine whether the LIV is relevant by computing the correlation between prestimulus and poststimulus values. Geenen and van de Vijver (1993) suggested that the LIV may be less common than perceived and correction methods may harm the data more than the phenomenon itself.
Generalization of Biofeedback Training: Will Skills Transfer?
The key muscle hypothesis and hand-warming specificity have important implications for biofeedback training.
The Key Muscle Hypothesis: A Tempting but Flawed Idea
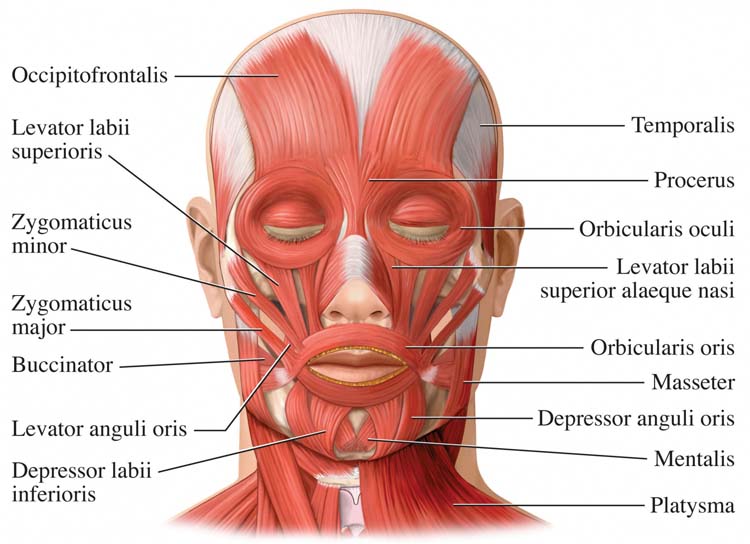
The key muscle hypothesis in biofeedback grew from the idea that certain skeletal muscles, especially the frontalis in the forehead, serve as a "master dial" for bodily tension. The logic was appealing: if forehead EMG drops, overall musculature is presumed to relax, and the person should feel globally calmer. Early EMG biofeedback studies on tension headache trained patients to reduce frontalis EMG via auditory or visual feedback and reported large reductions in headache activity, bolstering the intuition that relaxing one "key" muscle could unlock whole-body relaxation and symptom relief (Budzynski et al., 1973).
What EMG Biofeedback Actually Does Well
Biofeedback reliably reduces EMG in the specifically trained muscle and can increase people's ability to detect and modulate their own tension, suggesting genuine learning at the local muscular level (Sime & DeGood, 1977). More recent clinical and rehabilitation trials similarly show that EMG-based biofeedback can be effective when targeted to particular muscles. For example, researchers have successfully applied EMG biofeedback to masticatory muscles in temporomandibular disorders, pelvic floor muscles postpartum, and postural muscles in chronic low back pain and other musculoskeletal conditions (de la Barra Ortiz et al., 2022; Florjanski et al., 2019; Giggins et al., 2013; Handzlik-Waszkiewicz et al., 2025; Lazaridou et al., 2023). These findings support the value of site-specific muscle relaxation rather than a single global key.
The Evidence Against Generalization
When the core predictions of the key muscle hypothesis have been tested directly, however, the evidence has been largely negative. In a classic study, training frontalis EMG relaxation did not generalize to untrained forearm or lower-leg muscles, and reduced forehead EMG did not produce greater subjective relaxation than instructions without feedback (Alexander, 1975). A follow-up experiment similarly found that both feedback and non-feedback groups could lower EMG and that prior training on one muscle failed to facilitate training on another, undermining the idea of a privileged key muscle or a generalized relaxation skill emerging from it (Alexander et al., 1977).
Other work comparing EMG biofeedback, relaxation instructions, and adaptation effects showed that decreases in muscle activity were largely attributable to instructions to relax, not to the feedback signal itself. Again, no special generalization or superiority of key-muscle feedback was observed (Davis, 1980). The musculoskeletal system functions very specifically: we simultaneously contract and relax adjacent agonist-antagonist muscle pairs at a joint, which explains why training one site does not automatically transfer to others.
Current Status of the Key Muscle Hypothesis
Contemporary systematic reviews characterize EMG biofeedback as an effective adjunct for reducing activity and pain in specific muscles and improving function, but they do not support the notion that training a single "key" muscle reliably produces whole-body relaxation (de la Barra Ortiz et al., 2022; Florjanski et al., 2019; Giggins et al., 2013). In current science and practice, the key muscle hypothesis is therefore viewed as largely unsupported in its strong, generalized form, while localized EMG-guided relaxation remains a valuable, evidence-based tool.
Hand-Warming Specificity: Does Thermal Training Transfer?
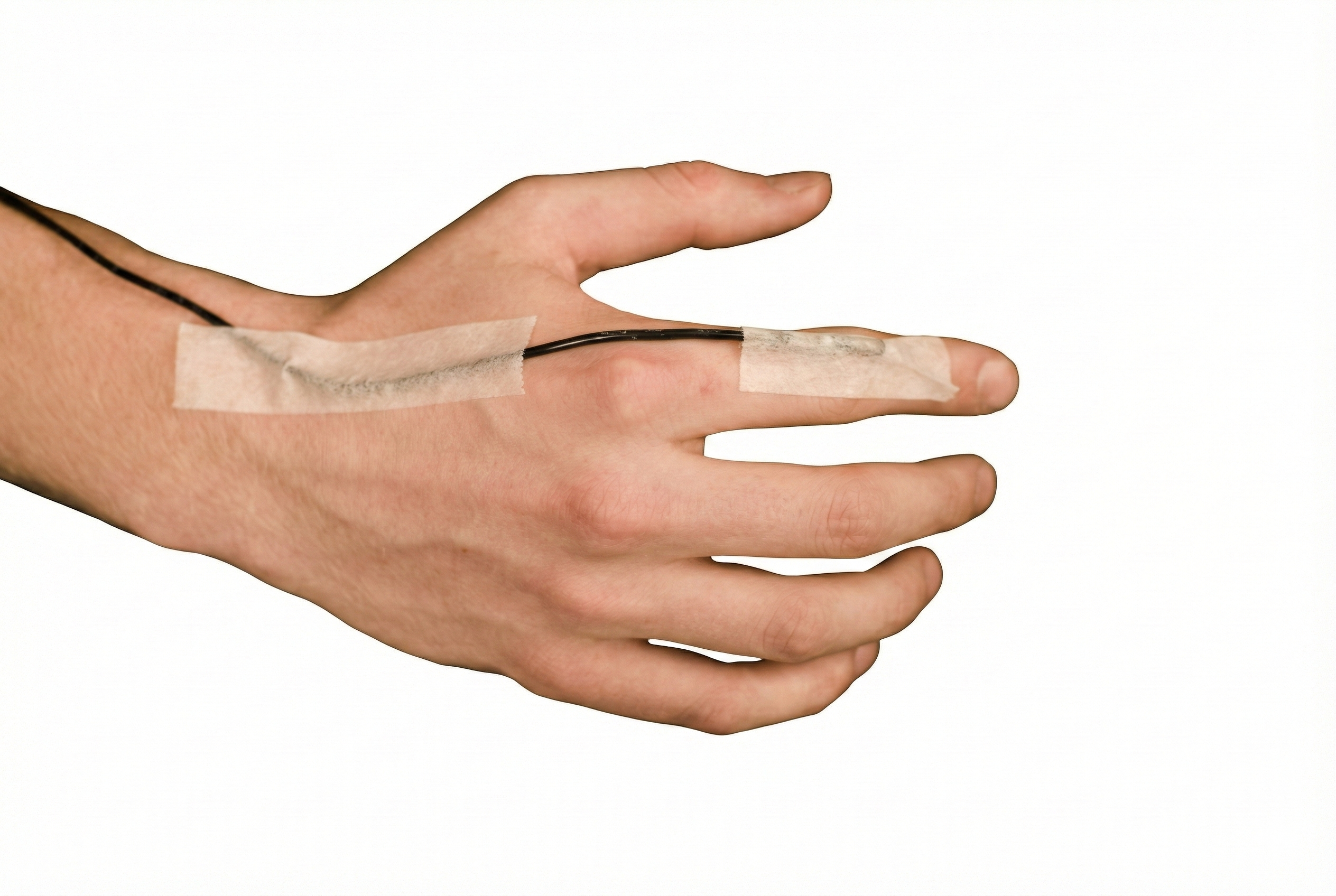
Situational specificity in thermal biofeedback is about how far a learned warming response "spreads." If someone learns to warm one finger using biofeedback, does that skill automatically generalize to the whole hand, other fingers, or even the feet? Early clinical enthusiasm often assumed "yes," treating hand-warming as a kind of global circulatory relaxation skill that would show up all over the body. More recent work paints a more cautious and nuanced picture: some spread across nearby sites is possible, but easy one-to-one transfer, especially from hand to feet, cannot be taken for granted.
The cardiovascular system can function very precisely, just like the musculoskeletal system. Temperature training can produce extremely site-specific changes. The picture below shows the placement of a thermistor (temperature sensor) on the index finger's dorsal surface.
Within-Hand Transfer: Local Control with Some Spillover
Within the hand, biofeedback studies show both local control and some local spillover. People can learn to raise or lower temperature in a targeted finger by a small but reliable amount, and training effects typically emerge within a few sessions (Keefe & Gardner, 1979; King & Montgomery, 1980). In diabetic patients, training on one hand increased temperature not only in the trained hand but also in the untrained hand, suggesting that part of the effect reflects more global autonomic changes or generalized relaxation rather than a perfectly isolated local response (Dean, 1984). More broadly, hand-temperature increase training tends to generalize to other physiological "relaxation" markers (like reduced muscle tension and lower arousal), indicating cross-system transfer of a general relaxation pattern rather than purely local vascular skill (Clemow, 1985).
The temperature map below shows mean undergraduate finger and web dorsum temperatures (Lammy et al., 2004).
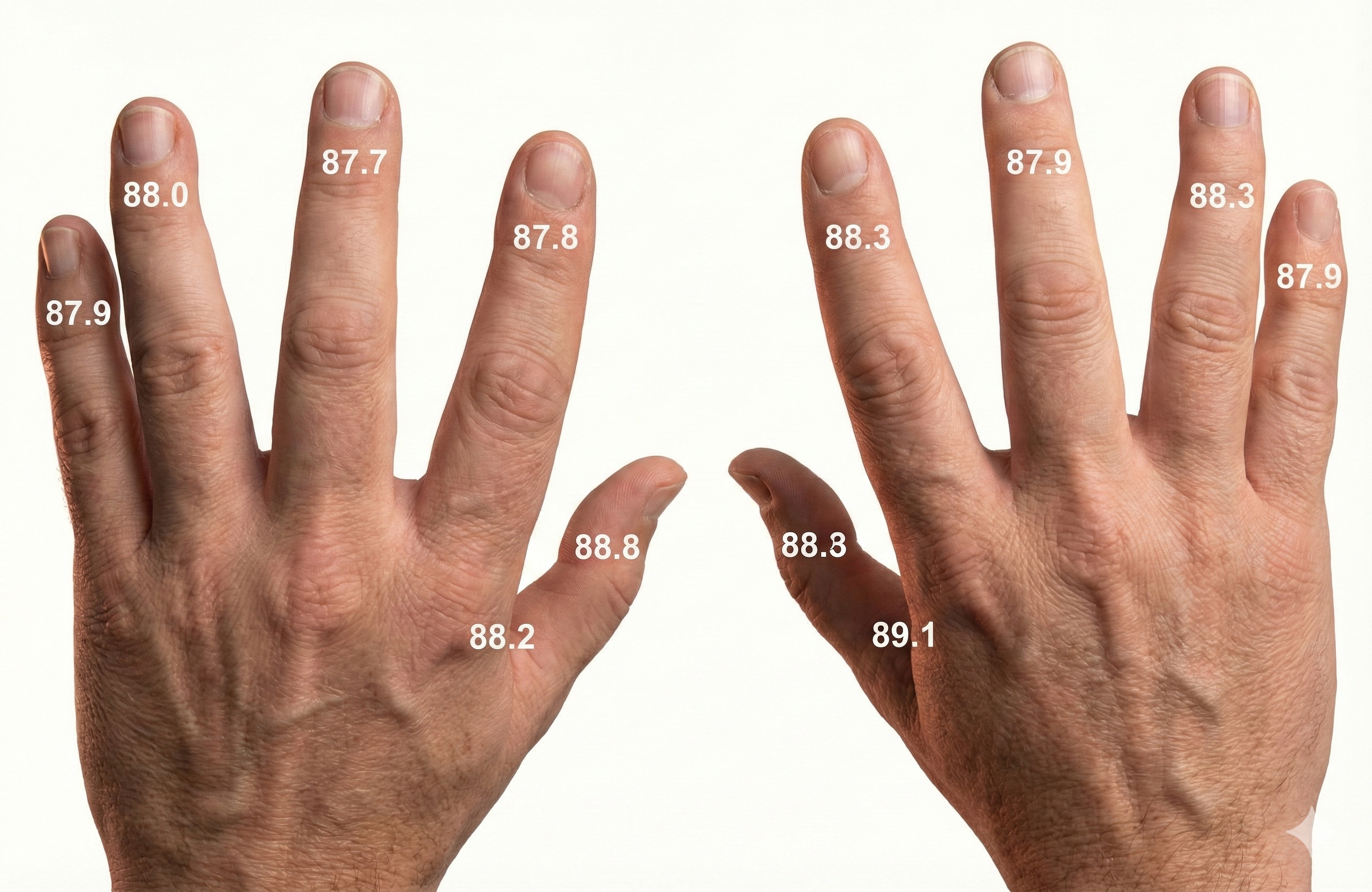
Hand-to-Foot Transfer: A Different Story
When researchers explicitly tested generalization from hand to foot, however, the story changes. In a Raynaud's syndrome case study, a patient learned a large and rapid hand-warming response, but foot temperature increases were modest and much slower to acquire; hand and foot temperature fluctuations were not significantly related across or within sessions, leading the authors to conclude that "an easy generalization of the hand-warming response cannot be assumed" (Crockett & Bilsker, 1984, p. 328).
Physiological studies of cold responses support this dissociation. Cold-induced vasodilation is highly variable across fingers and does not generalize reliably from fingers to toes or from hand to foot within the same person (Cheung & Mekjavic, 2007; Norrbrand et al., 2017). In other words, thermal control in one extremity tells you surprisingly little about another.
At the same time, targeted thermal biofeedback and relaxation focused directly on the feet can increase toe temperature and blood flow in people with diabetes, but that improvement comes from training the feet themselves, not from transfer of hand training (Rice & Schindler, 1992).
Clinical Implications for Temperature Training
Overall, current thinking is that hand-warming biofeedback can shape nearby or related systems but cannot be assumed to generalize automatically from one finger to the whole hand or from the hands to the feet; those regions likely need their own specific training. Clinicians should never expect hand-warming to generalize beyond the actual site trained. Depending on training procedure and individual differences, hand-warming might generalize to other digits on the same hand or to the other hand for some clients but not for others.
Key Takeaways: Psychophysiological Concepts
Homeostasis maintains your internal balance through feedback systems, while allostasis allows adaptation through anticipation. The law of initial values reminds us that starting points affect how much change is possible. The key muscle hypothesis, while intuitively appealing, has not been supported in its strong form: training one muscle does not reliably generalize to whole-body relaxation. However, site-specific EMG biofeedback remains a valuable clinical tool. Similarly, hand-warming at one site may not transfer to other sites. These specificity findings mean that your biofeedback training must target the actual physiological systems and specific muscle groups relevant to your client's presenting complaints.
Comprehension Questions: Measurements and Principles
- Why is it important to understand the difference between tonic and phasic activity when interpreting biofeedback data?
- How would the Law of Initial Values affect your expectations for a client whose resting heart rate is already very low?
- Your client asks, "If I can relax my forehead muscles, won't that relax my whole body?" How would you respond based on the key muscle hypothesis research?
- Explain how situational specificity might help you design a more effective treatment plan for a client with white-coat hypertension.
The Placebo Effect: The Power of Expectation
The placebo effect is no longer seen as "just in your head," but as a genuine set of mind-body responses to the whole treatment context: the rituals of medicine, the white coat, what the clinician says, and what the patient expects will happen (Finniss et al., 2010; Petrie & Rief, 2019; Price et al., 2008). When people receive an inert pill or sham procedure, their brains and bodies can change in measurable ways: pain decreases, Parkinson's symptoms improve, or mood lifts, especially when symptoms are subjective, like pain or nausea (Ashar et al., 2017; Ozpolat et al., 2025; Wager & Atlas, 2015).
Neuroscience links these effects to systems that use dopamine and endogenous opioids, which are the same chemicals involved in reward and natural pain control (Dodd et al., 2017; Knezevic et al., 2025; Wager & Atlas, 2015). Two core psychological mechanisms keep appearing across studies: expectation ("this will help me") and learning/conditioning (the body learns to respond to the look and feel of treatment the way it has responded to real drugs in the past) (Petrie & Rief, 2019; Price et al., 2008).
Ordinary (Deceptive) Placebos
Placebos in their classic form are sugar pills or sham procedures given as if they were real treatments. When compared with no treatment at all, these placebos can produce small-to-large improvements in symptoms across conditions such as pain, depression, irritable bowel syndrome, and Parkinson's disease (Ashar et al., 2017; Frisaldi et al., 2023; Price et al., 2008). In pain studies, placebo analgesia can be strong enough that sham injections or even sham surgeries sometimes match the benefit of active procedures (Ashar et al., 2017; Knezevic et al., 2025; Vase & Wartolowska, 2019).
Expectation is usually "built" by the doctor's confident words and the medical setting, then reinforced by experience when symptoms actually improve. Brain imaging shows activation of prefrontal and limbic regions that help people reappraise how threatening their symptoms feel, along with changes in pain-processing networks (Ashar et al., 2017; Wager & Atlas, 2015). Ordinary placebos also rely heavily on conditioning: over time, the body learns that pills, injections, and hospital smells predict relief, so these cues alone can trigger some of the same physiological responses as real drugs (Dodd et al., 2017; Petrie & Rief, 2019).
Active Placebos
Active placebos are special control treatments designed to mimic the side effects of a real drug, such as dry mouth or sleepiness, without having its specific therapeutic action. They are used because if only the real drug causes noticeable sensations, patients may "unblind" themselves and show bigger responses just from knowing they got the active treatment. Active placebos try to level that playing field by making both groups feel something (Vase & Wartolowska, 2019).
Mechanistically, active placebos still work through expectation and learning, but with an extra twist: once patients feel any bodily change, they often take it as a sign that "the medicine is working," which further boosts expectations and engages the same reward and pain-control circuits in the brain (Ashar et al., 2017; Petrie & Rief, 2019; Wager & Atlas, 2015). Umbrella reviews of pharmacological trials suggest that placebo (and nocebo) effects can be sizable, with effect sizes from about 0.08 up to 2.0, depending on the condition and how strongly expectations and conditioning are engaged (Frisaldi et al., 2023; Mishra & Bhargava, 2025).
Open-Label Placebos
In recent years, researchers have turned to open-label placebos (OLPs), pills honestly described as placebos, to avoid the ethical problem of deception. Surprisingly, across conditions such as irritable bowel syndrome, chronic back pain, depression, allergic rhinitis, and attention-deficit/hyperactivity disorder, randomized trials and meta-analyses show that open-label placebos outperform no treatment, with medium-to-large effects on self-reported symptoms (Charlesworth et al., 2017; Colloca & Howick, 2018; Schaefer et al., 2018). A network meta-analysis found that OLPs help both clinical and nonclinical populations, with stronger benefits in patients who are already suffering than in healthy volunteers (Buergler et al., 2023; Spille et al., 2023). These effects are usually largest for subjective outcomes such as pain, stress, or well-being, and less consistent for "hard" physiological measures like wound healing or lab values (Buergler et al., 2023; Spille et al., 2023).
How can open-label placebos work if people know they are taking an inert pill? One clue lies in the scripts used in OLP studies. Patients are typically told four things: that placebo effects are powerful, that the body can respond automatically to pill-taking (like Pavlov's dogs), that a positive attitude may help but is not required, and that taking the pills regularly is important (Colloca & Howick, 2018; Kaptchuk, 2018; Schaefer et al., 2018). This brief "education" seems to create a subtle but genuine expectation that "this might still help," even without outright belief that the pill is a drug.
Theories of Placebo Mechanisms
Because expectation alone does not fully explain OLPs, newer theories look to prediction and embodiment. Kaptchuk (2018) draws on predictive processing models, which suggest that the brain constantly guesses what is happening in the body and updates those guesses based on incoming signals. From this view, symptoms like pain or fatigue partly reflect "stories" the brain tells about the body's state. The ritual of pill-taking, even with full honesty, might nudge these predictions toward improvement and dampen exaggerated symptom signals, especially in conditions with central sensitization or symptom amplification (Ashar et al., 2017; Kaptchuk, 2018; Pagnini et al., 2024).
Embodied cognition theories add that the simple act of taking a pill, opening the bottle, swallowing with water, can itself shape thoughts and bodily states, because our minds are tightly linked to our physical routines (Pagnini et al., 2024; Schaefer et al., 2018).
Common Mechanisms and Clinical Implications
Underneath all three kinds of placebo, ordinary, active, and open-label, similar psychobiological machinery seems to be at work. Expectation, prior learning, and the meanings we assign to treatment change how the brain appraises symptoms and predicts the future, which in turn shapes activity in pain and emotion circuits, autonomic responses like heart rate, and even immune processes (Ashar et al., 2017; Dodd et al., 2017; Wager & Atlas, 2015). Placebos are strongest for subjective, fluctuating symptoms such as pain, insomnia, or fatigue, and weaker but still detectable for some objective outcomes (Frisaldi et al., 2023; Price et al., 2008). At the same time, not everyone responds: genetics, personality, prior experiences, and the quality of the clinician-patient relationship can all tilt placebo responses up or down (Dodd et al., 2017; Petrie & Rief, 2019).
Current thinking is that placebos are not magical cures and cannot replace effective drugs for serious diseases, but they are real amplifiers of healing built into every clinical encounter. Ordinary and active placebos show how powerful expectations and rituals can be when people believe they are getting active treatment. Open-label placebos show that those same mechanisms can sometimes be engaged without deception, by reframing how people understand and inhabit their symptoms and treatments (Buergler et al., 2023; Charlesworth et al., 2017; Kaptchuk, 2018). The challenge now is to use this knowledge ethically, designing trials that control for placebo effects fairly, and shaping everyday care so that communication, empathy, and ritual work with medicines rather than being ignored or wasted.
Psychophysiological Assessment: Building the Complete Picture
Client assessment is a collaborative process that starts with an orientation to biofeedback, client recording symptoms, a psychophysiological history, referral to physicians to investigate related medical complaints, and a psychophysiological stress profile (Moss & Shaffer, 2022).

Psychophysiological Stress Profile Overview
A psychophysiological stress profile (PSP) monitors multiple physiological variables under standardized resting, stress, and recovery conditions. Clinicians use a PSP to detect chronic problems that depress or elevate physiological measures, overactivation in response to stressors, and difficulty recovering from stress trials. A PSP provides a comprehensive picture of the client's psychophysiological health and identifies the biofeedback modalities to help clients achieve their training goals.
In a typical PSP, a practitioner will monitor hand temperature (TEMP), heart rate (HR) and HRV, respiration (RESP), skeletal muscle electrical activity (SEMG), skin conductance (SCL), and possibly brain electrical activity (EEG). The clients' presenting complaints may determine the placement of SEMG and EEG sensors. For example, a clinician may use left and right SEMG channels to record cervical paraspinal muscle activity in tension-type headaches.
PSP Structure
A clinician may divide the PSP into a 5-minute baseline (no feedback or breathing instructions), a 3-minute relaxation period (relaxation instructions), a 3-minute stress trial (visualizing a recent stressful event), a 3-minute recovery period, a second 3-minute stress trial, and a final 3-minute recovery period.
How the PSP Can Guide Treatment Planning
The PSP provides extensive information about a client's psychophysiology to guide treatment planning. Instead of a manualized "one size fits all" approach, the PSP enables a practitioner to tailor training to a client's unique strengths and needs.
First, the baseline reveals which values are depressed or elevated. This can help identify systems that are dysregulated in everyday life. Second, the relaxation trial evaluates a client's ability to self-regulate their physiology. Third, the stress trials show which systems over or under react to cognitive and emotional challenges. Fourth, the recovery trials assess a client's ability to restore relaxed physiology following stressors.
When the Psychophysiological History and PSP identify a distinct stereotypy, a practitioner can target the systems that may contribute to a client's symptoms. For example, a practitioner might recommend muscle relaxation training if a client's upper back and neck muscles were excessively tense during baseline, did not loosen during relaxation, tensed further during both stressors, and did not recover following the stressors.
Clinical Application: Using the PSP to Tailor Treatment
Consider a client named David who comes to you for stress-related symptoms. During his PSP, you notice that his baseline skin conductance is elevated, his hand temperature drops significantly during stress trials, and he shows poor recovery on all measures. His muscle tension, however, remains within normal limits throughout. This pattern suggests that David's stress response primarily involves his autonomic nervous system rather than muscular bracing. Rather than starting with EMG-based muscle relaxation (which might be a default protocol), you might prioritize HRV biofeedback and hand-warming training to address his specific response pattern.
Key Takeaways: Psychophysiological Assessment
The PSP is your roadmap for individualized treatment. Rather than applying a one-size-fits-all protocol, you use the PSP to identify which physiological systems are dysregulated at rest, which overreact to stress, and which recover poorly. This idiographic approach ensures that your biofeedback training targets the specific systems that contribute to your client's symptoms, making treatment more efficient and effective.
Comprehension Questions: Assessment and the Placebo Effect
- What are the four phases of information the PSP provides about a client's psychophysiology?
- How might understanding the placebo effect help you as a biofeedback practitioner?
- Your client shows normal baseline values but extreme physiological reactivity during stress trials with poor recovery. What does this pattern suggest about their treatment needs?
- Why is it important to monitor multiple physiological variables rather than just one during a PSP?
- How does the idiographic approach differ from a manualized treatment protocol?
Cutting Edge: Research Frontiers in Psychophysiology
The topics in this section represent some of the most exciting research frontiers in psychophysiology and biofeedback. These areas are still developing, with new studies appearing regularly, but they illustrate how the field is expanding beyond traditional applications to address complex health challenges.
The Inflammatory Reflex: When Your Nervous System Helps "Brake" Inflammation
Your nervous system and immune system are constantly talking to each other, and this conversation can shape how both systems behave. Scientists call this neuroimmune regulation, and it represents one of the most exciting frontiers in understanding how the body works. For a long time, researchers thought of inflammation as a slow chemical process that just runs on its own once it gets started. But influential research by Kevin Tracey (2002) proposed something different: the body can actually regulate inflammation quickly through what he called the inflammatory reflex.
Here is how the story goes. The vagus nerve helps carry information between the brain and organs throughout the body. When inflammation rises too high, the vagus can participate in turning that inflammatory signaling back down. Immune cells communicate through messenger proteins called cytokines, and one well-known example is tumor necrosis factor-alpha (TNF-α), a cytokine that tends to increase when the body is in a more inflammatory state. Research by Pavlov and Tracey (2005, 2012) described what they called the cholinergic anti-inflammatory pathway, through which neural signals can help keep inflammation from spiraling out of control.
Why does this matter for biofeedback? Many biofeedback methods aim to strengthen flexible regulation in body systems that connect closely to stress and inflammation. HRV provides a window into that flexibility because it reflects, in part, how dynamically the heart responds to changing demands, including signals related to calmer, restorative physiology. HRV biofeedback often trains people to increase their HRV using slow-paced breathing while watching real-time feedback and learning what patterns help their body settle. The health relevance is striking: if better autonomic regulation supports healthier immune "braking," then HRV biofeedback may not only help people feel calmer in the moment but could also influence a risk process that shows up across many chronic conditions.
Consistent with this logic, a randomized controlled trial in people with panic disorder found that HRV biofeedback with slow-paced breathing changed inflammatory markers, including TNF-α, compared with a sham condition (Herhaus et al., 2023). This is still a developing area, and more research is needed, but the possibility that practicing better autonomic regulation could influence inflammation opens exciting new doors for understanding how biofeedback works.
Long COVID and Autonomic "Misfires"
Many people who have had COVID-19 find that certain symptoms just will not go away. Their heart races unexpectedly, they feel dizzy when standing up, shortness of breath catches them off guard, fatigue seems to come from nowhere, and their thinking feels foggy. Scientists have given this collection of lingering symptoms a name: Long COVID. A leading explanation focuses on dysautonomia, which means that the autonomic nervous system is not adjusting smoothly to everyday demands like standing up, walking around, or handling stress (Tavee, 2024).
One especially important pattern that has emerged is postural orthostatic tachycardia syndrome (POTS). In POTS, the heart rate rises abnormally when a person stands up, often paired with lightheadedness, a "wired but tired" feeling, and difficulty tolerating exercise. In a study of highly symptomatic Long COVID patients, a substantial subgroup met criteria for POTS, and the condition created meaningful burdens in their daily lives (Björnson et al., 2025). An important point for anyone experiencing these symptoms: POTS is not "just anxiety," even though it can feel like panic in the body. It is a real physiology problem that can amplify distress, disrupt activities, and keep people stuck in a cycle of symptoms and avoidance.
This is where biofeedback becomes relevant. Biofeedback can train more stable "automatic" responses without medication side effects, and people can practice at home. HRV biofeedback uses real-time feedback, often paired with slow diaphragmatic breathing, to help people learn patterns associated with calmer, more flexible regulation. A small feasibility study in Long COVID found improvements in symptom and disability measures after a brief HRV biofeedback program (Corrado et al., 2024). Another open trial that combined HRV and temperature biofeedback reported improvements in self-reported symptoms and quality of life (Emerson et al., 2024). Related work suggests that slow-paced breathing can shift certain cardiovascular control signals in Long COVID toward a healthier response during practice (Mauro et al., 2025).
The cutting-edge clinical angle is not that biofeedback "cures" Long COVID. Rather, it may help a subgroup of people with dysautonomia-related symptoms regain day-to-day functioning by retraining regulation skills that the illness has destabilized. This is an active area of research, and studies are ongoing to understand who benefits most and why.
Inflammaging: When Slow-Burning Inflammation Meets Aging
As people get older, something interesting happens to their immune systems: there tends to be a slow, long-term rise in background inflammation, even when they are not sick with an obvious infection. Scientists have coined a term for this phenomenon: inflammaging (Franceschi & Campisi, 2014). This matters because chronic, low-grade inflammation is linked to many age-related diseases, so researchers increasingly treat it as a common "soil" in which multiple health problems can grow.
Here is where the story connects to what you have been learning about the autonomic nervous system. Researchers now emphasize that the immune system does not drift on its own. It is tightly connected to how the brain and body handle stress, sleep, activity, and recovery, all of which are targets for behavior-based health approaches. A second cutting-edge piece of this puzzle is the idea that autonomic nervous system balance may be part of what pushes inflammaging forward, and that we can track this balance using HRV (Olivieri et al., 2024; Shaffer & Ginsberg, 2017).
In simple terms, higher HRV at rest often reflects a nervous system that can shift gears more flexibly, while lower HRV can reflect reduced flexibility or higher physiological "wear and tear." This pattern becomes more common with aging. Because HRV is inexpensive and noninvasive to measure, scientists are discussing it as a practical biomarker that could help detect risk earlier and monitor whether lifestyle changes are moving someone toward healthier aging (Olivieri et al., 2024).
This is where HRV biofeedback enters the picture. The training helps people change their physiology in real time, most commonly by using slow-paced breathing at an individualized rhythm that boosts heart-breath coordination and engages reflexes that help regulate blood pressure and cardiac control (Lalanza et al., 2023). The clinical hope is not just "relaxation" but improving the body's capacity to recover from stress, which could matter for inflammation-related aging pathways over time. Controlled studies show HRV biofeedback can shift HRV upward and may influence brain systems involved in emotion regulation in both younger and older adults, supporting the idea that repeated training can produce real biological change rather than just a temporary feeling state (Yoo et al., 2022).
Interoception: Your Built-In Body Dashboard
Interoception is your ability to notice what is happening inside your body, things like your heartbeat, breathing, stomach feelings, temperature, and muscle tension. Researchers increasingly treat interoception as a "shared thread" across many mental health conditions because people often experience distressing body sensations in anxiety, depression, and psychosis, even when the diagnoses look different on paper. This way of thinking is called transdiagnostic because it cuts across traditional diagnostic categories (Jenkinson et al., 2024; Khalsa et al., 2018).
Why does interoception matter for health? How you notice and interpret body signals can shape your stress, emotions, and behavior. Whether a racing heart feels like "normal excitement" or "something is wrong" depends partly on how your brain reads those internal signals. A cutting-edge idea in this area proposes that the brain is always making educated guesses about what body signals mean, then updating those guesses using incoming information. Scientists call this predictive processing, and when it is applied specifically to body signals, it is called interoceptive inference (Nord & Garfinkel, 2022; Seth, 2013).
Here is what this means in practical terms. If your brain expects danger, normal sensations like faster breathing or a pounding heart can get "explained" as threat, which can amplify fear and make the body feel even more intense. This framework helps explain why body-focused treatments can be useful: the goal is not to erase sensations, but to help people read them more accurately and respond more flexibly.
Biofeedback fits into this picture because it gives you real-time information about your body so you can practice changing it on purpose, like learning a skill with a coach. When people practice HRV biofeedback with slow breathing, they strengthen vagal influences on the heart and improve self-regulation, which can make stressful body sensations feel more manageable and less mysterious (Lehrer & Gevirtz, 2014). Biofeedback may help because it turns vague internal feelings into clear, trackable signals, which can improve interoceptive accuracy, or at least improve confidence in reading body cues.
For example, resonance breathing (a slow breathing pace that tends to maximize heart-breath coordination) is linked to cardiovascular changes involving the baroreflex, and these changes may support stronger awareness of internal signals (Leganes-Fonteneau et al., 2021). A recent systematic review specifically evaluated HR and HRV biofeedback as ways to improve interoception across behavioral, physiological, and neural measures, highlighting this as an active research frontier rather than a settled conclusion (Wareing et al., 2024). The emerging picture suggests that training people to observe and influence their physiology may reshape how they experience their bodies from the inside out.
One reason interoceptive difficulties can be tricky to assess is that the concept itself has layers. Researchers distinguish between interoceptive sensibility, meaning how much attention you believe you pay to body signals (assessed through questionnaires), and interoceptive awareness, meaning how well your self-reported beliefs match your actual performance on tasks like heartbeat counting (Garfinkel et al., 2015). Imagine a student who is convinced she always knows when her heart speeds up but actually performs poorly on a lab test that asks her to count heartbeats. That gap between belief and ability is called miscalibration, and it can be a source of real distress. Someone might feel absolutely certain that their body is signaling danger while the body is doing nothing unusual, or they might miss genuine signals that something is wrong. The insula, a cortical region tucked beneath the frontal and temporal lobes, plays a central role here because it integrates bodily, emotional, and cognitive information, functioning as a kind of hub where body signals get interpreted and assigned meaning (Nord et al., 2021). Neuroimaging studies find altered insula activity across multiple psychiatric diagnoses during interoceptive tasks, suggesting that this brain region may be a shared vulnerability factor even when the surface symptoms look very different (Khalsa et al., 2018; Kwon, 2026).
This framework carries direct implications for biofeedback practice. When a client walks into a session reporting that their body constantly feels "wrong" or "unsafe," the problem may not be that their body signals are abnormal. The problem may be a learned pattern of misinterpreting those signals or attending to them in unhelpful ways. Biofeedback offers a potential corrective by externalizing normally private signals, making them visible on a screen, so clients can compare what they feel with what is actually happening (Critchley & Garfinkel, 2017). Over time, this process can help recalibrate the match between sensation and interpretation. A client practicing HRV biofeedback might notice that when they feel "anxious," their heart rate is actually within a normal range, or they might learn to recognize the difference between the heart rate pattern during genuine stress versus the pattern during calm alertness. This recalibration does not erase body signals. Instead, it helps the client read those signals more accurately and respond with greater flexibility, which may be exactly what "listening to your body" means when it works well.
Comprehension Questions: Cutting Edge Topics
- How does the inflammatory reflex challenge older ideas about inflammation as a purely slow chemical process?
- What is the difference between POTS and anxiety, and why is this distinction important for treatment?
- How might inflammaging connect autonomic flexibility to healthy aging?
- Explain how predictive processing and interoceptive inference could contribute to anxiety symptoms.
- In what ways might HRV biofeedback help someone with Long COVID-related dysautonomia?
- Why is interoception considered a "transdiagnostic" concept in mental health research?
Glossary
A-bar: Wenger's shorthand for the ratio of sympathetic to parasympathetic excitation. Patients with low A-bar scores are sympathetic-dominant, and those with high A-bar scores are parasympathetic dominant.
accentuated antagonism: the PNS's ability to directly oppose sympathetic action, such as slowing the heart by 20 or 30 beats.
acetylcholine: a neurotransmitter released by preganglionic neurons of both the sympathetic and parasympathetic divisions, and by postganglionic neurons of the parasympathetic division. At the heart, acetylcholine slows the natural pacemaker and reduces heart rate.
activation: Duffy's term for the degree to which an organism's stored energy is being released through metabolic and neural activity. Activation reflects the level of neural activity in central systems like the reticular activating system and is usually inferred from physiological measures such as brain waves or skin conductance.
activation theory: Elizabeth Duffy's theory that behavior varies along two dimensions (direction and intensity) and that intensity is best understood as internal activation levels rather than outward response strength. While Duffy's original single-dimension model is no longer a stand-alone theory, its core ideas survive in modern multidimensional models of arousal.
active placebo: a special control treatment designed to mimic the side effects of a real drug (such as dry mouth or sleepiness) without having its specific therapeutic action. Used in trials to prevent patients from "unblinding" themselves based on noticeable drug sensations.
afferent: nerve fibers that carry sensory information from the body's organs and tissues to the brain and spinal cord. Most vagus nerve fibers are afferent, providing the brain with information about the heart and other organs to help adjust autonomic output.
affective flexibility: the ability to adaptively shift emotional responses and attention to emotional stimuli based on context. Higher resting HRV is associated with greater affective flexibility, while lower HRV is linked to hypervigilance and less flexible responses to negative information.
allostasis: the maintenance of stability through change by mechanisms that anticipate challenges and adapt through behavioral and physiological change.
allostatic load: the cumulative "wear and tear" on the body that builds up when stress responses are activated too often, for too long, or do not shut off properly. Measured using a bundle of biomarkers from multiple systems including blood pressure, cholesterol, blood sugar, and inflammatory markers.
allostatic load model: McEwen and Seeman's framework proposing that chronic or repeated stress produces harmful multi-system physiological changes. Research supports its prediction that higher allostatic load scores are associated with cardiovascular disease, cognitive problems, and increased mortality risk.
anti-LIV pattern: a response pattern opposite to the law of initial values, where higher initial physiological values are followed by larger, not smaller, responses to stimulation.
autonomic balance: Wenger's concept of the dynamic relationship between sympathetic and parasympathetic activity. In the classic view, healthy functioning depends on coordinated activity between these two systems; too much sympathetic activity or too little parasympathetic tone leads to autonomic imbalance and vulnerability to disease.
autonomic imbalance: a pattern of chronic sympathetic dominance with reduced parasympathetic activity, linked to cardiovascular disease, increased mortality, and multiple risk factors such as hypertension, obesity, and chronic stress. Often indexed by reduced HRV.
autonomic nervous system: the subdivision of the peripheral nervous system that includes enteric, parasympathetic, and sympathetic branches.
autonomic space: a multi-dimensional framework for understanding autonomic function, recognizing that sympathetic and parasympathetic branches can change reciprocally, independently, or even rise and fall together, rather than operating as simple opposites on a single continuum.
boundary model: the position that setpoints are not rigid and that physiological processes are maintained within acceptable ranges.
cardiac autonomic balance (CAB): a composite measure that captures the relative dominance of sympathetic versus parasympathetic influence on the heart, providing a more nuanced assessment than simple ratio measures like LF/HF.
cardiac autonomic regulation (CAR): a composite measure that captures overall autonomic influence on the heart, distinguishing co-activation or co-inhibition of both branches from relative dominance of one branch over the other.
cardiac vagal tone: the degree of parasympathetic influence on the heart, primarily mediated by the vagus nerve. Most commonly used HRV measures, such as RMSSD and high-frequency power, are considered markers of cardiac vagal tone.
central nervous system: the division of the nervous system that includes the brain, spinal cord, and retina.
central autonomic network (CAN): a network of brain regions that links areas involved in attention, emotion, and autonomic control. The neurovisceral integration model proposes that vagally mediated HRV indexes the functional capacity of this network, connecting cognitive-emotional regulation with cardiac vagal tone.
central sensitization: a condition in which the central nervous system amplifies pain and other sensory signals, leading to heightened sensitivity even to normal stimuli. Placebo effects may help by dampening these exaggerated symptom signals through predictive processing mechanisms.
cholinergic anti-inflammatory pathway: a neural mechanism through which the vagus nerve can help regulate inflammation. When activated, vagal signals prompt the release of acetylcholine, which can dampen the production of pro-inflammatory cytokines by immune cells.
cold-induced vasodilation: the reflexive dilation of blood vessels in response to cold exposure, which is highly variable across fingers and does not generalize reliably from fingers to toes or from hand to foot within the same person.
crossover point: the baseline level at which physiological responses flip from increases to paradoxical decreases (or vice versa), as predicted by the law of initial values for certain autonomic measures.
cytokines: proteins that act as immune system messengers, helping coordinate inflammation and other immune responses. Examples include tumor necrosis factor-alpha (TNF-α) and interleukins.
defensive response: a very slowly habituating response pattern that limits harm from intense stimulation. This pattern includes reduced sensory sensitivity, a tendency to move away from the stimulus, heart rate increase, and both peripheral and cephalic vasoconstriction.
diathesis: vulnerability. For example, a genetic predisposition to gain weight.
directional fractionation: Lacey's concept of a complex pattern of physiological response to a stimulus where some indices increase and others decrease.
dorsal motor complex: in polyvagal theory, the brainstem region from which unmyelinated vagal fibers originate. Also called the dorsal vagal complex, this older system is associated with shutdown, freeze, and immobilization responses when fight or flight is not possible.
dysbiosis: an imbalance or disruption in the normal composition of the gut microbiota, associated with conditions including irritable bowel syndrome, anxiety, depression, autism spectrum conditions, and neurodegenerative diseases.
dysautonomia: a condition in which the autonomic nervous system does not adjust smoothly to everyday demands like standing up, walking, or handling stress. Dysautonomia can produce symptoms such as rapid heartbeat, dizziness, fatigue, and difficulty regulating blood pressure, and has been identified in a subgroup of people with Long COVID.
dynamic biomarker: a measurable signal that tracks ongoing changes in physiological or brain function over time rather than reflecting a fixed trait. HRV is considered a dynamic biomarker because it captures how the vagus nerve and central autonomic network manage repeated challenges across the lifespan.
efferent: nerve fibers that carry motor signals from the brain and spinal cord to the body's muscles and organs. Efferent fibers in the vagus nerve release acetylcholine at the heart, slowing the pacemaker and reducing heart rate on a beat-to-beat time scale.
embodied cognition: the theory that cognitive processes are deeply shaped by the body's physical actions and routines. In placebo research, this helps explain how the simple act of taking a pill (opening the bottle, swallowing with water) can itself influence thoughts and bodily states.
emotional response specificity: the hypothesis that primary emotions are associated with unique physiological changes.
energetic arousal: one of Thayer's two dimensions of activation, representing the awake-tired continuum. Energetic arousal is distinct from tense arousal and reflects feelings of energy, vigor, and alertness.
energetic cost: an expansion of the allostatic load model proposing that chronic stress forces the body to spend so much energy on survival responses that less energy remains for repair and healthy aging.
enteric nervous system (ENS): sometimes called the "second brain," a major division of the peripheral nervous system containing hundreds of millions of neurons organized into the myenteric and submucosal plexuses. The ENS coordinates gut motility, secretion, blood flow, and local immune responses, often independently of the brain, and communicates bidirectionally with the central nervous system via the gut-brain axis.
epinephrine: an adrenal medullary hormone that increases muscle blood flow, converts stored nutrients into glucose for use by skeletal muscles, and initiates cardiac muscle contraction when it binds to their receptors.
fragmented sinus rhythm: a disorganized pattern of heart rhythm variation that is not purely autonomic in origin. In older adults, fragmented sinus rhythm can contribute to increased HRV that does not reflect healthy parasympathetic function, requiring careful interpretation of HRV measures.
freeze response: immobilization when facing a threat you cannot fight or flee.
frontalis: the forehead muscle historically targeted in EMG biofeedback based on the key muscle hypothesis. Research has shown that training this muscle does not produce generalized whole-body relaxation.
function-depressing stimuli: interventions (such as certain drugs or relaxation techniques) that lower physiological measures like blood pressure or heart rate.
function-raising stimuli: interventions (such as certain drugs or stressors) that elevate physiological measures like blood pressure or heart rate.
functional MRI (fMRI): magnetic resonance imaging procedure that can detect small changes in brain metabolism.
gut-brain axis: the two-way communication system between the enteric nervous system, the central nervous system, and the gut environment including the microbiota. Signals travel via neural pathways (vagus nerve, spinal afferents, ENS circuits), hormonal systems (HPA axis), and immune/metabolic signaling through cytokines and microbial metabolites.
habituation: the weakening or disappearance of a response to a constant stimulus.
heart rate variability (HRV): the natural variation in the time between consecutive heartbeats, usually measured from R waves on an electrocardiogram and expressed as changing interbeat intervals. HRV reflects how flexibly the ANS influences the heart and is considered a dynamic biomarker of brain-heart communication, self-regulation capacity, and cardiovascular health.
high-frequency (HF) power: the spectral power in the 0.15-0.40 Hz band of HRV frequency-domain analysis. HF power is strongly driven by vagal (parasympathetic) influences and reflects breathing-related modulation of heart rate.
homeostasis: the state of dynamic constancy achieved by stabilizing conditions about a setpoint, whose value may change over time.
homeostat: device that maintains homeostasis. For example, the hypothalamus.
hypothalamus: the forebrain structure below the thalamus that dynamically maintains homeostasis controlling the ANS, endocrine system, survival behaviors, and interconnections with the immune system.
idiographic approach: a research strategy that emphasizes individual differences.
inflammaging: a slow, long-term rise in background inflammation that tends to occur with aging, even in the absence of obvious infection. Inflammaging is linked to many age-related diseases and may be influenced by autonomic nervous system balance and lifestyle factors.
inflammatory reflex: a proposed feedback-like control process in which neural signals, particularly through the vagus nerve, can help regulate inflammation. This concept frames inflammation as something the body can regulate quickly rather than a purely slow chemical process.
interbeat intervals: the time intervals between consecutive heartbeats, usually measured from R waves on an electrocardiogram. The variation in interbeat intervals over time constitutes HRV.
interoception: the sensing and awareness of internal bodily states such as heartbeat, breathing, stomach sensations, temperature, and muscle tension. Interoception is increasingly recognized as a shared thread across many mental health conditions.
interoceptive accuracy: a person's ability to correctly detect internal body signals, such as accurately perceiving one's own heartbeat. Biofeedback training may help improve interoceptive accuracy by turning vague internal feelings into clear, trackable signals.
interoceptive awareness: a metacognitive measure of how well a person's subjective beliefs about their bodily perception match their actual performance on interoceptive tasks such as heartbeat counting. Poor interoceptive awareness, or miscalibration, can contribute to clinical distress when someone feels certain they understand their body while being systematically misinformed by it.
interoceptive inference: a brain-based process of interpreting body signals by combining incoming sensations with prior expectations. When the brain expects danger, normal sensations can be interpreted as threatening, potentially amplifying anxiety and fear responses.
interoceptive sensibility: a self-reported tendency to attend to and value internal bodily sensations, typically assessed through questionnaires that ask how much attention a person pays to internal states. This measure captures subjective beliefs about bodily awareness rather than objective performance.
insula: a cortical brain region located beneath the frontal and temporal lobes that integrates interoceptive, emotional, and cognitive information. The insula functions as a central hub for body awareness and is consistently implicated in neuroimaging studies of interoceptive processing across multiple psychiatric conditions.
inverted-U curve: the curvilinear relationship between activation and performance proposed by the Yerkes-Dodson Law, where performance increases with activation up to an optimal level and then decreases with further activation.
Irritable Bowel Syndrome (IBS): a functional gastrointestinal disorder characterized by abdominal pain and altered bowel habits.
key muscle hypothesis: the proposition that training a single "key" muscle (such as the frontalis) to relax will produce generalized whole-body relaxation. Research has largely failed to support this hypothesis in its strong form, though site-specific EMG biofeedback remains clinically effective.
law of initial values (LIV): Wilder's principle that when the same stimulus is given to different individuals, those who start at higher baseline levels tend to show smaller changes in the expected direction and larger changes in the opposite direction. This principle has been most consistently demonstrated for heart rate and respiration rate under standardized laboratory conditions.
LF/HF ratio: the ratio of low-frequency to high-frequency power in HRV analysis, historically used as an index of sympathovagal balance. However, influential reviews argue this ratio does not reliably reflect sympathetic tone, as much of the HRV spectrum is shaped by the parasympathetic system.
local spillover: in thermal biofeedback, the phenomenon where training to warm a specific site (such as one finger) produces some temperature increase in nearby untrained sites, suggesting a mix of local control and more global autonomic or relaxation effects.
low-frequency (LF) power: the spectral power in the 0.04-0.15 Hz band of HRV frequency-domain analysis. Contrary to earlier beliefs, LF power reflects a mixture of baroreflex activity, sympathetic, and parasympathetic influences and should not be treated as a pure sympathetic marker.
Long COVID: a condition in which symptoms persist for weeks or months after a COVID-19 infection. Common symptoms include fatigue, rapid heartbeat, dizziness, shortness of breath, and cognitive difficulties ("brain fog"). A subgroup of people with Long COVID experience dysautonomia, including postural orthostatic tachycardia syndrome (POTS).
mass activation: the simultaneous stimulation of adjacent ganglia (cell bodies) in the sympathetic chain during emergencies allows the SNS to produce many coordinated changes at once. For example, increased heart rate, respiration rate, and sweat gland activity.
mathematical coupling: a statistical artifact that occurs when baseline values are embedded in the calculation of change scores, potentially creating spurious correlations between baseline and change.
medulla: a brainstem structure that regulates blood pressure, defecation, heart rate, respiration, vomiting, the ANS, and distributes signals between the brain and spinal cord.
microbiota: the trillions of microorganisms (bacteria, viruses, fungi) residing in the gut that produce short-chain fatty acids, neurotransmitter-like chemicals, and other metabolites influencing brain function and behavior through the gut-brain axis.
microbiota-gut-brain axis: the biologically grounded communication system through which gut microbes influence stress reactivity, mood, and cognition via their metabolites acting on enteric neurons, endocrine cells, and immune cells.
myelinated vagus: the newer, faster-conducting vagal fibers originating in the nucleus ambiguus that support social engagement and self-regulation.
myenteric plexus: one of the two main neural networks in the enteric nervous system, located between the longitudinal and circular muscle layers of the gut wall. Also called Auerbach's plexus, it primarily controls gut motility.
neuroception: in polyvagal theory, the nervous system's automatic, unconscious process of continuously scanning the environment for cues of safety or threat, which then shifts bodily state in ways that shape emotion, attention, and social openness.
neurovisceral integration model: a theoretical framework proposing that vagally mediated HRV indexes the functional capacity of the central autonomic network, a set of brain regions linking attention, emotion, and autonomic control. The model explains why higher resting HRV is associated with better executive functions and emotion regulation.
neuroimmune regulation: the two-way communication between the nervous system and immune system that can shape both stress physiology and inflammation. This interaction is central to understanding how the vagus nerve and autonomic function may influence inflammatory processes throughout the body.
nocebo: the negative counterpart to the placebo effect, where negative expectations or information about potential side effects lead to worsening symptoms or adverse experiences, even when receiving an inert treatment.
norepinephrine: an adrenal medullary hormone that increases muscle blood flow and converts stored nutrients into glucose for skeletal muscles.
open-label placebo (OLP): an inert treatment honestly disclosed as a placebo to patients. Research shows OLPs can still produce medium-to-large effects on self-reported symptoms for conditions like irritable bowel syndrome, chronic back pain, and depression, challenging assumptions that deception is required for placebo effects.
orienting response: Pavlov's "What is it?" reaction to stimuli like the sound of a vase crashing that includes increased sensory sensitivity, head (and ear) turning toward the stimulus, increased muscle tone (reduced movement), EEG desynchrony, peripheral constriction and cephalic vasodilation, a rise in skin conductance, heart rate slowing, and slower, deeper breathing.
parasympathetic division: the autonomic nervous system subdivision that regulates activities that increase the body's energy reserves, including salivation, gastric (stomach) and intestinal motility, gastric juice secretion, and increased blood flow to the gastrointestinal system.
parasympathetic dominance: Wenger's concept of greater parasympathetic activation than sympathetic activation.
peripheral nervous system: nervous system subdivision that includes autonomic and somatic branches.
phasic: a brief change in physiological activity in response to a discrete stimulus. For example, a single skin potential response in reaction to a sudden tone.
placebo: an inert intervention (such as a sugar pill or sham procedure) that can produce genuine mind-body responses through the treatment context, including medical rituals, clinician communication, and patient expectations. Placebo effects are mediated by dopamine and endogenous opioid systems.
placebo analgesia: pain reduction produced by placebo treatments, which can be strong enough that sham injections or surgeries sometimes match the benefit of active procedures. Mediated by activation of prefrontal and limbic brain regions that help reappraise the threat value of pain.
placebo effect: the genuine set of mind-body responses to the treatment context, including medical rituals, clinician communication, and patient expectations. Core mechanisms include expectation and conditioning, with neural substrates involving dopamine, endogenous opioids, and prefrontal-limbic circuits.
polyvagal theory: Stephen Porges' theory proposing three hierarchically arranged autonomic response systems: the oldest dorsal vagal complex (unmyelinated vagus) supporting shutdown and freeze; the sympathetic system supporting fight-or-flight; and the newest ventral vagal complex (myelinated vagus) supporting social engagement and calm states. The theory emphasizes neuroception (automatic scanning for safety cues) and has become influential in trauma therapy, though its detailed biological claims remain debated.
postural orthostatic tachycardia syndrome (POTS): a form of dysautonomia in which the heart rate rises abnormally when a person stands up, often accompanied by lightheadedness, fatigue, and exercise intolerance. POTS has been identified in a substantial subgroup of people with Long COVID and is not "just anxiety" but a real physiological condition.
predictive processing: a model of brain function suggesting that the brain constantly generates predictions about incoming sensory signals and updates these predictions based on new information. In placebo research, this model helps explain how treatment rituals can shift the brain's predictions toward improvement and dampen exaggerated symptom signals.
psychobiotics: probiotics or prebiotics specifically aimed at mental health benefits. Early research suggests modest but promising effects on mood and cognitive performance in some groups, though effects are strain- and context-dependent.
psychophysiological principle: Green, Green, and Walters' concept that there is a bidirectional relationship between physiological and psychological functioning. For example, facial muscle contraction can influence emotion, and emotion can influence facial muscle contraction.
Psychophysiological Stress Profile (PSP): structured assessment procedure that monitors multiple physiological variables under standardized resting, stress, and recovery conditions.
regression to the mean: a statistical phenomenon where extreme scores on a first measurement tend to drift toward the average on subsequent measurements, which can create spurious correlations between baseline and change scores.
respiratory sinus arrhythmia (RSA): the natural variation in heart rate that occurs with breathing, where heart rate increases during inhalation and decreases during exhalation. RSA is often used as a measure of vagal regulation and parasympathetic activity.
response stereotypy: a consistent pattern of physiological responses when an individual encounters stimuli with the same intensity and elicit similar emotions. For example, a patient may raise her heart rate and blood pressure when delivering a report during a business meeting or completing an assignment under time pressure.
resting baseline: a tonic measure of psychophysiological activity (such as temperature) without breathing or relaxation instructions and feedback.
reticular activating system: a network of neurons in the brainstem that plays a central role in regulating arousal, wakefulness, and attention. In activation theory, the reticular activating system is considered a key neural substrate for activation levels.
root mean square of successive differences (RMSSD): a time-domain HRV measure that captures beat-to-beat variation in heart rate. RMSSD is strongly driven by rapid vagus nerve effects on the heart and is considered a primary marker of cardiac vagal tone or parasympathetic influence.
short-chain fatty acids: metabolites produced by gut microbiota from dietary fiber, including acetate, propionate, and butyrate. These compounds can act on enteric neurons, endocrine cells, and immune cells, indirectly influencing brain function and behavior through the gut-brain axis.
situational specificity: the occurrence of a physiological response in specific situations. For example, blood pressure increase during a dental examination.
situational specificity in thermal biofeedback: the extent to which a learned warming response generalizes from the trained site to other body regions. Research shows that hand-warming may spread to nearby sites but does not reliably transfer from hands to feet, indicating that different body regions may require their own specific training.
social allostatic load: an extension of the allostatic load concept to relationships and groups, describing unhealthy interaction patterns that emerge when stressed groups get stuck in maladaptive dynamics that compound individual stress burdens.
somatic nervous system: the peripheral nervous system subdivision that receives external sensory and somatosensory information and controls skeletal muscle contraction.
spontaneous responses: physiological changes in the absence of detectable stimuli. For example, skin conductance responses when no tones are presented to a subject.
stimulus-response specificity: specific stimuli elicit a distinctive response pattern in most individuals instead of simply altering activation. For example, subjects may increase their skeletal muscle tone when challenged to compete.
submucosal plexus: one of the two main neural networks in the enteric nervous system, located in the submucosa of the gut wall. Also called Meissner's plexus, it primarily controls secretion and blood flow in the gastrointestinal tract.
sympathetic dominance: Wenger's concept of greater sympathetic activation than parasympathetic activation.
sympathetic nervous system: the autonomic nervous system branch that regulates activities that expend stored energy, such as when we are excited.
sympathicotonics: Wenger's term for sympathetic-dominant individuals who they hypothesized to suffer an elevated incidence of neurotic, psychotic, psychosomatic, and medical disorders.
tense arousal: one of Thayer's two dimensions of activation, representing the tense-calm continuum. Tense arousal is distinct from energetic arousal and reflects feelings of tension, anxiety, and nervousness.
thermistor: a temperature sensor used in biofeedback to measure skin temperature.
tonic: a background level of physiological activity. For example, a 5-minute average of hand temperature.
transdiagnostic: an approach that identifies common processes or mechanisms across multiple diagnostic categories rather than focusing on individual disorders. Interoception is considered a transdiagnostic factor because problems with body signal awareness appear in anxiety, depression, psychosis, and other conditions.
tumor necrosis factor-alpha (TNF-α): a cytokine that tends to rise when the body is in a more inflammatory state. TNF-α is often used as a marker of inflammatory activity and has been studied in relation to HRV biofeedback interventions.
tyrosine hydroxylase: an enzyme that marks sympathetic nerve fibers. Anatomical studies using this marker have shown that the human vagus nerve contains a small but real sympathetic component (about 4-14% of fibers), which may explain variable responses to vagus nerve stimulation therapies.
vagal tank theory: a conceptual framework suggesting that cardiac vagal activity functions like a "tank" of regulatory resources that can be filled, used, and refilled over time. Resting HRV reflects the current level in the tank, phasic changes during challenges reflect resource recruitment, and recovery HRV shows how well the tank is refilled.
vagal withdrawal: the inhibition of the myelinated vagus, often by daily stressors.
vagus nerve: the main parasympathetic nerve going from the brainstem to the heart, lungs, and digestive organs. Most vagus nerve fibers are afferent (sensory), carrying information from organs to the brain. Efferent (motor) fibers release acetylcholine at the heart, slowing heart rate on a beat-to-beat time scale. Human anatomical studies show the vagus also contains a small sympathetic component.
ventral vagal complex: in polyvagal theory, the newest of the three autonomic response systems, originating in the nucleus ambiguus. The ventral vagal complex is said to connect the heart with the face and voice muscles, supporting social engagement, calm states, and feelings of safety.
very-low-frequency (VLF) power: the spectral power in the band at or below 0.04 Hz in HRV frequency-domain analysis. VLF power increases during vagal withdrawal and reflects slow regulatory processes including thermoregulation and hormonal influences.
Test Yourself
Click on the ClassMarker logo to take 10-question tests over this unit without an exam password.

Review Flashcards on Quizlet
Click on the Quizlet logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Human Physiology, which satisfies BCIA's Human Anatomy and Physiology requirement, and Biofeedback100, which provides extensive multiple-choice testing over BCIA's Biofeedback Blueprint.

Essential Skills
By the end of this unit, you should be able to perform the following essential skills:
First, interview a client about the history and prior treatment of the presenting complaints, the individual's life situation, including stressors and resources, and their understanding of the cause(s) and consequences of these symptoms.
Second, explain the purpose and procedures of a psychophysiological profile to a client.
Third, administer a psychophysiological profile to a client utilizing several modalities and containing pre-baseline, stressor, recovery, and relaxation conditions. Explain your selection of the modalities and placements used.
Fourth, demonstrate how to detect and remove artifacts from the raw signals that were monitored.
Fifth, evaluate and summarize the findings of the psychophysiological profile, relate them to the client's presenting complaints, and identify training goals based on these data.
Sixth, explain the results from the psychophysiological profile and training goals to your client.
Seventh, develop a treatment plan for your client based on the interview and psychophysiological profile.
Eighth, explain the treatment plan to your client.
Assignment
Now that you have completed this unit, how might it change how you explain psychophysiological concepts like the autonomic nervous system to your clients?
References
Acharya, U. R., Joseph, K. P., Kannathal, N., Lim, C. M., & Suri, J. S. (2006). Heart rate variability: A review. Medical and Biological Engineering and Computing, 44(12), 1031-1051. https://doi.org/10.1007/s11517-006-0119-0
Advokat, C. D., Comaty, J. E., & Julien, R. M. (2019). Julien's primer of drug action (14th ed.). Worth Publishers.
AlJaroudi, W. (2018). Heart rate and ¹²³I-MIBG in heart failure with preserved ejection fraction: More variability and slower washout—a secret recipe for better survival. Journal of Nuclear Cardiology, 25(6), 2149-2153. https://doi.org/10.1007/s12350-018-1355-8
Alexander, A. B. (1975). An experimental test of assumptions relating to the use of electromyographic biofeedback as a general relaxation training technique. Psychophysiology, 12(6), 656-662. https://doi.org/10.1111/j.1469-8986.1975.tb00073.x
Alexander, A. B., White, P. D., & Wallace, H. J. (1977). Training and transfer of training effects in EMG biofeedback assisted muscular relaxation. Psychophysiology, 14(5), 476-482. https://doi.org/10.1111/j.1469-8986.1977.tb01292.x
Andreassi, J. L. (2007). Psychophysiology: Human behavior and physiological response (5th ed.). Lawrence Erlbaum Associates, Publishers.
Arakaki, X., Arechavala, R., Choy, E. H., Bautista, J., Bliss, B., Molloy, C., Wu, D. A., Shimojo, S., Jiang, Y., Kleinman, M. T., & Kloner, R. A. (2023). The connection between heart rate variability (HRV), neurological health, and cognition: A literature review. Frontiers in Neuroscience, 17, 1097851. https://doi.org/10.3389/fnins.2023.1097851
Ashar, Y. K., Chang, L. J., & Wager, T. D. (2017). Brain mechanisms of the placebo effect: An affective appraisal account. Annual Review of Clinical Psychology, 13, 73-98. https://doi.org/10.1146/annurev-clinpsy-021815-093015
Beauchaine, T. P., Gatzke-Kopp, L., & Mead, H. K. (2007). Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology, 74(2), 174-184. https://doi.org/10.1016/j.biopsycho.2005.08.008
Begley, S. (2005). How mirror neurons help us empathize, really feel others' pain. The Wall Street Journal, B1.
Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin, 114, 296-322. https://doi.org/10.1037/0033-2909.114.2.296
Berntson, G. G., Urchino, B. N., & Cacioppo, J. T. (1994). Origins of baseline variance and the Law of Initial Values. Psychophysiology, 31(2), 204-210. https://doi.org/10.1111/j.1469-8986.1994.tb01042.x
Berntson, G. (2018). Presidential Address 2011: Autonomic modes of control and health. Psychophysiology, 55(6), e13065. https://doi.org/10.1111/psyp.13065
Billman, G. E. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Frontiers in Physiology, 4, 26. https://doi.org/10.3389/fphys.2013.00026
Björnson, M., Wijnbladh, K., Törnberg, A., Svensson-Raskh, A., Svensson, A., Ståhlberg, M., Runold, M., Fedorowski, A., Nygren-Bonnier, M., & Bruchfeld, J. (2025). Prevalence and clinical impact of postural orthostatic tachycardia syndrome in highly symptomatic long COVID. Circulation: Arrhythmia and Electrophysiology, 18(10), e013629. https://doi.org/10.1161/CIRCEP.124.013629
Block, J. D., & Bridger, W. H. (1962). The law of initial value in psychophysiology: A reformulation in terms of experimental and theoretical considerations. Annals of the New York Academy of Sciences, 98(4), 1229-1241. https://doi.org/10.1111/j.1749-6632.1962.tb30668.x
Bobba-Alves, N., Juster, R.-P., & Picard, M. (2022). The energetic cost of allostasis and allostatic load. Psychoneuroendocrinology, 143, 105843. https://doi.org/10.1016/j.psyneuen.2022.105843
Brannon, L., Updegraff, J. A., & Feist, J. (2022). Health psychology: An introduction to behavior and health (10th ed.). Wadsworth.
Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc.
Budzynski, T. H., Stoyva, J. M., Adler, C. S., & Mullaney, D. J. (1973). EMG biofeedback and tension headache: A controlled outcome study. Psychosomatic Medicine, 35(6), 485-496. https://doi.org/10.1097/00006842-197311000-00004
Buergler, S., Sezer, D., Gaab, J., & Locher, C. (2023). The roles of expectation, comparator, administration route, and population in open-label placebo effects: A network meta-analysis. Scientific Reports, 13, 12079. https://doi.org/10.1038/s41598-023-39041-1
Cacioppo, J. T., Tassinary, L. G., & Berntson, G. G. (Eds.). (2007). Handbook of psychophysiology (3rd ed.). Cambridge University Press.
Carabotti, M., Scirocco, A., Maselli, M. A., & Severi, C. (2015). The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology, 28(2), 203-209. PMID: 25830558
Carbone, J. T., Clift, J., & Alexander, N. (2022). Measuring allostatic load: Approaches and limitations to algorithm creation. Journal of Psychosomatic Research, 160, 110973. https://doi.org/10.1016/j.jpsychores.2022.110973
Charlesworth, J. E. G., Petkovic, G., Kelley, J. M., Hunter, M., Onakpoya, I., Roberts, N., Miller, F. G., & Howick, J. (2017). Effects of placebos without deception compared with no treatment: A systematic review and meta-analysis. Journal of Evidence-Based Medicine, 10(2), 97-107. https://doi.org/10.1111/jebm.12251
Chen, Y., Xu, J., & Chen, Y. (2021). Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients, 13(6), 2099. https://doi.org/10.3390/nu13062099
Cheung, S. S., & Mekjavic, I. B. (2007). Cold-induced vasodilatation is not homogenous or generalizable across the hand and feet. European Journal of Applied Physiology, 99(6), 701-705. https://doi.org/10.1007/s00421-006-0380-0
Christensen, D. S., Zachariae, R., Amidi, A., & Wu, L. (2022). Sleep and allostatic load: A systematic review and meta-analysis. Sleep Medicine Reviews, 62, 101592. https://doi.org/10.1016/j.smrv.2022.101592
Clemow, L. (1985). Response transfer in biofeedback training. Unpublished manuscript.
Colloca, L., & Howick, J. (2018). Placebos without deception: Outcomes, mechanisms, and ethics. International Review of Neurobiology, 139, 299-315. https://doi.org/10.1016/bs.irn.2018.07.021
Corrado, J., Iftekhar, N., Halpin, S., Li, M., Tarrant, R., Grimaldi, J., Simms, A., O'Connor, R. J., Casson, A., & Sivan, M. (2024). HEART Rate Variability Biofeedback for LOng COVID Dysautonomia (HEARTLOC): Results of a feasibility study. Advances in Rehabilitation Science and Practice, 13, 27536351241227261. https://doi.org/10.1177/27536351241227261
Coull, J. A., Beggs, S., Boudreau, D., Boivin, D., Tsuda, M., Inoue, K., Gravel, C., Salter, M. W., & De Konink, Y. (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature, 438, 1017-1021. https://doi.org/10.1038/nature04223
Crockett, D. J., & Bilsker, D. (1984). Bringing the feet in from the cold: Thermal biofeedback training of foot-warming in Raynaud's syndrome. Biofeedback and Self-Regulation, 9(3), 323-331. https://doi.org/10.1007/BF00998851
Critchley, H. D., & Garfinkel, S. N. (2017). Interoception and emotion. Current Opinion in Psychology, 17, 7-14. https://doi.org/10.1016/j.copsyc.2017.04.020
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., Codagnone, M. G., Cussotto, S., Fulling, C., Golubeva, A. V., Guzzetta, K. E., Jaggar, M., Long-Smith, C. M., Lyte, J. M., Martin, J. A., Molinero-Perez, A., Moloney, G., Morelli, E., Morillas, E., ... Dinan, T. G. (2019). The microbiota-gut-brain axis. Physiological Reviews, 99(4), 1877-2013. https://doi.org/10.1152/physrev.00018.2018
Davis, P. (1980). Electromyograph biofeedback: Generalization and the relative effects of feedback, instructions, and adaptation. Psychophysiology, 17(5), 522-529. https://doi.org/10.1111/j.1469-8986.1980.tb00188.x
de la Barra Ortiz, H. A., Matamala, A., López Inostroza, F., Lobos Araya, C., & Núñez Mondaca, V. (2022). Efficacy of biofeedback in rehabilitation of musculoskeletal disorders: A systematic review. Advances in Rehabilitation, 36(1), 37-49. https://doi.org/10.5114/areh.2022.115971
Dean, E. (1984). Temperature biofeedback training to improve peripheral blood flow in the upper extremities of diabetic patients. Canadian Journal of Occupational Therapy, 51(5), 217-222. https://doi.org/10.1177/000841748405100507
Dhar, A. K., Lambert, G. W., & Barton, D. A. (2016). Depression and cardiovascular disease: Psychobiological mechanisms. In M. E. Alvarenga & D. Byrne (Eds.), Handbook of psychocardiology. Springer Singapore.
Ding, F., Tian, W., & Wei, L. (2025). Cardiac vagal activity dynamics during anxiety induction and the effects of short-term biofeedback training. International Journal of Psychophysiology. Advance online publication. https://doi.org/10.1016/j.ijpsycho.2025.103889
Dodd, S., Dean, O. M., Vian, J., & Berk, M. (2017). A review of the theoretical and biological understanding of the nocebo and placebo phenomena. Clinical Therapeutics, 39(3), 469-476. https://doi.org/10.1016/j.clinthera.2017.01.005
Doody, J. S., Burghardt, G. M., & Dinets, V. (2023). The evolution of sociality and the polyvagal theory. Biological Psychology, 181, 108518. https://doi.org/10.1016/j.biopsycho.2023.108518
Dowling, L. R., Strazzari, M. R., Keely, S. J., & Kaiko, G. E. (2022). Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. Journal of Allergy and Clinical Immunology, 150(3), 532-541. https://doi.org/10.1016/j.jaci.2022.05.021
Draghici, A. E., & Taylor, J. A. (2016). The physiological basis and measurement of heart rate variability in humans. Journal of Physiological Anthropology, 35(1), 22. https://doi.org/10.1186/s40101-016-0113-7
Duffy, E. (1957). The psychological significance of the concept of arousal or activation. Psychological Review, 64(5), 265-275. https://doi.org/10.1037/h0048837
Ehrlich, D. (1963). Activation and behavior by Elizabeth Duffy. Archives of General Psychiatry, 8(2), 212-213. https://doi.org/10.1001/archpsyc.1963.01720140090016
Emerson, N. D., Lavretsky, H., Pittman, W. Q., Viswanathan, N., & Siddarth, P. (2024). An open trial of biofeedback for long COVID. Journal of Psychosomatic Research, 179, 111625. https://doi.org/10.1016/j.jpsychores.2024.111625
Ernst, G. (2017). Heart-rate variability—More than heart beats? Frontiers in Public Health, 5, 240. https://doi.org/10.3389/fpubh.2017.00240
Fan, K.-C., Lin, C.-C., Chiu, Y.-L., Koh, S.-H., Liu, Y.-C., & Chuang, Y.-F. (2025). Compositional and functional gut microbiota alterations in mild cognitive impairment: Links to Alzheimer's disease pathology. Alzheimer's Research & Therapy, 17, 58. https://doi.org/10.1186/s13195-025-01591-5
Fava, G. A., Sonino, N., Lucente, M., & Guidi, J. (2022). Allostatic load in clinical practice. Clinical Psychological Science, 10(6), 1175-1185. https://doi.org/10.1177/21677026221088726
Finniss, D. G., Kaptchuk, T. J., Miller, F., & Benedetti, F. (2010). Biological, clinical, and ethical advances of placebo effects. The Lancet, 375(9715), 686-695. https://doi.org/10.1016/S0140-6736(09)61706-2
Florjanski, W., Małysa, A., Orzeszek, S., Smardz, J., Olchowy, A., Paradowska-Stolarz, A., & Więckiewicz, M. (2019). Evaluation of biofeedback usefulness in masticatory muscle activity management: A systematic review. Journal of Clinical Medicine, 8(5), 766. https://doi.org/10.3390/jcm8050766
Fox, E. (2025). Polyvagal theory in trauma-informed music therapy practice. Music Therapy Perspectives, 43(1), 25-36.
Fox, S. I., & Rompolski, K. (2025). Human physiology (2025 release). McGraw Hill.
Franceschi, C., & Campisi, J. (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Journal of Gerontology: Series A, Biological Sciences and Medical Sciences, 69(Suppl 1), S4-S9. https://doi.org/10.1093/gerona/glu057
Friedman, B. H. (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185-199. https://doi.org/10.1016/j.biopsycho.2005.08.009
Frisaldi, E., Shaibani, A., Benedetti, F., & Pagnini, F. (2023). Placebo and nocebo effects and mechanisms associated with pharmacological interventions: An umbrella review. BMJ Open, 13(10), e075099. https://doi.org/10.1136/bmjopen-2023-075099
Fröhlich, E. E., Farzi, A., Mayerhofer, R., Reichmann, F., Jačan, A., Wagner, B., Zinber, E., Borber, N., Kremer, C., Ber, P., Gorkiewicz, G., & Holzer, P. (2016). Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain, Behavior, and Immunity, 56, 140-155. https://doi.org/10.1016/j.bbi.2016.02.020
Gardner, D. G. (1986). Activation theory and task design: An empirical test of several new predictions. Journal of Applied Psychology, 71(3), 411-418. https://doi.org/10.1037/0021-9010.71.3.411
Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K., & Critchley, H. D. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65-74. https://doi.org/10.1016/j.biopsycho.2014.11.004
Geenen, R., & van de Vijver, F. J. R. (1993). A simple test of the Law of Initial Values. Psychophysiology, 30(5), 525-530. https://doi.org/10.1111/j.1469-8986.1993.tb02076.x
Gentile, F., Orlando, G., Montuoro, S., Ferrari Chen, Y. F., Macefield, V. G., Passino, C., Giannoni, A., & Emdin, M. (2024). Treating heart failure by targeting the vagus nerve. Heart Failure Reviews. Advance online publication. https://doi.org/10.1007/s10741-024-10423-z
Gevirtz, R. (2013). The nerve of that disease: The vagus nerve and cardiac rehabilitation. Biofeedback, 41, 32-38. https://doi.org/10.5298/1081-5937-41.1.01
Giggins, O. M., Persson, U. M., & Caulfield, B. (2013). Biofeedback in rehabilitation. Journal of NeuroEngineering and Rehabilitation, 10, Article 60. https://doi.org/10.1186/1743-0003-10-60
Green, E., Green, A. M., & Walters, E. D. (1970). Voluntary control of internal states: Psychological and physiological. Journal of Transpersonal Psychology, 2, 1-26.
Grol, M., & De Raedt, R. (2020). The link between resting heart rate variability and affective flexibility. Cognitive, Affective, & Behavioral Neuroscience, 20(3), 621-639. https://doi.org/10.3758/s13415-020-00781-0
Grossman, P. (2023). Fundamental challenges and likely refutations of the five basic premises of the polyvagal theory. Biological Psychology, 178, 108530. https://doi.org/10.1016/j.biopsycho.2023.108530
Guidi, J., Lucente, M., Sonino, N., & Fava, G. A. (2020). Allostatic load and its impact on health: A systematic review. Psychotherapy and Psychosomatics, 89(1), 11-27. https://doi.org/10.1159/000504341
Gurung, R. A. R. (2018). Health psychology: Well-being in a diverse world (4th ed.). Thompson Wadsworth.
Haeyen, S. (2024). A theoretical exploration of polyvagal theory in creative arts and psychomotor therapies for emotion regulation in stress and trauma. Frontiers in Psychology, 15, 1370399. https://doi.org/10.3389/fpsyg.2024.1370399
Hajjeh, O., Rajab, I., Bdair, M., Saife, S., Zahran, A., Abuzahra, M., Dweikat, I., Erekat, N., & Abuhilal, A. (2025). Enteric nervous system dysfunction as a driver of central nervous system disorders: The forgotten brain in neurological disease. Neuroscience, 558, 257-273. https://doi.org/10.1016/j.neuroscience.2024.12.019
Handzlik-Waszkiewicz, P., Sulowska-Daszyk, I., & Suder, A. (2025). The effect of BeBo® training and EMG-biofeedback-assisted therapy on pelvic floor muscle function in women after vaginal delivery and cesarean section: A randomized controlled trial. Journal of Clinical Medicine, 14(10), Article 1234.
Hattori, N., & Yamashiro, Y. (2021). The gut-brain axis. Annals of Nutrition and Metabolism, 77(Suppl. 2), 1-3. https://doi.org/10.1159/000512226
Hayano, J., & Yuda, E. (2021). Assessment of autonomic function by long-term heart rate variability: Beyond the classical framework of LF and HF measurements. Journal of Physiological Anthropology, 40(1), 21. https://doi.org/10.1186/s40101-021-00272-z
Herhaus, B., Conrad, R., & Petrowski, K. (2023). Effect of a slow-paced breathing with heart rate variability biofeedback intervention on pro-inflammatory cytokines in individuals with panic disorder: A randomized controlled trial. Journal of Affective Disorders, 326, 132-138. https://doi.org/10.1016/j.jad.2023.01.091
Hord, D. J., Johnson, L. C., & Lubin, A. (1964). Differential effect of the law of initial value (LIV) on autonomic variables. Psychophysiology, 1(1), 3-8. https://doi.org/10.1111/j.1469-8986.1964.tb02658.x
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Igboanugo, S., & Mielke, J. G. (2023). The allostatic load model: A framework to understand the cumulative multi-system impact of work-related psychosocial stress exposure among firefighters. Health Psychology and Behavioral Medicine, 11(1), 2190880. https://doi.org/10.1080/21642850.2023.2190880
Jin, P. (1992). Toward a reconceptualization of the law of initial value. Psychological Bulletin, 111(1), 176-184. https://doi.org/10.1037/0033-2909.111.1.176
Jenkinson, P. M., Fotopoulou, A., Ibañez, A., & Rossell, S. (2024). Interoception in anxiety, depression, and psychosis: A review. eClinicalMedicine, 73, 102673. https://doi.org/10.1016/j.eclinm.2024.102673
Juster, R.-P., McEwen, B. S., & Lupien, S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2-16. https://doi.org/10.1016/j.neubiorev.2009.10.002
Kaptchuk, T. J., Friedlander, E., Kelley, J. M., Sanchez, M. N., Kokkotou, E., Singer, J. P., Kowalczykowski, M., Miller, F. G., Kirsch, I., & Lembo, A. J. (2010). Placebos without deception: A randomized controlled trial in Irritable Bowel Syndrome. PLoS ONE, 5(12), e15591. https://doi.org/10.1371/journal.pone.0015591
Kaptchuk, T. J. (2018). Open-label placebo: Reflections on a research agenda. Perspectives in Biology and Medicine, 61(3), 311-334. https://doi.org/10.1353/pbm.2018.0045
Kara, T., Nykodym, J., & Somers, V. (2003). Heart rate variability. Journal of Cardiovascular Electrophysiology, 14(8 Suppl), S88-S90. https://doi.org/10.1046/j.1540-8167.90304.x
Karemaker, J. (2017). An introduction into autonomic nervous function. Physiological Measurement, 38(5), R89-R118. https://doi.org/10.1088/1361-6579/aa6782
Keefe, F. J., & Gardner, E. T. (1979). Learned control of skin temperature: Effects of short- and long-term biofeedback training. Behavior Therapy, 10(2), 202-210. https://doi.org/10.1016/S0005-7894(79)80044-3
Kemp, A. H., Koenig, J., & Thayer, J. F. (2017). From psychological moments to mortality: A multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neuroscience & Biobehavioral Reviews, 83, 547-567. https://doi.org/10.1016/j.neubiorev.2017.09.006
Khalsa, S. S., Adolphs, R., Cameron, O. G., Critchley, H. D., Davenport, P. W., Feinstein, J. S., Feusner, J. D., Garfinkel, S. N., Lane, R. D., Mehling, W. E., Meuret, A. E., Nemeroff, C. B., Oppenheimer, S., Petzschner, F. H., Pollatos, O., Rhudy, J. L., Schramm, L. P., Simmons, W. K., Stein, M. B., Stephan, K. E., Van den Bergh, O., Van Diest, I., von Leupoldt, A., & Paulus, M. P., Interoception Summit 2016 participants. (2018). Interoception and mental health: A roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 501-513. https://doi.org/10.1016/j.bpsc.2017.12.004
Khazan, I. Z. (2013). The clinical handbook of biofeedback: A step-by-step guide for training and practice with mindfulness. John Wiley & Sons, Ltd.
Khazan, I. Z. (2019). A guide to normal values for biofeedback. In D. Moss & F. Shaffer (Eds.), Physiological recording technology and applications in biofeedback and neurofeedback (pp. 2-6). Association for Applied Psychophysiology and Biofeedback.
Khan, A. A., Lip, G. Y. H., & Shantsila, A. (2019). Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system. European Journal of Clinical Investigation, 49(11), e13174. https://doi.org/10.1111/eci.13174
Khundrakpam, B. S., Segado, M., Pazdera, J., Gagnon Shaigetz, V., Granek, J. A., & Choudhury, N. (2025). An integrated platform combining immersive virtual reality and physiological sensors for systematic and individualized assessment of stress response (bWell): Design and implementation study. JMIR Formative Research, 9, e56944. https://doi.org/10.2196/56944
King, N. J., & Montgomery, R. W. (1980). A component analysis of biofeedback induced self-control of peripheral (finger) temperature. Biological Psychology, 11(2), 135-152. https://doi.org/10.1016/0301-0511(80)90022-2
Kingsley, R. E. (2000). Concise textbook of neuroscience (2nd ed.). Lippincott Williams & Wilkins.
Knezevic, N. N., Sič, A., Worobey, S., & Knezevic, E. (2025). Justice for placebo: Placebo effect in clinical trials and everyday practice. Medicines, 12(3), 34. https://doi.org/10.3390/medicines12030034
Koenig, S. B. (2025). Engaging polyvagal theory to inform a relational approach in clinical work with adolescent-parent dyads. Clinical Social Work Journal. Advance online publication. https://doi.org/10.1007/s10615-024-00962-5
Kwon, D. (2026). A distorted mind-body connection may explain common mental illnesses. Scientific American, 334(1), 50-57. https://doi.org/10.1038/scientificamerican012026-Y3Kvjhfs9IUIw9svFTsA6
Kronsteiner, B., Carrero-Rojas, G., Reissig, L., Moghaddam, A. S., Schwendt, K. M., Gerges, S., Maierhofer, U., Aszmann, O. C., Pastor, A., Kiss, A., Podesser, B., Birkfellner, W., Moscato, F., Blumer, R., & Weninger, W. J. (2024). Characterization, number, and spatial organization of nerve fibers in the human cervical vagus nerve and its superior cardiac branch. Brain Stimulation, 17(3), 510-524. https://doi.org/10.1016/j.brs.2024.04.016
Kulkarni, S., Ganz, J., Bayrer, J., Becker, L., Bogunovic, M., & Rao, M. (2018). Advances in enteric neurobiology: The "brain" in the gut in health and disease. Journal of Neuroscience, 38(44), 9346-9354. https://doi.org/10.1523/JNEUROSCI.1661-18.2018
Lalanza, J. F., Lorente, S., Bullich, R., García, C., Losilla, J.-M., & Capdevila, L. (2023). Methods for heart rate variability biofeedback (HRVB): A systematic review and guidelines. Applied Psychophysiology and Biofeedback, 48(3), 275-297. https://doi.org/10.1007/s10484-023-09582-6
Lazaridou, A., Paschali, M., Vilsmark, E., Sadora, J., Burton, D., Bashara, A., & Edwards, R. R. (2023). Biofeedback EMG alternative therapy for chronic low back pain (the BEAT-pain study). Digital Health, 9, 1-12. https://doi.org/10.1177/20552076231159347
LeDoux, J. (2002). Synaptic self. Viking Penguin.
Leganes-Fonteneau, M., Bates, M. E., Muzumdar, N., Pawlak, A., Islam, S., Vaschillo, E., & Buckman, J. F. (2021). Cardiovascular mechanisms of interoceptive awareness: Effects of resonance breathing. International Journal of Psychophysiology, 169, 71-87. https://doi.org/10.1016/j.ijpsycho.2021.09.003
Lehrer, P. M., & Gevirtz, R. (2014). Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology, 5, 756. https://doi.org/10.3389/fpsyg.2014.00756
Liu, L., Yang, S., Liu, X., Huang, M., Pei, Z., Wang, Y., Wang, Y., Han, Q., Mao, J., & Wang, L. (2025). Allostatic load in non-medical and medical college students. BMC Public Health, 25, 317. https://doi.org/10.1186/s12889-025-22222-4
Logan, J. G., & Barksdale, D. J. (2008). Allostasis and allostatic load: Expanding the discourse on stress and cardiovascular disease. Journal of Clinical Nursing, 17(7B), 201-208. https://doi.org/10.1111/j.1365-2702.2008.02347.x
Malta, L. S., Blanchard, E. B., Freidenberg, B. M., Galovski, T. E., Karl, A., & Holzapfel, S. R. (2001). Psychophysiological reactivity of aggressive drivers: An exploratory study. Applied Psychophysiology and Biofeedback, 26(2), 95-116.
Manzotti, A., Panisi, C., Pivotto, M., Vinciguerra, F., Benedet, M., Brazzoli, F., Zanni, S., Comassi, A., Caputo, S., Cerritelli, F., & Chiera, M. (2023). An in-depth analysis of the polyvagal theory in light of current findings in neuroscience and clinical research. Developmental Psychobiology, 65(8), e22516. https://doi.org/10.1002/dev.22516
Mauro, M., Cegolon, L., Bestiaco, N., Zulian, E., & Larese Filon, F. (2025). Heart rate variability modulation through slow-paced breathing in health care workers with long COVID: A case-control study. The American Journal of Medicine, 138(5), 870-883.e5. https://doi.org/10.1016/j.amjmed.2024.05.021
McCrory, C., McLoughlin, S., Layte, R., Ní Cheallaigh, C., O'Halloran, A. M., Barros, H., Berkman, L., Bochud, M., Crimmins, E., Farrell, M. T., Fraga, S., Grundy, E., Kelly-Irving, M., Petrovic, D., Seeman, T., Stringhini, S., Vollenweider, P., & Kenny, R. A. (2023). Towards a consensus definition of allostatic load: A multi-cohort, multi-system, multi-biomarker individual participant data meta-analysis. Psychoneuroendocrinology, 147, 105988. https://doi.org/10.1016/j.psyneuen.2022.105988
Mishra, P., & Bhargava, S. (2025). Unraveling the mystery of placebo effect in research and practice: An update. Perspectives in Clinical Research, 16(1), 3-11. https://doi.org/10.4103/picr.picr_134_24
Mohajeri, M. H., La Fata, G., Steinert, R. E., & Weber, P. (2018). Relationship between the gut microbiome and brain function. Nutrition Reviews, 76(7), 481-496. https://doi.org/10.1093/nutrit/nuy009
Moss, D., & Shaffer, F. (2022). A primer of biofeedback. Association for Applied Psychophysiology and Biofeedback.
Moss, D. (2025). Brain-heart interactions and optimizing psychotherapy. Applied Psychophysiology and Biofeedback. Advance online publication. https://doi.org/10.1007/s10484-025-09610-2
Myrtek, M., & Foerster, F. (1986). The law of initial value: A rare exception. Biological Psychology, 22(2), 141-157. https://doi.org/10.1016/0301-0511(86)90037-7
Nikolla, E., Grandberry, A., Jamerson, D., Flynn, C. R., & Sundaresan, S. (2025). The enteric neuronal circuitry: A key ignored player in nutrient sensing along the gut-brain axis. The FASEB Journal, 39(5), e23654. https://doi.org/10.1096/fj.202402365R
Nord, C. L., Garfinkel, S. N., Gray, M. A., & Critchley, H. D. (2021). Interoceptive processing in psychiatric disorders. Biological Psychiatry, 90(7), 476-486. https://doi.org/10.1016/j.biopsych.2021.05.006
Nord, C. L., & Garfinkel, S. N. (2022). Interoceptive pathways to understand and treat mental health conditions. Trends in Cognitive Sciences, 26(6), 499-513. https://doi.org/10.1016/j.tics.2022.03.004
Norrbrand, L., Kölegård, R., Keramidas, M. E., Mekjavic, I. B., & Eiken, O. (2017). No association between hand and foot temperature responses during local cold stress and rewarming. European Journal of Applied Physiology, 117(5), 971-980. https://doi.org/10.1007/s00421-017-3592-7
Nunan, D., Sandercock, G. R. H., & Brodie, D. A. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology, 33(11), 1407-1417. https://doi.org/10.1111/j.1540-8159.2010.02841.x
Olivieri, F., Biscetti, L., Pimpini, L., Pelliccioni, G., Sabbatinelli, J., & Giunta, S. (2024). Heart rate variability and autonomic nervous system imbalance: Potential biomarkers and detectable hallmarks of aging and inflammaging. Ageing Research Reviews, 98, 102331. https://doi.org/10.1016/j.arr.2024.102331
Olshansky, B., Sabbah, H. N., Hauptman, P. J., & Colucci, W. S. (2008). Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation, 118, 863-871. https://doi.org/10.1161/CIRCULATIONAHA.107.760405
O'Riordan, K. J., Moloney, G. M., Keane, L., Clarke, G., & Cryan, J. F. (2025). The gut microbiota-immune-brain axis: Therapeutic implications. Cell Reports Medicine, 6(3), 101538. https://doi.org/10.1016/j.xcrm.2024.101538
Ozpolat, C., Okcay, Y., Ulusoy, K., & Yildiz, O. (2025). A narrative review of the placebo effect: Historical roots, current applications, and emerging insights. European Journal of Clinical Pharmacology. Advance online publication. https://doi.org/10.1007/s00228-025-03619-4
Pagnini, F., Barbiani, D., Grosso, F., Cavalera, C., Volpato, E., Minazzi, G. A., Poletti, V., Riva, G., & Phillips, D. (2024). Enacting the mind/body connection: The role of self-induced placebo mechanisms. Humanities and Social Sciences Communications, 11, 367. https://doi.org/10.1057/s41599-024-02700-9
Park, G., & Thayer, J. F. (2014). From the heart to the mind: Cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Frontiers in Psychology, 5, 278. https://doi.org/10.3389/fpsyg.2014.00278
Pavlov, V. A., & Tracey, K. J. (2005). The cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity, 19(6), 493-499. https://doi.org/10.1016/j.bbi.2005.03.015
Pavlov, V. A., & Tracey, K. J. (2012). The vagus nerve and the inflammatory reflex, linking immunity and metabolism. Nature Reviews Endocrinology, 8(12), 743-754. https://doi.org/10.1038/nrendo.2012.189
Pennebaker, J. W., Kiecolt-Glaser, J. K., & Glaser, R. (1988). Disclosure of traumas and immune function: Health implications for psychotherapy. Journal of Consulting and Clinical Psychology, 56, 239-245. https://doi.org/10.1037//0022-006x.56.2.239
Pérez-Alcalde, A. I., Galán-del-Río, F., Fernández-Rodríguez, F. J., de la Plaza San Frutos, M., García-Arrabé, M., Giménez, M.-J., & Ruiz-Ruiz, B. (2024). The effects of a single vagus nerve's neurodynamics on heart rate variability in chronic stress: A randomized controlled trial. Sensors, 24(21), 6874. https://doi.org/10.3390/s24216874
Petrie, K. J., & Rief, W. (2019). Psychobiological mechanisms of placebo and nocebo effects: Pathways to improve treatments and reduce side effects. Annual Review of Psychology, 70, 599-625. https://doi.org/10.1146/annurev-psych-010418-102907
Petrovic, P., Kalso, E., Petersson, K. M., & Ingvar, M. (2002). Placebo and opioid analgesia: Imaging a shared neuronal network. Science, 295, 1737-1740. https://doi.org/10.1126/science.1067176
Petrut, S., Bragaru, A. M., Munteanu, A. E., Moldovan, A.-D., Moldovan, C., & Rusu, E. (2025). Gut over mind: Exploring the powerful gut-brain axis. Nutrients, 17(5), 1012. https://doi.org/10.3390/nu17051012
Pinel, P. J., & Barnes, S. (2017). Biopsychology (10th ed.). Pearson Education, Inc.
Pitz, G. (1964). Book review: Activation and behavior by Elizabeth Duffy. Educational and Psychological Measurement, 24(1), 226-228. https://doi.org/10.1177/001316446402400131
Poole, L., Dickens, C., & Steptoe, A. (2011). The puzzle of depression and acute coronary syndrome: Reviewing the role of acute inflammation. Journal of Psychosomatic Research, 71(2), 61-68. https://doi.org/10.1016/j.jpsychores.2010.12.009
Porges, S. W. (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology, 32(4), 301-318. https://doi.org/10.1111/j.1469-8986.1995.tb01213.x
Porges, S. W. (2003). The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiology & Behavior, 79(3), 503-513. https://doi.org/10.1016/S0031-9384(03)00156-2
Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116-143. https://doi.org/10.1016/j.biopsycho.2006.06.009
Porges, S. W. (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. W. W. Norton & Company.
Porges, S. W. (2017). The pocket guide to the Polyvagal Theory: The transformative power of feeling safe. W. W. Norton.
Porges, S. W. (2021). Polyvagal Theory: A biobehavioral journey to sociality. Comprehensive Psychoneuroendocrinology, 7, 100069. https://doi.org/10.1016/j.cpnec.2021.100069
Porges, S. W. (2022). Polyvagal theory: A science of safety. Frontiers in Integrative Neuroscience, 16, 871227. https://doi.org/10.3389/fnint.2022.871227
Porges, S. W. (2025). Polyvagal Theory: Current status, clinical applications, and future directions. Clinical Neuropsychiatry, 22(3), 148-163.
Pribram, K. H., & McGuinness, D. (1975). Arousal, activation, and effort in the control of attention. Psychological Review, 82(2), 116-149. https://doi.org/10.1037/h0076780
Price, D. D., Finniss, D. G., & Benedetti, F. (2008). A comprehensive review of the placebo effect: Recent advances and current thought. Annual Review of Psychology, 59, 565-590. https://doi.org/10.1146/annurev.psych.59.113006.095941
Quigley, K. M., & Moore, G. A. (2018). Development of cardiac autonomic balance in infancy and early childhood: A possible pathway to mental and physical health outcomes. Developmental Review, 49, 41-60. https://doi.org/10.1016/j.dr.2018.07.001
Rabey, M., & Moloney, N. (2022). "I don't know why I've got this pain!" Allostasis as a possible explanatory model. Physical Therapy, 102(4), pzac027. https://doi.org/10.1093/ptj/pzac027
Rajendran, P. S., Challis, R. C., Fowlkes, C. C., Hanna, P., Tompkins, J. D., Jordan, M. C., Hiyari, S., Gabris-Weber, B. A., Greenbaum, A., Deverman, B. E., Münzberg, H., Ardell, J. L., Salama, G., Gradinaru, V., & Shivkumar, K. (2018). Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nature Communications, 9, 1099. https://doi.org/10.1038/s41467-019-09770-1
Rajendran, P. S., Hadaya, J., Khalsa, S. S., Yu, C. D., Chang, R. B., & Shivkumar, K. (2023). The vagus nerve in cardiovascular physiology and pathophysiology: From evolutionary insights to clinical medicine. Seminars in Cell & Developmental Biology, 136, 60-77. https://doi.org/10.1016/j.semcdb.2023.02.007
Ramadan, Y. N., Alqifari, S., Alshehri, K., Alhowiti, A., Mirghani, H., Alrasheed, T., Albalawi, O., Alosaimy, S., & Hetta, H. F. (2025). Microbiome gut-brain axis: Impact on brain development and mental health. Molecular Neurobiology, 62(4), 1835-1859. https://doi.org/10.1007/s12035-024-03920-0
Rao, M., & Gershon, M. D. (2016). The bowel and beyond: The enteric nervous system in neurological disorders. Nature Reviews Gastroenterology & Hepatology, 13, 517-528. https://doi.org/10.1038/nrgastro.2016.107
Reyes del Paso, G. A., Langewitz, W., Mulder, L. J. M., van Roon, A., & Duschek, S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology, 50(5), 477-487. https://doi.org/10.1111/psyp.12027
Rice, B. L., & Schindler, J. V. (1992). Effect of thermal biofeedback-assisted relaxation training on blood circulation in the lower extremities of a population with diabetes. Diabetes Care, 15(7), 853-858.
Rizzolatti, G., & Sinigaglia, C. (2006). Mirrors in the brain. Oxford University Press.
Rodriguez, E. J., Kim, E. N., Sumner, A. E., Nápoles, A. M., & Pérez-Stable, E. J. (2019). Allostatic load: Importance, markers, and score determination in minority and disparity populations. Journal of Urban Health, 96(Supplement 1), 3-11. https://doi.org/10.1007/s11524-019-00345-5
Rusch, J., Layden, B. T., & Dugas, L. R. (2023). Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Frontiers in Endocrinology, 14, 1122334. https://doi.org/10.3389/fendo.2023.1122334
Saxbe, D. E., Beckes, L., Stoycos, S. A., & Coan, J. A. (2019). Social allostasis and social allostatic load: A new model for research in social dynamics, stress, and health. Perspectives on Psychological Science, 14(4), 469-482. https://doi.org/10.1177/1745691618814868
Schaefer, M., Sahin, T., & Berstecher, B. (2018). Why do open-label placebos work? A randomized controlled trial of an open-label placebo induction with and without extended information about the placebo effect in allergic rhinitis. PLoS One, 13(3), e0192758. https://doi.org/10.1371/journal.pone.0192758
Schimmack, U., & Reisenzein, R. (2002). Experiencing activation: Energetic arousal and tense arousal are not mixtures of valence and activation. Emotion, 2(3), 412-417. https://doi.org/10.1037/1528-3542.2.3.412
Schneider, E. M., O'Riordan, K. J., Clarke, G., & Cryan, J. F. (2024). Feeding gut microbes to nourish the brain: Unravelling the diet-microbiota-gut-brain axis. Nature Metabolism, 6(8), 1004-1024. https://doi.org/10.1038/s42255-024-01044-5
Schwartz, G. E., Weinberger, D. A., & Singer, J. A. (1981). Cardiovascular differentiation of happiness, sadness, anger, and fear following imagery and exercise. Psychosomatic Medicine, 43, 343-364. https://doi.org/10.1097/00006842-198108000-00007
Schwartz, M. S., & Andrasik, F. (Eds.). (2003). Biofeedback: A practitioner's guide (3rd ed.). The Guilford Press.
Schwartz, S. (2015). Viva vagus: Wandering nerve could lead to range of therapies. Science News, 188(11), 18.
Seki, A., Green, H. R., Lee, T.-D., Hong, L., Tan, J., Vinters, H. V., Chen, P.-S., & Fishbein, M. C. (2014). Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm, 11(8), 1411-1417. https://doi.org/10.1016/j.hrthm.2014.04.022
Seth, A. K. (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences, 17(11), 565-573. https://doi.org/10.1016/j.tics.2013.09.007
Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Frontiers in Psychology, 5, 01040. https://doi.org/10.3389/fpsyg.2014.01040
Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. https://doi.org/10.3389/fpubh.2017.00258
Shaffer, F., Meehan, Z. M., & Zerr, C. L. (2020). A critical review of ultra-short-term heart rate variability norms research. Frontiers in Neuroscience, 14, 594880. https://doi.org/10.3389/fnins.2020.594880
Sime, W. E., & DeGood, D. E. (1977). Effect of EMG biofeedback and progressive muscle relaxation training on awareness of frontalis muscle tension. Psychophysiology, 14(5), 529-536. https://doi.org/10.1111/j.1469-8986.1977.tb01297.x
Spille, L., Fendel, J. C., Seuling, P. D., Göritz, A. S., & Schmidt, S. (2023). Open-label placebos—a systematic review and meta-analysis of experimental studies with non-clinical samples. Scientific Reports, 13, 3608. https://doi.org/10.1038/s41598-023-30773-y
Stauss, H. M. (2003). Heart rate variability. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 285(5), R927-R931. https://doi.org/10.1152/ajpregu.00452.2003
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Stone, L. B., McCormack, C. C., & Bylsma, L. M. (2020). Cross-system autonomic balance and regulation: Associations with depression and anxiety symptoms. Psychophysiology, 57(8), e13601. https://doi.org/10.1111/psyp.13601
Sundas, A., Contreras, I., Navarro-Otano, J., Soler, J., Beneyto, A., & Vehi, J. (2025). Heart rate variability over the decades: A scoping review. PeerJ, 13, e19150. https://doi.org/10.7717/peerj.19150
Tavee, J. (2024). Current concepts in long COVID-19 brain fog and postural orthostatic tachycardia syndrome. Annals of Allergy, Asthma & Immunology, 133(5), 522-530. https://doi.org/10.1016/j.anai.2024.08.008
Tétrault, P., Mansour, A., Vachon-Pressau, E., Schnitzer, T. J., Apkarian, A. V., & Baliki, M. N. (2016). Brain connectivity predicts placebo response across chronic pain trials. PLoS Biology, 14(10), e1002570. https://doi.org/10.1371/journal.pbio.1002570
Thayer, J. F., & Brosschot, J. F. (2005). Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology, 30(10), 1050-1058. https://doi.org/10.1016/j.psyneuen.2005.04.014
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201-216. https://doi.org/10.1016/S0165-0327(00)00338-4
Thayer, J. F., & Lane, R. D. (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81-88. https://doi.org/10.1016/j.neubiorev.2008.08.004
Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122-131. https://doi.org/10.1016/j.ijcard.2009.09.543
Thayer, R. E. (1978). Toward a psychological theory of multidimensional activation (arousal). Motivation and Emotion, 2(1), 1-34. https://doi.org/10.1007/BF00992729
Thomas, B. L., Claassen, N., Becker, P., & Viljoen, M. (2019). Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology, 78(1), 14-26. https://doi.org/10.1159/000495519
Thompson, M. (2005). Intentions, empathy, and theory of mind: Implications for personality and psychotherapy. Presentation to the Annual Meeting of the American Association for the Advancement of Science (AAAS).
Tiwari, R., Kumar, R., Malik, S., Raj, T., & Kumar, P. (2020). Analysis of heart rate variability and implication of different factors on heart rate variability. Current Cardiology Reviews, 16(2), 87-100. https://doi.org/10.2174/1573403X15666191018112027
Tortora, G. J., & Derrickson, B. H. (2023). Principles of anatomy and physiology (16th ed.). John Wiley & Sons, Limited.
Tracey, K. J. (2002). The inflammatory reflex. Nature, 420(6917), 853-859. https://doi.org/10.1038/nature01321
Tracey, K. J. (2007). Physiology and immunology of the cholinergic anti-inflammatory pathway. Journal of Clinical Investigation, 117(2), 289-296. PMID: 17273548
VaezMousavi, S. M., Barry, R. J., Rushby, J. A., & Clarke, A. R. (2007). Evidence for differentiation of arousal and activation in normal adults. Acta Neurobiologiae Experimentalis, 67(2), 179-186. PMID: 17694861
van Weperen, V. Y. H., & Vaseghi, M. (2023). Cardiac vagal afferent neurotransmission in health and disease: Review and knowledge gaps. Frontiers in Neuroscience, 17, 1201964. https://doi.org/10.3389/fnins.2023.1201964
Vase, L., & Wartolowska, K. (2019). Pain, placebo, and test of treatment efficacy: A narrative review. British Journal of Anaesthesia, 123(2), e254-e262. https://doi.org/10.1016/j.bja.2019.03.024
von Rosenberg, W., Chanwimalueang, T., Adjei, T., Jaffer, U., Goverdovsky, V., & Mandic, D. P. (2017). Resolving ambiguities in the LF/HF ratio: LF-HF scatter plots for the categorization of mental and physical stress from HRV. Frontiers in Physiology, 8, 360. https://doi.org/10.3389/fphys.2017.00360
Wager, T. D., & Atlas, L. Y. (2015). The neuroscience of placebo effects: Connecting context, learning and health. Nature Reviews Neuroscience, 16(7), 403-418. https://doi.org/10.1038/nrn3976
Wareing, L., Readman, M. R., Longo, M. R., Linkenauger, S. A., & Crawford, T. J. (2024). The utility of heartrate and heartrate variability biofeedback for the improvement of interoception across behavioural, physiological and neural outcome measures: A systematic review. Brain Sciences, 14(6), 579. https://doi.org/10.3390/brainsci14060579
White, C., & Lammy, D. (2004). Infrared hand temperature mapping [Abstract]. Applied Psychophysiology and Biofeedback, 29(4), 306.
Wickramasekera, I. A. (1988). Clinical behavioral medicine: Some concepts and procedures. Plenum Press.
Wilder, J. (1962). Basimetric approach (law of initial value) to biological rhythms. Annals of the New York Academy of Sciences, 98(4), 1211-1220. https://doi.org/10.1111/j.1749-6632.1962.tb30664.x
Wilder, J. (2014). Stimulus and response: The law of initial value. Williams & Wilkins. (Original work published 1967)
Wilson, J. (2003). Biological foundations of human behavior. Wadsworth/Thompson Learning.
Wójcik, Z. M., Kamińska-Omasta, K., Omasta, B., Krupa, O., Pietrukaniec, P., Romańczuk, K. B., Jakimów, M., & Rybak, D. (2025). Gut microbiota and its impact on brain function and mental health. Quality in Sport, 11(1), 1-15. https://doi.org/10.12775/QS.2025.001
Yoo, H. J., Nashiro, K., Min, J., Cho, C., Bachman, S. L., Nasseri, P., Porat, S., Dutt, S., Grigoryan, V., Choi, P., Thayer, J. F., Lehrer, P. M., Chang, C., & Mather, M. (2022). Heart rate variability (HRV) changes and cortical volume changes in a randomized trial of five weeks of daily HRV biofeedback in younger and older adults. International Journal of Psychophysiology, 181, 50-63. https://doi.org/10.1016/j.ijpsycho.2022.08.006
Zoccali, C., Mallamaci, F., Kanbay, M., Tuttle, K. R., Kotanko, P., De Caterina, R., Grassi, G., & Mancia, G. (2025). The autonomic nervous system and inflammation in chronic kidney disease. Nephrology Dialysis Transplantation, 40(3), 345-355. https://doi.org/10.1093/ndt/gfae001